Abstract
Background
Transthyretin amyloid cardiomyopathy results from the accumulation of wild-type (ATTRwt) or variant (ATTRv) transthyretin amyloid fibrils in the myocardium. THAOS (Transthyretin Amyloidosis Outcomes Survey) is a global, longitudinal, observational survey of patients with ATTRv and ATTRwt amyloidosis and asymptomatic patients with transthyretin mutations.
Objectives
This study explored temporal trends in ATTRwt amyloidosis diagnoses using data from THAOS.
Methods
Using THAOS data from December 2007 to January 2020, the following comparisons were made according to year: ATTRwt amyloidosis diagnoses in the United States versus rest of the world, ATTRwt versus ATTRv amyloidosis with cardiac-associated mutations diagnoses, and ATTRwt amyloidosis diagnoses by tissue biopsy versus bone scintigraphy.
Results
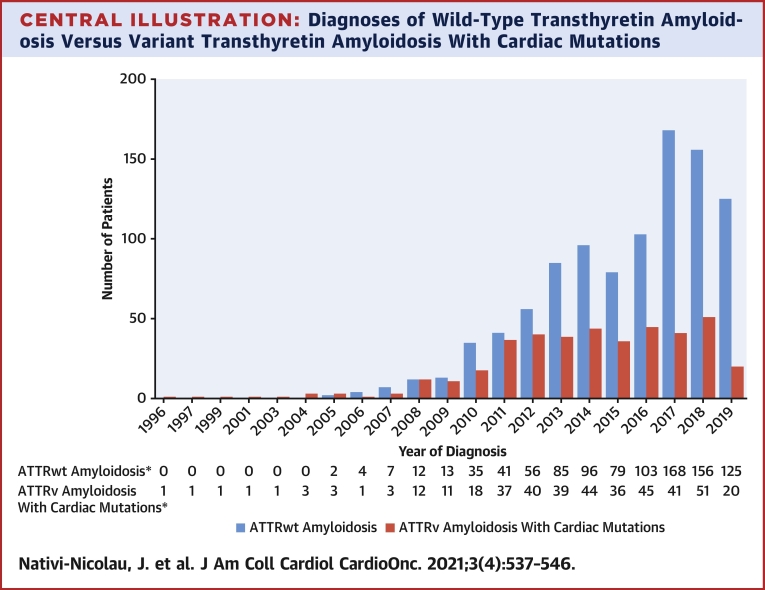
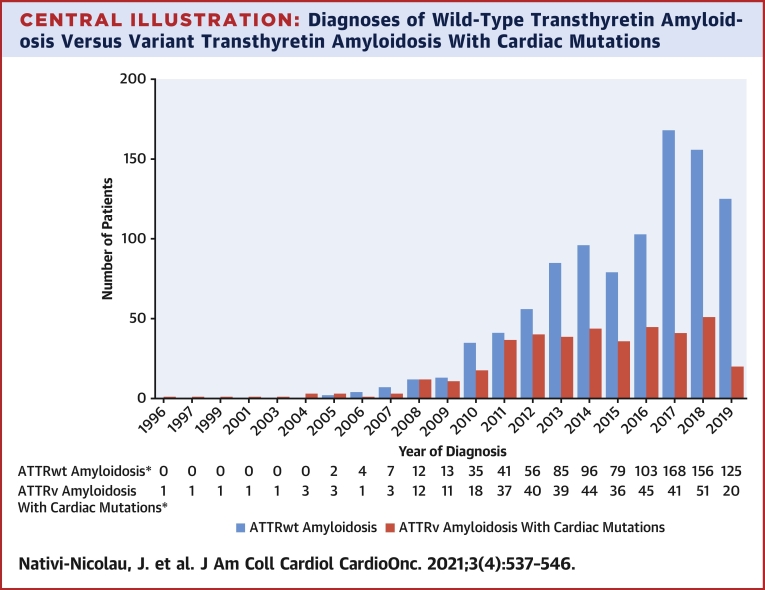
There were 1,069 patients with ATTRwt amyloidosis and 525 with ATTRv amyloidosis with cardiac mutations enrolled in THAOS. The median time from symptom onset to ATTRwt amyloidosis diagnosis did not change over the past 5 years (>60 months from 2015–2019). ATTRwt amyloidosis diagnoses increased from 2 in 2005 to >100 per year from 2016, with a more pronounced increase in the United States compared with the rest of the world. Diagnoses of ATTRwt amyloidosis by tissue biopsy increased yearly and peaked in 2014 before declining, whereas diagnoses by bone scintigraphy increased markedly since 2011. ATTRv amyloidosis with cardiac mutation diagnoses increased from 3 in 2005 to 37 in 2011, then plateaued. The proportion of patients with ATTRwt amyloidosis diagnosed with New York Heart Association functional class III/IV heart failure decreased from 2012 (46.4%) to 2019 (16.0%).
Conclusions
In the past decade, ATTRwt amyloidosis diagnoses increased worldwide. Despite the growing utilization of bone scintigraphy, patients are diagnosed several years after symptom onset. (Transthyretin Amyloidosis Outcomes Survey [THAOS]; NCT00628745)
Key Words: bone scintigraphy, registry, wild-type transthyretin amyloidosis
Abbreviations and Acronyms: ATTR amyloidosis, transthyretin amyloidosis; ATTR-CM, transthyretin amyloid cardiomyopathy; ATTRv amyloidosis, variant transthyretin amyloidosis; ATTRwt amyloidosis, wild-type transthyretin amyloidosis; NYHA, New York Heart Association; Q, quartile; TTR, transthyretin
Central Illustration
Transthyretin amyloidosis (ATTR amyloidosis) is a rare disease caused by the deposition of transthyretin-derived amyloid fibrils in the heart, peripheral nerves, and other organs (1). ATTR amyloidosis may arise from mutations in the transthyretin (TTR) gene (ATTRv amyloidosis), or from nonmutated, wild-type TTR (ATTRwt amyloidosis), which can deposit as amyloid fibrils in the extracellular matrix of the heart (2,3). This results in transthyretin amyloid cardiomyopathy (ATTR-CM), which is characterized by arrhythmias and heart failure (4,5). ATTRv amyloidosis can manifest as polyneuropathy, cardiomyopathy, or a mixed phenotype, depending on the particular TTR variant. ATTRwt amyloidosis predominantly manifests as ATTR-CM.
