Abstract
Young age is a risk factor for respiratory and gastrointestinal infections. Here, we compared infant and adult mice to identify age-dependent mechanisms that drive susceptibility to mucosal infections during early life. Transcriptional profiling of the upper respiratory tract (URT) epithelium revealed significant dampening of early life innate mucosal defenses. Epithelial-mediated production of the most abundant antimicrobial molecules, lysozyme and lactoferrin, and the polymeric immunoglobulin receptor (pIgR), responsible for IgA transcytosis, was expressed in an age-dependent manner. This was attributed to delayed functional development of serous cells. Absence of epithelial-derived lysozyme and the pIgR was also observed in the small intestine during early life. Infection of infant mice with lysozyme-susceptible strains of Streptococcus pneumoniae or Staphylococcus aureus in the URT or gastrointestinal tract, respectively, demonstrated an age-dependent regulation of lysozyme enzymatic activity. Lysozyme derived from maternal milk partially compensated for the reduction in URT lysozyme activity of infant mice. Similar to our observations in mice, expression of lysozyme and the pIgR in nasopharyngeal samples collected from healthy human infants during the first year of life followed an age-dependent regulation. Thus, a global pattern of reduced antimicrobial and IgA-mediated defenses may contribute to increased susceptibility of young children to mucosal infections.
Introduction
In children, acute respiratory and gastrointestinal infections are a significant cause of morbidity and mortality. In 2015, there were 5.941 million deaths worldwide in children younger than 5 years, and pneumonia and diarrhea accounted for 21.4% of all deaths in children aged 1–59 months1. Strikingly, a high proportion of deaths caused by either diarrhea (72%) or pneumonia (81%) occurred within the first 2 years of life2. This suggests that early age is associated with dampened mucosal defense responses.
As a first defense against colonization by opportunistic pathogens, epithelial cells lining the respiratory and gastrointestinal tracts produce numerous antimicrobial molecules. The secretion of lysozyme, lactoferrin, and secretory immunoglobulin A (SIgA) serves to disrupt bacterial cell membranes, sequester nutrients and block microbial attachment to epithelial cells, respectively. Together, these three antimicrobials form up to 50% of the total protein in human respiratory secretions3 and are also abundant in the gastrointestinal tract.
As the most abundant antimicrobial peptide, lysozyme exerts its bacteriolytic function through targeted hydrolysis of bacterial cell walls4. Previous work has shown that lysozyme contributes to the microbicidal activity of nasal fluid5. Additionally, enhanced production of lysozyme by respiratory epithelial cells from transgenic mice increased bacterial killing and reduced systemic dissemination following lung infection with Pseudomonas aeruginosa and group B streptococcus6. While humans encode a single lysozyme gene, the mouse genome encodes two genes: lysozyme 1 and lysozyme 2, also known as lysozyme P and lysozyme M, respectively. In the respiratory tract, lysozyme 2 is the predominant form and is produced by specialized epithelial cells and myeloid cells7,8. In contrast, lysozyme 1 is the dominant form found in the gastrointestinal tract and is produced by Paneth cells, which line the small intestine7,9.
Lactoferrin is an iron-binding glycoprotein that limits bacterial growth through sequestration of iron and also exerts direct antibacterial activity10–13. Studies identified a synergistic effect of lactoferrin and lysozyme in enhancing bacterial killing of gram-negative bacteria14–16. In addition to numerous antimicrobial peptides, SIgA contributes significantly to first-line defenses at mucosal sites17. In order for SIgA to exert its protective effect, dimeric IgA produced in the lamina propria is transcytosed across the epithelial barrier, a process mediated by the polymeric immunoglobulin receptor (pIgR)17. As the most prevalent immunoglobulin in mucosal secretions, SIgA impairs pathogen colonization by impeding access to epithelial receptors and enhances mucus entrapment to facilitate mucociliary clearance18,19. The abundance of these three proteins on mucosal surfaces highlights their important role in preventing and restricting growth of pathogenic bacteria.
Despite substantial evidence linking young age to mucosal infections, the identification of age-dependent factors that enhance acquisition and delay clearance of pathogens remains largely unexplored. This study was undertaken to identify age-dependent host factors critical for mediating resistance to mucosal infections by using infant and adult models.
Results
Baseline mucosal defenses in the URT are dampened in infant mice
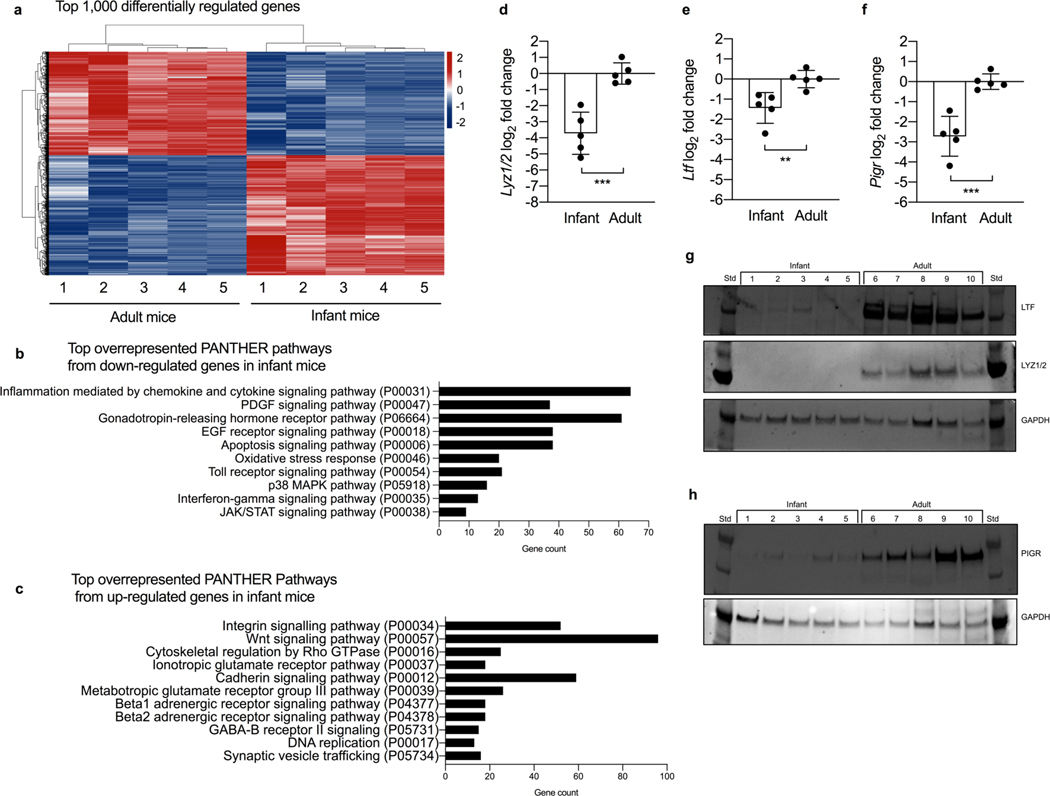
To elucidate age-dependent mechanisms that promote colonization of pathogens in the upper respiratory tract (URT), we performed a RNA-sequencing screen to assess gene expression profiles of the URT epithelia of infant and adult mice. RNA was isolated from nasal lavages collected from 7-day-old infant and 8-week-old adult mice. We identified over 7,000 differentially expressed genes (DEGs) between infant and adult mice with the top 1,000 DEGs shown to illustrate the distinct differences in expression (Fig. 1a). Pathway analysis showed that significantly down-regulated genes in infant mice were enriched for pathways involved in mucosal immune responses that included Inflammation mediated by chemokine and cytokine signaling, Toll receptor signaling and JAK/STAT signaling (Fig. 1b). Additionally, these genes were related to pathways involved in epithelial barrier function, such as EGF receptor signaling and PDGF signaling (Fig. 1b). In contrast, up-regulated genes in infant mice were enriched for cell fate determination and proliferation that included Wnt signaling, Integrin signaling and Cytoskeletal regulation by Rho GTPase (Fig. 1c). Of note genes involved in innate immune function were only identified in the down-regulated category, suggesting an age-dependent regulation.
Figure 1. Mucosal immune defenses in the URT are dampened in early life.
a, Heat-map of the top 1,000 differentially expressed genes in the upper respiratory tract between 7 day-old infant mice and 8 week-old adult mice (n=5). b, Top overrepresented PANTHER pathways from significantly down-regulated genes (FDR P >0.05) and (c) Top overrepresented PANTHER pathways from significantly up-regulated genes (FDR P >0.05) in 7 day-old infant mice compared to 8 week-old adult mice (n=5). d-f, Transcript levels of (d) lysozyme, Lyz1/2, (e) lactoferrin, Ltf, and (f) the pIgR, Pigr, from 7-day old infant mice and adult 8-week old mice (n=5). Data represent log2-transformed values and the mean ± SD. Statistical significance was determined using an unpaired Student’s t test and is indicated as **, p ≤ 0.01; ***, p ≤ 0.001. g, Protein levels of lactoferrin (LTF, top), lysozyme (LYZ1/2, middle), glyceraldehyde 3-phosphate dehydrogenase (GAPDH, bottom) and (h) pIgR (PIGR, top) and GAPDH (bottom) in nasal lavages collected from 7 day-old infant and adult mice (n=5). Std, protein standard. Data collected from two independent experiments.
