Abstract
Background:
This study examined patterns of physical activity and associations with pain, function, fatigue as well as sleep disturbance among individuals with knee or hip osteoarthritis (OA).
Methods:
Participants (n=54) were enrolled in a telephone-based physical activity coaching intervention trial; all data were collected at baseline. Self-reported measures of pain and function (WOMAC subscales), fatigue (10-point numeric rating scale), and PROMIS sleep disturbance were collected via telephone. Accelerometers were mailed to participants and were worn for at least 3 days. Proportion of time participants spent in sedentary behavior during the morning (from wake until 12:00 pm), afternoon (12:00 pm until 5:59 pm), and evening (6:00 pm until sleep) each day was averaged across all days of wear. Pearson correlations assessed associations between activity and self-reported measures.
Results:
Participants spent a large proportion of time in sedentary behavior: 65.6% of mornings, 70.0% of afternoons, and 76.6% of evenings. Associations between proportion of time spent in sedentary behavior and reported outcomes were generally strongest in the afternoon, strongest for WOMAC function, and lowest for PROMIS sleep disturbance. In the evening hours, sedentary time was most strongly associated with fatigue.
Conclusions:
Overall, findings stress the importance of reducing sedentary behavior among adults with OA and suggest behavioral interventions may be strengthened by considering patients’ within-day variation in symptoms and activity.
Keywords: Osteoarthritis, Physical Activity, Pain, Fatigue, Sleep, Function
BACKGROUND
Osteoarthritis (OA) is the most common joint disorder, affecting over 32.5 million adults in the US and rising in prevalence 1,2. Individuals with OA often struggle with daily pain, activity avoidance, lack of sleep, and overall decreased quality of life 3. Physical activity (PA) is a key component of improving function, pain, and other outcomes such as mood and sleep quality in the context of knee OA. The US Department of Health and Human Services and other organizations recommend 150 minutes of moderate to vigorous physical activity (MVPA) weekly5, but this can be difficult for many individuals with OA to obtain 6,7. There is some evidence that PA levels below general recommendations can still benefit individuals with OA, including reduced risk for functional limitations 8,9. However, we still know relatively little about how specific PA patterns are associated with outcomes like pain, sleep, and fatigue among individuals with OA.
Most PA studies have focused on total daily minutes spent in different behaviors, including MVPA and sedentary behavior 7,9,10. However, it is well recognized that PA patterns vary throughout the day 11, and some studies have evaluated PA behavior specifically during morning, afternoon, and evening periods 12–14. This is of interest because it can help to identify typical periods of sedentary behavior, which may be specific targets for intervention. In addition, some studies have shown that the relationship of PA with patient- and environmental-level characteristics varies by time of day13–15. From clinical and public health perspectives, this information is useful in tailoring interventions and forming specific recommendations for patients. However, little is known about PA patterns across the day among individuals with OA or other chronic pain conditions12,15,16.
In this study, we sought to describe PA patterns among individuals with hip and knee OA during morning, afternoon, and evening time periods. In addition, we were interested in the associations of these patterns with OA-related outcomes including pain, function, sleep, and fatigue, which have been associated with PA in previous studies 15,16. However, to our knowledge prior studies have not examined associations of these variables specifically with PA during morning, afternoon, and evening periods.
METHODS
Study Design
This was a secondary analysis of baseline data from a single-group, pre-post design pilot study; study methods and primary results have been published previously 19. Briefly, the study intervention involved three PA coaching calls (focused on goal setting), three check-in emails, and linkage with community-based or online resources to support PA. The study was approved by the Institutional Review Board of the University of North Carolina at Chapel Hill, the trial was registered at clinicaltrials.gov (NCT03780400) prior to study enrollment.
Study Sample
The OA-PCP program was developed to be compatible with delivery in the context of the Center for Medicare and Medicaid Services Chronic Care Management services, with the rationale that chronic care managers could deliver the content of OA-PCP within broader Chronic Care Management phone calls. Therefore, study eligibility criteria were designed with this in mind. Participants had to be age 65 or older, and have, in addition to a diagnosis of hip and/or knee OA, at least one other chronic health condition that qualified under Chronic Care Management guidelines. Participants had to report having current symptoms in a joint with OA, using the following validated item: “Do you have pain, aching or stiffness in your knees/hips on most days?” 20. Participants also had to self-report a pain score of ≥3 on a 0–10 numeric scale (0=no pain, 10=extreme pain) 21. Because the intervention was focused on increasing physical activity level, we only included individuals who self-reported not currently meeting the Department of Health and Human Services recommendation of at least 150 minutes MVPA per week 5. We used the PA Vital Sign measure to screen potential participants for PA level 22,23. Exclusion criteria, previously reported, were based on safety issues related to engaging in an independent exercise program, as well as health conditions that would make it infeasible to complete other aspects of the study 19.
