Abstract
Aim of the study
The coronavirus disease 2019 (COVID-19) pandemic significantly impacted cancer care. In this study, clinical patient characteristics related to COVID-19 outcomes and advanced care planning, in terms of non-oncological treatment restrictions (e.g. do-not-resuscitate codes), were studied in patients with cancer and COVID-19.
Methods
The Dutch Oncology COVID-19 Consortium registry was launched in March 2020 in 45 hospitals in the Netherlands, primarily to identify risk factors of a severe COVID-19 outcome in patients with cancer. Here, an updated analysis of the registry was performed, and treatment restrictions (e.g. do-not-intubate codes) were studied in relation to COVID-19 outcomes in patients with cancer. Oncological treatment restrictions were not taken into account.
Results
Between 27th March 2020 and 4th February 2021, 1360 patients with cancer and COVID-19 were registered. Follow-up data of 830 patients could be validated for this analysis. Overall, 230 of 830 (27.7%) patients died of COVID-19, and 60% of the remaining 600 patients with resolved COVID-19 were admitted to the hospital. Patients with haematological malignancies or lung cancer had a higher risk of a fatal outcome than other solid tumours. No correlation between anticancer therapies and the risk of a fatal COVID-19 outcome was found. In terms of end-of-life communication, 50% of all patients had restrictions regarding life-prolonging treatment (e.g. do-not-intubate codes). Most identified patients with treatment restrictions had risk factors associated with fatal COVID-19 outcome.
Conclusion
There was no evidence of a negative impact of anticancer therapies on COVID-19 outcomes. Timely end-of-life communication as part of advanced care planning could save patients from prolonged suffering and decrease burden in intensive care units. Early discussion of treatment restrictions should therefore be part of routine oncological care, especially during the COVID-19 pandemic.
Keywords: COVID-19, Cancer, Cancer treatment, Treatment restrictions, Advanced care planning
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic overwhelmed health-care systems worldwide [1]. Early reports from China showed an increased risk of a more severe course of COVID-19 in patients with cancer [2,3], which has led to adjustments in oncological treatment [4,5]. To date, the pandemic has significantly impacted cancer care [4,5].
The Dutch Oncology COVID-19 Consortium (DOCC) was initiated in March 2020. The main objective of this registry was to identify risk factors of a severe course of COVID-19 in patients with cancer. In September 2020, the first analysis was published [6]. Since then, the number of patients with cancer and COVID-19 in the Netherlands has increased rapidly. Although risk factors for these patients leading to a severe course of COVID-19 have partly been elucidated (e.g. age, male sex, haematological malignancies and lung cancer) [[6], [7], [8], [9], [10]], uncertainties regarding specific risks, especially regarding the safety of continuing cancer treatment, remain [11,12].
More knowledge of specific risks for patients with cancer could guide physicians to make informed decisions on continuing oncological treatment and treatment restrictions in case of severe COVID-19. In the Netherlands, advanced care planning, including patient-clinician communication about end-of-life care, is well-established in clinical practice, especially in the elderly or patients with severe medical conditions, such as cancer [13,14]. End-of-life care communication comprises mainly decisions regarding life-prolonging treatment restrictions, such as do-not-resuscitate codes. In patients with advanced cancer, conversations about end-of-life care often involve shared-decision making and usually take place in an elective setting at the outpatient clinic. As a result, treatment restrictions were already established for many patients with cancer before the COVID-19 pandemic, and if not, patients and treating physicians were motivated to discuss risks and benefits of invasive treatment in case of COVID-19 [15].
The initiation of COVID-19 vaccination programmes worldwide is leading to a decreased COVID-19 incidence and mortality [16,17]. However, as patients with cancer were often not included in vaccination trials [16,17], additional research is needed to ensure the efficacy of COVID-19 vaccination in patients with cancer [[18], [19], [20]].
It is expected that oncological care will still face issues regarding the vulnerability of patients with cancer and the safety of continuing cancer treatments during the COVID-19 pandemic. In this updated analysis, we studied clinical patient characteristics related to COVID-19 outcomes and advanced care planning in terms of treatment restrictions (e.g. do-not-intubate codes) in patients with cancer and COVID-19.
2. Methods
2.1. Study design and collection of data
The DOCC registry, consisting of medical oncologists, pulmonologists, haematologists, and neuro-oncologists, was initiated on 27th March 2020 in 45 hospitals in the Netherlands. The design of this registry and collection of the data have been described previously [6].
2.2. Inclusion criteria for this analysis
All patients registered within DOCC, with confirmed COVID-19 (either in the outpatient or in-hospital setting) and a cancer diagnosis ≤5 years, were eligible for the current analysis. In addition, patients with a history of cancer and/or treatments (e.g. bone marrow transplantation or chest radiation therapy) that could still affect the course of COVID-19 (as per the treating physician) were also eligible. Confirmed COVID-19 was defined as either a positive reverse transcription-polymerase chain reaction (PCR) test or the presence of antibodies in serology.
2.3. Data processing
For the current analysis, an update on the course and outcome of COVID-19 was requested for all patients diagnosed with COVID-19 before 1st October 2020 (>4 weeks before interim analysis on 29th October). For patients diagnosed after this date and registered before 4th February 2021, additional validation was performed in case the COVID-19 outcome was known. The applied methods for data validation have been described previously [6].
The use of steroids before COVID-19 was collected to evaluate whether their systemic use could affect COVID-19 outcomes. Data on type, dose, duration and indication for steroid use were obtained. To analyse the effect of duration and indication of steroid use on the course of COVID-19 independently, subgroup analyses were performed. The indication of steroid use was categorised in the following two groups: steroid use as part of the anticancer treatment regimen (e.g. as antiemetic treatment) versus steroid use not related to the anticancer treatment regimen. In addition, duration of steroid use was categorised as either <7 days or ≥7 days. As topical steroids are expected to have minimal systemic effects, and inhaled steroids are suggested to have beneficial effects [21] on COVID-19 outcomes, these types were excluded from this analysis. In addition, hydrocortisone suppletion in patients with adrenal insufficiency was not included.
To study the frequency of end-of-life communication within this cohort, data on life-prolonging treatment restrictions were collected. For this analysis, restrictions in oncological treatment were not taken into account. Life-prolonging treatment restrictions include a broad spectrum of limitations: ‘no hospital admission;’ ‘no admission to intensive care unit (ICU);’ ‘do not intubate/ventilate’ and ‘do not resuscitate.’ Any patient with at least one of these restrictions was considered to have life-prolonging treatment restrictions for this analysis. Treatment restrictions could have been discussed in the outpatient clinic (before or during the COVID-19 pandemic) or during hospital admission for COVID-19. The timing of the discussion was not accounted for in this analysis.