Upon further analysis of the DEGs, we observed that expression of the most abundant antimicrobial molecules, lysozyme and lactoferrin, were significantly decreased in infant mice. A similar observation was made for the pIgR, which is required for transport of secretory immunoglobulins. Using quantitative real time-PCR (qRT-PCR) we confirmed that expression of lysozyme (Lyz1/2) (Fig. 1d), lactoferrin (Ltf) (Fig. 1e), and the pIgR (Pigr) (Fig. 1f) was significantly reduced in the URT of infant mice compared to adult mice. In accordance with decreased expression, URT protein levels of lysozyme (Fig. 1g, S1a; S Table 1), lactoferrin (Fig. 1g, S1b; S Table 2) and the pIgR (Fig. 1h, S1c; S Table 3) were significantly lower in infant mice compared to adult mice. Additionally, we identified, and confirmed, numerous other antimicrobial molecules to be significantly decreased in infant mice compared to adult mice that included S100 calcium-binding protein A8 (S100a8) (S1d) and A13 (S100a13) (S1e), regenerating islet-derived 3 delta (Reg3d) (S1f), and complement component 3 (C3) (S1g). While expression of defensin beta 1 (Defb1) (S1h) was significantly decreased in our RNA-Sequencing screen, we were unable to confirm a statistically significant difference in expression using qRT-PCR. These results demonstrate that early age corresponds to reduced production of antimicrobial molecules that contribute to limit acquisition and colonization of URT pathogens.
Age-dependent regulation of lysozyme production and function
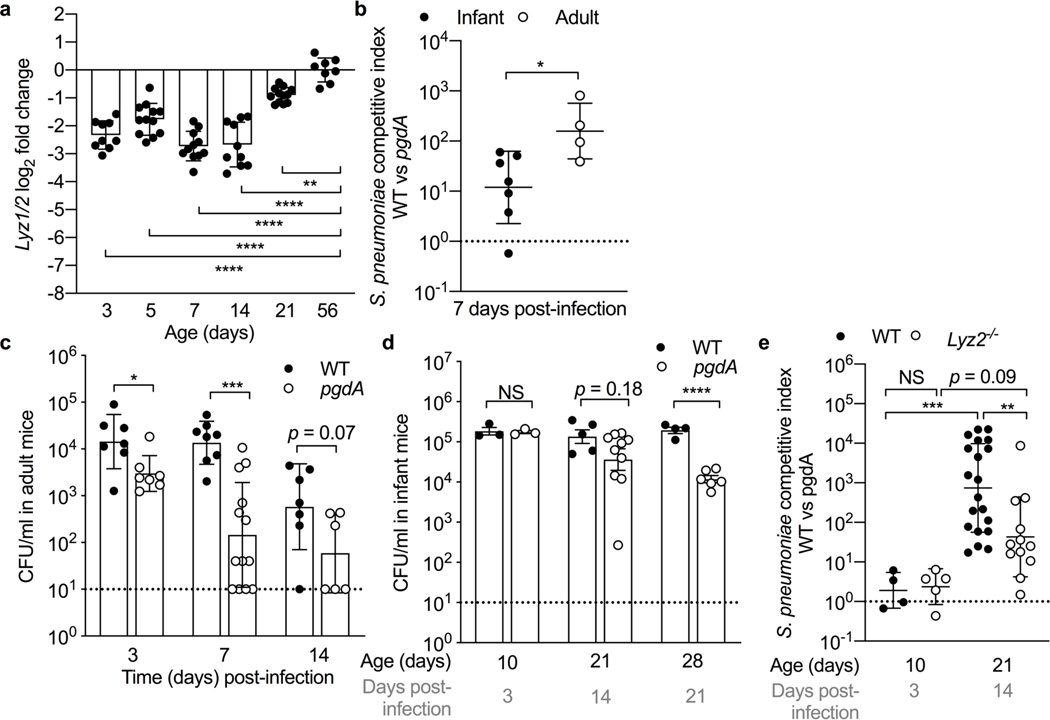
In human nasal secretions, lysozyme alone composes approximately 15–30% of the total protein3,20. To characterize lysozyme expression as a function of age, lysozyme transcripts were measured by qRT-PCR using RNA isolated from nasal lavage samples obtained from adult mice and infant mice at various ages (3, 5, 7, 14 and 21-days-old). We found that baseline levels of lysozyme transcripts in the URT remained significantly low during the first three weeks of life (Fig. 2a). Since both expression and protein levels of lysozyme in the URT were significantly lower in infant compared to adult mice, we wanted to determine whether early age also impacted lysozyme-mediated bactericidal activity. To test this, we utilized a clinically relevant infant mouse model of nasopharyngeal infection by Streptococcus pneumoniae (S. pneumoniae), a prominent URT pathogen. In order to control for mouse-to-mouse differences, we performed a competitive infection experiment in which both infant and adult mice were co-administered via the intranasal route with wild-type S. pneumoniae and an isogenic S. pneumoniae pgdA null mutant, which lacks a cell wall modification required for full lysozyme resistance21. While expression of pgdA conferred a significant fitness advantage in adult mice 7 days post-infection (100-fold), the fitness advantage conferred by pgdA was significantly reduced in infant mice (Fig. 2b; S2a). Similarly, in adult mice inoculated individually with either wild-type S. pneumoniae or the S. pneumoniae pgdA mutant, the lysozyme-susceptible strain consistently colonized at a lower density in the URT at 3, 7, and 14 days post-infection (Fig. 2c). In contrast, colonization levels of the wild-type S. pneumoniae and S. pneumoniae pgdA mutant in infant mice remained the same during the first 14-days post-infection and colonization levels of the S. pneumoniae pgdA mutant didn’t significantly decline until 21-days post-infection, corresponding to increased lysozyme expression (Fig. 2d). To further test the age-dependent regulation of lysozyme function in the URT, we competitively infected 7-day-old infant mice with wild-type S. pneumoniae and S. pneumoniae pgdA mutant and assessed URT colonization 3 and 14-days post-infection. We found that resistance to lysozyme-dependent killing did not provide a significant fitness advantage 3-days post-infection (Fig. 2e; S2b); whereas at 14-days post-infection, resistance to lysozyme conferred a significant colonization advantage (1,000-fold) in infant mice (Fig. 2e), a time-point that corresponded to increased lysozyme expression in infant mice (i.e. 21-days of life) (Fig. 2a). The competitive advantage mediated by pgdA at 14-days post-infection was significantly reduced in lysozyme 2-deficient infant mice (Lyz2−/−) (Fig. 2e; S2b). Loss of lysozyme expression in the URT of Lyz2−/− mice was confirmed in naïve adult mice (S2c). Taken together, these results suggest that young age impairs production and, subsequently, bactericidal activity of lysozyme in the URT.
Figure 2. Age-dependent regulation of lysozyme production and function in the URT.
a, Transcript levels of lysozyme (Lyz1/2) in nasal lavages collected from infant mice at 3, 5, 7, 14 and 21 days of age compared to adult mice (n=8–12). Data represent log2-transformed values with the mean ± SD. Statistical significance was determined using an ordinary one-way ANOVA and Dunnett’s multiple comparisons post-hoc test and is indicated as **, p ≤ 0.01; ****, p ≤ 0.0001. Data collected from two independent experiments. b, Competitive index of colony forming units (CFU) from nasal lavages of infant and adult mice 7 days after IN infection with a 1:1 mixture of wild-type S. pneumoniae and S. pneumoniae pgdA mutant (n=4–7). Data represent log10-transformed values with the mean ± SD. Statistical significance was determined using an unpaired Student’s t test and is indicated as *, p ≤ 0.05. Dotted line at competitive index of 1. Data collected from one experiment. c-d, CFU of nasal lavages at either 3, 7 and 14 days or 3, 14, and 21 days, after IN single infection with either wild-type S. pneumoniae or S. pneumoniae pgdA mutant in (c) adult mice (n=6–13) and (d) infant mice (n=3–10). Data represent log10-transformed values with the mean ± SD. Statistical significance was determined using an unpaired Students t test and is indicated as *, p ≤ 0.05; ***, p ≤ 0.001; ****, p ≤ 0.0001. NS, not significant. Dotted line at limit of detection. Adult data collected from 2–3 independent experiments. Infant data collected from one experiment, except D14 S. pneumoniae pgdA mutant data collected from two independent experiments. e, Competitive index of CFU in nasal lavages of wild-type or lysozyme-2 (Lyz2−/−)-deficient infant mice 3 and 14 days after intranasal infection with a 1:1 mixture of wild-type S. pneumoniae and S. pneumoniae pgdA mutant (n=4–20). Data represent log10-transformed values with the mean ± SD. Statistical significance was determined using an ordinary one-way ANOVA and Sidak’s multiple comparisons post-hoc test and is indicated by **, p ≤ 0.01; ***, p ≤ 0.001. NS, not significant. Dotted line at competitive index of 1. Day 3 data collected from one experiment. Day 14 data collected from 2–3 independent experiments.
