Abstract
Purpose
This study aimed to determine whether the use of drugs in the treatment of inflammatory bowel disease is related to the risk of colorectal cancer using a Cox proportional hazards model with the landmark method to minimize immortal time bias.
Materials and Methods
This study was conducted as national cohort-based study using data from Korea's Health Insurance Corporation. Newly diagnosed patients with inflammatory bowel disease from 2006 to 2010 were monitored for colorectal cancer until 2015. Hazard ratios and 95% confidence intervals were calculated and compared with the incidence of colorectal cancer with or without medications by applying various landmark points.
Results
In patients with Crohn's disease, the prevention of colorectal cancer in the group exposed to immunomodulators was significant in the basic Cox model; however, the effect was not statistically significant in the model using the landmark method. The preventive effect of 5-aminosalicylic acid in patients with ulcerative colitis was significant in the basic and 6-month landmark point application models, but not in the remaining landmark application models.
Conclusion
In patients with inflammatory bowel disease, the preventive effect of drug exposure on colorectal cancer varies depending on the application of the landmark method. Hence, the possibility of immortal time bias should be considered.
Keywords: Bias, cohort study, colorectal cancer, inflammatory bowel disease, proportional hazards models, survival analysis
INTRODUCTION
In studies on the effectiveness of drugs for certain diseases, the adoption of an observational research design using secondary databases has been increasing in recent times.1 Observational studies using cohort data have the advantage of being able to appraise the effectiveness and safety of drugs over a long period of time; however, caution is necessary because there is a very high risk of various types of biases.1,2,3 One common bias in cohort studies is immortal time bias. In epidemiology, the term“immortal time” refers to a follow-up period during which an event corresponding to an outcome has not yet occurred.4 In general, cohort data analysis classifies subjects into several groups based on whether they are exposed to a specific disease or treatment behavior, and then observes the time from the point of cohort entry until an event of interest occurs. In pharmacoepidemiology, the period between the time of inclusion in the cohort and the first prescription of a drug in the drug exposure group is “immortal time” by default, during which the event does not occur, thereby resulting in a time difference between the exposure and non-exposure groups.5,6 Immortal time biases are common in suboptimal survival analysis of exposed and non-exposed cohorts, often leading to significant overestimations of the benefits of the treatment.7 This phenomenon is particularly common in drug effectiveness studies based on secondary sources, such as claims data.8
Inflammatory bowel disease (IBD) is a chronic recurrent inflammatory disease of the intestine that is known to be caused by genetic susceptibility and intestinal bacteria, and it is clinically classified as Crohn's disease (CD) and ulcerative colitis (UC).9 Until recently, Asian countries have reported a relatively lower incidence of IBD than Western countries. However, the prevalence is now increasing, and the growth rate in Asian countries is higher than that in Western countries.10,11 IBD is known to be a strong risk factor for colorectal cancer;12,13 hence, colonoscopy is recommended for patients with IBD.14,15 However, patient reluctance makes it difficult to perform periodic colonoscopy regularly.16 Thus, the prevention of colorectal cancer by using certain drugs, such as 5-aminosalicylic acid (5-ASA), immunomodulators, glucocorticoids, and anti-tumor necrosis factor-α agents (anti-TNF), has been proposed as an alternative.17,18,19
Several studies have been conducted on patients with IBD concerning the preventive effect of these drugs on colorectal cancer; however, results vary widely between studies. For example, in 2012, a meta-analysis20 reported that the use of 5-ASA did not have a preventive effect on colorectal cancer, while a 2017 meta-analysis21 revealed significant preventive effects. A systematic review of observational studies on the relevance of anti-TNF drug use and the onset of lymphoma in patients with IBD reported that the results of these studies differed, which might be due to the use of inappropriate methodologies. Of the 14 studies selected for the review, 11 had a suboptimal definition of “exposure,” and seven had a very high probability of time-related bias.22 Targownik and Suissa7 have presented several studies as examples, highlighting the possibility of an immortal time bias in gastrointestinal observational research.
This study sought to analyze the effects of the medications used by patients with IBD on the risk of colorectal cancer using the landmark method,23 which is one approach employed to reduce immortal time bias. Additionally, we compared the results of various landmark time settings in the landmark analysis model.
