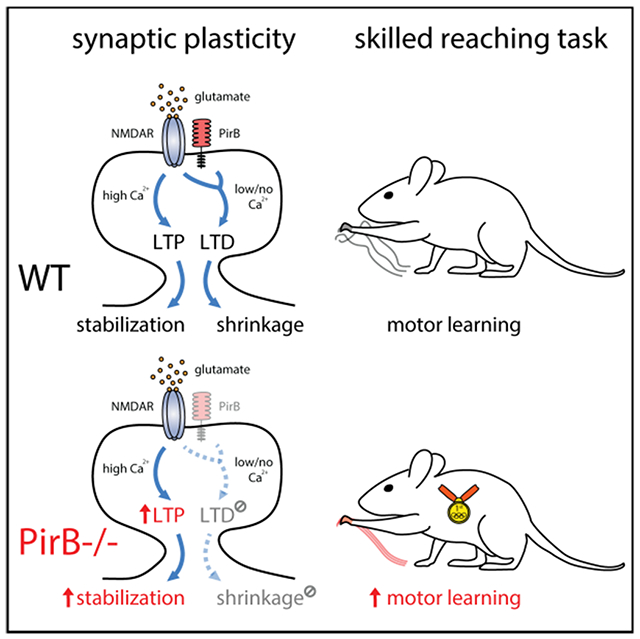
SUMMARY
Dendritic spine dynamics are thought to be substrates for motor learning and memory and altered spine dynamics often lead to impaired performance. Here we describe an exception to this rule by studying mice lacking Paired immunoglobulin receptor B (PirB−/−). Pyramidal neuron dendrites in PirB−/− mice have increased spine formation rates and density. Surprisingly, PirB−/− mice learn a skilled-reaching task faster than wild type (WT) littermates. Furthermore, the stabilization of learning-induced spines is elevated in PirB−/− mice. Mechanistically, single spine uncaging experiments suggest that PirB is required for NMDAR-dependent spine shrinkage. The degree of survival of newly-formed spines correlates with performance, suggesting that increased spine stability is advantageous for learning. Notably, inhibiting PirB function acutely in M1 of adult WT mice increases the survival of learning-induced spines and enhances motor learning. These results demonstrate that there are limits on motor learning that can be lifted by manipulating PirB, even in adulthood.
Graphical Abstract
eTOC Blurb
Dynamic changes of dendritic spines in motor cortex are critical for motor learning. Albarran et al. show that motor learning and performance can be enhanced by blocking PirB function, which disengages NMDAR-dependent spine shrinkage and LTD, resulting in increased spine stability and density.
INTRODUCTION
Animals have an incredible capacity for learning new motor skills. Is this capacity fully optimized, or are there limits on motor learning that could be removed to yield even better performance? One prevailing idea is that the acquisition of new skills involves formation and stabilization of new dendritic spines on motor cortical neurons (Xu et al., 2009; Yang et al., 2009; Peters et al., 2017).
Structural plasticity, the dynamic changes in spine size, shape, and numbers, is known to accompany experience-dependent changes in circuits (Yuste et al., 2001; Froemke et al., 2007 Yasumatsu et al., 2008; Holtmaat and Svoboda, 2009). In the visual and somatosensory systems, experience drives the formation and elimination of synapses and leads to lasting changes in spine densities (Trachtenberg et al., 2002; Zuo et al., 2005; Hofer et al., 2009). In the motor system, new spines on dendrites of Layer 5 pyramidal neurons (L5PNs) are generated when motor skills are learned (Harms et al., 2008; Xu et al., 2009). These new spines represent structural and functional circuit rewiring and involve changes in spine dynamics including stabilization, elimination, and turnover. It is thought that learning-induced spine formation and stabilization underlie the improved performance on motor tasks, a conclusion based on several important experiments. Firstly, spine dynamics correlate with motor performance (Xu et al., 2009; Yang et al., 2009). Secondly, animal models of neuropathology such as Alzheimer’s (Tsai et al., 2004; Spires et al., 2005; Shankar et al., 2007) and Parkinson’s disease (Stephens et al., 2005; Guo et al., 2015) exhibit spine destabilization and/or accelerated spine loss correlating with impaired learning and memory. Finally, directly eliminating task-specific spines abolishes motor cortical memories (Hayashi-Takagi et al., 2015; Frank et al., 2018).
All these experiments involve loss-of-function models and manipulations. Conversely, few studies have examined the effects on behavior of increasing spine stability and/or density. In Fragile-X syndrome mice, spine density is increased but learning is impaired (Padmashri et al., 2013). In contrast, enhanced learning on hippocampal memory tasks is associated with spine density in mice lacking CCR5 (Frank et al., 2018). Thus, spine density alone cannot predict learning and memory performance, raising the possibility that spine dynamics, the balance between elimination and maintenance, may be a key determinant of performance.
Here we investigate this possibility by studying mice lacking PirB, a receptor expressed on pyramidal neurons throughout neocortex and hippocampus. Germline (Syken et al, 2006; Djurisic et al., 2013) or conditional deletion of PirB exclusively from neurons (Bochner et al., 2014; Vidal et al., 2016; Djurisic et al., 2019) results in enhanced plasticity in visual cortex and hippocampus associated with significantly higher spine densities. Thus, PirB−/− mice offer an opportunity to examine the relationship between learning, increased spine density, and synaptic plasticity in the motor system. By manipulating PirB genetically and pharmacologically, we identify the stabilization of newly formed spines as a critical determinant of acquisition and maintenance of motor memory and show that there is a PirB-dependent limit that can be lifted to generate superior motor learning, even in adulthood.
RESULTS
Increased Spine Density and Functional Synapses in M1 L5 Pyramidal Neurons of PirB−/− Mice
To investigate the impact of PirB function on excitatory synapses in motor cortex in vivo, two-photon microscopy was used to image spines along the apical dendrites of L5PNs through cranial windows chronically implanted over the forelimb area of M1. L5PNs were labeled by expression of yellow fluorescent protein in Thy1-YFP-H;PirB−/− mice or Thy1-YFP-H;PirB+/+ wildtype (WT) littermate controls. Two weeks following cranial window implantation surgery, the same apical dendrites of L5PNs were imaged throughout a 6-day period at two-day intervals, allowing us to quantify the formation of new spines or elimination of existing ones (Figures 1A and 1B). The rate of spine formation but not elimination was significantly greater (~25%) in PirB−/− mice compared to WT littermates (Figures 1C,D and S1A), resulting in greater spine density in PirB−/− (~31%; Figures 1E and S1B). The elevated spine density on L5PNs in PirB−/− motor cortex is consistent with previous observations in visual cortex (Djurisic et al., 2013; Bochner et al, 2014), where it was shown to arise as a consequence of deficient pruning during development (Vidal et al, 2016). Next, survival of pre-existing and newly formed dendritic spines was monitored over the 6-day period and found to be greater in PirB−/− mice compared to WT controls (Figures S1C,D). Furthermore, when spine density changes are normalized to the density measured at the first imaging session, L5PNs in PirB−/− mice have significantly lower net spine loss over days compared to WT (Figure S1B), supporting the idea that the increased spine survival and rate of formation result in the greater spine density observed in PirB−/−. When clustering of spines along dendrites is compared between WT and PirB−/− (Figures S1E,F), PirB−/− mice have a greater proportion of spines located within 0.2-2.0 μm of each other, reflecting an even greater level of spine clustering than WT.
Figure 1. Increased Dendritic Spine Density and Improved Acquisition of Motor Skills in PirB−/− Mice.
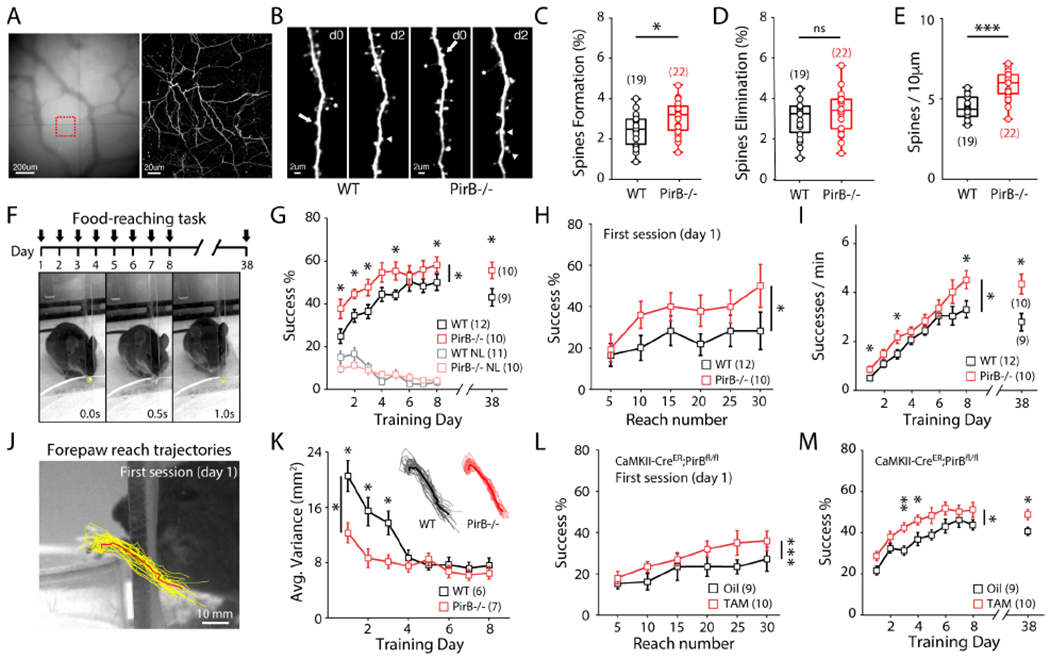
(A) Repeated imaging of dendritic spines (2-day interval). Left: vasculature in M1. Right: two-photon image of red square from left, revealing apical dendrites of L5PNs.
(B) Representative dendrites across 2 days in WT and PirB−/− mice. Arrows: elimination, arrow heads: formation, stars: filopodia.
(C-D) Increased baseline spine formation in PirB−/− mice (WT: 2.4445 ± 0.1856%; n = 19 mice; PirB−/−: 3.0625 ± 0.1732%; n = 22 mice; p = 0.024, Mann-Whitney), but not elimination (WT: 3.0544 ± 0.2175%; PirB−/−: 3.3660 ± 0.2112%; p = 0.353, Mann-Whitney).
(E) Increased spine density in PirB−/− mice (WT: 4.451 ± 0.1610; PirB−/−: 5.846 ± 0.1870; p = 1.7022 e-05, Mann-Whitney).
(F) Top: task timeline. Bottom: single trial of the reaching task. Yellow circle: food pellet.
(G) PirB−/− mice performed the reaching task better than WT mice (WT: n = 12; PirB−/−: n = 10; p = 0.018, 2-way repeated measures ANOVA), even when re-assessed 30 days later (WT: 43.056 ± 4.153%; n = 10; PirB−/−: 55.667 ± 3.818%; n = 9; p = 0.034, Mann-Whitney). NL: non-learners.
(H) PirB−/− mice achieved a higher success % than WT mice within the first day of training (p = 0.029, 2-way repeated measures ANOVA).
(I) PirB−/− mice made more successful reaches per minute than WT mice (p = 0.037, 2-way repeated measures ANOVA), even when re-assessed 30 days later (WT: 2.794 ± 0.357; PirB−/−: 4.343 ± 0.401; p = 0.025, Mann-Whitney).
(J) Reach trajectory kinematics analysis. Yellow: 30 individual reach trajectories. Red: average reach trajectory.
(K) PirB−/− mice achieve low-variance trajectories faster than WT mice (WT: n = 6; PirB−/−: n = 7; p = 0.019, 2-way repeated measures ANOVA).
(L) Conditional PirB excision resulted in increased success % within first day of training (Oil: n = 9; Tamoxifen: n = 10; p = 0.0008, 2-way repeated measures ANOVA).
(M) Conditional PirB excision resulted in enhanced motor learning throughout training (p = 0.022, 2-way repeated measures ANOVA) and when re-assessed 30 days later (p = 0.029, Mann-Whitney).
(C-E) Circles: individual mice. Box plots and mean ± s.e.m. are shown.
(G-I, K-M) Squares: mean (± s.e.m.).
* p < 0.05, ** p < 0.01, *** p < 0.001.
