Summary
Splenic stromal sarcomas are rarely reported tumours that were previously grouped as non-angiomatous, non-lymphomatous mesenchymal neoplasms of the canine spleen. Highly variable survival times have been reported probably due to their heterogeneous nature. The purpose of this study was to assess the outcome and prognostic factors in dogs with splenic stromal sarcoma after treatment by splenectomy. Clinical data were collected retrospectively and histopathology was reviewed for 47 patients. Histological classification, based on morphology in haematoxylin and eosin (HE)-stained sections, in conjunction with immunolabelling of macrophage scavenger receptor-A (CD204), desmin, factor VIII-related antigen and smooth muscle actin (SMA), yielded diagnoses of undifferentiated stromal sarcoma (n = 22), complex nodular hyperplasia (CNH, n = 9), sarcoma arising from benign complex nodular hyperplasia (n = 3), histiocytic sarcoma (n = 3), haemangiosarcoma (n = 1) and leiomyosarcoma (n = 1). Four samples were excluded from analysis due to extensive necrosis. An anti-podoplanin (PDPN) antibody was validated on canine tissue and used to assess expression of this protein as a potential indicator of the tissue of origin of the neoplasms (28/42 tumours were positive). There was a statistically significant difference in survival time between patients with stromal sarcoma (sarcoma from benign CNH and undifferentiated stromal sarcoma) and CNH (178 d versus 637 d, respectively; P = 0.027). Dogs with stromal sarcomas and high mitotic count (≥9 per 10 high-power fields) had a significantly shorter survival time (67 d versus 439 d; P = 0.01). Clinical diagnosis of splenic tumours should include evaluation for the presence of benign nodular hyperplasia morphology and immunohistochemistry to exclude more aggressive malignancies where adjuvant therapy is recommended. As in humans, PDPN may be an effective marker for stromal sarcomas of the canine spleen and immunopositivity suggests a fibroblastic reticular or follicular dendritic cell origin.
Keywords: complex nodular hyperplasia, splenic fibrohistiocytic nodule, splenic mass, stromal sarcoma
Introduction
Canine splenic stromal tumours are considered primary splenic mesenchymal neoplasms with spindle cell morphology and are suspected to originate from multipotent stem cell precursors (Valli et al, 2016). They were previously characterized as non-angiomatous, non-lymphomatous tumours, including fibrosarcoma, leiomyosarcoma, osteosarcoma, mesenchymoma, myxosarcoma, histiocytic sarcoma, liposarcoma and malignant fibrous histiocytoma (Spangler et al, 1994), and were reported to have a high metastatic rate and grave prognosis with a median survival time of 2.5 months after splenectomy (Weinstein et al, 1989). Biological behaviour has been most closely correlated with the mitotic count (MC) of these tumours, with a MC ≤9 associated with a significantly longer survival time (7 months versus 1–2 months; P <0.0001) (Spangler et al, 1994).
Categorization of a subset of these tumours into distinct histotypes based on standard light microscopy is exceptionally difficult, as there is significant histomorphological overlap between fibrosarcoma, leiomyosarcoma, histiocytic sarcoma and undifferentiated sarcoma (Spangler et al, 1994). Additionally, the appearance of splenic complex nodular hyperplasia (CNH), which can be dominated by stromal cells or histiocytes, can complicate attempts at classification (Moore et al, 2012). Not only can lesions of CNH and stromal sarcoma overlap morphologically with histiocytic sarcoma and lymphoma (collectively described as splenic fibrohistiocytic nodules [SFHNs]) (Spangler and Kass, 1998) but specifically, differentiating CNH with prominent fibrohistiocytic stromal expansion (ie, grade II SFHNs) from genuine but well-differentiated and inflamed stromal sarcomas (ie, grade III SFHNs) is particularly difficult.
This difficulty, in conjunction with adoption of the revised diagnostic terminology for canine nodular splenic masses (Moore et al, 2012; Sabattini et al, 2018), has led to increasing usage of the umbrella diagnosis of splenic stromal sarcoma in veterinary oncological pathology. This trend has led to confusion among veterinary oncologists and pathologists, and could have negative consequences for canine cancer patient management due to: (1) indirectly leading to omission of meaningful prognostic information provided by the original lesion morphology characterization, or (2) implying a tumour of known mixed and complex cellular origin whose diagnosis is not aided by the use of immunohistochemistry (IHC), thus potentially discouraging further immunohistochemical characterization of these masses, despite the known risk of their morphological overlap with more biologically aggressive splenic tumour entities.
Podoplanin (PDPN) is a transmembrane glycoprotein that is expressed on lymphatic endothelium and glomerular podocytes, and is a known marker for fibroblastic reticular cell subsets of murine and human lymph node and spleen (Yu et al, 2007; Astarita et al, 2012; Malhotra et al, 2012; Chai et al, 2013; Astarita et al, 2015; Cheng et al, 2019). Additionally, PDPN expression has also been reported in several different human cancers, including fibroblastic reticular cell and follicular dendritic cell sarcomas, which arise from stromal elements (Yu et al, 2007; Xie et al, 2008; Krishnan et al, 2018).
Thus, we aimed to conduct a retrospective study in dogs with previously diagnosed splenic stromal sarcoma in order to provide a more comprehensive histomorphological and immunohistochemical characterization of these splenic stromal sarcoma neoplasms, as well as evaluate clinical findings and outcomes in dogs treated by splenectomy in order to more specifically clinically define this poorly characterized canine tumour.
Materials and Methods
Patient Selection and Assessment
Dogs included in this study were identified through a search of the histopathology submissions to the Colorado State University Veterinary Diagnostic Laboratory (CSU VDL) from July 2010 to February 2017. Initial inclusion criteria were a histopathological diagnosis of stromal sarcoma, anaplastic sarcoma with concern for stromal sarcoma or malignant fibrohistiocytic nodule of splenic origin, and sufficient clinical data and follow-up for at least 1 day postoperatively. Cases that were immunohistochemically consistent with endothelial or histiocytic origin were removed from final data analysis. Medical records were reviewed retrospectively. Case information was requested by fax or e-mail from the submitting clinic if samples had been submitted from outside Colorado State University’s Veterinary Teaching Hospital. Data collected included breed, sex, age at the time of diagnosis, date of diagnosis, staging diagnostics performed, date and type of adjuvant therapy and date of last follow-up. Date of diagnosis was classified as the date of exploratory laparotomy. Outcome assessment was based on overall postoperative survival time.
Histopathology and Immunohistochemistry
Archived, formalin-fixed, paraffin-embedded (FFPE) tissue samples were obtained from the CSU VDL. Available blocks were routinely processed for haematoxylin and eosin (HE) staining and IHC. IHC was performed by routine, automated methods on the Leica Bond Max autostainer (Leica Biosystems, Buffalo Grove, Illinois, USA), with the following panel of primary antibodies: monoclonal mouse anti-human CD204 (1:200, clone SRA-E5; TransGenic, Chuo-ku, Kobe, Japan), monoclonal mouse anti-human desmin (RTU, clone DE-R-11; Leica Biosystems), polyclonal rabbit anti-human von Willebrand factor/factor VIII-related antigen (1:1,000, A008202–5; Dako, Santa Clara, California, USA), monoclonal mouse anti-human SMA (RTU, clone alpha sm-1/1A4; Leica Biosystems) and rabbit monoclonal anti-human PDPN/gp36 (1:100/2.9 μg/ml; clone EPR22182; Abcam, Waltham, Massachussetts, USA). For CD204, PDPN and von Willebrand factor, antigen retrieval was performed using Leica Epitope Retrieval 2 (Tris-EDTA buffer, pH 9) for 20 min (CD204 and PDPN) or 10 min (von Willebrand factor). For desmin and SMA, antigen retrieval was performed using Leica Epitope Retrieval 1 (sodium citrate buffer, pH 6), for 20 min and 10 min, respectively. Detection was performed with PowerVision IHC detection systems (Leica Biosystems), using either a polymeric horseradish peroxidase anti-mouse IgG (CD204) or anti-rabbit IgG (PDPN) and 3,3’-diaminobenzidine (DAB) chromogen, polymeric alkaline phosphatase anti-mouse IgG (desmin, SMA) or anti-rabbit IgG (von Willebrand factor) and Fast Red chromogen. Positive control tissues consisted of lung (factor VIII-related antigen), urinary bladder (SMA), skeletal muscle (desmin) and soft tissue sarcoma-tumour macrophages (CD204). Negative controls consisted of wash buffer used in place of the primary antibody for all markers. Non-immune rabbit IgG was also used as a negative control for the anti-PDPN antibody. All HE-stained slides were reviewed by a single pathologist (DPR), blinded to clinical outcome. For IHC analysis of all markers, tumours were considered positive if ≥25% of neoplastic cells (or stromal cell fraction between lymphoid follicles in cases of CNH) were immunopositive for the respective marker. Tumours were described by combining the criteria of Sabattini et al (2018), Spangler and Kass (1998) and Meuten (Valli et al, 2016) as follows:
CNH. Lymphoid component with characteristics similar to lymphoid nodular hyperplasia, associated with proliferation of splenic stromal elements (fibroblasts, smooth muscle cells, histiocytes) accounting for more than 30% of the nodule section (grade II and grade III SFHNs that fully retain nodular architecture). Mitotic activity and cellular pleomorphism absent (Fig. 1).
