Abstract
The skin is the first line of defense of our body, and it is composed of the epidermis and dermis with diverse immune cells. Various in vitro models have been investigated to recapitulate the immunological functions of the skin and to model inflammatory skin diseases. The simplest model is a two-dimensional (2D) co-culture system, which helps understand the direct and indirect cell-to-cell interactions between immune and structural cells; however, it has limitations when observing three-dimensional (3D) interactions or reproducing skin barriers. Conversely, 3D skin constructs can mimic the human skin characteristics in terms of epidermal and dermal structures, barrier functions, cell migration, and cell-to-cell interaction in the 3D space. Recently, as the importance of neuro-immune-cutaneous interactions in the inflammatory response is emerging, 3D skin constructs containing both immune cells and neurons are being developed. A microfluidic culture device called “skin-on-a-chip,” which simulates the structures and functions of the human skin with perfusion, was also developed to mimic immune cell migration through the vascular system. This review summarizes the in vitro skin models with immune components, focusing on two highly prevalent chronic inflammatory skin diseases: atopic dermatitis and psoriasis. The development of these models will be valuable in studying the pathophysiology of skin diseases and evaluating the efficacy and toxicity of new drugs.
Keywords: Atopic dermatitis, immune system, in vitro techniques, lab-on-a-chip devices, psoriasis, skin equivalent
INTRODUCTION
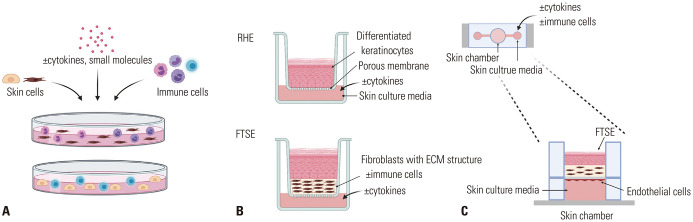
The skin is an essential barrier between the environment and the body to sustain life. It functions as a physical and immunological barrier.1 Acknowledging the importance of skin immunity, in vitro models, with structural and immune components, have been developed in the form of two-dimensional (2D) co-culture systems, three-dimensional (3D) skin constructs, and“skin-on-a-chip” with dynamic flow systems. The simplest model is a 2D co-culture system; it uses structural and immune cells, such as keratinocytes, fibroblasts, lymphocytes, eosinophils, basophils, and mast cells (Fig. 1A).2 These 2D co-culture systems are inexpensive and straightforward to maintain; however, they cannot mimic skin structures and do not represent cell-to-cell or cell-to-extracellular matrix (ECM) interactions in the 3D space.3 Additionally, the absence of stratified epidermis in 2D culture systems limits the testing of skin barrier functions. Ex vivo skin biopsies that display fundamental skin components are available; however, due to skin donor limitations and variability issues,4 there are restrictions in their use in laboratories. In vitro 3D culture systems, including immune components, have attracted substantial attention from investigators to recapitulate physiological skin properties, including its barrier function, 3D structure, and immune reactions, in one system (Fig. 1B).5,6 Recently, a microfluidic culture device called “skin-on-a-chip” was developed to simulate the vascular structures and perfusion of the human skin (Fig. 1C).
Fig. 1. Overview of in vitro models for mimicking cutaneous immune responses. (A) Two-dimensional co-culture systems: skin cells, immune cells, cytokines, and small molecules can be mixed and cultured together in the same culture medium. (B) Three-dimensional skin equivalents: RHE is a model of a fully differentiated epidermis without a dermal compartment; FTSE is a bi-layered skin model (epidermis and dermis). Dermis-like structures can be made from collagen gel mixed with fibroblasts, de-epidermized dermis, or self-assembled dermal sheets. Disease-associated cytokines can be added to the culture medium, and immune cells can be added inside or underneath the ECM structures. (C) Skin-on-a-chip: a microfluidic culture device that is composed of the skin and a perfusion system. Cytokines and/or immune cells can be added to the chamber of the device to reproduce inflammatory responses. RHE, reconstructed human epidermis; FTSE, full-thickness skin equivalent; ECM, extracellular matrix.
In this study, 2D and 3D culture systems and “skin-on-a-chip” that model inflammatory skin diseases were reviewed, focusing on atopic dermatitis (AD) and psoriasis, two highly prevalent chronic inflammatory skin diseases (Table 1). The availability of these new in vitro models with immune components is valuable in understanding the pathophysiology of skin diseases and for toxicity testing of cosmetic ingredients, biomaterials, and medications.
Table 1. Summary of In Vitro Culture Systems Modeling Inflammatory Skin Diseases.
| Atopic dermatitis | ||||||
|---|---|---|---|---|---|---|
| 2D models | Co-culture | Immune cells | FB+eosinophils or basophils+NOD2/TLR2 ligand | 65 | ||
| KC+eosinophil+IL-31 | 66 | |||||
| KC+T cell+Staphylococcal enterotoxin B | 67 | |||||
| KC+T cell+monocytes | ||||||
| 3D models | RHE | Cytokines | KC+IL-4, IL-14, IL-22, and TNF-a | 69 | ||
| KC+IL-4, IL-13, and IL-25 | 70 | |||||
| Gene-silenced KCs | FLG, FLG-2 gene silencing by shRNA interference | 73, 74 | ||||
| FTSE | Co-culture | Collagen model | KC+atopic FB | 76 | ||
| Cytokines | Collagen model | FTSE (KC+FB)+IL-4, IL-13 | 79 | |||
| FTSE (KC or FLG-deficient KC+FB)+IL-4, IL-13 | 80 | |||||
| DED model | KC seeded DED+IL-4, IL-13 | 78 | ||||
| Immune cells | Collagen model | HaCaT based FTSE-like model+T cells | 81 | |||
| FLG-deficient FTSE (KC+FB)+CD4+ T cells | 82 | |||||
| DED model | KC seeded DED+polarized Th2 cells | 44 | ||||
| Psoriasis | ||||||
2D, two-dimensional; 3D, three-dimensional; RHE, reconstructed human epidermis; FTSE, full-thickness skin equivalent; IL, interleukin; DED, de-epidermized dermis; FB, fibroblasts; iPSC, induced pluripotent stem cells; HUVEC, human umbilical vein endothelial cells; KC, keratinocytes; LPS, lipopolysaccharide; SDS, sodium dodecyl sulfate
GENERAL STRUCTURE OF THE SKIN-EMPHASIS ON IMMUNE CELLS
The skin is composed of the epidermis and dermis. As one of the primary immune barriers, each layer of the skin possesses diverse cells that regulate the immune response.
Epidermis
About 90% of the epidermis comprises keratinocytes that undergo differentiation and keratinization and form a stratified epidermis. One of the main functions of keratinocytes is to act as a physical barrier against environmental damages, such as ultraviolet (UV) radiation, heat, pathogenic bacteria, viruses, and fungi.7 Keratinocytes also participate in initiating the innate immune system. Keratinocytes secrete pro-inflammatory cytokines in response to environmental stimuli, such as UV, allergens, and microbes.8 They can also process and present peptide antigens to T cells, resulting in inflammatory cytokine and chemokine secretion.9
Langerhans cells are a subset of antigen-presenting cells commonly found between keratinocytes in the stratum spinosum.10 During skin infections or allergen entrance, they capture, process, and present antigens and migrate to lymph nodes to interact with naïve T cells.11
A normal skin accommodates more than twice the number of T cells found in the blood, and some can be found in the stratum basale and stratum spinosum.1,12 CD8+ resident memory T cells (TRM) in the epidermis are non-circulating memory T cells that persist at the sites of previous infection and inflammation.13 These CD8+TRM cells first encounter infections at the body surface, providing adequate protection against de novo infection by accelerating pathogen clearance.9,14
Dermis
In the dermis, various immune cells reside among structural cells; these include dendritic cells (DCs), macrophages, T cells, innate lymphoid cells (ILCs), and mast cells. Fibroblasts produce a structural framework by synthesizing ECM; they also initiate and regulate inflammation by producing pro-inflammatory cytokines and chemokines.15
DCs are efficient antigen-presenting cells abundant in peripheral tissues. Skin DCs take up antigens, generate major histocompatibility complex peptide complexes, and migrate to draining lymph nodes to interact with naïve T cells to induce immune responses.16
Most skin T cells are located in the dermis, especially near blood vessels or skin appendages.12 The primary types of CD4+ T helper cells observed in the skin during inflammatory diseases are Th1, Th2, Th17 cells, and regulatory T cells (Tregs). Th1 cells release IFN-γ and interleukin (IL)-2, and are associated with phagocytic activities and autoimmune diseases.17,18,19 Th2 cells, which produce IL-4, IL-5, and IL-13, are important against extracellular pathogens and contribute to the development of allergic diseases.20 Th17 cells, which produce IL-17A, IL-17F, and IL-22, are the first line of defense against bacterial and fungal infections,21,22,23 and they are involved in the pathogenesis of various inflammatory skin diseases, such as AD, psoriasis, and autoimmune skin diseases.24 Finally, Tresgs suppress skin inflammation by secreting inhibitory cytokines, initiating the cytolysis of target cells, inducing the metabolic disruption of effector T cells, and altering DC function.25,26
ILCs are tissue-resident innate immune cells that express no rearranged antigen receptors. Unlike T cells that undergo clonal expansion for several days to develop antigen-specific memory, ILCs immediately respond to pathogenic stimuli and contribute to early immune responses.27,28
Macrophages are white blood cells that initiate the innate immune response by phagocytosing pathogens. Skin macrophages are sessile in the dermis; however, they can migrate to lymph nodes under conditions like inflammation.29,30
Mast cells are granule-rich migrant cells that contain and release materials such as histamine and heparin.31 As the first line surveillance immune cells, mast cells release pro-inflammatory mediators rapidly upon activation.32 Neutrophils, eosinophils, and natural killer cells can also be found in the dermis and affect the induction and progression of inflammatory skin diseases.