It is possible that the increased dendritic spine density observed in PirB−/− motor cortex is accompanied by an increase in functional excitatory synapses. To confirm this possibility, whole-cell recordings were made from identified YFP+ L5PNs in acute coronal M1 brain slices prepared from Thy1-YFP+;PirB+/+ or Thy1-YFP+;PirB−/− mice (Figure S1G). A significant increase is present in the mean frequency of mEPSCs in PirB−/− mice (Figures S1H,J) but not in mean mEPSC amplitude (Figure S1K). These results imply that there is an increase in the number of functional excitatory inputs onto L5PNs in M1, consistent with the increase in spine density observed above. In addition, there is a significant increase in mIPSC frequency (but not mIPSC amplitude) recorded from M1 L5PNs in PirB−/− mice (Figure S1I,L,M), suggesting that despite a greater number of excitatory inputs, the overall balance of excitation and inhibition is maintained in these neurons.
Enhanced Motor Learning and Motor Performance in PirB−/− Mice
To determine if increased spine density and changes in spine dynamics in M1 impact the ability of mice to learn a new motor skill, adult (2-month-old) WT and PirB−/− mice were trained to perform a reaching task (Figures 1F and S1N) (Greenough et al., 1985; Rioult-Pedotti et al., 2000; Xu et al., 2009, Guo et al., 2015). During training, both WT and PirB−/− mice improved their reaching success rates (see Methods) in the initial 4 days (Figure 1G). However, throughout training, PirB−/− mice exhibited significantly higher rates of successful reaches compared to WT (Figure 1G). The same mice were then housed in their home cages for an additional 30 days following the training period and their performance on the same reaching task was re-evaluated. Although both pretrained WT and PirB−/− mice maintained their skillful performance, the enhanced success rate of PirB−/− mice persisted up to day 38 (Figure 1G, right). Even on day 1, within the first training session of the task, PirB−/− mice learned the task faster than the WT littermates (Figure 1H). Note that on day 1, novice WT and PirB−/− mice have the same low initial success rate (~20%) in the first 5 reaches; however, during subsequent reaches PirB−/− mice gradually outperformed their WT littermates. Consistent with a higher success rate, PirB−/− mice also completed the task significantly faster than WT throughout the 8-d training period, as well as during the re-evaluation on day 38 (Figure 1I). Moreover, a detailed analysis of reach trajectory kinematics using convolutional neural networks (DeepLabCut, see Methods) revealed that PirB−/− mice were faster to develop stereotyped, low-variability reaches compared to WT littermates (Figures 1J,K), demonstrating enhanced fine-motor adaptation in PirB−/− mice.
To examine if deletion of PirB specifically from forebrain pyramidal neurons is sufficient to endow mice with enhanced motor learning, a neuronal specific deletion of PirB was generated by crossing PirBfl/fl mice with a CaMKIIa-CreER line (Madisen et al., 2009), allowing for cell-type-specific excision of PirB in the pyramidal neurons upon administration of tamoxifen at P42. 2-month-old tamoxifen- or oil-treated control CaMKIIa-CreER;PirBfl/fl mice were then trained on the reaching task (Figures S1O,P). Tamoxifen-treated, PirB conditional deletion mice achieved a significantly higher success rate within the first training day compared to oil-treated controls (Figure 1L). Furthermore, conditional PirB deletion was sufficient to enhance motor learning throughout the 8-day training period and at re-evaluation 30-days later (Figure 1M), similar to germline knockout of PirB. These results demonstrate that specific deletion of PirB from forebrain pyramidal neurons is sufficient to enhance motor performance in this reaching task. In addition, because tamoxifen administration and behavioral training were performed in adult mice, these results reveal an ongoing repressive function of PirB on motor performance and they also rule out the possibility of early developmental effects and/or potential compensations caused by loss of PirB in the germline.
Survival of Learning-Induced Spines Correlates Best with Motor Performance
Previous studies have shown that dynamic changes in dendritic spines occur during motor learning (Xu et al., 2009; Peters et al., 2014; Yang et al., 2014; Roth et al. 2020). To investigate the relationship between increased spine formation, spine survival, and enhanced motor learning in PirB−/− mice, a longitudinal experiment that combined chronic in vivo two-photon imaging of dendritic spines with motor skill training was performed (Figure 2A). Throughout the training paradigm, the same dendritic segments of L5PNs were repeatedly imaged through a cranial window to monitor transient and long-term spine changes (Figures 2B and S2A). In WT mice that successfully learned to perform the reaching task, L5PNs in M1 contralateral to the reaching forelimb underwent a significant increase in spine formation during the early learning period (Figure 2C), followed by a significant increase in spine elimination (Figure 2F), as compared to mice undergoing the same training but were unable to learn the task which showed stable levels of spine formation and elimination (Figures 2C,F: non-learners, WT NL). These data are consistent with many previous reports showing that motor skill learning induces rapid dendritic spine formation followed by delayed and selective spine pruning in M1 L5PNs (Xu et al., 2009; Yang et al., 2009; Wang et al., 2010; Guo et al., 2015). Trained PirB−/− mice that learned the same motor skill task also underwent a significant increase in spine formation (Figure 2D). Notably however, in PirB−/− mice, this increase in formation was not accompanied by a significant increase in spine elimination (Figure 2G). Direct comparison of spine formation and elimination in WT and PirB−/− mice that learned the task revealed that motor learning induces similar increases in spine formation despite the fact that baseline spine formation is higher in PirB−/− mice (Figure 2E). Remarkably, what differs between the genotypes is that learning-induced spine elimination is significantly lower in PirB−/− learner mice compared to WT learners (Figure 2H).
Figure 2. More Stability of Newly Formed Spines During Early Learning in PirB−/− Mice Correlates with Learning Performance.
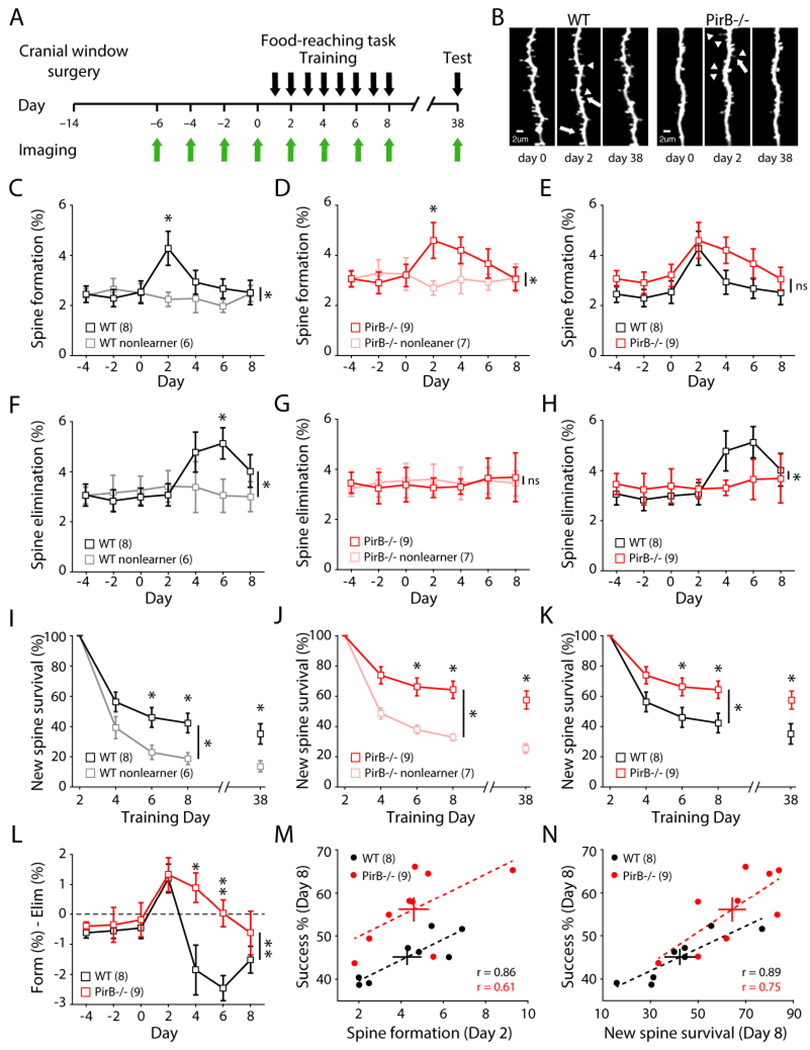
(A) Experimental timeline.
(B) Representative dendrites repeatedly imaged throughout training. Arrows: elimination, arrow heads: formation.
(C) Increased spine formation rates in WT learners during early training (day 0: 2.54 ± 0.45%; day 2: 4.28 ± 0.68%; n = 8; p = 0.016, Wilcoxon), but not in non-learners (day 0: 2.51 ± 0.37%; day 2: 2.26 ± 0.29%; n = 6; p = 0.688, Wilcoxon). Significant difference between learners and non-learners (p = 0.0084, 2-way repeated measures ANOVA).
(D) Increased spine formation rates in PirB−/− learners during early training (day 0: 3.20 ± 0.45%; day 2: 4.60 ± 0.72%; n = 9; p = 0.008, Wilcoxon), but not in non-learners (day 0: 3.26 ± 0.65%; day 2: 2.70 ± 0.28%; n = 7; p = 0.375, Wilcoxon). Significant difference between learners and non-learners (p = 0.0063, 2-way repeated measures ANOVA).
(E) Learning-induced spine formation rates in PirB−/− learners were not significantly different from WT learners (p = 0.228, 2-way repeated measures ANOVA).
(F) Increased spine elimination rates in WT learners during training (day 0: 2.99 ± 0.35%; day 6: 5.13 ± 0.62%; p = 0.008, Wilcoxon), but not in non-learners (day 0: 3.26 ± 0.56%; day 6: 3.05 ± 0.66%; p = 0.438, Wilcoxon). Significant difference between learners and non-learners (p = 0.0144, 2-way repeated measures ANOVA).
(G) No increase in spine elimination rate in PirB−/− learners (day 0: 3.38 ± 0.69%; day 6: 3.65 ± 0.80%; p = 0.734, Wilcoxon) or non-learners (day 0: 3.56 ± 0.69%; day 2: 3.56 ± 0.52%; p = 0.813, Wilcoxon). Spine elimination throughout training was not different between PirB−/− learners and non-learners (p = 0.952, 2-way repeated measures ANOVA).
(H) Impaired learning-induced spine elimination rates in PirB−/− learners (p = 0.035, 2-way repeated measures ANOVA).
(I) Survival of learning-induced spines (formed on day 2). Learning stabilized new spines in WT learners compared to non-learners (p = 0.0283, 2-way repeated measures ANOVA). Difference persisted 30 days later (WT learners: 35.143 ± 6.735%; WT non-learners: 13.667 ± 3.685%; p = 0.0221, Mann-Whitney).
(J) Learning stabilized newly formed spines in PirB−/− mice (p = 0.001, 2-way repeated measures ANOVA). Difference persisted 30 days later (PirB−/− learners: 57.556 ± 6.005%; PirB−/− non-learners: 25.333 ± 2.894%; p = 0.0028, Mann-Whitney).
(K) PirB−/− learners have increased survival of learning-induced spines relative to WT learners (p = 0.0333, 2-way repeated measures ANOVA). This difference persisted even after 30 days (p = 0.033, Mann-Whitney).
(L) Net dendritic spine dynamics were significantly different between WT and PirB−/− mice (p = 0.007, 2-way repeated measures ANOVA).
(M) Correlation between early learning spine formation rates and last day performance on reaching task (WT: r = 0.8637; p = 0.0057, Pearson correlation; PirB−/−: r = 0.6071; p = 0.0830, Pearson correlation).
(N) Correlation between survival of learning-induced spines and last day performance on reaching task (WT: r = 0.8900; p = 0.0031, Pearson correlation; PirB−/−: r = 0.7455; p = 0.0211, Pearson correlation).
(C-L) Squares depict mean (± s.e.m.).
(M, N) Circles depict individual animals, crosses depict mean ± s.e.m., dotted lines depict linear fit of each correlation.
* p < 0.05, ** p < 0.01; ns: non-significant.
