Abstract
Background:
The temporal association of delirium during critical illness with mortality is unclear, along with the associations of hypoactive and hyperactive motoric subtypes of delirium with mortality. We aimed to evaluate the relationship of delirium during critical illness, including hypoactive and hyperactive motoric subtypes, with mortality in the hospital and after discharge up to 1 year.
Methods:
We analyzed a prospective cohort study of adults with respiratory failure and/or shock admitted to university, community, and Veterans Affairs hospitals. We assessed patients’ using the Richmond Agitation-Sedation Scale and the Confusion Assessment Method for the ICU and defined the motoric subtype according to corresponding Richmond Agitation-Sedation Scale if delirium was present. We used Cox proportional hazard models, adjusted for baseline characteristics, coma, and daily hospital events, to determine whether delirium on a given day predicted mortality the following day in patients in the hospital and also to determine whether delirium presence and duration predicted mortality after discharge up to 1 year in patients who survived to hospital discharge. We performed similar analyses for hypoactive and hyperactive subtypes of delirium.
Results:
Among 1,040 critically ill patients, 214 (21%) died in the hospital, and 204 (20%) died out-of-hospital by 1 year. Delirium was common, occurring in 740 (71%) patients for a median [IQR] of 4 [2-7] days. Hypoactive delirium occurred in 733 (70%) patients, and hyperactive occurred in 185 (18%) patients, lasting a median of 3 [2-7] days and 1 [1-2] days, respectively. Delirium on a given day (HR 2.87, 95% CI [1.32-6.21], P=0.008), in particular the hypoactive subtype (HR 3.35, 95% CI [1.51-7.46], P=0.003), was independently associated with increased risk of death the following day in the hospital. Hyperactive delirium was not associated with increased risk of death in the hospital (HR 4.00, 95% CI [0.49-32.51], P=0.19). Among hospital survivors, neither delirium presence (HR 1.01, 95% CI [0.82-1.24], P=0.95) nor duration (HR 0.99, 95% CI [0.97-1.01], P=0.56), regardless of motoric subtype, was associated with mortality after hospital discharge up to 1 year.
Conclusions:
Delirium during critical illness is associated with nearly a three-fold increased risk of death the following day for patients in the hospital but is not associated with mortality after hospital discharge. This finding appears primarily driven by the hypoactive motoric subtype. The independent relationship between delirium and mortality occurs early during critical illness but does not persist after hospital discharge.
Keywords: delirium, mortality, critical illness, coma, shock, acute respiratory failure
INTRODUCTION
Delirium occurs commonly in critically ill patients and is independently associated with worse clinical outcomes, including increased duration of mechanical ventilation and hospital stay, higher cost, and risk of long-term cognitive impairment.1–10 Numerous studies have demonstrated associations between delirium and increased mortality.8,10–13 Our 2004 prospective study of Intensive Care Unit (ICU) patients showed that delirium was predictive of a 3-fold higher risk of mortality within 6 months.14 A subsequent prospective study demonstrated that each additional day with ICU delirium was predictive of a 10% higher risk of death by 12 months.15 More recently, a retrospective multicenter study of >30,000 septic patients found that acute neurologic dysfunction was the organ dysfunction most associated with both increased in-hospital and long-term mortality.16
A large single-center prospective study, however, examined the attributable mortality of delirium across a single ICU17. Their results suggested that the increase in overall mortality in patients with delirium was primarily mediated through a prolonged length of stay in the ICU (and not from a direct association between delirium and ICU mortality), although delirium episodes persisting longer than 2 days did increase risk of ICU mortality beyond prolonging the length of stay. A secondary analysis of a randomized controlled trial found that delirium was not associated with mortality up to 90 days but was unable to adjust for evolving disease course.18 Also in a large prospective study restricted to ICU survivors, the development of delirium during the ICU stay was not associated with post-discharge mortality up to 1 year.19 Additional data indicate potential mortality differences between the hypoactive and hyperactive motoric subtypes of delirium,20–22 but these relationships have not been adequately evaluated in recent large cohorts. Advancements in critical illness and delirium management23 and research, as well as in statistical methods that account for the time varying nature of critical illness,17,19,24 require us to revisit our understanding of the relationship between delirium and mortality.
We, therefore, performed a secondary analysis of a large multicenter multi-ICU prospective cohort of civilians and veterans with daily delirium assessments to test the hypothesis that delirium during critical illness, including hypoactive and hyperactive motoric subtypes, is associated with increased risk of mortality while in the hospital and after discharge.
METHODS
Study Design and Population
This study is a secondary analysis of the multicenter prospective cohort study Bringing to Light the Risk Factors and Incidence of Neuropsychological Dysfunction in ICU Survivors (BRAIN-ICU) study (NCT00392795) conducted at Vanderbilt University Medical Center and Saint Thomas Hospital (both Nashville, TN, USA) and the parallel (identical protocol) MIND-ICU Study: Delirium and Dementia in Veterans Surviving ICU Care study (NCT00400062) conducted at the Tennessee Valley Healthcare System Nashville VA Medical Center (Nashville, TN, USA), George E. Wahlen Department of VA Medical Center in VA Salt Lake City Health Care System (Salt Lake City, UT, USA), and Seattle Division of the VA Puget Sound Health Care System (Seattle, WA, USA). Results from these studies have been previously reported, but the hypotheses and findings presented herein are original and have not been previously published.4,6,9,25 This manuscript adheres to the applicable STROBE guidelines.