Untreated patients with ATTR-CM generally have a poor prognosis, with median survival between 2 and 4 years following diagnosis (6, 7, 8, 9). Early diagnosis of ATTR-CM is critical, given its progressive nature. Effective treatments have recently become available, and treatment early in the disease course is more likely to be effective (10, 11, 12). Traditionally, a definitive diagnosis of ATTR-CM was obtained through endomyocardial biopsy, an invasive procedure that requires expertise and carries potential risks (10,12). Bone scintigraphy in the absence of a monoclonal protein has more recently emerged as a diagnostic tool with high sensitivity and specificity for ATTR-CM, offering greater ease of access than a tissue biopsy (13,14).
ATTR-CM is both underdiagnosed and frequently misdiagnosed and, consequently, its prevalence remains difficult to establish (12). Increased use of bone scintigraphy may help identify a greater proportion of patients with ATTR-CM. ATTRwt amyloidosis is the most common form of ATTR-CM, with 13% of older patients with heart failure with preserved ejection fraction and increased wall thickness having evidence of previously undiagnosed ATTRwt amyloidosis (15).
The primary aim of this study was to characterize temporal trends in the diagnosis of ATTRwt amyloidosis in the United States and the rest of the world using real-world data from THAOS (the Transthyretin Amyloidosis Outcomes Survey). THAOS is a global, longitudinal, observational survey of patients with ATTR amyloidosis, including both inherited and wild-type disease, and asymptomatic patients with TTR mutations (NCT00628745) (3,16, 17, 18, 19, 20).
Methods
Study design and population
The study design and eligibility criteria from THAOS have been reported (20). In this analysis, data from THAOS (from initiation in December 2007 to the data cutoff on January 6, 2020) were used to evaluate, on a yearly basis, the number of patients with: 1) a diagnosis of ATTRwt amyloidosis in the United States versus the rest of the world; 2) a diagnosis of ATTRwt amyloidosis versus a diagnosis of ATTRv amyloidosis with cardiac mutations; and 3) a diagnosis of ATTRwt amyloidosis by tissue biopsy versus bone scintigraphy. All patients with ATTRwt amyloidosis and ATTRv amyloidosis with cardiac mutations enrolled in THAOS as of the data cutoff date were included in this analysis, with the number of patients diagnosed (recorded retrospectively at enrollment, or at diagnosis) in each year also compared with the number enrolled in THAOS each year. Patients with ATTRv amyloidosis with cardiac mutations were those patients with symptomatic ATTRv amyloidosis and a mutation predominantly associated with cardiac disease (either Val122Ile [p.Val142Ile] [21], Leu111Met [p.Leu131Met] [22], Thr60Ala [p.Thr80Ala] [23], or Ile68Leu [p.Ile88Leu] [24]), and were included as a comparison group. All study sites received ethical or institutional review board approval before patient enrollment, and each patient provided written informed consent. The study followed the Good Pharmacoepidemiology Practice guidelines and the principles of the Declaration of Helsinki.
Demographic information, clinical characteristics, and diagnostic method were collected at enrollment in THAOS and are presented here for the ATTRwt amyloidosis population. Symptom onset was based on any symptom (cardiac or noncardiac) defined as ATTR amyloidosis–related by the investigator.
Patients with ATTRwt amyloidosis diagnosed by tissue biopsy were defined as those who were diagnosed by recorded TTR amyloid in cardiac (or noncardiac) biopsy tissue by mass spectrometry or immunohistochemistry (in addition to echocardiogram with mean left ventricular wall thickness >12 mm). Patients with ATTRwt amyloidosis diagnosed by bone scintigraphy were defined as those with technetium-99m (99mTC) scintigraphy indicating TTR amyloid in cardiac tissue with no evidence of light-chain amyloidosis (in addition to echocardiogram with mean left ventricular wall thickness of >12 mm).
Statistical analysis
Continuous data are presented as mean ± SD and median (25th, 75th percentiles [quartile (Q)1, Q3]), whereas categorical data are presented using count (percentages) unless stated otherwise. SAS version 9.4 was used to summarize the data. Qualitative rather than quantitative comparisons were made throughout the study.
Data statement
Pfizer provides secure access to anonymized patient-level data to qualified researchers in response to scientifically valid research proposals. Further details can be found on the Pfizer website (25).
Results
Demographics and clinical characteristics of patients with ATTRwt amyloidosis
A total of 1,069 patients with ATTRwt amyloidosis were enrolled in THAOS as of January 6, 2020. Of these, 94.6% were men and 94.3% White patients (Table 1). Median age (Q1, Q3) at enrollment was 77.2 (72.6, 81.9) years. Median (Q1, Q3) age at symptom onset was 69.8 (62.4, 76.6) years, and diagnosis was 76.4 (71.5, 81.0) years. These were similar in patients in the United States and patients in the rest of the world (Table 2).
Table 1.