Potential participants were first identified from among patients of participating primary care providers in three University of North Carolina clinics, using the electronic medical record. We identified patients age 65 and older with diagnosis codes for knee, hip or generalized OA, a diagnosis code for at least one qualifying comorbid health condition under Chronic Care Management guidelines, and no diagnosis codes for exclusionary health conditions. Because we included the diagnosis code for generalized OA (to avoid missing a large number of patients with knee or hip OA), we also conducted a chart review to verify the presence of OA in a knee or hip. Primary care providers reviewed lists of patients eligible based on the electronic medical record and approved a final list of patients to contact. We mailed these patients an introductory letter, signed by their primary care provider, and then a study team member called patients to further assess eligibility. Patients who were eligible and interested in participating completed a verbal consent process and were mailed a Health Insurance Portability and Accountability Act waiver form to sign and return. Then participants completed baseline assessments via telephone and were mailed an accelerometer for PA assessment. Participants were paid $25 for completing each phone-based assessment and $15 for returning the accelerometer at each time point. Following return of the accelerometer at baseline, participants began the OA-PCP intervention, although post OA-PCP data are not used in this analysis.
Measures
Physical Activity Assessment
Prior to receiving the OA-PCP intervention, each participant was asked to wear an Actigraph GT3X+ (Pensacola, FL) on an elastic belt, or belt clip, during waking hours for 7 days. Instructions were provided over the phone and mailed with the accelerometer in a pre-stamped / addressed return envelope. Upon return, accelerometer data were downloaded, summarized to 60-second epochs and processed to identify wear, non-wear, and wake periods using current algorithms, participant logs, and visual inspection of data 24,25. Minutes classified as non-wear were removed before estimating total daily waking wear and intensity outcomes. A valid day needed 8+ hours of waking wear, and participants with 3+ valid days were included in the analysis. Most participants (96%) had 5 or more days of wear. Data for the 3 participants with 3 or 4 days were examined and determined to be complete, consistent, and acceptable. Participants averaged 13.7 (standard deviation=2.3) hours of wear per day. Total minutes spent in sedentary behavior (< 100 counts per minute), light intensity PA (100–2019 counts per minute), and MVPA (2020+ counts per minute) were determined using common cut-points 25,26.
Minutes spent at each activity level were calculated overall and for three distinct time periods for each valid day of measurement: morning (from wake until 12:00 pm), afternoon (from 12:00 pm until 5:59 pm), and evening (from 6:00 pm until sleep). To account for differences in wear time across participants during each of these time periods, we converted raw minutes to the proportion of time spent in each behavior (e.g., sedentary, light intensity PA, and MVPA). In order to determine if day of wear should be considered in the analysis, participants’ patterns of time spent in sedentary behavior, light PA, and MVPA were examined across days. We observed a high degree of within-person consistency of time spent in PA and sedentary behavior over both days of observation (e.g. day1 vs day6) and day of week (e.g. weekday vs weekend). Therefore, we averaged minutes spent in each behavior across days for each participant. Due to the very low amount and variability of MVPA in this sample (less than 5 minutes per day), sedentary time was selected as the primary outcome for analysis.
Self-Report Measures
Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC):
We assessed self-reported pain and function using WOMAC subscales 27. The pain subscale includes 5 items, and the function subscale includes 17 items, all rated on a Likert scale of 0 (no pain or difficulty) to 4 (extreme pain or difficulty). Higher scores indicate worse pain and function. The reliability and validity of the WOMAC total score and subscales have been confirmed 29. In patients with hip or knee OA, Bellamy et al. reported internal consistency coefficient (Cronbach’s alpha) between 0.86 and 0.95 on WOMAC pain, stiffness, and function subscales. Construct validity has been confirmed by a significant association with the Lequesne Algofunctional Index for Knees 29.
PROMIS Sleep Disturbance Short Form 4a:
This scale assesses sleep quality, sleep depth, and restoration associated with sleep 30. It includes 4 items, with 5 response options ranging in value from 1 to 5. Results are presented as T-scores, with higher values indicating greater sleep disturbance. This scale was shown high correlation with the full PROMIS Sleep Disturbance bank score, high reliability, and construct validity related to general health and quality of life 31.
Stanford Visual Numeric Rating Scale (NRS) for Fatigue:
This is a single item measure, rated on a Likert scale of 0 (no fatigue) to 10 (severe fatigue) in the past 2 weeks 29. This measure is based on a pain visual NRS, which has strong correlations with health distress and general health 33.
Participant Characteristics:
We collected the following self-reported information to characterize the study sample: age, race / ethnicity, gender, education, work status, marital status, body mass index, comorbidities30, number of joints with arthritis symptoms, and duration of knee / hip OA symptoms.
Statistical Analyses
Descriptive statistics were calculated for participant characteristics using means and standard deviations for continuous variables and frequencies and percentages for categorical variables. Pearson’s correlation coefficients were calculated to determine the strength of associations between proportion of sedentary time (during morning, afternoon, and evening intervals) and each self-report measure (WOMAC pain, WOMAC function, PROMIS sleep disturbance, and fatigue NRS). As an exploratory analysis, we examined period of the day (morning, afternoon, evening) as a repeated effect to see if significant interactions existed between OA outcomes and time of day. To illustrate the relationship of WOMAC pain, WOMAC function, and fatigue scores with sedentary behaviors, we present box plots showing the proportion of sedentary time by quartile of each OA outcome.
RESULTS
Participants and Recruitment
We previously reported details regarding recruitment and enrollment into this study, and these data are summarized in Figure 1. Briefly, we identified 823 potentially eligible patients in the University of North Carolina Healthcare System electronic medical records and mailed introductory letters to 442 of these individuals who were eligible based on chart review. Among these patients, 70 screened eligible and 60 consented and completed baseline assessments. An additional 6 participants were excluded from these analyses because they did not have adequate accelerometer data based on metrics described above; there were no substantial differences in demographic or clinical characteristics between participants with and without adequate accelerometer data (data not shown). Of the remaining 54 participants in these analyses, 80% were women, 69% were White, the mean age was 72.8 ± 5.7 years old, and the mean BMI was 33.1 ± 7.2 kg/m2 (Table 2).