Adult medical and surgical ICU patients with respiratory failure and/or shock were eligible for enrollment into the BRAIN-ICU and MIND-ICU studies as previously described. Given their interest in examining new cognitive impairment after critical illness, these studies excluded patients with prolonged critical illness prior to evaluation, including those who had been in the ICU >5 days in the month prior to current admission, mechanical ventilation in the past 2 months, patients who were unlikely to survive for 24 hours or who had >72 hours with organ dysfunction during the current ICU admission prior to enrollment. These studies also excluded patients at high risk for preexisting cognitive deficits owing to neurodegenerative disease, cardiac surgery within the previous 3 months, suspected anoxic brain injury, or severe dementia. Patients who could not be reliably assessed for delirium or cognitive impairment owing to deafness or inability to speak English, and patients in whom it would be difficult to complete follow-up were also excluded. We obtained written informed consent from all patients or their authorized surrogates; if consent was initially obtained from a surrogate, we obtained consent from the patient once the patient was deemed competent. Each local institutional review board approved the study protocol.
Exposures
Trained research assistants evaluated each patient’s mental status after enrollment for delirium and level of consciousness using the Confusion Assessment Method for the ICU (CAM-ICU)26 and Richmond Agitation-Sedation Scale (RASS).27 We performed assessments twice daily during ICU admission and once daily after ICU discharge while on the ward until hospital discharge (for up to 30 days). We considered delirium present for that day if the patient was not comatose (i.e., had a RASS of -3 or more awake) and was CAM-ICU positive on either of the assessments. We defined motoric subtype presentation according to the corresponding RASS score on assessments when the CAM-ICU was positive. We considered hypoactive delirium present if the corresponding RASS score was ≤ 0. We considered hyperactive delirium present if the corresponding RASS score was > 0. Because we considered each delirium assessment as independent for these motoric subtypes, a patient could have a day with neither hypoactive nor hyperactive delirium, with either hypoactive or hyperactive delirium, or with both hypoactive and hyperactive delirium on a given day for statistical analyses. This provided increased granularity to test associations compared to classifying patients as ‘mixed’ motoric subtype. We considered coma present for that day if the patient had a RASS of -4 (responsive to physical stimulus only) or -5 (completely unresponsive) on at least one assessment and did not have delirium on that day. We considered normal mental status present if the patient had neither delirium nor coma on a given day.
Outcomes
We examined daily mortality during the hospital stay among all patients who were enrolled in the study and mortality at 1 year among those patients who survived to hospital discharge.
Covariates
We collected sociodemographic data upon enrollment and hospital course data from admission until discharge, death, or a maximum of 30 days after enrollment. We chose the covariates a priori based on clinical judgment and previous research suggesting they may confound the associations between delirium and mortality. Baseline covariates included age, race, sex, years of education, comorbid disease burden per the Charlson comorbidity index, pre-existing cognitive deficit per the Short Form Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE-SF) score, frailty per the Canadian Study on Health & Aging (CSHA) Clinical Frailty Scale, Agency for Healthcare Research and Quality socioeconomic status (AHRQ SES) index, and hospital type (i.e., Veteran’s Affairs hospital or civilian hospital). In-hospital covariates collected daily included severity of illness per the mean modified Sequential Organ Failure Assessment (SOFA) score (excluding the Glasgow Coma Scale score since we adjusted for delirium and coma separately), severe sepsis (presence of infection and ≥2 systemic inflammatory response syndrome features, plus signs of organ dysfunction, including mechanical ventilation, cardiovascular or renal SOFA score > 2, or delirium or coma), mechanical ventilation, exposure to intravenous benzodiazepines (in midazolam equivalents in mg where 2.5 mg midazolam = 1 mg lorazepam = 5 mg diazepam), exposure to propofol (mg), and exposure to intravenous opioids (in fentanyl equivalents in mcg where 100 mcg fentanyl = 0.75 mg hydromorphone = 5 mg morphine). Exposure to dexmedetomidine was too infrequent in this cohort to be included as a covariate.
Statistical Analysis
We designed analyses to account for the time-varying nature of critical illness, the competing risk of death, and that patients must be alive to have delirium with longer survival providing increased opportunity for delirium development or for longer delirium duration. To test the association between delirium status on a given day and risk of death the following day for patients in the hospital (i.e., daily mortality during the hospital), we performed Cox proportional hazard analyses with time-dependent exposure including delirium (yes/no) on the given day, coma (yes/no) on the given day, and adjusting for baseline covariates and the value of the daily covariates on the given day. To test the association between delirium and risk of death after discharge up to 1 year (i.e., mortality between discharge and 1 year for those who survived to hospital discharge), we conducted two Cox proportional hazard analyses including delirium, coma, and adjusting for baseline covariates and the mean value of the daily covariates in patients who survived to hospital discharge. First, we determined the association between delirium presence in the hospital (i.e., ever/never) and mortality after discharge up to 1 year. Second, we determined the association between duration of delirium in the hospital (i.e., the overall number of days with delirium) and mortality after discharge up to 1 year.
