Abstract
Immunoglobulin light chain (AL) amyloidosis is an incurable plasma cell disorder characterized by deposition of fibrils of misfolded immunoglobulin free light chains (FLC) in target organs, leading to failure. Cardiac involvement is common in AL amyloidosis and represents the single most adverse prognostic feature. A high index of clinical suspicion with rapid tissue diagnosis and commencement of combinatorial, highly effective cytoreductive therapy is crucial to arrest the process of amyloid deposition and preserve organ function. The clinical use of molecularly targeted drugs, such as proteasome inhibitors and immunomodulatory agents, monoclonal antibodies such as daratumumab, and risk-adjusted autologous stem cell transplant in eligible patients, has radically changed the natural history of AL amyloidosis. Here, we review the state-of-the-art treatment landscape in AL amyloidosis with an eye toward future therapeutic venues to impact the outcome of this devastating illness.
Key Words: AL amyloidosis, cardiomyopathy, chemotherapy, fibrils, immunotherapy, organ failure, plasma cell disorders
Abbreviations and Acronyms: AL, immunoglobulin light chain; ASCT, autologous stem cell transplant; FLC, free light chains; Ig, immunoglobulin; MM, multiple myeloma; PC, plasma cell
Central Illustration
Highlights
-
•
Cardiac involvement in AL amyloidosis is common and represents the single most adverse prognostic factor.
-
•
Chemo-immunotherapies and autologous stem cell transplant lead to prolonged remission and survival in low-stage patients.
-
•
Early diagnosis is critical in AL amyloidosis to avoid irreversible organ damage.
-
•
Rapid and deep FLC reduction is necessary to ensure long-term survival and functional recovery of affected organs.
Amyloidoses are a heterogeneous group of diseases characterized by organized deposition of a misfolded protein in repetitive β-pleated sheets in target organs. The identity of the precursor protein distinguishes the various amyloidoses and guides organ tropism and the therapeutic approach (1). In immunoglobulin light chain (AL) amyloidosis, the amyloidogenic protein is a misfolded immunoglobulin (Ig) free light chain (FLC), typically produced by clonal plasma cells (PC), less often by a more immature B cell neoplasm (2). FLC amyloid deposition can occur in any organ except the central nervous system, underscoring the variable and often complex clinical presentation. A high index of clinical suspicion is crucial to rapidly pursue diagnostic studies and definitive tissue diagnosis in an effort to preserve organ function and maximize likelihood of short-term survival. AL amyloidosis treatment relies on cytoreductive, PC-directed chemotherapy and/or immune-therapy with the goal of achieving rapid and deep hematologic remission to halt progression of end-organ damage. Drugs that are active in the PC cancer multiple myeloma (MM) are typically efficacious in AL amyloidosis, albeit the therapeutic index may substantially differ. For decades a neglected disease with significant limitations in clinical trial execution and accrual, it was not until early 2021 that the U.S. Food and Drug Administration (FDA) approved the first treatment for newly diagnosed AL amyloidosis: a combination of cyclophosphamide, bortezomib, and dexamethasone with the monoclonal antibody (MoAb) daratumumab hyaluronidase (Dara-CyBorD). Dara-CyBorD is the only FDA-approved treatment for AL amyloidosis, with the remainder of PC-directed therapies being used off-label.
The clinical use of proteasome inhibitors (PIs), the scrupulous selection of patients eligible for high-dose chemotherapy followed by autologous stem cell transplant (ASCT) rescue, and most recently, the introduction of CD38-targeting MoAbs have profoundly impacted the outcomes of AL amyloidosis patients. Intensification of upfront treatment with a combination of targeted drugs, immunotherapy, and alkylating agents has resulted in rapid and deep responses even in patients with advanced cardiac involvement, allowing for increased consideration of solid organ transplantation.
In this state-of-the-art review, we briefly discuss the pathogenesis, epidemiology, clinical presentation, and diagnostic criteria of AL amyloidosis as the foundation, and then detail the current therapeutic approaches, with an eye toward promising therapeutic agents.
AL Pathogenesis
AL amyloidosis pathogenesis is invariably related to deposition of FLC amyloid fibrils, a distinctive feature from other PC disorders, including MM (Table 1). Unorganized amyloidogenic FLCs are directly cytotoxic while deposited FLC fibrils, as they amass, cause distortion of histological architecture, resulting in progressive organ failure and, eventually, death. Amyloidogenic FLC are skewed toward λ Ig light chain (about 75% of cases), and specific Ig light chain genotypes are associated with particular organ tropism (3). It is thought that beyond the intrinsic instability of certain Ig light chain sequences, somatic hypermutation of the variable region and dysfunction of extracellular proteostasis mechanisms both contribute to amyloid formation (4,5). Although AL amyloidosis has been generally regarded as a particularly ominous MM variant or, alternatively, a monoclonal gammopathy of undetermined significance (MGUS) with a mischievous protein, a recently surfaced genetic and functional observation suggests that AL amyloidosis cells and their surrounding bone marrow microenvironment are intrinsically distinct from both these entities (6).
Table 1.
Comparison of Diagnostic Criteria for Common PC Disorders
| FLC | M protein | BM PC | Presence of Disease-Related Organ Damage | |||
|---|---|---|---|---|---|---|
| AL amyloidosis | Abnormal FLC ratioa | Absent/present in SPEP/UPEP with IFE | AND | Typically present, at highly variable % | AND | Yes. Always caused by deposition of FLC organized in amyloid fibrils. |
| MGUSe | Abnormal/normal FLC ratiob | <3 g/dL (serum) | AND | <10% | AND | Noc |
| SMMe | Abnormal/normal FLC ratiob | ≥3 g/dL (serum) or ≥500 min/24 h (urine) | OR | 10%-60% | AND | Noc |
| MMe | Abnormal/normal FLC ratio | Absent/present in SPEP/UPEP with IFE | AND | ≥10% or plasmacytoma | AND | Yes. Generally secondary to expansion of PC clone.d
|
The table outlines the diagnostic criteria and distinctive feature for the most common PC disorders.
BM = bone marrow invasion by monoclonal malignant plasma cells; CMR = cardiac magnetic resonance; FLC = serum free light chains; M = monoclonal; MGUS = monoclonal gammopathy of undetermined significance; MM = multiple myeloma; PC = plasma cells; SMM = smoldering multiple myeloma; SPEP = serum protein electrophoresis; UPEP = urine protein electrophoresis.
Ratio may be spuriously normal in patients with λ FLC AL amyloidosis and advanced renal failure caused by disproportionate elevation of κ FLC compared with λ FLC.
FLC ratio must be <100 based on most recent diagnostic criteria.
Occasionally, MGUS/SMM may present clinical manifestations directly related to FLC/Ig pathogenicity, such as in monoclonal gammopathy of renal significance (MGRS). The term monoclonal gammopathy of clinical significance (MGCS) has been proposed to classify these conditions.
Occasionally caused by direct FLC/M protein pathogenicity (cast nephropathy, hyperviscosity, and so on). At least 1 MM-defining event or 1 biomarker of malignancy must be present to fulfill MM diagnostic criteria, according to Rajkumar et al (13).
Congo red staining must be negative to exclude amyloidosis.
Epidemiologic Considerations
Historically considered a rare disorder, modern epidemiologic studies suggest that AL amyloidosis is rather underdiagnosed because of the vague nature of early symptoms and variable, often multisystemic clinical presentation. The median age of AL amyloidosis patients at diagnosis is 63 years, and there is a slight male predominance (55%) (7). A pre-existing diagnosis of MGUS or smoldering MM is a risk factor for AL amyloidosis (8,9). Further, 15% of newly diagnosed MM patients are concurrently diagnosed with AL, and an extra 1% will be diagnosed with AL amyloidosis throughout the course of their care. The current prevalence of AL amyloidosis is estimated at about 12,000 patients in the United States, and although data regarding impact of race on AL are lacking, the increased incidence of MGUS in Black American individuals suggests that AL amyloidosis may also be more frequent in this patient population (10).
Diagnosis, staging, and prognostic factors
A high index of suspicion and prompt diagnostic work-up is imperative to diagnose AL amyloidosis at early stages, before extensive and irreversible and cardiac damage occurs. Heart and/or kidneys are frequently affected, with three-quarters of patients presenting with involvement of both. As AL PC are immunophenotypically indistinguishable from MGUS or MM PC, clinicians should carefully evaluate for the presence of signs/symptoms concerning for AL amyloidosis in patients with an underlying PC dyscrasia (Table 2). Consideration should be given to screen these patients with N-terminal pro–B-type natriuretic peptide and albuminuria, as biomarkers that raise suspicion for cardiac and renal involvement by AL amyloidosis, respectively. Although N-terminal pro–B-type natriuretic peptide is not a biomarker specific for AL amyloidosis, its elevation in a patient with unexplained heart failure and biopsy-proven AL amyloidosis in an organ other than the heart is highly suggestive of cardiac amyloidosis. Excitingly, the use of genomic, FLC proteomics and high-throughput competition assay are being investigated for use in early diagnosis of AL in MGUS/SMM patients (11,12). According to the International Myeloma Working Group, 4 criteria must be fulfilled to render a definitive diagnosis of AL amyloidosis: 1) clinical presentation compatible with AL pattern of injury; 2) evidence of a PC (or less often lymphoproliferative) disorder based on bone marrow aspirate/biopsy and serologic parameters; 3) histopathological identification of amyloidosis deposition in periumbilical fat or affected organ; and 4) amyloidosis typing for identification of Ig light chain precursor protein via LC-MS or immunoelectronmicroscopy (13). In a small minority of patients, an underlying lymphoproliferative disorder may not be identified. It is important to note that histopathological evidence of amyloidosis in a patient with a PC dyscrasia does not automatically equal AL amyloidosis, because other amyloidoses, particularly transthyretin, can coexist with MGUS (14).
Table 2.
System-Based, Clinical Presentation of AL Amyloidosis Patients and Key Diagnostic Findings
| Organ | Frequency of Involvement | Common Presenting Signs/Symptoms | Diagnostic Findings | Consensus Criteria for Involvementa |
|---|---|---|---|---|
| Heart | 60%-75% |
|
ECG
|
NT-proBNP > 332 ng/Lc OR Mean IVSd >12 mm |
| Kidney | 50%-70% |
|
|
Proteinuria ≥0.5 g/24 h (mostly glomerular proteinuria, thus albumin) |
| Liver | 20% |
|
|
Liver span >15 cme OR Alkaline phosphatase elevation >1.5 times upper limit of normal |
| Gastrointestinal tract | 10%-20% |
|
Direct biopsy verification | |
| Lungf | 30%-90%f |
|
|
Direct biopsy verification |
| Peripheral nervous system | 10%-20% |
|
|
Clinical diagnosis |
| Autonomic nervous system | 10%-20% |
|
|
Clinical diagnosis |
| Soft tissue | 10%-20% |
|
Clinical diagnosis |
The table outlines incidence of organ involvement and frequent signs/symptoms and diagnostic findings in patients with AL amyloidosis based on pattern of organ involvement (125,126). Consensus criteria for diagnosis also reported.
CMR = cardiac magnetic resonance; ECG = electrocardiogram; EMG = electromyography. GLS = global longitudinal strain; IVSd = interventricular septal wall thickness at end diastole; LV = left ventricular; LVEF = left ventricular ejection fraction; NT-proBNP = N-terminal pro–B-type natriuretic peptide; PN = peripheral neuropathy; RHC = right heart catheterization; TTE = transthoracic echocardiogram.
Alternative etiologies must be excluded.
Typical of patients with amyloid deposition in the smaller vessels within the heart wall, mimicking coronary artery disease in the absence of large-vessel disease.
In the absence of renal failure or atrial fibrillation.
Factor X deficiency can occur independently of liver involvement caused by direct absorption of factor X by amyloid fibrils.
In the absence of congestive hepatopathy secondary to heart failure.
Depending on single institution series, often asymptomatic and detected postmortem.
Presumed related to vascular deposition of amyloid.
Table 3 outlines the diagnostic and staging studies recommended for patients with suspected/confirmed AL amyloidosis. Because cardiac involvement has a major prognostic impact by driving early mortality, current staging systems incorporate the extent of cardiac involvement as measured by serum markers (Table 4) (15, 16, 17). A staging system for renal involvement predicting renal survival has also been validated (Table 5) (18). An overlapping diagnosis of MM based on the presence of CRAB criteria, or a bone marrow clonal plasmacytosis exceeding 10% both represent adverse prognostic factors in AL amyloidosis (19). Translocation t(11;14) resulting in the juxtaposition of the cyclin D1 gene locus to the Ig heavy chain locus is the most common cytogenetic abnormality in AL, present in 40%-60% of patients, and portends a worsened prognosis (Table 6). This is in contrast to MM where t(11;14) is a standard-risk cytogenetic abnormality. Furthermore, t(11;14) is a predictive factor of poor response to bortezomib (20). The molecular basis for the biological impact of t(11;14) on prognosis and response to therapy remain obscure.
Table 3.
