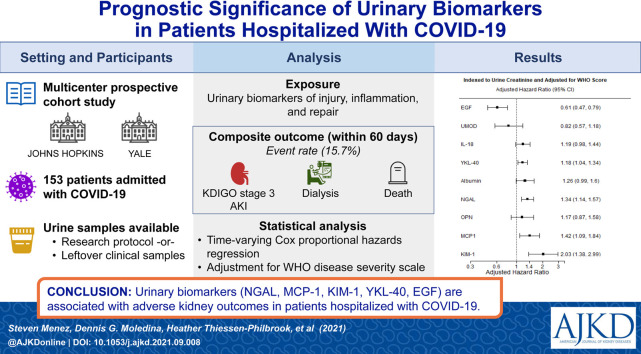
Abstract
Rationale & Objective
Acute kidney injury (AKI) is common in patients with coronavirus disease 2019 (COVID-19) and associated with poor outcomes. Urinary biomarkers have been associated with adverse kidney outcomes in other settings and may provide additional prognostic information in patients with COVID-19. We investigated the association between urinary biomarkers and adverse kidney outcomes among patients hospitalized with COVID-19.
Study Design
Prospective cohort study.
Setting & Participants
Patients hospitalized with COVID-19 (n = 153) at 2 academic medical centers between April and June 2020.
Exposure
19 urinary biomarkers of injury, inflammation, and repair.
Outcome
Composite of KDIGO (Kidney Disease: Improving Global Outcomes) stage 3 AKI, requirement for dialysis, or death within 60 days of hospital admission. We also compared various kidney biomarker levels in the setting of COVID-19 versus other common AKI settings.
Analytical Approach
Time-varying Cox proportional hazards regression to associate biomarker level with composite outcome.
Results
Out of 153 patients, 24 (15.7%) experienced the primary outcome. Twofold higher levels of neutrophil gelatinase-associated lipocalin (NGAL) (HR, 1.34 [95% CI, 1.14-1.57]), monocyte chemoattractant protein (MCP-1) (HR, 1.42 [95% CI, 1.09-1.84]), and kidney injury molecule 1 (KIM-1) (HR, 2.03 [95% CI, 1.38-2.99]) were associated with highest risk of sustaining primary composite outcome. Higher epidermal growth factor (EGF) levels were associated with a lower risk of the primary outcome (HR, 0.61 [95% CI, 0.47-0.79]). Individual biomarkers provided moderate discrimination and biomarker combinations improved discrimination for the primary outcome. The degree of kidney injury by biomarker level in COVID-19 was comparable to other settings of clinical AKI. There was evidence of subclinical AKI in COVID-19 patients based on elevated injury biomarker level in patients without clinical AKI defined by serum creatinine.
Limitations
Small sample size with low number of composite outcome events.
Conclusions
Urinary biomarkers are associated with adverse kidney outcomes in patients hospitalized with COVID-19 and may provide valuable information to monitor kidney disease progression and recovery.
Index Words: Acute kidney injury (AKI), chronic kidney disease (CKD), coronavirus disease 2019 (COVID-19), COVID-19 prognosis, death, dialysis, epidermal growth factor (EGF), inflammatory marker, kidney injury molecule 1 (KIM-1), monocyte chemoattractant protein 1 (MCP-1), neutrophil gelatinase-associated lipocalin (NGAL), severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), subclinical AKI, tubular injury, urinalysis, urinary biomarkers, urine microscopy
Graphical abstract
Plain-Language Summary.
Acute kidney injury is a serious complication in patients hospitalized with coronavirus disease 2019 (COVID-19). We hypothesized that biomarkers measured in the urine that are more specific for kidney injury and inflammation than serum creatinine may add to our understanding of kidney injury in the setting of COVID-19. We found that certain biomarkers—including epidermal growth factor and kidney injury molecule 1, among others—were associated with severe acute kidney injury, dialysis, and death within 60 days in patients hospitalized with COVID-19. Our study results suggest that these studied biomarkers may help identify patients at particularly high risk for adverse kidney outcomes.
As of August 2021, over 200 million people have been infected with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) across 192 countries and territories.1 , 2 As many as 20% to 25% of patients admitted to the hospital with coronavirus disease 2019 (COVID-19) require intensive care unit admission, with an in-hospital mortality between 5% and 20%.3 , 4 Clinical acute kidney injury (AKI), defined by serum creatinine criteria established by KDIGO (Kidney Disease: Improving Global Outcomes), occurs in nearly 30% to 50% of hospitalized patients, among whom 19% require dialysis.5, 6, 7, 8 Furthermore, clinical AKI during hospital admission has been associated with greater need for intensive care unit admission9 and worse in-hospital mortality.10
In a consensus report of the 25th Acute Disease Quality Initiative Workgroup, Nadim et al11 concluded that the pathogenesis of COVID-19–associated AKI is not yet fully elucidated and is likely multifactorial. Endothelial dysfunction, coagulopathy, complement activation, systemic inflammation, and immune dysfunction may all play a role in disease pathogenesis affecting the kidney.11 Risk factors for the development of AKI in the setting of COVID-19 include Black race, male sex, age over 50 years, diabetes mellitus, hypertension, obesity, and heart failure.9 , 11 We showed, however, that even after adjusting for such risk factors, patients with COVID-19 have a more than 40% higher risk of AKI compared with patients without COVID-19.7 The mechanism for this higher AKI risk remains unexplained. The long-term impact of COVID-19 is also uncertain from both kidney-specific and more global standpoints, with growing concern for the disease’s long-term physiological and psychological effects.12
Biomarkers of kidney injury, inflammation, and repair may offer further insight beyond current standard methods of characterizing COVID-19–associated AKI.13 Biomarkers can help to differentiate various types of kidney injury14 , 15 and may be uniquely helpful in quantifying tubular injury in COVID-19. In this study, therefore, we explored the association of urinary biomarkers of kidney injury, inflammation, and repair with severe AKI and death in patients hospitalized with COVID-19. We additionally evaluated biomarkers in patients with serum creatinine levels in the reference range to identify the subset of patients with subclinical AKI, which has been consistently associated with adverse outcomes.16 , 17 To estimate the magnitude of tubular injury in COVID-19 and provide insight into pathogenesis, we compared biomarkers of injury, inflammation, and repair in the kidney across different AKI settings.
We hypothesized that several biomarkers across different biological pathways would be significantly elevated and that higher levels would be associated with an increased risk of severe AKI, requirement for dialysis, and death. We also hypothesized that the degree of injury to the tubules in hospitalized patients with COVID-19–associated AKI would be more severe than in other hospitalized AKI settings, given the general trend of severe critical illness requiring mechanical ventilation and maximum hemodynamic support seen in the first wave of the COVID-19 pandemic.