To test the association between delirium motoric subtype on a given day and risk of death the following day for patients in the hospital, we performed Cox proportional hazard analyses with time-dependent exposure including hypoactive delirium (yes/no) on the given day, hyperactive delirium (yes/no) on the given day, coma (yes/no) on the given day, and adjusting for baseline covariates and the value of the daily covariates on the given day. To test the association between delirium motoric subtype and risk of death after discharge up to 1 year for those who survived to hospital discharge, we conducted Cox proportional hazard analyses including hypoactive delirium, hyperactive delirium, coma, and adjusting for baseline covariates and the mean value of the daily covariates. We first determined the association between hypoactive delirium presence (i.e., ever/never), hyperactive delirium presence (i.e., ever/never), and mortality after discharge up to 1 year. We then determined the association between duration of hypoactive delirium (i.e., the total number of days with hypoactive delirium), duration of hyperactive delirium (i.e., the total number of days with hyperactive delirium), and mortality after discharge up to 1 year.
We transformed sedative drug doses by taking the cube root in order to improve model fit and reduce the influence of extreme values. The daily amount of each sedative drug exposure was used as a covariate in the daily mortality models, and the mean value of each sedative drug exposure in the ICU was used in the post-discharge mortality models. In all analyses, we modeled continuous variables with the use of restricted cubic splines to allow for nonlinear associations. To reduce bias from missing data, we used multiple imputation to account for missing covariates (<3% for all variables). Patients discharged alive were censored at hospital discharge for analyses of daily mortality in the hospital. We used R version 3.4.1 for all statistical analyses. We present estimated cause-specific hazard ratios (95% confidence interval [CI]), calculated all p-values using the likelihood method, and considered P<0.05 to be significant for independent variables.
Role of Funding Source
The funding agencies of the study had no role in study design, data collection, data analysis, data interpretation, writing, or submission of the report.
RESULTS
Between March 2007 and May 2010, we enrolled 1,046 patients; 6 patients withdrew consent (Figure 1). Data regarding screened and excluded patients were previously reported.4,6,9 Thus, we included 1,040 patients with a median (interquartile range) age of 62 (53-72) years and a high severity of illness for this study (Table 1). Of those enrolled, 214 (21%) died within the hospital and 204 (20%) died between discharge and 1-year follow-up, for an overall 1-year mortality of 418 (40%) patients.
Figure 1. Study Flow Diagram.
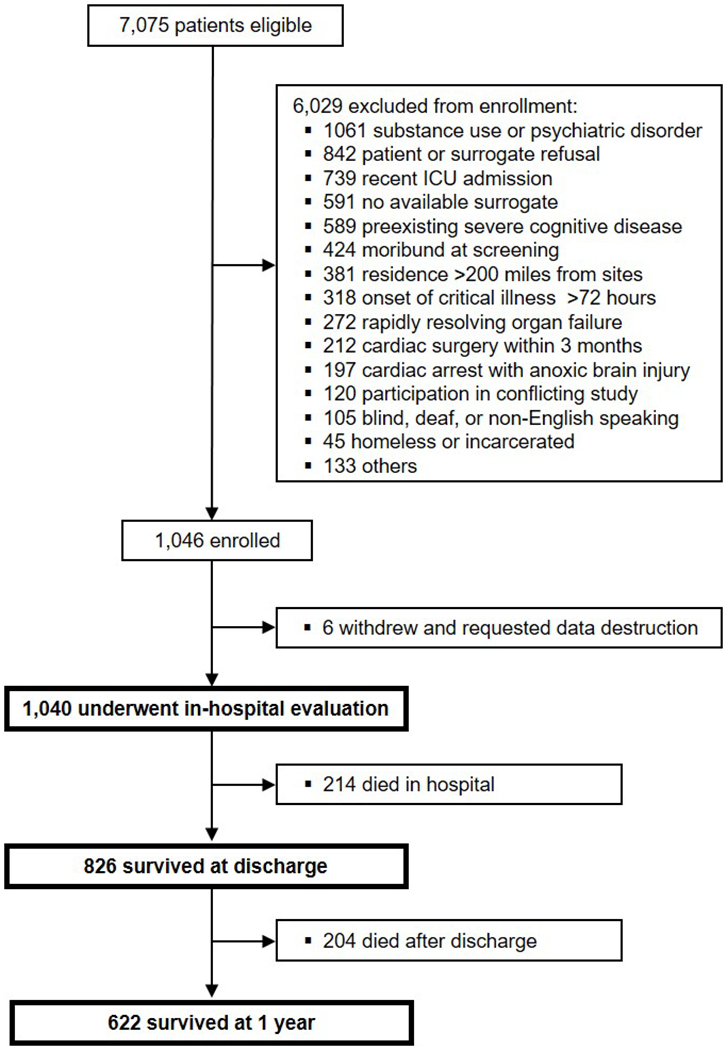
This multicenter prospective cohort study enrolled 1,040 patients who underwent in-hospital evaluation. Within this cohort, 214 (21%) died in-hospital, and another 204 (20%) died out-of-hospital by 1 year.
Table 1.
