Abstract
BACKGROUND
Stanniocalcin-1 (STC-1) is a widely expressed glycoprotein hormone involved in a diverse spectrum of physiological and pathophysiological processes including angiogenesis, mineral homeostasis, cell proliferation, inflammation and apoptosis. Over the last 20 years, numerous studies have reported STC-1 expression within female reproductive tissues including the uterus, ovaries and placenta and implicated STC-1 in processes such as ovarian follicular development, blastocyst implantation, vascular remodelling in early pregnancy and placental development. Notably, dysregulation of STC-1 within reproductive tissues has been linked to the onset of severe reproductive disorders including endometriosis, polycystic ovary syndrome, poor trophoblast invasion and placental perfusion in early pregnancy. Furthermore, significant changes in tissue expression and in maternal systemic concentration take place throughout pregnancy and further substantiate the vital role of this protein in reproductive health and disease.
OBJECTIVE AND RATIONALE
Our aim is to provide a comprehensive overview of the existing literature, to summarise the expression profile and roles of STC-1 within the female reproductive system and its associated pathologies. We highlight the gaps in the current knowledge and suggest potential avenues for future research.
SEARCH METHODS
Relevant studies were identified through searching the PubMed database using the following search terms: ‘stanniocalcin-1’, ‘placenta’, ‘ovary’, ‘endometrium’, ‘pregnancy’, ‘reproduction’, ‘early gestation’. Only English language papers published between 1995 and 2020 were included.
OUTCOMES
This review provides compelling evidence of the vital function that STC-1 plays within the female reproductive system. The literature presented summarise the wide expression profile of STC-1 within female reproductive organs, as well as highlighting the putative roles of STC-1 in various functions in the reproductive system. Moreover, the observed link between altered STC-1 expression and the onset of various reproductive pathologies is presented, including those in pregnancy whose aetiology occurs in the first trimester. This summary emphasises the requirement for further studies on the mechanisms underlying the regulation of STC-1 expression and function.
WIDER IMPLICATIONS
STC-1 is a pleiotropic hormone involved in the regulation of a number of important biological functions needed to maintain female reproductive health. There is also growing evidence that dysregulation of STC-1 is implicated in common reproductive and obstetric disorders. Greater understanding of the physiology and biochemistry of STC-1 within the field may therefore identify possible targets for therapeutic intervention and/or diagnosis.
Keywords: stanniocalcin-1 / STC1 / reproduction / placenta / ovary / endometrium / uterus / pregnancy / decidualisation / implantation
Introduction
Stanniocalcin (STC) is a homodimeric glycoprotein first isolated in bony fish (Wagner et al., 1986). It is secreted by the corpuscles of Stannius, small endocrine glands located on the ventral surface of the kidneys (Stannius, 1839). The function of these glands was elucidated in 1964 when surgical removal resulted in hypercalcaemia (Fontaine, 1964). In the late 1980s, the anti-hypercalcaemic factor secreted by the corpuscles of Stannius was purified and identified as STC (Wagner et al., 1986; Lafeber et al., 1988). In the 1990s, a mammalian orthologue was discovered following the successful cloning of mouse and human cDNAs by two independent groups (Chang et al., 1995, 1996; Olsen et al., 1996). The amino acid sequence of this orthologue shows ∼61% identity and ∼73% similarity with various fish STCs (Chang et al., 1995). Moreover, 2 years after the discovery of mammalian STC, several research groups identified a second member of the mammalian STC family (Chang and Reddel, 1998; DiMattia et al., 1998; Ishibashi et al., 1998). This finding resulted in the renaming of mammalian STC as STC-1 and the newly identified protein as STC-2 (Chang and Reddel, 1998).
Structure of STC-1
Human STC-1 cDNA encodes a protein of 247 amino acids (Chang et al., 1995). When compared to fish STC, the first 204 amino acids of human STC-1 show 92% similarity to that of salmon, however, the last 43 residues at the C-terminus differ immensely (Chang et al., 2003). In contrast, human STC-2 encodes a protein of 302 amino acids which shows just 34% identity to both human STC-1 and eel STC, with the greatest sequence similarity present at the N-terminus (Chang et al., 2003). At this terminus, a conserved cysteine motif is found in both fish and human STCs. In STC-1, there are 11 cysteine residues with the same spacing as those in fish STCs (Butkus et al., 1987; Wagner et al., 1992). STC-2 possesses 15 cysteine residues, but only 10 of which have the same spacing as fish STC and STC-1 (Moore et al., 1999). During translation of the protein, 10 out of the 11 cysteines form intrachain disulphide linkages and the 11th cysteine residue functions in forming the interchain dimer (Trindade et al., 2009), allowing STC-1 to exist as a homodimer in its native state (Lafeber et al., 1988). The lack of spatial conservation of the 11th cysteine residue in STC-2 compared to fish STC/STC-1, combined with the presence of four additional cysteine residues in this protein, leads to the prediction that STC-2 has a different tertiary structure when compared with other STCs. Despite some structural similarities between both STC-1 and STC-2, there is no evidence, thus far, to suggest that these proteins are capable of heterodimerisation (Joshi, 2020). Another defining feature of STC-1, which is also conserved amongst the STCs, is the presence of an N-linked glycosylation consensus sequence (Asn-X-Thr/Ser). In STC-1, this is present at residues 62–64; consistent with the position of this sequence in fish STC (Butkus et al., 1987; Wagner et al., 1992).
STC-1 appears to exist in several forms; the predicted molecular weight of STC-1 is 27 kDa, however, studies have pointed to the existence of di- or multimers (Zhang et al., 1998; Paciga et al., 2002, 2005a). The dimeric 56 kDa form of STC-1 is known as STC50 (Paciga et al., 2005b). In addition, a number of higher molecular weight STC-1 variants have been identified in ovarian cells, adrenocortical cells and adipocytes, and are collectively referred to as ‘big STC’. Within this category, at least three molecular weights: 84, 112 and 135 kDa have been described (Paciga et al., 2002, 2005a). STC-1 is secreted from most cells and contains a signal peptide sequence of ∼24 amino acids and a pro-sequence of ∼15 amino acids, both of which a processed to yield the mature forms of the protein (Moore et al., 1999). In most cell types, STC-1 is produced in large amounts intracellularly in lightly glycosylated forms, however, when secreted, STC-1 is highly glycosylated and phosphorylated by protein kinase C (Jellinek et al., 2000).
STC-1 mammalian expression pattern and pleiotropic roles
STC-1 has a wide expression pattern in mammals, with reported expression in many tissues including the heart, lungs, liver, adrenal gland, kidney, ovary, prostate, colon, bone and spleen (Chang et al., 1995; Olsen et al., 1996; Varghese et al., 1998; Yoshiko and Aubin, 2004; Liu et al., 2010; Law et al., 2011). In humans, the STC-1 gene is expressed predominantly as a 4 kb transcript and the highest expression levels are found in the ovary, kidney, prostate and thyroid (Moore et al., 1999).
Consistent with its wide expression profile, STC-1 appears to act in a pleiotropic manner in mammalian systems. It has been implicated in a diverse range of processes including mineral homeostasis (Olsen et al., 1996), angiogenesis (He et al., 2011; Law and Wong, 2013), organogenesis (Jiang et al., 2000; Stasko and Wagner, 2001), cell proliferation (Bai et al., 2017), apoptosis (Kim et al., 2013), retinal degeneration (Roddy et al., 2012), cerebral ischaemia (Zhang et al., 2000), inflammation (Mohammadipoor et al., 2016), tumourigenesis (Liu et al., 2010) and anti-oxidative activity (Kim et al., 2013; Wu et al., 2014; Bonfante et al., 2020). In addition, numerous studies have highlighted the role of STC-1 in the physiology and pathophysiology of the female reproductive system. These studies have outlined the wide expression of STC-1 amongst female reproductive tissues including the uterus (Stasko et al., 2001; Xiao et al., 2006; Allegra et al., 2009), placenta (Uusküla et al., 2012; Juhanson et al., 2016; Abid et al., 2020), and the developing maternal vasculature in early pregnancy (Wallace et al., 2013). Amongst all female reproductive tissues, the greatest expression is seen in the ovary (Varghese et al., 1998; Deol et al., 2000). STC-1 expression is virtually undetectable in the male testis (Varghese et al., 1998; Deol et al., 2000), indicating that it has a specific role in female reproduction. This review will provide an overview of the existing literature on the expression and putative functions of STC-1 within female reproduction and postulate future avenues of research (Table I).
Table I A summary of the expression pattern and roles of stanniocalcin-1 (STC-1) in different reproductive tissues across a number of species.
| Tissue | Species | Reported localisation of STC-1 gene expression | Reported localisation of STC-1 protein expression | Pathway regulating STC-1 expression | Demonstrated/ postulated role | Reference |
|---|---|---|---|---|---|---|
| Ovary | Human | Not reported | Follicular fluid | Not reported | Regulation of IGF activity through PAPP-A inhibition | Jepsen et al. (2016) |
| Mouse | Newborn ovary: Specific cell type not reported | Newborn ovary: Primordial follicles cells, oocytes, stromal cells | Not reported | Gestation and lactation | Deol et al. (2000) | |
| 5 days old: Stromal cells surrounding unilaminar and multilaminar follicles | 5 days old: Unilaminar, multilaminar and primordial follicle oocytes, granulosa cells | |||||
| 2 weeks old: Thecal and interstitial cells, granulosa cells | 2 weeks old: Unilaminar and multilaminar follicle oocytes | |||||
| During oestrous: Thecal cells surrounding peripheral follicles, interstitial cells, luteal cells | During oestrous: Luteal cells, preantral follicle oocytes, thecal and interstitial cells | |||||
| During gestation: Thecal and interstitial cells, luteal cells | During gestation: Primary and secondary follicle oocytes, thecal cells, interstitial cells, luteal cells | |||||
| Post-partum: Thecal and interstitial cells, luteal cells | Post-partum: Thecal and interstitial cells, luteal cells, primary, secondary and antral follicle oocytes | |||||
| Rat | Thecal interstitial cells | Thecal interstitial cells, granulosa cells | cAMP/PKA pathway | Granulosa cells: Steroidogenesis—decreases progesterone synthesis | Paciga et al. (2002) | |
| Luo et al. (2004) | ||||||
| Pig | Antral follicle thecal cells | Follicular fluid, granulosa cells | Not reported | Granulosa cells: ROS homeostasis—increases superoxide anion and catalase, decreases peroxidase | Basini et al. (2010) | |
| Baioni et al. (2011) | ||||||
| Bovine | Thecal interstitial cells, luteal cells (only when isolated from native environment) | Thecal interstitial cells, luteal cells | cAMP/PKA pathway | Thecal interstitial cells: Ovulation, luteinisation, apoptosis | Paciga et al. (2002) | |
| Luteal cells: Steroidogenesis—decreases progesterone synthesis | Paciga et al. (2003) | |||||
| Paciga et al. (2004) | ||||||
| Uterus | Human | Endometrial stromal fibroblasts | Endometrial epithelial cells, endometrial stromal fibroblasts, endometrial fluid | cAMP/PKA pathway | Blastocyst implantation, decidualisation, pathogenesis of endometriosis, pathogenesis of polycystic ovary syndrome | Aghajanova et al. (2016) |
| Khatun et al. (2020) | ||||||
| Allegra et al. (2009) | ||||||
| Mouse | Uterine epithelium, mesometrial stromal cells, mesometrial lateral sinusoids | Epithelial cells, stromal cells, decidual cells, anti-mesometrial cells | Not reported | Decidualisation, blastocyst implantation | Stasko et al. (2001) | |
| Rat | Luminal epithelium, glandular epithelium, stromal cells | Luminal epithelium, glandular epithelium, subluminal stroma | Not reported | Decidualisation, blastocyst implantation | Xiao et al. (2006) | |
| Pig | Luminal epithelium | Uterine luminal fluids | Not reported | Blastocyst implantation | Song et al. (2009) | |
| Bovine | Endometrium | Luminal epithelium, glandular epithelium, subepithelial stroma, uterine luminal fluid | Not reported | Not reported | Muñoz et al. (2017) | |
| Sheep | Endometrial glandular epithelial cells | Endometrial glands, uterine luminal fluids | Not reported | Blastocyst implantation | Song et al. (2006) | |
| Horse | Endometrial glands | Uterine luminal fluids | Not reported | Conceptus attachment and development | Kikuchi et al. (2011) | |
| Vasculature | Human | Endothelial cells, vascular smooth muscle cells | Not reported | Not reported | Spiral artery remodelling | Wallace et al. (2013) |
| Placenta | Human | Specific cell type not reported | Syncytiotrophoblast cells, cytotrophoblast cells, placental endothelial cells, stromal cells | cAMP/PKA/PI3-Kinase/Akt/SGK-1 pathway | Placental development | Uusküla et al. (2012) |
| Juhanson et al. (2016) | ||||||
| Abid et al. (2020) |
Note: Postulated roles are in grey text.
