Abstract
Background:
Computed tomography (CT) is the current gold standard for the detection of pulmonary nodules but has high radiation burden. In contrast, many radiologists tried to use magnetic resonance imaging (MRI) to replace CT because MRI has no radiation burden associated. Due to the lack of high-level evidence of comparison of the diagnostic accuracy of MRI versus CT for detecting pulmonary nodules, it is unknown whether CT can be replaced successfully by MRI. Therefore, the aim of this study was to compare the diagnostic accuracy of MRI versus CT for detecting pulmonary nodules.
Methods:
Electronic databases PubMed, EmBase, and Cochrane Library were systematically searched from their inception to September 2017 to identify studies in which CT/MRI was used to diagnose pulmonary nodules. According to true positive, true negative, false negative, and false positive extracted from the included studies, we calculate the pooled sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and area under the curve (AUC) using Stata version 14.0 software (STATA Corp, TX).
Results:
A total of 8 studies involving a total of 653 individuals were included. The pooled sensitivity, specificity, PLR, NLR, and AUC were 0.91 (95% confidence interval [CI]: 0.80–0.96), 0.76 (95%CI: 0.58–0.87), 3.72 (95%CI: 2.05–6.76), 0.12 (95%CI: 0.06–0.27), and 0.91 (95%CI: 0.88–0.93) for MRI respectively, while the pooled sensitivity, specificity, PLR, NLR, and AUC for CT were 1.00 (95%CI: 0.95–1.00), 0.99 (95%CI: 0.78–1.00), 79.35 (95%CI: 3.68–1711.06), 0.00 (95%CI: 0.00–0.06), and 1.00 (95%CI: 0.99–1.00), respectively. Further, we compared the diagnostic accuracy of CT versus MRI and found that compared with MRI, CT shows statistically higher sensitivity (odds ratio [OR] for MRI vs CT: 0.91; 95%CI: 0.85–0.98; P value .010), specificity (OR: 0.82; 95%CI: 0.69–0.97; P value .019), PLR (OR: 0.29; 95%CI: 0.10–0.83; P value 0.02), AUC (OR: 0.91; 95%CI: 0.89–0.94; P value < .001), and lower NLR (OR: 8.72; 95%CI: 1.57–48.56; P value .013).
Conclusion:
Our study suggested both CT and MRI have a high diagnostic accuracy in diagnosing pulmonary nodules, while CT was superior to MRI in sensitivity, specificity, PLR, NLR, and AUC, indicating that in terms of the currently available evidence, MRI could not replace CT in diagnosing pulmonary nodules.
Keywords: computed tomography, magnetic resonance imaging, meta-analysis, pulmonary nodules
1. Introduction
Pulmonary nodules are a common disease including viral pulmonary nodule,[32] bacterial pulmonary nodule, and malignant pulmonary nodule, which can result in several complications involving pulmonary fibrosis and pulmonary hypertension.[33] A solitary pulmonary nodule is widely encountered in multi detector computed tomography (CT),[1] and the screening of a high-risk peoples with low-dose CT was associated with 20% reduction of lung cancer mortality.[2] Nevertheless, although frequent use of CT in screening and follow-up to observe the growth rate of pulmonary nodules is acceptable,[3,4] it was associated with considerable cumulative radiation exposure, which could stimulate the progression of cancer even most of individuals were diagnosis with benign pulmonary disease.[5–7] Therefore, it is necessary to find additional alternative technique without radiation exposure for detecting pulmonary nodules.
Pulmonary magnetic resonance imaging (MRI) has been introduced and increased using in parenchymal evaluation, including bronchopulmonary dysplasia, cystic fibrosis, cardio-pulmonary vascular abnormalities, and intra-thoracic tumors, and without using of ionizing radiation.[8,9] The value of MRI in patients with pulmonary nodules have already illustrated in several meta-analyses.[10–12] Jiang et al[10] pooled 12 studies with 524 malignant and 284 benign nodules and indicated the diagnosis parameters for discrimination of benign from malignant pulmonary nodules were relative higher (sensitivity: 0.95; specificity: 0.87; positive likelihood ratio [PLR]: 7.60; negative likelihood ratio [NLR]: 0.06; and area under receiver operating characteristic [ROC] curves: 0.94), while this study could not provide the direct comparison with the diagnostic value of CT. Similar limitations are detected in the study conducted by Li et al,[11] which just provide the diagnosis parameters for MRI detection of malignant pulmonary nodules and masses. Further, these studies main focused on discriminating benign and malignant pulmonary nodules and not studied the detecting accuracy rate for pulmonary nodules. Cronin et al[12] evaluate the diagnostic value of CT, MRI, positron emission tomography, and single photon emission CT for the evaluation of solitary pulmonary nodule, while the comparisons of the diagnostic value directly were not calculated and provided relative synthetic results. Clarifying the diagnostic value of alternative technique namely MRI is particularly important for detecting pulmonary nodules, as it has not been definitively determined. Therefore, we attempted examination of the available studies to compare the diagnostic value between MRI and CT for detecting pulmonary nodules directly. The results of our study will provide evidence-based summaries for whether CT can be replaced successfully by MRI for detection of pulmonary nodules, which may assist the clinicians in making decisions about the selection of diagnostic methods.
