Abstract
Tendinopathy is a debilitating disease that causes as much as 30% of all musculoskeletal consultations. Existing treatments for tendinopathy have variable efficacy, possibly due to incomplete characterization of the underlying pathophysiology. Mechanical load can have both beneficial and detrimental effects on tendon, as the overall tendon response depends on the degree, frequency, timing, and magnitude of the load. The clinical continuum model of tendinopathy offers insight into the late stages of tendinopathy, but it does not capture the subclinical tendinopathic changes that begin before pain or loss of function. Small animal models that use high tendon loading to mimic human tendinopathy may be able to fill this knowledge gap. The goal of this review is to summarize the insights from in-vivo animal studies of mechanically-induced tendinopathy and higher loading regimens into the mechanical, microstructural, and biological features that help characterize the continuum between normal tendon and tendinopathy.
Keywords: Tendon, Tendinopathy, Small Animal, Mechanics, Biology
Graphical Abstract
1. Introduction
The prevalence of tendinopathy has been reported up to 3.8%[1] in the general population with 30% of visits for musculoskeletal pain in general practice resulting from tendinopathy[2, 3]. Clinically, tendinopathy is characterized by tendon pain during activity, localized tenderness upon palpation, swelling, and impaired performance[4]. Treatment strategies for tendinopathy vary[2, 5], and efficacy may depend on the tendon injured[6]. Exercise rehabilitation is broadly accepted as a primary strategy, but the most effective treatment protocol depends on the patient factors such as pain, kinesiophobia (the fear of motion), range of motion, and muscle capacity[7]. Prevention, diagnosis, and treatment of tendinopathy depend on improving our understanding of the pathophysiology and etiology of tendon injury.
Models of early-stage tendinopathy are limited because human data are restricted to late-stage tendinopathy, as clinical presentation occurs after the initiation of pain or restricted function. Although novel measurement techniques permit analysis of earlier timepoints[8–10], the lack of clinical presentation during early-stage tendinopathy limits investigation into the underlying etiology. Tendinopathy is thought to be caused by repeated overload of the tendon with insufficient recovery time, leading to an inadequate healing response[7] and incomplete recovery of preinjury material strength and function[2, 11]. A sudden exposure to elevated activity may expose the tendon to high risk for damage[12].
The leading clinical model of tendinopathy formulated by Cook et al suggests that the local mechanical environment around tendon cells provokes a cascade of biochemical responses that alter the extracellular matrix (ECM)[4, 13–17], and incorporates pain and the concept of “reactive-on-degenerative” tendon[18]. The Cook model describes overuse tendinopathy as a continuum with three stages: reactive tendinopathy, tendon disrepair, and degenerative tendinopathy[13]. Reactive tendinopathy is the initial response to overload characterized by tendon thickening and a non-inflammatory proliferative response, and the second stage, tendon disrepair, includes microstructural disruption[13]. Ultrasound imaging in humans demonstrates that reactive and disrepair tendons may revert to normal tendons as defined by the absence of hypoechoic regions and/or the reduction of fusiform swelling following loading reduction[10, 19–21]. The final stage is degenerative tendinopathy, characterized by permanent, maximum accumulation of fatigue damage, in which localized areas of cell death and structural degeneration appear[13]. Although purely overloading tendon is considered an unlikely cause of tendinopathy[22], it is clear that tendon loading is a contributing factor that affects the healing response[12].
An increase in tendon-focused research has improved our understanding of tendinopathy. This increase has led to definitions of tendinopathy that implicate a wide variety of factors including accumulated damage[2, 22, 23], altered ECM[15, 24], decreased time to heal[2, 25–27], and increased cell number and cell rounding[13, 28, 29]. More recently, research has identified other possible factors, such as metabolic disease[12, 30], age[22, 31], tendon compression[32, 33], tendon location or function[34], and genetics[22] as possible additions to this list. One perspective regarding the reparability of tendon separates tendon into two discrete parts, intrinsic and extrinsic compartments, that perform different, but coordinated functions during tendon healing[12]. These factors likely all contribute to the development and progression of tendinopathy, but how these factors culminate in altered tendon function and tendon’s inability to recover remains unclear.
Studies performed in animals are commonly designed to mimic central mechanical and structural features of tendinopathy identified from human tendon samples[35–37]. Animal studies aim to recapitulate similar features of tendinopathy in humans, but also enable more mechanistic insight into disease mechanism and careful control of experimental parameters. Onset of tendinopathy typically involves progression of microdamage with repeated loading, which has motivated study of tendons experiencing higher loading (e.g., Achilles tendon (AT) [38], patellar tendon (PT)[39], and rotator cuff [40]). Although tendon is an active tissue that normally responds to various loads[17, 41], the thresholds that define the difference between normal tendon response[2] and the initiation of a healing or reparative response are not well-defined[16]. Some animal studies use an injection, such as TGF-β1 to mimic the biological response of tendinopathy[42, 43], but these studies are not covered in this review. In-vivo small animal models of mechanically-induced tendinopathy offer an attractive framework to examine the cascade of processes that occur throughout tendon pathology and repair[44], and may be invaluable for understanding the interplay between mechanics, biology, and structure.
The goal of this review is to summarize the insights gained from in-vivo animal studies of mechanically-induced tendinopathy. In addition, we examine the impacts that higher loading regimens have on the mechanical, microstructural, and biological features in tendons. High loading was defined as any loading regimen aimed to represent an increase from normal activity. A better understanding of the interplay between these realms could lead to improved patient management, especially in the presence of painful tendon.
2. Mechanical Changes with High Loading
2.1. Tendinopathy Induction – Is Overloading Enough?
Loading can have restorative or detrimental effects on tendon based on multiple factors including magnitude, frequency, duration, and the specific tendon of interest. Physical therapy regimens with eccentric exercises (which lengthen the muscle as resistance increases) have been shown to improve patient symptoms and are central to the treatment of tendinopathy in humans [45–47]. Although eccentric training alone may not improve symptoms for all patients, it is thought that muscle alteration that reduces tendon load due to eccentric training may improve tendon health[48]. Similarly, animal models have shown possible therapeutic effects of eccentric exercise models of tendon loading[49, 50]. However, given that tendon overuse (defined as higher load level and frequency) is accepted as a causative factor for tendinopathy,[12] it is counterintuitive that additional loading through eccentric exercise would improve tendon health.
Although altered response due to higher loading has been explored in multiple studies, a precise threshold between normal use and overuse has proven elusive[15]. The inability to define a threshold may be due to the natural variation of tendon structure for a given tendon within a single species. These differences could explain the varying degree of damage induced by a consistent loading protocol[51, 52]. In comparison to human studies, animal models of tendon loading offer the ability to more precisely control loading and document the effect of the loading regimen on tendon health.
2.2. Effect of High Loading on Cross-Sectional Area
The best characterized macroscopic property of tendinopathy is variation in cross-sectional area (CSA). Normally, healthy tendon adapts to loading without change in CSA[53], but with tendinopathy, tendon CSA increases in order to decrease tendon stress[54]. Reactive tendons may exhibit increased CSA, but further thickening of degenerative tendons is variable, suggesting that the tendon’s response during the late stage of tendinopathy affects CSA differently compared to the earlier stages[13].
Mechanical overuse models in small animals with multiple timepoints have demonstrated sustained significant increases in CSA for two to sixteen weeks of declined treadmill and eccentric loading (Supraspinatus tendon (SS)[55–57], AT [58, 59]). These results suggest that tendons become reactive with only two weeks of overuse loading. However, some studies examining the long term effects of overuse loading using single timepoints at 12 weeks or later have shown no significant change in CSA (AT: inclined treadmill [60] running wheel/ vibration [61], SS: horizontal treadmill [62], calcaneal tendon (CT), superficial flexor tendon (SFT), and deep flexor tendon (DFT): climbing[63]). Unlike reactive tendons, which display homogenous thickening, human tendons in disrepair and degeneration stages exhibit inhomogeneous thickening,[13] which suggest that single timepoint studies may have captured tendon degeneration.
It remains unclear if loading direction, frequency, and magnitude variation affect changes in CSA at different stages of tendinopathy. Studies that applied normal tendon loading (not tendinopathy) have seen different CSA responses for different types of loading. One study found that 5 weeks of eccentric or concentric loading had no effect on rat AT or PT CSA, but increased CSA of tricipital tendons[50]. In separate studies using vibrational loading, different levels of vibration strength training did not affect AT tendon CSA [61]. Eccentric training (AT[50, 58]) and downhill treadmill running (SS[55–57]) provoke an increase in tendon CSA in rats. Although tendon CSA is not altered during uphill treadmill running or climbing (CT, SFT, DFT[63] and AT[60, 61]), the results are mixed for horizontal treadmill running. (AT[49, 64] and SS[62]). The relationship between type and degree of loading as it relates to CSA change may signal the start of tendinopathy, but overall this single measurement alone is insufficient to confirm the presence of tendinopathy or its stage.
2.3. Effect of High Loading on Sub-Failure Mechanical Response
Healthy tendon is known to stiffen in response to normal levels of loading[13], to allow better load transmission without additional change in strain[65]. Tendinopathy often occurs in the absence of trauma, implying a gradual accumulation of micro-injuries[12, 66]. In addition to increased CSA, tendinopathic tendon is expected to stiffen[13], implicating subrupture fatigue damage in the initiation and progression of tendinopathy[2, 23, 67]. Studies applying direct loading to PTs show that higher loading intended to provoke tendinopathy increases tendon modulus[51, 52, 68], but downhill treadmill running decrease tendon modulus (AT[69] and SS[56, 57]). Time may also be a factor, as SS elastic modulus decreases at 4 weeks of downhill running in one study[57], but not until 8 weeks in another[56].
Some studies have investigated the change of tendon stiffness following cyclic loading. The loading and unloading stiffnesses of rat PT exhibit cycle-dependent changes[51]. Initially, unloading stiffness decreases, but decreases with further loading[51]. The initial stiffness reduction is hypothesized to result from an early stage of damage where fibers under greater tension are loaded, whereas the subsequent increase above initial stiffness may result from impaired tendon recoil, in which less tendon deformation is recovered during a loading cycle[51]. Comparable results were found employing a similar protocol, in which loading stiffness increased by more than 24% during the last 100 cycles of a fatigue regimen compared to the initial measurement[70].
Hysteresis is a viscoelastic mechanical property that quantifies the damping capacity of the tissue[51, 71], and is attributed to changes in non-collagenous components in the interfibrillar space[72] and loss of fibril crimp[51]. Studies of fatigue loading of rat PT have examined hysteresis and quantified the change in damping capacity of tendon during the accumulation of subrupture fatigue damage. With high levels of fatigue damage, there is a loss of hysteresis in rat PT[51, 67, 68] and murine PT[70]. Further, the initial hysteresis loss (the change in hysteresis measured at the end of the fatigue loading regimen) is correlated with the stiffness measured seven days later[51], suggesting that the tendon responds to loss of damping capacity during loading by increasing stiffness.
Stiffness and cross-sectional area change do not fully characterize the multifactorial mechanical response of tendinopathic tendon. Further studies are needed to characterize the changes to the complex viscoelastic properties of tendinopathic tendon that include the response to different loading rates and non-zero mean stress for cyclic tests [73].
2.4. Effect of High Loading on Tendon Failure
The ultimate tensile strength (UTS) of tendon reflects the maximum load that a tendon can bear prior to failure. Clinically, loading is thought to increase tendon strength to accommodate higher load. This notion is largely supported by small animal studies[49, 50, 62–64, 74]. Downhill treadmill exercise uniformly decreases UTS of the rat SS[56] and AT[75] after overuse loading, but some forms of exercise do not alter the UTS in one or more groups[50, 61, 63, 64, 74, 76]. Direct fatigue loading to rat PT did not alter failure load[70]. These results suggest that UTS alone may not be an indicator of tendon damage accumulation, and that detrimental tendon overload is unique for different tendons. With the exception of a recent study that used a specimen-specific bone fixation method[77], load-to-failure testing has been criticized for errors imposed by clamping methods.
An unloaded tendon is exposed to a mechanical environment that is similar to ligaments - structures that require static strength from increased collagen matrix deposition[71]. Although not explicitly covered in this review, tendon’s response to unloading may coincide with the reactive-on-degenerative component to Cook’s model[18], where portions of tendon are stress-shielded[78]. In both cases, the portion of tendon under chronically lower loading develops decreased tendon damping capacity, thereby increasing risk of further damage[71].
Although Legerlotz et al[61] reported the animals’ body mass and food intake as a success-measure during training, most small animal studies applying higher load to tendon do not report functional deficits and pain. Given the relevance of pain and function to human tendinopathy, inclusion of these measurements in future small animal studies would improve our understanding of the etiology of tendinopathy.
In small animal studies applying high tendon load, the tendon CSA, modulus, and failure load increase, Table 1. yet, it is unclear whether these changes correspond with the natural response to higher load or the initiation of tendinopathy. The high loading threshold that triggers positive tissue adaptation distinct from tendinopathy remains controversial. Further, macroscopic mechanical properties are insufficient to confirm tendinopathy induction or define the load type and magnitude that initiates a tendinopathic response. In animal studies, pairing multiscale mechanical properties with pain, functional outcomes, structural changes, and biological response may provide a more comprehensive, quantitative measure of the state of tendon structure and the adaptive or pathological responses to higher loading.
Table 1.