Methods
This review was prepared by systematically searching the literature using the PubMed database with the search terms: ‘stanniocalcin-1’, ‘placenta’, ‘ovary’, ‘endometrium’, ‘pregnancy’, ‘reproduction’, ‘early gestation’. No restrictions were placed on the year published, but a focus was placed on papers published between 1995 and 2020 and only English language papers were included.
The expression pattern and physiological and pathophysiological roles of STC-1 in the mammalian female reproductive system
The expression pattern of STC-1 in the ovary and its role in folliculogenesis
The expression pattern of STC-1 in the mammalian ovary
Early studies in mice revealed that the highest levels of STC-1 expression are found in the ovary where expression commences postnatally and rises throughout development (Deol et al., 2000). Within the adult mouse ovary, STC-1 gene and protein expression are detectable in the secondary interstitial and theca interna cells, however, only STC-1 protein expression is found in oocytes and corpus luteal cells (Varghese et al., 1998). The discordance between gene and protein expression observed here could be indicative of a mechanism in which oocytes and corpus luteal cells do not express the STC-1 gene but are targets for STC-1 and therefore sequester the protein (Varghese et al., 1998).
Although ovarian STC-1 gene expression remains constant throughout the oestrous cycle in mice, STC-1 protein expression increases during metoestrus I in the corpus luteum and declines thereafter (Deol et al., 2000). During pregnancy and the post-partum period in mice, ovarian STC-1 mRNA increases 15-fold, reaching a peak between days 10–14 of gestation before dropping sharply at the time of birth and rising again postpartum (Deol et al., 2000). Co-incident with the rise in STC-1 gene expression, STC-1 protein levels also increase, and the protein is detectable in the circulation for the first time. Within the ovary, the spatial distribution of STC-1 mRNA remains relatively constant, where it is detectable in the interstitium and thecal compartment. In contrast, STC-1 protein localises to the oocytes and thecal cells of primary and secondary follicles, as well as to interstitial cells and corpora lutea (Deol et al., 2000). The active regulation of ovarian STC-1 expression during gestation suggests a key role for STC-1 in the physiology of this process. It is noteworthy that STC-1 is the only secreted protein produced by the ovarian thecal interstitial compartment that is significantly up-regulated during mouse pregnancy (Deol et al., 2000).
Ovarian STC-1 expression has also been reported in pigs, rats and bovines (Worthington et al., 1999; Paciga et al., 2002). Although not fully characterised in rats and bovines, in pigs, STC-1 protein expression was reported in follicular fluid and was shown to be produced by granulosa cells (Basini et al., 2010), while STC-1 gene expression was detectable in the thecal layer of antral follicles, consistent with findings in mice, rats and bovines (Varghese et al., 1998; Paciga et al., 2002). During follicle development, STC-1 expression in thecal layer cells in the swine ovaries begins to decrease, whilst STC-1 expression in granulosa cells increases (Basini et al., 2010). Granulosa cell STC-1 expression is also significantly increased under conditions of hypoxia and anoxia (Basini et al., 2010). The increase in STC-1 expression in ovarian follicles during follicular development observed here, combined with its induction under conditions of oxygen deprivation are strongly indicative of a role for STC-1 in ovarian follicle physiology, especially as progressive hypoxia is established during follicle growth (Basini et al., 2010).
Regulation of ovarian STC-1 expression
The mechanisms underlying the regulation of STC-1 expression in the mammalian ovary are beginning to be appreciated. In a superovulation mouse model, administration of human chorionic gonadotropin (hCG), which mimics the effects of luteinising hormone (LH) and induces ovulation, resulted in a 2.5-fold increase in steady-state STC-1 mRNA levels, suggesting that STC-1 may be regulated by LH (Deol et al., 2000). In rat and bovine thecal interstitial cells, hCG also stimulates STC-1 secretion (Paciga et al., 2002). This response was mimicked by treatment with forskolin, suggesting that STC-1 regulation is mediated through the cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA) pathway. The stimulatory effect of hCG on STC-1 expression was only completely suppressed following inhibition of PKA, demonstrating the requirement for PKA activation in hCG-induced STC-1 expression in thecal interstitial cells (Paciga et al., 2002).
Structure and sub-cellular targeting of ovarian STC-1
The ovarian form of STC-1 is physically distinct and has been referred to as ‘big STC’ (Paciga et al., 2002). In light of the discovery of STC-2 in 1998 (Chang and Reddel, 1998), big STC will hereafter be referred to as big STC-1 for clarity. Cellular and secreted STC-1 from thecal interstitial cells are substantially larger than STC50 which is found in other mammalian tissues. In the native state, STC-1 derived from thecal interstitial cells comprises three molecular mass species of 84, 112 and 135 kDa. Although still uncertain, it was postulated that the increased mass may be due to post-translational modifications, including glycosylation (Paciga et al., 2002). However, enzymatic treatment of thecal interstitial cell-derived STC-1 to remove N-glycosylation had no effect, indicating that the mass variation is not due to differential glycosylation (Paciga et al., 2002). Chemical reduction of big STC-1 resulted in a 45 kDa species, implying that the three molecular masses are polymers of two or more subunits which are stabilised by disulphide linkages. It is hypothesised that the unique structure of ovarian STC-1 may explain its presence in the circulation during gestation and lactation due to differences in clearance kinetics between big STC-1 and STC50 (Paciga et al., 2002).
Although STC-1 receptors in reproductive tissues have been little studied, ovarian big STC-1 receptors have been described (Paciga et al., 2003). Through the use of an STC-1 alkaline phosphatase fusion protein, composed of mouse STC-1 cDNA fused to the human placental alkaline phosphatase gene (McCudden et al., 2002; Paciga et al., 2003), high concentrations of STC-1 receptors have been identified in cultured bovine luteal cells. Within these cells, significant populations of receptors have been found in cholesterol/lipid storage droplets (Paciga et al., 2003). Some targeting to the luteal cell plasma membrane is also evident, but the affinity of the plasma membrane receptor is 10-fold lower than that of the cholesterol/lipid storage droplets receptor (Paciga et al., 2003). This receptor-mediated targeting in luteal cells provides an explanation for the observed discordances in the patterns of STC-1 mRNA and protein distribution in ovarian cells. For example, although both STC-1 gene and protein expression are detectable in the thecal interstitial compartment in mice, rats and bovines (Varghese et al., 1998; Deol et al., 2000; Paciga et al., 2002), only STC-1 protein expression is present in oocytes and corpus luteal cells (Varghese et al., 1998; Deol et al., 2000), thus, it is postulated that oocytes and luteal cells may be targets for STC-1 in this case and sequester the protein (Varghese et al., 1998).
The regulation of STC-1 expression in luteal cells and its function in steroidogenesis
Although in vivo luteal cells do not express the STC-1 gene, when isolated and maintained in culture, STC-1 gene expression can be induced (Paciga et al., 2004). In this context, bovine luteal cells are capable of expressing and secreting big STC-1 similarly to thecal interstitial cells (Paciga et al., 2004). Unlike in thecal interstitial cells, big STC-1 production by luteal cells is not regulated by LH but is regulated by prostaglandin E2. Similar to LH-induced STC-1 expression from thecal interstitial cells, positive regulation of STC-1 expression in luteal cells is mediated through the PKA pathway (Paciga et al., 2004).
Negative regulation of STC-1 expression in luteal cells is achieved by sex steroids; both progesterone and oestradiol suppress STC-1 release from luteal cells and STC-1 expression and secretion are reduced to undetectable levels in the presence of androstenedione (Paciga et al., 2004). Both progesterone and oestradiol are autocrine regulators of luteal cell function in vivo (Okuda et al., 2001; Berisha et al., 2002; Cassar et al., 2002; Van Den Broeck et al., 2002a,b), thus, it is possible that these steroids act in concert in vivo to suppress luteal cell STC-1 production (Paciga et al., 2004). Moreover, in vivo luteal cells are in close proximity to the source of androgens, such as androstenedione, which may further explain the observed absence of STC-1 in luteal cells in the intact ovary (Paciga et al., 2004). Though it will require further study to fully understand the regulation of luteal cell STC-1 expression in vivo, it is feasible that STC-1 production in this context is only suppressed under conditions of high sex steroid production, however, when these levels are reduced, for example in the luteal phase of the oestrous cycle or after parturition (Kanchev and Dobson, 1976; Mostl et al., 1981), luteal cell STC-1 production could take place in vivo (Paciga et al., 2004).
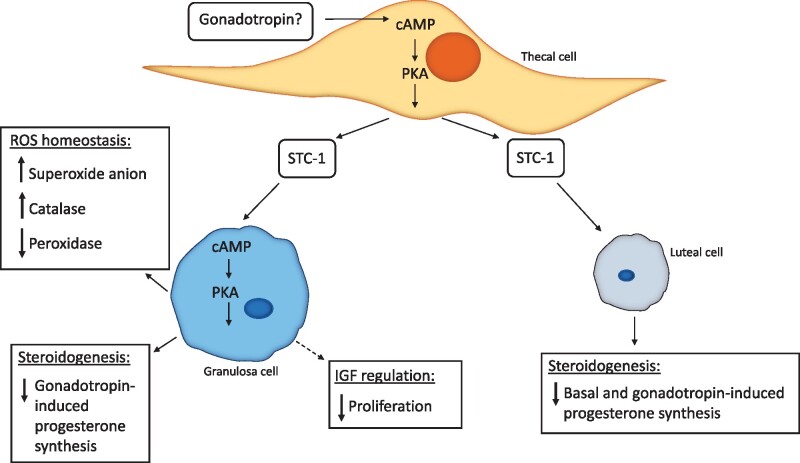
Although the regulation of STC-1 expression in luteal cells in vivo is not fully understood, it is apparent that thecal interstitial cell-derived STC-1 may be sequestered by luteal cells in vivo as they contain high levels of the STC-1 protein (Varghese et al., 1998). It appears that this relates to a role in progesterone synthesis. Basal and hCG-stimulated progesterone secretion from luteal cells is significantly decreased by big STC-1 (Paciga et al., 2003). Consistent with the requirement for PKA activation in hCG-induced expression of STC-1 in thecal interstitial cells (Paciga et al., 2002), big STC-1 receptors in luteal cells are up-regulated in response to PKA activation (Paciga et al., 2003). Thus, it is conceivable that in vivo big STC-1 may be targeted to luteal cells for the purpose of decreasing PKA-activated steroidogenesis (Paciga et al., 2003) (Fig. 1).
Figure 1.
A schematic illustration of the proposed regulation and roles of thecal cell-derived STC-1 in granulosa cells and luteal cells in the developing follicle. Demonstrated roles are shown with solid arrows, postulated roles are shown with dashed arrows. cAMP, cyclic adenosine monophosphate; IGF, insulin-like growth factor; PKA, protein kinase A; ROS, reactive oxygen species; STC-1, stanniocalcin-1.
