Abstract
Purpose
To describe longitudinal multimodal imaging findings of non-exudative choroidal neovascularization (CNV) in CTRP5 late-onset retinal degeneration (CTRP5-LORD).
Methods
Four patients with CTRP5-positive LORD underwent repeated ophthalmoscopic examination and multimodal imaging. All four patients (two siblings and their cousins, from a pedigree described previously) had the heterozygous S163R mutation.
Results
All four patients demonstrated large subretinal lesions in the mid-peripheral retina of both eyes. The lesions were characterized by confluent hypercyanescence with hypocyanescent borders on indocyanine green angiography, faintly visible branching vascular networks with absent/minimal leakage on fluorescein angiography, type 1 neovascularization on OCT angiography, and absent retinal fluid, consistent with non-exudative CNV. The neovascular membranes enlarged substantially over time and the birth of new membranes was observed, but all lesions remained non-/minimally exudative. Without treatment, all involved retinal areas remained free of atrophy and subretinal fibrosis.
Conclusion
We report the existence of massive advancing non-exudative type 1 CNV in CTRP5-LORD. These findings have implications for age-related macular degeneration (AMD). They provide a monogenic model system for studying the mechanisms underlying the distinct events of CNV development, enlargement, progression to exudation, and atrophy in AMD. They suggest that choroidal hypoperfusion precedes neovascularization and that non-exudative neovascularization may protect against atrophy.
Summary:
Massive advancing non-exudative type 1 choroidal neovascularization in patients with CTRP5-LORD is characterized using multimodal imaging. The neovascular membranes begin as small foci in the mid-peripheral retina. Despite enlarging progressively to enormous sizes, exudation is minimal. These findings in CTRP5-LORD, a monogenic condition, have implications for age-related macular degeneration.
Introduction
CTRP5-late onset retinal degeneration (CTRP5-LORD) is a rare monogenic disorder that has important parallels with age-related macular degeneration (AMD).1–8 CTRP5-LORD is an autosomal dominant retinal disease with complete penetrance.1–8 It results from a single base pair substitution in the gene encoding C1q and tumor necrosis factor-related protein 5 (C1QTNF5), also known as CTRP5.5,6 The onset of visual symptoms occurs typically in the fifth decade of life with nyctalopia and dark adaptation difficulties.1–4,6,8 Clinical findings include elongated anterior zonules, peripapillary iris atrophy, and widespread yellow-white drusenoid deposits (particularly in the macula and mid-peripheral retina).1–6,8 These deposits are similar to the subretinal drusenoid deposits (SDD) found frequently in AMD and other macular diseases9,10; they are accompanied in both CTRP5-LORD and AMD by a thin choroid.9,11 Functional testing at this stage in CTRP5-LORD reveals prolonged dark adaptation despite relatively normal visual acuity1–8, similar to findings in non-advanced AMD with SDD.12
Patients with CTRP5-LORD usually progress to bilateral central visual loss within 20 years of presentation.8 Characteristic signs include scalloped chorioretinal atrophy in the temporal retina, which spreads towards the central macula.8 In common with some other monogenic retinal diseases, such as Sorsby fundus dystrophy and dominant drusen, CTRP5-LORD frequently leads to neovascularization (CNV).8 Central visual loss may occur through either atrophy or CNV affecting the central macula.8 Hence, as a monogenic condition, CTRP5-LORD recapitulates many important clinical features of AMD (specifically the AMD phenotype with SDD12), including prolonged dark adaptation, prominent SDD, abnormalities in the Bruch’s membrane/retinal pigment epithelial (RPE) complex, and slow progression to central RPE atrophy and/or CNV.1–9
In AMD, the advent of optical coherence tomography angiography (OCT-A) has led to renewed appreciation of non-exudative CNV.13 Its existence in AMD was known from early histological reports and clinical reports based on indocyanine green angiography (ICG-A), where hypercyanescent foci or plaques with late staining were observed.14,15 The prevalence and natural history of non-exudative CNV in AMD have recently been examined in a systematic review.16 These lesions are characterized by neovascularization (typically type 1, i.e., sub-RPE location) in the absence of intraretinal, subretinal, or sub-RPE fluid on OCT or leakage on fluorescein angiography (FA). In AMD, in the absence of progression to exudation, their long-term effects on overlying RPE and retinal tissue are suspected to be relatively benign.16 Indeed, some authors hypothesize they may even protect against RPE atrophy by enhancing transport between the RPE and choroid and compensating for areas of choriocapillaris degeneration.16–18 A recent histological study of a non-exudative type 1 lesion observed that the CNV appeared structurally similar to native choriocapillaris and the overlying outer retina had preserved structure.19
In this study, we describe the novel finding of massive advancing non-exudative type 1 CNV in four out of four individuals with genetically confirmed CTRP5-LORD, who underwent multimodal imaging including OCT-A and ICG-A. The CNV membranes had a characteristic location in the mid-peripheral retina but demonstrated progressive centripetal enlargement. These observations have implications for our understanding of neovascular disease in AMD.
