Abstract
Coronaviruses are evolutionarily successful RNA viruses, common to multiple avian, amphibian and mammalian hosts. Despite their ubiquity and potential impact, knowledge of host immunity to coronaviruses remains incomplete, partly owing to the lack of overt pathogenicity of endemic human coronaviruses (HCoVs), which typically cause common colds. However, the need for deeper understanding became pressing with the zoonotic introduction of three novel coronaviruses in the past two decades, causing severe acute respiratory syndromes in humans, and the unfolding pandemic of coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This renewed interest not only triggered the discovery of two of the four HCoVs, but also uncovered substantial cellular and humoral cross-reactivity with shared or related coronaviral antigens. Here, we review the evidence for cross-reactive B cell memory elicited by HCoVs and its potential impact on the puzzlingly variable outcome of SARS-CoV-2 infection. The available data indicate targeting of highly conserved regions primarily in the S2 subunits of the spike glycoproteins of HCoVs and SARS-CoV-2 by cross-reactive B cells and antibodies. Rare monoclonal antibodies reactive with conserved S2 epitopes and with potent virus neutralising activity have been cloned, underscoring the potential functional relevance of cross-reactivity. We discuss B cell and antibody cross-reactivity in the broader context of heterologous humoral immunity to coronaviruses, as well as the limits of protective immune memory against homologous re-infection. Given the bidirectional nature of cross-reactivity, the unprecedented current vaccination campaign against SARS-CoV-2 is expected to impact HCoVs, as well as future zoonotic coronaviruses attempting to cross the species barrier. However, emerging SARS-CoV-2 variants with resistance to neutralisation by vaccine-induced antibodies highlight a need for targeting more constrained, less mutable parts of the spike. The delineation of such cross-reactive areas, which humoral immunity can be trained to attack, may offer the key to permanently shifting the balance of our interaction with current and future coronaviruses in our favour.
Keywords: Human coronaviruses, Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), Coronavirus disease 2019 (COVID-19), Antibodies, Humoral immunity, Immunological memory, Heterologous immunity, Heterotypic immunity
1. Introduction
A cardinal property of adaptive immunity is the ability to tailor its response to each invading pathogen, discriminating it from self-antigens or other pathogens [1]. The basis of this ability is a repertoire of T cell and B cell antigen receptors (TCRs and BCRs, respectively), which are somatically generated, clonally distributed and, in the case of BCRs, further somatically hypermutated [[2], [3], [4]]. Clonally expanded lymphocytes with TCRs or BCRs specific to a given foreign antigen need to be accommodated in the finite homeostatic space of the host, and their elevated frequency over time after infection constitutes the cellular basis of immunological memory [5,6].
Despite the exquisite specificity that characterises adaptive immunity, the response elicited by one pathogen can also recognise another related or unrelated pathogen. Such heterologous immunity may arise through recognition of identical antigenic epitopes shared by different pathogens as part of larger proteins, or through recognition of unrelated epitopes owing to cross-reactivity of individual TCRs and BCRs [7]. These two extremes are not exclusive, and heterologous immunity often arises from recognition of non-identical but related epitopes with sequence or structure differences that fall within the tolerance thresholds of the inherently degenerate recognition by the antigen receptors [7].
Here, we review the evidence for heterologous immunity to human and emerging zoonotic coronaviruses, and the extent to which it may diversify the adaptive immune responses to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Evidence for both T cell and B cell cross-reactivity between endemic common cold human coronaviruses (HCoVs) and SARS-CoV-2 has recently been provided [8]. T cell cross-reactivity is observed far more commonly than B cell cross-reactivity, often in over half of individuals, and has been recently reviewed elsewhere [8]. Instead, we will focus primarily on antibody cross-reactivity and the limits humoral immunity may provide against HCoVs or SARS-CoV-2.
The immensity of the unfolding coronavirus disease 2019 (COVID-19) pandemic has underscored the pathogenic potential of SARS-CoV-2 but also the need for deeper understanding of coronavirus infection and host immunity [9]. In addition to SARS-CoV-2, six other coronaviruses have made humans their host, albeit not all equally successfully [[10], [11], [12], [13], [14]]. Since the isolation of the first HCoVs, the betacoronavirus HCoV-OC43 (Organ Culture 43) and the alphacoronavirus HCoV-229E around 1965 [15], it took nearly four decades and the zoonotic introduction of SARS-CoV in 2002 for further coronaviruses to be discovered. Although SARS-CoV outbreaks were limited and the virus disappeared by 2004, this renewed interest in coronaviruses led to the discovery of two other HCoVs: the alphacoronavirus HCoV-NL63 (Netherlands 63) and the betacoronavirus HCoV-HKU1 (Hong Kong University 1) in 2004–2005 [15]. It was several years later, in 2012, when another zoonotic coronavirus, Middle East respiratory syndrome coronavirus (MERS-CoV), emerged with significant pathogenic potential but limited transmissibility.
The main process targeted by virus-neutralising antibodies is entry into the host cell, which in all coronaviruses is carried out by the spike protein (S), a homotrimeric membrane-bound glycoprotein that is proteolytically processed into the S1 and S2 subunits which mediate receptor binding and membrane fusion respectively [15]. Although not all coronaviruses use the same cellular receptor, parallels in receptor use can be observed, with a choice between a membrane-bound peptidase and sialoglycan-based receptors [15]. SARS-CoV, SARS-CoV-2, and HCoV-NL63 all share the angiotensin-converting enzyme 2 (ACE2) as their cellular receptor, whereas MERS-CoV and HCoV-229E use dipeptidyl peptidase 4 (DPP4) and aminopeptidase N (APN), respectively [[15], [16], [17], [18]]. In contrast, the two other betacoronaviruses, HCoV-OC43 and HCoV-HKU1, use 9-O-acetylated sialic acids on glycoproteins and glycolipids and also employ the homodimeric hemagglutinin-esterase (HE) in the entry process, which is specific to this clade [15].
In addition to HCoVs, which have become endemic globally, humans may also be exposed to animal coronaviruses in more localised settings. These relatively frequent coronavirus zoonoses suggest frequent exposure of humans to animal coronaviruses which may not necessarily break the species barrier. For example, bats harbour a diverse array of coronaviruses and are considered an important source of coronavirus zoonoses [19,20]. In addition to the presence of live bats in wet markets, close and frequent contact with tree-roosting fruit bats may occur in agricultural areas. Moreover, bat guano, used as fertiliser in several countries in at least three continents, can expose guano miners to bat coronaviruses [21]. The avian coronavirus infectious bronchitis virus (IBV) causes disease in birds including farmed chickens, and can be a significant problem for the poultry industry [22]. Historically, IBV was controlled by a number of measures, which include spray and drinking water vaccination with live attenuated IBV strains [23,24], potentially exposing chicken farmers to avian coronaviruses. Similarly, detection of canine and feline coronaviruses has been reported in humans with acute respiratory symptoms [25,26].
Thus, humans are variably exposed to HCoVs as well as diverse animal coronaviruses, all of which may have the potential to induce adaptive immune responses that cross-react with other coronaviruses and SARS-CoV-2. Indeed, at least one study of SARS-CoV-2 reactive T cell responses in individuals with no history of SARS-CoV or SARS-CoV-2 infection incriminated unknown animal coronaviruses rather than HCoVs as inducers of such cross-reactive T cells [27]. This was based on low homology of the identified epitopes with HCoVs but high conservation in animal betacoronaviruses [27]. As the precise source of coronavirus exposure or the inducers of cross-reactive immunological memory may not always be clear, even among HCoVs, we will consider all potential sources as one.
2. Detection of coronavirus cross-reactive antibodies
2.1. Main targets of humoral immunity
Emerging zoonotic coronavirus outbreaks have necessitated the development of assays for the detection of infection and monitoring viral spread, and serology assays have been a vital part of such efforts. It was during the development of serology assays that antibody cross-reactivity between HCoVs and zoonotic coronaviruses was frequently observed and confounded interpretation of serology results. Indeed, the earlier attempts to develop serology assays for SARS-CoV infection demonstrated cross-reactivity of human and immunised animal sera with the nucleoproteins (N) of SARS-CoV and of HCoVs [[28], [29], [30]].
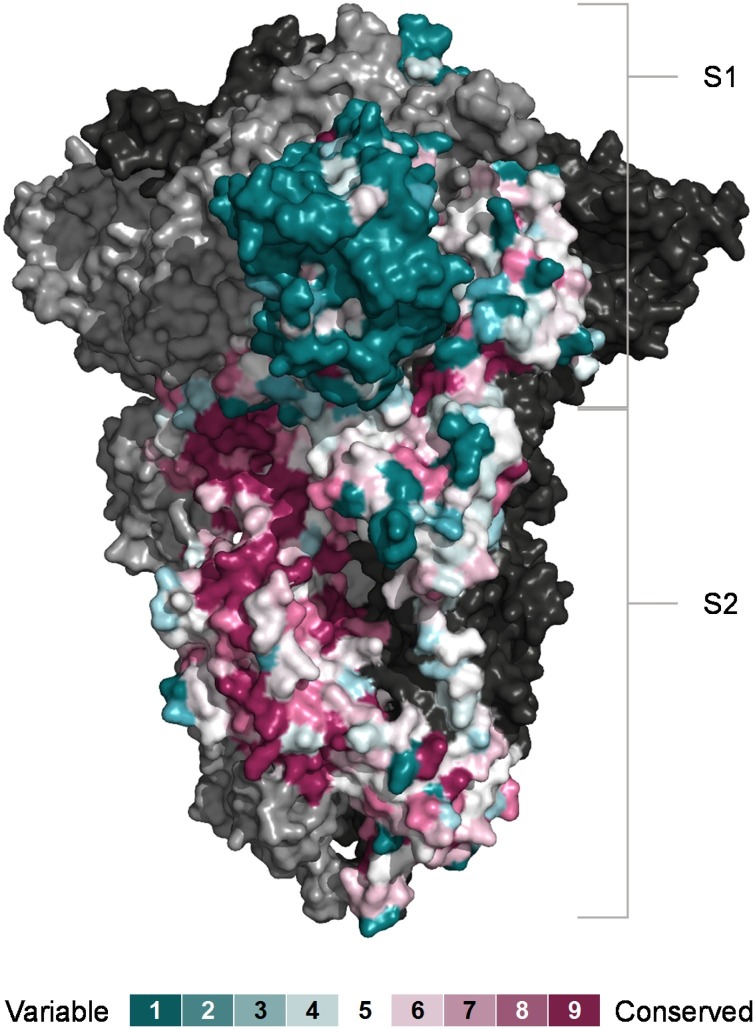
Both the S and N proteins are dominant targets of the antibody response to coronaviruses and have both been implicated in antibody cross-reactivity between SARS-CoV-2 and HCoVs [31,32]. Over the entire protein length, SARS-CoV-2 N shares higher amino acid identity (between 23.1 % and 31.1 %) than the SARS-CoV-2 S (between 20.5 % and 26.4 %), with their respective HCoV proteins. However, the S1, which contains the receptor binding domain (RBD), is considerably more divergent than the S2, and several regions in the S2 exhibit a higher degree of conservation among human and animal coronaviruses (Fig. 1 ) which could underlie its cross-recognition.
Fig. 1.
Conservation of SARS-CoV-2 S subunits. Sequence conservation visualised on the closed structure of SARS-CoV-2 spike (PDB ID 6ZGE) [119]. Chain A was coloured according conservation calculated with the ConSurf algorithm (https://consurf.tau.ac.il) from the sequences of 24 animal and human coronaviruses. The other two chains are depicted in ling and dark grey respectively.
The sequence divergence of both S and N proteins between SARS-CoV-2 and HCoVs is sufficiently large that the de novo response to unique SARS-CoV-2 epitopes appears to outweigh cross-reactive responses [31,32]. Deep serological profiling of the SARS-CoV-2 proteome using VirScan [31] revealed more extensive cross-reactivity of the SARS-CoV-2 replicase polyprotein 1ab (ORF1ab), which was targeted less frequently than S or N but equally well by sera from both SARS-CoV-2 infected and uninfected donors.
Whilst S as well as N and ORF1ab may carry epitopes that are similar among coronaviruses, an important distinction between cross-reactive antibodies to S and those to either N or ORF1ab is that only antibodies to surface S protein may interfere with viral entry and, therefore, affect viral spread, whereas no clear protective function has been described for antibodies to the internal N or ORF1ab proteins. For this reason, the focus here will be on cross-reactive antibodies to S, with emphasis on this distinction where it has been clearly made in individual studies. Moreover, antibody levels to S and N in response to SARS-CoV-2 or HCoV-OC43 infection [[32], [33], [34], [35], [36]] do not necessarily correlate, and therefore antibodies to N are not a reliable surrogate for antibodies to S.
2.2. Pre-existing SARS-CoV-2 S1 and RBD cross-reactive serum antibodies
Anderson et al. employed standard ELISA to test pre-pandemic samples and reported the presence of SARS-CoV-2 RBD cross-reactive antibodies in 0.9 % of a large cohort of patients spanning the age spectrum [35]. By comparison, antibodies cross-reactive with SARS-CoV-2 N were found in 16 % of the same patients [35]. In a separate cohort of adults, they detected SARS-CoV-2 RBD and N cross-reactive antibodies in 0.6 % and 24 % of samples, respectively [35].
Using a modified ELISA method that allowed use of higher concentrations of sera, Yuen et al. detected reactivity with SARS-CoV-2 RBD in 44 % of pre-pandemic samples and titres of these antibodies correlated with antibodies to HCoV-NL63 and HCoV-229E [33]. In the same study, 100 % of the samples contained SARS-CoV-2 N cross-reactive antibodies [33].
Selva et al. used both a multiplex assay and standard ELISA to detect cross-reactive antibodies to SARS-CoV-2 S1 and RBD in pre-pandemic samples across the age spectrum [37]. Differences in cross-reactive antibodies were higher when assessed by the multiplex assay than by ELISA, which the authors partly attributed to higher sensitivity of the former [37].
