Abstract
Staphylococcus aureus asymptomatically colonizes a third of the world’s population, but is an opportunistic pathogen that can cause life threatening diseases. To diagnose S. aureus infections, it is necessary to differentiate S. aureus from the ubiquitous human commensal Staphylococcus epidermidis, which beneficially colonizes the skin of all humans. Efforts are underway to identify volatile biomarkers for diagnosing S. aureus infections, but to-date no studies have investigated whether S. aureus and S. epidermidis can be reliably differentiated under a variety of growth conditions. The overall goal of this study was to evaluate the influence of growth medium on the ability to differentiate S. aureus and S. epidermidis based on their volatile profiles. We used headspace solid-phase microextraction (HS-SPME) and comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry (GC×GC-TOFMS) to examine the headspace volatiles of S. aureus and S. epidermidis when aerobically grown in four different complex media. We detected 337 volatile features when culturing S. aureus and S. epidermidis in four complex media, termed the staph volatiles, and found only 20 – 40% concurrence in the volatiles produced by these two species in any single medium. Using principal components analysis and hierarchical clustering analysis on the staph volatiles, we observed that S. aureus and S. epidermidis clustered independently from each other, and distinctly clustered by growth medium within species. Removing volatiles that are species and/or media specific from the analyses reduced the resolution between species clusters, but in all models clustering by species overrode clustering by media type. These analyses suggest that, while volatile profiles are media-specific, species differences dominate the staph volatilome. These data enable future investigations into the identification of volatile biomarkers to discriminate staphylococcal pathogens versus commensals, which will improve staph diagnoses and provide insights into the biochemistry of staph infections and immunity.
Keywords: Staphylococcus aureus, Staphylococcus epidermidis, commensal, opportunistic pathogen, metabolomic profiling, volatile organic compounds, GC×GC-TOFMS
Introduction
Staphylococcus aureus and Staphylococcus epidermidis reign as the most prevalent human nasal and skin bacteria, respectively, both at the global and individual population levels [1–4]. S. epidermidis is a true commensal in immune-sufficient persons, with skin colonization beginning at birth, and increasing until day 3 when it plateaus and remains the predominant skin commensal bacterium [4, 5]. Several studies suggest that S. epidermidis in its normal skin habitat contributes beneficial qualities to this niche, such as improved immunity against pathogens and cancer [2, 6, 7], as well as the modulation of inflammation in conditions such as atopic dermatitis [5]. In contrast to S. epidermidis as a commensal, the S. aureus genome encodes numerous and diverse virulence factors and toxins, and to date, there is no record of S. aureus presenting beneficial effects to a human host. Carriage of S. aureus – or asymptomatic bacterial colonization on or within the host – is prevalent, but not universal; S. aureus persistently colonizes one-third of the population, transiently colonizes one-third, and rarely, if ever colonizes the remaining third of the population [3]. Though most people who are colonized by S. aureus will remain asymptomatic, S. aureus is an opportunistic pathogen that can cause life-threatening diseases. In 2017 nearly 120,000 cases of septicemia and 20,000 deaths occurred in the United States due solely to S. aureus infections [8]. While the average cost for in-hospital stays is nearly $11,000 [9], the average cost to treat S. aureus infections in-hospital ranges from $9,100 to nearly $55,000 based on the type and severity of toxicity [10]. Furthermore, the S. aureus bacteremia 30-day readmission rate is 17% and averages over $20,000 per incident [11]. Early intervention in S. aureus infections significantly reduces length of stay, total health-care costs, and rates of readmission and mortality [12], but the increased surveillance and defensive therapeutic measures recommended by the Healthcare-Associated Infection National Action Plan adds to the economic burden of S. aureus infections [8, 10, 13]. Therefore, the development of rapid, non-invasive, sensitive and specific diagnostics for S. aureus would have significant clinical and financial impacts on healthcare delivery.
The current standard-of-care for diagnosing S. aureus infections and distinguishing S. aureus from S. epidermidis and other coagulase-negative staphylococci (CONS) is via bacterial culturing on selective and differential media for staph, requiring 24 h for diagnosis. Alternatively, rapid detection methods such as the API RAPIDEC staph test strip, the tube coagulase test, or the AdvanDx fluorescent in situ hybridization test can be used and will yield a diagnosis in 0.5 – 4 h [14]. While these culture-dependent and culture-independent methods have excellent specificity (≥ 99%) and acceptable sensitivities (84 – 99%), they all suffer the constraints of retrieving viable bacteria or bacterial products (coagulase enzymes, DNA) from infection sites. We and others are working toward the identification of volatile metabolic biomarkers or diagnostic patterns of volatile features for the detection of S. aureus bacterial infections (see review [15]). These diagnostic volatiles can be detected directly from primary specimens such as bronchoalveolar lavage fluid, milk, or urine [16–18], and are easily retrieved from breath [19–24]. Prior investigations into volatile biomarkers of S. aureus have demonstrated that S. aureus can be readily differentiated from other common human bacterial pathogens [16, 20, 23], and that important clinical traits such as methicillin resistance [19] or enterotoxin production [25] can be detected. Thus far, however, there have been only a few published studies on differentiating S. aureus from S. epidermidis in clinical specimens [18, 26–29], and therefore many of the previously identified S. aureus volatile biomarkers have not been tested for specificity against S. epidermidis or other CONS. We are beginning to more robustly characterize the volatile metabolome of S. epidermidis in order to fill this information gap.
While our ultimate goals are to identify volatile compounds to discriminate S. epidermidis from S. aureus, our first step, developed herein, is to determine if volatile profiles can be used to differentiate the species at all, or if other variables, such as medium choice, dictate the volatile features we are able to detect. Several in vitro analyses of bacterial mono-cultures, including some that analyzed S. aureus, have shown that medium choice plays a significant role in the production of volatile metabolites [30–33], and differences in S. aureus lipid membrane composition upon growth in different rich media have also been described [34]. A significant question, though, is whether media-dependent differences in the volatilome will influence the ability to differentiate S. aureus from other bacteria. Dryahina and colleagues used SIFT-MS to compare the volatile metabolites produced by four major bacterial taxa that cause cystic fibrosis lung infections (Pseudomonas aeruginosa, Stenotrophomonas maltophilia, Burkholderia cepacia, and S. aureus) grown in three different rich media (brain heart infusion (BHI), Mueller-Hinton broth (MHB), and NB). They, too, demonstrated that there are media-specific volatiles for each organism, but that the differences between these four bacteria – all from different taxonomic orders – overrode the media-dependent differences in their experiments [31]. By contrast, in an analysis of the volatiles produced by clinical strains of Klebsiella pneumoniae grown in BHI, MHB, lysogeny broth (LB), and tryptic soy broth (TSB), Rees et al. showed that the volatiles produced were more strongly dependent on the rich medium used rather than the bacterial strain [30]. This collection of studies suggests that the variance in bacterial volatilomes that is introduced by medium selection is greater than the variance between strains of the same species, and less than the volatilome differences between orders of bacteria. Where the medium-specific variance lies relative to variance between taxonomic family, genus, or species is yet to be determined.