Diagnostic Work-Up in Patients With Suspected AL Amyloidosis
| Test/Procedure | |
|---|---|
| Blood/serum testsa | CBC with manual differential |
| Basic metabolic panel | |
| Liver function tests | |
| SPEP+IFE | |
| FLC | |
| LDH | |
| β2 microglobulin | |
| Albumin | |
| High-sensitivity troponin | |
| NT-proBNP | |
| TSH and free T4 | |
| Cholesterol panel | |
| PT and PTTb | |
| Urine tests | Albumin/creatinine ratio |
| UPEP+IFE | |
| Imaging studies and diagnostic procedures | Bone survey inclusive of long bones and skull and/or PET/CT |
| ECG | |
| TTE | |
| CMRc | |
| Right heart catheterization (with endomyocardial biopsy if indicated)c | |
| Chest x-ray/CT chestc | |
| Abdominal imagingc | |
| EMGc | |
| GI transitc | |
| Upper and lower endoscopiesc | |
| Pathology specimens | Unilateral bone marrow aspirate and biopsy for IHC, Congo red stain, flow cytometry, and CD138-selected cytogenetics and FISH |
| Biopsy of plasmacytoma, if present | |
| Target organ or fat pad, minor salivary gland or rectum aspirate, for Congo red stain, immunofluorescence and, if available, EM. Typing of amyloid needs to be performed for accurate precursor protein identification. |
CBC = cell blood count; EM = electron microscopy; FISH = fluorescent in situ hybridization; FLC = serum free light chain; IFE = immunofixation; IHC = immunohistochemistry; LC-MS = liquid chromatography mass spectrometry; LDH = lactate dehydrogenase; MM = multiple myeloma; PET/CT = positron emission tomography/computed tomography; SPEP = serum protein electrophoresis; UPEP = urine protein electrophoresis; other abbreviations as in Table 2.
Transthyretin (TTR) gene sequencing should be performed in patients where familial transthyretin amyloidosis is in the differential diagnosis and/or if transthyretin amyloidosis is diagnosed.
Factor X absorption onto amyloidosis can lead to PTT prolongation and bleeding diathesis.
As needed depending on clinical presentation.
Table 4.
Prognostic Impact of AL Amyloidosis Staging Systems
| Mayo Clinic (2004) Integrating European Collaborative Study 3B Staging |
Mayo Clinic (2012) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Risk Factors | Risk Factors Present (n) | Stage | Patients (%) | Median OS (months) | Risk Factors | Risk Factors Present (n) | Stage | Patients (%) | Median OS (Months) |
| cTnT >0.035 ng/mL NT-proBNP >332 pg/mL |
0 | 1 | 33 | 86 | cTnT >0.025 ng/mL NT-proBNP >1,800 pg/mL dFLC >180 mg/L |
0 | 1 | 25 | 94.1 |
| 1 | 2 | 30 | 43 | 1 | 2 | 27 | 40.4 | ||
| 2 | 3A | 18 | 17 | 2 | 3 | 25 | 14 | ||
| NT-proBNP ≥8,500 pg/mL | 3B | 19 | 4.6 | 3 | 4 | 23 | 5.8 | ||
Table 5.
Renal Staging in AL Amyloidosis and Impact on Renal Survival (18)
| Risk Factors | Risk Factors Present (n) | Stage | Patients (%) | % of Patients on Renal Replacement Therapy at 3 y |
|---|---|---|---|---|
| Proteinuria >5 g/24 h eGFR <50 ml/min/1.73 m2 | 0 | 1 | 23 | 4 |
| 1 | 2 | 60 | 30 | |
| 2 | 3 | 17 | 85 |
eGFR = estimated glomerular filtration rate.
Table 6.
Frequency and Clinical Impact of Common Genetic Abnormalities in AL Amyloidosis
| Incidence, % | Clinical Impact | |
|---|---|---|
| t(11;14) | 40-60 | Adverse prognostic factor Predictive factor of poor response to bortezomib-based therapy Melphalan may abrogate unfavorable prognosis |
| Del(13)/(13q) | 30-40 | - |
| Trisomy of a single chromosome | 25-30 | Shorter OS in patients treated with melphalan |
| Gain(1q21) | 15-20 | Standard risk in patients treated with bortezomib |
| Hyperdiploid | 12 | Standard risk |
| t(14;16) and t(4;14) | 3-4 each | Standard risk in patients treated with bortezomib Associated with adverse outcome after high dose melphalan and ASCT |
| Del(17p)/17 | 2-6 | Associated with higher BM plasmacytosis Cardiac involvement almost universally present Circa 45% with stage III cardiac involvement Associated with adverse outcome after high dose melphalan and ASCT |
ASCT = autologous stem cell transplantation; BM = bone marrow; OS = overall survival.
Treatment Considerations
AL amyloidosis patients often present with complex syndromes and multisystemic disease that require a multidisciplinary treatment approach to optimize supportive care. Although the goal of therapy in AL amyloidosis is rapid and deep reduction of circulating FLC through PC-directed therapy, intensive supportive care and treatment of underlying organ dysfunction is of paramount importance to improve not only quality but also quantity of life. Although MM patients generally die from complications of widespread, relapsed/refractory cancer, patients with AL die as a consequence of irreversible, progressive organ failure, most commonly cardiac failure, caused by ongoing amyloid deposition. Thus, time is truly of the essence in AL amyloidosis. Although PC-directed therapy does not directly affect the amyloid deposits, when successful, it stops, or significantly reduces, FLC secretion, thus indirectly halting amyloid deposition and progressive organ dysfunction. A major branching point when a clinician is faced with a newly diagnosed AL amyloidosis patient is deciding whether ASCT is an appropriate treatment strategy. Historically, ASCT has been the only therapy resulting in sustained remission and, thus, long-term survival in selected AL amyloidosis patients. The availability of highly effective chemo-immunotherapies has radically changed this paradigm, and clinicians should take into consideration not just whether a patient fulfills criteria for ASCT candidacy (Table 7), but also what the personal preferences of each individual are. The lack of randomized data regarding the impact of ASCT in the era of modern chemo-immunotherapy limits our counselling, but also offers patients more flexibility regarding treatment options. Extrapolating from MM, it is likely that achievement of minimal residual disease negative hematologic response has the most impact on progression free survival (PFS), regardless of the treatment strategy pursued to achieve it (21).
Table 7.
Criteria for Autologous Stem Cell Transplant Eligibility in AL Amyloidosis in Our Centers
| Transplant Eligible (All Criteria Must Be Met) | Transplant Ineligiblea (Any Criteria) | |
|---|---|---|
| Age, y | ≤70 | >70 |
| ECOG PS | 0-2 | >2 |
| Staging (revised Mayo 2004) | I-II | III |
| LVEF, % | >45 | ≤45 |
| NYHA functional class | I-II | III-IV |
| eGFR | ≥30 ml/min/1.73 m2 | <30 ml/min/1.73 m2 |
| SBP | ≥90 mm Hg without orthostatic hypotension | <90 mm Hg or untreated orthostatic hypotension |
| DLCO, % | >50 | <50 |
The table outlines criteria implemented to determine transplant eligibility in AL amyloidosis in our centers.
DLCO = diffusing capacity for carbon monoxide; ECOG PS = Eastern Cooperative Oncology Group Performance Status; eGFR = estimated glomerular filtration rate; LVEF = left ventricular ejection fraction; NYHA = New York Heart Association; SBP = systolic blood pressure.
Consideration can be given to risk-stratified, dose-reduced melphalan conditioning and ASCT for selected patients, including patients with end-stage renal disease if all other eligibility criteria are satisfied.
Response Criteria
Although not sufficient, a deep and sustained hematologic remission is necessary for organ response to occur. The exact molecular mechanisms underlying organ response remain largely obscure. Because achievement of a deep hematologic remission translates into higher chances of organ response, it is reasonable to speculate that a significant removal of circulating FLC is necessary for organ response. Further, reabsorption of amyloid fibrils over time has been observed in patients who achieve a hematologic remission, although it remains unknown what extent of amyloid reabsorption is needed to elicit organ response. Importantly, as the removal of deposited amyloidosis by intrinsic mechanisms is rather inefficient, organ response typically follows a hematologic response by months, sometimes years, although significant interpatient variability in the extent and kinetics of amyloid reabsorption exists (22). The causes underlying inefficient amyloid clearance remain poorly understood, and MoAbs directly targeting amyloid fibrils are currently being evaluated in clinical trials (23,24). Tables 8 and 9 summarize the validated hematologic and organ response criteria, respectively, in AL. Emerging data support the notion that the deeper the hematologic response, the better the outcome in terms of major organ improvement and/or PFS (25). In recent retrospective studies, deep suppression of AL-PC clone as reflected in suppression of involved FLC (≤10 mg/L) and difference between involved and uninvolved FLC ≤10 mg/L was shown to associate with improved PFS, organ remission and overall survival (OS) (26,27). Similarly, minimal residual disease negativity as measured by next generation flow cytometry was shown to associate with PFS prolongation and increased organ response in several studies (25,28,29). The definition of progressive disease in AL amyloidosis is evolving. Under the premises that even low levels of circulating FLC may be sufficient to precipitate progressive organ dysfunction over time, a recent paper examined a high-risk difference between involved and uninvolved FLC progression criteria and showed its impact on OS, supporting its use as a trigger for treatment in patients who previously achieved a hematologic remission (30). Although both hematologic and organ remission affect long-term survival, advanced cardiac involvement is the single most important factor in driving early mortality, highlighting the necessity to raise awareness about AL to improve early diagnosis (31).
Table 8.
Hematologic Response Criteria
| Hematologic Response Parameters |
Complete Response (CR)a | Very Good Partial Response (VGPR) | Partial Response (PR) | Progression From CR | Progression From PR |
|---|---|---|---|---|---|
| FLC | Normal ratio | dFLC <40 mg/L | dFLC >50% | Abnormal FLCb | >50% increase in affected FLC AND >100 mg/L absolute value |
| SPEP+IFE | No M spike. Negative IFE | Not applicable | Not applicable | Positive | >50% increase in M spike AND >0.5 g/dL M spike |
| UPEP+IFE | No M spike. Negative IFE | Not applicable | Not applicable | Not applicable | >50% increase in urinary M spike AND >200 min/24 h M spike |
The table synthesizes the most updated hematologic response criteria in AL amyloidosis (31).
dFLC = difference between involved and uninvolved serum free light chains; IFE = immunofixation; M spike = monoclonal spike; SPEP = serum protein electrophoresis.
All criteria must be met.
Affected serum free light chains must double in absolute value.
Table 9.
Validated Organ Response Criteria
| NT-proBNP Response | NYHA Functional Class Response | NT-proBNP Progression | ||
|---|---|---|---|---|
| CARDIAC RESPONSE | NT-proBNP decrease of >30% AND >300 pg/mL from baselinea | 2 NYHA functional class improvement from baselineb | CARDIAC PROGRESSION | NT-proBNP increase of >30% AND >300 ng/L from baseline |
| Proteinuria | eGFR | |||
| RENAL RESPONSE | Decrease in proteinuria by >30% or to <0.5 g/24 hc | RENAL PROGRESSION | >25% increase in eGFR | |
Therapeutic Agents
Historically, AL amyloidosis therapy has followed in the footsteps of anti-MM therapies, and patients have been treated with anti-PC drugs off label. For decades, oral melphalan in combination with steroids constituted the mainstay treatment for AL. It was not until the late 1990s that high-dose melphalan with ASCT was pioneered. Molecularly targeted agents such as immunomodulatory drugs (IMiDs) and PIs were evaluated in AL in the late 2000s (Figure 1). The past 5 years have seen an acceleration in novel therapies for AL, including the investigational use of antifibrillary antibodies. In January 2021, the FDA approved Dara-CyBorD for the treatment of newly diagnosed AL (excluding stage IIIB patients) based on the results of the Andromeda study, a randomized, open-label, active-controlled trial in 388 newly diagnosed AL amyloidosis patients. Although Dara-CyBorD is the first and only FDA-approved therapy for AL amyloidosis, several chemo-immunotherapy, multidrug regimens as well as ASCT in selected patients are effective in AL amyloidosis (32,33). We will review the most commonly used agents in AL amyloidosis starting from preclinical data of mechanism of function and resistance and then outlining clinical evidence (prospective studies are reported in Table 10). The lack of reliable, preclinical models of AL amyloidosis limits basic-translational research on the molecular bases of effectiveness of distinct drugs. For the purpose of this review, data obtained in MM systems will be cited instead.
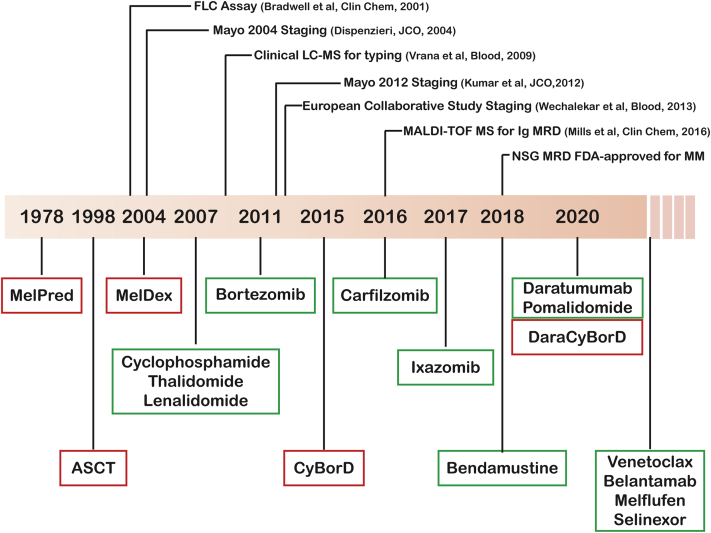
Figure 1.
