Abstract
Investigations within the Human Connectome Project have expanded to include studies focusing on brain disorders. This paper describes one of the investigations focused on psychotic psychopathology: The psychosis Human Connectome Project (P-HCP). The data collected as part of this project were multimodal and derived from clinical assessments of psychopathology, cognitive assessments, instrument-based motor assessments, blood specimens, and magnetic resonance imaging (MRI) data. The dataset will be made publicly available through the NIMH Data Archive. In this report we provide specific information on how the sample of participants was obtained and characterized and describe the experimental tasks and procedures used to probe neural functions involved in psychotic disorders that may also mark genetic liability for psychotic psychopathology. Our goal in this paper is to outline the data acquisition process so that researchers intending to use these publicly available data can plan their analyses. MRI data described in this paper are limited to data acquired at 3 Tesla. A companion paper describes the study’s 7 Tesla image acquisition protocol in detail, which is focused on visual perceptual functions in psychotic psychopathology.
Keywords: Psychosis, Schizophrenia, Connectomics, Neuroimaging, Brain, MRI
1. Introduction
The psychosis Human Connectome Project (P-HCP) is part of the series of Connectomes Related to Human Disease funded by the National Institutes of Health. The original Human Connectome Project was an effort aimed at mapping normative brain structure and function and making the resulting data publicly available. This work was extended to apply similar measures to populations diagnosed with various disorders that affect the brain. The project described in this paper, titled, “Neural Disconnection & Errant Visual Perception in Psychotic Psychopathology” was focused on psychotic psychopathology in adults.
Psychosis is characterized by altered perceptual experiences (hallucinations) and delusional beliefs that distort one’s sense of reality and, as a result, significantly impair a person’s ability to function in their social and occupational roles (Raij et al., 2009). Neural abnormalities, including gray matter loss (De Peri et al., 2012), compromised white matter connectivity (Pettersson-Yeo et al., 2011; Wheeler and Voineskos, 2014), and aberrant functional connectivity (Baker et al., 2019; Calhoun et al., 2012) have been consistently documented in psychotic disorders by a large body of research. However few studies examine the relation between neural connectivity and symptom severity. Thus, the specific neural circuitry underlying symptoms is not yet known. Evidence suggests that the compromised neural connectivity in psychosis may be related to hallucinatory experiences (Ashtari et al., 2007; Whitford et al., 2012). In particular, connectivity between low-level sensory and high-level cognitive areas of the brain demonstrate abnormal interactions in people with psychosis (PwP) which may contribute to the experience of hallucinations (Allen et al., 2008; Pokorny et al., 2020). However, information is limited on neural connections that integrate low- and high-level perceptual processing. Further, psychotic illnesses may share common pathophysiology (Zwicker et al., 2018) and are characterized by cognitive deficits such as reward processing (Esslinger et al., 2012; Hanssen et al., 2015; Juckel et al., 2006a, 2006b; Murray et al., 2008; Nielsen et al., 2012; Schlagenhauf et al., 2009), cognitive control (Poppe et al., 2016; Smucny et al., 2020), and social cognition deficits (Das et al., 2012); Martin et al., 2016). A long history of twin and family studies reveal schizophrenia as a highly heritable condition (Gottesman, 1991). Finally, heritability estimates and evidence of subtle neural alterations among healthy biological relatives of people with psychotic disorders suggest a genetic predisposition towards abnormalities typically observed in psychotic disorders (de Zwarte et al., 2019, 2020). Together, this previous research provides the groundwork for transdiagnostic investigations aimed at studying brain connectivity in psychosis using a family study design. Thus, the current study was designed to measure neural mechanisms of aberrant perception, cognition, and other characteristics of psychosis by implementing Human Connectome Project imaging protocols with a sample of individuals affected by psychotic psychopathology and their first-degree biological relatives.
The goal of the psychosis Human Connectome Project (P-HCP) was to collect multimodal data that allows for testing of a wide range of hypotheses related to connectomics, genetics, and various cognitive processes relevant to psychosis. Specific hypotheses about visual function are described in our companion paper (Schallmo et al. (2021) in preparation). The P-HCP is well poised to link connectivity measures to dimensional variation in perceptual anomalies and other psychotic symptomatology by collecting multimodal data from a transdiagnostic sample of people with a history of psychosis, who meet diagnostic criteria for one of several psychotic disorders (i.e., schizophrenia, schizoaffective disorder, bipolar I disorder with psychotic features). This approach is in line with the NIMH Research Domain Criteria (RDoC) framework and allows for examination of neurophysiological mechanisms involved in psychotic symptomatology across the psychosis spectrum. Data from the P-HCP study will be made publicly available, as is done with other Human Connectome Projects as well as similar psychosis-focused open source projects such as the Biomedical Informatics Research Network (BIRN; http://www.nbirn.net; Glover et al. 2012) or the Mind Research Network’s Centers of Biomedical Research Excellence (COBRE) and MIND Clinical Imaging Consortium (MCIC; www.mrn.org; Gollub et al. 2013), among others. Further, because P-HCP was designed as a family study that included first-degree biological relatives of the participants with psychotic psychopathology, the neural abnormalities associated with aberrant perception could be tested as markers of genetic liability for psychotic psychopathology.
The P-HCP was designed to acquire multimodal data from three groups with the following recruitment goals: 150 PwP, 100 of their first-degree biological relatives, and 50 healthy control participants. Clinical, cognitive, motor, blood, and 3 Tesla neuroimaging data, including structural, diffusion weighted imaging (dMRI), resting state fMRI (rfMRI) and task fMRI (tfMRI) data were used to test study hypotheses related to psychotic psychopathology, cognitive function, genetic liability for psychosis, and neural connectivity and function. All data were collected at the University of Minnesota, with neuroimaging scans conducted at the Center for Magnetic Resonance Research in accordance with HCP protocols (Harms et al., 2018). Neuroimaging data were also collected on a Siemens 7 Tesla scanner, the specific goals and acquisition parameters of which are described in a companion paper (Schallmo et al. (2021) in preparation).
2. Objectives
The aim of this project was to use state-of-the-art brain imaging techniques from the Human Connectome Project in concert with cognitive tasks to develop and test neurophysiological models of aberrations in basic and complex functions of the brain related to psychotic psychopathology. The data contributed by the P-HCP informs our understanding of the neural circuitry related to distorted cognition and perception in psychosis. This information may facilitate interventions by uncovering neural mechanisms central to the development and maintenance of psychotic symptoms, which could serve as novel treatment targets. The goal of this paper is to describe how our data were collected such that other research groups can understand the nature of the dataset and plan their analyses.
3. Participants
The goal of the study is to enroll a total of 300 adult participants. Due to the Coronavirus Disease 2019 (COVID-19) pandemic, data collection was suspended in March 2020, with planned completion of data collection in the near future. To date, 247 participants completed the study (see Table 1 for demographic characteristics). Of the 131 PwP, 79 met criteria for schizophrenia, 16 for schizoaffective disorder, and 36 for bipolar I disorder with psychotic features. Of the 74 relatives, 41 were relatives of a person with schizophrenia, 9 were relatives of someone with schizoaffective disorder, and 24 had a first-degree family member with bipolar disorder with psychotic features. The majority of relatives were siblings (58.1%) with the remaining relatives being either parents (32.4%) or offspring (9.5%) of someone with a psychotic disorder.
Table 1.
Participant characteristics of the first 247 participants with complete data.
| Healthy Controls (n = 42) | Relatives(n = 74) | People with Psychosis (n = 131) | Statistic | Pairwise comparison | |
|---|---|---|---|---|---|
| Mean age (SD) | 39.19 (13.58) | 44.49 (13.98) | 38.48 (12.59) | F (2244) = 5.12, p =.007 | PwP < Rel** |
| Female sex | 21 (50.0%) | 49 (66.2%) | 57 (43.5%) | χ2 (2247) = 9.80, p =.007 | PwP < Rel** |
| Racial identity | - | - | - | χ2 (10,247) = 15.44, p =.117 | - |
| Caucasian, not of Hispanic origin | 36 (85.7%) | 67 (90.5%) | 91 (69.5%) | - | - |
| African American, not of Hispanic origin | 3 (7.1%) | 4(5.4%) | 24 (18.3%) | - | - |
| Asian or Pacific Islander | 1 (2.4%) | 0 | 5 (3.8%) | - | - |
| Hispanic | 1 (2.4%) | 1(1.4%) | 4 (3.1%) | - | - |
| American Indian or Alaska Native | 0 | 0 | 1 (0.8%) | - | - |
| Other | 1 (2.4%) | 2(2.7%) | 6 (4.6%) | - | - |
| Participant years of education | 15.88 (2.48) | 15.05 (2.33) | 14.04 (2.09) | F (2244) = 12.51, p <.001 | PwP < Con***PwP < Rel** |
| Parent education | 5.43 (1.38) | 5.13 (1.15) | 5.03 (1.15) | F (2237) = 1.70, p =.185 | - |
| WAIS IQ estimate | 106.36 (11.77) | 102.03 (11.01) | 97.24 (11.74) | F (2244) = 11.25, p <.001 | PwP < Con***PwP < Rel* |
| BPRS total | 27.40 (4.05) | 32.01 (6.47) | 45.63 (11.92) | F (2244) = 82.13, p <.001 | PwP > Con***PwP > Rel***Con < Rel* |
| SANS total (global scores) | - | - | 6.81 (3.96) | - | - |
| SAPS total (global scores) | - | - | 5.21 (4.32) | - | - |
| SPQ total | 6.86 (6.94) | 14.04 (13.00) | 30.27 (15.02) | F (2244) = 64.91, p <.001 | PwP > Con***PwP > Rel***Con < Rel* |
Note:
p < .0
p < .01
p < .001
Con = Healthy Controls; Rel = Relatives; PwP = People with Psychosis; Parent education (coded: 1 = 7th grade or less, 2 = 7th –9th grade, 3 = 10th –12th grade, 4 = high school graduate/ GED, 5 = partial college/ vocational/technical school, 6 = 4-year college/ university graduate, 7 = graduate degree, 9 = unknown); WAIS = Wechsler Adult Intelligence Scale; BPRS = Brief Psychiatric Rating Scale; SANS = Scale for the Assessment of Negative Symptoms (completed by PwP only); SAPS = Scale for the Assessment of Positive Symptoms (completed by PwP only); SPQ = Schizotypal Personality Questionnaire
3.1. Recruitment
Potential participants with psychosis were recruited from the community through advertisements and in-person announcements at community mental health support agencies, referrals from other research studies conducted at the University of Minnesota or collaborating institutions, referrals from local mental health care providers, and informational mailings following medical record review. Additionally, participants from our group’s previous studies who consented to being contacted for future research were also invited to participate. Once enrolled, participants with a history of psychotic symptomatology were asked to provide contact information for their first-degree relatives for recruitment outreach. This was not required and not doing so did not preclude people with a history of psychosis from participation. If the participant agreed, letters announcing the opportunity for participation were sent to first-degree relatives with follow-up by telephone if the letter went unanswered.
To enroll healthy control subjects, advertisements and postings announcing the study were placed in organizational newsletters and public places (e.g., hospital clinics, foyers and hallways of public and private institutions). Control participants from our group’s previous studies who agreed to follow-up communication regarding additional studies were also contacted.
3.2. Inclusion/exclusion criteria
To be eligible for enrollment, all participants spoke English as their primary language and did not have: a legal guardian (or otherwise lack capacity to provide informed consent), alcohol/drug abuse in the past month or alcohol/drug dependence in the last 6 months, a diagnosed Learning Disability or estimated IQ lower than 70 (if either condition was diagnosed based on testing by a trained professional or the latter by research staff), a current or past central nervous system disease (including: seizures, epilepsy, encephalitis, MS, Parkinson’s, stroke), history of head injury with skull fracture or loss of consciousness greater than 30 min, history of electro-convulsive therapy (ECT) in the last year, tardive dyskinesia (as evidenced by medical record), obstructed or compromised vision (e.g., lazy eye that is uncorrected or was corrected after age 17 / strabismus / cross eyes / permanent eye injury / abnormality in visual field / cataract), hearing problems (e.g., cannot hear without hearing aid / severe tinnitus), or a condition likely making it impossible to perform tasks (e.g., paralysis, severe arthritis). Additionally, PwP were between the ages of 18 and 65 years old with a diagnosis of schizophrenia, schizoaffective disorder, or bipolar I disorder with a history of psychotic symptomatology (i.e., delusions or hallucinations) with no indication that symptoms were caused by substance use or a general medical condition. While PwP were screened and excluded for current substance use issues, a history of such issues as well as current/lifetime comorbidities of any kind were permitted for enrollment in the study in order to have a sample representative of patients with psychosis in the general population while simultaneously limiting nuisance effects. Biological relatives were 18–69 years old with a first-degree biological relative with schizophrenia, schizoaffective disorder, or bipolar I disorder with psychosis, and living within one day’s drive or planning to visit the vicinity of the University of Minnesota. Because relatives included parents of PwP, we expanded the age range of the relatives group to accommodate recruitment efforts. Relatives were enrolled regardless of psychopathology. Approximately half (45.95%) of the first-degree biological relatives in the study carried their own mental health diagnosis (e.g., major depression). Occasionally, relatives with substance dependence in partial remission (3 participants, 3.9%), current substance dependence (1 participant, 1.3%), or psychotic psychopathology (1 participant, 1.3%) were included in the study. Controls were aged 18–65 and had no history of schizophrenia, schizoaffective disorder, or bipolar I disorder with psychotic features, or other psychotic symptoms or history of major depressive disorder. Additionally, controls had no first-degree biological relative with a history of psychiatric hospitalization for a psychotic or affective disorder. Finally, as this was a family study, PwP and relatives were not adopted.