In this study, we aimed to determine the role of medium choice on differentiating two species of the same genus, S. aureus and S. epidermidis, by their volatile profiles, with a secondary goal of determining optimal growth media for future work on volatile biomarker discovery and identification. We hypothesized that S. aureus and S. epidermidis would differentially produce volatile features that reflect the differences in species, more so than differences in the medium in which they were cultured. To test this hypothesis, we grew each Staphylococcus spp. in four rich media – BHI, MHB, LB, and TSB – and characterized the volatilomes of the culture headspace using comprehensive two-dimensional gas chromatography – time-of-flight mass spectrometry (GC×GC-TOFMS). We utilized clustering analyses to determine whether volatile patterns can differentiate the two species, or whether other factors such as the selection of medium are the source of volatilome differences. These data represent the first comparative analysis of the GC×GC volatilomes of two species of the same genus – one predominantly a human commensal and one an opportunistic pathogen – and the influence of medium choice on differentiating the two.
Methods and Materials
Bacterial strains, culture conditions, and growth curves
Two ATCC staphylococcal strains, Staphylococcus aureus (ATCC 12600) [35] and Staphylococcus epidermidis (ATCC 12228; Table 1) [36, 37] were cultured in four filter-sterilized complex media for metabolomics analysis: Brain Heart Infusion broth (BHI; Bacto; 7.7 g Calf Brain, 9.8 g Beef Heart, 10.0 g Proteose Peptone, 2.0 Dextrose, 5.0 g NaCl, 2.5 Na2PO4, per liter); lysogeny broth Lennox (LB; Fisher Scientific; 10 g Tryptone, 5 g Yeast Extract, 5 g NaCl, per liter); Mueller Hinton broth (MHB; Difco; 2.0 g Beef Extract, 17.5 g Acid Digest of Casein, 1.5 g Soluble Starch per liter); and Tryptic Soy broth (TSB; Bacto; 17.0 g Pancreatic Digest of Casein, 3.0 g Papaic Digest of Soybean, 2.5 g Dextrose, 5.0 g NaCl, 2.5 g K2PO4, per liter). Each species in each medium was prepared for growth and metabolomics analyses by inoculating single colonies grown on LB agar into 5 mL of medium in 50 mL conical tubes, which were incubated aerobically at 37 °C overnight. The overnight cultures were diluted 1000-fold into 25 mL of fresh media in 250 mL Erlenmeyer flasks with foam stoppers. Growth rates of each species in each medium were assessed in technical triplicates via optical density at 600 nm (OD600), and doubling times (Td) were calculated using data from exponential growth phase and the equation
Table 1.
Genetic information for S. aureus and S. epidermidis strains used in this study
| S. aureus | S. epidermidis | |
|---|---|---|
| ATCC | 12600 | 12228 |
| AKA | NCTC 8532 DSM 20231 |
NCTC 13360 DSM 1798 |
| Taxon ID | 1280 | 176280 |
| Size | 2.76 Mb | 2.49 Mb |
| Genes | 2831 | 2545 |
| GC% | 32.86 | 32.08 |
| # Strain Assemblies | 1 | 3 |
| Plasmids | 1 | 5 |
| NCBI BioProject Accession # |
PRJNA283471 5836 others |
PRJNA392321 PRJNA57861 PRJNA375108 |
| GOLD | Gp0120765 | Gp0258829 |
Sample preparation for metabolomics analysis
For volatile metabolomics analysis, overnight cultures and subcultures were prepared as described above in biological triplicates, and the subcultures were incubated aerobically at 37 °C with shaking for 24 h. Uninoculated media-only controls were also prepared in six replicates for BHI, LB, and MHB, and in triplicate for TSB, following the same procedures as the bacterial samples. After culturing, the samples were chilled on ice to slow growth and metabolism, then centrifuged at 4 °C to pellet the cells. Ten milliliters of each supernatant were transferred to Macrosep Advance centrifugal filter devices (Pall, 0.20 μm Supor® membrane) that were pre-rinsed with 10 mL filter-sterilized media. The samples were centrifuged at 4 °C and 2 mL of the sterile filtrates were transferred into 10 mL glass GC sample vials with PTFE/silicone septum screw caps. The GC vials and caps were pre-treated by heating at 100 °C for 24 h to reduce volatiles contamination. Uninoculated media controls were prepared in the same manner for chromatographic analysis. The samples were stored at −20 °C for approximately two weeks prior to analysis.
Chromatographic and mass spectrometric analysis
Prior to analysis, the bacterial culture supernatants and media blanks were thawed overnight at 4 °C. Volatile metabolites sampling was performed using a Gerstel® Multipurpose Sampler directed by Maestro® software. Prior to headspace volatiles extraction, the samples were incubated at 50 °C for 5 min, with agitation pulses at 600 rpm of 10 s on, and 1 s off. Volatiles were sampled for 10 min at 50 °C with agitation via head space solid phase microextraction (HS-SPME) using a 2 cm divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) coated fiber, 50/30 μm (Supelco) and a fiber penetration depth of 30 mm. For injection, the fiber inlet penetration was 60 mm, with a 600 s desorption at 250 °C.
Analysis by GC×GC-TOFMS was performed on a Pegasus 4D (LECO), using an Rxi-624Sil MS (60 m × 250 μm × 1.40 μm (length × internal diameter × film thickness); Restek) for the first-dimension column and a Stabilwax (1 m × 250 μm × 0.5 μm; Restek) for the second-dimension column, coupled by a press-fit connection. The columns were independently heated, with the primary oven initiated at 35 °C (0.5 min hold) and ramped at 5 °C/min to 230 °C (5 min hold); the secondary oven containing the second-dimension column was offset by +5 °C relative to the primary oven. A quad-jet modulator was used with a 2 s modulation period (0.5 s hot and cold pulses) and a +15 °C offset relative to the secondary oven. The helium carrier gas flow rate was 2 mL/min, with splitless injection. The transfer line and ion source temperatures were 250 °C. Compounds were ionized by electron impact at −70 eV and mass spectra were collected over the range of 35 – 400 m/z, at 100 spectra/s.
External alkane standards (C8 - C20 in hexane; Sigma-Aldrich) were used for retention indexing (RI). The alkane mix was incubated for 2 min at 50 °C, with agitation pulses at 600 rpm of 10 sec on and 1 sec off, then sampled via SPME fiber for 2 min at 50 °C with agitation and desorbed for 180 s at 250 °C. All other GC×GC-TOFMS parameters were as described, above.