It is known that newly formed task-specific dendritic spines are stabilized during training and have a significantly higher chance of survival even after motor skill training ends (Xu et al., 2009; Yang et al., 2009), forming a structural substrate that may underlie long-lasting motor memory (Hayashi-Takagi et al., 2015; Frank et al., 2018). To investigate if spine stabilization might contribute to the superior motor memory observed in PirB−/− mice, the fate of newly formed spines during early motor training was analyzed. Consistent with previous studies, in WT mice, newly-formed learning-induced spines are more stable in expert mice compared to non-learners (Figure 2I). Expert PirB−/− mice also have higher spine survival compared to non-learners (Figure 2J). However, the percentage of newly formed spines that survive in expert PirB−/− mice is significantly greater compared with that of expert WT mice not only during the training period but also at re-evaluation one month later (Figure 2K). When the net effect of spine turnover (spine formation - spine elimination) is compared between WT and PirB−/− mice, a rather remarkable difference emerges: spine dynamics during learning in PirB−/− mice are significantly shifted towards formation and away from elimination (Figure 2L). The net result of diminished spine elimination plus enhanced spine survival in PirB−/− mice during learning is a significant increase in spine density following training, as compared to WT (Figure S2B). These results show that learning-induced stabilization of newly formed spines is significantly enhanced in PirB−/− mice, suggesting that enhanced spine stability can serve as a mechanism for the improved motor learning.
Previous studies have shown that spine formation, spine elimination, and stabilization of newly formed spines correlate with motor learning and motor memory (Xu et al., 2009; Yang et al., 2009; Hayashi-Takagi et al., 2015). Here we have an example where both spine dynamics and learning are altered in PirB−/− mice (specifically, increased survival of learning-induced spines and enhanced motor learning). Therefore, by comparing the relationship between spine dynamics and motor learning across individual animals, it is possible to discern which factors might be the strongest predictors of motor performance. In accordance with previous studies, peak motor learning performance (% success on training day 8) correlates significantly with early training (day 2) spine formation rates in both genotypes (Figure 2M: r = 0.86 WT; 0.61 PirB−/−). However, because learning-induced spine formation is not different between the two genotypes (Figure 2E), it is unlikely to account for the superior performance present in PirB−/ mice. Neither pre-training spine density nor pre-training spine formation rates correlate significantly with motor learning performance in either WT or PirB−/− mice (Figures S2Cand S2D). In WT mice, learning-induced spine elimination (day 6) is significantly correlated with learning success (day 8) as previously reported, but in PirB−/− mice there is no correlation between spine elimination and motor learning (Figure S2E: r = 0.73 WT; 0.02 PirB−/−). Thus, this variable also cannot account for the superior motor learning exhibited by PirB−/− mice. Instead, by far the strongest predictor of motor learning ability is the survival of spines formed during early training (Figure 2N: r = 0.89 WT; 0.75 PirB−/−). Indeed, the survival of these spines significantly correlates with motor performance even 30 days after the end of motor training (Figure S2F: r = 0.87 WT; 0.69 PirB−/−), and spine survival is the strongest predictor even when motor performance is measured as the variability of reach trajectory kinematics (Figures S2G–I). Together, these observations point to increased stabilization of newly formed spines as the most likely explanation for enhanced motor learning in PirB−/− mice. Furthermore, they suggest that the survival of task-specific spines is the greatest general indicator of motor learning because it is not only the strongest correlation observed, but it is consistent across genotypes.
Increased LTP and Deficient LTD in M1 L5 Pyramidal Neurons of PirB−/− Mice
Structural plasticity has been shown to depend on Hebbian synaptic plasticity (Dudek and Bear, 1993; Engert and Bonhoeffer, 1999; Matsuzaki et al., 2004; Nagerl et al., 2004; Zhou et al., 2004; Harvey and Svoboda, 2007; Bastrikova et al., 2008; Murakoshi and Yasuda, 2012). Conversely, pharmacological manipulation of long-term potentiation (LTP) and long-term depression (LTD) via targeting key signaling pathways such as Ca2+, NMDA, and dopamine, results in significant changes to dendritic spine formation, elimination, and stability (Zuo et al., 2005; Guo et al., 2015; Stein et al., 2021). In particular, LTP is thought to be critical for dendritic spine stabilization (Hill and Zito, 2013; Guo et al., 2015), and LTP stimulates spine formation, whereas LTD promotes spine elimination (Dudek and Bear, 1993; Engert and Bonhoeffer, 1999; Harvey and Svoboda, 2007; Bastrikova et al., 2008; Steiner et al., 2008; Kwon and Sabatini, 2011; Wiegert and Oetner, 2013). Our observations above that spine formation at baseline and stabilization following motor learning are enhanced in L5PNs in PirB−/− M1 cortex suggest that there might be alterations in Hebbian synaptic plasticity.
To determine if cellular mechanisms of synaptic plasticity are altered, coronal slices of M1 from 2-month-old Thy1-YFP;PirB+/+ or Thy1-YFP;PirB−/− mice were prepared, and whole-cell recordings from identified YFP+ M1 L5PNs were made. A stimulating electrode was placed at the boundary between L1 and L2/3 of cortex and minimal stimulation was used to activate superficial layer synaptic inputs onto L5PNs (Figure S3A). Using an LTP induction protocol in which presynaptic stimulation is paired with postsynaptic depolarization (Guo et al., 2015), LTP could be reliably induced in L5PNs from WT M1 (Figure S3A and 3A), as expected. The same induction protocol in slices from PirB−/− M1 generated significantly larger LTP (Figures S3B and 3B,C). In both genotypes, LTP could be completely blocked by the NMDA receptor blocker 3-((R)-2-carboxypiperazin-4-yl)propyl-1-phosphonic acid (R-CPP, 10 μM) (Figure 3A–C).
Figure 3. Shifts in Hebbian Synaptic Plasticity Rules and Single Spine Structural Plasticity in PirB−/− Mice Towards Synaptic Potentiation and Spine Stabilization Related to Altered NMDAR Function.
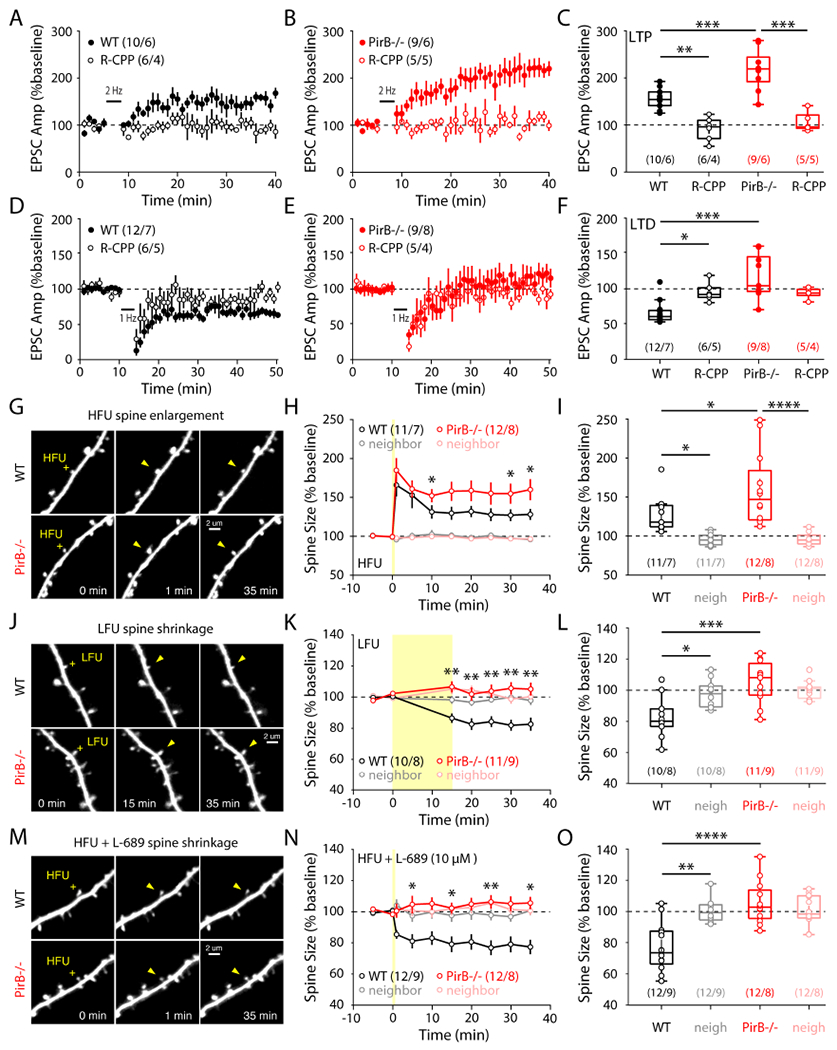
(A, B) Average evoked EPSC amplitudes following 2 Hz LTP induction in WT and PirB−/− mice. A subset of recordings included the NMDAR antagonist R-CPP.
(C) LTP was significantly increased in PirB−/− mice (WT: 155.127 ± 6.946%; n = 10 cells / 6 mice; PirB−/−: 217.592 ± 14.639%; n = 9 cells / 6 mice; p = 8.777e-4, 1-way ANOVA multiple comparisons). Observed LTP was NMDAR-dependent for both WT (WT: 155.127 ± 6.946%; WT + R-CPP: 91.849 ± 10.238%; n = 6 cells / 4 mice; p = 0.0027, 1-way ANOVA multiple comparisons) and PirB−/− mice (PirBKO: 217.592 ± 14.639%; PirB−/− + R-CPP: 107.266 ± 9.622%; n = 5 cells / 5 mice; p = 4.950e-6, 1-way ANOVA multiple comparisons).
(D, E) Average evoked EPSC amplitudes following 1 Hz LTD induction in WT and PirB−/− mice. A subset of recordings included the NMDAR antagonist R-CPP.
(F) LTD was significantly impaired in PirB−/− mice (WT: 66.345 ± 4.686%; n = 12 cells / 7 mice; PirB−/−: 117.504 ± 10.581%; n = 9 cells / 8 mice; p = 3.245e-5, 1-way ANOVA multiple comparisons). Observed LTD was NMDAR-dependent for WT (WT: 66.345 ± 4.686%; WT + R-CPP: 95.462 ± 5.490%; n = 6 cells / 5 mice; p = 0.043, 1-way ANOVA multiple comparisons).
(G) Representative Thy1-YFP+ dendrite images in WT and PirB−/− slices before and after HFU stimulation (yellow crosses) at single spines (yellow arrowheads).
(H) HFU stimulation led to significant long-term growth of WT and PirB−/− spines, with increased volume stabilization in PirB−/− spines (WT: n = 11 spines / 7 mice; PirB−/−: n = 12 spines / 8 mice; p = 0.0337, 2-way repeated measures ANOVA). Note: post-HFU spine enlargement (t = 1min) was not different between WT and PirB−/− spines (WT: 165.874 ± 4.35%; PirB−/−: 184.879 ± 4.53%; p = 0.4417, Mann-Whitney).
(I) WT and PirB−/− spines targeted with HFU underwent significant growth compared to unstimulated neighboring spines (WT targeted: 128.11 ± 6.91%; WT neighbors: 95.80 ± 2.20%; p = 0.0376, 2-way ANOVA with multiple comparisons; PirB−/− targeted: 159.98 ± 13.57%; PirB−/− neighbors: 96.69 ± 2.47%; p = 5.931e-6, 2-way ANOVA with multiple comparisons). Stabilization of spine growth was increased in HFU-targeted PirB−/− spines compared to HFU-targeted WT spines (WT targeted: 128.11 ± 6.91%; PirB−/− targeted: 159.98 ± 13.57%; p = 0.0357, 2-way ANOVA with multiple comparisons).
(J) Representative dendrite images showing single spine targeting of LFU (yellow crosses) to induce spine shrinkage (yellow arrowheads).
(K) LFU stimulation led to significant long-term shrinkage of WT spines, but not PirB−/− spines (WT: n =10 spines / 8 mice; PirB−/−: n =11 spines / 9 mice; p = 0.0035, 2-way repeated measures ANOVA).
(L) WT spines targeted with LFU underwent significant shrinkage compared to unstimulated neighboring spines (WT targeted: 82.57 ± 4.21%; WT neighbors: 97.79 ± 2.83%; p = 0.0181, 2-way ANOVA with multiple comparisons). PirB−/− spines targeted with LFU did not significantly shrink, unlike LFU-targeted WT spines (WT targeted: 82.57 ± 4.21%; PirB−/− targeted: 105.13 ± 4.14; p = 1.872e-4, 2-way ANOVA with multiple comparisons), and instead resembled the unstimulated neighboring spines (PirB−/− neighbors: 100.27 ± 1.83%; p = 0.729, 2-way ANOVA with multiple comparisons).
(M) Representative dendrite images showing single spine targeting of HFU (yellow crosses) in the presence of L-689 (10 μM) to induce spine shrinkage (yellow arrowheads).