Figure 1.
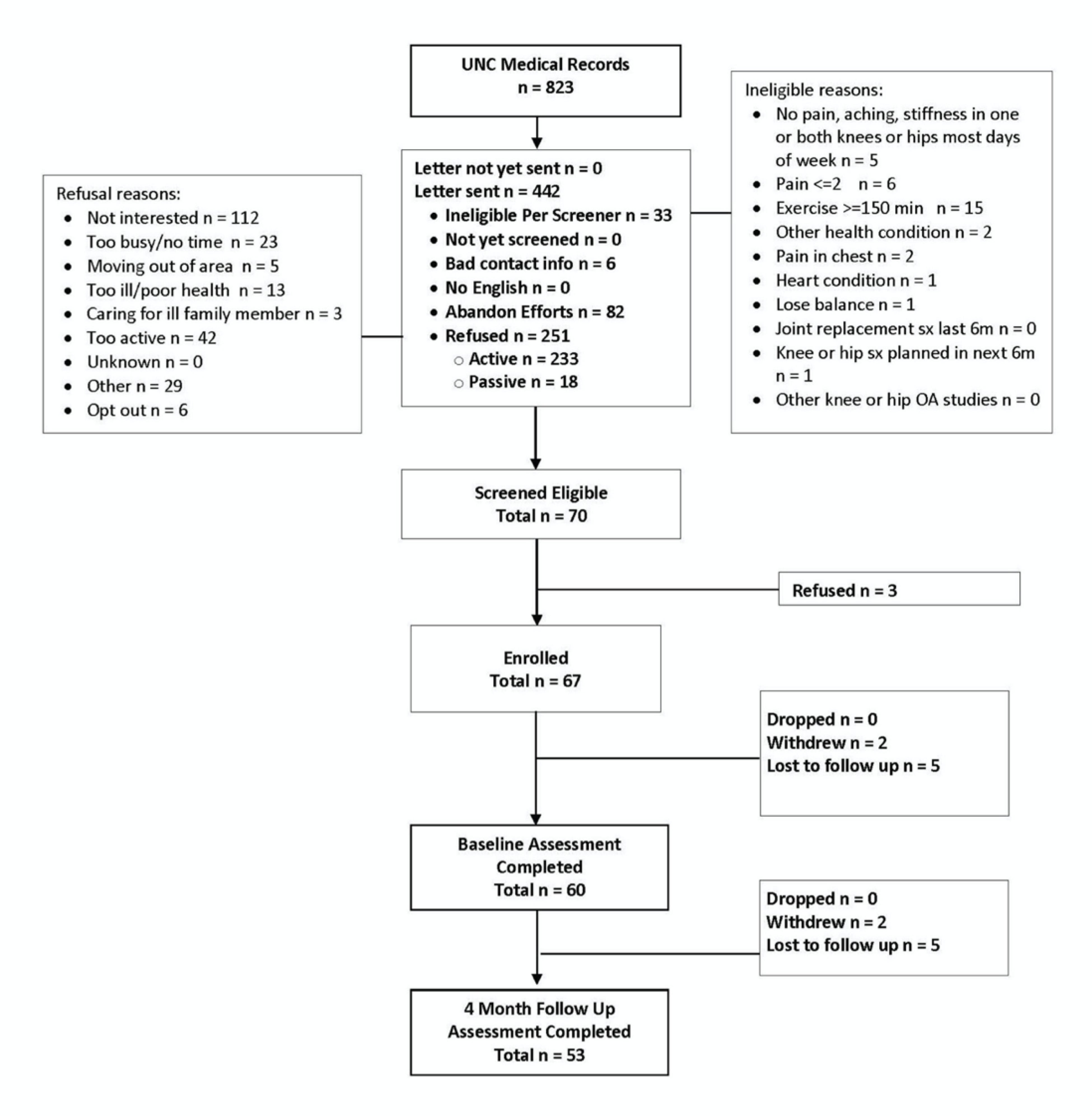
OA-PCP Study Participant Flow
Table 2.
Average times spent in each activity type throughout the day.
| Morning | Afternoon | Evening | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Activity Type | Avg. Activity Mins (SD) | Avg. Mins of Wear (SD) | % of time activity in wear (range) | Avg. Activity Mins (SD) | Avg. Mins of Wear (SD) | % of time activity in wear (range) | Avg. Activity Mins (SD) | Avg. Mins of Wear (SD) | % of time activity in wear (range) |
| Sedentary | 145.7 (55.3) | 222.2 (71.0) | 65.6 (35.2–91.6) | 243.6 (34.0) | 348.2 (17.4) | 70.0 (54.4–90.4) | 201.7 (65.6) | 262.0 (74.4) | 76.6 (52.7–90.8) |
| Light Activity | 73.2 (32.8) | 222.2 (71.0) | 33.0 (8.4–54.4) | 101.5 (33.5) | 348.2 (17.4) | 29.1 (9.6–45.6) | 59.3 (24.9) | 262.0 (74.4) | 23.0 (9.2–46.0) |
| MVPA | 3.3 (5.7) | 222.2 (71.0) | 1.4 (0–10.4) | 3.0 (4.8) | 348.2 (17.4) | 0.9 (0–6.2) | 1.0 (1.3) | 262.0 (74.4) | 0.4 (0–1.9) |
Physical Activity Patterns
Table 2 shows minutes spent in each PA category, accelerometer wear time, and percent time spent in each PA category for morning, afternoon, and evening hours. Low levels of MVPA were noted throughout the day (1.4% of mornings, 0.9% of afternoons, and 0.4% of evenings). Most of the day was spent in sedentary behavior, with some variation across times of day. Participants spent less time engaging in sedentary behavior in the morning with a gradual increase as the day continued (65.6% of mornings, 70% of afternoons, and 76.6% of evenings).