Methods
Study Design, Population, and Data Sources
We obtained patient data and biosamples for the present study from 2 academic medical centers, the Johns Hopkins Hospital in Baltimore, Maryland, and Yale New Haven Hospital in New Haven, Connecticut, between April and June 2020 as part of the Translational Investigation of Kidney Disease in COVID-19 (TRIKIC) Consortium. Both health care systems used similar institutional protocols to prospectively collect data and biosamples in hospitalized adult inpatients with SARS-CoV-2 infection confirmed by polymerase chain reaction testing. Inpatients who did not have urine samples available for biomarker measurement before the onset of stage 3 AKI were excluded. Inpatients with baseline serum creatinine ≥ 4 mg/dL were also excluded.
At Johns Hopkins, all patient data were obtained from JH-CROWN (COVID-19 Precision Medicine Analytics Platform Registry), which includes demographic characteristics, baseline medical comorbidities, and inpatient clinical variables in the Johns Hopkins Health System.18 At Yale, similar data were obtained from the Joint Data Analytics Team (JDAT) via a structured data query from the Clarity database of the Epic Electronic Health Record.
Baseline comorbidities were defined by mapping ICD-10 codes to the Elixhauser comorbidity index.19 Laboratory values and vital signs on admission were defined as the first available values after admission.
Sample Collection and Biomarker Measurement
Urine samples were collected after a patient’s admission with a confirmed SARS-CoV-2 test, with repeat urine sample collections attempted weekly thereafter for patients who remained hospitalized. Samples were collected either after informed consent was obtained or as leftover residuals from clinical samples. Urine samples were collected at a median of 6 (interquartile range [IQR], 2-10) days after hospital admission for all patients. For patients who experienced the primary outcome, samples were collected at a median of 9.5 (IQR, 4.5-17) days after hospital admission and in patients who did not experience the primary outcome at a median of 5 (IQR, 2-9) days after hospital admission. Only urine samples collected before the time of the primary outcome were included in analysis. Details on sample processing and biomarker measurement can be found in the Item S1.
Interassay and intra-assay coefficients of variability were evaluated for all measured biomarkers (Table S1). We selected 9 candidate urinary biomarkers as primary exposure variables based on our prior work demonstrating associations of such biomarkers with adverse short and long-term kidney outcomes in other clinical settings.14 , 20, 21, 22 These 9 urinary biomarkers were as follows: albumin, epidermal growth factor (EGF), kidney injury molecule 1 (KIM-1), monocyte chemoattractant protein 1 (MCP-1), neutrophil gelatinase-associated lipocalin (NGAL), osteopontin (OPN), uromodulin (UMOD), interleukin 18 (IL-18), and chitinase-3-like protein 1 (YKL-40). Given the marked systemic inflammatory response associated with COVID-19, we additionally investigated the association of 10 inflammatory biomarkers with the primary outcome: urinary IL-1β, 2, 4, 6, 8, 10, 12, and 13, tumor necrosis factor α (TNF-α), and interferon γ (IFN-γ). All biomarker measurements obtained from all urine samples were used in our analyses.
Urine Microscopy
Urine microscopy scoring was completed in a subset of 59 patients at the Johns Hopkins site, using the IDEXX SediVue Dx platform,23 with all 59 urine microscopy samples generating an automated report with 70 images each, which were reviewed manually. All urine microscopy measurements were made on the same urine samples for which biomarkers were measured in patients at the Johns Hopkins Hospital. The urine microscopy score was determined based on the number of renal tubular epithelial cells per high-power field, and number of granular casts per low-power field.24 We used reverse transcriptase–polymerase chain reaction (RT-PCR) testing to evaluate for the presence of the SARS-CoV-2 virus in urine samples of 55 patients from the Johns Hopkins Hospital.
Kidney Function Evaluation and Outcome Definitions
Baseline kidney function was defined using the median of all outpatient serum creatinine measurements 7 to 365 days before hospitalization, if available. This definition was chosen given the concern that serum creatinine values measured closest to the time of hospitalization in patients with COVID-19 may already be elevated above baseline. In the 17% of patients without any outpatient serum creatinine measurements available, the lowest serum creatinine value obtained during the COVID-19 hospitalization was used as the baseline creatinine. In patients who were started on dialysis during hospitalization, we ensured that no serum creatinine values were used to calculate baseline kidney function after the start of dialysis. Otherwise, when needed we used the lowest value of serum creatinine at any point during hospitalization to determine baseline. Estimated glomerular filtration rates (eGFRs) were calculated using the CKD-EPI equation.25 Clinical AKI was defined as a ≥ 50% increase in serum creatinine from baseline or dialysis at any time during the index hospitalization. AKI severity was classified by modified KDIGO staging criteria on the basis of the peak serum creatinine during the index hospitalization.26 Stage 3 AKI was defined as a 3-fold increase in serum creatinine, a peak serum creatinine value > 4 mg/dL with at least a 50% increase in serum creatinine, or dialysis at any time during the index hospitalization. If multiple serum creatinine measurements were available within the same day, the mean value for the day was used. The primary outcome of the study was the time to a composite of stage 3 AKI, dialysis, or death within 60 days of hospital admission.
Statistical Analysis
Descriptive characteristics were reported using mean ± SD or median (IQR) for continuous variables, and frequency (percentage) for categorical variables. All biomarkers were modeled continuously after log2 transformation. Vital signs and clinical laboratory values were primarily obtained on the day of admission with the exception of urinalysis (Table S2).
Time-varying Cox proportional hazards regression was used to examine the association between urinary biomarker levels and the primary outcome using all available biomarker measurements before the development of the primary outcome, with time of each biomarker measurement taken into consideration. We performed all analyses after log2 transformation of biomarker measurements, first with univariable analysis and then with indexing to the urine creatinine concentration to account for any influence that urinary dilution or concentration based on a patient’s hydration status may have on biomarker level. We further adjusted for World Health Organization (WHO) disease severity scale at the time of sample collection, taking into account activity level, ventilatory requirement, end-organ failure including need for vasopressors and extracorporeal membrane oxygenation, and death to reflect COVID-19 disease severity.27 If a patient did not experience the primary outcome, it was treated as a censored event, and censoring time was assigned as 60 days. Kolmogorov-type supremum tests were used to evaluate proportional hazards assumptions for all models. To evaluate the discriminatory ability of the biomarkers, the concordance index (C-index) was estimated for individual biomarkers using all available urinary biomarkers per patient.28
As a supplementary analysis to demonstrate the degree of injury or inflammation in patients with COVID-19 in comparison to other clinical settings, we compared the biomarker levels of urinary albumin, KIM-1, IL-18, UMOD, EGF, MCP-1, NGAL, and YKL-40 in our patient population with the postoperative biomarker levels in patients with cardiac surgery–associated AKI from the Translational Research Investigating Biomarker Endpoints in Acute Kidney Injury (TRIBE-AKI) Study,20 in brain-dead donors at the time of nephrectomy for organ retrieval for kidney transplantation in the Deceased Donor Study (DDS),29 and marathon runners with exercise stress-associated AKI.30 Specifically, for this analysis, we used biomarker measurements in any patient with KDIGO stage 1 or greater AKI across all comparison groups.