2. Methods
2.1. Data sources, search strategy, and selection criteria
The Research Ethics Committee of Central South University Xiangya School of Medicine Affiliated Haikou Hospital approved this study. This review was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Statement issued in 2009.[13] The study compared the diagnostic value between MRI and CT for detecting pulmonary nodules were eligible for inclusion in this study, and no restriction was placed on publication status (published, in press, or in progress). Further, we restricted the study published in English. We searched the PubMed, EmBase, and Cochrane Library electronic databases for articles published with inception to September 2017 and used ((“nodules” or “nodule”) AND (“lung” or “pulmonary”)) AND ((“computed tomography” or “CT”) and (“Magnetic Resonance Imaging” or “MRI”)) AND “human” AND “English” as the search terms. Manual searches of reference lists from relevant studies were performed to identify any potential eligible studies. The study topic, diagnostic tool, control, patient's status, and the outcomes reported were employed to select potential relevant studies.
The literature search was independently undertaken by 2 authors using a standardized approach. Any inconsistencies between these 2 authors were settled by the primary author until a consensus was reached. The study was eligible for inclusion if the following criteria were met: patients with pulmonary lesions or with high risk of pulmonary nodules; patients with MRI and CT for detecting pulmonary nodules; and the study provided true positive, false positive, false negative, true negative for MRI, and CT diagnostic results.
2.2. Data collection and quality assessment
The data collected included the first author's name, publication year, country, sample size, mean age, and number of men and women, true and false positive and negative for MRI and CT, respectively. The Quality Assessment of Diagnostic Accuracy Studies (QUADAS), which is quite comprehensive and has been partially validated for evaluating the quality of studies in diagnosis meta-analysis, was used to evaluate methodological quality.[14,15] The QUADAS is based on the following sub-scales: representative patient spectrum, reporting of selection criteria, reference standard, absence of disease progression bias, absence of partial verification bias, absence of differential verification bias, absence of incorporation bias, description of index text execution, description of reference standard execution, reference standard blinded, index test blinded, absence of clinical review bias, reporting of uninterpretable/intermediate results, and withdrawal. Each of sub-scale was regarded as “yes”, “no”, or “NA”. The data extraction and quality assessment were conducted independently by 2 authors. Information was examined and adjudicated independently by an additional author referring to the original studies.
2.3. Statistical analysis
We calculated the summary sensitivity, specificity, PLR, NLR, and corresponding 95% confidence intervals (CIs) by bivariate random effects for MRI and CT respectively on the basis of true positive, false positive, false negative, and true negative in each study.[16] Further, the summary receiver operating characteristic curve and the area under the curve (AUC) for MRI and CT respectively by using the hierarchical regression model.[17] In addition, the diagnosis parameters with corresponding 95%CI were abstracted for MRI and CT in each study, and the summary ratio between MRI and CT and 95% CIs for were sensitivity, specificity, PLR, NLR by using the random-effects model.[18]
Heterogeneity between studies was investigated by using the Q statistic, and we considered P values < .10 as indicative of significant heterogeneity.[19,20] Subgroup analyses were conducted for sensitivity, specificity, PLR, NLR on the basis of publication year, country, and mean age. Deeks asymmetry test for MRI and CT were calculated and presented as funnel plots.[21] All reported P values are 2-sided, and P values < .05 were considered statistically significant for all included studies. Statistical analyses were performed using STATA software (version 10.0; Stata Corporation, College Station, TX).
3. Results
The results of study-selection process are shown in Figure 1. We identified 631 articles in our initial electronic search, of which 589 were identified after duplicates and irrelevant studies were excluded. A total of 42 potentially eligible studies were selected. After detailed evaluations, 8 prospective studies with a total of 2628 individuals were selected for the final meta-analysis.[22–29] A manual search of the reference lists of these studies did not yield any new eligible studies. The general characteristics of the included studies are presented in Table 1. The assessment outcome of the each study by using QUADAS are listed in Table 2.
Figure 1.
Flow diagram of the literature search and trials selection process.
Table 1.