2D CO-CULTURE SYSTEMS TO UNDERSTAND CELL-TO-CELL INTERACTIONS
2D co-culture systems are useful to evaluate each cell type’s specific role and understand direct and indirect cell-to-cell interactions. A study that used a co-culture system of primary keratinocytes and T cell subsets revealed that a co-culture of keratinocytes with CD4+ and CD8+ T cells stimulated keratinocytes to produce chemokines, CCL2, CCL20, and CXCL10, and skewed T cells to IL-17+CCR6+RORγt+ Th17/Tc17 cells.33 In another study, a co-culture of CD8+ T cells with keratinocytes increased granzyme B expression in keratinocytes and IL-18 concentration in culture supernatants.34 These keratinocyte-T cell co-culture systems suggested that keratinocyte-T cell communication can recruit immune cells into the skin by secreting chemokines and regulate T cell differentiation. When DCs were co-cultured with keratinocytes and treated with weak and strong allergens, DC maturation varied with the type and potency of allergens.35
3D SKIN CONSTRUCTS MIMICKING SKIN IMMUNE RESPONSES
Two models adequately mimic the characteristics of the human epidermis: reconstructed human epidermis (RHE) and full-thickness human skin equivalents (FTSE). Both models reproduce the in vivo characteristics of the human skin in terms of epidermal morphology, differentiation, and barrier function.6,36,37 RHE comprises the epidermis alone, since only keratinocytes are grown at the air-liquid interface. FTSE has both the epidermis and dermis. In FTSE models, keratinocytes are seeded on top of dermal-like structures, such as collagen type I gel mixed with fibroblasts, de-epidermized dermis (DED), or self-assembled dermal sheets. When keratinocytes are fully grown to make confluent monolayers, keratinocytes with dermal structures are exposed to air for epidermal stratification and keratinization.38,39,40
To mimic immune responses in the skin, the simplest method is to add relevant cytokines to 3D skin equivalents. Otherwise, immune cells can be co-cultured with 3D skin for more physiological models. For example, Langerhans cells have been incorporated into FTSE models,41,42,43 where they express CD1a and Lag and contain Birbeck granules as in epidermal Langerhans cells. They can also migrate in response to stimuli, such as UV radiation or allergen exposure.42,43 Other immune cells of the dermis, such as dermal DCs,42 T cells,44 and macrophages45 can also be incorporated into FTSE models. Recently, disease-relevant immune cells, such as polarized Th1 and Th17 cells, have been used to model inflammatory skin diseases.44,46 These models reproduced some disease phenotypes and demonstrated their potential for drug screening and elucidating pathogeneses.
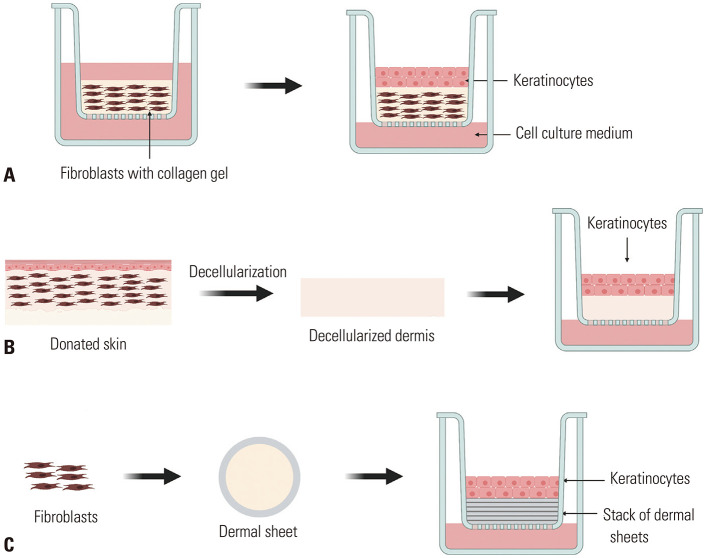
FTSE models can be divided into three groups depending on how the dermal part of the skin is reconstructed: 1) collagen-based model, 2) dead DED model, and 3) self-assembled skin substitute (SASS) model (Fig. 2).
Fig. 2. Three FTSE models depending on how the dermal part of the skin is reconstructed. (A) Collagen-based model: collagen matrix and fibroblasts are co-cultured; when a dermal matrix is formed, keratinocytes are seeded on the top. (B) Dead de-epidermized dermis model: donated human skin undergoes decellularization; on top of the decellularized dermis, keratinocytes are seeded on the top. (C) Self-assembled skin substitute model: dermal fibroblasts construct dermal sheets; keratinocytes are seeded on top of the stack of dermal sheets. FTSE, full-thickness human skin equivalents.
1) Collagen-based model (Fig. 2A) is made of epidermal keratinocytes grown on a dermal matrix comprising collagen and dermal fibroblasts.47,48 The collagen gel provides scaffolding, delivers nutrients, and makes cell-to-cell and cell-to-matrix interaction possible.49 The advantages of the collagen-based model include excellent biocompatibility and cell adhesion; however, it has a weak mechanical strength and limited lifespan.50
2) DED model (Fig. 2B) is made from donated human skin. Samples are cut into squares and decellularized to be used as a dermal substitute that provides ECM scaffolds for keratinocytes to attach and proliferate.51,52
3) SASS model is made of keratinocytes and fibroblasts without exogenous ECM materials.53 Dermal fibroblasts are grown to confluence while secreting their own ECM to build a dermal sheet; then, keratinocytes are seeded on top of stacked dermal sheets to undergo differentiation and cornification.54 Owing to its advantage of using only autologous cells and its similarities to the native human skin, various studies on the clinical use of SASS are in progress. There is an ongoing clinical trial that uses SASS as a permanent skin graft for full-thickness burn wounds (ClinicalTrials.gov NCT02350205).
IN VITRO MODELS OF AD
Pathophysiology of AD
AD is a chronic relapsing skin disease characterized by skin barrier defects, Th2-skewed inflammation, and chronic pruritus.55 In AD skin lesions, both lesional and non-lesional skin exhibit upregulated Th2 inflammatory genes.56 Th2-skewed immune responses induce isotype switches to immunoglobulin E in B cells, impair the skin barrier function, and increase the susceptibility to bacterial and viral infections.57,58 Lymphocytes, mast cells, and eosinophils are also commonly found in AD skin, while basophils and neutrophils are rare.59
Increased pH, transepidermal water loss, and changes in epidermal lipid profile demonstrate epidermal barrier dysfunction, an essential characteristic of AD pathogenesis. Patients with AD have significantly lower filaggrin (FLG) levels in the skin, contributing to epidermal barrier dysfunction.60 Not every patient with AD carries FLG genotype mutations; however, FLG deficiency can be acquired secondary to Th2 inflammation.61
Chronic pruritus, which negatively affects the quality of life of patients with AD, is associated with the crosstalk between the nervous system and cutaneous immune system.62 IL-4, IL-13, and IL-31, which are released by Th2 cells, and thymic stromal lymphopoietin (TSLP), which is released by keratinocytes, directly activate pruritoceptive neurons called histamine-independent C fibers (non-histaminergic sensory nerve fibers).63 As the importance of neuro-immune-cutaneous interaction in itch and inflammatory responses of the skin has been highlighted, studies revealing new interactions between these components are being actively conducted.64
Since various factors and complicated mechanisms are involved in AD, replicating all aspects of AD pathology may be impossible. Several in vitro models have been developed to mimic at least one aspect of AD immune reaction and can be useful to study AD if researchers choose an appropriate model tailored to the aims of their research.
