Systemic amyloidosis is a rare disease secondary to the extracellular deposition of insoluble fibrils in tissues, potentially leading to multiorgan dysfunction. Amyloid cardiomyopathy (AC) is associated with a poor prognosis and is caused mainly by 2 precursor proteins, light chain (AL) and transthyretin (ATTR) (1, 2, 3).
AC is underdiagnosed and associated with increased morbidity and a high risk of mortality. For decades, it was considered an untreatable cause of heart failure, but recent improvements in diagnosis and treatment have changed the course of the disease, especially if recognized early and promptly treated (3,4). In middle- and low-income countries, data on AC are scarce, and physicians are increasingly aware of the disease with the growing number of publications mainly from American, European, and Asian centers. In Brazil, amyloidosis referral centers have emerged in the recent years, with an aim to improve diagnosis and treatment of patients. By reporting the experience of a public university health service in Brazil, the Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo and its subunits (the Cancer and Heart Institutes), that are focused on the care of patients with AC from their diagnostic work-up to eventual outcomes, we highlight the real-life challenges faced in a middle-income country and emphasize the importance of establishing not only a referral center in amyloidosis, but also a patient registry.
After approval by our Institutional Research Ethics Committee, the medical records of patients with AL and ATTR AC were retrospectively reviewed. AC was defined either by histologic evidence of amyloid deposit in cardiac biopsy or by at least 1 of the following imaging and/or biomarker criteria, in patients with a previous diagnosis of systemic amyloidosis and no other identifiable cause of the cardiomyopathy: interventricular septum thickness ≥12 mm by echocardiogram or cardiac magnetic resonance (CMR), longitudinal strain >−18%, late gadolinium enhancement on CMR, and alterations in natriuretic peptides (B-type natriuretic peptide, N-terminal pro–B-type natriuretic peptide) or troponin (5). All patients with an AL amyloidosis required a biopsy-proven diagnosis (cardiac or noncardiac specimen), and the diagnosis in patients with the ATTR subtype was established either by histologic confirmation or by a noninvasive algorithm (6). ATTR AC was additionally classified as wild-type (ATTRwt) or variant (ATTRv). The time from symptom onset to diagnosis and the number and types of specialists consulted were also recorded. Cardiac staging was assessed in AL patients using the Mayo Clinic standard and European staging systems and in ATTR by the National Amyloidosis Center staging (7, 8, 9). The data were compared between AL and ATTR subtypes, and results were reported in frequencies and median, with 25th and 75th percentiles (Q1-Q3). Continuous and categorical variables were compared by the Mann-Whitney and chi-square test with the Yate continuity correction or the Fisher exact test, respectively. Overall survival was evaluated by the Kaplan-Meier method. Survival data were censored on the date of last visit or last contact with the patient, and survival curves were compared using the log-rank test. Statistical tests were 2-sided with P values <0.05 denoting statistical significance. The analyses were performed using R software studio version 1.3.959 (R Foundation for Statistical Computing).
Between 2006 and 2021, 176 patients were identified with systemic amyloidosis, and 125 were included in the analyses (66%; n = 82 AL; and 34%; n = 43 ATTR). Excluded patients had other amyloid subtypes (12, serum amyloid A; 6, fibrinogen; 10, inconclusive) or no cardiac involvement (13, AL; and 10, ATTR). The ATTR subgroup comprised 28 (65%) patients with ATTRv, 6 (14%)patients with ATTRwt, and 9 (21%) patients who did not have available mutational status. Among ATTRv patients, Val142Ile (46%) and Val50Met (39%) were the predominant mutations, but other mutations were also present, such as Glu109Lys, Thr80Ala, Phe84Leu, and Ala39Asp (3.5% each).
