Abstract
Background:
Chronic migraine headaches affect nearly 30 million Americans every year and are responsible for roughly 1.2 million emergency department visits annually. Many of the standard therapies commonly used to treat migraines are often unsuccessful and may furthermore introduce unwanted side effects. The purpose of this study was to identify independent predictors of clinical improvement in patients undergoing surgical nerve decompression for migraine.
Methods:
A retrospective chart review between 2010 and 2020 was conducted. The primary endpoint was clinical improvement at 1-year follow-up, defined as an independence from prescription medications. Patients were stratified into two groups: clinical improvement and treatment failure. Backward multivariable logistic regression was used to examine the associations between migraine improvement and different patient characteristics.
Results:
A total of 153 patients were included. In total, 129 (84.3%) patients improved and 24 (15.7%) did not. Significant associations with clinical improvement at multivariable logistic regression were found with acellular dermal matrix nerve wrap (OR = 10.80, 95%CI: 6.18–16.27), and operation of trigger sites four (OR = 37.96, 95%CI: 2.16–73.10) and five (OR = 159, 95%CI: 10–299).
Conclusion:
The use of acellular dermal matrix nerve wraps in surgery was significantly associated with clinical migraine improvement, as was operation at trigger sites four and five.
INTRODUCTION
Chronic migraine headaches affect nearly 30 million Americans every year and are responsible for roughly 1.2 million emergency department visits annually.1 It is well known that migraines are associated with a wide range of comorbidities, including psychiatric and gastrointestinal disorders.2,3 If left untreated, comorbid conditions can increase the severity of migraine episodes and cause a reduced quality of life.2,3 Many of the standard therapies commonly used to treat migraines are often unsuccessful and furthermore, may introduce unwanted side effects, including nervous system dysfunction, cardiovascular complications, weight gain, and cognitive impairment.4
Numerous studies in the literature support that surgical intervention is an effective method to treat migraine patients that do not respond to traditional therapies.5–15 We set out to examine the association of various patient characteristics, including the use of acellular dermal matrix (ADM) nerve wraps, trigger site, prescription and nonprescription medication use, and comorbidities with migraine improvement in patients undergoing surgical intervention. Many of these variables have been studied by Dr. Bahman Guyuron, who pioneered the surgical treatment of migraines. However, there are no studies evaluating clinical improvement rates and predictors thereof, and defining the improvement criterion as “complete independence from any prescriptive medication.” We picked this endpoint because it is a very objective means of concluding whether the patient suffers enough postsurgery to continue to take prescriptive medications. We posit that patients who have a significant improvement after surgery will be liberated from the chronic need for prescriptive medications. The aim of this study was to assess potential associations between baseline and perioperative characteristics and clinical migraine improvement following surgical decompression of various trigger points.
METHODS
Study Design, Eligibility Criteria, and Definitions
This was a retrospective cohort study on patients with migraine who underwent surgical nerve decompression by the senior author over a 10-year period (from April 2010 through April 2020). Institutional review board approval was obtained from New York Medical College. All patients were evaluated, and the diagnosis was determined based on the International Headache Society criteria by a board-certified neurologist or pain specialist. Inclusion criteria for the study were all patients who underwent nerve decompression surgery at one or more of the six clinically determined migraine trigger sites and evaluated at the minimum of 1-year follow-up following surgery. Exclusion criteria were all patients who did not have surgery and patients who underwent surgery but were not followed for at least 1 year following surgery. At 1-year follow-up, patients were stratified into one of two categories: failure of treatment and clinical improvement. Clinical improvement was defined as complete independence from prescription medications, whereas failure was defined as persisting symptoms requiring prescription medications at 1-year follow-up. All the patients included in this cohort were dependent on prescription medication for migraine or other types of headaches. This allowed us to have an objective means of following our patients over a 1-year period. Our main premise is based on the notion that if any patient does not require prescription pain medication after our surgical intervention, they are considered a success because they no longer require medical intervention for their headaches like most other people with over-the-counter medications. This allowed objective and independent measurement of success from our chart review because we could determine exactly which patients had received medications before and after surgery.
Collected Data and Definitions
Data were collected in predefined Microsoft Excel tables (Microsoft Corp, Redmond, Wash.). Collected data included demographic variables (age at surgery, sex, and general medical and surgical history), migraine course (location, triggers, medication use, length, and type of treatment before surgery), surgical details (location, use of ADM nerve wrapping, removal of superficial temporal artery, Doppler site, and time from surgery to outcome), and postoperative outcomes based on patients’ need for prescriptive medications at 1-year follow-up.
Study Interventions
All patients underwent specific decompressive surgery of their trigger sites, as described by Guyuron et al.5 After 2015, a cohort of patients underwent application of AlloDerm ADM (Allergan, Inc.) thin 2 × 4 cm wrap around the great and lesser occipital nerves at the time of surgery by placing the dermal side against the epineurium and sewing it as a tube graft along the path of neuroplasty and dissection (Fig. 1). (See Video [online], which demonstrates the surgical wrapping of the nerve with ADM.) All patients had a Jackson Pratt drain for approximately 1 week and were started on physical therapy within 1 month after surgery and followed up for a minimum of five times from the time of surgery to ascertain their progress and document their outcome.
