Abstract
Background
Endothelial dysfunction is one of the underlying mechanisms to vascular and cardiac complications in patients with COVID-19. We sought to investigate the systemic vascular endothelial function and its temporal changes in COVID-19 patients from a non-invasive approach with reactive hyperemia peripheral arterial tonometry (PAT).
Methods
This is a prospective, observational, case-control and blinded study. The population was comprised by 3 groups: patients investigated during acute COVID-19 (group 1), patients investigated during past COVID-19 (group 2), and controls 1:1 matched to COVID-19 patients by demographics and cardiovascular risk factors (group 3). The natural logarithmic scaled reactive hyperemia index (LnRHI), a measure of endothelium-mediated dilation of peripheral arteries, was obtained in all the participants and compared between study groups.
Results
144 participants were enrolled (72 COVID-19 patients and 72 matched controls). Median time from COVID-19 symptoms to PAT assessment was 9.5 and 101.5 days in groups 1 and 2, respectively. LnRHI was significantly lower in group 2 compared to both group 1 and controls (0.53 ± 0.23 group 2 vs. 0.72 ± 0.26 group 1, p = 0.0043; and 0.79 ± 0.23 in group 3, p < 0.0001). In addition, within group 1, it was observed a markedly decrease in LnRHI from acute COVID-19 to post infection stage (0.73 ± 0.23 vs. 0.42 ± 0.26, p = 0.0042).
Conclusions
This study suggests a deleterious effect of SARS-CoV-2 infection on systemic vascular endothelial function. These findings open new venues to investigate the clinical implication and prognostic role of vascular endothelial dysfunction in COVID-19 patients and post-COVID syndrome using non-invasive techniques.
Keywords: COVID-19, SARS-CoV-2, Systemic vascular endothelial function
1. Introduction
In addition to respiratory failure, patients with coronavirus disease-2019 (COVID-19) may suffer other life-threatening complications including acute cardiac failure, vascular thrombotic phenomena and multiorgan injury [1]. Severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) uses angiotensin-converting enzyme 2 (ACE2) receptor as a means to enter the host [2]. High density of ACE2 receptor in vascular endothelial cells may explain the development of vasculitis and endothelial dysfunction linked to vascular complications in COVID-19 [3], as documented in post-mortem studies [4]. In this study, we aimed to investigate in-vivo vascular endothelial function and its temporal changes in COVID-19 patients, compared to control subjects from a non-invasive approach with peripheral arterial tonometry (PAT).
2. Methods
2.1. Study design and population
This is a prospective, observational, case-control, and blinded study conducted from June 1 through November 20, 2020. The study included patients with history of COVID-19 that underwent assessment of vascular endothelial function in Hospital Clinico San Carlos, Madrid, Spain, at different stages of infection: group 1, constituted by patients assessed during acute phase of COVID-19 as documented by nasal swabs polymerase-chain-reaction assay (PCR); and group 2, constituted by patients assessed during post SARS-CoV-2 infection stage as documented by repeated negative PCR during follow-up. In addition, assessment of vascular endothelial function was repeated at post infection stage in patients within group 1. To assess the effect of SARS-CoV-2 infection on vascular endothelial function, the endothelial function in COVID-19 patients was compared with a matched control group comprised by subjects selected from an historical, pre-COVID 19 pandemic cohort of volunteers who underwent PAT evaluation as part of baseline assessment of cardiovascular risk at Mayo Clinic (Rochester, MN, USA). Written informed consent was obtained from all the participants, and the protocol was approved by local institutional review board (20/451-E COVID). The study was registered at ClinicalTrials (NCT04525443).
2.2. Assessment of vascular endothelial function
Systemic endothelial vascular function was assessed with non-invasive PAT using plethysmography-derived EndoPAT® technology (Itamar MedicalTm, Israel) as previously described [5]. Briefly, this method measures the changes in patient pulsatile arterial volume at the tip of the fingers from baseline to reactive hyperemia following upper arm occlusion of systolic blood pressure. From this technology, the natural logarithmic scaled reactive hyperemia index (LnRHI) is obtained an informs on the magnitude of endothelium-mediated dilation of peripheral arteries. All PAT studies from COVID-19 patients were analyzed blindly with respect to disease stage at a central corelab in Mayo Clinic, MN, USA.
2.3. Statistical analysis
Continuous variables are reported as median with interquartile range (IQR), and categorical variables as number and percentage. Comparison of continuous variables between study groups was performed with Mann-Whitney U test or Wilcoxon Rank test, as appropriate, whereas categorical variables were compared using Fisher's exact test or Pearson chi-square test, as appropriate. We aimed to select one control subject (group 3) for each COVID-19 patient in groups 1 and 2. Controls were individually matched to cases by age, sex, body mass index and cardiovascular risk factors including hypertension, dyslipidemia, diabetes mellitus, current smoking, and a history of coronary artery disease. Differences were considered statistically significant at p < 0.05 (2-sided). STATA software version 14 (StataCorp, College Station, TX) was used for statistical analyses.