Evolution of Treatment in AL Amyloidosis
The timeline outlines in chronological order the clinical use of distinct treatments in immunoglobulin light chain (AL) amyloidosis. Commonly used agents/regimens are in red boxes, less commonly used agents/regimens are in green boxes. The top of the figure outlines some of the most impactful technological/clinical advances in AL amyloidosis. ASCT = autologous stem cell transplant; CyBorD = cyclophosphamide-bortezomib-dexamethasone; Dara = daratumumab; Dex = dexamethasone; FLC = free light chain; LC-MS = liquid chromatography-mass spectrometry; MALDI-TOF = matrix-assisted laser desorption ionization time-of-flight; Mel = melphalan; MM = multiple myeloma; MRD = minimal residual disease; NSG = next generation sequencing; Pred = prednisone.
Table 10.
Prospective Studies Evaluating Current Treatment Approaches In AL Amyloidosis
| Therapy Regimens (Ref. #) | Study Phasea | N | Disease Setting | Hematologic Response | Organ Response | Median PFS/OS |
|---|---|---|---|---|---|---|
| Autologous transplant | ||||||
| ASCT (127) | R | 421 | — | — | 43% | 2.6 y/6.3 y |
| ASCT (128) | R | 434 | — | 76% | 53% | —/OS not reached |
| Risk-adapted ASCT (45) | 2 | 40 | NDAL | 79% | 47% | At 2 y: PFS: 69% OS: 82% |
| MelDex vs ASCT (41) | 3 | 50 vs 50 | NDAL | 68% vs 67% | 39% vs 45% | 2.7 y/4.7 y vs 2.7 y/1.8 y |
| Alkylators | ||||||
| MelDex (129) | 2 | 46 | — | 67% | 48% | 3.8/5.1 y |
| BendaPred (52) | R | 122 | NDAL: 12 RRAL: 110 |
35% | C: 12% R: 31% |
9 mo/21 mo |
| BendaDex (53) | 2 | 31 | RRAL | 57% | C: 13% R: 46% |
11.3 mo/18.2 mo |
| IMiD-based therapy | ||||||
| CTD (130) | R | 75 | NDAL: 31 RRAL: 44 |
74% | 27% | 1.7 y/3.4 y |
| RD (79) | 2 | 23 | NDAL: 10 RRAL: 13 |
41% | 23% | 1.6 y/— |
| CRD (51) | 2 | 35 | NDAL: 24 RRAL: 11 | 60% | 31% | 2.4 y/3.1 y |
| MelRD (83) | 1/2 | 26 | NDAL | 42% | 50% | At 2 y: PFS: 54% OS: 81% |
| PD (87) | R | 33 | RRAL | 48% | 15% | 1.2 y/2.3 y |
| Proteasome inhibitor-based therapy | ||||||
| Bortezomib (131) | 2 | 70 | RRAL | 67%-69% | C: 10% R: 27%-44%b |
At 1 y: PFS: 72%-75% OS: 84%-93%b |
| VDex (132) | R | 94 | NDAL: 18 RRAL: 76 |
72% | 30% | At 1 y: 76% OS |
| CyBorD (49) | R | 230 | NDAL | 60% | C: 17% R: 25% |
At 5 y: OS: 55% |
| RVd (66) | R | 34 | NDAL | 89% | 35% | At 1 y: OS: 73% |
| MelDex vs VelMelDex (133) | 3 | 56 vs 53 | NDAL | 52% vs 7% | C: 28% vs 38% R: 43% vs 44% |
At 2Y: PFS: 21% vs 47% OS: 45% vs 68% |
| K+/-D (134) | 1/2 | 28 | RRAL | 63% | 21% | Not applicable |
| IxaDex (69) | 1/2 | 27 | RRAL | 52% | 56% | At 2 y: 60%/85% |
| IRd (70) | R | 40 | RRAL | 66% | C: 6% R: 13% |
1.4 y/2.4 y |
| CD38-targeting MoAb-based therapy | ||||||
| Daratumumab (135) | 2 | 72 | RRAL | 77% | C: 55% R:52% |
At 2 y: 62%/97% |
| Dara-CyBorD vs CyBorD (103) | 3 | 195 vs 193 | NDAL | 92% vs 77% | C: 42% vs 22% R: 54% vs 27% |
Not applicable |
| Agents targeting amyloid fibrils | ||||||
| Doxycycline (113) | 2 | 25 | NDAL | 100%c | 36% | At 1 y: 100% OS |
| NEOD001 (136) | 1/2 | 27 | RRAL | — | C: 57% R:60% |
Not applicable |
| 11-1F4 (23) | 1a/b | 27 | RRAL | — | 67% | Not applicable |
The table summarizes prospective studies evaluating therapeutic approaches to AL amyloidosis.
ASCT = autologous stem cell transplant; C = cardiac response; CRD = cyclophosphamide, lenalidomide, dexamethasone; CTD = cyclophosphamide, thalidomide, dexamethasone; CyBorD = cyclophosphamide-bortezomib-dexamethasone; D = dexamethasone; Dex = dexamethasone; IRd = ixazomib, lenalidomide, and dexamethasone; K = carfilzomib; MelDex = melphalan-dexamethasone; MelRD = melphalan-lenalidomide-dexamethasone; MTD, maximum tolerated dose; NDAL, newly diagnosed AL amyloidosis; OHR = overall hematologic response; OS = overall survival; OW = once weekly; PD = pomalidomide-dexamethasone; PDex = pomalidomide, dexamethasone; PFS = progression-free survival; R = renal response; RD = lenalidomide-dexamethasone; RRAL = relapsed or refractory AL amyloidosis; RVd = lenalidomide, bortezomib, and dexamethasone; T = thalidomide; TW = twice weekly; VD = bortezomib-dexamethasone; VelMelDex = melphalan-bortezomib-dexamethasone.
R: retrospective.
Depending on once weekly 1.6 mg/m2 vs twice weekly 1.3 mg/m2 schedule.
In patients surviving 1 year or longer.
Alkylating agents
Melphalan, cyclophosphamide, and bendamustine are nitrogen mustard alkylating agents with antineoplastic activity secondary to the alkylation of guanines and formation of interstrand crosslinks (ICL), leading to DNA damage with consequent interference with DNA replication and DNA-to-RNA transcription. Recently, these agents have been shown to induce an immunogenic cell death and skew the cytokine milieu from a cancer-tolerant to a cancer-surveilling microenvironment (34). As alkylating agents are cell-cycle nonspecific agents, cytopenia and gastrointestinal toxicities are frequently observed.
Oral melphalan
Based on the detection of Ig fragments in deposited amyloid and extrapolating from MM, the combination of melphalan and prednisone was applied to the treatment of AL patients in the late 1970s (35). This regimen was shown to be superior to colchicine, a drug that was preliminarily reported effective in AL amyloidosis, and became a de facto standard of care treatment for AL amyloidosis patients for decades (36). Combination of melphalan with dexamethasone (MelDex) remains a highly effective treatment in AL amyloidosis and has been shown to abrogate the adverse prognostic impact of t(11;14) (37). Triplet combinations subsequently incorporating lenalidomide or bortezomib into MelDex backbone demonstrated good efficacy and a tolerable safety profile (38,39).
Autologous stem cell transplant
High-dose (200 mg/m2), intravenous melphalan followed by ASCT remains a mainstay of AL amyloidosis treatment in many centers since its pioneer use in the late 1990s (40). In a randomized, phase 3 study in newly diagnosed AL amyloidosis, MelDex resulted in superior OS compared with high-dose melphalan followed by ASCT (57 months vs 22 months; P = 0.04) (41). A prohibitive treatment-related mortality (TRM) of 24% in the ASCT arm as well as trend toward harm in the 6-month landmark analysis suggest that careful patient selection, intensive supportive care, and risk-adapted approaches are critical when deciding eligibility of AL amyloidosis patients for ASCT. Recently, an outcome analysis of the Center for International Blood and Marrow Transplant Research database showed a progressive improvement in 100-day transplant related mortality over time, reaching 5% across all centers and 3% in high-volume centers from 2007-2012 (42). Because patient staging was comparable over time, the improved outcome was attributed to increased expertise in caring for this complex patient population and enhanced supportive care rather than mere patient selection. A retrospective study from a large transplant center showed ASCT-TRM to be 8% in patients with stage 3 AL amyloidosis compared with 4% for the entire cohort, consistent with cardiac involvement driving TRM in ASCT (43). Risk-adapted strategies with dose reduction of melphalan to 140 or 100 mg/m2 based on extent of cardiac and/or renal involvement, number of organs involved, and age have resulted in improved outcome for high-risk patients (33).
Given the availability of highly effective, well-tolerated, combinatorial chemo-immunotherapies, the amyloidosis community is now faced with the task to understand the most appropriate sequencing of agents and role of ASCT in the treatment course of AL amyloidosis patients. Several studies have supported the use of ASCT as a tool to deepen the hematologic remission in patients with suboptimal response to induction chemotherapy (44,45). However, whether this approach is superior to chemo-immunotherapy-based treatment intensification remains unknown. Randomized clinical trials would be of utmost importance, and until high-quality modern data are available, ASCT remains a valuable therapeutic option to intensify therapy (Figure 2). Of note, although t(11;14) is a biomarker of suboptimal response to bortezomib, it is a favorable prognostic marker for hematologic response and PFS in patients undergoing ASCT (46). The opposite is true for t(4;14), t(14;16), and del(17p13), well-established high-risk cytogenetics in MM, that portend worse outcome in the setting of ASCT, but not bortezomib-containing regimens, in AL amyloidosis. These data outline the urgent need for a deeper understanding of AL amyloidosis biology and development of biomarkers to improve our patient stratification capabilities.
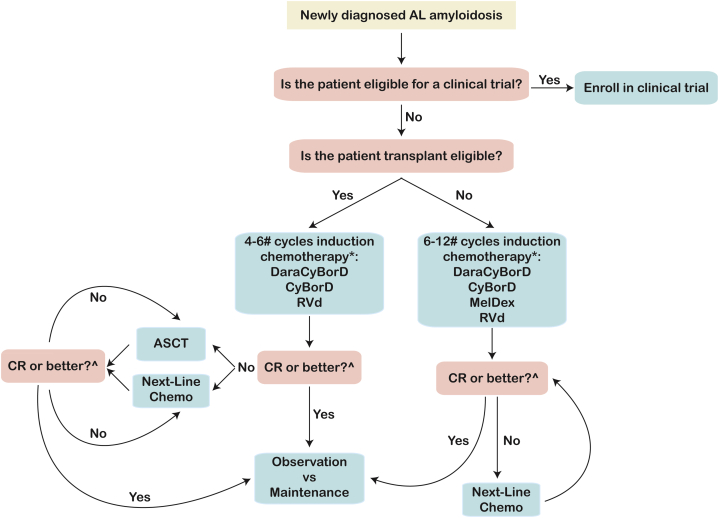
Figure 2.
Algorithm for Treatment Approach to Newly Diagnosed AL Amyloidosis Patients
The schema outlines an algorithm for therapeutic decisions in newly diagnosed AL amyloidosis patients with the goal of achieving a deep hematologic response. An early branching point is eligibility for high dose chemotherapy and ASCT. We recommend induction chemotherapy for all patients for 4-6 cycles with monthly assessment of disease response and change of therapy after 2 months if an optimal response is not achieved. ASCT and/or distinct chemotherapy regimens can be used to intensify treatment to achieve a hematologic CR. #Number of cycles is arbitrary and dependent on kinetic of response, tolerability, and indication for ASCT. ∗Monthly monitoring of hematologic and organ response is mandatory. If a VGPR is not achieved after 2 cycles, we recommend changing chemotherapy. ^Stem cells should be harvested even if ASCT is deferred to second remission. MRD assessment may be useful to aid in discussion regarding intensification of treatment. CR = complete response; other abbreviations as in Figure 1.
Cyclophosphamide
Differently from melphalan, cyclophosphamide is a prodrug and requires P450-mediated activation. Cyclophosphamide active metabolites are phosphoramide mustard and acrolein, the latter being responsible for the development of hemorrhagic cystitis. In AL amyloidosis, cyclophosphamide is administered by mouth or intravenously at low dose in combination with PIs, IMiDs, or MoAbs. Although high-dose, intravenous cyclophosphamide is routinely used for stem cell mobilization ahead of ASCT in MM, its use in AL amyloidosis is generally contraindicated because of added cardiac toxicity and increased risk of morbidity and mortality (47,48).
The combination of cyclophosphamide with bortezomib and dexamethasone (CyBorD) became the de facto standard of care regimen based on extensive retrospective data in newly diagnosed AL amyloidosis patients showing efficacy and good tolerability (32,49). Depth and length of response were superior with CyBorD compared with a combination of cyclophosphamide with thalidomide and dexamethasone (CTD), also an effective regimen in AL amyloidosis (50). In a phase 2, single arm, prospective study, in combination with lenalidomide and dexamethasone, cyclophosphamide was shown to elicit deep hematologic and organ responses (51).
Bendamustine
Bendamustine is administered intravenously and is metabolized via the cytochrome P450 liver system to its active metabolites. A multicenter, prospective, phase 2 study of bendamustine plus dexamethasone and a large, retrospective analysis of patients treated with bendamustine plus prednisone showed these combinations to be effective in inducing hematologic and organ responses with a tolerable pattern of side effects (52,53).