Sarcoma arising from CNH. Retains an overall architecture consistent with CNH. Most (>50%) of the expanded stromal component between lymphoid nodules displays a mixed morphology and lacks mitotic activity and cellular pleomorphism, consistent with CNH. However, a focal (<50%) region of this stromal component demonstrates a definitive transition to a monomorphic population of mesenchymal neoplastic cells similar to those of undifferentiated stromal sarcoma (Fig. 2).
Undifferentiated stromal sarcoma. Complete loss of nodular architecture, any remaining lymphocytes are present as small loosely aggregated fading follicular structures or diffuse infiltrates. There is a definitive and majority population of mesenchymal neoplastic cells characterized by dense interlacing, streaming bundles of pleomorphic polygonal to spindle-shaped cells, in conjunction with any combination of CD204 (positive due to reactive tumour-associated macrophages rather than neoplastic cell immunolabelling), desmin and PDPN labelling, regardless of SMA reactivity (Figs. 3 and 4).
Fig. 1.
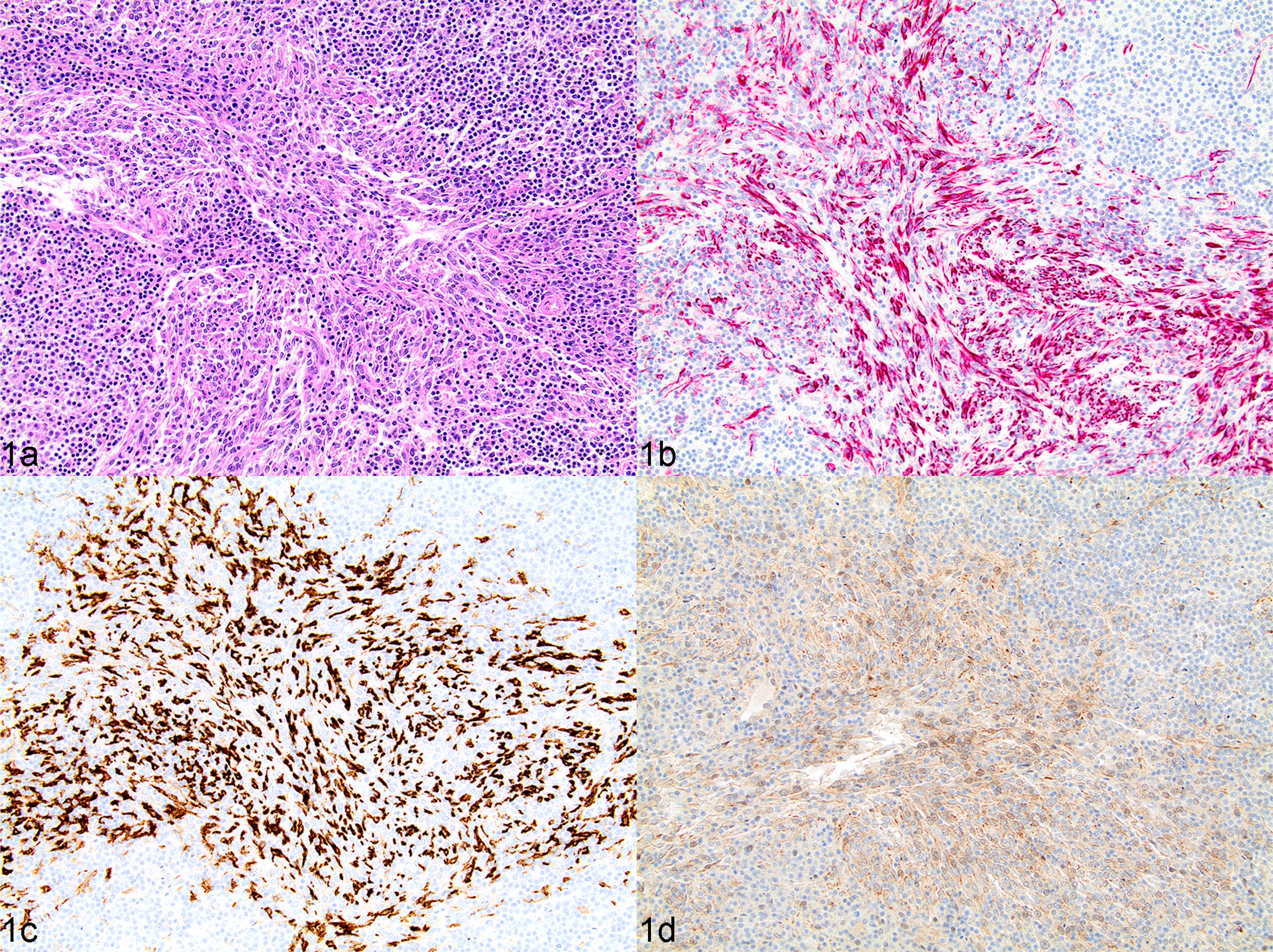
Complex nodular hyperplasia, spleen, dog, case 18. (a) Multinodular splenic lesion characterized by a mixture of approximately equal parts of fibrohistiocytic and lymphoid cells. HE. (b and c) Strong cytoplasmic immunolabelling of desmin (b) and CD204 (c) in most fibrohistiocytic cells present among lymphoid cells. IHC. (d) Fibrohistiocytic cells have uniformly weak to moderate cytoplasmic labelling of PDPN. IHC.
Fig. 2.
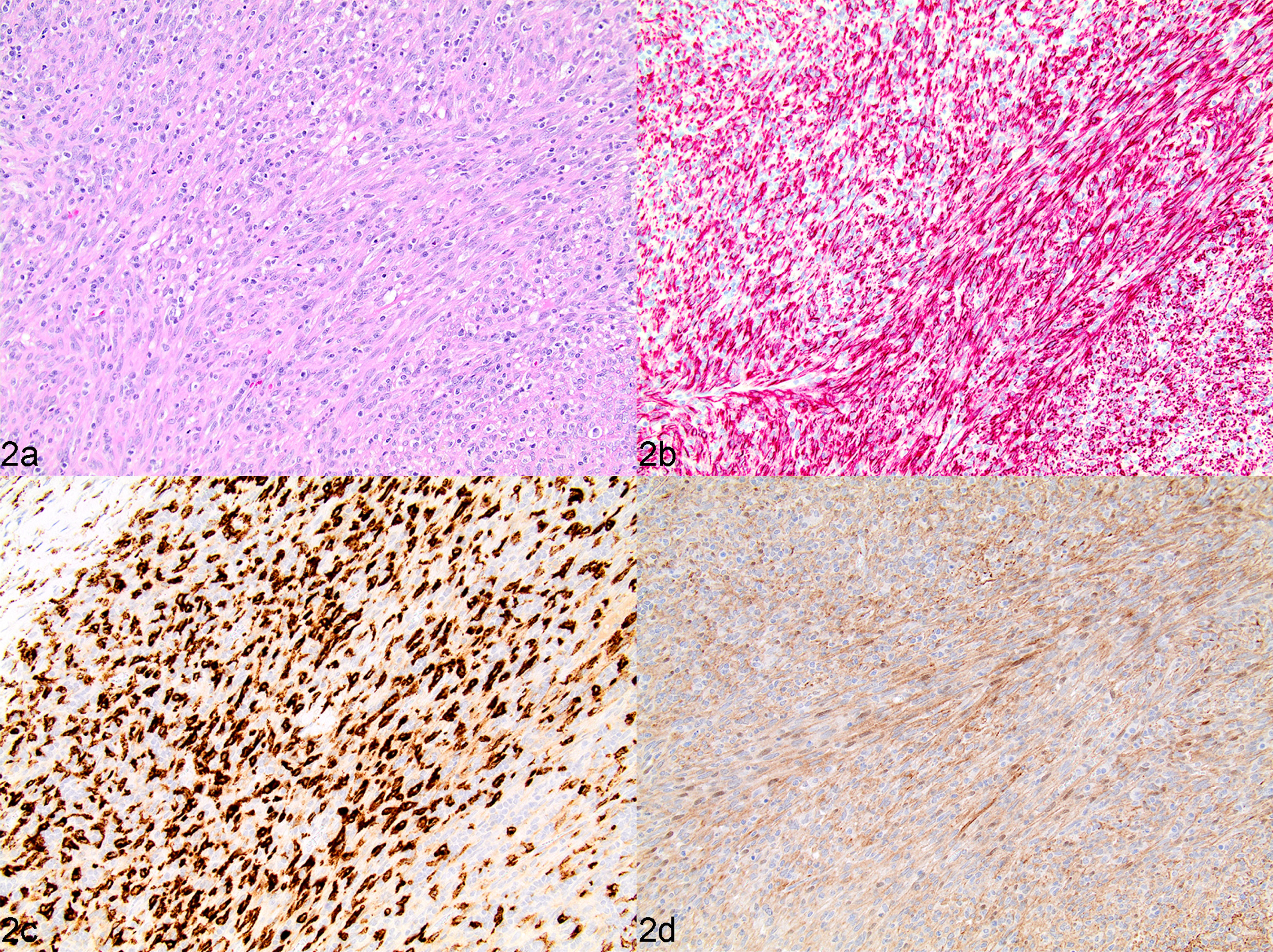
Sarcoma from complex nodular hyperplasia, spleen, dog, case 17. (a) Splenic mass characterized by a predominance of fibrohistiocytic cells admixed with a low percentage (<40%) of lymphoid cells, classified as a grade III splenic fibrohistiocytic nodule. HE. (b and c) Strong cytoplasmic immunolabelling of desmin (b) and CD204 (c) in vast majority of fibrohistiocytic cells. IHC. (d) Fibrohistiocytic cells have uniformly moderate to strong cytoplasmic labelling of PDPN. IHC.