Characteristics and Outcomes of Study Population
| Characteristic* | Without Delirium N=300 |
With Delirium N=740 |
Entire Cohort N=1040 |
|---|---|---|---|
| Age at enrollment, years | 61 (51-71) | 63 (54-72) | 62 (53-72) |
| White race, N (%) | 275 (92%) | 671 (91%) | 946 (91%) |
| Male sex, N (%) | 192 (64%) | 435 (59%) | 627 (60%) |
| Education, years | 12 (12-14) | 12 (12-14) | 12 (12-14) |
| AHRQ Socioeconomic Index | 50 (47-53) | 50 (47-53) | 50 (47-53) |
| IQCODE-SF at enrollment | 3.0 (3.0-3.1) | 3.0 (3.0-3.2) | 3.0 (3.0-3.2) |
| Katz ADL at enrollment | 0 (0-1) | 0 (0-1) | 0 (0-1) |
| FAQ at enrollment | 0 (0-2) | 0 (0-3) | 0 (0-3) |
| Clinical Frailty Scale at enrollment, N (%) | |||
| ▪ Very fit | 9 (3%) | 22 (3%) | 31 (3%) |
| ▪ Well | 36 (12%) | 97 (13%) | 133 (13%) |
| ▪ Well, treated comorbid disease | 108 (36%) | 247 (33%) | 355 (34%) |
| ▪ Apparently vulnerable | 61 (20%) | 153 (21%) | 214 (21%) |
| ▪ Mildly frail | 46 (15%) | 94 (13%) | 140 (13%) |
| ▪ Moderately frail | 30 (10%) | 105 (14%) | 135 (13%) |
| ▪ Severely frail | 10 (3%) | 22 (3%) | 32 (3%) |
| Charlson comorbidity index | 2 (1-4) | 2 (1-4) | 2 (1-4) |
| Framingham stroke risk | 10 (6-14) | 10 (6-15) | 10 (6-15) |
| SOFA score at enrollment | 8 (6-10) | 9 (7-12) | 9 (7-11) |
| APACHE II at ICU admission | 20 (15-26) | 25 (19-31) | 24 (18-30) |
| ICU type, N (%) | |||
| ▪ Medical | 237 (79%) | 488 (66%) | 725 (70%) |
| ▪ Surgical | 63 (21%) | 252 (34%) | 315 (30%) |
| Severe sepsis on enrollment, N (%) | 138 (47%) | 418 (57%) | 556 (54%) |
| Delirium duration, days | 0 (0-0) | 4 (2-7) | 4 (2-7) |
| Coma, N (%) | 98 (33%) | 522 (71%) | 620 (60%) |
| ▪ Duration among exposed, days | 2 (1-4) | 3 (2-6) | 3 (1-6) |
| ICU length of stay, days | 2.8 (1.5-4.9) | 6.9 (3.8-14.1) | 5.0 (2.8-11.1) |
| In-hospital mortality, N (%) | 65 (22%) | 149 (20%) | 214 (21%) |
| 12-month mortality, N (%) | 113 (38%) | 305 (41%) | 418 (40%) |
Median (interquartile range) or N (percentage).
Participant characteristics and mortality outcomes of the cohort are displayed. Summary statistics are reported for non-missing values. Percents may not total 100 because of rounding.
Abbreviations: ADL, activities of daily living; AHRQ, Agency for Healthcare Research and Quality; APACHE, Acute Physiology and Chronic Health Evaluation; FAQ, Functional Activities Questionnaire; ICU, intensive care unit; IQCODE-SF, Short Form Informant Questionnaire on Cognitive Decline in the Elderly; SOFA, Sequential Organ Failure Assessment.
Delirium was common, occurring in 740 (71%) patients for a median [IQR] of 4 [2-7] days. Patients with delirium were more commonly septic at enrollment, had more coma, and had longer ICU length of stay than those without delirium (Table 1). Hypoactive delirium occurred in 733 (70%) patients, and hyperactive occurred in 185 (18%) patients, lasting a median of 3 [2-7] days and 1 [1-2] days, respectively. Patient characteristics and outcomes stratified by motoric subtype are displayed in Supplemental Appendix Table 1. Patients with hypoactive delirium were more commonly female, had less coma, had shorter overall durations of delirium, and had shorter ICU length of stay compared to patients with hyperactive delirium.
Daily mortality in the hospital
In the hospital, delirium on a given day was independently associated with a three-fold increase in the risk of death the following day (HR 2.87, 95% CI [1.32-6.21], P=0.008) after adjusting for coma status and baseline and daily hospital course covariates (Table 2, Figure 2). Likewise, hypoactive delirium on a given day was associated with a three-fold increase in the risk of death the following day in the hospital (HR 3.35, 95% CI [1.51-7.46], P=0.003, Table 2, Figure 2). Hyperactive delirium, however, was not associated with an increased risk of death the following day in the hospital (HR 4.00, 95% CI [0.49-32.51], P=0.19), though the confidence interval was imprecise (Table 2, Figure 2).
Table 2.
