Abstract
Background:
This study reports a systematic review of association between meteorological parameters and hand, foot and mouth disease (HFMD) in mainland China.
Methods:
Using predefined study eligibility criteria, three electronic databases (PubMed, Web of Science, and Embase) were searched for relevant articles. Using a combination of search terms, including “Hand foot and mouth disease,” “HFMD,” “Meteorological,” “Climate,” and “China,” After removal of duplicates, our initial search generated 2435 studies published from 1990 to December 31, 2019. From this cohort 51 full-text articles were reviewed for eligibility assessment. The meta-analysis was devised in accordance with the published guidelines of the Cochrane Collaboration and Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA). Effect sizes, heterogeneity estimates and publication bias were computed using R software and Review Manager Software.
Results:
The meta-analysis of 18 eligible studies showed that the meteorological parameters played an important role in the prevalence of HFMD. Lower air pressure may be the main risk factor for the incidence of HFMD in Chinese mainland, and three meteorological parameters (mean temperature, rainfall and relative humidity) have a significant association with the incidence of HFMD in subtropical regions.
Conclusion:
Lower air pressure might be the main risk factor for the incidence of HFMD in Chinese mainland. The influence of meteorological parameters on the prevalence of HFMD is mainly through changing virus viability in aerosols, which may be different in different climate regions. In an environment with low air pressure, wearing a mask that filters the aerosol outdoors may help prevent HFMD infection.
Keywords: Hand, foot and mouth disease; Meteorological parameters; Meta-analysis
Introduction
Hand- foot-and-mouth disease (HFMD) is a common infectious disease, it was first identified in New Zealand in 1957 (1) and has been frequently reported worldwide (2–5). Since the late 1990s, the increasing reports of outbreaks HFMD confirm that it has become a serious public health concern in the Asia-Pacific region. In Chinese mainland, the first HFMD case was reported in Shanghai in the 1980s (6), and since 2010 HFMD has ranked first among the notifiable infectious diseases China accounted for 87% (9.8 million/11.3 million) of all hand, foot, and mouth disease (HFMD) cases reported to WHO by 2014 (7).
Significant seasonality in the incidence of HFMD has been observed in a number of countries (3, 8, 9), which indicates that meteorological parameters may play an important role on HFMD epidemiology. In the United States, a study of the seasonal pattern of enterovirus cases found that it exhibited a well-defined geographical structure very similar to that of historical poliomyelitis, and humidity such as the dew point temperature is a strong predictor of the intensity of enterovirus transmission (10). In Chinese mainland, a large number of studies have focused on the effects of meteorological parameters on the incidence of HFMD within the last decade (11–27).In previous studies, inconsistent correlations between meteorological parameters and HFMD have been found. The scope of this study was set as the HFMD outbreak in Chinese mainland. This disease was largely influenced by school terms and public holidays (e.g., Chinese New Year) in past studies, and this effect can be normalized (28).
The purpose of this meta-analysis was to evaluate the relationship between meteorological parameters and HFMD to help identify meteorological parameters critical for epidemics.
Methods
The meta-analysis was devised in accordance with the published guidelines of the Cochrane Collaboration and Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) (28).
Literature search
Literature searches were conducted using PubMed, Web of Science, and Embase by three independent reviewers. Medical Subject Heading terms included “hand foot and mouth disease (HFMD),” “meteorological,” “climate,” and “China,” and references from the retrieved documents were also checked to include any additional relevant articles. Reference lists of trials and reviews were also examined. No restrictions on language or publication year were applied. The last date of the search was December 31, 2019.
Selection criteria
Inclusion criteria for the studies were as follows: [1]studies written in English; [2]studies with a reported case size; and [3]studies provided clear correlation coefficient (COR), risk ratio (RR), odds ratio (OR) andincidence rate ratio (IRR) between meteorological parameters and the incidence of HFMD.
Exclusion criteria: [1] Review articles; [2] republished papers, [3] studies that cannot extract data for statistical analysis and [4] articles lacking the required information
Data collection and extraction
Each complete study report was thoroughly reviewed independently by two researchers to ensure that all data were collected entirely and accurately. In case of discrepancies a third author was consulted for consensus. Data abstraction was done on a modified Cochrane abstraction form. The following variables were extracted from the studies: [1] demographic characteristics, including first author’s name, year of publication, location, study period, case sample size [2]correlation coefficient (COR), risk ratio(RR), odds ratio (OR) and incidence rate ratio (IRR) between meteorological parameters and the incidence ofHFMD. These articles were evaluated using bias risk assessment tools developed specifically for epidemiological studies. The tool included ten items assessing reporting quality, external validity and bias.