Data processing, analysis, and statistics
ChromaTOF software, version 4.71.0.0 (LECO) was utilized for data acquisition and analysis. The signal baseline was set at the middle of the noise. A minimum signal-to-noise ratio (S/N) of 50 for a minimum of two apexing masses was used for initial peak finding, and subpeaks were combined using a 0.1 s maximum allowable second-dimension retention time shift and a 600 minimum mass spectral match score (out of 1000). Peak alignment was performed using the Statistical Compare package of ChromaTOF. A maximum 6.0 s (3 modulation period) retention time shift in the first dimension and 0.2 s maximum retention time shift in the second dimension and a minimum mass spectral match score of 600 was permitted. A second round of peak finding was performed during alignment using a S/N ≥ 5, if the same peak was present in at least one chromatogram at S/N ≥ 50. Peak areas were calculated using unique mass.
Peaks were putatively identified using published reporting standards [38]. Level 2 identifications were determined based on the following criteria: ≥ 800 (out of 1000) mass spectral forward and reverse matches to the NIST 2011 library and experimentally determined linear retention indices (LRI) consistent with published LRIs, as described previously [39, 40]. Level 3 identifications have ≥ 800 mass spectral forward and/or reverse match scores, but polar and non-polar LRI data were unavailable. Level 4 compounds had LRIs inconsistent with the compound identified by mass spectral matches. There were no level 1 identifications in this study (i.e., confirmed via standard injection).
Prior to statistical analyses, chromatographic artifacts (e.g., phthalates, siloxanes, atmospheric gasses) and peaks eluting prior to 358 s (i.e., acetone retention time) in the first chromatographic dimension were removed from the peak table. Peak areas were log10 transformed and normalized by probabilistic quotient normalization (PQN) [41] in R Studio version 3.5.1. Peaks were retained for further analysis if they met the following criteria: 1) present in all biological replicates, 2) mean peak area in the biological samples was two-fold or greater than in the media control samples, and 3) significantly greater in abundance in the biological samples versus media control using the student’s t-test and a significance threshold of p < 0.05. We used principal component analysis (PCA) score plots of PC1 versus PC2 and linear regressions of peak areas of biological replicates to identify potential outliers. One replicate of S. aureus in MHB was identified as an outlier by both methods, and was removed prior to further sample analysis. The relative abundances of volatiles were standardized (mean-centered and unit-scaled) and PCA and agglomerative hierarchical clustering analysis (HCA) with Canberra distance and McQuitty linkage was used to assess the relatedness of samples based on their volatiles. PCA and HCA analyses were performed in R Studio, and Venn Diagrams were generated using Venny [42].
Results and Discussion
Differential presence and absence of staph volatiles in complex media
For robust growth and expression of the volatilomes of two Staphylococcus species – Staphylococcus aureus and Staphylococcus epidermidis – we grew each organism in four complex media (BHI, LB, MHB, and TSB) and analyzed the volatile organic compounds (VOCs) of the cultures using headspace solid-phase microextraction and two-dimensional gas chromatography coupled to time-of-flight mass spectrometry (HS-SPME-GC×GC-TOFMS). After chromatographic alignment and the removal of artifacts, we detected 732 non-redundant chromatographic peaks across the eight sample types and four media controls. We describe 337 of these peaks as staph volatiles (Figure 1; Table S1), 242 produced by S. aureus and 250 produced by S. epidermidis, which met the following criteria: present in all biological replicates of an experimental condition (e.g., all replicates of S. aureus cultured in LB) and a statistically-significant two-fold or greater increase in the sample peak area in at least one bacterial culture condition versus the medium blank. Cultures grown in BHI produced the largest number of VOCs overall, as well as the largest number of VOCs unique to that medium. While growth in MHB was able to generate 40% of the total volatilome, only 13 VOCs (< 4%) were unique to this medium. MHB was also observed to produce the fewest bacterial volatiles in a HS-SPME-GC×GC-TOFMS analysis of K. pneumoniae, when compared to TSB, BHI, and LB [30].
Figure 1.

Distribution of 337 staph volatiles detected in cultures in four complex media.
Each medium has a unique influence on the production of volatiles for each staphylococcal species (Figures 2, S1). For S. aureus nearly half of the volatiles (n = 113, 47%) are produced exclusively within one medium (i.e., BHI or LB or MHB or TSB), though the contribution of BHI to the S. aureus volatilome is small, with only 19 unique volatiles (8%; Figure 2a). TSB is the medium that generated the largest proportion of volatiles for S. aureus (n = 105), followed by LB (n = 60). The complete TSB volatilome of S. aureus is equal in size and rather distinct from LB, which is interesting considering that the tryptic digest of casein makes up 75% of the organic dry weight of TSB, and 67% of LB. Together, volatiles produced in TSB and LB cover 81% of the S. aureus volatilome observed in this study (Figure 2a). For S. epidermidis, BHI produces the largest number of volatiles and also contributes the largest proportion of unique analytes (n = 66), constituting more than a quarter of the S. epidermidis volatilome (Figure 2b). Culturing in LB adds another 44 volatiles to the S. epidermidis volatilome, and together BHI and LB cover 90% of the 250 S. epidermidis volatiles.
Figure 2.
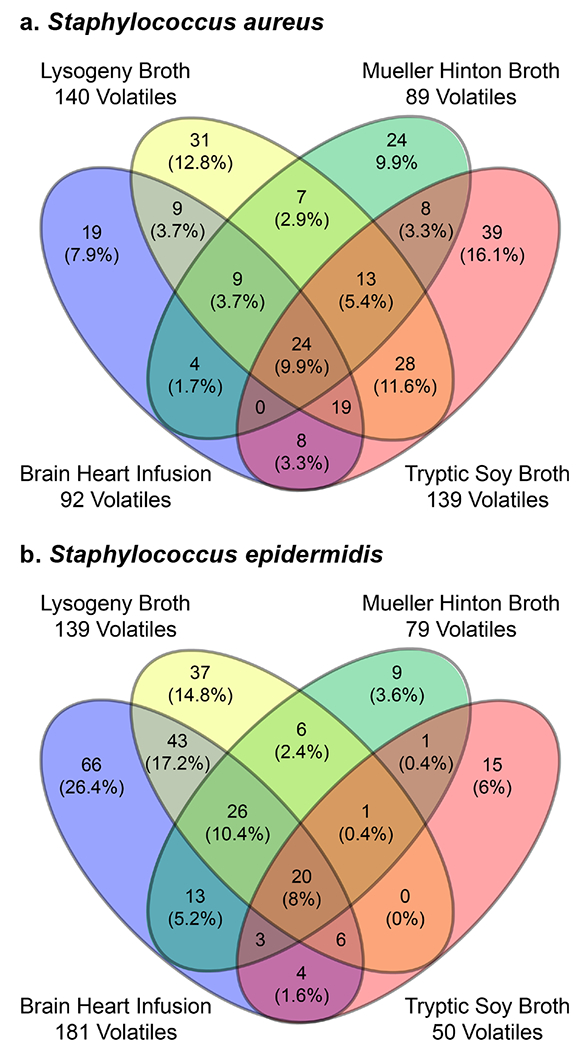
Distribution of 242 S. aureus (a) and 250 S. epidermidis (b) volatiles in four complex media.