Global reduction in lysozyme production and activity at mucosal surfaces
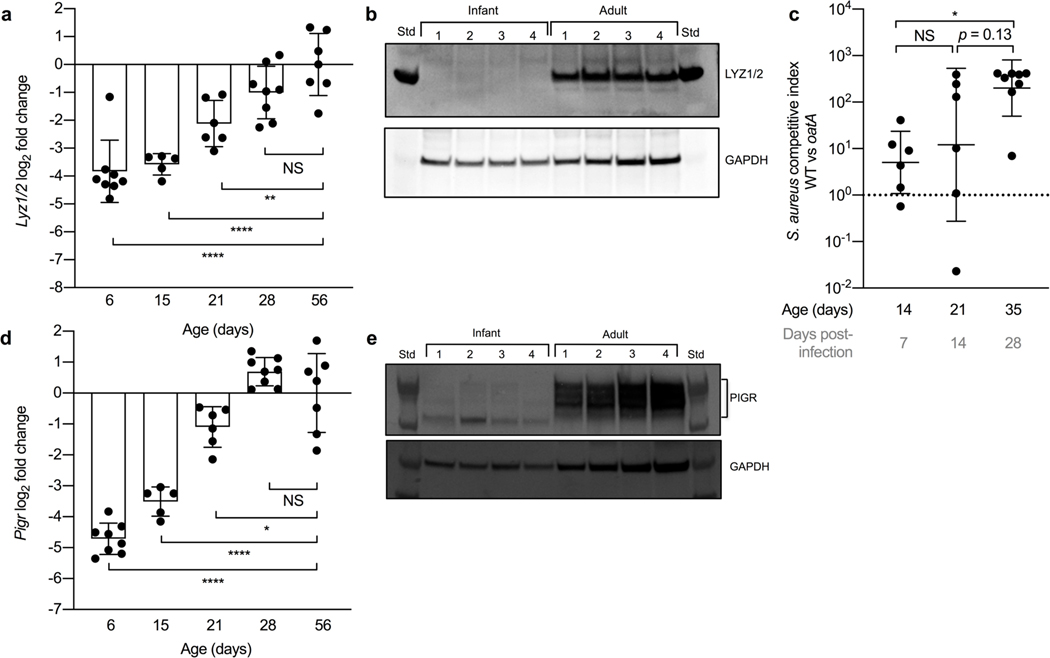
Considering the prevalence of lysozyme at multiple mucosal surfaces22, we wanted to determine whether the age-dependent regulation of lysozyme, observed in the URT, extended to the gastrointestinal tract. To test whether young age correlated with reduced lysozyme transcripts in the small intestine, RNA was isolated from the ileum of adult mice and infant mice at various ages (6, 15, 21, 28-days-old). Similar to the URT, lysozyme transcripts in the ileum were significantly decreased during the first three weeks of life and expression didn’t reach adult levels until 28-days of life (Fig. 3a). Correspondingly, protein levels of lysozyme in the small intestine of 7-day-old infant mice were significantly decreased compared to adult mice (Fig. 3b; S3a; S Table 4).
Figure 3. Global reduction of lysozyme production and activity at mucosal surfaces.
a, Transcript levels of lysozyme (Lyz1/2) in ileum lavages collected from infant mice at 6, 15, 21 and 28 days of age compared to adult 8 week-old mice (n=5–8). Data represent log2-transformed values with the mean ± SD. Statistical significance was determined using an ordinary one-way ANOVA and Dunnett’s multiple comparisons post-hoc test and is indicated as **, p ≤ 0.01; ****, p ≤ 0.0001. NS, not significant. Data collected from two independent experiments, except D15 data collected from one experiment. b, Protein levels of LYZ1/2 (top) and GAPDH (bottom) in small intestine collected from 7 day-old infant and adult mice (n=4). Infant samples were pooled n=2–3. Std, protein standard. Data collected from two independent experiments. c, Competitive index of CFU from cecum of infant mice at 7, 14, and 28 days after oral infection with a 1:1 mixture of wild-type S. aureus and S. aureus oatA mutant (n=6–8). Data represent log10-transformed values with the mean ± SD. Statistical significance was determined using an ordinary one-way ANOVA and Sidak’s multiple comparisons post-hoc test and is indicated by *, p ≤ 0.05. NS, not significant. Dotted line at competitive index of 1. Data collected from two independent experiments. d, Transcript levels of Pigr in ileum lavages collected from infant mice at 6, 15, 21 and 28 days of age compared to adult 8 week-old mice (n=5–8). Data represent log2-transformed values with the mean ± SD. Statistical significance was determined using an ordinary one-way ANOVA with a Dunnett’s multiple comparisons post-hoc test and is indicated as *, p ≤ 0.05; ****, p ≤ 0.0001. NS, not significant. Data collected from two independent experiments, except D15 data collected from one experiment. e, Protein levels of PIgR (top) and GAPDH (bottom) in small intestine collected from 7 day-old infant (n=4) and adult mice (n=4). Infant samples were pooled n=2–3. Std, protein standard. Data collected from two independent experiments.
To demonstrate that lysozyme activity in the intestine is also regulated in an age-dependent manner similar to the URT, we utilized an oral Staphylococcus aureus (S. aureus) infection model in infant mice, considering S. pneumoniae isn’t a significant colonizer of the gastrointestinal tract23. A previous study found that intestinal carriage of S. aureus in children is common and decreased in adults24. We competitively infected infant mice via the oral route with a wild-type methicillin-resistant S. aureus and lysozyme-sensitive S. aureus oatA mutant, an enzymatic equivalent to pgdA in S. pneumoniae25. Colonization levels were assessed from the cecum at multiple time-points post-infection. Similar to the URT, impaired lysozyme resistance had a greater impact on S. aureus colonization levels within the cecum later in life (i.e. 35-days) compared to earlier in life (i.e. 14 and 21-days) (Fig. 3c; S3b). The competitive advantage mediated by oatA corresponded to the gradual increase in intestinal lysozyme expression in infant mice during the first 4 weeks of life (Fig. 3a). Together, these results suggest that early life corresponds to a general reduction in lysozyme production and bactericidal activity at mucosal surfaces.
Next, we wanted to determine whether expression of lactoferrin and the pIgR also followed an age-dependent regulation in the intestinal tract. In contrast to lysozyme, lactoferrin expression was significantly increased during the first two weeks of life (S3c). However, similar to lysozyme, transcription (Fig. 3d) and protein levels (Fig. 3e; S3d; S Table 5) of the pIgR were significantly decreased in the small intestine of infant mice compared to adult mice. Since Paneth cells contribute significantly to the amount of lysozyme in the gastrointestinal tract7, we wanted to determine whether expression of another prominent Paneth cell-derived antimicrobial, regenerating islet-derived protein 3 gamma (Reg3g), was age-dependent. Similar to lysozyme and the pIgR, expression of Reg3g was significantly decreased during the first two weeks of life compared to adult mice (S3e). Thus, early life is characterized by variation in expression of antimicrobial molecules at the mucosal surface, with a significant reduction in expression of some of the most abundant proteins that protect mucosal barriers.
Non-hematopoietic cells are responsible for reduced lysozyme expression during early life
Studies in neonatal mice showed that during the first weeks of life, before weaning, the composition of the microbial community is dynamic and unstable26–28. Therefore we wanted to determine whether absence of a stable microbiome during early life contributes to decreased production of antimicrobial molecules in the URT. To test this, we collected nasal lavages from either germ free (GF) or specific pathogen free (SPF) infant and adult mice and isolated RNA to measure expression of antimicrobial proteins in the URT. Expression of lysozyme (S4a), lactoferrin (S4b), and pIgR (S4c) remained significantly reduced in both SPF and GF infant mice compared to adult SPF mice, while expression of these antimicrobials in GF adult mice were similar to SPF adult mice. These results indicate that complete absence of a microbial community does not significantly impact expression of these antimicrobials in the murine URT.
