Abstract
Background:
Damage caused by periodontitis not only affects periodontal tissues, but also increases the severity of various illnesses such as rheumatoid arthritis, diabetes, and liver diseases. The aim of this study is to investigate the association between induced periodontitis and damage caused through its systemic effects on the liver.
Methods:
Twenty rats were divided into two groups: control and periodontitis. The following parameters were evaluated: gingival bleeding index (GBI), probing depth (PD), myeloperoxidase (MPO) activity, alveolar bone loss (ABL) for periodontal tissues; histopathologic examination of gingival and liver tissues; immunohistochemistry to cells positive for neural/glial antigen 2 (NG2) expressed in hepatic pericytes, glutathione (GSH), and malondialdehyde (MDA) concentrations in liver; and serum levels of alanine aminotransferase and aspartate aminotransferase.
Results:
GBI, PD, MPO, ABL, and histopathologic examinations demonstrated the development of periodontitis. There was a significant increase in microvesicular steatosis accompanied by a marked reduction in NG2+ pericytes in the periodontitis group compared with the control group. The periodontitis group had significantly lower GSH and higher MDA concentration in the liver compared with the control group.
Conclusions:
The present study results link the systemic effects of induced periodontitis with changes in hepatic tissues such as microvesicular steatosis, likely caused by an increase in oxidative stress and lipid peroxidation. The findings from the present study implicate an association between a decrease of pericytes and liver disease caused by ligature-induced periodontitis in rats.
Keywords: Fatty Liver, histology, non-alcoholic fatty liver, oral medicine, oxidative stress, risk factors
Periodontitis is an infectious disease of the oral cavity with a prevalence of 4.2% in Oceania to 20.4% in Latin America.1 The disease destroys periodontal supporting and protecting tissues of the teeth.2 This process is complex and involves several components such as a host response influenced by genetics, cells, extracellular matrix, overproduction of free radicals, deregulation of cytokine production, and presence of periodontal pathogens.3–5 Damage caused by periodontitis affects not only periodontal tissues, but also increases the severity of different illnesses such as rheumatoid arthritis,6 diabetes,7 and liver diseases.8,9
Liver disease may be non-alcoholic fatty liver disease (NAFLD), characterized by fatty liver accumulation with a spectrum of liver damage ranging from simple steatosis to non-alcoholic steatohepatitis or severe cases of cancer.10 In Hong Kong, prevalence of NAFLD was 42.9%.11 Exacerbating the situation, 55.7% of NAFLD patients had normal liver enzyme tests.11 Steatosis is associated with metabolic syndrome in the general population and can be considered a multisystem disease associated with inflammation and overproduction of reactive oxygen species, whose byproduct is lipid peroxidation, such as malondialdehyde (MDA) and reduction of antioxidant molecules like glutathione (GSH).12
To evaluate liver disease, histologic analysis is the golden standard, which allows the detection of minimal changes in hepatic structure. Hepatic biology has shown the importance of mast cells that are increased in chronic C virus-related hepatitis.13 In addition, pericytes associated with portal vessels may be niches of cells with a high potential for cell differentiation, as has been demonstrated recently.14
Moreover, regarding liver disease, in previous years, studies8,9 have demonstrated an association with periodontitis. An animal study15 investigating this relationship used Escherichia coli lipopolysaccharide and Streptomyces griseus proteases applied into the gingival sulcus to induce periodontitis and the occurrence of steatosis. Porphyromonas gingivalis, an important periodontal pathogen, presented in the liver of patients with hepatic fibrosis and influenced progression of the liver disease.16
Other studies were conducted associating periodontitis with the progression of viral liver disease,8 with alcohol ingestion or not.17 Corroborating this relationship, recent studies have demonstrated that periodontal therapy may decrease levels of oxidative stress5,18 in the periodontium, saliva, and crevicular fluid and also reduce liver fibrosis.8
Although there are studies showing effects of periodontitis on liver disease,8,9,15–18 there are few studies on oxidative hepatic stress,9,15 and none, to the best knowledge of the authors, investigated lipid peroxidation through MDA levels. Furthermore, and again according to the best knowledge of the authors, there are no studies investigating the influence of mast cells and pericytes on hepatic tissues associated with periodontitis. To elucidate the association, a rat periodontitis model was used in this study.
MATERIALS AND METHODS
Animals
Twenty 6-week-old adult female Wistar rats (Rattus norvegicus), weighing 152.8 ± 17.8 g, were used. The rats were maintained under standard conditions, with a 12-hour light-dark cycle, temperatures at 24°C ± 2°C, and free access to water and food. All animal treatments and procedures were in accordance with both the Guide for Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, Maryland) and the guidelines of the Institutional Animal Ethics Committee (protocol number 0061/14).
Experimental Design
Rats were randomly divided into two groups (n = 10): 1) control (no ligature) and 2) periodontitis (ligated teeth). Periodontitis was induced under general intramuscular anesthesia by injection with a solution of 15 mg/kg of 2% xylazine hydrochloride# and 35 mg/kg of ketamine.** The ligatures were placed on the first day of experiment, using nylon 3–0,†† around the cervical region of the mandibular right first molar of each rat and then tightly knotted.19 Nylon ligature was selected to be more resistant, thereby avoiding replacing the ligature during the experiment. Twenty days later, samples of peripheral blood were collected from the retroorbital plexus for biochemical examinations. Animals subsequently were sacrificed, and total weights, as well as liver weights, were measured.