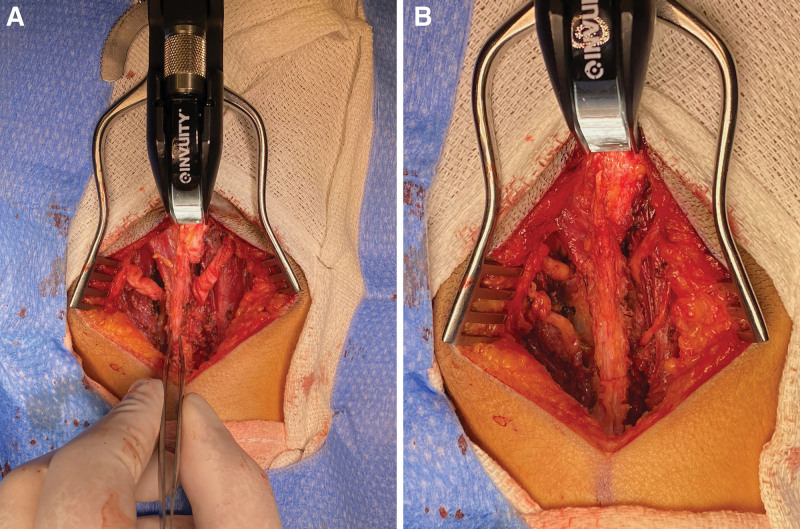
Fig. 1.
Appearance of occipital nerves before and after AlloDerm wrapping. A, Greater and lesser occipital nerve dissection. Note the hourglass appearance and the corkscrew trajectory of the diseased nerves. B, The nerves after being wrapped by AlloDerm; forceps outline the median raphe for orientation.
Video 1. Migraine Project. Video 1 from “Clinical Outcome of Nerve Decompression Surgery for Migraine Improves with Nerve Wrap.” Video 1 demonstrates the surgical wrapping of the nerve with ADM.
Statistical Analysis
Collected data were tested for normality using histograms. Continuous variables were expressed in means and SDs, whereas categorical variables were expressed in numbers, percentages, and ratios. Student t and chi square tests were used to compare continuous and categorical variables between the two groups, respectively. Univariate logistic regression was used to identify potential predictors to be used in the multivariable logistic regression. Backward logistic regression was used to evaluate associations between the primary endpoint and different patient characteristics in a multivariable model. A P value less than 0.05 was considered statistically significant. IBM SPSS (version 26; SPSS Inc., Chicago, Ill.) was utilized for statistical analysis.
RESULTS
Over 1000 adult patients who met the criteria for chronic migraine symptoms were evaluated over a 10-year period. A total of 180 patients underwent nerve decompression surgery during this period. These individuals ranged in age from 17 to 83 years, with a median age of 47. A total of 153 patients were included in the final analytic sample as 27 of the surgical patients were lost to follow-up. Of the 153 patients who underwent surgery, 129 (84.3%) were clinically improved at 1-year follow-up. Table 1 shows the patient characteristics stratified by the outcome, namely clinical improvement and treatment failure. There was no significant difference in terms of sex distribution between the two groups (Table 1). ADM nerve wraps were significantly more frequent in improved patients. The improvement group showed a significantly lower percentage of patients taking ergotamine and antidepressants. None of the other nonopioid medications showed a significant difference of distribution between the two groups. With regard to patient comorbidities, posttraumatic stress disorder (PTSD), obesity, fibromyalgia, and reoperation were significantly more frequent in the treatment failure group versus the patients who improved (Table 1).
Table 1.