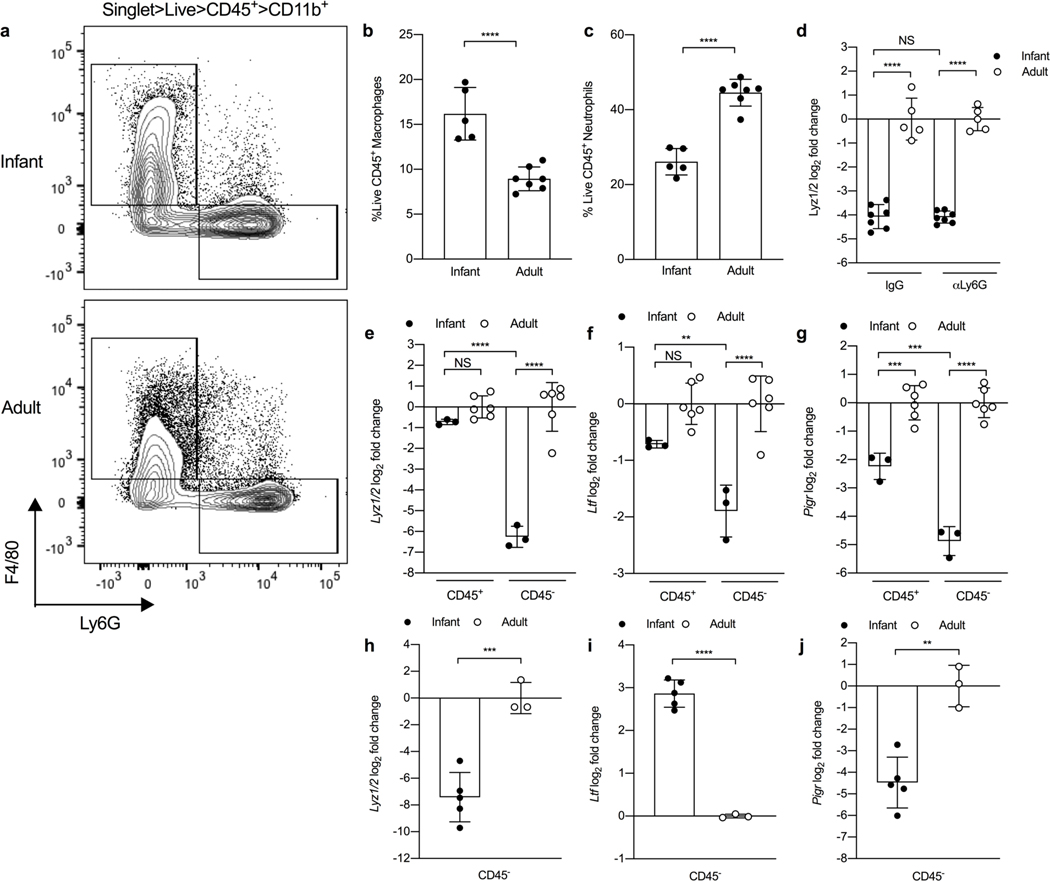
Next, we questioned whether alterations in the URT cellular composition between infant and adult mice could account for reduced lysozyme levels. In addition to epithelial cells, macrophages and neutrophils constitutively produce lysozyme in the URT8. To test this, we quantified myeloid cell numbers using whole nasal tissue isolated from infant and adult mice by flow cytometry. While the proportion of macrophages (CD45+CD11b+Ly6G-F4/80+) was significantly increased in the URT of infant mice compared to adult mice (Fig. 4a, 4b), the percentage of neutrophils (CD45+CD11b+Ly6G+F4/80-) was significantly decreased (Fig. 4a, 4c). This result indicated that reduced neutrophil numbers in the URT during early life could contribute to dampened lysozyme levels. To test this hypothesis, we depleted neutrophils in the URT by treating infant and adult mice with either a Ly6G-specific monoclonal antibody or isotype control and collected nasal lavages to assess lysozyme expression. While treatment with anti-Ly6G reduced neutrophil frequency in the URT of infant and adult mice (S4d; gating strategy S4e), expression of lysozyme remained significantly repressed in infant mice treated with either the isotype control or anti-Ly6G antibody compared to adult controls (Fig. 4d). This result suggested that alteration of neutrophil numbers in the URT during early life does not significantly impact lysozyme expression.
Figure 4. Non-hematopoietic cells are responsible for reduced lysozyme expression during early life.
a, Representative flow plots for frequency of macrophages (singlet live CD45+ CD11b+ F4/80+ Ly6G-) and neutrophils (singlet live CD45+ CD11b+ F4/80- Ly6G+) from uninfected 7 day-old infant and adult mice. b, Percentage of macrophages (singlet live CD45+ CD11b+ F4/80+ Ly6G-) and (c) neutrophils (singlet live CD45+ CD11b+ F4/80- Ly6G+) from naïve 7 day-old infant and adult mice (n=5–7). Data represent mean ± SD. Statistical significance was determined using a unpaired Student’s t test and is indicated as ****, p ≤ 0.0001. Data collected from two independent experiments. d, Transcript levels of Lyz1/2 in nasal lavages from infant and adult mice treated with 2 doses of either a neutrophil depleting antibody (αLy6G) or isotype control (n=5–7). Data represent log2-transformed values with the mean ± SD. Statistical significance was determined using an ordinary one-way ANOVA with a Sidak’s multiple comparisons post-hoc test and is indicated as ****, p ≤ 0.0001. NS, not significant. Data collected from two independent experiments. e-g, Transcript levels of (e) Lyz1/2, (f) Ltf and (g) Pigr in CD45+ and CD45- cells isolated from URT tissue of naïve 7 day-old infant and adult mice (n=3–6). Samples for infant mice were pooled n=3. Data represent log2-transformed values with the mean ± SD. Statistical significance was determined using an ordinary one-way ANOVA with a Sidak’s multiple comparisons post-hoc test and is indicated as **, p ≤ 0.01; ***, p ≤ 0.001; ****, p ≤ 0.0001. Adult data collected from two independent experiments and infant data collected from one experiment using two litters. h-j, Transcript levels of (h) Lys1/2, (i) Ltf and (j) Pigr in CD45- cells isolated from small intestine of naïve 7 day-old infant and adult mice (n=3–5). Samples for infant mice were pooled n=3–4 and samples for adult mice were pooled n=1–2. Data represent log2-transformed values with the mean ± SD. Statistical significance was determined using a unpaired Student’s t test and is indicated as **, p ≤ 0.01; ***, p ≤ 0.001; ****, p ≤ 0.0001. Data collected from two independent experiments.
In addition to myeloid cells, epithelial cells produce a number of antimicrobial proteins, including lysozyme8,29,30. To test whether a reduction in epithelial-mediated production of lysozyme accounted for the absence of lysozyme in infant mice, we isolated cells from infant and adult nasal tissue and separated the cells based on CD45 expression to distinguish between CD45+ hematopoietic (e.g. myeloid) and CD45- non-hematopoietic (e.g. epithelial) cells. While expression of lysozyme (Fig. 4e) and lactoferrin (Fig. 4f) were slightly, but not significantly, reduced in infant CD45+ cells compared to adult CD45+ cells, the expression of both antimicrobials were markedly dampened in infant CD45- cells compared to adult CD45- cells. In contrast, expression of the pIgR was significantly reduced in both infant CD45+ and CD45- cells compared to adult controls; however the reduction in expression of the pIgR was more pronounced in the infant CD45- cell population compared to adult CD45- cells (Fig. 4g). As expected, lysozyme expression was also significantly reduced in infant CD45- cells isolated from the whole small intestine compared to adult controls (Fig. 4h). While lactoferrin expression was significantly increased in infant CD45- cells compared to adult CD45- cells (Fig. 4i), expression of the pIgR was significantly decreased in infant CD45- cells (Fig. 4j). These results indicate that epithelial-mediated production of lysozyme and the pIgR in both the respiratory and intestinal tracts is impaired during young age.