Gingival Bleeding Index (GBI)
The periodontal pockets or gingival sulcus of the mandibular first molar were probed for 10 seconds and graded in accordance with Liu et al.20 with scores of 0, 1, 2, 3, 4, and 5.
Probing Depth (PD)
PD was performed using a round-ended probe with a 0.4-mm-diameter tip. Three points (mesio-buccal, disto-buccal, and mid-buccal) were evaluated in the ligated mandibular right first molar, and the mean of the values was used.20
Myeloperoxidase (MPO) Activity
MPO activity was evaluated through neutrophil accumulation in the gingival tissue around the mandibular first molar. Briefly, 45 mg of gingival tissue was homogenized at 55 mg/mL in potassium buffer containing 0.5% hexadecyltrimethylammonium bromide. The homogenate was centrifuged at 39,000 × g for 6 minutes at 4°C. The pellet was resuspended, and MPO activity was assayed by measuring the change in absorbance at 450 nm using o-dianisidinedihydrochloride and 1% hydrogen peroxide. MPO activity is reported as units/mg of tissue. A unit of MPO activity was defined by the conversion of 1 μmol hydrogen peroxide to water in 60 seconds at 23°C, as described by Chaves et al.21
Measurement of Alveolar Bone Loss (ABL)
To define the cemento-enamel junction (CEJ), mandibles were stained with aqueous methylene blue (1%) after the gingiva was dissected. A stereomicroscope equipped with a 30× objective was used to capture the image of alveolar bone height for each hemimandible. For evaluation of average alveolar bone height, three points were measured on the buccal and lingual parts. Measurements were made along the axis of the root20 by measuring the height from the CEJ to the alveolar crest in: 1) ABL-1, the anterior portion (mesial) of the mandibular first molar; 2) ABL-2, the mesial root of the mandibular first molar; 3) ABL-3, the intermediate root of the mandibular first molar; and 4) ABL-4, the distal root of the mandibular first molar.
These images, as well as the histopathologic assessment, were measured using an image analysis system.‡‡
Histopathologic Assessment of Gingival Tissue and Liver
Specimens were removed, cut into sections, and fixed in 10% buffered formaldehyde. After dehydration in increasing concentrations of alcohol and immersion in xylol, specimens were embedded in paraffin. The 6-μm-thick sections were stained with hematoxylin and eosin (H&E) as well as toluidine blue for mast cell density before assessment with light microscopy.§§
The polymorphonuclear leukocytes (PMNs) and blood vessels were quantified in two typical zones (50 × 50 μm each) of the connective tissue (CT) subjacent to the apical portion of the junctional epithelium (JE) under light microscopy∥∥ at 400× magnification. Additionally, the PMNs were counted in the JE, and its density per area was calculated in three sections (50 × 50 μm each) according to Tomofuji et al.15
Histologic assessment of liver fat and inflammation was scored for steatosis (the percentage of liver cells containing fat): 1+: <25%; 2+: <50%; 3+: <75%; and 4: >75%.22 In addition, for density of mast cells (DMCs) the number of positive cells on toluidine blue/mm2 was counted.
Immunohistochemistry for Pericytes
Pericytes appear as cells positive for neural/glial antigen 2 (NG2) expressed in hepatic tissues; NG2-positive cells were used to count the number of pericytes. After the previous routine histologic proceedings, sections were deparaffinized, and endogenous peroxidase activity was blocked with 4% hydrogen peroxide in water. After blocking non-specific reactivity with 3% bovine serum albumin (BSA) in phosphate-buffered saline (PBS), sections were reacted with each goat polyclonal primary antibody diluted in 1% BSA/PBS: antirat NG2,¶¶ 25 μg/mL, overnight at 4°C. After washing in PBS, sections were incubated with biotinylated secondary antibody,## followed by streptavidin–peroxidase complex*** for 30 minutes at 37°C. Brown staining was made by treating sections with diaminobenzidine solution (3,3 diaminobenzidine),††† and counter-staining was produced with hematoxylin. NG2 positive cells (pericytes) were counted in five sections (50 × 50 μm each). As a negative control, 1% BSA/PBS was used instead of primary antibodies.
GSH Levels
Liver samples were homogenized in 5 mL of cold 0.02-M EDTA solution (1 mL/100 mg tissue). Aliquots (400 μL) of tissue homogenate were mixed with 315 μL distilled water and 85 μL 50% (wt/vol) trichloroacetic acid in glass tubes and centrifuged at 3,000 rpm for 14 minutes. Four hundred microliters of each supernatant was mixed with 800 μL of Tris buffer (0.4 M, pH 8.8) and 25 μL 0.01-M 5,5-dithio-bis (2-nitrobenzoic acid). After shaking the preparation, absorbance was measured at 412 nm on a spectrophotometer. GSH concentration was determined via a reduced GSH standard curve, produced in parallel.23
MDA Concentration
Fragments of hepatic tissue weighing from 90 to 160 mg were homogenized with cold 1.2% KCl to prepare 10% homogenates. In summary, 260 μL of each homogenate was added to 1.5 mL of 1% H3PO4 and 0.5 mL of 0.6% tert-butyl alcohol (aqueous solution). This mixture was agitated and heated, for 50 minutes, in a boiling water bath. The preparation was cooled instantaneously in an ice water bath, followed by addition of 3.5 mL of n-butanol.‡‡‡ This mixture was shaken, and the butanol layer was separated by centrifugation at 1,200 × g for 9 minutes. Optical density was determined to be 535 and 520 nm, and the optical density difference between the two determinations was calculated as the tert-butyl alcohol value.24
Serum Alanine Aminotransferase (ALT) and Aspartate Aminotransferase (AST) Determination
Serum levels of ALT and AST were measured with a commercial enzyme-linked immunosorbent assay kit.§§§
Statistical Analyses
Data are expressed as mean ± standard error of the mean (SEM) and/or median. Distribution of the data was tested through Shapiro–Wilk test. Differences between the two groups were analyzed using Mann–Whitney U tests for non-parametric data and unpaired Student t tests for parametric data.
