Abstract
The hepatotoxicity of irinotecan is drawing wide concern nowadays due to the widespread use of this chemotherapeutic against various solid tumors, particularly metastatic colorectal cancer. Irinotecan-induced hepatotoxicity mainly manifests as transaminase increase and steatosis with or without transaminase increase, and is accompanied by vacuolization, and lobular inflammation. Irinotecan-induced steatohepatitis (IIS) increases the risk of morbidity and mortality in patients with colorectal cancer liver metastasis (CRCLM). The major risks and predisposing factors for IIS include high body mass index (BMI) or obesity, diabetes, and high-fat diet. Mitochondrial dysfunction and autophagy impairment may be involved in the pathogenesis of IIS. However, there is currently no effective preventive or therapeutic treatment for this condition. Thus, the precise mechanisms underlying the pathogenesis of IIS should be deciphered for the development of therapeutic drugs. This review summarizes the current knowledge and research progress on IIS.
Keywords: irinotecan, chemotherapy, hepatotoxicity, hepatic steatosis, steatohepatitis
Introduction
Irinotecan, also termed as CPT-11 or 7-ethyl-10-[4-(1-piperidino)-1-piperidino]-carbonyloxycamptothecine ( Figure 1 ), is an inhibitor of DNA topoisomerase I and has been used for 27 years since it was first approved in Japan in 1994 (1). As a crucial chemotherapeutic agent, it is widely used either alone or in combination against various solid tumors, particularly for the treatment of metastatic colorectal cancer, as recommended by the guidelines of the National Comprehensive Cancer Network and the European Society for Medical Oncology (1, 2). Notably, irinotecan-based neoadjuvant chemotherapy has improved the five-year survival rate in colorectal cancer liver metastasis (CRCLM) with unresectable tumors by approximately 58% (3, 4). However, there is a growing realization that irinotecan-induced hepatotoxicity, such as hepatic steatosis and steatohepatitis, can increase the risk of morbidity and mortality in patients with CRCLM (5, 6). Although irinotecan-induced steatohepatitis (IIS) has been known to be a clinicopathological symptom of irinotecan for decades, the mechanisms underlying this adverse effect are not exactly known. This review provides current insights into the clinical understanding of the epidemiology, risk factors, possible causal mechanisms, as well as preventive and therapeutic approaches regarding IIS.
Figure 1.
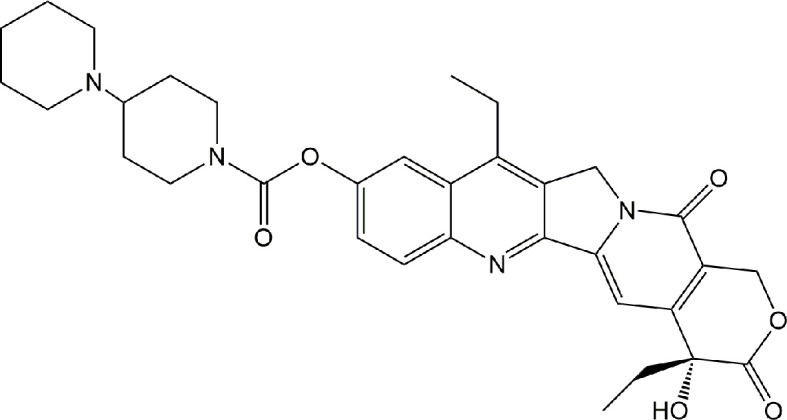
The structure of irinotecan.
Brief Description of Irinotecan
Irinotecan, which is a semi-synthetic and water-soluble camptothecin-derivative cytotoxic drug (2). It inhibits the DNA-topoisomerase I complex and causes DNA double-strand breaks, thereby inducing cytotoxicity (7, 8). As a prodrug, irinotecan is metabolized to the active metabolite SN-38, also termed as 7-ethyl-10-hydroxycamptothecin, in the blood and liver mainly by human carboxylesterase 2 (1, 9). Compared with irinotecan, SN-38 is a stronger inhibitor of DNA topoisomerase I (10, 11) and can induce lethal DNA double-strand breaks and eventually cell death (9, 11). SN-38 is inactivated upon its conversion into SN-38G (β-glucuronide conjugate) by uridine diphosphate-glucuronosyl transferase 1A1 (UGT1A1) in the liver. SN-38G can be converted back into SN-38 by bacterial β-glucuronidase in the intestinal tract, and the resulting SN-38 is absorbed into the systemic circulation, whereby the anti-tumor effect of irinotecan is extended (9, 12).