MATERIALS AND METHODS
Source of data and cohort definition
This study was approved by the Institutional Review Board of Wonju Severance Christian Hospital (IRB no. CR320304). The research data, including information on demographic characteristics, death, medical history, and prescription history from 2004 to 2015, were provided by the Korean National Health Insurance Database. Using the 7th Amendment Code of the Korean Standard Classification of Diseases (KCD-7), patients with IBD were classified as having CD (K50) or UC (K51) based on their medical history. The operational definition of patients with IBD was considered as patients with more than one outpatient visit for the disease codes “K50” and “K51” with a relevant drug being prescribed more than once.24 Drugs considered related to the treatment of IBD were 5-ASA, immunomodulators, anti-TNF, and corticosteroids (Supplementary Table 1, only online).
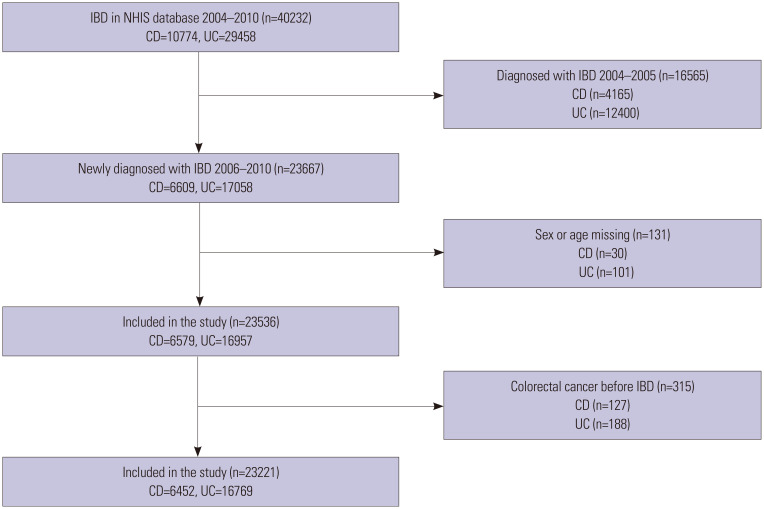
Newly diagnosed patients with IBD between January 1, 2006 and December 31, 2010 were selected as study participants. A washout period from January 2004 to December 2005 was considered, and patients diagnosed during this period were excluded. Patients whose age or sex was unknown and patients diagnosed with colorectal cancer before IBD diagnosis were also excluded from the analysis. A cutoff period until the occurrence of colorectal cancer was designated as December 31, 2015 (Fig. 1).
Fig. 1. Number of subjects according to disease diagnosis. IBD, inflammatory bowel disease; NHIS, National Health Insurance Service; CD, Crohn's disease; UC, ulcerative colitis.
Drug exposure and outcome
In the case of anti-TNF agents, provision of new health insurance benefits enacted in 2010 for patients with UC was considered to likely affect the results of our research, and since the number of prescriptions for anti-TNF agents was very small, they were excluded from the analysis. Using a Cox proportional hazards model, a preliminary analysis was conducted to compare the incidence of colorectal cancer following exposure to 5-ASA, immunomodulators, or corticosteroids among patients with CD and UC (Supplementary Table 2, only online). As one of the objectives of this study was to verify the effectiveness of controlling immortal time bias using the landmark method, the drugs selected for the final analysis were based on data indicating that drug exposure is significantly associated with colorectal cancer. The drugs selected for the final analysis were immunomodulatory agents for patients with CD and 5-ASA for those with UC. The outcomes were defined as patients diagnosed with C18 (malignant neoplasm of the colon), C19 (malignant neoplasm of the rectosigmoid junction), and C20 (malignant neoplasm of the rectum), referring to KCD-7 codes.
Confounding variables
Confounding variables, including sex, age, economic level (based on health insurance premiums), residence (urban and rural), Charlson comorbidity index (CCI), and underlying diseases (hypertension, diabetes, cerebrovascular disease, and cardiovascular disease), that could affect the occurrence of colorectal cancer in patients with IBD were selected. CCI was calculated excluding scores for the common disease code containing C (cancer).
Data analysis
In this study, the Kaplan-Meier method was used to estimate cumulative incidence, and the log-rank test was applied to compare differences in cumulative incidence between drug exposure and non-exposure groups. Using a Cox proportional hazards model adjusted for confounding variables, estimated hazard ratios (HR) and 95% confidence intervals (CIs) were determined. All analyses were performed using the SAS statistical software package for Windows (version 9.4; SAS Institute Inc., Cary, NC, USA), with a significance level of 0.05.