There was a predominance of male patients in the ATTR subgroup (81.4% vs 52.4%; P = 0.001), and ATTR patients were older than the AL group (median age 70 years [Q1-Q3: 65-78 years] vs 61 years [Q1-Q3: 55-68 years], respectively; P < 0.001). From the onset of symptoms, a median delay in diagnosis of 12.9 months (Q1-Q3: 6.9-33.2 months) was observed for the entire cohort. In patients with AL subtype, the diagnosis was established earlier than in patients with ATTR (median 9.2 months [Q1-Q3: 5.9-17.1 months] vs 34.5 months [Q1-Q3: 24.7-72.4 months], respectively; P < 0.001), although they had more specialist consultations than did patients with ATTR (61.7% consulted ≥3 medical specialties vs 15.8%; P < 0.001). The main initial clinical presentations differed between AL and ATTR subgroups (renal disorders, 63.4% vs 11.6%; P < 0.010; cardiac disorders, 45.1% vs 72.1%; P = 0.004; neurologic conditions, 20.7% vs 39.5%; P = 0.025; cachexia, 42.7% vs 20.9%; P = 0.015). Similarly, the types of specialists consulted also differed. AL patients were mainly seen by nephrologists (50%) and cardiologists (40%), whereas patients with ATTR were mainly seen by cardiologists (74%) and neurologists (26%).
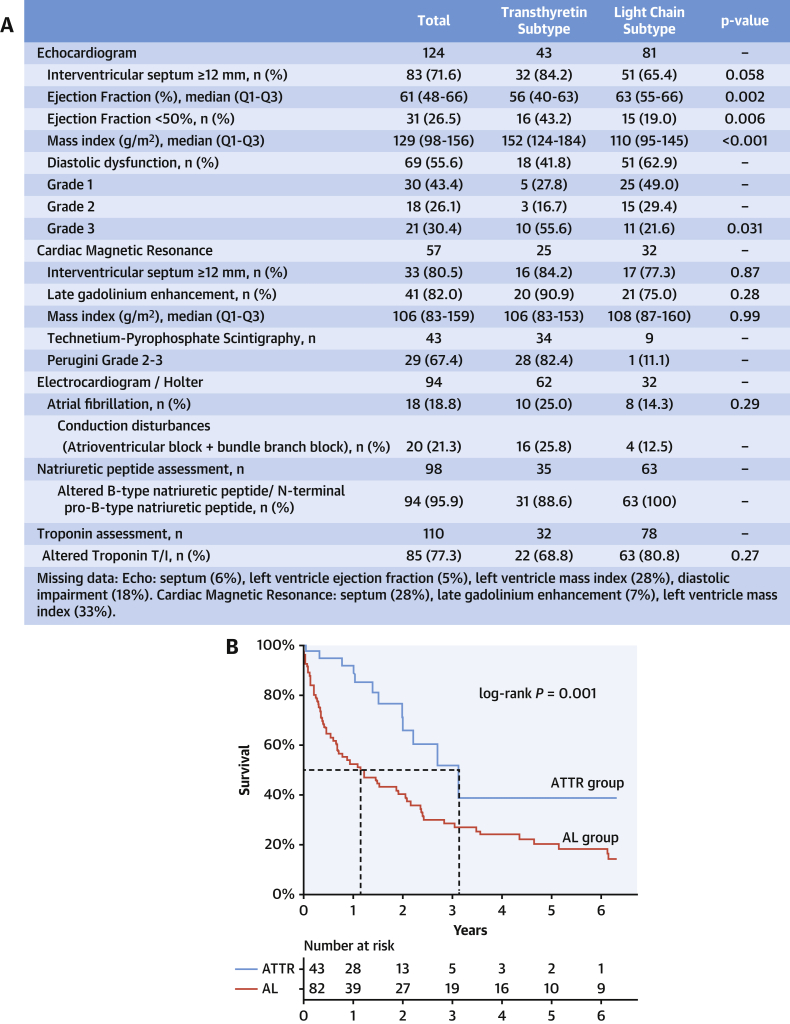
The kidney (48%) and abdominal fat pad (37%) were the most frequently biopsied sites in AL patients, and cardiac biopsy was performed in only 11% of cases. In the ATTR subgroup, 56% of patients had a diagnosis established by the noninvasive algorithm, and among those with histologic confirmation, 74% underwent fat pad biopsy and 42% required an endomyocardial biopsy (88% because of a previous negative fat pad biopsy result). A 60% positivity rate of fat pad biopsy was found in AL patients compared with 14% in ATTR. Endomyocardial biopsy results were 100% positive for amyloid deposition. Imaging and cardiac biomarkers are summarized in Figure 1A.
