Abstract
The emergence of the SARS-CoV-2 pandemic has imposed a multitude of complications on healthcare facilities. Healthcare professionals had to develop creative solutions to deal with resource shortages and isolation spaces when caring for COVID positive patients. Among many other solutions, facilities have utilized engineering strategies to mitigate the spread of viral contamination within the hospital environment. One of the standard solutions has been the use of whole room negative pressurization (WRNP) to turn a general patient room into an infection isolation space. However, this has not always been easy due to many limitations, such as direct access to the outdoors and the availability of WRNP units. In operating rooms where a patient is likely to go through aerosol-generating procedures, other solutions must be considered because most operating rooms use positive pressure ventilation to maintain sterility. The research team has designed, built, and tested a Covering for Operations during Viral Emergency Response (COVER), a low-cost, portable isolation chamber that fits over a patient's torso on a hospital bed to contain and remove the pathogenic agents at the source (i.e., patient's mouth and nose). This study tests the performance of the COVER system under various design and performance scenarios using particle tracing techniques and compares its efficiency with WRNP units. The results show that COVER can dramatically reduce the concentration of particles within the room, while WRNP is only effective in preventing the room-induced particles from migrating to adjacent spaces.
Keywords: Covid-19, Air quality, Healthcare, Airflow management, Aerosol containment
Graphical abstract
1. Introduction
The novel SARS-CoV-2 virus and its associated clinical presentation COVID-19 have strained hospital systems worldwide for over a year now [1]. The increased in-hospital treatment of COVID-19 has been paired with shortages of hospital personnel, equipment, and personal protection equipment, and an increased strain on hospital infrastructure. With COVID-19 showing both airborne and droplet transmission, this has required the hospital to protect both the healthcare worker and the clinical environment with still attempting to offer a continuum of care [2,3]. In this crisis, many hospitals were forced to delay most elective surgical procedures to focus their efforts on critically ill COVID-19 patients, and reduce the potential transmission of COVID-19 to and by any patients in the healthcare environment [4]. Unfortunately, delaying these urgent but elective surgical procedures has had serious ramifications on patient well-being [5,6], and the finances of the institution, with high-reimbursement elective procedures not being performed [[7], [8], [9]].
Aside from personal protective equipment, airborne isolation rooms are often the next line of defense to protect the clinical environment from additional contamination. Unfortunately, the need for these isolation rooms has frequently exceeded existing resources and infrastructure of hospitals [3], with some hospitals converting negative pressure operating rooms into COVID-19 isolation wards, further depleting the surgical infrastructure of the hospital. In an attempt to mitigate risk to others, something as straightforward as an aerosolizing procedure that would be typically performed in the emergency room has now been restricted to airborne isolation rooms [10,11]. This restriction can not only delay or limit the timely care of the patient [12] but also contaminates these rooms for hours after the in-room procedure and delays or prevents other surgical procedures from making use of these isolation rooms [13].
A variety of solutions have been developed to overcome the shortage of airborne isolation rooms and to reduce healthcare worker exposure during COVID-19. Whole rooms can be retrofitted to become negative pressure isolation rooms [14,15]. This strategy has the benefit of using existing physical space and protecting other patients and healthcare workers outside the room [16]. Like already existing negative pressure rooms, though, no protection is provided to staff members in the room, who will require PPE and, at times, N-95 masks for appropriate safety [17]. Temporary whole-body negative pressure isolation or temporary upper body negative pressure containment using a plastic sheet for containment, commercial air scrubbers to generate flow, and high efficiency particulate absorbing (HEPA) filters to purify viral-laden air have also been described [14,18]. Room pressurization has also been extensively used and recommended by standards as another engineering technique to limit the spread of pathogens within the hospital. However, pressurization alone does not provide a safer environment for those inside a negative room and cannot always be achieved for rooms that are initially designed with positive pressure (e.g., operating rooms) [19,20]. Several researchers studied air purifiers as another viable option to remove pathogenic agents from the space [21,22]. While these engineering approaches have shown promise, many still believe that a paradigm shift is needed in our understanding of the role of ventilation system and the built environment on the (mass) spread of disease [23,24].
Finally, physical devices such as the aerosol box have been developed to reduce healthcare worker risk during highly aerosolizing procedures such as intubation [25,26]. These physical barrier devices without negative pressure generation have been criticized as being not as protective as hoped and as being an impediment to successful procedure performance [27,28]. A study by Begley et al. though via a relatively small sample size (n = 12), showed that the use of such chambers could result in procedural complication (i.e., breaks and unsuccessful tries) while contributing to longer procedure durations [29]. Moreover, a review of 12 case studies further corroborated the findings of Begley's work [30]. Sorbello and colleagues published a scoping review of the aerosol boxes during the COVID-19 crisis. A total of 54 papers passed the eligibility criteria and made it to the final round of review. Of these, nine were labeled as original research, and only one (a work by Convissar et al.) investigated the role of air suction/filtration from the aerosol box [31]. Convissar and colleagues did not actually offer an approach to measure the efficiency of the air suction/filtration system [32]. However, another study published after the scoping review did offer measurements for system efficiency using a single particle counter [33]. Yet, the nuances of system design (i.e., filtration, air recirculation, etc.) were not fully studied.
