Abstract
Background
Transthyretin amyloid (ATTR) cardiomyopathy is slowed by tafamidis, which stabilizes the TTR molecule and reduces the formation of amyloidogenic oligomers. Stabilizers in clinical doses raise serum TTR, which may be a surrogate for the degree of stabilization.
Objectives
This study aims to determine, in a non-trial, unselected population of patients with ATTR cardiomyopathy, the effect of tafamidis on serum levels of TTR, and to compare these with published data of changes in TTR.
Methods
TTR levels were measured before therapy and 3 to 12 months following initiation of tafamidis therapy in all patients seen between May 20, 2019, and March 1, 2021, who had a follow-up visits within 12 months of therapy initiation.
Results
Among 72 patients with ATTR cardiomyopathy (67 patients with wild-type and 5 patients with variant TTR), administration of tafamidis increased serum TTR from 21.8 mg ± 0.7 mg/dL to 29.3 ± 0.86 mg/dL, an increase of 34.5%. In 5 patients with variant TTR, the increase was 70.9%, compared to 32.0% in the wild-type patients. Mean N-terminal pro-brain natriuretic peptide increased over a mean follow-up of 21 ± 1.2 weeks, but the change was not statistically significant. Over the same period there was a small increase in high-sensitivity troponin T that was of borderline statistical significance (P = 0.057).
Conclusions
Tafamidis consistently increases serum TTR levels in patients with ATTR cardiomyopathy, consistent with its effect on stabilizing TTR. Measurement of TTR level change post-TTR stabilizing therapy might be a surrogate for stabilization and could be a more accurate measure of drug efficacy than an in vitro nonphysiologic test of stabilization.
Key Words: amyloid cardiomyopathy, tafamidis, transthyretin
Abbreviations and Acronyms: ATTR, transthyretin amyloid; ATTRv, variant transthyretin amyloidosis; NT-proBNP, N-terminal pro-brain natriuretic peptide; TTR, transthyretin
Central Illustration
Transthyretin (TTR), a 55-kDa homotetramer is the second most ubiquitous protein, after albumin, in the body. In normal human serum it is found in a level of 20 to 40 mg/dL and has a half-life of approximately 48 hours. TTR-derived amyloid (ATTR) cardiomyopathy is an increasingly recognized disease characterized by infiltration of the myocardium with either variant or wild-type amyloid derived from transthyretin. One method of treatment is to use small molecules that stabilize TTR, thereby lessening breakdown into amyloidogenic monomers. To date, 3 main stabilizing drugs have been used in the treatment of either ATTR cardiomyopathy or neuropathy; diflunisal, tafamidis, and acoramidis (AG10) (1, 2, 3, 4, 5). Of these, only the first 2 are clinically available, and AG10 is still undergoing evaluation in a pivotal trial. Tafamidis binds to the thyroxine-binding site of the TTR tetramer and inhibits is dissociation into monomers. By doing so, the cascade leading to amyloid formation in susceptible patients is inhibited (3,6). The ATTR-ACT (Safety and Efficacy of Tafamidis in Patients With Transthyretin Cardiomyopathy) trial showed the efficacy of tafamidis in slowing the progression of amyloid cardiomyopathy, with decreased 30-month hospitalizations for congestive heart failure and decreased mortality in tafamidis-treated patients compared to placebo (4). There was a decrement in 6-minute walk test over time in both the placebo and treated group, but with a lesser decrement in the patients treated with tafamidis. N-terminal pro-brain natriuretic peptide (NT-proBNP) increased in both treated and placebo patients but with a statistically significant lower increase among patients receiving tafamidis than among those receiving placebo. Thus, although tafamidis slowed disease progression as measured by morbidity and mortality, it did not stop it. Two explanations may be given for the continued deterioration in clinical status among tafamidis-treated patients. One possibility is that tafamidis fails to fully stabilize the TTR molecule and therefore permits ongoing amyloid deposition in the heart, albeit at a slower rate than among untreated patients. Alternatively, decreased exercise tolerance and increasing NT-proBNP in the tafamidis-treated group may simply represent the natural progression of heart failure in a severely damaged ventricle, despite the absence of further amyloid deposition (7).