Figure 1.
Imaging, Biomarkers, and Survival in Amyloid Cardiomyopathy
(A) Imaging and biomarker parameters by amyloidosis subtypes. (B) Overall survival in AL (light chain) and ATTR (transthyretin amyloid) cardiomyopathy.
Cardiac staging was available in 52 (63%) patients with the AL subtype, and most of them had advanced stages: 67% had stage III by the Mayo Clinic system, and 63% of them were classified as IIIb or IIIC by the European model. Among the ATTR subgroup, only 11 (26%) patients had data for staging evaluation, and 64% of them had stages I to II.
The treatments performed in the AL subgroup consisted of chemotherapy in 70 (85%) patients; 8 (11%) of these patients underwent autologous stem cell transplantation, and 1 underwent kidney transplantation. Melphalan and cyclophosphamide-based combinations accounted for 50% of the chemotherapy regimens, and bortezomib was available in only 13% of the cases. The remaining patients (15%) exclusively received supportive measures. Patients with ATTR AC were mainly treated with supportive measures (51%). Tafamidis was prescribed to 7 (16%) and doxycycline to 5 (12%) patients. Among patients with ATTRv, liver transplantation was performed in 4 (14%), all carrying the Val50Met mutation, and 6 (21%) patients were included in a randomized clinical trial of patisiran vs placebo (A Study to Evaluate Patisiran in Participants With Transthyretin Amyloidosis With Cardiomyopathy [ATTR Amyloidosis With Cardiomyopathy] [APOLLO B]; NCT03997383).
With a median follow-up time of 4.2 years (Q1-Q3: 1.5-6.9 years), reduced median overall survival was observed in AL patients compared with the ATTR group: 1.1 years (95% confidence interval: 0.6-2.1) vs 3.1 years (95% confidence interval: 1.9 to not reached), respectively (P = 0.003), as shown in Figure 1B.
Our results provide evidence of the challenges in diagnosing and treating AC in a public health university center in a middle-income country. The median delay in diagnosis of more than 1 year may be explained by the low degree of amyloidosis suspicion by primary care physicians, the need for multiple specialists’ evaluation, and the complexity of the diagnostic work-up requiring specific tests. The lack of access and reimbursement of some diagnostic tools, such as free light chains and genetic testing, may also have contributed to our findings. Recently, both tests were incorporated into our routine diagnostic work-up.
The finding that AL patients had an earlier diagnosis, besides consulting with more specialists than patients with ATTR, may reflect the multiorgan character of AL amyloidosis, with cardiac involvement being part of a multisystemic disease with more rapid organ deterioration compared with ATTR (10). ATTR tends to be more insidious; it usually takes years for the deposited amyloid to manifest clinically, consistent with the delay of almost 3 years observed in the ATTR subgroup (2). Nonetheless, most of our AL patients received their diagnosis in advanced stages of disease. This finding reflects the low degree of clinical suspicion among physicians, the need for multiple consultations with specialists, the difficulties of access to a referral center in the Brazilian public health system, and the delay in diagnosis. The small proportion of ATTR patients who were evaluated by risk models limited this analysis in this subgroup.
At diagnosis, AL patients had a median age similar to that in published reports and were almost 10 years younger than the patients with ATTR. The composition of the ATTR subgroup may explain this finding because ATTRv caused by a Val142Ile mutation and ATTRwt accounted for 68% of the cases, both associated with older age (2). Regarding the sex distribution, our results were concordant with other published data, with a predominance of male patients in ATTR subtype and no sex predilection seen in AL (2,10).
The morphologic and electrical features observed in ATTR patients compared with patients with AL, such as greater septal thickening, higher mass index, and conduction disease, characterizing a more infiltrative pattern, denote advanced cardiac involvement. These findings probably reflect the more insidious progression of ATTR AC, with prolonged deposition of amyloid fibrils in the cardiac tissue and conduction system, resulting in symptoms later during the course of the disease. Moreover, the direct toxicity of light chains on cardiomyocytes may lead to changes in cardiac biomarkers before the occurrence of symptoms and structural alterations in AL (10).