In response, this article describes the design, construction, and testing of a Covering for Operations during Viral Emergency Response (COVER) device that was indigenously developed during the initial months of the COVID-19 pandemic. The primary goal of the COVER device was to facilitate standard patient care while keeping healthcare workers, patients, and the clinical environment safe through the generation of negative pressure micro-environment around the patient. In this paper, we will test and evaluate several configurations of the COVER device in an education healthcare simulation center and a standard hospital room. Specifically, COVER's performance in containing and removing surrogate aerosols from the inner COVER space, the room environment, and adjacent hallways will be evaluated under various scenarios and negative pressurization schemes at the room entrance.
2. Methods
2.1. The COVER system
The Covering for Operations during Viral Emergency Response (COVER) system is a low-cost, portable isolation chamber that fits over the torso of a supine patient on a hospital bed, and it was made solely with materials that could be found at an average hardware store [34]. The device uses an isolation chamber and directed airflow with filtration to create a physical and air-filtration barrier between the patient and the surrounding clinical environment. The first version of the device was designed for ultra-portability. It used two small, battery-powered fans to generate a negative pressure in the COVER unit, sucking air out through HEPA filters before returning it to the room. This version required no electrical cord connection and could move easily with the hospital bed through the clinical environment. In a second version, a portable vacuum system connected via 6-foot tubes to the ends of the COVER unit was used to suck air through HEPA filters before returning it to the room (Fig. 2-a and b). In both versions, the COVER frame remained the same, with the device's frame being constructed using PVC pieces and connectors, and a clear plastic sheet is used to cover the entire device. The rough dimensions of the COVER frame were 45.7 cm superior-inferiorly, 63.5 cm medial-laterally, and 63.5 cm anterior-posteriorly, with the bed completing the last side of the frame. The clear plastic sheet was connected with plastic clips and had marked indicator lines that guided the clinician in cutting potential access holes for patient treatment. The addition of access holes was hypothesized not to hinder the COVER device's function because it works on the premise of airflow into the COVER device, regardless of the access points. The two filter unit boxes on each side measured 30.5 × 30.5 cm and each contained a 25 × 25 cm HEPA filter on the interior side of a 5 cm deep box. The exterior-facing sides of the filter boxes each housed a portable fan, or a vacuum reservoir where the vacuum tube connected. The filter boxes could be easily removed for cleaning, fan battery replacement, or air filter changes.
Fig. 2.
Cover system and the sampling configurations.
2.2. Room Geometry and airflow measurements
In the summer of 2020 and amid the first local surge of COVID 19, the research team had the opportunity to test the performance of an aerosol control box designed indigenously by the research team. The COVER system was set up for air quality tests within a patient room that was equipped with a whole room negative pressure (WRNP) system (H2KM HEPA-AIRE, Abatement Technologies, Canada). The portable WRNP system could clean the room air at 2208 m3/h and 3400 m3/h rates in low and high modes, respectively. The air passed through a HEPA filtered embedded in the WRNP machine before discharging outside via flexible ducting and through the window in the wall opposite to the main room entrance. Duct connections at the wither end, namely the WRNP unit and the window, were carefully sealed. The patient room had an area of 31.3 m2 and 3.0 m of height, resulting in a space volume of 93.9 m3 (Fig. 1 ).
Fig. 1.
Room geometry, airflow values, and sampling locations.
Prior to the tests, airflow rates delivered by the hospital mechanical system were measured using an ALNOR EB-731 (TSI Incorporated, MN, USA) passive capture hood anemometer. The research team repeated these measurements three times during the experiments and once again when experiments ended. The results showed that 591 m3/h (σ = 54 m3/h) of conditioned air was supplied to the room, divided by the space volume (93.9 m3), the total ventilation rate for the room was 6 air changes per hour (ACH). This number is consistent with code requirements for a patient room, as recommended by The American Society of Heating, Refrigerating and Air-Conditioning Engineer (ASHRAE) and the Facility Guidelines Institute (FGI). Worthy to note, ASHRAE required a ventilation rate for an airborne infection isolation room that is twice this magnitude (i.e., 12 ACH) [35]. Furthermore, the facilities department had shut off the room air outlet to protect the HVAC system from being contaminated by the COVID-19 virus spread within the space, as verified by our measurements. In the absence of the WRNP unit, this configuration resulted in a positive pressurization scheme in the room. The research team continuously measured the pressure magnitude at the room using an Abatement PPM3-S portable pressure monitoring device. The research team read pressure magnitudes of +3.1 Pa when the WRNP unit was off, −1.3 Pa, and −2.6 Pa when the unit was running on the low and high modes. The COVER system was set up on the patient bed with a manikin representing the patient. It had two built-in HEPA filters on either side of the patient's head (Fig. 2 ). These filters could work in three different settings: 1) no air passed through, 2) two portable fans sucked the air through the filters and discharged it immediately into the room, 3) filters were connected to a vacuum pump inside the patient room via sealing tubing. These variations enabled the research team to define and test the COVER system under various scenarios, as outlined in the next section.