3. Results
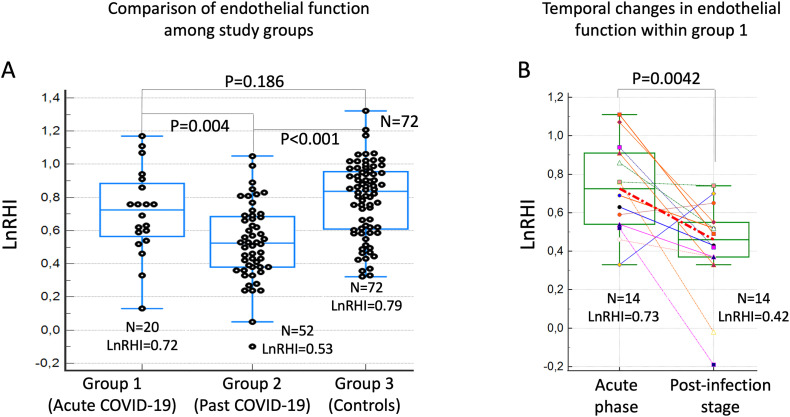
The study included a total of 144 participants: 72 COVID-19 patients (N = 20 in group 1, and N = 52 in group 2) and 72 matched controls (Group 3). After matching, no significant differences were found between COVID-19 patients and controls regarding age, gender, body mass index, hypertension, dyslipidemia, diabetes mellitus, smoking habit and history of coronary artery disease (Table 1 ). Median time from acute COVID-19 symptoms to PAT assessment was 9.5 days [interquartile range, 6] in group 1 and 101.5 [36] days in group 2. There was not statistically difference between acute COVID-19 patients and controls in PAT measurements (group 1 LnRHI 0.72 [0.26] vs. group 3 0.79 [0.23], respectively; p = 0.1868). However, compared to both acute COVID-19 group and controls subjects, patients with PAT assessment during post infection stage (group 2) had a significantly lower LnRHI (0.53 [0.23]): group 2 vs. group 1, p = 0.0043; group 2 vs. group 3, p < 0.0001 (Fig. 1A). Additionally, analysis of paired PAT data obtained in 14 COVID-19 patients within group 1 (median time interval 80 [22] days between acute and post-infection stage PAT assessment) showed a markedly decreased in LnRHI from acute to post infection stage: 0.73 [0.23] vs. 0.42 [0.26], p = 0.0042 (Fig. 1B).
Table 1.
Characteristics of the study population and reactive hyperaemia–peripheral arterial tonometry.
| Variable | GROUP 1 (Active COVID-19) |
Group 2 (Past COVID-19) |
Overall COVID-19 (Groups 1 and 2) | Group 3 (Matched controls) |
P value (COVID-19 vs matched controls) |
|---|---|---|---|---|---|
| Baseline characteristics | |||||
| Age | 54 (18) | 59 (25) | 54.8 (15.4) | 53.9 (14.7) | 0.717 |
| Female sex | 9 (45.0%) | 27 (52.9%) | 36 (50.7%) | 36 (50.0%) | 0.933 |
| Body mass index | 28.1 (6.7) | 27.4 (7.5) | 28.4 (5.1) | 28.6 (5.2) | 0.803 |
| Hypertension | 6 (30.0%) | 19 (37.3%) | 25 (35.2%) | 25 (34.7%) | 0.951 |
| Dyslipidaemia | 5 (25.0%) | 16 (31.4%) | 21 (29.6%) | 29 (40.3%) | 0.180 |
| Diabetes | 3 (15.0%) | 10 (19.6%) | 13 (18.3%) | 10 (13.9%) | 0.472 |
| Current smoker | 0 (0%) | 2 (3.92%) | 2 (2.8%) | 2 (2.8%) | 0.989 |
| Coronary artery disease |
0 (0%) | 5 (9.80%) | 5 (7.0%) | 8 (11.1%) | 0.397 |
| Atrial fibrillation | 0 (0%) | 1 (1.96%) | 1 (1.4%) | 6 (8.3%) | 0.055 |
| Peripheral artery disease |
0 (0%) | 1 (1.96%) | 1 (1.4%) | 1 (1.4%) | 0.992 |
| Reactive hyperaemia–peripheral arterial tonometry | |||||
| Time from symptoms to EndoPAT (days) |
9.5 (6) | 101.5 (36) | NA | NA | <0.001 |
| Time from admission to EndoPAT (days) |
3 (3) | 92 (27) | NA | NA | <0.001 |
| LnRHI | 0.72 (0.26) | 0.53 (0.23) | 0.58 (0.25) | 0.79 (0.23) | <0.001 |
Values are presented as n (%) or n (interquartile range). LnRHI = natural logarithmic scaled reactive hyperemia index; NA = not applicable.