Mechanical Changes Induced by High Loading in Small Animal Models
| Study Information |
Mechanical Measurements |
|||||||
|---|---|---|---|---|---|---|---|---|
| Animal | Tendon | Loading Mechanism | Comparison | CSA | Modulus | Hysteresis | Failure Load | Functional Outcome |
| Rats | ||||||||
| SD F Adult [68] | PT | Direct Tendon Loading | 7200 Cycles vs 100 Cycles | - | - | - | ||
| SD F Adult [51] | PT | Direct Tendon Loading | 7200 Cycles vs Other Groups | - | - | - | ||
| SD F Adult [87] | PT | Direct Tendon Loading | High Load Endpoint vs Baseline | - | - | - | ||
| SD M Adult [58] | AT | Eccentric Contraction | Loaded Tendon vs Unloaded Contralateral Thickness* Not CSA | - | - | - | - | |
| SD Adult * Gender not specified [50] | AT | Eccentric and Concentric Training | Eccentric (E) vs Untrained (U) | 3 | - | - | 3 | - |
| ” | AT, PT, or Tri | ” | Concentric (C) vs Untrained (U) | 3 | - | - | 3 | - |
| ” | PT | ” | Eccentric (E) vs Untrained (U) | 3 | - | - | - | |
| ” | Tri | ” | Eccentric (E) vs Untrained (U) | - | - | - | ||
| SD Adult * Gender Not Specified [75] | AT | Downhill Bipedal Running | Trained vs Untrained | - | - | - | ||
| SD M * Age Not Reported [55] | SS | Downhill Treadmill Running + Compression or Injury | Overuse (OV) vs Cage Activity | - | - | |||
| ” | SS | ” | Overuse plus Extrinsic Injury (OV+E) vs Cage Activity | 3 | - | 3 | - | |
| SD Adult * Gender not Specified [57] | SS | Downhill Treadmill Running | Overuse (OV) vs Cage Activity | - | - | |||
| ” | SS | ” | Overuse Plus Extrinsic Injury (OV+E) vs Cage Activity | - | - | |||
| ” | SS | ” | Extrinsic Injury vs Contralateral | 3 | 3 | - | 3 | - |
| SD F Adult [61] | AT | Running Wheel | Trained vs Non-Active Controls | 3 | 3 | 3 | 3 | |
| ” | ” | Vibration Strength-Trained | Vibration Strength-Trained (LVST) vs Non-Active Controls | 3 | 3 | 3 | 3 | 3 |
| ” | ” | High-Vibration Strength-Trained | High-Vibration Strength-Trained (HVST) vs Non-Active Controls | 3 | 3 | 3 | 3 | 3 |
| ” | ” | High Strength-Trained | High Strength-Trained (HST; n = 6) vs Non-Active Controls | 3 | 3 | 3 | 3 | 3 |
| W M Old and Adult [63] | CT | Climbing | Old Trained vs Old Sedentary | 3 | 3 | - | 3 | - |
| ” | SFT | Climbing | Old Trained vs Old Sedentary | 3 | 3 | - | - | |
| ” | DFT | Climbing | Old Trained vs Old Sedentary | 3 | 3 | - | 3 | - |
| ” | CT | Climbing | Young Trained vs Young Sedentary | 3 | 3 | - | - | |
| ” | SFT | Climbing | Young Trained vs Young Sedentary | 3 | 3 | - | 3 | - |
| ” | DFT | Climbing | Young Trained vs Young Sedentary | 3 | 3 | - | - | |
| SD M Adult [49] | AT | Treadmill Running (Horizontal) | Running vs Controls | 3 | - | - | ||
| W M Adult [64] | AT | Treadmill Running (Horizontal) | ST - Speed Exercise Running vs Cage Control | - | - | - | ||
| ” | ” | ” | IT - Endurance Exercise Running vs Cage Control | - | - | 3 | - | |
| ” | ” | ” | LT - Endurance Exercise Running vs Cage Control | 3 | - | - | 3 | - |
| SD M Adult [76] | TAT | Treadmill Running (Horizontal) | Running vs Cage Control Sedentary Controls | - | 3 | - | 3 | - |
| ” | FDLT | ” | Running vs Sedentary Controls | - | 3 | - | 3 | - |
| SD M Adult [62] | SS | Treadmill Running (Horizontal) | Exercise vs Cage Controls | 3 | - | - | - | |
| W M Adult [74] | AT | Strength Training | Strength Training vs Control | - | - | - | 3 | - |
| ” | AT | Swimming | Swimming vs Control | - | - | - | - | |
| Mice | ||||||||
| C57BL/6J F Adult [70] | PT | Direct Tendon Loading | 4 N Peak or 1 h vs Contralateral Control | - | 3 | - | 3 | - |
| ” | PT | ” | 6 N Peak for 1 h vs Contralateral Control | - | - | 3 | - | |
| ” | PT | ” | 4 N Peak for 2 h vs Contralateral Control | - | - | 3 | - | |
| ” | PT | ” | 4 N or 6N Peak or 1 h - Baseline vs Endpoint | - | - | - | ||
| ” | PT | ” | 4 N Peak for 2 h - Baseline vs Endpoint | - | - | - | ||
| C57BL/6 M Old and Adult [197] | Plant | Treadmill Running (Horizontal) | Running vs Sedentary | 3 | - | - | ||
Color Legend: Red - Increase; Blue - Decrease; Tan – No Change; Gray – Not Reported
SD – Sprague Dawley; W – Wistar; M – Male; F – Female; Coll – Collagan; Coll Org – Collagen Organization; GAG – gycosaminoglycan; Ag -Aggrecan; V – Versican; D – Decorin; Bn – Biglycan; Fn- Fibronectin; E – Elastin; Fmod – Fibromodulin; TNc – Tenascin-C; L - Leucin
3. Microstructural Changes with High Loading
Tendons can adopt a wide range of structural configurations[12], and recent research suggests that tendon structure may be related to the function of the muscle-tendon unit (i.e. force transmission versus energy storage)[79]. It is widely accepted that tendon’s structure adapts to mechanical load, which manifests as changes to the collagenous and non-collagenous matrix. Small animal studies applying high mechanical loading have shown temporal structural changes with different loading regimens.
3.1. Effect of High Loading on Collagen
A key component to tendon’s mechanical response to load is alteration of the collagenous matrix[80]. Collagen structure has been measured in excised human tissue primarily using histological methods, whereas animal studies offer the ability to measure structure nondestructively using electron microscopy and second harmonic imaging alongside collagen-related gene expression[81].
Changes in collagen microstructure have been observed with different types and levels of overuse training. An increase in collagen fiber content was demonstrated at 30 days for resistance training, and at 45 days for both resistance and endurance training[82]. Interestingly, resistance training (which involves higher intensity) shows an earlier increase suggesting the potential for faster progression of tendinopathy[82]. Increased collagen disorganization has been shown with the application of both increased repetition and increased force[83]. These changes suggest that microstructural damage accumulates faster not only due to higher loading duration, but also higher tendon stress.
Advantageous changes to collagen structure may occur as early as during the first week of loading. Within the first week of treadmill loading, flexor digitorum longus collagen fibril diameter, number, and overall fibril CSA increased relative to control[84, 85]. Similarly, for CT, the thickness of the peritendinous sheath increased, perhaps increasing load bearing capacity[86]. Increased Col I expression with high loading has been shown for fatigue loaded PT,[87] and SS[88]and AT[89] after treadmill running.
Multiple detrimental alterations to collagen microstructure occur as early as week 3 or 4 post loading; studies have observed increased fibril disorganization[57, 58, 90], microtears within collagen fibers[25, 27, 91, 92], decreased fibril diameter, number, and relative fibril CSA[84], and increased proportion of type III collagen fibers (reticular)[91]. Furthermore, from a microstructural perspective, collagen damage was invoked using electrical stimulation of the flexor digitorum profundus [27]. Changes to collagen in response to loading has also been studied in tendon fascicles ex-vivo, though this isn’t covered in our review[93–98].
3.2. Effect of High Loading on Glycosaminoglycans (GAGS)
The function of the non-collagenous tendon matrix has been of great interest recently. Human PT biopsies from patients with PT tendinopathy have indicated that increased GAG and proteoglycan content may be associated with tendon dysfunction[99–101]. Small animal studies offer a mechanism to explore the effects of high loading on GAG and proteoglycan content.
In rat models, glycosaminoglycan content increases with resistance training[102] and treadmill running[89, 91, 103, 104], which has been suggested to improve interfibrillar sliding[89, 105]. Furthermore, the relative proportions of GAGs may change with downhill running, as one study observed an increase in dermatan sulfate and chondroitin sulfate, a decrease in heparan sulfate, and increased proportion of chondroitin sulfate relative to dermatan sulfate.[106] Notably, Marqueti, et al demonstrated increased GAG content and increased proteoglycan content (proximal CT) for older animals at 12 weeks of resistance training[102], which suggests that resistance loading can help protect aging tendons from damage. Also, pathological GAG deposition and calcific tendinopathy may also be possible [107, 108].
Overall, high mechanical loading in small animal tendons leads to microstructural changes that differ depending on the magnitude and duration of loading, but overall includes increased Col I production, increased Col III/ Col I proportion, increased matrix disorganization, and altered proportions of matrix proteins.
4. Biological Changes with High Loading
4.1. Overview
Advances in imaging, sequencing, and transgenic mouse models have enabled mechanistic evaluation for studying the effect of mechanical loading on tendon biology. Recent studies have revealed interplay between complex changes in cell behavior, protein expression, gene expression, and vascular changes with the onset of high mechanical loading. Within the model systems commonly evaluated, in-vivo models can provide the most clinically relevant information about diseased biological mechanisms, due to the capacity to mechanically stimulate tissues within a living organism. The most common in-vivo loading models to study biological changes following high mechanical loading include treadmill activity[35, 109], forelimb reaching and pulling[83, 110], and electrical stimulation[111]. Since specific loading regimens in rodent models do not precisely control for the stresses and strains exhibited by animals during activities such as treadmill overuse, mechanical loading of tendon in-situ or ex-vivo can be advantageous due to the ability to precisely control applied stresses and strains while preserving the native architecture of the ECM[112]. For example, direct mechanical actuation is applied to tendons while animals are under anesthesia[68] or freshly harvested and maintained in a bioreactor[113].
4.2. Effect of High Loading on Cell Behavior
For decades, histology from tendinopathic human tendons has been well characterized, which has enabled benchmarks for load induced tendinopathy models in rodents. After high mechanical loading in rodent overuse models, cell number has been shown to increase in multiple tendons: the SS[55, 56, 103, 114], AT[60, 86, 115, 116], and FDL[110, 117]. Additionally, with high loading a rounder cell morphology is reported in the SS[56, 57, 103], FDL[110], and AT[115, 116]. Tendon cells may also show greater surface area[118], loose chromatin (which allows for increased replication)[82], and increased cytoplasm volume[82]. Multiscale strain transfer is also attenuated following high cyclic loading[113].
Broadly speaking, characterizing cell types within tendon has been an active area of research in the tendon community[119]. Morphologically, with high loading, both fibroblast and chondrocyte appearance become more abundant with time[103]. Loaded tendons may also have an increased quantity of myofibroblasts, as characterized by positive staining for α-smooth muscle actin (α-SMA), production of α-SMA stress fibers, and generation of large traction forces.[118] Although knowledge for specific cell markers in mechanical loading-induced tendinopathy model systems is lacking, available data suggest cells in the SS tendon-derived cells following high loading are positive for CD90.1, CD3.1, and CD94[103, 120]. Given the evidence that staining for macrophages, mast cells, T- cells, and myofibroblasts in subscapularis (SB) tendons in patients with torn SS[121] has provided, groups have been motivated to study immune cell populations[103].
The presence of apoptosis in tendinopathy remains controversial. Interest in studying the interplay of apoptosis and tendinopathy originates from human studies identifying elevated apoptosis in intact SB tendons in patients with torn SS[122]. In rodent models of high mechanical loading, apoptosis has been induced in both explant models of the PT[68] and rodent overuse of the AT[91], but not in the SS[103]. Tendinopathic SS from rats had elevated heat shock proteins and caspases with imbalance in antiapoptotic (heat shock protein) and proapoptotic (caspase) genes[123]. However, rats following 16 weeks of loading did not have evidence of apoptosis, but had increased IGF1[123], which can promote tenocyte proliferation[124, 125], collagen synthesis in tendon[126] and musculotendinous tissues[127], and tissue biomechanics following injury[128, 129].
Taken together, high loading models in rodents can create hypercellularity, cell roundness, apoptosis, elevated abundance of myofibroblasts, and a chondrogenic phenotype in most tendon types. Therapies to modulate cell rounding, multiscale strain transfer, aberrant differentiation, immune cell populations, and apoptosis in response to high loading are ripe areas for future study.
4.3. Effect of High Loading on Protein and Gene Expression
Advances in mechanobiology have uncovered relationships between forces applied to the ECM, multiscale strain transfer to the nucleus, and cell processes (e.g., transcription, inflammation, migration, proliferation, and differentiation)[43, 97, 130–136]. Here, we highlight specific changes within tendon following high mechanical loading in rodent studies. As high mechanical loads are applied, changes to tendon structure and mechanical properties may occur in concert with the generation of interstitial pressures surrounding the tendon and alterations in blood flow. These mechanosensory factors may activate mechano-transductive pathways[137] and promote non-tenogenic behavior in tendon cells[138, 139], secretion of non-tendinous proteins, release of growth factors and cytokines resulting in non-tendon like phenotypes, increase in the type III/I collagen ratio, and an imbalanced secretion of MMPs and tissue inhibitors of MMPs (TIMPs)[106, 140, 141]. Indeed, increased loading on the rat SS elevated expression of MMPs and TIMPS[142].
High treadmill activity affects tendon stem/progenitor cell differentiation in a tendon-dependent manner, as stem cell markers (Oct4, SEA1, Oct54, and NS) are upregulated in the PT, but not the AT[143]. Aberrant differentiation following loading may provide a mechanism to end-stage tendinopathy which progresses to calcific tendinopathy, with upregulation of genes associated with bone development and resorption[116, 144]. Other non-tendon like markers such as those associated with cartilage development have been identified through the presence of GAG staining in the AT[91, 115, 116, 145] and SS[103]. Overused shoulders in rodents express cartilage markers in the short term [140], that return to baseline after rest[146]. Proteoglycans and heparin affine regulatory peptide (chondrogenesis factor) are elevated with overuse[106].