3.3. Screening
Eligibility screening began with a semi-structured telephone survey prior to any in-person visits. The 15-to-25 min interview obtained basic demographics and information on medical conditions and past episodes of mood dysregulation, psychosis, and drug or alcohol use. Additionally, potential participants were screened for whether they could be safely scanned via MRI.
3.4. Study visits
Procedures were approved by the Institutional Review Board at the University of Minnesota, and followed the guidelines for human subjects research set by the Declaration of Helsinki. All participants completed an informed consent process, provided written consent, and were assessed for their capacity to provide informed consent using the University of California Brief Assessment of Capacity to Consent (Jeste et al., 2007). Demographic information was then collected, including current (e.g., living arrangements, work status, income, marital status) and lifetime variables (e.g., participant education, parent educational achievement; usual work status of parents; see Table 1). Participants then completed a clinical interview and self-report questionnaires, followed by cognitive and motor assessments, and additional study tasks. Clinical and cognitive assessments were often completed on two separate days to alleviate participant fatigue. Participants also provided a blood or saliva sample and then completed neuroimaging scans. Participants whose 3 T imaging data met acceptable data quality thresholds were invited to return to complete an ultra-high field MRI at a 7 T scanner. Finally, a subset of PwP completed a second 7 T scan and brief clinical interview follow-up. Overall, participants were able to tolerate the schedule of visits and only in rare instances were unable to complete all assessments due to fatigue, claustrophobia, excessive motion in the scanner, or time constraints.
3.5. Retention
Retention in the study was high, though several factors reduced the sample of complete data sets (n = 247). As of March 2020, 355 participants were initially consented and enrolled in the study, of which 284 participants (80%) completed the clinical assessments (the remaining 20% of participants did not complete the study for the following reasons: 15.49% were ineligible based on information gathered during clinical interview, 1.69% withdrew from the study, and 2.82% had their participation interrupted due to COVID-19). The majority (90.49%) of the 284 participants who completed clinical assessments went on to complete the 3T neuroimaging protocol. Reasons for not completing the scanning included: 2.82% became unreachable or moved out of state, 2.82% were excluded (due to the presence of significant motion artifacts, unable to fit in the scanner, or new information about meeting study eligibility criteria such as MRI contraindications), 2.46% withdrew (felt uncomfortable or claustrophobic in the scanner environment), and 1.41% had their participation interrupted due to COVID-19. The majority of participants (97.67%) who completed both clinical and 3 T scanning had usable data (only 6 participants - 2.33% - either had an MRI abnormality or significant motion artifacts that interfered with data quality) and the majority of those had their diagnoses confirmed during consensus review (4 participants were excluded after chart review; see description of this procedure in Clinical Assessment section below), yielding the sample of 247 participants described in this paper. Participants were provided with support to facilitate completion of study tasks (e.g., meals, transportation, breaks). The time to complete all study visits varied due to participant availability and study-related scheduling challenges (e.g., scanner upgrades). While some participants were able to complete all study visits within a few weeks, others required longer follow-up. On average, participants completed clinical, cognitive, and 3 T visits over the course of one month (mean = 33.82, SD = 55.89 days) and then, if eligible, returned to participate in 7 T scanning (see Schallmo et al. (2021) in preparation). Occasionally, participants decided to withdraw from the study, typically due to hesitations about confinement in the MRI scanner.
4. Clinical assessment
Diagnostic information was collected using the Structured Clinical Interview for DSM-IV-TR disorders (SCID; First et al., 2002). In place of the SCID module focused on psychosis, the psychosis section of the NIMH Diagnostic Interview for Genetic Studies (DIGS) version 3.0 revised 7 (Nurnberger et al., 1994) was used because the DIGS psychosis items more closely resemble the items queried using the study’s dimensional symptom measures (described below), which reduces participant burden. Staff training prior to independent administration of the diagnostic interview involved: reviewing the SCID user guide and DIGS manual, watching introductory videos provided by the SCID authors that describe the interview tool, engaging in a 9 session group training and (for trained staff) refresher meeting led by a PhD-level member of the team, completing practice ratings of interview videos provided by the SCID authors for the purpose of training, completing at least one mock interview with a fellow staff member, observing a trained rater administer at least two diagnostic interviews with study participants, and administering at least two diagnostic interviews with study participants while being observed by a trained rater. Interviewers regularly consulted with a doctorate-level study staff member regarding symptom classification and diagnosis. Clinical interviews and supporting materials (e.g., dimensional symptom measures, personality inventories, medical and work histories, medical records, when available) were reviewed by a team of at least 2 clinical psychology graduate students and / or postdoctoral associates to determine which diagnostic criteria were met and reach consensus on the most appropriate DSM diagnoses. Current and lifetime diagnoses were recorded, along with information for each of the five axes of the DSM-IV multiaxial diagnostic system. In addition, age of onset was collected for all PwP so that duration of illness can easily be calculated and examined in future analyze.
Further clinical information was provided by medical record review with consent and a participant-identified informant who could report on the participant’s current psychosocial functioning and symptomatology. Additional clinical measures are listed in Table 2 and described below. These include assessments of symptoms, functioning, personality, perception, physical health, and substance use. Behavioral measures, including assessments of cognition, handedness, emotion recognition, theory of mind, vision, and motor function, are listed in Table 3 and described below.
Table 2.
Clinical measures.
| Domain | Measure |
|---|---|
| Demographics questionnaire Pedigree |
|
| Symptoms | Structured Clinical Interview for DSM-IV-TR disorders (SCID) NIMH Diagnostic Interview for Genetic Studies - psychosis section (K-DIGS) Brief Psychiatric Rating Scale - 24 Item Version (BPRS) Scale for the Assessment of Negative Symptoms (SANS) & Scale for the Assessment of Positive Symptoms (SAPS)a Operational Criteria In Psychotic Illness (OPCRIT) a Temporal Experience of Pleasure Scale (TEPS) |
| Functioning | Global Functioning: Social and Role Scales Social Functioning Scale a (SFS) Social Adjustment Scale - Self Report (SAS-SR Short) Flanagan Quality of Life Scale (QOLS) |
| Personality | Modified Structured Interview for Schizotypy (SIS) b
Personality Inventory for DSM-5 (PID-5) Behavioral Approach/Inhibition Scale (BIS/BAS) Schizotypal Personality Questionnaire (SPQ) |
| Perception | Sensory Gating Inventory (SGI) / Structured Interview for Assessing Perceptual Anomalies (SIAPA) |
| Physical Health / Functioning | Pittsburgh Sleep Quality Index (PSQI) Medical History Medication List |
| Substance use | 7 Day Alcohol/Smoking questionnaire Drug Abuse Screening Test (DAST-10) Cannabis supplement |
Note:
denotes measures that are completed only by/for participants with psychosis
denotes measures that are completed only by relatives and healthy control participants
Table 3.
Behavioral measures.
| Domain | Measure |
|---|---|
| Handedness | Edinburgh Handedness Inventory |
| Cognition | Wide Range Achievement Test (WRAT-4) - word reading |
| Single word reading ability | |
| General cognitive ability | Wechsler Adult Intelligence Scale (WAIS-IV): |
| Similarities | |
| Matrix Reasoning | |
| Working Memory | Digit Span (forward and backward) |
| Sustained attention | Degraded Stimulus-Continuous Performance Task (DS-CPT) |
| Set shifting | Trails A and B |
| Visual processing speed | POSIT visual sweeps |
| Auditory processing speed | POSIT sound sweeps |
| Brief Assessment of Cognition in Schizophrenia (BACS): | |
| Verbal Memory; | Word list; |
| Working Memory; | Digit Sequencing; |
| Sensorimotor Processing Speed; | Token Motor Task; |
| Verbal Fluency; | Semantic Fluency; |
| Verbal Fluency; | Letter Fluency; |
| Speed of Processing; | Symbol Coding; |
| Reasoning/Problem Solving | Tower of London |
| Emotion | |
| Emotion Recognition | Penn Emotion Recognition Test (ER-40) POSIT PROID test |
| Affective state | Positive Affect Negative Affect Scale (PANAS) |
| Theory of Mind | Social Attribution Task–Multiple Choice (SAT-MC-II) |
| Vision | PhenX Vision Interview 1 |
| Vision health | |
| Visual acuity | Snellen Eye Chart |
| Contrast acuity | Mars Letter Contrast Sensitivity Test |
| Color vision | Farnsworth Dichotomous Color Vision Test: Panel D-15 |
| Motor | Abnormal Involuntary Movement Scale (AIMS) |
| Dyskinesia | |
| Dexterity | 9-Hole Pegboard Dexterity Test |
| Strength | Grip Strength Test 2 |
| Endurance | 2-Min Walk Endurance Test 2 |
| Locomotion | 4 M Walk Gait Speed Test 2 |
| Force variability | Force steadiness task |
| Balance | Postural sway task |
| Various motor variables | Handwriting task |
Note:
denotes measures from the NIH PhenXtoolkit (www.PhenXtoolkit.org)
denotes measures from the NIH toolbox (http://www.nihtoolbox.org/)
4.1. Dimensional assessment of symptoms
Symptoms of psychosis and mood dysregulation were measured with the Brief Psychiatric Rating Scale-24 Item Version (BPRS; Ventura et al., 2000) and the Scales for the Assessment of Negative/Positive Symptoms (SANS/SAPS; Andreasen 1981, 1983). Ratings on these measures reflect symptom severity over the previous 30 days. The BPRS and SANS/SAPS were repeated on the day of scanning if the initial assessment occurred over one month prior. Staff training on these measures involved a similar process as the training for diagnostic interview administration. The most recent inter-rater reliability check yielded intraclass correlations above 0.80 for all research staff administering the BPRS and SANS/SAPS measures. We will provide raw scores as well as summary scores for symptom domains or factors (e.g., see (Wilson and Sponheim, 2014) for BPRS factor solution). Preliminary examination of total BPRS symptoms shows an overall significant group difference as well as significant post-hoc comparisons such that, on average, relatives reported an intermediate level of symptoms in comparison to PwP and controls, as seen in Table 1 and Fig. 1 A. Following the diagnostic interview and symptom questionnaires, a further assessment of lifetime psychiatric symptoms, the Operational Criteria In Psychotic Illness (OPCRIT), was completed by research staff. The OPCRIT is a clinical rating form that uses semi-structured interview data to characterize a participant’s lifetime occurrence of symptoms across three domains (psychotic, depressed, manic) as well as other aspects related to the course of psychopathology in order to apply operational diagnostic criteria (McGuffin et al., 1991). Finally, the Temporal Experience of Pleasure Scale (TEPS; Gard et al., 2006) was used to assess affective-hedonic experiences.
Fig. 1.
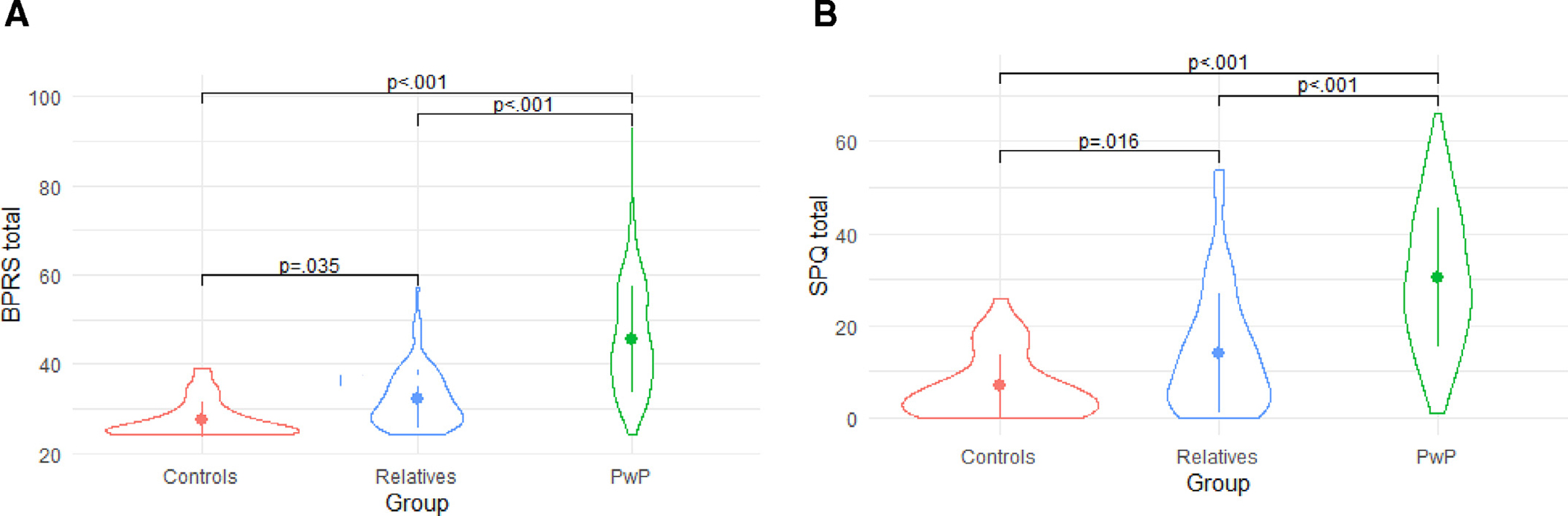
(A). Mean Brief Psychiatric Rating Scale (BPRS) symptom scores for preliminary sample (n = 247; error bars represent standard deviation). (B). Mean Schizotypal Personality Questionnaire (SPQ) symptom scores for preliminary sample (n = 247; error bars represent standard deviation).