We used preoperative biomarker levels from urine samples of patients in the TRIBE-AKI Study before cardiac surgery as the reference31; these measurements were chosen specifically because while not all of these patients were fully healthy, the preoperative urine samples were collected at the time of anesthesia clearance before the clear insult leading to AKI in this population. The other cohorts (post–cardiac surgery, brain death, exercise stress) were chosen to highlight different inpatient and outpatient clinical scenarios where biomarkers have been applied previously. For exercise stress-associated AKI, blood and urine biomarkers of injury and inflammation were measured from biosamples obtained within 30 minutes of completing a marathon in 23 individuals compared with their prerace values.30 Further details are provided in Item S1.
All analyses were performed in SAS (version 9.4; SAS Institute) and R (version 3.1.2; R Foundation for Statistical Computing). All tests of statistical significance were 2-sided, with P < 0 .05 considered statistically significant. Institutional review board approval was obtained for all patient samples as part of this study, either with consent for prospective urine sample collection or through waiver of consent for leftover clinical samples.
Results
Study Population
A total of 178 patients had urine samples collected during their hospital admission. Twenty-two patients who did not have urine samples obtained before the onset of the primary outcome were excluded. We also excluded 3 additional patients who had baseline serum creatinine levels above 4 mg/dL, resulting in a final analytic population of 153 patients with a total of 218 samples (Fig S1). Urine samples were available at a median of 6 (IQR, 2-10) days after hospital admission. Table 1 describes the baseline characteristics of participants in the combined cohort of patients from both study sites as well as key clinical and laboratory characteristics on admission and during hospitalization. The mean patient age on admission was 56.3 ± 19.5 years, and 78 patients (51%) were female. The mean baseline eGFR was 83.7 ± 36.0 mL/min/1.73 m2. Thirty-three patients (22%) had CKD, 57 had diabetes mellitus (37%), and 102 had hypertension (67%). The mean serum creatinine level at the time of sample collection was 1.09 ± 0.93 (SD) mg/dL.
Table 1.
Baseline and Inpatient Characteristics of Hospitalized Patients With COVID-19
| Characteristic | Value |
|---|---|
| Baseline Characteristics | |
| Demographics | |
| Age, y | 56.3 ± 19.5 |
| Black race | 63 (41%) |
| Female sex | 78 (51%) |
| Hispanic ethnicity | 37 (26%) |
| Comorbidities | |
| Chronic kidney disease | 33 (22%) |
| Hypertension (any) | 102 (67%) |
| Diabetes (any) | 57 (37%) |
| Elixhauser comorbidity score | 6.45 ± 4.94 |
| At Admission | |
| Vital signs | |
| Systolic blood pressure, mm Hg | 126.3 ± 25.5 |
| Diastolic blood pressure, mm Hg | 72.7 ± 14.9 |
| Pulse rate, beats per minute | 96.3 ± 21.9 |
| Pulse oximetry oxygen saturation, % | 95.4 ± 4.4 |
| Hematologic laboratory findings | |
| Hemoglobin, g/dL | 12.3 ± 2.1 |
| White blood cell count, × 103/μL | 7.5 ± 3.6 |
| Platelet count, × 103/μL | 211.7 ± 84.8 |
| Chemistry laboratory findings | |
| SUN-creatinine ratio | 20.11 ± 16.05 |
| Creatinine, mg/dL | 1.09 ± 0.62 |
| Estimated glomerular filtration rate, mL/min/1.73 m2 | 83.7 ± 36.0 |
| C-reactive protein, mg/dL | 73.2 ± 88.8 |
| Urinalysis | |
| Protein ≥2+ | 42 (34%) |
| Blood ≥2+ | 20 (16%) |
| Inpatient Characteristics | |
| Serum creatinine | |
| Baselinea | 0.87 ± 0.5 |
| Time of sample collection | 1.09 ± 0.93 |
| Peak | 1.46 ± 1.2 |
| Discharge | 0.98 ± 0.74 |
| No. of measurements | 11 [6-26] |
| AKI stage and duration | |
| No AKI | 91 (59%) |
| Stage 1 | 31 (20%) |
| Stage 2 | 17 (11%) |
| Stage 3 | 14 (9%) |
| Duration, db | 2.5 [1-6] |
| No. of days to peak serum creatinine measurement | 2 [1-8] |
| Urine microscopy scorec | |
| 0 | 36 (64%) |
| 1 | 17 (30%) |
| 2 | 3 (5%) |
| Dialysis | 7 (5%) |
| Mechanical ventilation | 38 (25%) |
| Death | 19 (12%) |
| Hospital length of stay, d | 12 [6-25] |
| Inpatient treatment before urine biomarker measurement | |
| Tocilizumab | 57 (37%) |
| Remdesivir | 24 (16%) |
| Hydroxychloroquine | 58 (38%) |
| Steroidd | 48 (31%) |
N = 153. Values given as mean ± standard deviation, median [interquartile range], or count (percentage).
Abbreviations: AKI, acute kidney injury; COVID-19, coronavirus disease 2019; SUN, serum urea nitrogen.
Baseline is within 7-365 days of hospital admission.
Number of days with AKI stage 1 or higher.
Based on number of renal tubular epithelial cells per high-power field, and number of granular casts per low-power field. Urine microscopy was completed in 59 participants (9 with the primary outcome).
Steroid is any administration of dexamethasone, methylprednisolone, or hydrocortisone.
The median length of COVID-19 hospitalization in this study was 11 (IQR, 6-25) days. In total, 24 patients (16%) developed the primary outcome. Of these patients, 14 developed stage 3 AKI, 7 required dialysis, and 19 died during the study period (Table 1). The primary outcome occurred at a median of 25 (IQR, 15-30) days after hospital admission (Fig S2). Before the first biomarker measurement, a total of 48 patients (31%) received some form of steroid therapy (dexamethasone, hydrocortisone, or methylprednisolone), 57 patients (37%) received tocilizumab, 24 patients (16%) received remdesivir, and 58 patients (38%) received hydroxychloroquine.
