Abstract
The Academy of Nutrition and Dietetics Evidence Analysis Center conducted a systematic review of the literature to develop an evidence-based practice guideline for primary nutrition issues in cystic fibrosis (CF). This guideline is designed to complement and build upon existing evidence-based CF nutrition guidelines. The objective of this guideline was to provide recommendations for registered dietitian nutritionists in the United States delivering medical nutrition therapy to individuals with CF and their families that fill gaps in current evidence-based guidelines on topics that are crucial in order to improve health and prevent disease progression. This guideline provides 28 nutrition recommendations to guide medical nutrition therapy, including nutrition screening, nutrition assessment, and dietary intake. For topics outside the scope of this guideline, practitioners are referred to external, evidence-based recommendations. The CF landscape is evolving rapidly with breakthroughs in cystic fibrosis transmembrane regulator modulators changing CF at a cellular level. Medical nutrition therapy for individuals with CF from infancy through advanced age requires novel and individualized approaches. The Academy Evidence Analysis Library CF guidelines provide a framework for expanding upon current knowledge to determine effective nutrition strategies for individuals with CF through long and healthy futures.
Nutrition management is an essential component of overall care for all individuals with cystic fibrosis (CF) in order to prevent and remediate malnutrition, which is strongly associated with pulmonary function and survival. Rapid advances in the understanding and treatment of CF, including the introduction of cystic fibrosis transmembrane regulator (CFTR) modulation therapies, necessitate evolution in medical nutrition therapy (MNT) for the many different manifestations of this complex disease.
Recently, 2 general nutrition care guidelines for CF were developed by the Thoracic Society of New Zealand and Australia and the European Society for Clinical Nutrition and Metabolism, the European Society for Paediatric Gastroenterology Hepatology and Nutrition and the European Cystic Fibrosis Society.1,2 In the United States, The Cystic Fibrosis Foundation (CFF) has sponsored numerous recommendations and guidelines that include nutrition-related topics.3–9
The Academy of Nutrition and Dietetics Evidence Analysis Center (EAC) conducted a systematic review of the literature to develop an evidence-based practice guideline for primary nutrition issues in CF. This guideline is designed to complement, build upon, and expand the existing Australian/New Zealand, European, and CFF recommendations and guidelines. The EAC CF guidelines are intended to inform the practice of registered dietitian nutritionists (RDNs) in the United States who provide individualized nutrition care for children and adults with CF and to identify gaps in the primary literature that require attention to improve nutrition practice.
In 1988, the seminal work of Corey and colleagues,10 elucidated the impact of achieving and maintaining better weight and height on lifespan for individuals with CF. The emphasis of MNT for CF shifted from a low-fat, high-protein diet prescribed to control symptoms of steatorrhea and abdominal discomfort to a high-energy, high-fat diet designed to promote weight gain. Nutritional management continued to focus on the attainment of body mass index (BMI; calculated as kg/m2) through the prescribed high-energy, high-fat, CF “legacy diet.” Diet quality and nutrient density received much less attention. Recent studies of the diet records of children with CF in Australia and the European Union demonstrated the achievement of CF estimated energy requirements through overconsumption of energy-dense, nutrient-poor foods, particularly sugars, saturated fats, and highly processed foods.11,12
The landscape of nutrition care is changing for those with CF, particularly since the introduction of CFTR modulation therapy, which may result in less-severe symptoms, longer lifespan, and less risk of undernutrition. The future health of adults and children with CF may be jeopardized by the continued reliance on a high-energy, nutrient-poor diet. Although a paucity of data suggest individuals with CF benefit from general population-based nutrition recommendations, there are currently no data to demonstrate adults and children with CF are exempt from these recommendations. For nutrition topics outside the scope of the current guideline, the workgroup has identified, reviewed, and approved of recommendations from external guidelines as a reference for practitioners (Figure 1). An executive summary of recommendation statements and ratings can be found in Figure 2.
Figure 1.
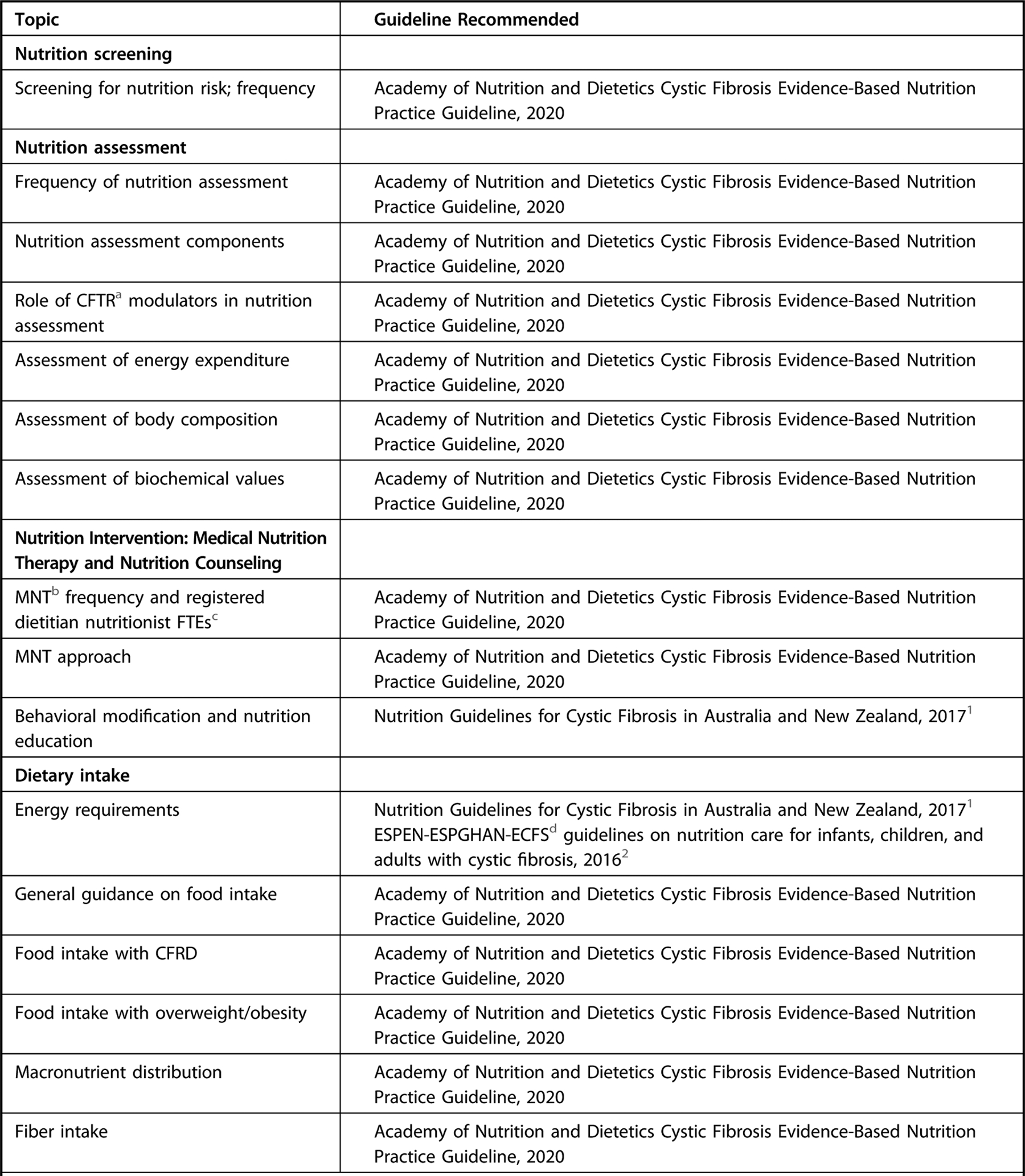
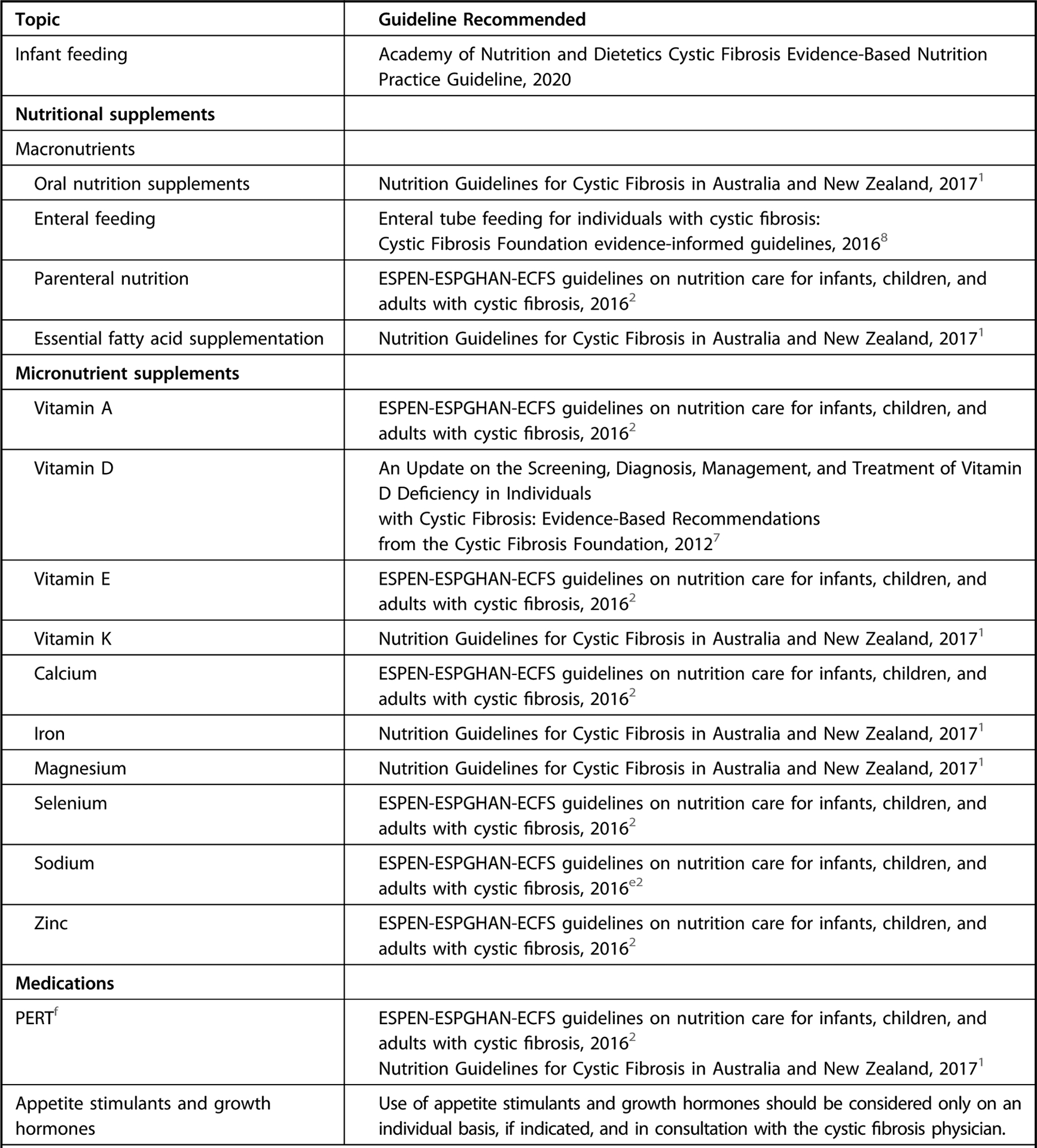
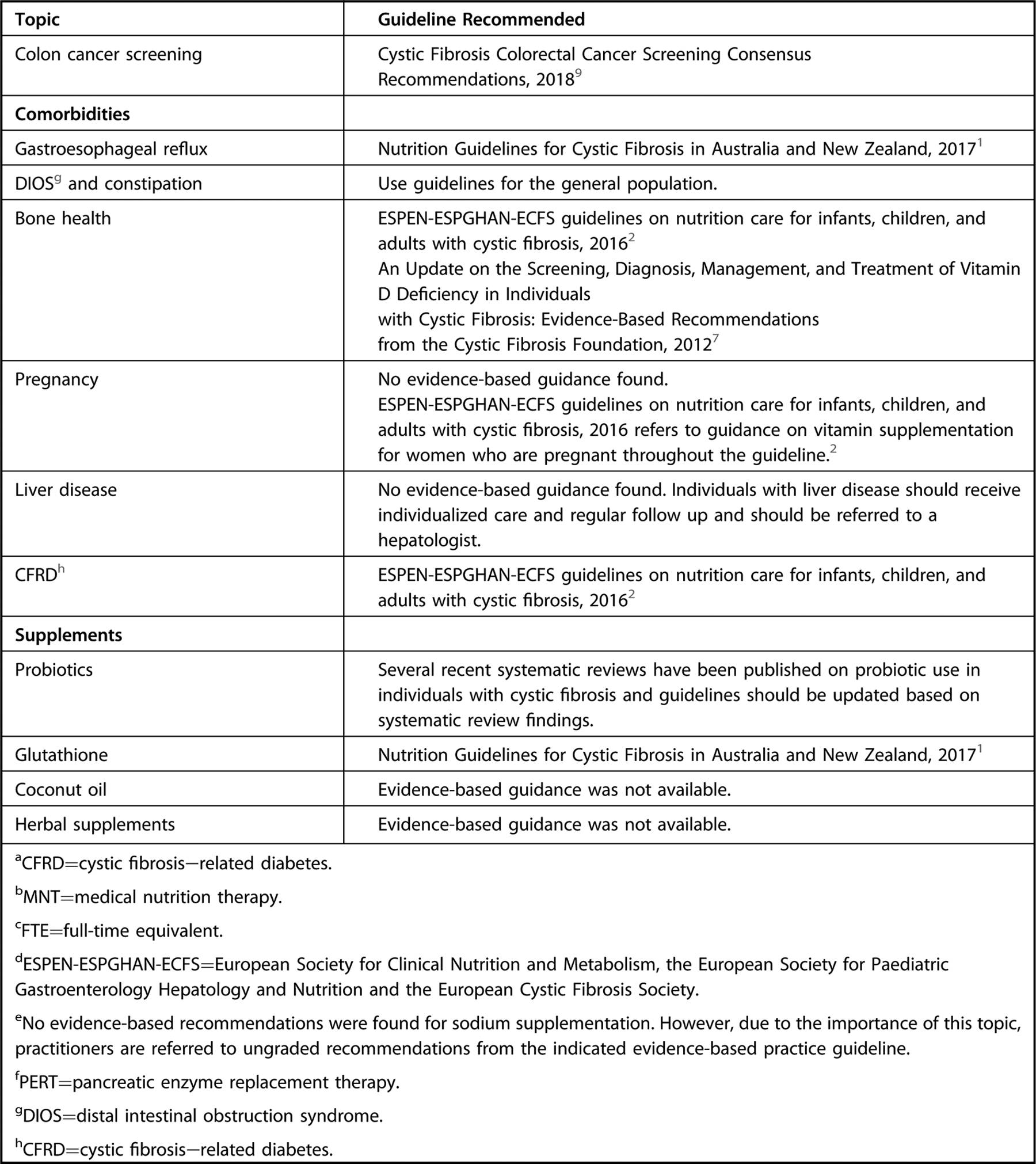