In the context of the only other GC×GC-MS study of S. aureus volatiles, in which the authors cultured S. aureus in BHI and detected 240 compounds across three strains [25], our observation that BHI produces a low number of volatiles in S. aureus may be due to the strain we studied, particularly since it was the largest producer of staph VOCs (Figure 1). Other possible reasons for the discrepancy are the cutoffs we used for selecting S. aureus peaks to include in our analyses, or the use of filter-sterilized vs. autoclaved BHI. A fourth option is that if Baptista and colleagues had used LB or TSB for their studies, their list of S. aureus volatiles may have been even larger. Additional GC×GC analyses of multiple strains of S. aureus in BHI, LB, and TSB would be required to test these hypotheses.
A subset of the bacterial volatiles (n = 155, 46%) were detected in both S. aureus and S. epidermidis. Included in this group are 116 volatiles that are detected in both S. aureus and S. epidermidis grown in the same medium, which we refer to as the common staph volatiles (Figure S2), and 39 volatiles we detected in at least one S. aureus experiment and one S. epidermidis experiment, but in different media. The highest proportion (69%) of common staph volatiles were observed in LB, while the fewest were observed in MHB and TSB, which each supported only 30% (n = 34) of the total. This ranking did not trend with the volatile productivity for all staph (Figure 1), but again MHB made the least contribution to the common staph volatiles (Figure S1).
Combining the information on the common and unique volatiles for each medium, we observe that the size of the volatilome of staphylococci in BHI is largely driven by the volatiles produced by S. epidermidis, which contributes 83% (Table S1, Figure S1). Additionally, the majority of the BHI volatiles were unique to S. epidermidis (58%), making this the medium of choice in our experiments for obtaining the largest number of total and unique S. epidermidis volatiles. TSB was best for enhancing the production of S. aureus unique volatiles (n = 105), representing 68% of the staphylococcal volatilome in this medium (Figure S1). The fewest number of staph volatiles were produced when growing S. aureus and S. epidermidis in MHB, but this medium simultaneously enhanced differences between S. aureus and S. epidermidis, with only 25% of the volatiles in common. LB generated the most commonalities between the species, with a 40% overlap in the volatilomes, and each species having the same sized set of unique volatiles (30%). Therefore, we observed that the optimal medium for culturing S. aureus and S. epidermidis to produce the most uniform suite of detectable VOCs across species is LB, but for disambiguation of S. aureus vs. S. epidermidis by the presence and absence of VOCs, TSB, BHI, or MHB may be more suitable choices.
Overall, we observed strong media-dependent differences in the VOCs, with 84% of the 337 staph volatiles produced by three or fewer media (Figure 1). We observed an even stronger dependence on medium in the common staph volatiles, with more than 90% of the VOCs observed in a subset of the media (Figure S2). Only ten VOCs were observed in S. aureus and S. epidermidis cultures in all four media, which we term the universal staph volatiles (Table 2). All of these compounds were observed in at least one replicate of the blanks for each medium, but were detected in significantly higher abundances in the headspace of the bacterial cultures. We were able to putatively identify four of the VOCs, two of which have been previously reported. 3-Methyl-1-butanol was reported by Lee et al. in 1995 as the most abundant identified VOC deriving from K. pneumoniae fermentation of TSB [43] and also found from clinical isolates[44], and since then this compound has been widely reported in other studies, including in the headspace of Acinetobacter radioresistans [45], Enterobacter agglomerans [40], Citrobacter freundeii [46], Corynebacterium xerosis [45], Escherichia coli [33], Pseudomonas aeruginosa [29], S. aureus [25, 33], Streptococcus pneumoniae [29], and the breath of COPD patients [47]. 5-Methyl-2-heptanone has recently been reported by Baptista et al. as a constituent of the volatile exometabolome of three enterotoxic and non-enterotoxic S. aureus strains [25].
Table 2:
Universal staph volatiles detected in the headspace of S. aureus and S. epidermidis cultured in all four rich media: LB, TSB, MHB, and BHI. RI: retention index; MW: molecular weight; MS Fwd and MS Rev: Forward and reverse mass spectral matches, respectively, to the NIST MS library; ID Level: confidence of identification based on mass spectra and retention indices (1 highest).
| Compound | 1tR (s) | 2tR (s) | RI | MW | MS Fwd | MS Rev | CAS # | Molecular Formula | ID Level (1-4) |
|---|---|---|---|---|---|---|---|---|---|
| 3-Tridecene | 1960 | 0.58 | 1293 | 182 | 808 | 839 | 41446-53-1 | C13H26 | 2 |
| 3-Methyl-1-butanol | 896 | 0.97 | 783 | 88 | 913 | 913 | 123-51-3 | C5H12O | 2 |
| 5-Methyl-2-heptanone | 1412 | 0.71 | 1011 | 128 | 709 | 812 | 18217-12-4 | C8H16O | 3 |
| 3,7-Dimethyl-1-octene | 1908 | 0.57 | 1262 | 140 | 852 | 852 | 4984-01-4 | C10H20 | 3 |
| Alkane | 1818 | 0.56 | 1215 | 184 | 890 | 890 | C13H28 | 3 | |
| Alkane | 1942 | 0.58 | 1282 | 140 | 861 | 874 | C10H20 | 3 | |
| Unknown | 1896 | 0.58 | 1255 | 711 | 797 | 4 | |||
| Unknown | 2378 | 0.58 | 1549 | 735 | 854 | 4 | |||
| Unknown | 1784 | 0.92 | 1197 | 846 | 858 | 4 | |||
| Unknown | 2334 | 0.59 | 1521 | 853 | 861 | 4 |
Relative abundance of staph volatiles in complex media
The preceding analyses have characterized the volatilomes of S. aureus and S. epidermidis based on the presence or absence of VOCs in the headspace of each complex medium. Additional information is carried by the relative abundance of the metabolites, and we hypothesized that regardless of the media type in which these microbes are cultured, S. aureus and S. epidermidis will differentially produce volatiles that will reflect the differences in species, rather than differences in the medium. We used two unsupervised clustering methods to test this hypothesis: Hierarchical clustering analysis (HCA; Figure 3) and principal components analysis (PCA; Figure 4). Using all 337 staph volatiles in both HCA and PCA, we observed that sample clustering was primarily driven by species, but the medium also played a role, creating clusters within each species. Importantly, the media blanks do not cluster as strongly by media type, demonstrating that the medium-dependent clusters within each species are not driven by inherent differences in the VOCs of the media, but rather differences in the metabolism of the bacteria in the media either as a function of unique anabolic or catabolic substrates in each medium and/or unique regulation of the bacterial metabolomes therein.
Figure 3.