The role of STC-1 in granulosa cells and in folliculogenesis
In addition to its role in modulating steroidogenesis in luteal cells, thecal interstitial cell-derived STC-1 has also been implicated in ovarian follicular development (Luo et al., 2004). Similar to the observed effects in bovine luteal cells, STC-1 causes a decrease in gonadotropin-induced progesterone production in granulosa cells (Luo et al., 2004). Treatment of rat granulosa cells with recombinant N-glycosylated STC-1 resulted in suppression of FSH-induced progesterone biosynthesis and LH receptor expression but, had little effect on oestradiol production (Luo et al., 2004) (Fig. 1). Specific inhibition of progesterone biosynthesis without effect on oestrogen production in this way has also been observed in other ovarian paracrine factors which are postulated as luteinisation inhibitors (Falck, 1959; Smith et al., 1975). It is, therefore, possible that STC-1 acts as a luteinisation inhibitor within ovarian follicles, but this will require further investigation. Treatment of rat granulosa cells with STC-1 also results in a decrease in expression of CYP11A mRNA, a rate-limiting enzyme in progesterone biosynthesis (Zlotkin et al., 1986; Goldring et al., 1987; Oonk et al., 1990), and this is the possible mechanism through which STC-1 mediates an effect on progesterone production in granulosa cells (Luo et al., 2004). To induce these cell-specific effects, STC-1 likely binds to several high specificity and affinity receptors located on the plasma membrane of granulosa cells, and when inside the cell, STC-1 acts downstream of adenylyl cyclase in the cAMP/PKA pathway to exert its effects on progesterone biosynthesis and LH receptor induction (Luo et al., 2004).
These findings are indicative of a follicular paracrine system in which thecal interstitial-derived STC-1 acts to dampen gonadotropin-induced granulosa cell differentiation (Luo et al., 2004). For STC-1 to act in this inhibitory manner in vivo, it is clear that stringent regulation of its expression must occur to permit granulosa cell differentiation. It was demonstrated that gonadotropin treatment of thecal interstitial cells suppresses STC-1 expression, suggesting a mechanism in which prior suppression of thecal interstitial STC-1 by gonadotropins is required to allow gonadotropin-induced granulosa cell differentiation (Luo et al., 2004). However, previous studies also report stimulation of STC-1 expression in thecal interstitial cells following gonadotropin treatment (Deol et al., 2000; Paciga et al., 2002), contrary to the gonadotropin-induced suppression of STC-1 from thecal interstitial cells reported by Luo et al. (2004). It is possible that differences in methodology could contribute to this discrepancy, therefore, further research is required to understand the regulation of STC-1 expression in thecal interstitial cells.
As well as modulating granulosa cell differentiation during ovarian follicular development, STC-1 has also been implicated in the regulation of granulosa cell redox status (Baioni et al., 2011). It is thought that the balance of pro-oxidant/anti-oxidant factors within the ovarian follicle plays an important role in folliculogenesis (Basini et al., 2008) and that some reactive oxygen species (ROS) induce the expression of genes involved in oocyte maturation and follicular development (Behrman et al., 2001; Agarwal et al., 2005). In granulosa cells, STC-1 stimulates the production of superoxide anion (Baioni et al., 2011), a ROS implicated in modulating ovarian steroidogenesis (Sawada and Carlson, 1996; Jain et al., 2000; Behrman et al., 2001) and in inducing ovulation (Behrman et al., 2001; Fujii et al., 2005). The process through which STC-1 stimulates superoxide anion production is unclear; however, as STC-1 is known to have stimulatory effects on the mitochondrial electron transport chain (Ellard et al., 2007), and the mitochondria are the main sites of superoxide anion generation (Murphy, 2009), it is possible that the action of STC-1 on mitochondria could explain this effect (Baioni et al., 2011). Although STC-1 does not affect the activity of superoxide dismutase, which catalyses the dismutation of superoxide anion to hydrogen peroxide, it has been shown to influence both catalase and peroxidase which scavenge hydrogen peroxide (Baioni et al., 2011). High concentrations of STC-1 stimulate catalase activity, thereby reducing levels of hydrogen peroxide. Conversely, elevated levels of STC-1 result in a decrease in peroxidase activity, potentially caused by the catalase-mediated reduction in its substrate, hydrogen peroxide (Baioni et al., 2011). These findings suggest that STC-1 may play an important role in ROS homeostasis in granulosa cells during folliculogenesis which may be essential to regulate their activity (Baioni et al., 2011) (Fig. 1), but the specific mechanisms underlying these processes, as well as their direct effect on granulosa cell function remain to be elucidated.
Regulation of pregnancy-associated plasma protein-A (PAPP-A) by STC-1/2 in ovarian follicles
The insulin-like growth factor (IGF) system is involved in several physiological ovarian functions including follicular growth, steroidogenesis and ovulation (Kwintkiewicz and Giudice, 2009). IGF signalling is regulated by the actions of the metzincin metalloproteinase, pregnancy-associated plasma protein-A (PAPP-A) (Oxvig, 2015) which is known to be secreted from granulosa cells (Conover et al., 2001). PAPP-A cleaves a subset of IGF binding proteins, counteracting their inhibitory effects and causing release of bioactive IGF (Oxvig, 2015). In vitro, STC-1 inhibits PAPP-A proteolytic activity through non-covalent, high affinity binding (Kløverpris et al., 2015). More recently, it has been established that these protein interactions also take place in vivo within human ovaries (Jepsen et al., 2016).
Coincident expression of both PAPP-A and STC-1 is evident throughout follicular development; PAPP-A and STC-1 are localised together in the primordial, late primary and antral follicles (Jepsen et al., 2016). Following follicle stimulation with hCG, the correlation between the intrafollicular concentration of PAPP-A and its activity is altered. hCG stimulation results in an increase in the intrafollicular concentration of PAPP-A, whilst a decrease in its activity is observed (Jepsen et al., 2016). Inhibitory complexes between PAPP-A and STC-1 are detectable in follicular fluid at this stage, explaining the decline in PAPP-A activity, despite an increase in its concentration (Jepsen et al., 2016). STC-2 is also expressed concurrently with STC-1 and PAPP-A and is found to form covalent inhibitory complexes with PAPP-A in follicular fluid (Jepsen et al., 2016). The finding of native complexes between PAPP-A and STC-1/2 in follicular fluid is strongly indicative of a model in which STC-1/2 inhibit the actions of PAPP-A, thus resulting in a decrease in bioactive IGF release (Jepsen et al., 2016). IGF activity in the mammalian ovaries has been linked to follicular growth via stimulation of the mitotic activity of granulosa cells (Kadakia et al., 2001; Spicer and Aad, 2007). At the midcycle peak, however, granulosa cell proliferation ceases, consistent with the decrease in PAPP-A activity in the follicular fluid (Jepsen et al., 2016). Thus, it is conceivable that STC-1/2 may be involved in the decline in granulosa cell proliferation during this stage of folliculogenesis through their actions in inhibiting PAPP-A (Jepsen et al., 2016) (Fig. 1). It remains to be determined whether STC-1/2-mediated inhibition of PAPP-A occurs in tissues other than the ovary.
The expression and postulated roles of STC-1 in the human endometrium and dysregulation in reproductive pathologies
The human endometrium is a dynamic tissue which undergoes a number of important histological and structural changes throughout the menstrual cycle in preparation for embryo implantation and subsequent shedding and regeneration in non-conception cycles (Talbi et al., 2006). Endometrial STC-1 expression has been described in several mammals, including rats, pigs, sheep and horses (Song et al., 2006, 2009; Xiao et al., 2006; Kikuchi et al., 2011). STC-1 expression in the human endometrium has been more recently described (Aghajanova et al., 2016; Khatun et al., 2020).
Several high-throughput gene expression profiling studies have implicated STC-1 in human endometrial functions and report fluctuating tissue expression levels throughout the menstrual cycle. Endometrial expression of STC-1 is high during the mid-secretory phase (MSE) (Talbi et al., 2006; Aghajanova et al., 2016; Boggavarapu et al., 2016; Suhorutshenko et al., 2018), correlating with the expression of receptivity markers during the window of implantation (Allegra et al., 2009). Aberrant endometrial expression of STC-1 has also been implicated in hypoxia-mediated abnormal uterine bleeding of long-acting progestin-only contraceptive users (Shapiro et al., 2015). The specific expression pattern and cellular localisation of STC-1 in the human endometrium in both normal and diseased states have been poorly studied and the existing literature suffers from restricted sample sizes. The findings, however, may still be clinically relevant but highlight the need for further research in this area.
Dysregulation of STC-1 in endometriosis
Endometriosis is an oestrogen-dependent gynaecological condition characterised by chronic inflammation and the presence and growth of ectopic endometrial tissue (Hickey et al., 2014). It affects between 10% and 15% of reproductive aged women and is often associated with severe and chronic pain and infertility (Mehedintu et al., 2014). Despite its prevalence, the aetiology of endometriosis is poorly understood. Altered endometrial expression of STC-1 in endometriosis has been described (Aghajanova et al., 2016).
In healthy women, endometrial STC-1 gene expression fluctuates throughout the menstrual cycle (Aghajanova et al., 2016). In the endometrium of women with endometriosis, however, STC-1 gene expression is significantly increased in MSE compared to the proliferative phase (PE) or early secretory phase (ESE). Interestingly, endometrial STC-1 gene expression in PE and MSE is 2-fold higher in women with endometriosis compared to healthy women (Aghajanova et al., 2016). Although not reported for women with endometriosis, up-regulation of STC-1 protein in endometrial fluid in MSE compared to ESE is observed in healthy women (Aghajanova et al., 2016). Abundance of STC-1 in the secretome of receptive MSE endometrium is highly indicative of a role in the process of implantation (Aghajanova et al., 2016), but further studies are required to confirm this role in the human endometrium.
In terms of cellular localisation in the MSE human endometrium, STC-1 protein is expressed in the cytoplasm of epithelial and stromal cells from both women with and without endometriosis (Aghajanova et al., 2016). In the endometrium of healthy women, STC-1 protein expression appears higher in the luminal epithelium and stroma compared to the glandular epithelium. In the endometrium of women with endometriosis, no difference in STC-1 protein expression was detectable between epithelial cells, however, endometrial stromal cell (eSC) expression of STC-1 was reduced in endometriosis compared to healthy cells (Aghajanova et al., 2016).
In endometrial stromal fibroblasts decidualised in vitro with cAMP, STC-1 gene expression was dramatically up-regulated over 230-fold in healthy women, however, in women with endometriosis, only a 45-fold increase is observed (Aghajanova et al., 2016). Of note, STC-1 gene expression is not significantly affected in endometrial stromal fibroblasts decidualised with oestradiol and progesterone in either the healthy or endometriosis group, suggesting that endometrial STC-1 expression is not regulated by sex steroids (Aghajanova et al., 2016), consistent with findings in the equine endometrium (Kikuchi et al., 2011). It does appear evident, however, that STC-1 expression in endometrial stromal fibroblasts is induced by cAMP and therefore may be mediated though the PKA pathway, but confirmation of this will require further investigation. The observed reduction in the up-regulation of STC-1 in cAMP-decidualised endometrial stromal fibroblasts from women with endometriosis may be indicative of a role in the pathogenesis of decidualisation defects (Aghajanova et al., 2016).
Dysregulation of STC-1 in polycystic ovary syndrome
In addition to endometriosis, STC-1 has also been implicated in the pathology of polycystic ovary syndrome (PCOS) (Khatun et al., 2020). PCOS is a common endocrine disorder which affects 8–12% of women of reproductive age. It is characterised by oligo-anovulation, hyperandrogenism and dysregulated metabolism (Teede et al., 2018). Moreover, women with PCOS present with subfertility and are at increased risk of developing pregnancy complications as well as endometrial cancer (Piltonen et al., 2013; Katulski et al., 2015; Palomba et al., 2015; El Hayek et al., 2016; McDonnell and Hart, 2017). Given the endometrial expression of STC-1, and its link to modulation of hypoxic and inflammatory responses essential for normal endometrial physiology (Westberg et al., 2007a,b; Mohammadipoor et al., 2016), it is postulated that STC-1 may play a role in the pathogenesis of PCOS.