RESULTS
GBI, PD, and MPO Activity
There were significant changes in the gingival tissues of the periodontitis group compared with the control group (Figs. 1A and 1B). Gingival papillae in the periodontitis group showed alterations in color, severe edema, ulceration, and intensive bleeding compared with controls. There were also significant differences in GBI scoring (P = 0.001) (Fig. 1C).
Figure 1.
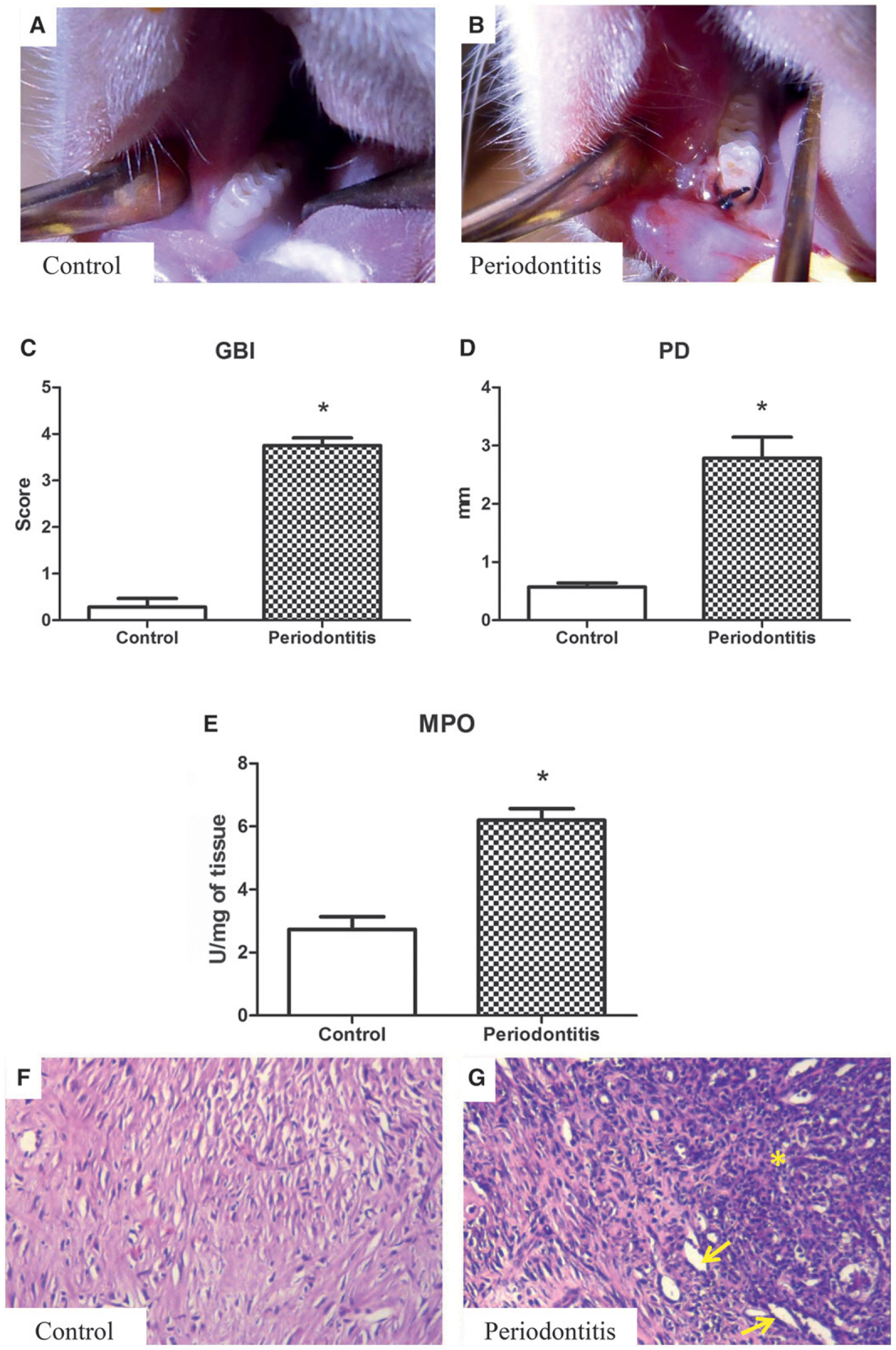
Clinical appearance of the control (A) and periodontitis (B) groups, with the latter showing changes in color, severe edema, ulcers, and bleeding after slight probing. C) GBI scores were significantly higher for the periodontitis group compared with the control group. D) PD is increased in the periodontitis group compared with the control group. E) MPO activity, with the periodontitis group showing significantly higher levels of MPO than the control group. C through E) Results are expressed as mean ± SEM. *P <0.05, periodontitis versus control group. F) Gingival tissue of the control group. G) Intense inflammatory infiltration of the PMNs (indicated by *) in the gingival CT, and increase in the blood vessel density (arrows). F and G) (H&E; original magnification ×300.)