Proteomic deconvolution of the SARS-CoV-2 S-reactive IgG antibody repertoire in COVID-19 convalescent donors revealed that antibodies targeting SARS-CoV-2 RBD comprise a small minority, with the bulk of the response (>80 %) directed against non-RBD domains such as the S2 and the N-terminal domain (NTD) in the S1 [38]. Using a flow cytometry-based assay, Liu et al. were able to detect antibodies targeting epitopes in the NTD in 3 of 48 pre-pandemic serum samples [39].
Although pre-existing antibodies that cross-react with SARS-CoV-2 S1 or RBD epitopes have been found in these and other studies [40], their levels and frequency are generally lower than of pre-existing antibodies to SARS-CoV-2 S2 or other SARS-CoV-2 antigens [31,32,35,41] and have not been extensively studied.
2.3. Pre-existing SARS-CoV-2 S2 cross-reactive serum antibodies
A flow cytometry-based assay, which measures binding of serum antibodies to the wild-type homotrimeric S, naturally presented on the surface of transfected cells, detected SARS-CoV-2 S cross-reactive antibodies in a proportion of pre-pandemic sera [32]. Pre-pandemic sera contained lower levels of SARS-CoV-2 S-binding antibodies predominantly of the IgG class and targeted the S2 subunit, whereas COVID-19 convalescent sera contained higher levels of IgG as well as IgM and IgA antibodies and targeted also the S1 subunit [32]. Cross-reactive epitopes in the SARS-CoV-2 S2 subunit in this study [32] mapped to several conserved regions, including epitope S810−824 in the fusion peptide (Table 1 ). For consistency, we will denote the epitopes identified in the S proteins of different coronaviruses according to the amino acid sequence of SARS-CoV-2. Notably, this method detected SARS-CoV-2 S-binding antibodies only in a small proportion of adults (∼5 %), but in a considerably higher proportion of children and adolescents (∼44 %) [32]. By comparison, standard ELISA with the stabilised ectodomain of SARS-CoV-2 S did detect cross-reactive antibodies in pre-pandemic sera but with a substantially reduced sensitivity and only identified approximately a third of the sera identified with the flow cytometry-based method in children and adolescents [32].
Table 1.
Cross-reactive epitopes in the S2 of SARS-CoV-2 and other coronaviruses.
| S2 amino acid residues1 | S2 region or domain2 | Virus strain | Mode of induction | Study |
|---|---|---|---|---|
| S785−840 | FP | SARS-CoV-2 | Natural infection | [46] |
| S809−826 | FP | SARS-CoV-2 | Natural infection | [80] |
| S809−834 | FP | SARS-CoV-2 | Natural infection | [81] |
| S810−816 | FP | SARS-CoV-2 | Natural infection | [32] |
| S811−830 | FP | SARS-CoV-2 | Natural infection | [31] |
| S813−868 | FP | SARS-CoV-2 | Natural infection | [46] |
| S817−824 | FP | SARS-CoV-2 | Natural infection | [32] |
| S819−824 | FP | SARS-CoV-2 | Natural infection | [47] |
| S851−856 | FP | SARS-CoV-2 | Natural infection | [32] |
| S910−1213 | HR1 to HR2 | SARS-CoV-2 | Immunisation | [82] |
| S980−1006 | HR1 to CH | MERS-CoV | Immunisation | [90] |
| S997−1002 | CH | SARS-CoV-2 | Natural infection | [32] |
| S1040−1044 | CH | SARS-CoV-2 | Natural infection | [32] |
| S1041−1207 | CD-SH-HR2 | SARS-CoV | Natural infection | [86] |
| S1073−1210 | CD-SH-HR2 | SARS-CoV | Immunisation | [83] |
| S1109−1148 | CD-SH | SARS-CoV | Immunisation | [84] |
| S1121−1176 | CD-SH-HR2 | SARS-CoV-2 | Natural infection | [46] |
| S1140−1168 | SH-HR2 | SARS-CoV-2 | Natural infection | [81] |
| S1144−1163 | SH | SARS-CoV-2 | Natural infection | [31] |
| S1147−1161 | SH | SARS-CoV-2 | Immunisation | [89] |
| S1148−1156 | SH | SARS-CoV-2 | Natural infection | [74] |
| S1149−1204 | SH-HR2 | SARS-CoV-2 | Natural infection | [46] |
| S1150−1157 | SH | MERS-CoV | Immunisation | [87] |
| S1150−1156 | SH-HR2 | SARS-CoV-2 | Natural infection | [47] |
| S1169−1188 | HR2 | SARS-CoV | Immunisation | [84] |
| S1169−1210 | HR2-MP | SARS-CoV | Immunisation | [84] |
| S1205−1212 | MP | SARS-CoV-2 | Natural infection | [32] |
Amino acid numbering refer to SARS-CoV-2 S.
FP: fusion peptide; HR1, heptad repeat 1; CH, central helix; CD, connecting domain; SH, stem helix; HR2, heptad repeat 2; MP, membrane proximal.
The higher sensitivity of flow cytometry-based detection may be expected, not only due to the more direct process of signal detection but also because it captures antibodies to the entire protein and its alternative conformations. Indeed, several alternative conformations of SARS-CoV-2 S have now been described [[42], [43], [44], [45]], exposing distinct epitopes that may not be presented in the fixed conformation of the stabilised ectodomain which also carries additional mutations necessary for its stability. Moreover, ELISA in this study or, indeed, other studies, would not have necessarily detected antibodies to epitopes in the membrane proximal region, as production of the soluble SARS-CoV-2 S ectodomain requires the removal of the transmembrane domain. Such modifications may alter or, in some cases, entirely remove epitopes in the membrane proximal region. SARS-CoV-2 N-binding antibodies were also found by standard ELISA in a comparable proportion of pre-pandemic sera, but not always in the same sera with SARS-CoV-2 S-binding antibodies [32], suggesting that the two are not always linked.
Using VirScan peptide libraries covering the proteomes of SARS-CoV-2, all four HCoVs, and other coronaviruses, Shrock et al. mapped an immunogenic region in SARS-CoV-2 S fusion peptide S811−830 (Table 1) that cross-reacted with the corresponding peptides from HCoV-OC43 and HCoV-229E in ∼20 % of pre-pandemic samples [31]. In a separate study using the same method, Morgenlander et al. detected cross-reactive antibodies in up to 8 % of pre-pandemic plasma samples, included as a control in the analysis of COVID-19 convalescent plasma [46]. These cross-reactive antibodies in pre-pandemic samples targeted epitopes in the fusion peptide and heptad repeat 2 (HR2) regions of SARS-CoV-2 S (Table 1) and in homologous regions of HCoVs as well as other coronaviruses in the library [46]. Ladner et al. also used peptide libraries and mapped two immunodominant epitopes in SARS-CoV-2 S2, one in the fusion peptide (S819−824) and one in HR2 (S1150−1156) (Table 1), which were targeted in 1.5 %–20 % of pre-pandemic samples [47].
Nguyen-Contant et al. employed standard ELISA for SARS-CoV-2 stabilised S ectodomain and the S2 subunit to detect cross-reactive antibodies in ∼24 % and ∼86 %, respectively, of pre-pandemic samples from adults [41]. No cross-reactive antibodies were detected against the SARS-CoV-2 RBD, underscoring preferential targeting of the S2 subunit [41]. The large difference in the proportion of cross-reactive antibodies detected in the same set of samples with each antigen suggested that the S2 subunit detects cross-reactive antibodies that are not necessarily detected by the stabilised S ectodomain [41]. Anderson et al. also used standard ELISA for SARS-CoV-2 stabilised S ectodomain and detected cross-reactive antibodies in 4.2 % and 2.2 % of two separate pre-pandemic cohorts [35]. The first cohort in this study included donors of all ages, which showed no obvious differences in SARS-CoV-2 S cross-reactive antibodies depending on birth year [35]. As with other studies, cross-reactive antibodies to SARS-CoV-2 S poorly correlated with those to SARS-CoV-2 N [35].
The multiplex assay employed by Selva et al. detected antibodies to SARS-CoV-2 S2 in a sizable fraction of pre-pandemic samples from donors of different ages [37]. Although the exact fraction of positive samples was not stated in the study, analysis of the source data indicated that 20 % of children and adolescents (ages 1–19), 12 % of adults (ages 22–63), and 7 % of elderly donors (ages 65–92) had SARS-CoV-2 S2 cross-reactive total IgG levels higher than the median of SARS-CoV-2 S2 reactive IgG levels in COVID-19 patients [37]. Lapp et al. detected SARS-CoV-2 cross-reactive IgG antibodies, but not SARS-CoV-2 neutralising antibodies, in pre-pandemic sera from 14 out of 14 children [48].
Using a microscopy-based immunofluorescence assay for detection of antibodies that bind the wild-type SARS-CoV-2 S, Tso et al. compared pre-pandemic plasma samples from sub-Saharan Africa (Tanzania and Zambia) and the USA and reported the presence of cross-reactive antibodies in 1.2 %, 2.9 % and 4 % of the respective samples [34]. These comparable frequencies of samples with SARS-CoV-2 S cross-reactive antibodies between sub-Saharan Africa and the USA contrasted with the prevalence of SARS-CoV-2 N cross-reactive antibodies, which were found to be much higher in sub-Saharan Africa [34], again highlighting the discordance between S and N cross-reactivity.
Together, these studies provide compelling evidence for antibody cross-reactivity with SARS-CoV-2 S and S2 in particular, detected by a diverse array of methods in a proportion of pre-pandemic samples and likely induced by exposure to HCoVs. Although these studies, as well as additional studies [40,49,50], detected pre-existing cross-reactive antibodies, the reported prevalence varied widely. Some of this variability may be related to geographic or regional patterns of HCoV spread, the season or year of sample collection, or the age of the donors (discussed in subsequent sections). However, most of this variability is likely generated by differences in sensitivity and specificity of distinct detection methods. As discussed above, this is particularly evident in studies comparing target antigen conformations and detection methods with the same samples [32,51].
2.4. Back-boosting of HCoV cross-reactive antibodies by SARS-CoV-2 infection or vaccination
Cross-reactivity between SARS-CoV-2 and other coronaviruses can also be demonstrated in reverse, by the ability of SARS-CoV-2 infection to induce or enhance titres of antibodies that cross-react with other coronaviruses. We will refer to this phenomenon as ‘back-boosting’, a term we consider neutral with respect to an impact on protective immunity [52]. Although mechanistically related, the term ‘original antigenic sin’ implies a negative effect on the antibody response to heterologous exposure, in this case to SARS-CoV-2, by ‘back-boosting’ of the response to previously encountered coronaviruses at the expense of a de novo response to SARS-CoV-2 [52].
Cross-reactivity between HCoVs and zoonotic coronaviruses that crossed the species barrier into humans was supported by back-boosting of pre-existing antibodies to HCoVs observed in patients who had recovered from SARS-CoV [53]. New studies during the COVID-19 pandemic have looked at the back-boosting effect in greater detail and identified specific targets of cross-reactive antibodies.
Using their comprehensive VirScan approach, Shrock et al. identified two cross-reactive epitopes in SARS-CoV-2 S2, S811−830 and S1144−1163 (Table 1), which were targeted much more frequently by COVID-19 patients than pre-pandemic controls, consistent with back-boosting [31]. Similarly, responses to the cross-recognised S819−824 and S1150−1156 epitopes identified by Ladner et al. (Table 1) were also elevated in COVID-19 convalescent samples compared with pre-pandemic samples [47]. In a separate study, antibodies cross-reactive with HCoV-OC43 S2 were also found to be increased in COVID-19 patients in the first seven days of hospitalisation [35].
Employing a bead-based Fc array method, Natarajan et al. found increased levels of HCoV-OC43 S2 cross-reactive antibodies in COVID-19 convalescent donors than in naïve donors [51]. Moreover, the antibodies boosted in COVID-19 convalescent donors cross-reacted with the wild-type HCoV-OC43 S but much less with the stabilised HCoV-OC43 S ectodomain, suggesting they recognised epitopes presented in the wild-type, but not mutated S [51]. A number of other studies documented increased titres of antibodies cross-reactive with HCoV-OC43 S as well as other HCoVs in COVID-19 convalescent samples than in SARS-CoV-2-naïve samples, supporting a back-boosting effect [41,[54], [55], [56], [57]].
In addition to back-boosting of antibody responses to previously encountered HCoVs, particularly HCoV-OC43, following infection with SARS-CoV-2, similar increases in cross-reactive antibodies have been reported following vaccination against SARS-CoV-2 S [[57], [58], [59]]. Indeed, one or two doses of the BNT162b2 vaccine significantly increased levels of antibodies to all four HCoVs compared with unvaccinated SARS-CoV-2 uninfected and even unvaccinated COVID-19 convalescent controls [58].
Collectively, these studies highlight the bidirectional nature of cross-reactive antibody induction between HCoVs and SARS-CoV-2. The strong back-boosting of HCoV cross-reactive antibodies following SARS-CoV-2 infection or vaccination greatly facilitates their detection, which, as discussed above, requires more sensitive and precise methods than standard ELISA in pre-pandemic samples.
2.5. Pre-existing SARS-CoV-2 S cross-reactive naïve and memory B cells
In addition to detecting serum antibodies that cross-react with SARS-CoV-2 and HCoV S, heterologous immunological memory can also be demonstrated by detection of B cells that can ultimately produce such antibodies.
By analysing the memory B cell repertoire of a SARS convalescent donor, Wec et al. identified 200 antibodies that cross-reacted with SARS-CoV-2 S [60]. Importantly, a sizable proportion of these antibodies also cross-reacted with HCoV S proteins and exhibited high somatic hypermutation levels, consistent with recall of pre-existing memory of HCoV infection [60]. Indeed, two SARS-CoV-2 S2 cross-reactive monoclonal antibodies also showed strong reactivity with HCoV-OC43 S [60]. Moreover, B cell repertoire analysis of a further 3 donors with no history of SARS-CoV or SARS-CoV-2 infection demonstrated that between 0.06 % and 0.12 % of total B cells displayed SARS-CoV-2 reactivity. Of note, ∼30 % of the cross-reactive antibodies identified in this study that failed to recognise recombinant SARS-CoV S ectodomain recognised the full SARS-CoV S expressed on the surface of transfected cells, reinforcing the notion that recombinant S forms do not display the full array of B cell epitopes presented by the intact S [60].