Characteristics of Improved Patients Compared with the Nonimproved Ones
| Outcome, N (%) | P | |||
|---|---|---|---|---|
| Treatment Failure | Clinical Improvement | |||
| Gender: women | 15 (65.2%) | 87 (66.9%) | 0.9 | |
| Removal of superficial temporal artery | 2 (9.1%) | 8 (6.2%) | 0.6 | |
| Doppler site | 1 (4.3%) | 8 (6.2%) | 0.7 | |
| Nerve wrap ADM | 6 (26.1%) | 73 (56.2%) | 0.008* | |
| Response to injection | None | 1 (5.6%) | 1 (1.0%) | 0.2 |
| Local | 3 (16.7%) | 31 (31.0%) | ||
| Botox/Mar/Triamcinolone | 14 (77.8%) | 68 (68.0%) | ||
| Trigger | 1 | 17 (77.3%) | 97 (75.2%) | 0.8 |
| 2 | 18 (81.8%) | 96 (74.4%) | 0.4 | |
| 3 | 9 (40.9%) | 55 (42.6%) | 0.9 | |
| 4 | 15 (68.2%) | 97 (75.2%) | 0.5 | |
| 5 | 6 (27.3%) | 56 (43.4%) | 0.1 | |
| 6 | 10 (45.5%) | 68 (52.7%) | 0.5 | |
| Drug | NSAIDs | 2 (8.7%) | 11 (8.5%) | 0.9 |
| Ergotamine | 7 (30.4%) | 9 (6.9%) | 0.001* | |
| Cox 2 inhibitors | 1 (4.3%) | 17 (13.1%) | 0.3 | |
| Propranolol | 6 (26.1%) | 19 (14.6%) | 0.2 | |
| Imitrex | 3 (13.0%) | 23 (17.7%) | 0.6 | |
| SSRI | 6 (26.1%) | 27 (20.8%) | 0.6 | |
| Steroids | 2 (8.7%) | 10 (7.7%) | 0.9 | |
| Antidepressants | 5 (21.7%) | 9 (6.9%) | 0.02* | |
| Anticonvulsant | 11 (47.8%) | 57 (43.8%) | 0.7 | |
| Benzodiazepine | 3 (13.0%) | 10 (7.7%) | 0.4 | |
| Antipsychotics | 6 (26.1%) | 37 (28.5%) | 0.8 | |
| Erenmalm | 0 (0%) | 7 (5.4%) | 0.3 | |
| Comorbidities | Arthritis | 0 (0%) | 1 (0.8%) | 0. 7 |
| Depression | 5 (21.7%) | 40 (30.8%) | 0.4 | |
| Anxiety | 0 (0%) | 1 (0.8%) | 0. 7 | |
| Bipolar | 2 (8.7%) | 1 (0.8%) | 0.06 | |
| PTSD | 2 (8.7%) | 0 (0%) | 0.02* | |
| Personality disorders | 1 (4.3%) | 0 (0%) | 0.1 | |
| Hypertension | 1 (4.3%) | 15 (11.5%) | 0.5 | |
| Whiplash injury | 0 (0%) | 14 (10.8%) | 0.1 | |
| Sports injury | 0 (0%) | 5 (3.8%) | 0.4 | |
| Thyroid | 0 (0%) | 1 (0.8%) | 0.7 | |
| Budd Chiari | 1 (4.3%) | 0 (0%) | 0.1 | |
| Trauma | 3 (13.0%) | 39 (30.0%) | 0.1 | |
| Diabetes | 1 (4.3%) | 10 (7.7%) | 0.6 | |
| Obesity | 2 (8.7%) | 0 (0%) | 0.02* | |
| POTTS | 1 (4.3%) | 1 (0.8%) | 0.3 | |
| Fibromyalgia | 4 (17.4%) | 0 (0%) | 0.001* | |
| Chronic spinal pain | 1 (4.3%) | 0 (0%) | 0.1 | |
| History of cervical surgery | 0 (0%) | 1 (0.8%) | 0.7 | |
| History of skull base surgery | 0 (0%) | 1 (0.8%) | 0.7 | |
| Back pain | 0 (0%) | 3 (2.3%) | 0.5 | |
| Eye injury | 0 (0%) | 1 (0.8%) | 0.7 | |
| Seizure | 0 (0%) | 1 (0.8%) | 0.7 | |
| Intracranial bleeding | 0 (0%) | 1 (0.8%) | 0.7 | |
| Eclampsia | 0 (0%) | 2 (1.5%) | 0.5 | |
| Reoperation | 12 (52.2%) | 16 (12.3%) | <0.001* | |
Categorical variables are presented here.
NSAIDs, Nonsteroidal antiinflammatory drugs; SSRI, Selective serotonin reuptake inhibitor.
*P < 0.05.
There was no significant difference in patient age between the failure and improvement groups (Table 2). Length of treatment before surgical intervention was not significantly different. The number of trigger points that were targets for surgery was also not significantly different between the two groups. There was no difference in the frequency of trigger site operations. The amount of time from surgery to follow-up to determine the outcome was greater for the improved patients, although the difference was not statistically different. The patients in the improvement group also used a smaller maximum morphine equivalent dosage, although it was not statistically different than that in the treatment failure group (Table 2).
Table 2.
Characteristics of Improved Patients Compared with the Nonimproved Ones
| Outcome, Mean (SD) | P | ||
|---|---|---|---|
| Treatment Failure | Clinical Improvement | ||
| Age (y) | 40.70 (17.76) | 43.38 (13.50) | 0.4 |
| Treatment before surgery (y) | 4.56 (3.66) | 4.74 (3.24) | 0.8 |
| No. triggers | 3.41 (1.68) | 3.64 (1.94) | 0.6 |
| Maximum morphine equivalent (mg) | 50.91 (66.43) | 36.45 (42.59) | 0.3 |
| Surgery to outcome (mo) | 19.52 (17.31) | 26.44 (17.69) | 0.08 |
The associations of patient characteristics and migraine improvement were determined using univariable and multivariable logistic regression (Table 3). (See figure, Supplemental Digital Content 1, which shows the forest plot of log OR (95%CI) depicting the findings of multivariate logistic regression. ADM, acellular dermal matrix. http://links.lww.com/PRSGO/B824.) Patients who received ADM nerve wraps were more likely to improve, as ADM nerve wraps were significantly associated with patient improvement from migraines in final multivariable logistic regression model. The time from surgery to follow-up also showed a significant association with migraine improvement. Surgical decompressions of trigger points four or five were significantly associated with migraine improvement in the model. Surgical decompression of trigger point five had the largest odds ratio associated with migraine improvement. Surgical management of trigger points two and six both had odds ratios less than one, denoting inverse association with migraine improvement in multivariable analysis. Using ergotamine and propranolol was also significantly and inversely associated with migraine improvement in the final model. Similarly, patients with bipolar disorder or those who underwent reoperation showed a significant and inverse association with migraine improvement in the model.