Data obtained from the investigation of endometrial biopsy samples has revealed increased endometrial expression of STC-1 mRNA in MSE and late secretory phase (LSE) in both healthy women and those with PCOS (Khatun et al., 2020). In younger women with a more severe PCOS phenotype and higher BMI, STC-1 gene expression did not increase in the secretory phase as it did in the other groups. STC-1 protein expression increased from PE to LSE in stromal cells, but only in healthy women and not in those with PCOS, which is potentially indicative of a stromal cell defect in women with PCOS (Khatun et al., 2020). Further investigation of eSCs challenged with cAMP, previously established as an inducer of STC-1 expression, has revealed that eSCs from healthy women displayed a strong increase in STC-1 expression (Khatun et al., 2020). eSCs from women with PCOS showed a significantly smaller increase in expression of STC-1 in response to cAMP compared to that in eSCs from healthy controls. Furthermore, reduced basal PKA activity was demonstrated in eSCs from women with PCOS, suggesting a defect in cAMP-mediated PKA signalling in PCOS stromal cells (Khatun et al., 2020). In healthy women, STC-1 expression from eSCs was also stimulated under hypoxic conditions (Khatun et al., 2020). In contrast, STC-1 expression in eSCs from women with PCOS was not triggered by hypoxia and its secretion from eSCs was also reduced in these conditions (Khatun et al., 2020). As hypoxia is a crucial factor in embryo implantation (Daikoku et al., 2003; Matsumoto et al., 2018), it is possible that the observed impairment in STC-1 expression under these conditions could contribute to the subfertility associated with PCOS.
The role of obesity in influencing endometrial STC-1 expression has also been established. Increased secretory phase endometrial STC-1 expression is present in overweight/obese women compared to women with a lower BMI, however, these BMI-specific differences are not apparent in women with PCOS (Khatun et al., 2020). Elevated STC-1 expression in the secretory phase endometrium in overweight/obese women suggests that obesity may be a stress factor which leads to the induction of endometrial STC-1 expression, however, this mechanism appears absent in women with PCOS (Khatun et al., 2020). A 3-month lifestyle intervention, commonly recommended as the first-line therapy for women with PCOS and obesity (Teede et al., 2018), was unable to restore STC-1 expression in the PCOS endometrium, corroborating the report of impaired STC-1 regulation in the PCOS endometrium, regardless of BMI (Khatun et al., 2020).
The expression and role of STC-1 in early gestation
The expression and role of STC-1 in the uterus during decidualisation and blastocyst implantation
Blastocyst implantation is a tightly controlled physiological process which requires complex interactions between the developing embryo and the uterus (Paria et al., 2002). Dramatic changes in gene expression accompany this process to aid decidualisation and remodelling of the uterine architecture (Stasko et al., 2001). Several studies have implicated STC-1 in this process in numerous species through the observation of dynamic changes in its gene and protein expression throughout both the decidualisation and implantation processes. The strategies underlying these processes vary significantly across species, thus STC-1 expression and localisation also appear to be species-specific. Herein, the uterine expression patterns and putative roles for STC-1 in decidualisation and implantation in several mammalian species are described.
Early studies in mice first suggested a role for STC-1 in decidualisation and blastocyst implantation (Stasko et al., 2001). Following blastocyst implantation, STC-1 gene expression in the mouse uterus shifts from the uterine epithelium to the mesometrial stromal cells. Between days 6.5–8.5 of pregnancy, STC-1 gene expression then moves to cells of the mesometrial lateral sinusoids and declines thereafter (Stasko et al., 2001). The localisation of STC-1 protein varies from its gene expression. STC-1 protein is reported to accumulate in epithelial, stromal and decidual cells, as well as in fetal giant trophoblast cells throughout the implantation process. In addition, it localises to decidualising anti-mesometrial cells (Stasko et al., 2001). The mRNA-protein disparities observed here indicate that STC-1 is transcribed in one cell type and then sequestered by target cells for action, suggestive of a paracrine/autocrine signalling role during these processes in the uterus (Stasko et al., 2001).
The pattern of STC-1 expression during implantation has been further investigated in rats (Xiao et al., 2006). Here, similar expression patterns to those seen in mice have been reported. In rats, STC-1 mRNA is present in the stromal cells surrounding the implanting blastocyst. It is notable, however, that in pseudopregnant rats, corresponding STC-1 gene expression is not observed suggesting that STC-1 expression in the uterus is specifically induced by developing embryos and implanting blastocysts. This hypothesis is further evidenced by the observation that STC-1 mRNA is maintained at a basal level during an induced delay in implantation but is elevated following termination of the delay. In addition, STC-1 gene and protein expression are concentrated at sites with the implanting blastocyst compared to other uterine segments (Xiao et al., 2006).
In contrast to rodents, studies in sheep have revealed that STC-1 gene expression is confined to endometrial glandular epithelial cells and is induced by progesterone (Song et al., 2006). Expression is detectable in the endometrial glands from day 16, consistent with the start of blastocyst implantation (Spencer et al., 2004), to day 120 of gestation and is stimulated by the placental hormones, ovine placental lactogen and ovine growth hormone (Song et al., 2006). STC-1 protein is also present in the endometrial glands, and is secreted into the uterine lumen where it is detectable in uterine secretions, allantoic fluid and placental areolae which transport uterine gland secretions across the placenta and into the fetal-placental circulation. Secretion of STC-1 from uterine glands into the fetal circulation and allantoic fluid in this manner may suggest an exocrine role in this system (Song et al., 2006).
In pigs, STC-1 mRNA is present exclusively in the luminal epithelium at the uterine-conceptus interface during the period of conceptus attachment prior to implantation, between days 12–25 of pregnancy (Song et al., 2009). Consistent with observations in sheep, STC-1 expression is also induced by progesterone and is initially stimulated by oestrogen in the luminal epithelium at implantation sites. Expression is later inhibited, however, by the combined effects of oestrogen and progesterone. STC-1 protein is also detectable but is found in uterine luminal fluids (Song et al., 2009). Although a specific role has yet to be elucidated, it is postulated that in pigs, STC-1 may be involved in implantation through effects on uterine receptivity and remodelling of the trophoectoderm for conceptus elongation (Song et al., 2009).
STC-1 expression in the equine uterus is remarkably similar to that seen in the pig uterus, where increased endometrial STC-1 expression coincides with conceptus attachment (Kikuchi et al., 2011). STC-1 protein is detectable in uterine luminal fluids, indicating secretion of STC-1 from the endometrial glands towards the conceptus (Kikuchi et al., 2011), which is consistent with observations in pigs and sheep (Song et al., 2006, 2009). In the equine uterus, it is also suggested that STC-1 expression is regulated by conceptus oestrogen (Kikuchi et al., 2011). As the STC-1 gene lacks any steroid receptor binding sites in its upstream region, it is likely that the effect of steroid hormones on STC-1 expression is indirect (Kikuchi et al., 2011). STC-1 possesses early growth response factor (Egr) binding sites in its upstream region and, since ligand-activated oestrogen receptors can up-regulate gene transcription indirectly through transcription factors such as the Egr family, it is possible that this represents an indirect mechanism for steroid hormone action on STC-1 expression (Almeida et al., 2006; Melamed et al., 2006). In addition, other factors present in the equine uterus, such as vascular endothelial growth factor A or hypoxia-inducible factor 1α, can be activated by oestrogen in vitro (Kazi and Koos, 2007; Bake et al., 2008) and are reported to induce expression of STC-1, possibly indicating another indirect mechanism (Wary et al., 2003; Yeung et al., 2005).
It is interesting that the uterine expression of STC-1 during early pregnancy appears to differ amongst species. STC-1 expression in pigs, horses and rodents is detectable earlier than in sheep, from the point of conceptus attachment (Stasko et al., 2001; Song et al., 2006, 2009; Xiao et al., 2006; Kikuchi et al., 2011). In pigs, STC-1 expression appears to be limited only to the peri-implantation period of pregnancy, unlike rodents and sheep where expression continues into the later stages of placentation (Stasko et al., 2001; Song et al., 2006, 2009; Xiao et al., 2006). The observed temporal and spatial expression of STC-1 in pigs and horses suggests that STC-1 may play an important role in conceptus attachment during implantation (Song et al., 2009; Kikuchi et al., 2011), whereas in rodents and sheep, STC-1 may be involved in later stages of implantation and decidualisation. It is intriguing that in bovines, endometrial STC-1 expression does not appear to be pregnancy-specific as comparable expression is detected in the non-pregnant, cyclic uterus (Muñoz et al., 2017). It is likely that these inter-species differences manifest from intrinsic differences in placental structure and development between species.
Despite our knowledge of the role of STC-1 in blastocyst implantation in non-human mammals, its corresponding role in humans has been little studied due to the challenges in investigating human pregnancy in vivo. A 2009 study by Allegra and colleagues is, thus far, the only study to implicate STC-1 in the process of blastocyst implantation in humans. By investigating endometrial gene expression in women undergoing IVF treatment who subsequently became pregnant it was found that STC-1 is one of only six genes which retains homogenous expression during the window of implantation (Allegra et al., 2009). Confirmation of STC-1 involvement in implantation will, however, require further study.
From the numerous studies discussed herein, it appears evident that STC-1 plays a role in the uterus during early pregnancy, particularly during the implantation/peri-implantation period. Therefore, data obtained from studies using genetically engineered mouse models are surprising. In STC-1 null mice, no important change in fertility was detectable (Chang et al., 2005). It is possible that there may be other gene products capable of fully compensating for STC-1 in this context. The potential for STC-2 to act in this way was investigated in the same study, however, no change in the expression of STC-2 in organs in the STC-1 null mice was detected, suggesting that this is not the compensatory factor (Chang et al., 2005). In contrast, transgenic overexpression of human STC-1 in mice results in reduced female reproductive capacity (Varghese et al., 2002). It is unclear what causes this, but from the data on uterine STC-1 expression in mice (Stasko et al., 2001), it is apparent that dramatic and precise shifts in expression accompany blastocyst implantation, thus, it is postulated that transgenic overexpression may interfere with this tightly controlled process (Varghese et al., 2002).
The expression of STC-1 in maternal spiral arteries in pregnancy
In addition to its dynamic regulation and putative essential function in establishing a healthy pregnancy, STC-1 has also been implicated in the remodelling of the maternal vasculature which occurs early in pregnancy (Wallace et al., 2013). During the second half of the menstrual cycle, under the influence of progesterone, highly coiled arteries begin to form at the myometrial-endometrial boundary of the uterus; these are known as spiral arteries (Ferenczy et al., 1979). These arteries supply blood to the endometrial layer and, during pregnancy, they span the inner myometrium and decidua. During early pregnancy, spiral arteries undergo a unique remodelling process in which they change from small, high resistance vessels to large, low resistance vessels (Burton et al., 2009). This remodelling results in large diameter vessels which are unresponsive to vasoconstrictors allowing an unimpeded increase in blood flow to the developing foetus (Osol and Mandala, 2009). During the remodelling process, vascular smooth muscle cells (VSMCs) and endothelial cells (ECs) are lost from the spiral arteries and are replaced by fetal trophoblast cells (Pijnenborg et al., 1983; Burton et al., 1999). Trophoblast cells disrupt the interactions of the VSMCs and ECs through processes including apoptosis and dedifferentiation and supplant the vascular cells (Whitley and Cartwright, 2009). The spiral artery remodelling process is completed by 20–22 weeks of gestation (Sato et al., 2012).
Defects in maternal vasculature remodelling have been associated with the onset of several pregnancy complications, including pre-eclampsia, fetal growth restriction and pre-term labour. Although these complications do not manifest until later in pregnancy, it is thought that their pathology is established during the early stages of pregnancy (Cartwright and Whitley, 2017). For example, spiral arteries derived from pre-eclamptic pregnancies have a reduced mean external diameter compared to those from normal pregnancies (200 µm compared to 500 µm) (Brosens et al., 1972). This defect in vascular remodelling dramatically reduces blood supply to the placenta resulting in poor placental perfusion (Zhou et al., 1993; Redman and Sargent, 2001). Despite our knowledge of insufficient vascular remodelling in early pregnancy, the specific mechanisms underlying the physiology and pathophysiology of the process are poorly understood. A potential role for STC-1 in this process, however, has been suggested.