Several cytokines have been implicated in load-induced tendinopathy (e.g., IL-6, IL-15, IL-11, IL-18). Rodent studies have demonstrated IL-6 increases in concert with continued loading[14, 117, 147] and aging[117] at both the protein and gene levels[120]. Inflammation related cytokines such as IL-11, IL-15, IL-18, and TNFα were also upregulated in overused tendons[120]. Interestingly, IL-33 expression may promote type-3 collagen synthesis and contribute to scar formation; however, this has only been shown in a tendon injury model[36]. In rat forelimb grasping models, repeated grasping has led to elevated TNAα, IL1α, and IL1β in the flexor digitorum muscle, tendon, and radius[83, 110]. High force tasks also increase spinal cord sensitization through substance P[83].
Several growth factors have been implicated in tendinopathy (e.g., TGF-β87, CTGF99, IGF-1[14], periostin-like factor[110], osteoactivin[148], PDGF[147], VEGF[88, 102, 141], FGF[149]). IGF-1 is known to stimulate collagen protein synthesis with loading in humans[14] and rodents[103], and is commonly identified to increase in tendinopathy[49, 88, 102], potentially to combat effects of apoptosis and heat shock signaling[123]. TGF-β and CTGF are known to stimulate collagen synthesis in human tendons[14], and in-vitro via stimulation of tendon fibroblasts[150]. Animal studies have demonstrated load-[102, 149] and repetition-[83] induced increased tendon tissue concentration of the isoform TGF-β1, and in gene expression, but this remains controversial[49, 97, 140]. Interestingly, this effect may be age dependent, as increased levels are observed in young rats, but decreased levels in old rats with loading[102]. Rodent studies of high loading also suggest elevated CTGF protein[110, 147] and gene expression[102], but this remains unclear[49, 97].
Taken together, high loading promotes non-tenogenic differentiation and protein expression (e.g., GAGs), and secretion of several proinflammatory cytokines and growth factors that occur in concert with tendon degeneration. Future studies are needed to mechanistically evaluate the role of each factor on tendon degeneration and whether their modulation can prevent or accelerate recovery from tendinopathy.
4.4. Effect of High Loading on Vascularization
Clinical studies of patients in the late stages of tendinopathy suggest neovascularization[151, 152] and intratendinous blood flow[153–157]. For these reasons, vascularization has been studied in several rodent models. Certain AT and SS overuse models have highlighted elevated vascularity[111, 141, 153]. Increased vascularity occurs in concert with elevated cellularity in the peritendinous sheath[86]. However, in another overuse rat running model, tendon neovascularization has not been observed continuously[103].
Neovascularization in tendon following overuse may be driven by VEGF[158], as it is known to increase the permeability of vessels[159]. Following treadmill overuse, VEGF increases after 3 days, but decreases after one week and remains high over 16 weeks[141]. This suggests that new blood vessel formation occurs early in the tendon disrepair stage and continues throughout[141]. These data are supported by another study using the same loading protocol, which highlights a low expression of VEGF after one week and an increase after 8 weeks[88]. In addition to VEGF, studies report increased expression of nitric oxide (NO) that may promote synthesis of collagen fibers and induce the replacement of injured cells[160].
4.5. Summary High Loading’s Effect on Tendon Biology
Although human models of disease provide the most accurate clinical representation of tendinopathy, they are limited by the availability of tissue sources and often require large study numbers. Therefore, animal studies can offer advantages due to being readily available.
Taken together, rodent high loading studies have highlighted several effects of loading on tendon pathology. Certain high loading models have recapitulated similar end-stage tendinopathy phenotypes commonly seen in humans. Overall, these studies provide insight into temporal patterns in gene and protein expression, aberrant differentiation pathways, cytokine secretion, and GF expression that are associated with the disease process. Harnessing these attributes in future studies will greatly add to our understanding and better enable identification of new treatments. It is noted that studies using tendon explants from large animals (e.g.,[135, 161–167]) and cells seeded on scaffolds (e.g.,[133, 168–172]) have also provided insight into mechanisms of tendinopathy, but are not discussed here in this review focused on learnings from small animals.
5. Future Perspectives
The precise etiology of tendinopathy has yet to be characterized, though from small animal studies of high loading, it is clear that a number of factors contribute to the progression of detrimental tendinopathic symptoms that may carry different weights depending on the tendon location and function. Small animal studies prescribing high tendon loading regimens show that tendon CSA, modulus, hysteresis change, and failure load may increase or decrease depending on the tendon location and the duration, frequency, and magnitude of loading. Collagen III production increases in response to high loading in small animals. GAG content increases with resistance and treadmill loading in rats, which may have protective qualities for tendon. Small animal studies provide insight into temporal patterns associated with the disease process, where growth factors, inflammation, and neovascularization are current areas of study. To date, none of these small animal studies directly contradict the clinical perspective that reducing load after potential overuse and subsequent physical therapy with eccentric exercise may have positive effects on tendinopathic symptoms. It is important to note that results from rodent tendon studies cannot be directly translated to humans. In specific instances, these animals have been shown to have increased healing rates compared to humans, [173, 174] which may include a higher capacity to respond to loading and prevent tendinopathy than humans.
As the inception of tendinopathy is multifactorial and seems to depend on the tendon studied, small animal model systems aiming to mimic the human tendinopathic condition should pay specific attention to the tendon location, age, and activity level of the patient population of clinical interest. Doing so will allow for prescription of loading that matches that seen in the patient and iterative feedback from the clinical setting to improve our understanding of the etiology and progress of the disease. This may provide indicators of how a specific tendon responds to the type and degree of loading seen in the patient.
An improved understanding of healthy tendon metabolism and homeostasis would allow a clearer baseline to detect tendon pathology. Mechanotransduction at the cellular and subcellular levels has been used to evaluate tensional homeostasis [175, 176], tendon development [177], gene expression [135], and collagen turnover [178, 179]. Knowledge from this setting have motivated more pointed studies in the small animal setting to better define the factors that define the temporal and load amplitude thresholds for overloading.
Future small animal studies applying high mechanical load to induce tendinopathy should consider quantifying pain [180–182] and function – such as measurements of dynamic weight bearing [183] and motion analysis [184] used in animal models to study different tendon diseases. Further, quantitative tendon imaging, especially using high-frequency ultrasound can be used to evaluate and quantify tendinopathy and treatment in small animals [185–187]. This may help indicate the onset of tendinopathic changes, aid in the staging of tendinopathy in small animals, and improve comparisons to clinical data. Also, it may be of interest to explore temporal changes in structure and biology during the early onset and throughout the training regimen. Further, studies that use data from these small animal studies to model the temporal material response as a function of the changes seen in the structure and the biological response may expose areas of interest along the continuum of tendinopathy.
This review offers an update on the realm of mechanically-induced tendinopathy with the hope that we, as a field, can make further progress to directly affect patient management. The studies of mechanically-induced tendinopathy included in this manuscript offer insight into the potential indicators of tendinopathy that differentiate therapeutic versus detrimental mechanical load. Though clinical measurement tools haven’t been established in humans, increased apoptosis may indicate the need for anti-apoptotic drugs. Increased matrix degeneration suggests that better imaging techniques are needed to assess matrix degeneration earlier. Recent studies using shear wave propagation [188] and ultrasound [189, 190]have shown promise in this area. For clinical translation, it may be necessary to correlate results from the clinical measurement techniques to the more invasive measurements that can be made in the small animal model setting.
Generally, small animal models that mimic the human condition offer a high throughput setting to establish the efficacy of biomaterial therepeutics that improve detriments associated with tendinopathy. Biomimetic biomaterials may be designed to deliver biomolecules to injured tendon to, for example, promote cell or matrix regeneration [191, 192], or mediate inflammation [35, 193]. Also, biomimetic biomaterials may be engineered to mimic the elasticity and material strength of tendon, [194, 195] or improve tendon-to-bone healing. [196] Overall, small animal studies of mechanically-induced tendinopathy have motivated more basic science research to improve our understanding of tendon’s response to loading to further improve the field’s diagnostic and therapeutic capability.
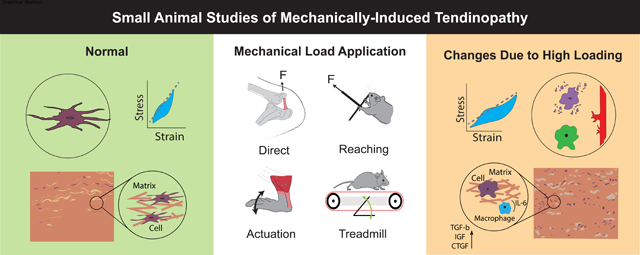
Figure 1. Mechanical Load Applied and Measurements of Induced Change in Small Animal Models.
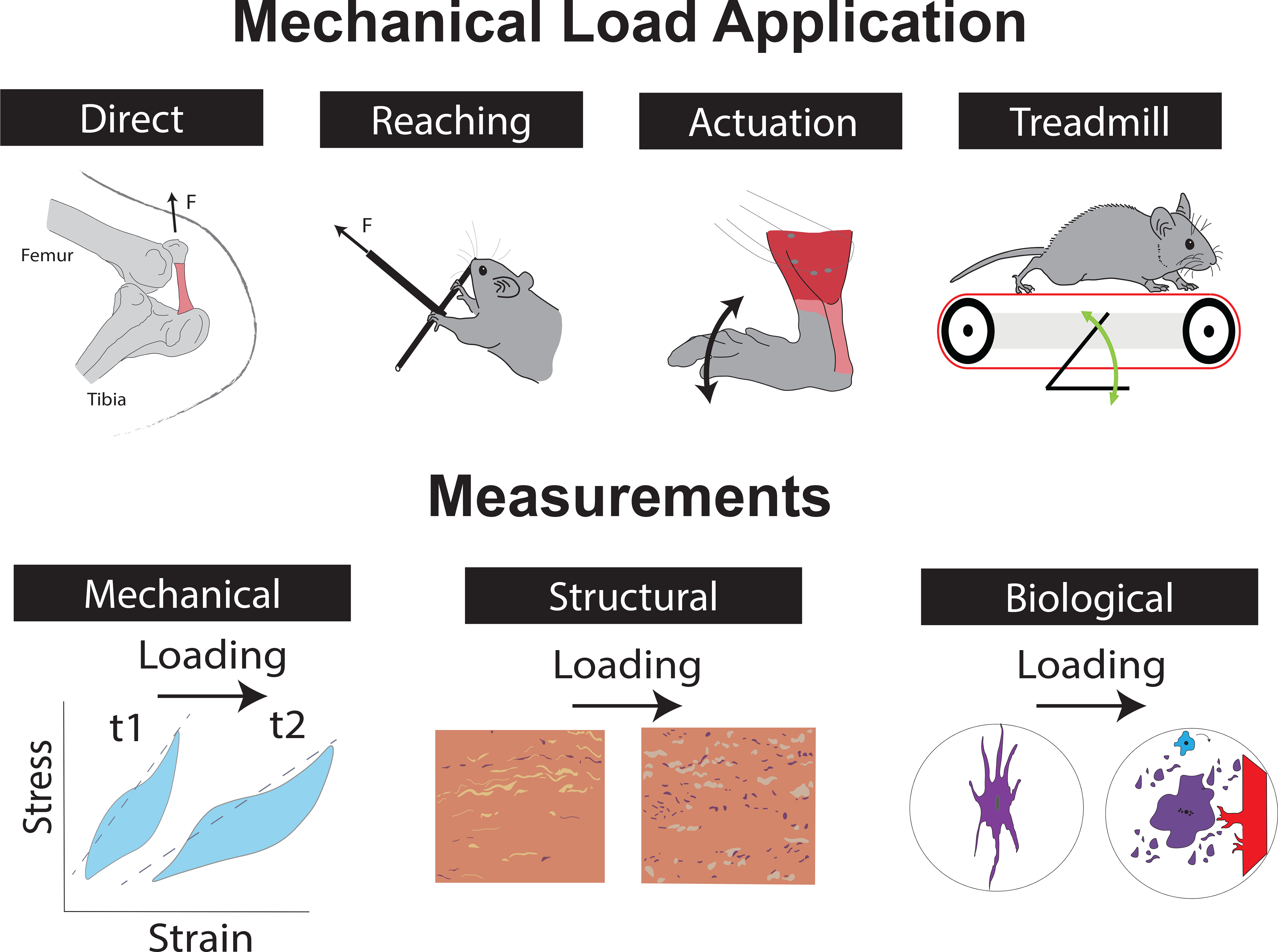
Studies use different methods to apply load to tendon experimentally, such as direct tendon loading applied to the patellar tendon, reaching and grasping tasks aimed at loading the flexor digitorum tendon, active (via muscle stimulation) or passive ankle dorsiflexion aimed to load the Achilles, and treadmill running in different configurations mainly loading the Achilles or rotator cuff tendons. Mechanical, structural, and biological changes to the tendon are measured to quantify the effects of loading. F – Force; t1 – timepoint 1; t2 – timepoint 2;
Figure 2. High Tendon Loading Leads to Mechanical, Structural, and Biological Changes in Small Animal Models.

High loading small animal studies induce multi-scale changes to tendon that are similar to tendinopathy. Increased cell rounding, apoptosis, vascularization, aberrant differentiation, inflammation, and altered growth factor production (increased TGF-b, IGF, and CTGF) are seen in some studies. Mechanically, increased hysteresis, and higher modulus may result. Structurally, increased collagen disorganization and altered non-collagenous matrix components are likely. Overall, between tendon types and loading protocols, there is no clear consensus for how high loading affects tendon. CSA – Cross-sectional Area; IL-6 – Interleukin-6; TGF-b – Transforming Growth Factor Beta; IGF – Insulin-like Growth Factor; CTGF - Connective Tissue Growth Factor
Table 2.