Mean PD value for the periodontitis group was 2.7 ± 0.8 mm versus 0.5 ± 0.1 mm in the control group (P = 0.001; Figure 1D).
MPO activity in the periodontitis group was twice that in the control group (6.1 ± 0.6 U/mg of tissue versus 2.7 ± 0.4 U/mg of tissue, P <0.001), shown in Figure 1E.
Histopathologic Assessment of Gingival Tissue
The control group exhibited healthy periodontal tissues (Fig. 1F). The periodontium of the periodontitis group revealed intense inflammatory cell infiltration within the JE and CT, as shown by H&E staining (Fig. 1G). In addition, the periodontitis group showed an increase in blood vessel density (Table 1 and Fig. 1G).
Table 1.
Histopathologic Assessment of Gingiva and Liver
| Parameters | Control | Periodontitis |
|---|---|---|
| Gingival | ||
| PMN density within the JE† (per 2,500 μm2) | 3.1 ± 0.5 | 8.3 ± 0.8* |
| PMN density within the CT† (per 2,500 μm2) | 2.1 ± 0.6 | 9.5 ± 0.6* |
| Blood vessel density† (per 2,500 μm2) | 1.3 ± 0.3 | 3.8 ± 0.7* |
| Liver | ||
| Liver median score‡ | 0 (0–1) | 3 (2–3)* |
| Mast celldensity† (per 2,500 μm2) | 1.9 ± 0.8 | 2.1 ± 0.4 |
| Pericytes† (equal to unity) | 4.9 ± 0.37 | 1.2 ± 0.29* |
Pericytes were counted by immunohistochemistry for NG2-positive cells. Results were expressed as mean ± SEM (t test) or median and range (Mann–Whitney U test).
Statistical difference (P <0.05) between periodontitis and control groups.
Unpaired t test.
Mann–Whitney U test.
Measurement of ABL
Figures 2A and 2B represent the changes in alveolar bone in the control and periodontitis group, respectively. There are statistically significant differences (P <0.05) in the four parameters evaluated (ABL1: P = 0.002; ABL2: P = 0.04; ABL3: P = 0.001; and ABL4: P <0.001) between the control and periodontitis groups (Fig. 2C).
Figure 2.
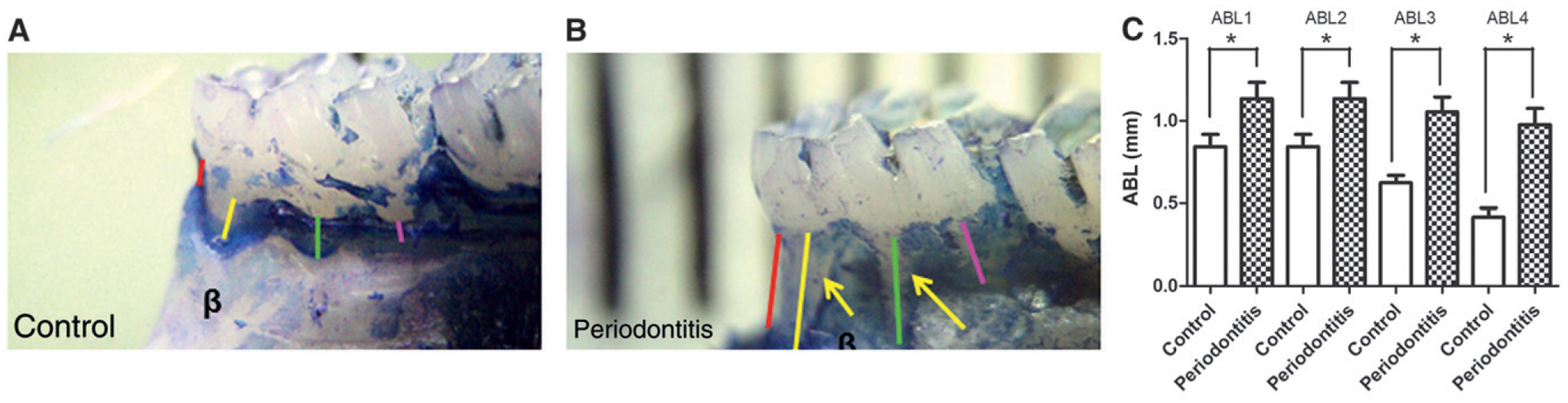
Clinical and statistical differences between alveolar bone in control and periodontitis groups. A and B) The clinical difference between the alveolar bone (β) in the control and periodontitis groups, respectively; arrows indicate ABL. Red line = ABL1; yellow line = ABL2; green line = ABL3; pink line = ABL4. C) Illustration of the significant difference between the four evaluated sites (ABL1, ABL2, ABL3, and ABL4), comparing the periodontitis group with the control group, with an increase in ABL for the periodontitis group (ligated teeth). Results are expressed as mean ± SEM. *P <0.05, periodontitis versus control group.
Values for GBI, PD, MPO, and ABLs, as well as the histopathology of the gingival tissue, were strong evidence for the presence of induced periodontitis, enabling subsequent systemic evaluations.
