Abstract
Background
To evaluate the impact of air pollution exposure on semen quality parameters during COVID-19 outbreak in China, and to identify potential windows of susceptibility for semen quality.
Methods
A retrospective observational study was carried out on 1991 semen samples collected between November 23, 2019 and July 23, 2020 (a period covering COVID-19 lock-down in China) from 781 sperm donor candidates at University-affiliated Sichuan Provincial Human Sperm Bank. Multivariate mixed-effects regression models were constructed to investigate the relationship between pollution exposure, windows of susceptibility, and semen quality, while controlling for biographic and meteorologic confounders.
Result(s)
The results indicated multiple windows of susceptibility for semen quality, especially sperm motility, due to ambient pollution exposure. Exposure to particulate matters (PM2.5 and PM10), O3 and NO2 during late stages of spermatogenesis appeared to have weak but positive association with semen quality. Exposure to CO late in sperm development appeared to have inverse relationship with sperm movement parameters. Exposure to SO2 appeared to influence semen quality throughout spermatogenesis.
Conclusion(s)
Potential windows of susceptibility for semen quality varied depending on air pollutants. Sperm motility was sensitive to pollution exposure. Findings from current study further elucidate the importance of sensitive periods during spermatogenesis and provide new evidence for the determinants of male fertility.
Keywords: Pollution exposure, Semen quality, Windows of susceptibility, Sperm motility, Computer sperm analysis
1. Introduction
Infertility, defined as the inability to conceive after one year of unprotected intercourse, is a global health issue and has far-reaching social influence (Choy and Eisenberg, 2018). Globally, approximately 15% of couples are affected by infertility, with male infertility contributing to 50% of the cause (Choy and Eisenberg, 2018). Numerous reports have indicated that semen quality in men is declining at a population level (Huang et al., 2017; Levine et al., 2017; Virtanen et al., 2017). Recent investigations on air pollution and semen quality have indicated that exposure to air pollutants, such as PM2.5, is likely linked with the declining trends in semen quality (Deng et al., 2016; Hammoud et al., 2010; Hansen et al., 2010), as indicated by clinically assessed sperm quantity and quality (Dias et al., 2019). The duration of spermatogenesis, or sperm development, is about 90 days (Clermont, 1963), during which exposure to ambient pollutants has been linked to changes in semen quality parameters (Huang et al., 2020; Qiu et al., 2020).
Ambient air pollution has been associated with various health effects, ranging from subclinical outcomes to death (Fang et al., 2016; Slama et al., 2008), and it is of particular concern in rapidly developing countries with severe pollution, such as China (Chan and Yao, 2008; Lin et al., 2013; Song et al., 2017; Wang et al., 2014). Recent studies on Chinese male population have indicated declining semen quality similar to the global trend (Deng et al., 2016; Huang et al., 2017), with potential linkage to air pollution (Han et al., 2011; Qiu et al., 2020; Zhou et al, 2014, 2018).
In the context of air pollution and the continuous decline of male fertility, the emergence and rapid development of the global coronavirus pandemic (COVID-19) pose a unique condition for studying male fertility. In order to control the spread of COVID-19, China implemented social distancing measures and imposed a nation-wide lock-down during the early part of 2020 (Liu et al., 2020; Pei et al., 2020). This lockdown period, following traditional Chinese New Year holiday, created an extended period of reduced traffic and industrial activities, resulting in a reduction of common ambient pollutants. In particular, pollutants related to industrial activities and traffic, such as PM2.5, PM10, NO2, and CO, were reduced during and/or post the lock-down period (Liu et al., 2020; Pei et al., 2020; Venter et al., 2020). The nearly three-month (90-day) lockdown period in China due to the COVID-19 epidemic provides a study opportunity to investigate the relationship between ambient pollutant exposure during sperm development (a 90-day process) and semen quality. Previous studies on pollution exposure and semen quality mostly analyzed the average exposure during entire spermatogenesis (Huang et al., 2020; Qiu et al., 2020; Sun et al., 2020), with limited investigation focused on specific exposure windows within the spermatogenesis period. There are three key periods in the entire spermatogenesis (90-day), namely epididymis storage (in cauda epididymidis), development of sperm motility (in caput epididymidis), spermatocytogenesis (in seminiferous tubule) (Huang et al., 2020; Zhang et al., 2019). To the best of our knowledge, there were only a few studies that considered these three sperm development stages as separate exposure periods (Huang et al., 2020; Zhang et al., 2019). Additionally, previous studies analyzed semen quality with a small number of clinical parameters, such as sperm concentration and progressive rate, with limited information on sperm movement parameters. Sperm movement parameters are indicators of sperm viability and potential fertility, and even were considered as sensitive biomarkers of human reproductive toxicity (Selevan et al., 2000; Zhou et al., 2014). Moreover, existing literature on pollution exposure and male fertility often utilized ambient pollution data matched from the closest pollution monitoring station, an approach that has limited ability to capture the specific exposure of individuals within the study population.
In this study, we investigated the relationship between semen quality and the following potential windows of susceptibility: 0–9 days before ejaculation (epididymis storage), 10–14 days before ejaculation (development of sperm motility), 70–90 days before ejaculation (spermatocytogenesis), and 0–90 days before ejaculation. Additionally, we were interested in understanding if the lock-down period influenced the outcome of sperm development. To better analyze semen quality, we included sperm movement parameters in addition to standard semen quality indicators. Lastly, to better reflect each individual's exposure condition, we utilized 0.1 ° × 0.1 ° (approximately 10 km × 10 km) ambient pollution data matched with specific exposure location. Findings from the current study could further enhance existing understanding of the relationship between pollution exposure and male fertility by highlighting potential sensitive periods during sperm formation.
2. Materials and methods
2.1. Study period
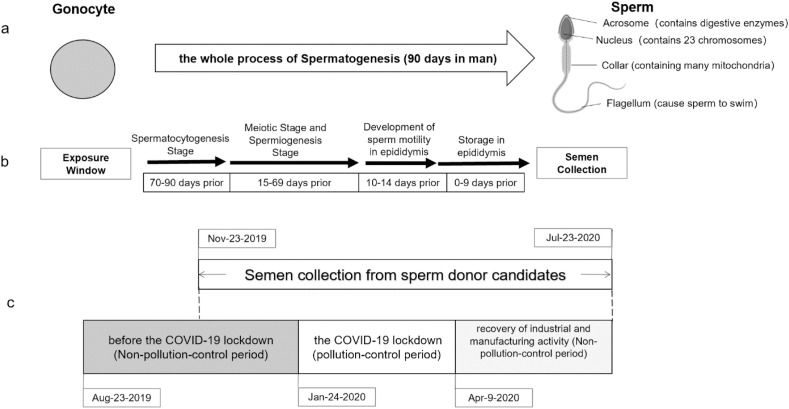
To investigate the potential effect of lock-down regulations on semen quality, we divided the overall study period into three sub-periods: before lock-down (2019-11-23 to 2020-01-23), during lock-down (2020-01-24 to 2020-04-08), and post lock-down (2020-04-09 to 2020-07-23) (Fig. 1 ). 2020-01-24 marks the beginning of Chinese New Year holiday period, during which industrial activities slowed down and people predominantly stayed at home. For 2020, the lock-down period due to COVID-19 pandemic extended the stay-at-home phenomenon with a national mandate issued on 2020-01-27 to limit activities (General Office of the State Council, 2020). On April 8, 2020, China's central government issued announcement to re-start economic activities and to resume “normality” (Joint Prevention and Control Mechanism of the State Council, 2020). We further extended the end of the study period to 2020-07-23 to have eight full months for analysis.
Fig. 1.
Spermatogenesis timeline (a), exposure windows (b) and study timeline (c).
2.2. Recruitment and data collection
Individuals included in this study were part of Sichuan Provincial Human Sperm Bank of China (SHSB) donor population. SHSB, located in Chengdu, capital city of Sichuan Province in southwest China, is the only official medical institution licensed to collect and store sperm from donors in Sichuan Province. The recruitment methods for sperm donors include handing out leaflets, conducting lectures in schools, and network publicity by technicians. The screening of sperm donors is conducted in strict accordance with the standard guidelines published by the Chinese Ministry of Health (China Ministry of Health, 2004). In this retrospective study, we recruited sperm donors who provided at least one semen sample between November 23, 2019 and July 23, 2020. Demographic information and clinical data were obtained from SHSB database. Demographic information included age, height, weight, ethnicity, education level, and duration of abstinence. Clinical data included semen parameters and the date of semen collection and analysis (occurring on the same day). Additional information on residential address at the time of sample collection, working address during study period, smoking, and drinking information were collected based on self-reported questionnaires. Ethics approval for this study was obtained from the ethics review board of West China Second University Hospital of Sichuan University, and all experimental protocols for human subjects were in accordance with guidelines approved by the Institutional Review Board of West China Second University Hospital of Sichuan University (WCSUH-SCU IRB, 2019–076).
