Corresponding Author

Key Words: amyloidosis, cardiomyopathy, diagnosis, nuclear imaging
Transthyretin (ATTR) amyloidosis is characterized by the progressive deposition of misfolded transthyretin protein, which polymerizes into amyloid fibrils in the extracellular matrix (1). These fibrils accumulate in the myocardium and lead to restrictive cardiomyopathy with a median untreated survival of approximately 3.5 years. Wild-type ATTR (ATTRwt) amyloidosis mostly affects older men and causes cardiomyopathy, whereas transthyretin gene variants may lead to hereditary ATTR (ATTRv), with variable combinations of cardiomyopathy and polyneuropathy. The cardiomyopathic variant Val122Ile is present in 3.4% of African Americans but has variable penetrance and typically causes symptoms later in life. ATTR amyloidosis has been traditionally considered a rare disease; in the absence of any effective treatment until recently, diagnosing ATTR amyloidosis had limited clinical consequences, and patients were not proactively screened for this disease. The transthyretin stabilizer tafamidis reduced mortality and rates of cardiovascular-related hospitalizations in ATTR cardiac amyloidosis (ATTR-CA) (2). In ATTRv amyloidosis, diflunisal, patisiran, and inotersen improved polyneuropathy symptoms, and CRISPR-Cas9 gene editing reduced transthyretin levels (1,3). Access to these highly effective therapies has motivated an interest in screening at-risk and symptomatic cohorts for ATTR amyloidosis.
In this issue of JACC: CardioOncology, Nativi-Nicolau et al (4) present the results of their study on temporal trends in the diagnosis of ATTR-CA from the THAOS (Transthyretin Amyloidosis Outcomes Survey). In this large, global database of 1,069 ATTRwt and 525 ATTRv amyloidosis patients (95% male), Nativi-Nicolau et al (4) found an impressive increase in annual ATTRwt patient inclusion, from 2 in 2005 to >100 in 2016. By contrast, annual ATTRv patient inclusion increased from 3 in 2005 to 37 in 2011 and then plateaued. These changes were not the result of the addition of new participating centers because the number of patients by center also increased. Thus, the findings of Nativi-Nicolau et al (4) highlight a steep increase in ATTR-CA diagnosis in the United States and worldwide, particularly for ATTRwt. Moreover, the proportion of patients in New York Heart Association functional class III or IV at the time of diagnosis diminished over time, suggesting earlier diagnosis. This rise in diagnosis was accompanied by a higher use of cardiac scintigraphy with bone-avid tracers and a lower use of endomyocardial biopsy. However, surprisingly, the median time from onset of symptoms to diagnosis remained more than 60 months in the last several years.
A potential limitation of this study may be a sampling bias reflecting patient recruitment from selected centers with expertise in ATTR-CA, mostly in the United States. However, these results mirror similar recent observations of a diagnostic surge in other cohorts across the world (5, 6, 7, 8). The newly increased awareness of ATTR-CA has spurred physicians to search for it actively, with a resulting wave of new diagnoses. Geographic discrepancies across the United States with more frequent cases in Minnesota and New England suggest a higher detection rate in specialized centers (6,9). This temporal and geographic evolution suggests that ATTR-CA was strongly underdiagnosed.
The proportion of women enrolled in the THAOS registry was only 5.4% and it was consistent with clinical experience, but whether this has changed temporally is not discussed in this study and may be valuable to understand. Numerous studies performing a systematic assessment of selected patients reported a high prevalence of ATTR-CA with much smaller sex differences. Among patients with heart failure with preserved ejection fraction (HFpEF), 6% to 17% had a diagnosis of ATTR-CA (10, 11, 12, 13, 14) including certain patients with severe aortic stenosis (15, 16, 17). A prevalence >20% was repeatedly found in patients older than 80 years of age, with or without HFpEF (13,14,18). Even retrospective studies of unselected patients with bone scintigraphy detected several unexpected cases of ATTR-CA (19,20). Because of underdiagnosis, the true prevalence of the disease is difficult to assess. However, it was estimated at between 70 and 191 per million when investigators used diagnostic codes in a large in-hospital Japanese database (21). Surprisingly, 45% to 54% of cases were women. Our group used Medicare fee for service data and reported an increase in prevalence (18.0 to 55.2 per 100,000 person years) of cardiac amyloidosis (International Classification of Diseases codes for heart failure and amyloidosis) from 2000 to 2012 (6); women comprised 50% of the cohort. Several other studies also showed much smaller sex differences. For example, Gonzalez-Lopez et al (11) performed technetium-99m–3,3-diphosphono-1,2-propanodicarboxylic acid (99mTc-DPD) scans in adults aged ≥60 years (59% women) who were hospitalized with HFpEF and left ventricular wall thickening (≥12 mm) and found that the proportion of women with positive 99mTc-DPD scan results was similar to the proportion of men. In an autopsy study, Mohammed et al (10) found that 15% (9/62) of women and 19% (9/47) of men with HFpEF had ATTRwt. These data suggest that the male predominance may not be as strong as reported in THAOS when systematic screening is performed. Thus, ATTR-CA should also be sought in older women with clinical or imaging red flags for amyloidosis.
