Abstract
Background
Allergic and nonallergic adverse reactions have been reported with global coronavirus disease 2019 (COVID-19) vaccination. It was previously hypothesized that polyethylene glycol (PEG) may be responsible for anaphylactic reactions to messenger RNA (mRNA) COVID-19 vaccines.
Objective
To report the workflow established at our institution, types, and frequency of adverse reactions to mRNA COVID-19 vaccines in patients presenting for allergy evaluation.
Methods
A COVID-19 vaccine adverse reaction registry was established. We used PEG prick skin testing, followed by PEG challenges in selected cases, to ensure PEG tolerance and encourage completion of COVID-19 vaccination series.
Results
A total of 113 patients were included. Most vaccine reactions (86.7%) occurred in women. Anaphylaxis occurred only in women, all of which had a history of allergic disease and two-thirds had asthma. Anaphylaxis rate was 40.6 cases per million. None of the anaphylactic cases developed hypotension, required intubation, or required hospital admission. Systemic allergic symptoms, not fulfilling anaphylaxis criteria, were significantly more common in Pfizer-BioNTech than Moderna-vaccinated patients (P = .02). We observed a higher incidence of dermatologic nonurticarial reactions in men (P = .004). Among first-dose reactors, 86.7% received and tolerated the second dose. We observed a high rate of false-positive intradermal skin test results and frequent subjective symptoms with oral PEG challenge.
Conclusion
Intradermal PEG testing has limited utility in evaluating anaphylaxis to mRNA vaccines. Most severe postvaccination allergic symptoms are not caused by hypersensitivity to PEG. Most people with reaction to the initial mRNA vaccine can be safely revaccinated. Patients with anaphylaxis to COVID-19 vaccines benefit from physician-observed vaccination.
Introduction
As of October 1, 2021, more than 233 million coronavirus disease 2019 (COVID-19) cases and more than 4.77 million COVID-19–related deaths have been reported. Highly immunogenic COVID-19 vaccines offer robust protection1 , 2 and are powerful tools to control the pandemic. Within the first few days of international vaccination efforts, systemic allergic reactions were reported at rates higher than those with other vaccines.3, 4, 5 Consequently, in December 2020, the Centers for Disease Control and Prevention (CDC) recommended against vaccinating people with severe or immediate allergic reactions after a dosage of a COVID-19 messenger RNA (mRNA) vaccine, or any of its components, and suggested referral to an allergist-immunologist for further evaluation.6 , 7 It has been hypothesized that polyethylene glycol (PEG) may be responsible for anaphylactic reactions to the COVID-19 mRNA vaccines,8 and initial guidance on risk stratification and management of patients with allergy to the mRNA vaccines, was based on PEG testing.9
Although multiple factors contribute to vaccine hesitancy, concern on adverse effects is one of the leading causes of vaccine refusal and delay in vaccination.10 As allergists, we addressed patients’ questions on allergic and other adverse reactions to COVID-19 vaccines to minimize vaccine hesitancy. We evaluated patients with reported reactions, performed testing and challenges where applicable, and provided counseling on risks and benefits of vaccination. Here, we report our experience and the types of adverse reactions to COVID-19 vaccines seen for consultation in the Division of Allergy and Immunology at Northwell Health, which employs more than 74,000 people in 23 hospitals and more than 700 outpatient settings.
Methods
Study Design
Patients referred to be seen in our office for COVID-19 vaccine reactions from January 2021 to May 2021 were prospectively evaluated. A COVID-19 vaccine adverse reaction registry for people who had adverse reactions and were evaluated in the Division of Allergy and Immunology was established. Demographic data, details of adverse reactions to COVID-19 vaccination and allergy evaluation, and previous allergic and relevant medical history were included in the registry. This study was approved by the Institutional Review Board of the Feinstein Institutes of Medical Research at Northwell Health. Written consent was obtained.
Referrals and Education
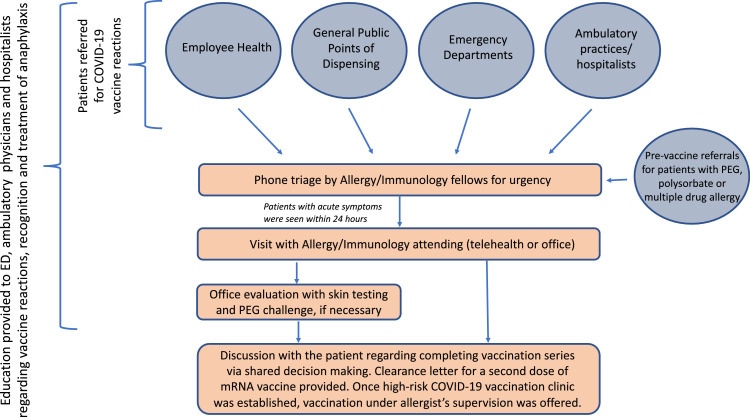
With the initiation of the national COVID-19 vaccination program, we established a referral process for vaccine reactions through collaboration with the Northwell Employee Health System, emergency departments, vaccination points of dispensing, ambulatory physicians, hospitalists, and the Northwell COVID Ambulatory Resource Support program (Fig 1 ). Education was provided by our faculty to the emergency department, ambulatory, and hospital medicine faculty regarding COVID-19 vaccine reactions, management, indications for allergy referrals, and recognition and treatment of anaphylaxis. An online educational module on identifying and treatment of anaphylaxis was developed as an additional resource. Our staff attempted to contact every patient referred. Individuals with a reported vaccine reaction were triaged over the phone by the allergy fellows-in-training, and telehealth or in-person visits were scheduled within a week with 1 of the 6 faculty attending physicians. People with acute allergic postvaccination symptoms were seen within 24 hours. A thorough history was obtained, and recommendations for further evaluation and COVID-19 vaccination, based on current knowledge, CDC guidance,11 and communications from the American Academy of Allergy, Asthma & Immunology and American College of Allergy, Asthma & Immunology,12 , 13 were provided. Depending on the type of reaction and history, office visits for skin testing were scheduled. In-office oral challenges to PEG (MiraLAX) were offered in select cases. Management algorithms for different types of COVID-19 vaccine reactions were developed and adapted as our knowledge evolved. A high-risk vaccination clinic was established for patients who had a reaction to the first dosage and patients with concerns on allergic reactions to COVID-19 vaccines, who preferred to receive vaccination under an allergist's supervision.