Recommendation overview table of nutrition topics for individuals with cystic fibrosis. This guideline focused primarily on the topics of medical nutrition therapy provided by a registered dietitian nutritionist or international equivalent, methods of nutrition screening and assessment, and dietary intake. For topics outside the scope of the current guideline, please refer to graded recommendations in the external guidelines indicated. This is not an exhaustive list of high-quality recommendations, but those referenced were from external guidelines assessed as having high quality using the Appraisal of Guidelines for Research and Evaluation II tool and were approved for use by the Academy’s Council on Research, and specific recommendations referenced were based on systematic review and reviewed, voted on, and approved by consensus by workgroup members.
Figure 2.
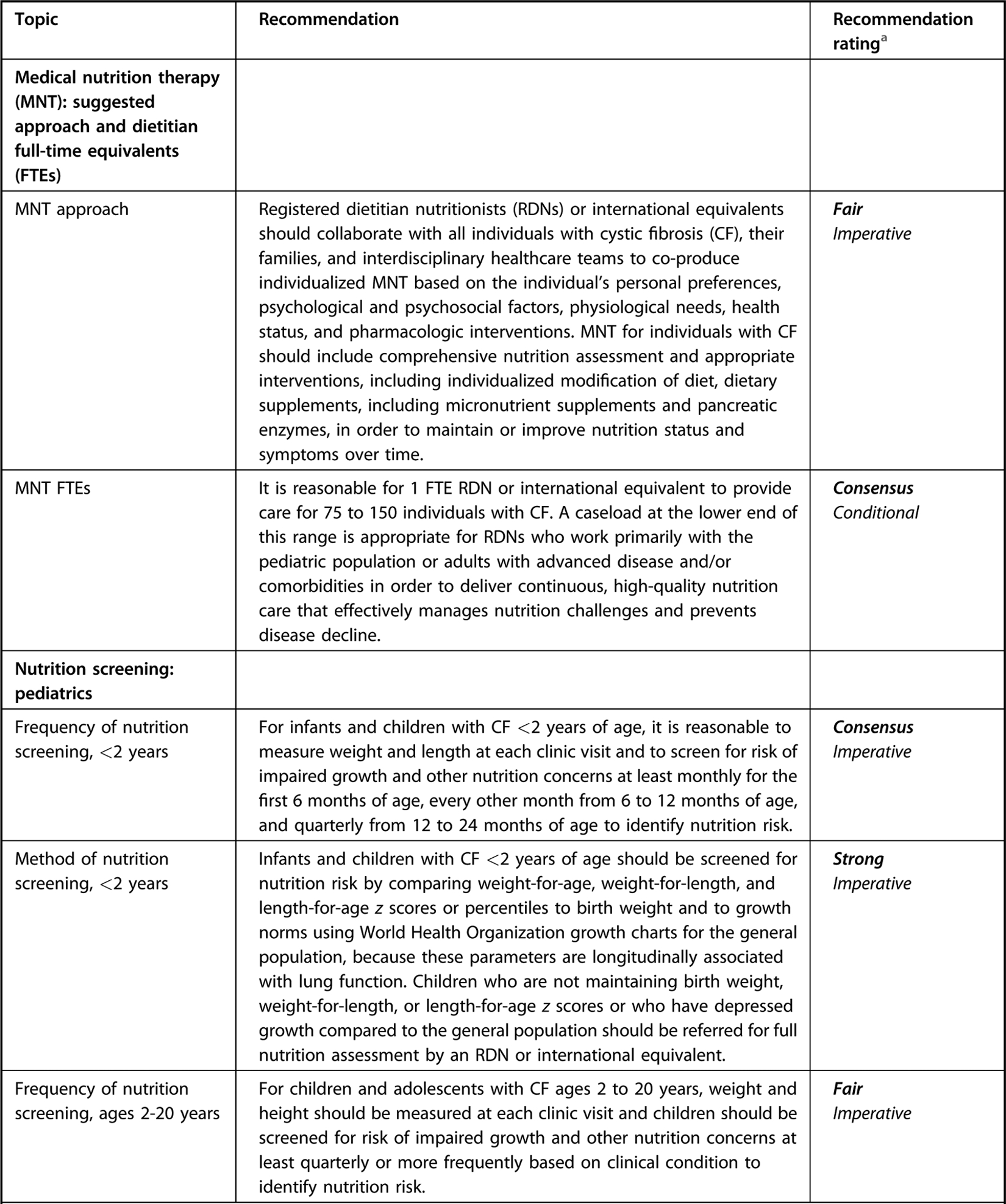
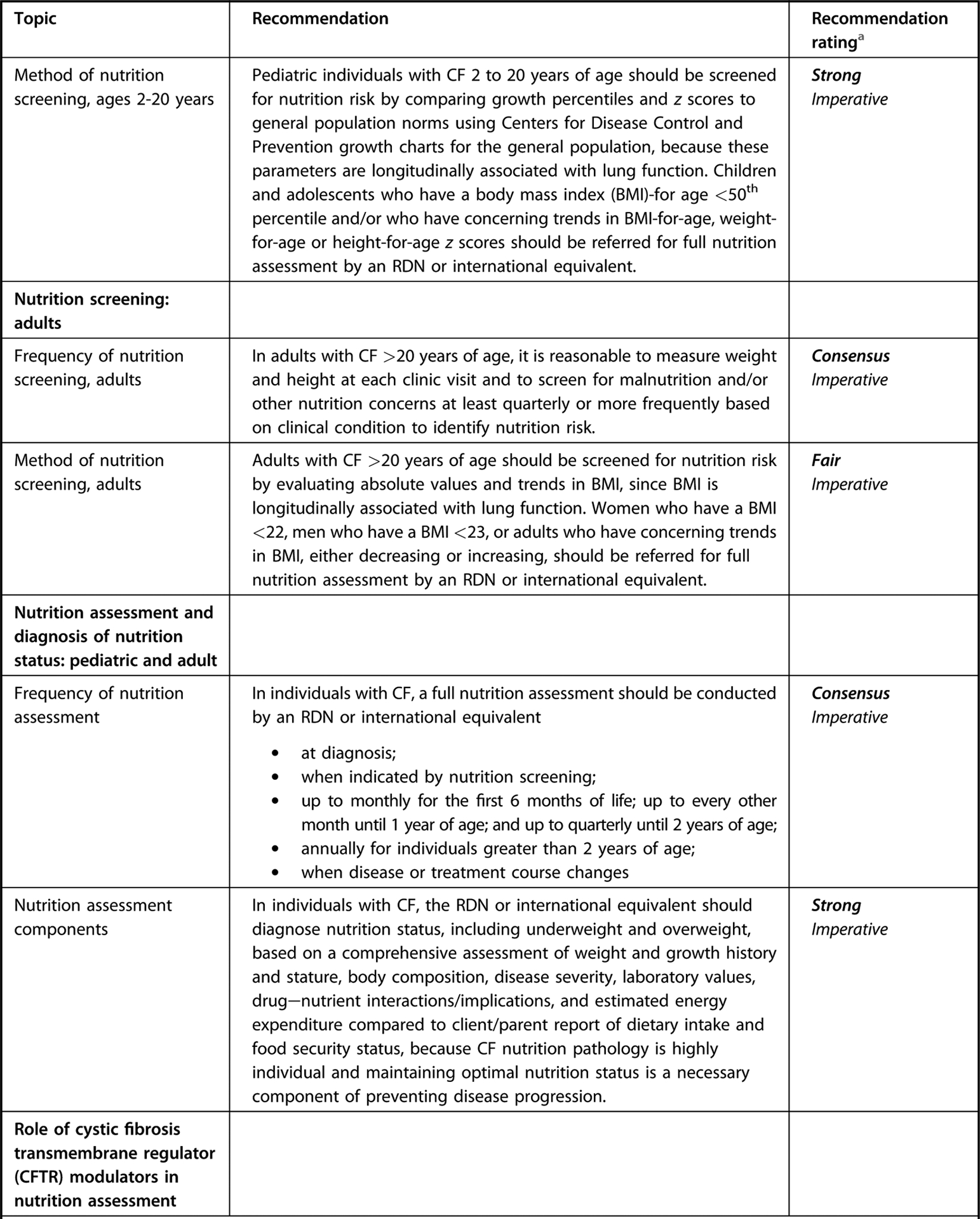
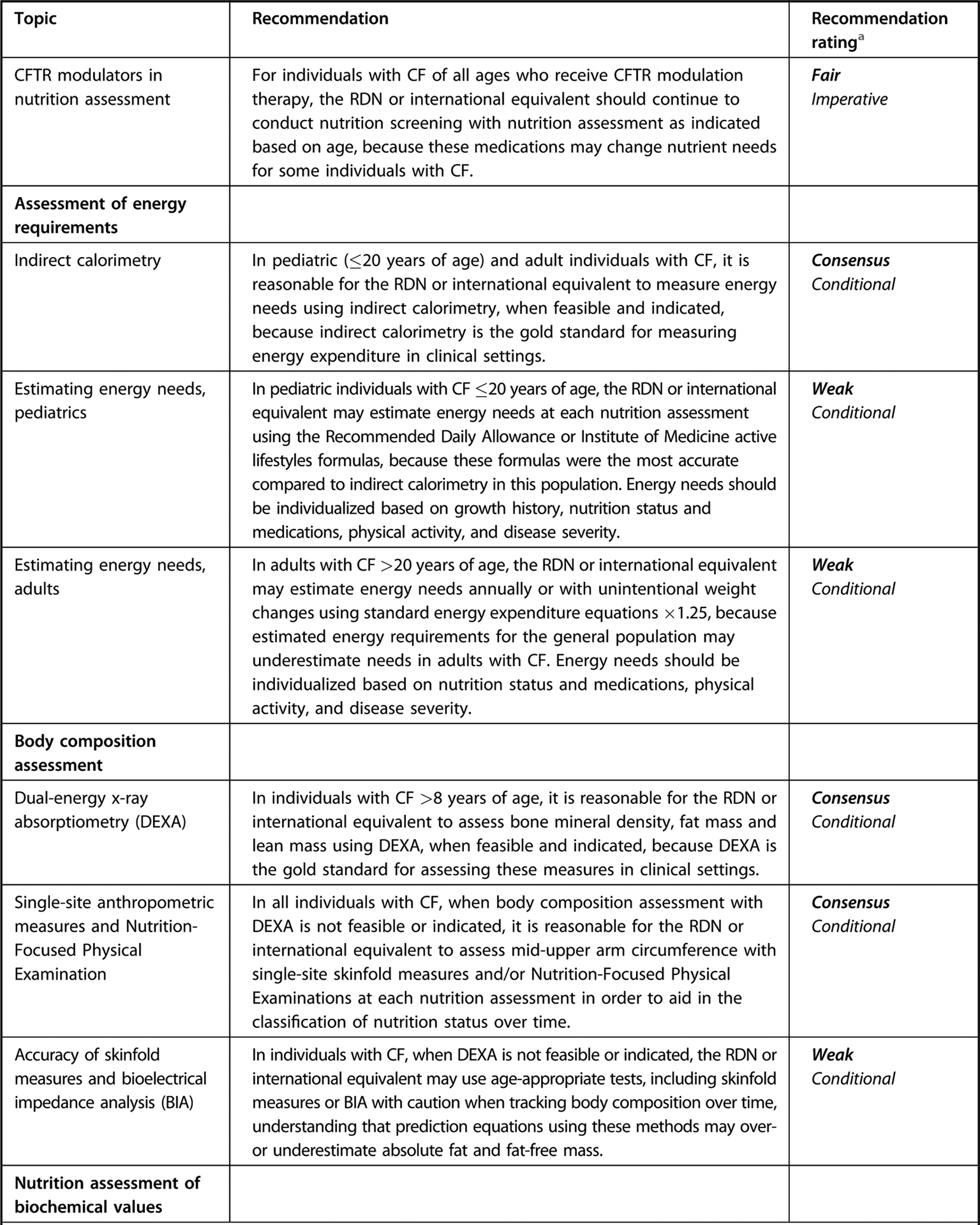
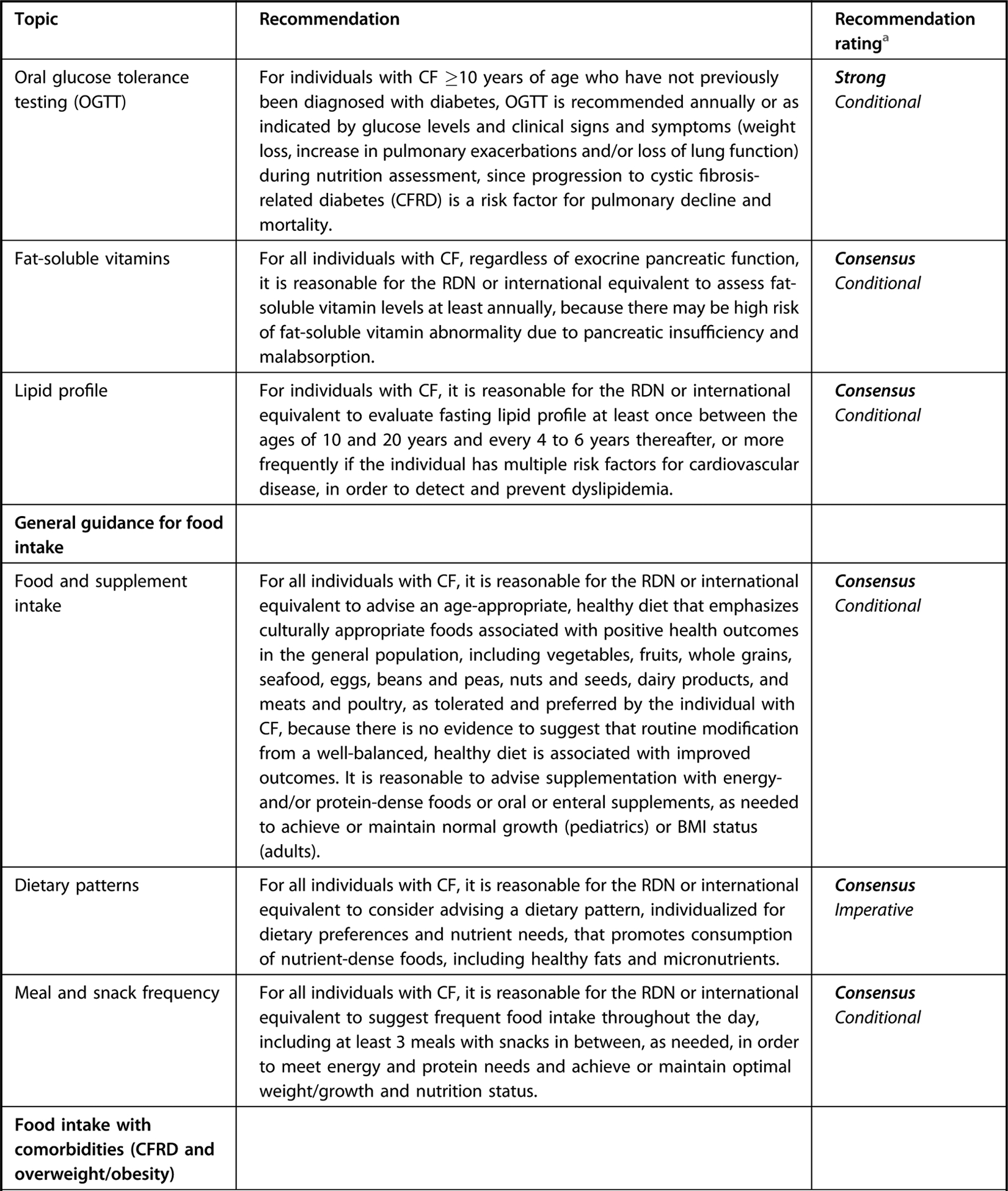
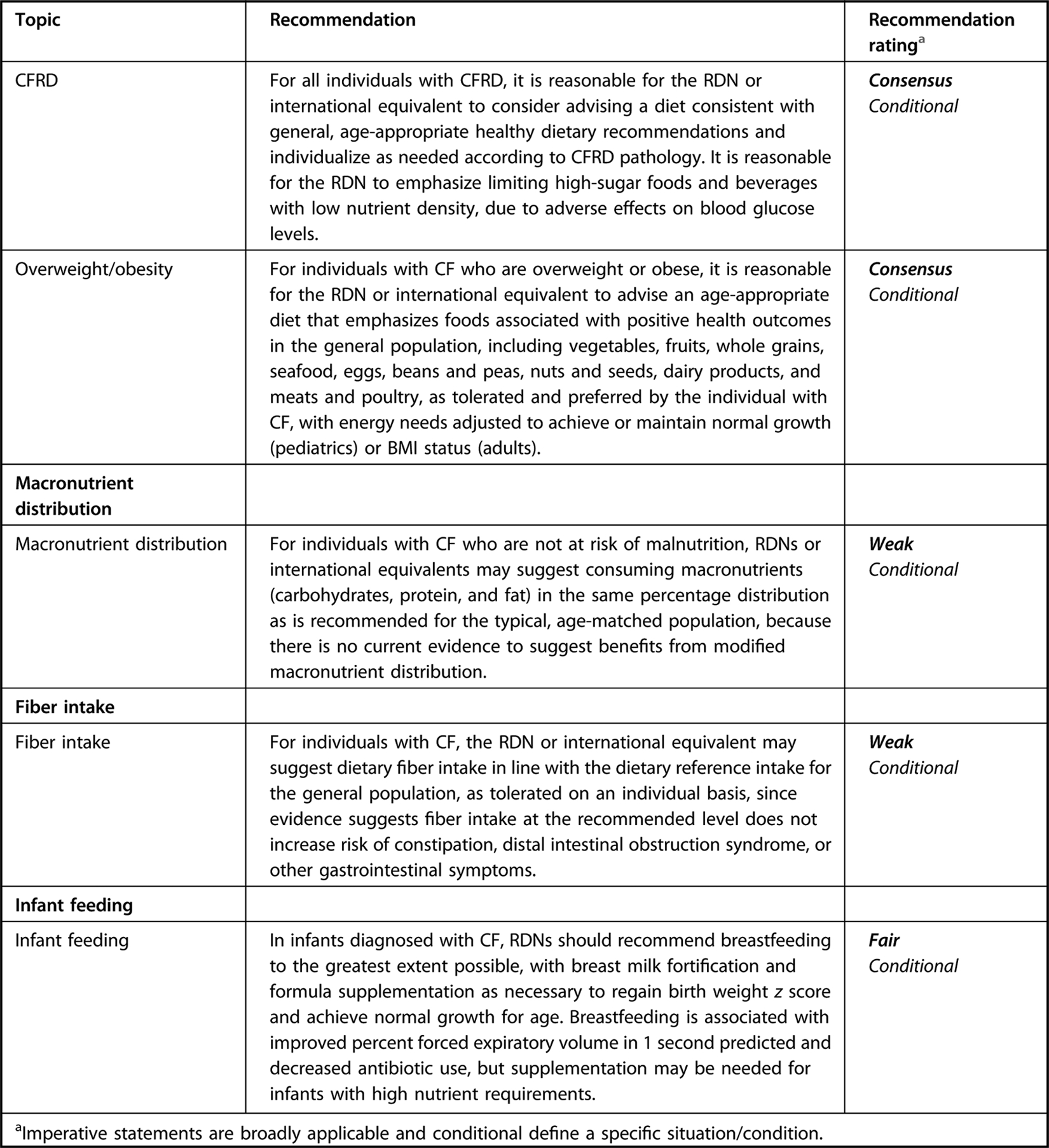
Executive summary table for Academy of Nutrition and Dietetics cystic fibrosis evidence-based nutrition practice guideline.
GUIDELINE OBJECTIVE
Our aim was to provide recommendations for RDNs delivering MNT to individuals with CF and their families that fill gaps in current evidence-based guidelines on topics that are crucial in order to improve health and prevents disease progression.
METHODS
Development of this evidence-based nutrition practice guideline and supporting systematic review followed rigorous processes established by the EAC.13,14 Methods specific to this guideline and the supporting systematic reviews are described briefly here, and in detail on the Evidence Analysis Library (EAL) website.15 For nutrition topics outside of the scope of this guideline, external evidence-based guidelines were reviewed using the Appraisal of Guidelines for Research and Evaluation II tool16 and graded recommendations were voted on by workgroup members in order to provide practitioners with a comprehensive guide to CF MNT (Figure 1).
Workgroup Description and Selection Process
In 2017, workgroup member applicants were reviewed and selected by the Academy’s Evidence-Based Practice Committee (now represented on the Academy’s Council on Research). Workgroup members included 6 RDNs with extensive experience in MNT and/or research with individuals with CF. Two parents of individuals with CF served as patient advocates. All team members participated in each step of the systematic review and guideline development process. Additional team members included systematic review and guideline methodologists, a medical librarian, a project manager, lead analysts, and trained evidence analysts. The workgroup met in a virtual workspace approximately twice per month to develop research questions, screen studies, analyze evidence, vote on and grade conclusion statements, and develop and discuss recommendations.
GUIDELINE SCOPE
Results of an evidence scoping review revealed that recent evidence-based nutrition guidelines for individuals with CF do not include guidance on frequency of MNT or give recommendations for staffing for RDNs working primarily in the United States. In addition, there was an absence of evidence-based recommendations describing valid and reliable nutrition screening and assessment methods to guide nutrition diagnosis and little evidence-based literature examining optimal food intake for individuals with CF.17 Therefore, in this guideline, the authors sought to fill gaps and address current changes in CF treatments and trends in nutrition status.
Systematic Review Process Summary
Question Development, Literature Search, and Study Selection.
This guideline followed the Academy of Nutrition and Dietetics systematic review methodology.13 The PICO (population [P], intervention or exposure [I], comparison or control [C], and outcome [O]) questions, eligibility criteria (Figure 3), and search plan for this systematic review were registered a priori on the PROSPERO database (CRD42018097373).18 The PICO questions examined in this guideline and supporting systematic review are listed in Figure 4.
Figure 3.
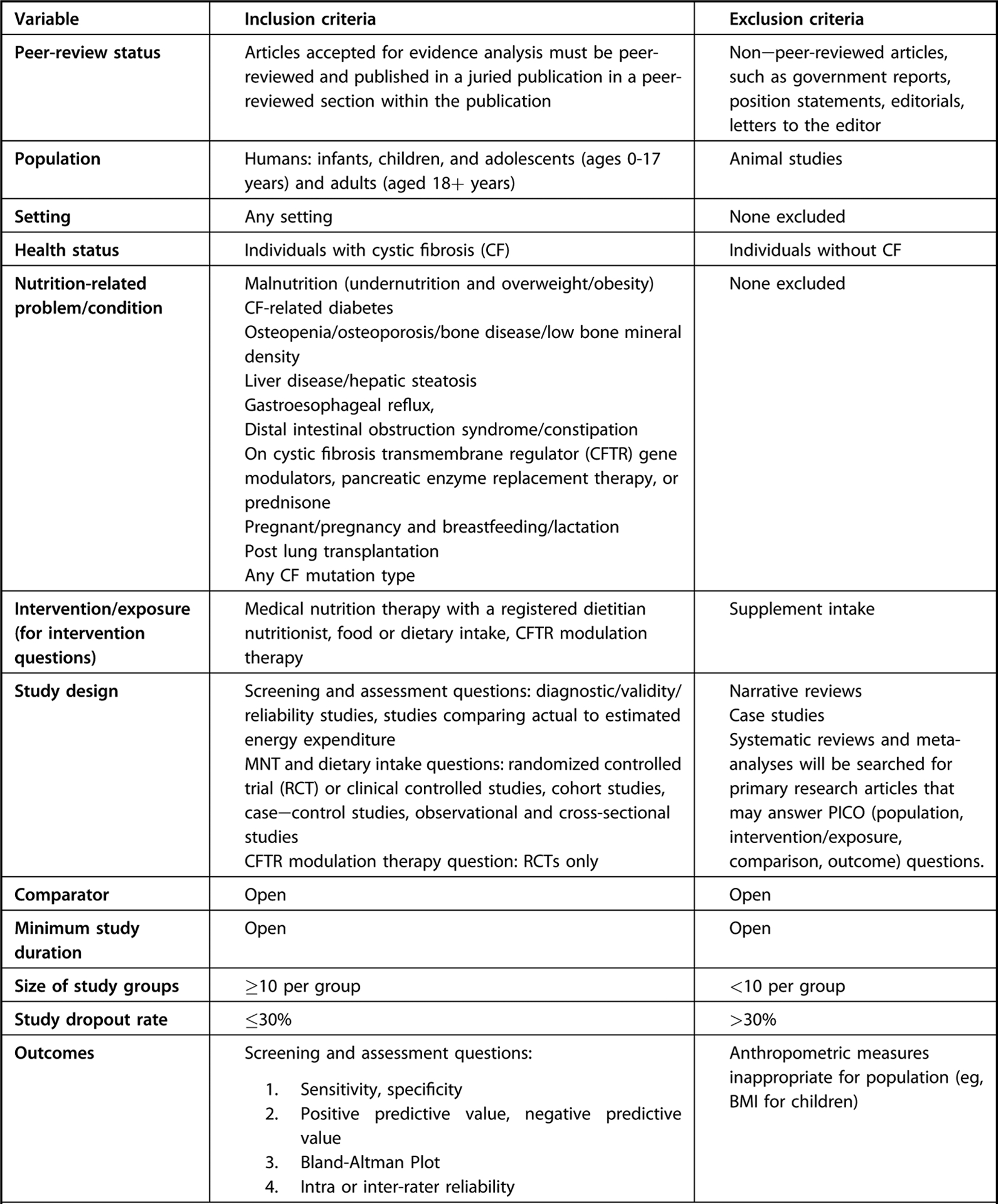
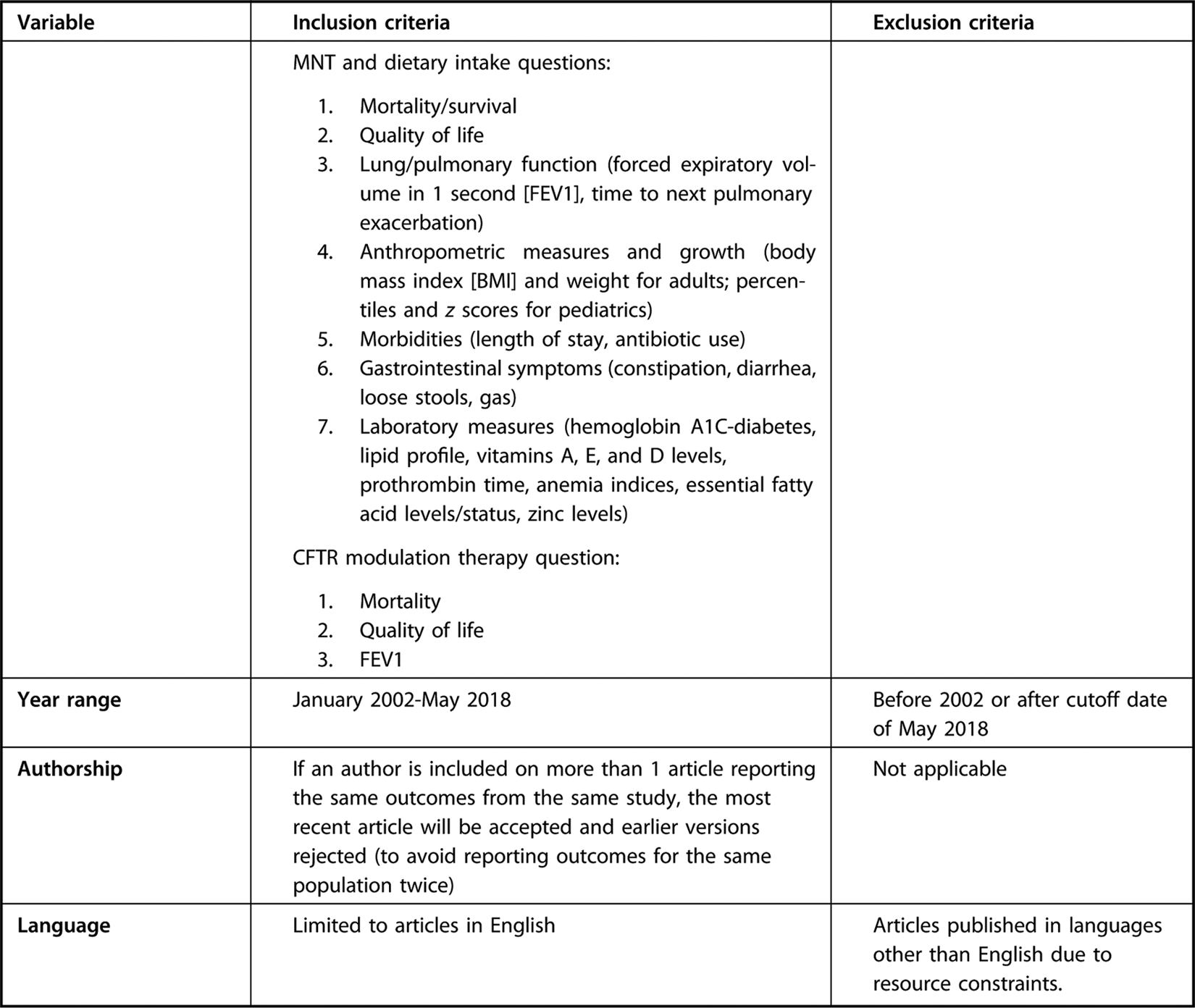
Eligibility criteria for systematic review questions supporting the evidence-based nutrition practice guideline for individuals with cystic fibrosis.
Figure 4.
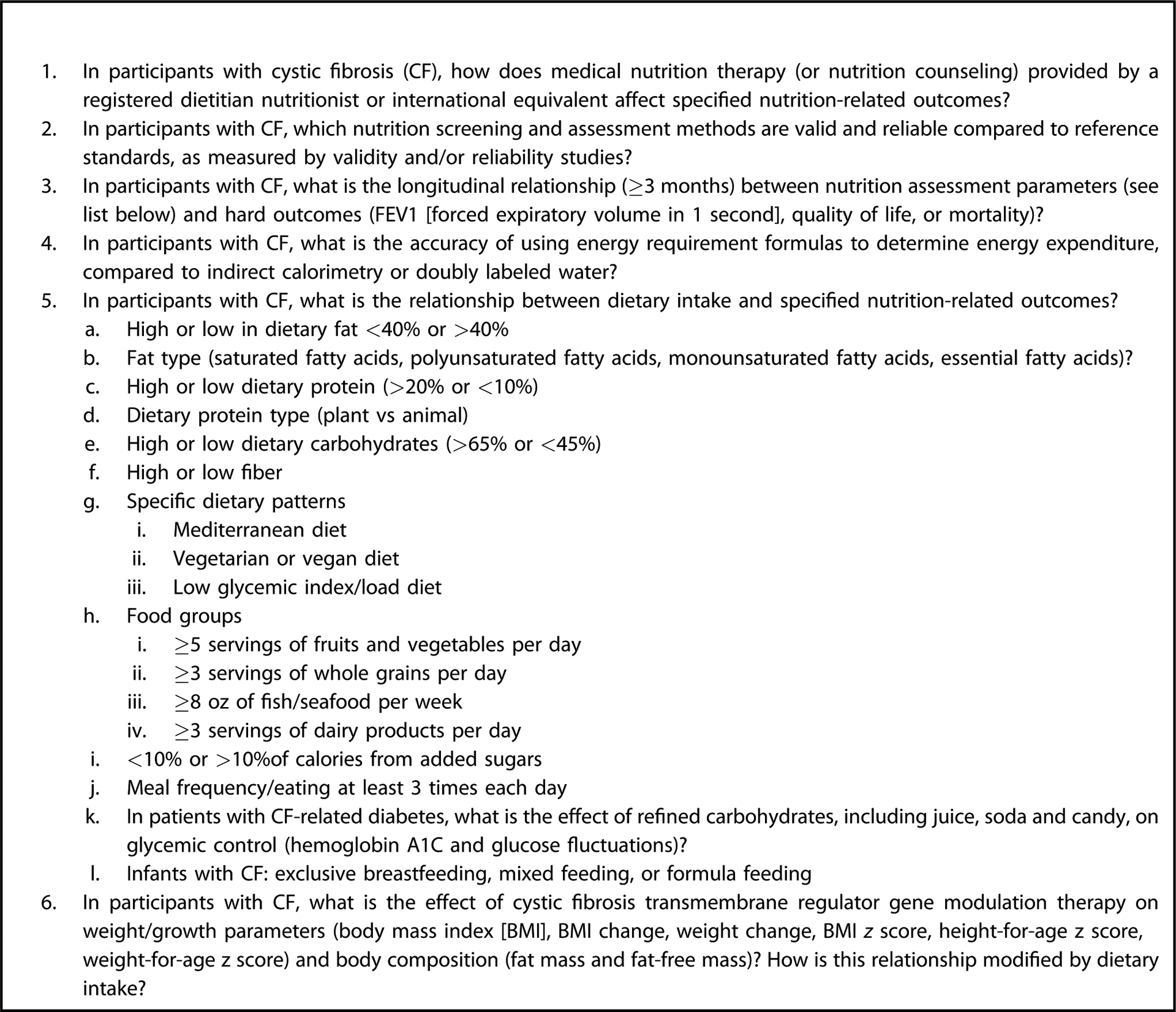
Research question list for cystic fibrosis systematic review supporting the Academy of Nutrition and Dietetics Evidence Analysis Center Cystic Fibrosis Guideline.
A comprehensive search of literature was conducted by a systematic review librarian using MEDLINE, EMBASE, and CINAHL databases. Search terms included terms to identify relevant nutrition screening and assessment tools, dietary intake, and relevant CFTR modulation therapy trials in individuals with CF. Details of the search plan can be found on the EAL website.15 Preferred Reporting Items for Systematic Reviews and Meta-Analyses19 flow diagrams for each of 3 literature searches can be found in Figure 5 (available at www.jandonline.org).
Figure 5.