In a separate study, Song et al. examined memory B cell responses to SARS-CoV-2 S in pre-pandemic and COVID-19 convalescent donors [61]. Although they found weak evidence for pre-existing cross-reactive serum antibodies, they did identify pre-existing cross-reactive memory B cells that were activated following SARS-CoV-2 infection [61]. The cross-reactive antibodies did not bind SARS-CoV-2 S1, suggesting reactivity with conserved epitopes in the S2 subunits of SARS-CoV-2 and one or more HCoVs [61]. Indeed, one such cloned cross-reactive antibody, CC40.8, targeted an epitope near the bottom of the S2 domain of the HCoV-HKU1 S trimer [61].
Nguyen-Contant et al. also investigated memory B cell responses to SARS-CoV-2 S in pre-pandemic and COVID-19 convalescent donors using an ELISPOT assay and although they did detect serum SARS-CoV-2 S2 cross-reactive antibodies, they did not detect memory B cells with the same specificity in pre-pandemic samples, which they attributed to insufficient frequency for this detection method [41]. Nevertheless, they did detect significantly higher numbers of memory B cells producing HCoV-OC43 S cross-reactive IgG in COVID-19 convalescent donors than in pre-pandemic donors, consistent with a recall of pre-existing B cell memory of HCoV-OC43 in COVID-19 convalescent donors [41]. A strong back-boosting of B cell memory to HCoVs and to HCoV-OC43 in particular was also observed by Westerhuis et al., who investigated the cross-reactive B cell repertoire in severe COVID-19 cases [62]. Recall of memory B cell responses reactive with shared epitopes in HCoV-OC43, HCoV-HKU1 and SARS-CoV-2 S proteins was seen, not only following SARS-CoV-2 infection but also after SARS-CoV-2 mRNA vaccination [63].
Yang et al. analysed convergent Ig heavy chain (IGH) repertoires and identified numerous B cell clones reactive with SARS-CoV-2 S in peripheral blood samples from pre-pandemic donors [64]. SARS-CoV-2 RBD and non-RBD S domain cross-reactive clones also displayed somatic hypermutation, with or without class-switching, consistent with B cell memory, particularly in the blood of young children [64]. Pre-pandemic donors also harboured SARS-CoV-2 S cross-reactive B cell clones with unmutated IgM or IgD, suggesting a relatively high precursor frequency in the naïve repertoire [64]. Similarly, Kim et al. reported the presence of a predominantly IgM and minimally hypermutated stereotypic antibody reactive with the SARS-CoV-2 RBD in the B cell repertoire of 6 out of 10 pre-pandemic donors [65].
Thus, SARS-CoV-2 RBD-reactive antibodies may pre-exist, albeit at very low frequency, in the naïve B cell repertoire, but class-switched, hypermutated antibodies cross-reactive with more conserved regions of SARS-CoV-2 and HCoVs S proteins are more commonly expanded in the memory B cell repertoire.
3. Age-dependent shifts in coronavirus-specific humoral immunity
Infection with HCoVs is more frequent in early life and progressively becomes rarer with age, although different HCoVs exhibit distinct patterns [[10], [11], [12], [13]]. Whilst behavioural factors may contribute to these patterns, progressive accumulation or maturation of immunity to HCoVs may also be expected to contribute.
By comparing children and adults using systems serology analysis of 196 antibody features, Selva et al. provided evidence for gradual maturation of the antibody response to HCoVs as well as cross-reactive antibodies to SARS-CoV-2 [37]. Vastly different serological signatures were observed between children and the elderly, supporting a model where the cumulative exposure to HCoVs with age progressively matures antibody class and function and focuses the specificity to less cross-reactive antigens [37]. In contrast, children exhibited a more adaptable and more polyreactive antibody response. On note, children showed elevated cross-reactive IgM responses, whereas the elderly had elevated IgA class-switched responses to a range of antigens including SARS-CoV-2 S2 and N [37].
In a cohort of pre-pandemic samples from healthy donors, Deakin et al. observed a significant age-dependent antibody class-switch in the response to HCoV-OC43 N [36]. Whereas children mounted almost exclusively an IgM response to HCoV-OC43 N, adults progressively acquired a mixed IgM and IgG response, reaching equal proportions in advanced age and supporting a model of age-dependent maturation [36]. In contrast, antibodies to HCoV-OC43 S and cross-reactive antibodies to SARS-CoV-2 S were predominantly of the IgG class [32,36].
Weisberg et al., compared antibody responses to SARS-CoV-2 S and N in adult COVID-19 and paediatric COVID-19 or MIS-C (multisystem inflammatory syndrome in children and adolescents) patients are found that whereas adults mounted IgG, IgM and IgA antibodies to SARS-CoV-2 S, children mounted predominantly an IgG response consistent with pre-existing immunological memory specifically in this age group [66]. In contrast, IgG responses to SARS-CoV-2 N were readily observed in adult but not paediatric COVID-19 patients [66].
These studies highlighted the notable differences in antibody class use between children and adults, as well as between S and N antigens, and underscored the importance of including all antibody classes when examining the response to SARS-CoV-2 or HCoV proteins.
In their extensive adult and paediatric pre-pandemic cohorts, Anderson et al. reported no correlation between age and IgG antibodies for HCoV-OC43 S or IgG cross-reactive antibodies for SARS-CoV-2 S or N, but noted lower levels of IgG antibodies for HCoV-229E and HCoV-NL63 S proteins in very young children [35]. A modest decrease in seroprevalence for HCoV-OC43, HCoV-HKU1 and HCoV-229E was also reported in younger individuals by Galipeau et al. [67].
Analysis of IGH repertoires for convergent SARS-CoV-2 S cross-reactive B cell clones in pre-pandemic donors indicated that class-switched antibodies or mutated but unswitched antibodies were found more commonly in very young children than in adults [64]. In contrast, unmutated, unswitched SARS-CoV-2 S cross-reactive B cell clones were present at similar frequencies in all age groups [64]. By expressing IGH genes as monoclonal antibodies and testing against a panel of HCoV and SARS-CoV-2 S proteins, Yang et al. obtained evidence that SARS-CoV-2 S cross-reactive B cell clones from children exhibit greater cross-reactivity in addition to higher frequencies [64].
Khan et al. also reported major qualitative differences in the HCoV-reactive pre-pandemic antibody repertoire between children and adults, with children harbouring more cross-reactive IgG antibodies targeting functionally important and structurally conserved regions of the S proteins [68].
Lastly, Dowell et al. provided evidence that in response to SARS-CoV-2 infection, children mounted a much broader humoral response against S antigens than adults, with back-boosting of antibodies to HCoV S proteins and cross-recognition of the S2 subunits [69].
Collectively, the emerging picture is one of progressive shaping of the B cell repertoire reactive with HCoVs and cross-reactive with SARS-CoV-2 through age-dependent changes in the frequency and accrued exposure to HCoVs. Antibodies for HCoV N appear to be largely of the unswitched IgM class in children that gradually, but incompletely class-switch to IgG with age. In contrast, antibodies for HCoV S, and particularly those for the more conserved S2 epitopes, are predominantly class-switched and are found more frequently and more often mutated in children than in adults.
4. Potential function of cross-reactive antibodies
4.1. Potential contribution to pathogenicity
Despite the wealth of evidence for the existence of antibodies that cross-react with SARS-CoV-2 S, a potential contribution, positive or negative, of such antibodies to the outcome of SARS-CoV-2 infection cannot be easily inferred from in vitro antibody binding assays. Many studies have also employed functional in vitro assays that measure correlates of potential in vivo function, including virus neutralisation, antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent enhancement (ADE) of virus infectivity.
Although most antibody functions contribute to protective immunity, heterologous immunity may also prove counterproductive or pathogenic. Partial or non-protective antibody cross-reactivity may hinder or diverge a typical protective response, with original antigenic sin representing a prime example [52,70]. Indeed, as the bulk of the antibody responses to HCoVs back-boosted after SARS-CoV-2 infection may not neutralise SARS-CoV-2, Westerhuis et al. hypothesised that such back-boosting, particularly of antibodies for HCoV-OC43 S, negatively impacts de novo SARS-CoV-2 immunity in severe COVID-19 patients [62]. Aydillo et al. provided evidence for a negative correlation between back-boosted antibodies for HCoVs and induction of IgG and IgM antibodies for SARS-CoV-2 S, consistent with immunological imprinting [55].
By comparing the antibody response to the different S protein subunits and domains, McNaughton et al. found that severe fatal COVID-19 cases exhibited lower levels of antibodies for SARS-CoV-2 S, RBD and NTD, but not for S2 or N, than severe non-fatal COVID-19 cases, and a strong positive correlation with antibodies to the S proteins of HCoV-OC43 and HCoV-HKU1 [56]. The altered balance of de novo responses to S1 and recall responses to S2 in severe fatal COVID-19 cases is reminiscent of original antigenic sin [56]. However, SARS-CoV-2 neutralising responses were not negatively impacted and all SARS-CoV-2 S antibody responses were still significantly higher in severe fatal COVID-19 cases than in asymptomatic SARS-CoV-2 infection cases, but perhaps not as high as it would be required to protect against the most severe SARS-CoV-2 infection [56].
Lapp et al. reported that priming of mice with HCoV-HKU1 induced SARS-CoV-2 S cross-reactive, non-neutralising antibodies and prevented the induction of SARS-CoV-2 neutralising antibodies following SARS-CoV-2 boost immunisation, consistent with original antigenic sin [48]. In contrast, the authors found that in children with COVID-19 or MIS-C, levels of antibodies for HCoV-HKU1 S showed a strong positive correlation with both SARS-CoV-2 S binding and SARS-CoV-2 neutralising antibodies [48], arguing that pre-existing HCoV-HKU1 antibodies did not inhibit the neutralising antibody responses to SARS-CoV-2.
By screening a series of SARS-CoV-2 S reactive monoclonal antibodies from COVID-19 patients, Liu et al. discovered some that dramatically enhanced S binding to ACE2 and thus increased infectivity [39]. These antibodies targeted an infectivity-enhancing site located in the NTD [39]. Notably, they also detected low levels of antibodies targeting the infectivity-enhancing site in the NTD in a small proportion of pre-pandemic serum samples [39], raising the possibility that pre-existing cross-reactive antibodies may contribute to ADE [71].
Whether heterologous immunity induced by HCoVs or heterotypic immunity induced by one of the emerging SARS-CoV-2 variants compromises the response to another SARS-CoV-2 variant or even enhances its replication will require further investigation.
4.2. Potential contribution to protection
Cross-reactive antibodies that bind SARS-CoV-2 S may, in principle, provide a certain degree of protection through one or more distinct modes of action. These include ADCC and other Fc-mediated effector functions, such as antibody-dependent cellular phagocytosis (ADCP) or complement-dependent cytotoxicity (CDC), which do not necessitate blocking of the interaction with the cellular receptor, as demonstrated with other viral pathogens [72,73], as well as with SARS-CoV-2 [74]. Nevertheless, virus neutralisation by interfering with cellular entry is a major action of protective antibodies and an aspect that is most commonly tested.
Given the very low prevalence of SARS-CoV-2 S cross-reactive antibodies in uninfected adults, as well as the low titres in cases where they are found, it is perhaps expected that neutralising activity in unselected sera will also be very rare and at low levels in the naïve population. Indeed, several studies detected no or minimal neutralising activity against SARS-CoV-2 in most pre-pandemic samples [75,76].
Prévost et al. detected SARS-CoV-2 S pseudotype neutralising antibodies in 2 of 10 pre-pandemic samples [54]. Ng et al. detected SARS-CoV-2 S pseudotype and authentic SARS-CoV-2 neutralising antibodies in a subset of pre-pandemic samples, pre-selected for the presence of cross-reactive antibodies for cell surface-presented SARS-CoV-2 S [32]. These were more frequently found in children, and Deakin et al. found similar activity in pre-pandemic sera from a subset of paediatric rheumatology patients [36].
Anderson et al. detected very low titres of SARS-CoV-2 S pseudotype neutralising activity in rare pre-pandemic samples, which, however, did not overlap with the presence of SARS-CoV-2 S and N binding antibodies detected by ELISA, and did not detect authentic SARS-CoV-2 neutralising activity in a smaller set of samples [35].
Weak SARS-CoV-2 neutralising activity has also been reported in pools of pre-pandemic plasma, but at levels that were two orders of magnitude lower than that of COVID-19 convalescent plasma [77], and low neutralising activity was detected in pre-pandemic intravenous immunoglobulin (IVIG) products in some studies but not in others [78,79]. By testing the in vitro functionality of convalescent plasma, Morgenlander et al. found a positive correlation between HCoV-NL63 antibodies and highly neutralising SARS-CoV-2 antibodies [46].
Given that most of the cross-reactivity in SARS-CoV-2 naïve sera is directed against the S2 subunit and would not prevent RBD-ACE2 interaction, virus neutralisation was unexpected. However, there is now clear evidence that polyclonal sera or monoclonal antibodies raised against the S2 subunit can exhibit considerable neutralising activity.
By depleting antibodies reactive with the S809−826 epitope in the S2, Poh et al. found that such antibodies contributed at least 20 % of the neutralising activity in COVID-19 convalescent sera [80]. Similarly, Garrett et al. found that substantial neutralising activity remained in COVID-19 convalescent sera after depletion of RBD-targeting antibodies, which correlated with S2 binding antibodies, with the most commonly publicly targeted epitopes mapping to the fusion peptide (S809−834) and stem helix and HR2 (S1140−1168) (Table 1) [81].