2D in vitro models of AD
Co-culture of immune cells with structural cells mimicking the microenvironment of AD
A study that used a co-culture model of human fibroblasts with eosinophils and basophils found that Staphylococcus aureus-associated NOD and TLR2 ligands increase cytokine and chemokine levels with different communication mechanisms, depending on the cell type. A direct intercellular contact was needed for the crosstalk between fibroblasts and basophils; however, soluble mediators could mediate the interactions between eosinophils and fibroblasts.65 Likewise, a co-culture of keratinocytes with eosinophils under IL-31 stimulation required a direct interaction between eosinophils and keratinocytes to secrete higher levels of pro-inflammatory cytokines (IL-1 β and IL-6) and AD-related chemokines (CXCL1, CXCL8, CCL2, and CCL18) mediated by the PI3K-Akt, ERK, p38 MAPK, and NF-κB pathways.66
When keratinocytes and T cells were co-cultured in the presence of staphylococcal enterotoxin B, there was a higher production of IFN-γ than when T cells were cultured alone. In the presence of activated T cells, keratinocyte-derived IL-1β enhanced the IFN-γ production. There was also a substantial enhancement of IFN-γ and Th1 cytokines when monocytes were added to a keratinocyte-T cell co-culture system as antigen-presenting cells.67
3D in vitro models of AD
RHE models of AD
RHE models can mimic AD conditions at the epidermal level in two ways: 1) by treating cocktails of cytokines that are over-expressed in AD skin or 2) by silencing the expressions of AD-related genes, such as FLG.
The RHE model cultivated in a medium supplemented with Th2 cytokines (IL-4, IL-13, and IL-31) and TNF-α showed some features of AD lesions, including spongiosis, augmented TSLP secretion, modified lipid organization, and alterations in epidermal barrier function.68 Similarly, the combination of IL-4, IL-13, IL-22, and TNF-α induced the overexpression of S100A7 and IL-13RA2 and decreased the expression of FLG,69 and the treatment of IL-4, IL-13, and IL-25 resulted in intercellular space widening and hypogranulosis.70 RHE models can be useful in evaluating the alterations in tight junctions caused by cytokine stimuli. The addition of Th2 cytokine cocktail (IL-4, IL-13, and IL-31) or IL-17 alone decreased tight junction function.71,72
Using a lentivirus-mediated shRNA interference in the RHE model, one study showed that FLG depletion caused hypogranulosis, impaired keratinocyte differentiation, altered corneocyte intercellular matrix, and reduction of natural moisturizing factor components; however, there was no difference in the skin surface pH.73 In another study, by silencing FLG2 in the RHE model, authors demonstrated that FLG-2 is essential for the proper cornification of a functional stratum corneum.74 However, the RHE model generated from FLG null keratinocytes of patients with ichthyosis vulgaris showed no altered skin barrier functions.75
FTSE models of AD
The FTSE models for AD can be built with keratinocytes and fibroblasts derived from patients with AD. Berroth, et al.76 reported the crosstalk between fibroblasts and keratinocytes in AD development by demonstrating that AD fibroblasts co-cultured with normal keratinocytes in FTSE can induce AD-like features, such as increased epidermal thickness and downregulated FLG gene expression. Healthy fibroblasts restored the morphology of the epidermis in FTSE cultured with AD keratinocytes.76
Like the RHE model, the FTSE model can be treated with Th2 cytokines to mimic the Th2-skewed inflammation in AD.77 The treatment of IL-4 and IL-13 induced the morphological and molecular characteristics of AD, including spongiosis, apoptosis, and increased expression of CA2 and NELL2 genes in the epidermis.78 These cytokine-induced FTSE models can be utilized to evaluate the possible effects of small molecules in alleviating AD inflammation. For example, Lee, et al.79 used dipotassium glycyrrhizinate in IL-4/IL-13-induced AD skin equivalents and showed that dipotassium glycyrrhizinate successfully suppressed cytokine release and ameliorated epidermal phenotype and gene expression patterns.
Another study evaluated the effects of Th2 cytokines in both normal and FLG-deficient skin equivalents. The study showed that FLG deficiency alone causes epidermal thickening, spongiosis, and parakeratosis, and these changes are augmented by Th2 cytokines. Additionally, incubation with Th2 cytokines elevated the skin surface pH and increased TSLP to a greater extent in FLG-deficient skin than in control skin.80
As a prototype of the FTSE model, including immune cells, an immortalized human keratinocyte (HaCaT)-based epidermis co-cultured with confluent fibroblasts and activated T cells in a transwell system was used to study the dynamic interactions between the skin and immune cells.81 In this study, the integration of activated T cells resulted in keratinocyte apoptosis, reduced E-cadherin, increased ICAM-1 and NT-4, and the disruption of barrier function. Interestingly, HaCaT cells produced IL-1α and TARC, whereas fibroblasts predominantly produced RANTES, eotaxin, IL-6, and IL-8. Later, the FTSE incorporated with T cells in the dermis allowed researchers to observe T cell migration in skin equivalents. For example, Wallmeyer, et al.82 showed that FLG-deficient FTSE induced CD4+ T cell migration in the skin and a T cell subtype shift from Th1/Th17 to Th2/Th22.
Innervated 3D skin models of AD
Since the importance of neuroimmune crosstalk for pruritus and cutaneous inflammation is increasingly emphasized, studies on in vitro skin models, including the nervous system, are ongoing. When human skin explants from healthy donors and sensory neurons extracted from the dorsal root ganglia (DRG) of rats were co-cultured, nerve fibers sprouted in both the epidermis and dermis.83 Later, an innervated FTSE model was built using a co-culture of porcine DRG with FTSE.84 The addition of neurons to FTSE induced proliferation of keratinocytes and increased epidermal thickness. Additionally, keratinocytes of the innervated skin model showed elevated gene expression of calcitonin gene-related peptide (CGRP) receptor components, clr and rmp1. When the atopic skin model composed of atopic keratinocytes and fibroblasts was incorporated with neurons, it revealed thicker epidermis, increased neurite density, and elevated CGRP levels compared to those of innervated healthy models.84
Recently, a more complex 3D skin model that includes neural[induced pluripotent stem cells (iPSCs)-induced neural stem cells], immune/endocrine (lipoaspirate), and cutaneous (keratinocytes and fibroblasts) components has been proposed. 85 Lipoaspirate-seeded hypodermis allowed this model to contain relevant hypodermal cell types, such as adipocytes, pre-adipocytes, endothelial cells, smooth muscle cells, pericytes, and inherent immune cells.86 This complex 3D skin model with neurons and hypodermis demonstrated the upregulation of genes for skin development, neuronal system processes, inflammatory responses, and adipogenesis pathways87 and a higher secretion of pro-inflammatory cytokines and higher complexity of identified proteins. Although the functionality of this model, including its epidermal barrier function, neural function, and immunologic response to stimuli, was not validated yet, it is meaningful that it included all neuro-immunecutaneous components. With further validation and optimization, this innervated FTSE model with immune components will be a useful tool to understand the interactions between the cutaneous, immune, and neural systems in the future.