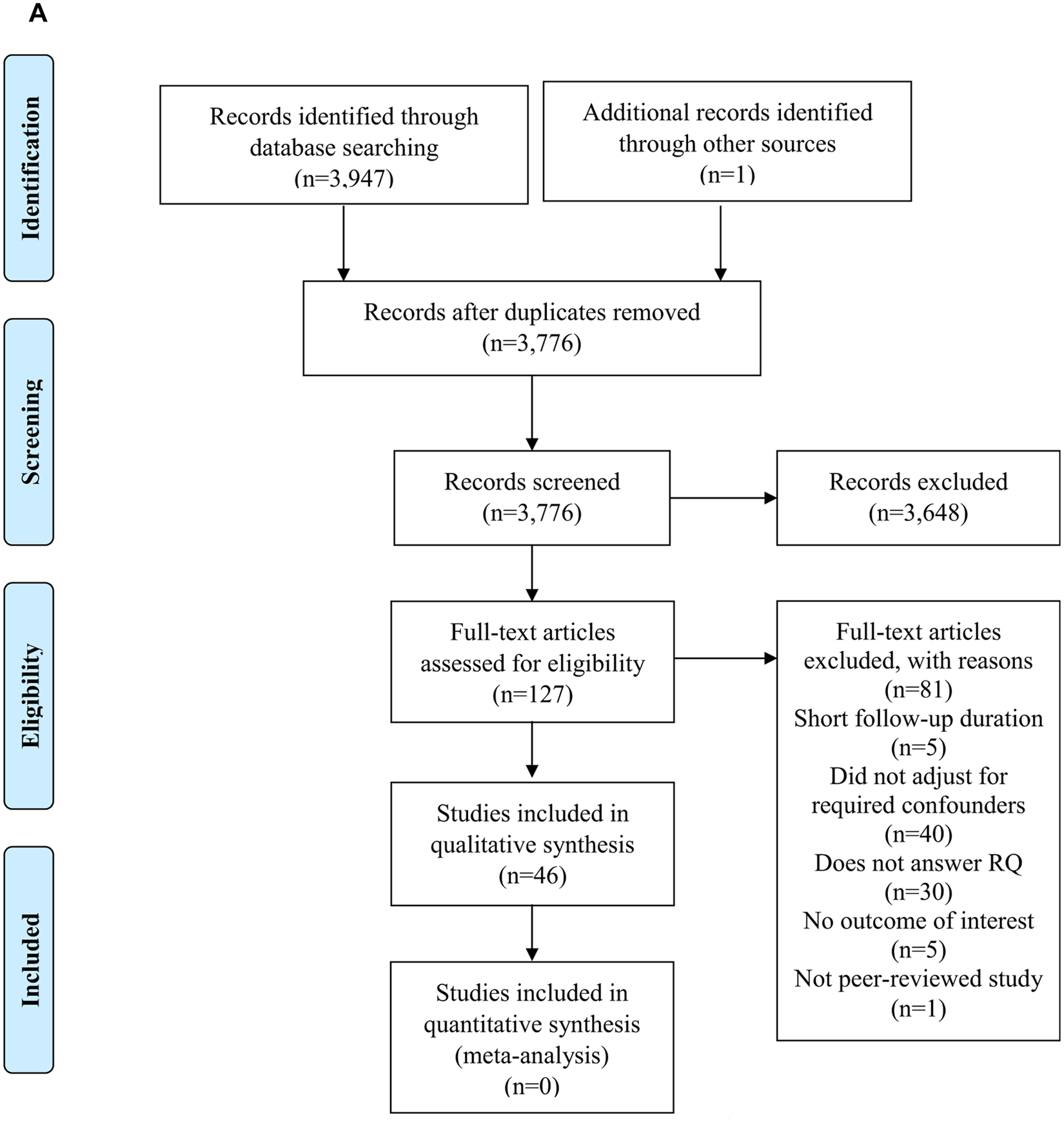
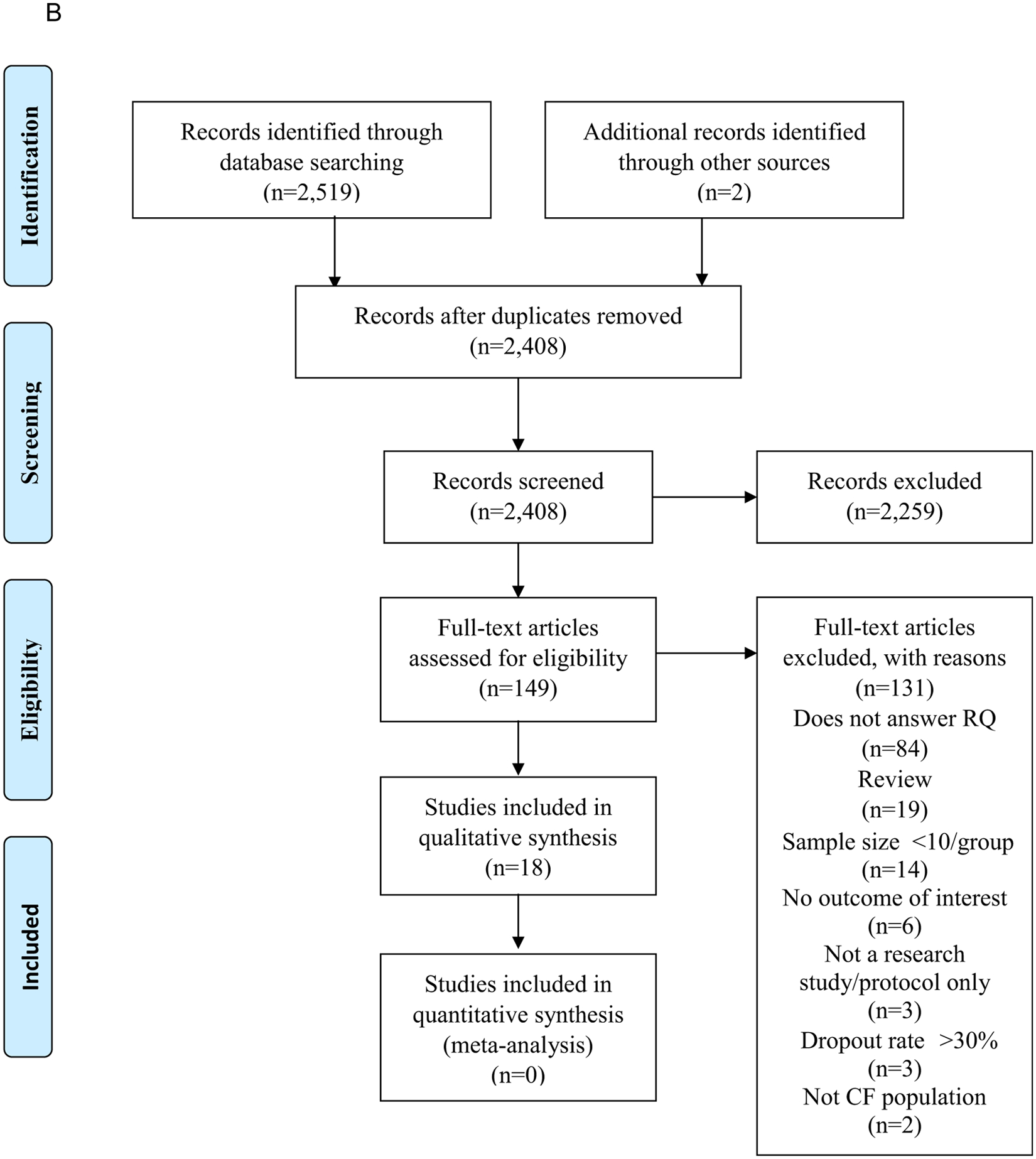
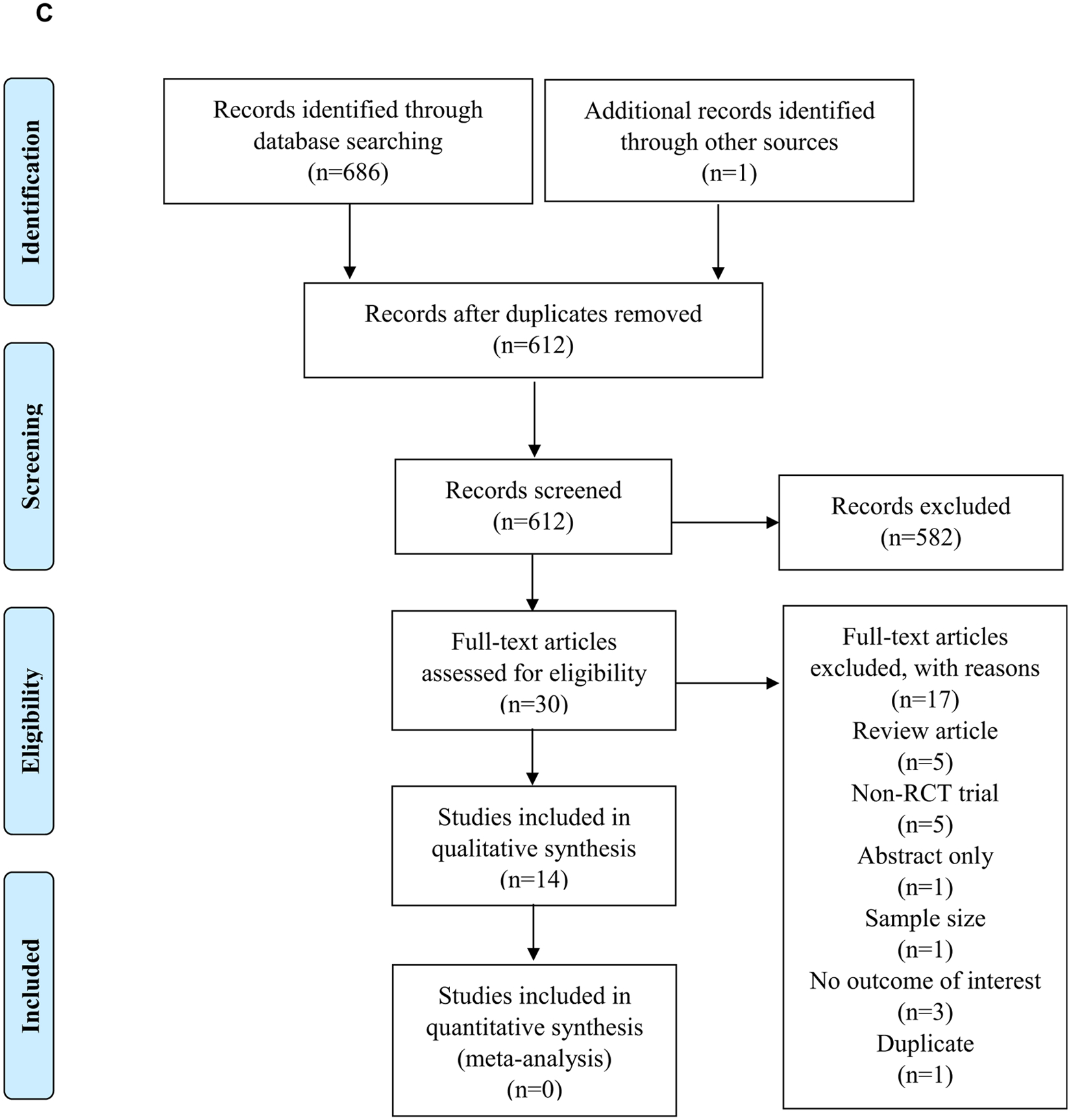
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)19 flow diagrams. (A) PRISMA flow diagram demonstrating the study selection process following the cystic fibrosis (CF) nutrition screening and assessment literature search. (B) PRISMA flow diagram demonstrating the study selection process following the cystic fibrosis medical nutrition therapy and dietary intake search. (C) PRISMA flow diagram demonstrating the study selection process following the cystic fibrosis cystic fibrosis transmembrane regulator (CFTR) modulation therapy literature search. RCT=randomized controlled trial. RQ=research question.
Study Quality Assessment.
Two independent reviewers assessed the risk of bias for each study using the Academy’s online risk of bias tool, the Quality Criteria Checklist.20 The questions of the Quality Criteria Checklist are based on quality constructs and risk of bias domains identified by the Cochrane Collaboration,21 including selection bias, performance bias, detection bias, attrition bias, and reporting bias. Any discrepancies between the 2 reviewers were resolved by consensus or a third reviewer.
Data Synthesis and Grading the Evidence.
Descriptive synthesis of evidence was conducted for all identified outcomes reported in included studies. Meta-analysis was considered for the RCTs examining effect of CFTR modulation therapy on nutrition status, but data were insufficient for meta-analysis for all other PICO questions/outcomes.
Study characteristics tables describing the included studies can be found on the EAL website.15 Conclusion statements were developed for each PICO question. Each of the conclusion statements was assigned a grade to reflect the quality of evidence by examining risk of bias of included studies, inconsistency of results, imprecision, indirectness of the evidence, and publication bias for each outcome reported in included studies. A Summary of Findings table was generated using GradePro and demonstrated how the strength of evidence (Grading of Recommendations Assessment, Development, and Evaluation [GRADE]) was derived for each outcome of interest.22 All Summary of Findings tables can be found on the EAL website.15 Using this method, the evidence for each outcome of interest was graded as I (Good/Strong), II (Fair/Moderate), III (Limited/Weak), or V (no evidence available) (Figure 6).
Figure 6.
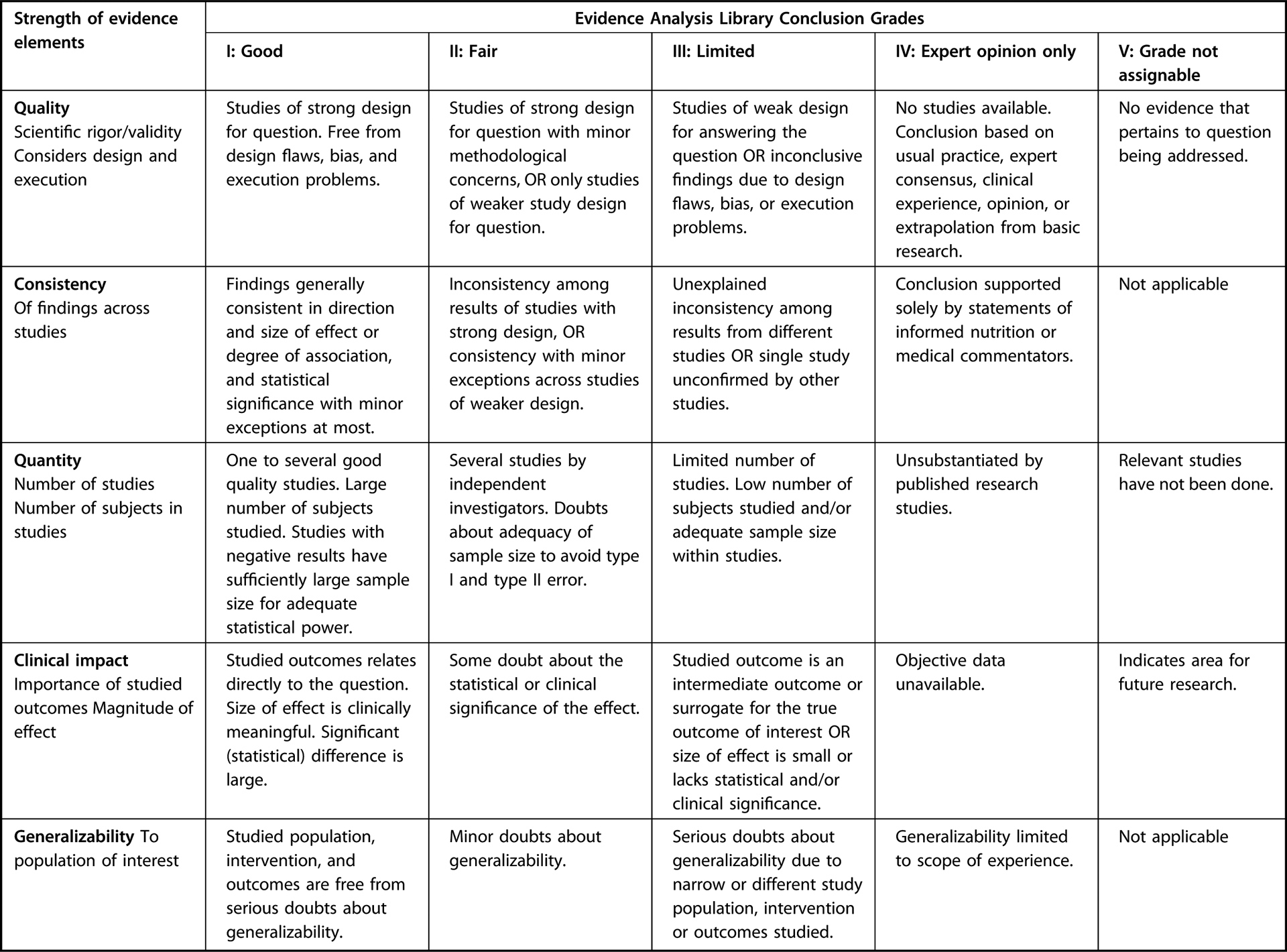
Academy of Nutrition and Dietetics Evidence Analysis Center systematic review conclusion statement grading.
Guideline Development
This guideline followed the Academy’s EAC process for guideline development.14 For each nutrition topic investigated for which evidence was available, workgroup members completed GRADE’s Evidence-to-Decision framework, which guides review of the balance of benefits and harms, certainty of evidence, outcome importance, resource use, equity, patient values, acceptability and feasibility based on available evidence, and clinical expertise in order to develop recommendations.23–25 In addition to evidence-based recommendations, in certain scenarios “Consensus” statements were developed based on clinical expertise; literature outside of the systematic review; and nutrition principles and growth goals for the general population, with specifications that all practice decisions should be individualized according to the specific client. All consensus recommendations were discussed and approved unanimously by the workgroup. The workgroup and staff used the Academy method for rating recommendations based on strength of evidence/confidence in findings and clinical experience (Figure 7).
Figure 7.
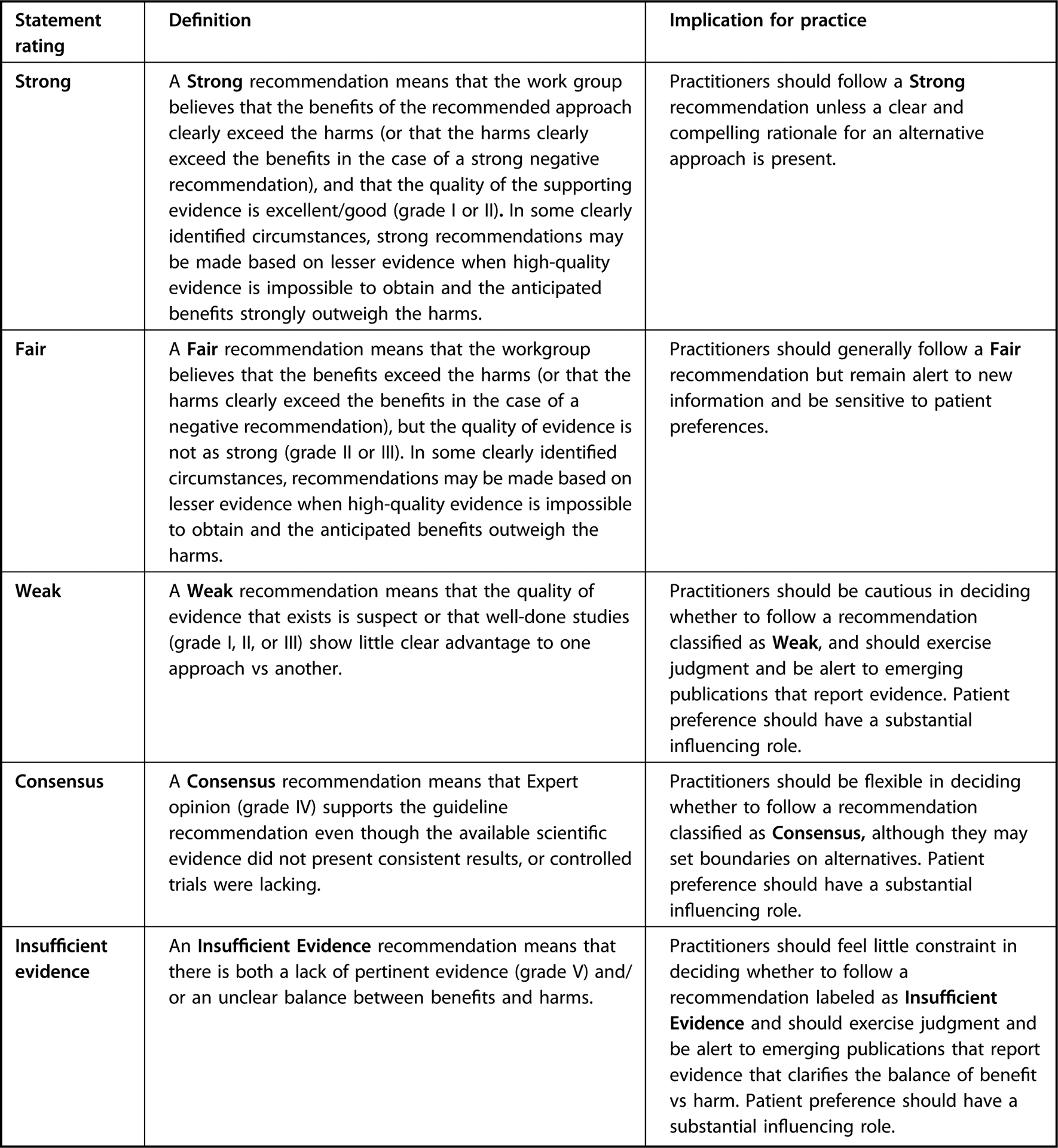
Academy of Nutrition and Dietetics Evidence Analysis Center Guideline Recommendation Ratings.20 Recommendations are categorized in terms of either imperative or conditional statements. Imperative statements are broadly applicable to the target population and do not impose restraints on their pertinence. Imperative recommendations may include terms such as “should” or “may” and do not contain conditional text that would limit their applicability to specified circumstances. Conditional statements clearly define a specific situation or population. Conditional recommendations are often presented in an if/then format, such that if condition than action(s) because reasons(s). Fulfillment of the condition triggers 1 or more guideline-specified actions.
Workgroup members and patient advocates participated in a final, blinded vote of recommendation statements, and a majority of votes approving the statement were necessary for each statement to be accepted into the final guideline. Each recommendation was approved unanimously by the workgroup members. For each set of recommendations, the workgroup members and patient advocates specified potentials risks and harms, conditions of application, costs, recommendation narrative/rationale, and rationale for the recommendation rating. In these sections, the workgroup members also cited additional references important to the respective topic, including discussion of studies published after our search dates or other systematic reviews on the topic. An abbreviated version of supporting evidence is included in this article and the complete version can be found on the EAL website.16
External Peer-Review Process.
These guidelines underwent a peer-review process. External review was conducted by 19 experienced RDNs and physicians in this field. The Appraisal of Guidelines for Research and Evaluation II tool criteria was used to assess the quality of guideline reporting.16 An additional external content review was conducted by the CFF in order to insure feedback from a variety of stakeholders in the CF community. The workgroup chair and project manager coordinated the final revision of the guideline document based on external review comments and any recommendation statements that were edited during external review were voted on and approved unanimously by workgroup members.
Guideline Updates.
Academy guidelines are revisited every 5 years. A scoping review will be conducted to examine the need for new and revised recommendations based on the available science. The Academy’s Council on Research determines whether the update will include modification to all, some, or no recommendations compared to the earlier version(s) of the guideline, or development of new recommendations.
RECOMMENDATION STATEMENTS AND SUPPORTING EVIDENCE
1.0. MNT: Suggested Approach and Dietitian Full-Time Equivalents
Recommendation 1.1.
RDNs or international equivalents should collaborate with all individuals with CF, their families, and interdisciplinary health care teams to co-produce individualized MNT based upon the individual’s personal preferences, psychological and psychosocial factors, physiological needs, health status, and pharmacologic interventions. MNT for individuals with CF should include comprehensive nutrition assessment and appropriate interventions, including individualized modification of diet, dietary supplements including micronutrient supplements, and pancreatic enzymes, in order to maintain or improve nutrition status and symptoms over time.
Strength: Fair
Imperative
Recommendation 1.2.
It is reasonable for 1 full-time equivalent RDN or international equivalent to provide care for 75 to 150 individuals with CF. A caseload at the lower end of this range is appropriate for RDNs who work primarily with the pediatric population or adults with advanced disease and/or comorbidities in order to deliver continuous, high-quality nutrition care that effectively manages nutrition challenges and prevents disease decline.
Strength: Consensus
Conditional
Rationale:
Anticipative, proactive nutritional management is essential to daily, preventive care and treatment in CF. Consistent contact between individuals with CF, their families and the RDN is essential for developing rapport and long-term relationships that facilitate addressing nutrition challenges and preventing adverse nutrition related outcomes throughout the life-span. The RDN is a critical member of an interdisciplinary team that includes the individual with CF, family, physicians, nurses, respiratory therapists, and other health care professionals, such as pharmacists, psychologists, social workers, research coordinators, and/or physical therapists.26 CF RDNs provide expertise and education on topics including but not limited to malnutrition and nutrient deficiencies, vitamin and mineral (including sodium) supplementation, pancreatic enzyme replacement therapy (PERT), oral and enteral nutrition supplementation, as well as CF-related diabetes (CFRD) and other comorbid conditions.
Evidence for Pediatrics.
There was little evidence describing optimal MNT frequency identified in the systematic review (Table), and there was no evidence examining effects of MNT on outcomes for infants with CF. Evidence generally demonstrated that increased or intensified MNT, as part of a greater intervention, was associated with improved growth outcomes in children and adolescents with CF (Table).27–29 This evidence, along with conclusions regarding the importance of weight/growth in preventing pulmonary decline,30–53 demonstrate the critical role of an adequate level of care delivered by an RDN to improve outcomes.
Table.