(N) HFU + L-689 led to significant long-term shrinkage of WT spines, but not PirB−/− spines (WT: n =12 spines / 9 mice; PirB−/−: n =12 spines / 8 mice; p = 0.0179, 2-way repeated measures ANOVA).
(O) WT spines targeted with HFU in the presence of L-689 underwent significant shrinkage compared to unstimulated neighboring spines (WT targeted: 77.36 ± 4.61%; WT neighbors: 100.69 ± 2.95%; p = 0.0014, 2-way ANOVA with multiple comparisons). In the presence of L-689 PirB−/− spines targeted with HFU did not significantly shrink compared to HFU-targeted WT spines (WT targeted: 77.36 ± 4.61%; PirB−/− targeted: 105.58 ± 3.98%; p = 1.664e-5, 2-way ANOVA with multiple comparisons) and were indistinguishable from unstimulated neighboring spines (PirB−/− neighbors: 100.75 ± 2.85%; p = 0.7882, 2-way ANOVA with multiple comparisons).
(A, B, D, E) Circles represent mean EPSC amplitude ± s.e.m.
(C, F) Circles represent individual cells. Box plots depict mean (± s.e.m.).
(H, K, N) Circles represent mean spine volumes ± s.e.m.
(I, L, O) Circles represent individual spines. Box plots and mean ± s.e.m. are shown.
* p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
Next, LTD was assessed. Coronal brain slices of Ml from 1-month-old mice were used because previous studies have indicated that LTD in L5PNs cannot be elicited in older mice using a pairing protocol (Guo et al., 2015). In our hands, this low-frequency (1 Hz) pairing LTD protocol successfully induced NMDA receptor-dependent LTD in WT mice (Figure S3C and 3D). However, the same protocol failed to induce LTD in PirB−/− mice (Figures S3D and 3E,F).
These observations demonstrate that L5PNs in PirB−/− mice have altered Hebbian synaptic plasticity rules in M1 that favor synaptic potentiation over synaptic depression, a result consistent with previous observations of changes in Hebbian synaptic plasticity present in PirB−/− in visual cortex (Djurisic et al., 2013) and hippocampus (Djurisic et al., 2019). These results also demonstrate that one function of PirB is to regulate cellular mechanisms of synaptic plasticity, which ultimately may account for the increases in stabilization of learning-induced spines and the enhancement in motor learning observed in PirB−/− mice.
Enhanced Spine Enlargement and Stabilization in PirB−/− Mice
Altered Hebbian synaptic plasticity rules may result from differences in baseline synaptic strengths, intrinsic excitability, or NMDAR signaling. For example, a disproportionately greater number of weaker synapses and/or thinner spines on PirB−/− neurons could result in a greater capacity for LTP (Arendt et al., 2013; Li et al., 2019). To test these possibilities, we first examined the proportion of different spine types (mushroom, stubby, and thin): no difference was seen in the relative proportion of spine types on L5PNs in PirB−/− mice compared to WT (Figures S3E,F), consistent with observations above that there is no difference in the mean amplitude of mEPSCs (Figures S1H,K). There was also no difference in the intrinsic excitability of L5PNs between PirB−/− and WT neurons (Figure S3G,H). Finally, we measured NMDAR currents and found no differences in AMPA/NMDA ratios (Figures S3I,J) or in NMDAR current kinetics (Figures S3K,L) between WT and PirB−/− neurons. Therefore, the altered synaptic plasticity in PirB−/− mice cannot be explained by differences in basal synaptic strengths, intrinsic excitability, or NMDAR currents.
To gain further mechanistic insight into the altered Hebbian synaptic plasticity rules and link them to our in vivo observations of increased learning-dependent spine stabilization in PirB−/− mice, we performed ex vivo single spine two-photon uncaging experiments. Previous reports have shown that activity can drive long-term structural changes in individual dendritic spines, including spine enlargement or spine shrinkage, coinciding with LTP or LTD respectively (Matsuzaki et al., 2004; Harvey and Svoboda, 2007; Lee et al., 2009; Ramiro-Cortés and Israely, 2013; Stein et al., 2021). To determine if activity-dependent enlargement of individual spines on L5PNs is regulated by PirB, individual spines of Thy1-YFP+ dendrites in acute slice preparations were first targeted with a high-frequency uncaging (HFU) protocol previously shown to induce spine enlargement (Matsuzaki et al., 2004; Harvey and Svoboda, 2007; Lee et al., 2009; Stein et al., 2015). Indeed, HFU elicited significant increases in spine volumes of targeted spines in both WT and PirB−/− mice (Figures 3G,H). However, in PirB−/− mice, long-term spine volume increases were greater than WT controls (Figure 3I). Next, we used a low-frequency uncaging (LFU) stimulation protocol designed for spine shrinkage (Oh et al., 2013; Stein et al., 2015; Stein et al., 2021). Remarkably, we found that LFU could not reliably shrink PirB−/− spines, although spines of WT mice underwent the expected significant long-term decrease in volume (Figures 3J–L). These results are consistent with our LTP and LTD findings, showing that PirB−/− synaptic plasticity rules are shifted such that synapse potentiation and stabilization are favored over depression and shrinkage. Unstimulated neighboring spines did not undergo significant volume changes (Figures 3I,L). Consistent with previous studies (Stein et al., 2015; Stein et al., 2021), we found that inhibition of NMDAR function by blockade of the glutamate binding site with R-CPP prevented both HFU-induced spine enlargement and LFU-induced spine shrinkage (Figures S3M,N).
Recent studies have shown that when ion flux through NMDARs is blocked, patterns of stimulation that would normally drive spine enlargement (HFU) instead drive spine shrinkage caused by non-ionotropic NMDAR signaling (Nabavi et al., 2013; Stein et al., 2015; Stein et al., 2021). Since LFU failed to drive spine shrinkage in PirB−/− mice, we tested if PirB is involved in this form of non-ionotropic NMDAR-dependent structural plasticity. In WT mice, specific blockade of the glycine-/D-serine-binding GluN1 subunits of NMDARs with L-689,560 (L-689, 10 μM) prevented HFU-induced spine enlargement and instead induced spine shrinkage (Figures 3M,N). However, in PirB−/− mice, spines did not shrink in response to HFU in the presence of L-689 (Figures 3M–O). Furthermore, in WT mice the HFU-induced spine shrinkage in the presence of L-689 was fully blocked with the NMDAR antagonist R-CPP (Figure S3O), confirming that this form of spine shrinkage requires glutamate binding (Stein et al., 2021). Together, these results suggest that a non-ionotropic function of NMDARs requires the presence of PirB, and that disengagement of this ion flux-independent form of NMDAR signaling may underlie the altered structural plasticity observed in PirB−/− mice.
Enhanced Motor Learning and Spine Survival with Acute Blockade of PirB Function in M1 of Wild Type Mice
The improved motor learning along with increased survival of learning-induced spines in PirB−/− mice suggests that PirB normally acts to limit motor learning. This consideration made us wonder if it might be possible to lift limits in adult WT mice by acutely blocking PirB. To accomplish this goal, a classic protein-based approach was used in which a recombinant soluble PirB decoy receptor (sPirB) was generated (Bochner et al., 2014). This decoy receptor consists of only the PirB ectodomain (containing all six immunoglobulin (Ig)-like domains) plus Myc- and His- tags for purification and detection. sPirB functions by binding all endogenous PirB ligands, thereby blocking activation of full length PirB downstream signaling. “Decoy receptors” have been used effectively as drugs in human clinical application (Davis et al., 1994; Cabelli et al., 1997; Holash et al., 2002), and previous work has demonstrated that a 2-week infusion of sPirB into visual cortex of adult WT mice effectively blocks endogenous PirB proximal signaling in vivo, reinstating juvenile-like ocular dominance plasticity and facilitating recovery from Amblyopia (Bochner et al., 2014).
To examine if pharmacological blockade of PirB can alter spine dynamics and improve motor learning, osmotic minipumps were implanted in 2-month-old Thy1-YFP-H mice and sPirB or BSA control (a similar sized protein to sPirB) was infused into M1 cortex contralateral to the imaged forelimb over a period of 2 weeks. As above, chronic two-photon imaging was then used over an additional 8 day infusion period to assess the effect of acute blockade of PirB on dendritic spine dynamics and motor learning (Figure 4A). At the completion of the experiment, in some animals the brain was removed, sectioned, and immunostained using an anti-Myc antibody to identify the cortical area infused with sPirB (Figure 4B). In all cases, Myc-immunostaining was restricted to a localized 3-4 mm3 region including M1.
Figure 4. Enhanced Stability of Newly Formed Spines and Improved Performance on Motor Skill Training with Chronic Infusion of sPirB in M1 of WT Mice.
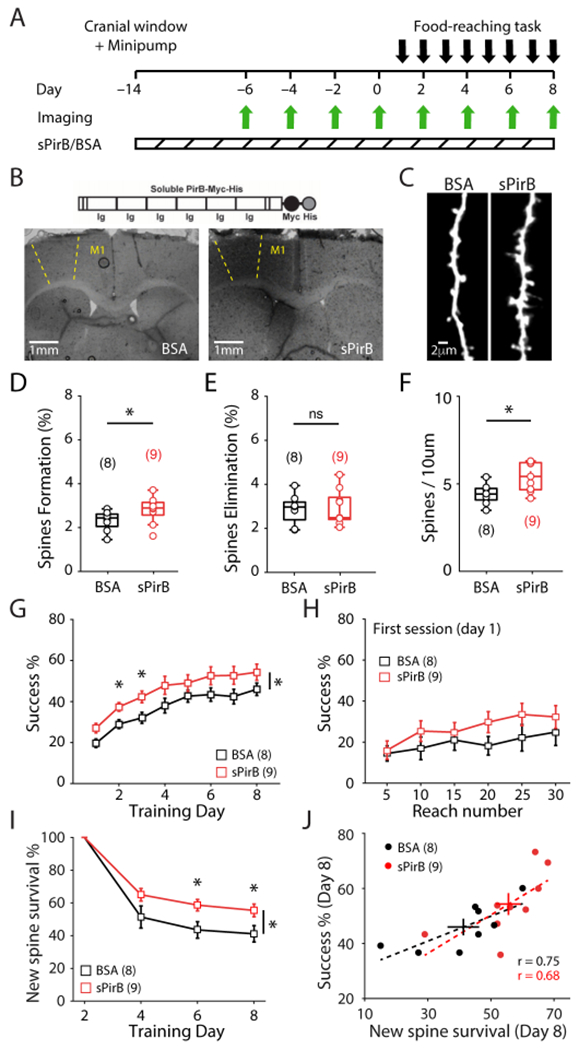
(A) Experimental timeline.
(B) (Top) Schematic of sPirB structure. (Bottom) Staining of sPirB-Myc depicting presence of sPirB in M1 of infused mouse (right) vs. a control mouse infused with BSA (left).
(C) Representative dendrites imaged after 2 weeks of BSA (left) or sPirB (right) infusion.
(D, E) Increased baseline spine formation after infusion of sPirB (BSA: 2.331 ± 0.162%; n = 8 mice; sPirB: 2.820 ± 0.204%; n = 9 mice; p = 0.036, Mann-Whitney), but not spine elimination (BSA: 2.844 ± 0.238%; sPirB: 2.910 ± 0.270%; p = 0.942, Mann-Whitney).
(F) Infusion of sPirB significantly increased dendritic spine density of WT mice (BSA: 4.310 ± 0.208; sPirB: 5.343 ± 0.285; p = 0.021, Mann-Whitney).
(G) WT mice infused with sPirB performed the reaching task better compared to BSA controls (p = 0.034, 2-way repeated measures ANOVA).
(H) sPirB infusion did not significantly enhance motor learning within the first training day (p = 0.051, 2-way repeated measures ANOVA).
(I) sPirB infusion significantly increased the survival of learning-induced spines (formed on day 2) (p = 0.0436, 2-way repeated measures ANOVA).
(J) Correlation between survival of learning-induced spines and last day performance on reaching task (BSA: r = 0.7506; p = 0.0319, Pearson correlation; sPirB: r = 0.6817; p = 0.0431, Pearson correlation). Circles depict individual mice, crosses depict mean ± s.e.m., dotted lines depict linear fit of each correlation.
(D, E, F) Circles represent individual mice. Box plots and mean ± s.e.m. are shown.
(G, H, I) Squares depict mean (± s.e.m.).
* p < 0.05, ns: non-significant.
