Background:
Genicular artery embolization (GAE) is a novel therapy to treat patients with symptomatic knee osteoarthritis (OA) by reducing synovial arterial hypervascularity. This study evaluates the safety and efficacy of GAE for the treatment of symptomatic knee OA.
Methods:
A prospective, single-center, open-label U.S. Food and Drug Administration-approved investigational device exemption study was conducted. Patients enrolled in the study were 40 to 80 years old, with moderate or severe knee OA (Kellgren-Lawrence grade 2, 3, or 4), who previously had failure of conservative therapy. Baseline pain (visual analog scale [VAS]) and symptom scores (Western Ontario and McMaster Universities Osteoarthritis Index [WOMAC]) were assessed. After femoral arterial access was achieved, GAE of 1, 2, or 3 genicular arteries supplying the location of the subject’s pain, as determined by digital subtraction angiography and cone-beam computed tomography, was performed using 100-μm particles. Adverse events and symptoms scores were assessed at 1 week, 1 month, 3 months, 6 months, and 1 year after GAE.
Results:
Over a 10-month period, 40 subjects were enrolled. The median age was 69 years (range, 49 to 80 years). The median body mass index was 29 kg/m2 (range, 19 to 44 kg/m2). Knee OA severity was grade 2 in 18% of the patients, grade 3 in 43%, and grade 4 in 40%. Technical success was achieved in 100% of the subjects. Transient skin discoloration and transient mild knee pain after the procedure were common and expected. Treatment-related adverse events included a groin hematoma requiring overnight observation in 1 subject, self-resolving focal skin ulceration in 7 subjects, and an asymptomatic small bone infarct on magnetic resonance imaging at 3 months in 2 subjects. The WOMAC total and VAS pain scores decreased by 61% and 67% at 12 months from a median baseline of 52 (of 96) and 8 (of 10), respectively. Twenty-seven patients (68%) had a reduction of ≥50% in both WOMAC total and VAS pain scores.
Conclusions:
This prospective trial demonstrates that GAE is effective and durable in reducing pain symptoms from moderate or severe knee OA that is refractory to other conservative therapy, with an acceptable safety profile.
Level of Evidence:
Therapeutic Level IV. See Instructions for Authors for a complete description of levels of evidence.
Osteoarthritis (OA) of the knee can be a debilitating condition with marked impact on a person’s overall quality of life1. Total knee arthroplasty (TKA) has a well-established track record with excellent overall outcomes and a relatively low complication rate2. A fair number of patients with knee OA, however, are suboptimal TKA surgical candidates. Certain comorbidities, such as diabetes mellitus, obesity, coronary artery disease, malnutrition, renal disease, cirrhosis, and immunosuppression, are associated with increased medical and surgical complications. Patient age is another factor to consider; there is concern in performing TKA in young patients who are at increased risk for aseptic loosening, requiring multiple future revision surgeries. Similarly, elderly patients may not be good surgical candidates, given their associated comorbidities and increased risk of periprosthetic fracture. Finally, some patients wish to postpone TKA until they are better able to dedicate time and effort to their postoperative recovery process3-5. Unfortunately, there is no currently available treatment alternative for patients who have exhausted conservative management (e.g., nonsteroidal anti-inflammatory drugs [NSAIDs], joint injections, and physical therapy), yet are not candidates for TKA6.
OA has historically been considered a “wear-and-tear” disease, resulting from years of stress-induced cartilage and meniscal degeneration. However, recent data have suggested that inflammation plays a role not only in the experience of pain secondary to OA but also as a driver of OA itself7. It is now understood that inflammatory mediators secreted from the synovium contribute to joint tissue destruction7. These mediators, including cytokines, nitric oxide, prostaglandin E2, and neuropeptides, are produced by the inflamed synovium, altering the balance of cartilage matrix degradation and restoration. Excess enzyme release ultimately contributes to cartilage destruction, which in turn results in synovial inflammation in a proverbial vicious cycle. As a sequela of synovial inflammation, vascular endothelial cell proliferation occurs, providing inflammatory cells access to the synovium and other joint tissues and promoting hyperplasia and inflammation in regional vessels, which furthers the destruction of bone and cartilage8. Angiogenesis may in fact contribute to chronic pain by enabling the growth of unmyelinated sensory nerves alongside newly formed blood vessels9.
As synovitis is associated with clinical symptoms and reflects joint degradation in OA, synovium-targeted therapy could help to alleviate the symptoms of the disease and perhaps prevent structural progression. Genicular artery embolization (GAE) is a minimally invasive procedure, in which the genicular arteries supplying the synovial lining of the knee are selectively catheterized during an angiogram to target aberrant neovasculature associated with knee OA. The injection of small, calibrated microspheres results in a reduction in arterial flow, which may in turn reduce the synovial inflammation.
The purpose of this clinical trial was to evaluate the safety and efficacy of GAE for patients with symptomatic moderate or severe knee OA who are not candidates for TKA.