Figure 1.
The referral process. COVID-19, coronavirus disease 2019; ED, emergency department; mRNA, messenger RNA; PEG, polyethylene glycol.
Types of Reactions
Given the wide variety of adverse reactions observed, we devised a system to classify the types of reactions (Table 1 ) into 6 different groups, using the most severe presenting symptom(s). This classification scheme was based on the types of reactions observed and intended to risk stratify patients, streamline management of vaccine reactions, and facilitate referrals for allergy evaluation, without delaying the second vaccination for people with mild reactions. Groups 1 and 2 included patients with immediate and delayed nonallergic reactions, respectively. Group 3 included patients with various dermatologic symptoms, except generalized urticaria, which was classified in group 5. Patients with subjective tongue and/or throat swelling and hoarseness were included in group 4. Although the exact mechanism for each patient is difficult to decipher, these symptoms are very disturbing to patients and necessitated an allergy evaluation to exclude hypersensitivity reactions. Group 5 included patients with allergic symptoms, suggestive of immunoglobulin (Ig)E-mediated sensitivity. To be assigned in group 6, the patient was required to meet the criteria for anaphylaxis according to the Brighton Collaboration case definitions,14 National Institute of Allergy and Infectious Diseases, and Food Allergy and Anaphylaxis Network criteria for anaphylaxis,15 or both. The designation of anaphylaxis was confirmed by 3 of the authors (B.K., G.C., S.F.).
Table 1.
Classification and Action Plan for Reactions to the First Dosage of a mRNA COVID-19 Vaccine
| Group # | Reaction to mRNA COVID-19 vaccine | Proceed with the second dosage without allergy consultation | Recommendations for second COVID-19 vaccine, aside of education and reassurance and shared decision-making |
|---|---|---|---|
| 1 | Immediate adverse reactions (eg, transient dyspnea, metallic taste, flushing, lip tingling, paresthesia, tachycardia, hypertension) | Yes |
|
| 2 | Delayed adverse reactions (eg, injection site reactions, axillar lymphadenopathy on the vaccinated site, expected adverse effects and neurologic symptoms) | Yes |
|
| 3 | Dermatologic reactions, excluding generalized urticaria (eg, mild transient pruritus and localized rashes: immediate and delayed and delayed eczematous and maculopapular rashes) | Yes |
|
| 4 | Throat symptoms (eg, tongue and throat swelling/fullness, hoarseness) | No |
|
| 5 | Systemic allergic symptoms, not fulfilling anaphylaxis criteria (eg, generalized intractable pruritus, generalized urticaria, angioedema, wheezing, stridor, need for hospitalization): immediate or delayed | ||
| 6 | Anaphylaxis |
Abbreviations: COVID-19, coronavirus disease 2019; mRNA, messenger RNA; OTC, over the counter.NOTE. Symptoms of reactions are italicised.
Polyethylene Glycol 3350 Skin Testing and (MiraLAX) Challenges for Messenger RNA Vaccine Reactions
Skin prick and intradermal testing was performed in patients when there was a concern for an IgE-mediated process after COVID-19 mRNA vaccination. Patients with no suspicion for immediate hypersensitivity reaction were not offered skin testing and were cleared for the next vaccine dosage. Testing protocols were adapted from Banerji et al.9 , 16 For patients with negative or inconclusive skin testing result to PEG3350, and concern for a risk of reaction to the mRNA vaccine, an in-office oral graded challenge to PEG3350 was offered. A total of 17 g of PEG3350 was diluted in 250 mL of water. Two-dose (10%, 90%) or three-dose (1%, 10%, and 89%) challenges were performed, with doses administered 30 minutes apart. Patients were observed for 60 to 90 minutes afterward for the development of signs or symptoms of an acute allergic reaction. We also used a single-blind, placebo-controlled PEG3350 challenges in some patients to minimize false-positive results. Skin testing to COVID-19 vaccines was not performed because the US Food and Drug Administration required an expanded-access investigational new drug application for COVID-19 vaccine skin testing, challenge, and desensitization owing to the emergency use authorization status of the vaccines. To comply, we requested letters of authorization from the manufacturers, which were not granted. Patch testing to PEG was performed in patients with delayed nonurticarial rashes.