Demographics in Patients With ATTRwt Amyloidosis (N = 1,069)
| Age at enrollment (y) | |
| n | 1,069 |
| Mean ± SD | 77.0 ± 7.2 |
| Median (Q1, Q3) | 77.2 (72.6, 81.9) |
| Min, Max | 48.0, 96.8 |
| Sex | |
| Male | 1,011 (94.6) |
| Female | 58 (5.4) |
| Race/ethnicity | |
| White | 894 (94.3) |
| Blacka | 31 (3.3) |
| Hispanic/Latinx | 8 (0.8) |
| Asian | 7 (0.7) |
| Other | 8 (0.8) |
| Country | |
| Belgium | 6 (0.6) |
| Brazil | 7 (0.7) |
| Canada | 13 (1.2) |
| Germany | 122 (11.4) |
| Denmark | 18 (1.7) |
| Spain | 59 (5.5) |
| France | 30 (2.8) |
| Italy | 98 (9.2) |
| Japan | 6 (0.6) |
| South Korea | 4 (0.4) |
| Netherlands | 5 (0.5) |
| Portugal | 19 (1.8) |
| Sweden | 4 (0.4) |
| Turkey | 1 (0.1) |
| United States | 677 (63.3) |
Values are n (%) unless otherwise indicated.
ATTRwt amyloidosis = wild-type transthyretin amyloidosis; Q = quartile.
Black includes Afro Caribbean and African American.
Table 2.
Clinical Characteristics in Patients With ATTRwt Amyloidosis by Region
| Overall (N = 1,027) | United States (n = 646) | Rest of the World (n = 381) | |
|---|---|---|---|
| Age at onset (y) | |||
| n | 1,027 | 646 | 381 |
| Mean ± SD | 68.7 ± 11.1 | 68.0 ± 11.2 | 69.8 ± 10.8 |
| Median (Q1, Q3) | 69.8 (62.4, 76.6) | 69.1 (62.0, 76.0) | 70.9 (63.1, 77.5) |
| Age at diagnosis (y) | |||
| n | 947 | 604 | 343 |
| Mean ± SD | 76.1 ± 7.1 | 75.8 ± 7.1 | 76.7 ± 7.2 |
| Median (Q1, Q3) | 76.4 (71.5, 81.0) | 76.1 (71.2, 80.4) | 77.4 (72.2, 81.7) |
| Time to diagnosis from symptom onseta (mo) | |||
| n | 947 | 604 | 343 |
| Mean ± SD | 90.4 ± 104.2 | 93.6 ± 112.0 | 84.8 ± 88.6 |
| Median (Q1, Q3) | 56.5 (12.0, 131.6) | 53.1 (11.8, 138.3) | 61.0 (13.6, 121.0) |
| Diagnostic modality, n (%) | |||
| Tissue biopsyb | 594 (57.8) | 388 (60.1) | 206 (54.1) |
| Cardiac tissue | 215 (20.9) | 142 (22.0) | 73 (19.2) |
| Noncardiac tissue | 45 (4.4) | 35 (5.4) | 10 (2.6) |
| Cardiac + noncardiac tissue | 28 (2.7) | 25 (3.9) | 3 (0.8) |
| Bone scintigraphy | 429 (41.8) | 257 (39.8) | 172 (45.1) |
| Missing/Unknown | 4 (0.4) | 1 (0.2) | 3 (0.8) |
Including all patients in THAOS (the Transthyretin Amyloidosis Outcomes Survey) with ATTRwt amyloidosis and any symptom at enrollment defined as ATTR amyloidosis–related by the investigator; 42 patients did not have ATTR amyloidosis–related symptoms at enrollment.
ATTR amyloidosis = transthyretin amyloidosis; other abbreviations as in Table 1.
Any symptom (cardiac or noncardiac) defined as ATTR amyloidosis–related by the investigator.
Tissue biopsy type not available for 306 patients.
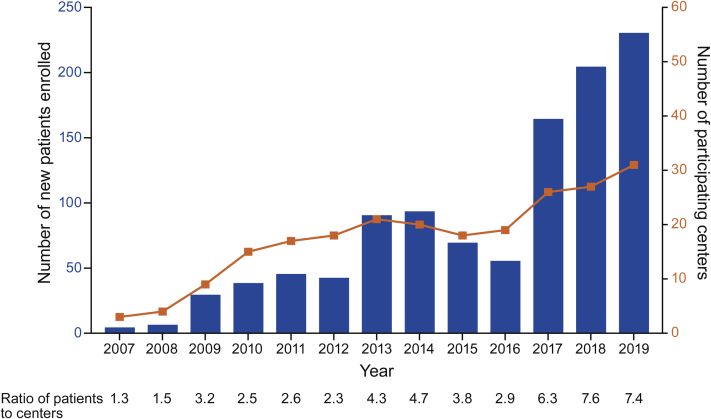
Patients were enrolled from 15 countries, most frequently from the United States (63.3%), followed by Germany (11.4%), Italy (9.2%), and Spain (5.5%) (Table 1). The number of participating centers with patients with ATTRwt amyloidosis grew from 3 in 2007 to 31 in 2019, whereas the number of new patient enrollments per year grew from 4 to 230 (Figure 1). The original 3 centers in 2007 (Germany, Japan, and the United States) had a total of 6 enrolled patients with ATTRwt amyloidosis in 2007 and 80 in 2018. The average number of patients with ATTRwt amyloidosis enrolled per year per participating center has increased from fewer than 2 in 2007 and 2008 to more than 7 in 2018 and 2019 (Figure 1).
Figure 1.
ATTRwt Amyloidosis Patient Enrollment and Participating Centers per Year
Participating centers in THAOS (the Transthyretin Amyloidosis Outcomes Survey) were centers with data available for patients with wild-type transthyretin amyloidosis (ATTRwt amyloidosis) in any given year. The number of participating centers with patients with ATTRwt amyloidosis grew from 3 in 2007 to 31 in 2019, and the number of new patient enrollments per year grew from 4 to 230. The average number of patients with ATTRwt amyloidosis enrolled per year per participating center has increased from <2 in 2007 and 2008 to >7 in 2018 and 2019.