Relationship of Delirium Presence and Duration with Mortality
| In-hospital Mortality the Following Day | Hazard Ratio (95% CI) |
P-value |
|---|---|---|
| Delirium on a given day, yes vs. no | 2.87 (1.32-6.21) | 0.008 |
|
| ||
| Hypoactive delirium | 3.35 (1.51-7.46) | 0.003 |
| Hyperactive delirium | 4.00 (0.49-32.51) | 0.19 |
| Post-discharge Mortality up to 1 Year | Hazard Ratio (95% CI) |
P-value |
|
| ||
| Delirium presence, yes vs. no | 1.01 (0.82-1.24) | 0.95 |
|
| ||
| Hypoactive delirium | 1.00 (0.81-1.23) | 0.99 |
| Hyperactive delirium | 0.78 (0.11-5.73) | 0.81 |
|
| ||
| Delirium duration, each additional day | 0.99 (0.97-1.01) | 0.56 |
|
| ||
| Hypoactive delirium | 1.00 (0.98-1.02) | 0.74 |
| Hyperactive delirium | 0.99 (0.82-1.19) | 0.89 |
The results of Cox proportional hazard analyses testing the independent associations between delirium on a given day and risk of death the following day for patients in the hospital are displayed, including for motoric subtypes. Delirium on a given day, in particular hypoactive delirium, was independently associated with approximately 3-fold increased risk of death the following day. In addition, the results of Cox proportional hazard analyses (restricted to those who survived to hospital discharge) testing the independent associations between delirium presence and duration in the hospital and risk of death after hospital discharge up to 1 year are shown. Neither delirium presence nor duration, regardless of subtype, was associated with post-discharge mortality up to 1 year. Analyses are adjusted for coma and baseline and daily hospital course covariates.
Figure 2. Relationship of Delirium Presence and Duration with Mortality.
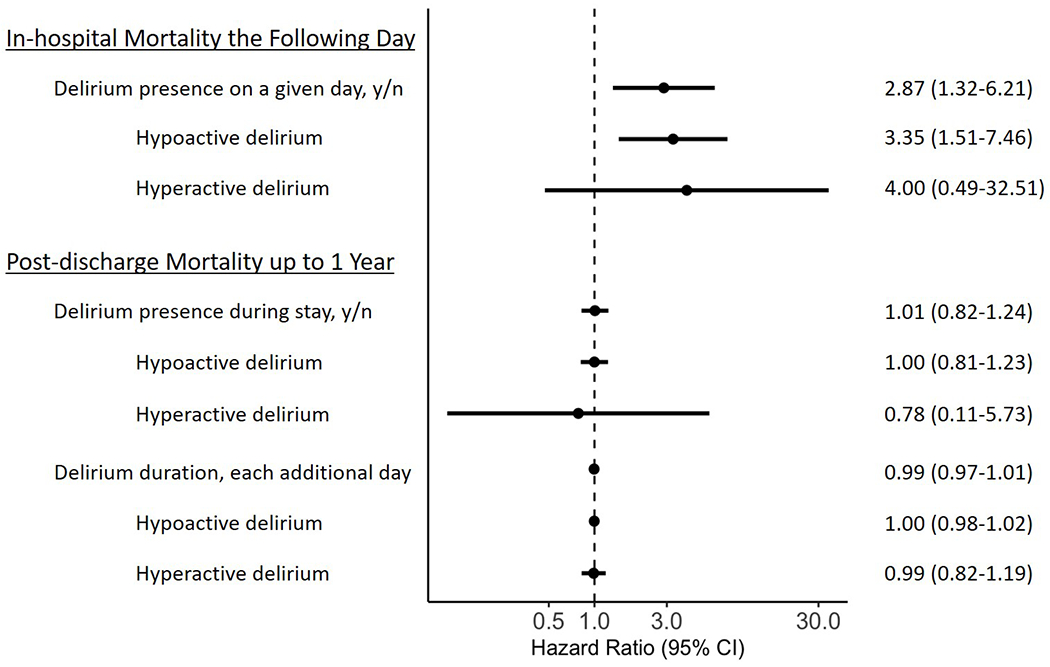
The figure displays the results of Cox proportional hazard analyses testing the independent associations between delirium on a given day and risk of death in the hospital the following day for patients in the hospital and the results of Cox proportional hazard analyses testing the independent associations between delirium presence and duration in the hospital and risk of death after discharge up to 1 year for those who survived to hospital discharge. Analyses are adjusted for coma and baseline and daily hospital course covariates. Delirium on a given day, in particular hypoactive delirium, was independently associated with approximately 3-fold increased risk of death the following day. Neither delirium presence nor duration, regardless of subtype, was associated with post-discharge mortality up to 1 year.
Mortality after discharge up to 1 year
In those patients surviving to hospital discharge, neither delirium presence (HR 1.01, 95% CI [0.82-1.24], P=0.95) nor delirium duration (HR 0.99, 95% CI [0.97-1.01], P=0.56) was associated with mortality after discharge up to 1 year after adjusting for coma and baseline and mean hospital course covariates (Table 2, Figure 2). Additionally, neither hypoactive delirium (HR 1.00, 95% CI [0.81-1.23], P=0.99 for presence and HR 1.00, 95% CI [0.98-1.02], P=0.74 for duration) nor hyperactive delirium (HR 0.78, 95% CI [0.11-5.73], P=0.81 for presence and HR 0.99, 95% CI [0.82-1.19], P=0.89 for duration) presence or duration was associated with mortality after discharge up to 1 year in hospital survivors (Table 2, Figure 2).