Proteasome inhibitors
Initially employed as research tools to investigate molecular mechanisms of proteolysis, PIs have completely revolutionized the treatment paradigm of PC disorders (54). In total, 3 PIs are FDA approved in MM: peptide boronic acids bortezomib and ixazomib, the former for parenteral, the latter for enteral use; and epoxyketone-derived carfilzomib (55). All 3 PIs mainly target the chymotryptic-like (CT-L, β5) catalytic activity of the proteasome, a large, barrel-shaped multicatalytic protease responsible for the degradation of most misfolded proteins tagged via polyubiquitin chains (54). Extensive research on the molecular mechanisms underlying PI activity in MM has shown that exacerbation of proteotoxicity, induction of immunogenic cell death, and modulation of the bone marrow microenvironment contribute to PI effectiveness (56). Inhibition of canonical NF-κB signaling caused by stabilization of IκB was initially thought to be the prime mechanism of action of PIs (57). Subsequently, exacerbation of baseline proteotoxicity and impairment in proteostasis emerged as the main drivers of PI-induced cytotoxicity, with the ratio between load on the proteasome and proteasome activity itself serving as a biomarker of PI sensitivity (58,59). Sensitivity of AL amyloidosis cells to PIs was subsequently shown to be caused by altered proteostasis in the setting of impaired autophagy (60). A reduction in proteasome load, an increase in proteasome capacity, or up-regulation of alternative proteolytic mechanisms, such as aggresome or autophagy, have been suggested as potential molecular mechanisms mediating PI resistance (55,61).
Although the research community was initially concerned that systemic administration of PIs would be too toxic for humans because of the ubiquitous expression and critical function of the proteasome, PIs are generally well tolerated, with distinct toxicities across different chemically-derived compounds (56).
Bortezomib
The first-in-class PI bortezomib is a peptide boronic acid that reversibly blocks the β5 proteasome subunit. It is approved for administration via intravenous or subcutaneous injection in MM (62). Its characteristic side effect is sensory peripheral neuropathy with a poorly defined etiology. Generally, dose reduction and treatment discontinuation lead to resolution or improvement in symptoms in the majority of patients (63).
Bortezomib radically changed the treatment landscape in AL amyloidosis. Since its use in combination with dexamethasone, bortezomib has shown improved outcome when added to any other chemo/immunotherapeutic agent as a multidrug regimen. Since its initial use in 2009, CyBorD has been commonly used as frontline treatment in AL amyloidosis because of its efficacy and safety, including in patients with end-stage renal disease (49). Cardiac toxicity is not a significant adverse event in AL amyloidosis, including in patients with advanced heart failure and systolic dysfunction. Recent data suggest that within this triplet, bortezomib and low-dose dexamethasone are the main drivers of efficacy (64). Translocation t(11;14) has emerged as a predictive factor of suboptimal response to bortezomib-containing regimens in AL amyloidosis, whereas cytogenetics considered high-risk in MM, such as t(4;14), t(14;16), del(17p), and/or gain of 1q21, had no impact on outcome (20). Interestingly, overexpression of cyclin D1 associates with increased expression of genes involved in endoplasmic reticulum quality control and protein homeostasis, suggesting lower baseline proteotoxicity as a mechanism of bortezomib resistance (59,60,65). In a multicenter, randomized phase 3 study of 109 patients, the combination of bortezomib, melphalan, and dexamethasone (VelMelDex) was superior to MelDex for frequency and depth of response. Importantly, the triplet treatment led to an improvement in overall survival by decreasing mortality 2-fold (38). The combination of bortezomib with lenalidomide and dexamethasone is also effective in inducing hematologic remission in the frontline setting; however, side effects and treatment discontinuation were more common compared with CyBorD (66).
Carfilzomib
Carfilzomib is an epoxyketone PI that irreversibly inhibits the β5 subunit. This critically distinct pharmacodynamic feature is likely at the base of the increased potency, but also broader toxicity of carfilzomib. In fact, cardiovascular side effects, including tachyarrhythmia, hypertension, systolic heart failure, and renal failure, have been reported in patients treated with carfilzomib (67). This pattern of toxicity makes the use of carfilzomib problematic in AL amyloidosis patients in whom cardiac and/or renal involvement is frequently present. However, in selected patients and with careful monitoring, carfilzomib monotherapy at low dosage was shown to elicit responses in 63% of patients, including bortezomib-refractory individuals. In patients with pre-existing peripheral neuropathy, carfilzomib represents an effective therapeutic alternative to bortezomib (68).
Ixazomib
Ixazomib is an orally bioavailable boronic acid PI. Similar to bortezomib, peripheral neuropathy can occur with ixazomib, and GI toxicities, including nausea and diarrhea, and rash are common adverse events (69). Ixazomib was effective at inducing both hematologic and organ responses in relapsed/refractory AL amyloidosis as a single agent and in combination with lenalidomide and dexamethasone (70). In the TOURMALINE-AL1 study, a randomized, phase 3 clinical trial of ixazomib plus dexamethasone vs clinician choice, the investigational arm did not meet the primary endpoint of overall hematologic response rate. However, vital organ response (36% vs 11%; P = 0.0001), median vital organ PFS (18 months vs 11 months; P = 0.019), and median time to vital organ deterioration or death (34.8 months vs 26.1 months; P = 0.0116) all favored ixazomib plus dexamethasone, suggesting that this doublet may more effectively mitigate AL amyloidosis-related organ damage compared with other regimens with similar hematologic activity (71).
Immunomodulatory drugs
Thalidomide and its derivatives, lenalidomide and pomalidomide, are FDA-approved oral agents for the treatment of MM. Their clinical effectiveness is based both on direct anti-MM cytotoxicity and modulation of cancer microenvironment, including antiangiogeneic and immunostimulatory properties (72). Cereblon (CRBN) has been recently identified as the molecular target mediating lenalidomide effectiveness in MM. Together with DNA damage-binding protein 1, CRBN is the substrate receptor of the cullin 4 ring E3 ubiquitin ligase complex (CRL4CRBN), which is responsible for proteasome-mediated degradation of IKZF1 (Ikaros) and IKZF3 (Aiolos). IKZF1 and IKZF3 are transcriptional repressors of interleukin (IL)-2, and their lenalidomide-mediated degradation results in increased IL-2 levels and T-cell immunostimulation (73, 74, 75).
All IMiDs are administered orally and are all considered class X drugs because of the infamously known teratogenic effect of thalidomide, whose administration in pregnant women as an effective antiemetic medication resulted in phocomelia. Other notable side effects include increased risk of venous thromboembolic events when administered as a combinatory regimen, thus warranting appropriate VTE prophylaxis; fatigue; rash; diarrhea; nausea; and cytopenia. In combination with melphalan, lenalidomide has been reported to increase the risk of secondary myeloid dyscrasias (76). IMiDs also carry a black box warning for arterial vascular events. Caution should be used in dosing lenalidomide in AL amyloidosis patients as the therapeutic index is significantly different than in MM. A starting dose of 5 mg D1-21 is recommended, and careful monitoring for side effects, including cardiac deterioration, is warranted.
Thalidomide
The use of thalidomide in the treatment of AL amyloidosis has been significantly limited by its narrow therapeutic index. In particular, peripheral neuropathy and symptomatic bradycardia emerged during a phase 2 clinical trial evaluating thalidomide in combination with dexamethasone (77). Although this doublet showed promising results in terms of both hematologic and organ response, toxicities were felt to be prohibitive. Thalidomide/dex was also evaluated as a fixed-duration treatment consolidation/maintenance for patients who failed to achieve a CR after risk-adapted ASCT with encouraging results, suggesting a potential value of IMiDs for long-term disease control (78).
Lenalidomide
Lenalidomide showed limited activity when used as single agent in AL amyloidosis, but hematologic and organ responses were observed in phase 3 studies of lenalidomide in combination with weekly dexamethasone. A very limited number of patients were able to tolerate the standard MM 25 mg daily D1-21 schedule caused by cytopenia, rash, and infections (79, 80, 81). Importantly, an increase in cardiac biomarkers of unclear etiology and not necessarily consistent with organ progression, as well as renal failure, were observed in patients enrolled in these studies, raising concerns for the routine use of lenalidomide in this patient population (82). Of note, lenalidomide is renally excreted, and thus requires dose adjustment based on renal function. Triplet combinations of PI, lenalidomide, and steroids, such as lenalidomide, bortezomib, and dexamethasone or ixazomib, lenalidomide, and dexamethasone, have significant activity in AL amyloidosis as measured by hematologic response; however, tolerability was limited with a high rate of treatment discontinuation despite low-dose lenalidomide administration (66,70). Lenalidomide proved to be effective also when used in combination with the alkylator agents cyclophosphamide or melphalan plus dexamethasone, although grade 3 cytopenias were common (51,83).
Pomalidomide
Pomalidomide is the most potent, FDA-approved IMiD and has shown activity in MM, including in patients with lenalidomide and/or bortezomib relapsed and/or refractory disease (84,85). In combination with dexamethasone, pomalidomide has been shown to be highly active in AL amyloidosis, inducing frequent hematologic remissions in highly refractory patients (86, 87, 88). Similar to lenalidomide, pomalidomide treatment was also linked to an increase in cardiac biomarkers without definitive evidence of cardiac progression (89). The significance and clinical impact of this finding remains unclear, and close monitoring of cardiac function is warranted. Based on its preclinical activity as an enhancer of immune response, the combination of pomalidomide with MoAbs targeting SLAMF7 or CD38 is currently being evaluated in clinical trials in AL amyloidosis (90, 91, 92).
Monoclonal antibodies targeting AL cells
Given its remarkable effectiveness in MM, MoAbs targeting PC markers have been evaluated in AL amyloidosis (93, 94, 95). In particular, daratumumab (DARA) proved highly effective in AL amyloidosis and has radically changed the natural history of this disease. DARA and isatuximab (ISA) are IgG1 MoAb targeting CD38, a universal marker of plasmablasts and PCs (96). Preclinical studies have demonstrated that DARA and ISA induce MM cell death through different mechanisms, including complement-dependent cytotoxicity (CDC), antibody-dependent cellular phagocytosis (ADCP), antibody-dependent cell-mediated cytotoxicity (ADCC), direct apoptosis, and modulation of CD38 enzymatic activity with subsequent impact on the soluble bone marrow milieu (97). Elotuzumab (ELO) is a humanized IgG1κ MoAb targeting the signaling lymphocytic activation molecule family member F7 (SLAMF7), a glycoprotein that is expressed by PCs as well as cytolytic lymphocyte subsets such as NK cells, NKT cells, or CD8+ T cells. SLAMF7 is located on chromosome 1q23 that is often amplified in MM, making it an attractive target for therapy. Differently from DARA/ISA, ELO does not elicit ADCC or direct cytotoxicity.
Daratumumab
Initially available as an intravenous infusion, a subcutaneous formulation of DARA was approved in 2020 (98). DARA is generally well-tolerated, with main adverse events being infusion-related reactions, infections, and cytopenia (99). Aggressive premedication with steroids, H2 blockers, and leukotriene receptor antagonist montelukast effectively abated incidence of severe infusion reactions. In AL amyloidosis patients with advanced cardiac or renal disease, heart failure exacerbations or worsening anasarca have been observed with intravenous administration, and the subcutaneous formulation is strongly preferred (100). Daratumumab in combination with lenalidomide or bortezomib has shown impressive activity in MM, achieving deep and durable responses in the relapsed and/or refractory population (93,94). DARA is currently approved for use in the frontline and relapsed/refractory setting in MM. In a phase 3, noninferiority, randomized clinical trial comparing intravenous vs subcutaneous DARA (daratumumab plus hyaluronidase), the latter showed decreased incidence of infusion reactions with preserved efficacy, resulting in its FDA approval (98). In AL amyloidosis, single-agent DARA proved highly effective in inducing deep and lasting hematologic and organ responses in heavily pretreated patients (100,101). Importantly, deep hematologic responses were observed after 1 single infusion of daratumumab (100,102). These remarkable results as monotherapy paved the way for upfront use of DARA in combination with standard of care CyBorD. ANDROMEDA is an ongoing, multicenter phase III study randomizing patients to CyBorD alone or in combination with subcutaneous DARA (Dara-CyBorD) (103). Addition of daratumumab results in significantly higher hematologic (92% vs 77%), cardiac (42% vs 22%), and renal (54% vs 27%) response rates compared with CyBorD alone. A complete hematologic response was observed in 53% of patients treated with DaraCyBorD compared with 18% of patients receiving CyBorD at a median follow-up of 11 months. Importantly, major organ deterioration progression-free survival (MOD-PFS) also favored the quadruple therapy with an HR of 0.58 (95% CI: 0.36-0.93; P = 0.02) and cardiac and renal responses were approximately doubled in the quadruplet arm (41% vs 22% and 53% vs 24%, respectively). Dara-CyBorD was well tolerated without unexpected safety concerns, and subcutaneous DARA formulation resulted in fewer infusion-related reactions compared with historic data with intravenous DARA. Based on these positive results, on January 15, 2021, the FDA granted accelerated approval to DARA-CyBorD, the first and only FDA-approved treatment in AL amyloidosis for newly diagnosed patients.
Isatuximab
ISA is a chimeric, IgG1 MoAb that binds with high affinity to a specific epitope on CD38 that is distinct from the daratumumab-binding site (104). ISA is approved as a third-line therapy in combination with pomalidomide and dexamethasone and a second-line therapy in combination with carfilzomib and dexamethasone based on the positive results of the ICARIA and IKEMA phase 3 studies, respectively (105,106). The preliminary result of a multicenter phase 2 study of isatuximab for patients with previously treated AL amyloidosis proved to be safe with encouraging efficacy based on 3% hematologic complete response, 54% very good partial response, and 1-year estimated PFS of 85% (107). A trial investigating isatuximab for the treatment of high-risk, newly diagnosed AL is currently ongoing, and results are eagerly awaited (NCT04754945).