Unfortunately, irinotecan can non-specifically damage any rapidly proliferating cell, including both tumor cells and non-tumor cells, such as bone-marrow cells and intestinal basal cells, as well as the commensal bacteria in the body (1). Consequently, hematotoxicity (neutropenia) and gastrointestinal toxicity (diarrhea) are common irinotecan-induced toxicities (8), with a large inter-individual variation (1). In recent years, an increasing body of evidence has demonstrated that irinotecan-induced hepatotoxicity, including hepatic steatosis and steatohepatitis can increase the risk of morbidity and mortality in patients with CRCLM (6). Therefore, IIS is nowadays drawing increasing attention in clinical practice.
Epidemiology of IIS
Long-term or high-dose administration of irinotecan may impair the liver parenchyma, thus leading to hepatotoxicity with or without transaminase increase (7, 13–16). There are numerous epidemiological reports on IIS, which mainly focus on patients with CRCLM. However, there are significant differences in IIS incidence among these studies. Morris-Stiff et al. summarized that up to 50% of patients with CRCLM who receive neoadjuvant irinotecan develop IIS (17). In a prospective study involving 45 patients with CRCLM who underwent hepatic resection, Gomez-Ramirez et al. observed that four out of the seven patients (57.2%) who had received neoadjuvant irinotecan developed IIS (18). Pawlik and colleagues analyzed 153 patients with CRCLM and reported that moderate or severe hepatic steatosis was dramatically more frequent in the patients who had received neoadjuvant irinotecan (n = 15, 27.3%) than in those without any chemotherapy (n = 2, 3.4%) or with 5-FU (n = 10, 14.9%) or oxaliplatin (n = 3, 9.6%) monotherapy (19). A meta-analysis found that one in every twelve patients with CRCLM under irinotecan-based regimens will ultimately develop IIS (20). By analyzing 406 patients with CRCLM who had undergone hepatic resection, Vauthey and co-workers showed that irinotecan is related to IIS (20.2% vs. 4.4% of the patients without chemotherapy) (21). Moreover, although liver biopsy, which is the gold standard in diagnosing steatosis or steatohepatitis (6), is recommended to diagnose IIS (22), sampling error and observational variations among pathologists can affect the diagnosis (23).
IIS is associated with the disruption of lipid homeostasis and with inflammation in hepatic cells. It may progressively increase the risk of fibrosis, cirrhosis and liver failure (24, 25), because irinotecan-based regimens have potentially harmful effects on liver parenchyma and associated with impaired liver regeneration (17, 26). Vauthey et al. found that IIS remarkably increased the 90-day mortality of patients with CRCLM (14.7% vs. 1.6% of those with no IIS) (21). The presence of IIS is more concerning than simple steatosis when undergoing major liver resection and has been demonstrated to be associated with increased surgical morbidity and mortality after resection of colorectal liver metastases. Morris-Stiff and colleagues found that IIS is related to increased morbidity and possibly to increased mortality in patients with CRCLM following hepatectomy because of the development of liver failure (17). The detrimental effect of hepatic steatosis in patients undergoing liver resection was also demonstrated in a meta-analysis by Robinson et al. (20). Therefore, it is crucial to emphasize that careful consideration needs to be given when performing extensive procedures on patients with IIS.
Risk Factors for IIS
Multiple studies have found that confounding factors could impact the development of chemotherapy-associated steatohepatitis (CASH) (including IIS) (26–28). High body mass index (BMI) or obesity is closely related to an increased risk of CASH (14, 29). Patients with BMI ≥ 25 kg/m2 under irinotecan-based chemotherapy have a 2.03-fold risk of IIS compared with those with BMI < 25 kg/m2 (21). Another report by Ryan et al. noted that both IIS and hepatic steatosis are correlated with a BMI of ≥ 30 kg/m2 (30). In a small cohort study, patients with a high BMI who had undergone irinotecan-based chemotherapy were found to be associated with a high IIS score according to the Brunt System (29). Fernandez et al. demonstrated that severe IIS is related to neoadjuvant irinotecan in patients, particularly obese patients, with CRCLM who had undergone hepatic resection (22). Animal experiments have shown that the decreased hepatic UGT1A1 and increased fecal β-glucuronidase levels in diet-induced obese mice compared with the levels in lean mice are responsible for the prolonged retention of SN-38, consequently increasing the occurrence of IIS, in these obese mice (31).