Urinary Biomarkers Associated With the Primary Outcome
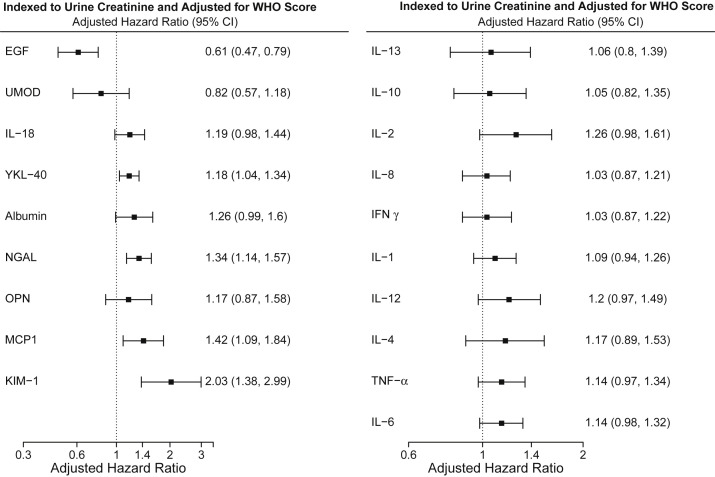
Table S3 details the range of urinary biomarker levels measured from all available urine samples in the analytic population. Figure S3 displays biomarker values indexed to urine creatinine from the time of biomarker measurement to event in those who developed the primary outcome. After indexing to urinary creatinine, each 2-fold increase in urinary EGF was associated with a significantly lower risk of the primary outcome (indexed hazard ratio [HR], 0.51 [95% CI, 0.39-0.67]) (Fig S4). In total, 8 biomarkers from the primary group were significantly associated with the primary outcome after indexing to urinary creatinine. However, after adjustment for WHO disease severity index, EGF remained significantly associated with a lower risk of the primary outcome (adjusted HR, 0.61 [95% CI, 0.47-0.79]), and YKL-40, NGAL, MCP-1, and KIM-1 remained significantly associated with a higher risk of the primary outcome (Fig 1 ). Of the 10 inflammatory biomarkers measured, only TNF-α (HR, 1.21 [95% CI, 1.04-1.41]) and IL-6 (HR, 1.19 [95% CI, 1.05-1.36]) were associated with the primary outcome after indexing to urinary creatinine, which was attenuated after adjustment for WHO scale (Fig 1). Additional analysis on the association of biomarkers with stage 3 AKI or dialysis, excluding death, yielded largely similar results.
Figure 1.
Risk of stage 3 AKI, new dialysis initiation, or death within 60 days of hospital admission by urinary biomarker level, indexed to urine creatinine and adjusted for World Health Organization disease severity scale. Abbreviations: AKI, acute kidney injury; EGF, epidermal growth factor; IFNγ, interferon γ; IL, interleukin; KIM-1, kidney injury molecule 1; MCP-1, monocyte chemoattractant protein 1; NGAL, neutrophil gelatinase-associated lipocalin; OPN, osteopontin; TFN-α, tumor necrosis factor α; UMOD, uromodulin; WHO, World Health Organization; YKL-40, chitinase-3-like protein 1.
Out of 59 patients at Johns Hopkins for whom urine microscopy was performed, 9 (15%) developed the primary outcome. Of these 9 patients, 5 (56%) had a urine microscopy score of 0, and only 1 (11%) had a score of 2 or higher. Of the 50 patients who did not develop the primary outcome, 3 (6%) had a score of 2 or higher. With RT-PCR testing for SARS-CoV-2 viral RNA in the urine samples of 55 patients, there was no virus detected in the urine of the 10 patients who developed the primary outcome whereas viral RNA was detected in 8 (18%) out of the 45 patients who did not develop the primary outcome.
Discriminatory Potential of Biomarkers and Primary Outcomes
We assessed the ability of a select number of individual biomarkers and combinations of biomarkers to discriminate for the primary outcome, using all available biomarker measurements (Table 2 ). Urinary albumin yielded a C-index of 0.62, whereas urinary NGAL and EGF yielded C-indexes of 0.80 and 0.75, respectively. Combinations of 2 biomarkers improved discrimination; the C-indexes for the combination of urinary EGF and NGAL and for the combination of EGF and MCP-1 were 0.84 and 0.85 respectively. The findings were similar for nonindexed or urinary biomarkers indexed to urinary creatinine.
Table 2.
Biomarker Association With Primary Outcome and Model Performance
| Urinary Biomarker | Hazard Ratio (95% CI) |
Model Performance of Biomarkers: C-Indexc | |
|---|---|---|---|
| Model 1a | Model 2b | ||
| Individual biomarkers | |||
| EGF (pg/mL) | 0.51 (0.39-0.67) | 0.61 (0.47-0.79) | 0.75 |
| YKL-40 (pg/mL) | 1.35 (1.19-1.53) | 1.18 (1.04-1.34) | 0.71 |
| Albumin (mg/dL) | 1.21 (1.02-1.43) | 1.26 (0.99-1.60) | 0.62 |
| NGAL (ng/mL) | 1.59 (1.37-1.84) | 1.34 (1.14-1.57) | 0.80 |
| MCP-1 (pg/mL) | 2.01 (1.58-2.56) | 1.42 (1.09-1.84) | 0.81 |
| KIM-1 (ng/mL) | 2.58 (1.84-3.61) | 2.03 (1.38-2.99) | 0.81 |
| Biomarker combinations | |||
| NGAL + albumin | 0.77 | ||
| MCP-1 + NGAL | 0.78 | ||
| EGF + albumin | 0.77 | ||
| EGF + NGAL | 0.84 | ||
| MCP-1 + EGF | 0.85 | ||
Primary outcome is a composite of KDIGO stage 3 AKI, short-term dialysis requirement, or death within 60 days of hospital discharge.
Abbreviations: AKI, acute kidney injury; EGF, epidermal growth factor; KIM-1, kidney injury molecule 1; MCP-1, monocyte chemoattractant protein 1; NGAL, neutrophil gelatinase-associated lipocalin; YKL-40, chitinase-3-like protein 1.
Model 1: Indexed to urine creatinine.
Model 2: Adjusted for World Health Organization disease severity scale.
Time-varying biomarkers used to determine concordance index (C-index).
Additional Analysis of Stage 2 or 3 AKI, Dialysis, and Death up to 60 Days
We performed additional analysis to look at an expanded composite outcome of stage 2 or 3 AKI, dialysis, or death up to 60 days of hospital admission. Because we excluded patients who had a biomarker measurement after the outcome occurred, our new analytic population for this analysis was 140 patients, of whom 26 (19%) developed the composite outcome. We observed largely similar results compared with our primary analysis, with the biomarkers EGF, IL-18, YKL-40, albumin, NGAL, MCP-1, and KIM-1 all significantly associated with the outcome after indexing to urine creatinine and adjusting for WHO disease severity scale (Table S4). Additional analysis on the association of biomarkers with stage 2-3 AKI or dialysis, excluding death, yielded largely similar results.