Materials and Methods
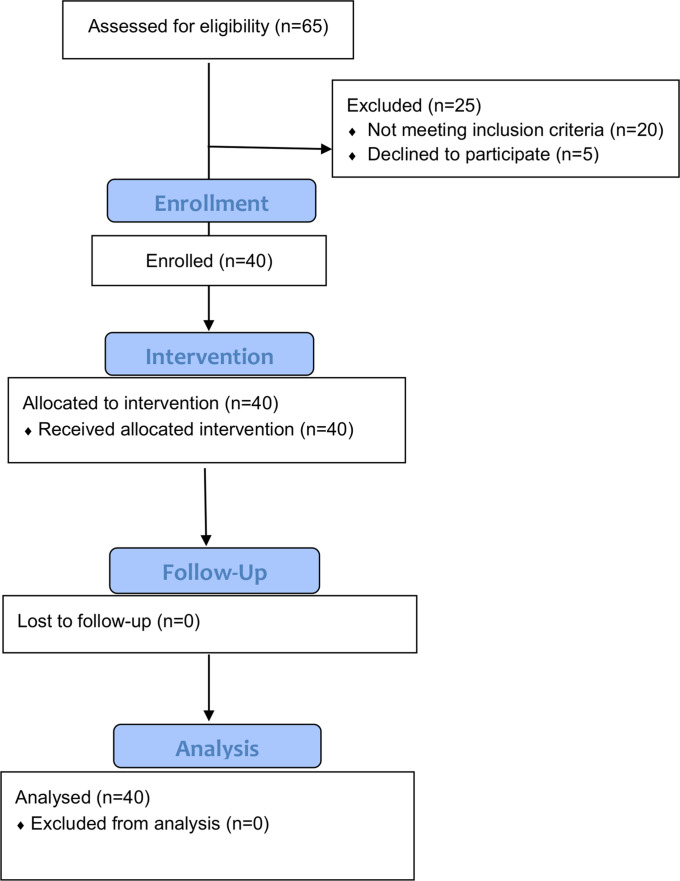
This was a single-center, single-arm prospective trial of GAE. The study received an investigational device exemption (IDE) from the U.S. Food and Drug Administration (FDA) (IDE G180048). The trial was registered with clinicaltrials.gov (NCT03491397). The study was approved by the institutional review board. Each subject signed an informed consent prior to enrollment. The primary end point was tabulation of adverse events (AEs) related to GAE, and the secondary end point was treatment efficacy. Study design is shown in Figure 1. Research coordination and the study device were funded by Varian Medical Systems. The GAE procedure and all study visits were self-funded by the authors’ institution.
Fig. 1.
CONSORT (Consolidated Standards of Reporting Trials) flow diagram.
Inclusion criteria are listed in Table I. After enrollment, subjects underwent a directed history and physical examination. Symptoms related to knee OA were quantified using the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC)10. This is a commonly utilized set of standardized questionnaires to assess pain, stiffness, and physical functioning of the joints. The severity of knee pain was also quantified using a visual analog scale (VAS) score ranging from 0 (indicating no pain) through 10 (indicating the worst pain imaginable). Baseline assessment included imaging with knee radiographs and magnetic resonance imaging (MRI) of the knee without contrast medium. Knee radiographs were used to confirm the presence of OA and grade the severity, based on the Kellgren-Lawrence score11. MRI was used to exclude other potential pathological conditions of the knee, such as a fracture, ligament tear, or malignant process. Image interpretation was performed by 2 separate board-certified radiologists with subspecialty training in musculoskeletal imaging.
TABLE I.
Study Eligibility*
| Inclusion criteria |
| Provided informed consent |
| Age of 40 to 80 yr |
| Ineligibility for or refusal of surgery |
| Moderate to severe knee pain (VAS of >4) |
| Kellgren-Lawrence grade of 2, 3, or 4 |
| Local knee tenderness |
| Resistance to or failure of conservative treatment (e.g., NSAIDs, PT, and joint injection) for at least 3 mo |
| Exclusion criteria |