4.2. Functioning
Real-world functioning was measured using the Global Assessment of Functioning scale (Hall, 1995) as part of the SCID, the Global Functioning Social and Role Scales (Auther et al., 2006; Niendam et al., 2006), the Social Adjustment Scale-Self Report (SAS-SR Short; Weissman and Bothwell, 1976), and the Quality of Life Scale (QOLS; Flanagan, 1978). Participants with psychosis additionally completed the Social Functioning Scale (SFS; Birchwood et al., 1990), which provides a thorough assessment of social adjustment over the past three months.
4.3. Personality
Personality and behavioral traits were assessed using the Personality Inventory for DSM-5 (PID-5; Krueger et al., 2012), the Behavioral Approach/Inhibition Scale (BIS/BAS; Carver and White, 1994), and the Schizotypal Personality Questionnaire (SPQ; Raine, 1991). The original 220-item PID-5 was slightly modified for the current study to include two validity items and 34 items from the absorption scale of the Multidimensional Personality Questionnaire (Tellegen, 1982). As with the BPRS, all study groups differed in SPQ total scores such that relatives had an intermediate level of symptoms compared to controls and PwP (see Table 1 and Fig. 1 B). Biological relatives and healthy control participants also completed the Modified Structured Interview for Schizotypy (SIS; Kendler et al., 1989; Nurnberger et al., 1994). These measures allow for dimensional variation on queried traits to be captured in this transdiagnostic sample of PwP and their biological relatives, who may themselves have some mental health concerns and/or mild to moderate levels of symptom expression.
4.4. Sensory/Perceptual phenomena
Perceptual aberrations were queried using the self-report Sensory Gating Inventory (SGI; Hetrick et al., 2012) and items from the Structured Interview for Assessing Perceptual Anomalies (SIAPA; Bunney et al., 1999) were administered in questionnaire format to assess everyday experiences with aberrant visual and auditory perception.
4.5. Physical health
To thoroughly characterize our sample, we obtained each participant’s account of their medical history, their current medication list, and their self-reported sleep habits via the Pittsburgh Sleep Quality Index (PSQI; Buysse et al., 1989), among other demographic and lifestyle information ascertained through the clinical interview.
4.6. Substance use
Problematic substance use was assessed during the diagnostic interview. In addition, participants completed the Drug Abuse Screening Test (DAST-10; Skinner, 1982) with study staff, as well as an in-house measure of lifetime cannabis use that allowed clinical evaluators to supplement the information gathered during the diagnostic interview, and an in-house questionnaire assessing recent use of alcohol and tobacco products (within the past 7 days prior to the clinical and MRI visits).
5. Cognitive assessment
General cognitive ability was estimated using the similarities and matrix reasoning subtests to calculate IQ (Denney, 2015) from the Wechsler Adult Intelligence Scale - fourth edition (Wechsler, 2008). The word reading subtest of the Wide Range Achievement Test, fourth edition (WRAT-4) was used as an estimate of reading ability (Wilkinson and Robertson, 2006). A variety of cognitive domains implicated in psychotic disorders were assessed using the paper version of the Brief Assessment of Cognition in Schizophrenia (BACS; Atkins et al., 2016), the digit span subtest of the WAIS-IV (Wechsler, 2008), the Trail Making Test of visual attention and task switching (Reitan and Wolfson, 1993), and the visual perceptual Degraded Stimulus-Continuous Performance Task (DS-CPT; Nuechterlein et al., 1983) of sustained attention which was administered via computer. Handedness was measured using the 10-item Edinburgh Handedness Inventory (Oldfield, 1971). Ratings were made on a scale of handedness preference (1: left hand only, 2: left hand preferred, 3: either hand/no preference, 4: right hand preferred, 5: right hand only) for each of the 10 actions queried. An item was added to the handedness inventory to assess how the wrist and hand are positioned when writing.
Speed of processing was measured in multiple modalities, using the symbol coding subtest of the BACS as well as two computer-based tasks from the battery provided by the PositScience Corporation: “Visual Sweeps” and “Sound Sweeps” (see https://www.brainhq.com). In the visual sweeps task, participants watched parallel, vertical bars moving across a portion of the screen and were instructed to identify the direction of the bars’ movement, as the bars were either compressing (moving inward toward each other) or expanding (moving outward, away from each other). This measure of basic visual processing speed engages several neural functions by alternating stimuli luminance and spatial frequency. The sound sweeps task required participants to determine the direction in which sounds “sweep” (rise or fall in pitch). Sounds were presented at different frequencies (500–5000 Hz) and with varying inter-stimulus intervals to simulate human speech and elicit common errors. This auditory task measures basic skills necessary for speech comprehension and sound differentiation. Performance on the Sound Sweeps and Visual Sweeps tasks among PwP and relatives was examined in a recent study from our group (Ramsay et al., 2020).
6. Emotion recognition
Emotion recognition abilities were measured using the 40 item Penn Emotion Recognition Test (ER-40) (Pinkham et al., 2008; Gur et al., 2002; Kohler et al., 2003; Kohler et al., 2004) and the PROID test, which is a computer-based task provided by the PositScience Corporation (see https://www.brainhq.com). The ER-40 presents photographs of male and female faces expressing mild or extreme happiness, sadness, anger, or fear, as well as neutral faces. The ER-40 faces include Black, White, and Asian individuals, as well as individuals of Hispanic origin. Participants indicated which emotion each face was expressing in a forced choice format. The PROID test of emotion recognition presents 21 trials of male and female speakers reading aloud a neutral sentence with either no emotional valence or to convey 1 of 6 different emotions (happiness, sadness, anger, fear, surprise, disgust). Participants were instructed to identify the emotional valence and rate the intensity on a Likert scale. Together, these two tasks provide a measure of emotion recognition based on visual and auditory input.
7. Theory of mind assessment
Theory of Mind, or mentalizing, was assessed using an alternate form of the multiple-choice version of the Social Attribution Task (SAT-MC-II; Johannesen et al., 2013; Johannesen et al., 2018; Bell et al., 2010; Klin, 2000; Heider and Simmel, 1944). In the SAT-MC-II, participants first view a short (approximately 1 min) animated video depicting three shapes (i.e., oval, rectangle, triangle) acting out a social drama, which is then played for participants again. Next, the video is shown again in shorter segments, after which a multiple-choice question is asked regarding the clip that was just viewed (Johannesen et al., 2013).
8. Vision screening
Participants completed a vision-related self-report questionnaire from the NIH PhenX Toolkit (www.PhenXtoolkit.org; Hamilton et al., 2011) to document self-reported vision health and personal or family history of strabismus and corrective lens use. Vision was also screened using behavioral measures of visual acuity, contrast acuity, and color vision. The Snellen eye chart is a measure of visual acuity that involved participants reading letters that decreased in size (Snellen, 1862). The Mars Letter Contrast Sensitivity test is similar, but the letters decrease in contrast in order to measure processing of low retinal spatial frequencies (Arditi, 2005). The Farnsworth Dichotomous Color Vision test screens for subtle abnormalities in color perception and tests color grading and discrimination abilities by requiring participants to arrange colored caps in order based on hue (Farnsworth, 1947). These measures were used to determine eligibility for neuroimaging tasks targeting visual functions.
9. Motor function
Gross and fine motor functioning were assessed using a battery of tests from the NIH Toolbox (http://www.nihtoolbox.org/). These included an assessment of dexterity using the 9-Hole Pegboard Dexterity Test, a measure of strength using the Grip Strength Test, a test of endurance using the 2 Min Walk Endurance Test, and a test of locomotion using the 4 Meter Walk Gait Speed Test (Reuben et al., 2013). Additionally, participants completed the Abnormal Involuntary Movement Scale (AIMS; Guy, 1976).
A subset of participants completed additional motor tasks including a force steadiness task (n = 240; 128 PwP, 69 relatives, 43 controls), a postural sway task (n = 212; 102 PwP, 66 relatives, 44 controls), and a handwriting task (n = 67; 31 PwP, 5 relatives, 31 controls).
The force steadiness task was similar to those used in previous studies (e.g., Mittal et al., 2011; Cortese et al., 2005; Caligiuri et al., 1997). In this task, participants attempt to apply constant pressure (approximately 300 centinewtons [cN] of force) to a load cell (visual feedback of both target force and force applied is provided) for two sets of three 15 s trials for both the dominant and non-dominant hand. Dyskinesia is reflected by increased variability in the force signal, which results from irregular muscle contractions (Mittal et al., 2011; Cortese et al., 2005; Caligiuri et al., 1997).
The postural sway task involves participants standing still on a Nintendo Wii Balance Board (Gilmore et al., 2015; Gilmore et al., 2017). Similar to Kent and colleagues (2012), participants were instructed to stand as still as possible for two minutes in each of four conditions that manipulated visual input and stance. Specifically, participants stood with their feet either together or shoulder-width apart and their eyes either closed or open (and looking straight ahead at an eye-level fixation cross).
For the handwriting task, participants used a Wacom Intuos Pro digitizing tablet (active area of 22.352 cm x 13.97 cm) (Wacom, Saitama, Japan) and a non-inking pen. Participants copied several patterns (e.g., overlaid circles, loops/repeated cursive letter “l”) under various conditions specifying size, speed, and handedness, with 5 trials for each condition. Movement of the pen was sampled using MovAlyzeR software (Neuroscript, LLC http://www.neuroscriptsoftware.com/ Tempe, AZ, USA). This task allows for the interrogation of a variety of motor variables, including for example movement fluidity (e.g., Teulings et al., 1997; Caligiuri et al., 2010; Caligiuri et al., 2015; Kent et al., 2019; Dean et al., 2013) and velocity scaling (e.g., Caligiuri et al., 2006; Caligiuri et al., 2009; Dean and Mittal, 2015).
10. Blood specimen collection
In order to harmonize biological data with the larger HCP database, blood samples were acquired in accordance with the HCP protocol. Participants provided a total of 20 mL (4 teaspoons) of blood via intravenous puncture. In accordance with HCP procedures, DNA and RNA material were extracted from whole blood and frozen and stored in a −70 °C freezer for future analyze. Serological samples were also extracted from whole blood by centrifuge at 2400 rpm and 24 °C for 16 min. If a participant was unable to provide a blood sample (e.g., needle discomfort, anemia), a saliva sample was obtained instead to be used for DNA analysis. For the first 247 participants with complete clinical and 3 T imaging data: 79% provided blood samples, 14% provided saliva samples, 2% declined to provide either, and a further 2% provided both types of samples, whereas 3% plan to provide a sample at a future 7 T imaging visit. There were no significant group differences in whether, or what type of, a DNA sample was provided (χ2 (8,247) = 11.16, p =.193).
11. MRI hardware
Imaging data were collected on a whole-body Siemens 3 T Prisma scanner (with the standard high gradient strength, 80 mT/m maximum amplitude and 200 T/m/s maximum slew rate) using a Siemens 32 channel head coil at the Center for Magnetic Resonance Research of the University of Minnesota.
12. Imaging data acquisition at 3 Tesla
We collected structural scans, diffusion weighted imaging, resting state fMRI, and task fMRI data across two scan sessions each of approximately 60–90 min duration. Typically, both scanning sessions were completed on the same day with a lunch intermission, but sessions occasionally occurred on separate days to accommodate participant fatigue and scheduling conflicts. The MRI sequence was split into two scan sessions to reduce participant burden for this clinical population spanning a wide age range, as was done in the HCP Lifespan protocol. This approach is not uncommon and standard co-registration procedures and options (such as AFNI’s align_epi_anat.py “-big_move” option; Cox 1996; Cox and Hyde 1997; Taylor et al. 2018) can be used in data processing to account for any participant placement differences between the two sessions, provided that gradient non-linearity correction is performed (Glasser et al., 2013). On the day of scanning, participants completed a brief questionnaire assessing current sleep and medications as well as recent use of caffeine, tobacco, alcohol, and other substances. Participants also completed the Positive Affect Negative Affect Scale (PANAS; Watson et al., 1988) to document their affective state prior to and after scanning. Finally, participants completed a MRI Debriefing Survey, in which participants reflected on the scanning experience. Preliminary analysis of this measure indicated that the imaging protocol was tolerable for this population and age range (average response agreeing with the statement, “I was comfortable while in the scanner” on a scale of 1–5 was 4.44, SD = 0.90; and 4.55, SD = 0.70 for the statement, “The length of time in the scanner was reasonable”).
12.1. Imaging protocol consistent with previous HCP studies
The P-HCP scanning protocol matches that of the Lifespan Human Connectome Projects, (see Harms et al., 2018), which collected data on healthy individuals across development. Specifically, structural T1- and T2-weighted imaging, diffusion weighted imaging, and resting state functional imaging remained consistent. The rationale for matching our imaging protocol to that of the Lifespan HCPs was that the adaptations (e.g., reduced scan duration, real-time motion correction for T1 and T2 scans) that were made to accommodate the youngest and oldest participants in the Lifespan HCPs also apply to our sample of vulnerable adults who, similarly, are likely to experience fatigue and increased motion. Additionally, the use of consistent imaging protocols across study populations allows for data to be integrated across HCPs, which is directly in line with the goals of the NIH.