Characteristics of the studies included in the meta-analysis.
| Magnetic resonance imaging | Computed tomography | ||||||||||||
| Author | Year | Country | N | Mean age | Men/women | True positive | False positive | True negative | False negative | True positive | False positive | True negative | False negative |
| Schaefer et al[22] | 2006 | Germany | 46 | 61.0 | 36/10 | 21 | 13 | 9 | 3 | 23 | 13 | 9 | 1 |
| Regier et al[23] | 2011 | Germany | 20 | 66.4 | 10/10 | 24 | 1 | 12 | 3 | 27 | 0 | 13 | 0 |
| Kim et al[24] | 2004 | Korea | 81 | 50.0 | 54/27 | 8 | 5 | 10 | 0 | 7 | 6 | 9 | 1 |
| Schroeder et al[25] | 2005 | Germany | 30 | 53.3 | 19/11 | 102 | 3 | 43 | 2 | 102 | 2 | 43 | 3 |
| Dewes et al[26] | 2016 | Germany | 54 | 60.8 | 27/27 | 121 | 3 | 14 | 16 | 137 | 0 | 14 | 0 |
| Sommer et al[27] | 2014 | Germany | 49 | 61.0 | 31/18 | 26 | 4 | 29 | 28 | 54 | 0 | 33 | 0 |
| Ohno et al[28] | 2015 | Japan | 198 | 75.4 | 111/87 | 123 | 25 | 60 | 10 | 133 | 0 | 85 | 0 |
| Ohno et al[29] | 2008 | Japan | 175 | 72.1 | 92/83 | 142 | 29 | 21 | 10 | 152 | 0 | 50 | 0 |
Table 2.
Quality evaluation of the included studies using the QUADAS tool.
| Question about study design characteristic | ||||||||||||||
| Study | Representative patient spectrum | Reporting of selection criteria | Reference standard | Absence of disease progression bias | Absence of partial verification bias | Absence of differential verification bias | Absence of incorporation bias | Description of index text execution | Description of reference standard execution | Reference standard blinded | Index test blinded | Absence of clinical review bias | Reporting of uninterpretable/intermediate results | Withdrawal |
| Schaefer | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes |
| Regier | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | NA | Yes |
| Kim | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | NA | Yes |
| Schroeder | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | NA | Yes |
| Dewes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes |
| Sommer | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | NA | Yes |
| Ohno | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | NA | Yes |
| Ohno | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | NA | Yes |
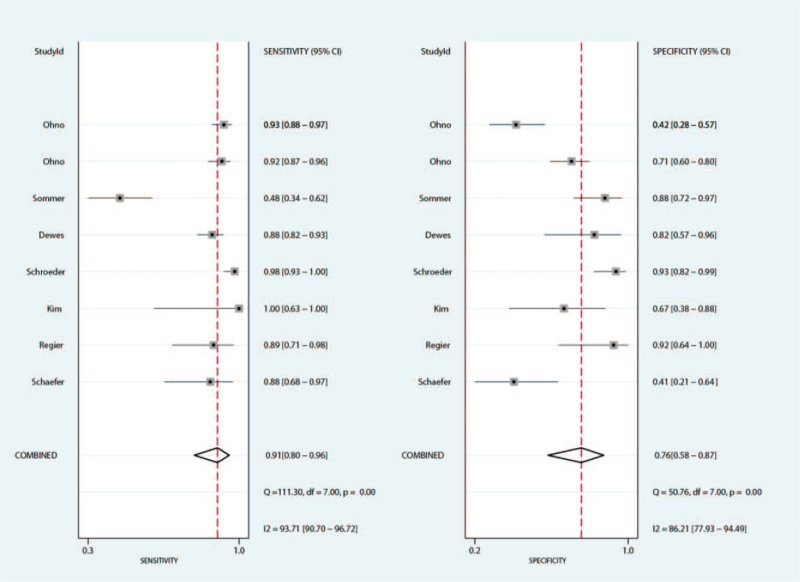
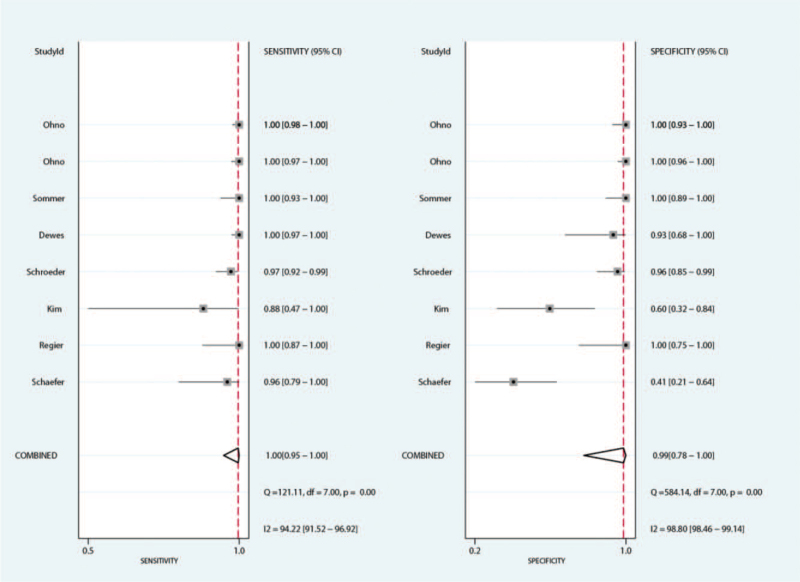
The summary sensitivity for MRI and CT for detecting pulmonary nodules were 0.91 (95%CI: 0.80–0.96) and 1.00 (95%CI: 0.95–1.00), respectively. We noted MRI with lower sensitivity for detecting pulmonary nodules when compared with CT (ratio between MRI and CT: 0.91; 95%CI: 0.85–0.98; P = .010; Fig. 2). Subgroup analyses suggested there was no significant difference for sensitivity between MRI and CT if the study published before 2010, or mean age of included patients <65.0 years (Table 3).