| Mild knee pain (VAS of ≤4) |
| Clinical evidence of peripheral arterial disease |
| Recent or active cigarette use |
| Prior knee arthroplasty in the involved knee |
| Renal insufficiency (serum creatinine of >1.5 mg/dL) |
PT = physical therapy.
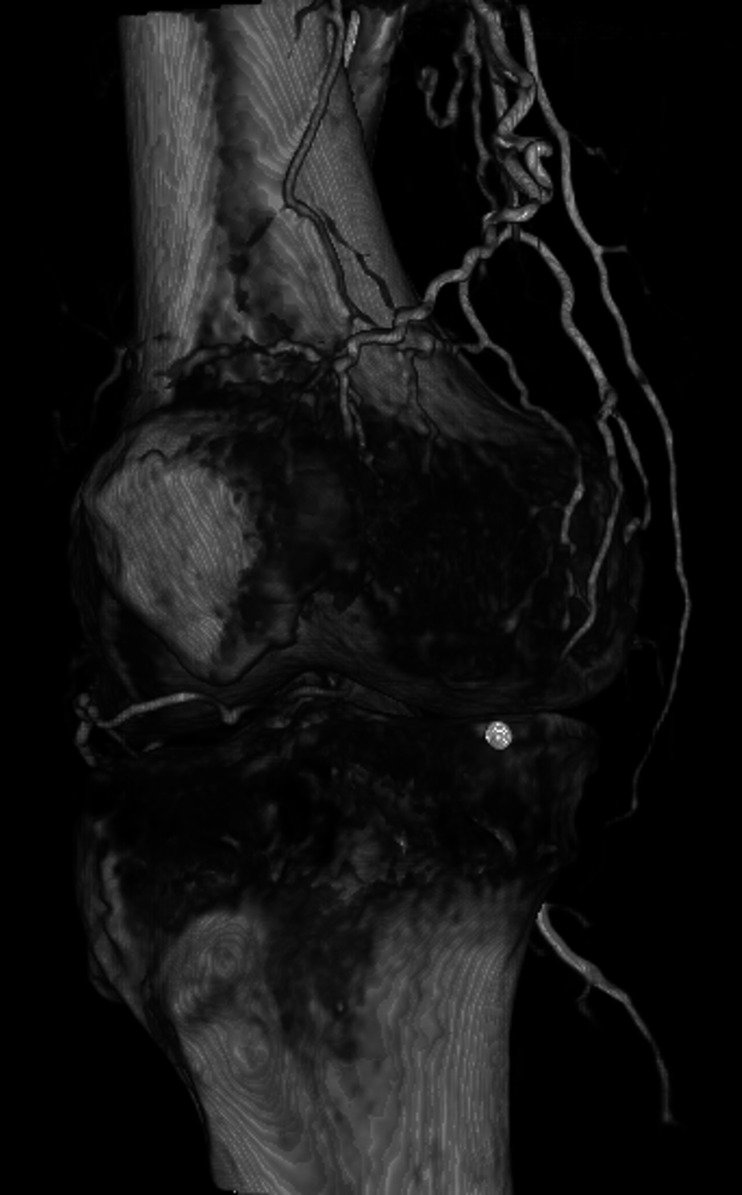
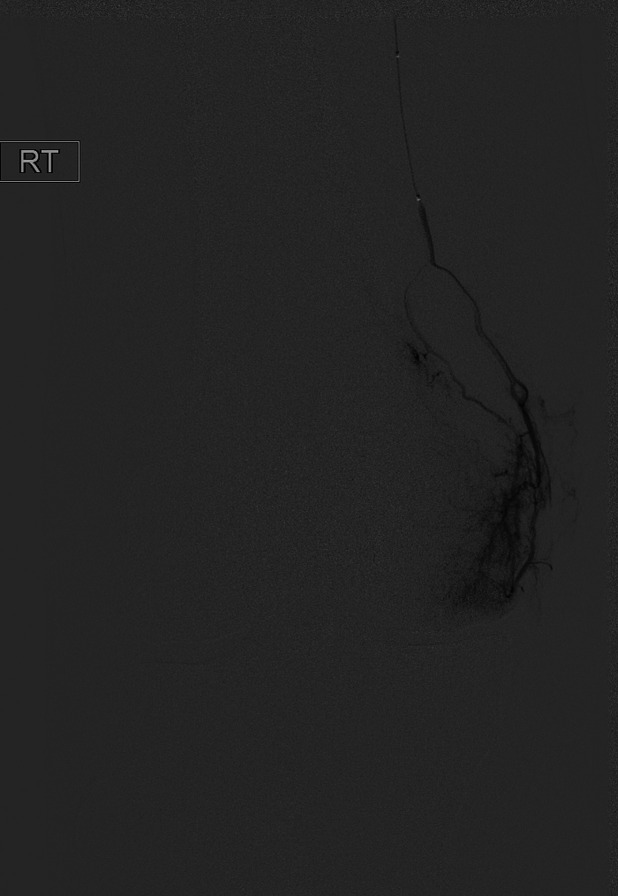
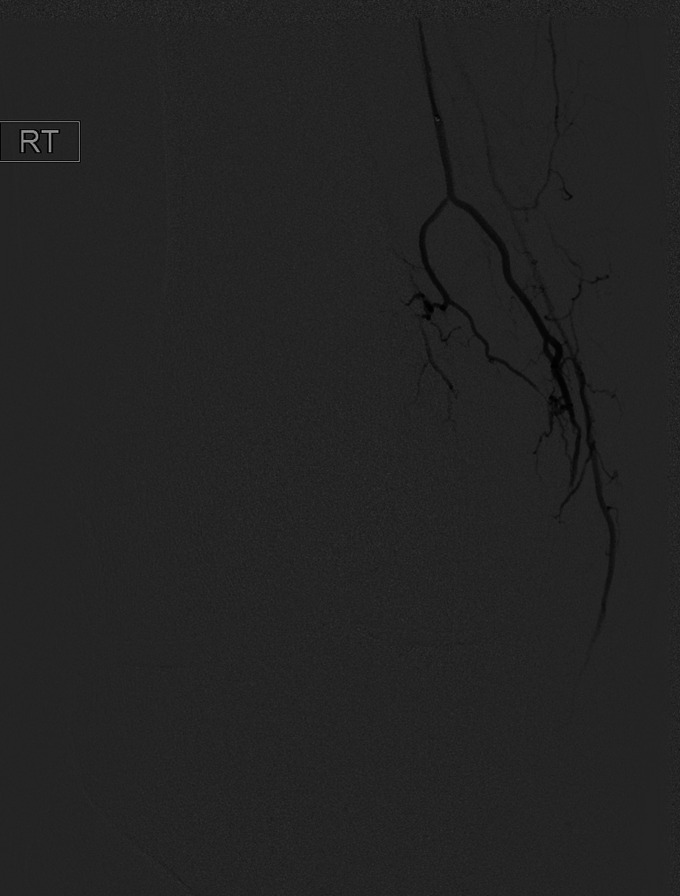
The GAE was performed by 1 of 3 board-certified interventional radiologists. An example GAE is shown in Figs. 2-A through 2-F. Procedures were performed with the patient under moderate sedation (intravenous midazolam and fentanyl) in an interventional radiology suite in the hospital. Immediately prior to the procedure, the focal areas of pain identified by the subject were marked with a radiopaque marker. Local anesthetic (1% lidocaine) was administered for a transfemoral approach. Access to the ipsilateral common femoral artery was performed with a 21-gauge needle, with exchange to a 3-French vascular sheath. In subjects with a high body mass index (BMI), contralateral femoral arterial access was obtained, and a 6-French sheath was then advanced over the iliac artery bifurcation to the common femoral artery of the target limb. A digital subtraction angiogram of the superficial femoral and popliteal arteries was performed through the sheath. This was followed by intraprocedural rotational cone-beam computed tomography (CT) of the knee in order to obtain a 3-dimensional (3D) assessment of the arterial supply to the knee. Using a 1.7 to 2.4-French microcatheter, the specific genicular arteries supplying the areas of the pain were catheterized. Embolization was performed with 100-µm Embozene particles (Varian Medical Systems). Embolization was performed until distal hypervascularity resolved, yet normal arterial flow was preserved within the selected artery. On completion of the procedure, hemostasis was achieved with manual compression or the use of an arterial closure device. Subjects were discharged from the hospital 4 hours after the procedure.
A Figs. 2-A through 2-F A 65-year-old man with pain in the medial aspect of the right knee secondary to OA.
Fig. 2-A.
Fig. 2-A Knee radiograph showing joint-space narrowing of the medial compartment, consistent with Kellgren-Lawrence grade-3 OA.