Diabetes may be another important risk factor for IIS. Wolf et al. demonstrated that hepatic steatosis or IIS is more common in diabetic patients treated with irinotecan-based regimens as neoadjuvant chemotherapy before hepatic resection of CRCLM (27). Moreover, a diet with high-fat content may also accelerate the development of IIS. A dietary study by Mallick et al. demonstrated that, upon irinotecan treatment, mice on a high-fat diet, such as lard, develop steatosis more easily than those on a regular diet (32). Although these reports are based on retrospective analyses or animal studies, the results indicate that, upon irinotecan-based chemotherapy, patients on a high-fat diet or with baseline obesity or diabetes may have a higher risk of developing IIS than non-diabetic and non-obese patients on a regular diet. However, this possibility should be verified via prospective controlled trials.
Mechanisms Underlying IIS
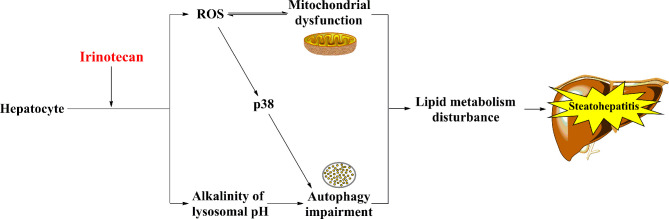
The exact mechanisms underlying IIS have not been fully elucidated. However, mitochondrial dysfunction and autophagy impairment have been proposed to be involved in the pathogenesis of IIS ( Figure 2 ).
Figure 2.
The potential mechanisms of IIS. Irinotecan-caused mitochondrial dysfunction and autophagy impairment result in lipid metabolism disturbance ultimately, which may involve in the pathogenesis of IIS.
Mitochondrial Dysfunction
Hepatocytes are rich in mitochondria, which are vulnerable to chemotherapeutic agents (33–35). In general, inhibition of β-oxidation of fatty acids, oxidative phosphorylation, and mitochondrial respiration primarily contributes to mitochondrial dysfunction (7). Irinotecan causes accumulation of lipids in hepatocytes via inhibiting the β-oxidation of fatty acids, which is one of the main pathways of the lipid metabolism, in the mitochondria of hepatocytes (7, 25). Moreover, irinotecan induces oxidative stress by uncoupling oxidative phosphorylation, restraining mitochondrial respiration, and facilitating the mitochondrial release of reactive oxygen species (ROS) in hepatocytes (7, 36). Interestingly, Bao et al. demonstrated that SN-38 upregulated ROS in cells derived from primary human hepatocytes but not in cancer cells (MDA-MB-231 and T47D) in vitro (8). Upregulation of ROS causes mitochondrial dysfunction (37) and stimulates the pathogenesis of IIS (14). It is worth noting that mitochondrial dysfunction usually improves when the chemotherapy is terminated (25).
Autophagy Impairment
Autophagy is a lysosome-mediated intracellular protein-degradation mechanism. It also regulates the lipid metabolism by metabolizing intracellular lipid droplets (triglycerides), which are the main form of lipid storage in the cell (38). Thus, impairment of this mechanism causes multiple metabolic diseases, such as obesity and hepatic steatosis (39). Mahli et al. found that irinotecan can weaken the autophagic flux by increasing the lysosomal pH to alkalinity, thereby contributing to lipid accumulation and steatosis in primary human hepatocytes (40). Furthermore, irinotecan impairs mitochondrial function and indirectly activates p38, thus inhibiting autophagosome formation (41, 42).
Potential Preventive and Therapeutic Approaches Against IIS
In general, there is still a lack of effective preventive and therapeutic strategies against IIS due to its complex mechanism of pathogenesis. However, several preclinical studies for IIS have suggested potential interventional drugs or measures, as described below.
Silymarin
Silymarin is a hepatoprotective agent, and it is derived from the seeds of Silybum marianum (43). As a natural flavonoid, silymarin has antioxidative effects and can decrease the oxidative stress in the liver (44). A study by Marcolino et al. about the effect of silymarin on IIS in mice reported that silymarin has a dual effect; low-dosage (1.5 mg/kg) of silymarin prevents irinotecan-induced hepatic injury, such as steatosis, vacuolization, lobular inflammation, and fibrosis, by suppressing the inflammatory factors and oxidative stress in the liver, whereas high-dosage (150 mg/kg) of silymarin exacerbates IIS and increases the mortality (45). Thus, the mechanisms whereby silymarin at different dosages result in different effects remain to be explored, and the specific clinical effects need to be confirmed, in future studies.