Methods
The four patients in this observational case series were enrolled in a protocol at the National Eye Institute, National Institutes of Health, Bethesda, MD. The protocol was approved by the National Institutes of Health Institutional Review Board and adheres to the tenets of the Declaration of Helsinki.
The patients underwent ophthalmoscopic examination including measurement of best-corrected visual acuity (BCVA), slit-lamp examination, and dilated fundus examination. Color fundus photographs (CFP) and ICG-A were obtained with the TRC-50DX camera (Topcon Medical Systems, Oakland, NJ). Fundus autofluorescence (FAF), near-infrared reflectance (NIR), spectral domain OCT (SD-OCT), and ICG-A were obtained with the Spectralis (Heidelberg Engineering, Heidelberg, Germany). Ultrawide-field false-color and FA images were obtained with the Optos California (Optos, Dunfermline, UK). OCT-A images were obtained with the Cirrus AngioPlex (Zeiss, Oberkochen, Germany). Patients repeated the ophthalmoscopic examination and multimodal imaging approximately yearly.
In order to measure CNV lesion area and assess enlargement, the Spectralis ICG-A late-phase images were assessed for CNV (EV and CC). The images from sequential time-points were spatially registered and overlaid using custom automatic algorithms.20 The lesion borders were recorded by outlining the hypercyanescent areas (excluding the hypocyanescent borders). The lesion areas were manually contoured using 3D Slicer software (v 4.10.2) and quantified on custom software implementations (using SimpleITK (v 1.2.2) and NumPy (v 1.16.5)) by calculating the number of pixels within the contoured region. The distance from fovea to nearest leading edge of the lesions was quantified (EV). The measurements were converted from pixels to millimeters (0.03642 mm/pixel conversion factor).
Results
The four patients in this report form part of a pedigree that has been described previously (V-33, V-34, V-41, and V-42)2. Patients 1 (V-33) and 2 (V-34) are female and male siblings, respectively, while patients 3 and 4 (V-41, and V-42) are female cousins of patients 1 and 2. All four have the same heterozygous S163R point mutation in C1QTNF5.5 All four individuals have classic features of CTRP5-LORD, including elongated anterior lens zonules, delayed dark adaptation, and SDD.
Asymptomatic Retinal Hemorrhages and Evidence of Type 1 Choroidal Neovascularization
Patient 1 (V-33) underwent regular retinal examination from age 50 years (baseline) to 65 years. BCVA was 20/20 in each eye throughout this period. The results of multimodal imaging up until 59 years have been described previously.9 However, at age 57 years, new asymptomatic retinal hemorrhages were present in the right eye outside the vascular arcades, at the inferonasal border of a large, dimly visible, subretinal lesion in the superotemporal mid-peripheral retina (Figure 1A). On FA, this lesion consisted of a faintly visible, branching vascular network suggestive of an occult CNV membrane, with absent or minimal leakage (Figure 1B–D). SD-OCT demonstrated the double-layer sign (i.e., a shallow, irregular pigment epithelial detachment consistent with type 1 CNV, more pronounced and irregular than the Bruch’s membrane-RPE separation already seen in CTRP5-LORD), a subretinal hemorrhage at the border between involved and uninvolved areas, but the absence of intraretinal or subretinal fluid (Figure 1E–F).
Figure 1.