2.3. Test procedure
In order to test the device efficiency, an oil-based substance (Bis-2-ethylhexyl sebacate, CAS: 122-62-3, density at 25 °C 0.914 g/mL) was aerosolized continuously in the test room from a patient bed. The COVER device's containment efficiency was tested with the aerosolization occurring within the plastic containment hood under various configurations as described below and compared to aerosolization from the bed without any containment structure in place. The process of aerosolization generated particles ranging from 150 nm to those in the range of 10.0 μm (Fig. 3 ). These particle sizes make it an appropriate simulation for COVID-19's generation of both droplets and aerosols and appropriately test the COVER device's ability to purify air from viral contaminants.
Fig. 3.
Size distribution of generated aerosols, measured and verified in the laboratory environment.
The COVER device's efficiency was tested in five different device configurations, ranging from using the plastic containment sheet without any negative pressure generation or air filtration (Test 4) to using a 3.5 hp vacuum with HEPA filtration on expelled air (Test 1), as indicated in Table 1 . The device's efficiency was also tested with the strongest vacuum while medical staff performed simulated aerosol-generating procedures such as intubation (Test 2). These tests were followed by another group of experiments where the COVER system was removed entirely. Testing the particulate spread without the COVER device present allowed for comparison of the device's purification efficiency with that of the HVAC system under both negative and positive pressures.
Table 1.
Testing scenarios description.
| TEST ID | Test Nickname | TEST Description |
|---|---|---|
| Test 1 | 3.5 VAC | A 3.5 hp vacuum filter air inside COVER and discharge into the room |
| Test 2 | 3.5 VAC + MSI | 3.5 hp vacuum working plus medical staff interaction |
| Test 3 | 2.5 VAC | A 2.5 hp vacuum filter air inside COVER and discharge into the room |
| Test 4 | NO_VAC | No Air filtration inside COVER |
| Test 5 | K_FAN | Portable Fans filter COVER and discharge immediately next to COVER's side |
| Test 6 | WRNP_High | No COVER. Isolation WRNP unit High ΔP = −2.6 Pa |
| Test 7 | WRNP_Low | No COVER. Isolation WRNP unit Low ΔP = −1.3 Pa |
| Test 8 | WRNP_Off | No COVER. WRNP unit Off ΔP = +3.1 Pa |
Finally, in order to measure the device and HVAC system efficiency in air purification, particle concentrations were measured inside the COVER device, inside the room from three locations, and in the corridor just external to the room. Measurements were collected using the EXTECH VPC-300 six-channel handheld particle counters (Fig. 1). These particle counters (PCs) recorded concentrations in six different size channels (0.3–10.0 μm) in 15-s intervals for the duration of the roughly 15-min tests. With the exception of Test 2, the room was empty during the testing procedure to minimize any potential effect that human movement might have on particle distributions. One member of the research team periodically stepped into the room to ensure that all the instruments were functioning correctly.
2.4. Statistical analysis
As the patient room was not entirely devoid of particles any time, the background concentration () of particles was measured before aerosolization for each test. Using Equation (1), we took a time-average of background concentrations and subtracted this value from the measured concentrations for each test to account only for the effect of synthetically generated particles.
| Equation 1 |
is the concentration at the time of sampling, t is the sampling time in minutes, and is the number of data points measured before aerosolization began. Admittedly, was different for each experiment. To avoid the effects of dissimilar background concentrations, was deducted from each data point.
A containment index (ε) was defined as the ratio between average concentrations of aerosols at any sampling station divided by the mean concentration recorded inside the COVER system (Equation (2)). This index is designed to quantify the COVER system's efficiency in containing the released particles to the COVER space and preventing them from spreading into the room and the adjoining corridor. By this definition, is always equal to 100%.
| Equation 2 |
The room concentration (CR) is defined for every test as the aggregate concentration of particles inside the room, that is, PC1, PC3, and PC5. The temporal sum of these concentrations embodies the overall concentration of particles inside the room. Mathematically,
| Equation 3 |
3. Results and discussion
Based on the above-mentioned test configurations, three major research questions were analyzed with their potential interrelations: (1) what is the efficiency of the COVER system in various configurations? (2) what is the effect of room pressurization by the WRNP unit, and how is it compared to the effectiveness of the COVER system as a source control strategy? and (3) what are the noise and temperature levels of the COVER device under working conditions? For each question, details of spatial and temporal distributions of contaminants and HEPA filtration's role are further assessed.
3.1. Noise and temperature measurements
Noise was an important design constraint for the COVER system in that it needed to be quiet enough to be tolerated by the patient and for communication between the hospital staff. The portable fans were designed for use in camping tents, so were relatively quiet during operation. The ShopVac systems required sound-dampening, so a custom sound-isolation box was constructed using a high-strength waterproof plastic case (Pelican 0370 Cube Cases, 67.3 × 67.3 × 64.1 cm). Air intake and outflow holes were made in the case, and a HEPA-rated filter was used in the ShopVac itself. The case was comprised of polypropylene, and the noise-reducing foam inside the case is made from polyurethane. Both materials could be readily sanitized through solutions as simple as bleach and water. Rubber caster wheels were installed to ensure ease of movement between patient rooms as needed.
