Abstract
Background
In patients with hip osteoarthritis (OA), pain at rest, unlike pain on activity, is due to pain mechanisms that cannot be explained by nociceptive pain. However, it remains unclear whether central sensitization (CS) is one of the causes of exacerbated pain at rest in patients with hip OA. Therefore, we investigated the differences in causative factors and postoperative course of total hip arthroplasty (THA) between preoperative pain at rest and on activity in patients with hip OA.
Methods
In total, 120 patients (125 hips, 22 men and 98 women, aged 64.5±1.0 years) with hip OA were included. Preoperative pain at rest and on activity and CS were assessed using the visual analog scale (VAS) and CS Inventory (CSI), respectively. Postoperative assessments were evaluated using the Japanese Orthopedic Association Hip Disease Evaluation Questionnaire (JHEQ); pain, satisfaction, function, and mental scores were evaluated 6 and 12 months after THA.
Results
Multivariate regression analysis indicated the CSI score as affecting VAS for pain at rest (β=0.067, P=0.002) but not VAS for pain on activity (β=0.036, P=0.073). VAS for pain at rest had a weak negative correlation with satisfaction and pain scores at both 6 and 12 months after THA (satisfaction, r=−0.309, −0.278; pain, r=−0.202, −0.22). VAS for pain on activity was not correlated with JHEQ. The CSI score had a weak or moderate negative correlation with three scores other than the function score at both 6 and 12 months after THA (satisfaction, r=−0.266, −0.213; pain, r=−0.332, −0.203, mental, r=−0.427, −0.370). The function score was weakly correlated with the CSI score only 6 months after THA (function, r=−0.231, −0.190).
Conclusion
A higher level of preoperative pain at rest, a CS-related symptom, may affect postoperative pain persistence and dissatisfaction in patients with hip OA.
Keywords: central sensitization, pain at rest, postoperative pain, hip osteoarthritis, total hip arthroplasty
Introduction
Osteoarthritis (OA) is a multifaceted disorder with synovitis, cartilage and osteophyte degeneration, and subchondral bone remodeling, resulting in persistent pain. Exacerbation of OA pain has a profound effect on activities of daily living and walking and significantly reduces the quality of life.1 Therefore, a comprehensive treatment, including surgical intervention for pain relief in OA, is important. Total hip arthroplasty (THA) is an effective surgical intervention for patients with hip OA who have complaints of chronic pain and functional disability.2,3 However, approximately 10% of the patients who undergo primary THA report persistent pain at long-term follow-up.4,5 Evidence suggests that central sensitization (CS), which can be defined as the amplification of neural signals within the central nervous system, is one of the causes of persistent postoperative pain in patients with hip OA who undergo THA.6 Understanding the pain characteristics of patients with hip OA with CS might help optimize treatment interventions and improve postoperative outcomes.
The CS Inventory (CSI), a new comprehensive self-reported inventory, was developed as a screening tool for clinicians to help identify patients with syndromes in which CS may be a root etiology.7 In recent years, the CSI has been used to evaluate CS components in patients with hip and knee OA.8,9 Studies using the CSI have indicated that the presence of preoperative CS components in patients with knee OA has a negative impact on postoperative outcomes, such as residual persistent pain, worsening patient satisfaction, and decreased quality of life.10,11 However, to the best of our knowledge, no study has evaluated the relationship between the CSI score and postoperative outcomes in patients with hip OA who underwent THA.
There is growing recognition of the importance of distinguishing between pain at rest and on activity to better understand the differing pathogenic pathways of pain among patients with OA.12,13 It has been reported that in patients with hip and knee OA, pain at rest, unlike pain on activity, is due to pain mechanisms that cannot be explained by only nociceptive pain.14,15 We previously reported a correlation between the presence of CS components and the level of pain at rest in patients with hip OA.9 Therefore, pain at rest may reflect the presence of CS and be a predictor of the postoperative outcomes. However, it remains unclear whether CS is one of the causes of exacerbated pain at rest and whether pain at rest affects the postoperative outcomes because our previous study was a univariable analysis of preoperative clinical scores.