Fig. 2-B.
Fig. 2-B Access was obtained in the right femoral artery with a 3-French sheath.
Fig. 2-C.
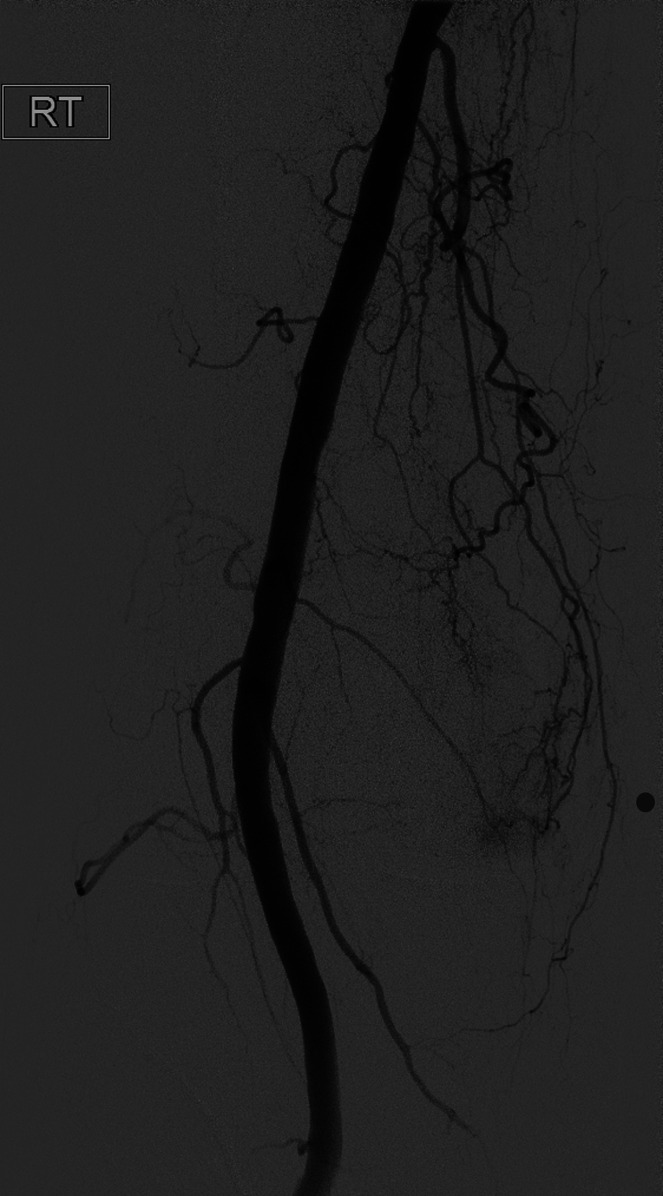
Fig. 2-C Angiogram of the distal superficial femoral artery with a radiopaque marker placed at the site of the pain, showing hypervascularity along the medial joint space.
Fig. 2-D.
Fig. 2-D Rotational 3D reconstructed angiogram identifies the descending genicular artery as coursing toward the region of pain.
Fig. 2-E.
Fig. 2-E Selective catheterization and digital subtraction angiogram of the descending genicular artery confirms the presence of hyperemia in the medial joint space.
Fig. 2-F.
Fig. 2-F After embolization with Embozene microspheres, a postembolization angiogram shows vessel patency with the absence of hyperemia.
Following treatment, subjects returned for follow-up visits at 1 week (±4 days), 1 month (±2 weeks), 3 months (±2 weeks), 6 months (±2 weeks), and 12 months (±2 weeks). At these visits, subjects completed the WOMAC and VAS questionnaires, underwent a directed physical examination, and reported any new AEs. At 3 months, subjects underwent a follow-up MRI of the knee without contrast. At 12 months, subjects had knee radiographs to assess the progression of OA. At 12 months, subjects also completed patient-reported outcomes (PROs) and patient global impression of change (PGIC) questionnaires.
Statistical Analysis
Cumulative rates of AEs were estimated with the 95% confidence interval (CI). AEs were analyzed as a composite of all AEs, composites based on major AE types or severity, and as individual AE types. An enrollment of 40 subjects achieved 83% power to test the difference between 10% of the upper bound of the 95% CI for an AE rate of <0.01% (i.e., no such events seen) and 10%. At this sample size, for AE rates of 0% to 50%, the difference between the upper bound of the 95% CI and the estimated AE rate ranged from 7.1% to 14.8%. These calculations were based on an exact binomial distribution, assuming no loss to follow-up.
Efficacy Analyses
The primary efficacy end point was the change in the WOMAC total score from before GAE to 12 months after GAE. Specifically, a 50% reduction in the WOMAC score was chosen as the definition of clinical success. Univariate logistic models were used to estimate an odds ratio of a reduction of ≥50% in WOMAC scores at month 12. Multivariable logistic models were used to estimate the important predictors of success, in which success was defined as a reduction of ≥50% in WOMAC scores at month 12. All statistical tests and CIs were 2-sided with a nominal significance level of p < 0.05 (95% confidence).
Results
Forty subjects were enrolled in the study, with the first enrollment in March 2019 and the final enrollment in January 2020. The 12-month study visit of the final subject was completed in January 2021. Baseline demographics are listed in Table II.
TABLE II.
Baseline Characteristics
| Variables | Findings |
|---|---|
| Age* (yr) | 69 (49-80) |
| Sex (no. [%]) | |
| Male | 9 (23) |
| Female | 31 (78) |
| BMI* (kg/m2) | 29.3 (18.8-43.7) |
| Baseline pain* (VAS) | 8 (5-10) |
| Baseline WOMAC pain score* | 11 (5-19) |
| Baseline WOMAC total score* | 52 (23-88) |
| Side (no. [%]) | |
| Right | 16 (40) |
| Left | 24 (60) |
| OA location (no. [%]) | |
| Medial | 27 (68) |
| Lateral | 12 (30) |
| Patellofemoral | 4 (10) |
| OA severity (Kellgren-Lawrence grade) (no. [%]) | |
| 2 | 7 (18) |
| 3 | 17 (43) |
| 4 | 16 (40) |
The values are given as the median, with the range in parentheses.