Structural imaging.
Structural scans included both a multi-echo T1w MPRAGE sequence and a variable-flip-angle, turbo-spin-echo T2w scan with volumetric navigators to aid real-time motion correction and selective reacquisition. As in the Lifespan HCP (Harms et al., 2018), up to 30 k-space lines for the T1w scan and up to 25 k -space lines for the T2w scan were allotted for reacquisition. Protocol details are provided in Table 4 and Inline Supplementary Table 1.
Table 4.
P-HCP 3 T neuroimaging protocol.
| Modality | Matrix | FOV (mm) | Resolu-tion (mm) | Flip Angle | TE (msec) | TR (msec) | Slices/Orient-ation | AF/ MB | Time (min: sec) |
|---|---|---|---|---|---|---|---|---|---|
| Session A | |||||||||
| Localizer | 256 | 300 | 1.72 | 15 | 3 | 40 | 1 axl,1 sag,1 cor | - | 0:10 |
| Auto Align Head Scout | 160 | 260 | 1.63 | 8 | 1.37 | 3.15 | 128 sag | - | 0:14 |
| Localizer, aligned | 256 | 300 | 1.17 | 15 | 3 | 104 | 1 axl,7 sag,1 cor | - | 0:21 |
| Spin echo field maps (AP then PA) | 104 | 208 | 2 | 90 | 66 | 8000 | 72 axl | - | 0:090:09 |
| Resting state fMRI (AP then PA) | 104 | 208 | 2 | 52 | 37 | 800 | 72 axl | - | 6:416:41 |
| T 1 w setter | 32 | 256 | 8 | 2 | 4.6 | 9.9 | 32 sag | - | 0:01 |
| T 1 w MPRAGE, multi-echo | 300 × 320 | 240 × 256 | 0.8 | 8 | 1.81, 3.6, 5.39, 7.18 | 2500 | 208 sag | AF = 2 | 8:22 |
| T 2 w setter | 32 | 256 | 8 | 2 | 6 | 13 | 32 sag | - | 0:01 |
| T 2 w turbo spin echo SPACE with volumetric navigators | 300 × 320 | 240 × 256 | 0.8 | VAR | 564 | 3200 | 208 sag | AF = 2 | 6:35 |
| Spin echo field maps (AP then PA) | 104 | 208 | 2 | 90 | 66 | 8000 | 72 axl | - | 0:090:09 |
| Task fMRI MID PA (2 runs) | 104 | 208 | 2 | 52 | 37 | 800 | 72 axl | MB = 8 | 13:4713:47 |
| Session B | |||||||||
| Localizer | 256 | 300 | 1.72 | 15 | 3 | 40 | 1 axl,1 sag,1 cor | - | 0:09 |
| AAHead Scout | 160 | 260 | 1.63 | 8 | 1.37 | 3.15 | 128 sag | - | 0:14 |
| Localizer, aligned | 256 | 300 | 1.17 | 15 | 3 | 104 | 1 axl,7 sag,1 cor | - | 0:21 |
| Spin echo field maps (AP then PA) | 104 | 208 | 2 | 90 | 66 | 8000 | 72 axl | - | 0:090:09 |
| Resting state fMRI (AP then PA) | 104 | 208 | 2 | 52 | 37 | 800 | 72 axl | MB = 8 | 6:416:41 |
| dMRI, dir98* (AP then PA) | 140 | 210 | 1.5 | 78 | 89.20 | 3230 | 92 axl | MB = 4 | 5:385:38 |
| dMRI, dir99 (AP then PA) | 140 | 210 | 1.5 | 78 | 89.20 | 3230 | 92 axl | MB = 4 | 5:425:42 |
| Spin echo field maps (AP then PA) | 104 | 208 | 2 | 90 | 66 | 8000 | 72 axl | - | 0:090:09 |
| Task fMRI DPX PA (3 runs) | 104 | 208 | 2 | 52 | 37 | 800 | 72 axl | MB = 8 | 6:116:116:11 “ |
| Task fMRI Social Cognition PA (3 runs) | 104 | 208 | 2 | 52 | 37 | 800 | 72 axl | MB = 8 | 4:074:074:07 |
Note: FOV = field of view; TE = echo time; TR = repetition time; AF = acceleration factor; MB = multi-band acceleration factor; AP = anterior-to-posterior phase encoding direction; PA = posterior-to-anterior phase encoding direction; axl = axial; sag = sagittal, cor = coronal; VAR = variable flip angle; MID = Monetary Incentive Delay; DPX = Dot Pattern Expectancy
a subset of the initial participants in the study completed the 98 direction dMRI in session A rather than session B, as described in the dMRI section above
Diffusion weighted imaging.
The diffusion weighted imaging (dMRI) protocol was identical to that of the HCP-development and HCP-aging projects (Harms et al., 2018). Briefly, we administered a total of 4 diffusion runs (186 b = 1500 s/mm2, 184 b = 3000 s/mm2 and 28 b = 0 s/mm2 volumes across all runs) using the HCP 98 and 99 “direction” tables with both AP and PA phase encoding directions (see Table 4). We chose to use the same dMRI protocol as HCP-development/aging given the relatively short scan duration as compared to other HCP protocols. At the start of the study, we were concerned that the protocol might be burdensome enough on participants that we would suffer from a high attrition rate in the second session. For this reason and to improve the chances that we would collect some dMRI data, the 98-direction dMRI data was collected in session A while the 99-direction data was acquired in session B. Contrary to our concerns our completion rate was very high and after 32 participants were scanned we moved the 98 direction dMRI data to the session B acquisition, as was done in the HCP development and aging studies. Using the same dMRI protocol also allows investigators to combine datasets across these studies.
Resting state fMRI.
To remain consistent with the Lifespan HCP, four resting state scans in total were administered, each lasting 6.5 min (488 volumes), in pairs of two runs with opposite phase encoding directions. Matching the Lifespan HCP approach, for each resting pair the scans were always administered with the anterior-to-posterior acquisition first followed by the posterior-to-anterior phase encoding). This decision may have introduced an order effect into the resting state data. Participants were instructed to keep their eyes open and fixated on a cross, to not think about anything in particular, and to try to stay awake. Real time evaluation of subject motion was performed using the Framewise Integrated Real-time MRI Monitoring (FIRMM) software (Dosenbach et al., 2017). When a resting state fMRI scan had excessive motion based on objective criteria (> 20% of data with framewise displacement > 0.4mm) from FIRMM, and/or visual monitoring of participant motion, we reran the scan if time allowed. A monitoring camera was trained on the participant’s eyes to ensure that their eyes were kept open and fixated on the cross. If subjects closed their eyes for prolonged periods we verbally prompted them between scans. Notes of subject compliance with task instructions were saved in the database and will be shared along with the data. Respiration and heart rate data were also collected.
12.2. Imaging protocol unique to P-HCP
Task fMRI.
Our imaging protocol differs from that of the Lifespan HCP in the tasks we selected for functional scans. The tasks we included were selected to probe processes specifically implicated in psychosis: reward processing, cognitive control, social cognition. The acquisition parameters (other than number of runs and volumes per run) for tfMRI are identical to those of the resting state fMRI scans (see Table 4). For all resting state and task fMRI runs, each scan begins with the acquisition of a single-band reference that is used for calibration and is reconstructed as a separate series. Each fMRI run then has 4 “dummy” volumes that the scanner runs through but does not reconstruct in order to allow steady-state magnetization to be reached before image reconstruction and saving occurs. For task fMRI, the task was triggered at the start of the first reconstructed volume. Participants practiced all tasks outside the scanner until performance reached a minimum standard to demonstrate their full comprehension of task instructions. Once inside the magnet, staff reviewed the task instructions with participants before each task scan was run. Task images were displayed to participants in the scanner via a mirror mounted to the head-coil that reflected a screen just at the edge of the bore of the magnet onto which images were projected. During task fMRI data acquisition, study staff monitored participants’ button presses to identify and correct technological issues (e.g., finger mis-alignment) or participant somnolence. The same, four-button, curve-right fiber optic response pad (Current Designs Inc. https://www.curdes.com/ Philadelphia, PA, USA) was used across tasks.
12.3. Task fMRI: monetary incentive delay (MID) task
The cued reinforcement reaction time task (CRRT) is a monetary incentive delay task that was developed to measure motivated actions in response to varying reinforcement likelihoods, while accounting for individual differences in reaction time by using adaptive learning technology (Cools et al., 2005). The CRRT allows researchers to evaluate responsiveness to rewards above and beyond the confound of processing speed deficits that are common in psychotic disorders.
Participants completed 25 practice trials outside of the scanner until a passing score of 20/25 was obtained. Only participants who successfully achieved this threshold in the practice session went on to complete the task in the scanner. The task practice was repeated one time inside the scanner. The CRRT consists of two types of cues (gain or loss), a 2.5–3 s delay, a target stimulus of three circles arranged in a row containing an outlier (odd-one-out), and feedback regarding the outcome of the trial (whether money was gained or lost; see Fig. 2 for stimuli). The CRRT was administered using Presentation (version 18.3, Neurobehavioral Systems, 2016, Inc., Berkeley, CA, www.neurobs.com). Two runs, each lasting 13 min (up to 1040 volumes) and containing 80 trials, were administered. Participants made responses with the response pad turned sideways and using their left thumb to press button 1 and their right thumb to press button 4. Participants were instructed to respond quickly and accurately. The amount earned on each trial depended on the participant’s reaction time (see Simon et al., 2015). Participants received payment commensurate with the amount of money they won in the task (up to $60). For the current study, the CRRT was revised slightly to include different colors for the types of reward stimuli (win = green; lose = red; see Fig. 2) and to display reward quantities in United States dollar currency (rather than Euros, as in Simon et al. 2015). Participants were instructed to respond quickly and accurately.
Fig. 2.
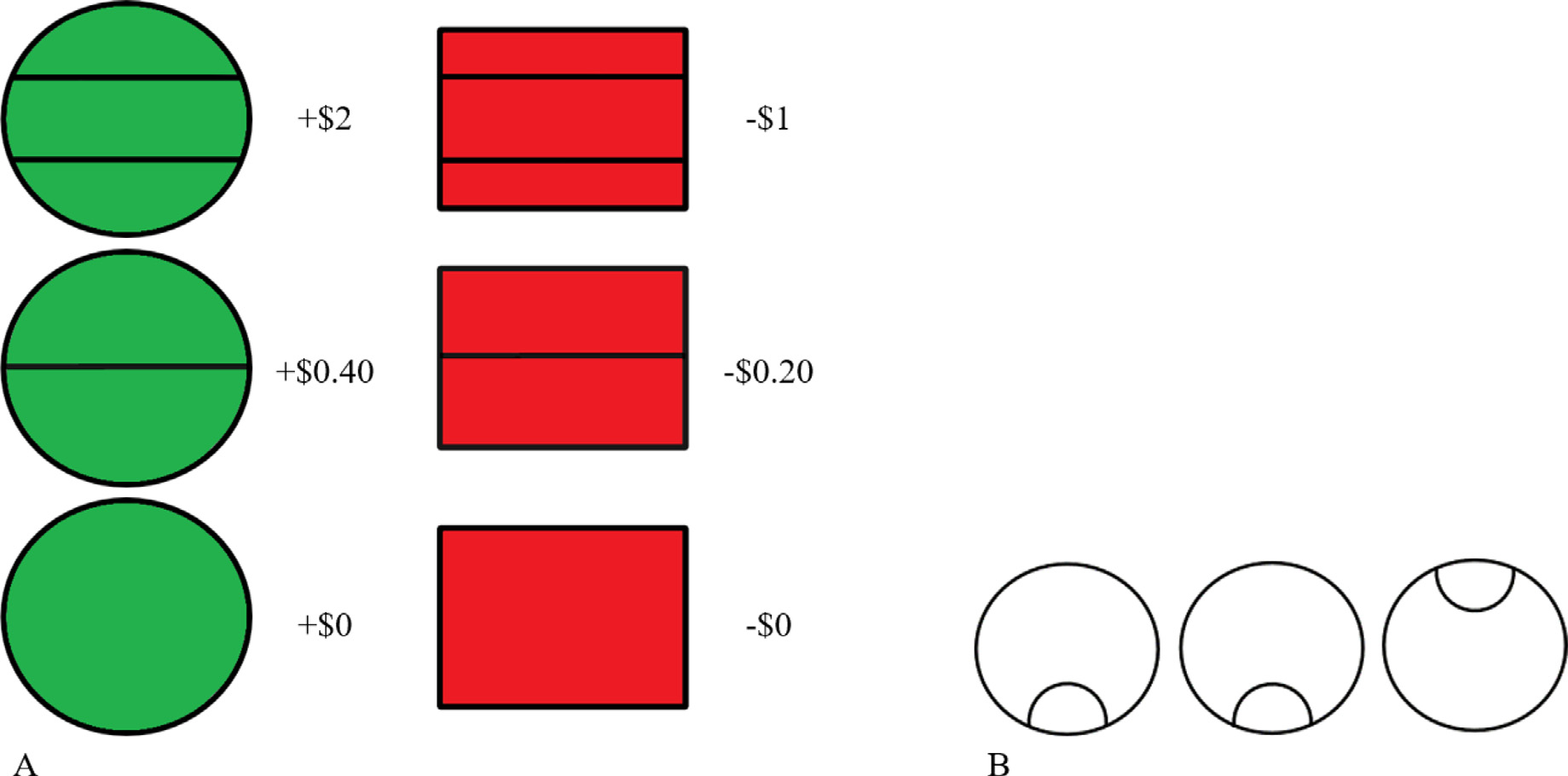
The reward cues (A) and the odd-one-out target stimulus (B) used for the cued reinforcement reaction time task (CRRT; adapted from Simon et al. 2015).