2.4. Statistical analysis
Descriptive statistics were used to analyse baseline characteristics. For univariable and multivariable analyses, patient characteristics between fatal versus resolved COVID-19 outcomes were analysed. Pearson's chi-square test was applied to identify risk factors associated with fatal COVID-19 outcome. Variables with p-values ≤0.10 were included in multivariable analyses. The multivariable logistic regression analyses were performed with backward selection, and variables with p-values <0.05 were considered significant. Data were analysed using IBM SPSS statistics 25, and statistical tests were performed two-sided. Missing data were not imputed. The impact of age on COVID-19 outcomes was studied categorically in the following age groups: <65 years; ≥65 to 75 years and ≥75 years.
To evaluate the effect of active cancer diagnosis or cancer treatment, different subgroups were analysed. Because cancer treatment-related predictive factors for fatal COVID-19 outcome are not yet established for patients with solid malignancies, subsequent analyses focussed on patients with solid tumours. Patients with an active solid tumour were defined as patients with metastatic disease, patients receiving cancer treatment ≤90 days before COVID-19 and patients not receiving treatment as they were still in a diagnostic phase or those receiving only best supportive care. In addition, patients with an active solid malignancy who received oncological treatment ≤30 days before COVID-19 were analysed to assess the impact of cancer treatment.
3. Results
3.1. Total patient population
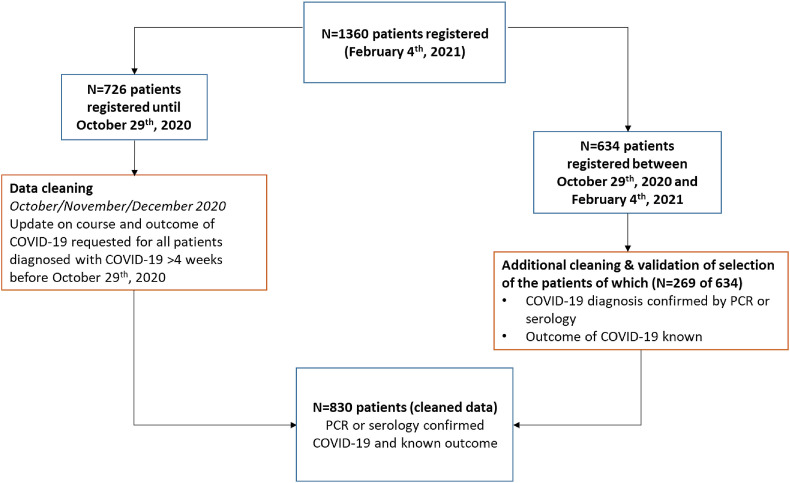
From March 2020 until 4th February 2021 (database lock), 1360 patients with cancer and COVID-19 were registered. During the fall of 2020, infection rates increased, and by 29th October, 726 patients had been registered. During the collection of the updated data, the second COVID-19 outbreak in the Netherlands had reached its peak, which resulted in an additional registration of 634 patients (Fig. 1 ).
Fig. 1.
Patient selection. Flowchart of patient selection for the current analysis
In total, data of 830 patients with confirmed and known outcomes of COVID-19 were validated for this analysis [6]. In summary, 53.3% of patients were men, the median age was 69 years [Interquartile Range (IQR) 60–76], and 20% of patients had a body mass index >30 (Table 1 ). In addition, almost 20% had been diagnosed with prior/other malignancies (Supplementary Table 1). In total, 230 of 830 (27.7%) patients died of COVID-19, of whom almost all (224/230) patients died in the hospital. Of the 600 patients with resolved COVID-19, 60% was admitted to the hospital (Table 1). As the database lock was set on 4th February 2021, and the first vaccinations were administered after 6th January 2021, in the Netherlands, none of the patients had received a COVID-19 vaccine before inclusion in the current analysis.
Table 1.
Clinical patients’ characteristics in DOCC registry. Clinical characteristics of patients with the DOCC registry with fatal outcome of COVID-19 (n = 230) and resolved COVID-19 (n = 600) in the total group of patients (n = 830).
| Variable | Resolved (n = 600) |
Fatal (n = 230)d | Total group (n = 830)d | |
|---|---|---|---|---|
| No hospital admission indicated (n = 233)d | Admitted to hospital (n = 367)d | |||
| Sex — n (%) | ||||
| Male | 87 (37.3) | 206 (56.1) | 149 (64.8) | 442 (53.3) |
| Female | 146 (62.7) | 161 (43.9) | 81 (35.2) | 388 (46.7) |
| Age | ||||
| Median age in years (interquartile range) | 60 (49.5–68) | 69 (61–76) | 75 (68–81) | 69 (60–76) |
| <65 years — n (%) | 153 (65.7) | 127 (34.6) | 29 (12.6) | 309 (37.2) |
| ≥65 years < 75 years — n (%) | 48 (20.6) | 122 (33.2) | 85 (37.0) | 255 (30.7) |
| ≥75 years — n (%) | 32 (13.7) | 118 (32.2) | 116 (50.4) | 266 (32.0) |
| Smoking — n (%) | ||||
| All smokers | 84 (36.0) | 190 (51.8) | 127 (55.2) | 401 (48.3) |
| Current smoker | 11 (4.7) | 16 (4.4) | 17 (7.4) | 44 (5.3) |
| History of smoking | 73 (31.3) | 174 (47.4) | 110 (47.8) | 357 (43.0) |
| Unknown | 36 (15.5) | 49 (13.4) | 38 (16.5) | 123 (14.8) |
| Presence of comorbidities - n (%) | ||||
| Cardiovascular disease | 75 (32.2) | 207 (56.4) | 156 (67.8) | 438 (52.8) |
| BMIa≥ 30 | 54 (23.2) | 75 (20.4) | 37 (16.1) | 166 (20.0) |
| COPDb | 11 (4.7) | 48 (13.1) | 37 (16.1) | 96 (11.6) |
| Diabetes mellitus | 27 (11.6) | 72 (19.6) | 54 (23.5) | 153 (18.4) |
| Autoimmune disease | 14 (6.0) | 21 (5.7) | 16 (7.0) | 51 (6.1) |
| Prior/other malignancy | 22 (9.4) | 64 (17.4) | 73 (31.7) | 159 (19.2) |
| Use of steroids at COVID-19 diagnosis | 36 (15.5) | 80 (21.8) | 68 (29.6) | 184 (22.2) |
| As part of cancer treatmente | 31 (86.1) | 43 (53.8) | 38 (55.9) | 112 (60.9) |