Hierarchical clustering analysis and heat map of 337 staphylococcal volatiles detected in cultures of S. aureus (gold), S. epidermidis (purple), and media controls (blue). Clustering is based on rows (samples) and abundances are mean-centered and scaled across columns (volatiles). SA, S. aureus; SE, S. epidermidis; BHI, brain heart infusion; LB, lysogeny broth; MHB, Mueller Hinton broth; TSB, tryptic soy broth.
Figure 4.
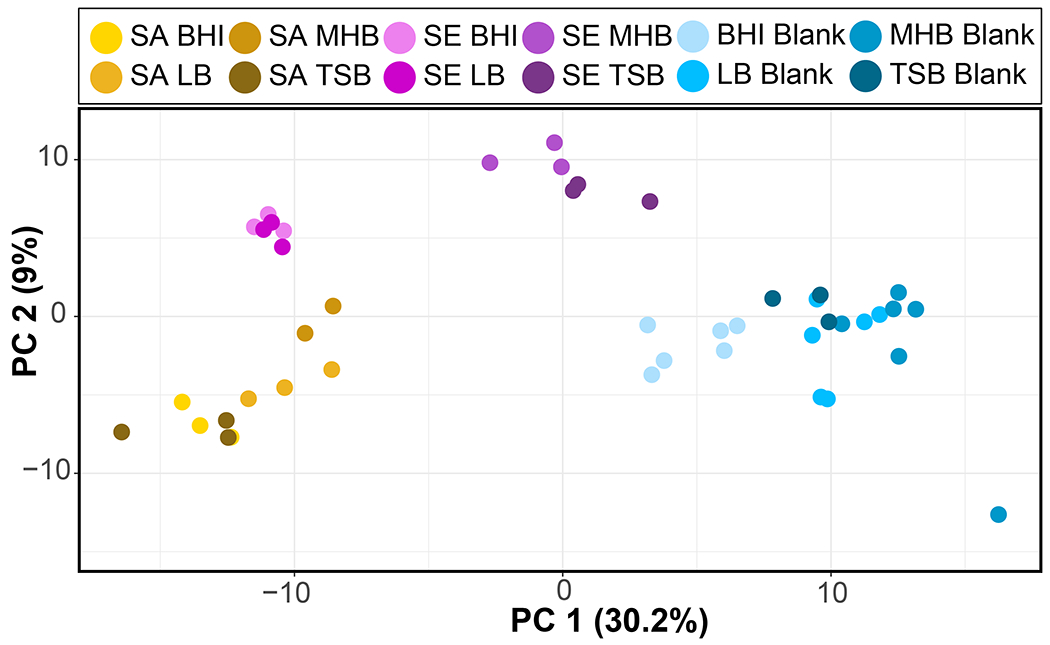
Principal component analysis score plot of 337 staph volatiles detected in cultures of S. aureus (gold), S. epidermidis (purple), and media controls (blue). Sa, S. aureus; Se, S. epidermidis; BHI, brain heart infusion; LB, lysogeny broth Lennox; MHB, Mueller Hinton broth; TSB, tryptic soy broth.
In the PCA we observed that S. aureus and S. epidermidis cluster separately from each other, but that there were also two media – MHB and TSB – in which S. epidermidis clustered more closely with the medium controls than with the bacterial cultures. In both cases these media produced a relatively small proportion of the overall S. epidermidis volatilome (Table S1), and the heat map indicates that the reduced number of detected volatiles is driving the observed clustering pattern (Figure 3). O’Hara and colleagues presented evidence that some of the media-dependent differences they observed in S. aureus volatiles may be attributable to differences in growth rates in the media [32], and therefore we performed growth analyses of S. aureus and S. epidermidis in all four media to determine if slow growth contributed to the reduced size and intensity of the S. epidermidis volatilome in MHB and TSB. The results reveal that all samples were in stationary phase and reached similar cell densities after 24 h, which was the sampling time for volatile metabolomics analyses (Table S2). Further, though we observed differences in growth rates, there were no clear trends in these data that explain the volatilome differences between S. epidermidis in MHB and TSB versus S. epidermidis in LB and BHI, nor the differences between S. epidermidis and S. aureus in MHB and TSB. While growth-dependent increases in microbial volatiles are expected during logarithmic phase growth, prior studies have shown that during bacterial stationary phase the concentrations of volatile metabolites can continue to change, and do not necessarily steadily increase or decrease with time [32, 48, 49]. Therefore, detailed time-course analyses of the dependence of staph volatiles on culture time and bacterial growth phase are required to fully elucidate the reasons that S. epidermidis VOC production was lower in MHB and TSB compared to the other six culture conditions we tested.
To determine how media influence S. aureus and S. epidermidis clustering when the volatiles unique to each species are removed, we evaluated clustering of the samples using only the 116 common staph volatiles (Figure S2), i.e., volatiles that are produced by both species when cultured in the same medium. We observed that the bacterial cultures cluster independently from the media controls, though resolution between S. aureus and S. epidermidis in BHI and LB is diminished (Figures 5 and 6). We also observe that S. epidermidis in MHB and TSB persist in clustering separately from the other two S. epidermidis data sets. Interestingly, though we removed the species-specific VOCs and therefore lose resolution between the species, we still do not observe clustering dominated by media type. Analyses using the 54 analytes produced by S. aureus or S. epidermidis in all four media (Figure 1) tightly clusters all staphylococcal cultures in the PCA, separate from the media controls (Figures S3 and S4). In the HCA we observe that the two species cluster separately from each other, and the media dependence of the volatilomes within each species is reduced (Figure S4). When removing the influence of any unique volatiles by using only the 10 universal staph volatiles that are produced by S. aureus and S. epidermidis in all media (Figure S2), we observe that the staph cultures cluster away from media controls (Figures S5, S6), even though all of the VOCs were detected in all media. In the HCA model we observe that the two species cluster separately, but the media-specific clustering is largely removed (Figure S5). Upon blank media exclusion from the PCA model, the largest proportion of variance among the bacterial cultures is explained by differences in biological replicates, though the two species are resolved along PC2 (Figure S7), and no clustering by medium is observed in PC1 or PC2.
Figure 5.

Hierarchical clustering analysis and heat map of 116 common staph volatiles detected in S. aureus and S. epidermidis cultures in four complex media. Clustering is based on rows (samples) and abundances are mean-centered and scaled across columns (volatiles). Sa, S. aureus; Se, S. epidermidis; BHI, brain heart infusion; LB, lysogeny broth Lennox; MHB, Mueller Hinton broth; TSB, tryptic soy broth.
Figure 6.

Principal component analysis score plot of 116 common staph volatiles detected in S. aureus and S. epidermidis cultures in four complex media. Sa, S. aureus; Se, S. epidermidis; BHI, brain heart infusion; LB, lysogeny broth Lennox; MHB, Mueller Hinton broth; TSB, tryptic soy broth.