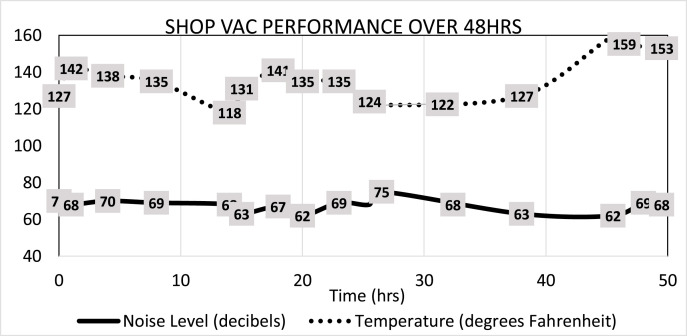
In order to test for the longevity of the vacuum inside the sound isolation box, temperature and sound measurements were recorded over an extended period of time. A remote Bluetooth-capable digital thermometer (Char-Griller Remote Grill Thermometer®) and digital sound meter (Apple Watch Series 5®) were used inside the sound box to gather temperature and sound data, respectively. Overall, no significant decreases in performance were determined after over 50 h of continuous use (Fig. 4 ).
Fig. 4.
Noise levels and temperature data inside the sound-isolation box over 50 h of continuous use.
3.2. Leakage analysis
Measuring the exact leakage of the COVER system was not practical, as the openings had irregular shapes (see Fig. 2) and their shape could easily change with the system's pump flowrate and pressure differential. Therefore, the team used indirect leakage measurements developed for buildings. From the theoretical perspective, it has been established that there is a correlation between flowrate, pressure differentials, and leakage area [37]. The Equivalent Leakage Area (ELA) is described by Equation (4).
| Equation 4 |
In Equation (4), Q is the flowrate [m3/s], Δp is the pressure differential [Pa], ρ is air density (1.125 kg/m3), ELA is the equivalent leakage area [m2], and α is a proportionality coefficient and is derived empirically. A two-step experimental design was set up to first estimate α, and then measure ELA for the COVER system with and without a patient. For the first experimental round, we fully sealed the COVER system by duct-taping all the boundaries and created a structured opening using a round aluminum duct with 10 cm diameter (Fig. 5). The structured opening made ELA a known parameter, therefore by changing the flowrate six times and reading pressure differential, we were able to estimate α = 0.6. To compare, Chan and colleagues estimated an for residential dwellings, with an acceptable range of 0.28–0.85 [38].
Fig. 5.
Connection of the capture flow hood to COVER through a structured hole.
With that measurement for the proportionality ratio (α), then the team arranged two different additional experimental setup to assess the COVER leakage with and without a patient (manikin) inside. Fig. 6 shows the variation between flow rate and . Fitting a linear regression to these data, the slope indicates ELA. As can be seen, the linear fit on the first round of experiments to estimate α = 0.6 is excellent (R-squared = 0.988). Furthermore, the intercept value for all three cases is near zero, which conceptually means that no flowrate should produce no pressure differential. The leakage analysis shows that the overall COVER leakage area is 84 cm2 or equivalent to a circle with 10 cm diameter when there is no patient, and it is 140 cm2 in the presence of a patient (manikin). These findings were consistent with the team's visual observation, also displayed in Fig. 2.
Fig. 6.
Estimated leakage area based on the experimental data.
3.3. COVER efficiency
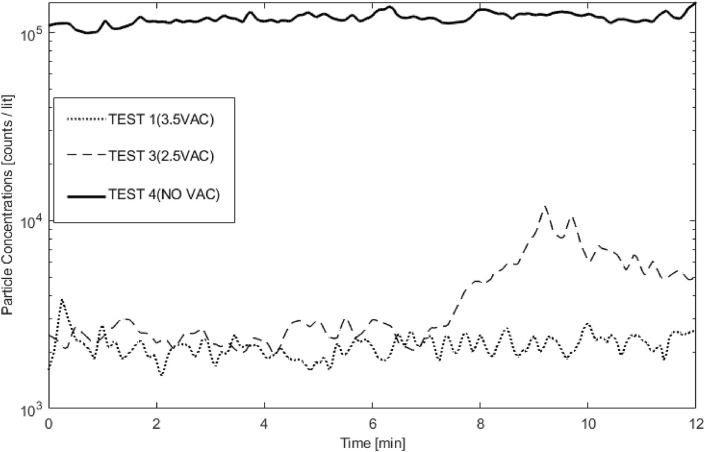
To fully understand the efficiency of the cover device under various conditions, we compared the concentration of particles inside the room (i.e., PC1, PC3, and PC5) for the tests with the COVER device set up (Tests 1–5). Also, these trends were compared to Test 8 as a baseline where there were no mitigation strategies (Fig. 7 ).
Fig. 7.
Efficiency of COVER under various configurations vs. baseline (Test 8).