2.3. Semen analysis
Semen collection and analysis followed the World Health Organization (WHO)'s 5th edition of the laboratory manual for the examination and processing of human semen (World Health Organization, 2010). Self-reported abstinence duration was recorded by clinical staff. Semen samples were collected in the laboratory by masturbation into a sterile container. After collection, the sample was immediately placed at 37 °C and evaluated directly. The sperm concentration and motility were determined according to the recommendation of the WHO 2010 and measured by the Makler chamber. The analysis was performed at room temperature and at a magnification of 200 × . Percentage of spermatozoa with progressive motility, non-progressive motility, and non-motile spermatozoa were assessed on 200 spermatozoa. Sperm motility was assessed within one-hour after sample collection. The following semen quality parameters were assessed by Computer Assisted Sperm Analysis system (CASA) from Beijing Suijia Software Co. LTD: sperm concentration (millions/mL), total mortility (%), percentage of progressive motile sperms (or progressive rate, %), and some movement characteristics of sperm, such as curvilinear velocity (VCL, μm/s), straight-line velocity (VSL, μm/s), average path velocity (VAP, μm/s), amplitude of lateral head displacement (ALH, μm/s), linearity (LIN, %), straightness (STR, %), wobble (WOB, %), beat-cross frequency (BCF, times/s). Data for semen volume (ml) and viability were obtained by weighing method and Eosin staining, respectively. Total sperm count (millions) was calculated as semen volume multiplied by sperm concentration.
2.4. Exposure assessment
2.4.1. Environmental data
Hourly data of six major air pollutants (PM2.5 in μg/m3, PM10 in μg/m3, SO2 in μg/m3, NO2 in μg/m3, CO in mg/m3, O3 in μg/m3) were obtained from China Network Environment Monitoring Center (CNEMC, http://www.cnemc.cn/). The aforementioned six pollutants were chosen for analysis because of their routine monitoring during the study period and previous reports of their association with fertility and semen quality (such as PM10, SO2 and NO2 in Zhou et al., 2014). Daily concurrent ground temperature, relative humidity (RH), and sunshine duration data were obtained from China's National Meteorological Information Center (http://data.cma.cn/) for all study population. Data quality control was conducted on all pollutant concentration and weather parameters to check for missingness, extreme values, distribution, and dispersion of measurements.
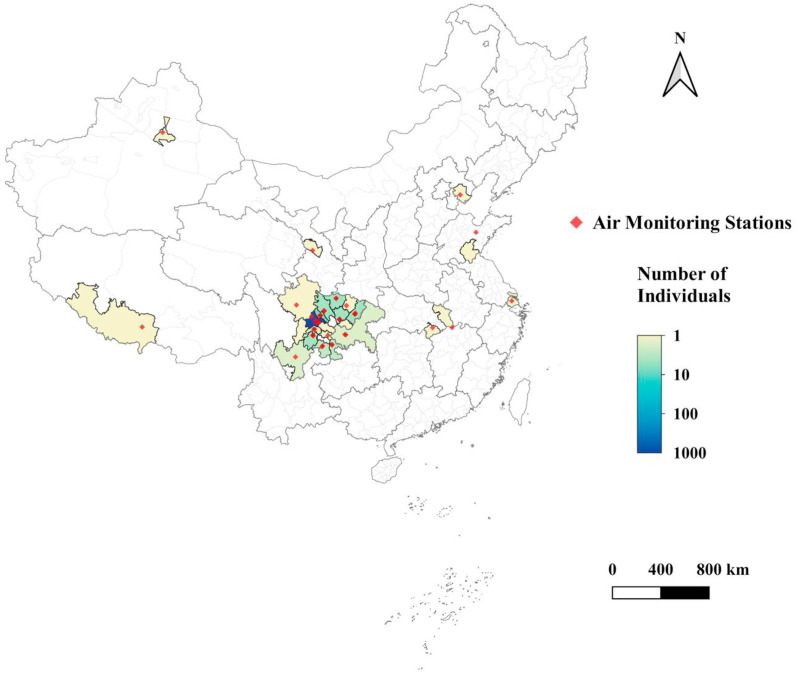
We extracted each study individual's residential address and standardized the addresses to district level to serve as spatial units. We then estimated the daily temperature and relative humidity, as well as daily concentrations of PM2.5, PM10, O3, NO2, SO2, and CO for each spatial unit by averaging its overlapped grid cells at 0.1° resolution. For weather conditions, we interpolated the daily observations at 839 meteorological stations to grid cells by using co-kriging with elevation (Kanevski, 2008). For air pollutants, we derived the gridded concentrations by using random forests (a machine learning algorithm) based on the data from the air quality monitoring network, satellite retrievals, meteorological conditions, and various geographic factors such as land use types. The air quality monitoring network consisted of approximately 1500 state-managed sites across Mainland China. For each air pollutant, a separate random forest was developed for estimating its daily concentrations. With respect to the satellite retrievals, we utilized the aerosol optical depth (AOD) from the Multi-Angle Implementation of Atmospheric Correction (MAIAC) for PM2.5 and PM10, the vertical column densities of NO2 (and SO2) from the Ozone Monitoring Instrument (OMI) for NO2 (and SO2), and the Measurements of Pollution in the Troposphere (MOPITT) CO retrievals for CO (Deeter et al., 2014; Lamsal et al., 2021; Li et al., 2013; Lyapustin et al., 2011). As NOx is an important precursor to O3, we used the vertical column densities of NO2 from OMI for deriving the O3 concentrations. Please refer to the previous studies for the details of the modeling procedure and predictive performance (Liu et al., 2019; Zhan et al, 2017, 2018a, 2018b; Zhang et al., 2019). The final exposure data for entire study population covered 24 cities in nine provinces across three time zones from UTC+6 to UTC+8 (Fig. 2 ).
Fig. 2.
Spatial distribution of study population during study period and respective ambient air quality monitoring stations.
During the study period, several individuals reported change of addresses in the questionnaire (7 before 2019-11-23, 3 between 2020-01–24 and 2020-04-08, and 5 after 2020-04-08). We checked each individual's questionnaire and followed-up with the individuals to verify the addresses and moving dates, then made adjustment accordingly.
2.5. Statistical analysis
2.5.1. Descriptive statistics
Demographic information, including age, height, weight, ethnicity, education level and duration of abstinence, was summarized for the study population. Data for key semen quality parameters were summarized for the following periods: before the government-mandated lock-down (2019-11-23 to 2020-01-23), during the lock-down (2020-01-24 to 2020-04-08, and after the lock-down (2020-04-09 to 2020-07-23). 2020-01-24 to 2020-02-02 was Chinese New Year and a 10-day national holiday, which was followed by extended homestay period due to COVID-19 pandemic. Chinese government issued “Epidemic Prevention and Proactive Restarting Production Activities Guidelines” (CPC Central Committee Leading Group on Novel Coronavirus, 2020) on April 9, 2020, signaling the restart of normality. Because spermatogenesis and spermiogenesis together is a 90-day process (Clermont, 1963; Heller et al., 1964), we included exposure data from 90 days prior to first semen collection record and 90 days after the last semen collection record as part of the analysis.
2.5.2. Regression analysis
Three multivariate mixed-effects models were constructed to investigate 1) if the government-initiated country-wide lock-down in China elucidated any effect on semen quality, and 2) the relationship between exposure windows and semen quality.
The first model included semen quality parameters (i.e., sperm concentration, progressive rate, ALH, BCF, LIN, STR, VAP, VCL, VSL, WOB), as outcome variables separately, while adjusting for weather (i.e., temperature, RH), individual's demographic condition (i.e., age, BMI, education level, ethnicity), and other habitual information (i.e., abstinence days, drinking and smoking status). A categorical variable was created for day of sample collection to indicate whether the sample was collected before, during, or after the lock-down period. As some individuals provided more than one semen sample during study period, individual (i.e., ID) was included as a random variable. The second model analyzed quality parameters aforementioned in the first model separately as outcome variables, while including single-pollutant analyses for each of the six pollutants (PM2.5, PM10, CO, O3, SO2, NO2) during different exposure windows (0–9 days preceding sample collection, 10–14 day preceding sample collection, 15–69 days preceding sample collection, 70–90 days preceding sample collection, and 0–90 days preceding sample collection). This model adjusted for weather (i.e., temperature, RH), individuals' demographic condition (i.e., age, BMI, education level, ethnicity), and other habitual information (i.e., abstinence days, drinking and smoking status). Individual (i.e., ID) was included as a random variable. The third model was built on the second model by further stratifying analysis by before, during, or after the lock-down period.
In addition, we conducted an analysis on the effect of temperature (lag 0–90 days ground temperature) on the following semen quality parameters: sperm concentration, total sperm number, total mortility, and progressive mortility. We first modeled temperature exposure as a natural cubic spline function with 4 degrees of freedom and set boundary knots to 1st and 99th percentile of temperature during the study period. Similar to Zhou et al. (2020), our model adjusted for potential counfounders including age, BMI, education, smoking, abstinence period and daily ground temperature on the day of semen collection, as well as lag 0–90 day PM2.5 concentration and lag 0–90 day relative humidity. Individual was included as the random effect.