STC-1 was first implicated in the process of spiral artery remodelling following investigation of the role of fetal trophoblast secreted factors on vascular cell gene expression (Wallace et al., 2013). The use of a three-dimensional spheroid co-culture of ECs and VSMCs to represent an inverted vessel lumen, revealed two-fold up-regulation of STC-1 following incubation with conditioned media from an extravillous trophoblast cell line (Wallace et al., 2013). The mechanisms underlying the up-regulation of STC-1 within this system are not yet understood, however, it is known that fetal trophoblast cells secrete a range of growth factors and cytokines, including hepatocyte growth factor, vascular endothelial growth factor, interleukin-1β, interleukin-6, platelet-derived growth factor and placental growth factor (Wallace et al., 2013). It is likely that a number of these factors will act either individually or in synchrony to stimulate STC-1 gene expression from vascular cells and this will require further investigation.
Despite clear up-regulation of STC-1 in the remodelling vessel, its specific function has yet to be described. It is evident from the current research that a number of tightly controlled cellular events take place during the vascular remodelling process (Whitley and Cartwright, 2010). The existing literature on the functional roles of STC-1 may suggest its potential mechanisms of action within the remodelling spiral artery. For example, STC-1 has been shown to inhibit the action of inflammatory cytokines, including tumour necrosis factor α (Chen et al., 2008; Sheikh-Hamad, 2010), an important factor in the regulation of vascular cell apoptosis in artery remodelling (Rastogi et al., 2012). In addition, STC-1 antagonises the activity of vascular reactive factors, including angiotensin II and hepatocyte growth factor (Zlot et al., 2003; Liu et al., 2012; Moreau et al., 2012). Both factors are known to be present within the environment of the remodelling spiral artery and could influence the behaviour of vascular cells. Furthermore, overexpression of STC-1 has been shown to promote angiogenesis in vivo and in vitro (Bell et al., 2001; Gerritsen et al., 2002; Liu et al., 2003; He et al., 2011), suggesting that it may have different roles depending on concentration or the local environment. It is crucial to maintain a balance of signals within the remodelling spiral artery to allow vessel change but also apply limits. Therefore, it is possible that altering levels of STC-1 acts as a local control mechanism to prevent excessive remodelling, but this will require further study.
The regulation of STC-1 secretion in the placenta during placental development
The placenta is a highly specialised, pregnancy specific organ that develops rapidly throughout gestation. The expression of placental genes varies significantly during pregnancy, with genes regulating the cell cycle, differentiation, motility and angiogenesis being up-regulated in early pregnancy, whereas genes involved in lipid metabolism, stress response and signal transduction are highly expressed at term (Winn et al., 2007). Transcriptome profiling of human placental gene expression dynamics from early to mid-gestation has revealed significant expression of STC-1 during this period with a peak of expression during mid-gestation. Interestingly, placental STC-1 expression also remains high at term, but only in complicated pregnancies (Uusküla et al., 2012). Several single-nucleotide polymorphisms (SNPs) in the STC-1 gene have been identified which appear to directly modulate placental STC-1 transcript levels (Juhanson et al., 2016). It is possible that these SNPs explain the observed variation in placental STC-1 gene expression levels between normal and complicated pregnancies, but this requires further study (Juhanson et al., 2016). Although the precise role of placental STC-1 in pregnancy has yet to be elucidated, current findings in the field suggest that it plays a role in maintaining a healthy pregnancy, but may also be implicated in the pathology of pregnancy complications (Abid et al., 2020).
Until recently, the cellular origin of STC-1 in the developing placenta had not been determined. Immunohistochemical staining of placental tissue has now revealed that STC-1 is predominantly expressed in the syncytiotrophoblast and cytotrophoblast cells of the first trimester placenta. STC-1 protein expression is also reported in placental endothelial and stromal cells but at lower expression levels (Abid et al., 2020). It was also reported that placental cells secrete STC-1 (Abid et al., 2020) and we are now beginning to understand the mechanisms underlying the regulation of this secretion.
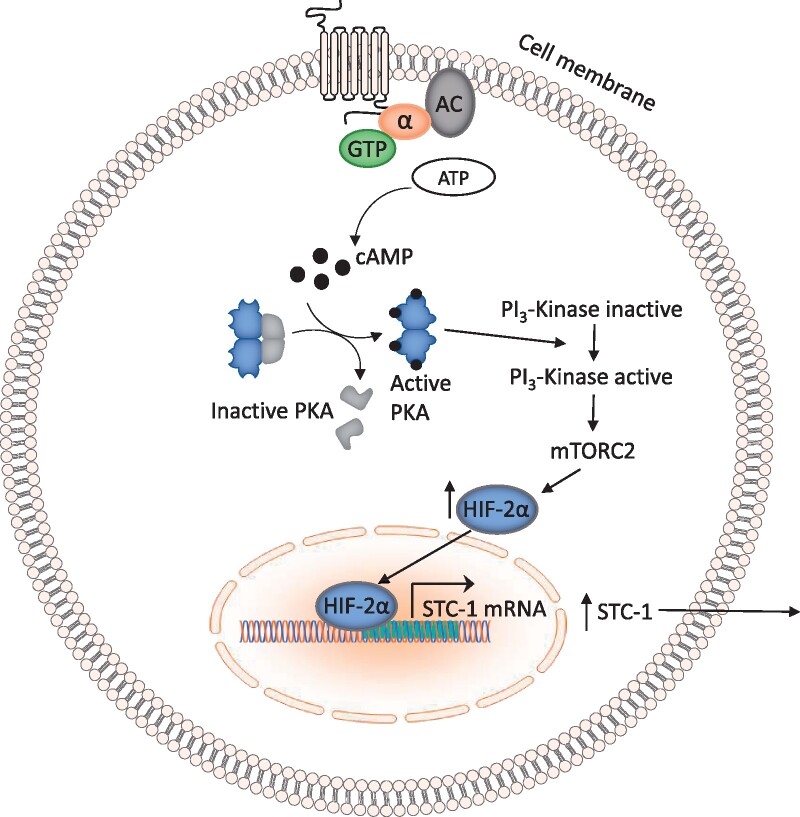
Low oxygen conditions (1% O2), combined with induction of intracellular cAMP, have been shown to increase STC-1 secretion from a choriocarcinoma-derived cytotrophoblast cell line (BeWo) (Abid et al., 2020). In this cell line, elevated intracellular cAMP induces cell fusion and differentiation (Wice et al., 1990). In vivo, differentiation of trophoblast cells is induced through the same mechanism where cAMP is stimulated by hCG (Weedon-Fekjær and Taskén, 2012). Pharmacological modulation of PKA has revealed that the secretion of STC-1 from BeWo cells is mediated through this pathway (Abid et al., 2020). Interestingly, elevation of intracellular cAMP alone had no effect on the secretion of STC-1 by BeWo cells, it was only in combination with low oxygen that a significant effect on secretion was observed. Similar data from first trimester chorionic villus tissue shows that STC-1 secretion was greater under conditions of low oxygen (Abid et al., 2020). Further investigation of the pathways underlying STC-1 secretion under these conditions has shown that this process is mediated through activation of the PI3-Kinase/Akt/SGK-1 pathway, primarily through activation of mTORC-2. In addition, inhibition of the transcription factor hypoxia-inducible factor 2α resulted in complete inhibition of STC-1 secretion in this system, suggesting a key role for this factor (Abid et al., 2020) (Fig. 2).
Figure 2.
A schematic overview of the pathway underlying STC-1 secretion from the choriocarcinoma-derived cytotrophoblast cell line, BeWo, under conditions of low oxygen. AC, adenylyl cyclase; cAMP, cyclic adenosine monophosphate; HIF-2α, hypoxia-inducible factor-2 alpha; mTORC2, mammalian target of rapamycin complex 2; PKA, protein kinase A; STC-1, stanniocalcin-1.
The observation of increased STC-1 secretion exclusively under low oxygen conditions is significant in relation to the in vivo situation. In early gestation, the oxygen concentration at the maternal–foetal interface is thought be around 1–2% (Rodesch et al., 1992). Around the 10th week of pregnancy, however, blood flow to the developing placenta changes significantly due remodelling of the maternal spiral arteries and the loss of trophoblast plugs (Burton et al., 2009). In pregnancies that later develop complications such as pre-eclampsia or fetal growth restriction, this remodelling is insufficient, resulting in placental under-perfusion and hypoxia (Redman and Sargent, 2001). It is notable that placental STC-1 expression and secretion are increased in pregnancies with these complications (Uusküla et al., 2012; Abid et al., 2020), therefore it is plausible that low oxygen may be a stimulus for STC-1 expression in the developing placenta. Correspondingly, increased STC-1 expression in response to low oxygen has also been observed in several pathological conditions including cerebral ischaemia (Zhang et al., 2000), carcinogenesis (Chang et al., 2003; Liu et al., 2010; Ma et al., 2015) and heart failure (Sheikh-Hamad et al., 2003).
In healthy, non-pregnant individuals, STC-1 is absent or undetectable in the circulation. During pregnancy, however, detectable levels of STC-1 have been reported, and are suggestive of an endocrine role in pregnancy (Deol et al., 2000). The source of circulating STC-1 in pregnancy is an area of uncertainty in the field. It is postulated that circulating STC-1 may originate from the ovary, due to the coincident rise in ovarian STC-1 gene expression with increased serum STC-1 levels (Deol et al., 2000). It is also thought, however, that the placenta may be the source of circulating STC-1 due to observed STC-1 secretion from placental cells (Abid et al., 2020). It is notable that STC-1 remains in the circulation post-partum after the placenta is delivered, therefore, it is conceivable that circulating STC-1 could originate from both the placenta and the ovaries, or that the tissue origin changes post-partum, perhaps to support the physiology of lactation. In pregnancies complicated by conditions whose aetiology is thought to occur in the first trimester, such as pre-eclampsia, fetal growth restriction and gestational diabetes, third trimester and post-partum serum levels of STC-1 are even further elevated (Uusküla et al., 2012; Abid et al., 2020). This is consistent with increased secretion of STC-1 seen in first trimester placentas derived from pregnancies at risk of developing pre-eclampsia (Abid et al., 2020). To fully understand the origin and pattern of STC-1 release into the circulation during pregnancy and pregnancy complications, however, more comprehensive analysis of maternal serum is required. As the ovarian STC-1 is structurally distinct (Paciga et al., 2002), it is plausible that mass spectrometric analysis of serum could identify the tissue-origin of circulating STC-1. Furthermore, the presence of STC-1 in the circulation in women during pregnancy has only been investigated in the third trimester and post-partum (Uusküla et al., 2012; Juhanson et al., 2016; Abid et al., 2020), therefore it is unclear at which stage of pregnancy STC-1 is released into the bloodstream. To understand the pattern of STC-1 release into the circulation in human pregnancy, longitudinal studies on maternal serum are required.
Conclusions and future perspectives
STC-1 was first implicated in the field of female reproduction over 20 years ago, but despite this, we are only now beginning to understand the mechanisms underlying its regulation in female reproductive tissues and its specific functions in this system. The current data suggest dynamic regulation of STC-1 within ovarian cells and indicate a paracrine system in which STC-1 is sequestered to target cells for action (Deol et al., 2000; Luo et al., 2004; Basini et al., 2010). STC-1 appears to function in the process of ovarian follicular development as well as in modulating ovarian steroidogenesis (Paciga et al., 2003; Luo et al., 2004; Baioni et al., 2011; Jepsen et al., 2016). In the human endometrium, the specific function of STC-1 has yet to be elucidated, however, clear dysregulation of STC-1 in endometrial pathologies indicates a further requirement for study in this area (Aghajanova et al., 2016; Khatun et al., 2020). Furthermore, uterine expression of STC-1 in early gestation may highlight a key role for this protein in the physiology and pathophysiology of pregnancy. Coincident expression of STC-1 during blastocyst implantation in most mammalian species, including humans, suggests its involvement in the establishment of pregnancy (Stasko et al., 2001; Song et al., 2006, 2009; Xiao et al., 2006; Allegra et al., 2009; Kikuchi et al., 2011). Moreover, expression of STC-1 in the developing placenta and altered expression in complicated pregnancies highlights the requirement of STC-1 for maintenance of a healthy pregnancy but suggests that its expression must be tightly regulated (Uusküla et al., 2012; Juhanson et al., 2016; Abid et al., 2020). It is evident that expression of STC-1 in most cells types is mediated through the PKA pathway and is induced by cAMP (Paciga et al., 2002, 2004; Aghajanova et al., 2016; Abid et al., 2020; Khatun et al., 2020). This is interesting as it indicates consistent regulation mechanisms amongst reproductive cell types, which could aid future research in this area.