All GAE procedures were technically successful (Table III). The procedure was performed with an ipsilateral femoral access approach in 65% of the patients and a contralateral approach in 35%. The median number of genicular arteries embolized was 2 (range, 1 to 3). Arteries embolized included the descending genicular, superior medial, inferior medial, superior lateral, inferior lateral, and recurrent ascending tibial artery. The median procedure time was 79 minutes, with a gradual improvement of procedure time with increasing operator experience.
TABLE III.
Technical Procedural Parameters
| Findings | |
|---|---|
| Duration of procedure* (min) | 78.5 (49-165) |
| Radiation dose* (gray × cm2) | 49.2 (13.6-158.2) |
| Amount of contrast used* (mL) | 70 (30-150) |
| Arteries embolized (no. [%]) | |
| Descending genicular | 21 (53) |
| Recurrent ascending tibial | 1 (3) |
| Superior lateral genicular | 8 (20) |
| Superior medial genicular | 11 (28) |
| Inferior lateral genicular | 12 (30) |
| Inferior medial genicular | 10 (25) |
| Median genicular | 0 (0) |
| No. of arteries embolized (no. [%]) | |
| 1 | 19 (48) |
| 2 | 19 (48) |
| 3 | 2 (5) |
The values are given as the median, with the range in parentheses.
One important AE that occurred was a small groin hematoma from the femoral arterial access site, necessitating an unplanned overnight hospital observation. Transfusion was not required, and follow-up ultrasound showed a normal femoral artery without sequelae. The remaining AEs related to GAE were few and minor. These included focal epidermal layer skin ulceration (1 cm in diameter) at the knee in 7 subjects (18%), likely due to embolization of nontarget cutaneous branches. These symptoms arose 7 to 10 days after the procedure and resolved with no treatment within 3 days. After the 7th case of ulceration, which occurred in the 25th study subject, the treatment protocol was modified to place an ice pack on the skin during the embolization. Following this modification, no further cases of ulceration occurred. Two cases of clinically asymptomatic focal bone infarct were identified on the 3-month MRI, 1 in the tibia and 1 in the patella. Both infarcts measured <2 cm in size and occurred in the non-weight-bearing portions of the joint. There was a single case of focal fat necrosis in the lower thigh, presenting as firmness of the deep tissues. Imaging with knee radiographs at 12 months showed 1 patient (3%) with mild progression of the OA, and the remaining patients had no progression.
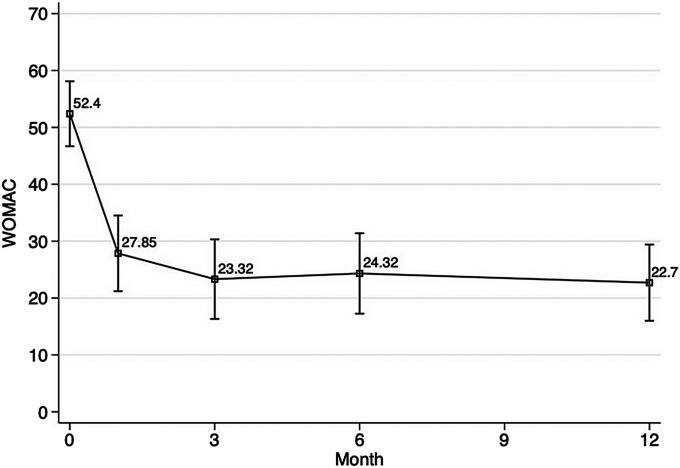
Efficacy outcomes are shown in Figure 3, Table IV, and Table V. Twenty-seven (68%) of the 40 subjects achieved clinical success from GAE, defined as a reduction of at least 50% in the WOMAC total score from baseline to 12 months. Furthermore, 17 (43%) of the 40 subjects had a reduction of ≥75% in the WOMAC score at 12 months. Using a lower threshold of a 25% reduction in the WOMAC, 34 (85%) of 40 subjects had clinical success at 12 months. The median WOMAC total score for the entire cohort decreased with time during the study period, from 52 at baseline to 19 at 12 months. The median of the percent changes in the WOMAC score decreased by 61%. Twenty-seven (68%) of the 40 subjects reported a reduction in pain on the VAS (a scale from 0 to 10) of ≥50% from baseline to 12 months. Of the 27 subjects who demonstrated clinical success during the study period at 3 months, only 1 subject (4%) reported partial symptom recurrence at 12 months. The remaining 26 subjects (96%) reported a sustained benefit at 12 months, demonstrated by a decrease of ≥50% in the WOMAC score. The median VAS pain score was 8 (of 10) at baseline, and then decreased to 3, 3, 3, and 3 at 1, 3, 6, and 12 months, respectively. PGIC, as part of the patient-reported outcome measure, demonstrated an overall positive patient experience regarding GAE (Table VI).
Fig. 3.
Mean WOMAC total scores over time. The I-bars indicate the upper and lower limits of the 95% confidence interval (CI), which were calculated with formula: 95% CI = the mean ± 1.96 × standard error of the mean.
TABLE IV.