Comparison of Urinary Biomarkers in COVID-19 and in Other Clinical Settings
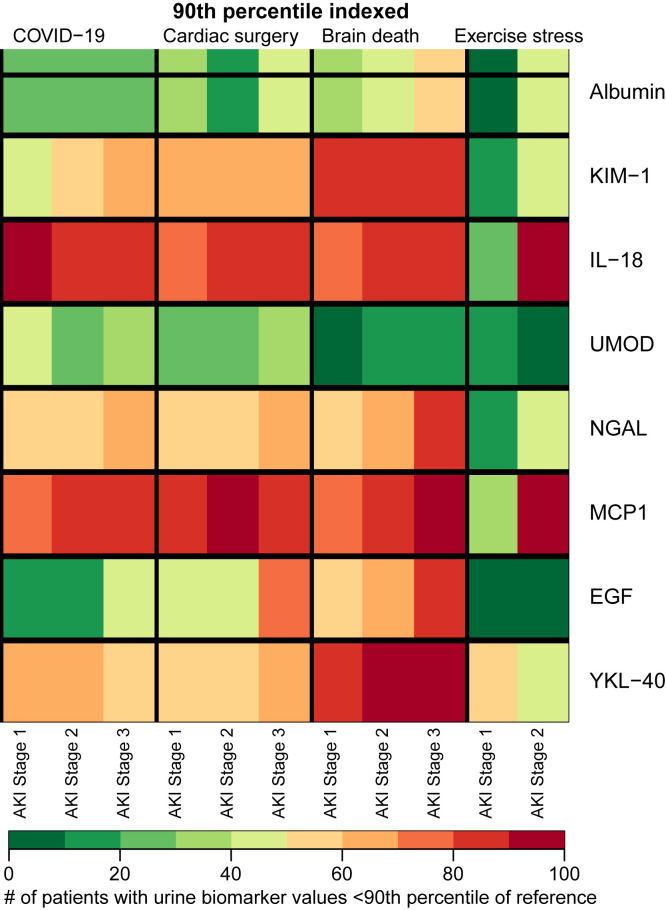
We used preoperative urinary biomarker levels in patients from the TRIBE-AKI Study as a reference group in our comparisons of biomarker levels across cohorts. Nearly 100% of patients with COVID-19–associated AKI, regardless of stage, had urinary IL-18 and MCP-1 levels above the 90th percentile of reference values (Fig 2 ). Similarly, over 50% of patients with COVID-19–associated AKI had urinary NGAL levels above the 90th percentile of reference values. In general, the degree of kidney injury based on biomarker levels above the reference preoperative level was generally similar in COVID-19–associated AKI compared with other AKI settings. Markers of injury and inflammation, namely IL-18 and MCP-1, were relatively more elevated than markers of glomerular injury (albumin) or tubular health (UMOD) in the setting of COVID-19. In general, the relative degree of kidney injury in those with COVID-19–associated AKI by NGAL or KIM-1 level was slightly higher than in those with exercise stress-induced AKI.
Figure 2.
Heat map showing the percentage of patients who have AKI in the setting of COVID-19, patients after cardiac surgery (TRIBE-AKI cohort), patients after brain death (DDS cohort), and patients in the setting of exercise stress (marathon-associated AKI). For exercise stress-associated AKI, blood and urine biomarkers were measured from biosamples obtained within 30 minutes of completing a marathon. The colors denote the percentage of patients by AKI stage who have biomarker levels above the 90th percentile of reference value, based on cardiac surgery preoperative values. Among patients with COVID-19–associated AKI, 40%, 50%, and 60% of patients with stage 1, 2, and 3 AKI, respectively, had KIM-1 levels above the 90th percentile of reference. Similarly, among kidney donors after brain death, 70%, 80%, and 90% of patients with stage 1, 2, and 3 AKI, respectively, had MCP-1 levels above the 90th percentile of reference. Note: Sample sizes by AKI stage: COVID-19: stage 1 (n = 56), stage 2 (n = 31), stage 3 (n = 20); cardiac surgery: stage 1 (n = 456), stage 2 (n = 34), stage 3 (n = 31); brain death: stage 1 (n = 275), stage 2 (n = 93), stage 3 (n = 76); exercise stress: stage 1 (n = 9), stage 2 (n = 2). Abbreviations: AKI, acute kidney injury; COVID-19, coronavirus disease 2019; DDS, Deceased Donor Study; EGF, epidermal growth factor; IL, interleukin; KIM-1, kidney injury molecule 1; MCP1, monocyte chemoattractant protein 1; NGAL, neutrophil gelatinase-associated lipocalin; TRIBE-AKI, Translational Research Investigating Biomarker Endpoints in Acute Kidney Injury; UMOD, uromodulin; YKL-40, chitinase-3-like protein 1.
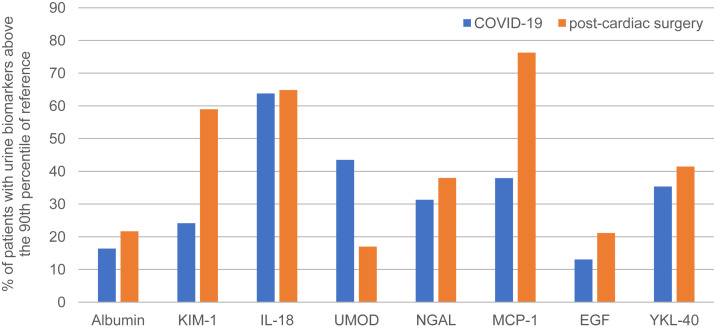
Compared with preoperative cardiac surgery patients, patients with COVID-19 who did not develop clinical AKI had higher levels of injury and inflammatory biomarkers, suggesting the presence of subclinical AKI (Fig 3 ). Within each cohort, roughly 30%-40% of patients had urinary NGAL levels above the 90th percentile of reference values, and between 60% and 65% of each cohort had IL-18 levels above the 90th percentile of reference values.
Figure 3.
The percentage of patients with COVID-19 (n = 116) and patients immediately after cardiac surgery (samples taken within 6 hours of end of surgery; n = 1,044) with urinary biomarker levels above the 90th percentile of reference values, defined by preoperative urinary biomarker levels in patients immediately before cardiac surgery. In both clinical settings, approximately 30%-40% of patients had urinary NGAL levels above the 90th percentile of reference values. Approximately 25% of patients with COVID-19 and no AKI had KIM-1 levels above reference, and nearly 60% of patients after cardiac surgery had KIM-1 levels above reference. Abbreviations: AKI, acute kidney injury; COVID-19, coronavirus disease 2019; EGF, epidermal growth factor; IL, interleukin; KIM-1, kidney injury molecule 1; MCP-1, monocyte chemoattractant protein 1; NGAL, neutrophil gelatinase-associated lipocalin; UMOD, uromodulin; YKL-40, chitinase-3-like protein 1.
Discussion
In this prospective study of patients hospitalized with COVID-19, we demonstrated that urinary biomarkers of injury, inflammation, and repair were associated with adverse kidney outcomes up to 60 days. We identified significant associations between urinary NGAL, KIM-1, MCP-1, and EGF with the primary outcome. The discriminatory ability of the individual biomarkers EGF, NGAL, and MCP-1 alone was higher than that of urinary albumin alone and improved in pairwise combinations of these 3 biomarkers. Neither urine microscopy score nor the presence of viral RNA in the urine of patients with COVID-19 was associated with the primary outcome. We also demonstrated that the degree of kidney injury by biomarker level was similar in COVID-19–associated AKI compared with AKI in other clinical settings and that a substantial proportion of patients had evidence of subclinical AKI (abnormal urinary biomarkers but no change in kidney function).