All analyses were conducted in R language (R Core Team, 2020), with packages: tidyverse, nlme, lme4, ggplot2, and zoo (Bates et al., 2015; Pinheiro et al., 2020; Wickham, 2016; Wickham et al., 2019; Zeileis and Grothendieck, 2005), and Python language (version 3.7.1), with packages: pandas 1.2.0, numpy 1.18.1, matplotlib 3.1.3 (Caswell et al., 2020; Harris et al., 2020; Reback et al., 2020).
3. Results
3.1. Study population and ambient pollution exposure during study period
The recruited study population was young, healthy, and overall well-educated (Table 1 ). Out of the 923 individuals recruited, the average age was 25.61 years (SD: 4.99 years), average height was 174.18 cm (SD: 5.14 cm), and average weight was 67.77 kg (SD: 9.05 kg). 95% of the study population was of Han ethnicity and 90% of them were single and never married. 90% of the study population received college or higher education. 73% of the study population never smoked and 56% never consumed alcohol. During the entire study period (2019-11-23 to 2020-07-23), the predominant portion of the study population resided within Sichuan Province (Fig. 2), with the dominant population residing within the Chengdu metropolitan (Fig. 2, dark blue).
Table 1.
Summary statistics of study population.
| Population Characteristics | Drinking and Smoking Habits | N (%) | |
|---|---|---|---|
| Mean (SD) | Smoking status | ||
| Age | 25.61 (4.99) | 1-3 times/week | 111(12%) |
| Height | 174.18 (5.14) | 4-6 times/week | 52(6%) |
| Weight | 67.77 (9.05) | More than 7 times/week | 83(9%) |
| Never | 677(73%) | ||
| Ethnicity | N (%) | Smoking frequency | |
| Han | 872 (95%) | 1-5/day | 165(18%) |
| Yi | 10 (1%) | 6-12/day | 71(8%) |
| Other | 41 (4%) | More than 12/day | 10(1%) |
| Education Level | N (%) | Alcohol consumption frequency | |
| High School and Below | 94 (10%) | 1-3 times/week | 403(44%) |
| College | 739 (80%) | 4-6 times/week | 3 (0.3%) |
| Graduate School | 90 (10%) | Never | 517(56%) |
| Marriage Status | N (%) | Alcohol consumption | |
| Single, Never Married | 734 (90%) | 50–150 mL/time | 11(1%) |
| Married | 173 (19%) | 150–250 mL/time | 366(40%) |
| Divorced | 16 (2%) | More than 250 mL/time | 29(3%) |
Abbreviations: N=number of unique individuals; SD=standard deviation.
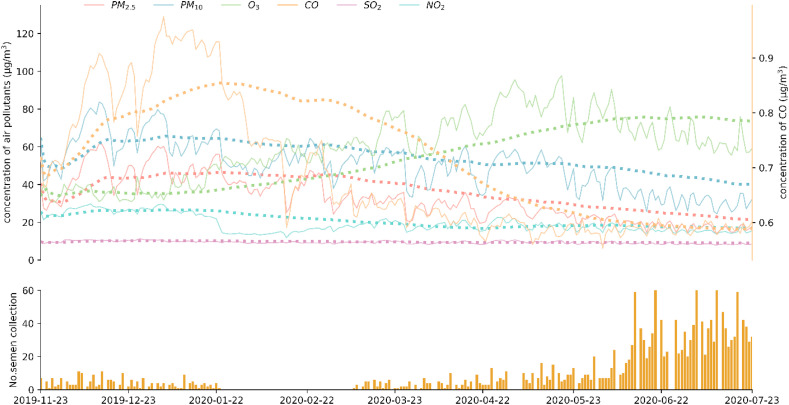
During study period, particulate matter (PM2.5 and PM10) and CO concentrations experienced by the study population showed a gradually decreasing trend (Fig. 3 , top panel). However, O3 concentration exhibited an overall increase. SO2 and NO2 concentrations experienced by the study population remained stable and at low concentration level. In the beginning of the study period (up until 2020-01-24 before lock-down), daily number of sample collection was low and remained below 10 samples/day for most days. During the early portion of the lock-down period, sample collection was halted, resulting in no sample collection until March 2020. During the latter half of the lock down period (up until 2020-04-08), sample collection resumed. During the post lock-down period, daily sample collection increased steadily. We conducted Spearman statistics to test for potential effect due to collinearity between pollutants and weather parameters. No strong collinearity was observed between pollutants and weather parameters except for PM2.5 and PM10 (Spearman rho cut off: 0.8; results not shown).
Fig. 3.
Temporal change in ambient pollution experienced by the study population and daily number of sample collection. Dotted line in top panel represents 90-day lag average of respective air pollutants. All concentrations in μg/m3 except for CO, which is in mg/m3.
3.2. Temporal variability of semen quality
Out of the 923 individuals recruited, 781 unique individuals with 1991 samples provided sufficient semen quality data to be included in subsequent analyses (Table 2 ). Sperm concentration differed significantly between three sub-periods, with during shut-down period exhibiting the highest average sperm concentration of 136.91 mil/mL. Progressive rate was significantly lower for samples collected post lock-down when compared with before and during lock-down periods (p < 0.001). Almost all parameters related to sperm motility exhibited significant temporal variability between study periods. The quality of sperm motion, as indicated by VCL, VAP, LIN, ALH, and BCF, was lower for samples collected post lock-down period (p=0.01, 0.02, <0.001, 0.02, respectively). Averages for WOB (an indicator for a measure of oscillation of the actual path about the average path, VAP/VCL), STR (an indicator for linearity of the average path, VSL/VAP), and ALH (an indicator for magnitude of lateral displacement of a sperm head about its average path) were lower for samples collected post lock-down period (p < 0.001 for all three).
Table 2.
Summary of semen quality parameters during study period.
| Semen Quality Parameters | Before (N*=74, n=219) |
During (N*=43, n=81) |
After (N*=726, n=1691) |
|
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | p value | |
| Abstinence (days) | 4.26 (1.19) | 4.48 (1.13) | 4.48 1.13) | 0.03 |
| Semen Volume (mL) | 3.95 (1.66) | 3.98 (1.69) | 3.86 (1.43) | 0.55 |
| Sperm Concentration (mil/mL) | 127.44 (65.24) | 136.91 (77.33) | 116.81 (73.58) | 0.01 |
| Total Sperm Count (millions) | 476.48(262.48) | 514.85(307.98) | 430.49(271.45) | <0.001 |
| Progressive Rate (%) | 70.31 (10.18) | 70.35 (8.57) | 66.52 (12.45) | <0.001 |
| Total Motility(%) | 0.73(0.10) | 0.73(0.10) | 0.70(0.13) | <0.001 |
| Round Cells(mil/mL) | 0.23 (0.20) | 0.22 (0.18) | 0.23 (0.52) | 0.97 |
| VCL (μm/s)a | 46.22 (8.62) | 46.52 (9.00) | 44.50 (10.05) | 0.01 |
| VAP (μm/s)a | 32.91 (6.34) | 33.32 (6.74) | 31.72 (7.36) | 0.02 |
| VSL (μm/s)a | 24.47 (5.53) | 25.00 (5.93) | 23.83 (6.19) | 0.10 |
| WOB (%)a | 0.63 (0.07) | 0.623 0.073) | 0.61 (0.09) | <0.001 |
| STR (%)a | 0.60 (0.07) | 0.61 (0.07) | 0.59 (0.09) | <0.001 |
| LIN (%)a | 0.44 (0.07) | 0.44 (0.07) | 0.43 (0.08) | 0.01 |
| ALH (μm/s)a | 4.82 (1.04) | 4.62 (0.90) | 4.44 (1.08) | <0.001 |
| BCF (times/s)a | 10.93 (1.71) | 11.48 (1.83) | 10.86 (2.04) | 0.02 |
Abbreviations: N=number of unique individuals; n=number of samples; SD=standard deviation; VCL=curvilinear velocity; VSL=straight-line velocity, VAP=average path velocity; ALH=amplitude of lateral head displacement; LIN=linearity; STR=straightness; WOB=wobble; BCF=beat-cross frequency.
* N is number of unique individuals during each time period, some individuals provided samples during different time periods; n is number of samples. Before is 2019–11-23 to 2020-01-23, During is 2020–01-24 to 2020-04-08, After is 2020–04-09 to 2020-07-23. **Test of significance at 0.05 level. N=unique individuals, n=number of samples.
Quality of sperm motion analyzed by CASA, only for samples with at least 25 sperm tracks, including VCL(μm/s), VAP(μm/s), VSL(μm/s), WOB(%), STR(%), LIN(%), ALH(μm/s), BCF(times/s).