Fig. 3.
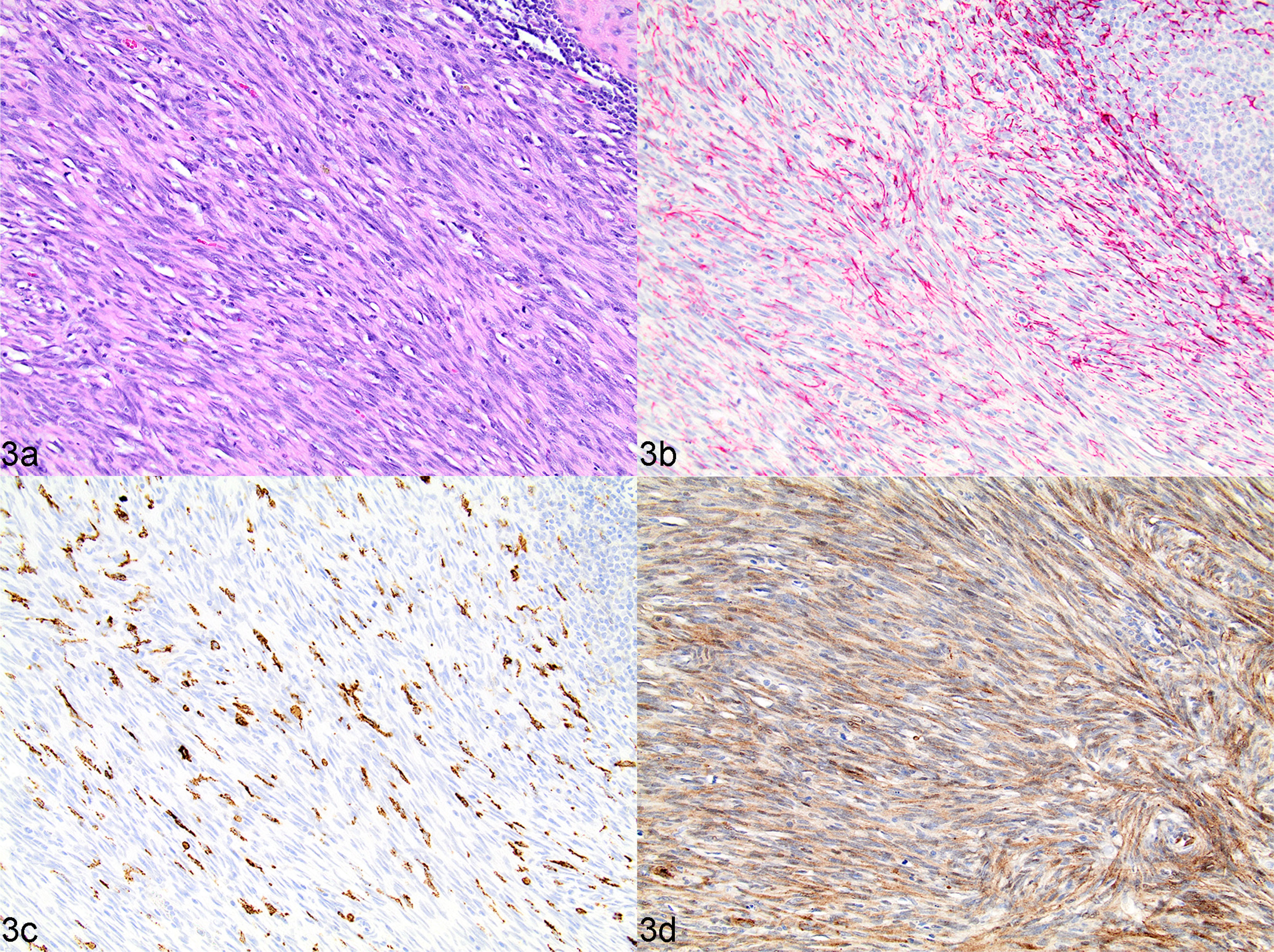
Undifferentiated stromal sarcoma (high-grade, fibroblastic reticular), spleen, dog, case 37. (a) Neoplasm composed of a uniform population of streaming bundles of spindle-shaped cells with indistinct cell borders and mild to moderate anisokaryosis, with multifocal infiltrates of lymphocytes and plasma cells. HE. (b) Neoplastic spindle cells variably immunopositive for desmin. IHC. (c) CD204 immunolabelling in a few cells scattered throughout the neoplasm, interpreted as remnant histiocytes or infiltrating tumour-associated macrophages. IHC. (d) Neoplastic spindle cells uniformly strongly immunolabelled for PDPN. IHC.
Fig. 4.