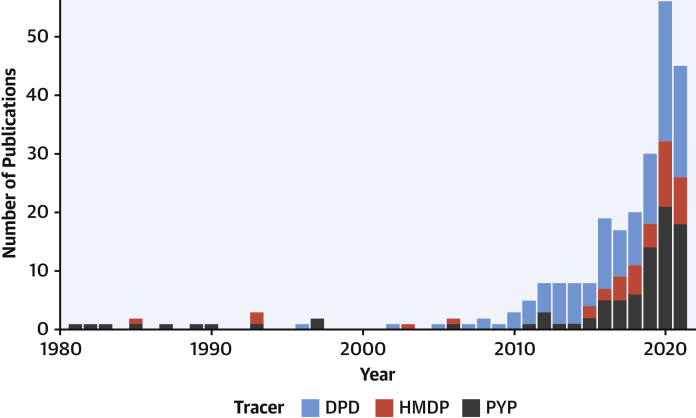
We congratulate Nativi-Nicolau et al (4) on adding these important data to the published reports on this topic, thereby showing that a major contributor to this diagnostic surge for ATTR-CA is the development of diagnostic imaging tests, specifically scintigraphy with bone-avid tracers (22). Traditionally, endomyocardial biopsy was required for every patient. Now, scintigraphy with bone-avid tracers permits a noninvasive, early, and accurate diagnosis of ATTR-CA; this imaging can be performed at a lower threshold of suspicion than biopsy, thus allowing for widespread early detection of ATTR-CA. The increased interest in bone scintigraphy in ATTR-CA is paralleled by the exponential progression of publications on PubMed in recent years (Figure 1). Quantitative imaging of amyloid burden with bone-avid tracer single-photon emission computed tomography combined with computed tomography (23) is emerging and may be crucial to evaluate prognosis, follow-up, and response to therapy.
Figure 1.
Annual Publications on Bone-Avid Tracer Scintigraphy for Amyloidosis
Data were extracted from PubMed on September 2, 2021. DPD = technetium-99m–3,3-diphosphono-1,2-propanodicarboxylic acid; HMDP = technetium-99m–hydroxymethylene diphosphonate; PYP = technetium-99m–pyrophosphate.
In conclusion, significant progress has been made in using bone-avid tracer scintigraphy to diagnose ATTR-CA, a relevant cause of HFpEF in older adult patients. More studies are needed to develop strategies to improve time to diagnosis and to study sex differences in the prevalence of ATTR-CA. Because this form of HFpEF can be specifically treated, identifying ATTR-CA in men and women is now an essential endeavor, to save and improve the lives of our patients.
Funding Support and Author Disclosures
Dr Dorbala has reported support from the National Institutes of Health (grants HL 130563, HL 150342, and AHA19SRG34950011); has received consulting fees from Pfizer, GE Health Care and Ionetix; and has received an investigator-initiated grant from Pfizer, Attralus, and GE Healthcare. Dr Clerc is supported by a research fellowship grant from the International Society of Amyloidosis and Pfizer.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Ruberg F.L., Grogan M., Hanna M., Kelly J.W., Maurer M.S. Transthyretin amyloid cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73:2872–2891. doi: 10.1016/j.jacc.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maurer M.S., Schwartz J.H., Gundapaneni B. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379:1007–1016. doi: 10.1056/NEJMoa1805689. [DOI] [PubMed] [Google Scholar]
- 3.Gillmore J.D., Gane E., Taubel J. CRISPR-Cas9 in vivo gene editing for transthyretin amyloidosis. N Engl J Med. 2021;385:493–502. doi: 10.1056/NEJMoa2107454. [DOI] [PubMed] [Google Scholar]
- 4.Nativi-Nicolau J., Siu A., Dispenzieri A. Temporal trends of wild-type transthyretin amyloid cardiomyopathy in the Transthyretin Amyloidosis Outcomes Survey. J Am Coll Cardiol CardioOnc. 2021;3:537–546. doi: 10.1016/j.jaccao.2021.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lane T., Fontana M., Martinez-Naharro A. Natural history, quality of life, and outcome in cardiac transthyretin amyloidosis. Circulation. 2019;140:16–26. doi: 10.1161/CIRCULATIONAHA.118.038169. [DOI] [PubMed] [Google Scholar]
- 6.Gilstrap L.G., Dominici F., Wang Y. Epidemiology of cardiac amyloidosis-associated heart failure hospitalizations among fee-for-service medicare beneficiaries in the United States. Circ Heart Fail. 2019;12 doi: 10.1161/CIRCHEARTFAILURE.118.005407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zampieri M., Nardi G., Del Monaco G. Changes in the perceived epidemiology of amyloidosis: 20 year-experience from a tertiary referral centre in Tuscany. Int J Cardiol. 2021;335:123–127. doi: 10.1016/j.ijcard.2021.04.023. [DOI] [PubMed] [Google Scholar]