Table 3.
Backward Logistic Regression Analysis to Evaluate the Associations between Migraine Improvement and Different Patient Characteristics
| Univariate Logistic Regression | Multivariable Logistic Regression | ||||
|---|---|---|---|---|---|
| OR (95% CI) | P | N = 151 | R 2 = 0.665 | ||
| OR (95% CI) | P | ||||
| Nerve wrap ADM | 3.62 (1.34, 9.79) | 0.01* | 10.80 (6.18, 16.27) | 0.01* | |
| Surgery to outcome (mo) | 1.03 (0.99, 1.07) | 0.09 | 1.10 (1.02, 1.19) | 0.01* | |
| Trigger | 2 | 0.64 (0.20, 2.04) | 0.5 | 0.01 (0.0001, 0.39) | 0.01* |
| 4 | 1.41 (0.53, 3.77) | 0.5 | 37.96 (2.16, 73.10) | 0.01* | |
| 5 | 2.04 (0.75, 5.56) | 0.1 | 159.46 (9.79, 299.56) | 0.02* | |
| 6 | 1.33 (0.54, 3.31) | 0.5 | 0.01 (0.0001, 0.79) | 0.04 | |
| Medications | Ergotamine | 0.17 (0.05, 0.51) | 0.02* | 0.03 (0.002, 0.48) | 0.01* |
| Propranolol | 0.48 (0.17, 1.38) | 0.2 | 0.02 (0.001, 0.28) | 0.004* | |
| Bipolar comorbidity | 0.08 (0.007, 0.93) | 0.04* | 0.001 (0.0001, 0.09) | 0.004* | |
| Reoperation | 0.12 (0.04, 0.34) | 0.001* | 0.007 (0.001, 0.10) | 0.001* | |
| Age (y) | 1.01 (0.98, 1.04) | 0.4 | Removed | ||
| Gender: women | 1.07 (0.42, 2.74) | 0.9 | via | ||
| Maximum morphine | 0.99 (0.98, 1.003) | 0.2 | backward | ||
| Trigger | 1 | 0.89 (0.30, 2.61) | 0.8 | elimination | |
| 3 | 1.07 (0.42, 2.69) | 0.9 | |||
| Antidepressants | 0.26 (0.08, 0.88) | 0.03* | |||
| Comorbidity: trauma | 2.85 (0.80, 10.17) | 0.1 | |||
| Fibromyalgia | 0.001 (0.01, 10.10) | 0.9 | |||
*P < 0.05.
According to multivariable model, the three main factors associated with improvement were ADM nerve wraps and surgical decompressions of trigger points four and five. In total, 92% of patients who met all three conditions, 88.5% of those who met two of the three, 82% of patients who met only one of the three, and 72% of patients who had none of the three conditions demonstrated clinical improvement.
DISCUSSION
The large number of patients who improved following surgical decompression in our study was consistent with other studies in the literature and supported the utilization of surgery as an effective treatment for chronic migraine headaches.5–15 This finding also supported that peripheral and central nerve sensitization and activation is one of the proposed pathophysiological mechanisms producing migraines.5,16,17 The significant positive association between ADM nerve wrap used during surgery and migraine improvement is intriguing. In the improved group, 56.2% of patients received ADM nerve wraps, whereas only 26.1% of patients in the failure group received ADM nerve wraps. Logistic regression model showed a significant positive association between ADM nerve wraps and migraine improvement after adjustment for several confounders. ADM is an acellular human dermis allograft that is processed from tissue-bank derived skin that is used in numerous plastic surgery procedures.18 More and more plastic surgeons have elected to use ADM grafts in reconstructive surgeries and numerous studies have reported improved outcomes.19–22 However, to our knowledge, this is the first study to explore the use of ADM nerve wraps in the context of surgical decompression of nerves in the head and neck to treat migraines.