Ma et al. found that sera from mice immunised with nanoparticles displaying both HR1 and HR2 domains of SARS-CoV-2 (S910−1213) potently neutralised diverse alphacoronaviruses and betacoronaviruses [82]. Sera from rabbits immunised with a recombinant SARS-CoV S2 fragment (corresponding to S1073−1210 of SARS-CoV-2 S, Table 1), exhibited strong neutralising activity against SARS-CoV [83]. The investigators also isolated monoclonal antibodies from immunised rabbits with potent neutralising activity against SARS-CoV that targeted three epitopes mapping to SARS-CoV-2 S stem helix, HR2 and membrane proximal regions (Table 1) [84].
Following immunisation of mice with human antibody genes, Elshabrawy et al. cloned several monoclonal antibodies reactive with SARS-CoV S2 with broad neutralising activity against different SARS-CoV isolates [85]. By cloning B cells from SARS convalescent patients, Duan et al. isolated a human monoclonal antibody B1, targeting a conserved epitope within SARS-CoV S2 (corresponding to S1041−1207 in SARS-CoV-2 S, Table 1), with potent neutralising activity [86].
More recently, Wang et al. isolated two neutralising monoclonal antibodies (1.6C7 and 28D9) from mice with human antibody genes sequentially immunised with S ectodomains of HCoV-OC43, SARS-CoV and MERS-CoV that cross-reacted with a stem helix epitope (corresponding to S1150−1157 of SARS-CoV-2 S, Table 1) [87]. Chi et al. cloned a neutralising monoclonal antibody, 0304−3H3, from a COVID-19 patient that targets SARS-CoV-2 S2 [88]. Similarly, the monoclonal antibody CC40.8, isolated by Song et al. that cross-reacted with a conserved epitope near the bottom of the S2 subunit of SARS-CoV-2 and HCoV-HKU1, neutralised both SARS-CoV and SARS-CoV-2, albeit weakly [61]. Using heterologous immunisation of mice, Sauer et al. isolated a monoclonal antibody B6, targeting the S1147−1161 epitope in SARS-CoV-2 S2 and cross-reacting with eight different betacoronavirus S proteins including HCoVs [89]. Structural data suggest that the S1147−1161 epitope (Table 1) is a conserved cryptic epitope, binding to which sterically interferes with conformational changes during membrane fusion [89]. Consequently, B6 neutralised betacoronaviruses from lineages A and C, but not from lineage B (SARS-CoV-2 and SARS-CoV), where the S1147−1161 epitope may be less accessible [89]. Pinto et al. isolated a broadly neutralising monoclonal antibody (S2P6) from a COVID-19 convalescent donor that cross-reacted with the S1148−1156 epitope (Table 1) in over twenty different betacoronavirus S proteins [74]. Moreover, the germline precursor of S2P6 reacted with the S1148−1156 epitope in HCoV-OC43, suggesting it likely arose in response to HCoV-OC43 infection and acquired mutation that broadened it specificity towards SARS-CoV-2 [74]. Finally, by immunising mice with stabilised MERS-CoV S2 recombinant trimers Huang et al. raised the monoclonal antibody 3A3 specific to an epitope on the S2 hinge (corresponding to S980−1006 of SARS-CoV-2 S, Table 1) with potent in vitro neutralising activity [90].
5. The limits of protection against coronaviruses afforded by immunological memory
Together, the above-mentioned studies establish that antibodies targeting conserved epitopes in the S2 subunit can indeed be elicited and exert potent neutralisation activity. Whether this activity can be detected in COVID-19 convalescent or even pre-pandemic sera ultimately depends on the type and sensitivity of the neutralisation assay used, similarly to detection of binding antibodies according to the assay used. However, even in rare cases when in vitro neutralisation can be demonstrated, it does not necessarily equate to in vivo protection from infection or disease, as immune correlates of protection from coronaviruses in general are still incompletely understood.
Several studies have examined possible correlations between measurable immune variables or historic exposure to heterologous coronaviruses and the risk or outcome of infection with SARS-CoV-2. In such epidemiological studies, cross-reactive T cell and B cell memory were not always assessed or distinguished. As any possible effect of heterologous immunity identified in these studies cannot be easily attributed to a particular arm of adaptive immunity, they will be considered collectively.
A study of SARS-CoV-2 infection or disease in children during the first wave of COVID-19 in France reported similar prevalence and levels of antibodies to HCoVs between SARS-CoV-2 seropositive hospitalised patients, MIS-C patients and SARS-CoV-2 uninfected controls [91], arguing that pre-existing antibodies for HCoV S proteins do not prevent disease after SARS-CoV-2 infection nor are they back-boosted.
In a study of MIS-C cases, Consiglio et al. found that antibodies for HCoVs, commonly found in other patient cohorts, were not present in sera from MIS-C patients, suggesting that they modulate the immune response to SARS-CoV-2 in a manner that leads to the development of MIS-C [92].
Anderson et al. reported no association between pre-existing antibodies cross-reactive with SARS-CoV-2 and protection from SARS-CoV-2 infection in a cohort of adults [35]. Antibodies cross-reactive with SARS-CoV-2 S were extremely rare in this cohort, which was therefore stratified mainly based on non-protective IgG antibodies cross-reactive with SARS-CoV-2 N instead [35]. In a separate cohort in the same study, Anderson et al. found that back-boosted antibodies cross-reactive with SARS-CoV-2 S2 and HCoV-CO43 S2 did not correlate with the outcome of COVID-19 [35]. In a follow-up study, Gouma et al. examined the course of SARS-CoV-2 infection in health care workers and found a strong negative association between pre-existing antibody levels for betacoronaviruses HCoV-CO43 and HCoV-HKU1 and the duration of COVID-19 symptoms, an association which they attributed to cellular immunological memory [93].
Similarly, Guo et al. reported a significant correlation between cross-reactive antibodies for HCoV-OC43 S and disease severity in COVID-19 patients [76], suggestive of cross-protection. In a study of hospitalised patients, Sagar et al. found that recent documented HCoV infection did not prevent SARS-CoV-2 infection but was associated with less severe disease [94], indicative of a protective effect. Henss et al. confirmed that levels of SARS-CoV-2 binding and neutralising antibodies correlated with COVID-19 severity in hospitalised patients [95]. In contrast, levels of HCoV-NL63 neutralising antibodies were lower in the most severe cases, suggesting the possibility of cross-reactive protection [95]. Using systems serology, Zohar et al. observed an early rise in SARS-CoV-2 S2-reactive FcR binding antibodies that correlated with COVID-19 survival and suggested that the ability to rapidly develop S2 cross-reactive immunity, rather than the level of pre-existing immunity to less cross-reactive RBDs, correlated with the neutralising response and disease recovery [96]. Wang et al. also observed elevated responses to SARS-CoV-2 S2 in mild than in severe COVID-19 cases [97].
Estimating any effect of pre-existing immunological memory induced by HCoVs on the risk of SARS-CoV-2 infection and disease is not free from challenges. Antibodies that cross-react with SARS-CoV-2 S are rare in uninfected individuals, particularly in adults, and are best detected in assays using natural S conformations. Although cross-reactive antibodies for SARS-CoV-2 N are more easily and frequently detected, and may therefore be chosen as a surrogate, they may not necessarily correlate with SARS-CoV-2 S antibodies, nor can they mediate protection. Similarly, antibodies with direct reactivity with HCoV S or N proteins may not be a reliable proxy for cross-reactive antibodies for SARS-CoV-2 S or for stratifying cohorts, as seropositivity for HCoVs is nearly universal whereas cross-reactivity with SARS-CoV-2 S is rare. Precise quantitation of an effect of pre-existing humoral or cellular immunological memory from HCoV exposure will require knowledge of HCoV immune status and SARS-CoV-2 S cross-reactivity shortly before infection with SARS-CoV-2, which may be difficult to obtain for cohorts where SARS-CoV-2 infection is not routinely diagnosed.
By detecting antibodies to the less conserved HCoV-OC43 RBD, Kaplonek et al. were able to determine the levels of pre-existing immunity to this HCoV in SARS-CoV-2 infected individuals without the confounding effect of back-boosting that affects antibodies to conserved domains such as the S2 [98]. Using this measure, the investigators uncovered significantly higher levels of pre-existing IgM and IgG1 antibodies to HCoV-OC43 RBD in COVID-19 survivors with severe and moderate disease, than in COVID-19 non-survivors [98]. Moreover, early development of cross-reactive antibody responses to S2 remained the top predictor of survival in COVID-19 [98].
Nevertheless, epidemiological considerations suggest that any effect of pre-existing immunological memory of HCoVs, whilst possibly modifying its outcome, is unlikely to prevent SARS-CoV-2 infection. For example, despite near universal HCoV seropositivity, both children and adults experience multiple HCoV reinfections over their lifetime. As antibodies directly reactive with HCoVs do not prevent re-infection with homologous HCoVs themselves, they would be unlikely to prevent heterologous infection with SARS-CoV-2 by virtue of cross-reactivity.
5.1. Protection against homologous or heterologous infection
Epidemiological evidence suggests that HCoV infection does provide a degree of protection from homologous reinfection, albeit incomplete or short-lived [10,11,99]. Indeed, reinfection with the same HCoV can frequently occur 12 months after the previous infection [100]. However, waning immunological memory may not be the only cause of homologous HCoV reinfections. Two recent studies provided evidence for antigenic drift of HCoV S proteins, particularly of the RBD, which enables HCoVs to evade adaptive immunity [101,102]. Such escape from antibody recognition is consistent with evolutionary pressure from otherwise protective longer-lived humoral immunity. Equally, immunological memory induced by SARS-CoV-2 infection or vaccination may be longer-lived that initially thought [[103], [104], [105], [106], [107]].
Epidemiological evidence also indicates partial heterologous immunity between HCoVs [10,11,99]. An important aspect of heterologous HCoV immunity is asymmetry. It has been suggested that, whereas HCoV-OC43 may elicit immunity against HCoV-HKU1, the reverse may not apply [11]. The alphacoronaviruses may also behave in a similar manner, with HCoV-NL63 eliciting immunity against HCoV-229E but not the reverse [11]. This hypothesis based on seroconversion data is also supported by transmission dynamics modelling [108].
Asymmetry and hierarchy may also characterise heterotypic immunity between SARS-CoV-2 variants. For example, whereas the parental SARS-CoV-2 strains induce robust immunity against the B.1.1.7 variant that emerged in the United Kingdom, natural immunity induced by infection with the B.1.1.7 variant is much weaker against the parental strains [109,110]. Neither variant induces strong immunity against the B.1.351 variant that emerged in South Africa, but infection with B.1.351 variant induces robust immunity against its parental strain, as well as the B.1.1.28 variant first emerged in Brazil [111,112]. Relative asymmetry in heterotypic immunity has also been observed following vaccination with SARS-CoV-2 variants in a mouse model [113].
5.2. Zoonotic introduction of new coronaviruses in the backdrop of endemic HCoVs
The devastating and rapid spread of SARS-CoV-2 in the human population since its introduction from suspected animal reservoirs is stark evidence that immunological memory induced by endemic HCoVs is insufficient to prevent zoonoses, epidemics, and eventual pandemics caused by new coronaviruses. Albeit far less successful than SARS-CoV-2, the initial spread of SARS-CoV or MERS-CoV was also unlikely to have been impacted by endemic HCoVs. These three recent zoonotic coronaviruses are phylogenetically more distant from HCoVs than HCoVs are amongst themselves. For example, the S glycoproteins of the alphacoronaviruses HCoV-229E and HCoV-NL63 share 54.7 % identity and of the betacoronaviruses HCoV-OC43 and HCoV-HKU1 share 63.1 % identity at the amino acid level. In contrast, the SARS-CoV-2 S and its closest relative in HCoVs, the HCoV-OC43 S, share only 26.4 % identity. It is, therefore, possible that heterologous immunity between the different HCoVs is stronger than between HCoVs and SARS-CoV-2. Indeed, there is epidemiological evidence that immunological memory elicited by one HCoV can reduce the likelihood of infection with another HCoV type [11,108].
Nevertheless, the fact that multiple HCoVs are concurrently endemic highlights the limits of heterologous immunity and further suggests that it played a limited role during the initial introduction of HCoVs into humans. For example, molecular clock analysis of the HCoV-OC43 S gene indicated a relatively recent zoonotic introduction, dating to around 1890 [114], whereas other HCoVs are estimated to have been introduced much earlier [[115], [116], [117]]. Therefore, HCoV-OC43 appears to have successfully established endemic infection in humans and is even hypothesised to have caused the 1889–1890 pandemic [114] despite the pre-existence of other HCoVs [117]. The same can also be argued for all endemic and new pandemic CoVs that were introduced in humans after the first endemic HCoV was established.
It is interesting to note, however, that the estimated order in which HCoVs first emerged in humans may be related to the degree of heterologous immunity they each elicit. In the alphacoronaviruses, HCoV-NL63, which has been suggested to induce stronger heterologous immunity against HCoV-229E than HCoV-229E does against HCoV-NL63 [11], is estimated to have emerged after HCoV-229E [117]. Similarly, in the betacoronaviruses, HCoV-OC43, which is thought to induce stronger heterologous immunity against HCoV-HKU1 than HCoV-HKU1 does against HCoV-OC43 [11,108], is estimated to have emerged after HCoV-HKU1 [117]. The ability to escape heterologous immunity elicited by their respective, pre-existing genus member may have contributed to the successful establishment of HCoV-NL63 and HCoV-OC43 in humans.
6. Concluding remarks
The initial introduction and continuous presence of multiple endemic HCoVs and the rapid spread of the new pandemic SARS-CoV-2 might suggest that humans are largely defenceless against infection with coronaviruses, if not associated disease. However, it is worth remembering that knowledge of our interaction with coronaviruses stems from the study of only those that have successfully crossed into our species – and this knowledge is still incomplete. We might not know, for example, of other coronaviruses that were less successful, perhaps owing to a certain degree of cross-reactivity with endemic HCoVs. The current HCoVs are typically thought of as harmless, but they may have not been as harmless when they first emerged [114], nor are they always harmless today. A relatively recent outbreak of HCoV-OC43 in a care facility resulted in infection mortality rates in vulnerable populations comparable with those of SARS-CoV or SARS-CoV-2 [30]. Thus, HCoVs may not be entirely tamed, but rather rendered less harmful by protective but not necessarily sterilising adaptive immune responses. Similarly, SARS-CoV-2 has an overall devastatingly high infection mortality rate, but the majority of infections, particularly in children and young adults, are not associated with severe disease.