IN VITRO MODELS OF PSORIASIS
Pathophysiology of psoriasis
Psoriasis is a chronic inflammatory skin disease characterized by erythematous, itchy, and scaly plaques with sharply demarcated borders.88 Histologically, the granular layer of the epidermis is significantly reduced or absent, the blood vessels are dilated and hyperplastic, and the mitotic rates of basal keratinocytes are excessive, leading to parakeratosis, acanthosis, and papillomatosis.89
Pro-inflammatory cytokines, secreted by activated T cells, can induce keratinocyte proliferation, which is a hallmark of psoriasis.90 As a positive feedback loop, psoriatic keratinocytes can recruit and activate immune cells, such as DCs, neutrophils, and T cells.91,92 The pathogenesis of psoriasis include the dysfunction of various T cell subsets, including Th1, Th2, Th17, Th22, and Tregs and an aberrant secretion of cytokines, such as IFN-γ, TNF-α, IL-17, IL-22, and IL-23.89 Among various types of T cells, IL-17-releasing Th17 cells contribute to the development of psoriasis, and the success of drugs that block the IL-23/Th17 axis underscores the importance of Th17 in the pathogenesis of psoriasis.93
2D models of psoriasis
Co-culture of immune cells with keratinocytes
Co-culture models of healthy T cells and psoriatic keratinocytes versus normal keratinocytes showed that T cells cooperate with psoriatic keratinocytes in an excessive production and a feedback loop of pro-inflammatory cytokines, TNF-α, IL-6, IL-8, GM-CSF, IP-10, and MCP-1.94 In another study, a co-culture of keratinocytes and activated CD4+ T cells resulted in a significant increase in IL-17 production when T cells were directly in contact with keratinocytes rather than indirectly via keratinocyte supernatants.95
3D skin models of psoriasis
RHE models of psoriasis
An RHE model treated with IL-20 subfamily cytokines (IL-19, IL-20, IL-22, and IL-24) showed epidermal acanthosis, S100A7 and K16 upregulation, and persistent STAT3 activation.96 Similarly, when a mixture of cytokines IL-17, IL-22, and TNF-α was treated in the RHE model, hypogranulosis, parakeratosis, and altered expression of psoriatic genes were reproduced in the epidermis.69
FTSE models of psoriasis
FTSE models of psoriasis can be made from psoriatic fibroblasts and keratinocytes. Psoriatic fibroblasts in FTSE models increased the proliferation of keratinocytes, epidermal thickness, and IL-8 expression compared to normal fibroblasts.97,98 When both psoriatic fibroblasts and keratinocytes were used in FTSE, psoriatic FTSE demonstrated higher proliferation rates and increased pro-inflammatory cytokine expression compared to normal skin equivalents.99 Likewise, when psoriatic keratinocytes and fibroblasts were grown using a self-assembly method, the skin equivalents revealed thickened epidermis and altered barrier proteins.100
Like the RHE models, the psoriatic FTSE model can be achieved by adding relevant cytokines to a culture medium. When IL-22 or various combinations of IL-1α, IL-6, and TNF-α were added to an FTSE with normal keratinocytes and fibroblasts, the epidermis demonstrated psoriatic phenotypes, such as hypogranulation, acanthosis, and upregulation of pSTAT3 and psoriasin.101,102 In another study, an FTSE treated with IL-17A, IL-22, and TNF-α displayed hyperkeratosis, parakeratosis, acanthosis, and IL20 and IL1B upregulation.103 The addition of IL-1α, IL-6, IL-22, and TNF-α or a combination thereof to DED-based skin equivalents also caused increased expressions of hBD-2, SKALP/elafin, IL-8, and TNF-α.52,104 More recently, a psoriatic FTSE model was built by the SASS method using psoriatic fibroblasts and keratinocytes and was treated with a cocktail of IL-1α, IL-6, IL-17A, and TNF-α. A psoriatic FTSE treated with a cytokine cocktail exhibited disorganized basal layer, thicker epidermis, and increased expression of S100A12, IL8, DEFB4A, and KYNU compared to the control FTSE without cytokine addition.105
When skin equivalents were treated with IL-17 and IL-22 separately, IL-17 induced a higher gene expression of CCL20, DEFB4, and CXCL8, while IL-22 altered the epidermal morphology, resulting in acanthosis, parakeratosis, and downward epidermal projections.106 Another FTSE model treated with IL-17 showed increases in IL-17-specific transcription factor C/EBPβ and psoriasis-associated inflammatory genes IL23A, STAT3, DEFB4, S100A7A, S100A12, SERBINB3, and SER-PINB4.107
A psoriatic FTSE incorporated with T cells was first developed by van den Bogaard, et al.44 by populating activated CD4+ T cells or Th1/Th17 cells under DED-based skin equivalents. The activated T cells or polarized Th1/Th17-populated skin equivalents exhibited migration of T cells in the dermis and increased psoriatic-associated markers and cytokine/chemokine gene expression. These T cell-incorporated models were further validated for drug response using cyclosporine, all-trans-retinoic acid (ATRA), and infliximab (TNF-α antibody). T cell-populated psoriatic skin models were later built in a collagen-based model, demonstrating the migration of Ki67+ T cells toward the epidermis.46 In addition to Th1/Th17-induced psoriatic phenotypes, this study added information regarding the role of epidermis on T cell migration by comparing skin equivalents with and without epidermis. Moreover, the potential use of patient-derived immune cell-populated FTSE is proposed for the testing of individualized drug responses.
SKIN-ON-A-CHIP
The vascular system is essential in mediating inflammatory responses, as it is a route for immune cells to travel between lymphoid organs and peripheral tissues. Organ-on-chips are microfluidic culture devices that mimic physiological circulations at the organ level. To recapitulate the structures and functions of the human skin with vasculature and perfusion, “skin-on-a chip” was developed.108
Cytokine-induced immune response in the microfluidic device
Wufuer, et al.109 developed a microfluidic device comprising three layers of HaCaT cells for the epidermis, fibroblasts for the dermis, and human umbilical vein endothelial cells (HUVECs) for endothelium. Each layer was divided by porous membranes to allow intercellular communications between the layers. To validate the device, the fibroblasts in the dermal layer were treated with TNF-α to induce inflammation. After 24 h of incubation, the expression of pro-inflammatory cytokines, such as IL-1β, IL-6, and IL-8, was elevated in the HUVECs and the medium. Treatment with dexamethasone, an anti-inflammatory drug, caused a significant decrease in TNF-α-induced cytokine secretion, and it also inhibited fluid transfer across vascular layers.
Immune cell-incorporated skin-on-a-chip models
Ramadan and Ting110 constructed a microfluidic-based keratinocyte and DC co-culture system. An epidermal barrier was constructed using HaCaT cells, and human leukemic monocyte lymphoma cells (U937) were used as a model of human DCs. Lipopolysaccharides (LPS) induced inflammatory reactions, and the expression of pro-inflammatory cytokines was evaluated for immune responses. Using syringe pumps, negative pressures were applied, resulting in a continuous perfusion of culture media through the cell culture chambers. When LPS was applied, U937 monocultures showed a more significant elevation of IL-6 and IL-1β compared to the HaCaT/U937 co-culture or HaCaT monoculture, suggesting the regulatory role of the epidermal layer to chemical and biological stimuli.
Ren, et al.111 described a microfluidic-based skin-on-a-chip model to illustrate the transendothelial and transepithelial migration of T cells from the bloodstream to skin inflammation sites. The device was a 3-inch silicon wafer consisting of a HaCaT cell layer, a type 1 collagen gel with porous fiber structure, and HUVEC layer as representative components for epithelium, ECM, and endothelium, respectively. When activated T cells were added to this system, the T cells tended to migrate toward the higher gradient of CXCL12 and CCL20. In contrast, CCL20 locked dimer (CCL20LD) significantly inhibited T cell transmigration. When TNF-α was injected into the well of the HaCaT cell layer, activated T cells transmigrated through the HUVEC layer across the collagen gel toward the HaCaT layer, demonstrating the potential use of this model for the study of T cell transmigration in response to inflammatory mediators or drugs.
Kwak, et al.112 developed a skin chip with fully-stratified skin and matured endothelial layers in a microfluidic chip device. The device has a center channel containing bi-layered skin (epidermis and dermis) and endothelial layers separated by a porous membrane. It allowed the skin to be fully differentiated in an air-liquid interface. The skin that grew in the device demonstrated a stratified epidermis histologically. After the maturation of the skin and endothelial cells, HL-60 cells (neutrophillike cells) were added to recapitulate the leukocyte migration into the skin. The production of the pro-inflammatory cytokine IL-6 was elevated following treatment with sodium dodecyl sulfate, and it was reduced after adding dexamethasone to the vascular endothelium of the skin chip. Under UV irradiation, leukocytes migrated from the fluidic channels to the skin across the vascular layer. This model successfully mimicked the immune response of the human skin in terms of cytokine production and recruitment of immune cells to inflammatory sites.
DRUG SCREENING POTENTIAL OF IMMUNOCOMPETENT 3D SKIN MODELS
The 3D skin models with immune components are useful in testing drugs for inflammatory skin diseases. When tofacitinib, a JAK1/3 inhibitor, was concurrently treated before IL-4 and IL-13, the epidermal morphology and FLG expression were restored in an AD FTSE model.103 Likewise, tofacitinib also suppressed the IL20 and IL1B expression in a psoriatic FTSE model induced by IL-17A, IL-22, and TNF-α.103 In other studies, calcipotriol treatment restored the disrupted epidermal morphology in an IL-22-induced psoriatic FTSE model,102 and ATRA significantly downregulated the SKALP/elafin and hBD-2 in FTSE treated with psoriatic cytokine cocktails (IL-1α, TNF-α, and IL-6).
However, when T cell-directed anti-inflammatory drugs, such as cyclosporine A, tacrolimus, and dexamethasone, were treated on the Th2 cytokine-induced AD FTSE model, none of the drugs inhibited the changes in epidermal morphology or gene expression patterns.78 To observe the drug response to these medications in vitro, T cell-incorporated models can be a useful tool. When ATRA and cyclosporine were tested in a T cell-incorporated DED model, both drugs downregulated the psoriasis-associated genes DEFB4, PI3, and S100A8 and reduced the secretion of IL-17A and IL-22; however, only cyclosporin suppressed the secreted IFN-γ levels and downregulated the epidermal expression of CXCL10 and CCL2. Likewise, infliximab (TNF-α antibody) significantly reduced the expression of TNF, DEFB4, and CXCL8 genes.44 Additionally, the T cell-incorporated FTSE model had more advantage in observing drug responses in T cell migration into the skin. Hydrocortisone treatment in polarized Th1/Th17 cell-incorporated 3D skin successfully inhibited the T cell migration into the skin and reversed the psoriatic epidermal phenotypes and cytokine secretions.46 If researchers want to observe immune cell migration across the vascular system, a microfluidic system will be a suitable model to use. Since each model has a different composition of cell types and functions, it is important to select an adequate immunocompetent 3D skin model depending on the research purpose and target mechanism of the drug being tested.