The associations of urinary biomarkers with stage 3 AKI, dialysis, and death are consistent with previous studies from our group and others15 , 32 , 33 and may reflect the kidney’s response to injury and inflammation due to COVID-19. Moreover, our study findings may localize kidney injury in COVID-19 to specific segments of the nephron, particularly within the renal tubule. Based on the significant association between higher levels of urinary NGAL and the primary outcome, patients with COVID-19 may be at increased risk for either proximal or distal tubular injury, as NGAL serves as a marker of global tubular injury that is rapidly up-regulated in the setting of ischemia and tubular stress.34, 35, 36
Patients who developed stage 3 AKI, dialysis, or death had relatively higher levels of KIM-1, primarily associated with proximal tubular injury, and IL-18, strongly associated with proximal tubular injury.37 These findings are consistent with biopsy studies of patients undergoing kidney biopsy in the setting of COVID-19, showing histological evidence of proximal tubular injury.38 , 39
However, we also saw relatively higher levels of urinary MCP-1 and lower levels of EGF in patients with COVID-19. CCL2, the gene that encodes MCP-1, is expressed primarily in the ascending loop of Henle and distal tubule.40 Higher levels of both plasma and urinary MCP-1 were shown to be important biomarkers of AKI,41 , 42 and we have previously shown that gene expression of CCL2 and urinary MCP-1 levels are elevated in the setting of cardiac surgery–associated AKI.43 Higher urinary MCP-1 levels have also been found to be associated with chronic tubule-interstitial damage and CKD progression in the context of various conditions such as IgA nephropathy, diabetes mellitus, and obstructive uropathy.40 , 44, 45, 46
EGF, a 53-amino-acid polypeptide, is similarly expressed in the loop of Henle and the distal tubule.47 EGF is a growth factor that serves as a marker for repair after either muscle and kidney tissue injury.48 Urinary EGF levels correlate well with creatinine clearance,49 and lower urinary EGF levels have been associated with AKI.49 Therefore, we provide evidence that both proximal and distal tubular damage may be occurring. Our analysis of inflammatory markers, including urinary TNF-α and IL-6, and their significant associations with the primary outcome, corroborates the view that the global systemic inflammatory response to SARS-CoV-2 leads to significant end-organ injury.50 , 51
Inflammatory marker elevations much higher than reference values confirm findings in prior studies50 and also indicate that the inflammation in COVID-19–associated AKI is significant even at stage 1 and 2 and comparable to other clinical settings. Furthermore, we demonstrated that even among patients with COVID-19 who did not develop clinical AKI based on serum creatinine, biomarkers were significantly elevated above reference values, similar to patients after cardiac surgery.20 , 52 Given the clear associations between subclinical AKI and long-term outcomes in other settings, the subset of patients with subclinical kidney injury may represent a very high-risk group who will require close follow-up of kidney function upon discharge.17
Our study also has some limitations. We did not have consistent data on daily or hourly urine output trends in these patients, so we defined AKI only using serum creatinine criteria. We measured several biomarkers because we wanted to identify candidates who can be validated in future studies. Thus, there is a risk of type 1 error from multiple comparisons, but all the primary biomarkers have shown an association in other AKI studies.
To limit chance findings from multiple comparisons, we selected biomarkers with high pretest probability based on our experience with other AKI settings. Although our primary composite outcome included death up to 60 days from admission, deaths that occurred after hospital discharge but within the 60-day window may have been incompletely captured.
Also, there was significant heterogeneity in the timing of urine processing. However, the majority of biomarkers that were significant are robust to handling procedures such as lack of centrifugation and being left at 4°C or room temperature for up to 48 hours (Table S5).53 Furthermore, the relatively few events limited our ability to adjust for potential confounders in our analyses. However, the discriminatory potential of these biomarkers for severe events suggests that these biomarkers can be used by themselves or in combination to predict future events. However, the interpretation of C-indexes in this study is somewhat limited given the small cohort size, and along with the lack of a validation cohort there is a risk for overfitting.
Our study offers a number of strengths with important clinical implications for the care of patients with COVID-19. We were able to obtain urine samples from 2 different study sites with different demographic characteristics, allowing for a more diverse patient population and more generalizable results. Despite the significant heterogeneity in the preprocessing of urine samples, the biomarker measurements were robust with excellent analytical precision. Our study can therefore offer insights into a pragmatic approach for the use of urinary biomarker measurements that can be implemented in clinical care.
In summary, we have shown that urinary biomarkers can help identify the patients who are hospitalized with COVID-19 who are at high risk for adverse kidney outcomes and death. These findings may improve the risk stratification of patients admitted with COVID-19. The degree of tubular injury and inflammation does not appear to be overtly higher than in other common settings of AKI. Subclinical AKI, as defined by elevations in urinary biomarkers, was present in 30%-50% of individuals who did not manifest clinical AKI. Further studies are needed to validate these findings and assess the association of the biomarkers with risk for CKD as well as the potential long-term consequences associated with subclinical AKI after COVID-19.
Article Information
Translational Research Investigating Kidney Outcomes in COVID-19 (TRIKIC) Consortium Investigators
Jie Deng and the Johns Hopkins COVID-19 and Data Research Evaluation Committee (Johns Hopkins University School of Medicine); Albert Ko, Akiko Iwasaki, Shelli Farhadian, Allison Nelson, Arnau Casanovas-Massana, Elizabeth B. White, Wade Schulz, Andreas Coppi, Patrick Young, Angela Nunez, Denise Shepard, Irene Matos, Yvette Strong, Kelly Anastasio, Kristina Brower, Maxine Kuang, Michael Chiorazzi, Santos Bermejo, Pavithra Vijayakumar, Bertie Geng, John Fournier, Maksym Minasyan, M. Catherine Muenker, Adam J. Moore (Yale University School of Medicine, Yale IMPACT Biorepository team; also includes author CDC); and Girish Nadkarni (Icahn School of Medicine at Mount Sinai).
Authors’ Full Names and Academic Degrees
Steven Menez, MD, MHS, Dennis G. Moledina, MBBS, PhD, Heather Thiessen-Philbrook, MMath, F. Perry Wilson, MD, MSCE, Wassim Obeid, PhD, Michael Simonov, MD, Yu Yamamoto, MS, Celia P. Corona-Villalobos, MD, MS, Crystal Chang, BS, Brian T. Garibaldi, MD, William Clarke, PhD, Shelli Farhadian, MD, PhD, Charles Dela Cruz, MD, PhD, Steven G. Coca, DO, MS, and Chirag R. Parikh, MD, PhD.
Authors’ Contributions
Data analysis: HTP, MS, YY; specimen collection, processing, and biomarker measurement: CPC, CC, WO; development and principal investigator of the JH-CROWN Registry: BTG; specimen retrieval for remnant biorepository for JH-CROWN Registry: WC; contributed to IMPACT Biorepository: SF, CDC; supervision/mentorship: CRP, SM, DGM, HTP, FPW, SGC. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
This work was supported by NIDDK supplemental award R01DK093770-09S1 for COVID-19 research (to CRP). These studies were supported through the generosity of the collective community of donors to the Johns Hopkins University School of Medicine for COVID research as part of the JH-CROWN: The COVID PMAP Registry (IRB00253168, IRB00252317) and with funding from the JHU COVID-19 Research Response Program. Data presented here were also obtained through the Yale Department of Medicine’s COVID Explorer data repository, which is funded by the Department of Medicine, the George M. O’Brien Kidney Center at Yale, and resources from the Clinical and Translational Research Accelerator (IMPACT Study IRB 2000027690). The funders had no direct role in the study design, data collection, analysis, reporting, or the decision to submit the manuscript for publication.