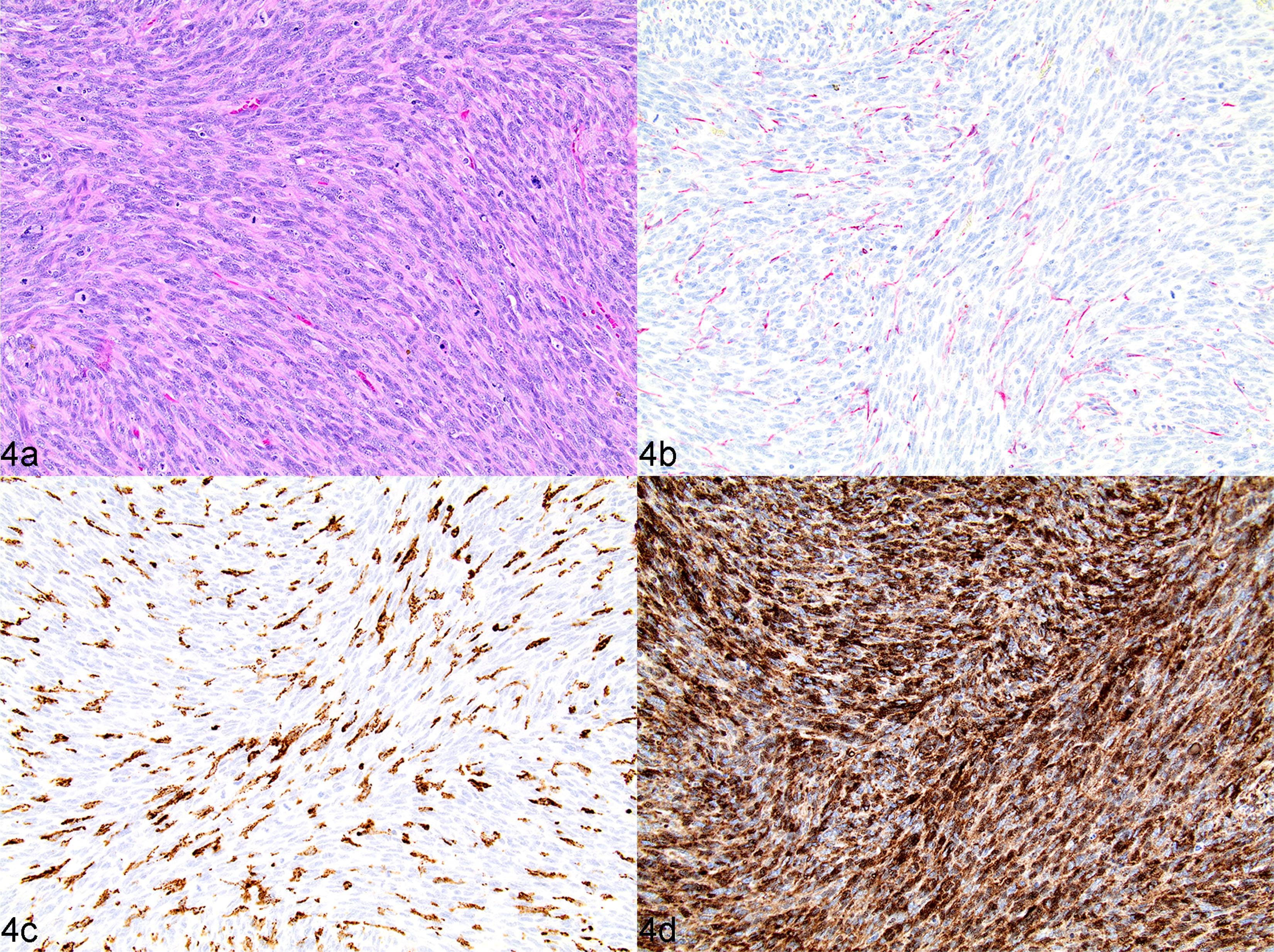
Undifferentiated stromal sarcoma (high-grade, fibroblastic reticular), spleen, dog, case 2. (a) Neoplasm composed of a uniform population of streaming bundles of polygonal to spindle-shaped mesenchymal cells with indistinct cell borders, mild to moderate anisokaryosis and a high mitotic rate. HE. (b) Neoplastic spindle cells immunonegative for desmin. IHC. (c) CD204 labelling in a minority of individualized cells throughout neoplasm, interpreted as remnant histiocytes or infiltrating tumour-associated macrophages. IHC. (d) Neoplastic spindle cells have uniformly strong cytoplasmic immunolabelling of PDPN. IHC.
MC was calculated as the number of mitotic figures per 10 high-power fields (HPFs; ×400; 2.59 mm2) and was evaluated in the tumour region of highest mitotic activity. The MC was standardized to 2.37 mm2 using the multiplication strategy reported by Meuten (Meuten et al, 2016).
Western Blot Validation of Anti-Podoplanin Antibody on Canine Tissue
The human U2OS osteosarcoma and canine DH82 histiocytic sarcoma cell lines were used as positive controls for western blot assays. The U2OS cell line (HTB-96) was purchased from the American Type Culture Collection (Manassas, Virginia, USA). The DH82 cell line (CRL-10389) was provided by Dawn Duval (Flint Animal Cancer Center) and had been originally purchased from the American Type Culture Collection. Cells were maintained in Dulbecco’s Modified Eagle Medium (Gibco, Grand Island, New York, USA) supplemented with 10% fetal bovine serum (Atlas Biologicals, Fort Collins, Colorado, USA), penicillin (100 U/ml), streptomycin (100 μg/ml), L-glutamine (2mM) and non-essential amino acids (0.1mM) (all obtained from Gibco). Cells were grown on standard plastic tissue culture flasks (Cell Treat, Shirley, Massachusetts, USA), incubated under standard conditions of 37°C, 5% CO2 and humidified air. Cells were grown to ~80% confluence and lysates were generated by removing the media from the flasks, washing ×1 in cold phosphate buffered saline, and incubating with 1 ml M-PER lysate buffer (7 ml M-PER; ThermoFisher, Waltham, Massachusetts, USA), 140 mg sodium dodecyl sulfate (ThermoFisher), 70 μl of 100mM phenylmethylsulfonyl fluoride (ThermoFisher), 35 μl of 200mM sodium orthovanadate (Sigma-Aldrich, St. Louis, Missouri, USA) and half a tablet of cOmplete Mini Protease Inhibitor (Roche, Basel, Switzerland) for 5 min on ice. Lysates were removed from the flasks and homogenized using a pipette before protein concentrations were measured using a Pierce BCA Protein Assay Kit (ThermoFisher).
37.5 μg DH82 cell lysate protein and 15 μg U2OS cell lysate protein were mixed 1:4 with 4× Laemelli sample buffer containing 5% 2-mercaptoethanol (BioRad Laboratories, Hercules, California, USA), boiled for 5 min, cooled on ice and then loaded in a 50 μl volume and 20 μl volume, respectively, into a Mini-Protean TGX 4–20% pre-cast polyacrylamide gel (BioRad Laboratories) for sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). SDS-PAGE was performed at 180V for approximately 40 min. Protein was then transferred wet to nitrocellulose membranes using the Trans-blot Turbo Transfer System (1.3A, 25V, 7 min) (BioRad Laboratories) and the membranes were blocked for 1 h at room temperature (RT) in a 5% non-fat dry milk in Tris-buffered saline Tween 20 solution (TBST). After three washes in TBST, membranes were incubated with rabbit monoclonal anti-human PDPN antibody (Abcam), diluted 1:300 in 5% non-fat dry milk in TBST, overnight at 4°C. The following day, membranes were rinsed (×3 with TBST), incubated with the secondary antibody (HRP-linked goat anti-rabbit IgG; ThermoFisher) diluted 1:3000 in 5% milk-TBST for 1 h at RT. Membranes were rinsed again (×3) with TBST. Lastly, membranes were imaged with chemiluminescent substrate (Clarity Western ECL Substrate; BioRad Laboratories) using a Chemi Doc XES + system (BioRad Laboratories).
Statistical Analysis
Continuous data were expressed as means ± standard deviation and categorical data as frequencies and percentages. Overall postoperative survival (OST) was calculated from the date of splenectomy to the date of death. Dogs that were alive at the time of data analysis were censored at the last date of follow up. Dogs that had died were assumed to have died as a result of their tumour, even if this was not confirmed.
Statistical analysis was performed using commercial software (Prism 6; GraphPad Software, San Diego, California, USA). Survival curves were generated using the Kaplan–Meier product limit method; the logrank test was used for delineation of significance between categorical subpopulations, with significance set at P ≤0.05. Factors assessed for prognostic value included tumour type, grade, MC and treatment with adjuvant chemotherapy.
The data analysed in this study are available in the Supplementary Material.
Results
Patient Characteristics
Fifty-three dogs with diagnoses matching our search criteria were identified through the CSU VDL, of which 47 had adequate clinical information for analysis. Original histological diagnoses included: 44 stromal sarcomas, and one each of malignant fibrous histiocytoma, anaplastic sarcoma and malignant sarcoma. The median age was 10.5 years (range 4 to 14) and the median weight was 24.2 kg (range 2.6 to 46.3). There were 27 males (26 castrated) and 20 females (19 spayed). Golden Retrievers were the most common (n = 8) followed by mixed breed (n = 4), English Cocker Spaniels, Maltese Terriers (n = 3 each) and other breeds (n <2 each).
The initial diagnostic tests performed were variable among dogs. Complete blood count and serum chemistry were performed on 41 dogs; 25 were anaemic (haematocrit ≤30%) and 16 were neutrophilic (39%). Thirty-one animals had thoracic radiographs and two had thoracic computed tomography (CT) scans (one had both radiographs and CT), none of which revealed pulmonary metastasis. Abdominal imaging was performed, including radiographs (n = 27), abdominal CT (n = 1) and abdominal ultrasound or abdominal-focused assessment with sonography for trauma (AFAST) (n = 36), from which 41 dogs were identified as having splenic nodules or masses.
Diagnosis was made by exploratory laparotomy and splenectomy. Eight dogs had haemoabdomen and seven had evidence of metastasis at the time of surgery (liver, n = 6; abdominal lymph node, n = 2; omentum, n = 1) confirmed by histopathology. Concurrent neoplasia was documented in five dogs at the time of surgery including mammary adenocarcinoma (n = 2, cases 11 and 33), low to intermediate splenic lymphoma (n = 1, case 12), portal lymph node mast cell tumour (n = 1, case 8) and small intestinal mural sarcoma (n = 1, case 38). The median OST for all dogs was 322 days (range 1 to 959 days).