LIMITATIONS AND FUTURE PERSPECTIVES
Donor mismatch
3D skin equivalents are powerful tools for investigating functional barriers, epidermal morphology, and epidermal-dermal interactions. Additionally, studies to develop a more advanced 3D skin model, including hair,113 nerves,85 pigments,114 blood vessels,115 subcutaneous fat layer,116 and immune cells, are ongoing. When making these 3D skins, most studies have not yet matched the donor of each cell type, but as the composition of the 3D skin becomes more complex, the importance of donor match might emerge. In the case of 3D constructs with immune cells, such as T cells, it may be important to use donor-matched cells to avoid unexpected inflammatory responses. For experiments where donor match is necessary or in cases of difficult primary cell cultures, utilizing iPSC can be an alternative source of each cell type.117,118,119 Additionally, since disease phenotypes and drug responses are different in each patient, using patient-derived iPSC technology can have a further advantage of establishing personalized medicine in the future.
Reproducibility and efficiency
Building a 3D skin model is time-consuming and labor-intensive, as it uses various types of primary cells; therefore, depending on the proficiency of researchers or sources of primary cells (aged or young, etc.), the quality of the 3D skin might vary. As the 3D skin models become more complex with various cell types, an increase in technical and biological variances is unavoidable. The recent development of bioprinting technologies that allow small-scale and constant quality of engineered skin models will reduce these technical variances and enhance the reproducibility and efficiency of 3D skin models. To overcome biological variances, it is recommended to utilize multiple different donors. The awareness of these technical and biological variances and efforts to reduce them will significantly improve the reproducibility of the experiments.
CONCLUSION
Various in vitro models that mimic the in vivo structure and environment of the human skin have been developed and used to understand skin physiology, uncover disease pathogenesis, and test drug toxicities. The inclusion of immune components in the 3D skin model is valuable in understanding the pathogenesis of inflammatory skin diseases and in evaluating the effects of drugs. If researchers understand the usefulness and limitations of immunocompetent 3D skin models and choose an appropriate model according to the purpose of the study, it will be a valuable tool that can overcome the weakness of 2D cell culture.
ACKNOWLEDGEMENTS
This work has supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2020R1C1C1005293).
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Dong Hyun Kim and Jung U Shin.
- Data curation: Sujin Moon and Dong Hyun Kim.
- Formal analysis: Sujin Moon and Jung U Shin.
- Funding acquisition: Jung U Shin.
- Investigation: Sujin Moon and Dong Hyun Kim.
- Methodology: Sujin Moon and Jung U Shin.
- Project administration: Jung U Shin.
- Resources: Sujin Moon.
- Software: Sujin Moon.
- Supervision: Dong Hyun Kim and Jung U Shin.
- Validation: Sujin Moon and Dong Hyun Kim.
- Visualization: Sujin Moon and Jung U Shin.
- Writing—original draft: Sujin Moon and Jung U Shin.
- Writing—review & editing: Dong Hyun Kim and Jung U Shin.
- Approval of final manuscript: all authors.
References
- 1.Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ. Skin immune sentinels in health and disease. Nat Rev Immunol. 2009;9:679–691. doi: 10.1038/nri2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Vuyst E, Salmon M, Evrard C, Lambert de Rouvroit C, Poumay Y. Atopic dermatitis studies through in vitro models. Front Med (Lausanne) 2017;4:119. doi: 10.3389/fmed.2017.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kapałczyńska M, Kolenda T, Przybyła W, Zajączkowska M, Teresiak A, Filas V, et al. 2D and 3D cell cultures - a comparison of different types of cancer cell cultures. Arch Med Sci. 2018;14:910–919. doi: 10.5114/aoms.2016.63743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reus AA, Usta M, Krul CA. The use of ex vivo human skin tissue for genotoxicity testing. Toxicol Appl Pharmacol. 2012;261:154–163. doi: 10.1016/j.taap.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 5.Ackermann K, Borgia SL, Korting HC, Mewes KR, Schäfer-Korting M. The Phenion Full-Thickness Skin model for percutaneous absorption testing. Skin Pharmacol Physiol. 2010;23:105–112. doi: 10.1159/000265681. [DOI] [PubMed] [Google Scholar]
- 6.Frankart A, Malaisse J, De Vuyst E, Minner F, de Rouvroit CL, Poumay Y. Epidermal morphogenesis during progressive in vitro 3D reconstruction at the air-liquid interface. Exp Dermatol. 2012;21:871–875. doi: 10.1111/exd.12020. [DOI] [PubMed] [Google Scholar]
- 7.Murphy KM, Weaver C. Janeway's immunobiology. 9th ed. New York: Garland Science, Taylor & Francis Group; 2016. [Google Scholar]
- 8.Barker JN, Mitra RS, Griffiths CE, Dixit VM, Nickoloff BJ. Keratinocytes as initiators of inflammation. Lancet. 1991;337:211–214. doi: 10.1016/0140-6736(91)92168-2. [DOI] [PubMed] [Google Scholar]
- 9.Black AP, Ardern-Jones MR, Kasprowicz V, Bowness P, Jones L, Bailey AS, et al. Human keratinocyte induction of rapid effector function in antigen-specific memory CD4+ and CD8+ T cells. Eur J Immunol. 2007;37:1485–1493. doi: 10.1002/eji.200636915. [DOI] [PubMed] [Google Scholar]
- 10.Kabashima K, Honda T, Ginhoux F, Egawa G. The immunological anatomy of the skin. Nat Rev Immunol. 2019;19:19–30. doi: 10.1038/s41577-018-0084-5. [DOI] [PubMed] [Google Scholar]
- 11.Bennett CL, Ambler CA. Editorial: langerhans cells and how skin pathology reshapes the local immune environment. Front Immunol. 2019;10:139. doi: 10.3389/fimmu.2019.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark RA, Chong B, Mirchandani N, Brinster NK, Yamanaka K, Dowgiert RK, et al. The vast majority of CLA+ T cells are resident in normal skin. J Immunol. 2006;176:4431–4439. doi: 10.4049/jimmunol.176.7.4431. [DOI] [PubMed] [Google Scholar]
- 13.Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature. 2012;483:227–231. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mackay LK, Stock AT, Ma JZ, Jones CM, Kent SJ, Mueller SN, et al. Long-lived epithelial immunity by tissue-resident memory T (T[RM]) cells in the absence of persisting local antigen presentation. Proc Natl Acad Sci U S A. 2012;109:7037–7042. doi: 10.1073/pnas.1202288109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith RS, Smith TJ, Blieden TM, Phipps RP. Fibroblasts as sentinel cells. Synthesis of chemokines and regulation of inflammation. Am J Pathol. 1997;151:317–322. [PMC free article] [PubMed] [Google Scholar]
- 16.Stingl G, Bergstresser PR. Dendritic cells: a major story unfolds. Immunol Today. 1995;16:330–333. doi: 10.1016/0167-5699(95)80148-0. [DOI] [PubMed] [Google Scholar]
- 17.Skapenko A, Leipe J, Lipsky PE, Schulze-Koops H. The role of the T cell in autoimmune inflammation. Arthritis Res Ther. 2005;7 Suppl 2:S4–S14. doi: 10.1186/ar1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coias J, Marzuka A, Hosler GA, Chong BF. T-cell polarization differs in various stages of discoid lupus erythematosus skin. Br J Dermatol. 2020;182:1291–1293. doi: 10.1111/bjd.18704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jäger A, Dardalhon V, Sobel RA, Bettelli E, Kuchroo VK. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J Immunol. 2009;183:7169–7177. doi: 10.4049/jimmunol.0901906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396:345–360. doi: 10.1016/S0140-6736(20)31286-1. [DOI] [PubMed] [Google Scholar]
- 21.Saravia J, Chapman NM, Chi H. Helper T cell differentiation. Cell Mol Immunol. 2019;16:634–643. doi: 10.1038/s41423-019-0220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel DD, Kuchroo VK. Th17 cell pathway in human immunity: lessons from genetics and therapeutic interventions. Immunity. 2015;43:1040–1051. doi: 10.1016/j.immuni.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Park CO, Fu X, Jiang X, Pan Y, Teague JE, Collins N, et al. Staged development of long-lived T-cell receptor αβ TH17 resident memory T-cell population to Candida albicans after skin infection. J Allergy Clin Immunol. 2018;142:647–662. doi: 10.1016/j.jaci.2017.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cesare AD, Meglio PD, Nestle FO. A role for Th17 cells in the immunopathogenesis of atopic dermatitis? J Invest Dermatol. 2008;128:2569–2571. doi: 10.1038/jid.2008.283. [DOI] [PubMed] [Google Scholar]