Time to ATTRwt amyloidosis diagnosis
Median (Q1, Q3) time from the onset of symptoms to ATTRwt amyloidosis diagnosis was 56.5 (12.0, 131.6) months in the total cohort, 53.1 (11.8, 138.3) months in the United States, and 61.0 (13.6, 121.0) months in the rest of the world (Table 2). Overall, the time to diagnosis of ATTRwt amyloidosis has not changed substantially in the past 5 years, with a median time from symptom onset to diagnosis of 63.1 months in 2015, 67.5 months in 2016, 61.6 months in 2017, 73.0 months in 2018, and 71.5 months in 2019.
Diagnoses of ATTR-CM by year
The number of ATTRwt amyloidosis diagnoses in THAOS increased steadily from 2 in 2005 to more than 100 per year from 2016 to 2019 (Central Illustration). There was a relative decline in new diagnoses of ATTRwt amyloidosis in 2018 and 2019, but there was no decline in the number of patients with ATTRwt amyloidosis enrolling in those years (164 in 2017, 204 in 2018, and 230 in 2019) (Figure 1).
Central Illustration.
Diagnoses of Wild-Type Transthyretin Amyloidosis Versus Variant Transthyretin Amyloidosis With Cardiac Mutations
Diagnoses in THAOS (the Transthyretin Amyloidosis Outcomes Survey) are shown by year. Wild-type transthyretin amyloidosis (ATTRwt amyloidosis) diagnoses increased from 2 in 2005 to >100 per year starting in 2016. Diagnoses of variant transthyretin amyloidosis (ATTRv amyloidosis) with cardiac mutations increased from 3 in 2005 to 37 in 2011, after which they plateaued. ∗Year of diagnosis missing for 87 patients with ATTRwt amyloidosis and 116 patients with ATTRv amyloidosis with cardiac mutations.
Diagnoses of ATTRv amyloidosis with cardiac mutations increased from 3 in 2005 to 37 in 2011, after which point the number of new patients plateaued, with between 36 and 51 each year (Central Illustration). A total of 525 patients were diagnosed with ATTRv amyloidosis with cardiac mutations as of the data cutoff date.
Diagnoses of ATTRwt amyloidosis in THAOS in the united states compared with the rest of the world
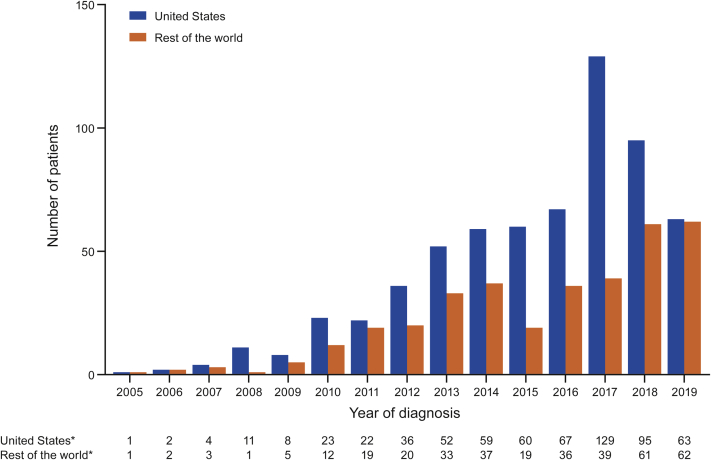
ATTRwt amyloidosis diagnoses in the United States increased steadily from 2005, with the increase being almost 2-fold from 2016 (n = 67) to 2017 (n = 129), after which diagnoses appeared to decrease (Figure 2). In contrast, ATTRwt amyloidosis diagnoses in the rest of the world increased less steadily but continued to increase in 2018 (n = 61) and 2019 (n = 62) (Figure 2).
Figure 2.
Diagnoses of ATTRwt Amyloidosis by Region
Diagnoses in the United States versus the rest of the world in THAOS (the Transthyretin Amyloidosis Outcomes Survey) are shown by year. Wild-type transthyretin amyloidosis (ATTRwt amyloidosis) diagnoses in the United States in THAOS increased steadily from 2005 to 2017, after which diagnoses appeared to decrease. In the rest of the world, ATTRwt amyloidosis diagnoses increased less steadily, but continued upward in 2018 and 2019. ∗Year of diagnosis missing for 45 patients in the United States and 42 in the rest of the world.
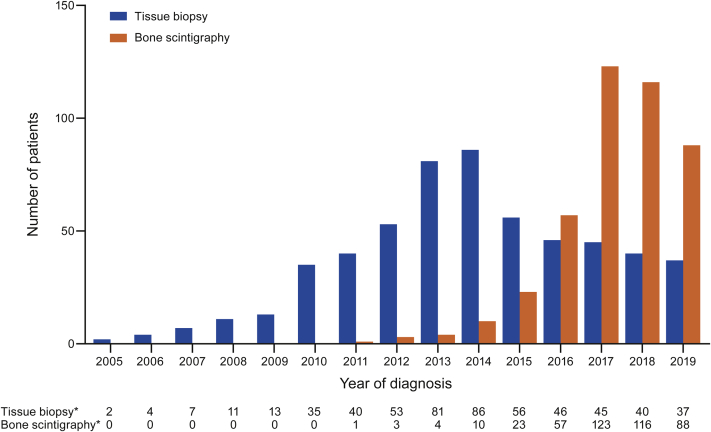
Diagnoses of ATTRwt amyloidosis with tissue biopsy compared with bone scintigraphy
The number of patients with ATTRwt amyloidosis diagnosed by tissue biopsy increased yearly and peaked in 2014 (n = 86), before declining (Figure 3). The number of patients with ATTRwt amyloidosis diagnosed by bone scintigraphy increased steadily from 2011 to 2017, with more patients diagnosed by bone scintigraphy than tissue biopsy every year from 2016. In 2018 and 2019, the number of patients with ATTRwt amyloidosis diagnosed by bone scintigraphy declined. However, this corresponded with the decline in the total number of patients diagnosed in 2018 and 2019 (Central Illustration). The proportion of patients with ATTRwt amyloidosis diagnosed by bone scintigraphy was 55.3% in 2016, 73.2% in 2017, 74.4% in 2018, and 70.4% in 2019.