Validation of an Anti-Podoplanin Antibody on Canine Tissue and Expression in Undifferentiated Splenic Stromal Sarcomas
Western blot, performed using a polyclonal anti-human PDPN antibody, detected a band of approximately 40 kDa, consistent with the predicted molecular weight for PDPN, in the canine histiocytic sarcoma cell line (DH-82) and the positive control human osteosarcoma cell line U2OS (Supplementary Fig. 1a). IHC, performed with this antibody on FFPE normal canine spleen and kidney positive control tissues, demonstrated appropriate positive labelling of podocytes (Supplementary Fig. 1b) and lymphatic endothelium and the associated reticular cell network of the spleen (Supplementary Fig. 1d). PDPN expression in the spleen significantly overlapped with observed desmin localization (Supplementary Fig. 1c), suggesting co-expression of these markers by a common cell type in the splenic reticular cell network.
Histological and Immunohistochemical Evaluation and Tumour Reclassification
Forty-three FFPE tumour samples were available for retrospective histopathological and IHC review. Tumours were classified as undifferentiated stromal sarcoma (n = 22), CNH (n = 9), sarcoma arising from CNH (n = 3), histiocytic sarcoma (n = 3), haemangiosarcoma (n = 1) and leiomyosarcoma (n = 1). Four cases were excluded due to extensive necrosis or haemorrhage confounding interpretation. The overall morphology of the undifferentiated stromal sarcomas in HE-stained sections varied from nodular masses with many features of sarcoma from CNH (previously grade 3 SFHNs), but which demonstrated focal transition to a population of true pleomorphic malignant stromal cells, to non-discrete masses composed almost entirely of sheets of polygonal to spindle-shaped cells, with primarily diffuse infiltrates of individual lymphocytes and plasma cells. As compared with sarcoma from CNH, neoplastic cells of undifferentiated stromal sarcomas had increasing features of malignancy (Figs. 2A and 3A), with indistinct cell borders and moderate to marked anisokaryosis. Of the undifferentiated stromal sarcomas, 19/22 (86%) were PDPN positive, 12/22 (54%) CD204 positive and 12/22 (54%) were desmin positive (Table 1).
Table 1.
Immunolabelling profiles of 23 canine undifferentiated stromal sarcomas*
| Case no. | Antigen | ||||
|---|---|---|---|---|---|
| CD204 | Desmin | Factor VIII | PDPN | SMA | |
| 1 | − | + | − | + | + |
| 2 | − | − | − | + | + |
| 3 | + | + | − | + | − |
| 5 | + | + | − | + | − |
| 6 | + | + | − | + | + |
| 7 | + | − | − | + | + |
| 8 | + | + | − | + | + |
| 12 | + | + | − | + | − |
| 15 | + | + | − | − | − |
| 16 | + | + | − | + | − |
| 19 | + | − | − | − | + |
| 21 | + | + | − | − | + |
| 23 | + | + | − | + | + |
| 26 | − | + | − | + | + |
| 27 | − | − | + | + | + |
| 28 | − | − | − | + | − |
| 29 | − | + | + | + | + |
| 30 | − | − | − | + | + |
| 33 | + | − | − | + | − |
| 34 | + | − | − | − | + |
| 35 | − | − | − | + | − |
| 37 | − | − | − | + | + |
| 43 | − | + | − | + | − |
Tumours were classified as stromal sarcoma on the basis of positive immunolabelling for any combination of two or more of the following markers: CD204, desmin and podoplanin (PDPN), regardless of smooth muscle actin (SMA) immunoreactivity.
+, immunopositive; −, immunonegative.
Clinical Outcome Based on Revised Classification
Undifferentiated stromal sarcomas were stratified on the basis of MC (≥9 per 10 HPFs) as reported for assessing outcome (Spangler et al, 1994). Of the 34 dogs diagnosed with either undifferentiated stromal sarcoma, sarcoma from CNH or CNH, 33 were included in survival analysis (the patient euthanized at the time of surgery was censored). Median time to follow up was 250 days. The median survival time was 439 days (range 1–959). Three dogs were alive 2 years after diagnosis.
There was a statistically significant difference in survival time between patients diagnosed with stromal sarcoma (sarcoma from CNH or undifferentiated stromal sarcoma) and CNH (178 d versus 637 d, respectively; Gehan-Breslow-Wilcoxon test, P = 0.027). When comparing undifferentiated stromal sarcomas with sarcoma from CNH (Fig. 5), there was no difference in survival time (236 d versus 466 d, respectively; P = 0.852). Patients with stromal sarcoma were also evaluated on the basis of MC (Fig. 6). Those dogs with a low MC (≤9 per 10 HPFs) had a significantly longer survival time (439 d versus 67 d; logrank test P = 0.01). Patients diagnosed with metastatic disease at the time of surgery had a significantly shorter survival time (27 d versus 279 d; logrank test P = 0.31). Diagnosis of concurrent neoplasia was statistically insignificant (649 days with versus 161 days without; logrank test P = 0.3).
Fig. 5.
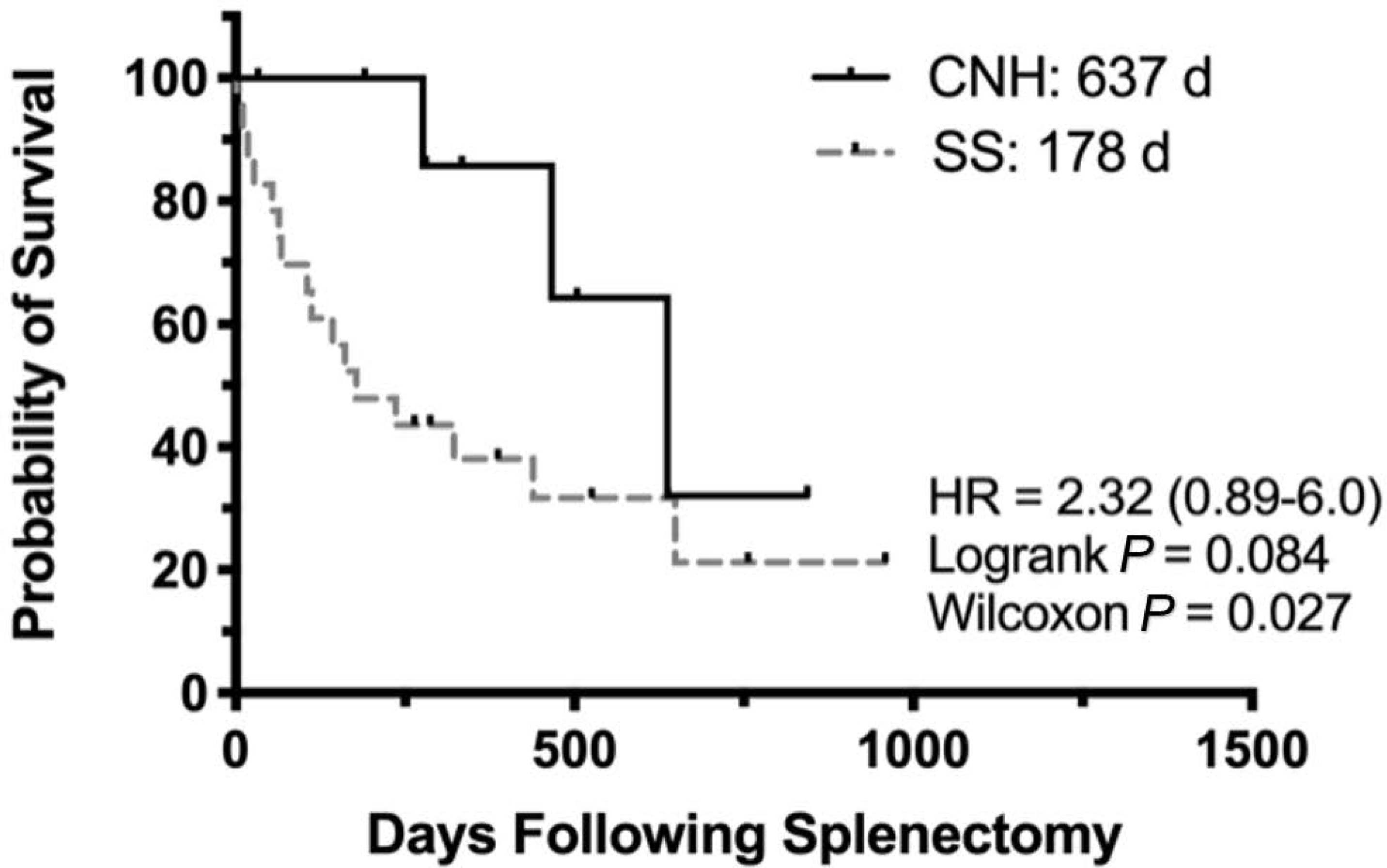
Kaplan–Meier survival curve for 33 dogs diagnosed with complex nodular hyperplasia (CNH) (n = 9) versus sarcoma from CNH and undifferentiated stromal sarcoma (SS) (n = 24). Probability of survival (%). HR, hazard ratio.