The properties underlying such protection from disease may hold the key to a favourable equilibrium with coronaviruses and this may well include shaping of our immune repertoires by repeated exposure to endemic and pandemic coronaviruses. The current successes of SARS-CoV-2 vaccination campaigns also highlight that adaptive immunity can indeed be trained to provide effective control of coronaviruses. As SARS-CoV-2 still retains much of its bat coronavirus origins, vaccination against SARS-CoV-2 S should provide a certain degree of protection against other bat coronaviruses attempting to cross the species barrier. Indeed, cross-reactive antibodies against bat coronavirus RBDs are induced by vaccination with SARS-CoV-2 RBDs [118]. Given the significantly higher conservation of the S2 than the RBD among animal coronaviruses as well as between animal and HCoVs, it may prove the Achilles heel for a broader range of coronaviruses that can escape antibody recognition by antigenic drift of the RBD [101,102]. Such cross-reactive immunity may also constrain the available space for SARS-CoV-2 escape. For example, certain mutations in the fusion peptide of SARS-CoV-2 S2 improve binding by pre-existing HCoV-induced antibodies [81].
As the SARS-CoV-2 pandemic is evolving, we should soon be able to fill the gaps in understanding of the interplay of coronaviruses and the human immune response, the interaction between coronaviruses, and the ultimate impact of the first global coronavirus vaccination campaign on the equilibrium with endemic and any emerging new coronaviruses.
Funding
This work was supported by the Francis Crick Institute (FC001099 and FC001078), which receives its core funding from Cancer Research UK, the UK Medical Research Council, and the Wellcome Trust.
Declaration of Competing Interest
The authors report no declarations of interest.
References
- 1.Cohn M. The immune system: a weapon of mass destruction invented by evolution to even the odds during the war of the DNAs. Immunol. Rev. 2002;185:24–38. doi: 10.1034/j.1600-065x.2002.18504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohn M. The common sense of the self-nonself discrimination. Springer Semin. Immunopathol. 2005;27(1):3–17. doi: 10.1007/s00281-005-0199-1. [DOI] [PubMed] [Google Scholar]
- 3.Davis M.M., Bjorkman P.J. T-cell antigen receptor genes and T-cell recognition. Nature. 1988;334(6181):395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- 4.Klinman N.R. The "clonal selection hypothesis" and current concepts of B cell tolerance. Immunity. 1996;5(3):189–195. doi: 10.1016/s1074-7613(00)80314-3. [DOI] [PubMed] [Google Scholar]
- 5.Stockinger B., Kassiotis G., Bourgeois C. CD4 T-cell memory. Semin. Immunol. 2004;16(5):295–303. doi: 10.1016/j.smim.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Weisel F., Shlomchik M. Memory B cells of mice and humans. Annu. Rev. Immunol. 2017;35:255–284. doi: 10.1146/annurev-immunol-041015-055531. [DOI] [PubMed] [Google Scholar]
- 7.Cohn M. Degeneracy, mimicry and crossreactivity in immune recognition. Mol. Immunol. 2005;42(5):651–655. doi: 10.1016/j.molimm.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Lipsitch M., Grad Y.H., Sette A., Crotty S. Cross-reactive memory T cells and herd immunity to SARS-CoV-2, Nature reviews. Immunology. 2020;20:709–713. doi: 10.1038/s41577-020-00460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu B., Guo H., Zhou P., Shi Z.-L. Characteristics of SARS-CoV-2 and COVID-19, nature reviews. Microbiology. 2020;19:141–154. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aldridge R.W., Lewer D., Beale S., Johnson A.M., Zambon M., Hayward A.C., Fragaszy E.B. Seasonality and immunity to laboratory-confirmed seasonal coronaviruses (HCoV-NL63, HCoV-OC43, and HCoV-229E): results from the Flu Watch cohort study. Wellcome Open Res. 2020;5(52 % U) doi: 10.12688/wellcomeopenres.15812.1. https://wellcomeopenresearch.org/articles/5-52/v1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dijkman R., Jebbink M.F., Gaunt E., Rossen J.W., Templeton K.E., Kuijpers T.W. L. Van der Hoek, the dominance of human coronavirus OC43 and NL63 infections in infants. J. Clin. Virol. 2012;53(2):135–139. doi: 10.1016/j.jcv.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedman N., Alter H., Hindiyeh M., Mendelson E., Shemer Avni Y., Mandelboim M. Human coronavirus infections in Israel: epidemiology, clinical symptoms and summer seasonality of HCoV-HKU1. Viruses. 2018;10(10):515. doi: 10.3390/v10100515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Principi N., Bosis S., Esposito S. Effects of coronavirus infections in children. Emerging Infect. Dis. 2010;16(2):183–188. doi: 10.3201/eid1602.090469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Wit E., van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses, Nature reviews. Microbiology. 2016;14(8):523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu D.X., Liang J.Q., Fung T.S. Encyclopedia of Virology. 2021. Human Coronavirus-229E, -OC43, -NL63, and -HKU1 (Coronaviridae) pp. 428–440. [Google Scholar]
- 16.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Muller M.A., Drosten C., Pohlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2) doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and Antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281–292.e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong A.C.P., Li X., Lau S.K.P., Woo P.C.Y. Global epidemiology of bat coronaviruses. Viruses. 2019;11(2) doi: 10.3390/v11020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith I., Wang L.F. Bats and their virome: an important source of emerging viruses capable of infecting humans. Curr. Opin. Virol. 2013;3(1):84–91. doi: 10.1016/j.coviro.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wacharapluesadee S., Sintunawa C., Kaewpom T., Khongnomnan K., Olival K.J., Epstein J.H., Rodpan A., Sangsri P., Intarut N., Chindamporn A., Suksawa K., Hemachudha T. Group C betacoronavirus in bat guano fertilizer, Thailand. Emerging Infect. Dis. 2013;19(8):1349–1351. doi: 10.3201/eid1908.130119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cavanagh D. Coronavirus avian infectious bronchitis virus. Vet. Res. 2007;38(2):281–297. doi: 10.1051/vetres:2006055. [DOI] [PubMed] [Google Scholar]
- 23.Winterfield R.W., Fadly A.M., Hoerr F.J. Immunity to infectious bronchitis virus from spray vaccination with derivatives of a Holland strain. Avian Dis. 1976;20(1):42–48. [PubMed] [Google Scholar]
- 24.Guzmán M., Hidalgo H. Live attenuated infectious bronchitis virus vaccines in poultry: modifying local viral populations dynamics. Animals (Basel) 2020;10(11) doi: 10.3390/ani10112058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silva C.S., Mullis L.B., Pereira O., Jr., Saif L.J., Vlasova A., Zhang X., Owens R.J., Paulson D., Taylor D., Haynes L.M., Azevedo M.P. Human respiratory coronaviruses detected in patients with influenza-like illness in Arkansas, USA. Virol. Mycol. 2014;2014(Suppl 2) doi: 10.4172/2161-0517.S2-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiu L., Binder R.A., Alarja N.A., Kochek K., Coleman K.K., Than S.T., Bailey E.S., Bui V.N., Toh T.H., Erdman D.D., Gray G.C. A RT-PCR assay for the detection of coronaviruses from four genera. J. Clin. Virol. 2020;128 doi: 10.1016/j.jcv.2020.104391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Bert N., Tan A.T., Kunasegaran K., Tham C.Y.L., Hafezi M., Chia A., Chng M.H.Y., Lin M., Tan N., Linster M., Chia W.N., Chen M.I.C., Wang L.-F., Ooi E.E., Kalimuddin S., Tambyah P.A., Low J.G.-H., Tan Y.-J., Bertoletti A. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020 doi: 10.1038/s41586-020-2550-z. [DOI] [PubMed] [Google Scholar]
- 28.Che X.Y., Qiu L.W., Liao Z.Y., Wang Y.D., Wen K., Pan Y.X., Hao W., Mei Y.B., Cheng V.C., Yuen K.Y. Antigenic cross-reactivity between severe acute respiratory syndrome-associated coronavirus and human coronaviruses 229E and OC43. J. Infect. Dis. 2005;191(12):2033–2037. doi: 10.1086/430355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maache M., Komurian-Pradel F., Rajoharison A., Perret M., Berland J.L., Pouzol S., Bagnaud A., Duverger B., Xu J., Osuna A., Paranhos-Baccalà G. False-positive results in a recombinant severe acute respiratory syndrome-associated coronavirus (SARS-CoV) nucleocapsid-based western blot assay were rectified by the use of two subunits (S1 and S2) of spike for detection of antibody to SARS-CoV. Clin. Vaccine Immunol. 2006;13(3):409–414. doi: 10.1128/CVI.13.3.409-414.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patrick D.M., Petric M., Skowronski D.M., Guasparini R., Booth T.F., Krajden M., McGeer P., Bastien N., Gustafson L., Dubord J., Macdonald D., David S.T., Srour L.F., Parker R., Andonov A., Isaac-Renton J., Loewen N., McNabb G., McNabb A., Goh S.H., Henwick S., Astell C., Guo J.P., Drebot M., Tellier R., Plummer F., Brunham R.C. An outbreak of human coronavirus OC43 infection and serological cross-reactivity with SARS coronavirus. Can. J. Infect. Dis. Med. Microbiol. 2006;17(6):330–336. doi: 10.1155/2006/152612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shrock E., Fujimura E., Kula T., Timms R.T., Lee I.-H., Leng Y., Robinson M.L., Sie B.M., Li M.Z., Chen Y., Logue J., Zuiani A., McCulloch D., Lelis F.J.N., Henson S., Monaco D.R., Travers M., Habibi S., Clarke W.A., Caturegli P., Laeyendecker O., Piechocka-Trocha A., Li J., Khatri A., Chu H.Y., Villani A.-C., Kays K., Goldberg M.B., Hacohen N., Filbin M.R., Yu X.G., Walker B.D., Wesemann D.R., Larman H.B., Lederer J.A., Elledge S.J. Viral epitope profiling of COVID-19 patients reveals cross-reactivity and correlates of severity. Science (New York, N.Y.) 2020:eabd4250. doi: 10.1126/science.abd4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ng K.W., Faulkner N., Cornish G.H., Rosa A., Harvey R., Hussain S., Ulferts R., Earl C., Wrobel A.G., Benton D.J., Roustan C., Bolland W., Thompson R., Agua-Doce A., Hobson P., Heaney J., Rickman H., Paraskevopoulou S., Houlihan C.F., Thomson K., Sanchez E., Shin G.Y., Spyer M.J., Joshi D., O’Reilly N., Walker P.A., Kjaer S., Riddell A., Moore C., Jebson B.R., Wilkinson M., Marshall L.R., Rosser E.C., Radziszewska A., Peckham H., Ciurtin C., Wedderburn L.R., Beale R., Swanton C., Gandhi S., Stockinger B., McCauley J., Gamblin S.J., McCoy L.E., Cherepanov P., Nastouli E., Kassiotis G. Preexisting and de novo humoral immunity to SARS-CoV-2 in humans. Science. 2020;370(6522):1339–1343. doi: 10.1126/science.abe1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuen R.R., Steiner D., Pihl R.M.F., Chavez E., Olson A., Smith E.L., Baird L.A., Korkmaz F., Urick P., Sagar M., Berrigan J.L., Gummuluru S., Corley R.B., Quillen K., Belkina A.C., Mostoslavsky G., Rifkin I.R., Kataria Y., Cappione A.J., Gao W., Lin N.H., Bhadelia N., Snyder-Cappione J.E. Novel ELISA protocol links pre-existing SARS-CoV-2 reactive antibodies with endemic coronavirus immunity and age and reveals improved serologic identification of acute COVID-19 via multi-parameter detection. Front. Immunol. 2021;12(826) doi: 10.3389/fimmu.2021.614676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tso F.Y., Lidenge S.J., Peña P.B., Clegg A.A., Ngowi J.R., Mwaiselage J., Ngalamika O., Julius P., West J.T., Wood C. High prevalence of pre-existing serological cross-reactivity against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in sub-Saharan Africa. Int. J. Infect. Dis. 2021;102:577–583. doi: 10.1016/j.ijid.2020.10.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson E.M., Goodwin E.C., Verma A., Arevalo C.P., Bolton M.J., Weirick M.E., Gouma S., McAllister C.M., Christensen S.R., Weaver J., Hicks P., Manzoni T.B., Oniyide O., Ramage H., Mathew D., Baxter A.E., Oldridge D.A., Greenplate A.R., Wu J.E., Alanio C., D’Andrea K., Kuthuru O., Dougherty J., Pattekar A., Kim J., Han N., Apostolidis S.A., Huang A.C., Vella L.A., Kuri-Cervantes L., Pampena M.B., Betts M.R., Wherry E.J., Meyer N.J., Cherry S., Bates P., Rader D.J., Hensley S.E. Seasonal human coronavirus antibodies are boosted upon SARS-CoV-2 infection but not associated with protection. Cell. 2021;184:1858–1864. doi: 10.1016/j.cell.2021.