Multimodal imaging of patient 1 (OD) at age 57 years, showing asymptomatic retinal hemorrhages outside the vascular arcades, at the inferonasal border of a large, dimly visible, subretinal lesion in the superotemporal mid-peripheral retina. Multimodal imaging of this lesion was consistent with a large non-exudative type 1 choroidal neovascular membrane. A. Color fundus photography; B-D. fluorescein angiography (early, mid, and late phases), showing a faintly visible, branching vascular network with minimal leakage; E-F. near-infrared reflectance and spectral domain optical coherence tomography, showing the double-layer sign, a subretinal hemorrhage at the border, and the absence of intraretinal or subretinal fluid.
A similar lesion was subsequently observed in the left eye (Figure 2). At age 61 years, new asymptomatic retinal hemorrhages were observed in the left eye (Figure 2A); these were inferior to the vascular arcades, at the posterior border of a large, dimly visible lesion. On FA, again, a faintly visible, branching vascular network was present, with absent leakage apart from at one area near the hemorrhages (Figure 2B–C). On SD-OCT, the double-layer sign was present, with a subretinal hemorrhage at the border, but no intraretinal fluid (Figure 2F–G); a trace of subretinal fluid was present, but this was absent six months and one year later.
Figure 2.
Multimodal imaging of patient 1 (OS) at age 61 years, showing asymptomatic retinal hemorrhages outside the vascular arcades, at the posterior border of a large, dimly visible, subretinal lesion in the mid-peripheral retina. Multimodal imaging of this lesion was consistent with a large non-exudative type 1 choroidal neovascular membrane. A. Color fundus photography; B-C. fluorescein angiography (early and late phases), showing a faintly visible, branching vascular network with minimal leakage; D-E indocyanine green angiography (early and late phases), showing a large hypercyanescent plaque with irregular but well-demarcated edges and a hypocyanescent border; F-G. near-infrared reflectance and spectral domain optical coherence tomography, showing the double-layer sign, a subretinal hemorrhage at the border, and the absence of intraretinal or subretinal fluid.
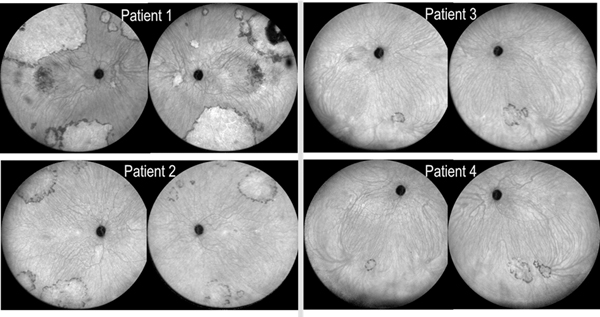
These large vascular lesions were visualized most clearly on ICG-A (Figures 2D–E and 3) as massive hypercyanescent plaques with irregular but well-demarcated edges, consistent with type 1 CNV membranes. Several small hypercyanescent foci were observed on ICG-A that were not readily apparent on FA. Multiple similar lesions were observed in both eyes, and a high degree of symmetry was present between eyes, with the superotemporal and inferior retina as the sites of preference (Figure 3). In all cases, a pronounced hypocyanescent border surrounded most of the lesions, consistent with choroidal hypoperfusion at the CNV edges; in support of this, flow voids on OCT angiography were present at similar locations (Figure 4).
Figure 3.
Wide-field indocyanine green angiography (late phase) in patients 1–4 (both eyes), showing multiple hypercyanescent plaques (of varying sizes, from small to massive) with irregular but well-demarcated edges and hypocyanescent borders, consistent with type 1 choroidal neovascularization. The lesions appeared most commonly in the superotemporal and inferior mid-peripheral retina, with a high degree of symmetry present between both eyes of each patient.
Figure 4.