Academy of Nutrition and Dietetics Evidence Analysis cystic fibrosis systematic review questions, conclusions, number of studies, evidence quality, and respective recommendations and ratings
| 1.0 MNTa: Suggested approach and registered dietitian nutritionist (RDN) full-time equivalents (FTEs) | |
|---|---|
| SRb question 1: In participants with cystic fibrosis (CF), how does MNT (or nutrition counseling) provided by an RDN or international equivalent on nutrition-related outcomes? | |
| Conclusions | |
| • In pediatric participants with CF ages 2 to 20 years, the preponderance of observational evidence suggests that increasing individualized nutrition therapy from an RDN to a minimum of at least quarterly may improve body mass index (BMI) percentile or decrease decline in BMI z score, although baseline MNT frequency was not described. | 4 observational studies,27–29,60 Grade III |
| • In participants with moderate-to-severe CF ages 4 to 15 years, a small quality-improvement study demonstrated a 23% decrease in days required for inpatient intravenous antibiotic treatment and a 20% decrease in days required for outpatient antibiotic treatment when physiotherapy and individualized dietetics therapy were increased for 1 year, although duration of MNT sessions and previous MNT care were not reported. | 1 observational study,60 Grade III |
| SR question 3: In participants with CF, what is the longitudinal relationship (at least 3 months) between weight and growth parameters and hard outcomes (FEV1,c quality of life, or mortality)? | |
| Conclusions | |
| • In pediatric participants with CF, regaining birth weight z score by age 2 years and maintaining BMI and height z score throughout childhood was associated with the highest FEV1% predicted later in life compared to those who did not regain and maintain z scores in studies referencing Centers for Disease Control and Prevention (CDC) growth charts. Participants who maintained a weight, length, weight-for-length (WFL), and BMI >50th percentile from infancy and early childhood had better FEV1% predicted values, although there was no added improvement for those who maintained growth parameters >85th percentile compared with >50th percentile In general, normal growth parameters during childhood were associated with increased FEV1% predicted in long-term follow-up studies (4–16 years). | 16 observational studies,31–46 Grade I |
| • In pediatric and adult participants combined, underweight (BMI >10th to 12th percentile for pediatric and <18.5 for adult) was associated with increased risk of mortality (hazard ratio [HR]=2.12) after a median of 12 years. There was a linear relationship between each unit increase in BMI z score at baseline and mortality after a median of 13.4 years (HR=1.2). Stunting (defined as height <5th percentile) was associated with increased odds of mortality (odds ratio [OR]=2.2). | 3 observational studies,50–52 Grade II |
| • In adults with CF, data were mixed, but 1 large retrospective cohort study suggested that BMI ≥25 is associated with decreased decline in FEV1% predicted and BMI <18.5 is associated with increased decline in FEV1% predicted after a follow-up of up to 13 years. Baseline FEV1% predicted was also associated with change in BMI over time. | 2 observational studies,33,47 Grade II |
| • In adults with CF, baseline BMI of ≤18 to 19 was associated with higher risk of mortality after up to 24 years of follow-up (HR range=1.52–1.57). | 2 observational studies,48,49 Grade II |
| Recommendations | |
| 1.1 RDNs or international equivalents should collaborate with all individuals with CF, their families, and interdisciplinary health care teams to co-produce individualized MNT based on the individual’s personal preferences, psychological and psychosocial factors, physiological needs, health status, and pharmacologic interventions. MNT for individuals with CF should include comprehensive nutrition assessment and appropriate interventions, including individualized modification of diet, dietary supplements, including micronutrient supplements, and pancreatic enzymes, in order to maintain or improve nutrition status and symptoms over time. | Fair Imperative |
| 1.2 It is reasonable for 1 FTE RDN or international equivalent to provide care for 75 to 150 individuals with CF. A caseload at the lower end of this range is appropriate for RDNs who work primarily with the pediatric population or adults with advanced disease and/or comorbidities in order to deliver continuous, high-quality nutrition care that effectively manages nutrition challenges and prevents disease decline. | Consensus Conditional |
| 2.0 Nutrition screening: pediatrics | |
| SR question 2: In pediatric participants with CF, which nutrition screening methods are valid and reliable compared to reference standards, as measured by validity and/or reliability studies? | |
| Conclusion | |
| In children with CF ≤2 years of age World Health Organization (WHO) growth charts were less likely to classify children as being underweight based on weight-for-age (WFA) and WFL <50th percentile compared to CDC growth charts. Validity was low for growth standards or velocities at 4 to 12 months for predicting WFA and length-for-age (LFA) growth failure at 24 months. | 2 validity/reliability studies,37,56 Grade II |
| SR question 1: In participants with CF, how does MNT (or nutrition counseling) provided by an RDN or international equivalent on nutrition-related outcomes? | |
| Conclusions | |
| • In pediatric participants with CF ages 2 to 20 years, the preponderance of observational evidence suggests that increasing individualized nutrition therapy from an RDN to a minimum of at least quarterly may improve BMI percentile or decrease decline in BMI z score, although baseline MNT frequency was not described. | 4 observational studies27–29,60 Grade III |
| • In participants with moderate-to-severe CF ages 4 to 15 years, a small quality-improvement study demonstrated a 23% decrease in days required for inpatient intravenous antibiotic treatment and a 20% decrease in days required for outpatient antibiotic treatment when physiotherapy and individualized dietetics therapy were increased for 1 year, although duration of MNT sessions and previous MNT care were not reported. | 1 observational study,60 Grade III |
| SR question 3: In participants with CF, what is the longitudinal relationship (at least 3 months) between weight and growth parameters and hard outcomes (FEV1, quality of life, or mortality)? | |
| Conclusions | |
| • In pediatric participants with CF, regaining birth weight z score by age 2 years and maintaining BMI and height z score throughout childhood was associated with the highest FEV1% predicted later in life compared to those who did not regain and maintain z scores in studies referencing CDC growth charts. Participants who maintained a weight, length, WFL, and BMI >50th percentile from infancy and early childhood had better FEV1% predicted values, although there was no added improvement for those who maintained growth parameters >85th percentile compared with >50th percentile. In general, normal growth parameters during childhood were associated with increased FEV1% predicted in long-term follow-up studies (4–16 years). | 16 observational studies,31–46 Grade I |
| • In pediatric and adult participants combined, underweight (BMI <10th to 12th percentile for pediatric and <18.5 for adult) was associated with increased risk of mortality (HR=2.12) after a median of 12 years. There was a linear relationship between each unit increase in BMI z score at baseline and mortality after a median of 13.4 years (HR=1.2). Stunting (defined as height <5th percentile) was associated with increased odds of mortality (OR=2.2). | 3 observational studies,50–52 Grade II |
| Recommendations | |
| 2.1 For infants and children with CF <2 years of age, it is reasonable to measure weight and length at each clinic visit and to screen for risk of impaired growth and other nutrition concerns at least monthly for the first 6 months of age, every other month from 6 to 12 months of age, and quarterly from 12 to 24 months of age to identify nutrition risk. | Consensus Imperative |
| 2.2 Infants and children with CF <2 years of age should be screened for nutrition risk by comparing WFA, WFL, and LFA z scores or percentiles to birth weight and to growth norms using WHO growth charts for the general population because these parameters are longitudinally associated with lung function. Children who are not maintaining birth weight, WFL, or LFA z scores or who have depressed growth compared to the general population should be referred for full nutrition assessment by an RDN or international equivalent. | Strong Imperative |
| 2.3 For children and adolescents with CF ages 2 to 20 years, weight and height should be measured at each clinic visit and children should be screened for risk of impaired growth and other nutrition concerns at least quarterly or more frequently based on clinical condition to identify nutrition risk. | Fair Imperative |
| 2.4 Pediatric individuals with CF 2 to 20 years of age should be screened for nutrition risk by comparing growth percentiles and z scores to general population norms using CDC growth charts for the general population, because these parameters are longitudinally associated with lung function. Children and adolescents who have a BMI-for-age <50th percentile and/or who have concerning trends in BMI-for-age, WFA, or height-for-age z scores should be referred for full nutrition assessment by an RDN or international equivalent. | Strong Imperative |
| 3.0 Nutrition screening: adults | |
| SR question 2: In adults with CF, which nutrition screening methods are valid and reliable compared to reference standards, as measured by validity and/or reliability studies? | |
| Conclusion: In adult participants with CF, using a BMI cutoff of ≤18.5 to identify malnutrition may misclassify those who have a BMI >18.5 but are malnourished based on low fat-free mass. | 3 validity/reliability studies,58,114,115 Grade II |
| SR question 3: In adults with CF, what is the longitudinal relationship (at least 3 months) between weight parameters and hard outcomes (FEV1, quality of life, or mortality)? | |
| Conclusions | |
| • In adults with CF, data was mixed, but 1 large retrospective cohort study suggested that BMI ≥25 is associated with decreased decline in FEV1% predicted and BMI <18.5 is associated with increased decline in FEV1% predicted after a follow-up of up to 13 years. Baseline FEV1% predicted was also associated with change in BMI over time. | 2 observational studies,33,47 Grade II |
| • In adults with CF, baseline BMI of ≤18 to 19 was associated with higher risk of mortality after up to 24 years of follow-up (HR range=1.52–1.57). | 2 observational studies,48,49 Grade II |
| Recommendations | |
| 3.1 In adults with CF >20 years of age, it is reasonable to measure weight and height at each clinic visit and to screen for malnutrition and/or other nutrition concerns at least quarterly or more frequently based on clinical condition to identify nutrition risk. | Consensus Imperative |
| 3.2 Adults with CF >20 years of age should be screened for nutrition risk by evaluating absolute values and trends in BMI, because BMI is longitudinally associated with lung function. Women who have a BMI <22, men who have a BMI <23, or adults who have concerning trends in BMI, either decreasing or increasing, should be referred for full nutrition assessment by an RDN or international equivalent. | Fair Imperative |
| 4.0 Nutrition assessment and diagnosis of nutrition status: pediatric and adult | |
| SR question 2: In participants with CF, which nutrition assessment methods are valid and reliable compared to reference standards, as measured by validity and/or reliability studies? | |
| Conclusions | |
| • In children with CF ≤2 years of age WHO growth charts were less likely to classify children as being underweight based on WFA and WFL <50th percentile compared to CDC growth charts. Validity was low for growth standards or velocities at 4 to 12 months for predicting WFA and LFA growth failure at 24 months. | 2 validity/reliability studies,37,56 Grade II |
| • In pediatric participants with CF, dual energy x-ray absorptiometry (DEXA) is considered the gold standard to measure body composition in clinical practice. Bioelectrical impedance analysis (BIA) estimates may be accurate for measuring body composition compared to DEXA if CF-specific equations are used, but evidence is limited. There were differences in fat mass and fat-free mass measured by equations utilizing 2- or 4-site skinfold measurements compared to gold standards and the degree and direction of bias was variable according to individuals’ sex and body composition. | 4 validity/reliability studies,108–110,113 Grade III |
| • In adult participants with CF, using a BMI cutoff of ≤18.5 to identify malnutrition may misclassify those who have a BMI >18.5 but are malnourished based on low fat-free mass. | 3 validity/reliability studies,58,114,115 Grade II |
| • In adults with CF, DEXA is considered the gold standard to measure body composition in clinical practice. Compared to DEXA, assessment of fat-free mass using skinfold measures or bioelectrical impedance with equations intended for the general population under- or overestimated fat-free mass by a mean of −3.3 kg to 2.9 kg for adults with CF (low validity). | 2 validity/reliability studies,114,115 Grade II |
| SR question 1: In participants with CF, how does MNT (or nutrition counseling) provided by an RDN or international equivalent on nutrition-related outcomes? | |
| Conclusions | |
| • In pediatric participants with CF ages 2 to 20 years, the preponderance of observational evidence suggests that increasing individualized nutrition therapy from an RDN to a minimum of at least quarterly may improve BMI percentile or decrease decline in BMI z score, although baseline MNT frequency was not described. | 4 observational studies,27–29,60 Grade III |
| • In participants with moderate-to-severe CF ages 4 to 15 years, a small quality-improvement study demonstrated a 23% decrease in days required for inpatient intravenous antibiotic treatment and a 20% decrease in days required for outpatient antibiotic treatment when physiotherapy and individualized dietetics therapy were increased for 1 year, although duration of MNT sessions and previous MNT care were not reported. | 1 observational study,60 Grade III |
| SR question 3: In participants CF, what is the longitudinal relationship (at least 3 months) between nutrition parameters and hard outcomes (FEV1, quality of life, or mortality)? | |
| Conclusions | |
| • In pediatric participants with CF, regaining birth weight z score by age 2 years and maintaining BMI and height z score throughout childhood were associated with the highest FEV1% predicted later in life compared to those who did not regain and maintain z scores in studies referencing CDC growth charts. Participants who maintained a weight, length, WFL, and BMI >50th percentile from infancy and early childhood had better FEV1% predicted values, although there was no added improvement for those who maintained growth parameters >85th percentile compared with >50th percentile In general, normal growth parameters during childhood were associated with increased FEV1% predicted in long-term follow-up studies (4–16 years). | 16 observational studies,31–46 Grade I |
| • In pediatric and adult participants combined, underweight (BMI <10th to 12th percentile for pediatric and <18.5 for adult) was associated with increased risk of mortality (HR=2.12) after a median of 12 years. There was a linear relationship between each unit increase in BMI z score at baseline and mortality after a median of 13.4 years (HR=1.2). Stunting (defined as height <5th percentile) was associated with increased odds of mortality (OR=2.2). | 3 observational studies,50–52 Grade II |
| • In pediatric participants with CF, 1 cohort study found no longitudinal relationship between 25-hydroxyvitamin D levels in 6- to 18-year-olds who were pancreatic insufficient (PI) and FEV1% predicted 4 years later. | 1 observational study,129 Grade III |
| • Pediatric participants with CF who were 5 to 20 years old with cystic fibrosis-related diabetes (CFRD) had significantly greater decline in FEV1% predicted after at least 10 years of follow-up, compared to those without CFRD. | 2 observational studies,33,44 Grade II |
| • In adults with CF, data were mixed, but 1 large retrospective cohort study suggested that body mass index (BMI) ≥25 is associated with decreased decline in FEV1% predicted and BMI <18.5 is associated with increased decline in FEV1% predicted after a follow-up of up to 13 years. Baseline FEV1% predicted was also associated with change in BMI over time. | 2 observational studies,33,47 Grade II |
| • In adults with CF, baseline BMI of ≤18 to 19 was associated with higher risk of mortality after up to 24 years of follow-up (HR range=1.52–1.57). | 2 observational studies,48,49 Grade II |
| • In adults with CF, presence of CFRD was associated with increased decline in FEV1% predicted after a follow-up of 5 to up to 13 years. In adults with CF, 1 large cohort study determined that presence of CFRD was associated with higher risk of mortality after a follow-up of up to 24 years. However, this increased risk was not significant in another smaller study. | 4 observational studies,33,47–49 Grade II |
| • In pediatric participants with CF, 1 cohort study suggests that linoleic acid levels ≥21mol% may be associated with higher FEV1% predicted after 1 year, but there was no relationship with essential fatty acid (EFA) deficiency measured by triene to tetraene ratio and FEV1% predicted after 1 year. In participants with CF ranging from 1 to 41 years and homozygous for F508del mutation, 1 cohort study demonstrated that docosahexaenoic acid levels were positively associated with FEV1% predicted 3 years later. | 2 observational studies,134,139 Grade III |
| • In participants with CF ranging from 1 to 41 years and homozygous for F508del mutation, 1 cohort study detected no longitudinal relationship between triacylglyceride levels and later FEV1% predicted. | 1 observational study,134 Grade III |
| • In a combined group of adult and pediatric participants with CF, the longitudinal relationship between CFRD and association with later mortality was mixed, with the largest study suggesting an association with increased risk of mortality for those with CFRD at baseline (HR=1.31) after 2.9 years, but other studies reporting no difference in mortality according to CFRD. | 2 observational studies,50,52 Grade III |
| Recommendations | |
| 4.1 In individuals with CF, a full nutrition assessment should be conducted by an RDN or international equivalent | Consensus Imperative |
| • at diagnosis; | |
| • when indicated by nutrition screening; | |
| • up to monthly for the first 6 months of life; up to every other month until 1 year of age;and up to quarterly until 2 years of age; | |
| • annually for individuals >2 years of age; | |
| • when disease or treatment course changes | |
| 4.2. In individuals with CF, the RDN or international equivalent should diagnose nutrition status, including underweight and overweight, based on a comprehensive assessment of weight and growth history and stature, body composition, disease severity, laboratory values, drug–nutrient interactions/implications, and estimated energy expenditure compared to client/parent report of dietary intake and food security status, because CF nutrition pathology is highly individual and maintaining optimal nutrition status is a necessary component of preventing disease progression. | Strong Imperative |
| 5.0 Role of CFTR modulators in nutrition assessment | |
| SR question 6: In participants with CF, what is the effect of CFTR modulation therapy on weight/growth parameters and body composition? | |
| Conclusions | |
| • In participants with CF ≤20 years of age with at least 1 copy of the G551D mutation (class III), 48 weeks of 150 mg ivacaftor twice daily increased WFA and BMI-for-age z scores by 0.35 and 0.39, respectively, compared to placebo, when mean baseline BMI z score was −0.199 and WFA z score was −0.292. The same dose may increase BMI-for-age z score after 8 weeks in 6- to 17-year-olds with other gating mutations, but evidence was limited. Effect of ivacaftor on body composition in pediatric participants was not described, nor was the role of diet. | 3 RCTs,d,71,72,76 Grade II |
| • In adults with CF with at least 1 copy of the G551D mutation and with optimal or low mean BMI at baseline, 4 to 48 weeks of treatment with 150 mg ivacaftor twice daily increased weight and BMI by a mean of 2.9 kg and 0.58 to 1.2, respectively. There was no statistically significant effect of CFTR modulation therapy on fat-free mass. Effect of dietary intake on the relationship of interest was not described. | 3 RCTs,71,76,77 Grade II |
| • In adults with CF with at least 1 copy of the R117H mutation (class IV) and optimal mean BMI, 150 mg of ivacaftor twice daily for 24 weeks had no effect on BMI compared to placebo. Effect of ivacaftor on body composition in pediatric participants was not described, nor was the role of diet. | 1 RCT,78 Grade III |
| • In children with CF homozygous for the F508del mutation and ages 6 to 11 years, there was no effect of lumacaftor with ivacaftor on BMI-for-age z score after 24 weeks. Effect of treatment on body composition was not described, nor was the role of diet. | 1 RCT,79 Grade II |
| • In adults with CF with class II mutations, results were mixed regarding the effect of lumacaftor with ivacaftor on BMI. Eight weeks of 400 mg lumacaftor with 250 mg ivacaftor twice daily had no effect on BMI in participants heterozygous for the F508del mutation who had an optimal mean BMI at baseline. However, 24 weeks of this regimen significantly increased BMI in participants homozygous for F508del, and a dose of 600 mg/day lumacaftor daily and 250 mg ivacaftor twice daily had a similar effect, although nutritional status at baseline could not be determined. Effect of treatment on body composition was not described, nor was the role of diet. | 3 RCTs,81,82 Grade II |
| • In participants with CF 12 to 20 years of age who were homozygous for F508del mutation (class II), there was no effect of 100 mg of tezacaftor once daily with 150 mg of ivacaftor twice daily for 24 weeks, compared to placebo, on change in BMI-for-age z score, although baseline nutritional status for pediatric participants could not be determined. Effect of treatment on body composition in pediatric participants was not described, nor was the role of diet. | 1 RCT,83 Grade III |
| Recommendation | |
| 5.1 For individuals with CF of all ages who receive CFTR modulation therapy, the RDN or international equivalent should continue to conduct nutrition screening with nutrition assessment as indicated based on age, because these medications may change nutrient needs for some individuals with CF. | Fair Imperative |
| 6.0 Assessment of Energy Requirements | |
| SR question 4: In participants with CF, what is the accuracy of using energy requirement formulas to determine energy expenditure, compared to indirect calorimetry or doubly labeled water? | |
| Conclusions | |
| • In 1 study of children with CF 6 to 9 years of age who were primarily PI, CF-specific energy equations overestimated needs (122%−136%) compared to doubly labeled water. Energy estimated with the Institute of Medicine (IOM) active lifestyle formula and Recommended Dietary Allowance (RDA) were similar to measured values, although these equations did not adjust for individuals’ activity level. | 1 observational study,93 Grade III |
| • In adults with end-stage CF, Harris-Benedict, Schofield, and WHO 1985 energy expenditure equations underestimated energy needs compared to indirect calorimetry (76%−78% in pre-lung transplantation and 90% to 91% in post-lung transplantation). Underestimation was greater in those with more severe disease. | 3 observational studies,91,92,94 Grade III |
| Recommendations | |
| 6.1 In pediatric (<20 years of age) and adult individuals with CF, it is reasonable for the RDN or international equivalent to measure energy needs using indirect calorimetry, when feasible and indicated, because indirect calorimetry is the gold standard for measuring energy expenditure in clinical settings. | Consensus Conditional |
| 6.2 In pediatric individuals with CF ≤20 years of age, the RDN or international equivalent may estimate energy needs at each nutrition assessment using the RDA or IOM active lifestyles formulas, because these formulas were the most accurate compared to indirect calorimetry in this population. Energy needs should be individualized based on growth history, nutrition status and medications, physical activity and disease severity. | Weak Conditional |
| 6.3 In adults with CF >20 years of age, the RDN or international equivalent may estimate energy needs annually or with unintentional weight changes using standard energy expenditure equations ×1.25, because estimated energy requirements for the general population may underestimate needs in adults with CF. Energy needs should be individualized based on nutrition status and medications, physical activity, and disease severity. | Weak Conditional |
| 7.0 Body composition assessment | |
| SR sub-question 2: In participants with CF, which body composition parameters are valid and reliable compared to reference standards, as measured by validity and/or reliability studies? | |
| Conclusions | |
| • In pediatric participants with CF, DEXA is considered the gold standard to measure body composition in clinical practice. BIA estimates may be accurate for measuring body composition compared to DEXA if CF-specific equations are used, but evidence is limited. There were differences in fat mass and fat-free mass measured by equations utilizing 2- or 4-site skinfold measurements compared to gold standards and the degree and direction of bias was variable according to individuals’ sex and body composition. | 4 validity/reliability studies,108–110,113 Grade III |
| • In adults with CF, DEXA is considered the gold standard to measure body composition in clinical practice. Compared to DEXA, assessment of fat-free mass using skinfold measures or bioelectrical impedance with equations intended for the general population under- or overestimated fat-free mass by a mean of −3.3 kg to 2.9 kg for adults with CF (low validity). | 2 validity/reliability studies,114,115 Grade II |
| Recommendations | |
| 7.1 In individuals with CF >8 years of age, it is reasonable for the RDN or international equivalent to assess bone mineral density, fat mass, and lean mass using DEXA, when feasible and indicated, because DEXA is the gold standard for assessing these measures in clinical settings. | Consensus Conditional |
| 7.2 In all individuals with CF, when body composition assessment with DEXA is not feasible or indicated, it is reasonable for the RDN or international equivalent to assess mid-upper arm circumference with single site skinfold measures and/or Nutrition-Focused Physical Examinations at each nutrition assessment in order to aid in the classification of nutrition status over time. | Consensus Conditional |
| 7.3 In individuals with CF, when DEXA is not feasible or indicated, the RDN or international equivalent may use age- appropriate tests including skinfold measures or BIA with caution when tracking body composition over time, understanding that prediction equations using these methods may over- or underestimate absolute fat and fat-free mass. | Weak Conditional |
| 8.0 Nutrition assessment of biochemical values | |
| SR sub-question 3: In participants with CF, what is the longitudinal relationship (at least 3 months) between CFRD and hard outcomes (mortality, FEV1, and quality of life)? | |
| Conclusions | |
| • Pediatric participants with CF who were 5 to 20 years old with CFRD had significantly greater decline in FEV1% predicted after at least 10 years of follow-up compared to those without CFRD. | 2 observational studies,33,44 Grade II |
| • In adults with CF, presence of CFRD was associated with increased decline in FEV1% predicted after a follow-up of 5 to up to 13 years. In adults with CF, 1 large cohort study determined that presence of CFRD was associated with higher risk of mortality after a follow-up of up to 24 years. However, this increased risk was not significant in another smaller study. | 4 observational studies,33,47–49 Grade II |
| • In a combined group of adult and pediatric participants with CF, the longitudinal relationship between CFRD and association with later mortality was mixed, with the largest study suggesting an association with increased risk of mortality for those with CFRD at baseline (HR=1.31) after 2.9 years, but other studies reporting no difference in mortality according to CFRD. | 2 observational studies,50,52 Grade II |
| Recommendation | |
| 8.1 For individuals with CF ≥10 years of age who have not previously been diagnosed with diabetes, oral glucose tolerance testing (OGTT) is recommended annually or as indicated by glucose levels and clinical signs and symptoms (weight loss, increase in pulmonary exacerbations, and/or loss of lung function) during nutrition assessment, because progression to CFRD is a risk factor for pulmonary decline and mortality. | Strong Conditional |
| SR sub-question 3: In participants with CF, what is the longitudinal relationship (at least 3 months) between fat-soluble vitamin levels and hard outcomes (mortality, FEV1, and quality of life)? | |
| Conclusion | |
| In pediatric participants with CF, 1 cohort study found no longitudinal relationship between 25-hydroxyvitamin D levels in 6- to 18-year-olds with PI and FEV1% predicted 4 years later. | 1 observational study,129 Grade III |
| Recommendation | |
| 8.2 For all individuals with CF, regardless of exocrine pancreatic function, it is reasonable for the RDN or international equivalent to assess fat-soluble vitamin levels at least annually, because there may be high risk of fat-soluble vitamin abnormality due to PI and malabsorption. | Consensus Conditional |
| SR sub-question 3: In participants with CF, what is the longitudinal relationship (at least 3 months) between lipid profile and hard outcomes (mortality, FEV1, and quality of life)? | |
| Conclusion | |
| In participants with CF ranging from 1 to 41 years and homozygous for F508del mutation, 1 cohort study detected no longitudinal relationship between triacylglyceride levels and later FEV1% predicted. | 1 observational study,134 Grade III |
| Recommendation | |
| 8.3 For individuals with CF, it is reasonable for the RDN or international equivalent to evaluate fasting lipid profile at least once between the ages of 10 and 20 years and every 4 to 6 years thereafter, or more frequently if the individual has multiple risk factors for cardiovascular disease, in order to detect and prevent dyslipidemia. | Consensus Conditional |
| 9.0 General guidance for food intake | |
| SR sub-question 5: In participants with CF, what is the relationship between dietary intake of food groups, dietary patterns and meal frequency and nutrition-related outcomes? | |
| Conclusion | |
| In participants with CF, there were no studies identified that reported on the relationships between dietary intake of food groups, dietary patterns or meal frequency and mortality, lung function, quality of life, anthropometric measures and growth, included morbidities, gastrointestinal symptoms, or included laboratory measures. | 0 studies, Grade V |
| Recommendations | |
| 9.1 For all individuals with CF, it is reasonable for the RDN or international equivalent to advise an age-appropriate, healthy diet that emphasizes culturally appropriate foods associated with positive health outcomes in the general population, including vegetables, fruits, whole grains, seafood, eggs, beans and peas, nuts and seeds, dairy products, and meats and poultry, as tolerated and preferred by the individual with CF, because there is no evidence to suggest that routine modification from a well-balanced, healthy diet is associated with improved outcomes. It is reasonable to advise supplementation with energy- and/or protein-dense foods or oral or enteral supplements, as needed to achieve or maintain normal growth (pediatrics) or BMI status (adults). | Consensus Conditional |
| 9.2 For all individuals with CF, it is reasonable for the RDN or international equivalent to consider advising a dietary pattern, individualized for dietary preferences and nutrient needs, that promotes consumption of nutrient-dense foods, including healthy fats and micronutrients. | Consensus Imperative |
| 9.3 For all individuals with CF, it is reasonable for the RDN or international equivalent to suggest frequent food intake throughout the day, including at least 3 meals with snacks in between, as needed, in order to meet energy and protein needs and achieve or maintain optimal weight/growth and nutrition status. | Consensus Conditional |
| 10.0 Food intake with comorbidities (CFRD and overweight/obesity) | |
| SR sub-question 5: In participants with CF, what is the relationship between dietary intake of food groups, dietary patterns, and meal frequency and nutrition-related outcomes? | |
| Conclusion | |
| In participants with CF, there were no studies identified that reported on the relationships between dietary intake of food groups, dietary patterns or meal frequency and mortality, lung function, quality of life, anthropometric measures and growth, included morbidities, gastrointestinal symptoms, or included laboratory measures. | 0 studies, Grade V |