Structural Changes induced by High Loading in Small Animal Studies
| Study Information |
Structural Measurements |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Animal | Tendon | Loading Mechanism | Comparison | Coll Type |
Coll Org | Non-Coll Tendon Matrix |
||||||||||
| Coll 1 | Coll 3 | GAG | Ag | V | D | Bn | Fn | E | Fmod | TNc | L | |||||
| Rats | ||||||||||||||||
| SD F * Age not reported [87] | PT | Direct Tendon Loading | Mid-level Fatigue (Day 1 post-Fatigue) | |||||||||||||
| ’ ’ | PT | ’ ’ | Mid-level Fatigue (Day 3 post-Fatigue) | |||||||||||||
| ’ ’ | PT | ’ ’ | High-level Fatigue (Day 1 post-Fatigue) | |||||||||||||
| ’ ’ | PT | ’ ’ | High-level Fatigue (Day 3 post-Fatigue) | |||||||||||||
| SD F * Age not reported [98] | PT | Direct Tendon Loading | High-level Fatigue vs Control (Sham and Naive) | |||||||||||||
| SD Adult F * Age not reported [198] | PT | Direct Tendon Loading | Tendon Elongation Increased by 0.6% or 1.7% vs Control | |||||||||||||
| SD F * Age not reported [199] | AT | Concentric, Eccentric or Isometric Training by Nerve Stimulation | Concentric (CON), Ecccentric (ECC), or Isometric (ISO) Loading vs Contralateral Control | |||||||||||||
| SD M * Age not reported [140] | SS | Downhill Treadmill Running | Running vs Baseline | |||||||||||||
| SD M * Age not reported [55] | SS | Downhill Treadmill Running + Compression or Injury | Overuse + Intrinsic or Extrinsic | |||||||||||||
| SD (n = 14); Adult [75] | AT | Downhill Bipedal Running | Running vs Cage Control | |||||||||||||
| SD (n=20); M Adult [88] | SS | Downhill Treadmill Running | Acute Exercise vs Control | |||||||||||||
| ’ ’ | SS | ’ ’ | Chronic Exercise vs Control | |||||||||||||
| SD M * Age not reported [103] | SS | Downhill Treadmill Running | Running vs Cage Control | |||||||||||||
| SD Adult * Gender not reported [57] | SS | Downhill Treadmill Running | Extrinsic Compression (E), Overuse (O/V), or Both vs Control | |||||||||||||
| SD M * Age not reported [56] | SS | Downhill Treadmill Running | Exercise vs Cage Control | |||||||||||||
| SD M Adult [145] | AT | Uphill Treadmill Running | Runners vs Sedentary Controls | |||||||||||||
| SD Adult *Gender not reported [60] | AT | Uphill Treadmill Running | Running vs Non-running Control | |||||||||||||
| SD M * Age not reported [49] | AT | Uphill Treadmill Running | Running vs Control | |||||||||||||
| SD M Adult [200] | PT | Uphill Treadmill Running | LR11 vs Control | |||||||||||||
| ’ ’ | PT | ’ ’ | HR11 vs Control | |||||||||||||
| W Adult and Old [102] | CT | Resistance Training | Young Trained (YT) vs Young Sedentary (YS) | |||||||||||||
| ’ ’ | CT | ’ ’ | Old Trained (OT) vs Old Sedentary (OS) | |||||||||||||
| W M Adult [115] | CT | Uphill Treadmill Running | Running vs Cage Control | |||||||||||||
| W M Adult [91] | AT | Uphill Treadmill Running | Running vs Cage Control | |||||||||||||
| SD M Adult [116] | AT | Uphill Treadmill Running | Runnning vs Cage Control | |||||||||||||
| SD M Adult [89] | AT | Treadmill Running (Horizontal) | Runner (RUN) vs Sedentary (SED) | |||||||||||||
| SD F Adult [92] | FDT | Reaching + Grasping | Reach Limb vs Non-Reach Limb | |||||||||||||
| SD F Adult [83] | FDT | Reaching + Pulling | 12-week HRLF vs Normal Control (NC) | |||||||||||||
| ’ ’ | FDT | ’ ’ | 12-week LRHF vs Normal Control (NC) | |||||||||||||
| ’ ’ | FDT | ’ ’ | 12-week HRHF vs LRLF | |||||||||||||
| SD F Adult [110] | FDT | Reaching + Grasping | HRHF vs Control | |||||||||||||
| SD F Adult [147] | FDT | (HRLF) High Repetition Low Force Grip Tasks | High Repetition Low Force Task (HRLF) vs Trained Only Controls (TR0 and TR24) | |||||||||||||
| SD F Old and Adult [117] | FDT | High Repetition, Low Force (HRLF) Reaching and Handle-Pulling Task | Old HRLF vs Control | |||||||||||||
| ’ ’ | FDT | ’ ’ | Young HRLF vs Control | |||||||||||||
| ’ ’ | SS | ’ ’ | Old HRLF vs Control | |||||||||||||
| ’ ’ | SS | ’ ’ | Young HRLF vs Control | |||||||||||||
| SD M Adult [111] | AT | Electrical Muscle Stimulation | Eccentric vs Untrained Controls | |||||||||||||
| Rabbit | ||||||||||||||||
| NZW F Adult [201] | AT | Electrical Muscle Stimulation | Loaded vs Contralateral Control | |||||||||||||
| Mice | ||||||||||||||||
| C57BL/6J F Adult [70] | PT | Direct Tendon Loading | Loading Regimens vs Contralateral Control | |||||||||||||
| C57BL/6 M Old and Adult [197] | Plant | Uphill Treadmill Running | Old Trained vs Old Cage Control | |||||||||||||
| ’ ’ | ’ ’ | ’ ’ | Old Trained vs Young Cage Control | |||||||||||||
| C57BL/6J F Adult [143] | PT or AT | Treadmill Running (Horizontal) | Moderate or Intense Training vs Cage Control | |||||||||||||
Color Legend: Red - Increase; Blue - Decrease; Tan – No Change; Gray – Not Reported
SD – Sprague Dawley; W – Wistar; M – Male; F – Female; CSA – Cross-sectional Area
Table 3.
Biological Changes Induced by Small Animal Studies.
| Study Information |
Biological Measurements |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Animal | Tendon | Loading Mechanism | Comparison | Cell Rounding | Abberant Differentiation | Inflammation | Apoptosis | Growth Factors | Matrix Degradation Enzymes | Vascularization |
| Rat | ||||||||||
| SD F Adult [68] | PT | Direct Tendon Loading | High-level Fatigue vs Naive Control (3 Days Post-Fatigue) | |||||||
| ’ ’ | ’ ’ | ’ ’ | High-level Fatigue vs Naive Control (7 Days Post-Fatigue) | |||||||
| SD F * Age not reported [98] | PT | Direct Tendon Loading | High-level Fatigue vs Control (Sham and Naive) | |||||||
| SD F Adult * Age not reported[198] | PT | Direct Tendon Loading | Tendon Elongation Increased by 0.6% vs Control | |||||||
| ’ ’ | PT | ’ ’ | Tendon Elongation Increased by 1.7% vs Control | IDK WHY THIS WON'T STAY BLUE | IDK WHY THIS WON'T STAY BLUE | |||||
| SD F * Age not reported [199] | AT | Concentric, Eccentric or Isometric Training by Nerve Stimulation | Concentric (CON) or Isometric (ISO) Loading vs Contralateral Control | TGF-b higher in all 3 grps but CTGF no change | ||||||
| ’ ’ | AT | ’ ’ | Eccentric (ECC) Loading vs Contralateral Control | TGF-b higher in all 3 grps but CTGF no change | 3 | |||||
| SD F Adult * Age not reported [202] | AT | Concentric, Eccentric or Isometric Training by Nerve Stimulation | Concentric (CON), Eccentric (ECC), or Isometric (ISO) Loading vs Contralateral Control | |||||||
| SD Adult *Gender not specified [50] | Tri, PT, or AT | Eccentric and Concentric Training | Concentric Loading vs Untrained Controls | (“epitendon”) | ||||||
| ’ ’ | Tri, PT, or AT | ’ ’ | Eccentric Loading vs Untrained Controls | (“epitendon”) | ||||||
| SD M * Age not reported [140] | SS | Downhill Treadmill Running | Running vs Baseline | 3 | 3 | (midsubstance) | ||||
| SD M * Age not reported [55] | SS | Downhill Treadmill Running + Compression or Injury | Overuse + Intrinsic or Overuse + Extrinsic vs Control | |||||||
| SD M Adult [120] | SS | Downhill Treadmill Running | Running vs control | |||||||
| SD M Adult [149] | SS | Downhill Treadmill Running | Exercise vs Control | Of Note was the differential expression of inflammation-related genes, representing 12% (11 genes) of the significantly upregulated genes and 19% (7 genes) of significantly downregulated genes. | ||||||
| SD Adult [75] | AT | Downhill Bipedal Running | Running vs Cage Control | |||||||
| SD M Adult [88] | SS | Downhill Treadmill Running | Acute Exercise vs Control | 3 | (NR) | |||||
| ’ ’ | SS | ’ ’ | Chronic Exercise vs Control | 3 | Casp3 downreg after 1 Week, no mention of down/upreg after 8 wks | (NR) | ||||
| SD M * Age not reported [103] | SS | Downhill Treadmill Running | Running vs Cage Control | 3 | 3 | (NR) | ||||
| SD Adult * Gender not reported [57] | SS | Downhill Treadmill Running | Extrinsic Compression (E), Overuse (O/V) or Both vs Control | |||||||
| SD M * Age not reported [56] | SS | Downhill Treadmill Running | Exercise vs Cage Control | |||||||
| SD M Adult [145] | AT | Uphill Treadmill Running | Runners vs Sedentary Controls | |||||||
| W M Adult [86] | CT | Vertical Jump | Vertical Jump (VJ) vs Control | 3 | (“peritendinous sheath”) | |||||
| ’ ’ | CT | Uphill Treadmill Running | Treadmill Running (TR) vs Control | 3 | (“peritendinous sheath”) | |||||
| SD Adult *Gender not reported [60] | AT | Uphill Treadmill Running | Running vs Non-Running Control | 3 | (midsubstance) | |||||
| SD M * Age not reported [49] | AT | Uphill Treadmill Running | Running vs Control | 3 |
TGF-b, CTGF: Down
IGF: Up |
3 | ||||
| SD M Adult [200] | PT | Uphill Treadmill Running | LR11 vs Control | |||||||
| ’ ’ | PT | ’ ’ | HR11 vs Control | |||||||
| W M Adult and Old [102] | CT | Resistance Training (Climbing with Weights) | Young Trained (YT) vs Young Sedentary (YS) | (NR) | ||||||
| ’ ’ | CT | ’ ’ | Old Trained (OT) vs Old Sedentary (OS) | (NR) | ||||||
| W (n = 24); M Adult [115] | CT | Uphill Treadmill Running | Running vs Cage Control | (NR) | ||||||
| W M Adult [91] | AT | Uphill Treadmill Running | Running vs Cage Control | 3 | 3 | (NR) | ||||
| SD M Adult [116] | AT | Uphill Treadmill Running | Runnning vs Cage Control | |||||||
| SD F Adult [92] | FDT | Reaching + Grasping Task | Reach Limb vs Non-Reach LImb | |||||||
| SD F Adult [83] | FDT | Reaching + Pulling | 12-Week HRLF vs Normal Control (NC) | 3 | ||||||
| ’ ’ | FDT | ’ ’ | 12-Week LRHF vs Normal Control (NC) | 3 | 3 | |||||
| ’ ’ | FDT | ’ ’ | 12-Week HRHF vs LRLF | 3 | ||||||
| SD F Adult [110] | FDT | Reaching + Grasping | HRHF vs Control | (“peritendon”) | ||||||
| SD F Adult [148] | FDT | High Repetition Negligible Force (HRNF) Food Retrieval Task | HRNF vs FRC (3 Week Timepoint) | |||||||
| ’ ’ | ’ ’ | ’ ’ | HRNF vs FRC (6 Week Timepoint) | 123 | ||||||
| SD F Adult[147] | FDT | (HRLF) High Repetition Low Force Grip Tasks | HRLF vs Control | |||||||
| SD F Old and Adult [117] | FDT | High Repetition, Low Force (HRLF) Reaching + Handle-Pulling Task | Old HRLF vs Control | 3 | (“endotendon”) | |||||
| ’ ’ | FDT | ’ ’ | Young HRLF vs Control | 3 | (“endotendon”) | |||||
| ’ ’ | SS | ’ ’ | Old HRLF vs Control | (“endotendon”) | ||||||
| ’ ’ | SS | ’ ’ | Young HRLF vs Control | 3 | 3 | (“endotendon”) | ||||
| SD M Adult [89] | AT | Treadmill Running (Horizontal) | Runner (RUN) vs Sedentary (SED) | no chane | MMP13 decreases | |||||
| SD and W Both Sexes * Age not reported [203] | AT | Treadmill Running (Horizontal) | Contralateral-Impaired vs Control | |||||||
| ’ ’ | Tibialis Anterior | ’ ’ | Running vs Untrained Control | |||||||
| SD M Adult [111] | AT | Electrical Muscle Stimulation | Eccentric vs Untrained Controls | (“epitendon”) | ||||||
| W M Adult [86] | CT | Swimming | Adapted Swimming ADP vs Control | 3 | 3 | (“peritendinous sheath”) | ||||
| Rabbit | ||||||||||
| NZW F Adult [204] | AT | Electrical Muscle Stimulation | Trained vs Control (At Week 1) | both | ||||||
| ’ ’ | AT | ’ ’ | Trained vs Control (At Week 3 or 6) | both | ||||||
| NZW F Adult [201] | AT | Electrical Muscle Stimulation | Loaded vs Contralateral Control | 3 | 3 | IGF-II expression significantly Lower | ||||
| NZW Both Sexes Adult [205] | AT | Electrical Muscle Stimulation | Loaded vs Baseline | (paratenon) | ||||||
| Mice | ||||||||||
| C57BL/6 M Old and Adult [197] | Plant | Uphill Treadmill Running | Old Trained vs Old Cage Control | |||||||
| ’ ’ | ’ ’ | ’ ’ | Old Trained vs Young Cage Control | |||||||
| C57BL/6J F Adult [118] | PT | Treadmill Running (Horizontal) | Running vs Cage Control | |||||||
| C57BL/6J F Adult [143] | PT or AT | Treadmill Running | Moderate Training vs Cage Control | (paratenon) | ||||||
| ’ ’ | PT or AT | ’ ’ | Intense Training vs Cage Control | (paratenon) | ||||||
Color Legend: Red - Increase; Blue - Decrease; Tan – No Change; Gray – Not Reported
SD – Sprague Dawley; W – Wistar; M – Male; F – Female;
Statement of Significance.