The results show that the COVER device reduces environmental particulate spread when compared to no containment device. Even when not using vacuums or fans to generate airflow within the COVER device, the room concentration was an order of magnitude lower in the presence of the COVER system, as shown in Test 4 compared to Test 8. The COVER device without airflow also delayed the detection of particulates by the in-room PCs by nearly 2 min. This delay increased to 4 min with the use of portable fans to generate airflow across HEPA filters. As the test time continued with portable fans, though, the particulates detected in the room increased, exceeding the in-room particulates without any airflow. These fans resulted in significant leakage of contamination from the COVER space into the room, as recorded by PC1 and PC3, both located near the outlet of the portable fan. Results show that using portable fans to generate airflow across HEPA filters did not successfully reduce in-room particular spread after a brief initial delay in particulate detection. On the contrary, both configurations with 2.5 and 3.5 HP shop vacuums demonstrated high efficiency in particulate reduction, reducing the detected concentration of in-room particulates by two orders of magnitude when compared to the baseline.
Simulated patient care and medical staff interactions affected the COVER device's efficiency, as shown in Fig. 8 . Specifically, medical staff interactions initially did not change the device's performance in limiting in-room particulate detection. After roughly 6 min of continuous patient care, though, in-room particulate detection increased compared to the device's performance without medical staff interaction. The observed level of particulate detection remained roughly one-tenth of the particulate detection without the COVER device, even with prolonged medical staff interactions.
Fig. 8.
The effect of medical staff interactions on cover efficiency.
3.4. Room pressurization and HEPA filtration
In order to examine the effect of the whole room portable negative pressurization unit, only the tests without the COVER unit were included in the analysis, plus the COVER with no filtration mechanism (i.e., test 4) as a baseline for comparison. As shown in Fig. 9 the WRNP unit on the low mode did not provide sufficient air scrubbing to remove the released pathogens from the space. However, when the WRNP unit was turned on the high mode, it exhibited a similar performance to the COVER system with no filtration mechanism. Worthy to note, this similar outcome was a result of two distinctly different mechanisms. The WRNP unit works on the premise of particle removal through active filtration of air, while COVER solely relies on containing particles at the source of generation. Admittedly, the latter demands much less energy, and thus, is a less expensive strategy.
Fig. 9.
The effect of negative HEPA unit on particle removal.
Moreover, results show that the negative pressurization created by the WRNP unit reduced the migration of particles from the room. To that end, two indices were analyzed. First, the concentrations of particles outside the room were compared to those inside the COVER (i.e., the Containment Index). This index was formally defined as εOUTSIDE in the methods section and is reported in Table 2 . The results show that εOUTSIDE was substantially lower for cases where some mitigation strategy was in place. For example, this index is lowest for COVER with the Shop Vac for vacuum generation (i.e., Tests 1 and 2), followed by the case where the WRNP unit ran on the high mode (Test 6).
Table 2.
Containment index for various tests and sampling locations.
| TEST Nickname | TEST ID | PC2 |
PC1 |
PC3 |
PC5 |
PC4 |
PC4/CR |
|---|---|---|---|---|---|---|---|
| inside COVER | ROOM 1 | ROOM 2 | ROOM3 | OUTSIDE | |||
| 3.5 VAC | Test 1 | 100% | 2.9% | 3.7% | 2.3% | 1.9% | 21.3% |
| 3.5 VAC + MSI | Test 2 | 100% | 6.7% | 6.1% | 3.9% | 2.3% | 13.8% |
| 2.5 VAC | Test 3 | 100% | 12.5% | 11.9% | 10.4% | 8.5% | 24.4% |
| NO_VAC | Test 4 | 100% | 34.2% | 28.1% | 23.0% | 17.3% | 20.3% |
| K_FAN | Test 5 | 100% | 32.9% | 40.8% | 25.0% | 15.4% | 15.6% |
| HEPA_High | Test 6 | 100% | 42.7% | 17.3% | 34.7% | 3.4% | 3.6% |
| HEPA_Low | Test 7 | 100% | 37.1% | 31.1% | 27.8% | 5.0% | 5.2% |
| HEPA_Off | Test 8 | 100% | 39.5% | 23.3% | 33.1% | 18.8% | 21.3% |
The next index was defined as a ratio between the corridor concentrations and the room concentrations (shown as PC4/CR in Table 2). This index was used to assess, specifically, the effect of pressurization across the room entrance. For this purpose, corridor concentrations are normalized by those inside the room (i.e., CR) to quantify what portion of particles that could escape COVER (i.e., room concentration) could further migrate into the corridor. Hence, the effect of the COVER system is entirely bypassed by this index. Based on the pressure across the room entrance, the experiments can be clustered into three categories: (1) Positive pressure: these are all the tests in which the portable WRNP unit was shut off, namely, Tests 1–5 and Test 8, (2) Slightly negative pressure: this category includes Test 7, where the WRNP unit was on the low mode (ΔP = −1.3 Pa), and (3) Negative pressure: this includes Test 6 where the pressure difference across the door matched the CDC and ASHRAE current requirements (P = −2.6 Pa). As can be seen in Table 2, the room-to-corridor particle migration was significantly higher for the positive pressure tests (19.5% on average), where the same value was reduced considerably (∼70%) by when some negative pressure was introduced, and it was further improved, disproportionally, by the increase in the negative pressure magnitude. Worthy to note, PC4/CR seeks to specifically measure the migration ratio of those particles that could escape the COVER device, while the PC4 containment index (reported in Table 2) depicts the overall migration. Table 2 shows that while the overall likelihood of migration into the space is lowest for Test 1 (1.9%), once particles escaped the COVER boundary, they could further move to the adjacent corridor.