Figure 2.
The summary results for sensitivity between MRI and CT. CT = computed tomography, MRI = magnetic resonance imaging.
Table 3.
Subgroup analyses for diagnostic accuracy comparison of MRI versus CT by publication year, country, and mean age.
| Outcomes | Group | Gender | Odds ratio between MRI and CT and 95%CI | P value | I2 (%) | P value for heterogeneity |
| Sensitivity | Publication year | 2010 or after | 0.84 (0.75–0.95) | .007 | 83.0 | .001 |
| Before 2010 | 0.97 (0.92–1.03) | .313 | 35.8 | .197 | ||
| Country | Europe | 0.86 (0.76–0.99) | .033 | 86.2 | <.001 | |
| Asia | 0.94 (0.90–0.97) | <.001 | 0.0 | .685 | ||
| Mean age | ≥65.0 | 0.93 (0.90–0.97) | <.001 | 0.0 | .875 | |
| <65.0 | 0.87 (0.75–1.01) | .073 | 86.3 | <.001 | ||
| Specificity | Publication year | 2010 or after | 0.83 (0.72–0.95) | .007 | 43.3 | .152 |
| Before 2010 | 0.80 (0.48–1.31) | .369 | 84.2 | <.001 | ||
| Country | Europe | 0.93 (0.86–1.02) | .122 | 0.0 | .928 | |
| Asia | 0.66 (0.43–1.03) | .065 | 79.0 | .009 | ||
| Mean age | ≥65.0 | 0.67 (0.47–0.95) | .026 | 83.3 | .003 | |
| <65.0 | 0.94 (0.86–1.03) | .166 | 0.0 | .883 | ||
| PLR | Publication year | 2010 or after | 0.12 (0.02–0.65) | .014 | 45.1 | .141 |
| Before 2010 | 0.57 (0.19–1.70) | .310 | 70.7 | .017 | ||
| Country | Europe | 0.78 (0.49–1.26) | .311 | 0.0 | .424 | |
| Asia | 0.08 (0.00–2.31) | .143 | 89.2 | <.001 | ||
| Mean age | ≥65.0 | 0.04 (0.01–0.23) | <.001 | 29.7 | .241 | |
| <65.0 | 0.85 (0.51–1.40) | .512 | 9.6 | .352 | ||
| NLR | Publication year | 2010 or after | 30.84 (7.69–123.61) | <.001 | 24.2 | .266 |
| Before 2010 | 1.90 (0.28–13.03) | .512 | 55.2 | .082 | ||
| Country | Europe | 7.77 (1.02–59.17) | .048 | 79.8 | .001 | |
| Asia | 14.44 (0.20–1019.62) | .219 | 73.0 | .025 | ||
| Mean age | ≥65.0 | 22.21 (2.39–206.20) | .006 | 27.3 | .253 | |
| <65.0 | 4.86 (0.46–51.72) | .190 | 82.9 | <.001 |
The summary specificity for MRI and CT for detecting pulmonary nodules were 0.76 (95%CI: 0.58–0.87) and 0.99 (95%CI: 0.78–1.00), respectively. The summary results suggested MRI with lower specificity for detecting pulmonary nodules when compared with CT specificity (ratio between MRI and CT: 0.82; 95%CI: 0.69–0.97; P = .019; Fig. 3). This significantly difference was observed in the study published in 2010 or after, and the mean age of included patients ≥65.0 years (Table 3).
Figure 3.
The summary results for specificity between MRI and CT. CT = computed tomography, MRI = magnetic resonance imaging.