Statistical Analysis
Summary descriptive statistics were calculated for demographics, vaccine type, and reaction classification for the mRNA vaccines. Incidence of anaphylaxis was calculated based on employee immunization data within the Northwell Health System. The difference between 2 independent proportions was used to compare the proportion of vaccine reactions in each category between the Pfizer-BioNTech and Moderna vaccine groups, using an “N-1” χ2 test as recommended by Campbell (2007) and Richardson (2011) with MedCalc.17
Results
Types of Reactions to Coronavirus Disease 2019 Vaccines
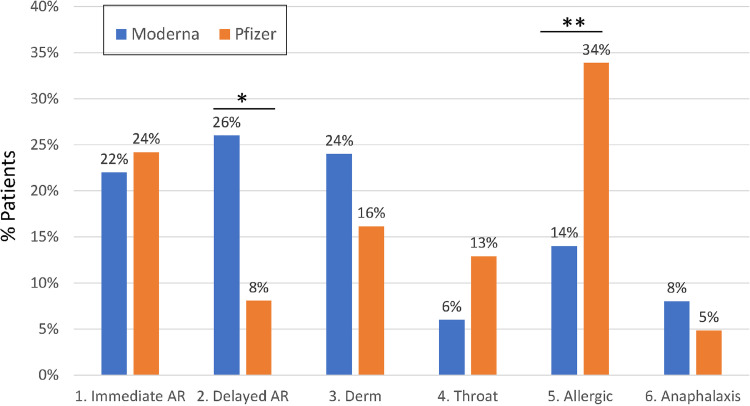
Since December 2020, 212 individuals were referred for COVID-19 vaccine reactions and 114 were evaluated by the Division of Allergy & Immunology faculty. Among the remaining 98 patients, we were unable to reach 83, 10 did not show up, and 5 cancelled their appointments. Of the 114 evaluated, 1 patient with anaphylaxis was excluded from our analysis because her symptoms started on day 4 after receiving her first Pfizer-BioNTech vaccine, making direct association with the vaccination unclear. Of the 113 patients, 62 had reactions after receiving the Pfizer-BioNTech, 50 after Moderna, and 1 after receiving the Johnson & Johnson's/Janssen (J&J/Janssen) COVID 19 vaccines (eTable 1). Mean age was 48 years (range, 19-89 years), and most of the patients were of female sex at 98 of 113 (86.7%). Among 112 patients who received mRNA vaccines, 105 presented after the reaction to the first dosage and 7 patients reacted to their second dosage (4 to Pfizer-BioNTech and 3 to Moderna) after tolerating the first. Some differences were noted between the Moderna and Pfizer-BioNTech vaccines with respect to the types of reactions (Fig 2 ). Delayed nonallergic adverse reactions occurred more frequently in Moderna-vaccinated patients (P = .01). This difference was primarily because of injection site reactions that were observed in 10 of 13 Moderna-vaccinated patients, but in none of the patients who received Pfizer-BioNtech. Systemic allergic symptoms (generalized pruritus, urticaria, angioedema, wheezing) were higher in Pfizer-BioNtech–vaccinated patients (P = .02). None of the patients had stridor or required hospitalization. We did not identify any significant differences between mRNA vaccines for other types of adverse reactions. In our cohort, 47% (7/15) of the male participants developed dermatologic nonurticarial reactions, whereas only 15% (15/97) of the female participants had these symptoms (P = .004).
eTable 1.
Demographic and Vaccination Data
| Demographic categories | Demographics | Moderna | Pfizer | J&J/Janssen | Total | |
|---|---|---|---|---|---|---|
| Sex | Female | 44 | 53 | 1 | 98 | |
| Male | 6 | 9 | 15 | |||
| Age | 15-24 | 3 | 3 | |||
| 25-34 | 4 | 13 | 17 | |||
| 35-44 | 12 | 11 | 23 | |||
| 45-54 | 16 | 24 | 1 | 41 | ||
| 55-64 | 9 | 8 | 17 | |||
| 65-74 | 6 | 2 | 8 | |||
| 75-84 | 2 | 1 | 3 | |||
| 85-94 | 1 | 1 | ||||
| Ethnicity or race | Hispanic or Latino | |||||
| White | 4 | 9 | 13 | |||
| Non-Hispanic or Latino | ||||||
| White | 29 | 30 | 59 | |||
| Asian | 5 | 6 | 11 | |||
| Blacka | 2 | 7 | 9 | |||
| Other race | 5 | 6 | 1 | 12 | ||
| Unknown | ||||||
| Unknown | 6 | 3 | 9 | |||
Abbreviation: J&J, Johnson & Johnson.
Black or African American.
Figure 2.
Differences in types of allergic and nonallergic reactions between the Moderna and Pfizer-BioNTech vaccines. Reaction types are defined in Table 1. AR, adverse reaction; Derm, dermatologic reactions.
Anaphylaxis
There were 8 patients, all women, who had anaphylaxis based on Brighton or the National Institute of Allergy and Infectious Diseases criteria, or both (Table 2 ). In addition, 6 cases (75%) of anaphylaxis occurred after the first dosage of mRNA vaccines and 1 each (12.5%) after the second vaccination (Moderna) and single-dose J&J/Janssen vaccine, respectively. None of the patients who had anaphylaxis developed hypotension or required intubation or hospital admission. The anaphylaxis rate was calculated based on 4 Northwell Health System employees only, given available data. Combined anaphylaxis rate for both Moderna and Pfizer-BioNTech vaccines was 40.6 cases per million doses administered, with 28.3 and 71.6 cases per million doses administered for Pfizer-BioNTech and Moderna, respectively (Table 3 ). The anaphylaxis rate did not differ between the 2 mRNA vaccines.