| Use >1 weeke | 13 (36.1) | 33 (41.3) | 31 (45.6) | 77 (41.8) |
| Cancer type — n (%) | ||||
| Breast cancer | 61 (26.2) | 35 (9.5) | 21 (9.1) | 117 (14.1) |
| Non small-cell lung cancer | 14 (6.0) | 52 (14.2) | 41 (17.8) | 107 (12.9) |
| Colorectal cancer | 23 (9.9) | 39 (10.6) | 16 (7.0) | 78 (9.4) |
| Non-Hodgkin lymphoma | 13 (5.6) | 35 (9.5) | 21 (9.1) | 69 (8.3) |
| Prostate cancer | 12 (5.2) | 31 (8.4) | 25 (10.9) | 68 (8.2) |
| Cancer subgroups — n (%) | ||||
| Haematological malignancies | 31 (13.3) | 109 (29.7) | 79 (34.3) | 219 (26.4) |
| Lung cancer | 16 (6.9) | 57 (15.5) | 44 (19.1) | 117 (14.1) |
| Neuro-oncological malignancies | 9 (3.9) | 12 (3.3) | 4 (1.7) | 25 (3.0) |
| Other solid tumours | 177 (75.9) | 189 (51.5) | 103 (44.8) | 469 (56.5) |
| Last cancer treatment — n (%) | ||||
| Surgery | 33 (14.2) | 34 (9.3) | 17 (7.4) | 84 (10.1) |
| Radiotherapy | 29 (12.4) | 65 (17.7) | 28 (12.2) | 122 (14.7) |
| Thoracic radiotherapy | 17 (7.3) | 28 (7.6) | 21 (9.1) | 66 (8.0) |
| Chemotherapy | 93 (39.9) | 153 (41.7) | 93 (40.4) | 339 (40.8) |
| Immunotherapy | 46 (19.7) | 58 (15.8) | 36 (15.7) | 140 (16.9) |
| Targeted therapy | 41 (17.6) | 60 (16.3) | 30 (13.0) | 131 (15.8) |
| Hormonal therapy | 37 (15.9) | 32 (8.7) | 27 (11.7) | 96 (11.6) |
| Disease stage solid tumours — n (%) | ||||
| Metastatic | 90 (38.6) | 120 (32.7) | 86 (37.4) | 288 (34.7) |
| Intention most recent cancer treatment given — n (%) | ||||
| Curative | 117 (50.2) | 147 (40.1) | 81 (35.2) | 345 (41.6) |
| Non-curative | 114 (48.9) | 202 (55.0) | 139 (60.4) | 455 (54.8) |
| Unknown | 2 (0.9) | 18 (4.9) | 10 (4.3) | 30 (3.6) |
| Diagnostic confirmation SARS-CoV-2cinfection | ||||
| PCR | 229 (98.3) | 354 (96.5) | 223 (97.0) | 806 (97.1) |
| Serology (presence of antibodies) | 4 (1.7) | 13 (3.5) | 7 (3.0) | 24 (2.9) |
| Treatment restrictions — n (%) | 39 (16.7) | 180 (49.0) | 199 (86.5) | 418 (50.4) |
COVID-19, coronavirus disease 2019; DOCC, Dutch Oncology COVID-19 Consortium; PCR, polymerase chain reaction.
BMI, body mass index
COPD, chronic obstructive pulmonary disease
SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2).
Percentage is expressed as total number of patients with particular variable within group of patients with similar outcomes of COVID-19-.
Within group of patients with steroids.
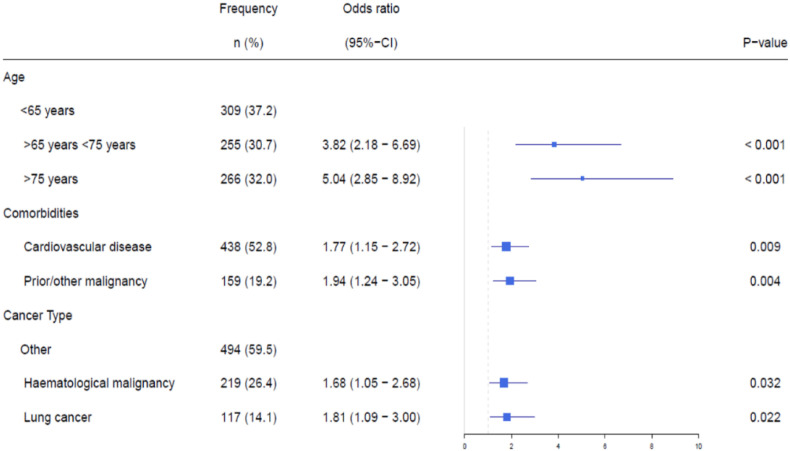
In univariable analysis, male sex, older age, (a history of) smoking, cardiovascular disease, chronic obstructive pulmonary disease, diabetes mellitus, prior/other malignancies and treatment with steroids before COVID-19 were all associated with a higher risk of fatal COVID-19 outcome (Table 2 ). In addition, lung cancer and haematological malignancies were associated with an increased risk of fatal outcome compared with other solid tumours (Table 2). In multivariable analysis, age, cardiovascular disease, and prior/other malignancies were associated with a higher risk of a fatal outcome of COVID-19 (Fig. 2 ). For patients with haematological malignancies or lung cancer, the risk of fatal COVID-19 outcome was higher than in patients with other solid tumours (Fig. 2).
Table 2.
Univariable analysis of patient characteristics related to fatal outcome of COVID-19. Risk (expressed in odds ratio) of a fatal outcome of COVID-19 for the different patients’ characteristics (n = 830).
| All patients (n = 830) |
||
|---|---|---|
| Odds ratio (95% CI) | p-value | |
| Sex (male) | 1.93 (1.41–2.64) | <0.001 |
| Age | ||
| <65 years | – | – |
| ≥65 years < 75 years | 4.83 (3.04–7.67) | <0.001 |
| ≥75 years | 7.47 (4.75–11.74) | <0.001 |
| Smoking | 1.47 (1.08–1.99) | 0.014 |
| Comorbidities | ||
| Cardiovascular disease | 2.38 (1.73–3.27) | <0.001 |
| BMIa ≥ 30 | 0.70 (0.47–1.05) | 0.081 |
| COPDb | 1.94 (1.23–3.07) | 0.004 |
| Diabetes mellitus | 1.55 (1.07–2.26) | 0.020 |
| Autoimmune disease | 1.21 (0.65–2.23) | 0.546 |
| Prior/other malignancy | 2.78 (1.94–3.98) | <0.001 |
| Use of steroids at COVID-19 diagnosis | 1.75 (1.24–2.48) | 0.002 |
| As part of cancer treatmentc | 0.96 (0.44–2.06) | 0.910 |
| Use >1 weekc | 1.27 (0.60–2.71) | 0.536 |
| Cancer type | ||
| Other | – | – |
| Haematological malignancy | 2.01 (1.40–2.89) | <0.001 |
| Lung cancer | 1.99 (1.28–3.11) | 0.002 |
| Last cancer treatment | ||
| Surgery | 0.64 (0.36–1.11) | 0.107 |
| Radiotherapy | 0.75 (0.48–1.17) | 0.203 |
| Thoracic radiotherapy | 1.24 (0.72–2.13) | 0.437 |
| Chemotherapy | 0.98 (0.72–1.33) | 0.882 |
| Immunotherapy | 0.89 (0.59–1.34) | 0.563 |
| Targeted therapy | 0.74 (0.48–1.15) | 0.180 |
| Hormonal therapy | 1.02 (0.64–1.64) | 0.923 |
| Disease stage for solid tumours | ||
| Metastatic | 0.95 (0.69–1.31) | 0.768 |
| Intention most recent cancer treatment given | ||
| Non-curative | 1.27 (0.91–1.76) | 0.154 |
COVID-19, coronavirus disease 2019.