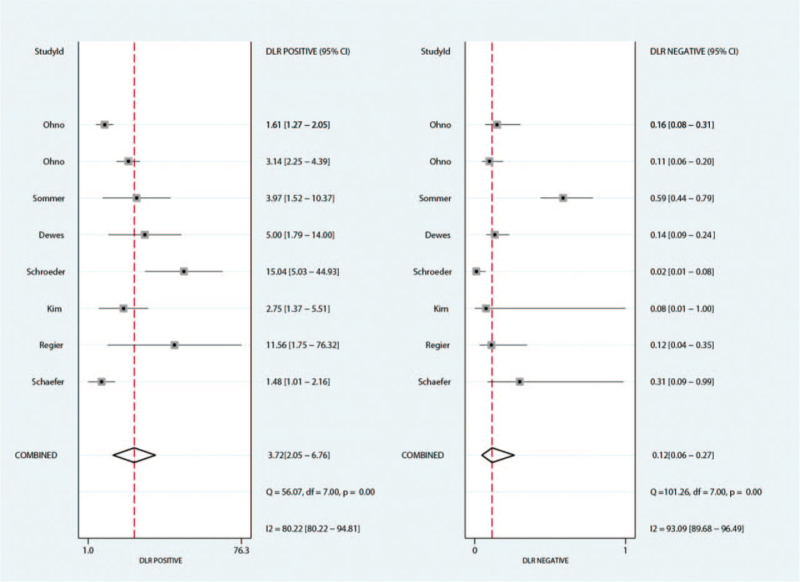
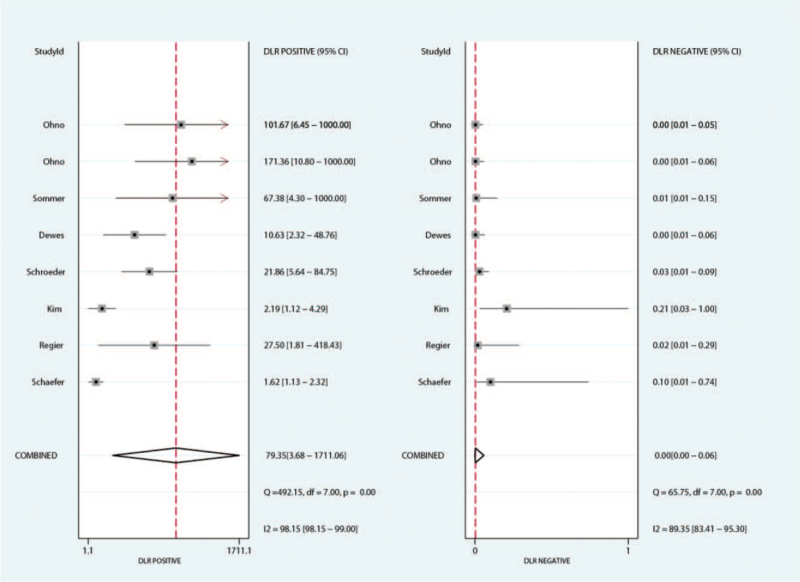
The summary PLR for MRI and CT for detecting pulmonary nodules were 3.72 (95%CI: 2.05–6.76) and 79.35 (95%CI: 3.68–1711.06), respectively. We noted MRI was associated with lower PLR for detecting pulmonary nodules (ratio between MRI and CT: 0.29; 95%CI: 0.10–0.83; P = .020; Fig. 4). The findings of subgroup analysis found the significant difference between MRI and CT for PLR if the study published in 2010 or after, and the mean age of included patients ≥65.0 years (Table 3).
Figure 4.
The summary results for PLR between MRI and CT. CT = computed tomography, MRI = magnetic resonance imaging, PLR = positive likelihood ratio.
The summary NLR for MRI and CT for detecting pulmonary nodules were 0.12 (95%CI: 0.06–0.27) and 0.00 (95%CI: 0.00–0.06), respectively. The summary results indicated MRI with higher NLR for detecting pulmonary nodules when compared with CT (ratio between MRI and CT: 0.29; 95%CI: 0.10–0.83; P = .020). Subgroup analysis suggested the significant difference between MRI and CT was prominent for NLR in study published in 2010 or after, the study conducted in Europe, and the mean age of included patients ≥65.0 years (Table 3).
The summary area under ROC for MRI and CT for detecting pulmonary nodules were 0.91 (95%CI: 0.88–0.93) and 1.00 (95%CI: 0.99–1.00), respectively. We noted MRI was associated with lower diagnostic value for detecting pulmonary nodules as compared with CT (ratio between MRI and CT: 0.91; 95%CI: 0.89–0.94; P < .001).
Review of the funnel plots could not rule out the potential for publication bias for MRI and CT for detecting pulmonary nodules. The Deeks asymmetry test results showed no evidence of publication bias for MRI (P = .84), and potential evidence of publication bias for CT (P = .02).