Statistical methods and analysis
R software (R Foundation for Statistical Computing, Vienna, Austria) and Review Manager Software (RevMan 5.3; Cochrane Collaboration) were used for meta-analysis. Prior to beginning meta-analyses, the sample COR (summary r value) of each study was converted to Fisher’s Z to avoid excessive dependence of COR’s variance on correlation (29). The formulas are as follows.
The standard error of Z is n is the case sample size.
Between-study heterogeneity will be explored using the Q and I2 statistic. The hypothesis test was used to judge whether the correlation was statistically significant. The data were calculated and transformed using R software. The meta-analysis was performed using Review Manager Software to construct forest plots used to indicate the effect size. The significance test of publication bias was interpreted by funnel plot.
Results
Description of studies
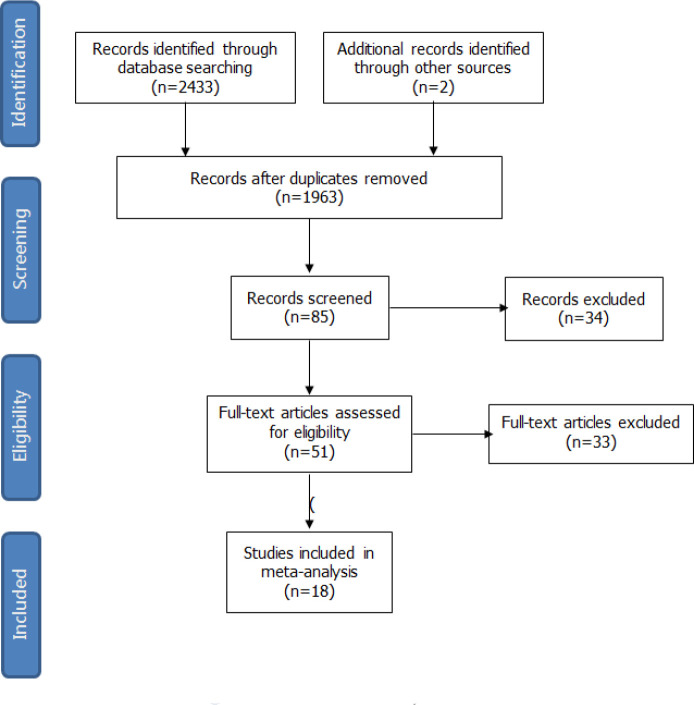
After removal of duplicates, our initial search generated 2435 studies. From this cohort 51 full-text articles were reviewed for eligibility assessment and 18 studies between 2008 and 2017 were included for synthesis in this review finally. The screening process for study selection was listed in the flow diagram (Fig. 1).
Fig. 1:
Flowchart of study selection
Detailed information regarding the meta-analysis is provided in Table 1. Collectively, the articles provided the data on the association between meteorological parameters and the incidence of HFMD in a total of 3,939,023 cases between 2008 and 2017 in Chinese mainland; the time unit was month, week and day. The number of included studies were as follows: average relative humidity (16 studies), mean temperature (15 studies), rainfall (14 studies), hours of sunshine (9 studies) and mean wind speed (9 studies), mean air pressure (7 studies). The quality of included studies were reviewed and judged according to the STROBE guidelines (30). All included studies in this paper were related to “research design”, “research area”, “research period”, “research variables”, “data source”, “statistical method”, “conflict of interest” etc.