Table 2.
Characteristics of Patients Who Experienced Anaphylaxis
| Group # | Age(y) | Past pertinent allergic history | Symptoms ofanaphylaxis | Confirmed anaphylaxis |
COVID-19 vaccine | PEG prick skin test | PEG challenge result | Tryptase: reaction/basal (μg/L) | Notes | |
|---|---|---|---|---|---|---|---|---|---|---|
| Brighton criteria | NIAAD criteria | |||||||||
| 1 | 27 | Latex allergy, lip fillers, silicone breast implants | Generalized hives, SOB, throat and chest tightness, eyelid swelling | Level 2 | Yes | Pfizer | Positive at 1:1000 and 1:100 | ND | ND/4.2 |
|
| 2 | 38 | Asthma, AR, FA | SOB, chest tightness, cough, tracheal pruritus, dizziness | No | Yes | Pfizer | Negative | Passed | ND/ND |
|
| 3 | 50 | Asthma, FA | Generalized pruritus, SOB, tongue and throat swelling, difficulty swallowing, | No | Yes | Pfizer | Negative | Passed | ND/7 |
|
| 4 | 40 | Asthma, latex allergy, drug allergy | Generalized hives, SOB, chest tightness, tachycardia | Level 2 | Yes | Moderna | Negative | Passed | ND/4 |
|
| 5 | 46 | Asthma, AR, OAS | Generalized hives, chest tightness, abdominal pain, diarrhea, rhinorrhea, facial and eyelid swelling | Level 2 | Yes | Moderna | Negative | Passed | ND/ND |
|
| 6 | 32 | Asthma, AR, chronic urticaria, FA | Generalized itching, hives, SOB | No | Yes | Moderna | ND | ND | ND/ND |
|
| 7 | 34 | IVC | Generalized pruritus, urticaria, nausea, felt very lightheaded and weak | Level 2 | Yes | Moderna | ND | ND | 6/ND |
|
| 8 | 48 | IVC, FA | Generalized pruritus, lip swelling, SOB, chest and throat tightness, hoarseness | No | Yes | J & J | ND | ND | ND/ND |
|
Abbreviations: AR, allergic rhinitis; FA, food allergy; J & J, Johnson & Johnson COVID 19 vaccine; IVC, intravenous contrast; ND, not done; NIAID, National Institute of Allergy and Infectious Diseases; OAS, oral allergy syndrome; PEG, polyethylene glycol 3350; SOB, shortness of breathing.
Table 3.
Rate of Anaphylaxis
| Anaphylaxis cases and rates | After first dosage |
After second dosage |
Combined, after both doses |
|||
|---|---|---|---|---|---|---|
| Moderna | Pfizer | Moderna | Pfizer | Moderna | Pfizer | |
| Number of anaphylaxis cases, n | 1 | 2 | 1 | 0 | 2 | 2 |
| Total doses administered, n | 14,185 | 36,050 | 13,766 | 34,521 | 27,929 | 70,571 |
| Anaphylaxis rate, cases per million doses administered | 70.5 | 55.5 | 72.6 | 0 | 71.6 | 28.3 |
This analysis assumes that each reaction is an independent event.
Polyethylene Glycol Testing and Challenges
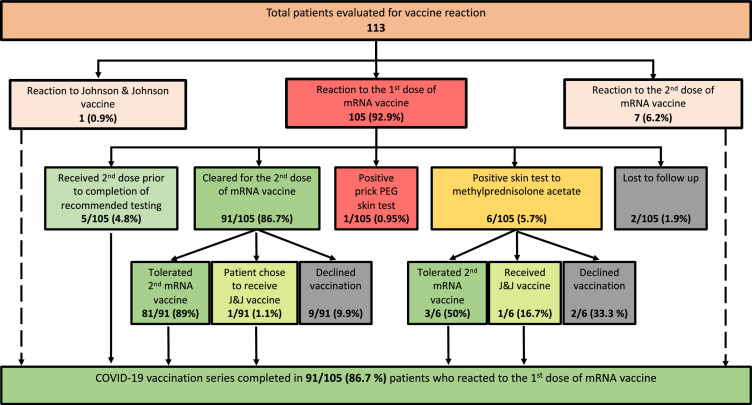
We initially implemented the suggested skin testing algorithm.9 One patient with anaphylaxis to Pfizer-BioNTech vaccine had positive prick skin test result to PEG (Table 2). There were 18 patients who had methylprednisolone acetate skin testing performed at recommended concentrations, and in 6 of them (33%), the skin test result was positive. Of these 6 patients, 3 (50%) had a subsequent negative PEG challenge result and tolerated their second mRNA vaccine (Fig 3 ). Seeing a high rate of false-positive test results to methylprednisolone acetate, we decided to proceed with graded PEG challenges after negative PEG prick skin test result for patients with an unconvincing history of PEG allergy (Table 4 ). Of 23, 10 (43.5%) patients developed mild, transient symptoms during the PEG3350 challenge. Symptoms included scratchy throat, tingling, numbness of the tongue and palate, sweet taste in the mouth, feeling of swollen tongue, lips, lump in the throat, throat closure sensation, shortness of breath, skin itching and blotching, lightheadedness, and dizziness. All symptoms were subjective, except skin blotching that occurred only in people with history of long-term and intermittent hives. These symptoms were transient, mostly subjective and were not treated, except in 1 patient with history of urticaria, who developed pruritus and received fexofenadine 180 mg with resolution of symptoms. In some cases, symptoms experienced by an individual during PEG challenge were similar to those experienced by that patient with vaccination. Patients were reassured, educated, and cleared for their second dosage with premedication. PEG patch testing was performed, and results were negative in 3 patients with delayed rashes.
eTable 2.