A two-week sPirB infusion into M1 of WT mice can increase spine formation rate (Figures 4C,D) with no detectable effect on spine elimination (Figure 4E), resulting in a significant increase in spine density (~24%; Figures 4C,F). Next, sPirB or BSA- infused Thy1-YFP-H mice were trained on the reaching task and apical dendritic segments of L5PNs in M1 were repeatedly imaged (Figure 4A). Remarkably, there was a significant enhancement in motor learning in mice receiving sPirB infusion compared to mice infused with BSA throughout the 8-d training period (Figure 4G). The first training session on day 1 did not reveal a significant enhancement in performance in sPirB vs BSA treated mice (Figure 4H), possibly because sPirB infusion had only started 14 days earlier and time is needed for levels to achieve an effective blockade in M1. Nevertheless, by training day 6 a significant increase in survival of newly formed spines by training day 6 was observed in sPirB infused mice compared to BSA infused controls (Figure 4I), recapitulating our findings in germline PirB−/− mice. Consistent with those findings, a significant correlation between the magnitude of new spine survival and motor performance was also observed (Figure 4J) and once again served as the strongest relationship between spine dynamics and motor learning (Figure S4A–C). These observations extend our study beyond genetic manipulations of PirB to show that a relatively simple functional blockade of PirB using a protein-based approach yields similar results in adult WT mice: not only is an increase in spine survival by acute and localized blockade of PirB in M1 adult mice sufficient to enhance motor learning, but also the survival of learning-induced spines is the strongest structural correlate of motor learning.
DISCUSSION
Our findings provide key evidence supporting the idea that increasing the stability of newly formed spines is sufficient to enhance motor learning. By investigating PirB−/− mice, we have identified a neuronal receptor that regulates dendritic spine dynamics and motor learning. Our work adds to a growing theory that the formation and stabilization of dendritic spines are the chief mechanisms by which motor skills are acquired and maintained (Yang et al., 2009; Peters et al., 2017). Furthermore, our study demonstrates that artificial increases in spine stability during learning correlate with enhanced performance, suggesting that motor learning is limited by the number of stable spines formed during learning and that this limit can be lifted by manipulating PirB. Finally, our results also provide an alluring target – PirB – for increasing dendritic spine stability: here we have discovered that PirB blockade lifts the limits of motor learning in the healthy brain, suggesting that blockade of this receptor may also counteract or even prevent phenotypes of spine instability and accelerated elimination observed in various neurological and psychiatric disorders.
Stability of learning-induced spines as a strong candidate for a structural correlate of motor learning
Here we find that for each genotype, the magnitude of learning-induced spine formation predicts performance (Figure 2M), a result that is consistent with previous studies (Xu et al., 2009; Yang et al., 2009). However, spine formation alone cannot predict the enhanced motor learning present in PirB−/− mice because learning-induced spine formation rates do not differ between WT and PirB−/− mice (Figure 2E). Instead, the survival of learning-induced newly formed spines stands out as the major factor correlating with motor learning and is sufficient to predict performance of individual animals regardless of genotype (Figure 2N). These observations therefore allow a more complete understanding of the relative importance of different parameters of spine dynamics to motor learning (Ziv and Brenner, 2018). Indeed, in WT mice, simply increasing the stability of newly formed spines by infusing sPirB is sufficient to enhance motor learning in a predictable manner (Figures 4I,J).
It cannot be argued that merely increasing spine density is sufficient to improve motor learning, as a significant relationship between spine density and motor learning is not evident across the animals used in this study (Figure S2C). Instead, we find that enhanced learning in PirB−/− mice is more likely due to the increased survival of newly formed spines. A corollary of this logic is consistent with the observation that spine instability accompanies learning deficits in Fragile X mice. Furthermore, dendritic spines in Fragile X mouse cortex are significantly thinner and smaller in size (Cruz-Martin et al., 2010), whereas we do not observe significant differences in the distributions of spine types between WT and PirB−/− mice (Figures S3E,F). Lastly, deeper characterization of the behavior of PirB−/− mice could help uncover potential deleterious effects of increased spine stability. Indeed, we do find that PirB−/− mice develop stereotyped reaching kinematics at a faster rate than WT littermates (Figures 1J,K), which may translate to behavioral inflexibility in specific contexts (Wolpert et al., 2001; Hamilton and Brigman, 2015; Yin et al., 2021).
Enhanced motor learning can occur even when spine elimination is deficient
Previous studies have shown that spine elimination correlates with, and may even be required, for motor learning (Yang et al., 2009; Chen et al., 2015). Although a significant correlation between learning-induced spine elimination and motor learning is observed here in WT mice as expected (Figure S2E), such a correlation was notably absent in PirB−/− mice, despite their enhanced performance on the reaching task. It is important to note that at baseline, spine elimination on L5PNs in PirB−/− mice is comparable to that seen in WT mice. In fact, the difference appears to lie specifically in the learning-induced elimination that is engaged during training. Although the overall magnitude of spine elimination in PirB−/− is diminished, it remains possible that selective elimination of specific spines still occurs. Thus, our results do not contradict the notion that spine elimination is required for motor learning. Nevertheless, the spine dynamics observed here in PirB−/− mice demonstrate that superior acquisition of a motor skill can occur even in the face of diminished spine elimination.
Altered Hebbian plasticity and NMDAR function as a mechanism for increased spine stabilization and enhanced learning
Results from this study of L5PNs in PirB−/− mice link altered learning and spine dynamics with altered mechanisms of Hebbian synaptic plasticity. Studies have connected altered learning to altered mechanisms of LTP and LTD (Clem et al., 2008; Zhou et al., 2016), and previous work has shown that PirB−/− mice display altered Hebbian plasticity rules that favor synaptic potentiation over synaptic depression in visual cortex and hippocampus (Djurisic et al., 2013; Djurisic et al., 2019). Therefore, one might expect that altered Hebbian plasticity rules in PirB−/− cortex would translate to altered spine dynamics favoring stabilization, which is a likely downstream readout of enhanced LTP (see below). Indeed, we demonstrate that L5PNs in M1 of PirB−/− mice also exhibit greater LTP and impaired LTD. Furthermore, the structural plasticity of single PirB−/− spines favors stabilization of activity-dependent spine enlargement along with a disengagement of activity-dependent spine shrinkage; all of these changes are associated with the significantly increased in vivo spine stability and enhanced motor learning.
The significant increase in spine clustering observed in PirB−/− mice (Figures S1E,F) could also be related to the shift in Hebbian synaptic plasticity favoring LTP. Indeed, there is growing evidence that synaptic clustering can lower local thresholds for LTP (Harvey and Svoboda, 2007; Murakoshi and Yasuda, 2012). Clustering of synaptic inputs can lead to nonlinear integration of functionally similar or dissimilar inputs, resulting in greater influence on the overall information encoding of the neuron (Froemke et al., 2005; Larkum and Nevian, 2008; Wilson et al., 2016; Iacaruso et al., 2017; El-Boustani et al., 2018; Frank et al., 2018). A similar increase in spine clustering is also observed in visual cortex of PirB−/− mice using an automated spine analysis algorithm (Xiao et al., 2018; Xiao et al., unpublished). Though more is known about the role of spine clustering in sensory areas such as visual cortex (Lee et al., 2019), further studies are needed to characterize the functional consequence of clustered spines on L5PNs in M1 (Fu et al., 2012; Gokce et al., 2016).
The PirB receptor has intracellular signaling domains that interact with SHP2 (Takai, 2005; Syken et al., 2006) and also with the serine-threonine phosphatases PP2A and PP2B/calcineurin, and their target, cofilin (Kim et al., 2013). PirB downstream signaling via cofilin dephosphorylation is thought to drive postsynaptic actin filament disassembly and synapse collapse, a mechanism that is accentuated in AD mouse models and disengaged in the visual cortex and hippocampus of PirB−/− mice (Kim et al., 2013; Djurisic et al., 2019). Our observations in M1 L5PNs show that NMDAR-dependent spine shrinkage, which also activates cofilin (Nishiyama and Yasuda, 2015; Noguchi et al., 2016; Stein et al., 2021), is disengaged in PirB−/− spines. Thus, PirB and non-ionotropic NMDAR signaling are both required for activity-dependent structural disassembly of dendritic spines. Consistent with these requirements, we found that LTD induction protocols that are sufficient to induce robust synaptic depression in WT mice appear entirely ineffective in PirB−/− mice. In total, the loss of LTD and spine shrinkage observed in PirB−/− mice may account for reduced learning-induced spine elimination and enhanced spine stabilization, which then would account for faster and enhanced motor learning.
Increased excitatory and inhibitory tone in PirB−/− L5PNs
Although we observe an increase in the number of functional excitatory inputs to L5PNs in PirB−/− mice, net excitation is unchanged because there is also a significant increase in inhibition (frequency of mIPSCs; Figures S1I,L). This increase may be a result of altered inhibitory plasticity, and/or a consequence of a homeostatic upscaling of inhibitory inputs in response to the increased number of excitatory inputs to stabilize excitation and E/I balance (Froemke et al., 2007; Maffei and Turrigiano, 2008). Spine dynamics, elimination in particular, have been shown to be regulated by inhibitory inputs. GABA signaling along dendrites locally promotes LTD and spine elimination (Hayama et al., 2013) and inhibitory inputs undergo dramatic structural and functional plasticity during learning (Donato et al., 2013; Chen et al., 2015). Whatever the mechanism, E/I balance is preserved with loss of PirB function and the elevated number of functional GABAergic inputs may compensate for increased excitation to prevent circuit instability.
Specificity of enhanced motor learning revealed by infusing sPirB into adult WT M1
Motor learning is a distributed process involving many structures and modalities (Jin and Costa, 2010; Guo et al., 2015; Makino et al., 2017; Lemke et al., 2019; Roth et al., 2020). Here we made a focal blockade of endogenous PirB restricted to M1, demonstrating that targeting this site with local infusion of sPirB in WT mice is sufficient to enhance motor learning. But it is also important to consider the identity of the presynaptic partners of dendritic spines, as there is a great heterogeneity of inputs to L5PNs that remains to be explored. Notably, previous work has shown that motor learning induces plasticity of horizontal connections within motor cortex (Rioult-Pedotti et al., 2000), as well as of thalamocortical synapses onto a subset of functionally relevant L5PNs (Biane et al., 2016). Dopaminergic inputs to M1 L5PNs have also been shown to regulate synaptic plasticity and spine dynamics, emphasizing the multitude of levels of regulation surrounding this process (Guo et al., 2015). Further characterization of these various inputs, and their own plasticity mechanisms, will provide a more complete picture of the dynamic synaptic rewiring during motor learning. Nevertheless, we show that manipulating spine stability locally in M1 is sufficient to result in significant enhancements to motor performance, even in WT adult. These results imply that intervening in one place can improve performance and may result in a cascade of changes throughout the motor system. These considerations also suggest that sPirB or other approaches that decrease PirB function within cortex may be leveraged to counteract, or even prevent, the detrimental spine instability and loss that accompanies many neurological disorders such as PD (Stephens et al., 2005; Guo et al., 2015) and AD (Tsai et al., 2004; Spires et al., 2005; Shankar et al., 2007). Learning and memory deficits are hallmarks of such pathologies involving dendritic spines loss, and it may be possible to restore or protect these functions by infusion of sPirB into the affected brain regions.
STAR METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact: Jun B. Ding (dingjun@stanford.edu).
Materials availability
This study did not generate new unique reagents.
Data and code availability
All data reported in this paper will be shared by the lead contact upon request.