Pioglitazone
Pioglitazone is a thiazolidinedione antidiabetic agent. It modulates the lipid metabolism and ameliorates the glycemic control in patients with type-2 diabetes via activating the peroxisome proliferator-activated receptor (46, 47). A study in rats demonstrated that pioglitazone has a hepatoprotective effect against chemotherapy (irinotecan and 5-fluorouracil)-induced steatohepatitis, but no effect on histopathological changes (24). Therefore, the hepatoprotective effect of pioglitazone against IIS should be further explored.
Sorafenib
Sorafenib, a multityrosine-kinase inhibitor, is used for the treatment of unresectable hepatocellular carcinoma and advanced renal cell carcinoma (48). Mahli and co-workers reported that sorafenib has a protective effect against IIS by decreasing irinotecan-induced ERK activation and pro-inflammatory gene expression in hepatocytes and murine models of IIS (40). Nevertheless, the hepatoprotective effect of sorafenib against IIS should be confirmed in patients via clinical studies.
Glycine
Glycine is a nonessential amino acid with remarkable protective effects against liver injury (49, 50). Mikalauskas et al. found that glycine markedly reduces the levels of transaminases and microvesicular steatosis in rats treated with FOLFIRI, presumably by inhibiting the activation of Kupffer cells and enhancing the hepatic microcirculation (51).
Grain-Based Chow Diet
Phytoestrogens (especially isoflavones) and polyunsaturated fatty acids (PUFA) in diet may be effective in suppressing non-alcoholic fatty liver disease (NAFLD) or IIS. Isoflavones can be beneficial against NAFLD by reducing the lipogenesis, lipolysis, and fat deposition in adipocytes (52, 53). PUFA can decrease hepatic storage of triglycerides and has a significant protective effect against hepatic steatosis (54). A dietary study by Mallick et al. reported that a grain-based chow diet, which included a low level of fat (vegetable-based, such as soybean oil, which is especially rich in PUFA) and high levels of carbohydrate (fiber), phytoestrogen, and protein, had a notably protective effect against irinotecan-induced mixed hepatic steatosis (micro & macrovesicular) in mice (32). Thus, similar dietary studies involving specific ingredients should be performed on cancer patients undergoing irinotecan treatment.
Conclusions
Overall, IIS is a crucial adverse effect of irinotecan and can increase the risk of morbidity and mortality in patients with CRCLM. The major risks and predisposing factors for IIS include high BMI or obesity, diabetes, and high-fat diet. Although mitochondrial dysfunction and autophagy impairment may be involved in the pathogenesis of IIS, the exact causal mechanisms of IIS have not been fully elucidated. Till now, liver biopsy is the gold standard in diagnosing hepatotoxicity, including IIS, but it is a highly invasive, complex, and painful operation. Thus, biomarkers of IIS, are urgently needed to precisely evaluate irinotecan-induced hepatotoxicity. Besides, we should explore more risk factors for the development of IIS which can help oncologists to identify the patients at risk. Furthermore, effective preventive and therapeutic approaches are still lacking. Potential interventional drugs or measures have been reported in multiple preclinical studies and the medications susceptible to be active in steatosis such as fibroblast growth factor 21 agonists, obeticholic acid or glucagon-like peptide-1 agonists deserve considerations. Thus, further investigations involving humans, especially clinical trials, are required to develop feasible preventive and therapeutic approaches against IIS.
Author Contributions
CZ designed this work. JH and JZ wrote this manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the scientific research project of Wuhan NO.6 hospital (No. LX19013 to JH).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
BMI, body mass index; CASH, chemotherapy-associated steatohepatitis; CRCLM, colorectal cancer liver metastasis; IIS, irinotecan-induced steatohepatitis; NAFLD, non-alcoholic fatty liver disease; PUFA, polyunsaturated fatty acids; ROS, reactive oxygen species; UGT1A1, uridine diphosphate-glucuronosyl transferase 1A1.