Associations of Physical Activity Patterns with Self-Report Measures
Table 3 shows the correlations between proportion of time spent in sedentary behavior and self-report measures for morning, afternoon, and evening; overall these correlations were small to moderate. Correlations with proportion of sedentary time were highest for the WOMAC function subscale (r=0.11–0.40). On the other hand, PROMIS Sleep Disturbance scores were the least correlated with sedentary behavior throughout the day (r= −0.10–0.11). Overall, sedentary behavior in the afternoon was more strongly correlated with self-report measures compared to morning or evening sedentary time; evening activity patterns had the lowest correlations with self-report measures overall. In repeated measures analyses (results not shown), sedentary behavior was not associated with the WOMAC pain subscale, PROMIS Sleep Disturbance scores, or fatigue, nor were interactions with the time period. A significant time-by-WOMAC function interaction term (p=0.004) indicated that the effect of WOMAC function differed across time periods, which supports our findings in simpler analyses. However, these results are exploratory because the study is not sufficiently powered to test for an interaction.
Table 3.
Associations between proportion of sedentary time and patient outcomes.
| Morning | Afternoon | Evening | ||||
|---|---|---|---|---|---|---|
| Patient Outcome | Pearson Correlation | p-Value | Pearson Correlation | p-Value | Pearson Correlation | p-Value |
| WOMAC Function | 0.30 | 0.03 | 0.40 | 0.003 | 0.11 | 0.42 |
| WOMAC Pain | 0.18 | 0.19 | 0.30 | 0.02 | 0.05 | 0.73 |
| Fatigue | 0.26 | 0.06 | 0.30 | 0.03 | 0.21 | 0.13 |
| *PROMIS Sleep Disturbance | −0.10 | 0.49 | 0.04 | 0.79 | 0.11 | 0.42 |
PROMIS Sleep Disturbance score missing for 1 participant (n=53)
Figure 2 illustrates the proportion of afternoon time spent in sedentary behavior, with participants grouped by quartiles of WOMAC function score, WOMAC pain score, and fatigue score. While it is difficult to identify a strong linear pattern in our small sample, the proportion of sedentary behavior tended to increase in participants having the poorest outcomes. These results should be viewed with caution due to the small numbers of participants in the second quartiles (n = 8 for WOMAC pain, n = 12 for WOMAC Function, and n = 5 for Fatigue) and high variation of afternoon PA in all groups. There was some consideration to illustrate these results as tertiles rather than quartiles due to the limited data present across the second quartiles, however, we felt it was more important to include the second quartiles in order to fully represent our sample.
Figure 2.
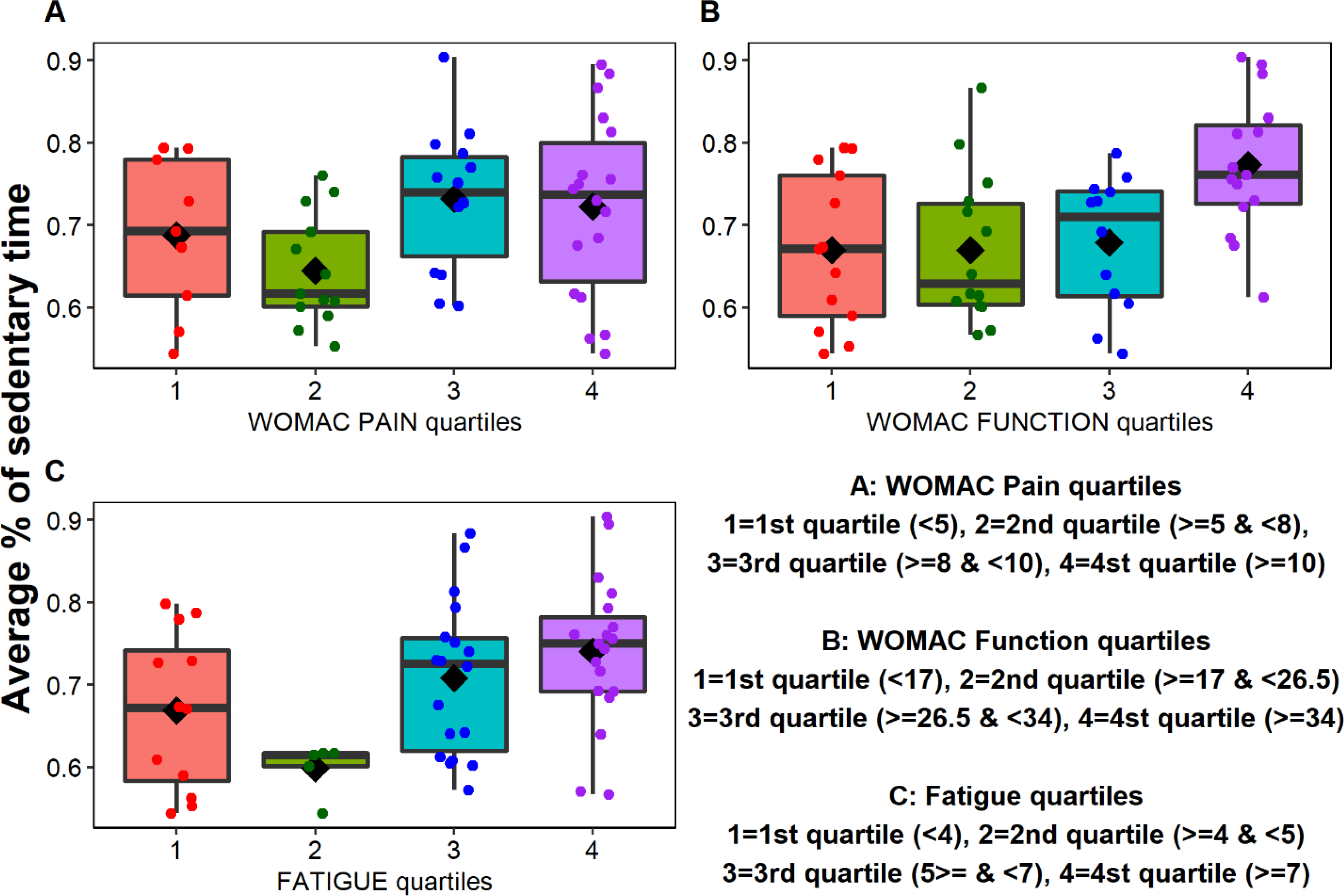
Proportion of afternoon time spent in sedentary behavior, based on quartiles of (A) WOMAC pain scores, (B) WOMAC function scores, and (C) fatigue scores. The boxplots show the mean (◇) and median (horizontal line drawn within the box); the dots are individual data points. The “whiskers” (the lines extending parallel from the boxes) indicate variability outside the upper and lower quartiles.