4. Discussion
Our current study compared the diagnostic value of MRI and CT for detecting pulmonary nodules. This comprehensive quantitative study included 653 individuals from 8 published studies with a broad range of populations. The findings from our current meta-analysis indicated MRI has relative higher diagnostic value for detecting pulmonary nodules with in terms of higher sensitivity, specificity, PLR, AUC, and lower NLR. However, when compared with the diagnostic value of CT for detecting pulmonary nodules, MRI was associated with lower sensitivity, specificity, PLR, AUC, and higher NLR, which indicate in terms of the currently available evidence, MRI could not replace CT in diagnosing pulmonary nodules. Next, we performed a subgroup analysis for diagnostic accuracy comparison of MRI versus CT by publication year, country, and mean age, and MRI has even poorer diagnostic accuracy than CT regardless of publication year, country, and mean age. The probable reasons of lower diagnostic accuracy are as follows: on the one hand, the lung is composed primarily of trachea, bronchus, alveolus pulmonis, blood vessels, and lymphatic vessels, and alveolus pulmonis which harbor a significant amount of air is the major component of lung, which indicate low diagnostic accuracy of MRI because MRI is a functional imaging technique based on water diffusivity. On the other hand, there is a sharp density difference between normal tissue and lung lesion which can be accurately detected by CT.
A previous meta-analysis suggested that patients with dynamic contrast-enhanced MRI is valuable for distinguish benign or malignant pulmonary nodules, and especially for discrimination of malignant pulmonary nodules, while the study just reported the summary diagnosis parameters for MRI, and the comparison of MRI versus CT were not available. Further, it is unknown whether the diagnostic value is different in specific subpopulation patients.[10] Li et al[11] conducted a meta-analysis based on 17 studies involving 855 malignant and 322 benign lesions. They indicated MRI is valuable for differentiating malignant from benign pulmonary nodules or masses, and the summary results of diagnostic performance in retrospectively designed studies was higher than prospectively designed studies. The study did not compare MRI and CT for detecting pulmonary nodules directly. Cronin et al[12] reported the summary results of diagnostic performance in CT, MRI, positron emission tomography, and single photon emission CT for detecting solitary pulmonary nodules, respectively. However, the summary results was based on various multiple studies including the study evaluate single diagnostic tool for detecting pulmonary nodules, and the direct comparison of diagnostic performance between MRI and CT for detecting pulmonary nodules were not calculated. Further, subgroup analyses for diagnostic performance based on study or participants characteristics were not evaluated. Therefore, we conducted a meta-analysis to directly compare the diagnostic performance between MRI and CT for detecting pulmonary nodules.
The findings of this study suggested MRI was associated with lower diagnostic performance for detecting pulmonary nodules when compared with CT, whereas numerous studies did not provide the comparison of MRI and CT diagnostic performance. Schaefer et al[22] reported the mean sensitivity, specificity, and accuracy of observers for MRI were 89%, 42%, and 66%, respectively. They concluded no significant difference of accuracy between MRI and CT. The reason for this could be the optimized signal gain using a proton density weighted GE with a short echo time and a low flip angle. Further, the study used ECG-triggering might contribute an important reason for this high accuracy of MRI. Regier et al[23] suggested patients with diffusion-weighted imaging MRI for detection of nodules ≥6 mm with reasonably high sensitivity rates, while the results of false positive decreases the accuracy of MRI as compared with CT. Kim et al[24] reported similar diagnostic performance for differentiation between benign and malignant solitary pulmonary nodule. The reason for this could be different threshold values were correlated with different MRI techniques, which include pulse sequence and imaging acquisition time. Schroeder et al[25] suggested the sensitivity of HASTE MRI was 73% for lesions less than 3 mm, 86.3% for lesions between 3 and 5 mm, 95.7% for lesions between 6 and 10 mm, and 100% for lesions greater than 10 mm. They concluded HASTE MRI could be employed screen pulmonary lesions that are 5 mm and bigger, and the lesions smaller than 5 mm needed to be validated by CT. Dewes et al[26] reported similar findings as compared with the study conducted by Schroeder et al,[25] which used non-contrast Controlled Aliasing In Parallel Imaging Results In Higher Acceleration volumetric interpolated breath-hold examination 3T MRI. Sommer et al[27] suggested MRI has greater valuable for detecting malignant nodules than to benign ones, and should employ to detect early stage lung cancer. The reason for this is due to inherent soft tissue contrast of MRI. The findings of Ohno et al[28,29] reported similar findings contrast with the current meta-analysis, and suggested CT is more sensitive than MRI for detecting solitary pulmonary nodules in routine clinical practice. The possible reason for this could be that time-density and time-signal intensity course curves in wish-in phase were associated with the combination of perfusion (blood flow per unit of tissue), microvascular density (tumor angiogenesis), extracellular space for accumulation of contrast material, and permeability of capillaries, which might contribute an important role for the diagnosis performance of MRI.[30,31]
Subgroup analysis suggested publication year, country, and mean age might affect the diagnostic performance between MRI and CT for detecting pulmonary nodules. The possible reason for this could be that the diagnostic techniques are different across the study published years. Further, the diagnostic techniques in Europe and Asia might differ and affect the diagnostic performance for detecting pulmonary nodules. In addition, the mean age of patients might affect the progression of pulmonary nodules, which associated with different size and morphologic features. Finally, although diagnostic performance is different according to published year, country, and mean age of included patients, while these conclusions may be variable since smaller studies were included in such subset. Therefore, we just gave a relative result and provided a synthetic and comprehensive review.