Table 1:
Characteristics of the 18 publications included
| Reference | Location | Study period | Case | Resolution |
|---|---|---|---|---|
| Tian, Liang et al. 2018(11) | Beijing | 2010–2012 | 114777 | Monthly |
| Chen, Sun et al. 2015(12) | Suzhou | 2012–2013 | 1730 | Monthly |
| Feng, Duan et al. 2014(13) | Zhengzhou | 2008–2012 | 2932 | Monthly |
| Gou, Liu et al. 2017(14) | Gansu | 2010 | 12428 | Monthly |
| Huang, Deng et al. 2013(15) | Guangzhou | 2008–2011 | 100875 | Weekly |
| Jiang, Yang et al. 2016 (17) | Qingdao | 2007–2014 | 78641 | Weekly |
| Li, Qiu et al. 2018(19) | Shandong | 2008 | 6439 | Weekly |
| Wu, Hu et al. 2017(22) | Hunan | 2009–2015 | 895429 | Monthly |
| Xu, Yu et al. 2015(26) | Beijing | 2010–2012 | 14152 | Daily |
| Zhao, Wang et al. 2017(39) | Huainan | 2009–2014 | 113475 | Weekly |
| Zheng, Cao et al. 2014(25) | Shenzhen | 2008–2010 | 39046 | Monthly |
| Li, Zhang et al. 2019(18) | Ningxia | 2009–2013 | 917285 | Monthly |
| Liu, Bao et al. 2019(21) | Jiangsu | 2009–2016 | 13 928 | Monthly |
| Hong, Hao et al. 2018(40) | Inner Mongolia Autonomous Region | 2016 | 114777 | Monthly |
| Liu, Chen et al. 2018(20) | Nanjing | 2010–2015 | 1105117 | Weekly |
| Xu, Hu et al. 2019(27) | Guangdong | 2010–2013 | 357238 | Daily |
| Yan, Wei et al. 2019(23) | Shenzhen | 2009–2017 | 4873 | Daily |
| Huang, Ning et al. 2019(16) | Ningbo | 2012–2016 | 59809 | Daily |
Correlation between meteorological parameters and HFMD
The heterogeneity test results demonstrated that there was significant statistical heterogeneity in all the six meteorological parameters included in this study, and the random effect model was used to merge effect values (Table 2). The relatively high positive correlation with mean temperature (COR: 0.21, 95% CI: 0.26–0.55) and negative correlation with air pressure (COR:−0.27, 95% CI: −0.51– −0.03) were found. The correlation coefficients of the other four meteorological parameters (Average relative humidity, rainfall, sunshine and mean wind speed) and the incidence of HFMD very close (0.07–0.1).
Table 2:
Meta-analysis of the correlation between meteorological factors and HFMD
| Meteorological parameter | Studies | COR | 95% CI | P | I2 |
|---|---|---|---|---|---|
| Average relative humidity | 16 | 0.08 | [0.05,0.12] | <0.00001 | 100% |
| Mean temperature | 15 | 0.21 | [0.15,0.25] | <0.00001 | 100% |
| Rainfall | 14 | 0.10 | [0.07,0.13] | <0.00001 | 100% |
| Sunshine | 9 | 0.07 | [0.04,0.08] | <0.00001 | 100% |
| Meanwind speed | 9 | 0.07 | [0.02,0.11] | <0.00001 | 100% |
| Mean air pressure | 7 | −0.26 | [−0.65, −0.02] | <0.00001 | 100% |
Subgroup analysis
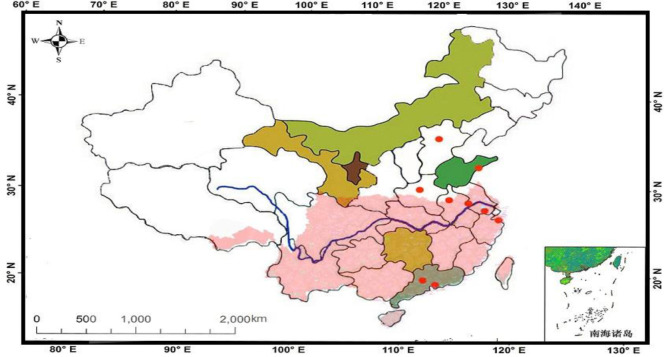
The geographical distribution of the studies included were divided into subtropical and temperate climate zones as shown in Fig. 2. In subgroup analysis by regional climate, no significant change was observed in the heterogeneity after stratification, as shown in Table 3. The correlation between the three meteorological parameters (average temperature, average rainfall and relative humidity) and HFMD was significantly stronger in subtropical zone than temperate zone. The effect of rainfall on the incidence of HFMD was very small (COR=0.007) in temperate zones.