The argument that a greater drug-induced TTR stability may improve outcomes seen with has tafamidis has prompted interest in AG10, currently being evaluated in clinical trials (5,8). AG10 has a somewhat different mechanism of stabilization from tafamidis in that it was designed to mimic the structural form of the TTR “super-stabilizer” variant, T119M. AG10 forms hydrogen bonds with serine residues at the same site as the T119M variant and, based on assays of stabilization, it is believed to be a more potent stabilizer of TTR than is tafamidis (9,10). However, a major problem in theorizing improved efficacy of TTR stabilizers based on assays of stabilization is that there are several assays and all are performed under nonphysiologic conditions. In addition, even if a specific drug produces more stabilization in vitro than another, the efficacy in a human or animal model will depend upon the half-life of the drug and its degree of absorption.
As TTR stabilizers do not appear to affect the rate of production of TTR by the liver, an increase in TTR levels after tafamidis use most likely represents a greater half-life of TTR. The 48-hour half-life of TTR in the human is similar to the half-life of tafamidis, and maximal stabilization of TTR after initiation of the bioequivalent formulations tafamidis meglumine 80 mg daily or tafamidis 61 mg daily occurs by 4 weeks after drug initiation (3,11). Therefore, it would seem reasonable that measurement of TTR levels before and after initiation of a clinically effective TTR stabilizer would be a consistent, even if indirect, measure of drug efficacy that potentially overcomes the problems of different in vitro nonphysiologic assays of stabilization. Here, we report the effect of tafamidis meglumine 80 mg or tafamidis 61 mg on TTR levels in an unselected group of patients with amyloid cardiomyopathy seen since the release of tafamidis for general clinical use in the United States.
Methods
The records of all patients seen at our institution who were prescribed tafamidis since its approval by the U.S. Food and Drug Administration from May 20, 2019, until March 1, 2021, were reviewed. Our practice is to routinely measure serum TTR (prealbumin), NT-proBNP, and high-sensitivity troponin T (measured on Roche Diagnostics cobas analyzer by photometrics [prealbumin] and chemiluminescence [NT-proBNP and high-sensitivity troponin]) at each visit to our amyloidosis program. Patients who were initiated on tafamidis were generally asked to return in 3 months to evaluate how they were tolerating the drug, and for a repeat TTR level. Since tafamidis meglumine 80 mg and tafamidis 61 mg are bioequivalent, patients receiving either of these formulations were included and analyzed together (11). Patients were included in this study if they had had a baseline TTR level within 3 months of initiating tafamidis and a repeat level was drawn at their return visit. Not all patients were able to return at 3 months (particularly during the coronavirus disease-2019 pandemic); therefore, we accepted measurement of a post-tafamidis TTR level up to 12 months after initiating therapy. The primary purpose of the study was to determine the effect of tafamidis in clinically approved doses (80 mg or 61 mg) on TTR levels, as well as to determine any changes in the cardiospecific biomarkers, NT-proBNP and high-sensitivity troponin T. Patients were excluded from the study if they had been receiving diflunisal immediately before tafamidis initiation, if they were receiving a lower dose of tafamidis meglumine (which is supplied as 20-mg tablets), or if they had had prior treatment with a TTR-silencing agent.
Transthyretin amyloidosis (ATTR) was diagnosed when either by a positive endomyocardial biopsy or, more commonly, a positive technetium pyrophosphate scan in the absence of a plasma cell dyscrasia and in the presence of an echocardiographic or cardiac magnetic resonance appearance was strongly suggestive of amyloid infiltration. Analysis of data from these patients was approved by Partners Institutional Review Board.
Statistical analysis was performed using GraphPad Prism version 8.0.0 for Windows (GraphPad Software). The changes from baseline were analyzed using the paired Student's t-test, with a P value <0.05 considered as statistically significant. Values are expressed as mean ± SEM unless otherwise specified as mean ± SD. For data not normally distributed (ie, troponin T values and NT-proBNP values), data are presented as median (25th and 75th percentiles [Q1-Q3]) and comparison of the paired data was made with the Wilcoxon signed rank test.
Results
Between May 20, 2019, and March 1, 2021, 135 consecutive patients seen at the Brigham and Women’s Hospital Amyloidosis Program received a prescription for tafamidis. Approximately 18 had previously been taking diflunisal, 12 patients did not start treatment, 2 opted to decrease the dose from 80 mg to 20 mg because of the cost of the drug and 1 received only 20 mg daily caused by the policy of the U.S. Veteran’s Administration. Of the remaining 102 patients, 25 either did not return within the 12-month window or had not reached their 3-month return appointment at the time of data collection. These patients were excluded from analysis, as were 5 patients who had been started on tafamidis by an outside physician without baseline TTR levels. Thus, the study group consisted of 72 patients. The mean age was 79 ± 6 years, and all but 3 were men. Sixty-seven patients had wild-type TTR and the remaining 5 had variant ATTR (4 with V122Ile and 1 woman with Thr60Ala).