Delayed development of specialized epithelial cells in the URT during early life
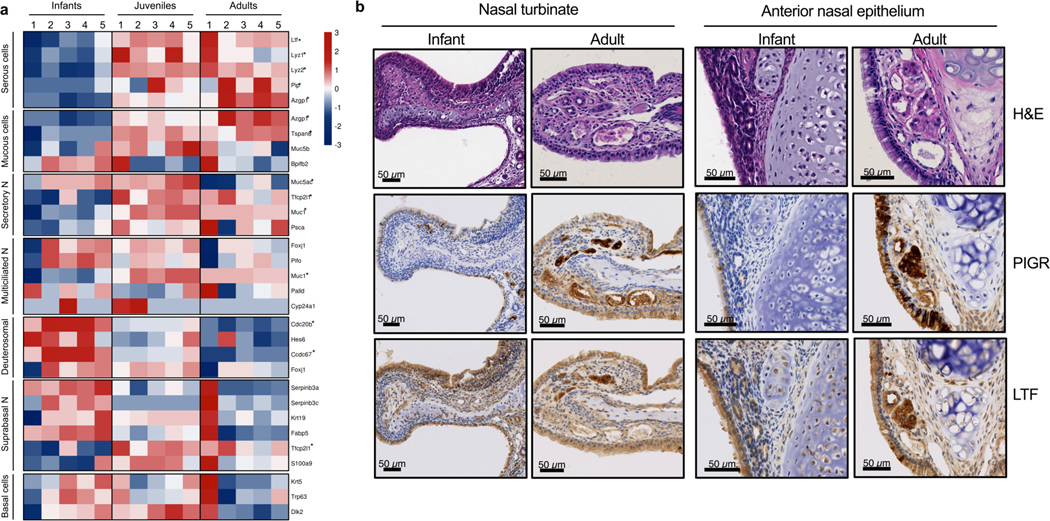
Previous studies showed that Paneth cell numbers in the mouse small intestine are significantly reduced during the first three weeks of life31–33, suggesting that delayed development of these specialized epithelial cells likely accounts for the absence of intestinal lysozyme in infant mice. In the URT, serous cells, located within the submucosal glands, contribute significantly to the levels of antimicrobials, including lysozyme and lactoferrin, at the mucosal surface29,34–36. However, unlike Paneth cells, the effect of age on serous cell development in the URT is unknown. To test whether serous cell differentiation and/or function is specifically impaired during early life, we utilized our RNA-seq screen to determine transcript levels of the most highly expressed genes in seven epithelial cell subsets in the respiratory tract: serous, mucous, secretory, multiciliated, deuterosomal, suprabasal and basal. The target genes for each cellular subset were identified from a recent study that used single-cell RNA-seq to profile the human respiratory tract, which included sampling of the nose, of healthy adults37. Strikingly, we found that only in the serous cell subset were all of the cell specific genes (Ltf, Lyz1, Lyz2, Pip and Azgp1) significantly repressed in infants compared to adult mice (Fig. 5a). Additionally, we observed a significant reduction in expression of cell specific genes of mucous (Azgp1 and Tspan8) and secretory (Tfxp2l1 and Muc1) cell subsets from infants compared to adult mice (Fig. 5a). In contrast, deuterosomal cell specific genes (Cdc20b and Ccdc67), which are precursors of multiciliated cells, were significantly increased in infants (Fig. 5a). For each of these cellular subsets, the expression of cell specific genes from juvenile mice (25 days old) showed an intermediate phenotype, which indicated an age-dependent affect on epithelial cell development (Fig. 5a).
Figure 5. Delayed development of specialized epithelial cells in the URT during early life.
a, Heatmap of log2-transformed gene expression values in nasal lavages collected from infant (7 day old), juvenile (25 day old) and adult (8 week old) mice. Genes are representative of specific epithelial subsets from the respiratory tract and include: serous (Ltf, Lyz1, Lyz2, Pip, and Azgp1), mucous (Azgp1, Tspan8, Muc5b and Bpifb2), secretory (Muc5ac, Tfcp2l1, Muc1 and Psca), multiciliated (Foxj1, Pifo, Muc1, Palld, and Cyp24a1), deuterosomal (Cdc20b, Hes6, Ccdc67, and Foxj1), suprabasal (Serpinb3a, Serpinb3c, Krt19, Fabp5, Tfcp2l1 and S100a9) and basal (Krt5, Trp63 and Dlk2). Genes found to be statistically different between infant and adult mice are designated *, p-adjusted value <0.05. b, Representative coronal URT tissue sections of a naive infant (6 day old) and adult (8 week old) mouse. Sections were stained with hematoxylin and eosin (H&E; top row), anti-pIgR antibody (middle row) or anti-lactoferrin antibody (bottom row). Levels of pIgR and lactoferrin were detected by IHC using DAB substrate (brown precipitate) and counterstained with hematoxylin.
Our transcriptional profiling of epithelial subsets in the URT, suggested that functional development of serous cells, in particular, was delayed in infant mice. To more fully understand the effect of age on development of this secretory population, we examined haematoxylin and eosin (H&E) and serous cell specific (lactoferrin and pIgR38) stained URT tissue sections from infant and adult mice. We were unable to definitively assess lysozyme levels due to low and non-specific staining using our antibody. Serous cells are found within the submucosa of the URT where they form glandular structures39. Given the size differences in the nasal cavity between infant and adult mice, we focused on the nasal turbinates and the anterior nasal epithelium near the septum to assess gland structure and staining of specific markers. We observed more prominent staining of pIgR and lactoferrin within the submucosal gland structures of both the nasal turbinate and anterior nasal epithelium from adult mice relative to infants (Fig. 5b). Additionally, infant nasal tissue sections were hypercellular compared to adults, indicating increased proliferation. The increase in cellularity at the nasal epithelium from infant mice corresponded with the general trend of increased expression of genes associated with progenitor populations (i.e. basal and suprabasal) and decreased expression of genes associated with some differentiated cell populations (i.e. serous, mucous and secretory) (Fig. 5a). Together, these results suggest that early life corresponds with a significant alteration in epithelial cell heterogeneity that is specifically marked by delayed functional development of serous cells in the URT submucosal tissue.
Maternal milk partially compensates for reduced production of lysozyme in infant mice
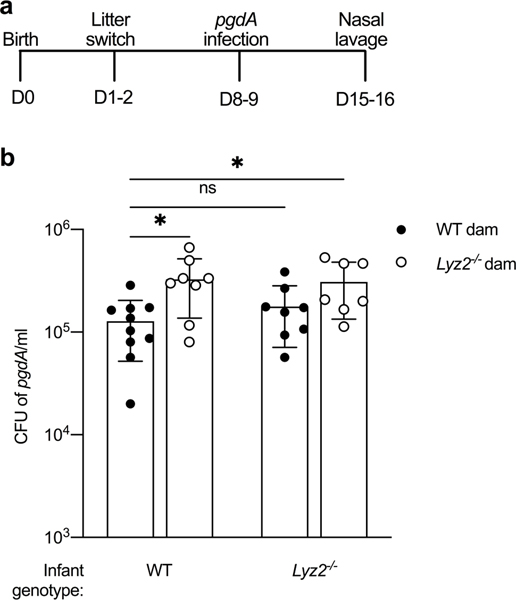
In addition to providing essential macronutrients for neonatal development, maternal milk contains numerous non-nutritive components - the most abundant being lysozyme, lactoferrin, and SIgA40. To test whether maternal milk compensates for the absence of lysozyme in infants, we switched half of the pups from a wild-type and lysozyme 2-deficient litter within 24–48 hours of birth, and then 7 days after the switch, we infected all pups with a lysozyme sensitive S. pneumoniae pgdA mutant and assessed URT colonization 7 days post-infection (Fig. 6a). We confirmed that lysozyme expression in mammary gland tissue was significantly reduced in lysozyme 2-deficient dams compared to wild type dams. Colonization of the S. pneumoniae pgdA mutant was unchanged between wild-type and lysozyme 2-deficient pups fed by a wild-type dam (Fig. 6b) In contrast, when wild-type or lysozyme 2-deficient pups were fed by a lysozyme 2-deficient dam, colonization levels of the S. pneumoniae pgdA mutant significantly increased compared to wild-type pups fed by a wild-type dam (Fig. 6b). This result indicates that exogenous lysozyme provided in maternal milk can partially compensate for the absence of endogenous production of lysozyme in infant mice.
Figure 6. Maternal milk partially compensates for reduced lysozyme production in infant mice.
a, Experimental design for assessment of maternal milk-derived lysozyme in clearance of a lysozyme sensitive S. pneumoniae strain (pgdA). At 1–2 days after birth, half of the pups from a wild-type dam and half of the pups from a lysozyme-2 deficient dam were switched. After 7 days all pups (8–9 days-old) were infected with S. pneumoniae pgdA mutant. b, CFU of nasal lavages at 7 days after IN infection with S. pneumoniae pgdA mutant in wild-type and lysozyme-2 (Lyz2−/−)-deficient infant mice (n=7–10). Data represent log10-transformed values with the mean ± SD. Statistical significance was determined using an ordinary one-way ANOVA and Dunnett’s multiple comparisons post-hoc test and is indicated as *, p ≤ 0.05.