Financial Disclosure
Drs Coca and Parikh have served as members of the advisory board of and own equity in RenalytixAI. Dr Coca has received consulting fees from Goldfinch Bio, CHF Solutions, Quark Biopharma, Janssen Pharmaceuticals, Takeda Pharmaceuticals, and Relypsa in the past 3 years. Dr Garibaldi is a member of the Food and Drug Administration Pulmonary and Asthma Drug Advisory Committee and is a consultant for Janssen Research and Development, LLC. These arrangements have been reviewed and approved by Johns Hopkins University in accordance with its conflict-of-interest policies. Dr Menez has received consulting fees from the Dedham Group. The other authors declare that they have no relevant financial interests.
Peer Review
Received April 30, 2021. Evaluated by 2 external peer reviewers, with direct editorial input from a Statistics/Methods Editor and an Associate Editor, who served as Acting Editor-in-Chief. Accepted in revised form September 1, 2021. The involvement of an Acting Editor-in-Chief was to comply with AJKD’s procedures for potential conflicts of interest for editors, described in the Information for Authors & Journal Policies.
Footnotes
Complete author and article information (including a list of the TRIKIC Consortium Investigators) provided before references.
Figure S1: Flow diagram for study.
Figure S2: Cumulative incidence curve for stage 3 AKI, dialysis, death up to 60 days from hospital admission.
Figure S3: Biomarker levels indexed to urine creatinine as a function of time from measurement to event.
Figure S4: Risk of stage 3 AKI, new dialysis initiation, or death within 60 days of hospital admission by urinary biomarker level, indexed to urine creatinine.
Item S1: Supplemental methods.
Table S1: Interassay and intra-assay coefficients of variability for urinary biomarker measurements.
Table S2: Vital signs and laboratory values reported on the day of admission.
Table S3: Urinary biomarker levels.
Table S4: Biomarker association with stage 2 or 3 AKI, dialysis, or death.
Table S5: Measurement reliability of urinary biomarker measurement.
Contributor Information
the TRIKIC Consortium Investigators:
Albert Ko, Akiko Iwasaki, Shelli Farhadian, Allison Nelson, Arnau Casanovas-Massana, Elizabeth B. White, Wade Schulz, Andreas Coppi, Patrick Young, Angela Nunez, Denise Shepard, Irene Matos, Yvette Strong, Kelly Anastasio, Kristina Brower, Maxine Kuang, Michael Chiorazzi, Santos Bermejo, Pavithra Vijayakumar, Bertie Geng, John Fournier, Maksym Minasyan, M. Catherine Muenker, Adam J. Moore, and Girish Nadkarni
Supplementary Material
Figures S1-S4. Item S1. Tables S1-S5.
References
- 1.Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johns Hopkins Center for Systems Science and Engineering Johns Hopkins global coronavirus 2019 map. https://coronavirus.jhu.edu/map.html
- 3.Grasselli G., Zangrillo A., Zanella A., et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Argenziano M.G., Bruce S.L., Slater C.L., et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996. doi: 10.1136/bmj.m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan L., Chaudhary K., Saha A., et al. AKI in hospitalized patients with COVID-19. J Am Soc Nephrol. Sep 3 2020;32(1):151–160. doi: 10.1681/asn.2020050615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirsch J.S., Ng J.H., Ross D.W., et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moledina D.G., Simonov M., Yamamoto Y., et al. The association of COVID-19 with acute kidney injury independent of severity of illness: a multicenter cohort study. Am J Kidney Dis. 2021;77(4):490–499. doi: 10.1053/j.ajkd.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowe B., Cai M., Xie Y., Gibson A.K., Maddukuri G., Al-Aly Z. Acute kidney injury in a national cohort of hospitalized US veterans with COVID-19. Clin J Am Soc Nephrol. 2021;16(1):14–25. doi: 10.2215/CJN.09610620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher M., Neugarten J., Bellin E., et al. AKI in hospitalized patients with and without COVID-19: a comparison study. J Am Soc Nephrol. 2020;31(9):2145–2157. doi: 10.1681/asn.2020040509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ng J.H., Hirsch J.S., Hazzan A., et al. Outcomes among patients hospitalized with COVID-19 and acute kidney injury. Am J Kidney Dis. 2021;77(2):204–215.e1. doi: 10.1053/j.ajkd.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nadim M.K., Forni L.G., Mehta R.L., et al. COVID-19-associated acute kidney injury: consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup. Nat Rev Nephrol. 2020;16(12):747–764. doi: 10.1038/s41581-020-00356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Del Rio C., Collins L.F., Malani P. Long-term health consequences of COVID-19. JAMA. 2020;324(17):1723–1724. doi: 10.1001/jama.2020.19719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delanaye P., Cavalier E., Pottel H. Serum creatinine: not so simple. Nephron. 2017;136(4):302–308. doi: 10.1159/000469669. [DOI] [PubMed] [Google Scholar]
- 14.Moledina D.G., Wilson F.P., Pober J.S., et al. Urine TNF-alpha and IL-9 for clinical diagnosis of acute interstitial nephritis. JCI Insight. 2019;4(10) doi: 10.1172/jci.insight.127456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belcher J.M., Garcia-Tsao G., Sanyal A.J., et al. Urinary biomarkers and progression of AKI in patients with cirrhosis. Clin J Am Soc Nephrol. 2014;9(11):1857–1867. doi: 10.2215/cjn.09430913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ronco C., Kellum J.A., Haase M. Subclinical AKI is still AKI. Crit Care. 2012;16(3):313. doi: 10.1186/cc11240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haase M., Kellum J.A., Ronco C. Subclinical AKI—an emerging syndrome with important consequences. Nat Rev Nephrol. 2012;12:735–739. doi: 10.1038/nrneph.2012.197. [DOI] [PubMed] [Google Scholar]
- 18.Garibaldi B.T., Fiksel J., Muschelli J., et al. Patient trajectories among persons hospitalized for COVID-19. Ann Intern Med. 2020;174(1):33–41. doi: 10.7326/M20-3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elixhauser A., Steiner C., Harris D.R., Coffey R.M. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Coca S.G., Garg A.X., Thiessen-Philbrook H., et al. Urinary biomarkers of AKI and mortality 3 years after cardiac surgery. J Am Soc Nephrol. 2014;25(5):1063–1071. doi: 10.1681/ASN.2013070742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tidbury N., Browning N., Shaw M., Morgan M., Kemp I., Matata B. Neutrophil gelatinase-associated lipocalin as a marker of postoperative acute kidney injury following cardiac surgery in patients with preoperative kidney impairment. Cardiovasc Hematol Disord Drug Targets. 2019;19(3):239–248. doi: 10.2174/1871529X19666190415115106. [DOI] [PubMed] [Google Scholar]
- 22.Parikh C.R., Mishra J., Thiessen-Philbrook H., et al. Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2006;70(1):199–203. doi: 10.1038/sj.ki.5001527. [DOI] [PubMed] [Google Scholar]