The present data, combined with the known roles of STC-1 in diverse biological processes, are strongly suggestive of a pivotal role in female reproduction. The obvious limitations in studying the human reproductive system in vivo and the lack of adequate models have hindered our progression in the field. It is clear, however, that further research is required to elucidate the specific roles of STC-1 within various reproductive tissues, as this will help shed light on the pathogenesis of reproductive pathologies in which STC-1 is dysregulated.
Data availability
No new data were generated or analysed in support of this research.
Acknowledgements
Figures 1 and 2 of this manuscript were prepared using the Motifolio drawing toolkit.
Authors’ roles
A.B. wrote the first draft of the manuscript; G.S.W. and J.E.C. edited and approved the final manuscript.
Funding
A.B. is funded by a PhD Studentship from the British Heart Foundation (FS/17/72/33181). G.S.W. and J.E.C. are funded by the Higher Education Funding Council for England.
Conflict of interest
The authors declare no conflicts of interest.
References
- Abid N, Embola J, Tryfonos Z, Bercher J, Ashton SV, Khalil A, Thilaganathan B, Cartwright JE, Whitley GS.. Regulation of stanniocalcin‐1 secretion by BeWo cells and first trimester human placental tissue from normal pregnancies and those at increased risk of developing preeclampsia. FASEB J 2020;34:6086–6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A, Gupta S, Sharma RK.. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol 2005;3:28–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajanova L, Altmäe S, Kasvandik S, Salumets A, Stavreus-Evers A, Giudice LC.. Stanniocalcin-1 expression in normal human endometrium and dysregulation in endometriosis. Fertil Steril 2016;106:681–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allegra A, Marino A, Coffaro F, Lama A, Rizza G, Scaglione P, Sammartano F, Santoro A, Volpes A.. Is there a uniform basal endometrial gene expression profile during the implantation window in women who became pregnant in a subsequent ICSI cycle? Hum Reprod 2009;24:2549–2557. [DOI] [PubMed] [Google Scholar]
- Almeida M, Han L, O'brien CA, Kousteni S, Manolagas SC.. Classical genotropic versus kinase-initiated regulation of gene transcription by the estrogen receptor α. Endocrinology 2006;147:1986–1996. [DOI] [PubMed] [Google Scholar]
- Bai Y, Xiao Y, Dai Y, Chen X, Li D, Tan X, Zhang X.. Stanniocalcin 1 promotes cell proliferation via cyclin E1/cyclin-dependent kinase 2 in human prostate carcinoma. Oncol Rep 2017;37:2465–2471. [DOI] [PubMed] [Google Scholar]
- Baioni L, Basini G, Bussolati S, Grasselli F.. Stanniocalcin 1 affects redox status of swine granulosa cells. Regul Pept 2011;168:45–49. [DOI] [PubMed] [Google Scholar]
- Bake S, Ma L, Sohrabji F.. Estrogen receptor-α overexpression suppresses 17β-estradiol-mediated vascular endothelial growth factor expression and activation of survival kinases. Endocrinology 2008;149:3881–3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basini G, Baioni L, Bussolati S, Grolli S, Kramer LH, Wagner GF, Grasselli F.. Expression and localization of stanniocalcin 1 in swine ovary. Gen Comp Endocrinol 2010;166:404–408. [DOI] [PubMed] [Google Scholar]
- Basini G, Simona B, Santini SE, Grasselli F.. Reactive oxygen species and anti-oxidant defences in swine follicular fluids. Reprod Fertil Dev 2008;20:269–274. [DOI] [PubMed] [Google Scholar]
- Behrman HR, Kodaman PH, Preston SL, Gao S.. Oxidative stress and the ovary. J Soc Gynecol Investig 2001;8:S40–S42. [DOI] [PubMed] [Google Scholar]
- Bell SE, Mavila A, Salazar R, Bayless KJ, Kanagala S, Maxwell SA, Davis GE.. Differential gene expression during capillary morphogenesis in 3D collagen matrices: regulated expression of genes involved in basement membrane matrix assembly, cell cycle progression, cellular differentiation and G-protein signaling. J Cell Sci 2001;114:2755–2773. [DOI] [PubMed] [Google Scholar]
- Berisha B, Pfaffl MW, Schams D.. Expression of estrogen and progesterone receptors in the bovine ovary during estrous cycle and pregnancy. Endocrine 2002;17:207–214. [DOI] [PubMed] [Google Scholar]
- Boggavarapu NR, Lalitkumar S, Joshua V, Kasvandik S, Salumets A, Lalitkumar PG, Gemzell-Danielsson K.. Compartmentalized gene expression profiling of receptive endometrium reveals progesterone regulated ENPP3 is differentially expressed and secreted in glycosylated form. Sci Rep 2016;6:33811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfante S, Della Giustina A, Danielski LG, Denicol T, Joaquim L, Biehl E, Scopel G, de Carli RJ, Hubner M, Cardoso T. et al. Stanniocalcin-1 ameliorates cerebral ischemia by decrease oxidative stress and blood brain barrier permeability. Microvasc Res 2020;128:103956. [DOI] [PubMed] [Google Scholar]
- Brosens IA, Robertson WB, Dixon HG.. The role of the spiral arteries in the pathogenesis of preeclampsia. Obstet Gynecol Annu 1972;1:177–191. [PubMed] [Google Scholar]
- Burton GJ, Jauniaux E, Watson AL.. Maternal arterial connections to the placental intervillous space during the first trimester of human pregnancy: the Boyd collection revisited. Am J Obstet Gynecol 1999;181:718–724. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Woods AW, Jauniaux E, Kingdom JCP.. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta 2009;30:473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butkus A, Roche PJ, Fernley RT, Haralambidis J, Penschow JD, Ryan GB, Trahair JF, Tregear GW, Coghlan JP.. Purification and cloning of a corpuscles of Stannius protein from Anguilla australis. Mol Cell Endocrinol 1987;54:123–133. [DOI] [PubMed] [Google Scholar]
- Cartwright JE, Whitley GSJ.. Strategies for investigating the maternal-fetal interface in the first trimester of pregnancy: what can we learn about pathology? Placenta 2017;60:145–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassar CA, Dow MPD, Pursley JR, Smith GW.. Effect of the preovulatory LH surge on bovine follicular progesterone receptor mRNA expression. Domest Anim Endocrinol 2002;22:179–187. [DOI] [PubMed] [Google Scholar]
- Chang AC-M, Cha J, Koentgen F, Reddel RR.. The murine stanniocalcin 1 gene is not essential for growth and development. Mol Cell Biol 2005;25:10604–10610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AC-M, Dunham MA, Jeffrey KJ, Reddel RR.. Molecular cloning and characterization of mouse stanniocalcin cDNA. Mol Cell Endocrinol 1996;124:185–187. [DOI] [PubMed] [Google Scholar]
- Chang AC-M, Janosi J, Hulsbeek M, de Jong D, Jeffrey KJ, Noble JR, Reddel RR.. A novel human cDNA highly homologous to the fish hormone stanniocalcin. Mol Cell Endocrinol 1995;112:241–247. [DOI] [PubMed] [Google Scholar]
- Chang AC-M, Jellinek DA, Reddel RR.. Mammalian stanniocalcins and cancer. Endocr Relat Cancer 2003;10:359–373. [DOI] [PubMed] [Google Scholar]
- Chang ACM, Reddel RR.. Identification of a second stanniocalcin cDNA in mouse and human: Stanniocalcin 2. Mol Cell Endocrinol 1998;141:95–99. [DOI] [PubMed] [Google Scholar]
- Chen C, Jamaluddin MS, Yan S, Sheikh-Hamad D, Yao Q.. Human stanniocalcin-1 blocks TNF-α-induced monolayer permeability in human coronary artery endothelial cells. Arterioscler Thromb Vasc Biol 2008;28:906–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover CA, Faessen GF, Ilg KE, Chandrasekher YA, Christiansen M, Overgaard MT, Oxvig C, Giudice LC.. Pregnancy-associated plasma protein-A is the insulin-like growth factor binding protein-4 protease secreted by human ovarian granulosa cells and is a marker of dominant follicle selection and the corpus luteum. Endocrinology 2001;142:2155–2155. [DOI] [PubMed] [Google Scholar]
- Daikoku T, Matsumoto H, Gupta RA, Das SK, Gassmann M, DuBois RN, Dey SK.. Expression of hypoxia-inducible factors in the peri-implantation mouse uterus is regulated in a cell-specific and ovarian steroid hormone-dependent manner. Evidence for differential function of HIFs during early pregnancy. J Biol Chem 2003;278:7683–7691. [DOI] [PubMed] [Google Scholar]
- Deol HK, Varghese R, Wagner GF, DiMattia GE.. Dynamic regulation of mouse ovarian stanniocalcin expression during gestation and lactation. Endocrinology 2000;141:3412–3421. [DOI] [PubMed] [Google Scholar]
- DiMattia GE, Varghese R, Wagner GF.. Molecular cloning and characterization of stanniocalcin-related protein. Mol Cell Endocrinol 1998;146:137–140. [DOI] [PubMed] [Google Scholar]
- El Hayek S, Bitar L, Hamdar LH, Mirza FG, Daoud G.. Poly cystic ovarian syndrome: an updated overview. Front Physiol 2016;7:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellard JP, McCudden CR, Tanega C, James KA, Ratkovic S, Staples JF, Wagner GF.. The respiratory effects of stanniocalcin-1 (STC-1) on intact mitochondria and cells: STC-1 uncouples oxidative phosphorylation and its actions are modulated by nucleotide triphosphates. Mol Cell Endocrinol 2007;264:90–101. [DOI] [PubMed] [Google Scholar]
- Falck B. Site of production of oestrogen in the ovary of the rat. Nature 1959;184(Suppl 14):1082. [DOI] [PubMed] [Google Scholar]
- Ferenczy A, Bertrand G, Gelfand MM.. Proliferation kinetics of human endometrium during the normal menstrual cycle. Am J Obstet Gynecol 1979;133:859–867. [DOI] [PubMed] [Google Scholar]
- Fontaine M. Stannius’ Corpuscles and Ionic (Ca, K, Na) of the Interior Environment of the Eel (Anguilla Anguilla L.). C R Hebd Seances Acad Sci 1964;259:875–878. [PubMed] [Google Scholar]
- Fujii J, Iuchi Y, Okada F.. Fundamental roles of reactive oxygen species and protective mechanisms in the female reproductive system. Reprod Biol Endocrinol 2005;3:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerritsen ME, Peale FV Jr, Wu T.. Gene expression profiling in silico: relative expression of candidate angiogenesis associated genes in renal cell carcinomas. Exp Nephrol 2002;10:114–119. [DOI] [PubMed] [Google Scholar]
- Goldring NB, Durica JM, Lifka J, Hedin L, Ratoosh SL, Miller WL, Orly J, Richards JS.. Cholesterol side-chain cleavage p450 messenger ribonucleic acid: Evidence for hormonal regulation in rat ovarian follicles and constitutive expression in corpora lutea. Endocrinology 1987;120:1942–1950. [DOI] [PubMed] [Google Scholar]
- He L, Wang T, Gao Q, Zhao G, Huang Y, Yu L, Hou Y.. Stanniocalcin-1 promotes tumor angiogenesis through up-regulation of VEGF in gastric cancer cells. J Biomed Sci 2011;18:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey M, Ballard K, Farquhar C.. Endometriosis. BMJ 2014;348:g1752. [DOI] [PubMed] [Google Scholar]
- Ishibashi K, Miyamoto K, Taketani Y, Morita K, Takeda E, Sasaki S, Imai M.. Molecular cloning of a second human stanniocalcin homologue (STC2). Biochem Biophys Res Commun 1998;250:252–258. [DOI] [PubMed] [Google Scholar]