We hypothesize that the 10 universal staph volatiles (Table 2), and the 54 VOCs produced by S. aureus or S. epidermidis in all four media (Figure 1), are the compounds most likely to be produced in vivo – that is, they are the least condition-dependent. The detection of the 10 universal staph volatiles in this study are both media and species independent, though differences in their relative abundances facilitated separation between the species. The 54 VOCs common to staph cultures include the 10 universal staph volatiles, but also an additional 44 compounds that were detected in a media-independent but species-dependent manner. Therefore, we propose that this subset of compounds are viable candidates for translational species-specific biomarkers to differentiate S. aureus from S. epidermidis, warranting further investigation.
This study included only a single strain of each species, and therefore it could be argued that the differences we observe between S. aureus and S. epidermidis are no greater than differences we may observe between different strains of S. aureus. Additional experiments with multiple strains of S. aureus and S. epidermidis are required to directly test this. However, experiments performed by Rees and colleagues on the VOCs produced by multiple clinical strains of K. pneumoniae in these same four complex media suggest that at the sub-species level of biological similarity, differences in the volatilomes are greater between media than between strain [30]. We posit, therefore, that we would observe the same trend, with multiple strains of S. aureus clustering together by media type rather than by strain, but that strains of S. aureus in any medium would cluster separately from strains of S. epidermidis. That is, that volatilome variance follows the trend of species > medium > strain.
In the development of volatile biomarkers to detect staph infections, it should be noted that the categorization of S. epidermidis as a beneficial commensal and S. aureus as a harmful pathogen is clinically useful, but an oversimplification. S. aureus and S. epidermidis can exchange genetic material (e.g., antibiotic resistance genes, SaPI, virulence factors) [50–53] and metabolites (heme, thiolactone peptide pheromones) [54, 55], enabling S. epidermidis to become pathogenic, especially in neonates, the elderly, the immunocompromised, and persons with other chronic infections [56, 57]. S. epidermidis isolates that are methicillin resistant (MRSE) are a growing concern, and are so because they carry the staphylococcal chromosome cassette mec (SCCmec), a mobile genetic element responsible for the methicillin resistant Staphylococcus aureus (MRSA) epidemic [58]. Embedded in this cassette resides the gene for a surfactant-like toxin that causes sepsis, enabling S. epidermidis to become pathogenic [59]. Therefore, while the vast majority of S. epidermidis detected in a clinical setting is benign, future work on VOC-based staph diagnostics could include the development of biomarkers that can detect S. epidermidis strains that pose a risk of infection, especially MRSE strains.
Conclusions
To our knowledge, this is the first study to compare the volatile metabolomes of Staphylococcus aureus and Staphylococcus epidermidis using GC×GC, and the first to evaluate the role of growth medium on the volatile profiles of two species of the same genus. We observed that the volatile profiles of S. aureus and S. epidermidis are media-dependent within species, but that larger differences in the volatilomes were observed between species. When we removed volatiles that are species and/or media specific from the HCA and PCA analyses, we observed a reduction in the resolution between clusters, but in all models clustering by species overrode clustering by media type. LB generated the most commonalities between the species, and therefore was the optimal medium for uniform VOC production by S. aureus and S. epidermidis, but TSB or BHI may be better for differentiating the two species while covering large proportions of the bacterial volatilomes. The next steps in our investigation will be to measure the strain-to-strain variation in the volatile profiles of S. aureus and S. epidermidis in these selected rich media, and identify putative biomarkers that robustly discriminate between the two species. This and future work to develop volatile profiles that can differentiate pathogenic S. aureus from commensal S. epidermidis will improve the specificity of diagnostics for prevailing community- and hospital-associated staph infections, and the chemical identification of any discriminating volatile biomarkers will yield deeper understanding of the biochemistry of staph infections and immunity.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge Dr. Stephen Pratt and Trenton J. Davis for their input on the statistical analyses used in this manuscript.
References
- [1].Kaspar U, Kriegeskorte A, Schubert T, Peters G, Rudack C, Pieper DH, Wos-Oxley M and Becker K 2016. The culturome of the human nose habitats reveals individual bacterial fingerprint patterns Environ Microbiol. 18(7) 2130–42 [DOI] [PubMed] [Google Scholar]
- [2].Naik S, Bouladoux N, Linehan JL, Han SJ, Harrison OJ, Wilhelm C, Conlan S, Himmelfarb S, Byrd AL, Deming C, et al. 2015. Commensal-dendritic-cell interaction specifies a unique protective skin immune signature Nature. 520(7545) 104–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].von Eiff C, Becker K, Machka K, Stammer H, Peters G and Study Group 2001 Nasal carriage as a source of Staphylococcus aureus bacteremia. N Engl J Med. 344(1) 11–6 [DOI] [PubMed] [Google Scholar]
- [4].Sarkany I and Gaylarde CC 1967. Skin flora of the newborn Lancet. 1(7490) 589–90 [DOI] [PubMed] [Google Scholar]
- [5].Kennedy EA, Connolly J, Hourihane JO, Fallon PG, McLean WHI, Murray D, Jo JH, Segre JA, Kong HH and Irvine AD 2017. Skin microbiome before development of atopic dermatitis: Early colonization with commensal staphylococci at 2 months is associated with a lower risk of atopic dermatitis at 1 year J Allergy Clin Immunol. 139(1) 166–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Iwase T, Uehara Y, Shinji H, Tajima A, Seo H, Takada K, Agata T and Mizunoe Y 2010. Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization Nature. 465(7296) 346–9 [DOI] [PubMed] [Google Scholar]
- [7].Nakatsuji T, Chen TH, Butcher AM, Trzoss LL, Nam SJ, Shirakawa KT, Zhou W, Oh J, Otto M, Fenical W, et al. 2018. A commensal strain of Staphylococcus epidermidis protects against skin neoplasia Sci Adv. 4(2) eaao4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kourtis AP, Hatfield K, Baggs J, Mu Y, See I, Epson E, Nadle J, Kainer MA, Dumyati G, Petit S, et al. 2019. Vital signs: Epidemiology and recent trends in methicillin-resistant and in methicillin-susceptible Staphylococcus aureus bloodstream infections - United States MMWR Morb Mortal Wkly Rep. 68(9) 214–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].McDermott KW, Elixhauser A and Sun R. Trends in hospital inpatient stays in the United States, 2005–2014. Rockville, MD: Agency for Healthcare Research and Quality; 2017 June 2017. Report No.: 07438079 Contract No.: 10.