Therefore, we investigated the differences in causative factors between preoperative pain at rest and on activity in patients with hip OA. Further, we investigated the impact of the two different pain symptoms and their predictors on the clinical outcomes after THA.
Materials and Methods
Patients
This study received ethical approval from our Institutional Review Board. All patients provided informed consent, and this study was conducted in accordance with the Declaration of Helsinki. In total, 138 patients with hip OA undergoing primary THA at a single center between December 2018 and March 2020 were enrolled in this study. Four patients with American Society of Anesthesiologists physical status III or higher, eight patients who used centrally acting agents within 1 month before surgery, four patients with a history of hip surgery on the same side as the THA, and two patients with postoperative complications requiring reoperation were excluded. Accordingly, a total of 120 patients (125 hips) who were able to evaluate all the clinical items required for this study were finally included. There were 22 men and 98 women, and the mean age was 64.5±1.0 years.
Clinical Assessments
Preoperative Assessments
The preoperative pain level was assessed using the visual analog scale (VAS). We assessed pain at rest and on activity separately, based on our previous study.9 Pain during walking on level ground was assessed as pain on activity in this study. Furthermore, the participants’ pain at rest and on activity could be a minimum of 0 mm and a maximum of 100 mm. The preoperative levels of these two pain symptoms were represented by the VAS for pain at rest and the VAS for pain on activity. We assessed hip OA status according to the Tönnis classification system.16 All patients had hip OA, categorized as advanced or end-stage (Tönnis grade 2–3). Two observers (YO and KF) independently performed the evaluation using the Tönnis classification system and reached a consensus. To determine the existence of CS components in preoperative patients with hip OA, the patients were assessed using the CSI (part A).7 The CSI, designed to evaluate symptoms associated with CS, comprises 25 self-reported items on somatic and emotional symptoms, scored from 0 to 100 points, with 0 and 100 being the best and worst scores, respectively. Each item was graded on a 5-point Likert scale (0 = never, 1 = rarely, 2 = sometimes, 3 = often, 4 = always). The Japanese version of the CSI was completed by the patients themselves.17 All preoperative clinical assessments (VAS for pain at rest, VAS for pain on activity, radiographic OA status, and CSI scores) were conducted 1 month before THA.
Postoperative Assessments
All operations were performed under general anesthesia using a minimally invasive anterolateral supine approach. Cementless fixation type implants were used in all cases. Three types of stems (fully hydroxyapatite-coated, Zweymüller type, and modular Wagner-cone type) were used according to the shape of the patient’s medullary cavity. Postoperative assessments were evaluated using the scoring system of the Japanese Orthopedic Association Hip-Disease Evaluation Questionnaire (JHEQ).18 The JHEQ has been widely used as a patient-reported outcome measure (PROM) in Japan to evaluate preoperative and postoperative conditions in patients with hip diseases. JHEQ completion is based on a patient’s self-report or the report of guardians caring for the patient. The JHEQ consists of items on pain (0–28 points), function (0–28 points), and mental status (0–28 points). Furthermore, the patient’s satisfaction level is evaluated by a VAS assessment in the JHEQ. In this study, each item of the JHEQ was evaluated at 6 and 12 months postoperatively, and the relationships between JHEQ scores and preoperative clinical evaluations were investigated.