DISCUSSION
In this study, we assessed patterns of PA and sedentary behavior, as well as their associations with patient outcomes among individuals with knee or hip OA, with particular attention to patterns across different times of day. The first main finding was that participants spent the majority of their time in sedentary behavior (71% overall), with very little MVPA (mean 7.4 minutes/ day). These findings are consistent with other studies of PA patterns among individuals with lower extremity OA 32–34. Among individuals with or at risk for knee OA in the Osteoarthritis Initiative, 66% of waking time was spent in sedentary behavior, and the average amount of daily moderate or vigorous activity was less than 20 minutes 32. Activity levels were somewhat lower among participants in our study, and this may be due to a higher symptom burden compared with Osteoarthritis Initiative, some of whom did not have symptomatic OA. In a small study of German patients with knee OA, sedentary time was also high, with 88% of time spent in non-locomotion behavior 34. Results of these studies, along with ours, indicate the urgency of efforts to increase activity and reduce sedentary time among individuals with knee OA. This is particularly important given the associations of sedentary behavior on future functional limitations and frailty in these patients9,32.
Participants in our study were somewhat more active in the morning, with the greatest average proportion of sedentary behavior occurring in the evening; however, differences in the proportion of sedentary time across times of day were modest. Few studies have examined PA patterns across times of day among individuals with OA. However, findings of our study are consistent with prior research in this area. For example, in a sample of individuals with hip or knee OA, average minutes of activity was highest from waking time until 11:00 am, decreasing as the day progressed, with lower activity levels from 7:00 pm until bedtime 15; similar patterns were observed in a sample of women with hip or knee OA 34. In studies of older adults, PA was also highest in the morning, with increasing sedentary behavior throughout the day 36.37. Findings of these studies suggest that for older individuals and those with chronic pain, it may be easier to be active in the morning, as pain and/or fatigue may worsen as the day continues 35. While relationships of pain- and fatigue-related activity interference with PA behaviors are complex and may vary throughout the day, results of our study and others suggest it may be useful to address time of day in behavioral interventions aimed at increasing PA among individuals with OA. Specifically, PA counselors can help patients to consider times of day when pain and fatigue are lowest, as these may be the best times to engage in purposeful activity. However, patients should also be encouraged to consider feasible strategies for decreasing sedentary behavior during times when this tends to be most common (e.g., evening), given the detrimental impacts of high levels of sedentary behavior.