References
- 1. Bailly C. Irinotecan: 25 Years of Cancer Treatment. Pharmacol Res (2019) 148:104398. doi: 10.1016/j.phrs.2019.104398 [DOI] [PubMed] [Google Scholar]
- 2. Kciuk M, Marciniak B, Kontek R. Irinotecan-Still an Important Player in Cancer Chemotherapy: A Comprehensive Overview. Int J Mol Sci (2020) 21(14):4919. doi: 10.3390/ijms21144919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, et al. Trends in Long-Term Survival Following Liver Resection for Hepatic Colorectal Metastases. Ann Surg (2002) 235(6):759–66. doi: 10.1097/00000658-200206000-00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fernandez FG, Drebin JA, Linehan DC, Dehdashti F, Siegel BA, Strasberg SM. Five-Year Survival After Resection of Hepatic Metastases From Colorectal Cancer in Patients Screened by Positron Emission Tomography With F-18 Fluorodeoxyglucose (FDG-PET). Ann Surg (2004) 240(3):438–47; discussion 447-50. doi: 10.1097/01.sla.0000138076.72547.b1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chun YS, Laurent A, Maru D, Vauthey JN. Management of Chemotherapy-Associated Hepatotoxicity in Colorectal Liver Metastases. Lancet Oncol (2009) 10(3):278–86. doi: 10.1016/S1470-2045(09)70064-6 [DOI] [PubMed] [Google Scholar]
- 6. Cai Z, Yang J, Shu X, Xiong X. Chemotherapy-Associated Hepatotoxicity in Colorectal Cancer. J BUON (2014) 19(2):350–6. [PubMed] [Google Scholar]
- 7. Schumacher JD, Guo GL. Mechanistic Review of Drug-Induced Steatohepatitis. Toxicol Appl Pharmacol (2015) 289(1):40–7. doi: 10.1016/j.taap.2015.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bao X, Wu J, Kim S, LoRusso P, Li J. Pharmacometabolomics Reveals Irinotecan Mechanism of Action in Cancer Patients. J Clin Pharmacol (2019) 59(1):20–34. doi: 10.1002/jcph.1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mathijssen RH, Loos WJ, Verweij J, Sparreboom A. Pharmacology of Topoisomerase I Inhibitors Irinotecan (CPT-11) and Topotecan. Curr Cancer Drug Targets (2002) 2(2):103–23. doi: 10.2174/1568009023333890 [DOI] [PubMed] [Google Scholar]
- 10. Li F, Jiang T, Li Q, Ling X. Camptothecin (CPT) and Its Derivatives Are Known to Target Topoisomerase I (Top1) as Their Mechanism of Action: Did We Miss Something in CPT Analogue Molecular Targets for Treating Human Disease Such as Cancer? Am J Cancer Res (2017) 7(12):2350–94. [PMC free article] [PubMed] [Google Scholar]
- 11. Liu LF, Desai SD, Li TK, Mao Y, Sun M, Sim SP. Mechanism of Action of Camptothecin. Ann N Y Acad Sci (2000) 922:1–10. doi: 10.1111/j.1749-6632.2000.tb07020.x [DOI] [PubMed] [Google Scholar]
- 12. Hasegawa Y, Ando Y, Ando M, Hashimoto N, Imaizumi K, Shimokata K. Pharmacogenetic Approach for Cancer Treatment-Tailored Medicine in Practice. Ann N Y Acad Sci (2006) 1086:223–32. doi: 10.1196/annals.1377.020 [DOI] [PubMed] [Google Scholar]
- 13. Pessaux P, Chenard MP, Bachellier P, Jaeck D. Consequences of Chemotherapy on Resection of Colorectal Liver Metastases. J Visc Surg (2010) 147(4):e193–201. doi: 10.1016/j.jviscsurg.2010.06.004 [DOI] [PubMed] [Google Scholar]
- 14. European Association for the Study of the Liver. Electronic address, C. Clinical Practice Guideline Panel, m. Panel and E. G. B. representative . EASL Clinical Practice Guidelines: Drug-Induced Liver Injury. J Hepatol (2019) 70(6):1222–61. doi: 10.1016/j.jhep.2019.02.014 [DOI] [PubMed] [Google Scholar]