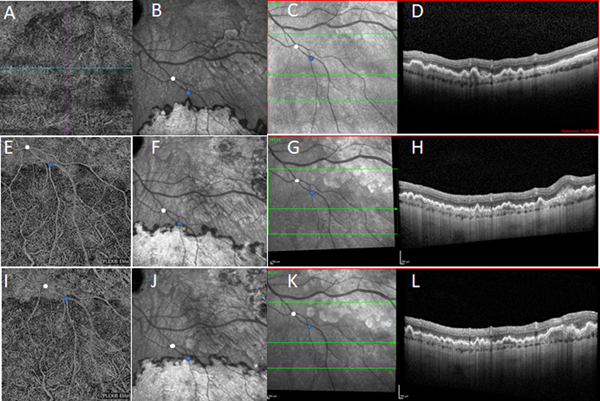
Enlargement over time in a choroidal neovascular lesion in patient 1 (OS). Tracked near-infrared reflectance and spectral domain optical coherence tomography (OCT) (C, D, G, H, K, L), together with OCT angiography (swept-source in 2018 and 2019) (A, E, I) and indocyanine green (ICG) angiography (late phase) (B, F, J), at ages 61 (A-D), 64 (E-H), and 65 (I-L), showing enlargement over time in the furthest extent of the double-layer sign on OCT and of the choroidal neovascularization on OCT angiography and ICG angiography.
No further retinal hemorrhage or OCT sign of exudation was present in either eye on regular imaging studies over the following years, including the most recent imaging (65 years), nor any progression to subretinal fibrosis or RPE atrophy in the affected areas. No treatment with anti-VEGF injection or photocoagulation was administered at any time.
Evidence of Massive Non-Exudative Type 1 Choroidal Neovascularization in Additional Patients
Patient 2 (V-34) underwent regular retinal examination from age 45 years to 59 years. BCVA was 20/20 in each eye throughout this period. Multimodal imaging findings up until 53 years have been described previously.9 Wide-field ICG-A was performed at 55 years, following the diagnosis of asymptomatic CNV in his sibling (patient 1). This demonstrated strikingly similar findings to those observed in patient 1: massive hypercyanescent plaques were present in the mid-peripheral retina of both eyes, with a high degree of symmetry between eyes (Figures 3 and 5). Again, the preferred locations were the superotemporal and inferior retina, and prominent borders of hypocyanescence encircled most lesions. Over 14 years of follow-up, no clinical or OCT signs of exudation were present at any point, nor progression to fibrosis or RPE atrophy in the affected areas.
Figure 5.
Multimodal imaging of patient 2 (OD) at age 55 years, consistent with non-exudative choroidal neovascular membrane. A-C. Fluorescein angiography (early, mid, and late phases); D. color fundus photography; E-G. indocyanine green angiography (early, mid, and late phases), showing a large hypercyanescent plaque with irregular but well-demarcated edges and a hypocyanescent border
Patient 3 (V-41) underwent retinal examination at 49 years and 60 years. BCVA at 49 years was OD 20/32 and OS 20/20; by 60 years, BCVA was OD 20/500 (from macular atrophy without CNV) and OS 20/32. Wide-field ICG-A performed at 60 years showed similar but smaller lesions to those observed in her cousins, consistent with multiple foci of occult CNV (Figure 3). Again, the lesions were present at the superotemporal, superior, and inferior retina, near the insertion of the vortex veins. No history or signs of any exudative process or fibrosis were present in either eye.
Patient 4 (V-42) underwent retinal examination at age 58 years and 60 years. BCVA was 20/20 OU at both time points. Wide-field ICG-A performed at 60 years again showed similar lesions in both character and location (Figure 3). As before, no evidence of an exudative process was observed. For comparison between patients, wide-field ICG-A is shown for all four patients (Figure 3).
Progressive Centripetal Enlargement of Choroidal Neovascularization
Comparison of wide-field ICG-A of patient 1 over at a 3-year interval (Figure 6; ages 57 and 62 years) demonstrated a substantial increase in the size of all lesions in both eyes: the posterior borders showed progressive centripetal movement towards the vascular arcades, with the hypocyanescent border acting as a leading edge. At the later time-point, three foci of isolated hypocyanescence were present, suggesting that the CNV membranes might arise from nidi of choroidal hypoperfusion. Progressive enlargement over this period was also observed on tracked SD-OCT imaging (Figure 4), where the furthest extents of the double-layer sign were seen to move.
Figure 6.
Enlargement and posterior extension over time in the choroidal neovascular lesions in both eyes of patient 1. Ultrawide-field indocyanine green angiography (late phase) at ages 61 (A) and 64 (B).