Temporal increase in lysozyme expression during the first year of life in children
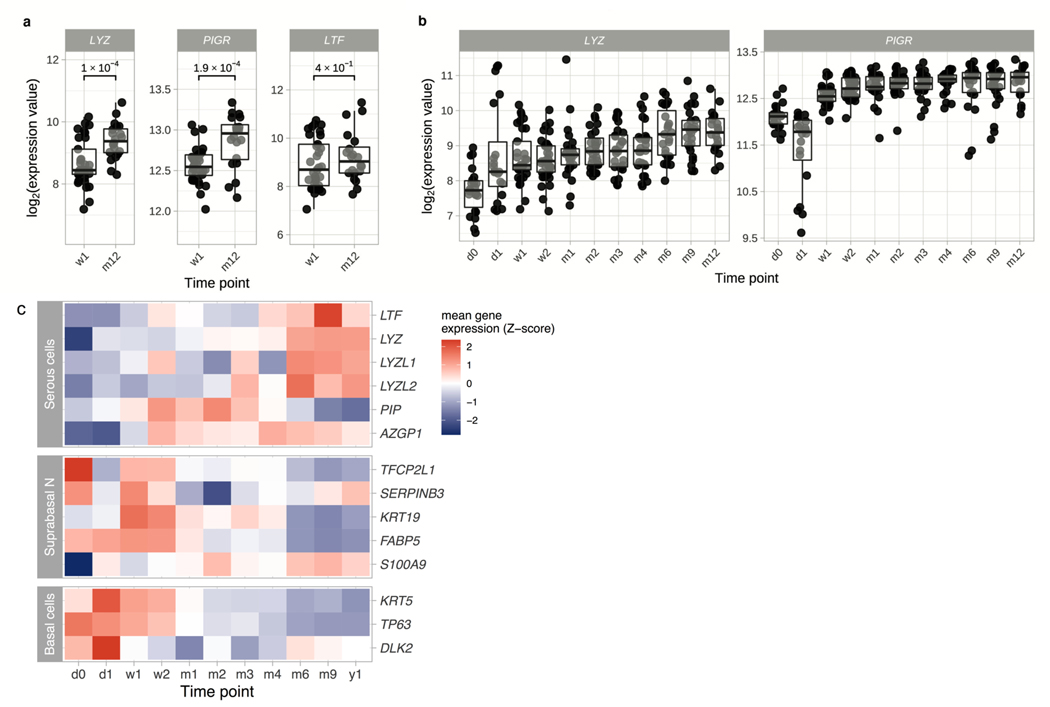
The absence of mucosal lysozyme during the first weeks of life in mice led us to question whether humans also exhibited an age-dependent regulation of lysozyme during early life. To test this we assessed the gene expression signature of nasopharyngeal samples collected from healthy infants at up to 11 time-points in the first year of life (2 hours after birth, at 24 hours, 7 and 14 days, and at 1, 2, 3, 4, 6, 9 and 12 months of age). Strikingly, lysozyme expression was significantly reduced in 7-day-old children compared to 12-month-old children (Fig. 7a). Additionally, children displayed a gradual increase in lysozyme transcripts during the first year of life (Fig. 7b), complementing our observations in infant mice (Fig. 2a). Although lactoferrin transcripts (Fig. 7a) were similar between 7-day-old and 12-month-old children, expression of the pIgR was also significantly reduced in 7-day-old children (Fig. 7a). Further, transcripts of the pIgR followed a temporal increase in expression during the first year of life similar to what was observed with lysozyme (Fig. 7b). Considering we found that serous cell development was impaired in the URT of infant mice, we wanted to determine whether development of this epithelial cell subset was also impacted in human infants. To test this, we assessed the expression of serous cell specific genes (Fig. 5a) from human infants at multiple time points during the first year of life. Additionally, we assessed expression of genes specific for basal and suprabasal cells since immunohistochemical staining of nasal sections from infant mice demonstrated increased cellularity at the mucosal epithelium. During the first year of life, we observed that the average expression of the majority of transcripts specific to serous cells gradually increased, whereas the average expression of progenitor cell (i.e. basal and suprabasal) specific genes generally decreased throughout the first year (Fig. 7c). The inverse relationship of proliferating vs. differentiating cells in the URT epithelium of human infants corresponded with our observations in infant mice. Together, our findings suggest that the delay in serous cell function during early life significantly contributes to the absence of key antimicrobial defenses and may contribute to enhanced susceptibility to infection.
Figure 7. Temporal increase in lysozyme and pIgR expression during the first year of life in children.
a, Transcript levels of lysozyme (LYZ; left), the pIgR (PIGR; middle) and lactoferrin (LTF; right) in nasopharyngeal samples from healthy infants at 7 days (w1) and 12 months (m12) of age. b, Transcript levels of LYZ and PIGR in nasopharyngeal samples from healthy infants at 11 time-points (2 hours after birth (d0), at 24 hours (d1), at 7 (w1) and 14 days (w2) and at 1, 2, 3, 4, 6, 9, and 12 months (m1, m2, m3, m4, m6, m9 and m12)). n = 286 samples. Data represent log2-transformed intensity values generated using microarray. Statistical significance was determined using a linear mixed effects model to correct for repeated measures (lmerTest R-package) and included time point as fixed effect and subject as a random intercept. c, Heatmap of the Z-transformed mean gene expression values at each time-point for serous (LTF, LYZ, LYZL1, LYZL2, PIP and AZGP1), suprabasal (TFCP2L1, SERPINB3, KRT19, FABP5 and S100A9) and basal (KRT5, TP63 and DLK2) specific genes. The log2-transformed gene expression values were averaged per time-point and then Z-scores for these mean expression values were determined.
Discussion
While studies have shown that young age increases susceptibility to viral and bacterial infections, the specific mechanisms that mediate this susceptibility are incompletely understood33. Using an infant mouse model, we discovered that epithelial-mediated production of key mucosal defense components was significantly impaired in the URT during early life. Due to their abundance and known function we focused on lysozyme, lactoferrin, and the pIgR, which is involved in mediating levels of SIgA. Similar to the URT, the infant gastrointestinal tract was devoid of lysozyme and the pIgR, indicating an overall reduction in antimicrobial and IgA-mediated defenses. Further, we showed that in nasopharyngeal samples collected from infants during the first year of life, expression of lysozyme and the pIgR increased with age. Together, these data demonstrate that early life is associated with diminished first-line defenses at mucosal barriers, which may impact colonization of potential pathogens.
In this study, we demonstrated an age-dependent regulation of multiple antimicrobial molecules at the respiratory and gastrointestinal surfaces. While impaired production of antimicrobial molecules in the nasopharynx of infant mice and newborn infants have yet to be shown, absence of Paneth cell-derived antimicrobial peptides in the small intestine of neonatal mice has been observed32,33. However, not all antimicrobial peptides found in the small intestine follow this phenotype. In our study, we found that lactoferrin expression showed an opposite phenotype to lysozyme and the pIgR, and was significantly increased in the small intestine of infant mice. Similar to lactoferrin, a previous study demonstrated that intestinal production of the cathelin-related antimicrobial peptide (CRAMP) was enhanced during the first two weeks of life32. Clearly, these results illustrate that each antimicrobial protein maintains a distinct expression pattern during postnatal development, which is influenced, in part, by cellular source and anatomical site.
In contrast to some antimicrobial molecules in the gastrointestinal tract, expression of lysozyme has been shown to be independent of the microbiota41. In this study, we also found that URT expression of lysozyme, lactoferrin, and the pIgR was unaffected in mice lacking an intact microbiota. However, this does not preclude the possibility that differences in the composition of microbial communities affects antimicrobial production. Conversely, dampened production of these antimicrobials could affect the composition of the commensal flora. In particular, lysozyme and SIgA are known to modulate the gut microbiota and improve intestinal barrier function in animals when provided exogenously through milk42–46. Multiple studies demonstrated a significant reduction in SIgA levels at mucosal surfaces during the first weeks of life, in both mice and humans44,47,48. While impaired B cell development during early life48 certainly contributes to reduced SIgA levels, delayed induction of the pIgR in epithelial cells could also negatively impact the amount of SIgA found at mucosal surfaces. Considering that the commensal flora protects the host by limiting the introduction of opportunistic pathogens, alteration of local microbial composition due to reduced lysozyme and/or IgA-mediated defenses during early life could indirectly enhance susceptibility to infection.
The absence of the most abundant antimicrobial molecules in infant mice suggests that an exogenous source of these factors may compensate for defective production during early life. Many studies have illustrated the protective effects of breastfeeding in reducing the prevalence of acute respiratory and gastrointestinal illness during infancy49. The most abundant proteins found in the whey fraction of human breast milk include lysozyme, lactoferrin, and SIgA40. In this study, we demonstrated that exogenous lysozyme provided by maternal milk facilitated clearance of a lysozyme-susceptible strain of S. pneumoniae in the URT in infant mice. However, cecal colonization of a lysozyme-susceptible strain of S. aureus remained unchanged regardless of whether pups were fed by a wild-type dam or lysozyme-2 deficient dam (data not shown). It is unclear whether our bioassay for an effect of lysozyme in the gastrointestinal tract with S. aureus was sensitive enough to detect a contribution of maternal milk lysozyme as in the URT.