Number of Patients for Each Type of Allergic and Nonallergic Reactions to mRNA Vaccines
| Reaction type | Moderna | Pfizer | Total |
|---|---|---|---|
| 1. Immediate AR | 11 | 15 | 26 |
| 2. Delayed AR | 13 | 5 | 18 |
| 3. Derm | 12 | 10 | 22 |
| 4. Throat | 3 | 8 | 11 |
| 5. Allergic | 7 | 21 | 28 |
| 6. Anaphylaxis | 4 | 3 | 7 |
| Grand total | 50 | 62 | 112 |
Abbreviations: AR, adverse reaction; Derm, dermatologic reaction; mRNA, messenger RNA.
Figure 3.
Results of allergy evaluation after COVID-19 vaccination. COVID-19, coronavirus disease 2019; mRNA, messenger RNA; PEG, polyethylene glycol.
Table 4.
Completing Vaccination After PEG3350 Challenge
| PEG-challenged patients | All patients challenged | Developed symptoms during PEG challenge | No symptoms during PEG challenge |
|---|---|---|---|
| Total number of PEG-challenged patients | 23 | 10/23 (43.5) | 13/23 (56.5) |
| Tolerated second mRNA vaccine | 20/23 (87.0) | 8/10 (80.0) | 12/13 (92.3) |
| Patient chose to receive J&J | 1/23 (4.3) | 0 | 1/13 (7.7) |
| Lost to follow-up | 2/23 (8.7) | 2/10 (20.0) | 0 |
Data are presented as number (percentage).
Abbreviations: COVID-19, coronavirus disease 2019; J&J, Johnson & Johnson COVID-19 vaccine; mRNA, messenger RNA; PEG, polyethylene glycol
Pretreatment Before Vaccination
As per published guidance,9 patients with mild allergic symptoms to the first COVID-19 mRNA vaccine were pretreated with nonsedating antihistamines (eg, cetirizine 10-20 mg or fexofenadine 180-360 mg 1 hour before vaccination). Asthma and chronic urticaria medications were optimized to control underlying conditions. Patients were instructed to take additional doses of cetirizine or fexofenadine for postvaccination pruritus and rashes. Patients with delayed rashes were recommended to continue antihistamines for a few days (1-2 days longer than duration of the rash after the first dosage). Patients with history of subjective feeling of throat and tongue swelling were premedicated with antireflux medications (H2 blockers or proton pump inhibitors). Patients with asthma, who had respiratory symptoms with the first dosage, were instructed to take albuterol HFA 2 puffs before their second dosage.
Administration of the Second Dose
Among 105 patients who reacted to the first dosage of COVID-19 mRNA vaccines, 91 (86.7%) completed their vaccination series without any severe immediate reactions (Fig 3). Among 6 patients with anaphylaxis to the first dosage of mRNA vaccine, 5 received the second dosage of the same mRNA vaccine (Table 2). Of these patients, 4 tolerated vaccination without any allergic symptoms and 1 developed isolated cough (patient 4). In addition, 1 patient with history of anaphylaxis to the first dosage had a positive PEG prick skin test result and a positive intradermal test result to triamcinolone acetonide (which contains polysorbate 80) and did not complete the 2-dose vaccination series (patient 1). Notably, the latter 2 patients had protracted allergic symptoms for days after the first dosage.
Discussion
Despite the extremely low rate of anaphylaxis to COVID-19 vaccines, public concern on adverse effects, including allergic reactions, remains high, contributing to vaccine hesitancy. Our experience underscores that most people with allergic symptoms after their initial mRNA vaccine can be safely revaccinated with premedication and hypersensitivity to PEG is not the culprit in most cases of severe allergic symptoms.
The rate of anaphylaxis in our cohort is 40.6 cases per million, whereas previously reported rates of anaphylaxis to COVID-19 mRNA vaccines vary from 4.5 per million, as reported by CDC,18 to 247 cases per million doses administered after the first vaccination, as reported at Mass General Brigham.19 The estimated rate of anaphylaxis to non–COVID-19 vaccines is significantly lower, at 1.31 per million vaccine doses.5 The differences in reported anaphylaxis rates can be explained by the extremely low incidence of anaphylaxis to vaccines, variability in applying anaphylaxis criteria, and different populations studied. We believe that misdiagnosis and mistakenly assumed causality lead to overestimation of anaphylaxis rate. For example, a patient who developed shortness of breath 2 hours after vaccination and required intubation was referred for evaluation of presumed COVID-19 vaccine anaphylaxis. This patient did not have any associated allergic symptoms and was diagnosed with having bilateral pneumonia, asthma, and acute or chronic respiratory failure. Respiratory failure alone does not fulfill anaphylaxis criteria, tryptase was not checked, and the relationship to vaccination is unclear. Furthermore, causality is uncertain for a patient who developed anaphylaxis 4 days after vaccination and was thus excluded from our analysis. The reported rate of anaphylaxis to COVID-19 vaccines has been decreasing as the vaccination rate increases and concerns regarding new vaccines diminish, suggesting anxiety and panic attacks can present with symptoms mimicking allergic reactions, such as shortness of breath, globus sensation, tachycardia, and hypertension, and therefore may contribute to a higher reported rate of allergic reactions to COVID-19 vaccines.