Fig. 2:
Locations of studies included
Table 3:
Subgroup analysis of the correlation between meteorological factors and HFMD (regional climate)
| Variable | Temperate | ||||
|---|---|---|---|---|---|
| Studies | COR | 95% CI | I2% | P | |
| Mean temperature | 4 | 0.035 | [−0.01,0.12] | 100 | <0.00001 |
| Rainfall | 3 | 0.007 | [−0.00,0.03] | 97 | <0.00001 |
| Relative humidity | 4 | 0.014 | [0.00,0.04] | 97 | <0.00001 |
| Subtropical | |||||
| Studies | COR | 95% CI | I2% | P | |
| Mean temperature | 5 | 0.221 | [0.32,0.60] | 100 | <0.00001 |
| Rainfall | 4 | 0.166 | [0.14,0.47] | 100 | <0.00001 |
| Relative humidity | 4 | 0.073 | [0.02,0.20] | 99 | <0.00001 |
Sensitivity analysis and publication bias
Sensitivity analyses were performed to evaluate the effect of each study on the pooled results by excluding single studies sequentially. The results showed that there was no significant difference in the stability of the results after excluding individual studies. The funnel plots of all meteorological parameters are shown in Fig. 3. No publication bias existed in the meta-analysis.
Fig. 3:
The funnel plots of all meteorological parameters
Discussion
The current study found that the meteorological parameters played an important role in the prevalence of HFMD. Past studies have suggested that the mechanism of its effects is unclear, but we believe that among the common transmission routes of infectious diseases, only aerosols are considered to be affected by meteorological factors. Among the six meteorological parameters included in the analysis, only mean air pressure is negatively correlated with the incidence of HFMD, while the other five meteorological parameters (including mean temperature, average relative humidity, rainfall, sunshine and mean wind speed) are positively correlated with it, and the correlation coefficient with average temperature is the largest.
In this meta-analysis, the effect of mean wind speed on the incidence of HFMD was relatively weak, so transmission dynamics may not play an important role in HFMD outbreaks.
The aerosol transmission of influenza virus is affected by relative humidity and temperature in the environment. Aerosol spread of influenza virus is dependent upon both ambient relative humidity and temperature (31). Laboratory studies have shown the stability of enteric viruses are influenced by environmental factors such as temperature and relative humidity (32–34). In past studies, ambient humidity has been a concern. The relationship between relative humidity (RH) and viability has been thoroughly reviewed in a WHO report by Sobsey and Meschke (35). However, there are many exceptions that remain unexplained. But in this meta-analysis, the negative correlation between air pressure and the incidence of HFMD is prominent. As a nonenveloped virus, the survival of HFMD in aerosol is more susceptible to environmental factors. The lower pressure may accelerate aerosol evaporation by making water more diffused. But for the water activity of the aerosol, the effect of humidity should be greater than the average pressure. The main reason is probably lower air pressure reduce the damage of viruses dispersed on aerosol surfaces due to conformational rearrangement caused by surface tension shear stress and hydrophobicity, and eventually leads to relatively high concentration of viruses in the aerosol and increases the risk of infection among the population. Because this study is a systematic review and meta-analysis, the effect of air pressure on the transmission of virus in aerosol is only a hypothesis, which has not been verified experimentally.
We also conducted subgroup analysis by regional climate. Three meteorological parameters (mean temperature, rainfall and relative humidity) have significant association with the incidence of HFMD in subtropical regions. Subtropical climate is more suitable for the survival of enteroviruses (36) and outdoor activities of humans (37), while increasing the chance of exposure to pathogens. In temperate regions, the weak effect of the three meteorological parameters (mean temperature, rainfall and relative humidity) on the incidence of HFMD were found. In particular, the correlation between rainfall and the incidence of HFMD was not statistically significant. This may be because the temperate zone has less annual rainfall, making the impact on disease harder to show.
Conclusion
The results of the meta-analysis provided an epidemiological evidence that lower air pressure may be the main risk factor for the incidence of HFMD in Chinese mainland. The influence of meteorological parameters on the prevalence of HFMD is mainly through changing virus viability in aerosols, which may be different in different climate regions. Monitoring of meteorological parameters can be used to provide early warning of the occurrence and prevalence of HFMD and provide useful information for the development of preventive and control measures. In an environment with low air pressure, wearing a mask that filters the aerosol outdoors may help prevent HFMD infection.
Ethical considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
No funding was received in this study.