- 15. Okusaka T, Ikeda M, Fukutomi A, Ioka T, Furuse J, Ohkawa S, et al. Phase II Study of FOLFIRINOX for Chemotherapy-Naive Japanese Patients With Metastatic Pancreatic Cancer. Cancer Sci (2014) 105(10):1321–6. doi: 10.1111/cas.12501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chung MJ, Kim YJ, Park JY, Bang S, Song SY, Chung JB, et al. Prospective Phase II Trial of Gemcitabine in Combination With Irinotecan as First-Line Chemotherapy in Patients With Advanced Biliary Tract Cancer. Chemotherapy (2011) 57(3):236–43. doi: 10.1159/000328021 [DOI] [PubMed] [Google Scholar]
- 17. Morris-Stiff G, Tan YM, Vauthey JN. Hepatic Complications Following Preoperative Chemotherapy With Oxaliplatin or Irinotecan for Hepatic Colorectal Metastases. Eur J Surg Oncol (2008) 34(6):609–14. doi: 10.1016/j.ejso.2007.07.007 [DOI] [PubMed] [Google Scholar]
- 18. Gomez-Ramirez J, Martin-Perez E, Amat CG, Sanz IG, Bermejo E, Rodriguez A, et al. Influence of Pre-Surgical Chemotherapy on Liver Parenchyma and Post-Surgical Outcome of Patients Subjected to Hepatectomy Due to Colorectal Carcinoma Metastases. Cir Esp (2010) 88(6):404–12. doi: 10.1016/j.ciresp.2010.09.005 [DOI] [PubMed] [Google Scholar]
- 19. Pawlik TM, Olino K, Gleisner AL, Torbenson M, Schulick R, Choti MA. Preoperative Chemotherapy for Colorectal Liver Metastases: Impact on Hepatic Histology and Postoperative Outcome. J Gastrointest Surg (2007) 11(7):860–8. doi: 10.1007/s11605-007-0149-4 [DOI] [PubMed] [Google Scholar]
- 20. Robinson SM, Wilson CH, Burt AD, Manas DM, White SA. Chemotherapy-Associated Liver Injury in Patients With Colorectal Liver Metastases: A Systematic Review and Meta-Analysis. Ann Surg Oncol (2012) 19(13):4287–99. doi: 10.1245/s10434-012-2438-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vauthey JN, Pawlik TM, Ribero D, Wu TT, Zorzi D, Hoff PM, et al. Chemotherapy Regimen Predicts Steatohepatitis and an Increase in 90-Day Mortality After Surgery for Hepatic Colorectal Metastases. J Clin Oncol (2006) 24(13):2065–72. doi: 10.1200/JCO.2005.05.3074 [DOI] [PubMed] [Google Scholar]
- 22. Fernandez FG, Ritter J, Goodwin JW, Linehan DC, Hawkins WG, Strasberg SM. Effect of Steatohepatitis Associated With Irinotecan or Oxaliplatin Pretreatment on Resectability of Hepatic Colorectal Metastases. J Am Coll Surg (2005) 200(6):845–53. doi: 10.1016/j.jamcollsurg.2005.01.024 [DOI] [PubMed] [Google Scholar]
- 23. Fiorini RN, Kirtz J, Periyasamy B, Evans Z, Haines JK, Cheng G, et al. Development of an Unbiased Method for the Estimation of Liver Steatosis. Clin Transplant (2004) 18(6):700–6. doi: 10.1111/j.1399-0012.2004.00282.x [DOI] [PubMed] [Google Scholar]
- 24. Celik S, Kartal K, Ozseker H, Hayran M, Hamaloglu E. Hepatoprotective Effect of Pioglitazone in Cases of Chemotherapy Induced Steatohepatitis. Chirurgia (Bucur) (2015) 110(1):49–55. [PubMed] [Google Scholar]
- 25. Meunier L, Larrey D. Chemotherapy-Associated Steatohepatitis. Ann Hepatol (2020) 19(6):597–601. doi: 10.1016/j.aohep.2019.11.012 [DOI] [PubMed] [Google Scholar]
- 26. Bower M, Wunderlich C, Brown R, Scoggins CR, McMasters KM, Martin RC. Obesity Rather Than Neoadjuvant Chemotherapy Predicts Steatohepatitis in Patients With Colorectal Metastasis. Am J Surg (2013) 205(6):685–90. doi: 10.1016/j.amjsurg.2012.07.034 [DOI] [PubMed] [Google Scholar]
- 27. Wolf PS, Park JO, Bao F, Allen PJ, DeMatteo RP, Fong Y, et al. Preoperative Chemotherapy and the Risk of Hepatotoxicity and Morbidity After Liver Resection for Metastatic Colorectal Cancer: A Single Institution Experience. J Am Coll Surg (2013) 216(1):41–9. doi: 10.1016/j.jamcollsurg.2012.08.030 [DOI] [PubMed] [Google Scholar]