Statistical Analysis
Results are expressed as the mean and standard error of the mean, unless otherwise indicated. Multivariate regression analysis was used to identify the predictors of preoperative pain at rest and on activity. Age, body mass index (BMI), pain duration, and the CSI score were analyzed as continuous variables. Sex and hip OA grade by the Tönnis classification system were analyzed as nominal scale variables. Statistical significance was set at 0.05 in multivariate regression analysis. Correlation analyses between the preoperative VAS for pain at rest, VAS for pain on activity, and CSI scores and the postoperative clinical scores were performed using non-parametric Spearman correlation coefficient because the data did not meet the normality test examined by the Shapiro–Wilk test. The correlation coefficient is represented by r. Correlation results were interpreted according to r values: |r|<0.2, r=±0.2–0.4, r=±0.4–0.6, r=±0.6–0.8, and |r|>0.8 is none or very weak correlation, weak correlation, moderate correlation, strong correlation, and very strong correlation, respectively.19 The priori power analysis for multiple regression and correlation analyses were conducted using G*Power 3 to determine a sufficient sample size.20 The minimum sample size for the multiple regression analysis was estimated using an f-test table and selecting a statistical significance of 0.05, medium variable effect size of 0.15, and statistical power of 0.9, which indicated 123 samples. The minimum sample size for the correlation analysis was estimated using a t-test table and selecting a statistical significance of 0.05, medium variable effect size of 0.3, and statistical power of 0.9, which indicated 109 samples. Therefore, the sample size of 120 patients (125 hips) was deemed adequate in this study. Statistical analyses were performed using SPSS for Windows (version 19.0; IBM Corp, Armonk, NY, USA).
Results
Participant and Clinical Assessment Data
Preoperatively, the mean VAS for pain at rest was 32.2±0.3 mm, mean VAS for pain on activity was 57.6±0.2 mm, and mean CSI score was 21.1±1.1. For all items in the JHEQ, the scores at 12 months after THA were higher than those at 6 months after THA. Details of the demographic factors of the participants as well as pre- and post-operative clinical assessments are shown in Table 1.
Table 1.
Patients’ Demographics and Clinical Assessment Data (N=120 Patients, 125 Hips)
| Mean±SE or N (%) | |
|---|---|
| Demographic factors | |
| Sex, male/female, N | 22 (18.3)/98 (81.7) |
| Age (years) | 64.5±1.0 |
| Height (cm) | 156.0±0.7 |
| Weight (kg) | 59.5±1.4 |
| BMI (kg/m2) | 24.3±0.4 |
| Tönnis grade (2/ 3), N | 31 (24.8)/94 (75.2) |
| Pain duration (years) | 4.5±0.5 |
| Preoperative assessments | |
| VAS for pain at rest (0–100 mm) | 32.2±0.3 |
| VAS for pain on activity (0–100 mm) | 57.6±0.2 |
| CSI score (0–100 points) | 21.1±1.1 |
| Postoperative assessments | |
| JHEQ score 6/ 12 months after THA | |
| VAS for satisfaction (0–100 mm) | 89.5±1.2/ 92.6±1.1 |
| Pain (0–28 points) | 24.5±0.4/ 25.6±0.3 |
| Function (0–28 points) | 16.5±0.7/ 17.2±0.6 |
| Mental (0–28 points) | 20.5±0.6/ 21.1±0.6 |
| Total (0–84 points) | 61.5±1.5/ 63.7±1.3 |
Abbreviations: SE, standard error; VAS, visual analog scale; CSI, central sensitization inventory; JHEQ, Japanese Orthopedic Association Hip-Disease Evaluation Questionnaire; THA, total hip arthroplasty.
Predictors of Preoperative Pain at Rest and on Activity in Patients with Hip OA
The results of the multiple regression analysis are shown in Table 2. Of the six variables (sex, age, BMI, hip OA grades, pain term, and CSI score) in the multivariate regression model, BMI was the only factor significantly influencing VAS for pain on activity (β=0.127, P=0.018). Conversely, the factors significantly influencing VAS for pain at rest comprised BMI and the CSI score (BMI, β=0.113, P=0.049; CSI score, β=0.067, P=0.002).
Table 2.