Elotuzumab
ELO was shown to lyse SLAMF7-expressing MM cells via ADCC. Importantly, ELO has no clinical efficacy as monotherapy, but has been shown to increase activity of IMiDs in randomized clinical trials, leading to its approval in combination with lenalidomide-dexamethasone and pomalidomide-dexamethasone (92,95). The potential activity of ELO in AL amyloidosis stems from a single case report of a woman with heavily pretreated, overlapping MM/AL amyloidosis who achieved hematologic and organ response upon treatment with ELO-lenalidomide-dexamethasone (EloRD) (108). An ongoing phase 2 trial is evaluating EloRD with or without cyclophosphamide followed by EloRD maintenance as second line in AL amyloidosis.
Agents targeting amyloid fibrils
Early mortality is a major hurdle in AL amyloidosis, with a mortality risk of 35%-60% within the first 12 months from diagnosis, depending on the extent of cardiac involvement. Even highly effective, combinatorial chemo-immunotherapy such as Dara-CyBorD appears not to affect early mortality in AL amyloidosis because it lacks a direct effect on amyloid reabsorption. There has been great interest in the development of agents specifically targeting amyloid fibrils, with hope that preventing fibril deposition and/or removing deposited fibrils could improve the outcome of amyloidosis patients. However, data from randomized clinical trials of antifibrillary antibodies either have been negative or are not yet mature, perhaps reflecting the complexity of the deposition process and microenvironmental responses to it in different organs even in the same patient.
Anti–serum amyloid P component
Human serum amyloid P component (SAP) is an abundant plasma protein that binds to amyloid fibril regardless of its precursor protein, being an invariably present component of amyloid deposits. Given its function in stabilizing and shielding fibrils from degradation, SAP was postulated to be an ideal therapeutic target for antiamyloid therapy (109). Dezamizumab, a fully humanized IgG1 anti-SAP MoAb, was shown to successfully bind and eliminate amyloid-bound SAP after serum SAP was cleared via Miridesap, a small-molecule drug. Excitingly, in a phase 1 study, miridesap followed by dezamizumab resulted in significant reductions in hepatic amyloid load and liver responses in patients with amyloidosis, including AL amyloidosis (110). Based on these results, a phase 2 trial was initiated, but was then closed because of the emergence of previously unknown side effects and changes in the risk/benefit ratio.
Doxycycline
Preclinical studies in vitro and in animal models have shown antiamyloidogenic activity of doxycycline across the spectrum of amyloidoses with most data in transthyretin amyloidosis (111). The molecular mechanisms underlying the antifibrillary activity of doxycycline remain largely obscure, but inhibition of matrix metalloproteinase has been suggested as a potential mechanism (112). Retrospective studies in AL amyloidosis patients treated with chemotherapy or ASCT showed improved OS in patients receiving doxycycline, prompting interest in prospective studies focused on cardiac protection. The DUAL (Doxycycline to Upgrade response in AL) study is a phase 2 prospective clinical trial that evaluated the safety and activity of doxycycline for 1 year in combination with standard of care chemotherapy in newly diagnosed AL amyloidosis (113). The 1-year OS was 80% in the study population, with 60% of patients undergoing ASCT with 0% 100-day mortality. Importantly, organ responses were observed in 36% of patients in the absence of significant adverse events.
Birtamimab (NEOD001)
Birtamimab is a humanized derivative of the murine MoAb 2A4 that recognizes a cryptic epitope on AL amyloid fibril thought to be exposed selectively by misfolded and aggregated FLC. In vitro, 2A4 specifically binds to both soluble and insoluble FLC aggregates and induces the clearance of insoluble aggregates by macrophage phagocytosis mediated by the Fc receptor (114). NEOD001 showed promising activity in a phase 1/2 trial in 69 relapsed AL amyloidosis patients with high organ response rates. However, subsequent placebo-controlled studies failed to show clinical benefit, and a randomized, phase 3 study of NEOD001 vs placebo in combination with standard of care chemotherapy was halted because of futility at interim analysis (24). As a post hoc analysis showed a significant improvement in all-cause mortality in patients with stage IV AL amyloidosis, a randomized clinical trial of birtamimab in combination with standard of care chemotherapy in this high-risk patient population is currently ongoing (NCT04973137).
CAEL-101
11-1F4 (CAEL-101) is an amyloid fibril-reactive murine MoAb that binds directly to an epitope present only on misfolded, human light-chain amyloid fibrils, but not properly folded FLC. CAEL-101 binds to AL amyloidosis fibrils and enhances their Fcγ receptor-mediated opsonization and proteolysis. A phase 1a/b study in relapsed or refractory AL amyloidosis showed CAEL-101 to be safe and effective with 67% renal and/or cardiac response and improvement in mean global longitudinal strain observed in 9 of 10 patients (23). A phase 2 trial with 13 patients demonstrated that CAEL-101 administered at doses of up to 1,000 mg/m2, in combination with standard of care CyBorD, was well-tolerated, setting the stage for 2 phase 3 studies in patients with cardiac stage 3A and 3B AL amyloidosis that are currently recruiting (115).
Promising Investigational Agents
Having discussed agents most advanced in clinical development, we now focus on the most promising investigational agents in AL amyloidosis. All of these drugs are FDA approved or in advanced clinical development in MM (Central Illustration).
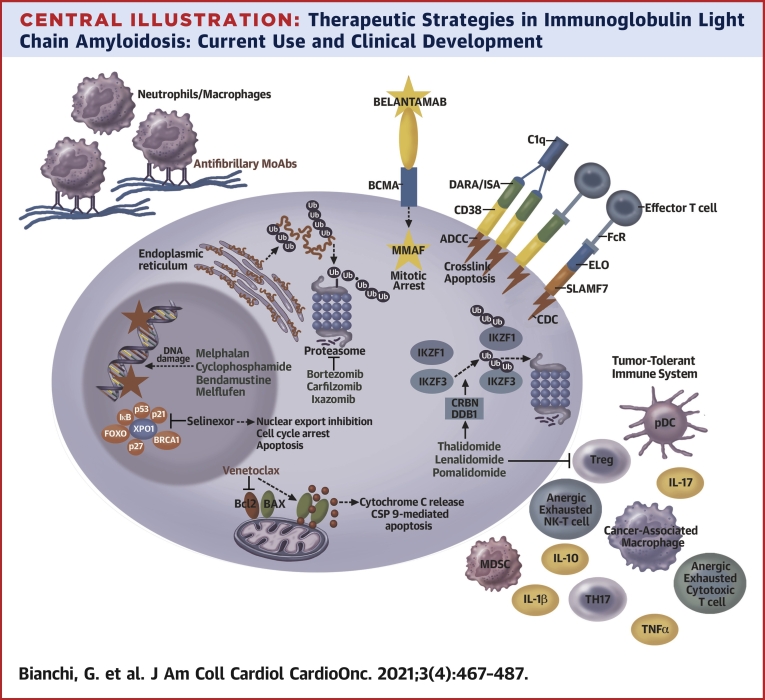
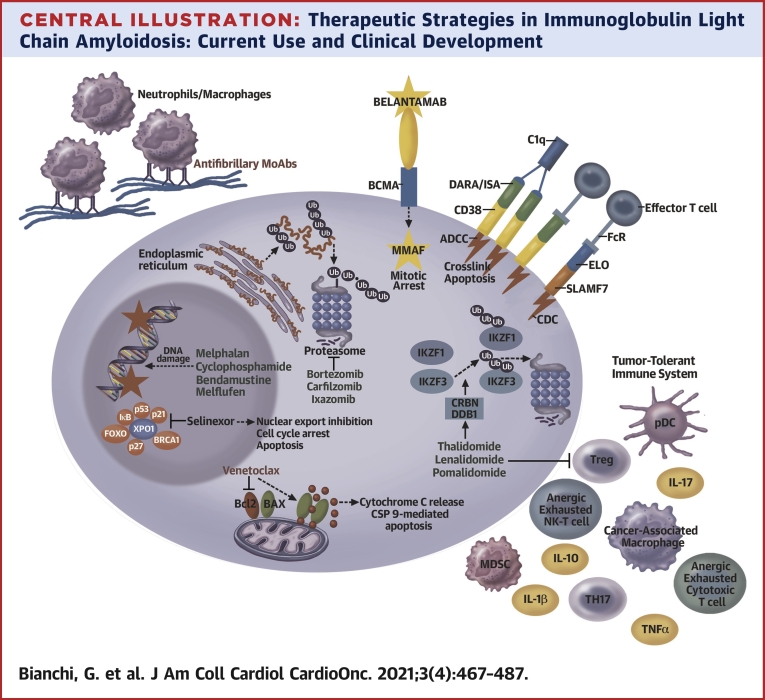
Central Illustration.
Therapeutic Strategies in Immunoglobulin Light Chain Amyloidosis: Current Use and Clinical Development
The Figure outlines the target and/or mechanisms of action of the most frequently used drugs in immunoglobulin light chain amyloidosis and agents in advanced clinical development. Proteasome inhibitors block the function of the proteasome, inducing polyubiquitinated protein accumulation. IMiDs induce Ikaros and Aiolos (IKZF1 and IKZF2, respectively) proteasome-mediated degradation and enhance T-cell and NK-T-cell function. MoAbs DARA and ISA cause complement-dependent cytotoxicity (CDC), antibody-dependent cell cytotoxicity (ADCC), and direct cytotoxicity from crosslinking. ELO triggers ADCC, and the antibody drug conjugated (ADC) targeting BCMA, belantamab mafodotin, induces DNA damage via MMAF. Alkylating agents similarly induce DNA damage and selinexor blocks XPO1. Venetoclax binds BCL2, releasing BAX and triggering cytochrome C release and caspase 9-mediated apoptosis. Antifibrillary antibodies facilitating macrophage-mediated amyloid reabsorption are depicted in the top left corner. U.S. Food and Drug Administration–approved drugs in MM therapy are green, whereas investigational agents are in red. DARA = daratumumab; ELO = elotuzumab; IL = interleukin; ISA = isatuximab; MMAF = monomethyl auristatin F; TNFα = tumor necrosis factor alpha; MDSC = myeloid derived suppressor cell; pDC = plasmacytoid dendritic cell; TH17 = T helper 17; Treg = regulatory T cells; Ub = ubiquitin; XPO1 = exportin 1.
Venetoclax
BCL2 is a mitochondrial, antiapoptotic protein whose overexpression/overactivity has been shown to drive several hematologic malignancies, particularly B lymphoproliferative disorders (116). Although MM cells are generally dependent on a distinct antiapoptotic BCL2 family member, MCL1, t(11;14) is a biomarker for a BCL2 high state, translating into increased BCL2 dependency and predicting venetoclax efficacy (117). Consistent with MM data, t(11;14) AL amyloidosis patients showed high sensitivity to venetoclax with rapid and deep responses in heavily pretreated patients (118,119). Although clinical studies of venetoclax in AL amyloidosis were designed, the FDA put a hold on the BELLINI study, a phase 3 clinical trial comparing bortezomib/dexamethasone plus/minus venetoclax, because of excess mortality from infectious complications noted in the experimental arm (120). Because clinical benefit outweighed the risk for t(11;14) patients, the hold was lifted with a plan for increased surveillance of patients and prophylactic antimicrobials. Considering that t(11;14) is the most common cytogenetic abnormality in AL amyloidosis and portends poor prognosis, much-awaited clinical studies of these agents are finally at the horizon.
BCMA-targeting immuno and cellular therapies
The BCMA-targeting antibody drug conjugate belantamab mafodotin has shown activity in heavily pretreated MM and is FDA approved for triple class (PI, IMiD, and CD38-targeting MoAbs) relapsed/refractory MM patients. Belantamab mafodotin is an afucosylated IgG1 MoAb conjugated to the antitubular agent monomethyl auristin-F (MMAF). It targets BCMA, a surface receptor for BAFF/APRIL that is universally and rather specifically expressed in PCs and triggers anti-MM activity via direct cytotoxicity of intracellularly released MMAF, inhibition of pro-survival BAFF/APRIL signaling, and ADCC via enhanced Fc/FcγR binding (121). Belantamab mafodotin induces deep and sustained hematologic remissions in heavily pretreated MM patients with dose-limiting toxicity being reversible keratopathy and is FDA approved in relapsed and/or refractory MM. Clinical trials are eagerly awaited in AL amyloidosis (122). Trials exploring safety and efficacy of bispecific T-cell engagers (BiTEs) and chimeric T-cell receptor (CAR) T-cell therapy targeting BCMA in AL amyloidosis are being considered, carefully pondering risk/benefit ratio.
Melphalan flufenamide
Melphalan flufenamide (melflufen) is a peptide-conjugated, melphalan derivative that has shown encouraging activity in MM, leading to its FDA approval in combination with dexamethasone in triple class relapsed/refractory MM. It leverages the increased expression of aminopeptidases in cancer cells, including MM, restricting the release of active alkylator payload in aminopeptidase-rich cancer cells and thereby reducing off-target and increasing on-target activity. The FDA put a partial clinical hold on all melflufen studies, including a phase 1/2 trial examining melflufen/dexamethasone in relapsed and/or refractory AL amyloidosis, in light of decreased OS in patients receiving melflufen/dexamethasone compared with pomalidomide/dexamethasone in the context of the OCEAN phase III noninferiority study (123).