All original code has been deposited at Zenodo and is publicly available as of the date of publication. DOIs are listed in the key resources table.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-Myc/c-Myc Antibody (9E10) | Santa Cruz | Cat# sc-40 |
| Alkaline phosphatase goat anti-mouse IgG | Jackson | Cat# 115-055-062; RRID: AB_2338533 |
| NBT/BCIP stock solution | Roche | Cat# 11681451001 |
| Chemicals, peptides, and recombinant proteins | ||
| Tamoxifen | Sigma-Aldrich | Cat# T5648 |
| sPirB | Bochner et al., 2014 | N/A |
| Tetrodotoxin citrate | A.G. Scientific | Cat# T-2785 |
| Picrotoxin | Tocris | Cat# 1128 |
| NBQX | Tocris | Cat# 0373 |
| (R)-CPP | Tocris | Cat# 0247 |
| DNI-glutamate trifluoroacetate | Femtonics Ltd. | Cat# 1951 |
| L-689,560 | Tocris | Cat# 0742 |
| Experimental models: Organisms/strains | ||
| Mouse: PirB−/− | Syken et al., 2006 | N/A |
| Mouse: PirBfl/fl | Djurisic et al., 2019 | N/A |
| Mouse: CaMKIIa-CreER | The Jackson Laboratory | Jax# 012362 |
| Software and algorithms | ||
| DeepLab Cut | Mathis et al., 2018 | N/A |
| ImageJ | NIH ImageJ | RRID: SCR_003070 |
| MATLAB | Mathworks | RRID: SCR_001622 |
| Custom MATLAB scripts for analysis | Custom | DOI: 10.5281/zenodo.5146405 |
| Other | ||
| Micro-osmotic pump | Alzet | Cat# 0004317 |
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Animals
All experiments were performed in accordance with protocols approved by the Stanford University Animal Care and Use Committee in keeping with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals. Animals were kept at a 12hr:12hr light/dark cycle. All behavior and imaging experiments were carried out at consistent times of day (behavior: 7pm-10pm, every day; imaging: 7am-10am, every 2 days) to avoid circadian and sleep related effects. Both male and female mice were used for all experiments (P60-P80, with the exception of LTD recordings performed at P20-22). PirB−/− and PirBfl/fl mice were generated as previously described (Syken et al., 2006; Djurisic et al., 2019). PirB+/− lines were maintained to generate littermate PirB+/+ and PirB−/− mice used in all experiments (P60-P80). The CaMKIIa-Cre ER line was obtained from Jackson (JAX 012362; Madisen et al., 2009). CaMKIIa-CreER;PirBfl/fl mice were crossed to PirBfl/fl mice to yield 50% Cre+ animals.
METHODS DETAILS
Cranial window surgery
Chronic cranial window was implanted over M1 of P45-P50 as previously described (Holtmaat et al., 2009). Briefly, mice were anesthetized with isoflurane and anti-inflammatory drugs Carprofen and Dexamethasone were administered. The head of the animal was stabilized in a stereotaxic frame. The skin and periosteum were removed to expose the skull from the olfactory bulb to the cerebellum. A high-speed drill was used to drill a circular groove in the skull over motor cortex. Drilling was intermittent for heat dissipation and cool sterile saline was periodically added to the skull to avoid damage to the underlying cortex due to friction-induced heat. When the remaining island of cranial bone moved easily in response to light touch it was removed and a sterile circular 3mm round window was placed over the exposed cortex. Animals with compromised dura and/or vasculature were excluded. A thin layer of cyanoacrylate was applied to the surface of the skull and the edge of the cover glass to seal off the exterior, followed by dental resin to cover both the exposed skull and wound edge. Lastly, a titanium bar with threaded screw holes was attached to the skull postlateral to the cranial window for stabilizing the head during subsequent imaging sessions. Animals recovered from surgery for 2 weeks before imaging experiments. For experiments combining imaging with behavioral training, only mice with cranial windows contralateral to the dominant reaching forelimb were analyzed.
Single-pellet reaching task
The single-pellet reaching task for testing motor learning was conducted as previously described (Xu et al., 2009). Briefly, before training, animals (male and female, P45-P55) were food restricted to 90% of their free-feeding body weight. The training chamber was constructed using transparent Plexiglas. A vertical slit was made on the front side of the chamber for the mouse to reach through for millet seeds. The experiment involved a shaping phase followed by a training phase. In the shaping phase, mice were placed in the reaching chambers to familiarize with the new environment and task requirements. On the last day of shaping, the dominant forelimb of the animal was determined by placing a pile of seeds in front of the slit and letting the animal reach using either limb. When at least 20 reaches were conducted within 20 min, and limb preference met 70% or greater, dominant forelimb was determined. All mice finished shaping within 3-7 days. Every training day consisted of 30 trials with the dominant forelimb, or 20 min. Reaches were classified as a ‘success’ if the animal used the dominant forelimb to grasp the seed and bring it to its mouth, a ‘drop’ if the animal used the dominant forelimb to grasp the seed but dropped it when retrieving it, or a ‘fail’ if the animal used the dominant forelimb to reach for the seed but failed to grasp it. Success rates was quantified as the percentage of ‘success’ reaches over total reaches. Speed of success was quantified as the number of ‘success’ reaches divided by the total session time (20 min max) as many of the animals saturated their ‘success’ rate performance but continued to increase the speed of their ‘success’. The training phase lasted 8 days, with a final session conducted 30 days after the last training session. All shaping and training sessions were conducted at similar times of day (7pm – 10pm). Controls were ‘non-learners’: mice that underwent shaping but failed to reach a minimum of 20% success rate throughout training (including mice that began ‘reaching in vain’ and mice that slowed down or stopped attempting reaches). Mice that underwent complications after surgery or during food restriction were excluded from the study.
Tamoxifen administration
Tamoxifen (Sigma T5648) was dissolved at 20mg/ml in com oil (Sigma C8267) by 60 second vortex and overnight inversion at 37°C and stored in aliquots at 4°C. 2 mg (100ul) tamoxifen (or oil vehicle) was injected intraperitoneally into P42 CaMKIIa-CreER;PirBfl/fl mice at 8am and again at 8pm daily (4 mg / day), for 5 consecutive days, with the last injection being ≥ 2wks before behavioral training, at which point the mice were 2-months old.
Analysis of reaching kinematics
Videos of reaching sessions were recorded at 100 Hz using a Grasshopper3 camera (FLIR Systems) and chopped to include only reaching bouts (150-300 frames each). X-Y paw positions were then extracted using DeepLab Cut (Mathis et al., 2018) using a Linux workstation utilizing an NVIDIA GeForce RTX 2080TI graphics card. Briefly, the DeepLab Cut model was trained using ~500 manually annotated frames, after which the model output was checked for errors and corrected. After X-Y data was extracted for each bout, trajectory variance was quantified as the average of the X and Y trajectory variances, which were calculated by taking the average pairwise variances of reaches in the X and Y dimensions across all reaches for that training session (~30 reaches / day).
In vivo two-photon imaging
Chronic two-photon imaging experiments were performed as previously described (Xu et al., 2009; Holtmaat et al., 2009; Guo et al., 2015). Repeated imaging of apical dendritic stretches of L5 pyramidal neurons 10-100μm below the cortical surface was performed through the cranial window using a custom-built two-photon microscope or an Olympus microscope (FV1200) with a Mai Tai Ti:sapphire laser (Spectra-Physics) at 920 nm with a low laser power (output optical power <40 mW) to avoid phototoxicity. Image stacks were taken with a z-step of 0.7 μm using a water-immersion Olympus objective. In order to re-locate the same region across imaging days, lower magnification dendritic structures of interest were taken with a 2.0 μm step size and brain vasculature of the area was imaged with a CCD camera to serve as landmark guides. After imaging sessions, the titanium bar was detached from the metal base and the animal was returned to the original home cage. Imaged regions were determined using stereotaxic coordinates (Tennant et al., 2011) (1.2 mm lateral from the midline, 1.3 mm anterior to the bregma).
Identification and presentation of dendritic spines
As previously described (Xu et al., 2009; Guo et al., 2015), individual dendritic protrusions (minimum length of 1/3 the dendrite diameter) were manually identified and tracked along dendritic segments. Spine changes (i.e. formation and elimination) were determined by comparing two temporally adjacent images of the same spines. Spines were classified as stable if they were present in both images, using their spatial relationship to landmarks and/or their position relative to other spines. An eliminated spine was one that was present in the first image but not detected on the second image. A newly formed spine was one that was absent in the first image but appeared in the second image. Representative dendrites were created by making 2D projections of 3D stacks containing in-focus dendritic segments. Projections were thresholded, contrast-enhanced, and median filtered to create the final images.
Dendritic spine clustering analysis
Spine clustering was analyzed by quantifying nearest neighbor (NN) distance: for each spine, the distance along the dendrite to the nearest adjacent spine (Fu et al., 2012; Gokce et al., 2016). Expected NN distributions were generated by simulating dendrites with shuffled spine positions and spine densities matching the observed values from imaging experiment, and extracting the corresponding NN values. Repeating this process through 1000 simulations generated expected NN distributions for WT and PirB−/− mice given their respective spine densities (Figure S1D, shaded). Spine clustering was then quantified as significant differences between the observed NN distributions and the expected NN distributions for each genotype.
Whole-cell slice electrophysiology
Mice (male and female, 2 months old) were anesthetized with isoflurane, decapitated, and brains were extracted and briefly submerged into chilled artificial cerebrospinal fluid (ACSF) containing 125 mM NaCl, 2.5 mM KCl, 1.25 mM NaH2PO4, 25 mM NaHCO3, 15 mM glucose, 2 mM CaCl2, and 1 mM MgCl2, oxygenated with 95% O2 and 5% CO2 (300-305 mOsm, pH 7.4). Coronal slices (300 μm thickness) containing M1 were then prepared using a tissue vibratome (VT1200S, Leica), incubated in chambers containing 34°C ACSF for 30 min, and then allowed to recover at room temperature for 30 min. After recovery, slices were transferred to a submerged recording chamber perfused with ACSF at a rate of 2-3 ml/min at a temperature of 30-31°C. Motor cortex M1 layer V pyramidal neurons were identified by YFP fluorescence (BX51, Olympus). Whole-cell voltage clamp recordings were made with glass pipettes (3-4 MΩ) filled with internal solution containing 126 mM CsMeSO3, 10 mM HEPES, 1 mM EGTA, 2 mM QX-314 chloride, 0.1 mM CaCl2, 4 mM Mg-ATP, 0.3 mM Na3-GTP, and 8 mM disodium phosphocreatine (280-290 mOsm, pH 7.3 with CsOH), and cells were voltage clamped at −70 mV. Access resistance was 15-25 MΩ for all recordings and only cells with a change in access resistance <20% throughout the entire experiment were included in the analysis. Miniature excitatory postsynaptic currents (mEPSCs) were measured by continuously recording for 10 min in the presence of 1 μM Tetrodotoxin to prevent action potential firing and 50 μM Picrotoxin to block GABAA receptor-mediated currents. For miniature inhibitory postsynaptic currents (mIPSCs), 10 μM NBQX and 10 μM (R)-CPP were added to block glutamate receptors and NMDA receptors, respectively. For pairing LTP, induction stimuli were delivered within 13 min of achieving whole-cell configuration. After a stable baseline (5 min), presynaptic stimulation (2 Hz, 360 pulses) was paired with postsynaptic depolarization to +10 mV. For pairing LTD (3-week old mice), induction stimuli were delivered within 20 min of achieving whole-cell configuration. After establishing a stable baseline (10 min), low-frequency stimulation (1 Hz, 200 pulses) was paired with postsynaptic depolarization to −30 mV. EPSCs were induced by stimulating the superficial layer of M1 via a concentric bipolar stimulating electrode (FHC) located at the border between L1 and L2/3 (Guo et al., 2015). EPSCs were evoked at 0.05 Hz and three successive EPSCs were averaged and quantified relative to the normalized baseline. Tissue surrounding the recording area were cut to prevent polysynaptic responses. Picrotoxin (50 μM) was present in the ACSF perfusion for LTP and LTD experiments. To measure intrinsic excitability, an internal solution was used containing 135 mM KMeSO3, 8.1 mM KC1, 10 mM HEPES, 1 mM EGTA, 0.1 mM CaCl2 4 mM Mg-ATP, 0.3 mM Na3-GTP, and 8 mM disodium phosphocreatine (280-285 mOsm, pH 7.3 with KOH). Current injections ranging from 30 pA to 360 pA (30 pA step size) were used to evoke voltage responses in the presence of blocked synaptic transmission (10 μM NBQX, 10 μM R-CPP, 50 μM picrotoxin) and firing rates were quantified as the number of action potential evoked over the current step duration. For IAMPA/INMDA ratio experiments, AMPAR currents were elicited at −70 mV in the presence of 50 μM picrotoxin, then 10 μM NBQX was added and NMDAR currents were elicited at +40 mV. IAMPA/INMDA were quantified as the ratio of amplitudes of IAMPA of INMDA responses. To measure τfast and τslow decay constants, double exponentials were fitted to INMDA decay responses. Whole-cell patch clamp recordings were performed using a Multiclamp 700B (Molecular Devices), monitored with WinWCP (Strathclyde Electrophysiology Software) and analyzed offline using Clampfit 10.0 (Molecular Devices) and custom-made MATLAB (Mathworks) software. Signals were filtered at 2 kHz and digitized at 10 kHz (NI PCIe-6259, National Instruments).