Multivariate Regression Analysis for the Preoperative Pain at Rest/on Activity in Patients with Hip OA
| VAS for Pain at Rest | VAS for Pain on Activity | |||||||
|---|---|---|---|---|---|---|---|---|
| Predictors | β | 95% Confidence Interval | P value | β | 95% Confidence Interval | P value | ||
| Lower | Upper | Lower | Upper | |||||
| Sex | −0.299 | −1.697 | 1.099 | 0.672 | −0.599 | −1.897 | 0.698 | 0.362 |
| Age (years) | −0.008 | −0.054 | 0.039 | 0.746 | 0.016 | −0.026 | 0.059 | 0.451 |
| BMI (kg/m2) | 0.113 | −0.001 | 0.226 | 0.049 | 0.127 | 0.022 | 0.231 | 0.018 |
| Tönnis grade (2 or 3) | 0.988 | −0.224 | 2.201 | 0.109 | 0.222 | −0.904 | 1.347 | 0.697 |
| Pain duration (years) | 0.050 | −0.043 | 0.144 | 0.289 | −0.023 | −0.110 | 0.064 | 0.601 |
| CSI score | 0.067 | 0.025 | 0.110 | 0.002 | 0.036 | −0.003 | 0.076 | 0.073 |
Notes: Age, BMI, pain duration, and CSI score were analyzed as continuous variables. Sex, hip OA grade by the Tönnis classification were analyzed as nominal scale variables. Statistically significant P-values (P<0.05) are indicated in bold.
Abbreviations: OA, osteoarthritis; BMI, body mass index; CSI, central sensitization inventory; β, standardized partial regression coefficient.
Correlations Between Preoperative Pain at Rest and on Activity and Postoperative Clinical Outcomes After THA
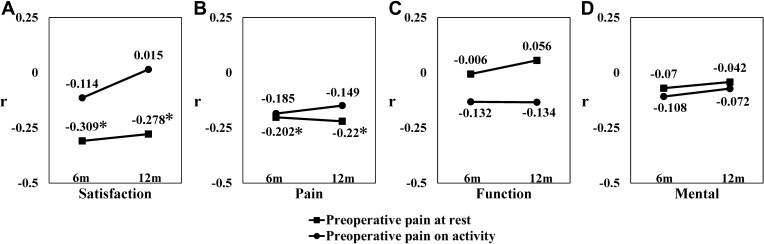
Correlations between preoperative VAS for pain at rest and on activity and postoperative clinical outcomes at 6 and 12 months are shown in Figure 1. VAS for pain at rest had a weak negative correlation with JHEQ satisfaction and pain scores at both 6 and 12 months after THA (6 months: satisfaction, r =−0.309; pain, r =−0.202; 12 months: satisfaction, r=−0.278; pain, r =−0.22; Figure 1A and B). On the contrary, VAS for pain on activity was not correlated with all scores of the postoperative JHEQ (Figure 1A–D). VAS for pain at rest did not correlate with postoperative functional and mental scores on the JHEQ (Figure 1C and D).
Figure 1.
Correlations between preoperative pain at rest and on activity and clinical evaluations at 6 and 12 months after THA in patients with osteoarthritis. Correlations between preoperative VAS scores for pain at rest and on activity and postoperative (A) satisfaction, (B) pain, (C) function, and (D) mental scores on the Japanese Orthopedic Association Hip-Disease Evaluation Questionnaire. The correlation coefficient is represented by r. * |r|≥0.2.
Abbreviations: 6m, 6 months; 12m, 12 months; VAS, visual analog scale; THA, total hip arthroplasty.
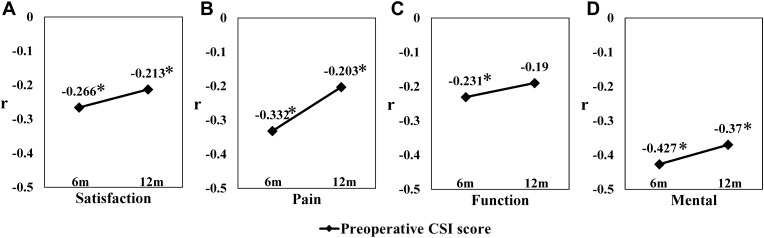
Correlations Between the Preoperative CSI Score and Clinical Outcomes After THA
Correlations between the preoperative CSI score and postoperative clinical outcomes at 6 and 12 months are shown in Figure 2. The CSI score had a weak or moderate negative correlation with all scores at 6 months after THA (satisfaction, r =−0.266; pain, r =−0.332; function, r =−0.231; mental, r =−0.427; Figure 2A–D). At 12 months after THA, the CSI score had a weak negative correlation with the three scores other than the function score (satisfaction, r=−0.213; pain, r =−0.203; function, r =−0.190; mental, r =−0.370; Figure 2A–D).