Although recent advances in the diagnosis and treatment of AC have improved patients’ survival and quality of life, the AL subtype is still associated with reduced survival compared with ATTR, and this was also observed in our cohort (1,2,10). Late diagnosis in advanced stages of disease, the unavailability of some treatment options such as proteasome inhibitors and monoclonal antibodies in the Brazilian public health system, and the small number of patients eligible for stem cell transplantation may have further limited management of AL AC in our center. Moreover, specific treatments for the ATTR subtype are also lacking because tafamidis is approved in the public system only for amyloid neuropathy at a dose of 20 mg once daily, and diflunisal is not available in Brazil.
In conclusion, AC remains a challenging and underrecognized disease. The nonspecific symptoms and overlapping signs with other conditions result in a low degree of suspicion, and patients undergo a complex diagnostic journey, with late diagnosis in advanced stages of cardiac involvement. Because AC is a treatable condition, the establishment of referral centers and a registry of patients with amyloidosis in middle-income countries is essential to raise awareness of the disease and improve access to specific diagnostic tools and therapies, with the fundamental aim of optimizing outcomes for patients in the future.
Funding Support and Author Disclosures
Dr Szor has received lecture honoraria from Alnylam, Pfizer, and Janssen-Cilag Pharmaceuticals; and has reported membership on the advisory board of Janssen-Cilag Pharmaceuticals. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Gertz M.A., Dispenzieri A. Systemic amyloidosis recognition, prognosis, and therapy: a systematic review. JAMA. 2020;324(1):79–89. doi: 10.1001/jama.2020.5493. [DOI] [PubMed] [Google Scholar]
- 2.Muchtar E., Dispenzieri A., Magen H. Systemic amyloidosis from A (AA) to T (ATTR): a review. J Intern Med. 2021;289(3):268–292. doi: 10.1111/joim.13169. [DOI] [PubMed] [Google Scholar]
- 3.Ruberg F.L., Grogan M., Hanna M., Kelly J.W., Maurer M.S. Transthyretin amyloid cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(22):2872–2891. doi: 10.1016/j.jacc.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palladini G., Milani P., Merlini G. Management of AL amyloidosis in 2020. Blood. 2020;136(23):2620–2627. doi: 10.1182/blood.2020006913. [DOI] [PubMed] [Google Scholar]
- 5.Castiglione V., Franzini M., Aimo A. Use of biomarkers to diagnose and manage cardiac amyloidosis. Eur J Heart Fail. 2021;23(2):217–230. doi: 10.1002/ejhf.2113. [DOI] [PubMed] [Google Scholar]
- 6.Gillmore J.D., Maurer M.S., Falk R.H. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation. 2016;133(24):2404–2412. doi: 10.1161/CIRCULATIONAHA.116.021612. [DOI] [PubMed] [Google Scholar]
- 7.Dispenzieri A., Gertz M.A., Kyle R.A. Serum cardiac troponins and N-terminal pro-brain natriuretic peptide: a staging system for primary systemic amyloidosis. J Clin Oncol. 2004;22(18) doi: 10.1200/JCO.2004.03.029. 3751-2757. [DOI] [PubMed] [Google Scholar]
- 8.Wechalekar A.D., Schonland S.O., Kastritis E. A European collaborative study of treatment outcomes in 346 patients with cardiac stage III AL amyloidosis. Blood. 2013;121(17):3420–3427. doi: 10.1182/blood-2012-12-473066. [DOI] [PubMed] [Google Scholar]
- 9.Gillmore J.D., Damy T., Fontana M. A new staging system for cardiac transthyretin amyloidosis. Eur Heart J. 2018;39(30):2799–2806. doi: 10.1093/eurheartj/ehx589. [DOI] [PubMed] [Google Scholar]
- 10.Falk R.H., Alexander K.M., Liao R., Dorbala S. AL (light-chain) cardiac amyloidosis: a review of diagnosis and therapy. J Am Coll Cardiol. 2016;68(12):1323–1341. doi: 10.1016/j.jacc.2016.06.053. [DOI] [PubMed] [Google Scholar]