Correlations of patient outcomes with sedentary behavior in this study were relatively small. This is consistent with a prior study finding relatively small associations between pain and fatigue with average weekly PA 15. However, we observed somewhat stronger associations of function with sedentary time, particularly in the morning and afternoon. Because this was a cross-sectional study, the direction of this association cannot be determined. It is plausible that lower function led to greater sedentary behavior or the converse, and it is likely a bi-directional relationship 38. It is also interesting that associations of patient outcomes with sedentary behavior tended to be strongest in the afternoon. Murphy et al. found that among individuals with knee OA, differences in activity between those with low and high pain tended to be more accentuated during the afternoon hours, particularly relative to evening hours 15. Again, it is not possible to ascertain causality from our cross-sectional data; however, it is possible that symptoms are more likely to influence PA patterns during the afternoon time period compared with other times of day. During evening hours, fatigue was most strongly associated with the proportion of time spent in sedentary behavior. Other research has also found fatigue to be an important predictor of physical activity among individuals with OA 17,35. Our study’s finding that fatigue may be particularly relevant in the evening hours has implications for PA counseling. In particular, fatigue may be a particularly important barrier to address with respect to reducing sedentary behavior in the evening, when it is most common. The small correlation of sleep disturbance with PA was surprising, giving prior research showing that poor sleep quality was associated with less activity among individuals with or at risk for OA 16. This may be due to different measures of sleep quality or the overall lower level of PA in our study sample.
There are several limitations to this study. This was a small sample of 54 patients, and individuals who consented to participate in a trial of PA intervention may differ in their activity patterns relative to the general population of patients with OA. In addition, many patients who were contacted chose not to participate in the study; although this is common in clinical trials, there may have been differences between participants and non-participants with respect to baseline PA patterns. Along with the small sample size, the majority of the sample were female, Caucasian, and had some college education, which may also limit generalizability. Because wear time varied, there are some differences in the total number of minutes each person contributed to the 3 time periods examined. However, participants contributed substantial data to each time period, averaging 3.5 to 5.8 hours of wear. In addition, our approach of examining the proportion of time (rather than raw minutes) helps to account for differences in wear. Finally, other studies of PA in patients with knee OA have identified predictors that were not measured in our study, such as coping, self-efficacy, and mood 39,40.
CONCLUSION
In summary, we observed high levels of sedentary behavior among patients with knee or hip OA, which was somewhat accentuated during the evening hours, and small to modest associations of patient outcomes with activity, with strongest associations for physical function. This study is one of few to explore PA and sedentary behavior patterns across times of day among patients with OA, and further research in this area could help to inform behavioral interventions 12,41. Continued and more complex analyses of how outcomes such as pain, function, and fatigue influence PA patterns at different times of day could be particularly fruitful. Overall, our findings stress the importance of continuing efforts to enhance PA among individuals with knee OA and suggest that patient outcomes (e.g., pain, function, and fatigue), as well as daily variations in activity, should be considered within behavioral interventions.
Table 1.
Baseline Participant Characteristics (N=54)
| Mean age (SD), years | 72.8 (5.7) |
|
| |
| Gender | |
| N(%) Female | 43 (80%) |
|
| |
| Race | |
| N(%) White | 37 (69%) |
| N(%) Black / African American | 14 (26%) |
| N(%) Asian | 1 (2%) |
| N(%) Other | 1 (2%) |
|
| |
| Ethnicity | |
| N(%) Hispanic | 2 (4%) |
|
| |
| Education | |
| N(%) with at least some college | 44 (82%) |
|
| |
| Marital Status | |
| N(%) Married or living with partner | 35 (65%) |
|
| |
| Work Status | |
| N(%) Working full or part time | 16 (30%) |
|
| |
| Mean (SD) Body mass index, kg/m2 | 33.1 (7.2) |
|
| |
| Mean (SD) Number of comorbid illnesses* | 4.2 (1.7) |
|
| |
| Mean (SD) Years with arthritis symptoms | 11.1 (9.6) |
|
| |
| Mean (SD) Number joints with arthritis symptoms | 7.8 (2.8) |
|
| |
| Mean (SD) WOMAC Function Score | 26.3 (13.3) |
|
| |
| Mean (SD) WOMAC Pain Score | 8.0 (3.7) |
|
| |
| Mean Fatigue Score | 5.2 (2.4) |
|
| |
| Mean PROMIS Sleep Disturbance Score | 53.1 (2.6) |
Possible range: 0–16. Missing data: Race: n=1; Ethnicity: n=8; PROMIS Sleep Disturbance n=1
Acknowledgments:
The authors wish to thank all the participants in the OA-PCP study. All views expressed are those of the authors and do not reflect the official policy or views of the US Government, the Department of Veterans Affairs, or the University of North Carolina.
Funding: This study was funded by the National Institute on Aging (NIA), “UNC-CH Summer Research Training in Aging for Medical Students” Award Number Grant 2T35AG038047. The funding agency did not have a role in the analysis of data or interpretation of results.
ABBREVIATIONS
- MVPA
Moderate to Vigorous Physical Activity
- NRS
Numeric Rating Scale
- OA
Osteoarthritis
- OA-PCP
Osteoarthritis Physical Activity Care Pathway
- PA
Physical Activity
- PROMIS
Patient Reported Outcomes Measurement Information System
- WOMAC
Western Ontario and McMaster Universities Osteoarthritis Index
Footnotes
Competing interests: The authors’ institution received funding to conduct this project from the National Institutes of Health. No other conflicts of interest declared.
Declarations
Ethics approval and consent to participate: This study was reviewed and approved by the Institutional Review Board of the University of North Carolina at Chapel Hill. All participants provided written informed consent and HIPAA authorization.
Trial registration: NCT03780400, December 19, 2018
Contributor Information
Tyler Beauchamp, Medical College of Georgia at Augusta University, 1301 R.A. Dent Blvd, Augusta, GA 30901.