In our cohort, anaphylaxis occurred only in women, which is similar to previously reported data.19 , 20 There was no statistically significant difference in the rate of anaphylaxis between the Pfizer-BioNTech and Moderna vaccines, but the rate of systemic allergic symptoms (generalized pruritus, urticaria, angioedema, wheezing) was significantly higher in the former and the rate of delayed nonallergic reactions was significantly higher in the latter. Delayed injection site reactions in Moderna-vaccinated patients accounted for higher rate of delayed nonallergic symptoms after Moderna. This is easily managed with reassurance and anticipatory guidance with clear recommendations on symptomatic treatment (Table 1). The reason for higher rate of systemic allergic symptoms in Pfizer-BioNTech–vaccinated patients in our cohort may be because of different excipients in the mRNA vaccines, but these findings should be confirmed by larger studies. Every patient with anaphylaxis to a COVID-19 vaccine had a history of atopy, and two-thirds had asthma. Underlying asthma and urticaria may contribute to postvaccination respiratory and cutaneous symptoms, which highlights the importance of optimizing asthma control and that of other underlying diseases before immunization. We want to emphasize that in our cohort no one required intensive care or hospital admission. Interestingly, although most allergic and nonallergic vaccine reactions occurred in women, our data indicate that delayed nonurticarial cutaneous reactions may be higher in men. Further research is needed to uncover the etiology for this observed difference in presentation between sexes, but experience from other vaccinations has documented higher rates of adverse reactions, including anaphylaxis, in women.21 , 22
Skin testing to the culprit vaccine and its components is the reference standard in evaluating patients with vaccine allergy.23 Because the predictive value of COVID-19 vaccine skin testing is unknown, and PEG, which is reported to cause rare cases of IgE-mediated reactions, including anaphylaxis, is the only component of mRNA COVID vaccines that can be tested at this time, initial risk stratification pathways were based on excipient skin testing to PEG. Skin testing to PEG is not standardized, and intradermal PEG skin testing can cause anaphylaxis24; therefore, PEG-containing methylprednisolone acetate is recommended for intradermal testing. Although PEG and PEG-containing steroid skin testing has been helpful for evaluating immediate hypersensitivity to PEG-containing medications,25 , 26 its value in predicting tolerance to COVID-19 mRNA vaccines is unknown. False-positive skin test results may interfere with the ability to clear patients for their subsequent vaccine dosage and lead to labeling patients with new drug allergies. Therefore, we used oral PEG challenges to clear and encourage patients to receive their second vaccine doses. Although only 0.06% of oral PEG is absorbed, this results in 10.2 mg of a 17-g dosage, which is much greater than the amount in mRNA vaccines.27 , 28 We observed that at least half of the patients with positive methylprednisolone acetate test results went on to tolerate oral PEG and their second dosage of an mRNA vaccine, indicating low specificity of this intradermal skin test. Furthermore, negative results from PEG challenges after presumed hypersensitivity reactions to mRNA vaccines indicate that PEG is an unlikely cause of severe allergic symptoms in people who do not have a history of anaphylaxis to PEG. Nevertheless, 1 of our patients who developed anaphylaxis to the first dosage of mRNA vaccine and had protracted symptoms had positive PEG prick skin test result, suggesting PEG can be a rare culprit and PEG prick skin test may be helpful for evaluating anaphylaxis to mRNA vaccines in selected cases.
Some patients had mild symptoms during their PEG challenge, which sometimes mimicked their symptoms to the initial vaccine. These patients were carefully observed and reassured, and maintenance medications for patients with suboptimally controlled asthma and urticaria were adjusted. Patients were then cleared for their second vaccine dosage with premedication. Patients with subjective feeling of “throat symptoms” were also empirically premedicated with antireflux medications (H2 blockers and/or proton pump inhibitors). All patients (n = 91) who went on to receive their second dosage, including those with mild symptoms during PEG challenge, tolerated subsequent vaccination with premedication, suggesting these symptoms are either not allergic, hypersensitivity to PEG is very mild, or there is another allergen within the vaccine that could be triggering reactions.
We established a high-risk vaccination clinic to provide a safe and comfortable setting where patients would receive their second dosage under the supervision of an allergist trained to identify and treat allergic reactions. All patients tolerated vaccination, administered as a single-dose, with appropriate premedication. Most of our patients with anaphylaxis to the first COVID-19 vaccine received second dosage at the high-risk clinic. None of these patients had a severe reaction to the second vaccination. Tolerance of the second dosage after reported anaphylaxis to the first one has been reported by others20 , 29 and suggests a non–IgE-mediated mechanism for most of postvaccination reactions. Other immune mechanisms, including direct mast cell activation by vaccine components and various host factors, have been proposed.30
On the basis of our experience, we believe that intradermal PEG testing has limited utility. Prick PEG testing may be helpful in people with history of anaphylaxis to mRNA vaccines. Most severe allergic symptoms, associated with mRNA vaccines, are not caused by hypersensitivity to PEG. Special attention should be given to patients with anaphylaxis, followed by protracted symptoms. Although successful administration of the second dosage of the mRNA vaccine by graded dosing protocol after immediate hypersensitivity reaction to the first dosage has been described,31 our data suggest that with premedication, the full dose of mRNA vaccines can be administered safely and is well tolerated in most patients. Obviously, this approach will not be appropriate for rare patients with history of anaphylaxis to PEG. Emerging data on mRNA COVID-19 vaccine skin testing suggest that it is safe32 , 33 but may cause delayed reactions at the skin test sites, which were not predictive of immediate hypersensitivity reactions to the vaccines.34 Sensitivity and specificity of vaccine skin testing are still unknown.