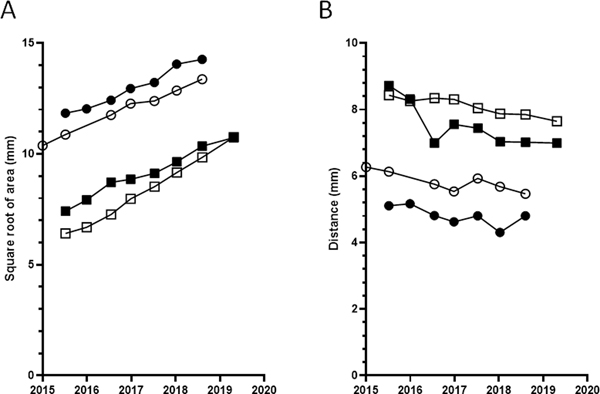
For the four eyes with frequent longitudinal data (patients 1 and 2), the change in total CNV area over approximately four years is shown in Figure 7, together with the change in distance from the fovea. The square root of CNV area appeared to increase monotonically and at a relatively similar rate in all eyes; the mean rate of change was 0.92 mm/year. Similarly, the distance from the fovea appeared to decrease monotonically and at a relatively similar rate in all eyes; the mean rate of change was 0.23 mm/year.
Figure 7.
Plots of change in square root of choroidal neovascular membrane area over time (A) and change in distance from fovea (B), for the four eyes with frequent longitudinal data, based on wide-field (102°) indocyanine green angiography. The square root area appeared to increase monotonically and at a relatively similar rate in all eyes, with a mean rate of 0.92 mm/year. The distance from the fovea appeared to decrease monotonically and at a relatively similar rate in all eyes, with a mean rate of 0.23 mm/year. The circles indicate participant 1 and the squares indicate participant 2 (OD filled and OS unfilled).
Discussion
Massive type 1 non-exudative CNV appears common in CTRP5-LORD. In our observational case series of four patients, this phenomenon was present in both eyes of all four patients. We are not aware of previous reports of similar findings in CTRP5-LORD. If this phenomenon does represent a common or even universal feature of CTRP5-LORD in its more advanced stages, it may previously have been overlooked owing to the generally asymptomatic nature of the lesions, their peripheral location prior to enlargement, their apparently benign natural history, and the need for ultrawide-field ICG-A for clear visualization. The lesions are characterized by confluent hypercyanescence with a hypocyanescent border on ICG-A, faintly visible branching vascular network with absent or minimal leakage on FA, type 1 CNV on OCT-A, and double-layer sign with absent and retinal fluid on OCT. Other characteristic features appear to include their birth as small foci in the mid-peripheral retina (with predilection for the superior and inferior quadrants) and progressive circumferential enlargement and coalescence over years to massive sizes (including posterior extension towards the arcades), with a striking hypocyanescent border at the leading edge.
The hypocyanescent border, with a corresponding flow void on OCT-A, suggests that choroidal hypoperfusion precedes progression to neovascularization. This may have parallels with observations of choriocapillaris flow voids in eyes with neovascular AMD.21–23 In neovascular AMD, the presence of a hypoperfused ring concentric to neovascular lesions has often been reported in OCT-A studies.21–23 Possible explanations have included choriocapillaris hypoperfusion or dropout, mechanical compression of the choriocapillaris by exudative changes, a vascular ‘steal’ phenomenon, or artefact.23 However, in our patients with CTRP5-LORD, we observed new lesions appearing first as tiny foci of isolated hypocyanescence, prior to enlargement. This argues against artefact as an explanation and supports genuine choriocapillaris hypoperfusion or dropout as a temporal and/or causal prelude to neovascularization.
Importantly, aside from very rare subretinal hemorrhage or fluid near the borders, the lesions in this cohort demonstrate a striking absence of exudation or tendency towards subretinal fibrosis or atrophy of the RPE or retina, despite years of follow-up without treatment. In that sense, the natural history in this cohort seems benign; the preserved RPE and retinal structure over years might even argue for a protective role. Indeed, as described above for AMD, some authors hypothesize that non-exudative CNV might protect against RPE atrophy.16–19
In a previous report of a relative from the same pedigree (IV-11), the natural history of CNV affecting the macula was exudative and not benign.2 At 58 years, this patient (IV-11) developed exudative CNV in the temporal macula of the left eye that responded poorly to laser photocoagulation, the only therapy available at the time. The right eye soon followed a similar course, even though laser was deferred, leading to foveal involvement and a permanent decrease in acuity to counting fingers. A similar course occurred in two sisters of this individual (IV-8 and IV-13).2 In those cases, the tissue response to laser photocoagulation was atypical, in that the choriocapillaris and RPE appeared to be ablated.2 This suggests a structural tissue abnormality in CTRP5-LORD that makes the Bruch’s membrane/RPE/choriocapillaris complex more susceptible to insult. Overall, it seems likely that the CNV tendency towards exudation, together with its natural history and need for treatment, may differ strikingly according to CNV location in the macular versus extramacular retina. However, more data is needed to understand the factors contributing to the manifestations of the CNV lesions.