Advances in medical technology may enable ADM to serve as a useful tool in the repair of nerves of various sizes with relatively inexpensive cost.23 ADM wraps have been used on nerves in various locations in the body, but have not been explored for nerve sheath protection in the head and neck.24–26 As seen in previous studies, the application of ADM nerve wraps has been successful in relieving pain from damaged nerves, which is most likely due to its prevention of scar adhesions developing around the nerve.18,24,25 There are also reports of surgeons performing a neurolysis without any nerve wrap with improved outcomes although they are limited due to the small number of participants.27 We were intrigued by the ability of ADM to prevent re-scarring and started applying ADM first to re-operative cases followed by adapting this technique in every primary case. Recent research has also supported that nerve wrapping is effective in treating neuropathic pain; however, this was used in the treatment of sciatic nerve compression following hamstring repair.18 Similarly, Peterson et al. demonstrated that the use of ADM allografts in patients with posttraumatic neuropathic pain at the wrist improved their pain scores.25 The likely mechanism of these wraps in preventing pain is increased padding between the nerve and surrounding tissue to diminish nerve stimulation, which is likely the case in our study as well. We placed the dermal side of the ADM against the nerve epineurium using an atraumatic technique because we did not want any adhesion between the nerve and the ADM. We used a 4 -cm segment of the ADM to make sure the entire path of nerve dissection is covered, which we believe is much better than the original proposed fat or fascial flaps limited by size and extent of soft tissue in each individual. We have documented reoperative patients who had minimal scarring of the nerve upon intraoperative examination (Fig. 2). To our knowledge, all the reconstructive cases utilizing ADMs have been covered by both government and private insurance. Our findings support the use of ADM nerve wraps in the treatment of neuropathic pain and may help improve patient outcomes in those undergoing surgical decompression for migraines.
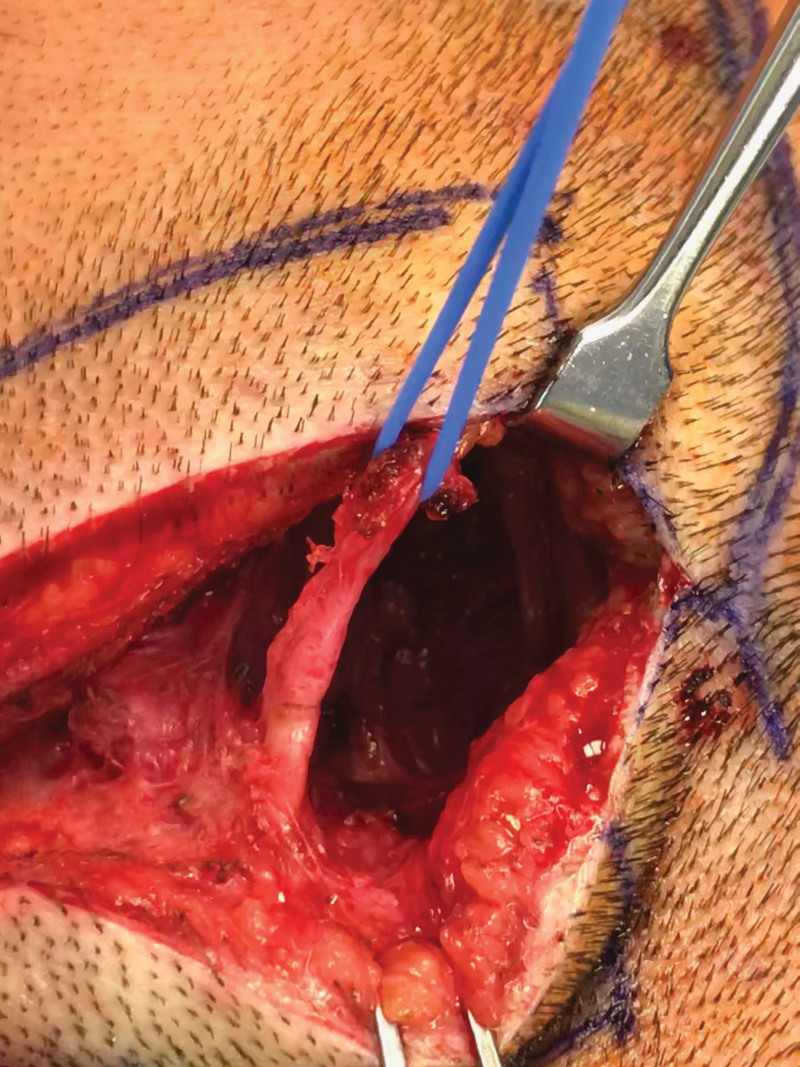
Fig. 2.
An example of reoperative exploration of the greater occipital nerve (trigger site 4) where the ADM has protected the nerve from re-scarring.
The patients who did not demonstrate symptomatic improvement and required persistent use of medications were referred back to the neurologist and pain specialist on our team to manage their symptoms. All the patients who required reoperation had not had any nerve wrap placed, and upon reoperation were noted to have re-scarring around the nerve. Since our original compilation of data in 2020, we had one patient who needed a reoperation. Upon surgical exploration of the occipital nerves, we noted that the nerve was pristine within the AlloDerm blanket but was scarred immediately distal to it. We assume that there was possible bleeding distal to the nerve wrap that could have resulted in the neuroma distally.