Figure 3.
Diagnoses of ATTRwt Amyloidosis by Tissue Biopsy Versus Bone Scintigraphy
Diagnoses in THAOS (the Transthyretin Amyloidosis Outcomes Survey) are shown by year. The number of patients with wild-type transthyretin amyloidosis (ATTRwt amyloidosis) diagnosed by tissue biopsy increased yearly and peaked in 2014 before declining. The number of patients with ATTRwt amyloidosis diagnosed by bone scintigraphy increased steadily from 2011 to 2017, with more patients diagnosed by bone scintigraphy than tissue biopsy every year from 2016. ∗Year of diagnosis missing for 66 patients diagnosed by biopsy and 18 patients diagnosed by scintigraphy.
Diagnoses of ATTRwt amyloidosis by new york heart association Functional class
From 2012 onward, there was a trend toward a greater proportion of patients diagnosed in New York Heart Association (NYHA) functional class I or II, compared with the proportion diagnosed in NYHA functional class III or IV (Table 3). In 2012, 24 (42.9%) patients were diagnosed in NYHA functional class I or II and 26 (46.4%) in NYHA functional class III or IV, whereas 78 (62.4%) and 20 (16.0%) were diagnosed in the respective classes in 2019.
Table 3.
NYHA Functional Class at Diagnosis According to Year of Diagnosis
| Year | NYHA Functional Class I | NYHA Functional Class II | NYHA Functional Class III | NYHA Functional Class IV | Missing | Total |
|---|---|---|---|---|---|---|
| 2005 | 0 | 1 (50.0) | 1 (50.0) | 0 | 0 | 2 |
| 2006 | 0 | 1 (25.0) | 2 (50.0) | 0 | 1 (25.0) | 4 |
| 2007 | 1 (14.3) | 3 (42.9) | 2 (28.6) | 1 (14.3) | 0 | 7 |
| 2008 | 1 (8.3) | 9 (75.0) | 1 (8.3) | 0 | 1 (8.3) | 12 |
| 2009 | 1 (7.7) | 4 (30.8) | 6 (46.2) | 1 (7.7) | 1 (7.7) | 13 |
| 2010 | 4 (11.4) | 15 (42.9) | 11 (31.4) | 2 (5.7) | 3 (8.6) | 35 |
| 2011 | 3 (7.3) | 20 (48.8) | 11 (26.8) | 1 (2.4) | 6 (14.6) | 41 |
| 2012 | 4 (7.1) | 20 (35.7) | 23 (41.1) | 3 (5.4) | 6 (10.7) | 56 |
| 2013 | 6 (7.1) | 36 (42.4) | 25 (29.4) | 4 (4.7) | 14 (16.5) | 85 |
| 2014 | 5 (5.2) | 51 (53.1) | 23 (24.0) | 2 (2.1) | 15 (15.6) | 96 |
| 2015 | 1 (1.3) | 51 (64.6) | 14 (17.7) | 3 (3.8) | 10 (12.7) | 79 |
| 2016 | 9 (8.7) | 52 (50.5) | 28 (27.2) | 1 (1.0) | 13 (12.6) | 103 |
| 2017 | 23 (13.7) | 88 (52.4) | 34 (20.2) | 2 (1.2) | 21 (12.5) | 168 |
| 2018 | 11 (7.1) | 96 (61.5) | 25 (16.0) | 2 (1.3) | 22 (14.1) | 156 |
| 2019 | 19 (15.2) | 59 (47.2) | 19 (15.2) | 1 (0.8) | 27 (21.6) | 125 |
Values are n (%). Percentages based on total patients diagnosed in that year.
NYHA = New York Heart Association.
Discussion
Over the past 10 years, the number of patients with ATTRwt amyloidosis enrolled in THAOS has increased substantially, reaching more than 1,000 worldwide. The number of ATTRwt amyloidosis diagnoses for patients enrolled in the United States peaked in 2017 while continuing to rise in the rest of the world, which may reflect recent improvements in medical education and increased availability of bone scintigraphy outside the United States (3,10,19). After an initial increase, the number of patients diagnosed with ATTRv amyloidosis with cardiac mutations has remained largely stable since 2011.
ATTR-CM remains underdiagnosed, despite evidence that it is likely a more common cause of cardiovascular disease in the aged than previously thought (1,12). Studies have suggested that as many as 15% of older patients with heart failure with preserved ejection fraction and increased wall thickness have evidence of previously undiagnosed ATTR-CM (15,26,27). Interest in bone scintigraphy to diagnose ATTR-CM was renewed in the past 2 decades when studies confirmed the usefulness of 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy to differentiate ATTR-CM from light-chain amyloidosis and to identify ATTR-CM across a wide spectrum of morphologic and functional cardiac involvement (28,29). In addition, a 4-stage grading system was devised to score the degree of cardiac uptake (28). Over the past 5 years, the use of bone scintigraphy to diagnose ATTR-CM has increased worldwide, whereas the use of tissue biopsy has decreased. It has been suggested that an increase in the use of a minimally invasive diagnostic tool like bone scintigraphy in the absence of a monoclonal protein (to exclude light-chain amyloidosis) will help to identify a greater proportion of patients with ATTR-CM (1,12). These data from THAOS appear to support this claim, with large increases in the numbers of patients diagnosed with ATTRwt amyloidosis in recent years, with more diagnoses having been made by bone scintigraphy.