Figure 2.
Correlations between the preoperative CSI score and clinical evaluations at 6 and 12 months after THA in patients with osteoarthritis. Correlations between the preoperative CSI score and postoperative (A) satisfaction, (B) pain, (C) function, and (D) mental scores on the Japanese Orthopedic Association Hip-Disease Evaluation Questionnaire at 6 and 12 months after THA. The correlation coefficient is represented by r. * |r|≥0.2.
Abbreviations: 6m, 6 months; 12m, 12 months; CSI, central sensitization inventory; THA, total hip arthroplasty.
Discussion
The present study revealed the CSI score as a factor influencing the degree of preoperative pain at rest in patients with hip OA. Further, the adverse effects of higher preoperative pain at rest and CSI score on outcomes after THA were indicated. These results can aid in identifying patients with hip OA with CS components. Additionally, these results suggest that the presence of CS might be one of the reasons why some patients with uncomplicated hip OA who undergo THA experience persistent postoperative pain.
The current study results elucidate the factors that influence pain at rest and on activity in hip OA. Pain at rest, but not pain on activity, was reflected in the CSI score. Higher pain at rest in patients with knee and hip OA is one of the pain features associated with CS.9,14,15,21 Power et al reported that pain at rest was associated with painDETECT questionnaire scores to a greater extent than pain on activity in patients with end-stage knee and hip OA.14 PainDETECT questionnaire scores are associated with CS pain and neuropathic pain.22 We found that the higher the preoperative CSI score, the higher the preoperative pain at rest. In addition, preoperative pain at rest had a weak negative correlation with patient satisfaction and pain scores at 6 and 12 months postoperatively, while pain on activity had not. Lundblad et al reported that a high preoperative VAS for pain at rest was related to less pain relief and a lower pain threshold 18 months after total knee arthroplasty (TKA).21 Thus, higher preoperative pain at rest is associated with CS and may be associated with postoperative pain persistence and reduced satisfaction in patients with hip OA.
Several studies have reported that CS in patients with knee OA, as assessed by the CSI, has a negative impact on the postoperative course.10,11,23 Koh et al reported that patients in the CS group (CSI score ≥40) showed worse VAS pain, Knee Society Score, and Western Ontario and McMaster Universities Osteoarthritis Index scores than did patients in the non-CS group (CSI score <40) 2 years after primary TKA.11 Kim et al reported that higher preoperative VAS pain in patients in the CS group (CSI score ≥40) was maintained at 3, 6, 12, and 24 months after revision TKA compared with that in patients in the non-CS group (CSI score <40).23 We found that the CSI score had at least weak negative correlation with satisfaction, pain, function, and mental scores as assessed by PROM (ie, the JHEQ) after THA in patients with hip OA. CS components in patients with hip OA may affect not only persistent pain but also functional and psychological factors, postoperatively, resulting in reduced patient satisfaction. Thus, the CSI score may be a prediction tool for postoperative outcomes.
BMI, but not radiological severity, affected both of these pain symptoms. Consistent with this, several studies have reported increased BMI as an aggravating factor in hip and knee OA pain.24,25 However, it has been reported that radiographic structural changes in knee OA are poorly correlated with pain levels.26 Furthermore, Wylde et al demonstrated that pain severity as assessed by the pressure pain threshold did not differ between radiographically evaluated Kellgren and Lawrence grades 3 and 4 in hip OA.27 The results of the present analysis, which divided pain assessments into pain at rest and on activity, were similar to those of previous reports.