4. Conclusion
The COVER device successfully reduces the in-room particulate spread of both droplets and aerosols when used with a vacuum to generate negative pressure within the plastic containment sheet. While portable fans were employed as one of the initial designs due to their portability and low noise generation, particulate containment efficiency was poor and sometimes worse than the plastic containment sheet without any airflow generation. One proposed reason for worsened particulate containment with portable fans is that they fail to generate substantial airflow across the HEPA filters—failing, in turn, to generate a negative pressure environment—and, instead, propel particulate-laden air leaked from within the plastic sheet farther than would be possible when the fans are absent. Therefore, a vacuum-based system is more effective in reducing particulate spread.
When the whole room has appropriately negative pressure, particulate spread outside of the room is very low, which supports its use as a protective measure for HCWs and patients outside the room. Unfortunately, the particulate levels remain quite high within the room itself, consistent with the current HCW practice of using PPE in the presence of droplet and aerosol-transmitting diseases. The COVER device, by contrast, significantly reduces particulate spread outside of the room and within the room, potentially leading to greater HCW safety when in the patient's room. Further study would be required to determine if the COVER device particulate containment is sufficient to prevent clinical infection for specific pathogens such as SARS-CoV-2 in the absence of PPE use.
While the COVER device reduced particulate spread effectively, medical staff interaction greater than 6 min resulted in increased particulate spread. While this increased particulate spread did not exceed the spread when no device was used, it suggests that prolonged and continuous patient care activities would increase the risk of exposure to infectious particles even with the COVER device in use. Providing more frequent patient care activities, each lasting less than 5 min, may decrease this particulate spread as the risk of particulate spread is greatest with prolonged and continuous medical staff interactions. Furthermore, the use of COVER could lead to patient anxiety, surface condensation, and other potential complications. Since a manikin was used to embody the patient, these data were not collected during experiments. Anecdotally, the team has heard from medical collaborators that using the COVER does not create a major behavioral problem with most patients. They also indicate that, often, patients are on sedation (maybe unconscious), which means they do not fully notice what is around them. Although a couple of papers studied the effects of aerosol boxes on anxiety and performance of the medical team [29,36], these effects on the patient have remained a critical line of research in the future. The COVER device is effective in containing aerosols and droplets, and it is built entirely from non-medical components. The COVER device likely increases HCW safety by reducing the spread of infectious agents from infected patients, especially when PPE and negative-pressure isolation rooms supply are insufficient. Below are some particular conclusions of this study:
-
1.
Both of the mitigation strategies, namely WRNP filtration and the COVER system, are efficient approaches to reduce the spread and migration of particles.
-
2.
The COVER system is efficient even without active HEPA filtration. The addition of built-in HEPA filtration to the COVER system further reduces particulate migration. However, the suction fan should be split from the COVER system. Our experiments revealed substantial leakage when kitchen fans were used to discharge air directly from the boundary of the COVER system.
-
3.
Medical staff interaction results in additional leakage of the COVER system, increasing particulate migration. However, increased migration was observed to happen after about 6 min of medical staff interaction. As a result, if the medical staff can potentially finish their interaction with the COVER system within approximately 5–6 min, it is unlikely that the risk of their exposure to the virus is substantially increased.
-
4.
As far as the distribution of particles inside the room, the portable WRNP unit's performance was comparable to that of the COVER system with no filtration. The built-in HEPA filtration with 2.5–3.5 shop vacuum far outperformed the portable HEPA unit in limiting in-room particulate spread. This is especially critical when the goal is to limit the medical staff's exposure in the room to the virus.
-
5.
The portable WRNP unit inside the room did not significantly affect the spread of particles inside the room, but it did significantly reduce the migration from the room to the adjacent corridor. The negative pressurization across the room entrance is necessary in preventing particulate spread, and it is particularly important when the goal is to protect the rest of the ward or hospital from exposure to the virus.