This review summarizes the insights gained from in-vivo animal studies of mechanically-induced tendinopathy by evaluating the effect high loading regimens have on the mechanical, structural, and biological features of tendinopathy. A better understanding of the interplay between these realms could lead to improved patient management, especially in the presence of painful tendon.
7. Acknowledgements
The authors would like to thank Alana Kelley for her assistance with figure preparation.
Funding Source:
This work was supported by the National Institutes of Health [NIH/NIA (1K99AG065495–01), NIH (2T32AR055885–11A1), and departmental funding.
Footnotes
8. Competing Interests
The authors declare no competing interests.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Shiri R, Varonen H, Heliovaara M, Viikari-Juntura E, Hand dominance in upper extremity musculoskeletal disorders, The Journal of rheumatology 34(5) (2007) 1076–82. [PubMed] [Google Scholar]
- [2].Lipman K, Wang C, Ting K, Soo C, Zheng Z, Tendinopathy: injury, repair, and current exploration, Drug Des Devel Ther 12 (2018) 591–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kaux JF, Forthomme B, Goff CL, Crielaard JM, Croisier JL, Current opinions on tendinopathy, J Sports Sci Med 10(2) (2011) 238–53. [PMC free article] [PubMed] [Google Scholar]
- [4].Magnusson SP, Langberg H, Kjaer M, The pathogenesis of tendinopathy: balancing the response to loading, Nat Rev Rheumatol 6(5) (2010) 262–8. [DOI] [PubMed] [Google Scholar]
- [5].Beyer R, Kongsgaard M, Hougs Kjaer B, Ohlenschlaeger T, Kjaer M, Magnusson SP, Heavy Slow Resistance Versus Eccentric Training as Treatment for Achilles Tendinopathy: A Randomized Controlled Trial, Am J Sports Med 43(7) (2015) 1704–11. [DOI] [PubMed] [Google Scholar]
- [6].Waugh CM, Morrissey D, Jones E, Riley GP, Langberg H, Screen HR, In vivo biological response to extracorporeal shockwave therapy in human tendinopathy, European cells & materials 29 (2015) 268–80; discussion 280. [DOI] [PubMed] [Google Scholar]
- [7].Silbernagel KG, Hanlon S, Sprague A, Current Clinical Concepts: Conservative Management of Achilles Tendinopathy, J Athl Train (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Heinemeier KM, Kjaer M, Magnusson SP, Methods of Assessing Human Tendon Metabolism and Tissue Properties in Response to Changes in Mechanical Loading, Advances in experimental medicine and biology 920 (2016) 97–106. [DOI] [PubMed] [Google Scholar]
- [9].Chiu YH, Chang KV, Chen IJ, Wu WT, Özçakar L, Utility of sonoelastography for the evaluation of rotator cuff tendon and pertinent disorders: a systematic review and meta-analysis, Eur Radiol (2020). [DOI] [PubMed] [Google Scholar]
- [10].Ooi CC, Schneider M, Malliaras P, Png MA, Chadwick M, Jones D, Venkatanarasimha N, Connell D, Real-time sonoelastography evaluation of the Achilles tendon following ultrasound-guided platelet-rich plasma injection and eccentric exercise for the treatment of refractory Achilles tendinopathy, Ultrasound 27(3) (2019) 138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nichols AEC, Best KT, Loiselle AE, The cellular basis of fibrotic tendon healing: challenges and opportunities, Transl Res 209 (2019) 156–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Snedeker JG, Foolen J, Tendon injury and repair - A perspective on the basic mechanisms of tendon disease and future clinical therapy, Acta Biomater 63 (2017) 18–36. [DOI] [PubMed] [Google Scholar]
- [13].Cook JL, Purdam CR, Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy, Br J Sports Med 43(6) (2009) 409–16. [DOI] [PubMed] [Google Scholar]
- [14].Kjaer M, Langberg H, Heinemeier K, Bayer ML, Hansen M, Holm L, Doessing S, Kongsgaard M, Krogsgaard MR, Magnusson SP, From mechanical loading to collagen synthesis, structural changes and function in human tendon, Scand J Med Sci Sports 19(4) (2009) 500–10. [DOI] [PubMed] [Google Scholar]
- [15].Shepherd JH, Screen HR, Fatigue loading of tendon, Int J Exp Pathol 94(4) (2013) 260–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Riley GP, Gene expression and matrix turnover in overused and damaged tendons, Scand J Med Sci Sports 15(4) (2005) 241–51. [DOI] [PubMed] [Google Scholar]
- [17].Riley G, Chronic tendon pathology: molecular basis and therapeutic implications, Expert Rev Mol Med 7(5) (2005) 1–25. [DOI] [PubMed] [Google Scholar]
- [18].Cook JL, Rio E, Purdam CR, Docking SI, Revisiting the continuum model of tendon pathology: what is its merit in clinical practice and research?, Br J Sports Med 50(19) (2016) 1187–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cook JL, Khan KM, Kiss ZS, Purdam CR, Griffiths L, Prospective imaging study of asymptomatic patellar tendinopathy in elite junior basketball players, J Ultrasound Med 19(7) (2000) 473–9. [DOI] [PubMed] [Google Scholar]
- [20].Khan KM, Cook JL, Kiss ZS, Visentini PJ, Fehrmann MW, Harcourt PR, Tress BW, Wark JD, Patellar tendon ultrasonography and jumper’s knee in female basketball players: a longitudinal study, Clin J Sport Med 7(3) (1997) 199–206. [DOI] [PubMed] [Google Scholar]
- [21].Ohberg L, Lorentzon R, Alfredson H, Eccentric training in patients with chronic Achilles tendinosis: normalised tendon structure and decreased thickness at follow up, Br J Sports Med 38(1) (2004) 8–11; discussion 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Abat F, Alfredson H, Cucchiarini M, Madry H, Marmotti A, Mouton C, Oliveira JM, Pereira H, Peretti GM, Spang C, Stephen J, van Bergen CJA, de Girolamo L, Current trends in tendinopathy: consensus of the ESSKA basic science committee. Part II: treatment options, J Exp Orthop 5(1) (2018) 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Archambault J, Tendon micromechanics and research methods in tendinopathy, J Musculoskelet Neuronal Interact 3(4) (2003) 326–8; discussion 333–4. [PubMed] [Google Scholar]
- [24].Andarawis-Puri N, Flatow EL, Soslowsky LJ, Tendon basic science: Development, repair, regeneration, and healing, J Orthop Res 33(6) (2015) 780–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Nakama LH, King KB, Abrahamsson S, Rempel DM, Evidence of tendon microtears due to cyclical loading in an in vivo tendinopathy model, J Orthop Res 23(5) (2005) 1199–205. [DOI] [PubMed] [Google Scholar]
- [26].Arndt AN, Komi PV, Bruggemann GP, Lukkariniemi J, Individual muscle contributions to the in vivo achilles tendon force, Clin Biomech (Bristol, Avon) 13(7) (1998) 532–541. [DOI] [PubMed] [Google Scholar]
- [27].Nakama LH, King KB, Abrahamsson S, Rempel DM, Effect of repetition rate on the formation of microtears in tendon in an in vivo cyclical loading model, J Orthop Res 25(9) (2007) 1176–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Killian ML, Cavinatto L, Galatz LM, Thomopoulos S, The role of mechanobiology in tendon healing, J Shoulder Elbow Surg 21(2) (2012) 228–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Choi R, Smith M, Clarke E, Little C, Cellular, matrix, and mechano-biological differences in load-bearing versus positional tendons throughout development and aging: a narrative review, Connect Tissue Res 59(5) (2018) 483–494. [DOI] [PubMed] [Google Scholar]
- [30].Oliva F, Misiti S, Maffulli N, Metabolic diseases and tendinopathies: the missing link, Muscles Ligaments Tendons J 4(3) (2014) 273–4. [PMC free article] [PubMed] [Google Scholar]
- [31].Little D, Multiscale molecular interactions of tendon, Connect Tissue Res 59(5) (2018) 393–395. [DOI] [PubMed] [Google Scholar]
- [32].Cook JL, Purdam C, Is compressive load a factor in the development of tendinopathy?, Br J Sports Med 46(3) (2012) 163–8. [DOI] [PubMed] [Google Scholar]
- [33].Docking S, Samiric T, Scase E, Purdam C, Cook J, Relationship between compressive loading and ECM changes in tendons, Muscles Ligaments Tendons J 3(1) (2013) 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Thomopoulos S, Parks WC, Rifkin DB, Derwin KA, Mechanisms of tendon injury and repair, J Orthop Res 33(6) (2015) 832–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Abraham AC, Shah SA, Golman M, Song L, Li X, Kurtaliaj I, Akbar M, Millar NL, Abu-Amer Y, Galatz LM, Thomopoulos S, Targeting the NF-kappaB signaling pathway in chronic tendon disease, Sci Transl Med 11(481) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Millar NL, Gilchrist DS, Akbar M, Reilly JH, Kerr SC, Campbell AL, Murrell GA, Liew FY, Kurowska-Stolarska M, McInnes IB, MicroRNA29a regulates IL-33-mediated tissue remodelling in tendon disease, Nature communications 6 (2015) 6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Klatte-Schulz F, Minkwitz S, Schmock A, Bormann N, Kurtoglu A, Tsitsilonis S, Manegold S, Wildemann B, Different Achilles Tendon Pathologies Show Distinct Histological and Molecular Characteristics, International journal of molecular sciences 19(2) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Longo UG, Franceschi F, Ruzzini L, Rabitti C, Morini S, Maffulli N, Denaro V, Characteristics at haematoxylin and eosin staining of ruptures of the long head of the biceps tendon, Br J Sports Med 43(8) (2009) 603–7. [DOI] [PubMed] [Google Scholar]
- [39].Rudavsky A, Cook J, Physiotherapy management of patellar tendinopathy (jumper’s knee), J Physiother 60(3) (2014) 122–9. [DOI] [PubMed] [Google Scholar]
- [40].Factor D, Dale B, Current concepts of rotator cuff tendinopathy, Int J Sports Phys Ther 9(2) (2014) 274–88. [PMC free article] [PubMed] [Google Scholar]
- [41].Yang G, Rothrauff BB, Tuan RS, Tendon and ligament regeneration and repair: clinical relevance and developmental paradigm, Birth Defects Res C Embryo Today 99(3) (2013) 203–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Bell R, Li J, Gorski DJ, Bartels AK, Shewman EF, Wysocki RW, Cole BJ, Bach BR Jr., Mikecz K, Sandy JD, Plaas AH, Wang VM, Controlled treadmill exercise eliminates chondroid deposits and restores tensile properties in a new murine tendinopathy model, Journal of biomechanics 46(3) (2013) 498–505. [DOI] [PubMed] [Google Scholar]
- [43].Maeda T, Sakabe T, Sunaga A, Sakai K, Rivera AL, Keene DR, Sasaki T, Stavnezer E, Iannotti J, Schweitzer R, Ilic D, Baskaran H, Sakai T, Conversion of mechanical force into TGF-beta-mediated biochemical signals, Current biology : CB 21(11) (2011) 933–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hast MW, Zuskov A, Soslowsky LJ, The role of animal models in tendon research, Bone Joint Res 3(6) (2014) 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Schwartz A, Watson JN, Hutchinson MR, Patellar Tendinopathy, Sports Health 7(5) (2015) 415–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Dimitrios S, Exercise for tendinopathy, World J Methodol 5(2) (2015) 51–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Galloway MT, Lalley AL, Shearn JT, The role of mechanical loading in tendon development, maintenance, injury, and repair, The Journal of bone and joint surgery. American volume 95(17) (2013) 1620–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].O’Neill S, Watson PJ, Barry S, Why Are Eccentric Exercises Effective for Achilles Tendinopathy?, Int J Sports Phys Ther 10(4) (2015) 552–62. [PMC free article] [PubMed] [Google Scholar]
- [49].Heinemeier KM, Skovgaard D, Bayer ML, Qvortrup K, Kjaer A, Kjaer M, Magnusson SP, Kongsgaard M, Uphill running improves rat Achilles tendon tissue mechanical properties and alters gene expression without inducing pathological changes, J Appl Physiol (1985) 113(5) (2012) 827–36. [DOI] [PubMed] [Google Scholar]
- [50].Kaux JF, Drion P, Libertiaux V, Colige A, Hoffmann A, Nusgens B, Besancon B, Forthomme B, Le Goff C, Franzen R, Defraigne JO, Cescotto S, Rickert M, Crielaard JM, Croisier JL, Eccentric training improves tendon biomechanical properties: a rat model, J Orthop Res 31(1) (2013) 119–24. [DOI] [PubMed] [Google Scholar]
- [51].Andarawis-Puri N, Sereysky JB, Jepsen KJ, Flatow EL, The relationships between cyclic fatigue loading, changes in initial mechanical properties, and the in vivo temporal mechanical response of the rat patellar tendon, Journal of biomechanics 45(1) (2012) 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Andarawis-Puri N, Sereysky JB, Sun HB, Jepsen KJ, Flatow EL, Molecular response of the patellar tendon to fatigue loading explained in the context of the initial induced damage and number of fatigue loading cycles, J Orthop Res 30(8) (2012) 1327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Magnusson SP, Narici MV, Maganaris CN, Kjaer M, Human tendon behaviour and adaptation, in vivo, J Physiol 586(1) (2008) 71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Magnusson SP, Beyer N, Abrahamsen H, Aagaard P, Neergaard K, Kjaer M, Increased cross-sectional area and reduced tensile stress of the Achilles tendon in elderly compared with young women, J Gerontol A Biol Sci Med Sci 58(2) (2003) 123–7. [DOI] [PubMed] [Google Scholar]