- [10].Klein EY, Jiang W, Mojica N, Tseng KK, McNeill R, Cosgrove SE and Perl TM 2019. National costs associated with methicillin-susceptible and methicillin-resistant Staphylococcus aureus hospitalizations in the United States, 2010-2014 Clin Infect Dis. 68(1) 22–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Inagaki K, Lucar J, Blackshear C and Hobbs CV 2019. Methicillin-susceptible and methicillin-resistant Staphylococcus aureus bacteremia: Nationwide estimates of 30-day readmission, in-hospital mortality, length of stay, and cost in the United States Clin Infect Dis. [DOI] [PubMed] [Google Scholar]
- [12].Schmitt S, MacIntyre AT, Bleasdale SC, Ritter JT, Nelson SB, Berbari EF, Burdette SD, Hewlett A, Miles M, Robinson PA, et al. 2019. Early infectious diseases specialty intervention is associated with shorter hospital stays and lower readmission rates: A retrospective cohort study Clin Infect Dis. 68(2) 239–46 [DOI] [PubMed] [Google Scholar]
- [13].HAI Steering Committee. National action plan to prevent health care-associated infections: Road map to elimination. Washington, DC: The Department of Health & Human Services (HHS), (HHS) USDoHHS; 2013 April 2013. [Google Scholar]
- [14].Chapin K and Musgnug M 2003. Evaluation of three rapid methods for the direct identification of Staphylococcus aureus from positive blood cultures J Clin Microbiol. 41(9) 4324–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hong-Geller E and Adikari S. Volatile organic compound and metabolite signatures as pathogen identifiers and biomarkers of infectious diseases. In: Biosensing technologies for the detection of pathogens: A prospective way for rapid analysis. Rinken T and Kivirand K, editors. London, UK: IntechOpen; 2017. p. 1–17. [Google Scholar]
- [16].Nasir M, Bean HD, Smolinska A, Rees CA, Zemanick ET and Hill JE 2018. Volatile molecules from bronchoalveolar lavage fluid can ‘rule-in’ Pseudomonas aeruginosa and ‘rule-out’ Staphylococcus aureus infections in cystic fibrosis patients Sci Rep. 8(1) 826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hettinga KA, van Valenberg HJ, Lam TJ and van Hooijdonk AC 2009. The influence of incubation on the formation of volatile bacterial metabolites in mastitis milk J Dairy Sci. 92(10) 4901–5 [DOI] [PubMed] [Google Scholar]
- [18].Storer MK, Hibbard-Melles K, Davis B and Scotter J 2011. Detection of volatile compounds produced by microbial growth in urine by selected ion flow tube mass spectrometry (SIFT-MS) J Microbiol Methods. 87(1) 111–3 [DOI] [PubMed] [Google Scholar]
- [19].Bean HD, Zhu J, Sengle JC and Hill JE 2014. Identifying methicillin-resistant Staphylococcus aureus (MRSA) lung infections in mice via breath analysis using secondary electrospray ionization-mass spectrometry (SESI-MS) J Breath Res. 8(4) 041001–41001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bean HD, Jimenez-Diaz J, Zhu J and Hill JE 2015. Breathprints of model murine bacterial lung infections are linked with immune response Eur Respir J. 45(1) 181–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Joensen O, Paff T, Haarman EG, Skovgaard IM, Jensen PO, Bjarnsholt T and Nielsen KG 2014. Exhaled breath analysis using electronic nose in cystic fibrosis and primary ciliary dyskinesia patients with chronic pulmonary infections PLoS ONE. 9(12) e115584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Neerincx AH, Geurts BP, van Loon J, Tiemes V, Jansen JJ, Harren FJ, Kluijtmans LA, Merkus PJ, Cristescu SM, Buydens LM, et al. 2016. Detection of Staphylococcus aureus in cystic fibrosis patients using breath VOC profiles J Breath Res. 10(4) 046014. [DOI] [PubMed] [Google Scholar]
- [23].Zhu J, Bean HD, Wargo MJ, Leclair LW and Hill JE 2013. Detecting bacterial lung infections: In vivo evaluation of in vitro volatile fingerprints J Breath Res. 7(1) 016003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhu J, Jimenez-Diaz J, Bean HD, Daphtary NA, Aliyeva MI, Lundblad LK and Hill JE 2013. Robust detection of P. aeruginosa and S. aureus acute lung infections by secondary electrospray ionization-mass spectrometry (SESI-MS) breathprinting: From initial infection to clearance J Breath Res. 7(3) 037106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Baptista I, Santos M, Rudnitskaya A, Saraiva JA, Almeida A and Rocha SM 2019. A comprehensive look into the volatile exometabolome of enteroxic and non-enterotoxic Staphylococcus aureus strains Int J Biochem Cell Biol. 108 40–50 [DOI] [PubMed] [Google Scholar]
- [26].Dolch ME, Hornuss C, Klocke C, Praun S, Villinger J, Denzer W, Schelling G and Schubert S 2012. Volatile organic compound analysis by ion molecule reaction mass spectrometry for Gram-positive bacteria differentiation Eur J Clin Microbiol Infect Dis. 31(11) 3007–13 [DOI] [PubMed] [Google Scholar]
- [27].Julák J, Stránska E, Procházková-Francisci E and Rosová V 2000. Blood cultures evaluation by gas chromatography of volatile fatty acids Med Sci Monit. 6(3) 605–10 [PubMed] [Google Scholar]
- [28].Julák J, Procházková-Francisci E, Stránska E and Rosová V 2003. Evaluation of exudates by solid phase microextraction-gas chromatography J Microbiol Methods. 52(1) 115–22 [DOI] [PubMed] [Google Scholar]
- [29].Jünger M, Vautz W, Kuhns M, Hofmann L, Ulbricht S, Baumbach JI, Quintel M and Perl T 2012. Ion mobility spectrometry for microbial volatile organic compounds: A new identification tool for human pathogenic bacteria Appl Microbiol Biotechnol. 93(6) 2603–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Rees CA, Nordick KV, Franchina FA, Lewis AE, Hirsch EB and Hill JE 2017. Volatile metabolic diversity of Klebsiella pneumoniae in nutrient-replete conditions Metabolomics. 13(2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Dryahina K, Sovova K, Nemec A and Spanel P 2016. Differentiation of pulmonary bacterial pathogens in cystic fibrosis by volatile metabolites emitted by their in vitro cultures: Pseudomonas aeruginosa, Staphylococcus aureus, Stenotrophomonas maltophilia and the Burkholderia cepacia complex J Breath Res. 10(3) 037102. [DOI] [PubMed] [Google Scholar]
- [32].O’Hara M and Mayhew CA 2009. A preliminary comparison of volatile organic compounds in the headspace of cultures of Staphylococcus aureus grown in nutrient, dextrose and brain heart bovine broths measured using a proton transfer reaction mass spectrometer J Breath Res. 3(2) 027001. [DOI] [PubMed] [Google Scholar]
- [33].Tait E, Perry JD, Stanforth SP and Dean JR 2014. Identification of volatile organic compounds produced by bacteria using HS-SPME-GC-MS J Chromatogr Sci. 52(4) 363–73 [DOI] [PubMed] [Google Scholar]