- Jain S, Saxena D, Kumar GP, Laloraya M.. NADPH dependent superoxide generation in the ovary and uterus of mice during estrous cycle and early pregnancy. Life Sci 2000;66:1139–1146. [DOI] [PubMed] [Google Scholar]
- Jellinek DA, Chang AC, Larsen MR, Wang X, Robinson PJ, Reddel RR.. Stanniocalcin 1 and 2 are secreted as phosphoproteins from human fibrosarcoma cells. Biochem J 2000;350(Pt 2):453–461. [PMC free article] [PubMed] [Google Scholar]
- Jepsen MR, Kløverpris S, Bøtkjær JA, Wissing ML, Andersen CY, Oxvig C.. The proteolytic activity of pregnancy-associated plasma protein-A is potentially regulated by stanniocalcin-1 and-2 during human ovarian follicle development. Hum Reprod 2016;31:866–874. [DOI] [PubMed] [Google Scholar]
- Jiang W, Chang A, Satoh M, Furuichi Y, Tam P, Reddel R.. The distribution of stanniocalcin 1 protein in fetal mouse tissues suggests a role in bone and muscle development. J Endocrinol 2000;165:457–466. [DOI] [PubMed] [Google Scholar]
- Joshi AD. New insights into physiological and pathophysiological functions of stanniocalcin 2. Front Endocrinol (Lausanne) 2020;11:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhanson P, Rull K, Kikas T, Laivuori H, Vaas P, Kajantie E, Heinonen S, Laan M.. Stanniocalcin-1 hormone in nonpreeclamptic and preeclamptic pregnancy: clinical, life-style, and genetic modulators. J Clin Endocrinol Metab 2016;101:4799–4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadakia R, Arraztoa JA, Bondy C, Zhou J.. Granulosa cell proliferation is impaired in the Igf1 null ovary. Growth Horm IGF Res 2001;11:220–224. [DOI] [PubMed] [Google Scholar]
- Kanchev LN, Dobson H.. Plasma concentration of androstenedione during the bovine oestrous cycle. J Endocrinol 1976;71:351–354. [DOI] [PubMed] [Google Scholar]
- Katulski K, Czyzyk A, Podfigurna-Stopa A, Genazzani AR, Meczekalski B.. Pregnancy complications in polycystic ovary syndrome patients. Gynecol Endocrinol 2015;31:87–91. [DOI] [PubMed] [Google Scholar]
- Kazi AA, Koos RD.. Estrogen-induced activation of hypoxia-inducible factor-1α, vascular endothelial growth factor expression, and edema in the uterus are mediated by the phosphatidylinositol 3-kinase/Akt pathway. Endocrinology 2007;148:2363–2374. [DOI] [PubMed] [Google Scholar]
- Khatun M, Arffman RK, Lavogina D, Kangasniemi M, Laru J, Ahtikoski A, Lehtonen S, Paulson M, Hirschberg AL, Salumets A. et al. Women with polycystic ovary syndrome present with altered endometrial expression of stanniocalcin-1. Biol Reprod 2020;102:306–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi M, Nakano Y, Nambo Y, Haneda S, Matsui M, Miyake Y, Macleod JN, Nagaoka K, Imakawa K.. Production of calcium maintenance factor stanniocalcin-1 (STC1) by the equine endometrium during the early pregnant period. J Reprod Dev 2011;57:203–211. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Ko JH, Yun J-H, Kim J-A, Kim TE, Lee HJ, Kim SH, Park KH, Oh JY.. Stanniocalcin-1 protects retinal ganglion cells by inhibiting apoptosis and oxidative damage. PLoS One 2013;8:e63749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kløverpris S, Mikkelsen JH, Pedersen JH, Jepsen MR, Laursen LS, Petersen SV, Oxvig C.. Stanniocalcin-1 potently inhibits the proteolytic activity of the metalloproteinase pregnancy-associated plasma protein-A. J Biol Chem 2015;290:21915–21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwintkiewicz J, Giudice LC.. The interplay of insulin-like growth factors, gonadotropins, and endocrine disruptors in ovarian follicular development and function. Semin Reprod Med 2009;27:43–51. [DOI] [PubMed] [Google Scholar]
- Lafeber FP, Hanssen RG, Choy YM, Flik G, Herrmann-Erlee MP, Pang PK, Bonga SE.. Identification of hypocalcin (teleocalcin) isolated from trout Stannius corpuscles. Gen Comp Endocrinol 1988;69:19–30. [DOI] [PubMed] [Google Scholar]
- Law AYS, Wong CKC.. Stanniocalcin-1 and -2 promote angiogenic sprouting in HUVECs via VEGF/VEGFR2 and angiopoietin signaling pathways. Mol Cell Endocrinol 2013;374:73–81. [DOI] [PubMed] [Google Scholar]
- Law AYS, Yeung BHY, Ching LY, Wong CKC.. Sp1 is a transcription repressor to stanniocalcin-1 expression in TSA-treated human colon cancer cells, HT29. J Cell Biochem 2011;112:2089–2096. [DOI] [PubMed] [Google Scholar]
- Liu D, Huang L, Wang Y, Wang W, Wehrens XHT, Belousova T, Abdelrahim M, DiMattia G, Sheikh-Hamad D.. Human stanniocalcin-1 suppresses angiotensin II-induced superoxide generation in cardiomyocytes through UCP3-mediated anti-oxidant pathway. PLoS One 2012;7:e36994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Jia H, Holmes DIR, Stannard A, Zachary I.. Vascular endothelial growth factor-regulated gene expression in endothelial cells. Arterioscler Thromb Vasc Biol 2003;23:2002–2007. [DOI] [PubMed] [Google Scholar]
- Liu G, Yang G, Chang B, Mercado-Uribe I, Huang M, Zheng J, Bast RC, Lin S-H, Liu J.. Stanniocalcin 1 and ovarian tumorigenesis. J Natl Cancer Inst 2010;102:812–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C-W, Kawamura K, Klein C, Hsueh AJW.. Paracrine regulation of ovarian granulosa cell differentiation by stanniocalcin (STC) 1: mediation through specific STC1 receptors. Mol Endocrinol 2004;18:2085–2096. [DOI] [PubMed] [Google Scholar]
- Ma X, Gu L, Li H, Gao Y, Li X, Shen D, Gong H, Li S, Niu S, Zhang Y. et al. Hypoxia-induced overexpression of stanniocalcin-1 is associated with the metastasis of early stage clear cell renal cell carcinoma. J Transl Med 2015;13:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto L, Hirota Y, Saito-Fujita T, Takeda N, Tanaka T, Hiraoka T, Akaeda S, Fujita H, Shimizu-Hirota R, Igaue S. et al. HIF2a in the uterine stroma permits embryo invasion and luminal epithelium detachment. J Clin Invest 2018;128:3186–3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCudden CR, James KA, Hasilo C, Wagner GF.. Characterization of mammalian stanniocalcin receptors. Mitochondrial targeting of ligand and receptor for regulation of cellular metabolism. J Biol Chem 2002;277:45249–45258. [DOI] [PubMed] [Google Scholar]
- McDonnell R, Hart RJ.. Pregnancy-related outcomes for women with polycystic ovary syndrome. Womens Health (Lond) 2017;13:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehedintu C, Plotogea MN, Ionescu S, Antonovici M.. Endometriosis still a challenge. J Med Life 2014;7:349–357. [PMC free article] [PubMed] [Google Scholar]
- Melamed P, Zhu Y, Siew HT, Xie M, Koh M.. Gonadotropin-releasing hormone activation of C-jun, but not early growth response factor-1, stimulates transcription of a luteinizing hormone β-subunit gene. Endocrinology 2006;147:3598–3605. [DOI] [PubMed] [Google Scholar]
- Mohammadipoor A, Lee RH, Prockop DJ, Bartosh TJ.. Stanniocalcin-1 attenuates ischemic cardiac injury and response of differentiating monocytes/macrophages to inflammatory stimuli. Transl Res 2016;177:127–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore EE, Kuestner RE, Conklin DC, Whitmore TE, Downey W, Buddle MM, Adams RL, Bell LA, Thompson DL, Wolf A. et al. Stanniocalcin 2: characterization of the protein and its localization to human pancreatic alpha cells. Horm Metab Res 1999;31:406–414. [DOI] [PubMed] [Google Scholar]
- Moreau JM, Iqbal W, Turner JK, Wagner GF, Ciriello J.. Stanniocalcin-1 in the subfornical organ inhibits the dipsogenic response to angiotensin II. Am J Physiol Regul Integr Comp Physiol 2012;303:R921–R928. [DOI] [PubMed] [Google Scholar]
- Mostl E, Mostl K, Choi HS, Dreier HK, Stöckl W, Bamberg E.. Plasma levels of androstenedione, epitestosterone, testosterone and oestrogens in cows at parturition. J Endocrinol 1981;89:251–255. [DOI] [PubMed] [Google Scholar]
- Muñoz M, Martin D, Carrocera S, Alonso-Guervos M, Mora MI, Corrales FJ, Peynot N, Giraud-Delville C, Duranthon V, Sandra O. et al. Localisation of stem cell factor, stanniocalcin-1, connective tissue growth factor and heparin-binding epidermal growth factor in the bovine uterus at the time of blastocyst formation. Reprod Fertil Dev 2017;29:2127–2139. [DOI] [PubMed] [Google Scholar]
- Murphy MP. How mitochondria produce reactive oxygen species. Biochem J 2009;417:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda K, Uenoyama Y, Berisha B, Lange IG, Taniguchi H, Kobayashi SS, Kobayashi SS, Miyamoto A, Schams D.. Estradiol-17β is produced in bovine corpus luteum. Biol Reprod 2001;65:1634–1639. [DOI] [PubMed] [Google Scholar]
- Olsen HS, Cepeda MA, Zhang QQ, Rosen CA, Vozzolo BL, Wagner GF.. Human stanniocalcin: a possible hormonal regulator of mineral metabolism. Proc Natl Acad Sci USA 1996;93:1792–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oonk RB, Parker KL, Gibson JL, Richards JS.. Rat cholesterol side-chain cleavage cytochrome P-450 (P-450scc) gene structure and regulation by CAMP in vitro. J Biol Chem 1990;265:22392–22401. [PubMed] [Google Scholar]
- Osol G, Mandala M.. Maternal uterine vascular remodeling during pregnancy. Physiology (Bethesda) 2009;24:58–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxvig C. The role of PAPP-A in the IGF system: location, location, location. J Cell Commun Signal 2015;9:177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paciga M, DiMattia GE, Wagner GF.. Regulation of luteal cell big stanniocalcin production and secretion. Endocrinology 2004;145:4204–4212. [DOI] [PubMed] [Google Scholar]
- Paciga M, Hirvi ER, James K, Wagner GF.. Characterization of big stanniocalcin variants in mammalian adipocytes and adrenocortical cells. Am J Physiol Endocrinol Metab 2005a;289:E197–205. [DOI] [PubMed] [Google Scholar]
- Paciga M, James K, Gillespie JRJ, Wagner GF.. Evidence for cross-talk between stanniocalcins. Can J Physiol Pharmacol 2005b;83:953–956. [DOI] [PubMed] [Google Scholar]
- Paciga M, McCudden CR, Londos C, DiMattia GE, Wagner GF.. Targeting of big stanniocalcin and its receptor to lipid storage droplets of ovarian steroidogenic cells. J Biol Chem 2003;278:49549–49554. [DOI] [PubMed] [Google Scholar]
- Paciga M, Watson AJ, DiMattia GE, Wagner GF.. Ovarian stanniocalcin is structurally unique in mammals and its production and release are regulated through the luteinizing hormone receptor. Endocrinology 2002;143:3925–3934. [DOI] [PubMed] [Google Scholar]
- Palomba S, De Wilde MA, Falbo A, Koster MPH, Battista G, Sala L, Fauser BCJM.. Pregnancy complications in women with polycystic ovary syndrome. Hum Reprod Update 2015;21:575–592. [DOI] [PubMed] [Google Scholar]
- Paria BC, Reese J, Das SK, Dey SK.. Deciphering the cross-talk of implantation: advances and challenges. Science 2002;296:2185–2188. [DOI] [PubMed] [Google Scholar]
- Pijnenborg R, Bland JM, Robertson WB, Brosens I.. Uteroplacental arterial changes related to interstitial trophoblast migration in early human pregnancy. Placenta 1983;4:397–413. [DOI] [PubMed] [Google Scholar]