Liubov Arbeeva, Department of Medicine and Thurston Arthritis Research Center, University of North Carolina, 3330 Thurston Bldg, CB #7280, Chapel Hill, NC, USA..
Rebecca J. Cleveland, Department of Medicine and Thurston Arthritis Research Center, University of North Carolina, 3330 Thurston Bldg, CB #7280, Chapel Hill, NC, USA.
Yvonne M. Golightly, Department of Epidemiology, Injury Prevention Research Center, Division of Physical Therapy and Thurston Arthritis Research Center, University of North Carolina, 3330 Thurston Bldg, CB #7280, Chapel Hill, NC, USA.
Derek P. Hales, Department of Nutrition Gillings School of Global Public Health and Center for Health Promotion and Disease Prevention, University of North Carolina, 1700 M.L.K. Jr Blvd #7426, Chapel Hill, NC, USA.
David G. Hu, Department of Medicine and Thurston Arthritis Research Center, University of North Carolina, 3330 Thurston Bldg, CB #7280, Chapel Hill, NC, USA..
Kelli Allen, Department of Medicine and Thurston Arthritis Research Center, University of North Carolina, 3330 Thurston Bldg, CB #7280, Chapel Hill, NC, USA.
Availability of data and material:
The full data sets generated during the current study are not publicly available because they include personally identifiable data from study participants. However, de-identified data are available from the corresponding author on reasonable request.
References:
- 1.US Bone and Joint Initiative. The Burden of Musculoskeletal Diseases in the United States (BMUS), Fourth Edition Rosemont, IL: http://www.boneandjointburden.org. Accessed Sept 12, 2019. [Google Scholar]
- 2.Hootman JM, Helmick CG, Barbour KE, Theis KA, Boring MA. Updated projected prevalence of self-reported doctor-diagnosed arthritis and arthritis-attributable activity limitation among US adults, 2015–2040. Arthritis Rheumatol. 2016;68(7):1582–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Osteoarthritis Research Society International. Osteoarthritis: a Serious Disease, Submitted to the U.S. Food and Drug Administration. 2016. https://www.oarsi.org/research/oa-serious-disease. Accessed December 3, 2020.
- 4.Kolasinski SL, Neogi T, Hochberg MC, et al. 2019. American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res (Hoboken). 2020;72(2):149–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.US Department of Health and Human Services. Physical Activity Guidelines for Americans, 2nd Edition. 2018. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf. Accessed January 27, 2021.6.
- 6.Farr JN, Going SB, Lohman TG, et al. Physical activity levels in patients with early knee osteoarthritis measured by accelerometry. Arthritis Rheum. 2008;59(9):1229–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang AH, Song J, Lee J, Chang RW, Semanik PA, Dunlop DD. Proportion and associated factors of meeting the 2018 Physical Activity Guidelines for Americans in adults with or at risk for knee osteoarthritis. Osteoarthritis Cartilage. 2020;28(6):774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White DK, Tudor-Locke C, Zhang Y, et al. Daily walking and the risk of incident functional limitation in knee osteoarthritis: an observational study. Arthritis Care Res (Hoboken). 2014;66(9):1328–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunlop DD, Song J, Hootman JM, et al. One Hour a Week: Moving to Prevent Disability in Adults With Lower Extremity Joint Symptoms. Am J Prev Med. 2019;56(5):664–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thoma L, Dunlop D, Song J, et al. Are older adults with symptomatic knee osteoarthritis less active than the general population?: Analysis from the Osteoarthritis Initiative and NHANES. Arthritis Care and Research. 2018;70(10):1448–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy SL, Smith DM, Lyden AK. Type of activity pacing instruction affects physical activity variability in adults with symptomatic knee or hip osteoarthritis. J Phys Act Health. 2012;9(3):360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller MB, Roumanis MJ, Kakinami L, Dover GC. Chronic Pain Patients’ Kinesiophobia and Catastrophizing are Associated with Activity Intensity at Different Times of the Day. J Pain Res. 2020;13:273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Buul AR, Kasteleyn MJ, Chavannes NH, Taube C. Physical activity in the morning and afternoon is lower in patients with chronic obstructive pulmonary disease with morning symptoms. Respir Res. 2018;19(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cerin E, Mitas J, Cain KL, et al. Do associations between objectively-assessed physical activity and neighbourhood environment attributes vary by time of the day and day of the week? IPEN adult study. Int J Behav Nutr Phys Act. 2017;14(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy SL, Schepens Niemiec S, Lyden AK, Kratz AL. Pain, Fatigue, and Physical Activity in Osteoarthritis: The Moderating Effects of Pain- and Fatigue-Related Activity Interference. Arch Phys Med Rehabil. 2016;97(9 Suppl):S201–209. [DOI] [PubMed] [Google Scholar]
- 16.Gilbert AL, Lee J, Song J, et al. Relationship Between Self-Reported Restless Sleep and Objectively Measured Physical Activity in Adults With Knee Osteoarthritis. Arthritis Care Res (Hoboken). 2018(Online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy SL, Alexander NB, Levoska M, Smith DM. The relationship between fatigue and subsequent physical activity among older adults with symptomatic osteoarthritis. Arthritis Care Res (Hoboken). 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burrows NJ, Barry BK, Sturnieks DL, Booth J, Jones MD. The Relationship Between Daily Physical Activity and Pain in Individuals with Knee Osteoarthritis. Pain Med. 2020;21(10):2481–2495 [DOI] [PubMed] [Google Scholar]