| SR sub-question 5: In participants with CFRD, what is the relationship between refined carbohydrates, including juice, soda and candy, and glycemic control? | |
| Conclusion | |
| In participants with CFRD, there were no studies identified that reported on the relationship between refined carbohydrates and glycemic control. | 0 studies, Grade V |
| SR sub-question 3: In participants with CF, what is the longitudinal relationship (at least 3 months) between CFRD and hard outcomes (mortality, FEV1, and quality of life)? | |
| Conclusions | |
| • Pediatric participants with CF who were 5 to 20 years old with CFRD had significantly greater decline in FEV1% predicted after at least 10 years of follow-up compared to those without CFRD. | 2 observational studies,33,44 Grade II |
| • In adults with CF, presence of CFRD was associated with increased decline in FEV1% predicted after a follow-up of 5 to up to 13 years. In adults with CF, 1 large cohort study determined that presence of CFRD was associated with higher risk of mortality after a follow-up of up to 24 years. However, this increased risk was not significant in another smaller study. | 4 observational studies,33,47–49 Grade II |
| • In a combined group of adult and pediatric participants with CF, the longitudinal relationship between CFRD and association with later mortality was mixed, with the largest study suggesting an association with increased risk of mortality for those with CFRD at baseline (HR=1.31) after 2.9 years, but other studies reporting no difference in mortality according to CFRD. | 2 observational studies,50,52 Grade II |
| Recommendation | |
| 10.1 For all individuals with CFRD, it is reasonable for the RDN or international equivalent to consider advising a diet consistent with general, age-appropriate healthy dietary recommendations and individualize as needed according to CFRD pathology. It is reasonable for the RDN to emphasize limiting high-sugar foods and beverages with low nutrient density, due to adverse effects on blood glucose levels. | Consensus Conditional |
| SR sub-question 5: In participants with CF and overweight/obesity, what is the relationship between dietary intake of food groups, dietary patterns and meal frequency and nutrition-related outcomes? | |
| Conclusion | |
| In participants with CF and overweight/obesity, there were no studies identified that reported on the relationships between dietary intake of food groups, dietary patterns or meal frequency and mortality, lung function, quality of life, anthropometric measures and growth, included morbidities, gastrointestinal symptoms, or included laboratory measures. | 0 studies, Grade V |
| Recommendation | |
| For individuals with CF who are overweight or obese, it is reasonable for the RDN or international equivalent to advise an age- appropriate diet that emphasizes foods associated with positive health outcomes in the general population, including vegetables, fruits, whole grains, seafood, eggs, beans and peas, nuts and seeds, dairy products, and meats and poultry, as tolerated and preferred by the individual with CF, with energy needs adjusted to achieve or maintain normal growth (pediatrics) or BMI status (adults). | Consensus Conditional |
| 11.0 Macronutrient distribution | |
| SR sub-question 5: In participants with CF, what is the relationship between dietary macronutrient distribution and nutrition-related outcomes? | |
| Conclusions | |
| • In pediatric and adult participants with CF who were primarily PI and on pancreatic enzyme replacement therapy (PERT), limited, cross-sectional evidence suggests no association between macronutrient distribution and % of predicted FEV1. Estimated protein intake ranged from 10% to 23% of energy, fat intake ranged from 20% to 46% of energy and carbohydrate intake ranged from 32% to 67% of energy. | 3 observational studies,162–164 Grade III |
| • In adults with CF who were primarily PI and on PERT, limited, cross-sectional evidence suggests no association between macronutrient distribution and BMI when estimated protein intake ranged from 8% to 23% of energy, fat intake ranged from 20% to 49% of energy and carbohydrate intake ranged from 32% to 67% energy. In children with CF who were PI and on PERT, the relationship between the percent energy intake from fat and BMI z score was unclear because direction of correlation varied according to participant age. | 3 observational studies,164–166 Grade III |
| • In adults and pediatric participants with CF (ages 0–33.4 years) on PERT, limited, observational evidence suggests that dietary macronutrient distribution, specifically fat intake, was not associated with gastrointestinal symptoms or distal intestinal obstruction syndrome (DIOS). | 2 observational studies,167–168 Grade III |
| • In children and adolescents with CF ages 10 to 18 years, limited, cross-sectional evidence suggests no relationship between dietary macronutrient distribution and lipid profile (total cholesterol, high-density lipoprotein [HDL] cholesterol, low-density lipoprotein cholesterol, triglycerides levels, and the triglyceride to HDL-cholesterol ratio). Ranges of macronutrients measured with a 3-day food record were approximately 12% to 16% of energy from protein, 38% to 62% of energy from carbohydrate, and 25% to 45% of energy from fat. | 1 observational study,169 Grade III |
| • In adults with CF (31.4±9.1 years) of which 78% were PI and on PERT, 1 cross-sectional study suggested no relationship between dietary protein intake (11%−23% of energy) and serum 25-hydroxy vitamin D levels, based on interview of food frequency by an RDN. Relationships between intakes of other macronutrients and fat-soluble vitamin levels were not reported. | 1 observational study,164 Grade III |
| • In adults with CF, of whom 70% were PI (classified as having normal, impaired glucose tolerance, or CFRD), 1 small, cross-sectional study suggests no relationship between dietary macronutrient distribution and glucose fluctuations. Range of macronutrients measured via a 3-day food diary demonstrated fat intake was 21% to 47% of energy, protein intake was 10% to 22% of energy, and carbohydrate intake was 32% to 65% of energy. | 1 observational study,170 Grade III |
| Recommendation | |
| 11.1 For individuals with CF who are not at risk of malnutrition, the RDN or international equivalent may suggest consuming macronutrients (carbohydrates, protein, and fat) in the same percentage distribution as is recommended for the typical, age-matched population because there is no current evidence to suggest benefits from modified macronutrient distribution. | Weak Conditional |
| 12.0 Fiber intake | |
| SR sub-question 5: In participants with CF, what is the relationship between fiber intake and nutrition-related outcomes? | |
| Conclusion | |
| In participants with CF, limited, observational evidence suggests that fiber intake up to the Recommended Dietary Intake did not exacerbate gastrointestinal symptoms or DIOS. | 3 observational studies,167,168,174 Grade III |
| Recommendation | |
| 12.1 For individuals with CF, the RDN or international equivalent may suggest dietary fiber intake in line with the dietary reference intake for the general population, as tolerated on an individual basis because evidence suggests fiber intake at the recommended level does not increase risk of constipation, DIOS, or other gastrointestinal symptoms. | Weak Conditional |
| 13.0 Infant Feeding | |
| SR sub-question 5: In infants with CF, what is the relationship between exclusive breastfeeding, mixed feeding, or formula feeding and nutrition-related outcomes? | |
| Conclusions | |
| • Two observational studies reported that infants with CF breastfed for at least 4 to 6 months, exclusively or partially, may have higher FEV1 percentage of predicted for age at ≥6 years compared with infants who are formula-fed only. However, findings may be due to reverse causation (disease severity affected breastfeeding duration) or other confounding variables. | 2 observational studies,179,180 Grade III |
| • In infants with CF, limited evidence was mixed in regards to the influence of infant feeding modality on weight and length gain. Three cohort studies reported no significant differences in weight gain or WFA z scores between breastfed and formula-fed infants, even when considering exclusivity (2 studies). However, 1 study reported a significant decline in weight z scores from birth to 2 years of age, particularly from 2 to 6 months, for infants who had been exclusively breastfed for ≥2 months, although there was no change in other feeding groups. In infants with CF, 4 cohort studies concluded that breastfeeding duration and exclusivity did not influence length gain. | 4 observational studies,179,181–183 Grade III |
| • In infants with CF, 1 large, cross-sectional survey study reported that breastfeeding exclusively for ≥6 months was associated with a decrease in intravenous antibiotic use over the previous 2 years compared to no breastfeeding (P=0.03, adjusted for age), although there was no significant benefit noted with nonexclusive or shorter breastfeeding duration. However, findings may be due to reverse causation (disease severity affected breastfeeding duration) or other confounding variables. | 1 observational study,180 Grade II |
| • In infants with CF who were exclusively breastfed compared to exclusively formula-fed for the first 3 months of life, 1 cohort study reported a trend toward higher rates of anemia at 6 months, but this difference did not reach statistical significance. Incidence of anemia was twice as frequent in infants who were PI. | 1 observational study,181 Grade III |
| SR sub-question 3: In participants with CF, what is the longitudinal relationship (at least 3 months) between weight and growth parameters and hard outcomes (FEV1, quality of life, or mortality)? | |
| Conclusions | |
| • In pediatric participants with CF, regaining birth weight z score by age 2 years and maintaining BMI and height z score throughout childhood was associated with the highest FEV1% predicted later in life compared to those who did not regain and maintain z scores in studies referencing CDC growth charts. Participants who maintained a weight, length, WFL, and BMI >50th percentile from infancy and early childhood had better FEV1% predicted values, although there was no added improvement for those who maintained growth parameters >85th percentile compared with >50th percentile In general, normal growth parameters during childhood were associated with increased FEV1% predicted in long-term follow-up studies (4–16 years). | 16 observational studies,31–46 Grade I |
| • In pediatric and adult participants combined, underweight (BMI <10th to 12th percentile for pediatric and <18.5 for adult) was associated with increased risk of mortality (HR=2.12) after a median of 12 years. There was a linear relationship between each unit increase in BMI z score at baseline and mortality after a median of 13.4 years (HR=1.2). Stunting (defined as height <5th percentile) was associated with increased odds of mortality (OR=2.2). | 3 observational studies,50–52 Grade II |
| Recommendation | |
| 13.1 In infants diagnosed with CF, the RDN or international equivalent should recommend providing as much breast milk as possible, with breast milk fortification and formula supplementation as necessary for the first year of life, to regain birth weight z score and achieve normal growth for age. Breastfeeding is associated with improved FEV1% predicted and decreased antibiotic use, but supplementation may be needed for infants with high nutrient requirements. | Fair Conditional |
MNT=medical nutrition therapy.
SR=systematic review.
FEV1=forced expiratory volume in 1 second.
RCT=randomized controlled trial.
Components of successful MNT interventions included:
specified frequency of nutrition screening and conditions for assessment;
individualized energy and supplement prescriptions;
twice weekly to quarterly monitoring of weight and growth, with specified cut points for oral nutrition supplementation;
individualized nutrition education sessions regarding PERT, energy requirements, vitamin and mineral needs.
Evidence for Adults.
There was no evidence available examining the efficacy of MNT provided by an RDN or international equivalent in adults with CF.
Implementation.
The course of nutrition intervention will be highly dependent on the individuals with CF and their families, including their cultural values and individualized goals. Evidence-based recommendations addressing specific approaches to behavioral modification and nutrition education are addressed in Nutrition Guidelines for Cystic Fibrosis in Australia and New Zealand.1 RDNs must work with the entire interdisciplinary CF team and family to facilitate implementation of nutrition prescriptions that:
are feasible;
foster long-term enjoyment of and a healthy relationship with food;
increase intake of essential nutrients;
decrease intake of high-energy, low nutrient density foods, especially those that may contribute to impaired glucose or lipid homeostasis; and
optimize nutrition status while aligning with each individual’s personal health goals.
RDN Effort.
Providing appropriate, proactive, individualized nutrition care in the modern age of CF, in which 90% of individuals will be on CFTR modulation therapy, will require increased involvement and care from an RDN. Therefore, based on clinical experience, anticipation of increasing heterogeneity in the CF population and external reviewer feedback, the workgroup elected to corroborate the guidance from Australia and recommend a caseload of 75 to 150 CF clients per RDN full-time equivalent.1 The recommended caseload for CF RDNs should be considered as general guidance, because some individuals with CF may require more intensive nutrition care in order to manage nutrition status and comorbidities. These populations include individuals with a new CF diagnosis; pediatric individuals, including those transitioning to adult care; those with advanced lung disease or pulmonary exacerbations; those who are pre- or post-lung transplantation; those who have CFRD, liver disease, unintentional weight loss, or decline in nutrition status; those on enteral or parenteral nutrition or other significant comorbidities; and those who have lower socioeconomic status. RDNs working in both inpatient and outpatient settings should maintain caseloads at the lower end of the range recommended. As triple combination CFTR modulation therapy further changes the landscape of nutrition care, RDNs will have to take an even more individualized approach to nutrition care planning. It is likely that the lower end of the case range may be appropriate for all RDNs in all care settings moving forward. The recommendation describing the suggested case load for RDNs is based on standards from the United States and other comparable countries, as well as expert consensus/opinion.
2.0. Nutrition Screening: Pediatrics
Recommendation 2.1.
For infants and children with CF <2 years of age, it is reasonable to measure weight and length at each clinic visit and to screen for risk of impaired growth and other nutrition concerns at least monthly for the first 6 months of age, every other month from 6 to 12 months of age, and quarterly from 12 to 24 months of age to identify nutrition risk.
Rating: Consensus
Imperative
Recommendation 2.2.
Infants and children with CF <2 years of age should be screened for nutrition risk by comparing weight-for-age, weight-for-length, and length-for-age z-scores or percentiles to birth weight and to growth norms using WHO growth charts for the general population because these parameters are longitudinally associated with lung function. Children who are not maintaining birth weight, weight-for-length, or length-for-age z scores or who have depressed growth compared to the general population should be referred for full nutrition assessment by an RDN or international equivalent.
Rating: Strong
Imperative
Recommendation 2.3.
For children and adolescents with CF ages 2 to 20 years, weight and height should be measured at each clinic visit and children should be screened for risk of impaired growth and other nutrition concerns at least quarterly or more frequently based on clinical condition to identify nutrition risk.
Rating: Fair
Imperative
Recommendation 2.4.
Pediatric individuals with CF 2 to 20 years of age should be screened for nutrition risk by comparing growth percentiles and z scores to general population norms using Centers for Disease Control and Prevention (CDC) growth charts for the general population because these parameters are longitudinally associated with lung function. Children and adolescents who have a BMI-for-age <50th percentile and/or who have concerning trends in BMI-for-age, weight-for-age, or height-for-age z scores should be referred for full nutrition assessment by an RDN or international equivalent.
Rating: Strong
Imperative
Rationale:
Undernutrition is a common concern for individuals with CF due to increased energy expenditure and decreased nutrient absorption.54 In the systematic review of the literature, undernutrition demonstrated a clear longitudinal relationship with lung function decline and mortality.30–53 Participants who maintained a weight, length, weight-for-length, and BMI >50th percentile from infancy and early childhood had higher FEV1% (percent forced expiratory volume in 1 second) predicted values 4 to 16 years later, although there was no added improvement for those who maintained growth parameters >85th percentile compared with >50th percentile (Table).31–46
When monitoring clients, RDNs must use clinical judgment to determine whether a child with z scores below this cutoff is actually at nutrition risk or they are stable on a lower growth curve and assessment is not needed. While increasing or decreasing z scores will nearly always be an indication for full assessment, this may or may not be possible, depending on the other health concerns the client is managing at any given time.
World Health Organization vs CDC Growth Charts.
In accordance with recommendations for the general population, practitioners may use World Health Organization (WHO) growth standards for infants 0 to 24 months of age and begin using CDC growth charts at age 2 years.55 For infants and children 0 to 24 months of age, WHO growth charts, compared to CDC growth charts, were less likely to define children as <50th percentile for weight-for-age and weight-for-length.37,56 Therefore, if practitioners use WHO growth charts to screen for growth, percentiles <70th percentile may be used to identify nutrition risk rather than the <50th percentile recommended when using CDC growth charts.
Please see the EAL website for additional implementation considerations.15 Recommendations are based on systematic review (Table) as well as clinical experience and norms in clinical practice from the CF Foundation.57
3.0. Nutrition Screening: Adults
Recommendation 3.1.
In adults with CF >20 years of age, it is reasonable to measure weight and height at each clinic visit and to screen for malnutrition and/or other nutrition concerns at least quarterly or more frequently based on clinical condition to identify nutrition risk.
Rating: Consensus
Imperative
Recommendation 3.2.
Adults with CF >20 years of age should be screened for nutrition risk by evaluating absolute values and trends in BMI because BMI is longitudinally associated with lung function. Women who have a BMI <22, men who have a BMI <23, or adults who have concerning trends in BMI, either decreasing or increasing, should be referred for full nutrition assessment by an RDN or international equivalent.
Rating: Fair
Imperative
Rationale:
In adults with CF, data were mixed, but 1 large retrospective cohort study suggested that BMI 25 was associated with decreased decline in FEV1% predicted, and BMI <18.5 was associated with increased decline in FEV1% predicted compared to normal weight status after a follow-up of up to 13 years.33 Baseline FEV1% predicted was also associated with change in BMI over time,33 so it is likely that the relationship between weight parameters and lung function is bidirectional.
In adults with CF, unfortunately, there was no included literature examining health outcomes according to BMI cutoffs recommended by the CFF: 22 for women and 23 for men. However, evidence did demonstrate that using a BMI cutoff of 18.5 to identify malnutrition in adults with CF may misclassify those who have a BMI higher than 18.5, but are malnourished based on low fat-free mass.58,59 There is no literature available to suggest that individuals with CF are exempt from overweight-or obesity-related comorbidities, such as type 2 diabetes, cardiac disease, or hypertension.
These recommendations should be interpreted and implemented with consideration of client history and goals. Many adults will naturally fall beneath the BMI cut points. When monitoring clients, RDNs must use clinical judgment to determine whether adults with BMI scores below these cutoffs are actually at nutrition risk or they are stable at a lower BMI and assessment is not needed. While drastic increases or decreases in BMI will nearly always be an indication for full assessment, this may or may not be possible at any given time, depending on the other health concerns the client is managing.
Please see the EAL website for additional implementation considerations.15 The recommendation for adult participants is based on systematic review (Table), as well as clinical experience and norms in clinical practice, guided by the CFF.57
4.0. Nutrition Assessment and Diagnosis of Nutrition Status: Pediatric and Adult
Recommendation 4.1.
In individuals with CF, a full nutrition assessment should be conducted by an RDN or international equivalent
at diagnosis;
when indicated by nutrition screening;
up to monthly for the first 6 months of life; up to every other month until 1 year of age; and up to quarterly until 2 years of age;
annually for individuals >2 years of age; and
when disease or treatment course changes.
Rating: Consensus
Imperative
Recommendation 4.2.
In individuals with CF, the RDN or international equivalent should diagnose nutrition status, including underweight and overweight, based on a comprehensive assessment of weight and growth history and stature, body composition, disease severity, laboratory values, drugenutrient interactions/implications, and estimated energy expenditure compared to client/parent report of dietary intake and food security status, because CF nutrition pathology is highly individual and maintaining optimal nutrition status is a necessary component of preventing disease progression.
Rating: Strong
Imperative
Rationale:
Malnutrition can occur quickly and insidiously in individuals with CF. Consistent, individualized, and ongoing assessment of nutrition status may prevent or remediate the effects of malnutrition on the progression of pulmonary disease and improve quality of life for individuals with CF. There was no evidence that examined overall nutrition assessment protocols in isolation, but in quality-improvement studies, increased contacts between individuals with CF/family and an RDN, including nutrition screening and assessment, resulted in improved anthropometric outcomes and decreased antibiotic use in pediatric individuals with CF.27–29,60 There were no studies of this nature available that examined adults with CF.
The systematic review supporting this guideline clearly demonstrated that undernutrition, defined by weight and length/height measures, is longitudinally associated with pulmonary decline and mortality in pediatric individuals and adults with CF.30–53 However, evaluating weight and growth measures only may overlook other complex factors that contribute to poor nutritional status and ultimately poorer lung function. For example, in adult participants with CF, using a BMI cutoff of 18.5 to identify malnutrition led to classifying subjects with higher BMI values as well-nourished, despite having inadequate fat-free mass.58,59
For most nutrition assessment parameters examined (eg, handgrip strength, micronutrient deficiencies, lipid profile, and Nutrition-Focused Physical Examinations [NFPE]), there was little or no evidence available in either the pediatric or adult CF population describing validity and reliability compared to a reference standards,61 and longitudinal relationship with mortality, FEV1% predicted, or quality of life.62 Therefore, it is unclear exactly how or how often these nutrition parameters should be assessed. To review significant changes in disease or treatment course that warrant full nutrition assessment, please see the EAL website.15
Components of Comprehensive Nutrition Assessment.
For individuals with CF, when conducting nutrition assessment to determine whether and what nutrition interventions are necessary, RDNs should evaluate:
Anthropometrics
- Growth parameters: pediatrics
- Conducted at each clinic visit
- Infants <2 years: Head circumference percentiles and z scores, weight-for-age weight-for-length, length-for-age percentiles, and z scores using WHO growth charts (please see WHO vs CDC Growths Charts in 2.0 Nutrition Screening: Pediatrics)
- Pediatrics (18 years):Weight-for-age percentiles and z scores; height-for-age percentiles and z scores; BMI-for-age for children 2 years and older using CDC growth charts and weight status categories63
- CF centers and team members should collaborate to determine strategies for Tanner staging for adolescents, because self-reports can be inaccurate and Tanner examination can seem invasive
- Weight parameters: Adults
- Adult (>18 years): Weight, height, and BMI using CDC weight status categories66
- Body composition (please see 7.0 Body Composition Assessment section for details)
- Conducted during each nutrition assessment
- fat mass, fat-free mass, and bone mineral density according to external guidelines (please see Figure 1)
Biochemical (Please see “Nutrition Assessment of Biochemical Values” section for details)
- Glucose homeostasis measures, such as response to oral glucose tolerance testing (OGTT).
- Continuous glucose monitoring systems are not indicated for diagnosis of CFRD but may be ordered by an endocrinologist and data can be utilized by RDNs to guide nutrition care.
Fat-soluble vitamins status including for levels of vitamins A, D3 (25-OH-D), and E as well as prothrombin time for vitamin K)
Basic metabolic profile including sodium, chloride, potassium, blood urea nitrogen, glucose, and liver enzyme levels
Urine sodium, as indicated3
Clinical
Pulmonary function, including FEV1% predicted and pulmonary exacerbations
Lung transplantation and other medical history Pancreatic insufficiency and pancreatic enzyme replacement therapy
Other medications that may alter nutrient intake or needs, such as appetite stimulants, growth hormones, insulin, and/or CFTR modulation therapy
Gastrointestinal symptoms (constipation, symptoms of malabsorption including diarrhea/steatorrhea, distal intestinal obstruction syndrome [DIOS], gastroesophageal reflux disease, small intestinal bacterial overgrowth, Clostridium difficile infection)
Comorbidities, including CF-related diabetes, CF-related liver disease, CF-related bone disease, gallbladder/kidney issues, sinus disease, or other gastrointestinal comorbidities, such as celiac disease or inflammatory bowel disease
NFPE69
Dietary
Dietary intake history, including meal patterns. Quantitative assessment of dietary intake, including energy intake, may be assessed at least annually
Nutrient requirements, including energy expenditure using indirect calorimetry or appropriate prediction equations (please see section 6.0 Assessment of Energy Requirements)
Eating environment and challenges for the individual with CF and family, including level of food security and access to resources to implement RDN recommendations
Values/beliefs related to dietary choices (eg, vegetarian, vegan, religious observations)
Other
Beliefs about the impact of nutrition status on CF disease progression
Nutrition goals for the person with CF and family, such as weight status goals, dietary intake goals, reduction of nutrition-related symptoms, or improved body image
Social determinants of health
Values and beliefs about food and nutrition
After comprehensive nutrition assessment, the RDN should work with the individual, family, and interdisciplinary health care team to determine health care, including nutrition priorities. RDNs are poised to translate assessment findings into individualized education and counseling, in order to co-produce a nutrition care plan that works for the individual with CF. As more information is gathered, nutrition diagnoses may evolve, dictating a modification to the treatment plan.
Please see the EAL website for additional implementation considerations, including changes in disease course or treatment that would warrant additional or more frequent nutrition assessment. The “effect” of nutrition assessment is difficult to gauge in studies because it is the first step in Nutrition Care Process and subsequent steps also affect nutrition-related outcomes. Therefore, in addition to systematic review (Table), recommendations were also supported by clinical expertise, and guidance from the CF Foundation.5
5.0. Role of CFTR Modulators in Nutrition Assessment
Recommendation 5.1.
For individuals with CF of all ages who receive CFTR modulation therapy, the RDN or international equivalent should continue to conduct nutrition screening with nutrition assessment as indicated based on age because these medications may change nutrient needs for some individuals with CF.
Rating: Fair
Imperative
Rationale:
In the years since ivacaftor became the first commercially available CFTR restoration compound in 2012, the numbers of individuals with CF who qualify for this and subsequent modulation therapies has increased sharply. The goal of the CFF is to provide some type of CFTR modulation therapy for 100% of individuals with CF.70
The systematic review supporting this guideline elucidated that the effects of CFTR modulators on BMI and other anthropometric measures are dependent on formulation of the drug and the CFTR genotype of the individual (Table). Detailed results can be found on the EAL website.15,71–83 Anthropometric measures were not primary outcomes in these pharmacologic studies and some studies were not included because they did not report age-appropriate weight/growth measures. There were no included studies that examined the effect of CFTR modulators on body composition or the effect of diet on the relationship between CFTR modulators and weight/growth parameters.
Stallings and colleagues84 demonstrated that ivacaftor treatment in patients with CF with at least 1 CFTR gating mutation decreased resting energy expenditure, decreased fecal cal-protectin (a measure of gut inflammation), and increased fat absorption, especially in those with exocrine pancreatic insufficiency. The full nutrition impact of different CFTR modulation therapies in individuals with CF is not understood at this time. More studies are needed regarding how CFTR modulators and dietary intake interact to affect nutrition status. This topic is particularly important because new CFTR “triple combination” therapy (elexacaftor-tezacaftor-ivacaftor) is now available and will reach individuals with CF with at least one F508del mutation, which constitutes up to 90% of the CF population. Phase III studies of elexacaftor-tezacaftor-ivacaftor therapy have demonstrated improvement in BMI and BMI-for-age z scores in individuals who have either 1 or 2 copies of the F508del mutation.85,86 RCTs that have examined the nutrition implications of CFTR modulation therapy have not reported nutrition-related measures other than weight-based parameters, and, thus practitioners, may need to depend on observational studies that track effects of more patient-centered health outcomes over time.
Nutrition Prescription and PERT with CFTR Modulation Therapy.
None of the included CFTR modulation therapy studies reported dietary intake data in subjects with CF, therefore, it is unknown how (or whether) RDNs should modify nutrition recommendations once this therapy is started. In order to be well absorbed, genetic modulator agents need to be taken with an adequate amount of dietary fat87 and the appropriate dose of PERT, when applicable. Meals and snacks can be scheduled to accommodate CFTR modulation therapy, which is given every 12 hours. Therefore, the role of the RDN in assessing and counseling patients as they commence these therapies is paramount. Although there is limited evidence that biomarkers of intestinal absorption84 and pancreatic function88,89 are improved with ivacaftor, there is not enough evidence to warrant reduction or cessation of PERT therapy when patients begin taking CFTR modulators.90 In some cases, CFTR modulators may decrease energy expenditure and energy prescription may need to be decreased in order to avoid excessive weight gain and resulting health risks.84 Adherence to a diet that is closer to population norms may be challenging for individuals who have historically consumed high-calorie and high-fat, high-protein, high-salt diets. Therefore, individuals who begin CFTR modulation therapy must be fully and continually assessed to determine how these medications may be affecting nutrition status. Although it is not recommended at this time, if individuals/families consider stopping or have stopped PERT, RDNs should co-partner with individuals and health care team to monitor fecal elastase, weight changes, gastrointestinal symptoms, and fat-soluble vitamin levels and, when possible, 72-hour fecal fat.