Duloxetine is a potent selective serotonin-norepinephrine reuptake inhibitor that potentiates descending inhibitory pain pathways in the central nervous system.28 Several studies have reported that duloxetine is effective in reducing pain and improving the quality of life in patients with OA.29,30 Koh et al indicated that patients in the group receiving duloxetine from the day before surgery to 6 weeks after surgery had a greater reduction in pain than those in the control group, among centrally sensitized patients with knee OA who underwent TKA.31 Taken together, the results of these previous studies and the present results suggest that duloxetine administered preoperatively and postoperatively may improve the postoperative course of patients with hip OA with higher pain at rest and CSI scores.
The present study has some limitations. This study relied solely on the CSI to assess the presence of CS components. In other reports related to CS in patients with OA, several measurement instruments, such as quantitative sensory testing, have been used to identify patients with CS.32 However, there is no gold standard evaluation method to investigate CS, and assessment validation in patients with hip OA following THA remains uncertain. Further, this study used different scoring systems in the pre- and postoperative clinical assessment. An assessment using a similar scoring system should be performed. Moreover, multiple regression analysis to identify predictors of postoperative outcomes did not result in a normal distribution in assumption checks of residual analysis by Q-Q plot. Thus, the multiple regression analysis on postoperative outcomes was not examined. Despite these limitations, we believe that our study provides valuable information about preoperative CS components and their influence on postoperative outcomes following THA.
Conclusion
We indicated the CSI score as a causative factor of preoperative pain at rest in patients with hip OA. Higher preoperative CSI scores adversely affected postoperative pain persistence, satisfaction, and functional and mental factors. Preoperative pain at rest had a more negative impact on patient satisfaction and pain scores at 6 and 12 months postoperatively than pain on activity. Assessment of pain at rest may be important for more accurate characterization of pain in centrally sensitized patients with hip OA.
Acknowledgments
We would like to thank Editage for English language editing.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Abbreviations
THA, total hip arthroplasty; OA, osteoarthritis; CS, central sensitization; CSI, central sensitization inventory; VAS, visual analog scale; JHEQ, Japanese Orthopedic Association Hip-Disease Evaluation Questionnaire; PROM, patient-reported outcome measure; BMI, body mass index; TKA, total knee arthroplasty.
Data Sharing Statement
The datasets supporting the conclusions of this article are included within the article. The raw data can be requested from the corresponding author.
Ethics Approval
This study was approved by the Institutional Review Board of Kitasato University (the reference number, KMEO B20–096).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Martel-Pelletier J, Barr AJ, Cicuttini FM, et al. Osteoarthritis. Nat Rev Dis Primers. 2016;2:16072. doi: 10.1038/nrdp.2016.72 [DOI] [PubMed] [Google Scholar]
- 2.Ethgen O, Bruyere O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86(5):963–974. doi: 10.2106/00004623-200405000-00012 [DOI] [PubMed] [Google Scholar]
- 3.Judge A, Arden NK, Kiran A, et al. Interpretation of patient-reported outcomes for hip and knee replacement surgery: identification of thresholds associated with satisfaction with surgery. J Bone Joint Surg Br. 2012;94(3):412–418. doi: 10.1302/0301-620X.94B3.27425 [DOI] [PubMed] [Google Scholar]