- 19.Allen K, Vu MB, Callahan LF, et al. Osteoarthritis physical activity care pathway (OA-PCP): results of a feasibility trial. BMC Musculoskelet Disord. 2020;21(1):308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen US, Zhang Y, Zhu Y, Niu J, Zhang B, Felson DT. Increasing prevalence of knee pain and symptomatic knee osteoarthritis: survey and cohort data. Ann Intern Med. 2011;155(11):725–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McAlindon TE, Driban JB, Henrotin Y, et al. OARSI Clinical Trials Recommendations: Design, conduct, and reporting of clinical trials for knee osteoarthritis. Osteoarthritis Cartilage. 2015;23(5):747–760. [DOI] [PubMed] [Google Scholar]
- 22.Coleman KJ, Ngor E, Reynolds K, et al. Initial validation of an exercise “vital sign” in electronic medical records. Med Sci Sports Exerc. 2012;44(11):2071–2076. [DOI] [PubMed] [Google Scholar]
- 23.Ball TJ, Joy EA, Gren LH, Cunningham R, Shaw JM. Predictive Validity of an Adult Physical Activity “Vital Sign” Recorded in Electronic Health Records. J Phys Act Health. 2016;13(4):403–408. [DOI] [PubMed] [Google Scholar]
- 24.Choi L, Ward SC, Schnelle JF, Buchowski MS. Assessment of wear/nonwear time classification algorithms for triaxial accelerometer. Med Sci Sports Exerc. 2012;44(10):2009–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Medicine and Science in Sports and Exercise. 2008;40(181–188). [DOI] [PubMed] [Google Scholar]
- 26.Migueles JH, Cadenas-Sanchez C, Ekelund U, et al. Accelerometer Data Collection and Processing Criteria to Assess Physical Activity and Other Outcomes: A Systematic Review and Practical Considerations. Sports Med. 2017;47(9):1821–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matthews CE. Calibration of accelerometer output for adults. Med Sci Sports Exerc. 2005;37(11 Suppl):S512–522. [DOI] [PubMed] [Google Scholar]
- 28.Bellamy NA 20-year experiential review of a patient-centered self-reported health status questionnaire. Journal of Rheumatology. 2002;29(12):2473–2476. [PubMed] [Google Scholar]
- 29.Lorig KR, Ritter PL, Laurent DD, Plant K. The internet-based arthritis self-management program: a one-year randomized trial for patients with arthritis or fibromyalgia. Arthritis Rheum. 2008;59(7):1009–1017. [DOI] [PubMed] [Google Scholar]
- 30.Yu L, Buysse DJ, Germain A, et al. Development of short forms from the PROMIS sleep disturbance and Sleep-Related Impairment item banks. Behav Sleep Med. 2011;10(1):6–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The self-administered comorbidity questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis & Rheumatism. 2003;49(2):156–163. [DOI] [PubMed] [Google Scholar]
- 32.Song J, Lindquist LA, Chang RW, et al. Sedentary Behavior as a Risk Factor for Physical Frailty Independent of Moderate Activity: Results From the Osteoarthritis Initiative. Am J Public Health. 2015;105(7):1439–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verlaan L, Bolink SA, Van Laarhoven SN, et al. Accelerometer-based Physical Activity Monitoring in Patients with Knee Osteoarthritis: Objective and Ambulatory Assessment of Actual Physical Activity During Daily Life Circumstances. Open Biomed Eng J. 2015;9:157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sliepen M, Mauricio E, Lipperts M, Grimm B, Rosenbaum D. Objective assessment of physical activity and sedentary behaviour in knee osteoarthritis patients - beyond daily steps and total sedentary time. BMC Musculoskelet Disord. 2018;19(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy SL, Smith DM, Clauw DJ, Alexander NB. The impact of momentary pain and fatigue on physical activity in women with osteoarthritis. Arthritis Care and Research. 2008;59(6):849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sartini C, Wannamethee SG, Iliffe S, et al. Diurnal patterns of objectively measured physical activity and sedentary behaviour in older men. BMC Public Health. 2015;15:609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schrack JA, Zipunnikov V, Goldsmith J, et al. Assessing the “physical cliff”: detailed quantification of age-related differences in daily patterns of physical activity. J Gerontol A Biol Sci Med Sci. 2014;69(8):973–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Metti AL, Best JR, Shaaban CE, Ganguli M, Rosano C. Longitudinal changes in physical function and physical activity in older adults. Age Ageing. 2018;47(4):558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhaoyang R, Martire LM, Darnall BD. Daily pain catastrophizing predicts less physical activity and more sedentary behavior in older adults with osteoarthritis. Pain. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhaoyang R, Martire LM, Sliwinski MJ. Morning self-efficacy predicts physical activity throughout the day in knee osteoarthritis. Health Psychol. 2017;36(6):568–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song J, Dunlop DD, Semanik PA, et al. Reallocating time spent in sleep, sedentary behavior and physical activity and its association with pain: a pilot sleep study from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2018;26(12):1595–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The full data sets generated during the current study are not publicly available because they include personally identifiable data from study participants. However, de-identified data are available from the corresponding author on reasonable request.