DISCUSSION
In this large multicenter prospective cohort of adults admitted to the ICU for acute respiratory failure and/or shock with delirium assessments by trained research personnel and extensive daily in-hospital data collection, we found that delirium on a given day was independently associated with approximately 3-fold increased risk of death the following day in the hospital. The hypoactive motoric subtype of delirium, in particular, demonstrated an increased risk of death in the hospital. Among those who survived the index hospitalization, we did not find evidence that delirium presence or duration, regardless of motoric subtype, was associated with mortality after discharge up to 1 year. Thus, the independent relationship between delirium and mortality appears to occur early during critical illness but does not persist after hospital discharge.
Our findings build upon and extend those of prior studies of the relationship between ICU delirium and mortality.8,11,12,14,15,18 The first studies were important in demonstrating the potential long-lasting ramifications of acute brain dysfunction, but these studies occurred in a period of different sedation strategies, ICU management, and reduced delirium awareness. Moreover, statistical modeling in these early studies did not account for the time-varying nature of critical illness nor the competing risk of death. Further, it is important to account for immortal time bias in such analyses.28 Our study and other complementary investigations17,19 have tried to address these limitations but have important differences. We demonstrated an association between delirium and increased mortality while in the hospital, but in patients who survived to hospital discharge, neither the development of delirium nor the duration of delirium was associated with increased mortality after discharge up to 1 year. We used RASS and CAM-ICU assessments by trained research personnel to determine daily mental status, regardless of whether patients were receiving sedation. In our models, delirium and coma were considered separately, and we adjusted for daily sedation exposure. We additionally examined daily mortality throughout the hospital stay and not only in the ICU. Overall, our findings indicate that delirium is associated with increased mortality surrounding the critical illness but that this risk does not continue long-term in survivors.
Klouwenberg et al17 used marginal structural modeling to determine the ICU mortality attributable to delirium in a cohort of approximately 1,100 patients with critical illness. They found that overall delirium presence was associated with prolonged ICU stay without being associated with increased mortality in the ICU. Episodes of delirium that persisted for more than two days (which commonly occurs) were, however, independently associated with increased mortality in the ICU beyond prolonging the ICU stay. CAM-ICU assessments were performed by bedside nurses and additional delirium diagnoses were bestowed upon patients if there was a description in the medical record of fluctuating level of consciousness, agitation, disorientation, or hallucinations or if patients were administered haloperidol or quetiapine. If a patient received propofol or midazolam infusion within 48 hours of assessment, those patients were classified as sedated (i.e., not delirious). They further classified comatose patients (without sedation) as delirious. These methodological differences likely explain the contrasting results to our finding that delirium during critical illness was associated with increased mortality in the hospital. Our finding that delirium was not associated with post-discharge mortality in survivors of critical illness supports the results of Wolters et al19 who examined the association of delirium with post-discharge mortality in a prospective cohort of approximately 1,100 ICU survivors. They found that ICU delirium presence was not associated with post-discharge mortality up to 1 year. They also utilized CAM-ICU assessments by bedside nurses and considered haloperidol administration as indication of being positive for delirium and dichotomized patients by delirium presence. Despite some methodological differences, our study findings support and build upon those of the Wolters study.
We found that hypoactive delirium was associated with increased risk of mortality in the hospital, expanding current research into the potential disparate outcomes between motoric subtypes of delirium.20–22 It is possible that patients with hypoactive delirium have more profound pathophysiologic changes in the central nervous system or increased vulnerability factors that portend worse outcomes. In contrast, we did not find significant evidence that hyperactive delirium is associated with greater risk of mortality in the hospital. It is important to note that this cohort overall had fewer patients with hyperactive delirium compared to hypoactive delirium, reducing the ability to fully test the impact of hyperactive delirium. We attempted to capture the episodes of hyperactive delirium by accounting for motoric subtype per assessment and allowing patients to have both hypoactive and hyperactive delirium on a given day and not converge patients with both into a ‘mixed’ motoric subtype. As we assessed patients twice daily for delirium, we may have missed episodes of hyperactive delirium, including those that were treated with antipsychotics or sedatives and potentially converted to hypoactive delirium. Further research will be required to unravel the patient factors, hospital course, and physiology that contribute to different motoric presentations and outcomes, and future trials and patient care should consider extending delirium assessments to include motoric subtype and severity.29–31
Our present study has strengths and limitations that must be considered. Strengths include a large cohort of patients with respiratory failure and/or shock with a wide range of diagnoses who were admitted to an academic hospital, a community hospital, and 3 Veterans Affairs hospitals representing multiple ICUs. Results, however, may not apply to populations with high rates of substance abuse or psychiatric disorders or other groups excluded from this cohort, along with ICUs caring for patients with lower rates of sepsis or lower overall severity of illness. We used modern statistical techniques which account for the competing risk of death and adjusted for important potential confounders of the association between delirium and mortality, including coma, sedative exposure, and severity of illness. Research nurse evaluations of mental status provided us robust delirium assessments but might have led to missed episodes of delirium compared to more frequent bedside nursing assessments. We did not coordinate our delirium assessments with spontaneous awakening trials and, therefore, might have captured rapidly reversible sedation-related delirium. We did account for daily sedation exposure in all analyses, and if patients with rapidly reversible delirium have improved outcomes compared to those with persistent delirium,32 their inclusion would have biased our results towards the null. Although 18% of patients had hyperactive delirium during the study, the number of days with hyperactive delirium was 359 compared to 3978 days with hypoactive delirium, reducing our ability to draw conclusions between hyperactive delirium and mortality. Despite the reduced presence of documented hyperactive delirium, our study was still one of the largest to consider the association between hyperactive delirium and mortality and included 185 patients with hyperactive delirium. Finally, this observational cohort could not assess causality between delirium and mortality, and while we adjusted for potential confounders using robust statistical techniques to determine the independent effect of delirium on mortality, our results may still be subject to unknown confounding.