Fig. 6.
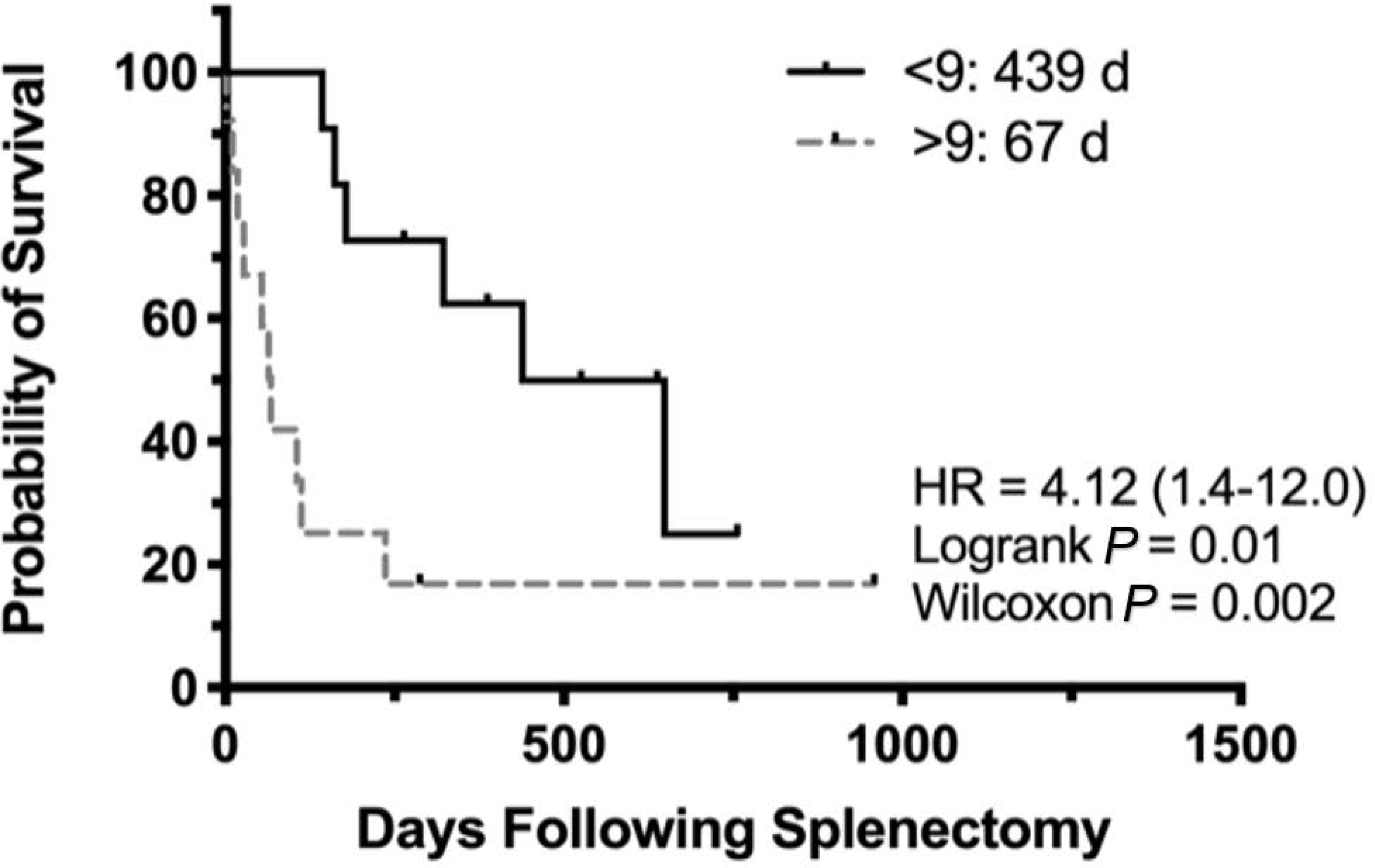
Kaplan–Meier product-limit estimates for 24 dogs diagnosed with splenic sarcoma from complex nodular hyperplasia (CNH) and undifferentiated stromal sarcoma (SS) grouped by mitotic count (<9 per 10 HPFs, solid line; >9 per 10 HPFs, dotted line). Probability of survival (%). HR, hazard ratio.
Postoperatively, seven dogs received adjuvant chemotherapy. One patient had CNH and six had undifferentiated stromal sarcoma (two with MC ≤9, four with MC >10). Treatment protocols included doxorubicin (n = 3), doxorubicin followed by cyclophosphamide (n = 2), doxorubicin followed by chlorambucil (n = 1) and toceranib phosphate (n = 1). The use of adjuvant chemotherapy was statistically insignificant (434 d with versus 178 d without; logrank test P = 0.31).
Discussion
In this study, patient characteristics were similar to previous reports of non-angiogenic, non-myogenic sarcomas with no sex, weight, breed or age predilection (Spangler et al, 1994; Weinstein et al, 1989). Most patients (84%) were diagnosed with a splenic mass on abdominal imaging prior to exploratory laparotomy. Palpable abdominal fluid wave and abdominal pain are also reported clinical signs (Valli et al, 2016). The median post-splenectomy survival time for patients with confirmed undifferentiated splenic stromal sarcoma was 178 days. Tumours with a MC ≤9 were found to have a significantly longer survival time, which is consistent with previous reports of stromal sarcomas and primary abdominal visceral soft tissue sarcomas (Weinstein et al, 1989; Spangler et al, 1994; Spangler and Kass, 1997; Linden et al, 2019). Our findings did not indicate that adjuvant chemotherapy provided a survival advantage, despite having four cases with a MC ≥10. However, this may have been due to the low number of treated cases.
Nine cases were reclassified as CNH after morphological and IHC evaluation. We had no samples morphologically consistent with lymphoma, although, as demonstrated by Moore et al (2012) and Sabattini et al (2018), some nodular lesions can be reclassified as marginal zone or diffuse large B-cell lymphomas. IHC for B-cell lineage markers such as CD79a and Pax-5 may be indicated to exclude these entities (Moore et al, 2012). Three tumours were reclassified on the basis of routine diagnostic IHC, and included one each of histiocytic sarcoma, haemangiosarcoma and leiomyosarcoma. Both splenic histiocytic sarcoma and haemangiosarcoma are highly metastatic neoplasms with a grave prognosis when left untreated, and chemotherapy can be efficacious in prolonging survival with reports of response in the context of gross or microscopic lesions (Clifford et al, 2007; Skorupski et al, 2007; Thamm, 2007; Cannon et al, 2015). A median survival time of 8 months has been reported for splenic leiomyosarcoma when treated with splenectomy, although there is no known effect of chemotherapy. Regardless, clinicians will often consider postoperative chemotherapy for high-grade sarcomas despite limited data showing improved survival (Liptak and Forrest, 2007).
The first large case series of primary mesenchymal neoplasms of the canine spleen was described in detail by Spangler and Kass (1994) and included a heterogeneous grouping of non-lymphoid and non-endothelial sarcomas. This series had three cases with anatomical characteristics suggestive of myoid origin. More recent histological and IHC characterization of these lesions suggested that SFHNs represent a variety of disease entities, including stromal sarcoma, and use of the SFHN term to describe this specific disease entity is not warranted. Our study also identified undifferentiated stromal sarcomas as having a morphological appearance distinct from forms of splenic nodular hyperplasia, characterized by interlacing streams and bundles of polygonal to spindle-shaped cells in a collagenous matrix, which exhibit characteristic criteria of malignancy including anisokaryosis, necrosis and multinucleation (Fig. 4) (Liptak and Forrest 2007; Moore et al, 2012). Some of these sarcomas are clearly defined by their standard morphological and IHC profile (ie, histiocytic sarcoma, osteosarcoma, myxosarcoma, liposarcoma, mesenchymoma), but overlapping prevalence of immunolabelled myoid features in others (fibrosarcoma, leiomyosarcoma, undifferentiated sarcoma) suggest a common cellular origin of splenic myofibroblasts or reticular cells (Spangler et al, 1994).