- 28. Pathak S, Tang JM, Terlizzo M, Poston GJ, Malik HZ. Hepatic Steatosis, Body Mass Index and Long Term Outcome in Patients Undergoing Hepatectomy for Colorectal Liver Metastases. Eur J Surg Oncol (2010) 36(1):52–7. doi: 10.1016/j.ejso.2009.09.004 [DOI] [PubMed] [Google Scholar]
- 29. Zorzi D, Laurent A, Pawlik TM, Lauwers GY, Vauthey JN, Abdalla EK. Chemotherapy-Associated Hepatotoxicity and Surgery for Colorectal Liver Metastases. Br J Surg (2007) 94(3):274–86. doi: 10.1002/bjs.5719 [DOI] [PubMed] [Google Scholar]
- 30. Ryan P, Nanji S, Pollett A, Moore M, Moulton CA, Gallinger S, et al. Chemotherapy-Induced Liver Injury in Metastatic Colorectal Cancer: Semiquantitative Histologic Analysis of 334 Resected Liver Specimens Shows That Vascular Injury But Not Steatohepatitis Is Associated With Preoperative Chemotherapy. Am J Surg Pathol (2010) 34(6):784–91. doi: 10.1097/PAS.0b013e3181dc242c [DOI] [PubMed] [Google Scholar]
- 31. Mallick P, Shah P, Gandhi A, Ghose R. Impact of Obesity on Accumulation of the Toxic Irinotecan Metabolite, SN-38, in Mice. Life Sci (2015) 139:132–8. doi: 10.1016/j.lfs.2015.08.017 [DOI] [PubMed] [Google Scholar]
- 32. Mallick P, Shah P, Ittmann MM, Trivedi M, Hu M, Gao S, et al. Impact of Diet on Irinotecan Toxicity in Mice. Chem Biol Interact (2018) 291:87–94. doi: 10.1016/j.cbi.2018.06.018 [DOI] [PubMed] [Google Scholar]
- 33. Begriche K, Massart J, Robin MA, Borgne-Sanchez A, Fromenty B. Drug-Induced Toxicity on Mitochondria and Lipid Metabolism: Mechanistic Diversity and Deleterious Consequences for the Liver. J Hepatol (2011) 54(4):773–94. doi: 10.1016/j.jhep.2010.11.006 [DOI] [PubMed] [Google Scholar]
- 34. Degli Esposti D, Hamelin J, Bosselut N, Saffroy R, Sebagh M, Pommier A, et al. Mitochondrial Roles and Cytoprotection in Chronic Liver Injury. Biochem Res Int (2012) 2012:387626. doi: 10.1155/2012/387626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McWhirter D, Kitteringham N, Jones RP, Malik H, Park K, Palmer D. Chemotherapy Induced Hepatotoxicity in Metastatic Colorectal Cancer: A Review of Mechanisms and Outcomes. Crit Rev Oncol Hematol (2013) 88(2):404–15. doi: 10.1016/j.critrevonc.2013.05.011 [DOI] [PubMed] [Google Scholar]
- 36. Labbe G, Pessayre D, Fromenty B. Drug-Induced Liver Injury Through Mitochondrial Dysfunction: Mechanisms and Detection During Preclinical Safety Studies. Fundam Clin Pharmacol (2008) 22(4):335–53. doi: 10.1111/j.1472-8206.2008.00608.x [DOI] [PubMed] [Google Scholar]
- 37. Fromenty B, Robin MA, Igoudjil A, Mansouri A, Pessayre D. The Ins and Outs of Mitochondrial Dysfunction in NASH. Diabetes Metab (2004) 30(2):121–38. doi: 10.1016/S1262-3636(07)70098-8 [DOI] [PubMed] [Google Scholar]
- 38. Saito T, Kuma A, Sugiura Y, Ichimura Y, Obata M, Kitamura H, et al. Autophagy Regulates Lipid Metabolism Through Selective Turnover of Ncor1. Nat Commun (2019) 10(1):1567. doi: 10.1038/s41467-019-08829-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Khawar MB, Gao H, Li W. Autophagy and Lipid Metabolism. Adv Exp Med Biol (2019) 1206:359–74. doi: 10.1007/978-981-15-0602-4_17 [DOI] [PubMed] [Google Scholar]
- 40. Mahli A, Saugspier M, Koch A, Sommer J, Dietrich P, Lee S, et al. ERK Activation and Autophagy Impairment Are Central Mediators of Irinotecan-Induced Steatohepatitis. Gut (2018) 67(4):746–56. doi: 10.1136/gutjnl-2016-312485 [DOI] [PubMed] [Google Scholar]
- 41. Corcelle E, Djerbi N, Mari M, Nebout M, Fiorini C, Fenichel P, et al. Control of the Autophagy Maturation Step by the MAPK ERK and P38: Lessons From Environmental Carcinogens. Autophagy (2007) 3(1):57–9. doi: 10.4161/auto.3424 [DOI] [PubMed] [Google Scholar]