In conclusion, it is consistent across the literature, even after adjusting for severity of illness markers and accounting for immortal time bias, that delirium during critical illness is independently associated with a higher likelihood of dying in the hospital. This predictive relationship appears primarily driven by hypoactive delirium, though lower capture of hyperactive delirium limits ability to test for its significance. Among survivors of hospitalization for critical illness, we did not find evidence that delirium presence or duration was associated with mortality after discharge up to 1 year, regardless of motoric subtype. Overall, the prognostic implications of delirium as a manifestation of acute organ dysfunction are important and drive recommendation of guidelines23 for daily monitoring in all ICU patients and diligent management towards reductions in onset and duration of this untoward development in the clinical course of our sickest patients.
Supplementary Material
Key Points Summary.
Question: The temporal association of delirium during critical illness with mortality is unclear, along with the associations of hypoactive and hyperactive motoric subtypes of delirium with mortality.
Findings: Delirium during critical illness, including the hypoactive but not the hyperactive subtype, is associated with nearly a three-fold increased risk of death the following day in the hospital but is not associated with mortality after hospital discharge.
Meaning: The independent relationship between delirium and mortality occurs early during critical illness, appears primarily driven by the hypoactive subtype, but does not persist after hospital discharge.
Acknowledgements
We acknowledge our research coordinators Leanne Boehm, PhD, RN, ACNS-BC; Brenda Truman Pun, RN, MSN; and Cayce Strength, RSN, BSN who performed project management, data acquisition, and data management. We acknowledge our neuropsychology staff Amy Kiehl, MA, LPC-MHSP who performed survival determination after discharge.
Funding
This project was supported by the National Institute on Aging (AG027472 and AG045085). In addition, Dr. Hughes received support from American Geriatrics Society Jahnigen Career Development Award and the National Institutes of Health (HL111111, AG045085, GM120484), Dr. Pandharipande received support from the National Institutes of Health (AG027472, AG035117, HL111111, GM120484, AG058639), Dr. Brummel received support from the National Institutes of Health TR00046, AG0450495, AG054864, the Doris Duke Foundation, and by the Vanderbilt Clinical and Translational Scholars Program, Dr. Girard received support from the National Institutes of Health (AG034257, AG035117, HL135144), Dr. Ely received support from the VA Clinical Science Research and Development Service and the National Institutes of Health (AG027472, AG035117, HL111111, GM120484, AG058639), Dr. Patel received support from the National Institutes of Health (HL111111, GM120484, AG058639). Drs. Ely and Girard also received support from the Veterans Affairs Tennessee Valley Geriatric Research, Education and Clinical Center. We used REDCap, a secure online database, supported in part by the National Institutes of Health TR000445.
Conflicts of Interest
Drs. Hughes, Jackson, and Ely received research grant from Dr. Franz Kohler Chemie GMBH. Dr. Pandharipande and Ely received research grant from Hospira Inc and Masimo. Dr. Brummel reports receiving honoraria for advisory board activities from Arjo and Pfizer. Dr. Ely reports receiving honoraria from Pfizer for educational programs. Dr. Patel received research support from Haemonetics.
Glossary of Terms
- ADL
activities of daily living
- AHRQ SES
Agency for Healthcare Research and Quality socioeconomic status
- APACHE
Acute Physiology and Chronic Health Evaluation
- BRAIN-ICU
Bringing to Light the Risk Factors and Incidence of Neuropsychological Dysfunction in ICU Survivors
- CAM-ICU
Confusion Assessment Method for the ICU
- CI
confidence interval
- CSHA
Canadian Study on Health & Aging
- FAQ
Functional Activities Questionnaire
- HR
hazard ratio
- ICU
intensive care unit
- IQCODE-SF
Short Form Informant Questionnaire on Cognitive Decline in the Elderly
- IQR
interquartile range
- MIND-ICU
Delirium and Dementia in Veterans Surviving ICU Care
- RASS
Richmond Agitation-Sedation Scale
- SOFA
Sequential Organ Failure Assessment
Footnotes
Trial Registration: NCT00392795 and NCT00400062
REFERENCES
- 1.Milbrandt EB, Deppen S, Harrison PL, et al. Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004;32(4):955–962. [DOI] [PubMed] [Google Scholar]
- 2.Ely EW, Gautam S, Margolin R, et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27(12):1892–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ouimet S, Kavanagh BP, Gottfried SB, Skrobik Y. Incidence, risk factors and consequences of ICU delirium. Intensive Care Med. 2007;33(1):66–73. [DOI] [PubMed] [Google Scholar]