The predilection for origin in the superior and inferior quadrants, together with the high degree of symmetry between eyes and patients, suggests some anatomical or other biological determination rather than a purely stochastic process. This might relate to the anatomy of choroidal watershed zones, though other explanations are possible. A choroidal watershed zone is the border between the territories distributed by different posterior ciliary arteries, and these areas are considered vulnerable to ischemia.24 Indeed, topographical relationships between choroidal watershed zones and CNV have been reported in AMD, polypoidal choroidal vasculopathy, and idiopathic CNV.25 The most common anatomical arrangement in humans is two posterior ciliary arteries, one medial and one lateral24; in this scenario, the watershed zone extends superiorly and inferiorly, which would fit with our observations in CTRP5-LORD.
Implications for age-related macular degeneration
CTRP5-LORD, as a monogenic condition, has important parallels with AMD, a multifactorial condition with more complex genetic contributions. During this period of renewed appreciation of the existence of non-exudative CNV in AMD, we have much to learn about its natural history. These findings in CTRP5-LORD emphasize that non-exudative CNV can have a highly benign natural history and might even play a protective role against atrophy. The mechanisms underlying the birth and propagation of neovascularization in CTRP5-LORD are likely to share important factors with those in AMD. However, the predilection for the mid-periphery and the substantial enlargement to massive sizes over years, without exudation, subretinal fibrosis, or atrophy, differ markedly from typical neovascular AMD. Indeed, the CNV enlargement rate measured in our study was substantially larger than that encountered in neovascular AMD. Interestingly, it was relatively similar to the enlargement rate for atrophy in CTRP5-LORD26; this might provide insights into the rate of RPE degeneration itself, which appears in CTRP5-LORD to instigate CNV in some areas but atrophy in others areas. Any insights into the mechanisms through which the lesions remain non-exudative could present useful therapeutic targets for AMD. Of note, CTRP5-LORD, as a monogenic disease, is presumably characterized by one relatively uniform and widespread abnormality throughout the RPE monolayer, whereas the RPE abnormalities in AMD may be more heterogeneous in both nature and location throughout the retina, with more heterogeneous changes in both the choriocapillaris and Bruch’s membrane.
CTRP5-LORD recapitulates many important clinical features of AMD. If the phenomenon of non-exudative CNV is indeed widespread in advanced CTRP5-LORD, then the retinal findings of CTRP5-LORD comprise: prominent SDD, thin choroid, and prolonged dark adaptation, leading over time to geographic atrophy (that enlarges more rapidly than that in AMD26), and the birth, enlargement, and confluence of non-exudative type 1 CNV to massive sizes. Interestingly, this constellation of findings may have similarities with a particular AMD phenotype: that driven principally by ARMS2/HTRA1. First, in AMD, SDD prevalence is higher in patients with ARMS2/HTRA1 risk variants10. Second, SDD are typically accompanied by a thin choroid and prolonged dark adaptation11,12, and ARMS2/HTRA1 risk variants have also been linked directly to thin choroid27 and prolonged dark adaptation.28 Third, in AMD, geographic atrophy enlargement is faster in patients with ARMS2/HTRA1 risk variants.29 Hence, it is possible that CTRP5-LORD, as a monogenic condition, recapitulates ARMS2/HTRA1-related AMD more closely than it does AMD overall. Indeed, recent basic science research has suggested that the pathology in CTRP5-LORD is mediated through HTRA1: unlike wild-type CTRP5, CTRP5 with the CTRP5-LORD mutation was resistant to HTRA1-mediated cleavage, and an abnormal accumulation of CTRP5 and HTRA1 was observed in the Bruch’s membrane/choroid of mice with the mutation.30
Conclusions
In conclusion, massive advancing non-exudative type 1 CNV appears common in CTRP5-LORD. The neovascular membranes begin as small foci in the mid-peripheral retina. Despite enlarging progressively to enormous sizes, exudation is minimal. The natural history appears benign and might even be protective against atrophy, though this differs substantially from the aggressive course of macular exudative CNV in this condition. We recommend multimodal imaging that includes wide-field ICG-A and OCT-A in other patients with CTRP5-LORD, to assess how widespread this feature may be. In all patients with this finding in the extramacular retina, we recommend close observation over time, to understand whether exudation, fibrosis, and atrophy are indeed absent or rare in the affected areas.