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Duff MF. (1968).Hand-foot-and-mouth syndrome in humans: coxackie A10 infections in New Zealand. Br Med J, 2(5606):661–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen KT, Chang HL, Wang ST, Cheng YT, Yang JY. (2007). Epidemiologic features of hand-foot-mouth disease and herpangina caused by enterovirus 71 in Taiwan, 1998–2005. Pediatrics, 120(2):e244–252. [DOI] [PubMed] [Google Scholar]
- 3.Ma E, Lam T, Chan KC, Wong C, Chuang SK. (2010). Changing epidemiology of hand, foot, and mouth disease in Hong Kong, 2001-2009. Jpn J Infect Dis, 63(6):422–426. [PubMed] [Google Scholar]
- 4.Ang LW, Koh BK, Chan KP, Chua LT, James L, Goh KT. (2009). Epidemiology and control of hand, foot and mouth disease in Singapore, 2001–2007. Ann Acad Med Singap, 38(2):106–112. [PubMed] [Google Scholar]
- 5.Nguyen NT, Pham HV, Hoang CQ, Nguyen TM, Nguyen LT, Phan HC, Phan LT, Vu LN, Tran Minh NN. (2014). Epidemiological and clinical characteristics of children who died from hand, foot and mouth disease in Vietnam, 2011. BMC Infect Dis, 14:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mao QY, Wang Y, Bian L, Xu M, Liang Z. (2016). EV71 vaccine, a new tool to control outbreaks of hand, foot and mouth disease (HFMD). Expert Rev Vaccines, 15(5):599–606. [DOI] [PubMed] [Google Scholar]
- 7.Wu JT, Jit M, Zheng Y, et al. (2016). Routine Pediatric Enterovirus 71 Vaccination in China: a Cost-Effectiveness Analysis. PLoS Med, 13(3):e1001975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Onozuka D, Hashizume M. (2011). The influence of temperature and humidity on the incidence of hand, foot, and mouth disease in Japan. Sci Total Environ, 410-411:119–125. [DOI] [PubMed] [Google Scholar]
- 9.Fujimoto T, Iizuka S, Enomoto M, et al. (2012). Hand, foot, and mouth disease caused by coxsackievirus A6, Japan, 2011. Emerg Infect Dis, 18(2):337–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pons-Salort M, Oberste MS, Pallansch MA, Abedi GR, Takahashi S, Grenfell BT, Grassly NC. (2018).The seasonality of nonpolio enteroviruses in the United States: Patterns and drivers. Proc Natl Acad Sci U S A, 115(12):3078–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tian L, Liang F, Xu M, Jia L, Pan X, Clements ACA. (2018). Spatio-temporal analysis of the relationship between meteorological factors and hand-foot-mouth disease in Beijing, China. BMC Infect Dis, 18(1):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Z, Sun H, Yan Y, et al. (2015). Epidemiological profiles of hand, foot, and mouth disease, including meteorological factors, in Suzhou, China. Arch Virol, 160(1):315–321. [DOI] [PubMed] [Google Scholar]
- 13.Feng H, Duan G, Zhang R, Zhang W. (2014).Time series analysis of hand-foot-mouth disease hospitalization in Zhengzhou: establishment of forecasting models using climate variables as predictors. PLoS One, 9(1):e87916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gou F, Liu X, Ren X, et al. (2017). Socio-ecological factors and hand, foot and mouth disease in dry climate regions: a Bayesian spatial approach in Gansu, China. Int J Biometeorol, 61(1):137–147. [DOI] [PubMed] [Google Scholar]
- 15.Huang Y, Deng T, Yu S, Gu J, Huang C, Xiao G, Hao Y. (2013). Effect of meteorological variables on the incidence of hand, foot, and mouth disease in children: a time-series analysis in Guangzhou, China. BMC Infect Dis, 13:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang R, Ning H, He T, Bian G, Hu J, Xu G. (2019). Impact of PM10 and meteorological factors on the incidence of hand, foot, and mouth disease in female children in Ningbo, China: a spatiotemporal and time-series study. Environ Sci Pollut Res Int, 26(18):17974–17985. [DOI] [PubMed] [Google Scholar]
- 17.Jiang FC, Yang F, Chen L, Jia J, Han YL, Hao B, Cao GW. (2016). Meteorological factors affect the hand, foot, and mouth disease epidemic in Qingdao, China, 2007–2014. Epidemiol Infect, 144(11):2354–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Zhang X, Wang L, Xu C, Xiao G, Wang R, Zheng F, Wang F. (2019). Spatial-temporal heterogeneity of hand, foot and mouth disease and impact of meteorological factors in arid/ semi-arid regions: a case study in Ningxia, China. BMC Public Health, 19(1):1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L, Qiu W, Xu C, Wang J. (2018). A spatiotemporal mixed model to assess the influence of environmental and socioeconomic factors on the incidence of hand, foot and mouth disease. BMC Public Health, 18(1):274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu S, Chen J, Wang J, Wu Z, Wu W, Xu Z, Hu W, Xu F, Tong S, Shen H. (2018). Predicting the outbreak of hand, foot, and mouth disease in Nanjing, China: a time-series model based on weather variability. Int J Biometeorol, 62(4):565–574. [DOI] [PubMed] [Google Scholar]