After adjusting for covariates, such as abstinence, age, and BMI, multivariate linear mixed-effects model results suggest that most of semen quality parameters did not differ significantly between the lock-down period and outside of the lock-down period (before lock-down or after lock-down), except for ALH and BCF (Table S2). Compared to lock-down period, ALH was 39% higher before lock-down (p < 0.005), whereas BCF was 74% lower before lock-down period (p < 0.005).
Additionally, we conducted regression analysis to test the potential effect of collinearity by removing one pollutant or one weather parameter at a time. The regression results were similar to that of the main models, suggesting that collinearity was not of concern (results not shown). We conducted sensitivity analysis by analyzing a subset of individuals who provided multiple semen samples during the study period. The results were similar to that obtained for the entire study population (results not shown).
3.3. Air pollution exposure during different exposure windows of spermatogenesis
For the study population, average exposures to ambient pollutants over the course of spermatogenesis differed during different developmental stages (Table 3 ). Exposures to PM2.5, PM10, NO2, and SO2 were at the highest 70–90 days before sample collection compared to other spermatogenesis periods (41.90 μg/m3, 69.97 μg/m3, 41.16 μg/m3, 6.51 μg/m3, respectively), whereas exposure to O3 was the lowest during that development window at 58.02 μg/m3.
Table 3.
Air pollution exposure during different time period.
| Pollutant and Weather Parameters | Days before Sample Collection (N=781, n=1991) |
||||
|---|---|---|---|---|---|
| 0–90 days |
0–9 days |
10–14 days |
70–90 days |
||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | p value | |
| PM2.5 (μg/m3) | 39.13 (7.73) | 32.96 (17.25) | 34.57 (17.61) | 41.90 (9.84) | <0.001 |
| PM10 (μg/m3) | 66.84 (10.55) | 54.81 (24.77) | 58.00 (25.69) | 69.97 (13.91) | <0.001 |
| O3 (μg/m3) | 69.08 (19.09) | 70.44 (23.18) | 74.05 (25.98) | 58.02 (18.17) | <0.001 |
| NO2 (μg/m3) | 39.49 (7.06) | 34.72 (10.91) | 35.88 (12.47) | 41.16 (10.04) | <0.001 |
| CO (mg/m3) | 0.66 (0.09) | 0.69 (0.16) | 0.68 (0.17) | 0.68 (0.11) | <0.001 |
| SO2 (μg/m3) | 6.21 (1.85) | 5.53 (2.41) | 5.69 (2.74) | 6.51 (1.96) | <0.001 |
| Ground Temperature(°C) | 22.24 (5.00) | 25.03 (6.74) | 24.86 (6.71) | 18.54 (4.60) | <0.001 |
| Relative humidity(%) | 75.64 (5.33) | 79.55 (6.89) | 78.34 (7.61) | 75.97 (7.27) | <0.001 |
Abbreviations: N=number of unique individuals; n=number of samples; SD=standard deviation; PM2.5=particulate matter with aerodynamic diameter ≤2.5 μm; PM10=particulate matter with aerodynamic diameter ≤10 μm; O3=ozone; NO2=nitrogen dioxide; CO=carbon monoxide; SO2=sulfur dioxide.
We found the association between semen quality parameters and ambient pollutants varied during different stages of spermatogenesis (Table 4 ). Ambient particulate matters (PM2.5 and PM10) had weak but significantly positive associations with progressive rate and multiple sperm motility parameters (such as VAP, VCL, WOB) during late stages of spermatogenesis (0–9 days and 10–14 days prior to semen sample collection). Average exposure to PM2.5 and PM10 during the entire sperm developmental period also appeared to be weakly but positively associated with progressive rate and BCF, VAP, VCL, VSL and WOB. However, sperm concentration did not appear to be associated with exposure to particulate matters (PM2.5 and PM10).
Table 4.
Summary of mixed effects model for exposure window.
| Exposure Window | Progressive Rate | Total Motility | Sperm Concentration | Total Sperm Count | ALH | BCF | LIN | STR | VAP | VCL | VSL | WOB | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PM2.5 | 0~9 | 0.10 *** | 0.00 ** | 0.22 | 0.67 | 0.01 ** | 0.01 | 0.00 | 0.00 * | 0.04 ** | 0.05 ** | 0.02 | 0.00 ** |
| 10~14 | 0.05 * | 0.00 * | 0.16 | 0.49 | 0.00 * | 0.00 | 0.00 | 0.00 | 0.02 | 0.02 | 0.01 | 0.00 * | |
| 15~69 | 0.09 | 0.00 | 0.03 | −0.89 | −0.00 | 0.03 *** | 0.00 | 0.00 | 0.07 * | 0.08 * | 0.05 * | 0.00 | |
| 70~90 | −0.01 | −0.00 | −0.29 | −1.33 | −0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.01 | 0.01 | 0.00 | |
| 0~90 | 0.14 * | 0.00 | −0.01 | −1.11 | 0.00 | 0.03 *** | 0.00 | 0.00 | 0.09 ** | 0.11 ** | 0.07 * | 0.00 * | |
| PM10 | 0~9 | 0.08 *** | 0.00 *** | 0.16 | 0.41 | 0.01 *** | 0.00 | 0.00 | 0.00 * | 0.03 *** | 0.05 *** | 0.02 * | 0.00 *** |
| 10~14 | 0.04 ** | 0.00 ** | 0.15 | 0.55 | 0.00 * | 0.00 | 0.00 | 0.00 | 0.02 * | 0.03 * | 0.01 | 0.00 ** | |
| 15~69 | 0.05 | 0.00 | 0.07 | −0.44 | −0.00 | 0.02 ** | 0.00 | 0.00 | 0.04 * | 0.05 * | 0.02 | 0.00 | |
| 70~90 | 0.01 | 0.00 | −0.19 | −0.62 | −0.00 | −0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | |
| 0~90 | 0.13 ** | 0.00 * | 0.07 | −0.48 | 0.00 | 0.02 ** | 0.00 | 0.00 | 0.08 ** | 0.09 ** | 0.05 * | 0.00 * | |
| O3 | 0~9 | 0.04 | 0.00 | −0.01 | −0.46 | −0.00 | 0.01 ** | 0.00 | 0.00 | 0.03 ** | 0.04 * | 0.03 * | 0.00 |
| 10~14 | 0.02 | 0.00 | 0.05 | 0.27 | −0.00 | 0.01 *** | 0.00 | 0.00 | 0.03 ** | 0.03 ** | 0.02 * | 0.00 | |
| 15~69 | −0.13 *** | −0.00 ** | −0.22 | −0.39 | −0.01 *** | −0.01 | −0.00 | −0.00 * | −0.07 *** | −0.10 *** | −0.04 ** | −0.00 *** | |
| 70~90 | −0.05 * | −0.00 | −0.06 | −0.03 | −0.00 | −0.01 *** | −0.00 | −0.00 | −0.03 ** | −0.04 ** | −0.02 * | −0.00 | |
| 0~90 | −0.13 ** | −0.00 * | −0.27 | −0.57 | −0.01 ** | −0.01 | −0.00 | −0.00 | −0.06 ** | −0.09 ** | −0.03 | −0.00 ** | |
| CO | 0~9 | 1.77 | 0.01 | −0.58 | −11.27 | 0.02 | −0.34 | 0.01 | 0.01 | −0.94 | −1.77 | −0.69 | 0.02 |
| 10~14 | −2.92 | −0.02 | −4.72 | −70.78 | 0.04 | −1.29 ** | −0.01 | −0.01 | −3.38 * | −4.55 * | −2.48 | −0.01 | |
| 15~69 | 13.11 * | 0.10 | −2.27 | −53.66 | 0.19 | 1.61 | 0.08 * | 0.08 * | 6.09 | 6.54 | 5.54 * | 0.08 * | |
| 70~90 | 1.75 | 0.01 | −10.69 | −95.89 | 0.14 | 0.89 | 0.01 | 0.01 | 2.25 | 3.38 | 1.69 | 0.02 | |
| 0~90 | 10.93 | 0.08 | −14.54 | −141.56 | 0.15 | 1.60 | 0.07 | 0.08 | 5.35 | 5.90 | 4.86 | 0.08 | |
| NO2 | 0~9 | 0.13 ** | 0.00 * | 0.38 | 1.12 | 0.01 * | 0.00 | 0.00 | 0.00 | 0.05 * | 0.07 * | 0.02 | 0.00 * |
| 10~14 | 0.05 | 0.00 | 0.28 | 0.53 | 0.01 | −0.00 | −0.00 | −0.00 | 0.01 | 0.02 | −0.00 | 0.00 | |
| 15~69 | 0.04 | 0.00 | 0.11 | 0.10 | 0.00 | −0.00 | −0.00 | −0.00 | 0.02 | 0.03 | 0.00 | 0.00 | |
| 70~90 | −0.02 | −0.00 | −0.45 * | −0.86 | −0.01 | −0.00 | 0.00 | −0.00 | 0.00 | −0.03 | 0.01 | 0.00 | |
| 0~90 | 0.06 | 0.00 | −0.05 | −0.15 | 0.00 | −0.00 | 0.00 | −0.00 | 0.03 | 0.03 | 0.01 | 0.00 | |
| SO2 | 0~9 | 0.81 *** | 0.01 * | 1.41 | 3.23 | 0.03 | 0.06 | 0.00 * | 0.00 * | 0.30 * | 0.36 * | 0.23 * | 0.00 ** |
| 10~14 | 0.66 *** | 0.00 * | 2.03 | 3.74 | 0.02 | 0.08 * | 0.00 * | 0.00 * | 0.25 * | 0.30 * | 0.18 * | 0.00 ** | |
| 15~69 | 0.92 *** | 0.01 * | 3.06 * | 3.96 | 0.02 | 0.13 ** | 0.00 | 0.00 * | 0.34 * | 0.50 * | 0.21 | 0.00 * | |
| 70~90 | 0.91 *** | 0.01 ** | 1.83 | 3.83 | 0.05 * | 0.06 | 0.00 | 0.00 * | 0.46 ** | 0.61 ** | 0.31 * | 0.00 * | |
| 0~90 | 1.39 *** | 0.01 *** | 3.43 * | 5.09 | 0.04 | 0.15 ** | 0.00 * | 0.01 ** | 0.55 ** | 0.78 ** | 0.36 * | 0.01 ** |
Abbreviations: VCL=curvilinear velocity; VSL=straight-line velocity, VAP=average path velocity; ALH=amplitude of lateral head displacement; LIN=linearity; STR=straightness; WOB=wobble; BCF=beat-cross frequency; PM2.5=particulate matter with aerodynamic diameter ≤2.5 μm; PM10= particulate matter with aerodynamic diameter ≤10 μm; O3=ozone; NO2=nitrogen dioxide; CO=carbon monoxide; SO2=sulfur dioxide; BMI= body mass index.