- [55].Carpenter JE, Fanagan CL, Thomopoulos S, Yian EH, Soslowsky LJ, The effects of overuse combined with intrinsic or extrinsic alterations in an animal model of rotator cuff tendinosis, American Journal of Sports Medicine 26(6) (1998) 801–807. [DOI] [PubMed] [Google Scholar]
- [56].Soslowsky LJ, Thomopoulos S, Tun S, Flanagan CL, Keefer CC, Mastaw J, Carpenter JE, Neer Award 1999. Overuse activity injures the supraspinatus tendon in an animal model: a histologic and biomechanical study, J Shoulder Elbow Surg 9(2) (2000) 79–84. [PubMed] [Google Scholar]
- [57].Soslowsky LJ, Thomopoulos S, Esmail A, Flanagan CL, Iannotti JP, Williamson JD 3rd, Carpenter JE, Rotator cuff tendinosis in an animal model: role of extrinsic and overuse factors, Annals of biomedical engineering 30(8) (2002) 1057–63. [DOI] [PubMed] [Google Scholar]
- [58].Cho NS, Hwang JH, Lee YT, Chae SW, Tendinosis-like histologic and molecular changes of the Achilles tendon to repetitive stress: a pilot study in rats, Clin Orthop Relat Res 469(11) (2011) 3172–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Huang TF, Perry SM, Soslowsky LJ, The effect of overuse activity on Achilles tendon in an animal model: a biomechanical study, Annals of biomedical engineering 32(3) (2004) 336–41. [DOI] [PubMed] [Google Scholar]
- [60].Glazebrook MA, Wright JR Jr., Langman M, Stanish WD, Lee JM, Histological analysis of achilles tendons in an overuse rat model, J Orthop Res 26(6) (2008) 840–6. [DOI] [PubMed] [Google Scholar]
- [61].Legerlotz K, Schjerling P, Langberg H, Bruggemann GP, Niehoff A, The effect of running, strength, and vibration strength training on the mechanical, morphological, and biochemical properties of the Achilles tendon in rats, J Appl Physiol (1985) 102(2) (2007) 564–72. [DOI] [PubMed] [Google Scholar]
- [62].Rooney SI, Loro E, Sarver JJ, Peltz CD, Hast MW, Tseng WJ, Kuntz AF, Liu XS, Khurana TS, Soslowsky LJ, Exercise protocol induces muscle, tendon, and bone adaptations in the rat shoulder, Muscles Ligaments Tendons J 4(4) (2014) 413–9. [PMC free article] [PubMed] [Google Scholar]
- [63].de Cassia Marqueti R, Almeida JA, Nakagaki WR, Guzzoni V, Boghi F, Renner A, Silva PE, Durigan JLQ, Selistre-de-Araujo HS, Resistance training minimizes the biomechanical effects of aging in three different rat tendons, Journal of biomechanics 53 (2017) 29–35. [DOI] [PubMed] [Google Scholar]
- [64].Sommer HM, The biomechanical and metabolic effects of a running regime on the Achilles tendon in the rat, International orthopaedics 11(1) (1987) 71–5. [DOI] [PubMed] [Google Scholar]
- [65].Wu F, Nerlich M, Docheva D, Tendon injuries: Basic science and new repair proposals, EFORT Open Rev 2(7) (2017) 332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Wang JH, Mechanobiology of tendon, Journal of biomechanics 39(9) (2006) 1563–82. [DOI] [PubMed] [Google Scholar]
- [67].Fung DT, Wang VM, Laudier DM, Shine JH, Basta-Pljakic J, Jepsen KJ, Schaffler MB, Flatow EL, Subrupture tendon fatigue damage, J Orthop Res 27(2) (2009) 264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Andarawis-Puri N, Philip A, Laudier D, Schaffler MB, Flatow EL, Temporal effect of in vivo tendon fatigue loading on the apoptotic response explained in the context of number of fatigue loading cycles and initial damage parameters, J Orthop Res 32(9) (2014) 1097–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Ng GYF, Chung PYM, Wang JS, Cheung RTH, Enforced Bipedal Downhill Running Induces Achilles Tendinosis in Rats, Connective Tissue Research 52(6) (2011) 466–471. [DOI] [PubMed] [Google Scholar]
- [70].Sereysky JB, Andarawis-Puri N, Jepsen KJ, Flatow EL, Structural and mechanical effects of in vivo fatigue damage induction on murine tendon, J Orthop Res 30(6) (2012) 965–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Khayyeri H, Blomgran P, Hammerman M, Turunen MJ, Lowgren A, Guizar-Sicairos M, Aspenberg P, Isaksson H, Achilles tendon compositional and structural properties are altered after unloading by botox, Sci Rep 7(1) (2017) 13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Eliasson P, Fahlgren A, Pasternak B, Aspenberg P, Unloaded rat Achilles tendons continue to grow, but lose viscoelasticity, J Appl Physiol (1985) 103(2) (2007) 459–63. [DOI] [PubMed] [Google Scholar]
- [73].Schechtman H, Bader DL, Dynamic Characterization of Human Tendons, Proceedings of the Institution of Mechanical Engineers, Part H: Journal of Engineering in Medicine 208(4) (1994) 241–248. [Google Scholar]
- [74].Simonsen EB, Klitgaard H, Bojsen-Moller F, The influence of strength training, swim training and ageing on the Achilles tendon and m. soleus of the rat, J Sports Sci 13(4) (1995) 291–5. [DOI] [PubMed] [Google Scholar]
- [75].Ng GY, Chung PY, Wang JS, Cheung RT, Enforced bipedal downhill running induces Achilles tendinosis in rats, Connect Tissue Res 52(6) (2011) 466–71. [DOI] [PubMed] [Google Scholar]
- [76].Nielsen HM, Skalicky M, Viidik A, Influence of physical exercise on aging rats. III. Life-long exercise modifies the aging changes of the mechanical properties of limb muscle tendons, 1(3) (1998) 243–60. [DOI] [PubMed] [Google Scholar]
- [77].Kurtaliaj I, Golman M, Abraham AC, Thomopoulos S, Biomechanical Testing of Murine Tendons, J Vis Exp (152) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Thornton GM, Hart DA, The interface of mechanical loading and biological variables as they pertain to the development of tendinosis, J Musculoskelet Neuronal Interact 11(2) (2011) 94–105. [PubMed] [Google Scholar]
- [79].Bojsen-Moller J, Magnusson SP, Mechanical properties, physiological behavior, and function of aponeurosis and tendon, J Appl Physiol (1985) 126(6) (2019) 1800–1807. [DOI] [PubMed] [Google Scholar]
- [80].Magnusson SP, Hansen P, Kjaer M, Tendon properties in relation to muscular activity and physical training, Scand J Med Sci Sports 13(4) (2003) 211–23. [DOI] [PubMed] [Google Scholar]
- [81].Andarawis-Puri N, Flatow EL, Tendon fatigue in response to mechanical loading, J Musculoskelet Neuronal Interact 11(2) (2011) 106–14. [PMC free article] [PubMed] [Google Scholar]
- [82].Barone R, Bellafiore M, Leonardi V, Zummo G, Structural analysis of rat patellar tendon in response to resistance and endurance training, Scand J Med Sci Sports 19(6) (2009) 782–9. [DOI] [PubMed] [Google Scholar]
- [83].Barbe MF, Gallagher S, Massicotte VS, Tytell M, Popoff SN, Barr-Gillespie AE, The interaction of force and repetition on musculoskeletal and neural tissue responses and sensorimotor behavior in a rat model of work-related musculoskeletal disorders, BMC Musculoskelet Disord 14 (2013) 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Michna H, Morphometric analysis of loading-induced changes in collagen-fibril populations in young tendons, Cell Tissue Res 236(2) (1984) 465–70. [DOI] [PubMed] [Google Scholar]
- [85].Michna H, Hartmann G, Adaptation of tendon collagen to exercise, International orthopaedics 13(3) (1989) 161–5. [DOI] [PubMed] [Google Scholar]
- [86].De Mello Malheiro OC, Giacomini CT, Justulin LA Jr., Delella FK, Dal-Pai-Silva M, Felisbino SL, Calcaneal tendon regions exhibit different MMP-2 activation after vertical jumping and treadmill running, Anat Rec (Hoboken) 292(10) (2009) 1656–62. [DOI] [PubMed] [Google Scholar]
- [87].Fung DT, Wang VM, Andarawis-Puri N, Basta-Pljakic J, Li Y, Laudier DM, Sun HB, Jepsen KJ, Schaffler MB, Flatow EL, Early response to tendon fatigue damage accumulation in a novel in vivo model, Journal of biomechanics 43(2) (2010) 274–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Rooney SI, Tobias JW, Bhatt PR, Kuntz AF, Soslowsky LJ, Genetic Response of Rat Supraspinatus Tendon and Muscle to Exercise, PLoS One 10(10) (2015) e0139880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Franchi M, Torricelli P, Giavaresi G, Fini M, Role of moderate exercising on Achilles tendon collagen crimping patterns and proteoglycans, Connect Tissue Res 54(4–5) (2013) 267–74. [DOI] [PubMed] [Google Scholar]
- [90].Carpenter JE, Flanagan CL, Thomopoulos S, Yian EH, Soslowsky LJ, The effects of overuse combined with intrinsic or extrinsic alterations in an animal model of rotator cuff tendinosis, Am J Sports Med 26(6) (1998) 801–7. [DOI] [PubMed] [Google Scholar]
- [91].Silva RD, Glazebrook MA, Campos VC, Vasconcelos AC, Achilles tendinosis: a morphometrical study in a rat model, Int J Clin Exp Pathol 4(7) (2011) 683–91. [PMC free article] [PubMed] [Google Scholar]
- [92].Barbe MF, Barr AE, Gorzelany I, Amin M, Gaughan JP, Safadi FF, Chronic repetitive reaching and grasping results in decreased motor performance and widespread tissue responses in a rat model of MSD, J Orthop Res 21(1) (2003) 167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Thorpe CT, Riley GP, Birch HL, Clegg PD, Screen HRC, Fascicles and the interfascicular matrix show decreased fatigue life with ageing in energy storing tendons, Acta Biomater 56 (2017) 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Freedman BR, Zuskov A, Sarver JJ, Buckley MR, Soslowsky LJ, Evaluating changes in tendon crimp with fatigue loading as an ex vivo structural assessment of tendon damage, J Orthop Res 33(6) (2015) 904–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Shepherd JH, Legerlotz K, Demirci T, Klemt C, Riley GP, Screen HR, Functionally distinct tendon fascicles exhibit different creep and stress relaxation behaviour, Proc Inst Mech Eng H 228(1) (2014) 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Iqbal SMA, Deska-Gauthier D, Kreplak L, Assessing collagen fibrils molecular damage after a single stretch-release cycle, Soft Matter 15(30) (2019) 6237–6246. [DOI] [PubMed] [Google Scholar]
- [97].Legerlotz K, Jones GC, Screen HR, Riley GP, Cyclic loading of tendon fascicles using a novel fatigue loading system increases interleukin-6 expression by tenocytes, Scand J Med Sci Sports 23(1) (2013) 31–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Sun HB, Andarawis-Puri N, Li Y, Fung DT, Lee JY, Wang VM, Basta-Pljakic J, Leong DJ, Sereysky JB, Ros SJ, Klug RA, Braman J, Schaffler MB, Jepsen KJ, Flatow EL, Cycle-dependent matrix remodeling gene expression response in fatigue-loaded rat patellar tendons, J Orthop Res 28(10) (2010) 1380–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Attia M, Scott A, Carpentier G, Lian O, Van Kuppevelt T, Gossard C, Papy-Garcia D, Tassoni MC, Martelly I, Greater glycosaminoglycan content in human patellar tendon biopsies is associated with more pain and a lower VISA score, Br J Sports Med 48(6) (2014) 469–75. [DOI] [PubMed] [Google Scholar]
- [100].Fu SC, Chan KM, Rolf CG, Increased deposition of sulfated glycosaminoglycans in human patellar tendinopathy, Clin J Sport Med 17(2) (2007) 129–34. [DOI] [PubMed] [Google Scholar]
- [101].Samiric T, Parkinson J, Ilic MZ, Cook J, Feller JA, Handley CJ, Changes in the composition of the extracellular matrix in patellar tendinopathy, Matrix Biol 28(4) (2009) 230–6. [DOI] [PubMed] [Google Scholar]
- [102].Marqueti RC, Durigan JLQ, Oliveira AJS, Mekaro MS, Guzzoni V, Aro AA, Pimentel ER, Selistre-de-Araujo HS, Effects of aging and resistance training in rat tendon remodeling, FASEB J 32(1) (2018) 353–368. [DOI] [PubMed] [Google Scholar]
- [103].Scott A, Cook JL, Hart DA, Walker DC, Duronio V, Khan KM, Tenocyte responses to mechanical loading in vivo: a role for local insulin-like growth factor 1 signaling in early tendinosis in rats, Arthritis Rheum 56(3) (2007) 871–81. [DOI] [PubMed] [Google Scholar]
- [104].Attia M, Huet E, Gossard C, Menashi S, Tassoni MC, Martelly I, Early events of overused supraspinatus tendons involve matrix metalloproteinases and EMMPRIN/CD147 in the absence of inflammation, Am J Sports Med 41(4) (2013) 908–17. [DOI] [PubMed] [Google Scholar]
- [105].Obuchowicz R, Ekiert M, Kohut P, Holak K, Ambrozinski L, Tomaszewski KA, Uhl T, Mlyniec A, Interfascicular matrix-mediated transverse deformation and sliding of discontinuous tendon subcomponents control the viscoelasticity and failure of tendons, J Mech Behav Biomed Mater 97 (2019) 238–246. [DOI] [PubMed] [Google Scholar]