Acknowledgments
Financial Support: NIH Intramural Research Program
Footnotes
Conflicts of interest: None
References
- 1.Kuntz CA, Jacobson SG, Cideciyan AV, et al. Sub-retinal pigment epithelial deposits in a dominant late-onset retinal degeneration. Invest Ophthalmol Vis Sci. 1996;37(9):1772–1782. [PubMed] [Google Scholar]
- 2.Ayyagari R, Griesinger IB, Bingham E, et al. Autosomal dominant hemorrhagic macular dystrophy not associated with the TIMP3 gene. Arch Ophthalmol. 2000;118(1):85–92. [DOI] [PubMed] [Google Scholar]
- 3.Milam AH, Curcio CA, Cideciyan AV, et al. Dominant late-onset retinal degeneration with regional variation of sub-retinal pigment epithelium deposits, retinal function, and photoreceptor degeneration. Ophthalmology. 2000;107(12):2256–2266. [DOI] [PubMed] [Google Scholar]
- 4.Jacobson SG, Cideciyan AV, Wright E, Wright AF. Phenotypic marker for early disease detection in dominant late-onset retinal degeneration. Invest Ophthalmol Vis Sci. 2001;42(8):1882–1890. [PubMed] [Google Scholar]
- 5.Hayward C, Shu X, Cideciyan AV, et al. Mutation in a short-chain collagen gene, CTRP5, results in extracellular deposit formation in late-onset retinal degeneration: a genetic model for age-related macular degeneration. Hum Mol Genet. 2003;12(20):2657–2667. [DOI] [PubMed] [Google Scholar]
- 6.Ayyagari R, Mandal MN, Karoukis AJ, et al. Late-onset macular degeneration and long anterior lens zonules result from a CTRP5 gene mutation. Invest Ophthalmol Vis Sci. 2005;46(9):3363–3371. [DOI] [PubMed] [Google Scholar]
- 7.Subrayan V, Morris B, Armbrecht AM, et al. Long anterior lens zonules in late-onset retinal degeneration (L-ORD). Am J Ophthalmol. 2005;140(6):1127–1129. [DOI] [PubMed] [Google Scholar]
- 8.Borooah S, Collins C, Wright A, Dhillon B. Late-onset retinal macular degeneration: clinical insights into an inherited retinal degeneration. Br J Ophthalmol. 2009;93(3):284–289. [DOI] [PubMed] [Google Scholar]
- 9.Cukras C, Flamendorf J, Wong WT, et al. Longitudinal Structural Changes in Late-Onset Retinal Degeneration. Retina. 2016;36(12):2348–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Domalpally A, Agron E, Pak JW, et al. Prevalence, Risk, and Genetic Association of Reticular Pseudodrusen in Age-related Macular Degeneration: Age-Related Eye Disease Study 2 Report 21. Ophthalmology. 2019;126(12):1659–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keenan TD, Klein B, Agron E, et al. Choroidal Thickness and Vascularity Vary with Disease Severity and Subretinal Drusenoid Deposit Presence in Nonadvanced Age-Related Macular Degeneration. Retina. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flamendorf J, Agron E, Wong WT, et al. Impairments in Dark Adaptation Are Associated with Age-Related Macular Degeneration Severity and Reticular Pseudodrusen. Ophthalmology. 2015;122(10):2053–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Capuano V, Miere A, Querques L, et al. Treatment-Naive Quiescent Choroidal Neovascularization in Geographic Atrophy Secondary to Nonexudative Age-Related Macular Degeneration. Am J Ophthalmol. 2017;182:45–55. [DOI] [PubMed] [Google Scholar]
- 14.Sarks SH. New vessel formation beneath the retinal pigment epithelium in senile eyes. Br J Ophthalmol. 1973;57(12):951–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang TS, Freund KB, de la Cruz Z, et al. Clinicopathologic correlation of choroidal neovascularization demonstrated by indocyanine green angiography in a patient with retention of good vision for almost four years. Retina. 1994;14(2):114–124. [DOI] [PubMed] [Google Scholar]