Landmark method
To effectively control for immortal time bias, the landmark method was used in this study. Landmark analysis is a method of establishing arbitrary landmark points and analyzing subjects exposed to drugs or treatments after that point by classifying them into non-exposure groups. Furthermore, if an event occurs before the landmark time, the subject is excluded from the observation. When analyzed without applying the landmark method in the example shown in Supplementary Fig. 1 (only online), patients 2 and 3 were classified in the exposure group and would exhibit a longer survival advantage than the nonexposure group during the time indicated by the dotted line. If the landmark point is set to L1 in the example presented in Supplementary Fig. 1 (only online), patient 2 remains in the exposure group, while patient 3 changes to the non-exposure group.
In this study, landmark points were varied. The majority (86.86%) of the 5-ASA group initiated dosing within 1 month of cohort entry; therefore, the landmark time points were set to 1 month, 2 months, 6 months, 1 year, 2 years, and 3 years (Supplementary Tables 3 and 4, only online). In patients with CD, 44.64% started using immunosuppressants within 6 months of cohort entry; therefore, landmark points were set for 6 months, 1 year, 2 years, and 3 years.
RESULTS
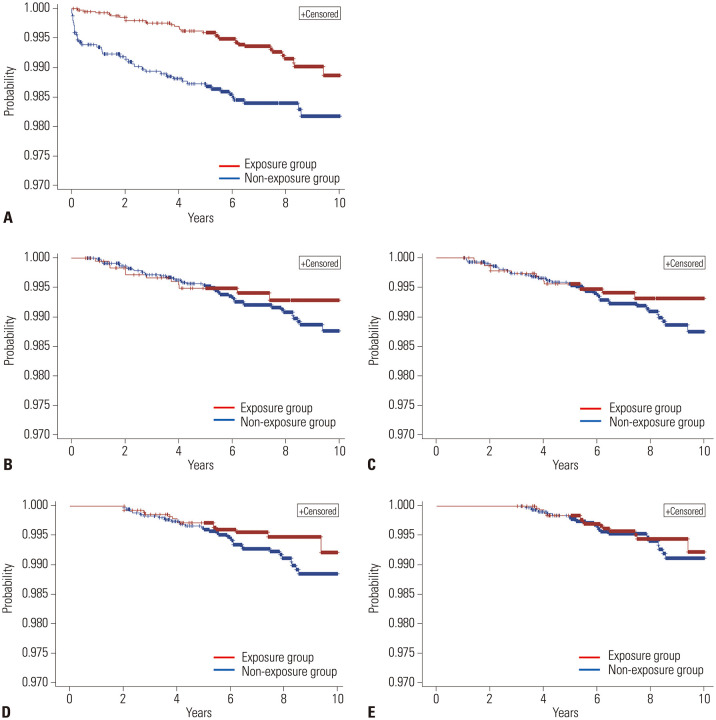
In this study, 71 (1.1%) of the 6452 patients with CD and 151 (0.9%) of the 16769 patients with UC developed colorectal cancer (Table 1). From the Cox proportional hazards model adjusted for confounding variables, we obtained an HR value (ref=“non-exposure”) for the use of immunomodulators in patients with CD of 0.47 (95% CI, 0.28–0.78), highlighting a statistically significant preventive effect against colorectal cancer (Table 2 and Fig. 2). However, upon applying the landmark method, HR values at each time point showed statistically insignificant results. The log-rank test results were also insignificant after applying the landmark method.
Table 1. Characteristics of the Study Participants.