It has been elucidated in the literature that there are many medical conditions that are more common in patients with migraines.28 Migraine has an especially high degree association with psychiatric comorbidities, including depression, bipolar disorder, and PTSD.28 Our findings showed that PTSD was significantly more frequent in patients who failed to improve following surgery compared with the group of patients who did improve. We also found that bipolar disorder was inversely associated with migraine improvement, further supporting the current literature that psychiatric comorbidities impact migraine prognosis.29 Neuroimaging studies propose that it may be due to inappropriate discharges of shared neurotransmitter pathways and stronger functional connectivity and activation of various brain regions.29–32 A further understanding of the comorbidities and their exact mechanism connecting them to migraines can help in the treatment of patients undergoing surgical decompression.
In a recent report, Onder et al suggest that the presence of fibromyalgia as a comorbidity indicates a more severe migraine.33 A proposed mechanism of interaction is due to the complex interplay between physiological pathways, including the dopaminergic, serotonergic, and inflammatory cytokine pathways.34 This may explain why the patients in our study who were diagnosed with fibromyalgia were all classified into the treatment failure group. Other authors support this finding that comorbid fibromyalgia is associated with increased migraine disability, which may underlie the lack of improvement following surgery.35–39
Our finding that ergotamine and propranolol use inversely impacted migraine improvement is thought-provoking. Although ergotamine use is commonly associated with nausea as an unfavorable side effect, it is unlikely that this would impact patient improvement following surgery. However, it is known that persistent ergotamine use can precipitate medication-overdose headache.40,41 Medication-overdose headache is a headache disorder as classified by the International Classification of Headache Disorders, and while it typically resolves upon cessation of the causative medication, it may transform into a chronic headache disorder.40 It is possible that some of these patients were chronically using ergotamine as a therapeutic treatment before surgery, thus making them more refractory to the benefits of the surgery. Long-term propranolol use has been shown to be associated with depression. Depression negatively impacts migraine improvement.
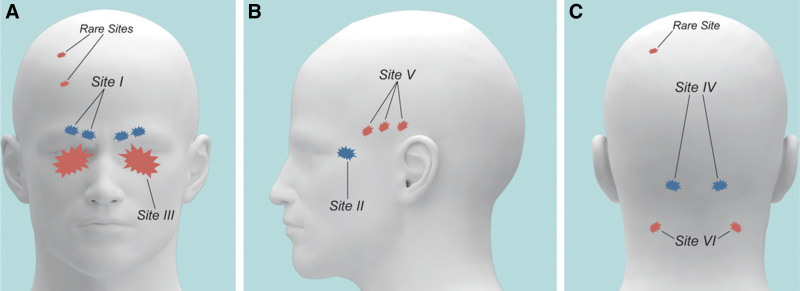
The migraine trigger site is defined as the site where the migraine headache starts.41 Identifying the migraine trigger site is crucial for successful surgery. It is possible for patients to have multiple trigger sites that are all surgically decompressed. This study included patients who had surgical decompression of one or a combination of six major trigger sites: trigger one (supraorbital and supratrochlear nerves), trigger two (zygomaticotemporal nerves), trigger three (nasal branches of the trigeminal nerve), trigger four (greater occipital or the third occipital nerves), trigger five (auriculotemporal nerve), and trigger six (lesser occipital nerve) (Fig. 3). The literature shows that there is generally a better outcome associated with a greater number of operative locations as it increases the chances of complete migraine elimination.37 Surgical decompression of trigger sites four and five showed the highest association with migraine improvement in our study. This is likely because these sites are major triggers of migraine pain, and surgical intervention resulted in large relief of pain and suggests that patients presenting with these trigger sites are good candidates for surgery. Conversely, Larson et al reported that operation of trigger sites one and two are more frequently associated with migraine improvement patients, owing to the fact that these are minor trigger sites and cause less intense pain before surgery.16 Our finding that operation at trigger site two is significantly associated with treatment failure may be due to incorrect trigger site identification preoperatively or patient interpretation of their pain starting at a high level. The relationship of migraine headache onset to location of trigger sites and response to surgery is still not fully understood.
Fig. 3.
Anatomy of common and rare migraine triggers: Site I, supraorbital and supratrochlear nerve; Site II, zygomaticotemporal nerve; Site III, nasal branches of the trigeminal nerve; Site IV, greater occipital or the third occipital nerves; Site V, auriculotemporal nerve; and Site VI, lesser occipital nerve. Reproduced with permission from Plastic and Reconstructive Surgery 2015;136(4):860–867.
STRENGTHS AND LIMITATIONS
One of the strengths of this study is that this is the first study to use prescriptive medication use as an endpoint of success. Migraine literature is often complicated by various subjective scores that are hard to validate across populations and locations. Our study allowed an objective means of seeing the impact of intervention. With the ongoing opioid epidemic, the ability of this surgical intervention to rid the patients from dependence on prescriptive pain medications is noteworthy.