- 42. Wu D, Cederbaum AI. Inhibition of Autophagy Promotes CYP2E1-Dependent Toxicity in Hepg2 Cells via Elevated Oxidative Stress, Mitochondria Dysfunction and Activation of P38 and JNK MAPK. Redox Biol (2013) 1:552–65. doi: 10.1016/j.redox.2013.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tvrdy V, Pourova J, Jirkovsky E, Kren V, Valentova K, Mladenka P. Systematic Review of Pharmacokinetics and Potential Pharmacokinetic Interactions of Flavonolignans From Silymarin. Med Res Rev (2021) 41(4):2195–246. doi: 10.1002/med.21791 [DOI] [PubMed] [Google Scholar]
- 44. Ghiasian M, Nafisi H, Ranjbar A, Mohammadi Y, Ataei S. Antioxidative Effects of Silymarin on the Reduction of Liver Complications of Fingolimod in Patients With Relapsing-Remitting Multiple Sclerosis: A Clinical Trial Study. J Biochem Mol Toxicol (2021) 35(8):e22800. doi: 10.1002/jbt.22800 [DOI] [PubMed] [Google Scholar]
- 45. Marcolino Assis-Junior E, Melo AT, Pereira VBM, Wong DVT, Sousa NRP, Oliveira CMG, et al. Dual Effect of Silymarin on Experimental non-Alcoholic Steatohepatitis Induced by Irinotecan. Toxicol Appl Pharmacol (2017) 327:71–9. doi: 10.1016/j.taap.2017.04.023 [DOI] [PubMed] [Google Scholar]
- 46. Leclercq IA, Lebrun VA, Starkel P, Horsmans YJ. Intrahepatic Insulin Resistance in a Murine Model of Steatohepatitis: Effect of Ppargamma Agonist Pioglitazone. Lab Invest (2007) 87(1):56–65. doi: 10.1038/labinvest.3700489 [DOI] [PubMed] [Google Scholar]
- 47. Laplante M, Festuccia WT, Soucy G, Gelinas Y, Lalonde J, Berger JP, et al. Mechanisms of the Depot Specificity of Peroxisome Proliferator-Activated Receptor Gamma Action on Adipose Tissue Metabolism. Diabetes (2006) 55(10):2771–8. doi: 10.2337/db06-0551 [DOI] [PubMed] [Google Scholar]
- 48. Abdelgalil AA, Alkahtani HM, Al-Jenoobi FI. Sorafenib. Profiles Drug Subst Excip Relat Methodol (2019) 44:239–66. doi: 10.1016/bs.podrm.2018.11.003 [DOI] [PubMed] [Google Scholar]
- 49. Zhong Z, Wheeler MD, Li X, Froh M, Schemmer P, Yin M, et al. L-Glycine: A Novel Antiinflammatory, Immunomodulatory, and Cytoprotective Agent. Curr Opin Clin Nutr Metab Care (2003) 6(2):229–40. doi: 10.1097/00075197-200303000-00013 [DOI] [PubMed] [Google Scholar]
- 50. Luntz SP, Unnebrink K, Seibert-Grafe M, Bunzendahl H, Kraus TW, Buchler MW, et al. HEGPOL: Randomized, Placebo Controlled, Multicenter, Double-Blind Clinical Trial to Investigate Hepatoprotective Effects of Glycine in the Postoperative Phase of Liver Transplantation [ISRCTN69350312]. BMC Surg (2005) 5:18. doi: 10.1186/1471-2482-5-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mikalauskas S, Mikalauskiene L, Bruns H, Nickkholgh A, Hoffmann K, Longerich T, et al. Dietary Glycine Protects From Chemotherapy-Induced Hepatotoxicity. Amino Acids (2011) 40(4):1139–50. doi: 10.1007/s00726-010-0737-6 [DOI] [PubMed] [Google Scholar]
- 52. Torre-Villalvazo I, Tovar AR, Ramos-Barragan VE, Cerbon-Cervantes MA, Torres N. Soy Protein Ameliorates Metabolic Abnormalities in Liver and Adipose Tissue of Rats Fed a High Fat Diet. J Nutr (2008) 138(3):462–8. doi: 10.1093/jn/138.3.462 [DOI] [PubMed] [Google Scholar]
- 53. Lephart ED, Porter JP, Lund TD, Bu L, Setchell KD, Ramoz G, et al. Dietary Isoflavones Alter Regulatory Behaviors, Metabolic Hormones and Neuroendocrine Function in Long-Evans Male Rats. Nutr Metab (Lond) (2004) 1(1):16. doi: 10.1186/1743-7075-1-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Levy JR, Clore JN, Stevens W. Dietary N-3 Polyunsaturated Fatty Acids Decrease Hepatic Triglycerides in Fischer 344 Rats. Hepatology (2004) 39(3):608–16. doi: 10.1002/hep.20093 [DOI] [PubMed] [Google Scholar]