| Confounding variables | CD (n=6452) | UC (n=16769) | |||
|---|---|---|---|---|---|
| Colorectal cancer | Colorectal cancer | ||||
| Yes | No | Yes | No | ||
| Total | 71 (1.1) | 6381 (98.9) | 151 (0.9) | 16618 (99.1) | |
| Sex, male | 49 (69.0) | 4462 (69.9) | 87 (57.6) | 9486 (57.1) | |
| Age group | |||||
| 0–19 years | 17 (23.9) | 1659 (26.0) | 6 (4.0) | 911 (5.5) | |
| 20–39 years | 32 (45.1) | 3131 (49.1) | 33 (21.9) | 6235 (37.5) | |
| 40–59 years | 16 (22.5) | 1192 (18.7) | 53 (35.1) | 6578 (39.6) | |
| ≥60 years | 6 (8.5) | 399 (6.3) | 59 (39.1) | 2894 (17.4) | |
| Residence (urban) | 64 (90.1) | 5963 (93.5) | 138 (91.4) | 15215 (91.6) | |
| Income level | |||||
| Low | 19 (26.8) | 1478 (23.2) | 42 (27.8) | 3855 (23.2) | |
| Middle | 24 (33.8) | 1957 (30.7) | 40 (26.5) | 4786 (28.8) | |
| High | 28 (39.4) | 2946 (46.2) | 69 (45.7) | 7977 (48.0) | |
| CCI without cancer | |||||
| 0 | 26 (36.6) | 2298 (36.0) | 32 (21.2) | 4299 (25.9) | |
| 1 | 20 (28.2) | 2220 (34.8) | 45 (29.8) | 5392 (32.5) | |
| 2 | 15 (21.1) | 1041 (16.3) | 34 (22.5) | 3457 (20.8) | |
| ≥3 | 10 (14.1) | 822 (12.9) | 40 (26.5) | 3470 (20.9) | |
| Comorbidity disease | |||||
| Hypertension | 13 (18.3) | 1078 (16.9) | 54 (35.8) | 5399 (32.5) | |
| Diabetes | 14 (19.4) | 1315 (20.6) | 46 (30.5) | 4804 (28.9) | |
| Cerebrovascular disease | 1 (1.4) | 68 (1.1) | 3 (2.0) | 353 (2.1) | |
| Cardiovascular disease | 0 (0.0) | 77 (1.2) | 3 (2.0) | 349 (2.1) | |
CD, Crohn's disease; UC, ulcerative colitis; CCI, Charlson comorbidity index.
Data are presented as n (%).
Table 2. HR and Log-Rank Test for Immunomodulator Use in CD.
| Landmark Point | Exposure | Colorectal cancer | HR (95% CI) | p value* | Log-rank test p value | |
|---|---|---|---|---|---|---|
| Yes | No | |||||
| Basic model | No | 40 | 2422 | ref | 0.003 | 0.002 |
| Yes | 31 | 3959 | 0.47 (0.28–0.78) | |||
| 6 months | No | 43 | 4595 | ref | 0.405 | 0.347 |
| Yes | 11 | 1763 | 0.75 (0.38–1.45) | |||
| 1 year | No | 38 | 4080 | ref | 0.296 | 0.297 |
| Yes | 14 | 2269 | 0.71 (0.38–1.34) | |||
| 2 years | No | 31 | 3505 | ref | 0.145 | 0.126 |
| Yes | 14 | 2813 | 0.61 (0.32–1.18) | |||
| 3 years | No | 20 | 3162 | ref | 0.737 | 0.599 |
| Yes | 15 | 3137 | 0.88 (0.43–1.81) | |||
CD, Crohn's disease; HR, hazard ratio; CI, confidence interval.
*By Cox proportional hazards models.
Fig. 2. Kaplan-Meier survival curves of patients with Crohn's disease depending on the use of immunomodulators. (A) Survival curve without adjustment for immortal time bias. (B-E) Survival curves after application of the landmark method with various landmark time points: (B) 6 months, (C) 1 year, (D) 2 years, and (E) 3 years.
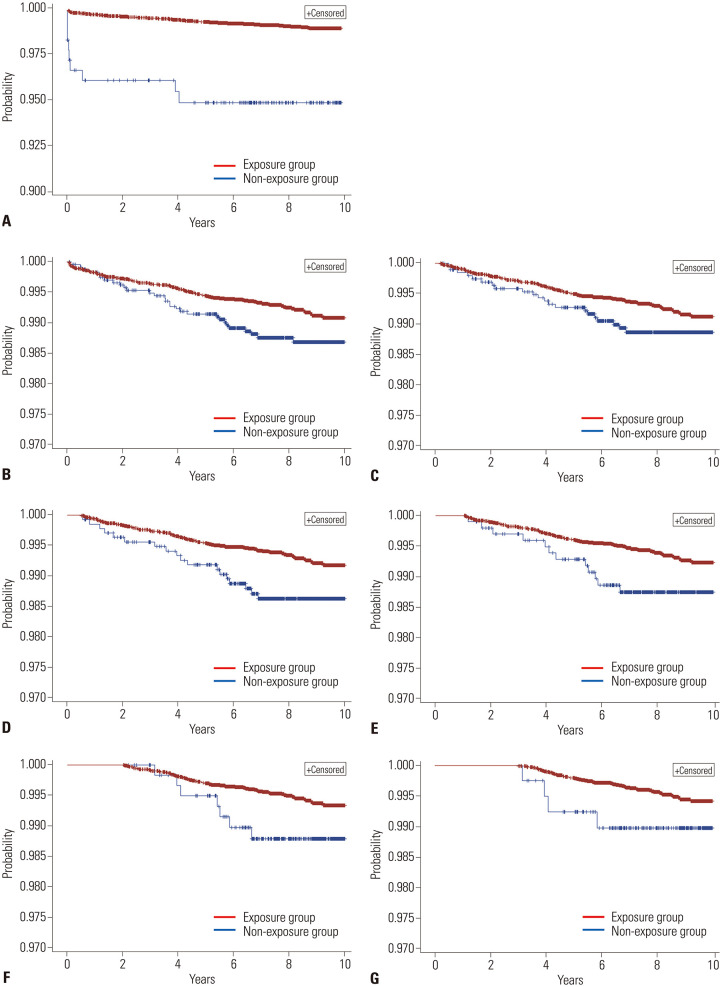
In patients with UC, the HR value for those treated with 5-ASA was 0.18 (95% CI, 0.09–0.35), and the HR value after application of the 6-month landmark point was 0.58 (95% CI, 0.35–0.97), indicating a statistically significant preventive effect (Table 3 and Fig. 3). However, 5-ASA had no significant effect on the prevention of colorectal cancer in the rest of the landmark application models.