In this study, we showed that alterations in epithelial-derived lysozyme and lactoferrin accounted for reduced production of these antimicrobial molecules at the respiratory surface of infant mice. Results from our RNA-seq screen revealed an overall reduction in serous cell specific genes, including lysozyme and lactoferrin, in the URT of infant mice compared to adults, which indicated that functional development of this secretory cell subset is delayed during early life. In accordance, nasopharyngeal gene expression profiles from human infants exhibited a similar trend with the average expression of serous cell specific markers gradually increasing during the first year of life. Corresponding with transcriptional differences, we also found that staining of serous cell markers, pIgR and lactoferrin, in the submucosal glands of nasal tissue was reduced from infant mice compared to adults. Together, these results suggest an age-dependent transition in serous cell development in the URT during postnatal development. Previous epithelial lineage studies demonstrate that basal cells, the main airway stem cell, differentiate first into secretory cells and then multiciliated cells at homeostasis50. However, during times of injury, lineage segregation of basal cells can occur creating one pool of secretory cells from Notch activation and another pool differentiating directly into ciliated cells due to increased c-myb expression50. Upon further analysis of our RNA-seq screen, we noted a significant reduction in Notch2 expression (p-adjusted value of 0.0027) and a significant increase in c-myb (Myb) expression (p-adjusted value of 0.00049) from infants compared to adult mice in the URT. This corresponded with an increase in deuterosomal markers, a precursor of multiciliated cells. The decreased expression of numerous serous cell markers from infant mice and humans suggests that maturation of this cell type occurs postnatally, similar to Paneth cell development in the intestine. Although comprehensive studies on serous cell development in submucosal glands of the URT are limited, a previous study demonstrated that secretory granules in serous glands of rat lingual tissue increased over the first three weeks of life, suggesting an age-dependent regulation51. Additionally, another study investigating rat parotid gland development identified a progressive increase in secretory granules during the first 12 days of life, which corresponded with acinar cell differentiation52. All together, our data suggests that delayed functional development of secretory cells at the respiratory surface in infant mice contributes to the absence of these key antimicrobial molecules.
While the delay in functional development of specific epithelial cell subsets in the URT during early life warrants further investigation, in this study we demonstrate that young age is associated with an absence of mucosal defense mechanisms. More specifically, epithelial cells, rather than myeloid cells, accounted for the reduced production of antimicrobial factors. Strikingly, similar to infant mice, we observed an age-dependent regulation of key antimicrobial molecules in the URT of human infants. Ultimately, additional studies focused on understanding age-related differences in mucosal defense mechanisms will facilitate development of novel therapeutic strategies to protect infants from prevalent respiratory and gastrointestinal pathogens.
Methods
Mouse strains
Male and female C57BL/6J (cat. 000664) and C57BL/6J Lyz2tm1(cre)lfo (cat. 004781) mice were purchased from Jackson Laboratory (Bar Harbor, Maine) and each colony was bred and maintained in a conventional animal facility. Pups were housed with the dam until 3-weeks of age. Following infection all mice appeared healthy and demonstrated normal weight gain similar to uninfected controls. The Institutional Animal Care and Use Committee of New York University Medical Center approved all animal experiments.
Bacterial strains
Streptococcus pneumoniae (S. pneumoniae) isolate serotype 23F (neomycinR) and a previously described isogenic S. pneumoniae pgdA mutant (kanamycinR and neomycinR) were used throughout the study21. For mouse infections, all pneumococcal strains were grown in tryptic soy (TS) broth (BD) at 37°C without aeration to an optical density of 1.0 at 620 nm. For in vivo bacterial colonization, pneumococci were incubated on TS plates supplemented with 100μl of catalase (30,000 U/ml; Worthington Biomedical) and either 5μg/ml neomycin (FisherScientific) or 125μg/ml kanamycin (Sigma) at 37°C in 5% CO2 overnight. Staphylococcus aureus (S. aureus) strain USA300-JE2 and S. aureus oatA::bursa USA300-JE2 (erythromycinR) were used for infant mouse oral infections. All S. aureus strains were grown overnight, with shaking, at 37°C in 5ml of TS broth, diluted 100-fold next day and subcultured for 4 hours in 5ml of TS broth. For in vivo bacterial colonization S. aureus strains were plated on CHROMID MRSA SMART II agar (bioMérieux), TS or TS+5μg/ml erythromycin and incubated overnight at 37°C.
Statistical analysis
All statistical analyses, excluding the human study, were performed using GraphPad Prism 9.0 (GraphPad Software Inc., San Diego, CA) and the statistical test is noted in figure legends.
Other methods
Additional methods are included in supplementary section.
Supplementary Material
Acknowledgements:
This work was supported by grants R01 AI150893 and R01 AI038446. KLLT was supported by NIH grants F32 AI143043 and T32 A1007180-35. R01 AI137336-01A1 and 1R01 AI140754-01A1 supported BS. We would like to thank the Applied Bioinformatics Center, Genome Technology Center and Experimental Pathology Research Laboratory at NYU. The core centers are partially supported by the Cancer Center Support Grant P30CA016087.
Footnotes
Disclosure: The authors have no conflicts of interest to disclose.
References
- 1.Liu L. et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet 388, 3027–3035, doi: 10.1016/S0140-6736(16)31593-8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker CLF et al. Global burden of childhood pneumonia and diarrhoea. Lancet 381, 1405–1416, doi: 10.1016/S0140-6736(13)60222-6 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raphael GD, Baraniuk JN & Kaliner MA How and why the nose runs. J Allergy Clin Immunol 87, 457–467, doi: 10.1016/0091-6749(91)90001-5 (1991). [DOI] [PubMed] [Google Scholar]
- 4.Ganz T. Antimicrobial polypeptides in host defense of the respiratory tract. J Clin Invest 109, 693–697, doi: 10.1172/JCI15218 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cole AM et al. Cationic polypeptides are required for antibacterial activity of human airway fluid. J Immunol 169, 6985–6991, doi: 10.4049/jimmunol.169.12.6985 (2002). [DOI] [PubMed] [Google Scholar]
- 6.Akinbi HT, Epaud R, Bhatt H. & Weaver TE Bacterial killing is enhanced by expression of lysozyme in the lungs of transgenic mice. J Immunol 165, 5760–5766, doi: 10.4049/jimmunol.165.10.5760 (2000). [DOI] [PubMed] [Google Scholar]
- 7.Cross M, Mangelsdorf I, Wedel A. & Renkawitz R. Mouse lysozyme M gene: isolation, characterization, and expression studies. Proc Natl Acad Sci U S A 85, 6232–6236, doi: 10.1073/pnas.85.17.6232 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Travis SM, Singh PK & Welsh MJ Antimicrobial peptides and proteins in the innate defense of the airway surface. Curr Opin Immunol 13, 89–95, doi: 10.1016/s0952-7915(00)00187-4 (2001). [DOI] [PubMed] [Google Scholar]
- 9.Hammer MF & Wilson AC Regulatory and structural genes for lysozymes of mice. Genetics 115, 521–533 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh PK, Parsek MR, Greenberg EP & Welsh MJ A component of innate immunity prevents bacterial biofilm development. Nature 417, 552–555, doi: 10.1038/417552a (2002). [DOI] [PubMed] [Google Scholar]
- 11.Arnold RR, Cole MF & McGhee JR A bactericidal effect for human lactoferrin. Science 197, 263–265, doi: 10.1126/science.327545 (1977). [DOI] [PubMed] [Google Scholar]
- 12.Arnold RR, Russell JE, Champion WJ, Brewer M. & Gauthier JJ Bactericidal activity of human lactoferrin: differentiation from the stasis of iron deprivation. Infect Immun 35, 792–799, doi: 10.1128/IAI.35.3.792-799.1982 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valenti P. & Antonini G. Lactoferrin: an important host defence against microbial and viral attack. Cell Mol Life Sci 62, 2576–2587, doi: 10.1007/s00018-005-5372-0 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellison RT 3rd & Giehl TJ Killing of gram-negative bacteria by lactoferrin and lysozyme. J Clin Invest 88, 1080–1091, doi: 10.1172/JCI115407 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raphael GD et al. Pathophysiology of rhinitis. Lactoferrin and lysozyme in nasal secretions. J Clin Invest 84, 1528–1535, doi: 10.1172/JCI114329 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leitch EC & Willcox MD Synergic antistaphylococcal properties of lactoferrin and lysozyme. J Med Microbiol 47, 837–842, doi: 10.1099/00222615-47-9-837 (1998). [DOI] [PubMed] [Google Scholar]
- 17.Brandtzaeg P. Secretory IgA: Designed for Anti-Microbial Defense. Front Immunol 4, 222, doi: 10.3389/fimmu.2013.00222 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boullier S. et al. Secretory IgA-mediated neutralization of Shigella flexneri prevents intestinal tissue destruction by down-regulating inflammatory circuits. J Immunol 183, 5879–5885, doi: 10.4049/jimmunol.0901838 (2009). [DOI] [PubMed] [Google Scholar]