Selinexor
Selinexor is a first-in-class inhibitor of the nuclear export protein, Exportin1 (XPO1). XPO1 is responsible for regulating export from the nucleus to the cytoplasm of cargo proteins, including oncosuppressors p53, RB1, and p27; cell cycle regulators; and antiapoptotic proteins. By blocking their nuclear export, selinexor inhibits the function of these factors, resulting in potent antitumor activity across a wide range of hematologic and solid malignancies. Selinexor showed activity in heavily pretreated MM patients and is FDA approved in relapsed and/or refractory MM. Selinexor activity comes at the expense of tolerability with cytopenia, nausea, diarrhea, and electrolyte abnormalities being frequently observed and necessitating careful ancillary care and patient selection. Case reports are emerging of activity of selinexor in AL amyloidosis, and clinical trials are expected to commence soon in this patient population (124).
Discussion
There has been tremendous progress over the last 3 decades in the treatment of AL amyloidosis, largely because of improved ancillary care, more effective PC-directed therapy, and improved patient stratification for ASCT. It is therefore an exciting time for the AL amyloidosis community. However, there are still obstacles to overcome in the care of AL amyloidosis patients. First, diagnostic delay leading to advanced cardiac involvement remains a major hurdle in the care of AL amyloidosis patients, negatively affecting outcome and driving early mortality. As a medical community, it is therefore of critical importance to raise awareness about AL amyloidosis, a great imitator and often overlooked systemic disease, and to invest in developing/validating tests and biomarkers for early diagnosis. Second, the lack of a deep understanding of AL amyloidosis biology and limited basic research efforts in this arena hampers the development of therapies targeting the intrinsic vulnerabilities of this disease. Mechanisms to better support scientists specifically studying AL amyloidosis biology are therefore welcome. Finally, AL amyloidosis patients and their families still face a significant financial burden during their treatment, largely caused by the off-label use of most therapies and frequent hospital admissions for disease-related complications. A stronger commitment from insurance companies, pharmaceutical companies, and regulatory bodies to support our patients as they embark on a long therapeutic journey is of outmost importance. We look forward to a time, not too far in the future, when AL amyloidosis patients will be rapidly diagnosed and effectively treated with innovative, molecularly-targeted drugs specifically tackling the Achilles’ heel of this devastating illness.
Funding Support and Author Disclosures
This work was supported in part by National Institutes of Health/National Institute of Aging grant R21-AG070502-01 (to Dr Comenzo). Dr Bianchi has participated in advisory boards (with personal payment) for Pfizer and Karyopharm. Dr Comenzo has received steering committee fees from Janssen Biotech; has received advisory board fees from Karyopharm Therapeutics; has received fees for serving on a data and safety monitoring committee from Sanofi; and holds patent WO2016187546A1 on anti-CD38 antibodies for treatment of light-chain amyloidosis and other CD38-positive hematologic cancers. Dr Zhang has reported that he has no relationships relevant to the contents of this paper to disclose.
Acknowledgments
Dr Bianchi is thankful to the Demarest Lloyd Jr Foundation and the Appleby Cardiac Amyloidosis Fund for their support of the Amyloidosis Program. Drs Zhang and Comenzo would like to thank the Amyloidosis and Myeloma Research Fund, the Sidewater Family Fund, the Amyloidosis Foundation, David and Barbara Levine (in memoriam), and the Demarest Lloyd Jr Foundation.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Merlini G., Dispenzieri A., Sanchorawala V. Systemic immunoglobulin light chain amyloidosis. Nat Rev Dis Primers. 2018;4:38. doi: 10.1038/s41572-018-0034-3. [DOI] [PubMed] [Google Scholar]
- 2.Bianchi G., Kumar S. Systemic amyloidosis due to clonal plasma cell diseases. Hematol Oncol Clin North Am. 2020;34:1009–1026. doi: 10.1016/j.hoc.2020.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Comenzo R.L., Zhang Y., Martinez C., Osman K., Herrera G.A. The tropism of organ involvement in primary systemic amyloidosis: contributions of Ig V(L) germ line gene use and clonal plasma cell burden. Blood. 2001;98:714–720. doi: 10.1182/blood.v98.3.714. [DOI] [PubMed] [Google Scholar]
- 4.Blancas-Mejia L.M., Tischer A., Thompson J.R. Kinetic control in protein folding for light chain amyloidosis and the differential effects of somatic mutations. J Mol Biol. 2014;426:347–361. doi: 10.1016/j.jmb.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wyatt A.R., Yerbury J.J., Dabbs R.A., Wilson M.R. Roles of extracellular chaperones in amyloidosis. J Mol Biol. 2012;421:499–516. doi: 10.1016/j.jmb.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Abraham R.S., Ballman K.V., Dispenzieri A. Functional gene expression analysis of clonal plasma cells identifies a unique molecular profile for light chain amyloidosis. Blood. 2005;105:794–803. doi: 10.1182/blood-2004-04-1424. [DOI] [PubMed] [Google Scholar]
- 7.Quock T.P., Yan T., Chang E., Guthrie S., Broder M.S. Epidemiology of AL amyloidosis: a real-world study using US claims data. Blood Adv. 2018;2:1046–1053. doi: 10.1182/bloodadvances.2018016402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kyle R.A., Larson D.R., Kurtin P.J. Incidence of AL amyloidosis in Olmsted County, Minnesota, 1990 through 2015. Mayo Clin Proc. 2019;94:465–471. doi: 10.1016/j.mayocp.2018.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kyle R.A., Larson D.R., Therneau T.M. Long-term follow-up of monoclonal gammopathy of undetermined significance. N Engl J Med. 2018;378:241–249. doi: 10.1056/NEJMoa1709974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landgren O., Graubard B.I., Katzmann J.A. Racial disparities in the prevalence of monoclonal gammopathies: a population-based study of 12,482 persons from the National Health and Nutritional Examination Survey. Leukemia. 2014;28:1537–1542. doi: 10.1038/leu.2014.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin E.B., Williams A.D., Heidel R.E. A functional assay to identify amyloidogenic light chains. Amyloid. 2018;25:93–100. doi: 10.1080/13506129.2018.1456425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou P., Kugelmass A., Toskic D. Seeking AL amyloidosis very early: the SAVE Trial — identifying clonal lambda light chain genes in patients with MGUS or smoldering multiple myeloma. Blood. 2018;132 1903-1903. [Google Scholar]
- 13.Rajkumar S.V., Dimopoulos M.A., Palumbo A. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–e548. doi: 10.1016/S1470-2045(14)70442-5. [DOI] [PubMed] [Google Scholar]
- 14.Geller H.I., Singh A., Mirto T.M. Prevalence of monoclonal gammopathy in wild-type transthyretin amyloidosis. Mayo Clin Proc. 2017;92:1800–1805. doi: 10.1016/j.mayocp.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 15.Dispenzieri A., Gertz M.A., Kyle R.A. Serum cardiac troponins and N-terminal pro-brain natriuretic peptide: a staging system for primary systemic amyloidosis. J Clin Oncol. 2004;22:3751–3757. doi: 10.1200/JCO.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 16.Wechalekar A.D., Schonland S.O., Kastritis E. A European collaborative study of treatment outcomes in 346 patients with cardiac stage III AL amyloidosis. Blood. 2013;121:3420–3427. doi: 10.1182/blood-2012-12-473066. [DOI] [PubMed] [Google Scholar]
- 17.Kumar S., Dispenzieri A., Lacy M.Q. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol. 2012;30:989–995. doi: 10.1200/JCO.2011.38.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palladini G., Hegenbart U., Milani P. A staging system for renal outcome and early markers of renal response to chemotherapy in AL amyloidosis. Blood. 2014;124:2325–2332. doi: 10.1182/blood-2014-04-570010. [DOI] [PubMed] [Google Scholar]
- 19.Kourelis T.V., Kumar S.K., Gertz M.A. Coexistent multiple myeloma or increased bone marrow plasma cells define equally high-risk populations in patients with immunoglobulin light chain amyloidosis. J Clin Oncol. 2013;31:4319–4324. doi: 10.1200/JCO.2013.50.8499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bochtler T., Hegenbart U., Kunz C. Translocation t(11;14) is associated with adverse outcome in patients with newly diagnosed AL amyloidosis when treated with bortezomib-based regimens. J Clin Oncol. 2015;33:1371–1378. doi: 10.1200/JCO.2014.57.4947. [DOI] [PubMed] [Google Scholar]
- 21.Perrot A., Lauwers-Cances V., Corre J. Minimal residual disease negativity using deep sequencing is a major prognostic factor in multiple myeloma. Blood. 2018;132:2456–2464. doi: 10.1182/blood-2018-06-858613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van G., II, van Rijswijk M.H., Bijzet J., Vellenga E., Hazenberg B.P. Histological regression of amyloid in AL amyloidosis is exclusively seen after normalization of serum free light chain. Haematologica. 2009;94:1094–1100. doi: 10.3324/haematol.2008.004119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edwards C.V., Bhutani D., Mapara M. One year follow up analysis of the phase 1a/b study of chimeric fibril-reactive monoclonal antibody 11-1F4 in patients with AL amyloidosis. Amyloid. 2019;26:115–116. doi: 10.1080/13506129.2019.1584892. [DOI] [PubMed] [Google Scholar]
- 24.Gertz M.A., Cohen A.D., Comenzo R.L. Results of the phase 3 VITAL study of NEOD001 (Birtamimab) plus standard of care in patients with light chain (AL) amyloidosis suggest survival benefit for Mayo stage IV patients. Blood. 2019;134:3166. [Google Scholar]
- 25.Palladini G., Paiva B., Wechalekar A. Minimal residual disease negativity by next-generation flow cytometry is associated with improved organ response in AL amyloidosis. Blood Cancer J. 2021;11:34. doi: 10.1038/s41408-021-00428-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Comenzo R.L., Kastritis E., Palladini G. Reduction in absolute involved free light chain and difference between involved and uninvolved free light chain is associated with prolonged major organ deterioration progression-free survival in patients with newly diagnosed al amyloidosis receiving bortezomib, cyclophosphamide, and dexamethasone with or without daratumumab: results from Andromeda (abstr) Blood. 2020;136(Suppl 1):48–50. [Google Scholar]
- 27.Muchtar E., Gertz M.A., Lacy M.Q. Refining amyloid complete hematological response: quantitative serum free light chains superior to ratio. Am J Hematol. 2020;95:1280–1287. doi: 10.1002/ajh.25940. [DOI] [PubMed] [Google Scholar]
- 28.Kastritis E., Kostopoulos I.V., Theodorakakou F. Next generation flow cytometry for MRD detection in patients with AL amyloidosis. Amyloid. 2021;28:19–23. doi: 10.1080/13506129.2020.1802713. [DOI] [PubMed] [Google Scholar]
- 29.Staron A., Burks E.J., Lee J.C., Sarosiek S., Sloan J.M., Sanchorawala V. Assessment of minimal residual disease using multiparametric flow cytometry in patients with AL amyloidosis. Blood Adv. 2020;4:880–884. doi: 10.1182/bloodadvances.2019001331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palladini G., Milani P., Foli A. Presentation and outcome with second-line treatment in AL amyloidosis previously sensitive to nontransplant therapies. Blood. 2018;131:525–532. doi: 10.1182/blood-2017-04-780544. [DOI] [PubMed] [Google Scholar]
- 31.Palladini G., Dispenzieri A., Gertz M.A. New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: impact on survival outcomes. J Clin Oncol. 2012;30:4541–4549. doi: 10.1200/JCO.2011.37.7614. [DOI] [PubMed] [Google Scholar]
- 32.Venner C.P., Lane T., Foard D. Cyclophosphamide, bortezomib, and dexamethasone therapy in AL amyloidosis is associated with high clonal response rates and prolonged progression-free survival. Blood. 2012;119:4387–4390. doi: 10.1182/blood-2011-10-388462. [DOI] [PubMed] [Google Scholar]
- 33.Comenzo R.L., Gertz M.A. Autologous stem cell transplantation for primary systemic amyloidosis. Blood. 2002;99:4276–4282. doi: 10.1182/blood.v99.12.4276. [DOI] [PubMed] [Google Scholar]
- 34.Lu X., Ding Z.C., Cao Y. Alkylating agent melphalan augments the efficacy of adoptive immunotherapy using tumor-specific CD4+ T cells. J Immunol. 2015;194:2011–2021. doi: 10.4049/jimmunol.1401894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kyle R.A., Greipp P.R. Primary systemic amyloidosis: comparison of melphalan and prednisone versus placebo. Blood. 1978;52:818–827. [PubMed] [Google Scholar]