CRediT authorship contribution statement
Ehsan S. Mousavi: Writing – review & editing, Writing – original draft, Visualization, Validation, Methodology, Formal analysis, Conceptualization. Ali Mohammadi Nafchi: Methodology, Investigation, Formal analysis. John D. DesJardins: Writing – review & editing, Supervision, Resources, Methodology, Funding acquisition, Formal analysis, Conceptualization. Amanda S. LeMatty: Resources, Methodology. Robert J. Falconer: Resources, Methodology, Investigation. Noah D. Ashley: Resources, Methodology, Investigation. Benjamin S. Roth: Writing – review & editing, Validation, Supervision, Methodology, Conceptualization. Phillip Moschella: Writing – review & editing, Validation, Supervision, Methodology, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Iyengar K., Mabrouk A., Jain V.K., Venkatesan A., Vaishya R. Learning opportunities from COVID-19 and future effects on health care system. Diabetes Metab. Syndr. Clin. Res. Rev. 2020;14:943–946. doi: 10.1016/j.dsx.2020.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saito T., Asai T. Aerosol containment device for airway management of patients with COVID-19: a narrative review. J. Anesth. 2021;35:384–389. doi: 10.1007/s00540-020-02879-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morawska L., Milton D.K. It is time to address airborne transmission of coronavirus disease 2019 (COVID-19) Clin. Infect. Dis. 2020;71:2311–2313. doi: 10.1093/cid/ciaa939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng N.S., Warner J.L., Osterman T.J., Wells Q.S., Shu X.O., Deppen S.A., Karp S.J., Dwyer S., Feng Q.P., Cox N.J., Peterson J.F., Stein C.M., Roden D.M., Johnson K.B., Wei W.Q. A retrospective approach to evaluating potential adverse outcomes associated with delay of procedures for cardiovascular and cancer-related diagnoses in the context of COVID-19. J. Biomed. Inf. 2021;113:103657. doi: 10.1016/j.jbi.2020.103657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byrnes M.E., Brown C.S., De Roo A.C., Corriere M.A., Romano M.A., Fukuhara S., Kim K.M., Osborne N.H. Elective surgical delays due to COVID-19: the patient lived experience. Med. Care. 2021;59:288–294. doi: 10.1097/MLR.0000000000001503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin B.I., Brodke D.S., Wilson F.A., Chaiyakunapruk N., Nelson R.E. The impact of halting elective admissions in anticipation of a demand surge due to the coronavirus pandemic (COVID-19) Med. Care. 2021;59:213–219. doi: 10.1097/MLR.0000000000001496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farooq I., Ali S. COVID-19 outbreak and its monetary implications for dental practices, hospitals and healthcare workers. Postgrad. Med. 2020;96:791–792. doi: 10.1136/postgradmedj-2020-137781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LoGiudice S.H., Liebhaber A., Schöder H. Overcoming the COVID-19 crisis and planning for the future. J. Nucl. Med. 2020;61:1096–1101. doi: 10.2967/jnumed.120.250522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mossa-Basha M., Deese J., Vincic D., Sahani D.V. Coronavirus disease 2019 (COVID-19): radiology department financial impact and planning for post-COVID recovery. J. Am. Coll. Radiol. 2020;17:894–898. doi: 10.1016/j.jacr.2020.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iacobucci G. Covid-19: emergency departments lack proper isolation facilities, senior medic warns. BMJ. 2020;368:m953. doi: 10.1136/bmj.m953. [DOI] [PubMed] [Google Scholar]
- 11.Her M. Repurposing and reshaping of hospitals during the COVID-19 outbreak in South Korea. One Heal. 2020;10:100137. doi: 10.1016/j.onehlt.2020.100137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mousavi E.S., Kananizadeh N., Martinello R.A., Sherman J.D. COVID-19 outbreak and hospital air quality: a systematic review of evidence on air filtration and recirculation. Environ. Sci. Technol. 2020 doi: 10.1021/acs.est.0c03247. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsui B.C.H., Pan S. Are aerosol-generating procedures safer in an airborne infection isolation room or operating room? Br. J. Anaesth. 2020;125:e485. doi: 10.1016/j.bja.2020.09.011. –e487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson J., Geeslin A., Streifel A. Airborne Infectious Disease management. Methods for temporary negative pressure isolation. Off. Emerg. Prep. Minnesota Dep. Heal. 2017:73–96. [Google Scholar]
- 15.Dyer J. COVID-19 Forced hospitals to build negative pressure rooms fast. Infect. Control Today. 2021 https://www.infectioncontroltoday.com/covid-19/h [Google Scholar]
- 16.Mousavi E.S., Godri Pollitt K.J., Sherman J., Martinello R.A. Performance analysis of portable HEPA filters and temporary plastic anterooms on the spread of surrogate coronavirus. Build. Environ. 2020 doi: 10.1016/j.buildenv.2020.107186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malik J.S., Jenner C., Ward P.A. Maximising application of the aerosol box in protecting healthcare workers during the COVID-19 pandemic. Anaesthesia. 2020;75:974–975. doi: 10.1111/anae.15109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adir Y., Segol O., Kompaniets D., Ziso H., Yaffe Y., Bergman I., Hassidov E., Eden A. COVID-19: minimising risk to healthcare workers during aerosol-producing respiratory therapy using an innovative constant flow canopy. Eur. Respir. J. 2020;55:3–5. doi: 10.1183/13993003.01017-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhattacharya A., Ghahramani A., Mousavi E. The effect of door opening on air-mixing in a positively pressurized room: implications for operating room air management during the COVID outbreak. J. Build. Eng. 2021;44:102900. doi: 10.1016/j.jobe.2021.102900. [DOI] [Google Scholar]