- 16.Laiginhas R, Yang J, Rosenfeld PJ, Falcao M. Nonexudative Macular Neovascularization - A Systematic Review of Prevalence, Natural History, and Recent Insights from OCT Angiography. Ophthalmol Retina. 2020;4(7):651–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang CK, Agron E, Domalpally A, et al. Progression of geographic atrophy with subsequent exudative neovascular disease in age-related macular degeneration: AREDS2 Report 24. Ophthalmol Retina. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfau M, Moller PT, Kunzel SH, et al. Type 1 Choroidal Neovascularization Is Associated with Reduced Localized Progression of Atrophy in Age-Related Macular Degeneration. Ophthalmol Retina. 2020;4(3):238–248. [DOI] [PubMed] [Google Scholar]
- 19.Chen L, Messinger JD, Sloan KR, et al. Nonexudative Macular Neovascularization Supporting Outer Retina in Age-Related Macular Degeneration: A Clinicopathologic Correlation. Ophthalmology. 2020. [DOI] [PubMed] [Google Scholar]
- 20.De Silva T, Chew EY, Hotaling N, Cukras CA. Deep-learning based multi-modal retinal image registration for the longitudinal analysis of patients with age-related macular degeneration. Biomed Opt Express. 2021;12(1):619–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keiner CM, Zhou H, Zhang Q, et al. Quantifying choriocapillaris hypoperfusion in patients with choroidal neovascularization using swept-source OCT angiography. Clin Ophthalmol. 2019;13:1613–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borrelli E, Uji A, Sarraf D, Sadda SR. Alterations in the Choriocapillaris in Intermediate Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci. 2017;58(11):4792–4798. [DOI] [PubMed] [Google Scholar]
- 23.Borrelli E, Sarraf D, Freund KB, Sadda SR. OCT angiography and evaluation of the choroid and choroidal vascular disorders. Prog Retin Eye Res. 2018;67:30–55. [DOI] [PubMed] [Google Scholar]
- 24.Hayreh SS. In vivo choroidal circulation and its watershed zones. Eye (Lond). 1990;4 ( Pt 2):273–289. [DOI] [PubMed] [Google Scholar]
- 25.Mendrinos E, Pournaras CJ. Topographic variation of the choroidal watershed zone and its relationship to neovascularization in patients with age-related macular degeneration. Acta Ophthalmol. 2009;87(3):290–296. [DOI] [PubMed] [Google Scholar]
- 26.Vanderford EK, De Silva T, Noriega D, et al. Quantitative analysis of longitudinal changes in multimodal imaging of Late-Onset Retinal Degeneration. Retina. 2020;Publish Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamashiro K, Hosoda Y, Miyake M, et al. Characteristics of Pachychoroid Diseases and Age-Related Macular Degeneration: Multimodal Imaging and Genetic Backgrounds. J Clin Med. 2020;9(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mullins RF, McGwin G, Jr., Searcey K, et al. The ARMS2 A69S Polymorphism Is Associated with Delayed Rod-Mediated Dark Adaptation in Eyes at Risk for Incident Age-Related Macular Degeneration. Ophthalmology. 2019;126(4):591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keenan TD, Agron E, Domalpally A, et al. Progression of Geographic Atrophy in Age-related Macular Degeneration: AREDS2 Report Number 16. Ophthalmology. 2018;125(12):1913–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chekuri A, Zientara-Rytter K, Soto-Hermida A, et al. Late-onset retinal degeneration pathology due to mutations in CTRP5 is mediated through HTRA1. Aging Cell. 2019;18(6):e13011. [DOI] [PMC free article] [PubMed] [Google Scholar]