Fig. 1.
Comparison of endothelial vasodilator function between study groups as determined by PAT.
Legend: A. Box-Whisker plots comparing LnRHI values between study groups. Patients with PAT assessment performed during post-infection stage (group 2) had a significantly reduced vasodilator function compared to the other groups. B. Pair PAT data with dot-line diagram and Box-Whisker plots showing a markedly decreased in the endothelial vasodilator function from acute to post-infection stage within same patients (group 1 only).
4. Discussion
Our findings provide in-vivo evidence on the presence and temporal evolution of endothelial vascular dysfunction associated to COVID-19. We found that, compared to controls, flow-mediated dilation of peripheral arteries is substantially lower in COVID-19 patients during post-infection stage. Temporal changes in LnRHI from acute COVID-19 to post-infection stage suggest that endothelial vascular dysfunction may be a chronic complication of the SARS-CoV-2 infection.
The vascular endothelium acts as an interface between blood cells and tissues, and as such, plays a key role in mediating vascular tone, homeostasis, inflammation, and coagulation. SARS-CoV-2, the etiological virus of COVID-19, bind to cells via ACE2 receptor, a single-pass transmembrane protein which is very abundant in vascular endothelial cells [2]. These facts have been proposed to explain post-mortem findings suggesting the development of vasculitis and endothelial dysfunction found in COVID-19 patients and the development of vascular complications of the disease [4].
Under normal conditions, the endothelial cells trigger vasodilatation through production of nitric oxide via the activity of endothelial nitric oxide synthase [6]. In the present study, indirect evaluation of this endothelial vasodilator function was performed non-invasively using PAT [5,7]. The consistent decrease observed over the time in LnRHI values from acute to past infection stage suggest a deleterious post infection effect of SARS-CoV-2 on systemic endothelial vascular function. These changes may mirror derangement of key functions of vascular endothelium caused by SARS-CoV-2 at a systemic level, which have been proposed as a cause of multiorgan injury and cardiovascular complications widely described in COVID-19 [1]. In support of this hypothesis, it has been recently reported that COVID-19 patients have increased rates of vascular complications after being discharged from hospital compared with the general population, as depicted by higher rates of readmission, respiratory disease, chronic kidney disease and major adverse cardiovascular event [8].
5. Study limitations
Our study has several limitations. First, we have assessed only one of the multiple functions of vascular endothelium, named mediation of vascular tone through reactive hyperemia. Changes in other parameters potentially related with endothelial function including inflammation and coagulation were not addressed in this study. Second, we cannot rule out potential bias in the results of PAT assessment during acute COVID-19 secondary to medication during admission including corticoids, antiviral drugs, and antibiotics among others. Third, our study lacks objective information on long-COVID-19 symptoms that can be correlated with long COVID-19 syndrome and PAT results. Finally, given the fact that our control group was constituted by an historical cohort of volunteers assessed with PAT several years before COVID-19 pandemic, we do not have access to raw data in the control group to compare separately the components of LnRHI (i.e., arterial tone at baseline vs. at reactive hyperemia) between groups, which could add important further information to elucidate the mechanisms of reduce LnRHI at post COVID-19 infection stage.
6. Conclusions
There is a deleterious effect of SARS-CoV-2 infection in the systemic vascular endothelial function from acute COVID-19 to post infection stage as determined by PAT. These findings open new venues to investigate the clinical implication and prognostic role of vascular endothelial dysfunction in COVID-19 patients and post COVID-19 syndrome using non-invasive techniques.
Declaration of Competing Interest
The authors state that they do not have any conflict of interest related with the submitted study.
References
- 1.Bikdeli B., Madhavan M.V., Jimenez D., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Libby P., Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur. Heart J. 2020;41:3038–3044. doi: 10.1093/eurheartj/ehaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varga Z., Flammer A.J., Steiger P., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet Lond. Engl. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubinshtein R., Kuvin J.T., Soffler M., et al. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur. Heart J. 2010;31:1142–1148. doi: 10.1093/eurheartj/ehq010. [DOI] [PubMed] [Google Scholar]
- 6.Furchgott R.F. Endothelium-derived relaxing factor: discovery, early studies, and Identifcation as nitric oxide (Nobel lecture) Angew. Chem. Int. Ed. Eng. 1999;38:1870–1880. doi: 10.1002/(SICI)1521-3773(19990712)38:13/14<1870::AID-ANIE1870>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 7.Al A., Fa G., Jp C. Assessing endothelial vasodilator function with the endo-PAT 2000. J. Vis. Exp. JoVE. 2010;44 doi: 10.3791/2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ayoubkhani D., Khunti K., Nafilyan V., et al. Post-covid syndrome in individuals admitted to hospital with covid-19: retrospective cohort study. BMJ. 2021;372:n693. doi: 10.1136/bmj.n693. [DOI] [PMC free article] [PubMed] [Google Scholar]