Our study parallels the subset of splenic nodular lesions evaluated by Sabattini et al (2018). However, this series evaluated the malignant mesenchymal expansion arising from stromal cells that are of probably of fibroblastic reticular cell/follicular dendritic cell origin instead of the lymphoid component described by Sabattini et al (2018). Based on the results of our IHC analysis, we also suggest that 15 of the 23 splenic stromal sarcomas evaluated in our study could be further differentiated into those of fibroblastic reticular cell (n = 11) or follicular dendritic cell (n = 4) origin. Ultrastructural and immunohistochemical demonstration of myoid cellular features had been defined in other non-myocyte cell types including a population of reticular cells present in spleen and lymph nodes (Toccanier-Pelte et al, 1987). Importantly, these data raised the possibility that those sarcomas of the canine spleen diagnosed as fibrosarcoma, leiomyosarcoma or undifferentiated sarcoma, which accounted for 60% of this original subset of splenic stromal sarcomas, may in fact arise from a common myoid subset of resident reticular cells in the spleen.
PDPN has been used in humans as a marker of follicular dendritic cells in the germinal centres of lymphoid follicles, as well as reticular fibroblasts in the para- and extrafollicular regions of the spleen (Yu et al, 2007; Xie et al, 2008; Astarita et al, 2012; Suárez-Vilela and Izquierdo, 2016) Desmin is also labelled in fibroblastic reticular cells and follicular dendritic cells (Zeng et al, 2011). Based on these observations and the results of our study, the observed morphology and IHC labelling profiles of these tumours as desmin+/PDPN+ or CD204+/PDPN+ are suggestive of fibroblastic reticular cell and follicular dendritic cell sarcomas, respectively. Due to the low power of this study, there was no statistical difference in outcome between lineage. While this differentiation may provide insight into the origin of this tumour type and potentially suggest that splenic hyperplastic nodules and stromal sarcomas represent a continuum from an inflammatory lesion to transformed fibroblastic reticular cells, larger studies are needed to determine the influence of this distinction on biological behaviour and clinical outcome.
Evaluation of the data presented in this retrospective study indicates that there is probably a continuum of disease from benign CNH, defined by reactive fibroblastic and histiocytic hyperplasia, to malignant undifferentiated stromal sarcoma, which includes all of the previously described ambiguous sarcomas of the spleen, while excluding those sarcomas that can be definitively classified based on distinct histomorphological and immunohistochemical features, including osteosarcoma, histiocytic sarcoma, haemangiosarcoma, myxosarcoma, liposarcoma and mesenchymoma. There is a clear distinction in morphological appearance between the original description of SFHNs in Spangler et al (1998), now termed lymphoid nodular hyperplasia (grade 1), benign CNH (grade 2) and sarcoma from CNH (grade 3) compared with true malignant stromal sarcomas, termed undifferentiated stromal sarcoma.
We propose a new diagnostic approach to separate low-grade lesions from distinctly neoplastic stromal sarcomas of the canine spleen (Fig. 7). Benign lymphoid and complex nodular hyperplastic splenic lesions should be termed as such and may be described in more detail according to Moore et al (2012) and Sabattini et al (2018). Dogs diagnosed with splenic stromal sarcoma on the basis of morphological and architectural evaluation of HE-stained sections should have IHC performed to exclude other malignant tumour types, including histiocytic sarcoma, haemangiosarcoma and lymphoma (diffuse large B-cell and marginal zone), as prognosis and chemotherapy recommendations would significantly vary based on these results. Patients diagnosed with sarcoma arising from CNH should be monitored more closely than those with CNH, as this disease process probably represents a continuum of malignant transformation and progression. Many of the undifferentiated stromal sarcomas appeared as CNH with a focal region of sarcomatous differentiation, which could be missed due to sampling variability. Patients with undifferentiated stromal sarcoma with a MC ≤9 can have prolonged survival with splenectomy alone as compared with the more mitotically active tumours.
Fig. 7.
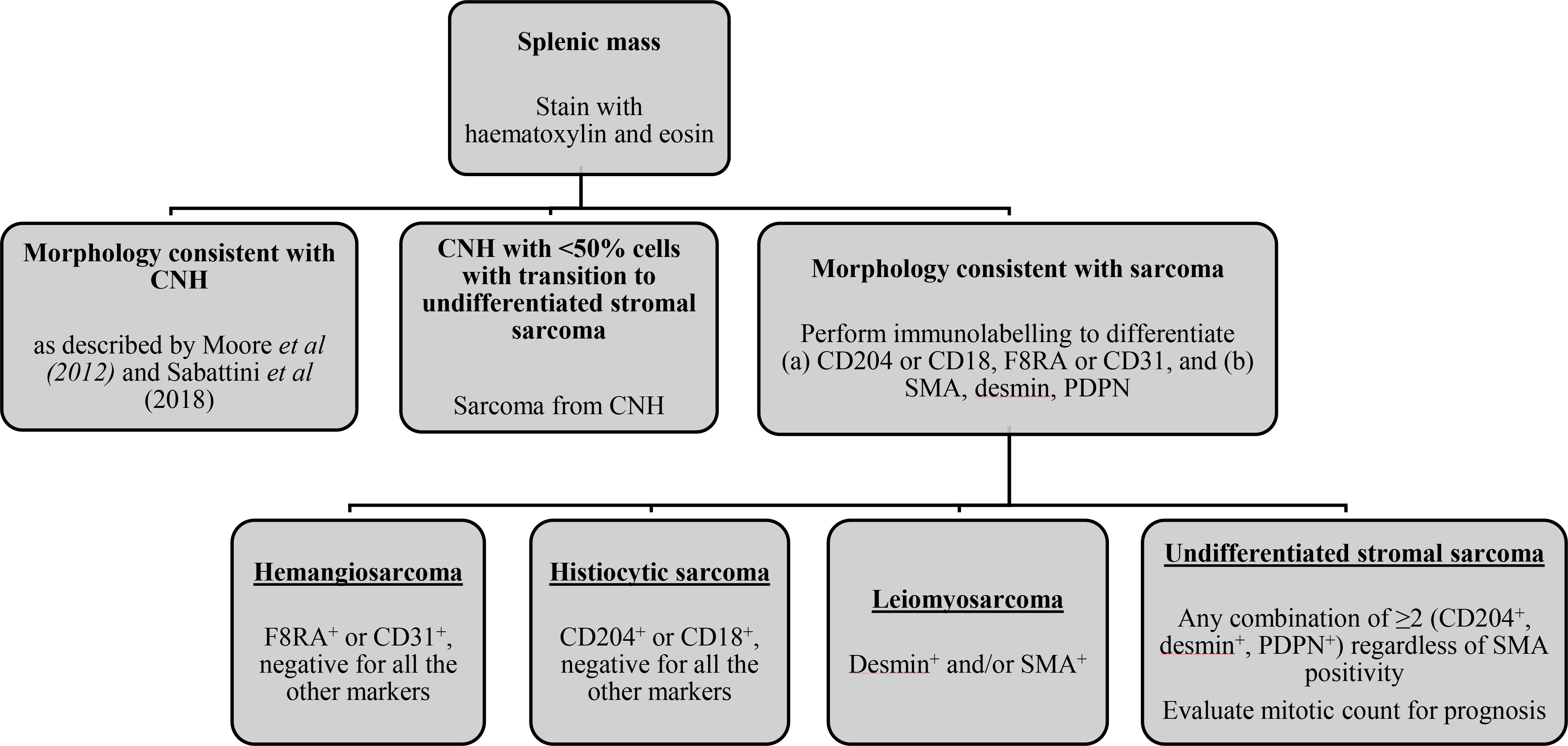
Proposed diagnostic algorithm for histopathological evaluation of splenic tumours in dogs. After diagnosis, immunohistohemical labelling should initially be performed to exclude haemangiosarcoma and histiocytic sarcoma.
Our study also validated the cross-reactivity of an anti-PDPN antibody on canine tissue and demonstrated PDPN expression in the normal canine spleen and mesenchymal splenic tumours. These results suggest PDPN may represent a useful diagnostic marker of undifferentiated splenic stromal sarcoma of the dog; however, studies evaluating PDPN expression in a significantly larger subset of clinically relevant canine splenic mesenchymal tumors, such as histiocytic sarcoma and haemangiosarcoma, would be necessary to fully determine its sensitivity and specificity for this tumour entity. Limitations of this study include small patient number, lack of lymphoid hyperplastic lesions, observations drawn from incomplete medical records and lack of standard diagnostic staging, treatment, follow-up protocol and post-mortem analysis to confirm cause of death. A standardized, prospective evaluation of dogs with undifferentiated splenic stromal sarcoma is indicated to fully appreciate if prolonged survival time is achieved with adjuvant chemotherapy.