6.0. Assessment of Energy Requirements
Recommendation 6.1.
In pediatric (<20 years of age) and adult individuals with CF, it is reasonable for the RDN or international equivalent to measure energy needs using indirect calorimetry, when feasible and indicated, because indirect calorimetry is the gold standard for measuring energy expenditure in clinical settings.
Rating: Consensus
Conditional
Recommendation 6.2.
In pediatric individuals with CF 20 years of age, the RDN or international equivalent may estimate energy needs at each nutrition assessment using the Recommended Dietary Allowance or Institute of Medicine active lifestyles formulas because these formulas were the most accurate compared to indirect calorimetry in this population. Energy needs should be individualized based on growth history, nutrition status and medications, physical activity, and disease severity.
Rating: Weak
Conditional
Recommendation 6.3.
In adults with CF >20 years of age, the RDN or international equivalent may estimate energy needs annually or with unintentional weight changes using standard energy expenditure equations 1.25 because estimated energy requirements for the general population may underestimate needs in adults with CF. Energy needs should be individualized based on nutrition status and medications, physical activity, and disease severity.
Rating: Weak
Conditional
Rationale:
Evidence in Pediatrics.
In 1 study of children with CF who were primarily pancreatic insufficient, CF-specific energy equations overestimated needs (122%−136%) compared to doubly labeled water. Energy expenditure estimated with the Institute of Medicine active lifestyle formula and Recommended Dietary Allowance were similar to measured values, although these equations did not adjust for individuals’ activity level. Evidence was only available for 6-to 20-year-old children and adolescents and, thus, may not be generalizable to younger children.91–93
Evidence in Adults.
In adults with end-stage CF, Harris-Benedict, Schofield, and WHO 1985 energy expenditure equations underestimated energy needs compared to indirect calorimetry (76%−78% pre-lung transplantation and 90%−91% post-lung trans- plantation). Underestimation was greater in those with more severe disease.91,92,94
When direct measurement by indirect calorimetry is available and feasible, measurement of energy expenditure may be indicated for individuals on enteral or parenteral nutrition, those struggling to maintain weight/growth, or according to patient preference. While indirect calorimetry can be conducted in pediatric clients, it may be difficult for them to cooperate for the duration of the procedure. These recommendations provide a starting point for estimating energy requirements, but will not be completely accurate for every individual with CF. Energy estimates may be decreased for those on certain medications (eg, CFTR modulation therapy) or with concerning increasing trends in weight and will need to be increased for those with concerning decreasing trends in weight/growth, and with physical activity. Increasing disease severity, including exocrine pancreatic insufficiency, may increase energy needs. Needs will also be increased to account for refeeding with severe malnutrition and initially after lung transplantation. Once an individual is stabilized and discharged after transplantation, however, energy needs may decrease.95–99 RDNs should individualize energy prescription by adjusting estimates according to growth and weight trends, individual disease status (including overweight/obesity), extent of malabsorption, and physical activity. Ultimately, weight loss or gain is the gold standard for increasing or decreasing energy prescription. Further research may result in changes to the above recommendations.
7.0. Body Composition Assessment
Recommendation 7.1.
In individuals with CF >8 years of age, it is reasonable for the RDN or international equivalent to assess bone mineral density, fat mass, and lean mass using dual-energy x-ray absorptiometry (DEXA), when feasible and indicated because DEXA is the gold standard for assessing these measures in clinical settings.
Rating: Consensus
Conditional
Recommendation 7.2.
In all individuals with CF, when body composition assessment with DEXA is not feasible or indicated, it is reasonable for the RDN or international equivalent to assess mid-upper arm circumference with single-site skinfold measures and/or NFPEs at each nutrition assessment in order to aid in the classification of nutrition status over time.
Rating: Consensus
Conditional
Recommendation 7.3.
In individuals with CF, when DEXA is not feasible or indicated, the RDN or international equivalent may use age-appropriate tests, including skinfold measures or bioelectrical impedance analysis (BIA) with caution when tracking body composition over time, understanding that prediction equations using these methods may over-or underestimate absolute fat and fat-free mass.
Rating: Weak
Conditional
Rationale:
Increased risk of malnutrition in CF may lead to alterations in body composition, including fat and lean mass as well as bone density. Compared to the general population, individuals with CF have less fat-free mass and bone mineral density,100 which increases risk for sarcopenia, protein-energy wasting, and osteopenia. While BMI is useful for screening for nutrition risk, it does not provide a clear indication of body composition (eg, fat-free mass, fat mass).
There were no studies available to indicate whether body composition, assessed by any means, is useful in predicting hard outcomes in either pediatric or adult individuals with CF.62 However, some cross-sectional studies have suggested that fat-free mass is more strongly associated with lung function than BMI.59,101–106 In the general population, fat-free mass is inversely associated with mortality and mediates the association between BMI and mortality.107
In validity and reliability studies, for pediatric individuals with CF, there were differences in fat mass and fat-free mass measured by equations utilizing 2- or 4-site skinfold measurements or BIA compared to gold standards. The amount of difference, along with whether the mass was overestimated vs underestimated, was variable according to individuals’ sex and body composition.108–110 These findings were in agreement with a 2019 systematic review conducted by Calella and colleagues, which described that accuracy of skinfold measures and BIA may have limited accuracy if individuals with CF have abnormal hydration status or if appropriate prediction methods are not used.111,112 BIA devices may be multifrequency or spectroscopy or single frequency, the latter of which is considered to be less accurate. Multifrequency BIA estimates were accurate for measuring body composition compared to DEXA if CF-specific equations were used, but evidence was limited to 1 study with the pediatric population.113
In adults with CF, compared to DEXA, assessment of fat-free mass using skinfold measures or BIA with equations intended for the general population under- or overestimated fat-free mass by a mean of e3.3 kg to 2.9 kg and, therefore, had low validity.114,115 Although body composition methods may have limited accuracy for individuals with CF, repeated measures may give an overall picture of trends in body composition changes over time.
There was no evidence to determine whether NFPEs or mid upper arm circumference (MUAC) can be used to validly and reliably assess body composition or whether it is longitudinally associated with health outcomes in individuals with CF.62 However, because the accuracy and reliability of other methods to assess body composition were unclear, NFPE and MUAC may contribute information regarding changes in nutrition status over time, even though they may not provide quantified values of fat mass or fat-fee mass or give specific information about changes in fat-free mass compared to fat mass. The Academy and the American Society for Parenteral and Enteral Nutrition suggest use of MUAC in children and adolescents to diagnose malnutrition in the general population,116 which has the advantage of not relying on prediction equations developed for the non-CF population. Use of raw measures for skinfolds, MUAC, and BIA, in comparison to available age- and sex- and ethnicity matched reference populations may provide useful information for nutrition assessment and are not limited by unvalidated assumptions of using prediction equations developed in healthy populations. Reference equations are available for anthropometric (skinfold and MUAC) measures across all ages.117–119 Various population reference values for raw bioimpedance parameters are also available,120–122 although specific BIA devices are variable.
Indications for DEXA Scans.
Consensus guidelines for individuals with CF specify that individuals with CF should have a DEXA scan beginning at 8 years of age when indicated.4,123–125 Please see the EAL website for details regarding indications for assessment with DEXA.15
Frequency of Skinfold Measures, MUAC, NFPE, and BIA.
Alternative methods of measuring body composition may not be validated for the CF population. However, when tracked over time, relative measurements can contribute to a malnutrition diagnosis over time. Therefore, body composition measures with these methods should be assessed at each nutrition assessment (please see Nutrition Assessment section).
Methods for Measuring BIA.
Given the variability in BIA devices and the sensitivity of BIA to hydration status and timing of meals (eg, fasting status), the same protocol and testing conditions should be used over time.126 Furthermore, manufacturer-provided estimates of fat mass and fat-free mass may be based on unknown proprietary equations, thus BIA devices that provide raw parameters, such as reactance and resistance should be used.
For more implementation considerations, please see the EAL website.15 These recommendations are based on systematic review (Table), external consensus guidelines, and clinical expertise.
8.0. Nutrition Assessment of Biochemical Values
Recommendation 8.1.
For individuals with CF 10 years of age who have not previously been diagnosed with diabetes, OGTT is recommended annually or as indicated by glucose levels and clinical signs and symptoms (weight loss, increase in pulmonary exacerbations, and/or loss of lung function) during nutrition assessment, because progression to CFRD is a risk factor for pulmonary decline and mortality.
Rating: Strong
Conditional
Recommendation 8.2.
For all individuals with CF, regardless of exocrine pancreatic function, it is reasonable for the RDN or international equivalent to assess fat-soluble vitamin levels at least annually because there may be high risk of fat-soluble vitamin abnormality due to pancreatic insufficiency and malabsorption.
Rating: Consensus
Conditional
Recommendation 8.3.
For individuals with CF, it is reasonable for the RDN or international equivalent to evaluate fasting lipid profile at least once between the ages of 10 and 20 years and every 4 to 6 years thereafter, or more frequently if the individual has multiple risk factors for cardiovascular disease, in order to detect and prevent dyslipidemia.
Rating: Consensus
Conditional
Rationale:
The RDNs role in recommending assessment of laboratory values outside of the typical blood panel will vary by facility policies, health care team, and specific RDN. In some cases, the RDN may recommend assessment of laboratory measures if a nutrition concern is suspected or in accordance with standard practices to prevent disease progressions (such as regular OGTTs). Correcting abnormal laboratory values will rarely be the sole responsibility of the RDN, who works within the interdisciplinary team to determine whether an abnormal laboratory value is a priority and, if so, what can be done to correct it that is in the best interest of the specific individual at the specific time.
OGTT.
Endocrine pancreatic insufficiency is a hallmark of disease pathology for many individuals with CF, which may ultimately result in glucose intolerance and CFRD. Rates of OGTT completion have traditionally been low,54 due to time commitment, multiple needle sticks, and taste aversion to the glucose solution.
In the systematic review supporting this guideline, pediatric individuals ages 5 to 20 years who had CFRD exhibited greater decline in FEV1% predicted after 10 or more years of follow-up compared to those who did not have CFRD.33,44 In adults with CF, presence of CFRD was associated with increased decline in FEV1% predicted after a follow-up of 5 to up to 13 years and was associated with higher risk of mortality after a follow-up of up to 24 years.33,47–49 Therefore, RDNs should emphasize the importance of early identification and management of CFRD in order to educate individuals on management with nutrition and exercise and to evaluate insulin needs. The recommended frequency for OGTT assessment is based on recommendations from the International Society for Pediatric and Adolescent Diabetes Clinical Practice Consensus Guidelines for Children and Adolescents with CF 2018.127 Continuous glucose monitoring systems are not indicated for diagnosis of CFRD, but may be ordered by an endocrinologist, and data can be utilized by RDNs to guide nutrition care. An OGTT is not clinically indicated for individuals already diagnosed with CFRD.
Fat-Soluble Vitamin Levels.
Due to pancreatic insufficiency and malabsorption, individuals with CF are at high risk of fat-soluble vitamin deficiency.128 There was very limited evidence examining the relationship between fat-soluble vitamin levels and hard outcomes (Table).62,129 However, the health consequences of fat-soluble vitamin deficiencies are well known, including susceptibility to infections, osteomalacia, muscle weakness or pain, neurologic symptoms, and impaired clotting.130 It is reasonable for practitioners to adhere to consensus recommendations that are currently utilized in CF practice that fat-soluble vitamin levels should be monitored at least annually.1,2 For additional information on indications for measuring fat-soluble vitamin levels, please see the EAL website.15
Lipid Profile.
Dyslipidemia is common in individuals with CF related to pancreatic insufficiency, malabsorption, inflammation, imbalanced macronutrient intake, and, in some cases, CF-related liver disease.131–133 However, the consequences of these abnormal triglyceride and cholesterol levels on hard outcomes, including FEV1, quality of life, and mortality, are unclear due to lack of available evidence.62,134 This systematic review did not investigate the relationship of plasma lipid levels with cardiovascular disease as an outcome in CF, although in the general population, dyslipidemia is associated with higher risk of cardiovascular morbidities and mortality.135 Limited research suggests that pediatric individuals (7–18 years) with CF may be at increased risk of endothelial dysfunction136 and other cardiovascular risk factors, such as increased inflammation and lipid abnormalities.137 However, evidence is lacking to determine whether these risk factors are likely to progress to atherosclerotic plaques.138
Recommendations adhered to guidance provided by the US Department of Health and Human Services and the American Academy of Pediatrics.67,68 Frequency of lipid profile assessment may be increased for individuals with heart disease or diabetes or who have a family history of high cholesterol.
Essential Fatty Acids.
Evidence examining the relationship between essential fatty acid levels and health outcomes in participants with CF was limited.134,139 There was no evidence to suggest an optimal frequency of assessing these measures or clear indications for when levels should be measured. Recent nutrition guidelines for CF have described that the effect of essential fatty acid supplementation is unclear.1,2 Thus, the workgroup determined that there was not adequate evidence to inform a specific protocol for assessing essential fatty acid levels.
These recommendations were based on systematic review (Table) and clinical expertise.
9.0. General Guidance for Food Intake
Recommendation 9.1.
For all individuals with CF, it is reasonable for the RDN or international equivalent to advise an age-appropriate, healthy diet that emphasizes culturally appropriate foods associated with positive health outcomes in the general population, including vegetables, fruits, whole grains, seafood, eggs, beans and peas, nuts and seeds, dairy products, and meats and poultry, as tolerated and preferred by the individual with CF, because there is no evidence to suggest that routine modification from a well-balanced, healthy diet is associated with improved outcomes. It is reasonable to advise supplementation with energy and/or protein dense foods or oral or enteral supplements, as needed to achieve or maintain normal growth (pediatrics) or BMI status (adults).
Strength: Consensus
Conditional
Recommendation 9.2.
For all individuals with CF, it is reasonable for the RDN or international equivalent to consider advising a dietary pattern, individualized for dietary preferences and nutrient needs, that promotes consumption of nutrient-dense foods, including healthy fats and micronutrients.
Strength: Consensus
Imperative
Recommendation 9.3.
For all individuals with CF, it is reasonable for the RDN or international equivalent to suggest frequent food intake throughout the day, including at least 3 meals with snacks in between, as needed, in order to meet energy and protein needs and achieve or maintain optimal weight/growth and nutrition status.
Strength: Consensus
Conditional
Rationale:
Due to increased energy expenditure and decreased nutrient absorption, particularly of fats and fat-soluble vitamins, the traditional dietary prescription for CF has been a high-energy, high-fat diet.1,2,5 In efforts to meet this nutrition prescription, individuals with CF often include energy-dense, nutrient-poor foods in their diets, such as fast food, desserts, packaged snacks, sweetened drinks, and fatty meats.11,12 This may translate to diets that are higher in trans-fatty acids and added sugars and lower in fiber in individuals with CF compared to healthy matched controls.140 While evidence is clear that maintaining adequate nutrition status protects against pulmonary decline,30–53 it is unclear how dietary patterns may differentially modify this relationship.
The systematic review supporting this guideline examined the relationship between food types, dietary patterns and meal patterns, and nutrition-related outcomes (ie, mortality, pulmonary function, quality of life, anthropometric and relevant laboratory values, antibiotic use, length of stay, gastrointestinal symptoms) in individuals with CF. However, there were no studies, even observational studies, which examined these nutrition exposures of interest in the pediatric or adult CF populations. Since the systematic review, 1 small cross-sectional study has been published that reported no relationships between intake of whole grains or Healthy Eating Index 2015 score and body composition or fasting glucose levels in adults with CF; however, intake of added sugars was positively associated with visceral adipose tissue in individuals with CF.140 In another small cross-sectional study of adults with CF, dietary glycemic index, and glycemic load were significantly positively associated with amount of time spent with blood in the hyperglycemic range, and larger glucose fluctuations, respectively, as determined by continuous blood glucose monitoring.141 Large, long-term nutrition trials are critically needed in the CF population to determine which dietary patterns are optimal for nutrition-related outcomes, including pulmonary function and glucose homeostasis.
Population Norms in the Absence of Evidence.
In the meantime, RDNs and other health professionals must rely on nutrition knowledge as it relates to the general population and adapted to what is known about CF pathology. In the general population, diets rich in energy-dense, nutrient-poor foods have been implicated for several chronic adverse health outcomes including metabolic syndrome,142 inflammation, cardiovascular disease and gut dysbiosis,143 and a variety of cancers.144 Fruit and vegetable consumption, conversely, is associated with reduction in pro-inflammatory mediators and an enhanced immune cell profile.145 Guidance for dietary recommendations for the general population can be found in the Dietary Guidelines for Americans and MyPlate146 or other country-specific guidelines for the general population. Various healthy dietary eating patterns, such as a Mediterranean-style diet, have been proposed and been shown to correlate with positive health outcomes in the general population.146 While a Mediterranean-style diet pattern does not have a strict definition, it generally consists of ample plant foods, olive oil as the primary source of fat, and moderate consumption of fish, poultry, and wine.147 In the general population, adherence to the Mediterranean Diet was associated with decreased risk of mortality,148,149 cardiovascular disease149,150 and cardiovascular disease risk factors,151 and glycemic control.152
One of the greatest challenges in nutrition care for individuals with CF will be to encourage a diet that is not only energy-dense, as needed, but is also nutrient-dense and relies on food-based nutrition when possible because nutrients in isolation may not have the same health benefits (eg, antioxidant supplementation trials in CF have provided mixed results; although the majority have used small sample sizes153–156). However, individuals with CF and exocrine pancreatic insufficiency have higher fat-soluble vitamin needs due to malabsorption, and it is not realistic to expect that all needs can be met by diet alone. Supplementation with fat-soluble vitamins is standard practice for individuals with CF. Guidance for supplementation of micronutrients and macronutrients (oral or enteral nutrition supplementation) are outside of the scope of this guideline but are addressed in other nutrition guidelines for individuals with CF (Figure 1).
MNT should always be tailored to the unique needs of the individual, including preferences and values, nutrition status and supplements, medications, disease exacerbations, comorbid conditions, such as food allergies, CFRD or gastrointestinal symptoms, inflammation, absorption, and exocrine pancreatic status and PERT. Individual dietary needs may vary widely depending on pancreatic insufficiency vs insufficiency, response to CFTR modulator therapy, physical activity, and/or other gastrointestinal-related issues. Sample menus can be found on the EAL website.15
These recommendations are based on expert consensus/opinion because there was no evidence included in the systematic review that examined the relationships between intake of foods or food groups, dietary patterns, or meal patterns on nutrition-related outcomes.
10.0. Food Intake with Comorbidities (CF-Related Diabetes and Overweight/Obesity)
Recommendation 10.1.
For all individuals with CFRD, it is reasonable for the RDN or international equivalent to consider advising a diet consistent with general, age-appropriate healthy dietary recommendations and individualize as needed according to CFRD pathology. It is reasonable for the RDN to emphasize limiting high-sugar foods and beverages with low nutrient density due to adverse effects on blood glucose levels.
Strength: Consensus
Conditional
Recommendation 10.2.
For individuals with CF who are overweight or obese, it is reasonable for the RDN or international equivalent to advise an age-appropriate diet that emphasizes foods associated with positive health outcomes in the general population, including vegetables, fruits, whole grains, seafood, eggs, beans and peas, nuts and seeds, dairy products, and meats and poultry, as tolerated and preferred by the individual with CF, with energy needs adjusted to achieve or maintain normal growth (pediatrics) or BMI status (adults).
Strength: Consensus
Conditional
CFRD
CFRD is the most common comorbidity in CF, with approximately 35% to 50% of people with CF affected by CFRD.127,157 CFRD increases progression of pulmonary dysfunction in pediatric individuals with CF33,44,50 and increases risk of pulmonary dysfunction and mortality in adults with CF.30,33,47–50 Despite the prevalence of this comorbidity, there were no primary research articles identified for the systematic review supporting this guideline that specifically examined the relationships between diet and CFRD-related outcomes in pediatric or adult individuals with CFRD. A cross-sectional study published in 2019 (after the systematic review) reported that in 24 adults with CF, 46% of whom had CFRD, and 25% of whom had impaired glucose tolerance, both added sugar intake and fasting glucose levels were both significantly associated with visceral adipose tissue.140 In a second cross-sectional study published in 2019, continuous blood glucose monitoring indicated that dietary glycemic index and glycemic load were significantly positively associated with amount of time spent with blood in the hyperglycemic range, and larger glucose fluctuations, respectively, in 18 adults with CF.141
Education for individuals with CFRD should focus on self-management of glycemic control and insulin therapy, adequacy of energy intake, and aerobic exercise.2,127 Intake of food and beverages with high added-sugar content and without other nutrients to counter rapid spikes in blood glucose (such as fat, protein, or fiber) should generally be discouraged.127 Due to high nutrient needs in individuals with CF, exclusion of sweet foods may not be warranted for some individuals with CFRD. In these cases, RDNs can encourage consumption of sweet foods with nutritive value (such as chocolate with nuts or desserts with fruit) or in combination with foods with nutritive values (ie, containing fat, protein, fiber, and/or micronutrients).1
Overweight/Obesity
Overweight and obesity63,66 traditionally have not been a concern for individuals with CF, but as CF treatments have improved, incidence of overweight and obesity have increased in the CF population, with estimates ranging from 13% to 18%.158,159 Individuals with CF who are more likely to be overweight or obese are those who are older and male, have less severe genotypes, better lung function, and are pancreatic sufficient.160
There were no studies included in the systematic review that examined how diet affected nutrition-related outcomes in pediatric or adult individuals with CF who are overweight or obese. While some evidence suggests that overweight status may be protective against pulmonary decline in adults,33 other evidence suggested no difference in pulmonary decline according to overweight status in adult44,51 or pediatric participants.36,40,42 However, BMI alone, which is typically used to classify overweight and obesity, does not describe body composition, which may be a stronger predictor of pulmonary function in adults.161
Weight and Dietary Goals.
It is unclear whether individuals with CF who are overweight or obese should be encouraged to attain a normal weight status. However, any kind of rapid weight loss or extreme weight loss plans should be discouraged in individuals with CF because it is unclear whether this would result in detrimental pulmonary effects or loss of fat-free mass. Individuals who begin treatments that lead to weight gain may have difficulty transitioning to a diet that emphasizes healthy foods, especially if the individual has historically focused intake on a high-fat, high-energy diet. RDNs must work with individuals with CF and their families to determine motivations and goals that will improve nutrition status and quality of life over time. Incorporation of healthy eating principles can be beneficial for all members of the family of an individual with CF. RDNs must use clinical expertise and co-produce dietary advice with the individual with CF and the interdisciplinary health care team.
For additional implementation considerations, please see the EAL website.15 This recommendation is based on expert consensus/opinion.
11.0. Macronutrient Distribution
Recommendation 11.1.
For individuals with CF who are not at risk of malnutrition, the RDN or international equivalent may suggest consuming macronutrients (carbohydrates, protein, and fat) in the same percentage distribution as is recommended for the typical, age-matched population because there is no current evidence to suggest benefits from modified macronutrient distribution.
Strength: Weak
Conditional
Rationale:
Traditionally, a high-fat diet has been recommended for individuals with CF to achieve high energy intake needs due to malabsorption of fats and fat-soluble fatty acids resulting from exocrine pancreatic insufficiency and a high risk of malnutrition. This CF “legacy diet” was implemented as a strategy to achieve high energy intakes required for individuals with CF and to compensate for fat malabsorption, as opposed to having a demonstrated, direct physiological effect. This diet was crucial during an era when individuals with CF commonly had major catch-up growth required at diagnosis. Undernutrition is still a significant concern in the CF population and has serious implications for long-term health.30–53 However, over time, treatment options for CF have improved, and there is a decline in the proportion of individuals with CF that are undernourished.54 Thus, health care professionals must balance immediate nutrition needs with potential long-term consequences of a diet that contains too many foods that are high in fat, but low in essential nutrients. Currently, it is more realistic for many individuals with CF to consume adequate energy to meet their needs on a mixed diet with less reliance on energy-dense, nutrient-poor foods than it was in the past.
There were no dietary trials for pediatric or adult individuals with CF examining the role of macronutrient distribution on nutrition-related outcomes identified in the systematic review. However, the observational literature that was included demonstrated no relationships between macronutrient distribution and FEV1% predicted in pediatric individuals162 or adults162–164; BMI z score (pediatrics)165 or BMI (adults)164,166; gastrointestinal symptoms including DIOS in pediatric individuals167,168 or adults167;or various laboratory measures in pediatrics169 or adults164,170 over a range of intakes, with mean intakes near population norms (Table). A 2019 cross-sectional study published after the cutoff date for the systematic review supporting this guideline concluded there was no association between the amount of fat, carbohydrate, or protein intake and body composition or fasting glucose levels in adults with CF.140 There were no studies that reported on the relationships between macronutrient distribution and mortality, quality of life, length of hospital stay, or antibiotic use. Therefore, the research examined in this systematic review does not indicate a benefit for altering macronutrient distribution without addressing overall energy content, micronutrient composition, or diet quality. Instead, RDNs should consider dietary patterns that are healthy for the general population and adjust as is necessary to address energy needs, protein energy wasting, malabsorption, and comorbidities, such as CFRD. The effects of types of dietary fats, whether beneficial or detrimental, have not been adequately investigated in the CF population.166,169,171–173 However,healthy diets suggested for the general population are generally low in trans and saturated fats.146
Counseling Individuals on a High-Fat Diet.
Many individuals with CF have been prescribed a high-fat diet, possibly for many years. For these individuals, RDNs should assess whether the individual is tolerating their current diet by assessing the individual’s gastrointestinal symptoms, energy levels, anthropometric and body composition measures, and relevant laboratory values, including inflammatory markers, glucose homeostasis measures, and blood lipid profile. Concerns about shifting dietary intake closer to that of the general population may also be met with concern by individuals with CF who have lower access to resources because high-fat foods that are low in other nutrients may be less expensive than foods that are high in essential nutrients. When appropriate, RDNs should provide education regarding inexpensive sources of nutrient-dense foods.