With expanding eligibility criteria for vaccination, we have been consulted in increasing numbers for patients with a history of anaphylaxis to medications and vaccines, multiple drug allergies, mast cell disorders, and other allergic concerns, before vaccination. We believe that establishing the referral process, educating the health care professionals who see the reactions first hand, and having an allergist evaluate patients early and thoroughly is essential. Initial allergy evaluation can be successfully done by telehealth visit. Close observation and reassurance for patients who developed mild symptoms during PEG challenges encouraged our patients to complete their COVID-19 vaccination. The ability to vaccinate patients under the close observation of a physician, trained in recognizing and managing allergic reactions, decreases vaccine hesitancy. As the science on the etiology of the immediate hypersensitivity to COVID-19 vaccines evolves, vaccine skin testing and physician-observed vaccination will likely become the standard of care for managing patients with COVID-19 vaccine reactions. As allergists, we are well positioned to educate patients on allergic issues and address them before and after vaccination.
Acknowledgments
We acknowledge the faculty, fellows, nursing, research and support staff of the Division of Allergy & Immunology, including Idil Ezhuthachan, MD, Yasmin Hamzavi, MD, Irina Katayeva, MD, Tyrone Coyle, MD, William Keefe, MD, Stefani Su, MD, Lorraine Campbell, Kyle Bauerschmidt, Gloria Mallia, Patricia McAdam, Heidi Youngling, Pinky Varghese, Aaqil Ali, Annica Bryson and Elizabeth Conroy for data collection and research assistance.
Footnotes
Dr Kaplan and Dr Farzan are co-first authors.
Disclosures: Dr Bonagura reports serving on the primary immunodeficiency disease advisory board for CSL Behring; serving as lecturer on primary immunodeficiency disease for Shire; and serving on the LINK advisory board for Grifols; however, these affiliations are not relevant to this research. The remaining authors have no conflicts of interest to report.
Funding: The authors have no funding sources to report.
References
- 1.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC COVID-19 Response Team; Food and Drug Administration Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine - United States, December 14-23, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(2):46–51. doi: 10.15585/mmwr.mm7002e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.CDC COVID-19 Response Team; Food and Drug Administration Allergic reactions including anaphylaxis after receipt of the first dose of Moderna COVID-19 vaccine - United States, December 21, 2020-January 10, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(4):125–129. doi: 10.15585/mmwr.mm7004e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McNeill MM, Weintraub ES, Duffy J. Risk of anaphylaxis after vaccination in children and adults. JACI. 2016;137(3):868–878. doi: 10.1016/j.jaci.2015.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. COVID-19 vaccines: update on allergic reactions, contraindications, and precautions. 2020. Available at: https://emergency.cdc.gov/coca/calls/2020/callinfo_123020.asp. Accessed May 20, 2021.
- 7.Centers for Disease Control and Prevention. Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. 2021. Available at: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html#Contraindications. Accessed August 18, 2021.
- 8.Castells MC, Phillips EJ. Maintaining safety with SARS-CoV-2 vaccines. N Engl J Med. 2021;384(7):643–649. doi: 10.1056/NEJMra2035343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banerji A, Wickner PG, Saff R, et al. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J Allergy Clin Immunol Pract. 2021;9(4):1423–1437. doi: 10.1016/j.jaip.2020.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rief W. Fear of adverse effects and COVID-19 hesitancy: recommendations of the Treatment Expectation Expert Group. JAMA Health Forum. 2021;2(4) doi: 10.1001/jamahealthforum.2021.0804. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Interim clinical considerations for use of mRNA COVID-19 vaccines currently authorized in the United States - appendix B. https://www.cdc.gov/vaccines/covid-19/info-byproduct/clinical-considerations.html#Appendix-B. Accessed January 15, 2021.
- 12.American College of Allergy, Asthma, and Immunology. ACAAI updates to guidance on risk of allergic reactions to COVID-19 vaccines. 2021. Available at: https://acaai.org/news/acaai-updates-guidance-risk-allergic-reactions-covid-19-vaccines. Accessed May 24, 2021.
- 13.American Academy of Allergy, Asthma, & Immunology. AAAAI COVID-19 response task force guidance on administration of COVID-19 vaccines related to concerns of allergic reactions. 2021. Available at: https://education.aaaai.org/resources-for-a-i-clinicians/reactionguidance_COVID-19. Accessed May 24, 2021.