Behavioral variables include total amount of money won, number of errors and response time for each condition. Further, “reinforcement-related speeding” can be calculated to give an estimate of whether participants respond faster to reward-relevant versus neutral stimuli and to cues indicating higher versus lower reward amounts (see Fig. 2 A). This is done by subtracting the response time to neutral trials from gain or loss trials, or extending this concept further by subtracting lower reward amounts from the higher amount of corresponding valence.
The literature on monetary incentive delay task activation shows reduced activation in the ventral striatum during anticipation of monetary rewards across the schizophrenia spectrum, including: people with chronic schizophrenia (Juckel et al., 2006a, 2006b), first-episode psychosis (Esslinger et al., 2012; Hanssen et al., 2015; Nielsen et al., 2012; Schlagenhauf et al., 2009), unaffected first-degree relatives (de Leeuw et al., 2015; Grimm et al., 2014) and individuals at ultrahigh risk for schizophrenia (Juckel et al., 2012). Other first-degree relative studies have identified hypoactivation of the dorsal striatum during reward anticipation (Li et al., 2018) and hyperactivation of the default mode network (Hanssen et al., 2015). In bipolar disorder, monetary incentive delay task studies have shown mood-congruent reward processing bias in the left lateral orbitofrontal cortex during anticipation of gains and losses in people in a state of mania despite intact task performance (Bermpohl et al., 2010). When utilizing the CRRT version, people with first-episode schizophrenia exhibit deficits in reward-related speeding (Murray et al., 2008), though this was not observed in people with schizophrenia or schizoaffective disorder of any illness duration (Simon et al., 2015). Taken together, these findings suggest reward-related activation and performance abnormalities across different phases of psychotic illness, positioning the CRRT well within the aims of the current project.
12.4. Task fMRI: dot pattern expectancy (DPX) task
The Dot Pattern Expectancy (DPX) Task serves as a measure of cognitive control and context processing deficits, which are implicated in schizophrenia (Poppe et al., 2016). Recent research suggests that cognitive control deficits are not specific to schizophrenia but also occur in schizoaffective disorder and bipolar I disorder (Smucny et al., 2020). Cognitive control involves both proactive and reactive control mechanisms to regulate thoughts and behaviors (Braver, 2012). Proactive control requires that information is actively maintained in memory in order to carry out goal-directed behavior. Reactive control requires recall of information when triggered by a stimulus that is relevant to the intended goal-directed behavior. The DPX task used in this study is a variant of the expectancy AX continuous performance task created by Cohen and colleagues (Cohen et al., 1999; MacDonald, 2008; Servan-Schreiber et al., 1996). The version used in the current study displays stimuli of dot patterns instead of Latin alphabet letters in an effort to prevent participants from forming verbal rules to complete the task. For a detailed examination of psychometric properties of the DPX task, see Jones et al. (2010).
The task consists of three runs each lasting 6 min (450 v) and containing 40 trials. Each trial displays a cue and a probe, both of which are dots arranged in varying Braille letters (see Fig. 3). Prior to scanning, participants were familiarized with the task stimuli and were provided the opportunity to practice making responses. During this orientation, participants were told that the white and blue dot patterns form pairs and that they were to respond to each pair but indicate the “special” (correct) pair with a different button press. Participants then practiced until they reached 80% accuracy for both cue and probe responses. Participants were instructed to fixate their gaze on a cross in between each trial. Participants were asked to respond to the target probe (X) only when it is preceded by the appropriate cue (A) such that only AX pairs are valid. The majority of trials are AX trials. Participants also performed button presses to the cue, indicating that it is not a target, which increases the prepotency of the motor response. The response pad was positioned lengthwise such that the buttons faced away from the participant and the cord ran towards the participant’s feet; the participant pressed button 4 with the index finger of their dominant hand to make a target response and button 3 with the middle finger of their dominant hand for non target responses. Invalid cues (B) and probes (Y) are incorporated into the task to elicit participant cognitive control responses. Proactive control is engaged when a participant must maintain in memory an invalid cue (B) that precedes the valid probe (X) in order to inhibit their response to the otherwise valid probe. Reactive control is engaged when a valid cue (A) is followed by an invalid probe (Y) and the participant successfully inhibits their response. The DPX task was administered using E-Prime version 2.0 (Psychology Software Tools, 2015, Pittsburgh, PA). Output variables include the error rates for each trial type (i.e.g, AX, AY, BX, BY) and response times.
Fig. 3.
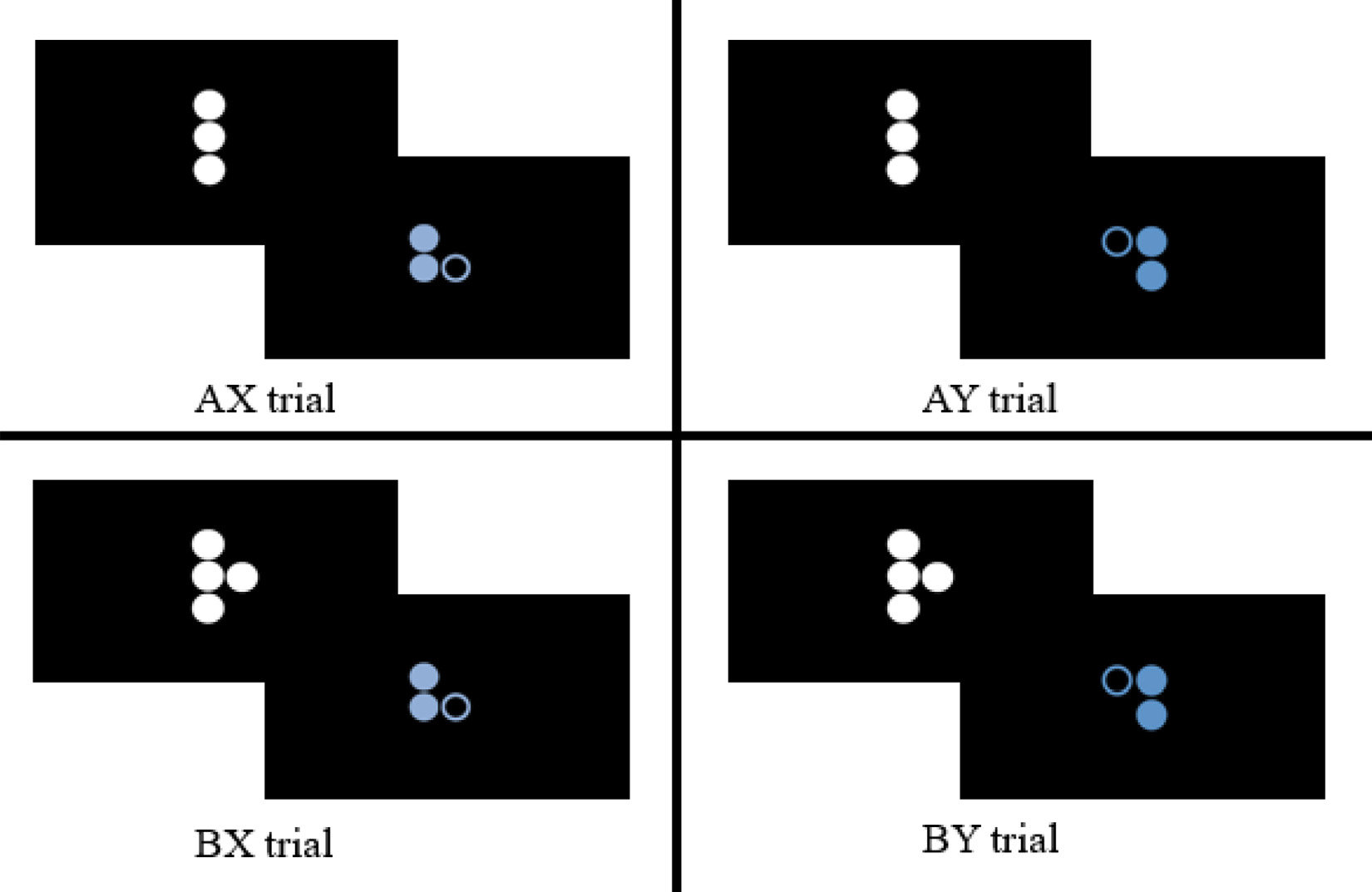
Cue-probe pairs of the dot pattern expectancy task.
12.5. Task fMRI: social cognition task
To measure theory of mind, a component of social cognition, the current study adapted the theory of mind task described in Barch et al. (2013) account of the Human Connectome Project task-fMRI battery. Our task used the Frith-Happé animations (see Fig. 4), wherein the movement of two triangles corresponds to one of three conditions: (1) theory of mind (the shapes were interacting socially, appearing to take each other’s mental states into account); (2) goal directed (movement of the shapes seemed related to each other, but they did not appear to be aware of each other’s thoughts and feelings); and (3) random (Abell et al., 2000; Castelli et al., 2000; White et al., 2011). Participants were familiarized with the task outside the scanner prior to scanning, and practiced viewing and responding to videos from each of the three conditions.
Fig. 4.
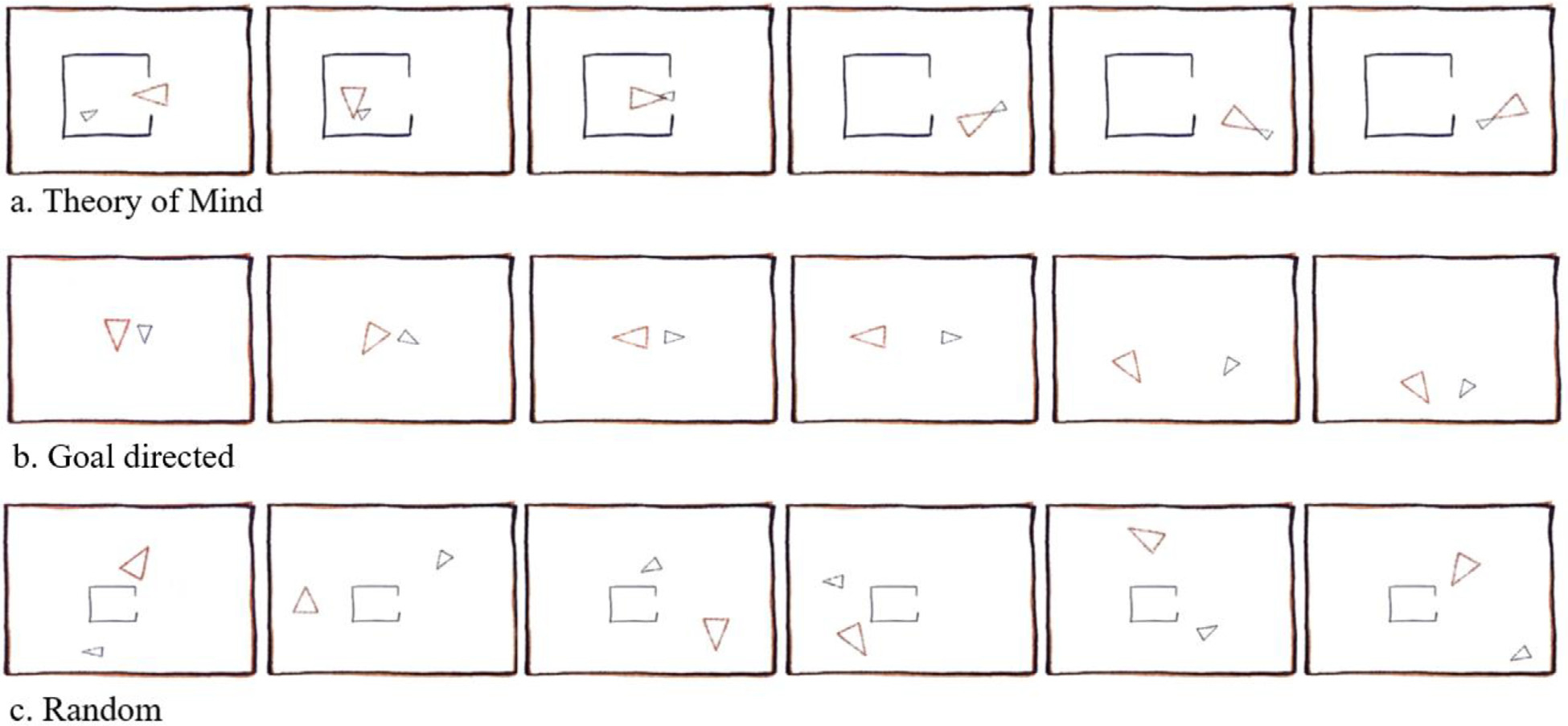
Social cognition task conditions.