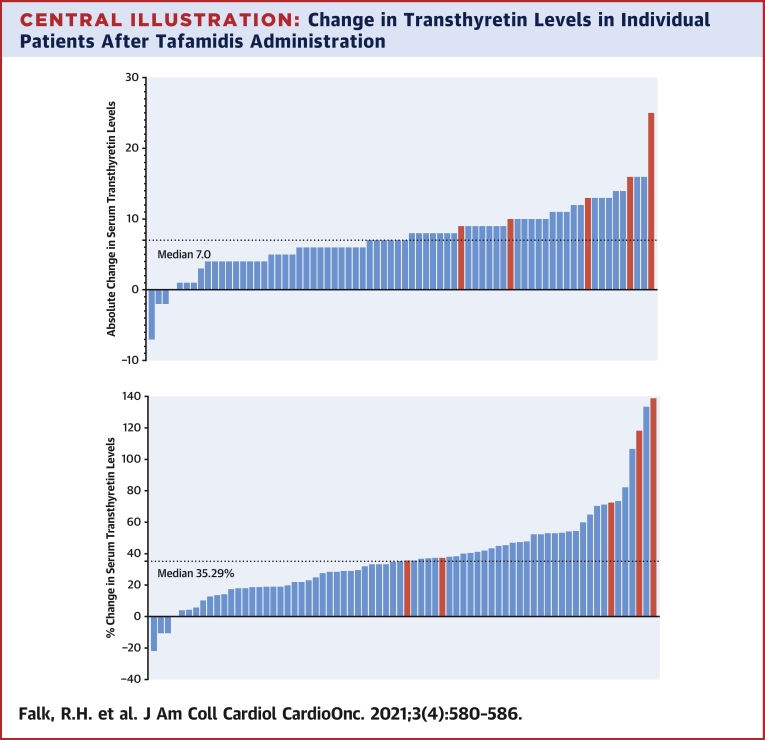
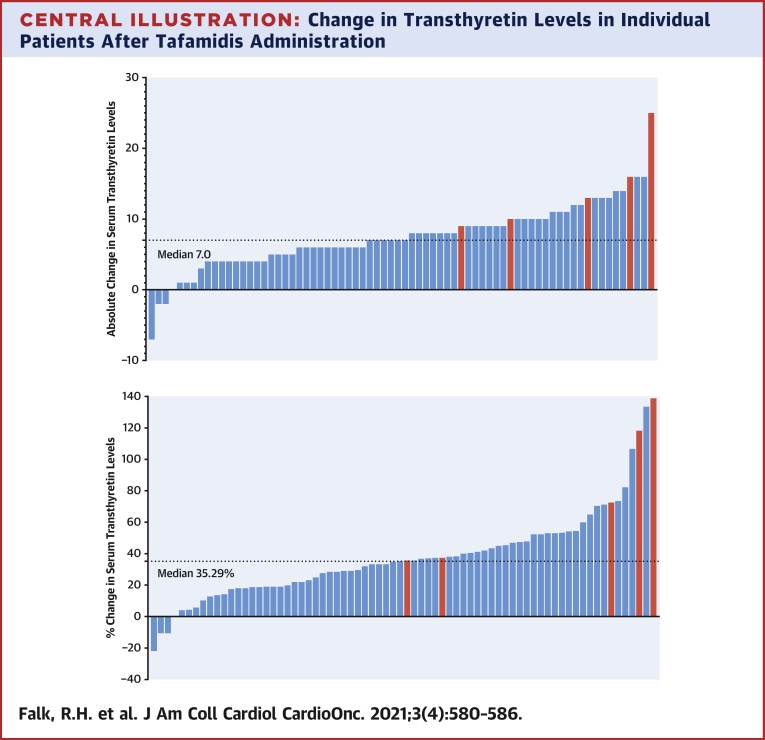
The key data are summarized in Table 1. Mean baseline TTR levels were 21.8 ± 0.7 mg/dL for the whole group of whom 27 (37.0%) had levels ranging from 11 to 19 mg/dL, which is below the lower limit of normal for our laboratory (20 mg/dL). After a mean of 21.0 ± 1.2 weeks therapy, the mean TTR levels for the whole group had increased to 29.3 ± 0.8 mg/dL, a mean increase of 34.5% (P < 0.0001). Twenty-three of 27 patients (85.2%) who fell <20 mg/dL normalized their TTR levels. After exclusion of the 5 patients with variant TTR, the mean baseline TTR in the wild-type TTR patients was 21.9 ± 0.7 mg/dL, increasing to 28.9 ± 0.8 mg/dL, a mean increase of 32.0%. In contrast, the 5 patients with variant ATTR cardiomyopathy had a baseline TTR level of 20.6 ± 2.9 mg/dL but a mean increase of 70.9% to 35.2 ± 3.20 mg/dL. The absolute and percentage changes of TTR levels are graphically shown in the Central Illustration.