Table 3. HR and Log-Rank Test for 5-Aminosalicylic Acid Use in UC.
| Landmark Point | Exposure | Colorectal cancer | HR (95% CI) | p value* | Log-rank test p value | |
|---|---|---|---|---|---|---|
| Yes | No | |||||
| Basic model | No | 9 | 173 | ref | 0.003 | <0.001 |
| Yes | 142 | 16445 | 0.18 (0.09–0.35) | |||
| 1 month | No | 29 | 2325 | ref | 0.052 | 0.029 |
| Yes | 106 | 14280 | 0.67 (0.44–1.00) | |||
| 2 months | No | 21 | 1928 | ref | 0.205 | 0.126 |
| Yes | 102 | 14665 | 0.74 (0.46–1.18) | |||
| 6 months | No | 18 | 1347 | ref | 0.036 | 0.015 |
| Yes | 99 | 15216 | 0.58 (0.35–0.97) | |||
| 1 year | No | 12 | 978 | ref | 0.091 | 0.050 |
| Yes | 93 | 15540 | 0.59 (0.32–1.09) | |||
| 2 years | No | 7 | 596 | ref | 0.130 | 0.071 |
| Yes | 79 | 15821 | 0.55 (0.25–1.19) | |||
| 3 years | No | 4 | 395 | ref | 0.294 | 0.166 |
| Yes | 68 | 15954 | 0.58 (0.21–1.60) | |||
UC, ulcerative colitis; HR, hazard ratio; CI, confidence interval.
*By Cox proportional hazards models.
Fig. 3. Kaplan-Meier survival curves of patients with ulcerative colitis depending on the use of 5-aminosalicylic acid. (A) Survival curve without adjustment for immortal time bias. (B-G) Survival curves after application of the landmark method with various landmark time points: (B) 1 months, (C) 2 months, (D) 6 months, (E) 1 year, (F) 2 years, and (G) 3 years.
DISCUSSION
This study adopted a retrospective cohort research design using data obtained from the Korean Health Insurance Database from 2006 to 2015. In a cohort of patients with IBD, our objectives were to determine whether there was a significant difference in the incidence of colorectal cancer depending on the use of drugs and to control for immortal time bias using the landmark method. A decade-long follow-up revealed colorectal cancer in 71 of 6452 patients (1.1%) with CD and 151 of 16769 patients (0.9%) with UC, which is similar to incidence rates reported in previous studies.18
A Cox proportional hazards model adjusted for confounding variables showed a 0.47 times lower risk ratio of colorectal cancer in patients with CD taking immunomodulators than in those not taking immunomodulators, which is consistent with the results of previous studies.18,25 However, after applying the landmark method, the result differed: HRs in models with various landmark points, from 6 months to 3 years, were not statistically significant. In the case of UC, the group exposed to 5-ASA showed a 0.18 times lower risk than the non-exposure group, which is consistent with previous studies.17,21 The landmark application results showed a significant preventive effect when set to 6 months; however, at other time points, the results were not statistically significant. Our findings indicate that cohort observational studies that seek to determine the efficacy of a drug might result in immortal time bias, which should be minimized. In support thereof, previous studies26,27 that conducted landmark analysis have also reported that results varied depending on the time of the landmark. One study26 that estimated association between inhaled corticosteroids and all-cause mortality among patients with chronic obstructive pulmonary disease reported a significant effect when the landmark time point was set to 3 months; however, at 6, 9, and 12 months, the results were not statistically significant.