Animal Total and Liver Weights
There was no significant difference in liver weight between the control and periodontitis groups (P >0.05), as shown in Table 2.
Table 2.
Body Weight and Absolute and Relative Liver Weight
| Group | Body Weight (g) | Liver Weight | |
|---|---|---|---|
| Absolute (g) | Relative (%) | ||
| Control | 192.6 ± 4.4 | 6.4 ± 0.9 | 3.35 ± 0.2 |
| Periodontitis | 189.3 ± 3.1 | 6.5 ± 0.8 | 3.49 ± 0.3 |
Relative weights are organ weight/body weight (g), mean of individual ratios ± SEM for values, measured at time of sacrifice. Comparison between the weights of organs in the control and periodontitis group showed no statistically significant difference in any parameter (P >0.05).
Histologic and Immunohistochemistry Assessment of Liver
Figure 3A shows the normal liver structure of the control group. Hepatic tissues in the periodontitis group showed a slight constriction of the central vein, partial loss of the radiating organization of hepatocytes in cordons, and degeneration and size variability of hepatocytes (Fig. 3B). Prevalence of steatosis in the periodontitis group, assayed by histopathologic scoring, was significantly higher compared with the control group (Table 1). In addition, extensive microvesicular steatosis was seen around the central vein (Fig. 3B). There was no fibrosis in the portal and sinusoidal areas of animals. In addition, there was no difference (P >0.05) in DMCs in the periodontitis and control groups (Table 1, Figs. 3C and 3D). The immunohistochemistry assessment of the liver, shown in Figures 3E and 3F, demonstrated that the induced periodontitis was associated with a significant (P <0.05) reduction of NG2-positive cells (pericytes) (4.9 ± 0.37, 1.2 ± 0.29 NG2-positive cells per 2,500 μm2 of the liver for control and periodontitis groups, respectively) compared with the control group (Table 1).
Figure 3.
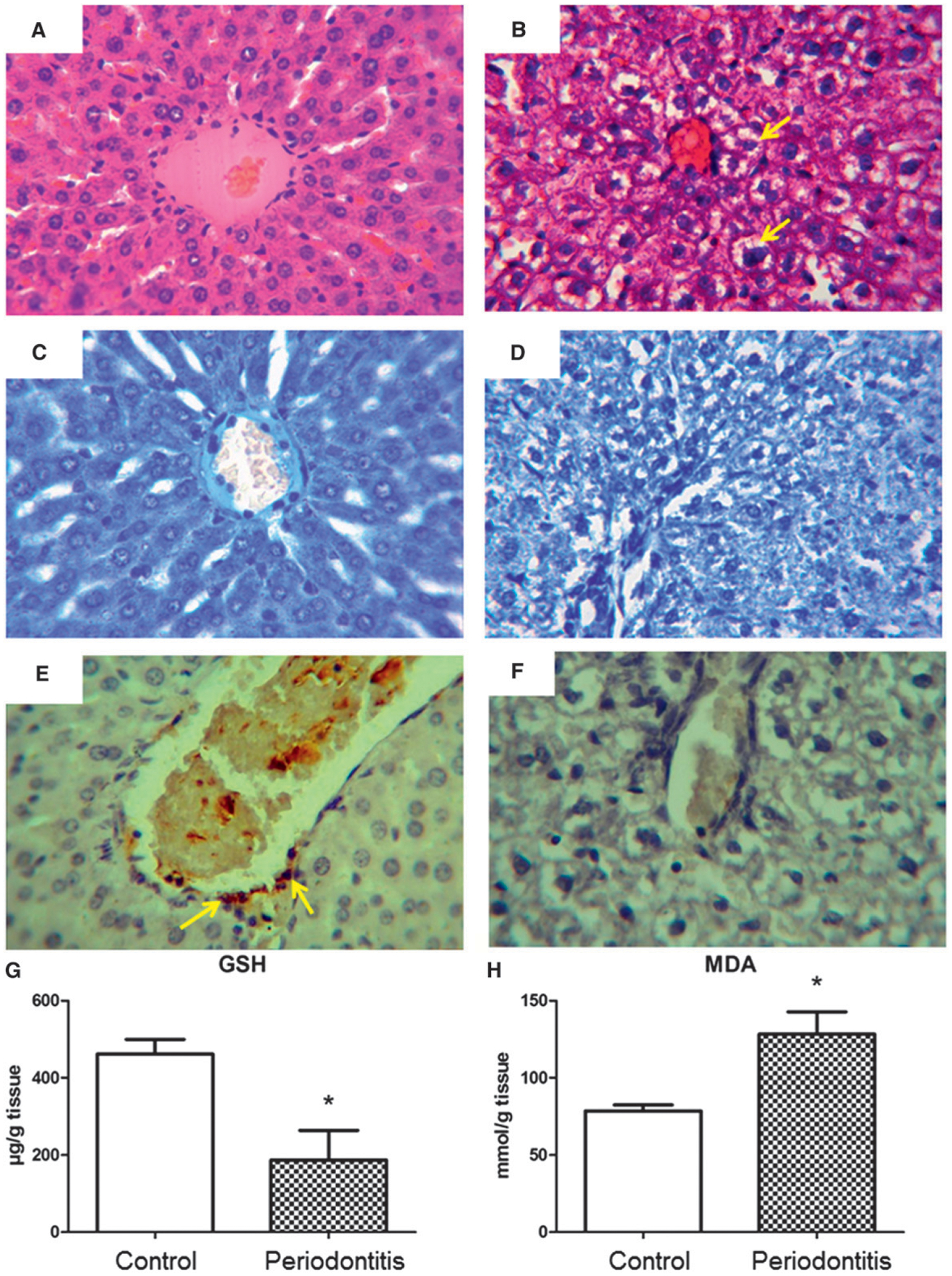
A and C) Liver of the control group, which did not show histologic changes, and the normal architecture of hepatocytes radiating from the central vein. B and D) Liver of the periodontitis group, presenting a slight constriction of the central vein and partial loss of radiating organization of hepatocytes in cordons, degeneration, and size variability of the hepatocytes, as well as microvesicular steatosis (arrows) around the vein area. A and B) (H&E; original magnification ×600.) C and D) (Toluidine blue; original magnification ×600.) E) NG2-positive cells (pericytes; arrows) in control group. F) Reduction in hepatic pericytes in the periodontitis group. E and F) (Immunostaining; original magnification ×600.) G) Liver GSH levels, with a significantly higher concentration for the control group compared with the periodontitis group. H) MDA liver concentration shows significantly higher lipid peroxidation for the periodontitis group compared with the control group. G and H) Results are expressed as mean ± SEM. *P <0.05, periodontitis versus control group.