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deakin C.T., Cornish G.H., Ng K.W., Faulkner N., Bolland W., Hope J., Rosa A., Harvey R., Hussain S., Earl C., Jebson B.R., Wilkinson M., Marshall L.R., O’Brien K., Rosser E.C., Radziszewska A., Peckham H., Patel H., Heaney J., Rickman H., Paraskevopoulou S., Houlihan C.F., Spyer M.J., Gamblin S.J., McCauley J., Nastouli E., Levin M., Cherepanov P., Ciurtin C., Wedderburn L.R., Kassiotis G. Favorable antibody responses to human coronaviruses in children and adolescents with autoimmune rheumatic diseases. Med (New York, N.Y.) 2021;2(9):1093–1109.e6. doi: 10.1016/j.medj.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Selva K.J., van de Sandt C.E., Lemke M.M., Lee C.Y., Shoffner S.K., Chua B.Y., Davis S.K., Nguyen T.H.O., Rowntree L.C., Hensen L., Koutsakos M., Wong C.Y., Mordant F., Jackson D.C., Flanagan K.L., Crowe J., Tosif S., Neeland M.R., Sutton P., Licciardi P.V., Crawford N.W., Cheng A.C., Doolan D.L., Amanat F., Krammer F., Chappell K., Modhiran N., Watterson D., Young P., Lee W.S., Wines B.D., Mark Hogarth P., Esterbauer R., Kelly H.G., Tan H.X., Juno J.A., Wheatley A.K., Kent S.J., Arnold K.B., Kedzierska K., Chung A.W. Systems serology detects functionally distinct coronavirus antibody features in children and elderly. Nat. Commun. 2021;12(1):2037. doi: 10.1038/s41467-021-22236-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Voss W.N., Hou Y.J., Johnson N.V., Delidakis G., Kim J.E., Javanmardi K., Horton A.P., Bartzoka F., Paresi C.J., Tanno Y., Chou C.-W., Abbasi S.A., Pickens W., George K., Boutz D.R., Towers D.M., McDaniel J.R., Billick D., Goike J., Rowe L., Batra D., Pohl J., Lee J., Gangappa S., Sambhara S., Gadush M., Wang N., Person M.D., Iverson B.L., Gollihar J.D., Dye J., Herbert A., Finkelstein I.J., Baric R.S., McLellan J.S., Georgiou G., Lavinder J.J., Ippolito G.C. Prevalent, protective, and convergent IgG recognition of SARS-CoV-2 non-RBD spike epitopes. Science (New York, N.Y.) 2021:eabg5268. doi: 10.1126/science.abg5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y., Soh W.T., Tada A., Arakawa A., Matsuoka S., Nakayama E.E., Li S., Ono C., Torii S., Kishida K., Jin H., Nakai W., Arase N., Nakagawa A., Shindo Y., Kohyama M., Nakagami H., Tomii K., Ohmura K., Ohshima S., Okada M., Matsuura Y., Standley D.M., Shioda T., Arase H. An infectivity-enhancing site on the SARS-CoV-2 spike protein is targeted by COVID-19 patient antibodies. Cell. 2021;184:3452–3466.e18. doi: 10.1016/j.cell.2021.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laing E.D., Sterling S.L., Richard S.A., Epsi N.J., Coggins S., Samuels E.C., Phogat S., Yan L., Moreno N., Coles C.L., Drew M., Mehalko J., Merritt S., Mende K., Munster V., de Wit E., Chung K.K., Millar E.V., Tribble D.R., Simons M.P., Pollett S.D., Esposito D., Lanteri C., Clifton G.T., Mitre E., Burgess T.H., Broder C.C. Antigen-based multiplex strategies to discriminate SARS-CoV-2 natural and vaccine induced immunity from seasonal human coronavirus humoral responses. medRxiv. 2021 [Google Scholar]
- 41.Nguyen-Contant P., Embong A.K., Kanagaiah P., Chaves F.A., Yang H., Branche A.R., Topham D.J., Sangster M.Y. S protein-reactive IgG and memory B cell production after human SARS-CoV-2 infection includes broad reactivity to the S2 subunit. mBio. 2020;11(5):e01991–20. doi: 10.1128/mBio.01991-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cai Y., Zhang J., Xiao T., Peng H., Sterling S.M., Walsh R.M., Rawson S., Rits-Volloch S., Chen B. Distinct conformational states of SARS-CoV-2 spike protein. Science. 2020;369(6511):1586–1592. doi: 10.1126/science.abd4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klein S., Cortese M., Winter S.L., Wachsmuth-Melm M., Neufeldt C.J., Cerikan B., Stanifer M.L., Boulant S., Bartenschlager R., Chlanda P. SARS-CoV-2 structure and replication characterized by in situ cryo-electron tomography. Nat. Commun. 2020;11(1):5885. doi: 10.1038/s41467-020-19619-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ke Z., Oton J., Qu K., Cortese M., Zila V., McKeane L., Nakane T., Zivanov J., Neufeldt C.J., Cerikan B., Lu J.M., Peukes J., Xiong X., Kräusslich H.-G., Scheres S.H.W., Bartenschlager R., Briggs J.A.G. Structures and distributions of SARS-CoV-2 spike proteins on intact virions. Nature. 2020;588(7838):498–502. doi: 10.1038/s41586-020-2665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turoňová B., Sikora M., Schürmann C., Hagen Wim J.H., Welsch S., Blanc Florian E.C., von Bülow S., Gecht M., Bagola K., Hörner C., van Zandbergen G., Landry J., de Azevedo Nayara Trevisan D., Mosalaganti S., Schwarz A., Covino R., Mühlebach Michael D., Hummer G., Krijnse Locker J., Beck M. In situ structural analysis of SARS-CoV-2 spike reveals flexibility mediated by three hinges. Science. 2020;370(6513):203–208. doi: 10.1126/science.abd5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morgenlander W.R., Henson S.N., Monaco D.R., Chen A., Littlefield K., Bloch E.M., Fujimura E., Ruczinski I., Crowley A.R., Natarajan H., Butler S.E., Weiner J.A., Li M.Z., Bonny T.S., Benner S.E., Balagopal A., Sullivan D., Shoham S., Quinn T.C., Eshleman S., Casadevall A., Redd A.D., Laeyendecker O., Ackerman M.E., Pekosz A., Elledge S.J., Robinson M.L., Tobian A.A.R., Larman H.B. Antibody responses to endemic coronaviruses modulate COVID-19 convalescent plasma functionality. J. Clin. Invest. 2021 doi: 10.1172/JCI146927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ladner J.T., Henson S.N., Boyle A.S., Engelbrektson A.L., Fink Z.W., Rahee F., D’Ambrozio J., Schaecher K.E., Stone M., Dong W., Dadwal S., Yu J., Caligiuri M.A., Cieplak P., Bjørås M., Fenstad M.H., Nordbø S.A., Kainov D.E., Muranaka N., Chee M.S., Shiryaev S.A., Altin J.A. Epitope-resolved profiling of the SARS-CoV-2 antibody response identifies cross-reactivity with endemic human coronaviruses, Cell reports. Medicine. 2021;2(1) doi: 10.1016/j.xcrm.2020.100189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lapp S.A., Edara V.V., Lu A., Lai L., Hussaini L., Chahroudi A., Anderson L.J., Suthar M.S., Anderson E.J., Rostad C.A. Original antigenic sin responses to heterologous <em>Betacoronavirus</em> spike proteins are observed in mice following intramuscular administration, but are not apparent in children following SARS-CoV-2 infection. medRxiv. 2021 doi: 10.1371/journal.pone.0256482. 2021.04.29.21256344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Freeman B., Lester S., Mills L., Rasheed M.A.U., Moye S., Abiona O., Hutchinson G.B., Morales-Betoulle M., Krapinunaya I., Gibbons A., Chiang C.-F., Cannon D., Klena J., Johnson J.A., Owen S.M., Graham B.S., Corbett K.S., Thornburg N.J. Validation of a SARS-CoV-2 spike protein ELISA for use in contact investigations and sero-surveillance. bioRxiv. 2020 2020.04.24.057323. [Google Scholar]
- 50.Hicks J., Klumpp-Thomas C., Kalish H., Shunmugavel A., Mehalko J., Denson J.-P., Snead K.R., Drew M., Corbett K.S., Graham B.S., Hall M.D., Memoli M.J., Esposito D., Sadtler K. Serologic Cross-Reactivity of SARS-CoV-2 with endemic and seasonal betacoronaviruses. J. Clin. Immunol. 2021 doi: 10.1007/s10875-021-00997-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Natarajan H., Crowley A.R., Butler S.E., Xu S., Weiner J.A., Bloch E.M., Littlefield K., Wieland-Alter W., Connor R.I., Wright P.F., Benner S.E., Bonny T.S., Laeyendecker O., Sullivan D.J., Shoham S., Quinn T., Larman H.B., Casadevall A., Pekosz A., Redd A., Tobian A.A., Ackerman M.E. SARS-CoV-2 antibody signatures robustly predict diverse antiviral functions relevant for convalescent plasma therapy. medRxiv. 2020 2020.09.16.20196154. [Google Scholar]
- 52.Zhang A., Stacey H.D., Mullarkey C.E., Miller M.S. Original antigenic sin: how first exposure shapes lifelong anti-influenza virus immune responses. J. Immunol. 2019;202(2):335–340. doi: 10.4049/jimmunol.1801149. [DOI] [PubMed] [Google Scholar]
- 53.Chan K.H., Cheng V.C., Woo P.C., Lau S.K., Poon L.L., Guan Y., Seto W.H., Yuen K.Y., Peiris J.S. Serological responses in patients with severe acute respiratory syndrome coronavirus infection and cross-reactivity with human coronaviruses 229E, OC43, and NL63. Clin. Diagn. Lab. Immunol. 2005;12(11):1317–1321. doi: 10.1128/CDLI.12.11.1317-1321.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prévost J., Gasser R., Beaudoin-Bussières G., Richard J., Duerr R., Laumaea A., Anand S.P., Goyette G., Benlarbi M., Ding S., Medjahed H., Lewin A., Perreault J., Tremblay T., Gendron-Lepage G., Gauthier N., Carrier M., Marcoux D., Piché A., Lavoie M., Benoit A., Loungnarath V., Brochu G., Haddad E., Stacey H.D., Miller M.S., Desforges M., Talbot P.J., Maule G.T.G., Côté M., Therrien C., Serhir B., Bazin R., Roger M., Finzi A. Cross-sectional evaluation of humoral responses against SARS-CoV-2 spike. Cell Rep. Med. 2020;1(7) doi: 10.1016/j.xcrm.2020.100126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aydillo T., Rombauts A., Stadlbauer D., Aslam S., Abelenda-Alonso G., Escalera A., Amanat F., Jiang K., Krammer F., Carratala J., Garcia-Sastre A. Antibody immunological imprinting on COVID-19 patients. medRxiv. 2020 doi: 10.1038/s41467-021-23977-1. 2020.10.14.20212662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McNaughton A.L., Paton R.S., Edmans M., Youngs J., Wellens J., Phalora P., Fyfe A., Belij-Rammerstorfer S., Bolton J.S., Ball J., Carnell G., Dejnirattisai W., Dold C., Eyre D.W., Hopkins P., Howarth A., Kooblall K., Klim H., Leaver S., Lee L., López-Camacho C., Lumley S.F., Macallan D., Mentzer A.J., Provine N., Ratcliff J., Slon-Compos J., Skelly D., Stolle L., Supasa P., Temperton N., Walker C., Wang B., Wyncoll D., Simmonds P., Lambe T., Baillie K., Semple M.G., Openshaw P.I., Obolski U., Turner M., Carroll M., Mongkolsapaya J., Screaton G., Kennedy S.H., Jarvis L., Barnes E., Dunachie S., Lourenço J., Matthews P.C., Bicanic T., Klenerman P., Gupta S., Thompson C.P. Fatal COVID-19 outcomes are associated with an antibody response targeting epitopes shared with endemic coronaviruses. medRxiv. 2021 doi: 10.1172/jci.insight.156372. 2021.05.04.21256571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dangi T., Palacio N., Sanchez S., Park M., Class J., Visvabharathy L., Ciucci T., Koralnik I.J., Richner J.M., Penaloza-MacMaster P. Cross-protective immunity following coronavirus vaccination and coronavirus infection. J. Clin. Invest. 2021 doi: 10.1172/JCI151969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Donal T.S., Adam C.H., Javier G.-J., Michael L.K., Stephanie L., AnthonyBrown, Sandra A., Emily A., Helen B., Medawar Laboratory T., TomTipton, Lizzie S., Síle A.J., Ali A., Group O.C., Tiong K.T., Lisa S., Kuan-Ying A.H., Pramila R., Group P.S., Group C.P.C., John F., Philip G., Christopher P.C., Katie J., Christina D., Andrew J.P., Alain R.T., Paul K., Susanna J.D., Eleanor B., Miles W.C., William S.J. Vaccine-induced immunity provides more robust heterotypic immunity than natural infection to emerging SARS-CoV-2 variants of concern. Research Square. 2021 [Google Scholar]
- 59.Amanat F., Thapa M., Lei T., Sayed Ahmed S.M., Adelsberg D.C., Carreno J.M., Strohmeier S., Schmitz A.J., Zafar S., Zhou J.Q., Rijnink W., Alshammary H., Borcherding N., Reiche A.G., Srivastava K., Sordillo E.M., van Bakel H., Turner J.S., Bajic G., Simon V., Ellebedy A.H., Krammer F. SARS-CoV-2 mRNA vaccination induces functionally diverse antibodies to NTD, RBD and S2. Cell. 2021 doi: 10.1016/j.cell.2021.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wec A.Z., Wrapp D., Herbert A.S., Maurer D.P., Haslwanter D., Sakharkar M., Jangra R.K., Dieterle M.E., Lilov A., Huang D., Tse L.V., Johnson N.V., Hsieh C.-L., Wang N., Nett J.H., Champney E., Burnina I., Brown M., Lin S., Sinclair M., Johnson C., Pudi S., Bortz R., Wirchnianski A.S., Laudermilch E., Florez C., Fels J.M., O’Brien C.M., Graham B.S., Nemazee D., Burton D.R., Baric R.S., Voss J.E., Chandran K., Dye J.M., McLellan J.S., Walker L.M. Broad neutralization of SARS-related viruses by human monoclonal antibodies. Science (New York, N.Y.) 2020;369(6504):731–736. doi: 10.1126/science.abc7424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Song G., He W.-t., Callaghan S., Anzanello F., Huang D., Ricketts J., Torres J.L., Beutler N., Peng L., Vargas S., Cassell J., Parren M., Yang L., Ignacio C., Smith D.M., Voss J.E., Nemazee D., Ward A.B., Rogers T., Burton D.R., Andrabi R. Cross-reactive serum and memory B cell responses to spike protein in SARS-CoV-2 and endemic coronavirus infection. bioRxiv. 2020 doi: 10.1038/s41467-021-23074-3. 2020.09.22.308965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Westerhuis B.M., Aguilar-Bretones M., Raadsen M.P., de Bruin E., Okba N.M.A., Haagmans B.L., Langerak T., Endeman H., van den Akker J.P.C., Gommers D.A.M.P.J., van Gorp E.C.M., Rockx B.H.G., Koopmans M.P.G., van Nierop G.P. Severe COVID-19 patients display a back boost of seasonal coronavirus-specific antibodies. medRxiv. 2020 2020.10.10.20210070. [Google Scholar]