5. Conclusion
In conclusion, the results of this meta-analysis indicated MRI has lower diagnostic performance for detecting pulmonary nodules when compared with CT. Further, according to the subgroup analysis results for diagnostic accuracy comparison of MRI versus CT by publication year, country, and mean age, MRI has even poorer diagnostic accuracy than CT regardless of publication year, country, and mean age. Therefore, our studies indicate in terms of the currently available evidence, MRI could not replace CT in diagnosing pulmonary nodules because of poorer diagnostic accuracy.
5.1. Limitations
The limitations of our study are as follows: the diagnostic performance based on size of pulmonary nodules of MRI versus CT were not calculated because most of the included studies did not report the diagnostic performance based on size of pulmonary nodules for MRI or CT; different diagnostic techniques in MRI and CT might affect the diagnostic accuracy for detecting pulmonary nodules; in a meta-analysis of published studies, publication bias is an inevitable problem; the meta-analysis is based on study level results but not original data of an individual patient, which restricted us to present a more comprehensive result. Therefore, more future studies are required to prove our conclusions.
Author contributions
Hui Liu and Xianwen Liang conceived and designed the study. All authors were responsible for data collection, analysis, interpretation, and generation of figures, and Hui Liu, Rihui Chen, and Chao Tong were involved in writing of the paper. Xianwen Liang took part in paper checking and modification, and gave final approval of the manuscript. All authors contributed to the article and approved the submitted version.
Formal analysis: Rihui Chen, Chao Tong.
Methodology: Chao Tong, Xianwen Liang.
Supervision: Xianwen Liang.
Validation: Xianwen Liang.
Writing – original draft: Hui Liu.
Footnotes
Abbreviations: AUC = area under the curve, CI = confidence interval, CT = computed tomography, MRI = magnetic resonance imaging, NLR = negative likelihood ratio, PLR = positive likelihood ratio, OR = odds ratio, QUADAS = Quality Assessment of Diagnostic Accuracy Studies.
How to cite this article: Liu H, Chen R, Tong C, Liang XW. MRI versus CT for the detection of pulmonary nodules: a meta-analysis. Medicine. 2021;100:42(e27270).
The authors have no conflicts of interest to disclose.
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
QUADAS = Quality Assessment of Diagnostic Accuracy Studies.
CI = confidence interval, CT = computed tomography, MRI = magnetic resonance imaging, NLR = negative likelihood ratio, PLR = positive likelihood ratio.
References
- [1].Eibel R, Turk TR, Kulinna C, Herrmann K, Reiser MF. Multidetector-row CT of the lungs: multiplanar reconstructions and maximum intensity projections for the detection of pulmonary nodules. Rofo 2001;173:815–21. [DOI] [PubMed] [Google Scholar]
- [2].National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Henschke CI. Early Lung Cancer Action Project: overall design and findings from baseline screening. Cancer 2000;89:2474–82. [DOI] [PubMed] [Google Scholar]
- [4].Yankelevitz DF, Reeves AP, Kostis WJ, Zhao B, Henschke CI. Small pulmonary nodules: volumetrically determined growth rates based on CT evaluation. Radiology 2000;17:251–6. [DOI] [PubMed] [Google Scholar]
- [5].Bach PB, Mirkin JN, Oliver TK, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA 2012;307:2418–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sodickson A, Baeyens PF, Andriole KP, et al. Recurrent CT, cumulative radiation exposure, and associated radiation-induced cancer risks from CT of adults. Radiology 2009;251:175–84. [DOI] [PubMed] [Google Scholar]
- [7].Diederich S, Lenzen H, Windmann R, et al. Pulmonary nodules: experimental and clinical studies at low-dose CT. Radiology 1999;213:289–98. [DOI] [PubMed] [Google Scholar]
- [8].Wielpütz M, Kauczor HU. MRI of the lung: state of the art. Diagn Interv Radiol 2012;18:344–53. [DOI] [PubMed] [Google Scholar]
- [9].Biederer J, Beer M, Hirsch W, et al. MRI of the lung (2/3). Why… when… how? Insights Imaging 2012;3:355–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jiang B, Liu H, Zhou D. Diagnostic and clinical utility of dynamic contrast- enhanced MR imaging in indeterminate pulmonary nodules: a metaanalysis. Clin Imaging 2016;40:1219–25. [DOI] [PubMed] [Google Scholar]