The task was administered using E-Prime version 2.0 (Psychology Software Tools, 2015, Pittsburgh, PA) and conducted across three runs, each run containing 295 v. Each run presented two videos from each condition in pseudorandom order. Participants watched a 20 s video, followed by a response screen and then 15 s of rest/fixation. At the end of each video, participants chose what type of interaction they believed was going on using their dominant hand and same response pad placement as during the DPX task: mental interaction (index finger), non-mental interaction (middle finger), or no interaction (ring finger; similar to White et al. 2011). Participants were instructed that there was no “right” or “wrong” answer, and to select the type of interaction they thought had occurred. Additionally, participants were instructed to press any of the three buttons during the video as soon as they thought they knew which type of interaction was occurring.
This task was selected for inclusion in the P-HCP protocol because of its focus on social cognition, and in particular theory of mind, which is an area of great interest in schizophrenia spectrum research. For example, people with schizophrenia have demonstrated diminished activity in the right superior temporal gyrus at the temporoparietal junction and bilaterally in the inferior frontal gyri during this task (Das et al., 2012). Further, people with schizophrenia have been shown to be less accurate at identifying the interactions and to have increased functional connectivity with the left inferior frontal gyrus and caudate nucleus during the theory of mind and goal directed interactions (Martin et al., 2016).
12.6. Imaging at 7 Tesla
Neuroimaging data are also collected on a Siemens 7 T scanner, including minimal structural data, functional MRI, and magnetic resonance spectroscopy. Data acquisition methods for 7 T imaging are described in detail in our companion paper (Schallmo et al. (2021) in preparation).
13. Data quality
Multiple, complementary quality assurance procedures were used to ensure that the highest quality imaging data were collected. At acquisition, all imaging data were visually inspected for overall quality. Further, scanner operators provided feedback to participants regarding excessive movement and reacquired data if necessary. For T1 and T2 weighted scans, operators performed a qualitative evaluation of motion artifact and data usability by assessing ghosting level and image sharpness, in particular in the gray/white boundary. This evaluation was performed right after acquisition completion, and reacquisition of the low quality T1 and /or T2 scans was typically attempted before proceeding with the scan sequence if the participant was willing and if time allowed, within session A (9 participants) and/or within session B (12 participants). For diffusion scans, data were evaluated for full brain coverage and slice drop out/dimming during acquisition. A total of 5 participants had one or more replacement diffusion scans acquired during data collection. For functional scans, participant motion and respiration artifacts were closely monitored during acquisition. We also used the freely available FIRMM software to collect and monitor motion-related data quality information of the fMRI during acquisition (Dosenbach et al., 2017). Preliminary results indicate good quality functional data given a FIRMM threshold of 0.3mm, as evidenced by mean percent of volumes meeting the threshold (standard deviation in parentheses): resting fMRI = 80.72 (20.87); MID task = 82.58 (18.95); DPX task = 80.91 (19.32); social cognition task = 81.11 (17.92). However, there was a significant effect of group for resting fMRI (F(2,123) = 5.62, p = .005) and the DPX task (F(2,106) = 5.79, p = .004) such that PwP had a lower proportion of retained volumes compared to their biological relatives. There was no significant difference between either group and healthy controls.
After participant imaging data were acquired, a more quantitative process was used to determine which data sets were of sufficient quality for inclusion in our analyses. For structural scans, a rating system was established that closely resembles that of the Connectome Coordination Facility (CCF). Scans were assigned a preliminary data quality score ranging from 1 to 4 (1 = poor, 2 = fair, 3 = good, 4 = excellent). Scan quality was judged based on the presence and severity of banding, ghosting, and poor resolution (as indicated by blurred gray/white matter boundaries). Of the 257 participants who completed MRI scanning, only 6 participants (2.33%) had unusable data due to the presence of pathology or poor quality images and a further 4 participants (1.56%) were excluded based on diagnostic review. The remaining 247 participants with usable structural imaging data are described in this paper. Preliminary examination of T1 data across these 247 participants revealed adequate signal-to-noise ratio on average (combined sample mean = 18.99, SD = 2.94) as measured in white matter using Freesurfer QA Tools. As of March 2020, there was no significant difference between study groups in signal-to-noise ratio of the T1 data (F(2,243) = 1.01, p = .365). For the diffusion data, the FSL tool eddy was applied to the data using the slice outlier replacement flag and the default outlier threshold for bad slices of 4 standard deviations. Total number of bad slices per subject and total number of volumes with 5 or more bad slices across subjects was tracked during the study. Based on criteria from the FSL eddy web page no completed diffusion data sets were excluded from analysis based on QA measures. The data quality of all fMRI scans was analyzed using quality assurance metrics generated by AFNI’s individual subject processing pipeline design tool afni_proc.py (see, e.g., Taylor et al., 2018). Preliminary analysis suggests good quality data overall, with only a small fraction of participants (task: 2.42%; rest: 9.09%) who have more than 20% of volumes exceeding the threshold. For task fMRI, volumes with motion parameters exceeding 0.5mm Euclidean norm (enorm) relative to the previous volume were marked for exclusion from analysis. A significant group difference in task fMRI data quality (F(2,204) = 6.04, p = .003) shows that PwP had more motion artifacts that led to more volumes being excluded in comparison to the control (p =.026) and biological relative groups (p = .008). Further, groups differed such that task fMRI signal-to-noise ratios were lower among PwP (F(2,198) = 3.25, p = .041) compared to relatives (p = .049) but not controls (p = .203) For resting fMRI, an enorm threshold of 0.4mm was used. A similar pattern of group differences emerged for resting fMRI such that PwP had more volumes with motion parameters that exceeded the threshold (F(2,228) = 8.46, p < .001) compared to controls (p = .005) and relatives (p = .002). However, there were no group differences in resting fMRI signal-to-noise ratios (F(2,228) = 0.72, p = .488). Finally, incidental findings were logged in the data set should investigators choose to exclude these scans from their analyses. All of the aforementioned data quality indices were used as criteria for: rescan, inclusion in the 7T arm of the study, and inclusion in specific analyses.
14. Data pre-processing
The Connectome Coordination Facility (CCF) performs minimal pre-processing of all imaging data (e.g., discarding the initial 10 data frames or 8 s of fMRI data, converting data files from DICOM to NIFTI format, processing data using the HCP minimal pre-processing pipeline; Glasser et al. 2013). All raw and pre-processed data are made publicly available so that individual investigators may select their own data quality thresholds and perform data processing using tools of their choosing.
15. Data sharing
All data collected as part of the P-HCP will be shared with the scientific community. For clinical and behavioral measures, both raw and scaled scores (e.g., estimated IQ; symptom rating measure factors calculated from individual scale items) will be made available upon completion of data collection in late 2021. The NDA release of these data is anticipated for summer 2022. A data dictionary that defines all clinical and behavioral variables will be shared along with the data to aid investigators in selecting their analysis approach. Imaging data are prepared by the Connectome Coordination Facility (CCF; intradb.humanconnectome.org) for consistency with other human connectome projects (e.g., data quality assurance, formatting, pre-processing) prior to being made publicly available in the NIMH Data Archive (NDA; nda.nih.gov) repository. Data release of all 3T imaging data is anticipated by early 2022, assuming adequate harmonization of data.
16. Intended use and limitations
Previous studies are limited by small sample sizes and varying neuroimaging protocols that make generalizing to the population and comparison across studies difficult. Further, much of the psychosis literature is confined to homogenous diagnostic categories with a significant focus on schizophrenia. The P-HCP applied standardized human connectome neuroimaging protocols to a transdiagnostic sample of people with psychotic disorders in order to test hypotheses related to psychotic symptoms that span across a number of clinical diagnoses. The neural functions underlying these psychotic illnesses are investigated using multimodal imaging techniques to measure brain connectivity, function, structure, and their interrelation. This study is unique in that the sample is large and also includes first-degree biological relatives of people with psychosis, which allows for an examination of the role of genetic liability (e.g., distinguishing predispositioned vulnerabilities from disease biomarkers and sequelae). The goals of the psychosis Human Connectome Project were to investigate neural abnormalities associated with distorted perception in psychotic disorders and biological first-degree relatives and to make the data available to the scientific community.
While the current study has many strengths, certain limitations should be acknowledged. First, even though there is a longitudinal component for a subset of participants who return for follow-up 7 Tesla imaging, the majority of the P-HCP data are cross-sectional in nature. This limits the conclusions that can be made regarding the predictive power of aberrations in neural structure and connectivity. Second, data collection was interrupted by the COVID-19 pandemic, which resulted in a smaller control group as of March 2020. However, the P-HCP was designed to maximize enrollment of people with psychotic psychopathology as well as their biological first-degree relatives to yield an enriched sample. Further, other Human Connectome Projects offer additional control samples that were assessed with similar, if not identical, measures. Third, fMRI data quality may differ across groups as preliminary data show a higher proportion of motion artifacts among PwP, per the fMRI data quality indices described earlier. However, there were no significant group differences in signal-to-noise ratios for resting fMRI, task fMRI (between PwP and controls) or structural scans, as well as no dMRI data quality issues (see details above). Nevertheless, we advise investigators to use the data quality metrics, particularly for fMRI data, that we will make available along with the imaging data and to employ data processing and statistical methods that reduce the impact of motion artifacts in the data.
17. Conclusions
By applying the Human Connectome Project Development and Aging imaging protocol to a thoroughly characterized clinical population, first degree biological relatives and demographically similar healthy controls, and adding a selection of illness-relevant tasks, this project is well poised to inform our understanding of neural mechanisms involved in cognitive and perceptual distortions of psychosis. Advances in delineating the mechanisms involved in perceptual and cognitive disturbances can inform novel treatments for psychotic disorders. The data from this project will be made publicly available so that other researchers can generate and test novel hypotheses regarding neural anomalies in psychosis.
Supplementary Material
Acknowledgment
We acknowledge the efforts of our talented team of co-Investigators, postdocs, research assistants, volunteers, and collaborators. We especially want to thank Yeliz Toker for her support with citation of the references and recruitment of the sample. We also want to thank Haven Hafar, Brianna Wenande, Jessica Arend, Evan Myers, and Alina Yasis for their careful and thoughtful approach to clinical assessment as well as Rohit Kamath, Elijah Lahud, Li Shen Chong, and Isaac Hatch-Gillette for their work to collect high quality neuroimaging data. We are grateful to the families who participated in this study.
Funding
This work was supported by the National Institutes of Health (U01MH108150 to S.R. Sponheim).
Footnotes
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.neuroimage.2021.118439.
Data availability
The dataset will be made publicly available. Imaging data are prepared by the Connectome Coordination Facility (CCF; intradb.humanconnectome.org) for consistency with other human connectome projects (e.g., data quality assurance, formatting, pre-processing) prior to being made available to the scientific community in the NIMH Data Archive (NDA; nda.nih.gov) repository upon completion of data collection. Thus, all data, including raw and pre-processed neuroimaging data, will be made publicly available so that other researchers can generate and test novel hypotheses regarding neural anomalies in psychosis.
References
- Abell F, Happé F, Frith U, 2000. Do triangles play tricks? Attribution of mental states to animated shapes in normal and abnormal development. J. Cognit. Dev 15 (1), 1–16. [Google Scholar]
- Allen P, Larøi F, McGuire PK, Aleman A, 2008. The hallucinating brain: a review of structural and functional neuroimaging studies of hallucinations. Neurosci. Biobehav. Rev 32 (1), 175–191. [DOI] [PubMed] [Google Scholar]
- Andreasen NC (1981). The scale for the assessment of negative symptoms (SANS). [PubMed]
- Andreasen NC (1983). The scale for the assessment of positive symptoms (SAPS).