Model adjusted for age, BMI, education level, ethnicity, abstinence duration (days), drinking status, smoking status, relatively humidity and temperature. Significance level: * p <0.05, ** p < 0.005, *** p < 0.001.
During all stages of spermatogenesis, SO2 had significant and positive association with progressive rate (all p < 0.001) and several sperm movement parameters, mainly ALH, VAP, VCL, and WOB. Meanwhile, average exposure to SO2 during the entire spermatogenesis (0–90 days) and for 15–69 days preceding semen collection was also positively associated with sperm concentration (p < 0.05). Notably, SO2 exposure for 10–14 days preceding semen collection was positively (but weakly) associated with progressive rate and all but one (ALH) motility parameters.
CO, compared to other pollutants, had the strongest relationship with semen quality parameters, especially with motility parameters (such as VAP, VCL, VSL), and the relationship between CO exposure and progressive rate was significantly positive for 15–69 days preceding collection. But later on, exposure to CO during 10–14 days before semen collection became negatively associated with sperm motility parameters BCF, VAP and VCL.
Average O3 exposure during the entire spermatogenesis period showed weak inverse relationship with various semen quality parameters, including progressive rate (all p < 0.05) and majority motility on parameters (including ALH, VAP, VCL, WOB). During earlier developmental stage, exposure windows such as 15–69 days and 70–90 days preceding sample collection (mainly during early developmental stages) appeared to have similar inverse relationship with aforementioned semen quality parameters. But O3 exposure during late development stages (10–14, 0–9 days preceding collection) appeared to become positive correlated with BCF, VAP, VCL, VSL (all p < 0.05).
Results from the first regression model with before, during, and after lock-down periods suggested that ALH from samples collected prior to lock-down period was 39% higher than that during lock-down period, whereas BCF was 74% lower (Table S2). Other semen quality (i.e., progressive rate, sperm concentration) and sperm movement parameters (LIN, STR, VAP, VCL, VSL, WOB) did not exhibit significant temporal difference. When stratified by study periods (before, during, and post lock-down period), pollutant exposures and semen quality exhibited weak to no association (Table S3).
Additional analysis on the effect of temperature (lag 0–90 days ground temperature) on semen quality parameters showed that our curve was not inversely-U shaped (Figure S1) as suggested previously by Zhou et al. (2020). The threshold of temperature in our data was around 18.5 °C.
4. Discussion
4.1. Pandemic and pollution
In this retrospective observational study, we examined the relationship between semen quality parameters and air pollution exposure based on semen samples collected between November 23, 2019 and July 23, 2020, a period that covered the on-going COVID-19 pandemic. Among young and healthy sperm donor candidates, we found the association of semen parameters with air pollutants to be weak in general. In response to the slowing of industrial activities during Chinese New Year (prior to COVID-19 lock-down control) and the subsequent nation-wide lock-down due to COVID-19, major ambient air pollutants (PM2.5, PM10, CO, SO2, NO2) experienced by the study population exhibited a steadily decreasing trend. Ozone (O3) was the only pollutant that showed an increase post lock-down period. Slowing of industrial activities and temporarily halting of social activities decreased primary emission of aforementioned major pollutants during the study period. Since O3 is a secondary pollutant from atmospheric reactions facilitated by sunshine (Warneck, 2000), the increasing amount of sunshine duration in late spring to early summer could have contributed to the increase in O3 level experienced by the study population. The concentrations of major ambient air pollutants were higher during the lock-down period than after the lock-down period, possibly due to seasonal variability and changes in pollution sources. The PM2.5 and PM10 concentrations in Chengdu were highest in winter, followed by spring and autumn, and lowest in summer, partly due to the lower temperature and boundary layer height, fewer precipitation, and slower wind speed in winter (Xiao et al., 2018). The lock-down period was between winter and spring, with more air pollution than the after-lock-down period. The major sources of PM2.5 in Chengdu is secondary aerosols and coal combustion, while vehicle and industrial emissions contribute less to PM2.5 (Kong et al., 2020). Therefore, the air quality improvement due to reduced industrial and traffic activities during the lock-down period might not be significant. Meanwhile, the average PM2.5 concentration in February–March 2020 was 8% lower than that in 2019, suggesting an overall improvement in local air quality (Chengdu Ecology and Environment Bureau, 2020).
4.2. Windows of susceptibility and sperm motility
The associations between different pollutants and semen quality parameters varied in different stages of spermatogenesis. Firstly, all six studied air pollutants appeared to affect progressive rate at multiple stages of sperm development. Progressive rate, defined as spermatozoa moving actively, either linearly or in a large circle, regardless of speed (World Health Organization, 2010), indicates a crucial capability for transport in the female tract (Sullivan and Mieusset, 2016). Our study results indicated that motility decline was significantly associated with the exposure of O3 in the entire (0–90 days) and early (15–90 days) stage of sperm development. Previous studies in Guangzhou (Huang et al., 2020), Wuhan (Sun et al., 2020), and Beijing (Zhang et al., 2019) suggested that O3 exposure during the entire period of sperm development (0–90 days before semen collection) was significantly associated with decreased semen motility. Other studies suggested several possible mechanisms, such as O3-induced oxidative stress, inflammatory reactions, and even the induction of the formation of circulating toxic species, could cause damage to semen parameters and sperm function, such as sperm concentration, sperm motility, sperm morphology, and even damage to sperm DNA (Agarwal et al, 2003, 2014; Diemer et al., 2003; Hansen et al., 2010; Sokol et al., 2006). Regarding exposure to SO2, our study showed that ambient SO2 exposure exhibited weak but positive association with progressive rate, sperm concentration and CASA motility parameters (such as STR, VAP, VCL) during multiple spermatogenesis stages, a finding that was in contrast with other studies (Liu et al., 2017; Sun et al., 2020; Zhou et al., 2014). Studies by Liu et al. and Sun et al. were from an infertility clinic or reproductive center with the study populations that sought assisted reproductive technology (ART) procedures, whereas our study population consisted of relatively healthy and young sperm donor candidates. Also, annual mean concentrations of SO2 declined steadily in China since 2015 (Kuerban et al., 2020), and in our study, average SO2 exposure during the entire sperm development was 6.21 μg/m3, far lower than earlier studies by Zhou et al. (69 μg/m3), Liu et al. (23 μg/m3) and Sun et al. (28.2 μg/m3) (Liu et al., 2017; Sun et al., 2020; Zhou et al., 2014). This difference in exposure level and the consequent difference in association with semen quality could suggest a non-linear relationship between SO2 exposure and semen quality, a study topic worth investigating in future studies.
Moreover, our results indicated a positive association between particulate matter (PM2.5 and PM10) exposure and progressive rate during multiple stages of spermatogenesis (such as 0–9, 10–14 and 0–90 lag days prior to semen collection). However, sperm concentration did not appear to be associated with exposure to particulate matters (PM2.5 and PM10). Previous studies showed inconsistent results with regard to the association between particular matter exposure and semen quality. While a couple of studies showed a negative association between air pollution and progressive rate (Guan et al., 2020; Huang et al., 2020), studies such as the one conducted by Lao et al. in Taiwan on 6475 male participants between 2001 and 2014 showed exposure to ambient PM2.5 air pollution was associated with higher level of sperm concentration (2018). Similarly, Zhou et al. found PM10 exposure has shown positive associations with progressive rate (2018). While our study did not investigate the biological mechanism, our results based on exposure windows suggested that the positive relationship between PM and semen quality was significant near the end of spermatogenesis, a period important for sperm motility development and storage in epididymis. Environmental stress posed by air pollutants experienced during this period of sperm development could elicit a compensatory response as suggested by Lao et al. (2018).