This is also the first study to evaluate the impact of ADM nerve wrap following migraine surgery. Moreover, this study evaluated long-term outcome, namely clinical improvement following surgery at 1 year or longer. Another strength was the fact that all procedures were performed by one experienced surgeon, thereby minimizing the risk of learning curve bias. We would also like to acknowledge the limitations of this study. First, retrospective observational design renders the study population subject to selection bias. An additional limitation was that 15% of the initial cohort was lost to follow-up, a fact that may further contribute to selection bias. Almost all the patients reported occipital neuralgia in addition to other pain triggers, which may explain the overwhelming significant change in positive outcome associated with decompression of the greater occipital nerve trigger.
CONCLUSIONS
The use of a nerve wrap has now become a standard part of our practice and is reinforced by the findings of this study. The use of ADM nerve wraps in surgery was significantly associated with surgical success and clinical migraine improvement, as was operation on the greater occipital and auriculotemporal nerves. Ergotamine treatment preoperatively was significantly associated with treatment failure following surgery. From a socioeconomic standpoint, this longitudinal cohort study suggests that migraine surgery can improve the lives of our patients 1 year after surgery by lowering their need for prescriptive medications.
Supplementary Material
Footnotes
Published online 22 October 2021.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
Disclosure: Dr. Kaveh Alizadeh has acted as advisory consultant for Allergan Inc. All the other authors have no financial interest in relation to the content of this article.
REFERENCES
- 1.Jesani J, Simerson D. Pharmacologic management of acute migraines in the emergency department. Adv Emerg Nurs J. 2019;41:150–162. [DOI] [PubMed] [Google Scholar]
- 2.Minen MT, Begasse De Dhaem O, Kroon Van Diest A, et al. Migraine and its psychiatric comorbidities. J Neurol Neurosurg Psychiatry. 2016;87:741–749. [DOI] [PubMed] [Google Scholar]
- 3.Talafi Noghani M, Namdar H. Migraine associated with gastrointestinal disorders: a pathophysiological explanation. Med Hypotheses. 2019;125:90–93. [DOI] [PubMed] [Google Scholar]
- 4.Ha H, Gonzalez A. Migraine headache prophylaxis. Am Fam Physician. 2019;99:17–24. [PubMed] [Google Scholar]
- 5.Guyuron B, Reed D, Kriegler JS, et al. A placebo-controlled surgical trial of the treatment of migraine headaches. Plast Reconstr Surg. 2009;124:461–468. [DOI] [PubMed] [Google Scholar]
- 6.Kung TA, Guyuron B, Cederna PS. Migraine surgery: a plastic surgery solution for refractory migraine headache. Plast Reconstr Surg. 2011;127:181–189. [DOI] [PubMed] [Google Scholar]
- 7.Guyuron B, Kriegler JS, Davis J, et al. Five-year outcome of surgical treatment of migraine headaches. Plast Reconstr Surg. 2011;127:603–608. [DOI] [PubMed] [Google Scholar]
- 8.Guyuron B, Harvey D, Reed D. A prospective randomized outcomes comparison of two temple migraine trigger site deactivation techniques. Plast Reconstr Surg. 2015;136:159–165. [DOI] [PubMed] [Google Scholar]
- 9.Guyuron B, Varghai A, Michelow BJ, et al. Corrugator supercilii muscle resection and migraine headaches. Plast Reconstr Surg. 2000;106:427–429. [DOI] [PubMed] [Google Scholar]
- 10.Guyuron B, Tucker T, Davis J. Surgical treatment of migraine headaches. Plast Reconstr Surg. 2002;109:2183–2189. [DOI] [PubMed] [Google Scholar]
- 11.Guyuron B, Kriegler JS, Davis J, et al. Comprehensive surgical treatment of migraine headaches. Plast Reconstr Surg. 2005;115:1–9. [PubMed] [Google Scholar]
- 12.Janis JE, Dhanik A, Howard JH. Validation of the peripheral trigger point theory of migraine headaches: single-surgeon experience using botulinum toxin and surgical decompression. Plast Reconstr Surg. 2011;128:123–131. [DOI] [PubMed] [Google Scholar]
- 13.Dirnberger F, Becker K. Surgical treatment of migraine headaches by corrugator muscle resection. Plast Reconstr Surg. 2004;114:652–657. [DOI] [PubMed] [Google Scholar]
- 14.Bink T, Duraku LS, Ter Louw RP, et al. The cutting edge of headache surgery: a systematic review on the value of extracranial surgery in the treatment of chronic headache. Plast Reconstr Surg. 2019;144:1431–1448. [DOI] [PubMed] [Google Scholar]
- 15.Hatef DA, Gutowski KA, Culbertson GR, et al. A comprehensive review of surgical treatment of migraine surgery safety and efficacy. Plast Reconstr Surg. 2020;146:187e–195e. [DOI] [PubMed] [Google Scholar]
- 16.Larson K, Lee M, Davis J, et al. Factors contributing to migraine headache surgery failure and success. Plast Reconstr Surg. 2011;128:1069–1075. [DOI] [PubMed] [Google Scholar]
- 17.Malick A, Burstein R. Peripheral and central sensitization during migraine. Funct Neurol. 2000;15(suppl 3):28–35. [PubMed] [Google Scholar]
- 18.Haus BM, Arora D, Upton J, et al. Nerve wrapping of the sciatic nerve with acellular dermal matrix in chronic complete proximal hamstring ruptures and ischial apophyseal avulsion fractures. Orthop J Sports Med. 2016;4:2325967116638484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.John YS, Kim M. Breast reconstruction with acellular dermis. Published 2020, updated July 29, 2021. Available at emedicine.medscape.com/article/1851090-overview. Accessed June 28, 2020.