- 8.Poterucha T.J., Elias P., Bokhari S. Diagnosing transthyretin cardiac amyloidosis by technetium Tc 99m pyrophosphate: a test in evolution. J Am Coll Cardiol Img. 2021;14:1221–1231. doi: 10.1016/j.jcmg.2020.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexander K.M., Orav J., Singh A. Geographic disparities in reported US amyloidosis mortality from 1979 to 2015: potential underdetection of cardiac amyloidosis. JAMA Cardiol. 2018;3:865–870. doi: 10.1001/jamacardio.2018.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohammed S.F., Mirzoyev S.A., Edwards W.D. Left ventricular amyloid deposition in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol HF. 2014;2:113–122. doi: 10.1016/j.jchf.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez-Lopez E., Gallego-Delgado M., Guzzo-Merello G. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J. 2015;36:2585–2594. doi: 10.1093/eurheartj/ehv338. [DOI] [PubMed] [Google Scholar]
- 12.Hahn V.S., Yanek L.R., Vaishnav J. Endomyocardial biopsy characterization of heart failure with preserved ejection fraction and prevalence of cardiac amyloidosis. J Am Coll Cardiol HF. 2020;8:712–724. doi: 10.1016/j.jchf.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maurizi N., Rella V., Fumagalli C. Prevalence of cardiac amyloidosis among adult patients referred to tertiary centres with an initial diagnosis of hypertrophic cardiomyopathy. Int J Cardiol. 2020;300:191–195. doi: 10.1016/j.ijcard.2019.07.051. [DOI] [PubMed] [Google Scholar]
- 14.AbouEzzeddine O.F., Davies D.R., Scott C.G. Prevalence of transthyretin amyloid cardiomyopathy in heart failure with preserved ejection fraction. JAMA Cardiol. Published online August 25, 2021 doi: 10.1001/jamacardio.2021.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nietlispach F., Webb J.G., Ye J. Pathology of transcatheter valve therapy. J Am Coll Cardiol Intv. 2012;5:582–590. doi: 10.1016/j.jcin.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Castano A., Narotsky D.L., Hamid N. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur Heart J. 2017;38:2879–2887. doi: 10.1093/eurheartj/ehx350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nitsche C., Scully P.R., Patel K.P. Prevalence and outcomes of concomitant aortic stenosis and cardiac amyloidosis. J Am Coll Cardiol. 2021;77:128–139. doi: 10.1016/j.jacc.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cornwell G.G., 3rd, Murdoch W.L., Kyle R.A., Westermark P., Pitkanen P. Frequency and distribution of senile cardiovascular amyloid. A clinicopathologic correlation. Am J Med. 1983;75:618–623. doi: 10.1016/0002-9343(83)90443-6. [DOI] [PubMed] [Google Scholar]
- 19.Longhi S., Guidalotti P.L., Quarta C.C. Identification of TTR-related subclinical amyloidosis with 99mTc-DPD scintigraphy. J Am Coll Cardiol Img. 2014;7:531–532. doi: 10.1016/j.jcmg.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Cuscaden C., Ramsay S.C., Prasad S., Goodwin B., Smith J. Estimation of prevalence of transthyretin (ATTR) cardiac amyloidosis in an Australian subpopulation using bone scans with echocardiography and clinical correlation. J Nucl Cardiol. Published online May 8, 2020 doi: 10.1007/s12350-020-02152-x. [DOI] [PubMed] [Google Scholar]
- 21.Winburn I., Ishii T., Sumikawa T., Togo K., Yasunaga H. Estimating the prevalence of transthyretin amyloid cardiomyopathy in a large in-hospital database in Japan. Cardiol Ther. 2019;8:297–316. doi: 10.1007/s40119-019-0142-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dorbala S., Cuddy S., Falk R.H. How to image cardiac amyloidosis: a practical approach. J Am Coll Cardiol Img. 2020;13:1368–1383. doi: 10.1016/j.jcmg.2019.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dorbala S., Park M.A., Cuddy S. Absolute quantitation of cardiac (99m)Tc-pyrophosphate using cadmium zinc telluride-based SPECT/CT. J Nucl Med. 2021;62:716–722. doi: 10.2967/jnumed.120.247312. [DOI] [PMC free article] [PubMed] [Google Scholar]