BMI, body mass index.
COPD, chronic obstructive pulmonary disease.
Within group of patients with steroids- -
Fig. 2.
Multivariable analysis of patient characteristics related to fatal outcomes of COVID-19. Multivariable analyses of a fatal outcome of COVID-19 for all patients (n = 830).
3.2. Active solid malignancies
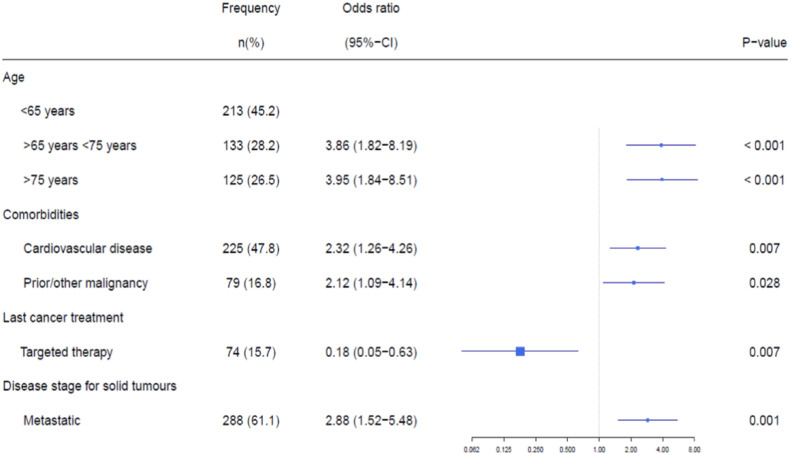
In total, 77% (471/611) of patients with solid tumours were considered having an active malignancy. The identified risk factors of fatal COVID-19 outcome were comparable with the overall group of patients in univariable analysis (Table 3 ). Treatment with targeted therapy (e.g. trastuzumab, bevacizumab and palbociclib) was associated with a decreased risk of a fatal COVID-19 outcome. The administered targeted therapies are presented in Supplementary Table 2. In multivariable analysis, age, cardiovascular disease, prior/other malignancies and presence of metastases were independent risk factors for fatal COVID-19 outcome (Fig. 3 ). Furthermore, treatment with targeted therapy remained associated with a decreased risk of a fatal COVID-19 outcome in multivariable analysis (Fig. 3).
Table 3.
Univariable analysis of subgroup of patients with active solid malignancy and COVID-19. Risk (expressed in odds ratio) of a fatal outcome of COVID-19 for the different patients’ characteristics in patients considered as having an active malignancy (n = 471).
| Patients with active malignancy (n = 471) |
|||
|---|---|---|---|
| Frequency n (%) | Odds ratio (95% CI) | p-value | |
| Sex (male) | 222 (47.1) | 1.63 (1.06–2.51) | 0.027 |
| Age | |||
| <65 years | 213 (45.2) | – | – |
| ≥65 years < 75 years | 133 (28.2) | 4.55 (2.50–8.27) | <0.001 |
| ≥75 years | 125 (26.5) | 6.37 (3.52–11.52) | <0.001 |
| Smoking | 230 (48.8) | 1.73 (1.12–2.67) | 0.014 |
| Comorbidities | |||
| Cardiovascular disease | 225 (47.8) | 2.60 (1.66–4.08) | <0.001 |
| BMIa ≥ 30 | 102 (21.7) | 0.84 (0.49–1.44) | 0.525 |
| COPDb | 52 (11.0) | 2.24 (1.20–4.20) | 0.010 |
| Diabetes mellitus | 88 (18.7) | 1.65 (0.99–2.76) | 0.055 |
| Autoimmune disease | 27 (5.7) | 1.19 (0.49–2.89) | 0.703 |
| Prior/other malignancy | 79 (16.8) | 2.30 (1.37–3.86) | 0.001 |
| Cancer type | |||
| Other solid tumours | 381 (80.9) | – | – |
| Lung cancer | 90 (19.1) | 2.21 (1.34–3.65) | 0.002 |
| Last cancer treatment | |||
| Surgery | 51 (10.8) | 0.80 (0.39–1.66) | 0.550 |
| Radiotherapy | 87 (18.5) | 0.59 (0.32–1.1) | 0.093 |
| Thoracic radiotherapy | 48 (10.2) | 1.14 (0.57–2.27) | 0.719 |
| Chemotherapy | 212 (45.0) | 0.84 (0.54–1.30) | 0.426 |
| Immunotherapy | 79 (16.8) | 0.68 (0.37–1.27) | 0.227 |
| Targeted therapy | 74 (15.7) | 0.42 (0.20–0.87) | 0.016 |
| Hormonal therapy | 86 (18.3) | 1.11 (0.64–1.91) | 0.716 |
| Disease stage for solid tumours | |||
| Metastatic | 288 (61.1) | 1.89 (1.18–3.03) | 0.007 |
| Intention most recent cancer treatment given | |||
| Non-curative | 292 (62.0) | 1.94 (1.19–3.15) | 0.007 |
CI, confidence interval; COVID-19, coronavirus disease 2019.
BMI, body mass index.
COPD, chronic obstructive pulmonary disease 2.
Fig. 3.
Multivariable analysis for the subgroup of patients with active malignancy and COVID-19. Multivariable analyses of a fatal outcome of COVID-19 in the group of patients considered having an active malignancy (n = 471).
A total of 318 patients with solid tumours were included in a subgroup analysis on active treatment. Outcomes were comparable to the analyses as shown previously. When focussing on different oncological treatments, none of the anticancer therapies had significant adverse effects on COVID-19 outcomes (data not shown). However, use of steroids before COVID-19 was associated with an increased risk of fatal COVID-19 outcome (odds ratio 2.07 [1.13–3.81] [95% confidence interval], p = 0.018). There were no significant differences in COVID-19 outcomes among indication or duration of steroid use.