- 36.Kyle R.A., Gertz M.A., Greipp P.R. A trial of three regimens for primary amyloidosis: colchicine alone, melphalan and prednisone, and melphalan, prednisone, and colchicine. N Engl J Med. 1997;336:1202–1207. doi: 10.1056/NEJM199704243361702. [DOI] [PubMed] [Google Scholar]
- 37.Sanchorawala V., Seldin D.C., Berk J.L., Sloan J.M., Doros G., Skinner M. Oral cyclic melphalan and dexamethasone for patients with AL amyloidosis. Clin Lymphoma Myeloma Leuk. 2010;10:469–472. doi: 10.3816/CLML.2010.n.081. [DOI] [PubMed] [Google Scholar]
- 38.Kastritis E., Leleu X., Arnulf B. Bortezomib, melphalan, and dexamethasone for light-chain amyloidosis. J Clin Oncol. 2020;38:3252–3260. doi: 10.1200/JCO.20.01285. [DOI] [PubMed] [Google Scholar]
- 39.Hegenbart U., Bochtler T., Benner A. Lenalidomide/melphalan/dexamethasone in newly diagnosed patients with immunoglobulin light chain amyloidosis: results of a prospective phase 2 study with long-term follow up. Haematologica. 2017;102:1424–1431. doi: 10.3324/haematol.2016.163246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Comenzo R.L., Vosburgh E., Falk R.H. Dose-intensive melphalan with blood stem-cell support for the treatment of AL (amyloid light-chain) amyloidosis: survival and responses in 25 patients. Blood. 1998;91:3662–3670. [PubMed] [Google Scholar]
- 41.Jaccard A., Moreau P., Leblond V. High-dose melphalan versus melphalan plus dexamethasone for AL amyloidosis. N Engl J Med. 2007;357:1083–1093. doi: 10.1056/NEJMoa070484. [DOI] [PubMed] [Google Scholar]
- 42.D'Souza A., Dispenzieri A., Wirk B. Improved outcomes after autologous hematopoietic cell transplantation for light chain amyloidosis: a Center for International Blood and Marrow Transplant research study. J Clin Oncol. 2015;33:3741–3749. doi: 10.1200/JCO.2015.62.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Girnius S., Seldin D.C., Meier-Ewert H.K. Safety and efficacy of high-dose melphalan and auto-SCT in patients with AL amyloidosis and cardiac involvement. Bone Marrow Transplant. 2014;49:434–439. doi: 10.1038/bmt.2013.192. [DOI] [PubMed] [Google Scholar]
- 44.Basset M., Milani P., Nuvolone M. Sequential response-driven bortezomib-based therapy followed by autologous stem cell transplant in AL amyloidosis. Blood Adv. 2020;4:4175–4179. doi: 10.1182/bloodadvances.2020002219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Landau H., Hassoun H., Rosenzweig M.A. Bortezomib and dexamethasone consolidation following risk-adapted melphalan and stem cell transplantation for patients with newly diagnosed light-chain amyloidosis. Leukemia. 2013;27:823–828. doi: 10.1038/leu.2012.274. [DOI] [PubMed] [Google Scholar]
- 46.Bochtler T., Hegenbart U., Kunz C. Prognostic impact of cytogenetic aberrations in AL amyloidosis patients after high-dose melphalan: a long-term follow-up study. Blood. 2016;128:594–602. doi: 10.1182/blood-2015-10-676361. [DOI] [PubMed] [Google Scholar]
- 47.Levesque J.P., Hendy J., Takamatsu Y., Simmons P.J., Bendall L.J. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J Clin Invest. 2003;111:187–196. doi: 10.1172/JCI15994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yeh J.C., Shank B.R., Milton D.R., Qazilbash M.H. Adverse prognostic factors for morbidity and mortality during peripheral blood stem cell mobilization in patients with light chain amyloidosis. Biol Blood Marrow Transplant. 2018;24:815–819. doi: 10.1016/j.bbmt.2017.11.040. [DOI] [PubMed] [Google Scholar]
- 49.Palladini G., Sachchithanantham S., Milani P. A European collaborative study of cyclophosphamide, bortezomib, and dexamethasone in upfront treatment of systemic AL amyloidosis. Blood. 2015;126:612–615. doi: 10.1182/blood-2015-01-620302. [DOI] [PubMed] [Google Scholar]
- 50.Venner C.P., Gillmore J.D., Sachchithanantham S. A matched comparison of cyclophosphamide, bortezomib and dexamethasone (CVD) versus risk-adapted cyclophosphamide, thalidomide and dexamethasone (CTD) in AL amyloidosis. Leukemia. 2014;28:2304–2310. doi: 10.1038/leu.2014.218. [DOI] [PubMed] [Google Scholar]
- 51.Kumar S.K., Hayman S.R., Buadi F.K. Lenalidomide, cyclophosphamide, and dexamethasone (CRd) for light-chain amyloidosis: long-term results from a phase 2 trial. Blood. 2012;119:4860–4867. doi: 10.1182/blood-2012-01-407791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Milani P., Schonland S., Merlini G. Treatment of AL amyloidosis with bendamustine: a study of 122 patients. Blood. 2018;132:1988–1991. doi: 10.1182/blood-2018-04-845396. [DOI] [PubMed] [Google Scholar]
- 53.Lentzsch S., Lagos G.G., Comenzo R.L. Bendamustine with dexamethasone in relapsed/refractory systemic light-chain amyloidosis: results of a phase ii study. J Clin Oncol. 2020;38:1455–1462. doi: 10.1200/JCO.19.01721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kisselev A.F., Goldberg A.L. Proteasome inhibitors: from research tools to drug candidates. Chem Biol. 2001;8:739–758. doi: 10.1016/s1074-5521(01)00056-4. [DOI] [PubMed] [Google Scholar]
- 55.Bianchi G., Anderson K.C. Contribution of inhibition of protein catabolism in myeloma. Cancer J. 2019;25:11–18. doi: 10.1097/PPO.0000000000000349. [DOI] [PubMed] [Google Scholar]
- 56.Bianchi G., Ghobrial I.M. Molecular mechanisms of effectiveness of novel therapies in multiple myeloma. Leuk Lymphoma. 2013;54:229–241. doi: 10.3109/10428194.2012.706287. [DOI] [PubMed] [Google Scholar]
- 57.Hideshima T., Ikeda H., Chauhan D. Bortezomib induces canonical nuclear factor-kappaB activation in multiple myeloma cells. Blood. 2009;114:1046–1052. doi: 10.1182/blood-2009-01-199604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Obeng E.A., Carlson L.M., Gutman D.M., Harrington W.J., Jr., Lee K.P., Boise L.H. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood. 2006;107:4907–4916. doi: 10.1182/blood-2005-08-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bianchi G., Oliva L., Cascio P. The proteasome load versus capacity balance determines apoptotic sensitivity of multiple myeloma cells to proteasome inhibition. Blood. 2009;113:3040–3049. doi: 10.1182/blood-2008-08-172734. [DOI] [PubMed] [Google Scholar]
- 60.Oliva L., Orfanelli U., Resnati M. The amyloidogenic light chain is a stressor that sensitizes plasma cells to proteasome inhibitor toxicity. Blood. 2017;129:2132–2142. doi: 10.1182/blood-2016-08-730978. [DOI] [PubMed] [Google Scholar]
- 61.Cenci S., Oliva L., Cerruti F. Pivotal advance: protein synthesis modulates responsiveness of differentiating and malignant plasma cells to proteasome inhibitors. J Leukoc Biol. 2012;92:921–931. doi: 10.1189/jlb.1011497. [DOI] [PubMed] [Google Scholar]
- 62.Richardson P.G., Sonneveld P., Schuster M.W. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 63.Richardson P.G., Briemberg H., Jagannath S. Frequency, characteristics, and reversibility of peripheral neuropathy during treatment of advanced multiple myeloma with bortezomib. J Clin Oncol. 2006;24:3113–3120. doi: 10.1200/JCO.2005.04.7779. [DOI] [PubMed] [Google Scholar]
- 64.Kastritis E., Gavriatopoulou M., Roussou M. Addition of cyclophosphamide and higher doses of dexamethasone do not improve outcomes of patients with AL amyloidosis treated with bortezomib. Blood. Cancer J. 2017;7 doi: 10.1038/bcj.2017.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou P., Hoffman J., Landau H., Hassoun H., Iyer L., Comenzo R.L. Clonal plasma cell pathophysiology and clinical features of disease are linked to clonal plasma cell expression of cyclin D1 in systemic light-chain amyloidosis. Clin Lymphoma Myeloma Leuk. 2012;12:49–58. doi: 10.1016/j.clml.2011.09.217. [DOI] [PubMed] [Google Scholar]
- 66.Kastritis E., Dialoupi I., Gavriatopoulou M. Primary treatment of light-chain amyloidosis with bortezomib, lenalidomide, and dexamethasone. Blood Adv. 2019;3:3002–3009. doi: 10.1182/bloodadvances.2019000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dimopoulos M.A., Moreau P., Palumbo A. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17:27–38. doi: 10.1016/S1470-2045(15)00464-7. [DOI] [PubMed] [Google Scholar]
- 68.Manwani R., Mahmood S., Sachchithanantham S. Carfilzomib is an effective upfront treatment in AL amyloidosis patients with peripheral and autonomic neuropathy. Br J Haematol. 2019;187:638–641. doi: 10.1111/bjh.16122. [DOI] [PubMed] [Google Scholar]
- 69.Sanchorawala V., Palladini G., Kukreti V. A phase 1/2 study of the oral proteasome inhibitor ixazomib in relapsed or refractory AL amyloidosis. Blood. 2017;130:597–605. doi: 10.1182/blood-2017-03-771220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cohen O.C., Sharpley F., Gillmore J.D. Use of ixazomib, lenalidomide and dexamethasone in patients with relapsed amyloid light-chain amyloidosis. Br J Haematol. 2020;189:643–649. doi: 10.1111/bjh.16401. [DOI] [PubMed] [Google Scholar]
- 71.Merlini G., Faller D.V., Liu G. Primary results from the Phase 3 Tourmaline-AL1 Trial of ixazomib-dexamethasone versus physician's choice of therapy in patients (pts) with relapsed/refractory primary systemic al amyloidosis (RRAL) Blood. 2019;134:139. [Google Scholar]
- 72.Corral L.G., Haslett P.A., Muller G.W. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-alpha. J Immunol. 1999;163:380–386. [PubMed] [Google Scholar]
- 73.Ito T.A.H., Suzuki T., Ogura T., Hotta K., Imamura Y., Yamaguchi Y., Handa H. Identification of a primary target of thalidomide teratogenicity. Science. 2010;327:1345–1350. doi: 10.1126/science.1177319. [DOI] [PubMed] [Google Scholar]
- 74.Lu G., Middleton R.E., Sun H. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science. 2014;343:305–309. doi: 10.1126/science.1244917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kronke J., Udeshi N.D., Narla A. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science. 2014;343:301–305. doi: 10.1126/science.1244851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Palumbo A., Bringhen S., Kumar S.K. Second primary malignancies with lenalidomide therapy for newly diagnosed myeloma: a meta-analysis of individual patient data. Lancet Oncol. 2014;15:333–342. doi: 10.1016/S1470-2045(13)70609-0. [DOI] [PubMed] [Google Scholar]
- 77.Palladini G., Perfetti V., Perlini S. The combination of thalidomide and intermediate-dose dexamethasone is an effective but toxic treatment for patients with primary amyloidosis (AL) Blood. 2005;105:2949–2951. doi: 10.1182/blood-2004-08-3231. [DOI] [PubMed] [Google Scholar]
- 78.Cohen A.D., Zhou P., Chou J. Risk-adapted autologous stem cell transplantation with adjuvant dexamethasone +/- thalidomide for systemic light-chain amyloidosis: results of a phase II trial. Br J Haematol. 2007;139:224–233. doi: 10.1111/j.1365-2141.2007.06783.x. [DOI] [PubMed] [Google Scholar]
- 79.Dispenzieri A., Lacy M.Q., Zeldenrust S.R. The activity of lenalidomide with or without dexamethasone in patients with primary systemic amyloidosis. Blood. 2007;109:465–470. doi: 10.1182/blood-2006-07-032987. [DOI] [PubMed] [Google Scholar]
- 80.Sanchorawala V., Wright D.G., Rosenzweig M. Lenalidomide and dexamethasone in the treatment of AL amyloidosis: results of a phase 2 trial. Blood. 2007;109:492–496. doi: 10.1182/blood-2006-07-030544. [DOI] [PubMed] [Google Scholar]
- 81.Palladini G., Milani P., Foli A. A phase 2 trial of pomalidomide and dexamethasone rescue treatment in patients with AL amyloidosis. Blood. 2017;129:2120–2123. doi: 10.1182/blood-2016-12-756528. [DOI] [PubMed] [Google Scholar]
- 82.Specter R., Sanchorawala V., Seldin D.C. Kidney dysfunction during lenalidomide treatment for AL amyloidosis. Nephrol Dial Transplant. 2011;26:881–886. doi: 10.1093/ndt/gfq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moreau P., Jaccard A., Benboubker L. Lenalidomide in combination with melphalan and dexamethasone in patients with newly diagnosed AL amyloidosis: a multicenter phase 1/2 dose-escalation study. Blood. 2010;116:4777–4782. doi: 10.1182/blood-2010-07-294405. [DOI] [PubMed] [Google Scholar]
- 84.Lacy M.Q., Hayman S.R., Gertz M.A. Pomalidomide (CC4047) plus low dose dexamethasone (Pom/dex) is active and well tolerated in lenalidomide refractory multiple myeloma (MM) Leukemia. 2010;24:1934–1939. doi: 10.1038/leu.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Leleu X., Attal M., Arnulf B. Pomalidomide plus low-dose dexamethasone is active and well tolerated in bortezomib and lenalidomide-refractory multiple myeloma: Intergroupe Francophone du Myelome 2009-02. Blood. 2013;121:1968–1975. doi: 10.1182/blood-2012-09-452375. [DOI] [PubMed] [Google Scholar]