- 25.Ali N, Rosenblum MD. Regulatory T cells in skin. Immunology. 2017;152:372–381. doi: 10.1111/imm.12791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sonnenberg GF, Hepworth MR. Functional interactions between innate lymphoid cells and adaptive immunity. Nat Rev Immunol. 2019;19:599–613. doi: 10.1038/s41577-019-0194-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells: 10 years on. Cell. 2018;174:1054–1066. doi: 10.1016/j.cell.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 29.Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 30.van Furth R, Nibbering PH, van Dissel JT, Diesselhoff-den Dulk MM. The characterization, origin, and kinetics of skin macrophages during inflammation. J Invest Dermatol. 1985;85:398–402. doi: 10.1111/1523-1747.ep12277056. [DOI] [PubMed] [Google Scholar]
- 31.Amin K. The role of mast cells in allergic inflammation. Respir Med. 2012;106:9–14. doi: 10.1016/j.rmed.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Pasparakis M, Haase I, Nestle FO. Mechanisms regulating skin immunity and inflammation. Nat Rev Immunol. 2014;14:289–301. doi: 10.1038/nri3646. [DOI] [PubMed] [Google Scholar]
- 33.Peters JH, Tjabringa GS, Fasse E, de Oliveira VL, Schalkwijk J, Koenen HJ, et al. Co-culture of healthy human keratinocytes and T-cells promotes keratinocyte chemokine production and RORγt-positive IL-17 producing T-cell populations. J Dermatol Sci. 2013;69:44–53. doi: 10.1016/j.jdermsci.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Akeda T, Yamanaka K, Tsuda K, Omoto Y, Gabazza EC, Mizutani H. CD8+ T cell granzyme B activates keratinocyte endogenous IL-18. Arch Dermatol Res. 2014;306:125–130. doi: 10.1007/s00403-013-1382-1. [DOI] [PubMed] [Google Scholar]
- 35.Galbiati V, Maddalon A, Iulini M, Marinovich M, Corsini E. Human keratinocytes and monocytes co-culture cell system: an important contribution for the study of moderate and weak sensitizers. Toxicol In Vitro. 2020;68:104929. doi: 10.1016/j.tiv.2020.104929. [DOI] [PubMed] [Google Scholar]
- 36.Asselineau D, Bernard BA, Bailly C, Darmon M, Pruniéras M. Human epidermis reconstructed by culture: is it “normal”? J Invest Dermatol. 1986;86:181–186. doi: 10.1111/1523-1747.ep12284237. [DOI] [PubMed] [Google Scholar]
- 37.Schäfer-Korting M, Mahmoud A, Lombardi Borgia S, Brüggener B, Kleuser B, Schreiber S, et al. Reconstructed epidermis and full-thickness skin for absorption testing: influence of the vehicles used on steroid permeation. Altern Lab Anim. 2008;36:441–452. doi: 10.1177/026119290803600405. [DOI] [PubMed] [Google Scholar]
- 38.Auger FA, Berthod F, Moulin V, Pouliot R, Germain L. Tissue-engineered skin substitutes: from in vitro constructs to in vivo applications. Biotechnol Appl Biochem. 2004;39:263–275. doi: 10.1042/BA20030229. [DOI] [PubMed] [Google Scholar]
- 39.Pennacchi PC, de Almeida ME, Gomes OL, Faião-Flores F, de Araújo Crepaldi MC, Dos Santos MF, et al. Glycated reconstructed human skin as a platform to study the pathogenesis of skin aging. Tissue Eng Part A. 2015;21:2417–2425. doi: 10.1089/ten.TEA.2015.0009. [DOI] [PubMed] [Google Scholar]
- 40.Poumay Y, Dupont F, Marcoux S, Leclercq-Smekens M, Hérin M, Coquette A. A simple reconstructed human epidermis: preparation of the culture model and utilization in in vitro studies. Arch Dermatol Res. 2004;296:203–211. doi: 10.1007/s00403-004-0507-y. [DOI] [PubMed] [Google Scholar]
- 41.Régnier M, Staquet MJ, Schmitt D, Schmidt R. Integration of Langerhans cells into a pigmented reconstructed human epidermis. J Invest Dermatol. 1997;109:510–512. doi: 10.1111/1523-1747.ep12336627. [DOI] [PubMed] [Google Scholar]
- 42.Bechetoille N, Dezutter-Dambuyant C, Damour O, André V, Orly I, Perrier E. Effects of solar ultraviolet radiation on engineered human skin equivalent containing both Langerhans cells and dermal dendritic cells. Tissue Eng. 2007;13:2667–2679. doi: 10.1089/ten.2006.0405. [DOI] [PubMed] [Google Scholar]
- 43.Ouwehand K, Spiekstra SW, Waaijman T, Scheper RJ, de Gruijl TD, Gibbs S. Technical advance: Langerhans cells derived from a human cell line in a full-thickness skin equivalent undergo allergen-induced maturation and migration. J Leukoc Biol. 2011;90:1027–1033. doi: 10.1189/jlb.0610374. [DOI] [PubMed] [Google Scholar]
- 44.van den Bogaard EH, Tjabringa GS, Joosten I, Vonk-Bergers M, van Rijssen E, Tijssen HJ, et al. Crosstalk between keratinocytes and T cells in a 3D microenvironment: a model to study inflammatory skin diseases. J Invest Dermatol. 2014;134:719–727. doi: 10.1038/jid.2013.417. [DOI] [PubMed] [Google Scholar]
- 45.Bechetoille N, Vachon H, Gaydon A, Boher A, Fontaine T, Schaeffer E, et al. A new organotypic model containing dermal-type macrophages. Exp Dermatol. 2011;20:1035–1037. doi: 10.1111/j.1600-0625.2011.01383.x. [DOI] [PubMed] [Google Scholar]
- 46.Shin JU, Abaci HE, Herron L, Guo Z, Sallee B, Pappalardo A, et al. Recapitulating T cell infiltration in 3D psoriatic skin models for patient-specific drug testing. Sci Rep. 2020;10:4123. doi: 10.1038/s41598-020-60275-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parenteau NL, Nolte CM, Bilbo P, Rosenberg M, Wilkins LM, Johnson EW, et al. Epidermis generated in vitro: practical considerations and applications. J Cell Biochem. 1991;45:245–251. doi: 10.1002/jcb.240450304. [DOI] [PubMed] [Google Scholar]
- 48.Stark HJ, Boehnke K, Mirancea N, Willhauck MJ, Pavesio A, Fusenig NE, et al. Epidermal homeostasis in long-term scaffold-enforced skin equivalents. J Investig Dermatol Symp Proc. 2006;11:93–105. doi: 10.1038/sj.jidsymp.5650015. [DOI] [PubMed] [Google Scholar]
- 49.Li L, Fukunaga-Kalabis M, Herlyn M. The three-dimensional human skin reconstruct model: a tool to study normal skin and melanoma progression. J Vis Exp. 2011;(54):2937. doi: 10.3791/2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parenteau-Bareil R, Gauvin R, Berthod F. Collagen-based biomaterials for tissue engineering applications. Materials (Basel) 2010;3:1863–1887. [Google Scholar]
- 51.Rehder J, Souto LR, Issa CM, Puzzi MB. Model of human epidermis reconstructed in vitro with keratinocytes and melanocytes on dead de-epidermized human dermis. Sao Paulo Med J. 2004;122:22–25. doi: 10.1590/S1516-31802004000100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tjabringa G, Bergers M, van Rens D, de Boer R, Lamme E, Schalkwijk J. Development and validation of human psoriatic skin equivalents. Am J Pathol. 2008;173:815–823. doi: 10.2353/ajpath.2008.080173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Germain L, Larouche D, Nedelec B, Perreault I, Duranceau L, Bortoluzzi P, et al. Autologous bilayered self-assembled skin substitutes (SASSs) as permanent grafts: a case series of 14 severely burned patients indicating clinical effectiveness. Eur Cell Mater. 2018;36:128–141. doi: 10.22203/eCM.v036a10. [DOI] [PubMed] [Google Scholar]
- 54.Climov M, Medeiros E, Farkash EA, Qiao J, Rousseau CF, Dong S, et al. Bioengineered self-assembled skin as an alternative to skin grafts. Plast Reconstr Surg Glob Open. 2016;4:e731. doi: 10.1097/GOX.0000000000000723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boguniewicz M, Leung DY. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev. 2011;242:233–246. doi: 10.1111/j.1600-065X.2011.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gittler JK, Shemer A, Suárez-Fariñas M, Fuentes-Duculan J, Gulewicz KJ, Wang CQ, et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol. 2012;130:1344–1354. doi: 10.1016/j.jaci.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cho SH, Strickland I, Tomkinson A, Fehringer AP, Gelfand EW, Leung DY. Preferential binding of Staphylococcus aureus to skin sites of Th2-mediated inflammation in a murine model. J Invest Dermatol. 2001;116:658–663. doi: 10.1046/j.0022-202x.2001.01331.x. [DOI] [PubMed] [Google Scholar]