- [106].Attia M, Scott A, Duchesnay A, Carpentier G, Soslowsky LJ, Huynh MB, Van Kuppevelt TH, Gossard C, Courty J, Tassoni MC, Martelly I, Alterations of overused supraspinatus tendon: a possible role of glycosaminoglycans and HARP/pleiotrophin in early tendon pathology, J Orthop Res 30(1) (2012) 61–71. [DOI] [PubMed] [Google Scholar]
- [107].Chen G, Jiang H, Tian X, Tang J, Bai X, Zhang Z, Wang L, Mechanical loading modulates heterotopic ossification in calcific tendinopathy through the mTORC1 signaling pathway, Mol Med Rep 16(5) (2017) 5901–5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Hatano M, Kitajima I, Yamamoto S, Nakamura M, Isawa K, Hirota Y, Suwabe T, Hoshino J, Sawa N, Ubara Y, New bone-like tissue formation in calcific tendinopathy: A case report, Bone Rep 14 (2021) 101062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Nuss CA, Huegel J, Boorman-Padgett JF, Choi DS, Weiss SN, Vournakis J, Soslowsky LJ, Poly-N-Acetyl Glucosamine (sNAG) Enhances Early Rotator Cuff Tendon Healing in a Rat Model, Annals of biomedical engineering 45(12) (2017) 2826–2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Fedorczyk JM, Barr AE, Rani S, Gao HG, Amin M, Amin S, Litvin J, Barbe MF, Exposure-dependent increases in IL-1beta, substance P, CTGF, and tendinosis in flexor digitorum tendons with upper extremity repetitive strain injury, J Orthop Res 28(3) (2010) 298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Messner K, Wei Y, Andersson B, Gillquist J, Rasanen T, Rat model of Achilles tendon disorder. A pilot study, Cells Tissues Organs 165(1) (1999) 30–9. [DOI] [PubMed] [Google Scholar]
- [112].Freedman BR, Rodriguez AB, Hillin CD, Weiss SN, Han B, Han L, Soslowsky LJ, Tendon healing affects the multiscale mechanical, structural and compositional response of tendon to quasi-static tensile loading, J R Soc Interface 15(139) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Freedman BR, Rodriguez AB, Leiphart RJ, Newton JB, Ban E, Sarver JJ, Mauck RL, Shenoy VB, Soslowsky LJ, Dynamic Loading and Tendon Healing Affect Multiscale Tendon Properties and ECM Stress Transmission, Sci Rep 8(1) (2018) 10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Abboud JA, Soslowsky LJ, Interplay of the static and dynamic restraints in glenohumeral instability, Clin Orthop Relat Res (400) (2002) 48–57. [DOI] [PubMed] [Google Scholar]
- [115].Pingel J, Wienecke J, Kongsgaard M, Behzad H, Abraham T, Langberg H, Scott A, Increased mast cell numbers in a calcaneal tendon overuse model, Scand J Med Sci Sports 23(6) (2013) e353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Xu SY, Li SF, Ni GX, Strenuous Treadmill Running Induces a Chondrocyte Phenotype in Rat Achilles Tendons, Med Sci Monit 22 (2016) 3705–3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Kietrys DM, Barr-Gillespie AE, Amin M, Wade CK, Popoff SN, Barbe MF, Aging contributes to inflammation in upper extremity tendons and declines in forelimb agility in a rat model of upper extremity overuse, PLoS One 7(10) (2012) e46954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Szczodry M, Zhang J, Lim C, Davitt HL, Yeager T, Fu FH, Wang JH, Treadmill running exercise results in the presence of numerous myofibroblasts in mouse patellar tendons, J Orthop Res 27(10) (2009) 1373–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Huang AH, Lu HH, Schweitzer R, Molecular regulation of tendon cell fate during development, J Orthop Res 33(6) (2015) 800–12. [DOI] [PubMed] [Google Scholar]
- [120].Millar NL, Wei AQ, Molloy TJ, Bonar F, Murrell GA, Cytokines and apoptosis in supraspinatus tendinopathy, J Bone Joint Surg Br 91(3) (2009) 417–24. [DOI] [PubMed] [Google Scholar]
- [121].Millar NL, Hueber AJ, Reilly JH, Xu Y, Fazzi UG, Murrell GA, McInnes IB, Inflammation is present in early human tendinopathy, Am J Sports Med 38(10) (2010) 2085–91. [DOI] [PubMed] [Google Scholar]
- [122].Lundgreen K, Lian OB, Engebretsen L, Scott A, Tenocyte apoptosis in the torn rotator cuff: a primary or secondary pathological event?, Br J Sports Med 45(13) (2011) 1035–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Millar NL, Wei AQ, Molloy TJ, Bonar F, Murrell GA, Heat shock protein and apoptosis in supraspinatus tendinopathy, Clin Orthop Relat Res 466(7) (2008) 1569–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Holladay C, Abbah SA, O’Dowd C, Pandit A, Zeugolis DI, Preferential tendon stem cell response to growth factor supplementation, J Tissue Eng Regen Med 10(9) (2016) 783–98. [DOI] [PubMed] [Google Scholar]
- [125].Caliari SR, Harley BA, Composite growth factor supplementation strategies to enhance tenocyte bioactivity in aligned collagen-GAG scaffolds, Tissue Eng Part A 19(9–10) (2013) 1100–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Nielsen RH, Holm L, Jensen JK, Heinemeier KM, Remvig L, Kjaer M, Tendon protein synthesis rate in classic Ehlers-Danlos patients can be stimulated with insulin-like growth factor-I, J Appl Physiol (1985) 117(7) (2014) 694–8. [DOI] [PubMed] [Google Scholar]
- [127].Ichinose T, Lesmana R, Yamamoto A, Kobayashi T, Shitara H, Shimoyama D, Takatsuru Y, Iwasaki T, Shimokawa N, Takagishi K, Koibuchi N, Possible involvement of IGF-1 signaling on compensatory growth of the infraspinatus muscle induced by the supraspinatus tendon detachment of rat shoulder, Physiol Rep 2(1) (2014) e00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Provenzano PP, Alejandro-Osorio AL, Grorud KW, Martinez DA, Vailas AC, Grindeland RE, Vanderby R Jr., Systemic administration of IGF-I enhances healing in collagenous extracellular matrices: evaluation of loaded and unloaded ligaments, BMC Physiol 7 (2007) 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Tang Y, Leng Q, Xiang X, Zhang L, Yang Y, Qiu L, Use of ultrasound-targeted microbubble destruction to transfect IGF-1 cDNA to enhance the regeneration of rat wounded Achilles tendon in vivo, Gene therapy 22(8) (2015) 610–8. [DOI] [PubMed] [Google Scholar]
- [130].Thomas CH, Collier JH, Sfeir CS, Healy KE, Engineering gene expression and protein synthesis by modulation of nuclear shape, Proc Natl Acad Sci U S A 99(4) (2002) 1972–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Lelievre SA, Weaver VM, Nickerson JA, Larabell CA, Bhaumik A, Petersen OW, Bissell MJ, Tissue phenotype depends on reciprocal interactions between the extracellular matrix and the structural organization of the nucleus, Proc Natl Acad Sci U S A 95(25) (1998) 14711–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Henderson JT, Shannon G, Veress AI, Neu CP, Direct measurement of intranuclear strain distributions and RNA synthesis in single cells embedded within native tissue, Biophys J 105(10) (2013) 2252–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Shi Y, Fu Y, Tong W, Geng Y, Lui PP, Tang T, Zhang X, Dai K, Uniaxial mechanical tension promoted osteogenic differentiation of rat tendon-derived stem cells (rTDSCs) via the Wnt5a-RhoA pathway, Journal of cellular biochemistry 113(10) (2012) 3133–42. [DOI] [PubMed] [Google Scholar]
- [134].Devkota AC, Tsuzaki M, Almekinders LC, Banes AJ, Weinhold PS, Distributing a fixed amount of cyclic loading to tendon explants over longer periods induces greater cellular and mechanical responses, J Orthop Res 25(8) (2007) 1078–86. [DOI] [PubMed] [Google Scholar]
- [135].Maeda E, Shelton JC, Bader DL, Lee DA, Differential regulation of gene expression in isolated tendon fascicles exposed to cyclic tensile strain in vitro, J Appl Physiol (1985) 106(2) (2009) 506–12. [DOI] [PubMed] [Google Scholar]
- [136].Maeda E, Ye S, Wang W, Bader DL, Knight MM, Lee DA, Gap junction permeability between tenocytes within tendon fascicles is suppressed by tensile loading, Biomechanics and modeling in mechanobiology 11(3–4) (2012) 439–47. [DOI] [PubMed] [Google Scholar]
- [137].Langberg H, Skovgaard D, Karamouzis M, Bulow J, Kjaer M, Metabolism and inflammatory mediators in the peritendinous space measured by microdialysis during intermittent isometric exercise in humans, J Physiol 515 ( Pt 3) (1999) 919–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, Li L, Leet AI, Seo BM, Zhang L, Shi S, Young MF, Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche, Nat Med 13(10) (2007) 1219–27. [DOI] [PubMed] [Google Scholar]
- [139].Zhang J, Wang JH, BMP-2 mediates PGE(2) -induced reduction of proliferation and osteogenic differentiation of human tendon stem cells, J Orthop Res 30(1) (2012) 47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Archambault JM, Jelinsky SA, Lake SP, Hill AA, Glaser DL, Soslowsky LJ, Rat supraspinatus tendon expresses cartilage markers with overuse, J Orthop Res 25(5) (2007) 617–24. [DOI] [PubMed] [Google Scholar]
- [141].Perry SM, McIlhenny SE, Hoffman MC, Soslowsky LJ, Inflammatory and angiogenic mRNA levels are altered in a supraspinatus tendon overuse animal model, J Shoulder Elbow Surg 14(1 Suppl S) (2005) 79S–83S. [DOI] [PubMed] [Google Scholar]
- [142].Thornton GM, Shao X, Chung M, Sciore P, Boorman RS, Hart DA, Lo IK, Changes in mechanical loading lead to tendonspecific alterations in MMP and TIMP expression: influence of stress deprivation and intermittent cyclic hydrostatic compression on rat supraspinatus and Achilles tendons, Br J Sports Med 44(10) (2010) 698–703. [DOI] [PubMed] [Google Scholar]
- [143].Zhang J, Wang JH, The effects of mechanical loading on tendons--an in vivo and in vitro model study, PLoS One 8(8) (2013) e71740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Oliva F, Barisani D, Grasso A, Maffulli N, Gene expression analysis in calcific tendinopathy of the rotator cuff, European cells & materials 21 (2011) 548–57. [DOI] [PubMed] [Google Scholar]
- [145].Abraham T, Fong G, Scott A, Second harmonic generation analysis of early Achilles tendinosis in response to in vivo mechanical loading, BMC Musculoskelet Disord 12 (2011) 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Jelinsky SA, Lake SP, Archambault JM, Soslowsky LJ, Gene expression in rat supraspinatus tendon recovers from overuse with rest, Clinical Orthopaedics and Related Research 466(7) (2008) 1612–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Gao HG, Fisher PW, Lambi AG, Wade CK, Barr-Gillespie AE, Popoff SN, Barbe MF, Increased serum and musculotendinous fibrogenic proteins following persistent low-grade inflammation in a rat model of long-term upper extremity overuse, PLoS One 8(8) (2013) e71875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Frara N, Abdelmagid SM, Tytell M, Amin M, Popoff SN, Safadi FF, Barbe MF, Growth and repair factors, osteoactivin, matrix metalloproteinase and heat shock protein 72, increase with resolution of inflammation in musculotendinous tissues in a rat model of repetitive grasping, BMC Musculoskelet Disord 17 (2016) 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Molloy TJ, Kemp MW, Wang Y, Murrell GA, Microarray analysis of the tendinopathic rat supraspinatus tendon: glutamate signaling and its potential role in tendon degeneration, J Appl Physiol (1985) 101(6) (2006) 1702–9. [DOI] [PubMed] [Google Scholar]
- [150].Yang G, Crawford RC, Wang JH, Proliferation and collagen production of human patellar tendon fibroblasts in response to cyclic uniaxial stretching in serum-free conditions, Journal of biomechanics 37(10) (2004) 1543–50. [DOI] [PubMed] [Google Scholar]
- [151].de Vos RJ, Weir A, Cobben LP, Tol JL, The value of power Doppler ultrasonography in Achilles tendinopathy: a prospective study, Am J Sports Med 35(10) (2007) 1696–701. [DOI] [PubMed] [Google Scholar]
- [152].Hoksrud A, Ohberg L, Alfredson H, Bahr R, Color Doppler ultrasound findings in patellar tendinopathy (jumper’s knee), Am J Sports Med 36(9) (2008) 1813–20. [DOI] [PubMed] [Google Scholar]
- [153].Pingel J, Harrison A, Simonsen L, Suetta C, Bulow J, Langberg H, The microvascular volume of the Achilles tendon is increased in patients with tendinopathy at rest and after a 1-hour treadmill run, Am J Sports Med 41(10) (2013) 2400–8. [DOI] [PubMed] [Google Scholar]
- [154].Boesen AP, Boesen MI, Koenig MJ, Bliddal H, Torp-Pedersen S, Langberg H, Evidence of accumulated stress in Achilles and anterior knee tendons in elite badminton players, Knee Surg Sports Traumatol Arthrosc 19(1) (2011) 30–7. [DOI] [PubMed] [Google Scholar]
- [155].Cook JL, Ptazsnik R, Kiss ZS, Malliaras P, Morris ME, De Luca J, High reproducibility of patellar tendon vascularity assessed by colour Doppler ultrasonography: a reliable measurement tool for quantifying tendon pathology, Br J Sports Med 39(10) (2005) 700–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Divani K, Chan O, Padhiar N, Twycross-Lewis R, Maffulli N, Crisp T, Morrissey D, Site of maximum neovascularisation correlates with the site of pain in recalcitrant mid-tendon Achilles tendinopathy, Man Ther 15(5) (2010) 463–8. [DOI] [PubMed] [Google Scholar]