- 63.Turner J.S., O’Halloran J.A., Kalaidina E., Kim W., Schmitz A.J., Zhou J.Q., Lei T., Thapa M., Chen R.E., Case J.B., Amanat F., Rauseo A.M., Haile A., Xie X., Klebert M.K., Suessen T., Middleton W.D., Shi P.-Y., Krammer F., Teefey S.A., Diamond M.S., Presti R.M., Ellebedy A.H. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature. 2021 doi: 10.1038/s41586-021-03738-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang F., Nielsen S.C.A., Hoh R.A., Röltgen K., Wirz O.F., Haraguchi E., Jean G.H., Lee J.-Y., Pham T.D., Jackson K.J.L., Roskin K.M., Liu Y., Nguyen K., Ohgami R.S., Osborne E.M., Nadeau K.C., Niemann C.U., Parsonnet J., Boyd S.D. Shared B cell memory to coronaviruses and other pathogens varies in human age groups and tissues. Science (New York, N.Y. 2021;372:738–741. doi: 10.1126/science.abf6648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim S.I., Noh J., Kim S., Choi Y., Yoo D.K., Lee Y., Lee H., Jung J., Kang C.K., Song K.-H., Choe P.G., Kim H.B., Kim E.S., Kim N.-J., Seong M.-W., Park W.B., Oh M.-d., Kwon S., Chung J. Stereotypic neutralizing V<sub>H</sub> antibodies against SARS-CoV-2 spike protein receptor binding domain in patients with COVID-19 and healthy individuals. Sci. Transl. Med. 2021;13(578) doi: 10.1126/scitranslmed.abd6990. eabd6990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weisberg S.P., Connors T.J., Zhu Y., Baldwin M.R., Lin W.-H., Wontakal S., Szabo P.A., Wells S.B., Dogra P., Gray J., Idzikowski E., Stelitano D., Bovier F.T., Davis-Porada J., Matsumoto R., Poon M.M.L., Chait M., Mathieu C., Horvat B., Decimo D., Hudson K.E., Zotti F.D., Bitan Z.C., La Carpia F., Ferrara S.A., Mace E., Milner J., Moscona A., Hod E., Porotto M., Farber D.L. Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum. Nat. Immunol. 2020;22:25–31. doi: 10.1038/s41590-020-00826-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Galipeau Y., Siragam V., Laroche G., Marion E., Greig M., McGuinty M., Booth R.A., Durocher Y., Cuperlovic-Culf M., Bennett S.A.L., Crawley A.M., Giguère P.M., Cooper C., Langlois M.-A. Relative ratios of human seasonal coronavirus antibodies predict the efficiency of cross-neutralization of SARS-CoV-2 spike binding to ACE2. medRxiv. 2021 doi: 10.1016/j.ebiom.2021.103700. 2021.07.16.21260079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khan T., Rahman M., Ali F.A., Huang S.S.Y., Ata M., Zhang Q., Bastard P., Liu Z., Jouanguy E., Béziat V., Cobat A., Nasrallah G.K., Yassine H.M., Smatti M.K., Saeed A., Vandernoot I., Goffard J.C., Smits G., Migeotte I., Haerynck F., Meyts I., Abel L., Casanova J.L., Hasan M.R., Marr N. Distinct antibody repertoires against endemic human coronaviruses in children and adults. JCI Insight. 2021;6(4) doi: 10.1172/jci.insight.144499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dowell A.C., Butler M.S., Jinks E., Tut G., Lancaster T., Sylla P., Begum J., Pearce H., Spalkova E., Margielewska-Davies S., Taylor G.S., Syrimi E., Baawuah F., Beckmann J., Okike I., Ahmad S., Garstang J., Brent A.J., Brent B., Ireland G., Aiano F., Amin-Chowdhury Z., Jones S., Borrow R., Linley E., Poh J., Saliba V., Amirthalingam G., Brown K.E., Ramsay M.E., Zuo J., Moss P., Ladhani S. Children develop strong and sustained cross-reactive immune responses against Spike protein following SARS-CoV-2 infection, with enhanced recognition of variants of concern. medRxiv. 2021 2021.04.12.21255275. [Google Scholar]
- 70.Welsh R.M., Che J.W., Brehm M.A., Selin L.K. Heterologous immunity between viruses. Immunol. Rev. 2010;235(1):244–266. doi: 10.1111/j.0105-2896.2010.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fierz W., Walz B. Antibody dependent enhancement due to original antigenic sin and the development of SARS. Front. Immunol. 2020;11:1120. doi: 10.3389/fimmu.2020.01120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saphire E.O., Schendel S.L., Gunn B.M., Milligan J.C., Alter G. Antibody-mediated protection against Ebola virus. Nat. Immunol. 2018;19(11):1169–1178. doi: 10.1038/s41590-018-0233-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vanderven H.A., Kent S.J. The protective potential of Fc-mediated antibody functions against influenza virus and other viral pathogens. Immunol. Cell Biol. 2020;98(4):253–263. doi: 10.1111/imcb.12312. [DOI] [PubMed] [Google Scholar]
- 74.Pinto D., Sauer M.M., Czudnochowski N., Low J.S., Tortorici M.A., Housley M.P., Noack J., Walls A.C., Bowen J.E., Guarino B., Rosen L.E., di Iulio J., Jerak J., Kaiser H., Islam S., Jaconi S., Sprugasci N., Culap K., Abdelnabi R., Foo C., Coelmont L., Bartha I., Bianchi S., Silacci-Fregni C., Bassi J., Marzi R., Vetti E., Cassotta A., Ceschi A., Ferrari P., Cippà P.E., Giannini O., Ceruti S., Garzoni C., Riva A., Benigni F., Cameroni E., Piccoli L., Pizzuto M.S., Smithey M., Hong D., Telenti A., Lempp F.A., Neyts J., Havenar-Daughton C., Lanzavecchia A., Sallusto F., Snell G., Virgin H.W., Beltramello M., Corti D., Veesler D. Broad betacoronavirus neutralization by a stem helix-specific human antibody. Science (New York, N.Y. 2021 doi: 10.1126/science.abj3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Poston D., Weisblum Y., Wise H., Templeton K., Jenks S., Hatziioannou T., Bieniasz P. Absence of SARS-CoV-2 neutralizing activity in pre-pandemic sera from individuals with recent seasonal coronavirus infection. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guo L., Wang Y., Kang L., Hu Y., Wang L., Zhong J., Chen H., Ren L., Gu X., Wang G., Wang C., Dong X., Wu C., Han L., Wang Y., Fan G., Zou X., Li H., Xu J., Jin Q., Cao B., Wang J. Cross-reactive antibody against human coronavirus OC43 spike protein correlates with disease severity in COVID-19 patients: a retrospective study. Emerg. Microbes Infect. 2021;10(1):664–676. doi: 10.1080/22221751.2021.1905488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Freedenberg A.T., Pan C.H., Diehl W.E., Romeiser J.L., Hwang G.R., Leiton C.V., Muecksch F., Shroyer K.R., Bennett-Guerrero E. Neutralizing activity to SARS-CoV-2 of convalescent and control plasma used in a randomized controlled trial. Transfusion. 2021 doi: 10.1111/trf.16283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Díez J.M., Romero C., Vergara-Alert J., Belló-Perez M., Rodon J., Honrubia J.M., Segalés J., Sola I., Enjuanes L., Gajardo R. Cross-neutralization activity against SARS-CoV-2 is present in currently available intravenous immunoglobulins. Immunotherapy. 2020;12(17):1247–1255. doi: 10.2217/imt-2020-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kubota-Koketsu R., Terada Y., Yunoki M., Sasaki T., Nakayama E.E., Kamitani W., Shioda T. Neutralizing and binding activities against SARS-CoV-1/2, MERS-CoV, and human coronaviruses 229E and OC43 by normal human intravenous immunoglobulin derived from healthy donors in Japan. Transfusion. 2021;61(2):356–360. doi: 10.1111/trf.16161. [DOI] [PubMed] [Google Scholar]
- 80.Poh C.M., Carissimo G., Wang B., Amrun S.N., Lee C.Y.-P., Chee R.S.-L., Fong S.-W., Yeo N.K.-W., Lee W.-H., Torres-Ruesta A., Leo Y.-S., Chen M.I.C., Tan S.-Y., Chai L.Y.A., Kalimuddin S., Kheng S.S.G., Thien S.-Y., Young B.E., Lye D.C., Hanson B.J., Wang C.-I., Renia L., Ng L.F.P. Two linear epitopes on the SARS-CoV-2 spike protein that elicit neutralising antibodies in COVID-19 patients. Nat. Commun. 2020;11(1):2806. doi: 10.1038/s41467-020-16638-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Garrett M.E., Galloway J., Chu H.Y., Itell H.L., Stoddard C.I., Wolf C.R., Logue J.K., McDonald D., Weight H., Matsen F.A.I.V., Overbaugh J. High resolution profiling of pathways of escape for SARS-CoV-2 spike-binding antibodies. Cell. 2021 doi: 10.1016/j.cell.2021.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ma X., Zou F., Yu F., Li R., Yuan Y., Zhang Y., Zhang X., Deng J., Chen T., Song Z., Qiao Y., Zhan Y., Liu J., Zhang J., Zhang X., Peng Z., Li Y., Lin Y., Liang L., Wang G., Chen Y., Chen Q., Pan T., He X., Zhang H. Nanoparticle vaccines based on the receptor binding domain (RBD) and heptad repeat (HR) of SARS-CoV-2 elicit robust protective immune responses. Immunity. 2020;53(6):1315–1330.e9. doi: 10.1016/j.immuni.2020.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Keng C.T., Zhang A., Shen S., Lip K.M., Fielding B.C., Tan T.H., Chou C.F., Loh C.B., Wang S., Fu J., Yang X., Lim S.G., Hong W., Tan Y.J. Amino acids 1055 to 1192 in the S2 region of severe acute respiratory syndrome coronavirus S protein induce neutralizing antibodies: implications for the development of vaccines and antiviral agents. J. Virol. 2005;79(6):3289–3296. doi: 10.1128/JVI.79.6.3289-3296.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lip K.M., Shen S., Yang X., Keng C.T., Zhang A., Oh H.L., Li Z.H., Hwang L.A., Chou C.F., Fielding B.C., Tan T.H., Mayrhofer J., Falkner F.G., Fu J., Lim S.G., Hong W., Tan Y.J. Monoclonal antibodies targeting the HR2 domain and the region immediately upstream of the HR2 of the S protein neutralize in vitro infection of severe acute respiratory syndrome coronavirus. J. Virol. 2006;80(2):941–950. doi: 10.1128/JVI.80.2.941-950.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Elshabrawy H.A., Coughlin M.M., Baker S.C., Prabhakar B.S. Human monoclonal antibodies against highly conserved HR1 and HR2 domains of the SARS-CoV spike protein are more broadly neutralizing. PLoS One. 2012;7(11) doi: 10.1371/journal.pone.0050366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Duan J., Yan X., Guo X., Cao W., Han W., Qi C., Feng J., Yang D., Gao G., Jin G. A human SARS-CoV neutralizing antibody against epitope on S2 protein. Biochem. Biophys. Res. Commun. 2005;333(1):186–193. doi: 10.1016/j.bbrc.2005.05.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang C., van Haperen R., Gutiérrez-Álvarez J., Li W., Okba N.M.A., Albulescu I., Widjaja I., van Dieren B., Fernandez-Delgado R., Sola I., Hurdiss D.L., Daramola O., Grosveld F., van Kuppeveld F.J.M., Haagmans B.L., Enjuanes L., Drabek D., Bosch B.J. A conserved immunogenic and vulnerable site on the coronavirus spike protein delineated by cross-reactive monoclonal antibodies. Nat. Commun. 2021;12(1):1715. doi: 10.1038/s41467-021-21968-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chi X., Yan R., Zhang J., Zhang G., Zhang Y., Hao M., Zhang Z., Fan P., Dong Y., Yang Y., Chen Z., Guo Y., Zhang J., Li Y., Song X., Chen Y., Xia L., Fu L., Hou L., Xu J., Yu C., Li J., Zhou Q., Chen W. A neutralizing human antibody binds to the N-terminal domain of the Spike protein of SARS-CoV-2. Science (New York, N.Y. 2020:eabc6952. doi: 10.1126/science.abc6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sauer M.M., Tortorici M.A., Park Y.J., Walls A.C., Homad L., Acton O.J., Bowen J.E., Wang C., Xiong X., de van der Schueren W., Quispe J., Hoffstrom B.G., Bosch B.J., McGuire A.T., Veesler D. Structural basis for broad coronavirus neutralization. Nat. Struct. Mol. Biol. 2021;28(6):478–486. doi: 10.1038/s41594-021-00596-4. [DOI] [PubMed] [Google Scholar]