- 20.Spear SL, Parikh PM, Reisin E, et al. Acellular dermis-assisted breast reconstruction. Aesthetic Plast Surg. 2008;32:418–425. [DOI] [PubMed] [Google Scholar]
- 21.Salzberg CA. Nonexpansive immediate breast reconstruction using human acellular tissue matrix graft (AlloDerm). Ann Plast Surg. 2006;57:1–5. [DOI] [PubMed] [Google Scholar]
- 22.Breuing KH, Warren SM. Immediate bilateral breast reconstruction with implants and inferolateral AlloDerm slings. Ann Plast Surg. 2005;55:232–239. [DOI] [PubMed] [Google Scholar]
- 23.Syu WZ, Hueng DY, Chen WL, et al. Adipose-derived neural stem cells combined with acellular dermal matrix as a neural conduit enhances peripheral nerve repair. Cell Transplant. 2019;28:1220–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masear VR. Nerve wrapping. Foot Ankle Clin. 2011;16:327–337. [DOI] [PubMed] [Google Scholar]
- 25.Peterson SL, Adham MN. Acellular dermal matrix as an adjunct in treatment of neuropathic pain at the wrist. J Trauma. 2006;61:392–395. [DOI] [PubMed] [Google Scholar]
- 26.Xu J, Varitimidis SE, Fisher KJ, et al. The effect of wrapping scarred nerves with autogenous vein graft to treat recurrent chronic nerve compression. J Hand Surg Am. 2000;25:93–103. [DOI] [PubMed] [Google Scholar]
- 27.Raposio E, Simonacci F. Frontal trigger site deactivation for migraine surgical therapy. Plast Reconstr Surg Glob Open. 2020;8:e2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scher AI, Bigal ME, Lipton RB. Comorbidity of migraine. Curr Opin Neurol. 2005;18:305–310. [DOI] [PubMed] [Google Scholar]
- 29.Noseda R, Kainz V, Borsook D, et al. Neurochemical pathways that converge on thalamic trigeminovascular neurons: potential substrate for modulation of migraine by sleep, food intake, stress and anxiety. PLoS One. 2014;9:e103929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwedt TJ, Chong CD, Chiang CC, et al. Enhanced pain-induced activity of pain-processing regions in a case-control study of episodic migraine. Cephalalgia. 2014;34:947–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lipchik GL, Penzien DB. Psychiatric comorbidities in patients with headache. Semin Pain Med. 2004;2:93–105. [Google Scholar]
- 32.Lipton RB, Silberstein SD, Saper JR, et al. Why headache treatment fails. Neurology. 2003;60:1064–1070. [DOI] [PubMed] [Google Scholar]
- 33.Onder H, Hamamci M, Alpua M, et al. Comorbid fibromyalgia in migraine patients: clinical significance and impact on daily life. Neurol Res. 2019;41:909–915. [DOI] [PubMed] [Google Scholar]
- 34.Singh L, Kaur A, Bhatti MS, et al. Possible molecular mediators involved and mechanistic insight into fibromyalgia and associated co-morbidities. Neurochem Res. 2019;44:1517–1532. [DOI] [PubMed] [Google Scholar]
- 35.Küçükşen S, Genç E, Yilmaz H, et al. The prevalence of fibromyalgia and its relation with headache characteristics in episodic migraine. Clin Rheumatol. 2013;32:983–990. [DOI] [PubMed] [Google Scholar]
- 36.Whealy M, Nanda S, Vincent A, et al. Fibromyalgia in migraine: a retrospective cohort study. J Headache Pain. 2018;19:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bond DS, Roth J, Nash JM, et al. Migraine and obesity: epidemiology, possible mechanisms and the potential role of weight loss treatment. Obes Rev. 2011;12:e362–e371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Couch JR, Lipton RB, Stewart WF, et al. Head or neck injury increases the risk of chronic daily headache: a population-based study. Neurology. 2007;69:1169–1177. [DOI] [PubMed] [Google Scholar]
- 39.Weiss HD, Stern BJ, Goldberg J. Post-traumatic migraine: chronic migraine precipitated by minor head or neck trauma. Headache. 1991;31:451–456. [DOI] [PubMed] [Google Scholar]
- 40.Tepper SJ. Acute treatment of migraine. Neurol Clin. 2019;37:727–742. [DOI] [PubMed] [Google Scholar]
- 41.Seyed Forootan NS, Lee M, Guyuron B. Migraine headache trigger site prevalence analysis of 2590 sites in 1010 patients. J Plast Reconstr Aesthet Surg. 2017;70:152–158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.