Despite the increase in the number of patients with ATTRwt amyloidosis in THAOS, including those diagnosed in NYHA functional class I or II, and the increase in use of bone scintigraphy, there remains an unmet clinical need, with patients waiting for several years (on average) from the onset of symptoms until diagnosis. The diagnostic delay observed here may even underestimate the real-world experience of patients with ATTRwt amyloidosis. By nature of the registry, THAOS patients are in a catchment area for specialized centers with a focus and interest in this disease, whereas many patients in the real world are not and may experience greater barriers to diagnosis. Overall, the observed discrepancy between increased use of bone scintigraphy and continued prolonged time to diagnosis highlights the need for improved education on the early symptoms of ATTR amyloidosis. For example, musculoskeletal symptoms, such as carpal tunnel syndrome and spinal stenosis, can manifest 5 to 15 years before cardiac manifestations, and screening for these symptoms in patients with heart failure can potentially decrease the diagnostic delay (12,30).
The plateau in the number of patients with ATTRv amyloidosis with cardiac mutations is multifactorial and might suggest that these patients remain underdiagnosed compared with ATTRwt amyloidosis. For example, patients with Val122Ile are predominantly of African and Caribbean descent (31). These patients suffer from other comorbidities, such as earlier arterial hypertension and heart failure, which can mask an evolving amyloidosis process (32). Also, Black communities can suffer from health disparities and limited access to care, which can further delay a rare disease diagnosis (33). Alternatively, the steadily increasing numbers of patients with ATTRwt amyloidosis could indicate that the growth will be ongoing and that ATTRwt amyloidosis remains considerably underdiagnosed compared with ATTRv amyloidosis. Patients with ATTRv amyloidosis inherit a genetic variant to manifest symptoms, whereas any older adult, predominantly of male sex, can develop ATTRwt amyloidosis.
Although this analysis was limited to patients enrolled in THAOS, there is evidence to suggest that THAOS is capturing a large proportion of all diagnosed patients with ATTRwt amyloidosis, at least in the United States, and that diagnostic patterns in ATTRwt amyloidosis are well represented in THAOS. In a recent retrospective study examining Medicare claims from 2010 to 2018, 726 patients with ATTRwt amyloidosis were identified in the United States (34), compared with 677 patients in the United States enrolled in THAOS as of this data cutoff.
Study limitations
Although improvements in diagnostic methodology can help identify more patients with ATTR-CM, the increase in ATTRwt amyloidosis diagnoses in this analysis may also reflect the changing characteristics of THAOS over time. THAOS has grown from 3 participating centers with patients with ATTRwt amyloidosis in 2007 to 31 participating centers in 2019, and the increase in diagnoses observed here could be attributable to the growing reach of THAOS. However, when controlling for the number of participating centers, the average number of patients with ATTRwt amyloidosis enrolled each year has been steadily increasing, suggesting that the increase in diagnoses does not solely reflect the growth of THAOS. It should be noted that for patients enrolled in another clinical trial while participating in THAOS, data collected during the period of that clinical trial participation may not be entered into THAOS. Additional restrictions as to data management post trial participation also apply. This may, in part, explain the decline in the number of new patients with ATTRwt amyloidosis enrolled in THAOS in 2015 and 2016, when there was significant recruitment for ongoing clinical trials in cardiomyopathy (11). The apparent decline in diagnoses in 2018 and 2019 was likely a consequence of most patients being diagnosed before entry into THAOS, since the number of enrollments in 2018 and 2019 increased. It may be that additional patients diagnosed with ATTR-CM in 2018 and 2019 will enroll in THAOS in the coming years. The fact that data are entered retrospectively into the THAOS registry by study sites and investigators suggests that perhaps not all data for the 2018-2019 period were added at the date of this analysis. Therefore, it is likely the increase observed here reflects both the growing reach of THAOS and advances in diagnostic approaches. Finally, as noted in the figures and tables, data were missing for some patients.
Conclusions
THAOS is a valuable resource for tracking the incidence of ATTR amyloidosis and monitoring temporal changes in diagnoses and diagnostic methodology used over time. This current analysis is one of the largest international evidence reports on ATTRwt amyloidosis and contributes to our understanding of this disease.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: In the past decade, ATTRwt amyloidosis diagnoses have increased worldwide and more diagnoses have been made by bone scintigraphy. However, patients still suffer from several years of delayed diagnosis.
TRANSLATIONAL OUTLOOK: The time to diagnosis of ATTRwt amyloidosis has not changed over the past 5 years despite the increased use of bone scintigraphy. Future research should further examine barriers to diagnosis of ATTRwt amyloidosis.
Funding Support and Author Disclosures
The THAOS registry and this analysis were sponsored by Pfizer. Dr Nativi-Nicolau has received research grants from Akcea/Ionis, Eidos, and Pfizer; has received consulting fees from Akcea, Alnylam, Eidos, and Pfizer; and has received educational grants from Pfizer. Dr Siu has received funding from Pfizer for meeting expenses (travel). Dr Dispenzieri has received research grants from Celgene, Millennium, Pfizer, Janssen, and Alnylam; and has received funding from Pfizer for meeting expenses (travel) and attended advisory boards for Akcea and Intellia. Dr Maurer has received grants from Pfizer during the conduct of the study; has received grants and personal fees from Pfizer, Eidos, Prothena, and Ionis; has received grants from Alnylam; and has received personal fees from GlaxoSmithKline and Akcea outside the submitted work. Dr Rapezzi has received research grants from Pfizer; and has received consultancy fees from Pfizer, Alnylam, and Prothena. Dr Kristen has received reimbursement for study visits from Pfizer during the conduct of the study. Dr Garcia-Pavia has received speaking fees from Pfizer, Eidos, Alnylam, and Akcea; has received consulting fees from Pfizer, Eidos, Neurimmune, Alnylam, Prothena, and Akcea; and has received research/educational support to his institution from Pfizer, Eidos, and Alnylam. Dr LoRusso has received support to her institution from Pfizer and Alnylam. Dr Waddington-Cruz has received research funding, consulting fees, and travel support for advisory boards and meetings from FoldRx Pharmaceuticals and Pfizer. Dr Lairez has received research grants from Pfizer; and has received consultancy fees from Pfizer and Alnylam. Dr Witteles has received honoraria for advisory board participation and funding for clinical trials from Pfizer, Alnylam, Eidos, and Ionis/Akcea. Drs Amass and Chapman are full-time employees of Pfizer and hold stock and/or stock options with Pfizer. Dr Grogan has received grants, and advisory board and consultancy fees paid to her institution from Alnylam, Eidos, Prothena, and Pfizer.