Additionally, we found that the exposure to CO had the strongest relationship with semen quality parameters, especially with progressive rate and motility parameters, and this relationship with semen quality parameters varied during different susceptible windows. The mechanism of how CO could affect the male reproduction system remains to be better understood. Animal model study showed that exposure to CO leads to differential distribution of CO in testes and semen, where CO level was lower in the testes but higher in semen in response to chronic restraint stress (Moustafa, 2021). A review on human male reproductive system by Mancuso et al. has suggested that CO not only could prevent excessive hypothalamic-pituitary-adrenal (HPA) axis activation and enhances reproductive processes, it could also be an important mediator of the pyrogenic response to neurogenic and immunoinflammatory stressors (Mancuso et al., 2010). More research is needed to clarify how such mechanisms influence the relationship between CO exposure and semen quality in human.
Moreover, our analysis revealed that exposure during late spermatogenesis (motility development stages) appeared to affect sperm motility. Human testicular sperm are mostly immotile, and it is in the epididymis where the acquisition of the potential for motility and development of the swimming pattern (Simerly et al., 2016). Windows of susceptibility for CO exposure appears to be near the end of spermatogenesis (10–14 days and 15–69 days preceding semen collection), whereas for O3 was evident earlier (10–14 days preceding semen collection). Ten-14 days preceding semen collection is the critical period for sperm motility development. Fifteen-69 days preceding semen collection includes both the meiotic stage (first and second meiotic divisions) and spermiogenesis stage, which is the final stage of spermatogenesis during which spermatids mature into motile spermatozoa (Heller and Clermont, 1964; Nobles et al., 2018; Griswold, 2016). The application of CASA enables relatively objective and quantitative descriptions of such changes in terms of velocity and other kinematic parameters (Yeung and Cooper, 2002). Earlier studies suggested that CASA motility parameters could be viewed as sensitive biomarkers of human reproductive toxicity (Selevan et al., 2000; Zhou et al., 2014).
Lastly, ambient pollutant concentrations during lock-down period did not seem to be associated with semen quality, as compared to before and after lock-down periods. This lack of association, in contrast with significant associations reported during the other two periods, could be due to unobserved factors that influence individual's exposure profile. During the lock-down period, the study population, like the rest of the country, stayed at home. The lack of association with ambient air pollution could be reflective of the fact that individuals during the lock-down period experienced less of outdoor air, and at the same time, indoor air quality potentially played a bigger role during this period. Mask-wearing outdoor during the COVID-19 pandemic could have further limited individual's exposure to ambient pollutants.
4.3. Strengths and limitations
In this retrospective observational study, our study design had a number of strengths. Firstly, we were able to go beyond traditional semen quality parameters (i.e., sperm concentration and progressive rate) to look at CASA parameters for sperm movement. Sperm movement parameters are better at indicating sperm viability and potential fertility. Secondly, in our analysis, we improved the resolution of exposure analysis by simulating 0.1 ° × 0.1 ° pollutant concentration for each individual in the study population. Increasing the spatio-temporal resolution of ambient pollutant concentration provides more accurate exposure condition for exposure assessment analysis and minimizes potential exposure misclassification. However, the weak (or the lack of) association between ambient pollutant exposure and semen quality outcomes suggested that there could still be unobserved factors influencing such relationship. Our study lacked information on indoor environmental quality, which could play a major role in individual's exposure profile during the lock-down period. Additionally, our current study did not have behavior information (i.e., sleeping schedule and diet) that could potentially influence the outcome of semen quality.
5. Conclusion
To understand the relationship between ambient pollution exposure and semen quality, we conducted a retrospective observational study to investigate window of susceptibility during spermatogenesis among young and healthy sperm donors. Out of 923 recruited individuals, a total of 781 individuals and 1991 samples between November 23, 2019 and July 23, 2020, a period covering a nation-wide lock-down due to COVID-19, were included in our analysis. Using simulated ambient pollution exposure data at a spatial resolution of 0.1 ° × 0.1 ° matched with residential address, we found that the nation-wide lock-down period did not play a significant role in semen quality outcomes, however, exposures during different developmental stages of spermatogenesis had varying associations with semen quality. Exposure to particulate matters (PM2.5 and PM10), O3, and NO2 during late stage spermatogenesis had weak but positive association with semen quality, whereas exposure to CO late in sperm development had inverse relationship with sperm motility parameters. Exposure to SO2 appeared to influence semen quality throughout spermatogenesis. Our results suggested that sperm motility could be susceptible to ambient pollution exposure. Findings from current study could help to further elucidate the importance of sensitive periods during spermatogenesis and provide more evidence for exposure susceptibility.
Credit author statement
Tingting Yang: Investigation, Conceptualization, Writing – original draft, Writing – review & editing. Li Deng: Visualization, Data curation. Boyu Sun: Visualization. Shifu Zhang: Formal analysis. Yang Xian: Data curation. Xiao Xiao: Data curation. Yu Zhan: Formal analysis. Kehui Xu: Supervision. Johnathan J. Buonocore: Writing – review & editing. Ya Tang: Supervision, Funding acquisition. Fuping Li: Project administration, Supervision, Funding acquisition. Yang Qiu: Methodology, Conceptualization, Writing – original draft, Writing – review & editing.
Funding
This research was funded partly by the National Natural Science Foundation of China (41929002).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envres.2021.111085.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Agarwal A., Saleh R.A., Bedaiwy M.A. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil. Steril. 2003;79:829–843. doi: 10.1016/s0015-0282(02)04948-8. [DOI] [PubMed] [Google Scholar]
- Agarwal A., Virk G., Ong C., du Plessis S.S. Effect of oxidative stress on male reproduction. World J. Men’s Health. 2014;32:1–17. doi: 10.5534/wjmh.2014.32.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D., Mächler M., Bolker B., Walker S. Fitting linear mixed-effects models using lme4. J. Stat. Software. 2015;67:1–48. [Google Scholar]
- Caswell T.A., Droettboom M., Lee A., Hunter J., Firing E., Stansby D., Klymak J., Hoffmann T., Andrade E.S.D., Varoquaux N., Nielsen J.H., Root B., Elson P., May R., Dale D., Lee Jae-Joon, Seppänen J.K., McDougall D., Straw A., Hobson P., Gohlke C., Yu T.S., Ma E., Vincent A.F., Silvester S., Moad C., Kniazev N., Ivanov P., Ernest E., Katins J. 2020. Matplotlib/Matplotlib v3.1.3. Zenodo. [Google Scholar]
- Chan C.K., Yao X. Air pollution in mega cities in China. Atmos. Environ. 2008;42:1–42. [Google Scholar]
- Chengdu Ecology and Environment Bureau . 2020. Ambient Air Quality Report.http://sthj.chengdu.gov.cn/cdhbj/c110802/list_1.shtml [WWW Document] 2.22.21. [Google Scholar]
- China Ministry of Health Basic standards and technical specifications for human sperm bank. Chin. J. Reprod. Health. 2004;15:68–71. [Google Scholar]
- Choy J.T., Eisenberg M.L. Male infertility as a window to health. Fertil. Steril. 2018;110:810–814. doi: 10.1016/j.fertnstert.2018.08.015. [DOI] [PubMed] [Google Scholar]