GSH and MDA for Liver
As shown in Figure 3G, induced periodontitis was associated with a significant (P = 0.02) decrease in liver GSH content (461.6 ± 38.1 and 186.8 ± 76.7 μg/g of tissue for control and periodontitis groups, respectively) compared with the control group.
There was also increased lipid peroxidation in the liver, as shown by MDA levels in the periodontitis group compared with the control group (128.3 ± 14.4 mmol/g of tissue versus 78.6 ± 3.8 mmol/g of tissue, P = 0.006) (Fig. 3H).
Serum ALT and AST
Serum levels of ALT (control group: 45.3 ± 17.3 U/L; periodontitis group: 40.0 ± 24.9 U/L; P >0.05), and AST (control group: 91.0 ± 13.1 U/L; periodontitis group: 88.0 ± 11.1 U/L; P >0.05) were not significantly different between the two groups.
DISCUSSION
In recent years there has been emerging evidence for an association between periodontitis and liver disease.8,9,15,25 It has been demonstrated that liver transplant candidates have significantly higher prevalence of apical periodontitis than healthy control patients.26 Earlier data has suggested the inflammatory condition present in periodontal disease contributes to the release of inflammatory cytokines, such as interleukin (IL)-1b, IL-6, and IL-17a,27,28 and increases oxidative stress and lipid peroxidation,29 not only in the periodontium, gingival fluid,30 blood serum, and saliva,4,5 but also in the liver. These finding have been, however, largely based on association studies in vitro or indirect findings from unrelated disease models. Hence, the mechanism underlying the association between periodontitis and liver disease had remained unclear. In the present study, the effect of induced periodontitis on liver parameters was examined in an in vivo induced periodontitis model.
GBI, PD, MPO activity, ABL, and gingival histopathologic findings were all indicative of a robust model of periodontal disease. Using this animal model it was possible to demonstrate that induced periodontitis can result in microvesicular steatosis. The induced periodontitis was also associated with altered structural organization of hepatocytes in cordons and degeneration and size variability of the hepatocytes, similar to injuries observed in response to long-time exposure to commercial chemicals.31 These changes are considerable, given the short period of induction. There was no difference in DMCs between periodontitis and control groups. This is in contrast to a previous study that observed an increase in DMCs in hepatic steatosis.13 Induced periodontal disease in the current model was also not associated with any alterations in body weights and liver function. Furthermore, some other features of non-alcoholic steatohepatitis,22 such as liver fibrosis or Mallory body formation, were absent in the current model. Overall, the hepatic histologic changes found in the periodontitis group were not severe. It is, however, conceivable that prolonged periodontitis could have potentially increased the risk for advanced liver disease.
Interestingly, the number of pericytes (NG2-positive cells) was decreased in the hepatic vasculature of the animals with induced periodontitis. To the best knowledge of the authors, this is the first study that demonstrates a correlation between pericyte number and steatosis induced by periodontitis. A reduction in pericytes in the kidney has been shown to be a critical step in the development of fibrosis.32 It has been suggested that NG2 positive cells may act as repository cells with great potential for differentiation.14 Similarly, findings from the current study suggest that reduction of hepatic pericytes is associated with microvesicular steatosis. Future studies are needed to clarify this association.
Liver diseases begin with accumulation of lipid into hepatocytes.12,15 These injuries may occur because of an overproduction of free radicals, nitrogen, and reactive oxygen species, commonly produced as byproducts of cell metabolism. Under physiologic conditions, GSH protects the liver from oxidative stress, whereas its depletion has been associated with lipid accumulation in hepatocytes.33 In the current periodontal disease model, there was a significant reduction of GSH compared with control animals. This finding is consistent with a previous report,9 which showed induced periodontitis increases 8-hydroxydeoxyguanosine by lipid peroxidation. In addition, the levels of malondialdehyde, which disrupts liver GSH, were significantly increased in the liver of the animals with periodontitis.