- 58.Nomura I, Gao B, Boguniewicz M, Darst MA, Travers JB, Leung DY. Distinct patterns of gene expression in the skin lesions of atopic dermatitis and psoriasis: a gene microarray analysis. J Allergy Clin Immunol. 2003;112:1195–1202. doi: 10.1016/j.jaci.2003.08.049. [DOI] [PubMed] [Google Scholar]
- 59.Mihm MC, Jr, Soter NA, Dvorak HF, Austen KF. The structure of normal skin and the morphology of atopic eczema. J Invest Dermatol. 1976;67:305–312. doi: 10.1111/1523-1747.ep12514346. [DOI] [PubMed] [Google Scholar]
- 60.Pellerin L, Henry J, Hsu CY, Balica S, Jean-Decoster C, Méchin MC, et al. Defects of filaggrin-like proteins in both lesional and nonlesional atopic skin. J Allergy Clin Immunol. 2013;131:1094–1102. doi: 10.1016/j.jaci.2012.12.1566. [DOI] [PubMed] [Google Scholar]
- 61.Jakasa I, Koster ES, Calkoen F, McLean WH, Campbell LE, Bos JD, et al. Skin barrier function in healthy subjects and patients with atopic dermatitis in relation to filaggrin loss-of-function mutations. J Invest Dermatol. 2011;131:540–542. doi: 10.1038/jid.2010.307. [DOI] [PubMed] [Google Scholar]
- 62.Yosipovitch G, Papoiu AD. What causes itch in atopic dermatitis? Curr Allergy Asthma Rep. 2008;8:306–311. doi: 10.1007/s11882-008-0049-z. [DOI] [PubMed] [Google Scholar]
- 63.Yosipovitch G, Berger T, Fassett MS. Neuroimmune interactions in chronic itch of atopic dermatitis. J Eur Acad Dermatol Venereol. 2020;34:239–250. doi: 10.1111/jdv.15973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tamari M, Ver Heul AM, Kim BS. Immunosensation: neuroimmune cross talk in the skin. Annu Rev Immunol. 2021;39:369–393. doi: 10.1146/annurev-immunol-101719-113805. [DOI] [PubMed] [Google Scholar]
- 65.Jiao D, Wong CK, Qiu HN, Dong J, Cai Z, Chu M, et al. NOD2 and TLR2 ligands trigger the activation of basophils and eosinophils by interacting with dermal fibroblasts in atopic dermatitis-like skin inflammation. Cell Mol Immunol. 2016;13:535–550. doi: 10.1038/cmi.2015.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheung PF, Wong CK, Ho AW, Hu S, Chen DP, Lam CW. Activation of human eosinophils and epidermal keratinocytes by Th2 cytokine IL-31: implication for the immunopathogenesis of atopic dermatitis. Int Immunol. 2010;22:453–467. doi: 10.1093/intimm/dxq027. [DOI] [PubMed] [Google Scholar]
- 67.Renne J, Schäfer V, Werfel T, Wittmann M. Interleukin-1 from epithelial cells fosters T cell-dependent skin inflammation. Br J Dermatol. 2010;162:1198–1205. doi: 10.1111/j.1365-2133.2010.09662.x. [DOI] [PubMed] [Google Scholar]
- 68.Danso MO, van Drongelen V, Mulder A, van Esch J, Scott H, van Smeden J, et al. TNF-α and Th2 cytokines induce atopic dermatitis-like features on epidermal differentiation proteins and stratum corneum lipids in human skin equivalents. J Invest Dermatol. 2014;134:1941–1950. doi: 10.1038/jid.2014.83. [DOI] [PubMed] [Google Scholar]
- 69.Bernard FX, Morel F, Camus M, Pedretti N, Barrault C, Garnier J, et al. Keratinocytes under fire of proinflammatory cytokines: bona fide innate immune cells involved in the physiopathology of chronic atopic dermatitis and psoriasis. J Allergy (Cairo) 2012;2012:718725. doi: 10.1155/2012/718725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.De Vuyst É, Giltaire S, Lambert de Rouvroit C, Malaisse J, Mound A, Bourtembourg M, et al. Methyl-β-cyclodextrin concurs with interleukin (IL)-4, IL-13 and IL-25 to induce alterations reminiscent of atopic dermatitis in reconstructed human epidermis. Exp Dermatol. 2018;27:435–437. doi: 10.1111/exd.13113. [DOI] [PubMed] [Google Scholar]
- 71.Gruber R, Börnchen C, Rose K, Daubmann A, Volksdorf T, Wladykowski E, et al. Diverse regulation of claudin-1 and claudin-4 in atopic dermatitis. Am J Pathol. 2015;185:2777–2789. doi: 10.1016/j.ajpath.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 72.Yuki T, Tobiishi M, Kusaka-Kikushima A, Ota Y, Tokura Y. Impaired tight junctions in atopic dermatitis skin and in a skin-equivalent model treated with interleukin-17. PLoS One. 2016;11:e0161759. doi: 10.1371/journal.pone.0161759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pendaries V, Malaisse J, Pellerin L, Le Lamer M, Nachat R, Kezic S, et al. Knockdown of filaggrin in a three-dimensional reconstructed human epidermis impairs keratinocyte differentiation. J Invest Dermatol. 2014;134:2938–2946. doi: 10.1038/jid.2014.259. [DOI] [PubMed] [Google Scholar]
- 74.Pendaries V, Le Lamer M, Cau L, Hansmann B, Malaisse J, Kezic S, et al. In a three-dimensional reconstructed human epidermis filaggrin-2 is essential for proper cornification. Cell Death Dis. 2015;6:e1656. doi: 10.1038/cddis.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Niehues H, Schalkwijk J, van Vlijmen-Willems IMJJ, Rodijk-Olthuis D, van Rossum MM, Wladykowski E, et al. Epidermal equivalents of filaggrin null keratinocytes do not show impaired skin barrier function. J Allergy Clin Immunol. 2017;139:1979–1981.e13. doi: 10.1016/j.jaci.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 76.Berroth A, Kühnl J, Kurschat N, Schwarz A, Stäb F, Schwarz T, et al. Role of fibroblasts in the pathogenesis of atopic dermatitis. J Allergy Clin Immunol. 2013;131:1547–1554. doi: 10.1016/j.jaci.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 77.Sriram G, Bigliardi PL, Bigliardi-Qi M. Full-thickness human skin equivalent models of atopic dermatitis. Methods Mol Biol. 2019;1879:367–383. doi: 10.1007/7651_2018_163. [DOI] [PubMed] [Google Scholar]
- 78.Kamsteeg M, Bergers M, de Boer R, Zeeuwen PL, Hato SV, Schalkwijk J, et al. Type 2 helper T-cell cytokines induce morphologic and molecular characteristics of atopic dermatitis in human skin equivalent. Am J Pathol. 2011;178:2091–2099. doi: 10.1016/j.ajpath.2011.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee SH, Bae IH, Choi H, Choi HW, Oh S, Marinho PA, et al. Ameliorating effect of dipotassium glycyrrhizinate on an IL-4-and IL-13-induced atopic dermatitis-like skin-equivalent model. Arch Dermatol Res. 2019;311:131–140. doi: 10.1007/s00403-018-1883-z. [DOI] [PubMed] [Google Scholar]
- 80.Hönzke S, Wallmeyer L, Ostrowski A, Radbruch M, Mundhenk L, Schäfer-Korting M, et al. Influence of Th2 cytokines on the cornified envelope, tight junction proteins, and β-defensins in filaggrin-deficient skin equivalents. J Invest Dermatol. 2016;136:631–639. doi: 10.1016/j.jid.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 81.Engelhart K, El Hindi T, Biesalski HK, Pfitzner I. In vitro reproduction of clinical hallmarks of eczematous dermatitis in organotypic skin models. Arch Dermatol Res. 2005;297:1–9. doi: 10.1007/s00403-005-0575-7. [DOI] [PubMed] [Google Scholar]
- 82.Wallmeyer L, Dietert K, Sochorová M, Gruber AD, Kleuser B, Vávrová K, et al. TSLP is a direct trigger for T cell migration in filaggrin-deficient skin equivalents. Sci Rep. 2017;7:774. doi: 10.1038/s41598-017-00670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lebonvallet N, Boulais N, Le Gall C, Pereira U, Gauché D, Gobin E, et al. Effects of the re-innervation of organotypic skin explants on the epidermis. Exp Dermatol. 2012;21:156–158. doi: 10.1111/j.1600-0625.2011.01421.x. [DOI] [PubMed] [Google Scholar]
- 84.Roggenkamp D, Köpnick S, Stäb F, Wenck H, Schmelz M, Neufang G. Epidermal nerve fibers modulate keratinocyte growth via neuropeptide signaling in an innervated skin model. J Invest Dermatol. 2013;133:1620–1628. doi: 10.1038/jid.2012.464. [DOI] [PubMed] [Google Scholar]
- 85.Vidal SEL, Tamamoto KA, Nguyen H, Abbott RD, Cairns DM, Kaplan DL. 3D biomaterial matrix to support long term, full thickness, immuno-competent human skin equivalents with nervous system components. Biomaterials. 2019;198:194–203. doi: 10.1016/j.biomaterials.2018.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Abbott RD, Wang RY, Reagan MR, Chen Y, Borowsky FE, Zieba A, et al. The use of silk as a scaffold for mature, sustainable unilocular adipose 3D tissue engineered systems. Adv Healthc Mater. 2016;5:1667–1677. doi: 10.1002/adhm.201600211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vidal Yucha SE, Tamamoto KA, Nguyen H, Cairns DM, Kaplan DL. Human skin equivalents demonstrate need for neuro-immuno-cutaneous system. Adv Biosyst. 2019;3:e1800283. doi: 10.1002/adbi.201800283. [DOI] [PubMed] [Google Scholar]