- 4.Jones CA, Beaupre LA, Johnston DW, Suarez-Almazor ME. Total joint arthroplasties: current concepts of patient outcomes after surgery. Rheum Dis Clin North Am. 2007;33(1):71–86. doi: 10.1016/j.rdc.2006.12.008 [DOI] [PubMed] [Google Scholar]
- 5.Slaven EJ. Prediction of functional outcome at six months following total hip arthroplasty. Phys Ther. 2012;92(11):1386–1394. doi: 10.2522/ptj.20110484 [DOI] [PubMed] [Google Scholar]
- 6.Izumi M, Petersen KK, Laursen MB, Arendt-Nielsen L, Graven-Nielsen T. Facilitated temporal summation of pain correlates with clinical pain intensity after hip arthroplasty. Pain. 2017;158(2):323–332. doi: 10.1097/j.pain.0000000000000764 [DOI] [PubMed] [Google Scholar]
- 7.Mayer TG, Neblett R, Cohen H, et al. The development and psychometric validation of the central sensitization inventory. Pain Pract. 2012;12(4):276–285. doi: 10.1111/j.1533-2500.2011.00493.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gervais-Hupe J, Pollice J, Sadi J, Carlesso LC. Validity of the central sensitization inventory with measures of sensitization in people with knee osteoarthritis. Clin Rheumatol. 2018;37(11):3125–3132. doi: 10.1007/s10067-018-4279-8 [DOI] [PubMed] [Google Scholar]
- 9.Ohashi Y, Fukushima K, Inoue G, et al. Central sensitization inventory scores correlate with pain at rest in patients with hip osteoarthritis: a retrospective study. BMC Musculoskelet Disord. 2020;21(1):595. doi: 10.1186/s12891-020-03630-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim SH, Yoon KB, Yoon DM, Yoo JH, Ahn KR. Influence of centrally mediated symptoms on postoperative pain in osteoarthritis patients undergoing total knee arthroplasty: a prospective observational evaluation. Pain Pract. 2015;15(6):E46–53. doi: 10.1111/papr.12311 [DOI] [PubMed] [Google Scholar]
- 11.Koh IJ, Kang BM, Kim MS, Choi KY, Sohn S, In Y. How does preoperative central sensitization affect quality of life following total knee arthroplasty? J Arthroplasty. 2020;35(8):2044–2049. doi: 10.1016/j.arth.2020.04.004 [DOI] [PubMed] [Google Scholar]
- 12.Hunter DJ, McDougall JJ, Keefe FJ. The symptoms of osteoarthritis and the genesis of pain. Rheum Dis Clin North Am. 2008;34(3):623–643. doi: 10.1016/j.rdc.2008.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sayers A, Wylde V, Lenguerrand E, et al. Rest pain and movement-evoked pain as unique constructs in hip and knee replacements. Arthritis Care Res. 2016;68(2):237–245. doi: 10.1002/acr.22656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Power JD, Perruccio AV, Gandhi R, et al. Neuropathic pain in end-stage hip and knee osteoarthritis: differential associations with patient-reported pain at rest and pain on activity. Osteoarthritis Cartilage. 2018;26(3):363–369. doi: 10.1016/j.joca.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 15.Satake Y, Izumi M, Aso K, Igarashi Y, Sasaki N, Ikeuchi M. Comparison of predisposing factors between pain on walking and pain at rest in patients with knee osteoarthritis. J Pain Res. 2021;14:1113–1118. doi: 10.2147/JPR.S298100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kovalenko B, Bremjit P, Fernando N. Classifications in brief: Tonnis classification of hip osteoarthritis. Clin Orthop Relat Res. 2018;476(8):1680–1684. doi: 10.1097/01.blo.0000534679.75870.5f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka K, Nishigami T, Mibu A, et al. Validation of the Japanese version of the central sensitization inventory in patients with musculoskeletal disorders. PLoS One. 2017;12(12):e0188719. doi: 10.1371/journal.pone.0188719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seki T, Hasegawa Y, Ikeuchi K, Ishiguro N, Hiejima Y. Reliability and validity of the Japanese Orthopaedic Association hip disease evaluation questionnaire (JHEQ) for patients with hip disease. J Orthop Sci. 2013;18(5):782–787. doi: 10.1007/s00776-013-0436-8 [DOI] [PubMed] [Google Scholar]