For studies that do not consider existing immortal time biases, the possibility of biased results has been consistently highlighted by many fellow researchers.28,29,30 Researchers sometimes assume that if the immortal period is significantly shorter than the overall follow-up period for the outcome in cohort studies, the impact of any immortal time bias would have a negligible effect on the results.31 However, in our study, in 57% of the patients with CD, the first immunomodulator was prescribed within a year of inclusion in the cohort, which was a very short period compared to the entire follow-up period of 10 years. Nevertheless, we confirmed the presence of immortal time bias and set a landmark time point to a short period of 6 months, which changed the outcome of the drug's preventive effects. Other studies have consistently reported that immortal time bias sometimes has a strong influence on research outcomes and might reverse the results of observational studies.5,6,32,33
To minimize immortal time bias when using the landmark method, time point selection is important. In the case of patients with UC in this study, the results for the preventive effect of 5-ASA according to the landmark point selection differed. Therefore, consistent criteria without subjective involvement of the researchers are necessary when setting the landmark points. Additionally, the strength of the treatment effect, sample size reduction, risk change over time, natural course of the disease, proportion of censored data, and length of follow-up should be considered.26 It is also good to use multiple landmark time points for sensitivity analysis.26
This study had the advantage of including data for the entire Korean population during the study period; however, detailed clinical information for each patient was unavailable. One limitation could be that we did not consider the possibility of two or more kinds of drugs being prescribed for one patient. Moreover, the landmark method is not the only means with which to control immortal time bias. Future studies will need to evaluate other methods, such as time-varying Cox models, and compare them with the landmark method. Lastly, we analyzed claims data with the possibility of coding errors. However, we believe that determining IBD and colorectal cancer using diagnostic codes shows favorable reliability in the Korean National Health Insurance Services database, as reported in a previous study.34
In conclusion, the use of immunomodulators in patients with CD had a significant effect on preventing colorectal cancer when analyzed using a Cox proportional hazards model; however, the effect was not statistically significant when landmark analysis was applied to minimize immortal time bias. The effect of preventing colorectal cancer following 5-ASA exposure in patients with UC was also significant in the basic model and at a 6-month landmark point; however, the effect was not significant in the subsequent five landmark points. Our study confirmed that immortal time could bias findings if not properly controlled. If immortal bias is suspected in cohort studies on the effects of a drug or treatment, it would be beneficial to perform a landmark analysis with the selection of the appropriate time points.
ACKNOWLEDGEMENTS
This research is based on the thesis submitted by JK Lee for a Master's degree at Yonsei University, Seoul, Korea in 2020.
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Yoon Young Choi, Jung Kuk Lee, Hyun-Soo Kim, Hee Man Kim, and Dae Ryong Kang.
- Data curation: Yoon Young Choi, Jung Kuk Lee, and Dong Wook Kim.
- Formal analysis: Jung Kuk Lee.
- Investigation: Yoon Young Choi, Jung Kuk Lee, and Dong Wook Kim.
- Methodology: Yoon Young Choi, Jung Kuk Lee, Dong Wook Kim, and Dae Ryong Kang.
- Project administration: Hyun-Soo Kim and Dae Ryong Kang.
- Resources: all authors.
- Software: Jung Kuk Lee and Dong Wook Kim.
- Supervision: Hyun-Soo Kim, Hee Man Kim, and Dae Ryong Kang.
- Validation: Yoon Young Choi, Jung Kuk Lee, and Dae Ryong Kang.
- Visualization: Yoon Young Choi and Jung Kuk Lee.
- Writing—original draft: Yoon Young Choi and Jung Kuk Lee.
- Writing—review & editing: Yoon Young Choi, Hyun-Soo Kim, Hee Man Kim, and Dae Ryong Kang.
- Approval of final manuscript: all authors.
SUPPLEMENTARY MATERIALS
Main Ingredient Codes for the Study Drugs
Cox Proportional Hazards Model Results
Descriptive Statistics on the Number of Cumulative Prescription Days
Patient Rates according to First Date of Prescription
Immortal time bias examples.