- Arditi A, 2005. Improving the design of the letter contrast sensitivity test. Invest. Ophthalmol. Vis. Sci 46 (6), 2225–2229. doi: 10.1167/iovs.04-1198. [DOI] [PubMed] [Google Scholar]
- Ashtari M, Cottone J, Ardekani BA, Cervellione K, Szeszko PR, Wu J, Kumra S, 2007. Disruption of white matter integrity in the inferior longitudinal fasciculus in adolescents with schizophrenia as revealed by fiber tractography. Arch Gen. Psychiatry 64 (11), 1270–1280. [DOI] [PubMed] [Google Scholar]
- Atkins AS, Davis V, Tseng T, Vaughan A, Harvey P, Patterson T, Narasimhan M, Keefe RSE, 2016. Validation of the tablet-administered brief assessment of cognition (BAC App). Schizophr. Res 181, 100–106. doi: 10.1016/j.schres.2016.10.010. [DOI] [PubMed] [Google Scholar]
- Auther AM, Smith CW, Cornblatt BA, 2006. Global Functioning: Social Scale (GF: Social). Zucker Hillside Hospital, Glen Oaks, NY. [Google Scholar]
- Baker JT, Dillon DG, Patrick LM, Roffman JL, Brady RO, Pizzagalli DA, Holmes AJ, 2019. Functional connectomics of affective and psychotic pathology. Proc. Natl. Acad. Sci 116 (18), 9050–9059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Burgess GC, Harms MP, Petersen SE, Schlaggar BL, Corbetta M, Van Essen DC, 2013. Function in the human connectome: task-fMRI and individual differences in behavior. NeuroImage 80, 169–189. doi: 10.1016/j.neuroimage.2013.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MD, Fiszdon JM, Greig TC, Wexler BE, 2010. Social attribution test-multiple choice (SAT-MC) in schizophrenia: comparison with community sample and relationship to neurocognitive, social cognitive and symptom measures. Schizophr Res. 122 (1–3), 164–171. doi: 10.1016/j.schres.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermpohl F, Kahnt T, Dalanay U, Hägele C, Sajonz B, Wegner T, Stoy M, Adli M, Krüger S, Wrase J, Ströhle A, Bauer M, Heinz A, 2010. Altered representation of expected value in the orbitofrontal cortex in mania. Hum. Brain Mapp 31 (7), 958–969. doi: 10.1002/hbm.20909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, 2012. The variable nature of cognitive control: a dual mechanisms framework. Trends Cogn. Sci 16 (2), 106–113. doi: 10.1016/j.tics.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchwood M, Smith J, Cochrane R, Wetton S, Copestake S, 1990. The social functioning scale: the development and validation of a new scale of social adjustment for use in family intervention programmes with schizophrenic patients. Br. J. Psychiatry 157 (6), 853–859. doi: 10.1192/bjp.157.6.853. [DOI] [PubMed] [Google Scholar]
- Bunney WE, Hetrick WP, Bunney BG, Patterson JV, Jin Y, Potkin SG, Sandman CA, 1999. Structured interview for assessing perceptual anomalies (SIAPA). Schizophr Bull. 25 (3), 577–592. doi: 10.1093/oxfordjournals.schbul.a033402. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ, 1989. The pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res 28, 193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Sui J, Kiehl K, Turner JA, Allen EA, Pearlson G, 2012. Exploring the psychosis functional connectome: aberrant intrinsic networks in schizophrenia and bipolar disorder. Front Psychiatry 2, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligiuri MP, Lohr JB, Rotrosen J, Adler L, Lavori P, Edson R, Tracy K, 1997. Reliability of an instrumental assessment of tardive dyskinesia: results from VA cooperative study #394. Psychopharmacology 132 (1), 61–66. doi: 10.1007/s002130050320. [DOI] [PubMed] [Google Scholar]
- Caligiuri MP, Teulings HL, Dean CE, Lohr JB, 2015. A quantitative measure of handwriting dysfluency for assessing tardive dyskinesia. J. Clin. Psychopharmacol 35, 168–174. doi: 10.1097/JCP.0000000000000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligiuri MP, Teulings HL, Filoteo JV, Song D, Lohr JB, 2006. Quantitative measurement of handwriting in the assessment of drug-induced parkinsonism. Hum. Mov. Sci 25 (4–5), 510–522. doi: 10.1016/j.humov.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Caligiuri MP, Teulings HL, Dean CE, Niculescu AB, Lohr J, 2009. Handwriting movement analyses for monitoring drug-induced motor side effects in schizophrenia patients treated with risperidone. Hum. Mov. Sci 28 (5), 633–642. doi: 10.1016/j.humov.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligiuri MP, Teulings HL, Dean CE, Niculescu AB, Lohr JB, 2010. Handwriting movement kinematics for quantifying extrapyramidal side effects in patients treated with atypical antipsychotics. Psychiatry Res. 177, 77–83. doi: 10.1016/j.psychres.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, White TL, 1994. Behavioral inhibition, behavioral activation, and affective response to impending rewarding and punishment: the BIS/BAS scales. J. Pers. Soc. Psychol 67, 319–333. [Google Scholar]
- Castelli F, Happé F, Frith U, Frith C, 2000. Movement and mind: a functional imaging study of perception and interpretation of complex intentional movement patterns. NeuroImage 12 (3), 314–325. doi: 10.1006/nimg.2000.0612. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Barch DM, Carter CS, Servan-Schreiber D, 1999. Context-processing deficits in schizophrenia: converging evidence from three theoretically motivated cognitive tasks. J. Abnorm. Psychol 108, 120–133. doi: 10.1037//0021-843x.108.1.120. [DOI] [PubMed] [Google Scholar]
- Cools R, Blackwell A, Clark L, Menzies L, Cox S, Robbins TW, 2005. Tryptophan depletion disrupts the motivational guidance of goal-directed behaviour as a function of trait impulsivity. Neuropsychopharmacology 30 (7), 1362–1373. doi: 10.1038/sj.npp.1300704. [DOI] [PubMed] [Google Scholar]
- Cortese L, Caligiuri MP, Malla AK, Manchanda R, Takhar J, Haricharan R, 2005. Relationship of neuromotor disturbances to psychosis symptoms in first-episode neuroleptic-naive schizophrenia patients. Schizophr Res. 75, 65–75. doi: 10.1016/j.schres.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Cox RW, 1996. AFNI: software for analyze and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res 29 (3), 162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox RW, Hyde JS, 1997. Software tools for analysis and visualization of FMRI Data. NMR Biomed. 10, 171–178. [DOI] [PubMed] [Google Scholar]
- Das P, Lagopoulos J, Coulston CM, Henderson AF, Malhi GS, 2012. Mentalizing impairment in schizophrenia: A functional MRI study. Schizophr. Res 134 (2–3), 158–164. [DOI] [PubMed] [Google Scholar]
- de Leeuw M, Kahn RS, Vink M, 2015. Fronto-striatal dysfunction during reward processing in unaffected siblings of schizophrenia patients. Schizophr. Bull 41 (1), 94–103. doi: 10.1093/schbul/sbu153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Peri L, Crescini A, Deste G, Fusar-Poli P, Sacchetti E, Vita A, 2012. Brain structural abnormalities at the onset of schizophrenia and bipolar disorder: a meta-analysis of controlled magnetic resonance imaging studies. Curr. Pharm. Des 18 (4), 486–494. [DOI] [PubMed] [Google Scholar]
- de Zwarte SM, Brouwer RM, Agartz I, Alda M, Aleman A, Alpert KI, van Haren NE, 2019. The association between familial risk and brain abnormalities is disease specific: an ENIGMA-relatives study of schizophrenia and bipolar disorder. Biol. Psychiatry 86 (7), 545–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zwarte SM, Brouwer RM, Agartz I, Alda M, Alonso-Lana S, Bearden CE, van Haren NE, 2020. Intelligence, educational attainment, and brain structure in those at familial high-risk for schizophrenia or bipolar disorder. Hum. Brain Mapp [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DJ, Mittal VA, 2015. Spontaneous parkinsonisms and striatal impairment in neuroleptic free youth at ultrahigh risk for psychosis. NPJ Schizophr 1 (1), 1–6. doi: 10.1038/npjschz.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DJ, Teulings HL, Caligiuri M, Mittal VA, 2013. Handwriting analysis indicates spontaneous dyskinesias in neuroleptic naïve adolescents at high risk for psychosis. J. Vis. Exp (81) e50852. doi: 10.3791/50852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Koller JM, Earl EA, Miranda-Dominguez O, Klein RL, Van AN, Snyder AZ, Nagel BJ, Nigg JT, Nguyen AL, Wesevich V, Greene DJ, Fair DA, 2017. Real-time motion analytics during brain MRI improve data quality and reduce costs. Neuroimage 161, 80–93. doi: 10.1016/j.neuroimage.2017.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esslinger C, Englisch S, Inta D, Rausch F, Schirmbeck F, Mier D, Kirsch P, Meyer-Lindenberg A, Zink M, 2012. Ventral striatal activation during attribution of stimulus saliency and reward anticipation is correlated in unmedicated first episode schizophrenia patients. Schizophr. Res 140 (1–3), 114–121. doi: 10.1016/j.schres.2012.06.025. [DOI] [PubMed] [Google Scholar]
- Farnsworth D, 1947. The Farnsworth Dichotomous Test for Color Blindness: Panel D-15; Manual. The Psychological Corp, New York. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Patient Edition (SCID-I/NP, 11/2002 Revision). Biometrics Research Department, New York: State Psychiatric Institution. [Google Scholar]
- Flanagan JC, 1978. A research approach to improving our quality of life. Am. Psychol 33 (2), 138–147. [Google Scholar]
- Gard DE, Gard MG, Kring AM, John OP, 2006. Anticipatory and consummatory components of the experience of pleasure: a scale development study. J. Res. Pers 40 (6), 1086–1102. [Google Scholar]
- Gilmore CS, Clancy D, Nelson BG, Gentz CL, Gierke M, Lim KO, 2015. Increased postural sway in veterans with mild traumatic brain injury measured with a force platform [poster presentation]. In: Proceedings of the Annual North American Brain Injury Society Conference. San Antonio, TX, United States. [Google Scholar]
- Gilmore C, Nelson B, Armstrong M, Lamberty G, Lim K, 2017. Engagement in a visual task increases postural stability in veterans with mild traumatic brain injury. Biol. Psychiatry 81 (10), S77. [Google Scholar]
- Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, Consortium, Wu-MHCP, 2013. The minimal preprocessing pipelines for the human connectome project. Neuroimage 80, 105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover GH, Mueller BA, Turner JA, van Erp TG, Liu TT, Greve DN, Potkin SG, 2012. Function biomedical informatics research network recommendations for prospective multicenter functional MRI studies. J. Magn. Reson. Imaging 36 (1), 39–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollub RL, Shoemaker JM, King MD, White T, Ehrlich S, Sponheim SR, Andreasen NC, 2013. The MCIC collection: a shared repository of multi-modal, multisite brain image data from a clinical investigation of schizophrenia. Neuroinformatics 11 (3), 367–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II, 1991. Schizophrenia Genesis: The Origins of Madness. W.H. Freeman & Co, New York. [Google Scholar]
- Grimm O, Heinz A, Walter H, Kirsch P, Erk S, Haddad L, Plichta MM, Romanczuk-Seiferth N, Pohland L, Mohnke S, Muhleisen TW, Mattheisen M, Witt SH, Schafer A, Cichon S, Nothen M, Rietschel M, Tost H, Meyer-Lindenberg A, 2014. Striatal response to reward anticipation: evidence for a systems-level intermediate phenotype for schizophrenia. JAMA Psychiatry 71 (5), 531–539. [DOI] [PubMed] [Google Scholar]
- Gur RC, Sara R, Hagendoorn M, Marom O, Hughett P, Macy L, Turner T, Bajcsy R, Posner A, Gur RE, 2002. A method for obtaining 3-dimensional facial expressions and its standardization for use in neurocognitive studies. J. Neurosci. Methods 115 (2), 137–143. doi: 10.1016/s0165-0270(02)00006-7. [DOI] [PubMed] [Google Scholar]
- Guy W, 1976. ECDEU Assessment Manual for Psychopharmacology, ADM 76–338. DHEW PublicationNational Institute of Mental Health, Rockville, MD. [Google Scholar]
- Hall RCW, 1995. Global assessment of functioning a modified scale. Psychosomatics 36 (3), 267–275. [DOI] [PubMed] [Google Scholar]
- Hamilton CM, Strader LC, Pratt JG, Maiese D, Hendershot T, Kwok RK, Hammond JA, Huggins W, Jackman D, Pan H, Nettles DS, Beaty TH, Farrer LA, Kraft P, Marazita ML, Ordovas JM, Pato CN, Spitz MR, Wagener D, Haines J, 2011. The PhenX toolkit: get the most from your measures. Am. J. Epidemiol 174 (3), 253–260. doi: 10.1093/aje/kwr193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanssen E, van der Velde J, Gromann PM, Shergill SS, de Haan L, Bruggeman R, Krabbendam L, Aleman A, van Atteveldt N, 2015. Neural correlates of reward processing in healthy siblings of patients with schizophrenia. Front. Hum. Neurosci 9. doi: 10.3389/fnhum.2015.00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms MP, Somerville LH, Ances BM, Andersson J, Barch DM, Bastiani M, Yacoub E, 2018. Extending the human connectome project across ages: imaging protocols for the lifespan development and aging projects. Neuroimage 183, 972–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heider F, Simmel M, 1944. An experimental study of apparent behavior. Am. J. Psychol 57 (2), 243–259. [Google Scholar]
- Hetrick WP, Erickson MA, Smith DA, 2012. Phenomenological dimensions of sensory gating. Schizophr. Bull 38 (1), 178–191. doi: 10.1093/schbul/sbq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeste DV, Palmer BW, Appelbaum PS, Golshan S, Glorioso D, Dunn LB, Kim K, Meeks T, Kraemer HC, 2007. A new brief instrument for assessing decisional capacity for clinical research. Arch Gen. Psychiatry 64 (8), 966–974. doi: 10.1001/arch-psyc.64.8.966. [DOI] [PubMed] [Google Scholar]