Changes in Pain and Function After GAE
| Outcome and Time | Score Change from Baseline* | Percent Change from Baseline* |
|---|---|---|
| VAS pain | ||
| Month 1 | −4.5 (−9 to 2) | −60.20 (−100 to 33.33) |
| Month 3 | −4.5 (−10 to 2) | −60.20 (−100 to 33.33) |
| Month 6 | −5.0 (−8.5 to −1) | −62.50 (−100 to −10) |
| Month 12 | −5.0 (−9 to 0) | −66.67 (−100 to 0) |
| WOMAC pain | ||
| Month 1 | −6.0 (−17 to 3) | −54.70 (−100 to 60) |
| Month 3 | −7 (−15 to 6) | −59.94 (−100 to 100) |
| Month 6 | −7 (−14 to 6) | −62.50 (−100 to100) |
| Month 12 | −7.5 (−14 to 6) | −65.69 (−100 to 100) |
| WOMAC total | ||
| Month 1 | −20.5 (−71 to 20) | −55.26 (−96 to 40) |
| Month 3 | −29.5 (−74 to 26) | −64.84 (−98 to 54) |
| Month 6 | −26.50 (−81 to 19) | −58.11 (−100 to 54) |
| Month 12 | −29.00 (−82 to19) | −60.54 (−100 to 54) |
The values are given as the median, with the range in parentheses.
TABLE V.
Effectiveness of GAE as Assessed by VAS and WOMAC Scores
| Month | Reduction in VAS Pain Score (no. [%]) | Reduction in WOMAC Pain Score (no. [%]) | Reduction in WOMAC Total Score (no. [%]) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ≥25% | ≥50% | ≥75% | ≥25% | ≥50% | ≥75% | ≥25% | ≥50% | ≥75% | |
| 1 | 31 (78) | 24 (60) | 12 (30) | 28 (70) | 21 (53) | 10 (25) | 28 (70) | 22 (55) | 8 (20) |
| 3 | 31 (78) | 25 (63) | 15 (38) | 30 (75) | 24 (60) | 15 (38) | 34 (85) | 27 (68) | 16 (40) |
| 6 | 36 (90) | 30 (75) | 16 (40) | 32 (80) | 25 (63) | 15 (38) | 33 (83) | 25 (68) | 14 (35) |
| 12 | 34 (85) | 27 (68) | 17 (43) | 35 (88) | 27 (68) | 15 (38) | 34 (85) | 27 (68) | 17 (43) |
TABLE VI.
Patient-Reported Outcomes and Global Impression of Change (N = 38)
| No. (%) of Patients | |
|---|---|
| 1. Since the procedure, how would you describe the change (if any) in activity limitations, symptoms, emotions, and overall quality of life related to your knee arthritis? | |
| a. No change (or condition has gotten worse) | 3 (8) |
| b. Almost the same, hardly any change at all | 2 (5) |
| c. A little better, but no noticeable change | 3 (8) |
| d. Somewhat better, but the change has not made any real difference | 2 (5) |
| e. Moderately better and a slight but noticeable change | 9 (24) |
| f. Better and a definite improvement that has made a real and worthwhile difference | 6 (16) |
| g. A great deal better and a considerable improvement that has made all the difference | 13 (34) |
| 2. Knowing what you know now, would you have the procedure done again? | |
| a. Yes | 29 (76) |
| b. No | 9 (24) |
Thirteen (33%) of 40 patients showed <50% improvement in the WOMAC score. Among the nonresponders, 5 subjects subsequently underwent TKA (4, 5, 6, 6, and 12 months after GAE). There were no reported complications from surgery in these patients. Specific attention was made to assess for wound and healing complications, such as tissue necrosis, wound dehiscence, and infection.
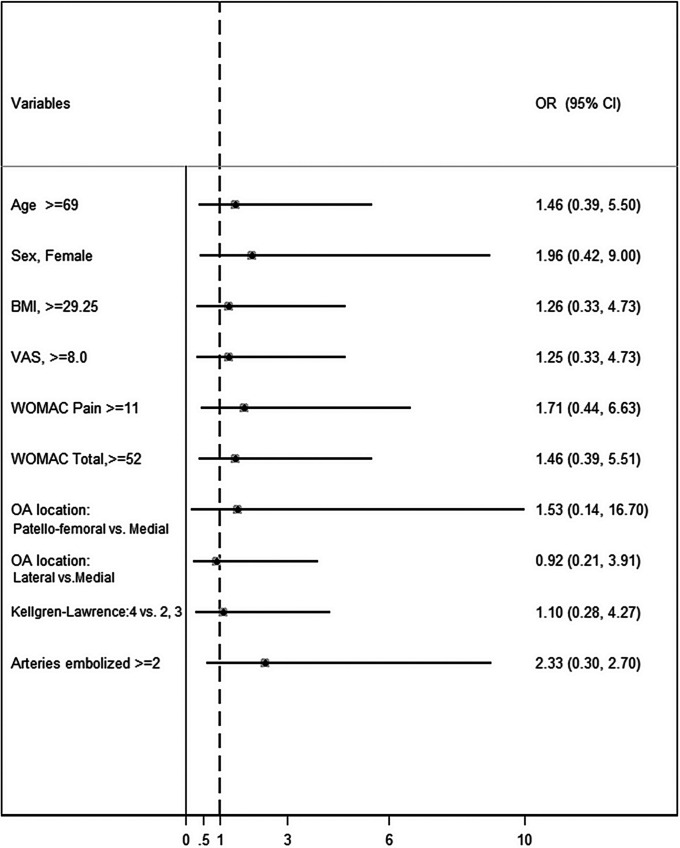
Univariate analysis was performed to identify baseline factors that could potentially signal a clinical success versus failure (Fig. 4). There were trends toward a better clinical response in females, with a mean 0.88-point change in the VAS and 17-point change in the WOMAC. A high severity score at baseline trended as a poor prognostic factor in improvement in all 3 measurements of the VAS, WOMAC pain, and WOMAC total score.
Fig. 4.
Univariate analysis. An odds ratio (OR) of >1 favors clinical success (defined as ≥50% reduction in WOMAC at 12 months).
Discussion
End-stage knee OA is successfully treated with TKA; however, effective conservative management of knee OA for people who are not surgical candidates or are trying to delay surgery remains a challenge.