Some individuals with CF may have very high energy needs, requiring an energy-dense diet. For these individuals, a diet higher in fat may be appropriate in order to attain high caloric intake. Individuals with CF may experience protein energy wasting, which may require higher intakes of high-protein foods. Those with CFRD may require modification of macronutrient distribution, including timing and types of carbohydrate. In all cases, RDNs should regularly assess nutrition status and symptoms of the person with CF and should adjust nutrition recommendations accordingly.
These recommendations were based on systematic review (Table). The understanding of how macronutrient distribution affects outcomes in CF may change with higher quality research, including dietary trials and large, long term cohort studies that follow relationships between dietary patterns and nutrition-related outcomes over time.
12.0. Fiber Intake
Recommendation 12.1.
For individuals with CF, the RDN or international equivalent may suggest dietary fiber intake in line with the dietary reference intake for the general population, as tolerated on an individual basis because evidence suggests fiber intake at the recommended level does not increase risk of constipation, DIOS, or other gastrointestinal symptoms.
Strength: Weak
Conditional
Rationale:
Limited, observational evidence suggests that fiber intake up to the recommended intake for the general population does not exacerbate gastrointestinal symptoms, including constipation and DIOS in pediatric participants167,168,174 or pediatric and adult participants combined.167 In 1 study with pediatric participants, the group with DIOS had actual fiber intake greater than the recommended intake, and those with no gastrointestinal symptoms consumed fiber intake at the recommended level.168
Fiber intake that is too high may exacerbate gastrointestinal symptoms in some individuals with CF. Fiber intake that is too low may increase risk of constipation. Dietary fiber recommendations should be adjusted according to gastrointestinal symptoms and individual tolerance, including past responses to fiber, dietary preferences, and meal patterns. Spreading fiber intake throughout the day may alleviate gastrointestinal symptoms associated with elevated fiber intake.
The recommendation for fiber was based on systematic review (Table).
13.0. Infant Feeding
Recommendation 13.1.
In infants diagnosed with CF, the RDN or international equivalent should recommend providing as much breast milk as possible, with breast milk fortification and formula supplementation as necessary for the first year of life, to regain birth weight z score and achieve normal growth for age. Breastfeeding is associated with improved FEV1% predicted and decreased antibiotic use, but supplementation may be needed for infants with high nutrient requirements.
Strength: Fair
Conditional
Rationale:
For the general population, exclusive breastfeeding during the first 6 months of life and any breastfeeding for up to 2 years of age and beyond is recommended to optimize infant health outcomes.175 Providing breast milk to infants with CF may be of particular importance because CF increases risk of inflammation and infection,176 and breastfeeding is known to enhance the immune system.177 However, higher nutrient requirements in infants with CF may require additional nutrients through breast milk fortification or supplementation with formula in order to prevent undernutrition,178 which is associated with pulmonary decline.30–53
Observational evidence reported that infants with CF who were breastfed for at least 4 to 6 months, exclusively or partially, had higher FEV1% predicted at 6 years compared with infants who were exclusively formula fed.179,180 Two cohort studies reported no significant differences in weight gain or weight-for-age z scores between breastfed and formula-fed infants,179,181,182 even when considering exclusivity.179,182 However, one study reported a significant decline in weight z scores from birth to 2 years of age, particularly from 2 to 6 months, for infants who had been exclusively breastfed for 2 months, although there were no changes in other feeding groups.183 Breastfeeding duration and exclusivity did not influence length gain.179,181–183
Strong evidence from the systematic review supporting this guideline demonstrated that individuals with CF who maintained a weight, length, weight-for-length, and BMI >50th percentile from infancy and early childhood had better FEV1% predicted values. In general, normal growth parameters during childhood were associated with increased FEV1% predicted in long-term follow-up studies.31–46 Because WHO growth charts are less likely to classify children as being underweight compared to CDC growth charts, it is suggested practitioners use a cutoff value of 70th percentile when using WHO growth charts.37,56
Newborn screening for CF has had a considerable impact on nutritional status, as most CF infants were previously diagnosed after failing to thrive. Evidence from eras before newborn screening may be biased in that infants who were breastfed had higher FEV1% predicted because infants with more severe disease and failure to thrive were more likely to begin formula feeding. Guidance on PERT for infants was outside the scope of this guideline but can be found in other contemporary guidelines.1,2
Breastfeeding Support.
Support systems in place for breastfeeding may be available through the hospital or through community services, such as the Special Supplemental Nutrition Program for Women, Infants, and Children program. For mothers of infants with CF who are willing and able to breastfeed, RDNs can encourage and support breastfeeding and can connect the family with resources, such lactation consultants or breastfeeding peer counselors. RDNs should work with the interdisciplinary health care team to frequently track the growth and evaluate the nutrition status of the infant with CF to determine whether breast milk fortification or supplementation is necessary to achieve or maintain growth that is consistent with birth weight z score and normal growth for age.
Please see the EAL website for more information.15
The evidence supporting the recommendation on infant feeding was based on systematic review (Table). There are many confounding relationships that need to be addressed in this literature, and conclusions may change with more high-quality evidence.
Strengths and Limitations
This evidence-based practice guideline was supported by rigorously conducted systematic reviews, with evidence translated to recommendations using an evidence-to-decision framework. This guideline is among the first to examine nutrition screening and assessment methods for individuals with CF in an evidence-based manner. This guideline also is the first of its kind to examine food/dietary intake and patterns through systematic review of the evidence to provide practical, relevant dietary recommendation for individuals with CF. For topics outside of the scope of this guideline, the workgroup reviewed and assessed the quality of other evidence-based nutrition guidelines in order to provide practitioners with a comprehensive resource for nutrition care in CF. The major limitation to this guideline is the lack of supporting evidence available to inform practice for some topics, particularly dietary intake. Paucity of evidence led the workgroup to write consensus recommendations based on supporting literature outside of the systematic review, including for the general population when necessary.
Development of this guideline, and the underlying systematic reviews, was done in close collaboration with 2 patient advocates, both of whom were parents of individuals with CF. However, the workgroup did not contain an individual with CF. Recommendations were written by RDNs experienced in the CF field for RDNs working in the CF field. However, other professionals that are crucial to the CF team, including endocrinologists, gastroenterologists, nurses and nurse practitioners, social workers, pharmacists, and many others, were not represented on the workgroup.
Future Research
There were considerable gaps in the literature supporting this systematic review. As understanding of CF pathology grows and CF treatments continue to improve, it will be crucial to conduct research that reflects the current landscape of nutrition needs and care in CF. New CFTR modulation therapy and other CF treatments in the pipeline will likely change nutrition status and needs for many individuals with CF, and more research is greatly needed that examines how these medications affect nutrition parameters outside of weight/growth and how dietary intake may modify the relationship between CFTR modulators and nutrition status. Intervention and long-term cohort studies are needed to determine the relationships between dietary intake and health outcomes in individuals with CF. Finally, there is an intimate relationship between nutrition status and health outcomes for individuals with CF, which is demonstrated in the CFF’s recommendation that all individuals with CF should have regular contact with an RDN. Limited evidence demonstrated that increasing contacts with an RDN resulted in improved nutrition status. More research is needed to determine which nutrition screening, assessment, intervention, and monitoring and evaluation methods performed by an RDN result in improved health outcomes for individuals with CF.
CONCLUSIONS
The Academy of Nutrition and Dietetics’ Evidence Analysis Library Cystic Fibrosis Systematic Review and Guideline committee aspired to develop evidence-based practice guidelines for primary nutrition issues in CF. Goals of this project were to inform the practice of RDNs who provide MNT for individuals with CF and to promote standardization of best practices in CF nutrition care. The Academy EAL CF guidelines were designed to complement and build upon existing guidelines from the Cystic Fibrosis Foundation and the international CF nutrition care community. Gaps in CF nutrition research literature requiring attention were identified.
Optimal nutrition continues to be an essential component of overall CF care. The CF landscape is evolving rapidly with breakthroughs in CFTR modulators changing CF at a cellular level. MNT for individuals with CF from infancy through advanced age requires novel and individualized approaches. The Academy EAC CF guidelines provide a framework for expanding upon current knowledge to determine effective nutrition strategies for individuals with CF through long and healthy futures.
ACKNOWLEDGEMENTS
The authors would like to acknowledge and thank the evidence analysts who extracted data for this systematic review.
FUNDING/SUPPORT
The Academy of Nutrition and Dietetics and the Medical Nutrition Dietetic Practice Group provided funding for this systematic review.
Footnotes
Supplementary materials: Figure 5 is available at www.jandonline.org
STATEMENT OF POTENTIAL CONFLICT OF INTEREST
J. A. Alvarez receives funding from the National Institutes of Health (R03 DK117246, K01 DK102851), and the Cystic Fibrosis Foundation (ALVARE19A0). There were no other conflicts of interest to disclose.
References
- 1.Saxby N, Painter C, Kench A, King S, Crowder T, van der Haak N; Australian and New Zealand Cystic Fibrosis Nutrition Guideline Authorship Group. Nutri- tion Guidelines for Cystic Fibrosis in Australia and New Zealand. Sydney: Thoracic Society of Australia and New Zealand; 2017. [DOI] [PubMed] [Google Scholar]
- 2.Turck D, Braegger CP, Colombo C, et al. ESPEN-ESPGHAN-ECFS guidelines on nutrition care for infants, children, and adults with cystic fibrosis. Clin Nutr. 2016;35(3):557–577. [DOI] [PubMed] [Google Scholar]
- 3.Borowitz D, Baker RD, Stallings V. Consensus report on nutrition for pediatric patients with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2002;35(3): 246–259. [DOI] [PubMed] [Google Scholar]
- 4.Aris RM, Merkel PA, Bachrach LK, et al. Guide to bone health and disease in cystic fibrosis. J Clin Endocrinol Metab. 2005;90(3):1888–1896. [DOI] [PubMed] [Google Scholar]
- 5.Stallings V, Stark L, Robinson K, Feranchak A, Quinton H. Evidence-based practice recommendations for nutrition related management of children and adults with cystic fibrosis and pancreatic insufficiency: Results of a systematic review. J Am Diet Assoc. 2008;108(5): 832–839. [DOI] [PubMed] [Google Scholar]
- 6.Borowitz D, Robinson KA, Rosenfeld M, et al. Cystic Fibrosis Foundation evidence-based guidelines for management of infants with cystic fibrosis. J Pediatr. 2009;155(6):S73–S93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tangpricha V, Kelly A, Stephenson A, et al. An update on the screening, diagnosis, management, and treatment of vitamin D deficiency in individuals with cystic fibrosis: Evidence-based recommendations from the Cystic Fibrosis Foundation. J Clin Endocrinol Metab. 2012;97(4):1082–1093. [DOI] [PubMed] [Google Scholar]
- 8.Schwarzenberg SJ, Hempstead SE, McDonald CM, et al. Enteral tube feeding for individuals with cystic fibrosis: Cystic Fibrosis Foundation evidence informed guidelines. J Cyst Fibros. 2016;15(6):724–735. [DOI] [PubMed] [Google Scholar]
- 9.Hadjiliadis D, Khoruts A, Zauber AG, Hempstead SE, Maisonneuve P, Lowenfels AB. Cystic Fibrosis colorectal cancer screening consensus recommendations. Gastroenterology. 2018;154(3): 736–745.e714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corey M, McLaughlin FJ, Williams M, Levison H. A comparison of survival, growth, and pulmonary function in patients with cystic fibrosis in Boston and Toronto. J Clin Epidemiol. 1988;41(6): 583–591. [DOI] [PubMed] [Google Scholar]
- 11.Sutherland R, Katz T, Liu V, et al. Dietary intake of energy-dense, nutrient-poor and nutrient-dense food sources in children with cystic fibrosis. J Cyst Fibros. 2018;17(6):804–810. [DOI] [PubMed] [Google Scholar]
- 12.Calvo-Lerma J, Hulst J, Boon M, et al. The relative contribution of food groups to macronutrient intake in children with cystic fibrosis: A European Multicenter Assessment. J Acad Nutr Diet. 2019;119(8):1305–1319. [DOI] [PubMed] [Google Scholar]
- 13.Handu D, Moloney L, Wolfram T, Ziegler P, Acosta A, Steiber A. Academy of Nutrition and Dietetics methodology for conducting systematic reviews for the Evidence Analysis Library. J Acad Nutr Diet. 2016;116(2):311–318. [DOI] [PubMed] [Google Scholar]
- 14.Papoutsakis C, Moloney L, Sinley RC, Acosta A, Handu D, Steiber AL. Academy of Nutrition and Dietetics methodology for developing evidence-based nutrition practice guidelines. J Acad Nutr Diet. 2017;117(5):794–804. [DOI] [PubMed] [Google Scholar]
- 15.Academy of Nutrition and Dietetics Evidence Analysis Library. Evidence Analysis Library Cystic Fibrosis Project. https://www.andeal.org/topic.cfm?menu=5876. Published 2019. Accessed February 5, 2020.
- 16.AGREE Next Steps Consortium. The AGREE II Instrument (electronic version). http://www.agreetrust.org. Published 2017. Accessed February 3, 2020.
- 17.Rozga M, Handu D. Nutrition Care for patients with cystic fibrosis: An Evidence Analysis Center scoping review. J Acad Nutr Diet. 2019;119(1):137–151. e131. [DOI] [PubMed] [Google Scholar]
- 18.Rozga M Nutritional assessment and dietary interventions in patients with cystic fibrosis. PROSPERO 2018 CRD42018097373. National Institute for Health Research. https://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42018097373&IDCRD42018097373. Published 2018. Accessed December 3, 2019. [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. [DOI] [PubMed] [Google Scholar]
- 20.Academy of Nutrition and Dietetics Evidence Analysis Library. Evidence Analysis Manual: Steps in the Academy Evidence Analysis Process: A Systematic Review and Guideline Manual. Chicago, IL: Academy of Nutrition and Dietetics; 2016. [Google Scholar]
- 21.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–394. [DOI] [PubMed] [Google Scholar]
- 22.GRADEpro GDT. GRADEpro Guideline Development Tool [computer program]. Hamilton, Ontario, Canada: McMaster University; 2015. [Google Scholar]
- 23.Moberg J, Oxman AD, Rosenbaum S, et al. The GRADE Evidence to Decision (EtD) framework for health system and public health decisions. Health Res Policy Syst. 2018;16(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alonso-Coello P, Schunemann HJ, Moberg J, et al. GRADE Evidence to Decision (EtD) frameworks: A systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016;353:i2016. [DOI] [PubMed] [Google Scholar]
- 25.American Academy of Pediatrics Steering Committee on Quality Improvement and Management. Classifying recommendations for clinical practice guidelines. Pediatrics. 2004;114(3):874–877s. [DOI] [PubMed] [Google Scholar]
- 26.Cystic Fibrosis Foundation. Your CF Care Team. Cystic Fibrosis Foundation. https://www.cff.org/Care/Your-CF-Care-Team/. Accessed October 15, 2019. [Google Scholar]
- 27.Ramirez I, Filbrun A, Hasan A, Kidwell KM, Nasr SZ. Improving nutritional status in a pediatric cystic fibrosis center. Pediatr Pulmonol. 2015;50(6): 544–551. [DOI] [PubMed] [Google Scholar]
- 28.Stark LJ, Opipari-Arrigan L, Quittner AL, Bean J, Powers SW. The effects of an intensive behavior and nutrition intervention compared to standard of care on weight outcomes in CF. Pediatr Pulmonol. 2011;46(1):31–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savant AP, Britton LJ, Petren K, McColley SA, Gutierrez HH. Sustained improvement in nutritional outcomes at two paediatric cystic fibrosis centres after quality improvement collaboratives. BMJ Qual Saf. 2014;23:81–89. [DOI] [PubMed] [Google Scholar]
- 30.Academy of Nutrition and Dietetics Evidence Analysis Library. In individuals with CF, what is the longitudinal relationship (at least 3 months) between weight and growth parameters and FEV1? Academy of Nutrition and Dietetics. Cystic Fibrosis Systematic Review web site. http://www.andeal.org/topic.cfm?menu=5876&cat=5979. Updated August 2019. Accessed September 10, 2019. [Google Scholar]
- 31.Darrah R, Nelson R, Damato EG, Decker M, Matthews A, Hodges CA. Growth deficiency in cystic fibrosis is observable at birth and predictive of early pulmonary function. Biol Res Nurs. 2016;18(5):498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emerson J, Rosenfeld M, McNamara S, Ramsey B, Gibson RL. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol. 2002;34(2):91–100. [DOI] [PubMed] [Google Scholar]
- 33.Goss CH, Sykes J, Stanojevic S, et al. Comparison of nutrition and lung function outcomes in patients with cystic fibrosis living in Canada and the United States. Am J Respir Crit Care Med. 2018;197(6):768–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keller BM, Aebischer CC, Kraemer R, Schoni MH. Growth in prepubertal children with cystic fibrosis, homozygous for the Delta F508 mutation. J Cyst Fibros. 2003;2(2):76–83. [DOI] [PubMed] [Google Scholar]
- 35.Konstan MW, Morgan WJ, Butler SM, et al. Risk factors for rate of decline in forced expiratory volume in one second in children and adolescents with cystic fibrosis. J Pediatr. 2007;151(2):134–139.e131. [DOI] [PubMed] [Google Scholar]
- 36.Lai HJ, Shoff SM, Farrell PM; ; Wisconsin Cystic Fibrosis Neonatal Screening Group. Recovery of birth weight z score within 2 years of diagnosis is positively associated with pulmonary status at 6 years of age in children with cystic fibrosis. Pediatrics. 2009;123(2):714–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Machogu E, Cao Y, Miller T, et al. Comparison of WHO and CDC growth charts in predicting pulmonary outcomes in cystic fibrosis. J Pediatr Gastroenterol Nutr. 2015;60(3):378–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McPhail GL, Acton JD, Fenchel MC, Amin RS, Seid M. Improvements in lung function outcomes in children with cystic fibrosis are associated with better nutrition, fewer chronic pseudomonas aeruginosa infections, and dornase alfa use. J Pediatr. 2008;153(6):752–757. [DOI] [PubMed] [Google Scholar]
- 39.Sanders DB, Li Z, Laxova A, et al. Risk factors for the progression of cystic fibrosis lung disease throughout childhood. Ann Am Thorac Soc. 2014;11(1):63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanders DB, Fink A, Mayer-Hamblett N, et al. Early life growth trajectories in cystic fibrosis are associated with pulmonary function at age 6 years. J Pediatr. 2015;167(5):1081–1088.e1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanders DB, Emerson J, Ren CL, et al. Early childhood risk factors for decreased FEV1 at age six to seven years in young children with cystic fibrosis. Ann Am Thorac Soc. 2015;12(8):1170–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanders DB, Zhang Z, Farrell PM, Lai HJ, Wisconsin CF Neonatal Screening Group. Early life growth patterns persist for 12 years and impact pulmonary outcomes in cystic fibrosis. J Cyst Fibros. 2018;17:528–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Usatin D, Yen EH, McDonald C, Asfour F, Pohl J, Robson J. Differences between WHO and CDC early growth measurements in the assessment of cystic fibrosis clinical outcomes. J Cyst Fibros. 2017;16(4):503–509. [DOI] [PubMed] [Google Scholar]
- 44.Welsh L, Robertson CF, Ranganathan SC. Increased rate of lung function decline in Australian adolescents with cystic fibrosis. Pediatr Pulmonol. 2014;49(9): 873–877. [DOI] [PubMed] [Google Scholar]
- 45.Woestenenk JW, Stellato RK, Terheggen-Lagro SW, van der Ent CK, Houwen RHJ. The relationship between body growth and pulmonary function in children with cystic fibrosis. Acta Paediatr. 2014;103(2):162–167. [DOI] [PubMed] [Google Scholar]
- 46.Yen EH, Quinton H, Borowitz D. Better nutritional status in early childhood is associated with improved clinical outcomes and survival in patients with cystic fibrosis. J Pediatr. 2013;162(3):530–535.e531. [DOI] [PubMed] [Google Scholar]
- 47.Amadori A, Antonelli A, Balteri I, Schreiber A, Bugiani M, De Rose V. Recurrent exacerbations affect FEV(1) decline in adult patients with cystic fibrosis. Respir Med. 2009;103(3):407–413. [DOI] [PubMed] [Google Scholar]
- 48.Ramos KJ, Quon BS, Heltshe SL, et al. Heterogeneity in survival in adult patients with cystic fibrosis with FEV1 <30% of Predicted in the United States. Chest. 2017;151(6):1320–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.George PM, Banya W, Pareek N, et al. Improved survival at low lung function in cystic fibrosis: Cohort study from 1990 to 2007. BMJ. 2011;342(8900488): d1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chamnan P, Shine BSF, Haworth CS, Bilton D, Adler AI. Diabetes as a determinant of mortality in cystic fibrosis. Diabetes Care. 2010;33(2):311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stephenson AL, Tom M, Berthiaume Y, et al. A contemporary survival analysis of individuals with cystic fibrosis: A cohort study. Eur Respir J. 2015;45(3): 670–679. [DOI] [PubMed] [Google Scholar]
- 52.Vieni G, Faraci S, Collura M, et al. Stunting is an independent predictor of mortality in patients with cystic fibrosis. Clin Nutr. 2013;32(3):382–385. [DOI] [PubMed] [Google Scholar]
- 53.McColley SA, Schechter MS, Morgan WJ, Pasta DJ, Craib ML, Konstan MW. Risk factors for mortality before age 18 years in cystic fibrosis. Pediatr Pulmonol. 2017;52(7):909–915. [DOI] [PubMed] [Google Scholar]
- 54.Cystic Fibrosis Foundation Patient Registry. 2017 Annual Data Report. Bethesda, MD: Cystic Fibrosis Foundation; 2018. [Google Scholar]
- 55.Grummer-Strawn LM, Reinold C, Krebs NF. Use of World Health Organization and CDC growth charts for children aged 0–59 months in the United States. MMWR Recomm Rep. 2010;59(RR-9):1–15. [PubMed] [Google Scholar]
- 56.Zhang Z, Shoff SM, Lai HJ. Comparing the use of Centers for Disease Control and Prevention and World Health Organization growth charts in children with cystic fibrosis through 2 years of age. J Pediatr. 2015;167(5):1089–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cystic Fibrosis Foundation. Nutritional basics. https://www.cff.org/Life-With-CF/Daily-Life/Fitness-and-Nutrition/Nutrition/Getting-Your-Nutrients/Nutritional-Basics/. Accessed September 25, 2019.
- 58.Hollander FM, van Pierre DD, de Roos NM, van de Graaf EA, Iestra JA. Effects of nutritional status and dietetic interventions on survival in cystic fibrosis patients before and after lung transplantation. J Cyst Fibros. 2014;13(2):212–218. [DOI] [PubMed] [Google Scholar]
- 59.King SJ, Nyulasi IB, Strauss BJG, Kotsimbos T, Bailey M, Wilson JW. Fat-free mass depletion in cystic fibrosis: Associated with lung disease severity but poorly detected by body mass index. Nutrition. 2010;26(7):753–759. [DOI] [PubMed] [Google Scholar]
- 60.Ledger SJ, Owen E, Prasad SA, Goldman A, Willams J, Aurora P. A pilot outreach physiotherapy and dietetic quality improvement initiative reduces IV antibiotic requirements in children with moderate-severe cystic fibrosis. J Cyst Fibros. 2013;12(6):766–772. [DOI] [PubMed] [Google Scholar]
- 61.Academy of Nutrition and Dietetics Evidence Analysis Library. In individuals with CF, what is the validity and reliability or nutrition assessment methods compared to reference standards, as measured by validity and/or reliability studies? Academy of Nutrition and Dietetics Evidence Analysis Library. https://www.andeal.org/topic.cfm?menu=5876&cat=5942. Published 2019. Accessed September 26, 2019. [Google Scholar]
- 62.Academy of Nutrition and Dietetics Evidence Analysis Library. In individuals with CF, what is the relationship between nutrition parameters and hard outcomes?. https://www.andeal.org/topic.cfm?menu=5876&cat=5979. Published 2019. Accessed September 25, 2019.
- 63.Centers for Disease Control and Prevention. Defining childhood obesity. https://www.cdc.gov/obesity/childhood/defining.html. Published 2019. Updated July 3, 2018. Accessed November 27, 2019.
- 64.EBMcalc. An educational medical reference. https://ebmcalc.com/HeightPotential.htm. Published 2019. Accessed January 9, 2020.
- 65.Tanner JM, Goldstein H, Whitehouse RH. Standards for children’s height at ages 2–9 years allowing for heights of parents. Arch Dis Child. 1970;45(244):755–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Centers for Disease Control and Prevention. Defining adult overweight and obesity. https://www.cdc.gov/obesity/adult/defining.html. Published 2019. Updated April 11, 2017. Accessed November 27, 2019.
- 67.Centers for Disease Control and Prevention. Getting your cholesterol checked. https://www.cdc.gov/cholesterol/cholesterol_screening.html. Published 2019. Accessed September 2019.
- 68.American Academy of Pediatrics. 2016. Recommendations for Preventive Pediatric Health Care. Pediatrics; 2016:137. [Google Scholar]
- 69.Academy of Nutrition and Dietetics. Pediatric Nutrition Focused Physical Exam Pocket Guide. Chicago, IL: Academy of Nutrition and Dietetics; 2016. [Google Scholar]
- 70.Cystic Fibrosis Foundation. Our mission. https://www.cff.org/About-Us/About-the-Cystic-Fibrosis-Foundation/Our-Mission/. Published 2019. Accessed September 25, 2019.