- 23.Hernandez A.M., Bilbrough G.E.A., DeNicola D.B., et al. Comparison of the performance of the IDEXX SediVue Dx® with manual microscopy for the detection of cells and 2 crystal types in canine and feline urine. J Vet Intern Med. 2019;33(1):167–177. doi: 10.1111/jvim.15341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perazella M.A., Coca S.G., Hall I.E., Iyanam U., Koraishy M., Parikh C.R. Urine microscopy is associated with severity and worsening of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol. 2010;5(3):402–408. doi: 10.2215/cjn.06960909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levey A.S., Stevens L.A., Schmid C.H., et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 27.World Health Organization World Health Organization COVID-19 clinical management. https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1
- 28.Bansal A., Heagerty P.J. A tutorial on evaluating the time-varying discrimination accuracy of survival models used in dynamic decision making. Med Decis Making. 2018;38(8):904–916. doi: 10.1177/0272989x18801312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reese P.P., Hall I.E., Weng F.L., et al. Associations between deceased-donor urine injury biomarkers and kidney transplant outcomes. J Am Soc Nephrol. 2016;27(5):1534–1543. doi: 10.1681/asn.2015040345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mansour S.G., Verma G., Pata R.W., Martin T.G., Perazella M.A., Parikh C.R. Kidney injury and repair biomarkers in marathon runners. Am J Kidney Dis. 2017;70(2):252–261. doi: 10.1053/j.ajkd.2017.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parikh C.R., Coca S.G., Thiessen-Philbrook H., et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol. 2011;22(9):1748–1757. doi: 10.1681/ASN.2010121302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen C., Yang X., Lei Y., et al. Urinary biomarkers at the time of AKI diagnosis as predictors of progression of AKI among patients with acute cardiorenal syndrome. Clin J Am Soc Nephrol. 2016;11(9):1536–1544. doi: 10.2215/cjn.00910116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hall I.E., Stern E.P., Cantley L.G., Elias J.A., Parikh C.R. Urine YKL-40 is associated with progressive acute kidney injury or death in hospitalized patients. BMC Nephrol. 2014;15:133. doi: 10.1186/1471-2369-15-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang W.R., Parikh C.R. Biomarkers of acute and chronic kidney disease. Annu Rev Physiol. 2019;81:309–333. doi: 10.1146/annurev-physiol-020518-114605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paragas N., Qiu A., Zhang Q., et al. The Ngal reporter mouse detects the response of the kidney to injury in real time. Nat Med. 2011;17(2):216–222. doi: 10.1038/nm.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuwabara T., Mori K., Mukoyama M., et al. Urinary neutrophil gelatinase-associated lipocalin levels reflect damage to glomeruli, proximal tubules, and distal nephrons. Kidney Int. 2009;75(3):285–294. doi: 10.1038/ki.2008.499. [DOI] [PubMed] [Google Scholar]
- 37.Parikh C.R., Jani A., Melnikov V.Y., Faubel S., Edelstein C.L. Urinary interleukin-18 is a marker of human acute tubular necrosis. Am J Kidney Dis. 2004;43(3):405–414. doi: 10.1053/j.ajkd.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 38.Koitka A., Cooper M.E., Thomas M.C., Tikellis C. Angiotensin converting enzyme 2 in the kidney. Clin Exp Pharmacol Physiol. 2008;35(4):420–425. doi: 10.1111/j.1440-1681.2008.04889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Werion A., Belkhir L., Perrot M., et al. SARS-CoV-2 causes a specific dysfunction of the kidney proximal tubule. Kidney Int. 2020;98(5):1296–1307. doi: 10.1016/j.kint.2020.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grandaliano G., Gesualdo L., Ranieri E., et al. Monocyte chemotactic peptide-1 expression in acute and chronic human nephritides: a pathogenetic role in interstitial monocytes recruitment. J Am Soc Nephrol. 1996;7(6):906–913. doi: 10.1681/asn.v76906. [DOI] [PubMed] [Google Scholar]
- 41.Munshi R., Johnson A., Siew E.D., et al. MCP-1 gene activation marks acute kidney injury. J Am Soc Nephrol. 2011;22(1):165–175. doi: 10.1681/asn.2010060641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moledina D.G., Isguven S., McArthur E., et al. Plasma monocyte chemotactic protein-1 is associated with acute kidney injury and death after cardiac operations. Ann Thorac Surg. 2017;104(2):613–620. doi: 10.1016/j.athoracsur.2016.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Menez S., Ju W., Menon R., et al. Urinary EGF and MCP-1 and risk of CKD after cardiac surgery. JCI Insight. 2021;6(11) doi: 10.1172/jci.insight.147464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grandaliano G., Gesualdo L., Bartoli F., et al. MCP-1 and EGF renal expression and urine excretion in human congenital obstructive nephropathy. Kidney Int. 2000;58(1):182–192. doi: 10.1046/j.1523-1755.2000.00153.x. [DOI] [PubMed] [Google Scholar]
- 45.Torres D.D., Rossini M., Manno C., et al. The ratio of epidermal growth factor to monocyte chemotactic peptide-1 in the urine predicts renal prognosis in IgA nephropathy. Kidney Int. 2008;73(3):327–333. doi: 10.1038/sj.ki.5002621. [DOI] [PubMed] [Google Scholar]
- 46.Satirapoj B., Dispan R., Radinahamed P., Kitiyakara C. Urinary epidermal growth factor, monocyte chemoattractant protein-1 or their ratio as predictors for rapid loss of renal function in type 2 diabetic patients with diabetic kidney disease. BMC Nephrol. 2018;19(1):246. doi: 10.1186/s12882-018-1043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gesualdo L., Paolo S.D., Calabró A., et al. Expression of epidermal growth factor and its receptor in normal and diseased human kidney: an immunohistochemical and in situ hybridization study. Kidney Int. 1996;49(3):656–665. doi: 10.1038/ki.1996.94. [DOI] [PubMed] [Google Scholar]
- 48.Harris R.C. Potential physiologic roles for epidermal growth factor in the kidney. Am J Kidney Dis. 1991;17(6):627–630. doi: 10.1016/s0272-6386(12)80336-2. [DOI] [PubMed] [Google Scholar]
- 49.Taira T., Yoshimura A., Iizuka K., Iwasaki S., Ideura T., Koshikawa S. Urinary epidermal growth factor levels in patients with acute renal failure. Am J Kidney Dis. 1993;22(5):656–661. doi: 10.1016/s0272-6386(12)80427-6. [DOI] [PubMed] [Google Scholar]
- 50.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20(6):355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Menez S., Moledina D.G., Garg A.X., et al. Results from the TRIBE-AKI Study found associations between post-operative blood biomarkers and risk of chronic kidney disease after cardiac surgery. Kidney Int. 2021;99(3):716–724. doi: 10.1016/j.kint.2020.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang C, Obeid W, Thiessen-Philbrook H, Parikh CR. Sample processing and stability for urine biomarker studies. J Appl Lab Med. Published online August 20, 2021. https://doi.org/10.1093/jalm/jfab082 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1-S4. Item S1. Tables S1-S5.