- 19.Phalipon A. et al. Secretory component: a new role in secretory IgA-mediated immune exclusion in vivo. Immunity 17, 107–115, doi: 10.1016/s1074-7613(02)00341-2 (2002). [DOI] [PubMed] [Google Scholar]
- 20.Cole AM, Dewan P. & Ganz T. Innate antimicrobial activity of nasal secretions. Infect Immun 67, 3267–3275, doi: 10.1128/IAI.67.7.3267-3275.1999 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis KM, Akinbi HT, Standish AJ & Weiser JN Resistance to mucosal lysozyme compensates for the fitness deficit of peptidoglycan modifications by Streptococcus pneumoniae. PLoS Pathog 4, e1000241, doi: 10.1371/journal.ppat.1000241 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masschalck B. & Michiels CW Antimicrobial properties of lysozyme in relation to foodborne vegetative bacteria. Crit Rev Microbiol 29, 191–214, doi: 10.1080/713610448 (2003). [DOI] [PubMed] [Google Scholar]
- 23.Copin R. et al. Sequential evolution of virulence and resistance during clonal spread of community-acquired methicillin-resistant Staphylococcus aureus. Proc Natl Acad Sci U S A 116, 1745–1754, doi: 10.1073/pnas.1814265116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindberg E. et al. High rate of transfer of Staphylococcus aureus from parental skin to infant gut flora. J Clin Microbiol 42, 530–534, doi: 10.1128/jcm.42.2.530-534.2004 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bera A, Herbert S, Jakob A, Vollmer W. & Gotz F. Why are pathogenic staphylococci so lysozyme resistant? The peptidoglycan O-acetyltransferase OatA is the major determinant for lysozyme resistance of Staphylococcus aureus. Mol Microbiol 55, 778–787, doi: 10.1111/j.1365-2958.2004.04446.x (2005). [DOI] [PubMed] [Google Scholar]
- 26.Hasegawa M. et al. Transitions in oral and intestinal microflora composition and innate immune receptor-dependent stimulation during mouse development. Infect Immun 78, 639–650, doi: 10.1128/IAI.01043-09 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim YG et al. Neonatal acquisition of Clostridia species protects against colonization by bacterial pathogens. Science 356, 315–319, doi: 10.1126/science.aag2029 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matamoros S, Gras-Leguen C, Le Vacon F, Potel G. & de La Cochetiere MF Development of intestinal microbiota in infants and its impact on health. Trends Microbiol 21, 167–173, doi: 10.1016/j.tim.2012.12.001 (2013). [DOI] [PubMed] [Google Scholar]
- 29.Masuda N. et al. Immunohistochemical study on the secretory host defense system with lysozyme and secretory phospholipase A2 throughout rat respiratory tract. J Vet Med Sci 80, 323–332, doi: 10.1292/jvms.17-0503 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tachibana M. et al. Lysozyme producers in nasal mucosa. An immunocytochemical study. Ann Otol Rhinol Laryngol 95, 193–195, doi: 10.1177/000348948609500218 (1986). [DOI] [PubMed] [Google Scholar]
- 31.Bry L. et al. Paneth cell differentiation in the developing intestine of normal and transgenic mice. Proc Natl Acad Sci U S A 91, 10335–10339, doi: 10.1073/pnas.91.22.10335 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menard S. et al. Developmental switch of intestinal antimicrobial peptide expression. J Exp Med 205, 183–193, doi: 10.1084/jem.20071022 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torow N, Marsland BJ, Hornef MW & Gollwitzer ES Neonatal mucosal immunology. Mucosal Immunol 10, 5–17, doi: 10.1038/mi.2016.81 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Ogawa H, Kami K, Suzuki T. & Mitsui T. An immunohistochemical study of lysozyme in the human nasal mucosa. Keio J Med 28, 73–79, doi: 10.2302/kjm.28.73 (1979). [DOI] [PubMed] [Google Scholar]
- 35.Tachibana M, Morioka H, Tsuruoka T, Machino M. & Mizukoshi O. Localization of lysozyme in the frozen section of nasal mucosa by protein A-gold technique. Auris Nasus Larynx 12, 23–26, doi: 10.1016/s0385-8146(85)80075-4 (1985). [DOI] [PubMed] [Google Scholar]
- 36.Bowes D, Clark AE & Corrin B. Ultrastructural localisation of lactoferrin and glycoprotein in human bronchial glands. Thorax 36, 108–115, doi: 10.1136/thx.36.2.108 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deprez M. et al. A Single-Cell Atlas of the Human Healthy Airways. Am J Respir Crit Care Med 202, 1636–1645, doi: 10.1164/rccm.201911-2199OC (2020). [DOI] [PubMed] [Google Scholar]
- 38.Hegab AE et al. Novel stem/progenitor cell population from murine tracheal submucosal gland ducts with multipotent regenerative potential. Stem Cells 29, 1283–1293, doi: 10.1002/stem.680 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.May A. & Tucker A. Understanding the development of the respiratory glands. Dev Dyn 244, 525–539, doi: 10.1002/dvdy.24250 (2015). [DOI] [PubMed] [Google Scholar]
- 40.Ballard O. & Morrow AL Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am 60, 49–74, doi: 10.1016/j.pcl.2012.10.002 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hooper LV et al. Molecular analysis of commensal host-microbial relationships in the intestine. Science 291, 881–884, doi: 10.1126/science.291.5505.881 (2001). [DOI] [PubMed] [Google Scholar]
- 42.Maga EA et al. Consumption of lysozyme-rich milk can alter microbial fecal populations. Appl Environ Microbiol 78, 6153–6160, doi: 10.1128/AEM.00956-12 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mirpuri J. et al. Proteobacteria-specific IgA regulates maturation of the intestinal microbiota. Gut Microbes 5, 28–39, doi: 10.4161/gmic.26489 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rogier EW et al. Secretory antibodies in breast milk promote long-term intestinal homeostasis by regulating the gut microbiota and host gene expression. Proc Natl Acad Sci U S A 111, 3074–3079, doi: 10.1073/pnas.1315792111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maga EA, Walker RL, Anderson GB & Murray JD Consumption of milk from transgenic goats expressing human lysozyme in the mammary gland results in the modulation of intestinal microflora. Transgenic Res 15, 515–519, doi: 10.1007/s11248-006-0014-3 (2006). [DOI] [PubMed] [Google Scholar]
- 46.Brundige DR, Maga EA, Klasing KC & Murray JD Lysozyme transgenic goats’ milk influences gastrointestinal morphology in young pigs. J Nutr 138, 921–926, doi: 10.1093/jn/138.5.921 (2008). [DOI] [PubMed] [Google Scholar]
- 47.Gopalakrishna KP et al. Maternal IgA protects against the development of necrotizing enterocolitis in preterm infants. Nat Med 25, 1110–1115, doi: 10.1038/s41591-019-0480-9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rognum TO, Thrane S, Stoltenberg L, Vege A. & Brandtzaeg P. Development of intestinal mucosal immunity in fetal life and the first postnatal months. Pediatr Res 32, 145–149, doi: 10.1203/00006450-199208000-00003 (1992). [DOI] [PubMed] [Google Scholar]
- 49.Clavano NR Mode of feeding and its effect on infant mortality and morbidity. J Trop Pediatr 28, 287–293, doi: 10.1093/tropej/28.6.287 (1982). [DOI] [PubMed] [Google Scholar]
- 50.Zaragosi LE, Deprez M. & Barbry P. Using single-cell RNA sequencing to unravel cell lineage relationships in the respiratory tract. Biochem Soc Trans 48, 327–336, doi: 10.1042/BST20191010 (2020). [DOI] [PubMed] [Google Scholar]
- 51.Hamosh M. & Hand AR Development of secretory activity in serous cells of the rat tongue. Cytological differentiation and accumulation of lingual lipase. Dev Biol 65, 100–113, doi: 10.1016/0012-1606(78)90183-5 (1978). [DOI] [PubMed] [Google Scholar]
- 52.Redman RS & Sreebny LM Morphologic and biochemical observations on the development of the rat parotid gland. Dev Biol 25, 248–279, doi: 10.1016/0012-1606(71)90030-3 (1971). [DOI] [PubMed] [Google Scholar]
- 53.Dobin A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21, doi: 10.1093/bioinformatics/bts635 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liao Y, Smyth GK & Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930, doi: 10.1093/bioinformatics/btt656 (2014). [DOI] [PubMed] [Google Scholar]
- 55.Love MI, Huber W. & Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 550, doi: 10.1186/s13059-014-0550-8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mi H. et al. PANTHER version 16: a revised family classification, tree-based classification tool, enhancer regions and extensive API. Nucleic Acids Res 49, D394–D403, doi: 10.1093/nar/gkaa1106 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bosch A. et al. Maturation of the Infant Respiratory Microbiota, Environmental Drivers, and Health Consequences. A Prospective Cohort Study. Am J Respir Crit Care Med 196, 1582–1590, doi: 10.1164/rccm.201703-0554OC (2017). [DOI] [PubMed] [Google Scholar]
- 58.Bosch A. et al. Development of Upper Respiratory Tract Microbiota in Infancy is Affected by Mode of Delivery. EBioMedicine 9, 336–345, doi: 10.1016/j.ebiom.2016.05.031 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kauffmann A. & Huber W. Microarray data quality control improves the detection of differentially expressed genes. Genomics 95, 138–142, doi: 10.1016/j.ygeno.2010.01.003 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.