- 71.Borowitz D, Lubarsky B, Wilschanski M, et al. Nutritional status improved in cystic fibrosis patients with the G551D mutation after treatment with ivacaftor. Dig Dis Sci. 2016;61(1):198–207. [DOI] [PubMed] [Google Scholar]
- 72.Davies JC, Wainwright CE, Canny GJ, et al. Efficacy and safety of ivacaftor in patients aged 6 to 11 years with cystic fibrosis with a G551D mutation. Am J Respir Criti Care Med. 2013;187(11): 1219–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Konstan MW, Plant BJ, Elborn JS, et al. Efficacy response in CF patients treated with ivacaftor: Post-hoc analysis. Pediatr Pulmonol. 2015;50(5):447–455. [DOI] [PubMed] [Google Scholar]
- 74.Ramsey BW, Davies J, McElvaney NG, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365(18):1663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stalvey MS, Pace J, Nikniar M, et al. Growth in prepubertal children with cystic fibrosis treated with ivacaftor. Pediatrics. 2017;139(2). 44–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.De Boeck K, Munck A, Walker S, et al. Efficacy and safety of ivacaftor in patients with cystic fibrosis and a non-G551D gating mutation. J Cyst Fibros. 2014;13(6):674–680. [DOI] [PubMed] [Google Scholar]
- 77.Edgeworth D, Keating D, Ellis M, et al. Improvement in exercise duration, lung function and well-being in G551D-cystic fibrosis patients: A double-blind, placebo-controlled, randomized, cross-over study with ivacaftor treatment. Clin Sci (Lond). 2017;131(15):2037–2045. [DOI] [PubMed] [Google Scholar]
- 78.Moss RB, Flume PA, Elborn JS, et al. Efficacy and safety of ivacaftor in patients with cystic fibrosis who have an Arg117His-CFTR mutation: A double- blind, randomised controlled trial. Lan- cet Respir Med. 2015;3(7):524–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ratjen F, Hug C, Marigowda G, et al. Efficacy and safety of lumacaftor and ivacaftor in patients aged 6–11 years with cystic fibrosis homozygous for F508delCFTR: A randomised, placebo-controlled phase 3 trial. Lancet Respir Med. 2017;5(7):557–567. [DOI] [PubMed] [Google Scholar]
- 80.Elborn JS, Ramsey BW, Boyle MP, et al. Efficacy and safety of lumacaftor/ivacaftor combination therapy in patients with cystic fibrosis homozygous for Phe508del CFTR by pulmonary function subgroup: A pooled analysis. Lancet Respir Med. 2016;4(8):617–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rowe SM, McColley SA, Rietschel E, et al. Lumacaftor/ivacaftor treatment of patients with cystic fibrosis heterozygous for F508del-CFTR. Ann Am Thorac Soc. 2017;14(2):213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wainwright CE, Elborn JS, Ramsey BW, et al. Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med. 2015;373(3):220–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Taylor-Cousar JL, Munck A, McKone EF, et al. Tezacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del. N Engl J Med. 2017;377(21): 2013–2023. [DOI] [PubMed] [Google Scholar]
- 84.Stallings VA, Sainath N, Oberle M, Bertolaso C, Schall JI. Energy balance and mechanisms of weight gain with ivacaftor treatment of cystic fibrosis gating mutations. J Pediatr. 2018;201:229–237. e224. [DOI] [PubMed] [Google Scholar]
- 85.Middleton PG, Mall MA, Drevinek P, et al. Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med. 2019;381(19):1809–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Heijerman HGM, McKone EF, Downey DG, et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: A double-blind, randomised, phase 3 trial. Lancet. 2019;394(10212):1940–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vertex Pharmaceuticals Incorporated. Prescribing information. TRIKAFTA (elexaccaftor/tezacaftor/ivacaftor; ivacaftor) tablets. Table 4: Pharmacokinetic parameters of TRIKAFTA components. Vertex Pharmaceuticals Incorporated; 2019. [Google Scholar]
- 88.Davies JC, Cunningham S, Harris WT, et al. Safety, pharmacokinetics, and pharmacodynamics of ivacaftor in patients aged 2–5 years with cystic fibrosis and a CFTR gating mutation (KIWI): An open-label, single-arm study. Lancet Respir Med. 2016;4(2):107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rosenfeld M, Cunningham S, Harris WT, et al. An open-label extension study of ivacaftor in children with CF and a CFTR gating mutation initiating treatment at age 2–5 years (KLIMB). J Cyst Fibros. 2019;18(6):838–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cystic Fibrosis Foundation. Clinical Considerations: TRIKAFTA. Bethesda, MD: Cystic Fibrosis Foundation; 2019. [Google Scholar]
- 91.Moudiou T, Galli-Tsinopoulou A, Vamvakoudis E, Nousia-Arvanitakis S. Resting energy expenditure in cystic fibrosis as an indicator of disease severity. J Cyst Fibros. 2007;6(2):131–136. [DOI] [PubMed] [Google Scholar]
- 92.Moudiou T, Galli-Tsinopoulou A, Nousia-Arvanitakis S. Effect of exocrine pancreatic function on resting energy expenditure in cystic fibrosis. Acta Paediatr. 2007;96(10):1521–1525. [DOI] [PubMed] [Google Scholar]
- 93.Trabulsi J, Ittenbach RF, Schall JI, et al. Evaluation of formulas for calculating total energy requirements of preadolescent children with cystic fibrosis. Am J Clin Nutr. 2007;85(1):144–151. [DOI] [PubMed] [Google Scholar]
- 94.Hollander FM, Kok A, de Roos NM, Belle-van Meerkerk G, van de Graaf EA. Prediction equations underestimate resting energy expenditure in patients with end-stage cystic fibrosis. Nutr Clin Pract. 2017;32(1):116–121. [DOI] [PubMed] [Google Scholar]
- 95.Brooks MJ, Melnik G. The refeeding syndrome: An approach to understanding its complications and preventing its occurrence. Pharmacotherapy. 1995;15(6):713–726. [PubMed] [Google Scholar]
- 96.Alpers DH, Klein S. Refeeding the malnourished patient. Curr Opin Gastro- enterol. 1999;15(2):151–153. [DOI] [PubMed] [Google Scholar]
- 97.Marik PE, Bedigian MK. Refeeding hypophosphatemia in critically ill patients in an intensive care unit. A prospective study. Arch Surg. 1996;131(10): 1043–1047. [DOI] [PubMed] [Google Scholar]
- 98.Afzal NA, Addai S, Fagbemi A, Murch S, Thomson M, Heuschkel R. Refeeding syndrome with enteral nutrition in children: A case report, literature review and clinical guidelines. Clin Nutr. 2002;21(6):515–520. [DOI] [PubMed] [Google Scholar]
- 99.Solomon SM, Kirby DF. The refeeding syndrome: A review. JPEN J Parenter Enteral Nutr. 1990;14(1):90–97. [DOI] [PubMed] [Google Scholar]
- 100.Calella P, Valerio G, Thomas M, et al. Association between body composition and pulmonary function in children and young people with cystic fibrosis. Nutrition. 2018;48:73–76. [DOI] [PubMed] [Google Scholar]
- 101.Pedreira CC, Robert RGD, Dalton V, et al. Association of body composition and lung function in children with cystic fibrosis. Pediatr Pulmonol. 2005;39(3): 276–280. [DOI] [PubMed] [Google Scholar]
- 102.Sheikh S, Zemel BS, Stallings VA, Rubenstein RC, Kelly A. Body composition and pulmonary function in cystic fibrosis. Front Pediatr. 2014;2(101615492):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ionescu AA, Nixon LS, Luzio S, et al. Pulmonary function, body composition, and protein catabolism in adults with cystic fibrosis. Am J Respir Criti Care Med. 2002;165(4):495–500. [DOI] [PubMed] [Google Scholar]
- 104.Olveira G, Olveira C, Gaspar I, et al. Fat-free mass depletion and inflammation in patients with bronchiectasis. J Acad Nutr Diet. 2012;112(12):1999–2006. [DOI] [PubMed] [Google Scholar]
- 105.Engelen MPKJ, Schroder R, Van der Hoorn K, Deutz NEP, Com G. Use of body mass index percentile to identify fat-free mass depletion in children with cystic fibrosis. Clin Nutr. 2012;31(6):927–933. [DOI] [PubMed] [Google Scholar]
- 106.Ionescu AA, Evans WD, Pettit RJ, Nixon LS, Stone MD, Shale DJ. Hidden depletion of fat-free mass and bone mineral density in adults with cystic fibrosis. Chest. 2003;124(6):2220–2228. [DOI] [PubMed] [Google Scholar]
- 107.Abramowitz MK, Hall CB, Amodu A, Sharma D, Androga L, Hawkins M. Muscle mass, BMI, and mortality among adults in the United States: A population-based cohort study. PLoS One. 2018;13(4):e0194697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wells GD, Heale L, Schneiderman JE, et al. Assessment of body composition in pediatric patients with cystic fibrosis. Pediatr Pulmonol. 2008;43(10):1025–1032. [DOI] [PubMed] [Google Scholar]
- 109.Williams JE, Wells JC, Benden C, et al. Body composition assessed by the 4-component model and association with lung function in 6–12-y-old children with cystic fibrosis. Am J Clin Nutr. 2010;92(6):1332–1343. [DOI] [PubMed] [Google Scholar]
- 110.Puiman PJ, Francis P, Buntain H, Wainwright C, Masters B, Davies PSW. Total body water in children with cystic fibrosis using bioelectrical impedance. J Cyst Fibros. 2004;3(4):243–247. [DOI] [PubMed] [Google Scholar]
- 111.Calella P, Valerio G, Brodlie M, Taylor J, Donini LM, Siervo M. Tools and methods used for the assessment of body composition in patients with cystic fibrosis: A systematic review. Nutr Clin Pract. 2019;34(5):701–714. [DOI] [PubMed] [Google Scholar]
- 112.Sheean P, Gonzalez MC, Prado CM, McKeever L, Hall AM, Braunschweig CA. American Society for Parenteral and Enteral Nutrition clinical guidelines: The validity of body composition assessment in clinical populations. JPEN J Parenter Enteral Nutr. 2020;44(1):12–43. [DOI] [PubMed] [Google Scholar]
- 113.Charatsi AM, Dusser P, Freund R, et al. Bioelectrical impedance in young patients with cystic fibrosis: Validation of a specific equation and clinical relevance. J Cyst Fibros. 2016;15(6):825–833. [DOI] [PubMed] [Google Scholar]
- 114.Hollander FM, De Roos NM, De Vries JHM, Van Berkhout FT. Assessment of nutritional status in adult patients with cystic fibrosis: Whole-body bioimpedance vs body mass index, skinfolds, and leg-to-leg bioimpedance. J Am Diet Assoc. 2005;105(4):549–555. [DOI] [PubMed] [Google Scholar]
- 115.King S, Wilson J, Kotsimbos T, Bailey M, Nyulasi I. Body composition assessment in adults with cystic fibrosis: Comparison of dual-energy X-ray absorptiometry with skinfolds and bioelectrical impedance analysis. Nutrition. 2005;21(11):1087–1094. [DOI] [PubMed] [Google Scholar]
- 116.Becker P, Carney LN, Corkins MR, et al. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: Indicators recommended for the identification and documentation of pediatric malnutrition (undernutrition). Nutr Clin Pract. 2015;30(1):147–161. [DOI] [PubMed] [Google Scholar]
- 117.Addo OY, Himes JH. Reference curves for triceps and subscapular skinfold thicknesses in US children and adolescents. Am J Clin Nutr. 2010;91(3):635–642. [DOI] [PubMed] [Google Scholar]
- 118.World Health Organization. Child growth standards, arm circumference-for-age. https://www.who.int/childgrowth/standards/ac_for_age/en/. Published 2020. Accessed January 13, 2020.
- 119.World Health Organization. Child growth standards, subscapular skinfold-for-age. https://www.who.int/childgrowth/standards/ssf_for_age/en/. Published 2020. Accessed January 13, 2020.
- 120.Schmidt SC, Bosy-Westphal A, Niessner C, Woll A. Representative body composition percentiles from bioelectrical impedance analyses among children and adolescents. The MoMo study. Clin Nutr. 2019;38(6):2712–2720. [DOI] [PubMed] [Google Scholar]
- 121.Mattiello R, Amaral MA, Mundstock E, Ziegelmann PK. Reference values for the phase angle of the electrical bioimpedance: Systematic review and meta-analysis involving more than 250, 000 subjects. Clin Nutr; 2019. July 29. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 122.Kuchnia A, Earthman C, Teigen L, et al. Evaluation of bioelectrical impedance analysis in critically ill patients: Results of a multicenter prospective study. JPEN J Parenter Enteral Nutr. 2017;41(7):1131–1138. [DOI] [PubMed] [Google Scholar]
- 123.Marquette M, Haworth CS. Bone health and disease in cystic fibrosis. Paediatr Respir Rev. 2016;20(100898941):2–5. [DOI] [PubMed] [Google Scholar]
- 124.Sermet-Gaudelus I, Bianchi ML, Garabedian M, et al. European cystic fibrosis bone mineralisation guidelines. J Cyst Fibros. 2011;10(suppl 2):S16–S23. [DOI] [PubMed] [Google Scholar]
- 125.Padula L, Reid E, Hanna M, Mascarenhas M, Kelly A, Mallowe A. DXA Screening Guidelines for CF Patients at CHOP. Philadelphia: Children’s Hospital of Pennsylvania; 2017. [Google Scholar]
- 126.Kyle UG, Earthman CP, Pichard C, CossBu JA. Body composition during growth in children: Limitations and perspectives of bioelectrical impedance analysis. Eur J Clin Nutr. 2015;69(12):1298–1305. [DOI] [PubMed] [Google Scholar]
- 127.Moran A, Pillay K, Becker D, Granados A, Hameed S, Acerini CL. ISPAD Clinical Practice Consensus Guidelines 2018: Management of cystic fibrosis-related diabetes in children and adolescents. Pediatr Diabetes. 2018;19(suppl 27):64–74. [DOI] [PubMed] [Google Scholar]
- 128.Rana M, Wong-See D, Katz T, et al. Fat-soluble vitamin deficiency in children and adolescents with cystic fibrosis. J Clin Pathol. 2014;67(7):605–608. [DOI] [PubMed] [Google Scholar]
- 129.McCauley LA, Thomas W, Laguna TA, Regelmann WE, Moran A, Polgreen LE. Vitamin D deficiency is associated with pulmonary exacerbations in children with cystic fibrosis. Ann Am Thorac Soc. 2014;11(2):198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Oregon State University Linus Pauling Institute. Micronutrient Information Center. https://lpi.oregonstate.edu/mic. Published 2019. Accessed December 2, 2019.
- 131.Rhodes B, Nash EF, Tullis E, et al. Prevalence of dyslipidemia in adults with cystic fibrosis. J Cyst Fibros. 2010;9(1): 24–28. [DOI] [PubMed] [Google Scholar]
- 132.Alves C, Lima D, Cardeal M, Santana A. Dyslipidemia in racially admixtured children with cystic fibrosis. Indian J Endocrinol Metabol. 2012;16(4):585–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Figueroa V, Milla C, Parks EJ, Schwarzenberg SJ, Moran A. Abnormal lipid concentrations in cystic fibrosis. Am J Clin Nutr. 2002;75(6):1005–1011. [DOI] [PubMed] [Google Scholar]
- 134.Ollero M, Astarita G, Guerrera IC, et al. Plasma lipidomics reveals potential prognostic signatures within a cohort of cystic fibrosis patients. J Lipid Res. 2011;52(5):1011–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kopin L, Lowenstein C. Dyslipidemia. Ann Intern Med. 2017;167(11). Itc81itc96. [DOI] [PubMed] [Google Scholar]
- 136.Poore S, Berry B, Eidson D, McKie KT, Harris RA. Evidence of vascular endothelial dysfunction in young patients with cystic fibrosis. Chest. 2013;143(4): 939–945. [DOI] [PubMed] [Google Scholar]
- 137.Cross CE, Reverri EJ, Morrissey BM. Joining the crowd: Cystic fibrosis and cardiovascular disease risk factors. Chest. 2013;143(4):882–884. [DOI] [PubMed] [Google Scholar]
- 138.Skolnik K, Levy RD, Wilcox PG, Quon BS. Coronary artery disease in cystic fibrosis: An emerging concern? J Cyst Fibros. 2016;15(6):e70–e71. [DOI] [PubMed] [Google Scholar]
- 139.Maqbool A, Schall JI, Garcia-Espana JF, Zemel BS, Strandvik B, Stallings VA. Serum linoleic acid status as a clinical indicator of essential fatty acid status in children with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2008;47(5):635–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bellissimo MP, Zhang I, Ivie EA, et al. Visceral adipose tissue is associated with poor diet quality and higher fasting glucose in adults with cystic fibrosis. J Cyst Fibros. 2019;18(3):430–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Armaghanian N, Atkinson F, Taylor N, et al. Dietary intake in cystic fibrosis and its role in glucose metabolism. Clin Nutr; 2019. November 19. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 142.Rodriguez-Monforte M, Sanchez E, Barrio F, Costa B, Flores-Mateo G. Metabolic syndrome and dietary patterns: A systematic review and meta-analysis of observational studies. Eur J Nutr. 2017;56(3):925–947. [DOI] [PubMed] [Google Scholar]
- 143.Casas R, Castro-Barquero S, Estruch R, Sacanella E. Nutrition and cardiovascular health. Int J Mol Sci. 2018;19(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Grosso G, Bella F, Godos J, et al. Possible role of diet in cancer: Systematic review and multiple meta-analyses of dietary patterns, lifestyle factors, and cancer risk. Nutr Rev. 2017;75(6):405–419. [DOI] [PubMed] [Google Scholar]
- 145.Hosseini B, Berthon BS, Saedisomeolia A, et al. Effects of fruit and vegetable consumption on inflammatory biomarkers and immune cell populations: A systematic literature review and meta-analysis. Am J Clin Nutr. 2018;108(1): 136–155. [DOI] [PubMed] [Google Scholar]
- 146.US Department of Health and Human Services and US Department of Agriculture. 2015e2020 Dietary Guidelines for Americans. 8th ed. http://health.gov/dietaryguidelines/2015/guidelines/. Published December 2015. Accessed April 2, 2020. [Google Scholar]
- 147.Widmer RJ, Flammer AJ, Lerman LO, Lerman A. The Mediterranean diet, its components, and cardiovascular disease. Am J Med. 2015;128(3):229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Soltani S, Jayedi A, Shab-Bidar S, Becerra-Tomas N, Salas-Salvado J. Adherence to the Mediterranean diet in relation to all-cause mortality: A systematic review and dose-response meta-analysis of prospective cohort studies. Adv Nutr. 2019;10(6):1029–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Becerra-Tomás N, Blanco Mejia S, Viguiliouk E, et al. Mediterranean diet, cardiovascular disease and mortality in diabetes: A systematic review and meta-analysis of prospective cohort studies and randomized clinical trials. Crit Rev Food Sci Nutr. 2020;60(7):1207–1227. [DOI] [PubMed] [Google Scholar]
- 150.Rosato V, Temple NJ, La Vecchia C, Castellan G, Tavani A, Guercio V. Mediterranean diet and cardiovascular disease: A systematic review and meta-analysis of observational studies. Eur J Nutr. 2019;58(1):173–191. [DOI] [PubMed] [Google Scholar]
- 151.Rees K, Takeda A, Martin N, et al. Mediterranean-style diet for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2019;3:Cd009825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Esposito K, Maiorino MI, Bellastella G, Chiodini P, Panagiotakos D, Giugliano D. A journey into a Mediterranean diet and type 2 diabetes: A systematic review with meta-analyses. BMJ Open. 2015;5(8):e008222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ciofu O, Lykkesfeldt J. Antioxidant supplementation for lung disease in cystic fibrosis. Cochrane Database Syst Rev. 2014:(8)-2014 Aug 2007. [DOI] [PubMed] [Google Scholar]
- 154.Sagel SD, Sontag MK, Anthony MM, Emmett P, Papas KA. Effect of an antioxidant-rich multivitamin supplement in cystic fibrosis. J Cyst Fibros. 2011;10(1):31–36. [DOI] [PubMed] [Google Scholar]
- 155.Sagel SD, Khan U, Jain R, et al. Effects of an antioxidant-enriched multivitamin in cystic fibrosis: Randomized, controlled, multicenter trial. Am J Respir Crit Care Med. 2018;(9421642). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Shamseer L, Adams D, Brown N, Johnson JA, Vohra S. Antioxidant micronutrients for lung disease in cystic fibrosis. Cochrane Database Syst Rev. 2010;12:CD007020. [DOI] [PubMed] [Google Scholar]
- 157.Kayani K, Mohammed R, Mohiaddin H. Cystic Fibrosis-related diabetes. Front Endocrinol. 2018;9(101555782):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Panagopoulou P, Fotoulaki M, Nikolaou A, Nousia-Arvanitakis S. Prevalence of malnutrition and obesity among cystic fibrosis patients. Pediatr Int. 2014;56(1):89–94. [DOI] [PubMed] [Google Scholar]
- 159.Hanna RM, Weiner DJ. Overweight and obesity in patients with cystic fibrosis: A center-based analysis. Pediatr Pulmonol. 2015;50(1):35–41. [DOI] [PubMed] [Google Scholar]
- 160.Stephenson AL, Mannik LA, Walsh S, et al. Longitudinal trends in nutritional status and the relation between lung function and BMI in cystic fibrosis: A population-based cohort study. Am J Clin Nutr. 2013;97(4):872–877. [DOI] [PubMed] [Google Scholar]
- 161.Alvarez JA, Ziegler TR, Millson EC, Stecenko AA. Body composition and lung function in cystic fibrosis and their association with adiposity and normal weight obesity. Nutrition. 2016;32(4): 447–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Forte GC, Pereira JS, Drehmer M, Simon MI. Anthropometric and dietary intake indicators as predictors of pulmonary function in cystic fibrosis patients. J Bras Pneumol. 2012;38(4):470–476. [DOI] [PubMed] [Google Scholar]
- 163.Moen IE, Nilsson K, Andersson A, et al. Dietary intake and nutritional status in a Scandinavian adult cystic fibrosis population compared with recommendations. Food Nutr Res. 2011;55(101488795). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gordon CM, Anderson EJ, Herlyn K, et al. Nutrient status of adults with cystic fibrosis. J Am Diet Assoc. 2007;107(12): 2114–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.White H, Wolfe SP, Foy J, Morton A, Conway SP, Brownlee KB. Nutritional intake and status in children with cystic fibrosis: Does age matter? J Pediatr Gas- troenterol Nutr. 2007;44(1):116–123. [DOI] [PubMed] [Google Scholar]
- 166.Olveira G, Dorado A, Olveira C, et al. Serum phospholipid fatty acid profile and dietary intake in an adult Mediterranean population with cystic fibrosis. Br J Nutr. 2006;96(2):343–349. [DOI] [PubMed] [Google Scholar]
- 167.Declercq D, Van Biervliet S, Robberecht E. Nutrition and pancreatic enzyme intake in patients with cystic fibrosis with distal intestinal obstruction syndrome. Nutr Clin Pract. 2015;30(1): 134–137. [DOI] [PubMed] [Google Scholar]
- 168.Proesmans M, De Boeck K. Evaluation of dietary fiber intake in Belgian children with cystic fibrosis: Is there a link with gastrointestinal complaints? J Pediatr Gastroenterol Nutr. 2002;35(5): 610–614. [DOI] [PubMed] [Google Scholar]
- 169.Woestenenk JW, Schulkes DA, Schipper HS, van der Ent CK, Houwen RHJ. Dietary intake and lipid profile in children and adolescents with cystic fibrosis. J Cyst Fibros. 2017;16(3): 410–417. [DOI] [PubMed] [Google Scholar]
- 170.Ziai S, Coriati A, St-Pierre D, et al. Glucose fluctuations are not modulated by the proportion of calories from macronutrients or spontaneous total energy expenditure in adults with cystic fibrosis. Can J Diabetes. 2016;40(5):389–392. [DOI] [PubMed] [Google Scholar]
- 171.Colombo C, Bennato V, Costantini D, et al. Dietary and circulating polyunsaturated fatty acids in cystic fibrosis: Are they related to clinical outcomes? J Pediatr Gastroenterol Nutr. 2006;43(5): 660–665. [DOI] [PubMed] [Google Scholar]
- 172.Maqbool A, Schall JI, Gallagher PR, Zemel BS, Strandvik B, Stallings VA. Relation between dietary fat intake type and serum fatty acid status in children with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2012;55(5):605–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Moukarzel S, Dyer RA, Innis SM. Complex relation between diet and phospholipid fatty acids in children with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2017;64(4):598–604. [DOI] [PubMed] [Google Scholar]
- 174.van der Doef HPJ, Kokke FTM, Beek FJA, Woestenenk JW, Froeling SP, Houwen RHJ. Constipation in pediatric cystic fibrosis patients: An underestimated medical condition. J Cyst Fibros. 2010;9(1):59–63. [DOI] [PubMed] [Google Scholar]
- 175.World Health Organization. Breastfeeding. https://www.who.int/topics/breastfeeding/en/. Published 2019. Accessed September 11, 2019. [Google Scholar]
- 176.Roesch EA, Nichols DP, Chmiel JF. Inflammation in cystic fibrosis: An update. Pediatr Pulmonol. 2018;53(S3):S30–S50. [DOI] [PubMed] [Google Scholar]
- 177.Palmeira P, Carneiro-Sampaio M. Immunology of breast milk. Rev Assoc Med Bras. 2016;62(6):584–593. [DOI] [PubMed] [Google Scholar]
- 178.Lai H, Chin L, Schoff S, Zhang Z, Greer FR. Impact of breastfeeding on the nutritional status of infants with cystic fibrosis (CF) in the first year of life results of the FIRST STUDY. FASEB J. 2016;30(suppl). 672.674–672.674. [Google Scholar]
- 179.Colombo C, Costantini D, Zazzeron L, et al. Benefits of breastfeeding in cystic fibrosis: A single-centre follow-up survey. Acta Paediatr. 2007;96(8):1228–1232. [DOI] [PubMed] [Google Scholar]
- 180.Parker EM, O’Sullivan BP, Shea JC, Regan MM, Freedman SD. Survey of breast-feeding practices and outcomes in the cystic fibrosis population. Pediatr Pulmonol. 2004;37(4):362–367. [DOI] [PubMed] [Google Scholar]
- 181.Munck A, Boulkedid R, Weiss L, et al. Nutritional status the first 2 years of life in cystic fibrosis diagnosed by newborn screening. J Pediatr Gastroenterol Nutr. 2018;67(1):123–130. [DOI] [PubMed] [Google Scholar]
- 182.Leung DH, Heltshe SL, Borowitz D, et al. Effects of diagnosis by newborn screening for cystic fibrosis on weight and length in the first year of life. JAMA Pediatr. 2017;171(6):546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Jadin SA, Wu GS, Zhang Z, et al. Growth and pulmonary outcomes during the first 2 y of life of breastfed and formula-fed infants diagnosed with cystic fibrosis through the Wisconsin Routine Newborn Screening Program. Am J Clin Nutr. 2011;93(5):1038–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]