- 86.Dispenzieri A., Buadi F., Laumann K. Activity of pomalidomide in patients with immunoglobulin light-chain amyloidosis. Blood. 2012;119:5397–5404. doi: 10.1182/blood-2012-02-413161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Milani P., Sharpley F., Schonland S.O. Pomalidomide and dexamethasone grant rapid haematologic responses in patients with relapsed and refractory AL amyloidosis: a European retrospective series of 153 patients. Amyloid. 2020;27:231–236. doi: 10.1080/13506129.2020.1767566. [DOI] [PubMed] [Google Scholar]
- 88.Sanchorawala V., Shelton A.C., Lo S., Varga C., Sloan J.M., Seldin D.C. Pomalidomide and dexamethasone in the treatment of AL amyloidosis: results of a phase 1 and 2 trial. Blood. 2016;128:1059–1062. doi: 10.1182/blood-2016-04-710822. [DOI] [PubMed] [Google Scholar]
- 89.Dispenzieri A., Dingli D., Kumar S.K. Discordance between serum cardiac biomarker and immunoglobulin-free light-chain response in patients with immunoglobulin light-chain amyloidosis treated with immune modulatory drugs. Am J Hematol. 2010;85:757–759. doi: 10.1002/ajh.21822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gorgun G., Calabrese E., Soydan E. Immunomodulatory effects of lenalidomide and pomalidomide on interaction of tumor and bone marrow accessory cells in multiple myeloma. Blood. 2010;116:3227–3237. doi: 10.1182/blood-2010-04-279893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chari A., Suvannasankha A., Fay J.W. Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood. 2017;130:974–981. doi: 10.1182/blood-2017-05-785246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dimopoulos M.A., Dytfeld D., Grosicki S. Elotuzumab plus pomalidomide and dexamethasone for multiple myeloma. N Engl J Med. 2018;379:1811–1822. doi: 10.1056/NEJMoa1805762. [DOI] [PubMed] [Google Scholar]
- 93.Palumbo A., Chanan-Khan A., Weisel K. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:754–766. doi: 10.1056/NEJMoa1606038. [DOI] [PubMed] [Google Scholar]
- 94.Dimopoulos M.A., Oriol A., Nahi H. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:1319–1331. doi: 10.1056/NEJMoa1607751. [DOI] [PubMed] [Google Scholar]
- 95.Lonial S., Dimopoulos M., Palumbo A. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med. 2015;373:621–631. doi: 10.1056/NEJMoa1505654. [DOI] [PubMed] [Google Scholar]
- 96.de Weers M., Tai Y.T., van der Veer M.S. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186:1840–1848. doi: 10.4049/jimmunol.1003032. [DOI] [PubMed] [Google Scholar]
- 97.van de Donk N.W., Janmaat M.L., Mutis T. Monoclonal antibodies targeting CD38 in hematological malignancies and beyond. Immunol Rev. 2016;270:95–112. doi: 10.1111/imr.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mateos M.V., Nahi H., Legiec W. Subcutaneous versus intravenous daratumumab in patients with relapsed or refractory multiple myeloma (COLUMBA): a multicentre, open-label, non-inferiority, randomised, phase 3 trial. Lancet Haematol. 2020;7:e370–e380. doi: 10.1016/S2352-3026(20)30070-3. [DOI] [PubMed] [Google Scholar]
- 99.Lokhorst H.M., Plesner T., Laubach J.P. Targeting CD38 with daratumumab monotherapy in multiple myeloma. N Engl J Med. 2015;373:1207–1219. doi: 10.1056/NEJMoa1506348. [DOI] [PubMed] [Google Scholar]
- 100.Sanchorawala V., Sarosiek S., Schulman A. Safety, tolerability, and response rates of daratumumab in relapsed AL amyloidosis: results of a phase 2 study. Blood. 2020;135:1541–1547. doi: 10.1182/blood.2019004436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sher T., Fenton B., Akhtar A., Gertz M.A. First report of safety and efficacy of daratumumab in 2 cases of advanced immunoglobulin light chain amyloidosis. Blood. 2016;128:1987–1989. doi: 10.1182/blood-2016-06-722496. [DOI] [PubMed] [Google Scholar]
- 102.Roussel M., Merlini G., Chevret S. A prospective phase 2 trial of daratumumab in patients with previously treated systemic light-chain amyloidosis. Blood. 2020;135:1531–1540. doi: 10.1182/blood.2019004369. [DOI] [PubMed] [Google Scholar]
- 103.Kastritis E., Palladini G., Minnema M.C. Daratumumab-based treatment for immunoglobulin light-chain amyloidosis. N Engl J Med. 2021;385:46–58. doi: 10.1056/NEJMoa2028631. [DOI] [PubMed] [Google Scholar]
- 104.Deckert J., Wetzel M.C., Bartle L.M. SAR650984, a novel humanized CD38-targeting antibody, demonstrates potent antitumor activity in models of multiple myeloma and other CD38+ hematologic malignancies. Clin Cancer Res. 2014;20:4574–4583. doi: 10.1158/1078-0432.CCR-14-0695. [DOI] [PubMed] [Google Scholar]
- 105.Attal M., Richardson P.G., Rajkumar S.V. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): a randomised, multicentre, open-label, phase 3 study. Lancet. 2019;394:2096–2107. doi: 10.1016/S0140-6736(19)32556-5. [DOI] [PubMed] [Google Scholar]
- 106.Moreau P., Dimopoulos M.A., Mikhael J. Isatuximab, carfilzomib, and dexamethasone in relapsed multiple myeloma (IKEMA): a multicentre, open-label, randomised phase 3 trial. Lancet. 2021;397:2361–2371. doi: 10.1016/S0140-6736(21)00592-4. [DOI] [PubMed] [Google Scholar]
- 107.Parker T.L., Rosenthal A., Sanchorawala V. A Phase II Study of Isatuximab (SAR650984) (NSC-795145) for Patients with Previously Treated AL Amyloidosis (SWOG S1702; NCT#03499808) (abstr) Blood. 2020;136(Suppl 1):20–21. [Google Scholar]
- 108.Iqbal S.M., Stecklein K., Sarow J., Krabak M., Hillengass J., McCarthy P. Elotuzumab in combination with lenalidomide and dexamethasone for treatment-resistant immunoglobulin light chain amyloidosis with multiple myeloma. Clin Lymphoma Myeloma Leuk. 2019;19:e33–e36. doi: 10.1016/j.clml.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 109.Tennent G.A., Lovat L.B., Pepys M.B. Serum amyloid P component prevents proteolysis of the amyloid fibrils of Alzheimer disease and systemic amyloidosis. Proc Natl Acad Sci U S A. 1995;92:4299–4303. doi: 10.1073/pnas.92.10.4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Richards D.B., Cookson L.M., Berges A.C. Therapeutic clearance of amyloid by antibodies to serum amyloid P component. N Engl J Med. 2015;373:1106–1114. doi: 10.1056/NEJMoa1504942. [DOI] [PubMed] [Google Scholar]
- 111.Ward J.E., Ren R., Toraldo G. Doxycycline reduces fibril formation in a transgenic mouse model of AL amyloidosis. Blood. 2011;118:6610–6617. doi: 10.1182/blood-2011-04-351643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cardoso I., Saraiva M.J. Doxycycline disrupts transthyretin amyloid: evidence from studies in a FAP transgenic mice model. FASEB J. 2006;20:234–239. doi: 10.1096/fj.05-4509com. [DOI] [PubMed] [Google Scholar]
- 113.D'Souza A., Flynn K., Chhabra S. Rationale and design of DUAL study: Doxycycline to Upgrade response in light chain (AL) amyloidosis (DUAL): a phase 2 pilot study of a two-pronged approach of prolonged doxycycline with plasma cell-directed therapy in the treatment of AL amyloidosis. Contemp Clin Trials Commun. 2017;8:33–38. doi: 10.1016/j.conctc.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Renz M., Torres R., Dolan P.J. 2A4 binds soluble and insoluble light chain aggregates from AL amyloidosis patients and promotes clearance of amyloid deposits by phagocytosis (dagger) Amyloid. 2016;23:168–177. doi: 10.1080/13506129.2016.1205974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Valent J., Silowsky J., Kurman M.R. Cael-101 Is well-tolerated in AL amyloidosis patients receiving concomitant cyclophosphamide-bortezomib-dexamethasone (CyborD): a phase 2 dose-finding study ( NCT04304144) (abstr) Blood. 2020;136(Suppl 1):26–27. [Google Scholar]
- 116.Souers A.J., Leverson J.D., Boghaert E.R. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19:202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 117.Kumar S., Kaufman J.L., Gasparetto C. Efficacy of venetoclax as targeted therapy for relapsed/refractory t(11;14) multiple myeloma. Blood. 2017;130:2401–2409. doi: 10.1182/blood-2017-06-788786. [DOI] [PubMed] [Google Scholar]
- 118.Pasquer H., Belhadj K., Dupuis J. Venetoclax induces profound and sustained responses in patients with relapsed/refractory light-chain amyloidosis. Br J Haematol. 2021;193:674–677. doi: 10.1111/bjh.17380. [DOI] [PubMed] [Google Scholar]
- 119.Sidiqi M.H., Al Saleh A.S., Leung N. Venetoclax for the treatment of translocation (11;14) AL amyloidosis. Blood Cancer J. 2020;10:55. doi: 10.1038/s41408-020-0321-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Moreau P., Harrison S., Cavo M. Updated analysis of Bellini, a phase 3 study of venetoclax or placebo in combination with bortezomib and dexamethasone in patients with relapsed/refractory multiple myeloma (abstr) Blood. 2019;134(Suppl 1):1888. [Google Scholar]
- 121.Trudel S., Lendvai N., Popat R. Targeting B-cell maturation antigen with GSK2857916 antibody-drug conjugate in relapsed or refractory multiple myeloma (BMA117159): a dose escalation and expansion phase 1 trial. Lancet Oncol. 2018;19:1641–1653. doi: 10.1016/S1470-2045(18)30576-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Godara A., Zhou P., Rosenthal B. B-cell maturation antigen (BCMA) in systemic light-chain amyloidosis (AL): association with disease activity and its modulation with gamma-secretase inhibition (abstr) Blood. 2019;134(Suppl 1):4409. [Google Scholar]
- 123.Palladini G., Schönland S.O., Lentzsch S. OP201: a phase 1/2 study of melflufen and dexamethasone in patients with immunoglobulin light chain (AL) amyloidosis (abstr) Blood. 2019;134(Suppl 1):3163. [Google Scholar]
- 124.Hughes D.M., DeMari S., Hassan H., Sanchorawala V., Sloan J.M. Safety, tolerability, and efficacy of selinexor in a patient with relapsed light chain (AL) amyloidosis. Clin Lymphoma Myeloma Leuk. 2021;21:e460–e463. doi: 10.1016/j.clml.2021.01.001. [DOI] [PubMed] [Google Scholar]
- 125.Merlini G. CyBorD: stellar response rates in AL amyloidosis. Blood. 2012;119:4343–4345. doi: 10.1182/blood-2012-03-413112. [DOI] [PubMed] [Google Scholar]
- 126.Gertz M.A., Comenzo R., Falk R.H. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18-22 April 2004. Am J Hematol. 2005;79:319–328. doi: 10.1002/ajh.20381. [DOI] [PubMed] [Google Scholar]
- 127.Cibeira M.T., Sanchorawala V., Seldin D.C. Outcome of AL amyloidosis after high-dose melphalan and autologous stem cell transplantation: long-term results in a series of 421 patients. Blood. 2011;118:4346–4352. doi: 10.1182/blood-2011-01-330738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gertz M.A., Lacy M.Q., Dispenzieri A. Autologous stem cell transplant for immunoglobulin light chain amyloidosis: a status report. Leuk Lymphoma. 2010;51:2181–2187. doi: 10.3109/10428194.2010.524329. [DOI] [PubMed] [Google Scholar]
- 129.Palladini G., Russo P., Nuvolone M., Lavatelli F., Perfetti V., Obici L., Merlini G. Treatment with oral melphalan plus dexamethasone produces long-term remissions in AL amyloidosis. Blood. 2007;110(2):787–788. doi: 10.1182/blood-2007-02-076034. [DOI] [PubMed] [Google Scholar]
- 130.Wechalekar A.D., Goodman H.J., Lachmann H.J., Offer M., Hawkins P.N., Gillmore J.D. Safety and efficacy of risk-adapted cyclophosphamide, thalidomide, and dexamethasone in systemic AL amyloidosis. Blood. 2007;109:457–464. doi: 10.1182/blood-2006-07-035352. [DOI] [PubMed] [Google Scholar]
- 131.Reece D.E., Hegenbart U., Sanchorawala V. Efficacy and safety of once-weekly and twice-weekly bortezomib in patients with relapsed systemic AL amyloidosis: results of a phase 1/2 study. Blood. 2011;118:865–873. doi: 10.1182/blood-2011-02-334227. [DOI] [PubMed] [Google Scholar]
- 132.Kastritis E., Wechalekar A.D., Dimopoulos M.A. Bortezomib with or without dexamethasone in primary systemic (light chain) amyloidosis. J Clin Oncol. 2010;28:1031–1037. doi: 10.1200/JCO.2009.23.8220. [DOI] [PubMed] [Google Scholar]
- 133.Palladini G., Milani P., Foli A. Melphalan and dexamethasone with or without bortezomib in newly diagnosed AL amyloidosis: a matched case-control study on 174 patients. Leukemia. 2014;28:2311–2316. doi: 10.1038/leu.2014.227. [DOI] [PubMed] [Google Scholar]
- 134.Cohen A.D., Landau H., Scott E.C. Safety and efficacy of carfilzomib (CFZ) in previously-treated systemic light-chain (AL) amyloidosis (abstr) Blood. 2016;128(22):645. [Google Scholar]
- 135.Chung A., Kaufman G.P., Sidana S. Organ responses with daratumumab therapy in previously treated AL amyloidosis. Blood Adv. 2020;4:458–466. doi: 10.1182/bloodadvances.2019000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gertz M.A., Landau H., Comenzo R.L. First-in-human phase i/ii study of NEOD001 in patients with light chain amyloidosis and persistent organ dysfunction. J Clin Oncol. 2016;34:1097–1103. doi: 10.1200/JCO.2015.63.6530. [DOI] [PMC free article] [PubMed] [Google Scholar]