- [157].Alfredson H, Ohberg L, Zeisig E, Lorentzon R, Treatment of midportion Achilles tendinosis: similar clinical results with US and CD-guided surgery outside the tendon and sclerosing polidocanol injections, Knee Surg Sports Traumatol Arthrosc 15(12) (2007) 1504–9. [DOI] [PubMed] [Google Scholar]
- [158].Vasta S, Di Martino A, Zampogna B, Torre G, Papalia R, Denaro V, Role of VEGF, Nitric Oxide, and Sympathetic Neurotransmitters in the Pathogenesis of Tendinopathy: A Review of the Current Evidences, Front Aging Neurosci 8 (2016) 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Astrom M, Westlin N, Blood flow in chronic Achilles tendinopathy, Clin Orthop Relat Res (308) (1994) 166–72. [PubMed] [Google Scholar]
- [160].Szomor ZL, Appleyard RC, Murrell GA, Overexpression of nitric oxide synthases in tendon overuse, J Orthop Res 24(1) (2006) 80–6. [DOI] [PubMed] [Google Scholar]
- [161].Maeda E, Fleischmann C, Mein CA, Shelton JC, Bader DL, Lee DA, Functional analysis of tenocytes gene expression in tendon fascicles subjected to cyclic tensile strain, Connect Tissue Res 51(6) (2010) 434–44. [DOI] [PubMed] [Google Scholar]
- [162].Maeda E, Shelton JC, Bader DL, Lee DA, Time dependence of cyclic tensile strain on collagen production in tendon fascicles, Biochemical and biophysical research communications 362(2) (2007) 399–404. [DOI] [PubMed] [Google Scholar]
- [163].Screen HR, Shelton JC, Bader DL, Lee DA, Cyclic tensile strain upregulates collagen synthesis in isolated tendon fascicles, Biochemical and biophysical research communications 336(2) (2005) 424–9. [DOI] [PubMed] [Google Scholar]
- [164].Hannafin JA, Arnoczky SP, Hoonjan A, Torzilli PA, Effect of stress deprivation and cyclic tensile loading on the material and morphologic properties of canine flexor digitorum profundus tendon: an in vitro study, J Orthop Res 13(6) (1995) 907–14. [DOI] [PubMed] [Google Scholar]
- [165].Jones ER, Jones GC, Legerlotz K, Riley GP, Cyclical strain modulates metalloprotease and matrix gene expression in human tenocytes via activation of TGFbeta, Biochimica et biophysica acta 1833(12) (2013) 2596–2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Yamamoto E, Kogawa D, Tokura S, Hayashi K, Effects of the frequency and duration of cyclic stress on the mechanical properties of cultured collagen fascicles from the rabbit patellar tendon, J Biomech Eng 127(7) (2005) 1168–75. [DOI] [PubMed] [Google Scholar]
- [167].Yamamoto E, Tokura S, Hayashi K, Effects of cyclic stress on the mechanical properties of cultured collagen fascicles from the rabbit patellar tendon, J Biomech Eng 125(6) (2003) 893–901. [DOI] [PubMed] [Google Scholar]
- [168].Wang JH, Jia F, Yang G, Yang S, Campbell BH, Stone D, Woo SL, Cyclic mechanical stretching of human tendon fibroblasts increases the production of prostaglandin E2 and levels of cyclooxygenase expression: a novel in vitro model study, Connect Tissue Res 44(3–4) (2003) 128–33. [DOI] [PubMed] [Google Scholar]
- [169].Cilli F, Khan M, Fu F, Wang JH, Prostaglandin E2 affects proliferation and collagen synthesis by human patellar tendon fibroblasts, Clin J Sport Med 14(4) (2004) 232–6. [DOI] [PubMed] [Google Scholar]
- [170].Rui YF, Lui PP, Ni M, Chan LS, Lee YW, Chan KM, Mechanical loading increased BMP-2 expression which promoted osteogenic differentiation of tendon-derived stem cells, J Orthop Res 29(3) (2011) 390–6. [DOI] [PubMed] [Google Scholar]
- [171].Chen CT, Malkus DS, Vanderby R Jr., A fiber matrix model for interstitial fluid flow and permeability in ligaments and tendons, Biorheology 35(2) (1998) 103–18. [DOI] [PubMed] [Google Scholar]
- [172].Lavagnino M, Arnoczky SP, Kepich E, Caballero O, Haut RC, A finite element model predicts the mechanotransduction response of tendon cells to cyclic tensile loading, Biomechanics and modeling in mechanobiology 7(5) (2008) 405–16. [DOI] [PubMed] [Google Scholar]
- [173].Weber B, Lackner I, Haffner-Luntzer M, Palmer A, Pressmar J, Scharffetter-Kochanek K, Knoll B, Schrezenemeier H, Relja B, Kalbitz M, Modeling trauma in rats: similarities to humans and potential pitfalls to consider, J Transl Med 17(1) (2019) 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Abdullahi A, Amini-Nik S, Jeschke MG, Animal models in burn research, Cell Mol Life Sci 71(17) (2014) 3241–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Gardner K, Arnoczky SP, Lavagnino M, Effect of in vitro stress-deprivation and cyclic loading on the length of tendon cell cilia in situ, J Orthop Res 29(4) (2011) 582–7. [DOI] [PubMed] [Google Scholar]
- [176].Lavagnino M, Gardner K, Sedlak AM, Arnoczky SP, Tendon cell ciliary length as a biomarker of in situ cytoskeletal tensional homeostasis, Muscles Ligaments Tendons J 3(3) (2013) 118–21. [PMC free article] [PubMed] [Google Scholar]
- [177].Asahara H, Inui M, Lotz MK, Tendons and Ligaments: Connecting Developmental Biology to Musculoskeletal Disease Pathogenesis, J Bone Miner Res 32(9) (2017) 1773–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Zhang C, Couppe C, Scheijen J, Schalkwijk CG, Kjaer M, Magnusson SP, Svensson RB, Regional collagen turnover and composition of the human patellar tendon, J Appl Physiol (1985) 128(4) (2020) 884–891. [DOI] [PubMed] [Google Scholar]
- [179].Choi H, Simpson D, Wang D, Prescott M, Pitsillides AA, Dudhia J, Clegg PD, Ping P, Thorpe CT, Heterogeneity of proteome dynamics between connective tissue phases of adult tendon, Elife 9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Elliott MB, Barr AE, Clark BD, Amin M, Amin S, Barbe MF, High force reaching task induces widespread inflammation, increased spinal cord neurochemicals and neuropathic pain, Neuroscience 158(2) (2009) 922–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Lukashevich IS, Golubev VP, Stcheslyenok EP, Finskaya NN, Stelmakh TA, L and S species of Machupo virion RNA contain a mixture of complementary strands. Brief report, Arch Virol 88(1–2) (1986) 151–6. [DOI] [PubMed] [Google Scholar]
- [182].Rio E, Moseley L, Purdam C, Samiric T, Kidgell D, Pearce AJ, Jaberzadeh S, Cook J, The pain of tendinopathy: physiological or pathophysiological?, Sports Med 44(1) (2014) 9–23. [DOI] [PubMed] [Google Scholar]
- [183].Sarver JJ, Dishowitz MI, Kim SY, Soslowsky LJ, Transient decreases in forelimb gait and ground reaction forces following rotator cuff injury and repair in a rat model, Journal of biomechanics 43(4) (2010) 778–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Wu PT, Hsu CH, Su FC, Jou IM, Chen SY, Wu CL, Su WR, Kuo LC, Dynamic weight bearing analysis is effective for evaluation of tendinopathy using a customized corridor with multi-directional force sensors in a rat model, Sci Rep 7(1) (2017) 8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Lee SY, Chieh HF, Lin CJ, Jou IM, Sun YN, Kuo LC, Wu PT, Su FC, Characteristics of Sonography in a Rat Achilles Tendinopathy Model: Possible Non-invasive Predictors of Biomechanics, Sci Rep 7(1) (2017) 5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Riggin CN, Schultz SM, Sehgal CM, Soslowsky LJ, Ultrasound Evaluation of Anti-Vascular Endothelial Growth Factor-Induced Changes in Vascular Response Following Tendon Injury, Ultrasound Med Biol 45(7) (2019) 1841–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Dallaudiere B, Lempicki M, Pesquer L, Louedec L, Preux PM, Meyer P, Hummel V, Larbi A, Deschamps L, Journe C, Hess A, Silvestre A, Sargos P, Loriaut P, Boyer P, Schouman-Claeys E, Michel JB, Serfaty JM, Efficacy of intra-tendinous injection of platelet-rich plasma in treating tendinosis: comprehensive assessment of a rat model, Eur Radiol 23(10) (2013) 2830–7. [DOI] [PubMed] [Google Scholar]
- [188].Martin JA, Brandon SCE, Keuler EM, Hermus JR, Ehlers AC, Segalman DJ, Allen MS, Thelen DG, Gauging force by tapping tendons, Nature communications 9(1) (2018) 1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Riggin CN, Sarver JJ, Freedman BR, Thomas SJ, Soslowsky LJ, Analysis of collagen organization in mouse achilles tendon using high-frequency ultrasound imaging, J Biomech Eng 136(2) (2014) 021029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Schmidt EC, Hullfish TJ, O’Connor KM, Hast MW, Baxter JR, Ultrasound echogenicity is associated with fatigue-induced failure in a cadaveric Achilles tendon model, Journal of biomechanics 105 (2020) 109784. [DOI] [PubMed] [Google Scholar]
- [191].Del Bakhshayesh AR, Asadi N, Alihemmati A, Tayefi Nasrabadi H, Montaseri A, Davaran S, Saghati S, Akbarzadeh A, Abedelahi A, An overview of advanced biocompatible and biomimetic materials for creation of replacement structures in the musculoskeletal systems: focusing on cartilage tissue engineering, J Biol Eng 13 (2019) 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Costa-Almeida R, Calejo I, Gomes ME, Mesenchymal Stem Cells Empowering Tendon Regenerative Therapies, International journal of molecular sciences 20(12) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Millar NL, Akbar M, Campbell AL, Reilly JH, Kerr SC, McLean M, Frleta-Gilchrist M, Fazzi UG, Leach WJ, Rooney BP, Crowe LA, Murrell GA, McInnes IB, IL-17A mediates inflammatory and tissue remodelling events in early human tendinopathy, Sci Rep 6 (2016) 27149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Coenen AMJ, Bernaerts KV, Harings JAW, Jockenhoevel S, Ghazanfari S, Elastic materials for tissue engineering applications: Natural, synthetic, and hybrid polymers, Acta Biomater 79 (2018) 60–82. [DOI] [PubMed] [Google Scholar]
- [195].Thayer PS, Verbridge SS, Dahlgren LA, Kakar S, Guelcher SA, Goldstein AS, Fiber/collagen composites for ligament tissue engineering: influence of elastic moduli of sparse aligned fibers on mesenchymal stem cells, J Biomed Mater Res A 104(8) (2016) 1894–901. [DOI] [PubMed] [Google Scholar]
- [196].Tang Y, Chen C, Liu F, Xie S, Qu J, Li M, Li Z, Li X, Shi Q, Li S, Li X, Hu J, Lu H, Structure and ingredient-based biomimetic scaffolds combining with autologous bone marrow-derived mesenchymal stem cell sheets for bone-tendon healing, Biomaterials 241 (2020) 119837. [DOI] [PubMed] [Google Scholar]
- [197].Wood LK, Brooks SV, Ten weeks of treadmill running decreases stiffness and increases collagen turnover in tendons of old mice, J Orthop Res 34(2) (2016) 346–53. [DOI] [PubMed] [Google Scholar]
- [198].Sun HB, Li Y, Fung DT, Majeska RJ, Schaffler MB, Flatow EL, Coordinate regulation of IL-1beta and MMP-13 in rat tendons following subrupture fatigue damage, Clin Orthop Relat Res 466(7) (2008) 1555–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Heinemeier KM, Olesen JL, Haddad F, Langberg H, Kjaer M, Baldwin KM, Schjerling P, Expression of collagen and related growth factors in rat tendon and skeletal muscle in response to specific contraction types, J Physiol 582(Pt 3) (2007) 1303–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Jarvinen TA, Jozsa L, Kannus P, Jarvinen TL, Kvist M, Hurme T, Isola J, Kalimo H, Jarvinen M, Mechanical loading regulates tenascin-C expression in the osteotendinous junction, Journal of cell science 112 Pt 18 (1999) 3157–66. [DOI] [PubMed] [Google Scholar]
- [201].Archambault JM, Hart DA, Herzog W, Response of rabbit Achilles tendon to chronic repetitive loading, Connect Tissue Res 42(1) (2001) 13–23. [DOI] [PubMed] [Google Scholar]
- [202].Heinemeier KM, Olesen JL, Schjerling P, Haddad F, Langberg H, Baldwin KM, Kjaer M, Short-term strength training and the expression of myostatin and IGF-I isoforms in rat muscle and tendon: differential effects of specific contraction types, J Appl Physiol (1985) 102(2) (2007) 573–81. [DOI] [PubMed] [Google Scholar]
- [203].Hansson HA, Engstrom AM, Holm S, Rosenqvist AL, Somatomedin C immunoreactivity in the Achilles tendon varies in a dynamic manner with the mechanical load, Acta Physiol Scand 134(2) (1988) 199–208. [DOI] [PubMed] [Google Scholar]
- [204].Andersson G, Forsgren S, Scott A, Gaida JE, Stjernfeldt JE, Lorentzon R, Alfredson H, Backman C, Danielson P, Tenocyte hypercellularity and vascular proliferation in a rabbit model of tendinopathy: contralateral effects suggest the involvement of central neuronal mechanisms, Br J Sports Med 45(5) (2011) 399–406. [DOI] [PubMed] [Google Scholar]
- [205].Backman C, Boquist L, Friden J, Lorentzon R, Toolanen G, Chronic achilles paratenonitis with tendinosis: an experimental model in the rabbit, J Orthop Res 8(4) (1990) 541–7. [DOI] [PubMed] [Google Scholar]