Single spine two-photon imaging and uncaging
Acute coronal brain slices containing M1 were prepared from p30-p60 Thy1-YFP+ WT and PirB−/− mice (as described above). Image stacks of Thy1-YFP+ apical dendrites (<100 μm from the pial surface, 10-60 μm of depth into the slice) were acquired with a Mai Tai Ti: sapphire (Spectra-Physics) laser at 920 nm wavelength with a 0.5 μm z-step size. Images stacks were acquired every 5 minutes in ACSF containing 0.7 mM DNI-glutamate and 1 μM Tetrodotoxin at 30-31°C. A second laser was used for glutamate uncaging at 720 nM wavelength. Uncaging laser was adjusted to deliver a 2 ms blast at the target (~7-10 mW) to evoke ~10 pA in whole-cell recording configuration. Laser blasts were parked ~0.5-1.0 μm from the target spine head (orthogonal to the dendrite). For HFU stimulation, 60 pulses were delivered at 2 Hz in ACSF containing 3 mM Ca2+ and 0 mM Mg2+. LFU stimulation consisted of 90 pulses delivered at 0.1 Hz in ACSF containing 1 mM Ca2+ and 0 mM Mg2+. In a subset of experiments, 10 μM L-689 and/or 10 μM R-CPP were included in the ACSF. Healthy cells / dendrites were identified using a test HFU stimulus to visualize enlargement of a test spine on a test dendrite (before application of drugs). Only cells / dendrites passing the test HFU stimulus were used for experiments. Unstimulated neighboring spines were used as controls.
Chronic minipump infusion of sPirB
For sPirB infusion experiments (Figures 4, S4), osmotic minipumps (Alzet, 0.2ul/hr, 100ul capacity) were implanted as described previously (Bochner et al., 2014) with cranial windows in the same surgery. Approximately 16-18 hours before cranial window surgeries, mini pumps were filled with either sPirB (1mg/ml) or BSA (1mg/ml) (VWR, EM-2930) in 1x PBS and incubated at 37°C overnight. The next day, mini pumps were implanted subcutaneously and connected to a cannula (PlasticsOne) which was inserted directly posterior to the cranial window. A thin layer of cryanoacrylate glue and dental cement resin was used to secure cannulas to stereotaxic frame for the duration of imaging sessions and behavioral testing.
Immunohistochemistry of sPirB
At the completion of live imaging experiments, mice were euthanized and whole brains were rapidly frozen in M-1 embedding matrix. Coronal sections were cut at a thickness of 15pm using a cryostat and mounted onto slides for processing. To stain for sPirB-myc, slices were fixed and stained as performed previously (Bochner et al., 2014). Briefly, slices were submerged in 4% PFA and fixed for 30 minutes. After washing in PBS, sections were incubated at 65°C for 30 minutes and then transferred to a blocking solution for 1 hour at room temperature. Sections were then stained with an anti-myc antibody (Santa Cruz, SC-40) and placed at 4°C overnight in the dark. Next day, sections were washed and probed with an anti-mouse secondary antibody conjugated to alkaline phosphatase (Jackson, 115-055-062) and incubated for 2 hours at room temperature. To develop the phosphatase signal, labeled sections were incubated for 1 hour in NBT/BCIP solution (Roche, 11681451001) and then washed and mounted in Prolong Gold mountant.
QUANTIFICATION AND STATISTICAL ANALYSIS
Analysis of in vivo two-photon spine dynamics
All spine image analysis was measured manually on the raw image stacks using ImageJ software (NIH ImageJ), blind to the experimental conditions. Only dendritic segments of high-quality image stacks were used for quantification to ensure that tissue rotation or movements between imaging sessions did not affect the identification of dendritic spines. All spines with saturated pixels were excluded. The spines and dendrites of each animal were pooled to calculate a single value of spine density, formation rate, elimination rate, and spine survival for each animal (per day). Spine formation rate: number of newly formed spines on image session Sx / total number of spines on session Sx-1, elimination rate: number of spines missing on session Sx (present on session Sx-1, but not Sx) / total number of spines on session Sx-1. Spine survival was quantified as the percentage of spines from session Sx that remained present on some future session Sx+Δx. Data on spine dynamics throughout this study are presented as mean ± s.e.m.
Analysis of uncaging-induced spine size changes
To quantify structural plasticity of individual spines, estimated spine volume was calculated using integrated intensity as previously described (Stein et al., 2015). Briefly, pixel intensity was integrated for ROIs containing z-projected spines (background subtracted). This quantity was compared for z-stack images taken across timepoints (normalized to baseline values).
Statistics
Repeated measurements (e.g. reaching task success across days, spine dynamics across days, etc.) were analyzed using 2-way repeated measures ANOVA with post-hoc tests. All two-sample comparisons (e.g. spine density comparisons, mEPSC frequency comparisons, etc.) were analyzed with nonparametric tests (Mann-Whitney or Wilcoxon). All correlations were calculated as Pearson correlation coefficient (r) with accompanying p-values. Unless otherwise specified, data is presented as mean ± SEM (standard error of the mean), with all statistical tests, statistical significance values, and sample sizes described in the figure legends. Statistical thresholds used: * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, ns: non-significant.
Supplementary Material
HIGHLIGHTS.
PirB−/− mice have enhanced acquisition and maintenance of motor skills
Learning-induced dendritic spines are more stable in motor cortex of PirB−/− mice
NMDAR-dependent spine shrinkage is disengaged in PirB−/− mice
Acute blockade of PirB increases spine stability and enhances motor learning in WT mice
ACKNOWLEDGMENTS
We thank Dr. Maja Djurisic and Dr. Richard Roth for their feedback on the manuscript, and Nora Sotelo-Kury, Peggy Kemper, Christina Chechelski, and Yu Liu for technical support. This work was funded by grants from the NINDS/NIH NS091144 (J.B.D.), the Klingenstein Foundation (J.B.D.), NIH EY02858 (C.J.S.), the Mathers Charitable Foundation (C.J.S.), HHMI Gilliam Fellowship (E.A.), NSF GRFP (E.A.), and DARE Fellowship (E.A.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
The authors declare no competing interests.
INCLUSION AND DIVERSITY
We worked to ensure sex balance in the selection of non-human subjects. One or more of the authors of this paper self-identifies as an underrepresented ethnic minority in science. One or more of the authors of this paper self-identifies as a member of the LGBTQ+ community. One or more of the authors of this paper received support from a program designed to increase minority representation in science. While citing references scientifically relevant for this work, we also actively worked to promote gender balance in our reference list.
REFERENCES
- Arendt KL, Sarti F, Chen L (2013). Chronic inactivation of a neural circuit enhances LTP by inducing silent synapse formation. J. Neurosci 33, 2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastrikova N, Gardner GA, Reece JM, Jeromin A, Dudek SM (2008). Synapse elimination accompanies functional plasticity in hippocampal neurons. PNAS 105, 3123–3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biane JS, Takashima Y, Scanziani M, Conner JM, Tuszynski MH (2016). Thalamocortical projections onto behaviorally relevant neurons exhibit plasticity during adult motor learning. Neuron 89, 1173–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner DN, Sapp RW, Adelson JD, Zhang S, Lee H, Djurisic M, Syken J, Dan Y, Shatz CJ (2014). Blocking PirB up-regultes spines and functional synapses to unlock visual cortex plasticity and faciliate recovery from amblyopia. Sci. Transl. Med 6, 258ra140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabelli RJ, Shelton DL, Segal RA, Shatz CJ (1997). Blockade of endogenous ligands of trkB inhibits formation of ocular dominance columns. Neuron 19, 63–76. [DOI] [PubMed] [Google Scholar]
- Chen SX, Kim AN, Peters AJ, Komiyama T (2015). Subtype-specific plasticity of inhibitory circuits in motor cortex during motor learning. Nat. Neurosci 18, 1109–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clem RL, Celikel T, Barth AL (2008). Ongoing in vivo experience triggers synaptic metaplasticity in the neocortex. Science 319, 101–104. [DOI] [PubMed] [Google Scholar]
- Cruz-Martin A, Crespo M, Portera-Cailliau C (2010). Delayed stabilization of dendritic spines in Fragile X mice. J. Neurosci 30, 7793–7803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S, Gale NW, Aldrich TH, Maisonpierre PC, Lhotak V, Pawson T, Goldfarb M, Yancopoulos GD (1994). Ligands for EPH-related recetpro tyrosine kinases that require membrane attachment or clustering for activity. Science 266, 816–819. [DOI] [PubMed] [Google Scholar]
- Djurisic M, Brott BK, Saw NL, Shamloo M, Shatz CJ (2019). Activity-dependent modulation of hippocampal synaptic plasticity via PirB and endocannabinoids. Mol. Psych 24, 1206–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djurisic M, Vidal GS, Mann M, Aharon A, Kim T, Santos AF, Zuo Y, Hubener M, Shatz CJ (2013). PirB regulates a structural substrate for cortical plasticity. PNAS 110, 20771–20776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato F, Rompani SB, Caroni P (2013). Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning. Nature 504, 272–276. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Bear MF (1993). Bidirectional long-term modification of synaptic effectiveness in the adult and immature hippocampus. J. Neurosci 13, 2910–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Boustani S, Ip JPK, Breton-Provencher V, Knott GW, Okuno H, Bito H, Sur M (2018). Locally coordinated synaptic plasticity of visual cortex neurons in vivo. Science 360, 1349–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engert F, Bonhoeffer T (1999). Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 399, 66–70. [DOI] [PubMed] [Google Scholar]
- Frank AC, Huang S, Zhou M, Gdalyahu A, Kastellakis G, Silva TK, Lu E, Wen X, Poirazi P, Trachtenberg JT, Silva AJ (2018). Hostpots of dendritic spine turnover facilitate clustered spine addition and learning and memory. Nat. Comm 9, 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke RC, Poo M.m., Dan Y (2005). Spike-timing-dependent synaptic plasticity depends on dendritic location. Nature, 434, 221–225. [DOI] [PubMed] [Google Scholar]
- Froemke RC, Merzenich MM, Schreiner CE (2007). A synaptic memory trace for cortical receptirve field plasticity. Nature, 450, 425–429. [DOI] [PubMed] [Google Scholar]
- Fu M, Yu X, Lu J, Zuo Y (2012). Repetitive motor learning induces coordinated formation of clustered dendritic spines in vivo. Nature 483, 92–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokce O, Bonhoeffer T, Scheuss V (2016). Clusters of synaptic inputs on dendrites of layer 5 pyramidal cells in mouse visual cortex. eLife 5:e09222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough WT, Larson JR, Withers GS (1985). Effects of unilateral and bilateral training in a reaching task on dendritic branching of neurons in the rat motor-sensory forelimb cortex. Behav. Neural Biol 44, 301–314. [DOI] [PubMed] [Google Scholar]
- Guo JZ, Graves AR, Guo WW, Zheng J, Lee A, Rodriguez-Gonzalez J, Li N, Macklin JJ, Phillips JW, Mensh BD, Branson K, Hantman AW (2015). Cortex commands the performance of skilled movement. eLife 4, e10774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Xiong H, Kim JI, Wu YW, Lalchandani RR, Cui Y, Shu Y, Xu T, Ding JB (2015). Dynamic rewiring of neural circuits in the motor cortex in mouse models of Parkinson’s disease. Nat. Neurosci 18, 1299–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DA, Brigman JL (2015). Behavioral flexibility in rats and mice: contributions of distinct frontocortical regions. Genes, Brain and Behavior 14, 4–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms KJ, Rioult-Pedotti MS, Carter DR, Dunaevsky A (2008). Transient spine expansion and learning-induced plasticity in layer 1 primary motor cortex. J. Neurosci 28, 5686–5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey CD, Svoboda K (2007). Locally dynamic synaptic learning rules in pyramidal neuron dendrites. Nature 450, 1195–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayama T, Noguchi J, Watanabe S, Takahashi N, Hayashi-Takagi A, Ellis-Davis GCR, Matsuzaki M, Kasai H (2013). GABA promotes the competitive selection of dendritic spines by controlling Ca2+ signaling. Nat. Neurosci 16, 1409–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi-Takagi A, Yagishita S, Nakamura M, Shirai F, Wu YI, Loshbaugh AL, Kuhlman B, Hahn KM, Kasai H (2015). Labelling and optical erasure of synaptic memory traces in the motor cortex. Nature 525, 333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill TC, Zito K (2013). LTP-induced long-term stabilization of individual nascent dendritic spines. J. Neurosci 33, 678–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer SB, Mrsic-Flogel TD, Bonhoeffer T, Hubener M (2009). Experience leaves a lasting structural trace in cortical circuits. Nature. 457, 313–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holash J, Davis S, Papadopoulos N, Croll SD, Ho L, Russell M, Boland P, Leidich R, Hylton D, Burova E, Ioffe E, Huang T, Radziejewski C, Bailey K, Fandl JP, Daly T, Wiegand SJ, Yancopoulos GD, Rudge JS (2002). VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc. Natl. Acad. Sci. U.S.A 99, 11393–11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat A, Svoboda K (2009). Experience-dependent structural synaptic plasticity in the mammalian brain. Nat. Rev. Neurosci 10, 647–658. [DOI] [PubMed] [Google Scholar]