Acknowledgments
The authors thank Dr Jan Kiszko for his contributions to earlier versions of this work. They also thank all THAOS patients and investigators for their important contributions to this study. Medical writing support was provided by Emily Balevich, PhD, and Paul Hassan, PhD, of Engage Scientific Solutions, and funded by Pfizer.
Footnotes
Michelle Hamilton, MD, served as the Guest Associate Editor for this paper. Anju Nohria, MD, served as the Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a list of additional THAOS investigators contributing to this analysis, please see the online version of this paper.
Contributor Information
Jose Nativi-Nicolau, Email: nativinicolau.jose@mayo.edu.
THAOS Investigators:
Fabio Adrian Barroso, Johan Van Cleemput, Nowell Fine, Hartmut Schmidt, Burkhard Gess, Henning Moelgaard, Violaine Planté-Bordeneuve, David Adams, Jocelyn Inamo, Giuseppe Vita, Calogero Lino Cirami, Marco Luigetti, Michele Emdin, Yoshiki Sekijima, Taro Yamashita, Eun-Seok Jeon, Maria Alejandra Gonzalez Duarte Briseno, Hans Nienhuis, Olga Azevedo, Josep Maria Campistol Plana, Juan Gonzalez Moreno, Jose Gonzalez Costello, Jonas Wixner, Yesim Parman, Sanjiv Shah, Dianna Quan, Tessa Marburger, Michael Polydefkis, Stephen Gottlieb, Jeffrey Ralph, Nitasha Sarswat, Jin Luo, Srinivas Murali, William Cotts, Brian Drachman, David Steidley, Scott Hummel, David Slosky, Hector Ventura, Daniel Jacoby, James Hoffman, James Tauras, Sasa Zivkovic, Jose Tallaj, Daniel Lenihan, and Christopher Mueller
Appendix
References
- 1.Ruberg F.L., Grogan M., Hanna M., Kelly J.W., Maurer M.S. Transthyretin amyloid cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73:2872–2891. doi: 10.1016/j.jacc.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ando Y., Coelho T., Berk J.L. Guideline of transthyretin-related hereditary amyloidosis for clinicians. Orphanet J Rare Dis. 2013;8:31. doi: 10.1186/1750-1172-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maurer M.S., Hanna M., Grogan M. Genotype and phenotype of transthyretin cardiac amyloidosis: THAOS (Transthyretin Amyloid Outcome Survey) J Am Coll Cardiol. 2016;68:161–172. doi: 10.1016/j.jacc.2016.03.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castaño A., Drachman B.M., Judge D., Maurer M.S. Natural history and therapy of TTR-cardiac amyloidosis: emerging disease-modifying therapies from organ transplantation to stabilizer and silencer drugs. Heart Fail Rev. 2015;20:163–178. doi: 10.1007/s10741-014-9462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rapezzi C., Quarta C.C., Obici L. Disease profile and differential diagnosis of hereditary transthyretin-related amyloidosis with exclusively cardiac phenotype: an Italian perspective. Eur Heart J. 2013;34:520–528. doi: 10.1093/eurheartj/ehs123. [DOI] [PubMed] [Google Scholar]
- 6.Gillmore J.D., Damy T., Fontana M. A new staging system for cardiac transthyretin amyloidosis. Eur Heart J. 2018;39:2799–2806. doi: 10.1093/eurheartj/ehx589. [DOI] [PubMed] [Google Scholar]
- 7.Ruberg F.L., Maurer M.S., Judge D.P. Prospective evaluation of the morbidity and mortality of wild-type and V122I mutant transthyretin amyloid cardiomyopathy: the Transthyretin Amyloidosis Cardiac Study (TRACS) Am Heart J. 2012;164:222–228.e221. doi: 10.1016/j.ahj.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 8.Givens R.C., Russo C., Green P., Maurer M.S. Comparison of cardiac amyloidosis due to wild-type and V122I transthyretin in older adults referred to an academic medical center. Aging Health. 2013;9:229–235. doi: 10.2217/ahe.13.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grogan M., Scott C.G., Kyle R.A. Natural history of wild-type transthyretin cardiac amyloidosis and risk stratification using a novel staging system. J Am Coll Cardiol. 2016;68:1014–1020. doi: 10.1016/j.jacc.2016.06.033. [DOI] [PubMed] [Google Scholar]
- 10.Maurer M.S., Bokhari S., Damy T. Expert consensus recommendations for the suspicion and diagnosis of transthyretin cardiac amyloidosis. Circ Heart Fail. 2019;12 doi: 10.1161/CIRCHEARTFAILURE.119.006075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maurer M.S., Schwartz J.H., Gundapaneni B. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379:1007–1016. doi: 10.1056/NEJMoa1805689. [DOI] [PubMed] [Google Scholar]
- 12.Witteles R.M., Bokhari S., Damy T. Screening for transthyretin amyloid cardiomyopathy in everyday practice. J Am Coll Cardiol HF. 2019;7:709–716. doi: 10.1016/j.jchf.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Gillmore J.D., Maurer M.S., Falk R.H. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation. 2016;133:2404–2412. doi: 10.1161/CIRCULATIONAHA.116.021612. [DOI] [PubMed] [Google Scholar]
- 14.Hanna M., Ruberg F.L., Maurer M.S. Cardiac scintigraphy with technetium-99m-labeled bone-seeking tracers for suspected amyloidosis: JACC review topic of the week. J Am Coll Cardiol. 2020;75:2851–2862. doi: 10.1016/j.jacc.2020.04.022. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez-Lopez E., Gallego-Delgado M., Guzzo-Merello G. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J. 2015;36:2585–2594. doi: 10.1093/eurheartj/ehv338. [DOI] [PubMed] [Google Scholar]