- Clermont Y. The cycle of the seminiferous epithelium in man. Am. J. Anat. 1963;112:35–51. doi: 10.1002/aja.1001120103. [DOI] [PubMed] [Google Scholar]
- CPC Central Committee Leading Group on Novel Coronavirus State Council: guidelines about advancing resumption of work and production on the premise of effective epidemic containment. Guo Fa Ming Dian. 2020;13 [Google Scholar]
- Deeter M.N., Martínez-Alonso S., Edwards D.P., Emmons L.K., Gille J.C., Worden H.M., Sweeney C., Pittman J.V., Daube B.C., Wofsy S.C. The MOPITT Version 6 product: algorithm enhancements and validation. Atmos. Meas. Tech. 2014;7:3623–3632. [Google Scholar]
- Deng Z., Chen F., Zhang M., Lan L., Qiao Z., Cui Y., An J., Wang N., Fan Z., Zhao X., Li X. Association between air pollution and sperm quality: a systematic review and meta-analysis. Environ. Pollut. 2016;208:663–669. doi: 10.1016/j.envpol.2015.10.044. [DOI] [PubMed] [Google Scholar]
- Dias T.R., Cho C.-L., Agarwal A. In: In Vitro Fertilization. Nagy Z.P., Varghese A.C., Agarwal A., editors. Springer; Cham: 2019. Sperm assessment: traditional approaches and their indicative value; pp. 249–263. [Google Scholar]
- Diemer T., Allen J.A., Hales K.H., Hales D.B. Reactive oxygen disrupts mitochondria in MA-10 tumor leydig cells and inhibits steroidogenic acute regulatory (StAR) protein and steroidogenesis. Endocrinology. 2003;144:2882–2891. doi: 10.1210/en.2002-0090. [DOI] [PubMed] [Google Scholar]
- Fang D., Wang Q., Li H., Yu Y., Lu Y., Qian X. Mortality effects assessment of ambient PM2.5 pollution in the 74 leading cities of China. Sci. Total Environ. 2016;569–570:1545–1552. doi: 10.1016/j.scitotenv.2016.06.248. [DOI] [PubMed] [Google Scholar]
- General Office of the State Council General Office of the state Council: notice to extend spring festival holiday. Guo Ban Fa Ming Dian. 2020;1 [Google Scholar]
- Griswold M.D. Spermatogenesis: the commitment to meiosis. Physiol. Rev. 2016;96:1–17. doi: 10.1152/physrev.00013.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Q., Chen S., Wang B., Dou X., Lu Y., Liang J., Ni R., Yang C., Wang H., Baktash M.B., Wu W., Wang X., Fu G., Xia Y. Effects of particulate matter exposure on semen quality: a retrospective cohort study. Ecotoxicol. Environ. Saf. 2020;193:110319. doi: 10.1016/j.ecoenv.2020.110319. [DOI] [PubMed] [Google Scholar]
- Hammoud A., Carrell D.T., Gibson M., Sanderson M., Parker-Jones K., Peterson C.M. Decreased sperm motility is associated with air pollution in Salt Lake City. Fertil. Steril. 2010;93:1875–1879. doi: 10.1016/j.fertnstert.2008.12.089. [DOI] [PubMed] [Google Scholar]
- Han X., Zhou N., Cui Z., Ma M., Li L., Cai M., Li Yafei, Lin H., Li Ying, Ao L., Liu J., Cao J. Association between urinary polycyclic aromatic hydrocarbon metabolites and sperm DNA damage: a population study in chongqing, China. Environ. Health Perspect. 2011;119:652–657. doi: 10.1289/ehp.1002340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen C., Luben T.J., Sacks J.D., Olshan A., Jeffay S., Strader L., Perreault S.D. The effect of ambient air pollution on sperm quality. Environ. Health Perspect. 2010;118:203–209. doi: 10.1289/ehp.0901022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris C.R., Millman K.J., van der Walt S.J., Gommers R., Virtanen P., Cournapeau D., Wieser E., Taylor J., Berg S., Smith N.J., Kern R., Picus M., Hoyer S., van Kerkwijk M.H., Brett M., Haldane A., del Río J.F., Wiebe M., Peterson P., Gérard-Marchant P., Sheppard K., Reddy T., Weckesser W., Abbasi H., Gohlke C., Oliphant T.E. Array programming with NumPy. Nature. 2020;585:357–362. doi: 10.1038/s41586-020-2649-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller C.H., Clermont Y. Kinetics of the germinal epithelium in man. Recent Prog. Horm. Res. 1964;20:545–575. [PubMed] [Google Scholar]
- Huang C., Li B., Xu K., Liu D., Hu J., Yang Y., Nie H., Fan L., Zhu W. Decline in semen quality among 30,636 young Chinese men from 2001 to 2015. Fertil. Steril. 2017;107:83–88. doi: 10.1016/j.fertnstert.2016.09.035. [DOI] [PubMed] [Google Scholar]
- Huang G., Zhang Q., Wu H., Wang Q., Chen Y., Guo P., Zhao Q. Sperm quality and ambient air pollution exposure: a retrospective, cohort study in a Southern province of China. Environ. Res. 2020;188:109756. doi: 10.1016/j.envres.2020.109756. [DOI] [PubMed] [Google Scholar]
- Joint Prevention and Control Mechanism of the State Council . vol. 16. Guo Ban Fa Ming Dian; 2020. (State Council: Circular about Making Arrangement on Epidemic Prevention and Control for Key Venues, Units and Groups). [Google Scholar]
- Kanevski M., editor. Advanced Mapping of Environmental Data, Geographical Information Systems Series. Wiley-ISTE; Hoboken, NJ: 2008. [Google Scholar]
- Kong L., Tan Q., Feng M., Qu Y., An J., Liu X., Cheng N., Deng Y., Zhai R., Wang Z. Investigating the characteristics and source analyses of PM2.5 seasonal variations in Chengdu, Southwest China. Chemosphere. 2020;243:125267. doi: 10.1016/j.chemosphere.2019.125267. [DOI] [PubMed] [Google Scholar]
- Kuerban M., Waili Y., Fan F., Liu Y., Qin W., Dore A.J., Peng J., Xu W., Zhang F. Spatio-temporal patterns of air pollution in China from 2015 to 2018 and implications for health risks. Environ. Pollut. 2020;258:113659. doi: 10.1016/j.envpol.2019.113659. [DOI] [PubMed] [Google Scholar]
- Lamsal L.N., Krotkov N.A., Vasilkov A., Marchenko S., Qin W., Yang E.-S., Fasnacht Z., Joiner J., Choi C., Haffner D., Swartz W.H., Fisher B., Bucsela E. Ozone Monitoring Instrument (OMI) Aura Nitrogen Dioxide Standard Product Version 4.0 with Improved Surface and Cloud Treatment. Atmos. Meas. Tech. 2021;14:455–479. [Google Scholar]
- Lao X.Q., Zhang Z., Lau A.K.H., Chan T.-C., Chuang Y.C., Chan J., Lin C., Guo C., Jiang W.K., Tam T., Hoek G., Kan H., Yeoh E., Chang L. Exposure to ambient fine particulate matter and semen quality in Taiwan. Occup. Environ. Med. 2018;75:148–154. doi: 10.1136/oemed-2017-104529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine H., Jørgensen N., Martino-Andrade A., Mendiola J., Weksler-Derri D., Mindlis I., Pinotti R., Swan S.H. Temporal trends in sperm count: a systematic review and meta-regression analysis. Hum. Reprod. Update. 2017;23:646–659. doi: 10.1093/humupd/dmx022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Joiner J., Krotkov N.A., Bhartia P.K. A fast and sensitive new satellite SO2 retrieval algorithm based on principal component analysis: application to the ozone monitoring instrument. Geophys. Res. Lett. 2013;40:6314–6318. [Google Scholar]
- Lin G., Fu J., Jiang D., Hu W., Dong D., Huang Y., Zhao M. Spatio-temporal variation of PM2.5 concentrations and their relationship with geographic and socioeconomic factors in China. IJERPH. 2013;11:173–186. doi: 10.3390/ijerph110100173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Di B., Luo Y., Deng X., Zhang H., Yang F., Grieneisen M.L., Zhan Y. Estimating ground-level CO concentrations across China based on the national monitoring network and MOPITT: potentially overlooked CO hotspots in the Tibetan Plateau. Atmos. Chem. Phys. 2019;19:12413–12430. [Google Scholar]
- Liu Y., Ni S., Jiang T., Xing S., Zhang Y., Bao X., Feng Z., Fan X., Zhang L., Feng H. Influence of Chinese new year overlapping COVID-19 lockdown on HONO sources in shijiazhuang. Sci. Total Environ. 2020;745:141025. doi: 10.1016/j.scitotenv.2020.141025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Zhou Y., Ma J., Bao W., Li J., Zhou T., Cui X., Peng Z., Zhang H., Feng M., Yuan Y., Chen Y., Huang X., Li Y., Duan Y., Shi T., Jin L., Wu L. Inverse association between ambient sulfur dioxide exposure and semen quality in wuhan, China. Environ. Sci. Technol. 2017;51:12806–12814. doi: 10.1021/acs.est.7b03289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyapustin A., Wang Y., Laszlo I., Kahn R., Korkin S., Remer L., Levy R., Reid J.S. Multiangle implementation of atmospheric correction (MAIAC): 2. Aerosol algorithm. J. Geophys. Res. Atmos. 2011;116 doi: 10.1002/2016JD025720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso C., Navarra P., Preziosi P. Roles of nitric oxide, carbon monoxide, and hydrogen sulfide in the regulation of the hypothalamic–pituitary–adrenal axis. J. Neurochem. 2010;113:563–575. doi: 10.1111/j.1471-4159.2010.06606.x. [DOI] [PubMed] [Google Scholar]