3.3. Treatment restrictions in the total patient population
Life-prolonging treatment restrictions were present in 50% (418/830) of all patients. Treatment restrictions were reported for 49.6% of patients with solid tumours and for 52.5% of patients with haematological malignancies (Table 4 ). Treatment restrictions varied from do-not-resuscitate restrictions (n = 179, 21.6%) to no ICU admissions (n = 148, 17.8%). They were almost fully constrained to treatment within the hospital as only 6 (0.7%) patients had a do-not-hospitalise restriction. Characteristics of patients with whom treatment limitations were discussed are shown in Table 4. Most patients with treatment restrictions had risk factors associated with a fatal COVID-19 outcome. Overall, treatment restrictions were mainly applied in the elderly (24.9% < 65 years of age vs 78.9% ≥ 75 years), patients with comorbidities (i.e. 70% of patients with cardiovascular disease had treatment restrictions) and patients treated with non-curative intent. In the group of patients with treatment restrictions (n = 418), 47.6% died (n = 199) of COVID-19, whereas 7% (n = 26) of patients had a fatal outcome in the group without treatment restrictions (n = 353).
Table 4.
Frequency of treatment restrictions in total group of patients with cancer and COVID-19. Number of patients in total group of patients (n = 830) with treatment restrictions, according to baseline characteristics.
| Variable | Solid tumours (n = 611) |
Haematological malignancies (n = 219) |
||
|---|---|---|---|---|
| Total n | Number of treatment restrictions — n (%)c | Total n | Number of treatment restrictions — n (%)c | |
| Age | ||||
| <65 years | 245 | 70 (28.6) | 64 | 7 (10.9) |
| ≥65 years < 75 years | 183 | 94 (51.4) | 72 | 37 (51.4) |
| ≥75 years | 183 | 139 (76.0) | 83 | 71 (85.5) |
| Sex | ||||
| Male | 308 | 173 (56.2) | 134 | 67 (50.0) |
| Female | 303 | 130 (42.9) | 85 | 48 (56.5) |
| Smoking | ||||
| Never smoked | 305 | 126 (41.3) | 94 | 46 (48.9) |
| Current smoker | 37 | 22 (59.5) | 7 | 3 (42.9) |
| History of smoking | 269 | 155 (57.6) | 88 | 50 (56.8) |
| Comorbidities | ||||
| Cardiovascular disease | 316 | 198 (62.7) | 122 | 75 (61.5) |
| BMIa≥ 30 | 135 | 64 (47.4) | 31 | 15 (48.4) |
| COPDb | 80 | 58 (72.5) | 16 | 9 (56.3) |
| Diabetes mellitus | 117 | 76 (65.0) | 36 | 23 (63.9) |
| Autoimmune disease | 35 | 17 (48.6) | 16 | 11 (68.8) |
| Prior/other malignancies | 115 | 74 (64.3) | 44 | 28 (63.6) |
| Cancer subgroups | ||||
| Lung cancer | 117 | 81 (69.2) | – | – |
| Other solid tumours | 494 | 222 (44.9) | – | – |
| Last cancer treatment | ||||
| Surgery | 84 | 33 (39.3) | – | – |
| Radiotherapy | 117 | 57 (48.7) | 5 | 3 (60.0) |
| Thoracic radiotherapy | 64 | 34 (53.1) | 2 | 1 (50.0) |
| Chemotherapy | 242 | 133 (55.0) | 97 | 56 (57.7) |
| Immunotherapy | 81 | 43 (53.1) | 59 | 30 (50.8) |
| Targeted therapy | 74 | 27 (36.5) | 57 | 38 (66.7) |
| Hormonal therapy | 95 | 44 (46.3) | – | – |
| Disease stage solid tumours | ||||
| Metastatic | 288 | 182 (63.2) | – | – |
| Outcome of COVID-19 | ||||
| Resolved | 460 | 174 (37.8) | 140 | 45 (32.1) |
| Discharged home | 247 | 79 (32.0) | 67 | 15 (22.4) |
| To revalidation centre | 31 | 24 (77.4) | 17 | 10 (58.8) |
| Fatal | 151 | 129 (85.4) | 79 | 70 (88.6) |
| Intention most recent cancer treatment given | ||||
| Curative | 285 | 98 (34.4) | 60 | 22 (36.7) |
| Non-curative | 308 | 194 (63.0) | 147 | 90 (61.2) |
| Total number of treatment restrictions | 611 | 303 (49.6) | 219 | 115 (52.5) |
COVID-19, coronavirus disease 2019.
BMI, body mass index.
COPD, chronic obstructive pulmonary disease 2.
Percentage is expressed as total number of patients with treatment restrictions within group of patients with the same variable.
4. Discussion
In total, 27.7% of patients in the DOCC registry had a fatal outcome of COVID-19. Patients with haematological malignancies and lung cancer had an increased risk of a fatal outcome of COVID-19. In addition, male sex, older age and the presence of comorbidities (cardiovascular disease and prior/other malignancies) are risk factors for a fatal COVID-19 outcome. These findings are comparable to the first DOCC analysis [6] and other registries of patients with cancer and COVID-19 [22]. In the overall cohort of patients, 418 of 830 patients (50.4%) had treatment restrictions. The identified patients with life-prolonging treatment restrictions all had risk factors associated with a fatal COVID-19 outcome. Treatment restrictions were not applied owing to Dutch ICU capacity issues, as the maximum capacity of patients who were hospitalised or admitted to the ICU was never reached during the time frame of this analysis.
Importantly, no correlation was found between specific forms of anticancer therapies and the risk of a severe or fatal outcome of COVID-19, which is supported by other publications [12,23]. However, it is important to note that oncological treatments may have been adjusted during this pandemic [4], possibly more frequently in patients with (multiple) comorbidities and patients treated within a non-curative setting [23].
Remarkably, a lower risk of a fatal outcome was observed in patients treated with targeted therapy, which mainly consisted of trastuzumab ± pertuzumab (Supplementary Table 2). As the effect was not significant in a multivariable model within the active treatment group, it is conceivable that the effect of targeted therapy is caused by multicollinearity. Treatment with trastuzumab ± pertuzumab is usually administered to patients with breast cancer, a population overrepresented by young (62.2% < 65 years) and female patients (63.5%), who have more favourable prognostic factors for COVID-19 outcomes.
For patients treated with steroids before COVID-19, an increased risk of a fatal COVID-19 outcome was found. Because steroids are often applied as part of anticancer treatment (to avoid allergic reactions or as antiemetic therapy), it is possible that steroid use potentially masked the negative impact of cancer treatments (e.g. chemotherapy) on the course of COVID-19. However, a subgroup analysis showed no significant differences in outcomes between steroids as part of anticancer treatment versus steroids for other indications. Therefore, the exact mechanism and significance of a possible severe outcome of COVID-19 in patients treated with steroids before COVID-19 remain unclear.