- Johannesen JK, Fiszdon JM, Weinstein A, Ciosek D, Bell MD, 2018. The social attribution task-multiple choice (SAT-MC): psychometric comparison with social cognitive measures for schizophrenia research. Psychiatry Res. 262, 154–161. doi: 10.1016/j.psychres.2018.02.011. [DOI] [PubMed] [Google Scholar]
- Johannesen JK, Lurie JB, Fiszdon JM, Bell MD, 2013. The social attribution task–multiple choice (SAT-MC): a psychometric and equivalence study of an alternate form. ISRN Psychiatry 2013, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JA, Sponheim SR, MacDonald III AW, 2010. The dot pattern expectancy task: reliability and replication of deficits in schizophrenia. Psychol. Assess 22 (1), 131. doi: 10.1037/a0017828. [DOI] [PubMed] [Google Scholar]
- Juckel G, Schlagenhauf F, Koslowski M, Filonov D, Wustenberg T, Villringer A, Knutson B, Kienast T, Gallinat J, Wrase J, Heinz A, 2006a. Dysfunction of ventral striatal reward prediction in schizophrenic patients treated with typical, not atypical, neuroleptics. Psychopharmacology 187 (2), 222–228. doi: 10.1007/s00213-006-0405-4. [DOI] [PubMed] [Google Scholar]
- Juckel G, Schlagenhauf F, Koslowski M, Wustenberg T, Villringer A, Knutson B, Wrase J, Heinz A, 2006b. Dysfunction of ventral striatal reward prediction in schizophrenia. NeuroImage 29 (2), 409–416. doi: 10.1016/j.neuroimage.2005.07.051. [DOI] [PubMed] [Google Scholar]
- Juckel G, Friedel E, Koslowski M, Witthaus H, Ozgurdal S, Gudlowski Y, Schlagenhauf F, 2012. Ventral striatal activation during reward processing in subjects with ultra-high risk for schizophrenia. Neuropsychobiology 66 (1), 50–56. doi: 10.1159/000337130. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Lieberman JA, Walsh D, 1989. The structured interview for schizotypy (SIS): a preliminary report. Schizophr. Bull 15 (4), 559–571. doi: 10.1093/schbul/15.4.559. [DOI] [PubMed] [Google Scholar]
- Kent JS, Caligiuri MP, Skorheim MK, Lano TJ, Mittal VA, Sponheim SR, 2019. Instrument-based assessment of motor function yields no evidence of dyskinesia in adult first-degree biological relatives of individuals with schizophrenia and schizoaffective disorder. Psychiatry Res. 272, 135–140. doi: 10.1016/j.psychres.2018.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent JS, Hong SL, Bolbecker AR, Klaunig MJ, Forsyth JK, O’Donnell BF, Hetrick WP, 2012. Motor deficits in schizophrenia quantified by nonlinear analysis of postural sway. PLOS ONE 7 (8), e41808. doi: 10.1371/journal.pone.0041808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin A, 2000. Attributing social meaning to ambiguous visual stimuli in higher-functioning autism and asperger syndrome: the social attribution task. J. Child Psychol. Psychiatry 41 (7), 831–846. [PubMed] [Google Scholar]
- Kohler CG, Turner TH, Bilker WB, Brensinger CM, Siegel SJ, Kanes SJ, Gur RE, Gur RC, 2003. Facial emotion recognition in schizophrenia: intensity effects and error pattern. Am. J. Psychiatry 160 (10), 1768–1774. doi: 10.1176/appi.ajp.160.10.1768. [DOI] [PubMed] [Google Scholar]
- Kohler CG, Turner TH, Gur RE, Gur RC, 2004. Recognition of facial emotions in neuropsychiatric disorders. CNS Spectr. 9 (4), 267–274. doi: 10.1017/s1092852900009202. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Derringer J, Markon KE, Watson D, Skodol AE, 2012. Initial construction of a maladaptive personality trait model and inventory for DSM-5. Psychol. Med 42 (9), 1879–1890. doi: 10.1017/S0033291711002674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Yan C, Lv QY, Yi ZH, Zhang JY, Wang JH, Lui S, Xu YF, Cheung E, Gur RE, Gur RC, Chan R, 2018. Striatal dysfunction in patients with schizophrenia and their unaffected first-degree relatives. Schizophr Res. 195, 215–221. doi: 10.1016/j.schres.2017.08.043. [DOI] [PubMed] [Google Scholar]
- MacDonald III AW, 2008. Building a clinically relevant cognitive task: case study of the AX paradigm. Schizophr Bull. 34, 619–628. doi: 10.1093/schbul/sbn038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AK, Dzafic I, Robinson GA, Reutens D, Mowry B, 2016. Mentalizing in schizophrenia: a multivariate functional MRI study. Neuropsychologia 93, 158–166. doi: 10.1016/j.neuropsychologia.2016.10.013. [DOI] [PubMed] [Google Scholar]
- McGuffin P, Farmer A, Harvey I, 1991. A polydiagnostic application of operational criteria in studies of psychotic illness: development and reliability of the OPCRIT system. Arch Gen. Psychiatry 48, 764–770. doi: 10.1001/archpsyc.1991.01810320088015. [DOI] [PubMed] [Google Scholar]
- Mittal VA, Dean DJ, Pelletier A, Caligiuri M, 2011. Associations between spontaneous movement abnormalities and psychotic-like experiences in the general population. Schizophr Res. 132, 194–196. doi: 10.1016/j.schres.2011.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray GK, Clark L, Corlett PR, Blackwell AD, Cools R, Jones PB, Robbins TW, Poustka L, 2008. Incentive motivation in first-episode psychosis: a behavioural study. BMC Psychiatry 8 (1), 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurobehavioral Systems, 2016. Presentation (Version 18.3) [Computer Software]. Neurobehavioral Systems, Inc., Berkeley, CA: https://www.neurobs.com/. [Google Scholar]
- Nielsen MO, Rostrup E, Wulff S, Bak N, Lublin H, Kapur S, Glenthoj B, 2012. Alterations of the brain reward system in antipsychotic naive schizophrenia patients. Biol. Psychiatry 71 (10), 898–905. doi: 10.1016/j.biopsych.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Niendam TA, Bearden CE, Johnson JK, Cannon TD, 2006. Global Functioning: Role Scale (GF: Role). University of California, Los Angeles, Los Angeles. [Google Scholar]
- Nuechterlein KH, Parasuraman R, Jiang Q, 1983. Visual sustained attention: image degradation produces rapid sensitivity decrement over time. Science 220 (4594), 327–329. doi: 10.1126/science.6836276. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T, 1994. Diagnostic interview for genetic studies. rationale, unique features, and training. NIMH genetics initiative. Arch Gen. Psychiatry 51, 849–859. doi: 10.1001/archpsyc.1994.03950110009002, discussion 863–864. [DOI] [PubMed] [Google Scholar]
- Oldfield RC, 1971. The Assessment and Analysis of Handedness: The Edinburgh Inventory. Neuropsychologia. Pergamon Press doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pettersson-Yeo W, Allen P, Benetti S, McGuire P, Mechelli A, 2011. Dysconnectivity in schizophrenia: where are we now? Neurosci. Biobehav. Rev 35 (5), 1110–1124. [DOI] [PubMed] [Google Scholar]
- Pinkham AE, Sasson NJ, Calkins ME, Richard J, Hughett P, Gur RE, Gur RC, 2008. The other-race effect in face processing among African American and caucasian individuals with schizophrenia. Am. J. Psychiatry 165 (5), 639–645. doi: 10.1176/appi.ajp.2007.07101604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokorny VJ, Espensen-Sturges TD, Burton PC, Sponheim SR, Olman CA, 2020. Aberrant cortical connectivity during ambiguous object recognition is associated with schizophrenia. Biol. Psychiatry Cog. Neurosci. Neuroimaging S2451-9022 (20), 30287–30291. doi: 10.1016/j.bpsc.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppe AB, Barch DM, Carter CS, Gold JM, Ragland JD, Silverstein SM, MacDonald AW, 2016. Reduced frontoparietal activity in schizophrenia is linked to a specific deficit in goal maintenance: a multisite functional imaging study. Schizophr Bull. 42 (5), 1149–1157. doi: 10.1093/schbul/sbw036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychology Software Tools, Inc. [E-Prime 2.0]. (2015). Retrieved from https://support.pstnet.com/. [Google Scholar]
- Raij TT, Valkonen-Korhonen M, Holi M, Therman S, Lehtonen J, Hari R, 2009. Reality of auditory verbal hallucinations. Brain 132 (11), 2994–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A, 1991. The SPQ: a scale for the assessment of schizotypal personality based on DSM-III-R. Schizophr Bull 17 (4), 555–564. [DOI] [PubMed] [Google Scholar]
- Ramsay IS, Schallmo M-P, Biagianti B, Fisher M, Vinogradov S, Sponheim SR, 2020. Deficits in auditory and visual sensory discrimination reflect a genetic liability for psychosis and predict disruptions in global cognitive functioning. Front Psychiatry 11, 638. doi: 10.3389/fpsyt.2020.00638, Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D, 1993. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. Neuropsychology Press, Tucson, AZ. [Google Scholar]
- Reuben DB, Magasi S, McCreath HE, Bohannon RW, Wang YC, Bubela DJ, Rymer WZ, Beaumont J, Rine RM, Lai JS, Gershon RC, 2013. Motor assessment using the NIH Toolbox. Neurology 80, S65–S75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallmo M-P, Weldon KB, Kamath RS, Moser HR, Killebrew KW, Demro C, Grant AN, Marjańska M, Sponheim SR, & Olman CA (In Preparation). Methods for investigating visual neurophysiology within the psychosis human connectome project. [Google Scholar]
- Schlagenhauf F, Sterzer P, Schmack K, Ballmaier M, Rapp M, Wrase J, Juckel G, Gallinat J, Heinz A, 2009. Reward feedback alterations in unmedicated schizophrenia patients: relevance for delusions. Biol. Psychiatry 65 (12), 1032–1039. doi: 10.1016/j.biopsych.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Servan-Schreiber D, Cohen J, Steingard S, 1996. Schizophrenic deficits in the processing of context: a test of a theoretical model. Arch Gen. Psychiatry 53, 1105–1112. doi: 10.1001/archpsyc.1996.01830120037008. [DOI] [PubMed] [Google Scholar]
- Simon JJ, Cordeiro SA, Weber MA, Friederich HC, Wolf RC, Weisbrod M, Kaiser S, 2015. Reward system dysfunction as a neural substrate of symptom expression across the general population and patients with schizophrenia. Schizophr Bull. 41 (6), 1370–1378. doi: 10.1093/schbul/sbv067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HA, 1982. The drug abuse screening test. Addict. Behav 7 (4), 361–371. [DOI] [PubMed] [Google Scholar]
- Smucny J, Iosif AM, Eaton NR, Lesh TA, Ragland JD, Barch DM, Gold JM, Strauss ME, MacDonald AW, Silverstein SM, Carter CS, 2020. Latent profiles of cognitive control, episodic memory, and visual perception across psychiatric disorders reveal a dimensional structure. Schizophr. Bull 46 (1), 154–162. doi: 10.1093/schbul/sbz025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snellen H, 1862. Probebuchstaben zur Bestimmung der Sehschärfe. Van de Weijer. [Google Scholar]
- Taylor PA, Chen G, Glen DR, Rajendra JK, Reynolds RC, Cox RW, 2018. FMRI processing with AFNI: some comments and corrections on “exploring the impact of analyze software on task fMRI results. BioRxiv doi: 10.1101/308643. [DOI] [Google Scholar]
- Tellegen A, 1982. Brief manual for the multidimensional personality questionnaire. Unpublished manuscript, University of Minnesota, Minneapolis: 8 1031–1010. [Google Scholar]
- Teulings HL, Contreras-Vidal JL, Stelmach GE, Adler CH, 1997. Parkinsonism reduces coordination of fingers, wrist, and arm in fine motor control. Exp. Neurol 146 (1), 159–170. doi: 10.1006/exnr.1997.6507. [DOI] [PubMed] [Google Scholar]
- Ventura J, Nuechterlein KH, Subotnik KL, Gutkind D, Gilbert EA, 2000. Symptom dimensions in recent-onset schizophrenia and mania: a principal components analysis of the 24-item brief psychiatric rating scale. Psychiatry Res. 97, 129–135. doi: 10.1016/s0165-1781(00)00228-6. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A, 1988. Development and validation of brief measures of positive and negative affect: the PANAS scales. J. Pers. Soc. Psychol 54 (6), 1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wechsler D, 2008. Wechsler Adult Intelligence Scale, 4th Ed. Pearson Assessment, San Antonio, TX. [Google Scholar]
- Weissman MM, Bothwell S, 1976. Assessment of social adjustment by patient self-report. Arch Gen. Psychiatry 33 (9), 1111–1115. doi: 10.1001/arch-psyc.1976.01770090101010. [DOI] [PubMed] [Google Scholar]
- Wheeler AL, Voineskos AN, 2014. A review of structural neuroimaging in schizophrenia: from connectivity to connectomics. Front Hum. Neurosci 8, 653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SJ, Coniston D, Rogers R, Frith U, 2011. Developing the frith-happé animations: a quick and objective test of theory of mind for adults with autism. Autism. Res 4 (2), 149–154. doi: 10.1002/aur.174. [DOI] [PubMed] [Google Scholar]
- Whitford TJ, Ford JM, Mathalon DH, Kubicki M, Shenton ME, 2012. Schizophrenia, myelination, and delayed corollary discharges: a hypothesis. Schiz Bull. 38 (3), 486–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson GS, Robertson GJ, 2006. WRAT4 Wide Range Achievement Test professional manual, 4th Ed. Psychological Assessment Resources, Lutz, FL. [Google Scholar]
- Wilson S, Sponheim SR, 2014. Dimensions underlying psychotic and manic symptomatology: Extending normal-range personality traits to schizophrenia and bipolar spectra. Compr. Psychiatry 55 (8), 1809–1819. [DOI] [PubMed] [Google Scholar]
- Zwicker A, Denovan-Wright E, Uher R, 2018. Gene-environment interplay in the etiology of psychosis. Psychol Med. 48 (12), 1925–1936. doi: 10.1017/S003329171700383X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset will be made publicly available. Imaging data are prepared by the Connectome Coordination Facility (CCF; intradb.humanconnectome.org) for consistency with other human connectome projects (e.g., data quality assurance, formatting, pre-processing) prior to being made available to the scientific community in the NIMH Data Archive (NDA; nda.nih.gov) repository upon completion of data collection. Thus, all data, including raw and pre-processed neuroimaging data, will be made publicly available so that other researchers can generate and test novel hypotheses regarding neural anomalies in psychosis.