The most recently published American Academy of Orthopaedic Surgeons (AAOS) clinical practice guidelines recommended the following conservative treatments for symptomatic knee osteoarthritis: low-impact aerobic exercises and strengthening, weight loss, and the use of NSAIDs or tramadol12. The guidelines were unable to recommend for or against the use of intra-articular corticosteroid injections and could not recommend the use of hyaluronic acid injections. Furthermore, recent data have suggested that injection of glucocorticoids may accelerate the progression of OA, and therefore treatments are generally limited to ≤3 injections13. A randomized trial of glucocorticoid injections versus physical therapy demonstrated that patients who underwent a regimented physical therapy program had less knee pain and functional disability at 1 year compared with patients who had joint injection14. Finally, intra-articular treatment with hyaluronic acid has shown mixed results, with several meta-analyses showing no significant benefit15. Regarding physical therapy, insurance barriers and lack of patient compliance because of intensive time requirements limit this option in the long term. These factors result in an important need for emerging therapies in this arena.
To date, 4 separate prospective trials of GAE have been completed16-19. The first GAE trial by Okuno et al. in 2017 studied 95 knees in 72 patients16. Imipenem-cilastatin sodium was used as the embolic material in most cases. The intent-to-treat clinical success rate at 6 months of follow-up was 86.3% (95% CI, 78% to 92%). The mean total WOMAC score decreased from 43 at baseline to 24, 14.8, 11.2, 8.2, and 6.2 at 1, 4, 6, 12, and 24 months, respectively (p < 0.001 for all). While the results from that initial trial showed a great deal of promise, most patients underwent embolization with imipenem-cilastatin, which is not readily available in the United States. Furthermore, the treatment population was relatively homogeneous with regard to ethnicity, the median BMI was lower than that typically seen in Western populations, and patients with Kellgren-Lawrence grade-4 OA were excluded from the study. The 3 other trials also demonstrated promising results with respect to safety and efficacy17-19. However, they were limited in their follow-up duration and had a relatively small sample size. In all 3 trials, patients with Kellgren-Lawrence grade-4 OA were excluded from the study.
Gaps in knowledge from the 4 previously described trials are answered by the current study. Forty percent of the patients in the current study had severe OA (Kellgren-Lawrence grade 4). Our study included patients with various ethnic backgrounds and a wide range of BMI values (range, 19 to 44 kg/m2). From a technical standpoint, the same embolic material (100 µm of Embozene) was used in all subjects, lending uniformity to the data. In previous studies, the mean number of genicular arteries embolized was 2.5 to 3.2. In our study, however, the use of 3D cone-beam CT provided us with a refined analysis of the vascular anatomy, which allowed us to embolize a mean of only 1.6 arteries (a median of 2 arteries) per procedure.
The trial showed a low rate of AEs. While focal skin necrosis occurred in 18% of the subjects secondary to nontarget embolization, protocol modification to include an application of an ice pack during the procedure resulted in no further skin complications. It should be noted that most patients had some degree of transient skin discoloration on the knee. The 2 instances of bone infarction were asymptomatic, and in fact both subjects had a clinical success based on a reduction in the WOMAC score. However, future trials should address this issue in case infarction is larger in area or occurs in the weight-bearing portion of the joint. No neurologic or distal vascular (i.e., below-the-knee) complications occurred. It should be noted, however, that people with active cigarette use or clinical evidence of peripheral arterial disease were excluded from this study and are likely poor candidates for GAE.
Our trial demonstrated marked reductions in pain and improvement in overall function (as shown by a decrease in WOMAC and VAS scores) in 68% of subjects. While the minimal clinically important difference for the WOMAC was reported as 16% (or a 10-point decrease), the present study chose a much higher threshold in order to mitigate a potential placebo effect20. Other randomized studies of surgery and joint injection have shown mild improvement in the WOMAC score in the placebo or observation arm15. Furthermore, the maximum response was seen at 3 months (not at 1 month) and persisted at 12 months. These features are not typically seen in a placebo arm, in which a mild effect on symptoms should wane over time.
For a minimally invasive treatment to be impactful, it must not preclude more invasive treatments in the future. Thirteen subjects (33%) did not meet the definition of clinical success at 12 months in our study. Four subjects subsequently underwent intra-articular joint injections, without any adverse sequelae. Two subjects underwent TKA during the study period, and 1 after the 12-month follow-up. All surgeries were uneventful, and the subjects made a full recovery without AEs or delayed healing.
Some limitations to this study exist. The patient population was heterogeneous with respect to age, degree of OA severity, and ethnic background. However, in a trial of 40 patients, multivariate analysis of these baseline demographics was limited. This was a single-arm trial, without a placebo or control arm. Therefore, it is possible that some degree of benefit is derived from the placebo effect in this trial21. However, by using a high threshold (a 50% reduction in the WOMAC) and follow-up to 12 months, the placebo effect can be somewhat mitigated. While a placebo arm may be ethically inappropriate as the angiogram itself carries some degree of potential risk, an observation arm would be ideal in future randomized studies.
In conclusion, this single-arm trial of GAE for knee OA demonstrated an acceptable safety profile. At 12 months after the GAE, 68% of subjects continued to have marked symptomatic improvement. The treatment effect was seen in both moderate and severe OA. Future comparative trials will determine the most appropriate nonsurgical treatment algorithm for knee OA.
Footnotes
Investigation performed at the David Geffen School of Medicine at University of California Los Angeles, Los Angeles, California
Disclosure: The Disclosure of Potential Conflicts of Interest forms are provided with the online version of the article (http://links.lww.com/JBJSOA/A326).