In the Netherlands, dialogues between patients and their treating physicians regarding treatment restrictions are part of daily clinical practice [[13], [14], [15]], as illustrated by the number of treatment restrictions that had been discussed in the DOCC registry. Nevertheless, the incidence and characteristics of fatal cases within this registry were comparable to other registries [22]. It is known that survival rates of patients with advanced cancer who are admitted to the ICU for non-elective purposes are lower compared with non-oncological patients [24,25]. These observations support that treatment restrictions do not necessarily cause an increased fatality rate. In the current registry, the frequency of treatment restrictions appeared to increase with the risk of having a fatal COVID-19 outcome. This indicates that treating physicians are well-experienced to identify patients who may not benefit from ICU submission. Prognostic models for the outcome of COVID-19 in patients with cancer could further support clinical decision making [26].
The design of this registry has some limitations [6]. Most importantly, the registry was only conducted in hospitals, which probably resulted in an overrepresentation of patients with a severe course of COVID-19. During the first wave, the Dutch testing policy for COVID-19 was restricted to patients with severe COVID-19, which initially resulted in an underestimation of the number of patients with COVID-19. At a later stage, PCR and serology tests were also conducted in patients with mild symptoms. However, as oncology physicians only maintained the registry, an overrepresentation of patients with a severe course of COVID-19 is also assumed during the second wave. In addition, for the current analysis, only patients with a known outcome were selected, which could also have led to an overestimation of patients with a severe or fatal COVID-19 outcome. Nevertheless, the possible overrepresentation of patients with severe COVID-19 should not be of great concern, as the main objective of this registry was to identify risk factors for a severe course of COVID-19 in patients with cancer.
Over a year into the COVID-19 pandemic, its impact on oncological healthcare is still significant. The initiation of vaccination programmes leads to decreases in both hospital admissions and mortality. However, vaccination efficacy against COVID-19 is reduced in patients with specific malignancies and/or cancer treatments [19,20]. Moreover, numerous variants are developing worldwide, and vaccines’ efficacy against these mutants remains uncertain [27,28]. Patients with cancer have an increased risk of a severe COVID-19 outcome, particularly patients with lung cancer, haematological malignancies and specific clinical characteristics [[7], [8], [9],11]. Despite the introduction of COVID-19 vaccines, a subgroup of patients with cancer will remain at high risk of a severe COVID-19 outcome and should therefore be identified. As a timely application of end-of-life communication as part of advanced care planning could decrease the burden on ICUs and, more importantly, save patients from prolonged suffering, early discussion of treatment restrictions should be part of routine oncology care, especially during the COVID-19 pandemic.
Author contributions
K.J., J.T., P.M., D.D., E.O., N.D., O.V., H.B., H.L., E.V., L.H., L.B., H.W., F.B., J.H., A.D. and A.V. have contributed to the design of the study. All authors except for E.O. contributed to data collection. G.H., D.D., P.M., A.D. and A.V. have checked all clinical data for inconsistencies. K.J., A.D. and A.V. have contributed to literature search, data analysis, data interpretation and writing of the article. K.J., J.T., P.H., M.C., E.K., J.B., V.N., Y.K., G.H., P.M., D.D., E.O., N.D., E.L., E.G., G.B., C.L., A.P., K.H., O.V., H.B., H.L., E.V., L.H., L.V., H.W., F.B., J.H., A.D. and A.V. participated in drafting the article and revising it critically for important intellectual content. All authors reviewed the and have given final approval of the submitted version.
The role of the funding source
This study was supported by a grant from the Dutch Cancer Society, a non-profit organisation. The Dutch Cancer Society had no role in study design, data collection, data analysis, data interpretation or writing of the report.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: P.H. reports consulting fees from Astellas, MSD, Pfizer, AstraZeneca, BMS, Ipsen, all outside the submitted work; D.D. reports personal speakers fees from MSD, Roche, AstraZeneca, BMS, Novartis, Pfizer, all outside the submitted work; EGEdV reports an advisory role at Daiichi Sankyo, NSABP and Sanofi (all outside the submitted work and paid to UMCG), and research funding from Amgen, AstraZeneca, Bayer, Chugai Pharma, Crescendo, CytomX Therapeutics, G1 Therapeutics, Genentech, Nordic Nanovector, Radius Health, Regeneron, Roche, Servier and Synthon (all outside the submitted work and paid to UMCG); L.H. reports others from boehringer ingelheim, others from BMS, others from Roche Genentech, others from BMS, grants from Roche Genentech, grants from Boehringer Ingelheim, others from AstraZeneca, personal fees from Quadia, grants from Astra Zeneca, others from Eli Lilly, others from Roche Genentech, others from Pfizer, others from MSD, others from Takeda, non-financial support from AstraZeneca, non-financial support from Novartis, non-financial support from BMS, non-financial support from MSD/Merck, non-financial support from GSK, non-financial support from Takeda, non-financial support from Blueprint Medicines, non-financial support from Roche Genentech, others from Amgen, all outside the submitted work; H.W. reports personal fees and non-financial support from Astellas, personal fees from roche, non-financial support from Ipsen, all outside the submitted work; JH has received financial compensation for advisory roles from Achilles Tx, BMS, BioNTech, Eisai, Immunocore, Instil Bio, Ipsen, MSD, Merk Serono, Molecular Partners, Neogene Tx, PokeAcel, Pfizer, Roche, Sanofi, T-knife, Third Rock Ventures, all outside the submitted work. JH received research grants from Amgen, Asher Bio, BMS, BioNTech, MSD and Novartis, all outside the submitted work. JH owns stock options from Neogene Therapeutics, outside the submitted work; A.D. reports personal fees from Roche, Eli Lily, Boehringer Ingelheim, Pfizer, BMS, Novartis, Takeda, Pharmamar, non-financial support from Abbvie, grants from BMS, grants from Amgen, all outside the submitted work; A.V. reports advisory board of BMS, MSD, Merck, Pfizer, Ipsen, Eisai, Pierre Fabre, Roche, Novartis, Sanofi, all paid to Erasmus MC and outside the submitted work; J.G. reports advisory board of Pierre Fabre, BMS, MSD, and Servier, all outside the submitted work; T.H. reports grants from Roche, Astra Zeneca, BMS, advisory board from MSD and BMS, congress support from Takeda, all outside the submitted work; K.S. reports grants and personal fees from Novartis, personal fees from Bristol Myers Squibb, MSD, Roche, Pierre Fabre, and Abbvie, all outside the submitted work; all remaining authors declare no competing interests.
Acknowledgements
The authors want to thank all the oncology physicians and healthcare staff for their participation in the DOCC registry during this COVID-19 pandemic. The authors thank the clinical trial center at the Erasmus MC, and in particular S. Aammari, for the administrative support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejca.2021.10.009.