- [11].Li B, Li Q, Chen C, Guan Y, Liu S. A systematic review and meta-analysis of the accuracy of diffusion-weighted MRI in the detection of malignant pulmonary nodules and masses. Acad Radiol 2014;21:21–9. [DOI] [PubMed] [Google Scholar]
- [12].Cronin P, Dwamena BA, Kelly AM, Bernstein SJ, Carlos RC. Solitary pulmonary nodules and masses: a meta-analysis of the diagnostic utility of alternative imaging tests. Eur Radiol 2008;18:1840–56. [DOI] [PubMed] [Google Scholar]
- [13].Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Whiting P, Rutjes AW, Reitsma JB, Bossuyt PMM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Whiting PF, Weswood ME, Rutjes AW, Reitsma JB, Bossuyt PNM, Kleijnen J. Evaluation of QUADAS, a tool for the quality assessment of diagnostic accuracy studies. BMC Med Res Methodol 2006;6:09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [17].Walter SD. Properties of the summary receiver operating characteristic (SROC) curve for diagnostic test data. Stat Med 2002;9:1237–56. [DOI] [PubMed] [Google Scholar]
- [18].Ades AE, Lu G, Higgins JP. The interpretation of random-effects metaanalysis in decision models. Med Decis Making 2005;25:646–54. [DOI] [PubMed] [Google Scholar]
- [19].Deeks JJ, Higgins JPT, Altman DG. Higgins J, Green S. Analyzing data and undertaking meta-analyses. Cochrane Handbook for Systematic Reviews of Interventions 5.0.1. Oxford, UK: The Cochrane Collaboration; 2008;chap 9. [Google Scholar]
- [20].Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 2005;58:882–93. [DOI] [PubMed] [Google Scholar]
- [22].Schaefer JF, Vollmar J, Wiskirchen J, et al. Differentiation between malignant and benign solitary pulmonary nodules with proton density weighted and ECG-gated magnetic resonance imaging. Eur J Med Res 2006;11:527–33. [PubMed] [Google Scholar]
- [23].Regier M, Schwarz D, Henes FO, et al. Diffusion-weighted MR-imaging for the detection of pulmonary nodules at 1.5 Tesla: intraindividual comparison with multidetector computed tomography. J Med Imaging Radiat Oncol 2011;55:266–74. [DOI] [PubMed] [Google Scholar]
- [24].Kim JH, Kim HJ, Lee KH, Kim KH, Lee HL. Solitary pulmonary nodules: a comparative study evaluated with contrast-enhanced dynamic MR imaging and CT. J Comput Assist Tomogr 2004;28:766–75. [DOI] [PubMed] [Google Scholar]
- [25].Schroeder T, Ruehm SG, Debatin JF, Ladd ME, Barkhausen J, Goehde SC. Detection of pulmonary nodules using a 2D HASTE MR sequence: comparison with MDCT. AJR Am J Roentgenol 2005;185:979–84. [DOI] [PubMed] [Google Scholar]
- [26].Dewes P, Frellesen C, Al-Butmeh F, et al. Comparative evaluation of non-contrast CAIPIRINHA-VIBE 3T-MRI and multidetector CT for detection of pulmonary nodules: in vivo evaluation of diagnostic accuracy and image quality. Eur J Radiol 2016;85:193–8. [DOI] [PubMed] [Google Scholar]
- [27].Sommer G, Tremper J, Koenigkam-Santos M, et al. Lung nodule detection in a high-risk population: comparison of magnetic resonance imaging and low-dose computed tomography. Eur J Radiol 2014;83:600–5. [DOI] [PubMed] [Google Scholar]
- [28].Ohno Y, Nishio M, Koyama H, et al. Solitary pulmonary nodules: comparison of dynamic first-pass contrast-enhanced perfusion area-detector CT, dynamic first-pass contrast-enhanced MR imaging, and FDG PET/CT. Radiology 2015;274:563–75. [DOI] [PubMed] [Google Scholar]
- [29].Ohno Y, Koyama H, Takenaka D, et al. Dynamic MRI, dynamic multidetector-row computed tomography (MDCT), and coregistered 2-[fluorine-18]-fluoro-2-deoxy-D-glucose-positron emission tomography (FDG-PET)/CT: comparative study of capability for management of pulmonary nodules. J Magn Reson Imaging 2008;27:1284–95. [DOI] [PubMed] [Google Scholar]
- [30].Littleton JT, Durizch ML, Moeller G, Herbert DE. Pulmonary masses: contrast enhancement. Radiology 1990;177:861–71. [DOI] [PubMed] [Google Scholar]
- [31].Sipkins DA, Cheresh DA, Kazemi MR, Nevin LM, Bednarski MD, Li KC. Detection of tumor angiogenesis in vivo by alpha Vbeta 3-targeted magnetic resonance imaging. Nat Med 1998;4:623–6. [DOI] [PubMed] [Google Scholar]
- [32].John AE, Joseph C, Jenkins G, Tatler AL. COVID-19 and pulmonary fibrosis: a potential role for lung epithelial cells and fibroblasts. Immunol Rev 2021;302:228–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Nicoleau S, Wojciak-Stothard B. Beyond thrombosis: the role of platelets in pulmonary hypertension. SciMed J 2020;2:243–71. [Google Scholar]