References
- 1.Dominick KL, Ahern FM, Gold CH, Heller DA. Health-related quality of life and health service use among older adults with osteoarthritis. Arthritis Rheum. 2004. Jun 15;51(3):326-31. [DOI] [PubMed] [Google Scholar]
- 2.Skou ST, Roos EM, Laursen MB, Rathleff MS, Arendt-Nielsen L, Simonsen O, Rasmussen S. A Randomized, Controlled Trial of Total Knee Replacement. N Engl J Med. 2015. Oct 22;373(17):1597-606. [DOI] [PubMed] [Google Scholar]
- 3.Delanois RE, Mistry JB, Gwam CU, Mohamed NS, Choksi US, Mont MA. Current Epidemiology of Revision Total Knee Arthroplasty in the United States. J Arthroplasty. 2017. Sep;32(9):2663-8. [DOI] [PubMed] [Google Scholar]
- 4.Mathis DT, Lohrer L, Amsler F, Hirschmann MT. Reasons for failure in primary total knee arthroplasty - An analysis of prospectively collected registry data. J Orthop. 2020. Dec 31;23:60-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharkey PF, Lichstein PM, Shen C, Tokarski AT, Parvizi J. Why are total knee arthroplasties failing today—has anything changed after 10 years? J Arthroplasty. 2014. Sep;29(9):1774-8. [DOI] [PubMed] [Google Scholar]
- 6.Bannuru RR, Osani MC, Vaysbrot EE, Arden NK, Bennell K, Bierma-Zeinstra SMA, Kraus VB, Lohmander LS, Abbott JH, Bhandari M, Blanco FJ, Espinosa R, Haugen IK, Lin J, Mandl LA, Moilanen E, Nakamura N, Snyder-Mackler L, Trojian T, Underwood M, McAlindon TE. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019. Nov;27(11):1578-89. [DOI] [PubMed] [Google Scholar]
- 7.Bonnet CS, Walsh DA. Osteoarthritis, angiogenesis and inflammation. Rheumatology (Oxford). 2005. Jan;44(1):7-16. [DOI] [PubMed] [Google Scholar]
- 8.Pap T, Distler O. Linking angiogenesis to bone destruction in arthritis. Arthritis Rheum. 2005. May;52(5):1346-8. [DOI] [PubMed] [Google Scholar]
- 9.Ashraf S, Wibberley H, Mapp PI, Hill R, Wilson D, Walsh DA. Increased vascular penetration and nerve growth in the meniscus: a potential source of pain in osteoarthritis. Ann Rheum Dis. 2011. Mar;70(3):523-9. [DOI] [PubMed] [Google Scholar]
- 10.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988. Dec;15(12):1833-40. [PubMed] [Google Scholar]
- 11.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957. Dec;16(4):494-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown GA. AAOS clinical practice guideline: treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013. Sep;21(9):577-9. [DOI] [PubMed] [Google Scholar]
- 13.Kompel AJ, Roemer FW, Murakami AM, Diaz LE, Crema MD, Guermazi A. Intra-articular Corticosteroid Injections in the Hip and Knee: Perhaps Not as Safe as We Thought? Radiology. 2019. Dec;293(3):656-63. [DOI] [PubMed] [Google Scholar]
- 14.Deyle GD, Allen CS, Allison SC, Gill NW, Hando BR, Petersen EJ, Dusenberry DI, Rhon DI. Physical Therapy versus Glucocorticoid Injection for Osteoarthritis of the Knee. N Engl J Med. 2020. Apr 9;382(15):1420-9. [DOI] [PubMed] [Google Scholar]
- 15.Jevsevar D, Donnelly P, Brown GA, Cummins DS. Viscosupplementation for Osteoarthritis of the Knee: A Systematic Review of the Evidence. J Bone Joint Surg Am. 2015. Dec 16;97(24):2047-60. [DOI] [PubMed] [Google Scholar]
- 16.Okuno Y, Korchi AM, Shinjo T, Kato S, Kaneko T. Midterm Clinical Outcomes and MR Imaging Changes after Transcatheter Arterial Embolization as a Treatment for Mild to Moderate Radiographic Knee Osteoarthritis Resistant to Conservative Treatment. J Vasc Interv Radiol. 2017. Jul;28(7):995-1002. [DOI] [PubMed] [Google Scholar]
- 17.Little MW, Gibson M, Briggs J, Speirs A, Yoong P, Ariyanayagam T, Davies N, Tayton E, Tavares S, MacGill S, McLaren C, Harrison R. Genicular artEry embolizatioN in patiEnts with oSteoarthrItiS of the Knee (GENESIS) Using Permanent Microspheres: Interim Analysis. Cardiovasc Intervent Radiol. 2021. Jun;44(6):931-940. Epub 2021 Jan 20. Erratum in: Cardiovasc Intervent Radiol. 2021 Apr 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bagla S, Piechowiak R, Hartman T, Orlando J, Del Gaizo D, Isaacson A. Genicular Artery Embolization for the Treatment of Knee Pain Secondary to Osteoarthritis. J Vasc Interv Radiol. 2020. Jul;31(7):1096-102. [DOI] [PubMed] [Google Scholar]
- 19.Landers S, Hely R, Page R, Maister N, Hely A, Harrison B, Gill S. Genicular Artery Embolization to Improve Pain and Function in Early-Stage Knee Osteoarthritis-24-Month Pilot Study Results. J Vasc Interv Radiol. 2020. Sep;31(9):1453-8. [DOI] [PubMed] [Google Scholar]
- 20.Clement ND, Bardgett M, Weir D, Holland J, Gerrand C, Deehan DJ. What is the Minimum Clinically Important Difference for the WOMAC Index After TKA? Clin Orthop Relat Res. 2018. Oct;476(10):2005-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sihvonen R, Paavola M, Malmivaara A, Itälä A, Joukainen A, Nurmi H, Kalske J, Järvinen TL; Finnish Degenerative Meniscal Lesion Study (FIDELITY) Group. Arthroscopic partial meniscectomy versus sham surgery for a degenerative meniscal tear. N Engl J Med. 2013. Dec 26;369(26):2515-24. [DOI] [PubMed] [Google Scholar]