Contributor Information
the DOCC investigators:
A. Becker-Commissaris, F. Terheggen, B.E.E.M. van den Borne, L.J.C. van Warmerdam, L. van Leeuwen, F.S. van der Meer, M.A. Tiemessen, D.M. van Diepen, L. Strobbe, J.A.F. Koekkoek, P. Brocken, J.C. Drooger, R. Heller, J.W.B. de Groot, J.A. Stigt, C.C.M. Pitz, M. Slingerland, F.J. Borm, B.C.M. Haberkorn, S.C. van 't Westeinde, M.J.B. Aarts, J.W.G. van Putten, M. Youssef, G.A. Cirkel, C.R. van Rooijen, E. Citgez, N.P. Barlo, B.M.J. Scholtes, R.H.T. Koornstra, N.J.M. Claessens, L.M. Faber, C.H. Rikers, R.A.W. van de Wetering, G.L. Veurink, B.W. Bouter, I. Houtenbos, M.P.L. Bard, G. Douma, M. Jalving, T.J.N. Hiltermann, O.C.J. Schuurbiers-Siebers, K.P.M. Suijkerbuijk, A.S.R. van Lindert, A.J. van de Wouw, V.E.M. van den Boogaart, S.D. Bakker, E. Looysen, W.K. de Jong, E.J.M. Siemerink, A.J. Staal, B. Franken, and W.H. van Geffen
Appendix 1. DOCC investigators
A. Becker-Commissaris1, F. Terheggen2, B.E.E.M. van den Borne3, L.J.C. van Warmerdam4, L. van Leeuwen5, F.S. van der Meer6, M.A. Tiemessen7, D.M. van Diepen7, L. Strobbe8, J.A.F. Koekkoek9, P. Brocken10, J.C. Drooger11, R. Heller12, J.W.B. de Groot13, J.A. Stigt14, C.C.M. Pitz15, M. Slingerland16, F.J. Borm17, B.C.M. Haberkorn18, S.C. van 't Westeinde19, M.J.B. Aarts20, J.W.G. van Putten21, M. Youssef22, G.A. Cirkel23, C.R. van Rooijen24, E. Citgez25, N.P. Barlo26, B.M.J. Scholtes27, R.H.T. Koornstra28, N.J.M. Claessens29, L.M. Faber30, C.H. Rikers31, R.A.W. van de Wetering32, G.L. Veurink33, B.W. Bouter34, I. Houtenbos35, M.P.L. Bard36, G. Douma37, M. Jalving38, T.J.N. Hiltermann39, O.C.J. Schuurbiers - Siebers40, K.P.M. Suijkerbuijk41, A.S.R. van Lindert42, A.J. van de Wouw43, V.E.M. van den Boogaart44, S.D. Bakker45, E. Looysen46, W.K. de Jong47, E.J.M. Siemerink48, A.J. Staal49, B. Franken50, W.H. van Geffen51
1Department of Pulmonary Diseases, Cancer Center Amsterdam, Amsterdam University Medical Center, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands; 2Department of Internal Medicine, Bravis Hospital, Bergen op Zoom, The Netherlands; 3Department of Pulmonary Diseases, Catharina Hospital, Eindhoven, Netherlands; 4Department of Internal Medicine, Catharina-Hospital, Eindhoven, The Netherlands; 5Department of Internal Medicine, Diakonessenhuis, Utrecht, The Netherlands; 6Department of Pulmonology, Diakonessenhuis, Utrecht, The Netherlands; 7Department of Pulmonology, Dijklander Hospital, Purmerend, The Netherlands; 8Department of Internal Medicine, Gelre Hospital, Zutphen, The Netherlands; 9Departments of Neurology, Haaglanden Medical Center and Leiden University Medical Center, Den Haag/Leiden, The Netherlands; 10Department of Pulmonary Diseases, Haga Ziekenhuis, Den Haag, The Netherlands; 11Department of Medical Oncology, Ikazia Hospital, Rotterdam, The Netherlands; 12Department of Pulmonology, Ikazia hospital, Rotterdam, The Netherlands; 13Department of Medical Oncology, Isala Oncology Center, Zwolle, The Netherlands; 14Department of Respiratory Medicine, Isala Hospital, Zwolle, The Netherlands; 15Department of Pulmonology, Laurentius Hospital, Roermond, The Netherlands; 16Department of Medical Oncology, Leiden University Medical Center, Leiden, The Netherlands; 17Department of Pulmonology, Leiden University Medical Center, Leiden, The Netherlands; 18Department of Medical Oncology, Maasstad Hospital, Rotterdam, The Netherlands; 19Department of Pulmonology, Maasstad Hospital, Rotterdam, The Netherlands; 20Division of Medical Oncology, GROW School for Oncology and Developmental Biology, Maastricht University Medical Center, Maastricht, The Netherlands; 21Department of Pulmonary Diseases, Martini Hospital, Groningen, The Netherlands; 22Department of Respiratory Medicine, Máxima Medical Center, Veldhoven, The Netherlands; 23Department of Internal Medicine, Meander Medical Center, Amersfoort, The Netherlands; 24Department of Internal Medicine, Medisch Spectrum Twente, Enschede, The Netherlands; 25Department of Pulmonary Medicine, Medisch Spectrum Twente, Enschede, The Netherlands; 26Department of Respiratory Medicine, Noordwest Ziekenhuisgroep, Alkmaar, the Netherlands; 27Department of Internal Medicine, Maasziekenhuis Pantein, Beugen, The Netherlands; 28Department of Internal Medicine, Rijnstate ziekenhuis, Arnhem, The Netherlands; 29Department of Respiratory Medicine, Rijnstate ziekenhuis, Arnhem, The Netherlands; 30Department of Internal Medicine, Rode Kruis Hospital, Beverwijk, The Netherlands; 31Department of Pulmonology, Rode Kruis Hospital, Beverwijk, The Netherlands; 32Department of Internal Medicine, Slingeland Hospital, Doetinchem, The Netherlands; 33Department of Medical Oncology, Saxenburgh, Hardenberg, The Netherlands; 34Department of Pulmonology, Saxenburgh, Hardenberg, The Netherlands; 35Department of Internal Medicine, Spaarne Gasthuis, Haarlem, The Netherlands; 36Department of Pulmonology, Spaarne Gasthuis, Haarlem, The Netherlands; 37Department of Pulmonary Diseases, Treant Zorggroep, Scheper hospital, Emmen, The Netherlands; 38Department of Medical Oncology, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands; 39Department of Pulmonary Diseases, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands; 40Department of Pulmonary Diseases, Radboud university medical center, Nijmegen, The Netherlands; 41Department of Medical Oncology, University Medical Center Utrecht, Utrecht, The Netherlands; 42Department of Respiratory Medicine, University Medical Center Utrecht, Utrecht, The Netherlands; 43Department of Internal Medicine, VieCuri Medical Center, Venlo, The Netherlands; 44Department of Respiratory Medicine, VieCuri Medical Center Venlo, The Netherlands; 45Department of Internal Medicine, Zaans Medical Center, Zaandam, The Netherlands; 46Department of Pulmonology, Zaans Medical Center, Zaandam, The Netherlands; 47Department of Pulmonology, Hospital Gelderse Vallei, Ede, The Netherlands; 48Department of Internal Medicine, Ziekenhuis Groep Twente (ZGT), Hengelo, The Netherlands; 49Department of Pulmonary Diseases, Ziekenhuis Groep Twente (ZGT), Hengelo, The Netherlands; 50Department of Hematology, Medical Center Leeuwarden, Leeuwarden, The Netherlands; 51Department of Respiratory Medicine, Medical Center Leeuwarden, Leeuwarden, The Netherlands