- [34].Sen S, Sirobhushanam S, Johnson SR, Song Y, Tefft R, Gatto C and Wilkinson BJ 2016. Growth-environment dependent modulation of Staphylococcus aureus branched-chain to straight-chain fatty acid ratio and incorporation of unsaturated fatty acids PLoS ONE. 11(10) e0165300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kim BS, Yi H, Chun J and Cha CJ 2014. Genome sequence of type strain of Staphylococcus aureus subsp. aureus Gut Pathog. 6(1) 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].MacLea KS and Trachtenberg AM 2017. Complete genome sequence of Staphylococcus epidermidis ATCC 12228 chromosome and plasmids, generated by long-read sequencing Genome Announc. 5(36) e00954–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhang YQ, Ren SX, Li HL, Wang YX, Fu G, Yang J, Qin ZQ, Miao YG, Wang WY, Chen RS, et al. 2003. Genome-based analysis of virulence genes in a non-biofilm-forming Staphylococcus epidermidis strain (ATCC 12228) Mol Microbiol. 49(6) 1577–93 [DOI] [PubMed] [Google Scholar]
- [38].Sumner LW, Amberg A, Barrett D, Beale MH, Beger R, Daykin CA, Fan TW, Fiehn O, Goodacre R, Griffin JL, et al. 2007. Proposed minimum reporting standards for chemical analysis chemical analysis working group (CAWG) metabolomics standards initiative (MSI) Metabolomics. 3(3) 211–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bean HD, Rees CA and Hill JE 2016. Comparative analysis of the volatile metabolomes of Pseudomonas aeruginosa clinical isolates J Breath Res. 10(4) 047102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Epsky ND, Heath RR, Dueben BD, Lauzon CR, Proveaux AT and MacCollom GB 1998. Attraction of 3-methyl-1-butanol and ammonia identified from Enterobacter agglomerans to Anastrepha suspensa Journal of Chemical Ecology. 24(11) 1867–80 [Google Scholar]
- [41].Dieterle F, Ross A, Schlotterbeck G and Senn H 2006. Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. Application in 1H NMR metabonomics Anal Chem. 78(13) 4281–90 [DOI] [PubMed] [Google Scholar]
- [42].Oliveros JC. Venny. An interactive tool for comparing lists with Venn’s diagrams 2007. [Available from: http://bioinfogp.cnb.csic.es/tools/venny/index.html.
- [43].Lee C-J, DeMilo AB, Moreno DS and Martinez AJ 1995. Analysis of the volatile components of a bacterial fermentation attractive to the Mexican fruit fly (Anastrepha ludens) Journal of agricultural and food chemistry. 43(5) 1348–51 [Google Scholar]
- [44].Rees CA, Franchina FA, Nordick KV, Kim PJ and Hill JE 2017. Expanding the Klebsiella pneumoniae volatile metabolome using advanced analytical instrumentation for the detection of novel metabolites J Appl Microbiol. 122(3) 785–95 [DOI] [PubMed] [Google Scholar]
- [45].Timm CM, Lloyd EP, Egan A, Mariner R and Karig D 2018. Direct growth of bacteria in headspace vials allows for screening of volatiles by gas chromatography mass spectrometry Front Microbiol. 9 491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Robacker DC and Bartelt RJ 1997. Chemicals attractive to Mexican fruit fly from Klebsiella pneumoniae and Citrobacter freundii. Cultures sampled by solid-phase microextraction Journal of Chemical Ecology. 23(12) 2897–915 [Google Scholar]
- [47].Pizzini A, Filipiak W, Wille J, Ager C, Wiesenhofer H, Kubinec R, Blaško J, Tschurtschenthaler C, Mayhew CA and Weiss G 2018. Analysis of volatile organic compounds in the breath of patients with stable or acute exacerbation of chronic obstructive pulmonary disease J Breath Res. 12(3) 036002. [DOI] [PubMed] [Google Scholar]
- [48].Filipiak W, Sponring A, Baur MM, Filipiak A, Ager C, Wiesenhofer H, Nagl M, Troppmair J and Amann A 2012. Molecular analysis of volatile metabolites released specifically by Staphylococcus aureus and Pseudomonas aeruginosa BMC Microbiol. 12 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bunge M, Araghipour N, Mikoviny T, Dunkl J, Schnitzhofer R, Hansel A, Schinner F, Wisthaler A, Margesin R and Märk TD 2008. On-line monitoring of microbial volatile metabolites by proton transfer reaction-mass spectrometry Appl Environ Microbiol. 74(7) 2179–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Forbes BA and Schaberg DR 1983. Transfer of resistance plasmids from Staphylococcus epidermidis to Staphylococcus aureus: Evidence for conjugative exchange of resistance J Bacteriol. 153(2) 627–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Méric G, Miragaia M, de Been M, Yahara K, Pascoe B, Mageiros L, Mikhail J, Harris LG, Wilkinson TS, Rolo J, et al. 2015. Ecological overlap and horizontal gene transfer in Staphylococcus aureus and Staphylococcus epidermidis Genome Biol Evoll. 7(5) 1313–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hanssen AM, Kjeldsen G and Sollid JU 2004. Local variants of staphylococcal cassette chromosome mec in sporadic methicillin-resistant Staphylococcus aureus and methicillin-resistant coagulase-negative staphylococci: Evidence of horizontal gene transfer? Antimicrob Agents Chemother. 48(1) 285–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Yu W, Kim HK, Rauch S, Schneewind O and Missiakas D 2017. Pathogenic conversion of coagulase-negative staphylococci Microbes Infect. 19(2) 101–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hammer ND, Cassat JE, Noto MJ, Lojek LJ, Chadha AD, Schmitz JE, Creech CB and Skaar EP 2014. Inter- and intraspecies metabolite exchange promotes virulence of antibiotic-resistant Staphylococcus aureus Cell Host Microbe. 16(4) 531–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Létoffé S, Audrain B, Bernier SP, Delepierre M and Ghigo JM 2014. Aerial exposure to the bacterial volatile compound trimethylamine modifies antibiotic resistance of physically separated bacteria by raising culture medium pH MBio. 5(1) e00944–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Becker K, Heilmann C and Peters G 2014. Coagulase-negative staphylococci Clin Microbiol Rev. 27(4) 870–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Dong Y, Speer CP and Glaser K 2018. Beyond sepsis: Staphylococcus epidermidis is an underestimated but significant contributor to neonatal morbidity Virulence. 9(1) 621–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Conlan S, Mijares LA, Program NCS, Becker J, Blakesley RW, Bouffard GG, Brooks S, Coleman H, Gupta J, Gurson N, et al. 2012. Staphylococcus epidermidis pan-genome sequence analysis reveals diversity of skin commensal and hospital infection-associated isolates Genome Biol. 13(7) R64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Qin L, Da F, Fisher EL, Tan DC, Nguyen TH, Fu CL, Tan VY, McCausland JW, Sturdevant DE, Joo HS, et al. 2017. Toxin mediates sepsis caused by methicillin-resistant Staphylococcus epidermidis PLoS Pathog. 13(2) e1006153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