MDA is one end-product of lipid peroxidation; thus, increased MDA content in the current periodontitis model compared with controls reflects increased oxidative stress. Moreover, it is well known that MDA plays a pivotal role in liver injury.12,33 Reduced GSH and increased MDA may, in part, explain changes observed in hepatic tissues in animals with periodontitis. Serum AST and ALT levels, two enzymes that indicate hepatocellular injury,34 were not increased in mice with induced periodontitis. This result is consistent with previously published data.15
The treatment of periodontal disease also has been linked to favorable local and systemic effects. Accordingly, the therapy for periodontal disease has been shown to be associated with both reduction of oxidative stress in the periodontum5,18 and reduced liver fibrosis.8 Periodontitis may be an important risk factor for liver disease, but further studies in animals and humans are necessary to consolidate this hypothesis.
CONCLUSIONS
The current study results link the systemic effects of induced periodontitis with changes in hepatic tissues such as microvesicular steatosis, likely caused by an increase in oxidative stress and lipid peroxidation. The current findings implicate that a decrease of pericytes is associated with liver disease caused by ligature-induced periodontitis in rats.
ACKNOWLEDGMENTS
The research was supported by the Federal University of Piaui (UFPI - Edital PIBIC 2014/2015 and BIAMA 03/2014; CNPq 455104/2014-0). The authors thank Professor Francisco Humberto Nociti Junior from the School of Dentistry, State University of Campinas, Piracicaba, Brazil for the insights on the paper. The authors thank Professor Marcelo Rocha Marques from School of Dentistry, State University of Campinas for collaboration regarding the antibodies. DFPV and JSO contributed to the concept, design, statistical analysis, and drafting of the manuscript. FRPS, MSCP, LABS, IGS, LKMS, NCMO, CAV, ALRB, JVRM, DFPV, and JSO contributed to the data collection and manuscript drafting. IM, PDN, and AM contributed to the drafting and critical review of the manuscript. All authors approved the final version for submission. The authors report no conflicts of interest related to this study.
Footnotes
Rompun, Bayer, São Paulo, Brazil.
Francotar, Virbac, Roseira, Brazil.
Shalon, Goiania, Brazil.
ImageJ v.1.48, US National Institutes of Health, Bethesda, MD.
Nova, Piracicaba, Brazil.
Nova, Piracicaba, Brazil.
Sigma-Aldrich, St. Louis, MO.
LSAB2 system, DAKO, Glostrup, Denmark.
LSAB2 system, DAKO.
LSAB2 system, DAKO.
Dinamica, Diadema, Brazil.
Labtest, Lagoa Santa, Brazil.
REFERENCES
- 1.Kassebaum NJ, Bernabé E, Dahiya M, Bhandari B, Murray CJL, Marcenes W. Global burden of severe periodontitis in 1990–2010: A systematic review and meta-regression. J Dent Res 2014;93:1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knight ET, Liu J, Seymour GJ, Faggion CM Jr., Cullinan MP. Risk factors that may modify the innate and adaptive immune responses in periodontal diseases. Periodontol 2000 2016;71:22–51. [DOI] [PubMed] [Google Scholar]
- 3.Martande SS, Kumari M, Pradeep AR, Singh SP, Suke DK, Guruprasad CN. Platelet-rich fibrin combined with 1.2% atorvastatin for treatment of intrabony defects in chronic periodontitis: A randomized controlled clinical trial. J Periodontol 2016;87:1039–1046. [DOI] [PubMed] [Google Scholar]
- 4.Tonguç MÖ,Öztürk O, Sütçü R, et al. The impact of smoking status on antioxidant enzyme activity and malondialdehyde levels in chronic periodontitis. J Periodontol 2011;82:1320–1328. [DOI] [PubMed] [Google Scholar]
- 5.Hendek MK, Erdemir EO, Kisa U, Ozcan G. Effect of initial periodontal therapy on oxidative stress markers in gingival crevicular fluid, saliva, and serum in smokers and non-smokers with chronic periodontitis. J Periodontol 2015;86:273–282. [DOI] [PubMed] [Google Scholar]
- 6.de Smit MJ, Westra J, Brouwer E, Janssen KM, Vissink A, van Winkelhoff AJ. Periodontitis and rheumatoid arthritis: What do we know? J Periodontol 2015;86:1013–1019. [DOI] [PubMed] [Google Scholar]
- 7.Sonnenschein SK, Meyle J. Local inflammatory reactions in patients with diabetes and periodontitis. Periodontol 2000 2015;69:221–254. [DOI] [PubMed] [Google Scholar]
- 8.Nagao Y, Kawahigashi Y, Sata M. Association of periodontal diseases and liver fibrosis in patients with HCV and/or HBV infection. Hepat Mon 2014;14:e23264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomofuji T, Ekuni D, Irie K, et al. Relationships between periodontal inflammation, lipid peroxide and oxidative damage of multiple organs in rats. Biomed Res 2011; 32:343–349. [DOI] [PubMed] [Google Scholar]
- 10.Zoller H, Tilg H. Nonalcoholic fatty liver disease and hepatocellular carcinoma. Metabolism 2016;65:1151–1160. [DOI] [PubMed] [Google Scholar]
- 11.Fung J, Lee CK, Chan M, Seto WK, Lai CL, Yuen MF; Hong Kong Liver Health Census Study Group. High prevalence of non-alcoholic fatty liver disease in the Chinese – Results from the Hong Kong liver health census. Liver Int 2015;35:542–549. [DOI] [PubMed] [Google Scholar]