- 16.Kristen A.V., Maurer M.S., Rapezzi C., Mundayat R., Suhr O.B., Damy T. Impact of genotype and phenotype on cardiac biomarkers in patients with transthyretin amyloidosis - Report from the Transthyretin Amyloidosis Outcome Survey (THAOS) PLoS One. 2017;12 doi: 10.1371/journal.pone.0173086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wixner J., Mundayat R., Karayal O.N., Anan I., Karling P., Suhr O.B. THAOS: gastrointestinal manifestations of transthyretin amyloidosis - common complications of a rare disease. Orphanet J Rare Dis. 2014;9:61. doi: 10.1186/1750-1172-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mundayat R., Stewart M., Alvir J. Positive effectiveness of tafamidis in delaying disease progression in transthyretin familial amyloid polyneuropathy up to 2 years: an analysis from the Transthyretin Amyloidosis Outcomes Survey (THAOS) Neurol Ther. 2018;7:87–101. doi: 10.1007/s40120-018-0097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Damy T., Kristen A.V., Suhr O.B. Transthyretin cardiac amyloidosis in continental Western Europe: an insight through the Transthyretin Amyloidosis Outcomes Survey (THAOS) Eur Heart J. 2019:ehz173. doi: 10.1093/eurheartj/ehz173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Planté-Bordeneuve V., Suhr O.B., Maurer M.S., White B., Grogan D.R., Coelho T. The Transthyretin Amyloidosis Outcomes Survey (THAOS) registry: design and methodology. Curr Med Res Opin. 2013;29:77–84. doi: 10.1185/03007995.2012.754349. [DOI] [PubMed] [Google Scholar]
- 21.Jacobson D.R., Pastore R.D., Yaghoubian R. Variant-sequence transthyretin (isoleucine 122) in late-onset cardiac amyloidosis in black Americans. N Engl J Med. 1997;336:466–473. doi: 10.1056/NEJM199702133360703. [DOI] [PubMed] [Google Scholar]
- 22.Svendsen I.H., Steensgaard-Hansen F., Nordvag B.Y. A clinical, echocardiographic and genetic characterization of a Danish kindred with familial amyloid transthyretin methionine 111 linked cardiomyopathy. Eur Heart J. 1998;19:782–789. doi: 10.1053/euhj.1997.0841. [DOI] [PubMed] [Google Scholar]
- 23.Sattianayagam P.T., Hahn A.F., Whelan C.J. Cardiac phenotype and clinical outcome of familial amyloid polyneuropathy associated with transthyretin alanine 60 variant. Eur Heart J. 2012;33:1120–1127. doi: 10.1093/eurheartj/ehr383. [DOI] [PubMed] [Google Scholar]
- 24.Almeida M.R., Hesse A., Steinmetz A. Transthyretin Leu 68 in a form of cardiac amyloidosis. Basic Res Cardiol. 1991;86:567–571. doi: 10.1007/BF02190707. [DOI] [PubMed] [Google Scholar]
- 25.Pfizer Inc Clinical trial data should be accessible and transparent. https://www.pfizer.com/science/clinical-trials/trial-data-and-results Accessed August 23 2021.
- 26.Mohammed S.F., Mirzoyev S.A., Edwards W.D. Left ventricular amyloid deposition in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol HF. 2014;2:113–122. doi: 10.1016/j.jchf.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hahn V.S., Yanek L.R., Vaishnav J. Endomyocardial biopsy characterization of heart failure with preserved ejection fraction and prevalence of cardiac amyloidosis. J Am Coll Cardiol HF. 2020;8:712–724. doi: 10.1016/j.jchf.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perugini E., Guidalotti P.L., Salvi F. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy. J Am Coll Cardiol. 2005;46:1076–1084. doi: 10.1016/j.jacc.2005.05.073. [DOI] [PubMed] [Google Scholar]
- 29.Rapezzi C., Quarta C.C., Guidalotti P.L. Role of (99m)Tc-DPD scintigraphy in diagnosis and prognosis of hereditary transthyretin-related cardiac amyloidosis. J Am Coll Cardiol Img. 2011;4:659–670. doi: 10.1016/j.jcmg.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 30.Nativi-Nicolau J.N., Karam C., Khella S., Maurer M.S. Screening for ATTR amyloidosis in the clinic: overlapping disorders, misdiagnosis, and multiorgan awareness. Heart Fail Rev. Published online February 20, 2021 doi: 10.1007/s10741-021-10080-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buxbaum J.N., Ruberg F.L. Transthyretin V122I (pV142I)∗ cardiac amyloidosis: an age-dependent autosomal dominant cardiomyopathy too common to be overlooked as a cause of significant heart disease in elderly African Americans. Genet Med. 2017;19:733–742. doi: 10.1038/gim.2016.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamath S.A., Drazner M.H., Wynne J., Fonarow G.C., Yancy C.W. Characteristics and outcomes in African American patients with decompensated heart failure. Arch Intern Med. 2008;168:1152–1158. doi: 10.1001/archinte.168.11.1152. [DOI] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention CDC Health Disparities and Inequalities Report - United States, 2013. MMWR Morb Mortal Wkly Rep. 2013;62(Suppl 3) [PubMed] [Google Scholar]
- 34.Nativi-Nicolau J., Vieira M.C., Bruno M. Demographic characteristics of Medicare beneficiaries with diagnosis of wild-type amyloidosis cardiomyopathy. Circulation. 2019;140:A14637. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.