- Piltonen TT, Chen J, Erikson DW, Spitzer TLB, Barragan F, Rabban JT, Huddleston H, Irwin JC, Giudice LC.. Mesenchymal stem/progenitors and other endometrial cell types from women with polycystic ovary syndrome (PCOS) display inflammatory and oncogenic potential. J Clin Endocrinol Metab 2013;98:3765–3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi S, Rizwani W, Joshi B, Kunigal S, Chellappan SP.. TNF-α response of vascular endothelial and vascular smooth muscle cells involve differential utilization of ASK1 kinase and p73. Cell Death Differ 2012;19:274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman CW, Sargent I.. The pathogenesis of pre-eclampsia. Gynécol Obs Fertil 2001;29:518–522. [DOI] [PubMed] [Google Scholar]
- Roddy GW, Rosa RH, Youn Oh J, Ylostalo JH, Bartosh TJ, Choi H, Lee RH, Yasumura D, Ahern K, Nielsen G. et al. Stanniocalcin-1 rescued photoreceptor degeneration in two rat models of inherited retinal degeneration. Mol Ther 2012;20:788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodesch F, Simon P, Donner C, Jauniaux E.. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet Gynecol 1992;80:283–285. [PubMed] [Google Scholar]
- Sato Y, Fujiwara H, Konishi I.. Mechanism of maternal vascular remodeling during human pregnancy. Reprod Med Biol 2012;11:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada M, Carlson JC.. Intracellular regulation of progesterone secretion by the superoxide radical in the rat corpus luteum. Endocrinology 1996;137:1580–1584. [DOI] [PubMed] [Google Scholar]
- Shapiro JP, Basar M, Kayisli UA, Guzeloglu-Kayisli O, Joseph Huang S, Suarez AA, Ozer HG, Schatz F, Lockwood CJ.. Mass spectrometry identification of potential mediators of progestin-only contraceptive-induced abnormal uterine bleeding in human endometrial stromal cells. Contraception 2015;91:253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh-Hamad D, Bick R, Wu GY, Christensen BM, Razeghi P, Poindexter B, Taegtmeyer H, Wamsley A, Padda R, Entman M. et al. Stanniocalcin-1 is a naturally occurring L-channel inhibitor in cardiomyocytes: relevance to human heart failure. Am J Physiol Heart Circ Physiol 2003;285:H442–8. [DOI] [PubMed] [Google Scholar]
- Sheikh-Hamad D. Mammalian stanniocalcin-1 activates mitochondrial antioxidant pathways: new paradigms for regulation of macrophages and endothelium. Am J Physiol Renal Physiol 2010;298:F248–F254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MS, Freeman ME, Neill JD.. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: Prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology 1975;96:219–226. [DOI] [PubMed] [Google Scholar]
- Song G, Bazer FW, Wagner GF, Spencer TE.. Stanniocalcin (STC) in the endometrial glands of the ovine uterus: regulation by progesterone and placental hormones. Biol Reprod 2006;74:913–922. [DOI] [PubMed] [Google Scholar]
- Song G, Dunlap KA, Kim J, Bailey DW, Spencer TE, Burghardt RC, Wagner GF, Johnson GA, Bazer FW.. Stanniocalcin 1 is a luminal epithelial marker for implantation in pigs regulated by progesterone and estradiol. Endocrinology 2009;150:936–945. [DOI] [PubMed] [Google Scholar]
- Spencer TE, Johnson GA, Bazer FW, Burghardt RC.. Implantation mechanisms: insights from the sheep. Reproduction 2004;128:657–668. [DOI] [PubMed] [Google Scholar]
- Spicer LJ, Aad PY.. Insulin-like growth factor (IGF) 2 stimulates steroidogenesis and mitosis of bovine granulosa cells through the IGF1 receptor: role of follicle-stimulating hormone and IGF2 receptor. Biol Reprod 2007;77:18–27. [DOI] [PubMed] [Google Scholar]
- Stannius H. Uber nebenniere bei knochenfischen. Arch Anat Physiol 1839;6:97–101. [Google Scholar]
- Stasko S, Wagner G.. Possible roles for stanniocalcin during early skeletal patterning and joint formation in the mouse. J Endocrinol 2001;171:237–248. [DOI] [PubMed] [Google Scholar]
- Stasko SE, DiMattia GE, Wagner GF.. Dynamic changes in stanniocalcin gene expression in the mouse uterus during early implantation. Mol Cell Endocrinol 2001;174:145–149. [DOI] [PubMed] [Google Scholar]
- Suhorutshenko M, Kukushkina V, Velthut-Meikas A, Altmäe S, Peters M, Mägi R, Krjutškov K, Koel M, Codoñer FM, Fco Martinez-Blanch J. et al. Endometrial receptivity revisited: endometrial transcriptome adjusted for tissue cellular heterogeneity. Hum Reprod 2018;33:2074–2086. [DOI] [PubMed] [Google Scholar]
- Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, Le Shay N, Nezhat CN, Kempson R, Lessey BA. et al. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology 2006;147:1097–1121. [DOI] [PubMed] [Google Scholar]
- Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, Piltonen T, Norman RJ; International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod 2018;33:1602–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trindade DM, Silva JC, Navarro MS, Torriani ICL, Kobarg J.. Low-resolution structural studies of human Stanniocalcin-1. BMC Struct Biol 2009;9:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uusküla L, Männik J, Rull K, Minajeva A, Kõks S, Vaas P, Teesalu P, Reimand J, Laan M.. Mid-gestational gene expression profile in placenta and link to pregnancy complications. PLoS One 2012;7:e49248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Broeck W, Coryn M, Simoens P, Lauwers H.. Cell-specific distribution of oestrogen receptor-alpha in the bovine ovary. Reprod Domest Anim 2002a;37:291–293. [DOI] [PubMed] [Google Scholar]
- Van den Broeck W, D'haeseleer M, Coryn M, Simoens P.. Cell-specific distribution of progesterone receptors in the bovine ovary. Reprod Domest Anim 2002b;37:164–170. [DOI] [PubMed] [Google Scholar]
- Varghese R, Gagliardi AD, Bialek PE, Yee S-P, Wagner GF, Dimattia GE.. Overexpression of human stanniocalcin affects growth and reproduction in transgenic mice. Endocrinology 2002;143:868–876. [DOI] [PubMed] [Google Scholar]
- Varghese R, Wong CKC, Deol H, Wagner GF, DiMattia GE.. Comparative analysis of mammalian stanniocalcin genes. Endocrinology 1998;139:4714–4725. [DOI] [PubMed] [Google Scholar]
- Wagner GF, Dimattia GE, Davie JR, Copp DH, Friesen HG.. Molecular cloning and cDNA sequence analysis of coho salmon stanniocalcin. Mol Cell Endocrinol 1992;90:7–15. [DOI] [PubMed] [Google Scholar]
- Wagner GF, Hampong M, Park CM, Copp DH.. Purification, characterization, and bioassay of teleocalcin, a glycoprotein from salmon corpuscles of Stannius. Gen Comp Endocrinol 1986;63:481–491. [DOI] [PubMed] [Google Scholar]
- Wallace AE, Cartwright JE, Begum R, Laing K, Thilaganathan B, Whitley GS.. Trophoblast-induced changes in C-x-C motif chemokine 10 expression contribute to vascular smooth muscle cell dedifferentiation during spiral artery remodeling. Arterioscler Thromb Vasc Biol 2013;33:e93–e 101. [DOI] [PubMed] [Google Scholar]
- Wary KK, Thakker GD, Humtsoe JO, Yang J.. Analysis of VEGF-responsive genes involved in the activation of endothelial cells. Mol Cancer 2003;2:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weedon-Fekjær MS, Taskén K.. Review: Spatiotemporal dynamics of hCG/cAMP signaling and regulation of placental function. Placenta 2012;33:S87–S91. [DOI] [PubMed] [Google Scholar]
- Westberg JA, Serlachius M, Lankila P, Andersson LC.. Hypoxic preconditioning induces elevated expression of stanniocalcin-1 in the heart. Am J Physiol Heart Circ Physiol 2007a;293:H1766–71. [DOI] [PubMed] [Google Scholar]
- Westberg JA, Serlachius M, Lankila P, Penkowa M, Hidalgo J, Andersson LC.. Hypoxic preconditioning induces neuroprotective stanniocalcin-1 in brain via IL-6 signaling. Stroke 2007b;38:1025–1030. [DOI] [PubMed] [Google Scholar]
- Whitley GSJ, Cartwright JE.. Cellular and molecular regulation of spiral artery remodelling: lessons from the cardiovascular field. Placenta 2010;31:465–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley GSJ, Cartwright JE.. Trophoblast-mediated spiral artery remodelling: a role for apoptosis. J Anat 2009;215:21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wice B, Menton D, Geuze H, Schwartz AL.. Modulators of cyclic AMP metabolism induce syncytiotrophoblast formation in vitro. Exp Cell Res 1990;186:306–316. [DOI] [PubMed] [Google Scholar]
- Winn VD, Haimov-Kochman R, Paquet AC, Yang YJ, Madhusudhan MS, Gormley M, Feng K-TV, Bernlohr DA, McDonagh S, Pereira L. et al. Gene expression profiling of the human maternal-fetal interface reveals dramatic changes between midgestation and term. Endocrinology 2007;148:1059–1079. [DOI] [PubMed] [Google Scholar]
- Worthington RA, Brown L, Jellinek D, Chang AC-M, Reddel RR, Hambly BD, Barden JA.. Expression and localisation of stanniocalcin 1 in rat bladder, kidney and ovary. Electrophoresis 1999;20:2071–2076. [DOI] [PubMed] [Google Scholar]
- Wu LM, Guo R, Hui L, Ye YG, Xiang JM, Wan CY, Zou M, Ma R, Sun XZ, Yang SJ. et al. Stanniocalcin-1 protects bovine intestinal epithelial cells from oxidative stress-induced damage. J Vet Sci 2014;15:475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao LJ, Yuan JX, Song XX, Li YC, Hu ZY, Liu YX.. Expression and regulation of stanniocalcin 1 and 2 in rat uterus during embryo implantation and decidualization. Reproduction 2006;131:1137–1149. [DOI] [PubMed] [Google Scholar]
- Yeung HY, Lai KP, Chan HY, Mak NK, Wagner GF, Wong CKC.. Hypoxia-inducible factor-1-mediated activation of stanniocalcin-1 in human cancer cells. Endocrinology 2005;146:4951–4960. [DOI] [PubMed] [Google Scholar]
- Yoshiko Y, Aubin JE.. Stanniocalcin 1 as a pleiotropic factor in mammals. Peptides 2004;25:1663–1669. [DOI] [PubMed] [Google Scholar]
- Zhang J, Alfonso P, Thotakura NR, Su J, Buergin M, Parmelee D, Collins AW, Oelkuct M, Gaffney S, Gentz S. et al. Expression, purification, and bioassay of human stanniocalcin from baculovirus-infected insect cells and recombinant CHO cells. Protein Expr Purif 1998;12:390–398. [DOI] [PubMed] [Google Scholar]
- Zhang K-Z, Lindsberg PJ, Tatlisumak T, Kaste M, Olsen HS, Andersson LC.. Stanniocalcin: a molecular guard of neurons during cerebral ischemia. Proc Natl Acad Sci USA 2000;97:3637–3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Damsky CH, Chiu K, Roberts JM, Fisher SJ.. Preeclampsia is associated with abnormal expression of adhesion molecules by invasive cytotrophoblasts. J Clin Invest 1993;91:950–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlot C, Ingle G, Hongo J, Yang S, Sheng Z, Schwall R, Paoni N, Wang F, Peale FV, Gerritsen ME.. Stanniocalcin 1 is an autocrine modulator of endothelial angiogenic responses to hepatocyte growth factor. J Biol Chem 2003;278:47654–47659. [DOI] [PubMed] [Google Scholar]
- Zlotkin T, Farkash Y, Orly J.. Cell-specific expression of immunoreactive cholesterol side-chain cleavage cytochrome p-450 during follicular development in the rat ovary. Endocrinology 1986;119:2809–2820. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.