- 90.Huang Y., Nguyen A.W., Hsieh C.-L., Silva R., Olaluwoye O.S., Wilen R.E., Kaoud T.S., Azouz L.R., Qerqez A.N., Le K.C., Bohanon A.L., DiVenere A.M., Liu Y., Lee A.G., Amengor D., Dalby K.N., D’Arcy S., McLellan J.S., Maynard J.A. Identification of a conserved neutralizing epitope present on spike proteins from all highly pathogenic coronaviruses. bioRxiv. 2021 doi: 10.7554/eLife.83710. 2021.01.31.428824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sermet-Gaudelus I., Temmam S., Huon C., Behillil S., Gajdos V., Bigot T., Lurier T., Chrétien D., Backovic M., Delaunay-Moisan A., Donati F., Albert M., Foucaud E., Mesplées B., Benoist G., Faye A., Duval-Arnould M., Cretolle C., Charbit M., Aubart M., Auriau J., Lorrot M., Kariyawasam D., Fertitta L., Orliaguet G., Pigneur B., Bader-Meunier B., Briand C., Enouf V., Toubiana J., Guilleminot T. S. Van der Werf, M. Leruez-Ville, M. Eloit, Prior infection by seasonal coronaviruses, as assessed by serology, does not prevent SARS-CoV-2 infection and disease in children, France, April to June 2020. Euro Surveill. 2021;26(13) doi: 10.2807/1560-7917.ES.2021.26.13.2001782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Consiglio C.R., Cotugno N., Sardh F., Pou C., Amodio D., Rodriguez L., Tan Z., Zicari S., Ruggiero A., Pascucci G.R., Santilli V., Campbell T., Bryceson Y., Eriksson D., Wang J., Marchesi A., Lakshmikanth T., Campana A., Villani A., Rossi P., Landegren N., Palma P., Brodin P. The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell. 2020;183(4):968–981.e7. doi: 10.1016/j.cell.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gouma S., Weirick M.E., Bolton M.J., Arevalo C.P., Goodwin E.C., Anderson E.M., McAllister C.M., Christensen S.R., Dunbar D., Fiore D., Brock A., Weaver J., Millar J., DerOhannessian S., Frank I., Rader D.J., Wherry E.J., Hensley S.E. Sero-monitoring of health care workers reveals complex relationships between common coronavirus antibodies and SARS-CoV-2 severity. medRxiv. 2021 doi: 10.1172/jci.insight.150449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sagar M., Reifler K., Rossi M., Miller N.S., Sinha P., White L., Mizgerd J.P. Recent endemic coronavirus infection is associated with less severe COVID-19. J. Clin. Invest. 2020;131 doi: 10.1172/JCI143380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Henss L., Scholz T., von Rhein C., Wieters I., Borgans F., Eberhardt F.J., Zacharowski K., Ciesek S., Rohde G., Vehreschild M., Stephan C., Wolf T., Hofmann-Winkler H., Scheiblauer H., Schnierle B.S. Analysis of humoral immune responses in SARS-CoV-2 infected patients. J. Infect. Dis. 2020;223:56–61. doi: 10.1093/infdis/jiaa680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zohar T., Loos C., Fischinger S., Atyeo C., Wang C., Slein M.D., Burke J., Yu J., Feldman J., Hauser B.M., Caradonna T., Schmidt A.G., Cai Y., Streeck H., Ryan E.T., Barouch D.H., Charles R.C., Lauffenburger D.A., Alter G. Compromised humoral functional evolution tracks with SARS-CoV-2 mortality. Cell. 2020;183(6):1508–1519.e12. doi: 10.1016/j.cell.2020.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang Y., Zhang L., Sang L., Ye F., Ruan S., Zhong B., Song T., Alshukairi A.N., Chen R., Zhang Z., Gan M., Zhu A., Huang Y., Luo L., Mok C.K.P., Al Gethamy M.M., Tan H., Li Z., Huang X., Li F., Sun J., Zhang Y., Wen L., Li Y., Chen Z., Zhuang Z., Zhuo J., Chen C., Kuang L., Wang J., Lv H., Jiang Y., Li M., Lin Y., Deng Y., Tang L., Liang J., Huang J., Perlman S., Zhong N., Zhao J., Malik Peiris J.S., Li Y., Zhao J. Kinetics of viral load and antibody response in relation to COVID-19 severity. J. Clin. Invest. 2020;130(10):5235–5244. doi: 10.1172/JCI138759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kaplonek P., Wang C., Bartsch Y., Fischinger S., Gorman Matthew J., Bowman K., Kang J., Dayal D., Martin P., Nowak Radoslaw P., Villani A.-C., Hsieh C.-L., Charland Nicole C., Gonye Anna L.K., Gushterova I., Khanna Hargun K., LaSalle Thomas J., Lavin-Parsons Kendall M., Lilley Brendan M., Lodenstein Carl L., Manakongtreecheep K., Margolin Justin D., McKaig Brenna N., Rojas-Lopez M., Russo Brian C., Sharma N., Tantivit J., Thomas Molly F., Sade-Feldman M., Feldman J., Julg B., Nilles Eric J., Musk Elon R., Menon Anil S., Fischer Eric S., McLellan Jason S., Schmidt A., Goldberg Marcia B., Filbin Michael R., Hacohen N., Lauffenburger Douglas A., Alter G. Early cross-coronavirus reactive signatures of humoral immunity against COVID-19. Sci. Immunol. 2021;0(0):eabj2901. doi: 10.1126/sciimmunol.abj2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Callow K.A., Parry H.F., Sergeant M., Tyrrell D.A. The time course of the immune response to experimental coronavirus infection of man. Epidemiol. Infect. 1990;105(2):435–446. doi: 10.1017/s0950268800048019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Edridge A.W.D., Kaczorowska J., Hoste A.C.R., Bakker M., Klein M., Loens K., Jebbink M.F., Matser A., Kinsella C.M., Rueda P., Ieven M., Goossens H., Prins M., Sastre P., Deijs M., Van der Hoek L. Seasonal coronavirus protective immunity is short-lasting. Nat. Med. 2020;26:1691–1693. doi: 10.1038/s41591-020-1083-1. [DOI] [PubMed] [Google Scholar]
- 101.Kistler K.E., Bedford T. Evidence for adaptive evolution in the receptor-binding domain of seasonal coronaviruses OC43 and 229e. eLife. 2021;10 doi: 10.7554/eLife.64509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Eguia R.T., Crawford K.H.D., Stevens-Ayers T., Kelnhofer-Millevolte L., Greninger A.L., Englund J.A., Boeckh M.J., Bloom J.D. A human coronavirus evolves antigenically to escape antibody immunity. PLoS Pathog. 2021;17(4) doi: 10.1371/journal.ppat.1009453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wajnberg A., Amanat F., Firpo A., Altman D.R., Bailey M.J., Mansour M., McMahon M., Meade P., Mendu D.R., Muellers K., Stadlbauer D., Stone K., Strohmeier S., Simon V., Aberg J., Reich D.L., Krammer F., Cordon-Cardo C. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science (New York, N.Y.) 2020:eabd7728. doi: 10.1126/science.abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dan J.M., Mateus J., Kato Y., Hastie K.M., Yu E.D., Faliti C.E., Grifoni A., Ramirez S.I., Haupt S., Frazier A., Nakao C., Rayaprolu V., Rawlings S.A., Peters B., Krammer F., Simon V., Saphire E.O., Smith D.M., Weiskopf D., Sette A., Crotty S. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science (New York, N.Y.) 2021;371(6529) doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hansen C.H., Michlmayr D., Gubbels S.M., Mølbak K., Ethelberg S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. Lancet. 2021;397(10280):1204–1212. doi: 10.1016/S0140-6736(21)00575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hall V.J., Foulkes S., Charlett A., Atti A., Monk E.J.M., Simmons R., et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN) Lancet. 2021;397(10283):1459–1469. doi: 10.1016/S0140-6736(21)00675-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Abu-Raddad L.J., Chemaitelly H., Coyle P., Malek J.A., Ahmed A.A., Mohamoud Y.A., Younuskunju S., Ayoub H.H., Kanaani Z.A., Kuwari E.A., Butt A.A., Jeremijenko A., Kaleeckal A.H., Latif A.N., Shaik R.M., Rahim H.F.A., Nasrallah G.K., Yassine H.M., Al Kuwari M.G., Romaihi H.E.A., Al-Thani M.H., Khal A.A., Bertollini R. SARS-CoV-2 reinfection in a cohort of 43,000 antibody-positive individuals followed for up to 35 weeks. medRxiv. 2021 2021.01.15.21249731. [Google Scholar]
- 108.Kissler S.M., Tedijanto C., Goldstein E., Grad Y.H., Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science (New York, N.Y.) 2020 doi: 10.1126/science.abb5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Faulkner N., Ng K.W., Wu M., Harvey R., Margaritis M., Paraskevopoulou S., Houlihan C.F., Hussain S., Greco M., Bolland W., Warchal S., Heaney J., Rickman H., Spyer M.J., Frampton D., Byott M., de Oliveira T., Sigal A., Kjaer S., Swanton C., Gandhi S., Beale R., Gamblin S.J., McCauley J., Daniels R., Howell M., Bauer D.L.V., Nastouli E., Kassiotis G. Reduced antibody cross-reactivity following infection with B.1.1.7 than with parental SARS-CoV-2 strains. bioRxiv. 2021 doi: 10.7554/eLife.69317. 2021.03.01.433314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brown J.C., Goldhill D.H., Zhou J., Peacock T.P., Frise R., Goonawardane N., Baillon L., Kugathasan R., Pinto A., McKay P.F., Hassard J., Moshe M., Singanayagam A., Burgoyne T., Barclay W.S. Increased transmission of SARS-CoV-2 lineage B.1.1.7 (VOC 2020212/01) is not accounted for by a replicative advantage in primary airway cells or antibody escape. bioRxiv. 2021 2021.02.24.432576. [Google Scholar]
- 111.Cele S., Gazy I., Jackson L., Hwa S.-H., Tegally H., Lustig G., Giandhari J., Pillay S., Wilkinson E., Naidoo Y., Karim F., Ganga Y., Khan K., Bernstein M., Balazs A.B., Gosnell B.I., Hanekom W., Moosa M.-Y.S., Lessells R.J., de Oliveira T., Sigal A., Ngs S.A., Team C.-K. Escape of SARS-CoV-2 501Y.V2 from neutralization by convalescent plasma. Nature. 2021 doi: 10.1038/s41586-021-03471-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Moyo-Gwete T., Madzivhandila M., Makhado Z., Ayres F., Mhlanga D., Oosthuysen B., Lambson B.E., Kgagudi P., Tegally H., Iranzadeh A., Doolabh D., Tyers L., Chinhoyi L.R., Mennen M., Skelem S., Marais G., Wibmer C.K., Bhiman J.N., Ueckermann V., Rossouw T., Boswell M., de Oliveira T., Williamson C., Burgers W.A., Ntusi N., Morris L., Moore P.L. SARS-CoV-2 501Y.V2 (B.1.351) elicits cross-reactive neutralizing antibodies. bioRxiv. 2021 doi: 10.1056/NEJMc2104192. 2021.03.06.434193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Amanat F., Strohmeier S., Meade P., Dambrauskas N., Mühlemann B., Smith D.J., Vigdorovich V., Sather D.N., Coughlan L., Krammer F. Vaccination with B.1.1.7, B.1.351 and P.1 variants protects mice from challenge with wild type SARS-CoV-2. bioRxiv. 2021 doi: 10.1371/journal.pbio.3001384. 2021.08.05.455212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vijgen L., Keyaerts E., Moës E., Thoelen I., Wollants E., Lemey P., Vandamme A.-M., Van Ranst M. Complete genomic sequence of human coronavirus OC43: molecular clock analysis suggests a relatively recent zoonotic coronavirus transmission event. J. Virol. 2005;79(3):1595–1604. doi: 10.1128/JVI.79.3.1595-1604.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pfefferle S., Oppong S., Drexler J.F., Gloza-Rausch F., Ipsen A., Seebens A., Müller M.A., Annan A., Vallo P., Adu-Sarkodie Y., Kruppa T.F., Drosten C. Distant relatives of severe acute respiratory syndrome coronavirus and close relatives of human coronavirus 229E in bats, Ghana. Emerging Infect. Dis. 2009;15(9):1377–1384. doi: 10.3201/eid1509.090224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Huynh J., Li S., Yount B., Smith A., Sturges L., Olsen J.C., Nagel J., Johnson J.B., Agnihothram S., Gates J.E., Frieman M.B., Baric R.S., Donaldson E.F. Evidence supporting a zoonotic origin of human coronavirus strain NL63. J. Virol. 2012;86(23):12816–12825. doi: 10.1128/JVI.00906-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Forni D., Cagliani R., Arrigoni F., Benvenuti M., Pontremoli C., Pozzoli U., Clerici M., De Gioia L., Sironi M. Human endemic coronavirus emergence in the context of past and recent zoonotic outbreaks. Research Square. 2021 [Google Scholar]
- 118.Saunders K.O., Lee E., Parks R., Martinez D.R., Li D., Chen H., Edwards R.J., Gobeil S., Barr M., Mansouri K., Alam S.M., Sutherland L.L., Cai F., Sanzone A.M., Berry M., Manne K., Bock K.W., Minai M., Nagata B.M., Kapingidza A.B., Azoitei M., Tse L.V., Scobey T.D., Spreng R.L., Rountree R.W., DeMarco C.T., Denny T.N., Woods C.W., Petzold E.W., Tang J., Oguin T.H., Sempowski G.D., Gagne M., Douek D.C., Tomai M.A., Fox C.B., Seder R., Wiehe K., Weissman D., Pardi N., Golding H., Khurana S., Acharya P., Andersen H., Lewis M.G., Moore I.N., Montefiori D.C., Baric R.S., Haynes B.F. Neutralizing antibody vaccine for pandemic and pre-emergent coronaviruses. Nature. 2021 doi: 10.1038/s41586-021-03594-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wrobel A.G., Benton D.J., Xu P., Roustan C., Martin S.R., Rosenthal P.B., Skehel J.J., Gamblin S.J. SARS-CoV-2 and bat RaTG13 spike glycoprotein structures inform on virus evolution and furin-cleavage effects. Nat. Struct. Mol. Biol. 2020;27(8):763–767. doi: 10.1038/s41594-020-0468-7. [DOI] [PMC free article] [PubMed] [Google Scholar]