- 88.Chiricozzi A, Romanelli P, Volpe E, Borsellino G, Romanelli M. Scanning the immunopathogenesis of psoriasis. Int J Mol Sci. 2018;19:179. doi: 10.3390/ijms19010179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Deng Y, Chang C, Lu Q. The inflammatory response in psoriasis: a comprehensive review. Clin Rev Allergy Immunol. 2016;50:377–389. doi: 10.1007/s12016-016-8535-x. [DOI] [PubMed] [Google Scholar]
- 90.Kim J, Krueger JG. The immunopathogenesis of psoriasis. Dermatol Clin. 2015;33:13–23. doi: 10.1016/j.det.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 91.Albanesi C, Scarponi C, Pallotta S, Daniele R, Bosisio D, Madonna S, et al. Chemerin expression marks early psoriatic skin lesions and correlates with plasmacytoid dendritic cell recruitment. J Exp Med. 2009;206:249–258. doi: 10.1084/jem.20080129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Albanesi C, De Pità O, Girolomoni G. Resident skin cells in psoriasis: a special look at the pathogenetic functions of keratinocytes. Clin Dermatol. 2007;25:581–588. doi: 10.1016/j.clindermatol.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 93.Bellinato F, Gisondi P, Girolomoni G. Latest advances for the treatment of chronic plaque psoriasis with biologics and oral small molecules. Biologics. 2021;15:247–253. doi: 10.2147/BTT.S290309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Martin G, Guérard S, Fortin MM, Rusu D, Soucy J, Poubelle PE, et al. Pathological crosstalk in vitro between T lymphocytes and lesional keratinocytes in psoriasis: necessity of direct cell-to-cell contact. Lab Invest. 2012;92:1058–1070. doi: 10.1038/labinvest.2012.69. [DOI] [PubMed] [Google Scholar]
- 95.Muhr P, Renne J, Schaefer V, Werfel T, Wittmann M. Primary human keratinocytes efficiently induce IL-1-dependent IL-17 in CCR6+ T cells. Exp Dermatol. 2010;19:1105–1107. doi: 10.1111/j.1600-0625.2010.01134.x. [DOI] [PubMed] [Google Scholar]
- 96.Sa SM, Valdez PA, Wu J, Jung K, Zhong F, Hall L, et al. The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis. J Immunol. 2007;178:2229–2240. doi: 10.4049/jimmunol.178.4.2229. [DOI] [PubMed] [Google Scholar]
- 97.Krueger GG, Jorgensen CM. Experimental models for psoriasis. J Invest Dermatol. 1990;95(5 Suppl):56S–58S. doi: 10.1111/1523-1747.ep12505791. [DOI] [PubMed] [Google Scholar]
- 98.Konstantinova NV, Duong DM, Remenyik E, Hazarika P, Chuang A, Duvic M. Interleukin-8 is induced in skin equivalents and is highest in those derived from psoriatic fibroblasts. J Invest Dermatol. 1996;107:615–621. doi: 10.1111/1523-1747.ep12584215. [DOI] [PubMed] [Google Scholar]
- 99.Barker CL, McHale MT, Gillies AK, Waller J, Pearce DM, Osborne J, et al. The development and characterization of an in vitro model of psoriasis. J Invest Dermatol. 2004;123:892–901. doi: 10.1111/j.0022-202X.2004.23435.x. [DOI] [PubMed] [Google Scholar]
- 100.Jean J, Lapointe M, Soucy J, Pouliot R. Development of an in vitro psoriatic skin model by tissue engineering. J Dermatol Sci. 2009;53:19–25. doi: 10.1016/j.jdermsci.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 101.Harvey A, Cole LM, Day R, Bartlett M, Warwick J, Bojar R, et al. MALDI-MSI for the analysis of a 3D tissue-engineered psoriatic skin model. Proteomics. 2016;16:1718–1725. doi: 10.1002/pmic.201600036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rüffer C. Psoriatic in vitro epidermis - a human tissue culture model for testing cosmetical and medical skin care products. H&PC Today. 2011;2:30–32. [Google Scholar]
- 103.Clarysse K, Pfaff CM, Marquardt Y, Huth L, Kortekaas Krohn I, Kluwig D, et al. JAK1/3 inhibition preserves epidermal morphology in full-thickness 3D skin models of atopic dermatitis and psoriasis. J Eur Acad Dermatol Venereol. 2019;33:367–375. doi: 10.1111/jdv.15301. [DOI] [PubMed] [Google Scholar]
- 104.Jansen PA, Rodijk-Olthuis D, Hollox EJ, Kamsteeg M, Tjabringa GS, de Jongh GJ, et al. Beta-defensin-2 protein is a serum biomarker for disease activity in psoriasis and reaches biologically relevant concentrations in lesional skin. PLoS One. 2009;4:e4725. doi: 10.1371/journal.pone.0004725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pouliot-Bérubé C, Zaniolo K, Guérin SL, Pouliot R. Tissue-engineered human psoriatic skin supplemented with cytokines as an in vitro model to study plaque psoriasis. Regen Med. 2016;11:545–557. doi: 10.2217/rme-2016-0037. [DOI] [PubMed] [Google Scholar]
- 106.Nograles KE, Zaba LC, Guttman-Yassky E, Fuentes-Duculan J, Suárez-Fariñas M, Cardinale I, et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br J Dermatol. 2008;159:1092–1102. doi: 10.1111/j.1365-2133.2008.08769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chiricozzi A, Nograles KE, Johnson-Huang LM, Fuentes-Duculan J, Cardinale I, Bonifacio KM, et al. IL-17 induces an expanded range of downstream genes in reconstituted human epidermis model. PLoS One. 2014;9:e90284. doi: 10.1371/journal.pone.0090284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bhatia SN, Ingber DE. Microfluidic organs-on-chips. Nat Biotechnol. 2014;32:760–772. doi: 10.1038/nbt.2989. [DOI] [PubMed] [Google Scholar]
- 109.Wufuer M, Lee G, Hur W, Jeon B, Kim BJ, Choi TH, et al. Skin-on-a-chip model simulating inflammation, edema and drug-based treatment. Sci Rep. 2016;6:37471. doi: 10.1038/srep37471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ramadan Q, Ting FC. In vitro micro-physiological immune-competent model of the human skin. Lab Chip. 2016;16:1899–1908. doi: 10.1039/c6lc00229c. [DOI] [PubMed] [Google Scholar]
- 111.Ren X, Getschman AE, Hwang S, Volkman BF, Klonisch T, Levin D, et al. Investigations on T cell transmigration in a human skin-on-chip (SoC) model. Lab Chip. 2021;21:1527–1539. doi: 10.1039/d0lc01194k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kwak BS, Jin SP, Kim SJ, Kim EJ, Chung JH, Sung JH. Microfluidic skin chip with vasculature for recapitulating the immune response of the skin tissue. Biotechnol Bioeng. 2020;117:1853–1863. doi: 10.1002/bit.27320. [DOI] [PubMed] [Google Scholar]
- 113.Abaci HE, Coffman A, Doucet Y, Chen J, Jacków J, Wang E, et al. Tissue engineering of human hair follicles using a biomimetic developmental approach. Nat Commun. 2018;9:5301. doi: 10.1038/s41467-018-07579-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zoio P, Ventura S, Leite M, Oliva A. Pigmented full-thickness human skin model based on a fibroblast-derived matrix for long-term studies. Tissue Eng Part C Methods. 2021;27:433–443. doi: 10.1089/ten.tec.2021.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ponec M, El Ghalbzouri A, Dijkman R, Kempenaar J, van der Pluijm G, Koolwijk P. Endothelial network formed with human dermal microvascular endothelial cells in autologous multicellular skin substitutes. Angiogenesis. 2004;7:295–305. doi: 10.1007/s10456-004-6315-3. [DOI] [PubMed] [Google Scholar]
- 116.Bellas E, Seiberg M, Garlick J, Kaplan DL. In vitro 3D full-thickness skin-equivalent tissue model using silk and collagen biomaterials. Macromol Biosci. 2012;12:1627–1636. doi: 10.1002/mabi.201200262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gledhill K, Guo Z, Umegaki-Arao N, Higgins CA, Itoh M, Christiano AM. Melanin transfer in human 3D skin equivalents generated exclusively from induced pluripotent stem cells. PLoS One. 2015;10:e0136713. doi: 10.1371/journal.pone.0136713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Itoh M, Umegaki-Arao N, Guo Z, Liu L, Higgins CA, Christiano AM. Generation of 3D skin equivalents fully reconstituted from human induced pluripotent stem cells (iPSCs) PLoS One. 2013;8:e77673. doi: 10.1371/journal.pone.0077673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Abaci HE, Guo Z, Coffman A, Gillette B, Lee WH, Sia SK. Human skin constructs with spatially controlled vasculature using primary and iPSC-derived endothelial cells. Adv Healthc Mater. 2016;5:1800–1807. doi: 10.1002/adhm.201500936. [DOI] [PMC free article] [PubMed] [Google Scholar]