- 12.Desai S, Baker SS, Liu W, et al. Paraoxonase 1 and oxidative stress in paediatric non-alcoholic steatohepatitis. Liver Int 2014;34:110–117. [DOI] [PubMed] [Google Scholar]
- 13.Franceschini B, Russo C, Dioguardi N, Grizzi F. Increased liver mast cell recruitment in patients with chronic C virus-related hepatitis and histologically documented steatosis. J Viral Hepat 2007;14:549–555. [DOI] [PubMed] [Google Scholar]
- 14.Khan JA, Mendelson A, Kunisaki Y, et al. Fetal liver hematopoietic stem cell niches associate with portal vessels. Science 2016;351:176–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomofuji T, Ekuni D, Yamanaka R, et al. Chronic administration of lipopolysaccharide and proteases induces periodontal inflammation and hepatic steatosis in rats. J Periodontol 2007;78:1999–2006. [DOI] [PubMed] [Google Scholar]
- 16.Furusho H, Miyauchi M, Hyogo H, et al. Dental infection of Porphyromonas gingivalis exacerbates high fat diet-induced steatohepatitis in mice. J Gastroenterol 2013; 48:1259–1270. [DOI] [PubMed] [Google Scholar]
- 17.Morita T, Yamazaki Y, Fujiharu C, et al. Serum g-glutamyltransferase level is associated with periodontal disease independent of drinking habits in Japanese adults. Med Sci Monit 2014;20:2109–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muniz FW, Nogueira SB, Mendes FL, et al. The impact of antioxidant agents complimentary to periodontal therapy on oxidative stress and periodontal outcomes: A systematic review. Arch Oral Biol 2015;60:1203–1214. [DOI] [PubMed] [Google Scholar]
- 19.Cai X, Li C, Du G, Cao Z. Protective effects of baicalin on ligature-induced periodontitis in rats. J Periodontal Res 2008;43:14–21. [DOI] [PubMed] [Google Scholar]
- 20.Liu R, Li N, Liu N, et al. Effects of systemic ornidazole, systemic and local compound ornidazole and pefloxacin mesylate on experimental periodontitis in rats. Med Sci Monit 2012;18:BR95–BR102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaves LdeS, Nicolau LA, Silva RO, et al. Antiinflammatory and antinociceptive effects in mice of a sulfated polysaccharide fraction extracted from the marine red algae Gracilaria caudata. Immunopharmacol Immunotoxicol 2013;35:93–100. [DOI] [PubMed] [Google Scholar]
- 22.Younossi ZM, Baranova A, Ziegler K, et al. A genomic and proteomic study of the spectrum of nonalcoholic fatty liver disease. Hepatology 2005;42:665–674. [DOI] [PubMed] [Google Scholar]
- 23.Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 1968;25:192–205. [DOI] [PubMed] [Google Scholar]
- 24.Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 1978;86:271–278. [DOI] [PubMed] [Google Scholar]
- 25.Saito T, Shimazaki Y, Koga T, Tsuzuki M, Ohshima A. Relationship between periodontitis and hepatic condition in Japanese women. J Int Acad Periodontol 2006; 8:89–95. [PubMed] [Google Scholar]
- 26.Castellanos-Cosano L, Machuca-Portillo G, Segura-Sampedro JJ, et al. Prevalence of apical periodontitis and frequency of root canal treatments in liver transplant candidates. Med Oral Patol Oral Cir Bucal 2013; 18:e773–e779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonçalves TO, Costa D, Brodskyn CI, Duarte PM, César Neto JB, Nogueira-Filho G. Release of cytokines by stimulated peripheral blood mononuclear cells in chronic periodontitis. Arch Oral Biol 2010;55:975–980. [DOI] [PubMed] [Google Scholar]
- 28.Matsuda Y, Kato T, Takahashi N, et al. Ligature-induced periodontitis in mice induces elevated levels of circulating interleukin-6 but shows only weak effects on adipose and liver tissues. J Periodontal Res 2016;51:639–646. [DOI] [PubMed] [Google Scholar]
- 29.Grant MM, Brock GR, Matthews JB, Chapple IL. Crevicular fluid glutathione levels in periodontitis and the effect of non-surgical therapy. J Clin Periodontol 2010;37:17–23. [DOI] [PubMed] [Google Scholar]
- 30.Palwankar P, Rana M, Arora K, Deepthy C. Evaluation of non-surgical therapy on glutathione levels in chronic periodontitis. Eur J Dent 2015;9:415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rui D, Daojun C, Yongjian Y. Liver and heart toxicity due to 90-day oral exposure of ICR mice to N,N-dimethylformamide. Environ Toxicol Pharmacol 2011; 31:357–363. [DOI] [PubMed] [Google Scholar]
- 32.Stefanska A, Eng D, Kaverina N, et al. Interstitial pericytes decrease in aged mouse kidneys. Aging (Albany, NY) 2015;7:370–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y, Dong H, Thompson DC, Shertzer HG, Nebert DW, Vasiliou V. Glutathione defense mechanism in liver injury: Insights from animal models. Food Chem Toxicol 2013;60:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang H, Xue L, Yan R, et al. Comparison of histologic characteristics of Chinese chronic hepatitis B patients with persistently normal or mildly elevated ALT. PLoS One 2013;8:e80585. [DOI] [PMC free article] [PubMed] [Google Scholar]