- Holtmaat A, Bonhoeffer T, Chow DK, Chuckowree J, De Paola V, Hofer SB, Hubener M, Keck T, Knott G, Lee WA, Mostany R, Mrsic-Flogel TD, Nedivi E, Portera-Cailliau C, Svoboda K, Trachtenberg JT, Wilbrecht L (2009). Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nature Protocols 4, 1128–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacaruso MF, Gasler IT, Hofer SB (2017). Synaptic organization of visual space in primary visual cortex. Nature 547, 449–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Costa RM (2010). Start/stop signals emerge in nigrostriatal circuits during sequence learning. Nature 466, 457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HB, Sabatini BL (2011). Glutamate induces de novo growth of functional spines in developing cortex. Nature 474, 100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkum ME, Nevian T (2008). Synaptic clustering by dendritic signaling mechanisms. Curr. Op. Neurobio 18, 321–331. [DOI] [PubMed] [Google Scholar]
- Lee KS, Vandemark K, Mezey D, Shultz N, Fitzpatrick D (2019). Functional synaptic architecture of callocal inputs in mouse primary visual cortex. Neuron 101(3): 421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SR, Escobedo-Lozoya Y, Szatmari EM, Yasuda R (2009). Activaiton of CaMKII in single dendritic spines during long-term potentiation. Nature 458, 299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke SM, Ramanathan DS, Guo L, Won SJ, Ganguly K (2019). Emergent modular neural control drives coordinated motor actions. Nat. Neurosci 22, 1122–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Park E, Zhong LR, Chen L (2019). Homeostatic synaptic plasticity as a metaplasticity mechanism – a molecular and cellular perspective. Curr. Op. Neurobio 54, 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H (2010). A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci 13, 133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei A, Turrigiano GG (2008). Multiple modes of network homeostasis in visual cortical layer 2/3. J. Neurosci 28, 4377–4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino H, Ren C, Liu H, Kim AN, Kondapaneni N, Liu K, Kuzum D, Komiyama T (2017). Transformation of cortex-wide emergent properties during motor learning. Neuron 94, 880–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GCR, Kasai H (2004). Structural basis of long-term potentiation in single dendritic spines. Nature 429, 761–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakoshi H, Yasuda R (2012). Postsynaptic signaling during plasticity of dendritic spines. Trends in Neurosci. 35, 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabavi S, Kessels HW, Alfonso S, Aow J, Fox R, Malinow R (2013). Metabotropic NMDA receptor function is required for NMDA receptor-dependent long-term depression. PNAS 110, 4027–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagerl UV, Eberhorn N, Cambridge SB, Bonhoeffer T (2004). Bidirectional activity-dependent morphological plasticity in hippocampal neurons. Neuron 44, 759–767. [DOI] [PubMed] [Google Scholar]
- Nishiyama J, Yasuda R (2015). Biochemical computation for spine structural plasticity. Neuron 87, 63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi J, Hayama T, Watanabe S, Ucar H, Yagishita S, Takahashi N, Kasai H (2016). State-dependent diffusion of actin-depolymerizing factor/cofilin underlies the enlargment and shrinkage of dendritic spines. Scientific Reports 6, 32897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh WC, Hill TC, Zito K (2012). Synapse-specific size-dependent mechanisms of spine structural plasticity accompanying synaptic weakening. PNAS 110, E305–E312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmashri R, Reiner BC, Suresh A, Spartz E, Dunaevsky A (2013). Altered structural and functional synaptic plasticity with motor skill learning in a mouse model of Fragile X Syndrome. J. Neurosci 33, 19715–19723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters AJ, Chen SX, Komiyama T (2014). Emergence of reproducible spatiotemporal activity during motor learning. Nature 510, 263–267. [DOI] [PubMed] [Google Scholar]
- Peters AJ, Liu H, Komiyama T (2017). Learning in the rodent motor cortex. Annu. Rev. Neurosci 40, 77–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramiro-Cortés Y, Israely I (2013). Long lasting protein synthesis- and activity-dependent spine shrinkage and elimination after synaptic depression. PLoS ONE 8(8): e71155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Friedman D, Donoghue JP (2000). Learning-induced LTP in neocortex. Science 290, 533–536. [DOI] [PubMed] [Google Scholar]
- Roth RH, Cudmore RH, Tan HL, Hong I, Zhang Y, Huganir RL (2020). Cortical synaptic AMPA receptor plasticity during motor learning. Neuron 105, 895–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL (2007). Natural oligomers of the Alzheimer Amyloid-β protein induce reversible synapse loss by modulating NMDA-type glutamate receptor-dependent signaling pathway. J. Neurosci 27, 2866–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires TL, Meyer-Luehmann M, Stern EA, McLean PJ, Skoch J, Nguyen PT, Bacskai BJ, Hyman BT (2005). Dendritic spine abnormalities in Amyloid Precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy. J. Neurosci 25, 7278–7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein IS, Gray JA, Zito K (2015). Non-ionotropic NMDA receptor signaling drives activity-induced dendritic spine shrinkage. J. Neurosci 35, 12303–12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein IS, Park DK, Claiborne N, Zito K (2021). Non-ionotripic NMDA receptor signaling gates bidirectional structural plasticity of dendritic spines. Cell Reports 34, 108664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner P, Higley MJ, Xu W, Czervionke BL, Malenka RC, Sabatini BL (2008). Destabilization of the postsynaptic density by PSD-95 serine 73 phosphorylation inhibits spine growth and synaptic plasticity. Neuron 60, 788–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens B, Mueller AJ, Shering AF, Hood SH, Taggart P, Arbuthnott GW, Bell JE, Kilford L, Kingsbury AE, Daniel SE, Ingam CA (2005). Evidence of a breakdown of corticostriatal connections in Parkinson’s disease. J. Neurosci 132, 741–754. [DOI] [PubMed] [Google Scholar]
- Syken J, GrandPre T, Kanold PO, Shatz CJ (2006). PirB restricts ocular-dominance plasticity in visual cortex. Science 313, 1795–1800. [DOI] [PubMed] [Google Scholar]
- Takai T (2005). Paired immunoglobulin-like receptors and their MHC class I recognition. Immunology 115, 433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant KA, Adkins DL, Donlan NA, Asay AL, Thomas N, Kleim JA, Jones TA (2010). The organization of the forelimb representation of the C57BL/6 mouse motor cortex as defined by intracortical microstimulation and cytoarchitecture. Cerebral Cortex 21, 865–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachtenberg JT, Chen BE, Knott GW, Feng G, Sanes JR, Welker E, Svoboda K (2002). Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature 420, 788–794. [DOI] [PubMed] [Google Scholar]
- Tsai J, Grutzendler J, Duff K, Gan WB (2004). Fibrillar amyloid deposition leads to local synaptic abnormalities and breakage of neuronal branches. Nat. Neurosci 7, 1181–1183. [DOI] [PubMed] [Google Scholar]
- Vidal GS, Djurisic M, Brown K, Sapp RW, Shatz CJ (2016). Cell-autonomous regulation of dendritic spine density by PirB. eNeuro. 0089-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Conner JM, Rickert J, Tuszynaski MH (2010). Structural plasticity within highly specific neuronal populations identifies a unique parcellation of motor learning in the adult brain. PNAS 108 2545–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegert JS, Oertner TG (2013). Long-term depression triggers the selective elimination of weakly integrated synapses. PNAS 110, 4510–4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DE, Whitney DE, Scholl B, Fitzpatrick D (2016). Orientation selectivity and the functional clustering of synaptic inputs in primary visual cortex. Nat. Neurosci 19, 1003–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z, Flanagan JR (2001). Perspectives and problems in motor learning. Trends in Cog. Sci 5, 487–494. [DOI] [PubMed] [Google Scholar]
- Xiao X, Djurisic M, Hoogi A, Sapp RW, Shatz CJ, Rubin DL (2018). Automated dendritic spine detection using convolutional neural networks on maximum intensity projected microscopic volumes. J. Neurosci. Methods 309:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Yu X, Perlik AJ, Tobin WF, Zweig JA, Tennant K, Jones T, Zuo Y (2009). Rapid formation and selective stabilization of synapses for enduring motor memories. Nature 462, 915–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Lai CSW, Cichon J, Ma L, Li W, Gan WG (2014). Sleep promotes branch-specific formation of dendritic spines after learning. Science 344, 1173–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Pan F, Gan WB (2009). Stably maintained dendritic spines are associated with lifelong memories. Nature 462, 920–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasumatsu N, Matsuzaki M, Miyazaki T, Noguchi J, Kasai H (2008). Principles of long-term dynamics of dendritic spines. J. Neurosci 28, 13592–13608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Jones N, Yang J, Asraoui N, Mathieu M, Cai L, Chen SX (2021). Delayed motor learning in a 16p11.2 deletion mosue model of autism is rescued by locus coeruleus activaiton. Nature Neurosci. 24, 646–657. [DOI] [PubMed] [Google Scholar]
- Yuste R, Bonhoeffer T (2001). Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu. Rev. Neurosci 24, 1071–1089. [DOI] [PubMed] [Google Scholar]
- Zhou M, Greenhill S, Huang S, Silva TK, Sano Y, Wu S, Cai Y, Nagaoka Y, Sehgal M, Cai DJ, Lee YS, Fox K, Silva AJ (2016). CCR5 is a suppressor for cortical plasticity and hippocampal learning and memory. eLife 5:e20985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Homma KJ, Poo MM (2004). Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron. 44, 749–757. [DOI] [PubMed] [Google Scholar]
- Ziv NE, Brenner N (2018). Synaptic tenacity or lack thereof: spontaneous remodeling of synapses. Trends in Neurosci. 41, 89–99. [DOI] [PubMed] [Google Scholar]
- Zuo Y, Yang G, Kwon E, Gan WB (2005). Long-term sensory deprivation prevents dendritic spine loss in primary somatosensory cortex. Nature 436, 261–265. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data reported in this paper will be shared by the lead contact upon request.
All original code has been deposited at Zenodo and is publicly available as of the date of publication. DOIs are listed in the key resources table.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-Myc/c-Myc Antibody (9E10) | Santa Cruz | Cat# sc-40 |
| Alkaline phosphatase goat anti-mouse IgG | Jackson | Cat# 115-055-062; RRID: AB_2338533 |
| NBT/BCIP stock solution | Roche | Cat# 11681451001 |
| Chemicals, peptides, and recombinant proteins | ||
| Tamoxifen | Sigma-Aldrich | Cat# T5648 |
| sPirB | Bochner et al., 2014 | N/A |
| Tetrodotoxin citrate | A.G. Scientific | Cat# T-2785 |
| Picrotoxin | Tocris | Cat# 1128 |
| NBQX | Tocris | Cat# 0373 |
| (R)-CPP | Tocris | Cat# 0247 |
| DNI-glutamate trifluoroacetate | Femtonics Ltd. | Cat# 1951 |
| L-689,560 | Tocris | Cat# 0742 |
| Experimental models: Organisms/strains | ||
| Mouse: PirB−/− | Syken et al., 2006 | N/A |
| Mouse: PirBfl/fl | Djurisic et al., 2019 | N/A |
| Mouse: CaMKIIa-CreER | The Jackson Laboratory | Jax# 012362 |
| Software and algorithms | ||
| DeepLab Cut | Mathis et al., 2018 | N/A |
| ImageJ | NIH ImageJ | RRID: SCR_003070 |
| MATLAB | Mathworks | RRID: SCR_001622 |
| Custom MATLAB scripts for analysis | Custom | DOI: 10.5281/zenodo.5146405 |
| Other | ||
| Micro-osmotic pump | Alzet | Cat# 0004317 |