- 21.Liu W, Bao C, Zhou Y, et al. (2019). Forecasting incidence of hand, foot and mouth disease using BP neural networks in Jiangsu province, China. BMC Infect Dis, 19(1):828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu X, Hu S, Kwaku AB, Li Q, Luo K, Zhou Y, Tan H. (2017). Spatio-temporal clustering analysis and its determinants of hand, foot and mouth disease in Hunan, China, 2009–2015. BMC Infect Dis, 17(1):645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan S, Wei L, Duan Y, et al. (2019). Short-Term Effects of Meteorological Factors and Air Pollutants on Hand, Foot and Mouth Disease among Children in Shenzhen, China, 2009–2017. Int J Environ Res Public Health, 16(19):3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Q, Zhou M, Yang Y, You E, Wu J, Zhang W, Jin J, Huang F. (2019). Short-term effects of extreme meteorological factors on childhood hand, foot, and mouth disease reinfection in Hefei, China: A distributed lag non-linear analysis. Sci Total Environ, 653:839–848. [DOI] [PubMed] [Google Scholar]
- 25.Zheng S, Cao CX, Cheng JQ, Wu YS, Xie X, Xu M. (2014). Epidemiological features of hand-foot-and-mouth disease in Shenzhen, China from 2008 to 2010. Epidemiol Infect, 142(8):1751–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu M, Yu W, Tong S, Jia L, Liang F, Pan X. (2015). Non-Linear Association between Exposure to Ambient Temperature and Children’s Hand-Foot-and-Mouth Disease in Beijing, China. PLoS One, 10(5):e0126171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu Z, Hu W, Jiao K, Ren C, Jiang B, Ma W. (2019).The effect of temperature on childhood hand, foot and mouth disease in Guangdong Province, China, 2010–2013: a multicity study. BMC Infect Dis, 19(1):969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. (2000). Improving the Quality of Reports of Meta-Analyses of Randomised Controlled Trials: The QUOROM Statement. Onkologie, 23(6):597–602. [DOI] [PubMed] [Google Scholar]
- 29.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. (2010).A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods, 1(2):97–111. [DOI] [PubMed] [Google Scholar]
- 30.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S. (2007). The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Epidemiology, 18(6):800–804. [DOI] [PubMed] [Google Scholar]
- 31.Lowen AC, Mubareka S, Steel J, Palese P. (2007). Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathog, 3(10):1470–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abad FX, Pinto RM, Bosch A. (1994). Survival of enteric viruses on environmental fomites. Appl Environ Microbiol, 60(10):3704–3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mbithi JN, Springthorpe VS, Sattar SA. (1991). Effect of relative humidity and air temperature on survival of hepatitis A virus on environmental surfaces. Appl Environ Microbiol, 57(5):1394–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGeady ML, Siak JS, Crowell RL. (1979). Survival of coxsackievirus B3 under diverse environmental conditions. Appl Environ Microbiol, 37(5):972–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MD S, JS M. (2003). Virus survival in the environment with special attention to survival in sewage droplets and other environmental media of fecal or respiratory origin. WHO, Geneva, Switzerland. [Google Scholar]
- 36.Suminski RR, Poston WC, Market P, Hyder M, Sara PA. (2008). Meteorological conditions are associated with physical activities performed in open-air settings. Int J Biometeorol, 52(3):189–197. [DOI] [PubMed] [Google Scholar]
- 37.Belanger M, Gray-Donald K, O’Loughlin J, Paradis G, Hanley J. (2009). Influence of weather conditions and season on physical activity in adolescents. Ann Epidemiol, 19(3):180–186. [DOI] [PubMed] [Google Scholar]