References
- 1.Prada-Ramallal G, Takkouche B, Figueiras A. Bias in pharmacoepidemiologic studies using secondary health care databases: a scoping review. BMC Med Res Methodol. 2019;19:53. doi: 10.1186/s12874-019-0695-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Walraven C, Austin P. Administrative database research has unique characteristics that can risk biased results. J Clin Epidemiol. 2012;65:126–131. doi: 10.1016/j.jclinepi.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Weiss NS. The new world of data linkages in clinical epidemiology: are we being brave or foolhardy? Epidemiology. 2011;22:292–294. doi: 10.1097/EDE.0b013e318210aca5. [DOI] [PubMed] [Google Scholar]
- 4.Rothman KJ, Greenland S. Modern epidemiology. 2nd ed. Philadelphia: Lippincott-Raven; 1998. [Google Scholar]
- 5.Suissa S. Effectiveness of inhaled corticosteroids in chronic obstructive pulmonary disease: immortal time bias in observational studies. Am J Respir Crit Care Med. 2003;168:49–53. doi: 10.1164/rccm.200210-1231OC. [DOI] [PubMed] [Google Scholar]
- 6.Suissa S. Immortal time bias in observational studies of drug effects. Pharmacoepidemiol Drug Saf. 2007;16:241–249. doi: 10.1002/pds.1357. [DOI] [PubMed] [Google Scholar]
- 7.Targownik LE, Suissa S. Understanding and avoiding immortal-time bias in gastrointestinal observational research. Am J Gastroenterol. 2015;110:1647–1650. doi: 10.1038/ajg.2015.210. [DOI] [PubMed] [Google Scholar]
- 8.Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol. 2008;167:492–499. doi: 10.1093/aje/kwm324. [DOI] [PubMed] [Google Scholar]
- 9.Danese S, Fiocchi C. Etiopathogenesis of inflammatory bowel diseases. World J Gastroenterol. 2006;12:4807–4812. doi: 10.3748/wjg.v12.i30.4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prideaux L, Kamm MA, De Cruz PP, Chan FK, Ng SC. Inflammatory bowel disease in Asia: a systematic review. J Gastroenterol Hepatol. 2012;27:1266–1280. doi: 10.1111/j.1440-1746.2012.07150.x. [DOI] [PubMed] [Google Scholar]
- 11.Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769–2778. doi: 10.1016/S0140-6736(17)32448-0. [DOI] [PubMed] [Google Scholar]
- 12.Bopanna S, Ananthakrishnan AN, Kedia S, Yajnik V, Ahuja V. Risk of colorectal cancer in Asian patients with ulcerative colitis: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2017;2:269–276. doi: 10.1016/S2468-1253(17)30004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Connor PM, Lapointe TK, Beck PL, Buret AG. Mechanisms by which inflammation may increase intestinal cancer risk in inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:1411–1420. doi: 10.1002/ibd.21217. [DOI] [PubMed] [Google Scholar]
- 14.Farraye FA, Odze RD, Eaden J, Itzkowitz SH, McCabe RP, Dassopoulos T, et al. AGA medical position statement on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138:738–745. doi: 10.1053/j.gastro.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 15.Cairns SR, Scholefield JH, Steele RJ, Dunlop MG, Thomas HJ, Evans GD, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002) Gut. 2010;59:666–689. doi: 10.1136/gut.2009.179804. [DOI] [PubMed] [Google Scholar]
- 16.Delaunoit T, Limburg PJ, Goldberg RM, Lymp JF, Loftus EV., Jr Colorectal cancer prognosis among patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2006;4:335–342. doi: 10.1016/j.cgh.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 17.Velayos FS, Terdiman JP, Walsh JM. Effect of 5-aminosalicylate use on colorectal cancer and dysplasia risk: a systematic review and metaanalysis of observational studies. Am J Gastroenterol. 2005;100:1345–1353. doi: 10.1111/j.1572-0241.2005.41442.x. [DOI] [PubMed] [Google Scholar]
- 18.Connell WR, Kamm MA, Dickson M, Balkwill AM, Ritchie JK, Lennard-Jones JE. Long-term neoplasia risk after azathioprine treatment in inflammatory bowel disease. Lancet. 1994;343:1249–1252. doi: 10.1016/s0140-6736(94)92150-4. [DOI] [PubMed] [Google Scholar]
- 19.Ha JE, Jang EJ, Im SG, Sohn HS. Medication use and drug expenditure in inflammatory bowel disease: based on Korean national health insurance claims data (2010-2014) Korean J Clin Pharm. 2019;29:79–88. [Google Scholar]