- 4.Pandharipande PP, Girard TD, Jackson JC, et al. Long-Term Cognitive Impairment after Critical Illness. The New England journal of medicine. 2013;369(14):1306–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive trajectories after postoperative delirium. The New England journal of medicine. 2012;367(1):30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes CG, Patel MB, Jackson JC, et al. Surgery and Anesthesia Exposure Is Not a Risk Factor for Cognitive Impairment After Major Noncardiac Surgery and Critical Illness. Ann Surg. 2017;265(6):1126–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lingehall HC, Smulter NS, Lindahl E, et al. Preoperative Cognitive Performance and Postoperative Delirium Are Independently Associated With Future Dementia in Older People Who Have Undergone Cardiac Surgery: A Longitudinal Cohort Study. Crit Care Med. 2017;45(8):1295–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salluh JI, Wang H, Schneider EB, et al. Outcome of delirium in critically ill patients: systematic review and meta-analysis. BMJ. 2015;350:h2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Girard TD, Thompson JL, Pandharipande PP, et al. Clinical phenotypes of delirium during critical illness and severity of subsequent long-term cognitive impairment: a prospective cohort study. Lancet Respir Med. 2018;6(3):213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abelha FJ, Luis C, Veiga D, et al. Outcome and quality of life in patients with postoperative delirium during an ICU stay following major surgery. Crit Care. 2013;17(5):R257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Rompaey B, Schuurmans MJ, Shortridge-Baggett LM, Truijen S, Elseviers M, Bossaert L. Long term outcome after delirium in the intensive care unit. J Clin Nurs. 2009;18(23):3349–3357. [DOI] [PubMed] [Google Scholar]
- 13.Shehabi Y, Riker RR, Bokesch PM, Wisemandle W, Shintani A, Ely EW. Delirium duration and mortality in lightly sedated, mechanically ventilated intensive care patients. Crit Care Med. 2010;38(12):2311–2318. [DOI] [PubMed] [Google Scholar]
- 14.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–1762. [DOI] [PubMed] [Google Scholar]
- 15.Pisani MA, Kong SY, Kasl SV, Murphy TE, Araujo KL, Van Ness PH. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009;180(11):1092–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuler A, Wulf DA, Lu Y, et al. The Impact of Acute Organ Dysfunction on Long-Term Survival in Sepsis. Crit Care Med. 2018;46(6):843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein Klouwenberg PM, Zaal IJ, Spitoni C, et al. The attributable mortality of delirium in critically ill patients: prospective cohort study. BMJ. 2014;349:g6652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duprey MS, van den Boogaard M, van der Hoeven JG, et al. Association between incident delirium and 28- and 90-day mortality in critically ill adults: a secondary analysis. Crit Care. 2020;24(1):161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolters AE, van Dijk D, Pasma W, et al. Long-term outcome of delirium during intensive care unit stay in survivors of critical illness: a prospective cohort study. Crit Care. 2014;18(3):R125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avelino-Silva TJ, Campora F, Curiati JAE, Jacob-Filho W. Prognostic effects of delirium motor subtypes in hospitalized older adults: A prospective cohort study. PLoS One. 2018;13(1):e0191092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim SY, Kim SW, Kim JM, et al. Differential Associations Between Delirium and Mortality According to Delirium Subtype and Age: A Prospective Cohort Study. Psychosom Med. 2015;77(8):903–910. [DOI] [PubMed] [Google Scholar]
- 22.Robinson TN, Raeburn CD, Tran ZV, Brenner LA, Moss M. Motor subtypes of postoperative delirium in older adults. Arch Surg. 2011;146(3):295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Devlin JW, Skrobik Y, Gelinas C, et al. Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit Care Med. 2018;46(9):e825–e873. [DOI] [PubMed] [Google Scholar]
- 24.Colantuoni E, Scharfstein DO, Wang C, et al. Statistical methods to compare functional outcomes in randomized controlled trials with high mortality. BMJ. 2018;360:j5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson JC, Pandharipande PP, Girard TD, et al. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. Lancet Respir Med. 2014;2(5):369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001;286(21):2703–2710. [DOI] [PubMed] [Google Scholar]
- 27.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338–1344. [DOI] [PubMed] [Google Scholar]
- 28.Shintani AK, Girard TD, Eden SK, Arbogast PG, Moons KG, Ely EW. Immortal time bias in critical care research: application of time-varying Cox regression for observational cohort studies. Crit Care Med. 2009;37(11):2939–2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inouye SK, Kosar CM, Tommet D, et al. The CAM-S: development and validation of a new scoring system for delirium severity in 2 cohorts. Annals of internal medicine. 2014;160(8):526–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khan BA, Perkins AJ, Gao S, et al. The Confusion Assessment Method for the ICU-7 Delirium Severity Scale: A Novel Delirium Severity Instrument for Use in the ICU. Crit Care Med. 2017;45(5):851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krewulak KD, Stelfox HT, Leigh JP, Ely EW, Fiest KM. Incidence and Prevalence of Delirium Subtypes in an Adult ICU: A Systematic Review and Meta-Analysis. Crit Care Med. 2018;46(12):2029–2035. [DOI] [PubMed] [Google Scholar]
- 32.Patel SB, Poston JT, Pohlman A, Hall JB, Kress JP. Rapidly reversible, sedation-related delirium versus persistent delirium in the intensive care unit. Am J Respir Crit Care Med. 2014;189(6):658–665. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