- 14.Ruggeberg JU, Gold MS, Bayas JM, et al. Anaphylaxis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25(31):5675–5684. doi: 10.1016/j.vaccine.2007.02.064. [DOI] [PubMed] [Google Scholar]
- 15.Manivannan V, Decker WW, Stead LG, Li JTC, Campbell RL. Visual representation of National Institute of Allergy and Infectious Disease and Food Allergy and Anaphylaxis Network Criteria for anaphylaxis. Int J Emerg Med. 2009;2(1):3–5. doi: 10.1007/s12245-009-0093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banerji A, Wolfson AR, Wickner PG, et al. COVID-19 vaccination in patients with reported allergic reactions: updated evidence and suggested approach. J Allergy Clin Immunol Pract. 2021;9(6):2135–2138. doi: 10.1016/j.jaip.2021.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MedCalc. Software Ltd. Comparison of proportions calculator. 2021. Available at: https://www.medcalc.org/calc/comparison_of_proportions.php. Accessed May 21, 2021.
- 18.Gee J, Marquez P, Su J, et al. First month of COVID-19 vaccine safety monitoring - United States, December 14, 2020-January 13, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(8):283–288. doi: 10.15585/mmwr.mm7008e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blumenthal KG, Robinson LB, Camargo CA, Jr, et al. Acute allergic reactions to mRNA COVID-19 vaccines. JAMA. 2021;325(15):1562–1565. doi: 10.1001/jama.2021.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krantz MS, Bruusgaard-Mouritsen MA, Koo G, Phillips EJ, Stone CA, Jr, Garvey LH. Anaphylaxis to the first dose of mRNA SARS-CoV-2 vaccines: don't give up on the second dose! Allergy. 2021;76(9):2916–2920. doi: 10.1111/all.14958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su JR, Moro PL, Ng CS, Lewis PW, Said MA, Cano MV. Anaphylaxis after vaccination reported to the Vaccine Adverse Event Reporting System, 1990-2016. J Allergy Clin Immunol. 2019;143(4):1465–1473. doi: 10.1016/j.jaci.2018.12.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halsey NA, Griffioen M, Dreskin SC, et al. Immediate hypersensitivity reactions following monovalent 2009 pandemic influenza A (H1N1) vaccines: reports to VAERS. Vaccine. 2013;31(51):6107–6112. doi: 10.1016/j.vaccine.2013.09.066. [DOI] [PubMed] [Google Scholar]
- 23.Broyles AD, Banerji A, Barmettler S, et al. Practical guidance for the evaluation and management of drug hypersensitivity: specific drugs. J Allergy Clin Immunol Pract. 2020;8(9S):S16–S116. doi: 10.1016/j.jaip.2020.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Sellaturay P, Nasser S, Ewan P. Polyethylene glycol-induced systemic allergic reactions (anaphylaxis) J Allergy Clin Immunol Pract. 2021;9(2):670–675. doi: 10.1016/j.jaip.2020.09.029. [DOI] [PubMed] [Google Scholar]
- 25.Jakubovic BD, Saperia C, Sussman GL. Anaphylaxis following a transvaginal ultrasound. Allergy Asthma Clin Immunol. 2016;12:3. doi: 10.1186/s13223-015-0106-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stone CA, Jr, Liu Y, Relling MV, et al. Immediate hypersensitivity to polyethylene glycols and polysorbates: more common than we have recognized. J Allergy Clin Immunol Pract. 2019;7(5) doi: 10.1016/j.jaip.2018.12.003. :1533-1540.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.US Food and Drug Administration. Fact Sheet for healthcare providers administering vaccine (vaccination providers) Emergency Use Authorization (EUA) of the MODERNA COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19). 2021. Available at: https://www.modernatx.com/covid19vaccine-eua/eua-fact-sheet-providers.pdf. Accessed June 4, 2021.
- 28.US Food and Drug Administration. Fact Sheet for healthcare providers administering vaccine (vaccination providers) Emergency Use Authorization (EUA) of the PFIZER-BIONTECH COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19). 2021. Available at: http://labeling.pfizer.com/ShowLabeling.aspx?id=14471&format=pdf&. Accessed June 4, 2021.
- 29.Wolfson AR, Robinson LB, Li L, et al. First-dose mRNA COVID-19 vaccine allergic reactions: limited role for excipient skin testing. J Allergy Clin Immunol Pract. 2021;9(9) doi: 10.1016/j.jaip.2021.06.010. :3308-3320.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Risma KA, Edwards KM, Hummell DS, et al. Potential mechanisms of anaphylaxis to COVID-19 mRNA vaccines. J Allergy Clin Immunol. 2021;147(6) doi: 10.1016/j.jaci.2021.04.002. :2075-2082.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mustafa SS, Ramsey A, Staicu ML. Administration of a second dose of the moderna COVID-19 vaccine after an immediate hypersensitivity reaction with the first dose: two case reports. Ann Intern Med. 2021;174(8):1177–1178. doi: 10.7326/L21-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marcelino J, Farinha S, Silva R, Didenko I, Proença M, Tomáz E. Nonirritant concentrations for skin testing with SARS-CoV-2 mRNA vaccine. J Allergy Clin Immunol Pract. 2021;9(6):2476–2477. doi: 10.1016/j.jaip.2021.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelso JM. Misdiagnosis of systemic allergic reactions to mRNA COVID-19 vaccines. Ann Allergy Asthma Immunol. 2021;127(1):133–134. doi: 10.1016/j.anai.2021.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bianchi L, Biondi F, Hansel K, Murgia N, Tramontana M, Stingeni L. Skin tests in urticaria/angioedema and flushing to Pfizer-BioNTech SARS-CoV-2 vaccine: limits of intradermal testing. Allergy. 2021;76(8):2605–2607. doi: 10.1111/all.14839. [DOI] [PMC free article] [PubMed] [Google Scholar]