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang L., Zhu F., Xie L., Wang C., Wang J., Chen R., et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;31:894–901. doi: 10.1016/j.annonc.2020.03.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liang W., Guan W., Chen R., Wang W., Li J., Xu K., et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Joode K., Dumoulin D.W., Engelen V., Bloemendal H.J., Verheij M., van Laarhoven H.W.M., et al. Impact of the coronavirus disease 2019 pandemic on cancer treatment: the patients' perspective. Eur J Cancer. 2020;136:132–139. doi: 10.1016/j.ejca.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.the European Society for Medical Oncology (ESMO) 2020. Cancer patient managment during the COVID-19 pandemic. [Google Scholar]
- 6.de Joode K., Dumoulin D.W., Tol J., Westgeest H.M., Beerepoot L.V., van den Berkmortel F., et al. Dutch Oncology COVID-19 consortium: outcome of COVID-19 in patients with cancer in a nationwide cohort study. Eur J Cancer. 2020;141:171–184. doi: 10.1016/j.ejca.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuderer N.M., Choueiri T.K., Shah D.P., Shyr Y., Rubinstein S.M., Rivera D.R., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garassino M.C., Whisenant J.G., Huang L.C., Trama A., Torri V., Agustoni F., et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21:914–922. doi: 10.1016/S1470-2045(20)30314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinana J.L., Martino R., Garcia-Garcia I., Parody R., Morales M.D., Benzo G., et al. Risk factors and outcome of COVID-19 in patients with hematological malignancies. Exp Hematol Oncol. 2020;9:21. doi: 10.1186/s40164-020-00177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Indini A., Rijavec E., Ghidini M., Cattaneo M., Grossi F. Developing a risk assessment score for patients with cancer during the coronavirus disease 2019 pandemic. Eur J Cancer. 2020;135:47–50. doi: 10.1016/j.ejca.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu C., Zhao Y., Okwan-Duodu D., Basho R., Cui X. COVID-19 in cancer patients: risk, clinical features, and management. Cancer Biol Med. 2020;17:519–527. doi: 10.20892/j.issn.2095-3941.2020.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee L.Y.W., Cazier J.B., Starkey T., Turnbull C.D., Kerr R., et al. Team UKCCMP COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395:1919–1926. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans N., Costantini M., Pasman H.R., Van den Block L., Donker G.A., Miccinesi G., et al. End-of-life communication: a retrospective survey of representative general practitioner networks in four countries. J Pain Symptom Manag. 2014;47:604–619 e3. doi: 10.1016/j.jpainsymman.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Kroon L.L., van Roij J., Korfage I.J., Reyners A.K.L., van den Beuken-van Everdingen M.H.J., den Boer M.O., et al. Perceptions of involvement in advance care planning and emotional functioning in patients with advanced cancer. J Cancer Surviv. 2021;15:380–385. doi: 10.1007/s11764-021-01020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Veer T., van der Sar-van der Brugge S., Paats M.S., van Nood E., de Backer I.C., Aerts J., et al. Do-not-intubate status and COVID-19 mortality in patients admitted to Dutch non-ICU wards. Eur J Clin Microbiol Infect Dis. 2021;40(10):2207–2209. doi: 10.1007/s10096-021-04223-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Veldt A.A.M., Oosting S.F., Dingemans A.C., Fehrmann R.S.N., GeurtsvanKessel C., Jalving M., et al. COVID-19 vaccination: the VOICE for patients with cancer. Nat Med. 2021;27:568–569. doi: 10.1038/s41591-021-01240-w. [DOI] [PubMed] [Google Scholar]
- 19.Bird S., Panopoulou A., Shea R.L., Tsui M., Saso R., Sud A., et al. Response to first vaccination against SARS-CoV-2 in patients with multiple myeloma. Lancet Haematol. 2021;8:e389–e392. doi: 10.1016/S2352-3026(21)00110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monin L., Laing A.G., Munoz-Ruiz M., McKenzie D.R., Del Molino Del Barrio I., Alaguthurai T., et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22:765–778. doi: 10.1016/S1470-2045(21)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramakrishnan S., Nicolau D.V., Jr., Langford B., Mahdi M., Jeffers H., Mwasuku C., et al. Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial. Lancet Respir Med. 2021;9(7):763–772. doi: 10.1016/S2213-2600(21)00160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee A.J.X., Purshouse K. COVID-19 and cancer registries: learning from the first peak of the SARS-CoV-2 pandemic. Br J Cancer. 2021;124:1777–1784. doi: 10.1038/s41416-021-01324-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinato D.J., Zambelli A., Aguilar-Company J., Bower M., Sng C., Salazar R., et al. Clinical portrait of the SARS-CoV-2 epidemic in European cancer patients. Cancer Discov. 2020;10(10):1465–1474. doi: 10.1158/2159-8290.CD-20-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martos-Benitez F.D., Soto-Garcia A., Gutierrez-Noyola A. Clinical characteristics and outcomes of cancer patients requiring intensive care unit admission: a prospective study. J Cancer Res Clin Oncol. 2018;144:717–723. doi: 10.1007/s00432-018-2581-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruckel J.T., Wong S.L., Chan P.S., Bradley S.M., Nallamothu B.K. Patterns of resuscitation care and survival after in-hospital cardiac arrest in patients with advanced cancer. J Oncol Pract. 2017;13:e821–e830. doi: 10.1200/JOP.2016.020404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghidini M., Indini A., Rijavec E., Bareggi C., Cattaneo M., Tomasello G., et al. The appropriateness of invasive ventilation in COVID-19 positive cancer patients: proposal of a new prognostic score. Viruses. 2021;13 doi: 10.3390/v13030508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Q., Wu J., Nie J., Zhang L., Hao H., Liu S., et al. The impact of mutations in SARS-CoV-2 Spike on viral infectivity and antigenicity. Cell. 2020;182:1284–12894 e9. doi: 10.1016/j.cell.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madhi S.A., Baillie V., Cutland C.L., Voysey M., Koen A.L., Fairlie L., et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 vaccine against the B.1.351 variant. N Engl J Med. 2021;384:1885–1898. doi: 10.1056/NEJMoa2102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.