Supplementary Material
Supplementary Fig. 1. Validation of canine cross-reactivity of an anti-human podoplanin antibody. (a) Western blot demonstrating antibody detection of identical protein bands of ~40 kDa, corresponding to the predicted molecular weight (36–43 kDa) of podoplanin, in both human (U2OS) and canine (DH82) control tumour cell lines. (b) Immunolabelling of podoplanin in podocytes of canine glomerulus. (c and d) Podoplanin immunolocalization in normal canine spleen (d) shares significant overlap with that of desmin (c) and is morphologically consistent with reticular cell network labelling.
Acknowledgments
The authors thank Jennifer Phinney and the Kansas State University Veterinary Diagnostic Laboratory, as well as Todd Bass and the Colorado State University Veterinary Diagnostic Laboratory, for performing the immunohistochemistry for this project.
Funding
Research reported in this publication was supported by The National Institutes of Health, Office of the Director, award no. K01ODO22982 and National Center for Advancing Translational Sciences, award no. L30 TR002126.
Footnotes
Conflict of Interest Statement
The authors declared no potential conflicts of interest with respect to the research, authorship or publication of this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Astarita JL, Acton SE, Turley SJ (2012) Podoplanin: emerging functions in development, the immune system, and cancer. Frontiers in Immunology, 3, 283–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astarita JL, Cremasco V, Fu J, Darnell MC, Peck JR et al. (2015) The CLEC-2-podoplanin axis controls the contractility of fibroblastic reticular cells and lymph node microarchitecture. Nature Immunology, 16, 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon C, Borgatti A, Henson M, Husbands B (2015) Evaluation of a combination chemotherapy protocol including lomustine and doxorubicin in canine histiocytic sarcoma. The Journal of Small Animal Practice, 56, 425–429. [DOI] [PubMed] [Google Scholar]
- Chai Q, Onder L, Scandella E, Gil-Cruz C, Perez-Shibayama C et al. (2013) Maturation of lymph node fibroblastic reticular cells from myofibroblastic precursors is critical for antiviral immunity. Immunity, 38, 1013–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HW, Onder L, Novkovic M, Soneson C, Lütge M et al. (2019) Origin and differentiation trajectories of fibroblastic reticular cells in the splenic white pulp. Nature Communications, 10, 1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford C, Skorupski K, Moore P (2007) Histiocytic diseases. In: Withrow and MacEwen’s Small Animal Clinical Oncology, 4th Edit, Withrow SJ, Vail DM, Page RL, Eds, Elsevier Saunders, St. Louis, pp 706–716. [Google Scholar]
- Krishnan H, Rayes J, Miyashita T, Ishii G, Retzbach EP et al. (2018) Podoplanin: an emerging cancer biomarker and therapeutic target. Cancer Science, 109, 1292–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden D, Liptak JM, Vinayak A, Grimes JA, Sandey M et al. (2019) Outcomes and prognostic variables associated with primary abdominal visceral soft tissue sarcomas in dogs: a Veterinary Society of Surgical Oncology retrospective study. Veterinary and Comparative Oncology, 17, 265–270. [DOI] [PubMed] [Google Scholar]
- Liptak L, Forrest L (2007) Soft tissue sarcomas. In: Withrow and MacEwen’s Small Animal Clinical Oncology, 4th Edit, Withrow SJ, Vail DM, Page, Eds, Elsevier Saunders, St. Louis, pp 356–380. [Google Scholar]
- Malhotra D, Fletcher AL, Astarita J, Lukacs-Kornek V, Tayalia P et al. (2012) Transcriptional profiling of stroma from inflamed and resting lymph nodes defines immunological hallmarks. Nature Immunology, 13, 499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuten DJ, Moore FM, George JW (2016) Mitotic count and the field of view area: time to standardize. Veterinary Pathology, 53, 7–9. [DOI] [PubMed] [Google Scholar]
- Moore AS, Frimberger AE, Sullivan N, Moore PF (2012) Histologic and immunohistochemical review of splenic fibrohistiocytic nodules in dogs. Journal of Veterinary Internal Medicine, 26, 1164–1168. [DOI] [PubMed] [Google Scholar]
- Sabattini S, Lopparelli RM, Rigillo A, Giantin M, Renzi A et al. (2018) Canine splenic nodular lymphoid lesions: immunophenotyping, proliferative activity, and clonality assessment. Veterinary Pathology, 55, 645–653. [DOI] [PubMed] [Google Scholar]
- Skorupski KA, Clifford CA, Paoloni MC, Lara-Garcia A, Barber L et al. (2007) CCNU for the treatment of dogs with histiocytic sarcoma. Journal of Veterinary Internal Medicine, 21, 121–126. [DOI] [PubMed] [Google Scholar]
- Spangler WL, Culbertson MR, Kass PH (1994) Primary mesenchymal (nonangiomatous/nonlymphomatous) neoplasms occurring in the canine spleen: anatomic classification, immunohistochemistry, and mitotic activity correlated with patient survival. Veterinary Pathology, 31, 37–47. [DOI] [PubMed] [Google Scholar]
- Spangler WL, Kass PH (1997) Pathologic factors affecting postsplenectomy survival in dogs. Journal of Veterinary Internal Medicine, 11, 166–171. [DOI] [PubMed] [Google Scholar]
- Spangler WL, Kass PH (1998) Pathologic and prognostic characteristics of splenomegaly in dogs due to fibrohistiocytic nodules: 98 cases. Veterinary Pathology, 35, 488–498. [DOI] [PubMed] [Google Scholar]
- Suárez-Vilela D, Izquierdo FM (2016) Podoplanin is positive not only on follicular dendritic cells and their tumoral counterparts, but also on reticular fibroblasts and in some tumors of reticular fibroblasts. Applied Immunohistochemistry and Molecular Morphology, 24, e11. [DOI] [PubMed] [Google Scholar]
- Thamm D (2007) Hemangiosarcoma. In: Withrow and MacEwen’s Small Animal Clinical Oncology, 4th Edit, Withrow SJ, Vail DM, Page RL, Eds, Elsevier Saunders, St. Louis, pp 679–688. [Google Scholar]
- Toccanier-Pelte MF, Skalli O, Kapanci Y, Gabbiani G (1987) Characterization of stromal cells with myoid features in lymph nodes and spleen in normal and pathologic conditions. The American Journal of Pathology, 129, 109–118. [PMC free article] [PubMed] [Google Scholar]
- Valli VE, Bienzle D, Meuten DJ (2016) Tumors of the hemolymphatic system. In: Tumors in Domestic Animals, Meuten DJ, Ed, John Wiley and Sons, Ames, pp 203–321. [Google Scholar]
- Weinstein MJ, Carpenter JL, Schunk CJ (1989) Nonangiogenic and nonlymphomatous sarcomas of the canine spleen: 57 cases (1975–1987). Journal of the American Veterinary Medical Association, 195, 784–788. [PubMed] [Google Scholar]
- Xie Q, Chen L, Fu K, Harter J, Young KH et al. (2008) Podoplanin (D2–40): a new immunohistochemical marker for reactive follicular dendritic cells and follicular dendritic cell sarcomas. International Journal of Clinical and Experimental Pathology, 1, 276–284. [PMC free article] [PubMed] [Google Scholar]
- Yu H, Gibson JA, Pinkus GS, Hornick JL (2007) Podoplanin (D2–40) is a novel marker for follicular dendritic cell tumors. American Journal of Clinical Pathology, 128, 776–782. [DOI] [PubMed] [Google Scholar]
- Zeng M, Smith AJ, Wietgrefe SW, Southern PJ, Schacker T et al. (2011) Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. The Journal of Clinical Investigation, 121, 998–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. Validation of canine cross-reactivity of an anti-human podoplanin antibody. (a) Western blot demonstrating antibody detection of identical protein bands of ~40 kDa, corresponding to the predicted molecular weight (36–43 kDa) of podoplanin, in both human (U2OS) and canine (DH82) control tumour cell lines. (b) Immunolabelling of podoplanin in podocytes of canine glomerulus. (c and d) Podoplanin immunolocalization in normal canine spleen (d) shares significant overlap with that of desmin (c) and is morphologically consistent with reticular cell network labelling.