- 20.Nguyen GC, Gulamhusein A, Bernstein CN. 5-aminosalicylic acid is not protective against colorectal cancer in inflammatory bowel disease: a meta-analysis of non-referral populations. Am J Gastroenterol. 2012;107:1298–1304. doi: 10.1038/ajg.2012.198. [DOI] [PubMed] [Google Scholar]
- 21.Qiu X, Ma J, Wang K, Zhang H. Chemopreventive effects of 5-aminosalicylic acid on inflammatory bowel disease-associated colorectal cancer and dysplasia: a systematic review with meta-analysis. Oncotarget. 2017;8:1031–1045. doi: 10.18632/oncotarget.13715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferraro S, Leonardi L, Convertino I, Blandizzi C, Tuccori M. Is there a risk of lymphoma associated with anti-tumor necrosis factor drugs in patients with inflammatory bowel disease? A systematic review of observational studies. Front Pharmacol. 2019;10:247. doi: 10.3389/fphar.2019.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eisenstein EL, Anstrom KJ, Kong DF, Shaw LK, Tuttle RH, Mark DB, et al. Clopidogrel use and long-term clinical outcomes after drug-eluting stent implantation. JAMA. 2007;297:159–168. doi: 10.1001/jama.297.2.joc60179. [DOI] [PubMed] [Google Scholar]
- 24.Lee CK, Ha HJ, Oh SJ, Kim JW, Lee JK, Kim HS, et al. Nationwide validation study of diagnostic algorithms for inflammatory bowel disease in Korean National Health Insurance Service database. J Gastroenterol Hepatol. 2020;35:760–768. doi: 10.1111/jgh.14855. [DOI] [PubMed] [Google Scholar]
- 25.Jung YS, Han M, Park S, Kim WH, Cheon JH. Cancer risk in the early stages of inflammatory bowel disease in Korean patients: a nationwide population-based study. J Crohns Colitis. 2017;11:954–962. doi: 10.1093/ecco-jcc/jjx040. [DOI] [PubMed] [Google Scholar]
- 26.Mi X, Hammill BG, Curtis LH, Lai EC, Setoguchi S. Use of the landmark method to address immortal person-time bias in comparative effectiveness research: a simulation study. Stat Med. 2016;35:4824–4836. doi: 10.1002/sim.7019. [DOI] [PubMed] [Google Scholar]
- 27.Gleiss A, Oberbauer R, Heinze G. An unjustified benefit: immortal time bias in the analysis of time-dependent events. Transpl Int. 2018;31:125–130. doi: 10.1111/tri.13081. [DOI] [PubMed] [Google Scholar]
- 28.Lewis JD. Immortal time bias in estimates of mortality among infliximab-treated patients with Crohn's disease. Gut. 2010;59:1586–1587. doi: 10.1136/gut.2009.191015. [DOI] [PubMed] [Google Scholar]
- 29.Targownik LE. Immortal time bias: a likely alternate explanation for the purported benefits of DXA screening in ulcerative colitis. Am J Gastroenterol. 2014;109:1689. doi: 10.1038/ajg.2014.234. [DOI] [PubMed] [Google Scholar]
- 30.Christensen DM, Gerds T, Gislason G, Torp-Pedersen C. Protective association of angiotensin blockade with influenza: a result of immortal time bias? Eur Heart J Cardiovasc Pharmacother. 2021;7:e58–e59. doi: 10.1093/ehjcvp/pvaa068. [DOI] [PubMed] [Google Scholar]
- 31.Beigel F, Steinborn A, Schnitzler F, Tillack C, Breiteneicher S, John JM, et al. Risk of malignancies in patients with inflammatory bowel disease treated with thiopurines or anti-TNF alpha antibodies. Pharmacoepidemiol Drug Saf. 2014;23:735–744. doi: 10.1002/pds.3621. [DOI] [PubMed] [Google Scholar]
- 32.Airaksinen J, Pentti J, Suominen S, Vahtera J, Kivimäki M. An example of how immortal time bias can reverse the results of an observational study. Epidemiology. 2020;31:e19–e20. doi: 10.1097/EDE.0000000000001103. [DOI] [PubMed] [Google Scholar]
- 33.Lévesque LE, Hanley JA, Kezouh A, Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ. 2010;340:b5087. doi: 10.1136/bmj.b5087. [DOI] [PubMed] [Google Scholar]
- 34.Park J, Kwon S, Choi EK, Choi YJ, Lee E, Choe W, et al. Validation of diagnostic codes of major clinical outcomes in a National Health Insurance database. Int J Arrhythm. 2019;20:5 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Main Ingredient Codes for the Study Drugs
Cox Proportional Hazards Model Results
Descriptive Statistics on the Number of Cumulative Prescription Days
Patient Rates according to First Date of Prescription
Immortal time bias examples.