- 19.Evans JD. Straightforward Statistics for the Behavioral Sciences. Belmont, CA, USA: Thomson Brooks/Cole Publishing; 1996. [Google Scholar]
- 20.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. doi: 10.3758/BF03193146 [DOI] [PubMed] [Google Scholar]
- 21.Lundblad H, Kreicbergs A, Jansson KA. Prediction of persistent pain after total knee replacement for osteoarthritis. J Bone Joint Surg Br. 2008;90(2):166–171. doi: 10.1302/0301-620X.90B2.19640 [DOI] [PubMed] [Google Scholar]
- 22.Hochman JR, Davis AM, Elkayam J, Gagliese L, Hawker GA. Neuropathic pain symptoms on the modified painDETECT correlate with signs of central sensitization in knee osteoarthritis. Osteoarthritis Cartilage. 2013;21(9):1236–1242. doi: 10.1016/j.joca.2013.06.023 [DOI] [PubMed] [Google Scholar]
- 23.Kim MS, Koh IJ, Sohn S, Kang BM, Kwak DH, In Y. Central sensitization is a risk factor for persistent postoperative pain and dissatisfaction in patients undergoing revision total knee arthroplasty. J Arthroplasty. 2019;34(8):1740–1748. doi: 10.1016/j.arth.2019.03.042 [DOI] [PubMed] [Google Scholar]
- 24.Bachmeier CJ, March LM, Cross MJ, et al. A comparison of outcomes in osteoarthritis patients undergoing total hip and knee replacement surgery. Osteoarthritis Cartilage. 2001;9(2):137–146. doi: 10.1053/joca.2000.0369 [DOI] [PubMed] [Google Scholar]
- 25.Gudbergsen H, Boesen M, Lohmander LS, et al. Weight loss is effective for symptomatic relief in obese subjects with knee osteoarthritis independently of joint damage severity assessed by high-field MRI and radiography. Osteoarthritis Cartilage. 2012;20(6):495–502. doi: 10.1016/j.joca.2012.02.639 [DOI] [PubMed] [Google Scholar]
- 26.Bedson J, Croft PR. The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet Disord. 2008;9:116. doi: 10.1186/1471-2474-9-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wylde V, Sayers A, Odutola A, Gooberman-Hill R, Dieppe P, Blom AW. Central sensitization as a determinant of patients’ benefit from total hip and knee replacement. Eur J Pain. 2017;21(2):357–365. doi: 10.1002/ejp.929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woolf CJ. Pain: moving from symptom control toward mechanism-specific pharmacologic management. Ann Intern Med. 2004;140(6):441–451. doi: 10.7326/0003-4819-140-8-200404200-00010 [DOI] [PubMed] [Google Scholar]
- 29.Enomoto H, Fujikoshi S, Ogawa K, Tsuji T, Tanaka S. Relationship between pain reduction and improvement in health-related quality of life in patients with knee pain due to osteoarthritis receiving duloxetine: exploratory post hoc analysis of a Japanese Phase 3 Randomized Study. J Pain Res. 2020;13:181–191. doi: 10.2147/JPR.S211072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Enomoto H, Fujikoshi S, Tsuji T, Sasaki N, Tokuoka H, Uchio Y. Efficacy of duloxetine by prior NSAID use in the treatment of chronic osteoarthritis knee pain: a post hoc subgroup analysis of a randomized, placebo-controlled, phase 3 study in Japan. J Orthop Sci. 2018;23(6):1019–1026. doi: 10.1016/j.jos.2018.07.008 [DOI] [PubMed] [Google Scholar]
- 31.Koh IJ, Kim MS, Sohn S, Song KY, Choi NY, In Y. Duloxetine reduces pain and improves quality of recovery following total knee arthroplasty in centrally sensitized patients: a prospective, randomized controlled study. J Bone Joint Surg Am. 2019;101(1):64–73. doi: 10.2106/JBJS.18.00347 [DOI] [PubMed] [Google Scholar]
- 32.den Boer C, Dries L, Terluin B, et al. Central sensitization in chronic pain and medically unexplained symptom research: a systematic review of definitions, operationalizations and measurement instruments. J Psychosom Res. 2019;117:32–40. doi: 10.1016/j.jpsychores.2018.12.010 [DOI] [PubMed] [Google Scholar]