- 20.National Center for Immunization and Respiratory Diseases, Division of Viral Diseases . Cdc; 2020. Interim Infection Prevention and Control Recommendations for Patients with Suspected or Confirmed Coronavirus Disease 2019 (COVID-19) in Healthcare Settings. [Google Scholar]
- 21.Chen C., Zhao B., Cui W., Dong L., An N., Ouyang X. The effectiveness of an air cleaner in controlling droplet/aerosol particle dispersion emitted from a patient's mouth in the indoor environment of dental clinics. J. R. Soc. Interface. 2010;7:1105–1118. doi: 10.1098/rsif.2009.0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao B., Liu Y., Chen C. Air purifiers: a supplementary measure to remove airborne SARS-CoV-2. Build. Environ. 2020;177 doi: 10.1016/j.buildenv.2020.106918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morawska L., Tang J.W., Bahnfleth W., Bluyssen P.M., Boerstra A., Buonanno G., Cao J., Dancer S., Floto A., Franchimon F., Haworth C., Hogeling J., Isaxon C., Jimenez J.L., Kurnitski J., Li Y., Loomans M., Marks G., Marr L.C., Mazzarella L., Melikov A.K., Miller S., Milton D.K., Nazaroff W., Nielsen P.V., Noakes C., Peccia J., Querol X., Sekhar C., Seppänen O., ichi Tanabe S., Tellier R., Tham K.W., Wargocki P., Wierzbicka A., Yao M. How can airborne transmission of COVID-19 indoors be minimised? Environ. Int. 2020 doi: 10.1016/j.envint.2020.105832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melikov A.K. COVID-19: reduction of airborne transmission needs paradigm shift in ventilation. Build. Environ. 2020;186 doi: 10.1016/j.buildenv.2020.107336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Canelli R., Connor C.W., Gonzalez M., Nozari A., Ortega R. Barrier enclosure during endotracheal intubation. N. Engl. J. Med. 2020;382:1957–1958. doi: 10.1056/nejmc1914262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown H., Preston D., Bhoja R. Thinking outside the box: a low-cost and pragmatic alternative to aerosol boxes for endotracheal intubation of COVID-19 patients. Anesthesiology. 2020;133:683–684. doi: 10.1097/ALN.0000000000003422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.FDA . United States Food Drug Adm; 2021. Protective Barrier Enclosures without Negative Pressure Used during the COVID-19 Pandemic May Increase Risk to Patients and Health Care Providers.www.fda.gov/medical-devices/letters-health-care-pr [Google Scholar]
- 28.Rosenblatt W.H., Sherman J.D., Kovatsis P.G., Matava C.T., Peyton J.M. More on barrier enclosure during endotracheal intubation. N. Engl. J. Med. 2020;382:e69. doi: 10.1056/nejmc2012960. [DOI] [PubMed] [Google Scholar]
- 29.Begley J.L., Lavery K.E., Nickson C.P., Brewster D.J. The aerosol box for intubation in coronavirus disease 2019 patients: an in-situ simulation crossover study. Anaesthesia. 2020;75:1014–1021. doi: 10.1111/anae.15115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim Z.J., Ponnapa Reddy M., Karalapillai D., Shekar K., Subramaniam A. Impact of an aerosol box on time to tracheal intubation: systematic review and meta-analysis. Br. J. Anaesth. 2021;126:e122–e125. doi: 10.1016/j.bja.2020.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sorbello M., Rosenblatt W., Hofmeyr R., Greif R., Urdaneta F. Aerosol boxes and barrier enclosures for airway management in COVID-19 patients: a scoping review and narrative synthesis. Br. J. Anaesth. 2020;125:880–894. doi: 10.1016/j.bja.2020.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Convissar D., Chang C.Y., Choi W.E., Chang M.G., Bittner E.A. The vacuum assisted negative pressure isolation hood (VANISH) system: novel application of the stryker NeptuneTM suction machine to create COVID-19 negative pressure isolation environments. Cureus. 2020;12:3–9. doi: 10.7759/cureus.8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simpson J.P., Wong D.N., Verco L., Carter R., Dzidowski M., Chan P.Y. Measurement of airborne particle exposure during simulated tracheal intubation using various proposed aerosol containment devices during the COVID-19 pandemic. Anaesthesia. 2020;75:1587–1595. doi: 10.1111/anae.15188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moschella P., Roth B., LeMatty A., Falconer R., Mousavi E.S., Nafchi A., Ashley N., DesJardins J.D. 22 flow and pressure differential results of a novel low-cost portable negative pressure patient enclosure for COVID-19. Ann. Emerg. Med. 2021;78:S11. doi: 10.1016/j.annemergmed.2021.07.023. [DOI] [Google Scholar]
- 35.ASHRAE Standard 170, Ventilation of healthcare facilities. ASHRAE Stand. 2013;170:1–26. [Google Scholar]
- 36.Katiyar R., Katiyar S., Acharya G., Yadava A. Impact of aerosol box on anxiety of the anaesthesiologist for intubation during COVID-19 pandemic: a single-blinded observational study. Indian J. Anaesth. 2021;65:554–556. doi: 10.4103/ija.ija-282-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han Guiyuan, Srebric Jelena, Enache-Pommer Elena. Different modeling strategies of infiltration rates for an office building to improve accuracy of building energy simulations. Energy Build. 2015;86:288–295. [Google Scholar]
- 38.Chan W.R., Nazaroff W.W., Price P.N., Sohn M.D., Gadgil A.J. Analyzing a database of residential air leakage in the United States. Atmos. Environ. 2005;39(19):3445–3455. [Google Scholar]