- Moustafa A. Changes in nitric oxide, carbon monoxide, hydrogen sulfide and male reproductive hormones in response to chronic restraint stress in rats. Free Radic. Biol. Med. 2021;162:353–366. doi: 10.1016/j.freeradbiomed.2020.10.315. [DOI] [PubMed] [Google Scholar]
- Nobles C.J., Schisterman E.F., Ha S., Kim K., Mumford S.L., Buck Louis G.M., Chen Z., Liu D., Sherman S., Mendola P. Ambient air pollution and semen quality. Environ. Res. 2018;163:228–236. doi: 10.1016/j.envres.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Z., Han G., Ma X., Su H., Gong W. Response of major air pollutants to COVID-19 lockdowns in China. Sci. Total Environ. 2020;743:140879. doi: 10.1016/j.scitotenv.2020.140879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J., Bates D., DebRoy S., Sarkar D., R Core Team . 2020. Nlme: Linear and Nonlinear Mixed Effects Models. [Google Scholar]
- Qiu Y., Yang T., Seyler B.C., Wang X., Wang Y., Jiang M., Liu B., Li F. Ambient air pollution and male fecundity: a retrospective analysis of longitudinal data from a Chinese human sperm bank (2013–2018) Environ. Res. 2020;186:109528. doi: 10.1016/j.envres.2020.109528. [DOI] [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2020. R: A Language and Environment for Statistical Computing. [Google Scholar]
- Reback J., McKinney W., Jbrockmendel Bossche J.V.D., Augspurger T., Cloud P., Gfyoung Hawkins S., Sinhrks Roeschke M., Klein A., Petersen Terji, Tratner J., She C., Ayd W., Naveh S., Garcia M., Schendel J., Hayden A., Saxton D., Jancauskas V., Patrick McMaster A., Battiston P., Seabold Skipper, Dong Kaiqi, Chris-B1 H-Vetinari, Hoyer S., Gorelli M. 2020. Pandas-Dev/pandas: Pandas 1.2.0. Zenodo. [Google Scholar]
- Selevan S.G., Borkovec L., Slott V.L., Zudová Z., Rubes J., Evenson D.P., Perreault S.D. Semen quality and reproductive health of young Czech men exposed to seasonal air pollution. Environ. Health Perspect. 2000;108:887–894. doi: 10.1289/ehp.00108887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly C., Castro C., Hartnett C., Lin C.C., Sukhwani M., Orwig K., Schatten G. Post-testicular sperm maturation: centriole pairs, found in upper epididymis, are destroyed prior to sperm's release at ejaculation. Sci. Rep. 2016;6:31816. doi: 10.1038/srep31816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slama R., Darrow L., Parker J., Woodruff T.J., Strickland M., Nieuwenhuijsen M., Glinianaia S., Hoggatt K.J., Kannan S., Hurley F., Kalinka J., Šrám R., Brauer M., Wilhelm M., Heinrich J., Ritz B. Meeting report: atmospheric pollution and human reproduction. Environ. Health Perspect. 2008;116:791–798. doi: 10.1289/ehp.11074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol R.Z., Kraft P., Fowler I.M., Mamet R., Kim E., Berhane K.T. Exposure to environmental ozone alters semen quality. Environ. Health Perspect. 2006;114:360–365. doi: 10.1289/ehp.8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C., Wu L., Xie Y., He J., Chen X., Wang T., Lin Y., Jin T., Wang A., Liu Y., Dai Q., Liu B., Wang Y., Mao H. Air pollution in China: status and spatiotemporal variations. Environ. Pollut. 2017;227:334–347. doi: 10.1016/j.envpol.2017.04.075. [DOI] [PubMed] [Google Scholar]
- Sullivan R., Mieusset R. The human epididymis: its function in sperm maturation. Hum. Reprod. Update. 2016;22:574–587. doi: 10.1093/humupd/dmw015. [DOI] [PubMed] [Google Scholar]
- Sun S., Zhao J., Cao W., Lu W., Zheng T., Zeng Q. Identifying critical exposure windows for ambient air pollution and semen quality in Chinese men. Environ. Res. 2020;189:109894. doi: 10.1016/j.envres.2020.109894. [DOI] [PubMed] [Google Scholar]
- Venter Z.S., Aunan K., Chowdhury S., Lelieveld J. COVID-19 lockdowns cause global air pollution declines. Proc. Natl. Acad. Sci. U.S.A. 2020;117:18984–18990. doi: 10.1073/pnas.2006853117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen H.E., Jørgensen N., Toppari J. Semen quality in the 21st century. Nat. Rev. Urol. 2017;14:120–130. doi: 10.1038/nrurol.2016.261. [DOI] [PubMed] [Google Scholar]
- Wang X.-B., Du J.-B., Cui H. Sulfur dioxide, a double-faced molecule in mammals. Life Sci. 2014;98:63–67. doi: 10.1016/j.lfs.2013.12.027. [DOI] [PubMed] [Google Scholar]
- Warneck P. Academic Press; San Diego: 2000. Chemistry of the Natural Atmosphere, 2nd Ed, International Geophysics. [DOI] [PubMed] [Google Scholar]
- Wickham H. Springer-Verlag; New York: 2016. ggplot2: Elegant Graphics for Data Analysis. [Google Scholar]
- Wickham H., Averick M., Bryan J., Chang W., McGowan L.D., François R., Grolemund G., Hayes A., Henry L., Hester J., Kuhn M., Pedersen T.L., Miller E., Bache S.M., Müller K., Ooms J., Robinson D., Seidel D.P., Spinu V., Takahashi K., Vaughan D., Wilke C., Woo K., Yutani H. Welcome to the tidyverse. J. Open Source Softw. 2019;4:1686. [Google Scholar]
- World Health Organization . fifth ed. World Health Organization; Geneva: 2010. WHO Laboratory Manual for the Examination and Processing of Human Semen. [Google Scholar]
- Xiao K., Wang Y., Wu G., Fu B., Zhu Y. Spatiotemporal characteristics of air pollutants (PM10, PM2.5, SO2, NO2, O3, and CO) in the inland basin city of Chengdu, southwest China. Atmosphere. 2018;9:74. [Google Scholar]
- Yeung C.-H., Cooper T.G. In: The Epididymis: from Molecules to Clinical Practice: A Comprehensive Survey of the Efferent Ducts, the Epididymis and the Vas Deferens. Robaire B., Hinton B.T., editors. Springer; Boston, MA: 2002. Acquisition and development of sperm motility upon maturation in the epididymis; pp. 417–434. [Google Scholar]
- Zeileis A., Grothendieck G. Zoo: S3 infrastructure for regular and irregular time series. J. Stat. Software. 2005;14:1–27. [Google Scholar]
- Zhan Y., Luo Y., Deng X., Chen H., Grieneisen M.L., Shen X., Zhu L., Zhang M. Spatiotemporal prediction of continuous daily PM2.5 concentrations across China using a spatially explicit machine learning algorithm. Atmos. Environ. 2017;155:129–139. [Google Scholar]
- Zhan Y., Luo Y., Deng X., Grieneisen M.L., Zhang M., Di B. Spatiotemporal prediction of daily ambient ozone levels across China using random forest for human exposure assessment. Environ. Pollut. 2018;233:464–473. doi: 10.1016/j.envpol.2017.10.029. [DOI] [PubMed] [Google Scholar]
- Zhan Y., Luo Y., Deng X., Zhang K., Zhang M., Grieneisen M.L., Di B. Satellite-based estimates of daily NO2 exposure in China using hybrid random forest and spatiotemporal kriging model. Environ. Sci. Technol. 2018;52:4180–4189. doi: 10.1021/acs.est.7b05669. [DOI] [PubMed] [Google Scholar]
- Zhang H.-T., Zhang Z., Cao J., Tang W.-H., Zhang H.-L., Hong K., Lin H.-C., Wu H., Chen Q., Jiang H. Ambient ozone pollution is associated with decreased semen quality: longitudinal analysis of 8945 semen samples from 2015 to 2018 and during pollution-control period in Beijing, China. Asian J. Androl. 2019;21:501–507. doi: 10.4103/aja.aja_116_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N., Cui Z., Yang S., Han X., Chen G., Zhou Z., Zhai C., Ma M., Li L., Cai M., Li Y., Ao L., Shu W., Liu J., Cao J. Air pollution and decreased semen quality: a comparative study of Chongqing urban and rural areas. Environ. Pollut. 2014;187:145–152. doi: 10.1016/j.envpol.2013.12.030. [DOI] [PubMed] [Google Scholar]
- Zhou N., Jiang C., Chen Q., Yang H., Wang X., Zou P., Sun L., Liu Jiaojiao, Li Ling, Li Lianbing, Huang L., Chen H., Ao L., Zhou Z., Liu Jinyi, Cui Z., Cao J. Exposures to atmospheric PM10 and PM10–2.5 affect male semen quality: results of MARHCS study. Environ. Sci. Technol. 2018;52:1571–1581. doi: 10.1021/acs.est.7b05206. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Meng T., Wu L., Duan Y., Li G., Shi C., Zhang H., Peng Z., Fan C., Ma J., Xiong C., Bao W., Liu Y. Association between ambient temperature and semen quality: a longitudinal study of 10 802 men in China. Environ. Int. 2020;135:105364. doi: 10.1016/j.envint.2019.105364. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.