Table 1.
Summary of the Effects of Tafamidis on Levels of Transthyretin and Cardiac Biomarkers
| Baseline | Post-Tafamidis | P Values | |
|---|---|---|---|
| Age, y | 79 ± 6.0 | n/a | n/a |
| TTR level all patients, mg/dL (n = 72) | 21.81 ± 0.70 | 29.33 ± 0.76 | <0.001 |
| TTR level ATTRwt (n = 67) | 21.90 ± 0.69 | 28.90 ± 0.76 | <0.001 |
| TTR level ATTRv (n = 5) | 20.6 ± 2.9 | 35.2 ± 3.2 | 0.060 |
| NT-proBNP, pg/mL (n = 70) | 3,301 ± 264 | 3,673 ± 324 | >0.05 |
| HS-troponin T, ng/L (n = 71) | 61.2 ± 3.8 | 66.9 ± 5.1 | 0.057 |
Values are mean ± SEM.
ATTRv = variant transthyretin cardiomyopathy; ATTRwt = wild-type transthyretin cardiomyopathy; HS troponin T = high-sensitivity (5th generation) troponin T; NT-proBNP = N-terminal B-type natriuretic peptide; n/a = not available; TTR = transthyretin.
Central Illustration.
Change in Transthyretin Levels in Individual Patients After Tafamidis Administration
(Top) Absolute change in transthyretin (TTR) levels in individual patients (represented by vertical bars) of TTR levels following administration of tafamidis. Blue bars represent patients with wild-type ATTR cardiomyopathy, and red bars represent those with variant TTR. (Bottom) Percentage change in TTR levels following administration of tafamidis. Color of bars as in top illustration.
To determine the stability of TTR levels in the absence of treatment with TTR stabilizers, 55 patients from the study group were identified who had had TTR levels drawn 3 to 6 months before the level immediately preceding the initiation of tafamidis. The mean TTR level for this group was 22.00 mg/dL immediately before initiating tafamidis compared to 22.04 mg/dL 3 to 6 months earlier (P = 0.95). There were 28 patients who had a second TTR level drawn after starting tafamidis, a mean of 20.5 weeks after the first post-tafamidis level. Among these patients, there was no statistically significant difference in TTR levels between the first post-tafamidis measurement (mean 27.8 ± 1.3 mg/dL) and the later measurement (26.9 ± 1.4 mg/dL).
For the 70 patients who had baseline and follow-up NT-proBNP values, the median baseline NT-proBNP value was 2,831 (1,758 to 4,560) pg/mL. After a mean period of 21 ± 1.2 weeks, the median value was 2,918 (1,688 to 5,537) pg/mL. There was no statistically significant difference in NT-proBNP at baseline and follow-up.
The corresponding numbers for the 70 patients with baseline and follow-up high-sensitivity troponin T were a baseline median high-sensitivity troponin T of 59 (34.0 to 83.8) ng/L and a follow-up median value of 61 (34.8 to 85.8) ng/L. The increase in high-sensitivity troponin between baseline and follow-up just failed to reach statistical significance (P = 0.057)
Discussion
These data, derived from a consecutive cohort of non-trial patients with cardiomyopathy caused by ATTR, show a significant increase in TTR levels after the initiation of tafamidis, with maintenance of this increase in the group of patients who had a second post-tafamidis visit at which a TTR level was drawn. This increase is consistent with the stabilizing effect of tafamidis on the TTR molecule. In contrast, although TTR is a negative acute-phase protein whose levels might be expected to decrease with poor nutritional status or an inflammatory state such as infection, there was a remarkably consistent level of TTR among 55 of the patients among whom 2 pretafamidis TTR levels had been measured between 3 and 6 months apart. This demonstrates that the increase in TTR post-tafamidis was, as anticipated, a result of the stabilizing effect of tafamidis on TTR.
Comparison with other stabilizers
To our knowledge, there are no extensive data on the effect of tafamidis on TTR levels when administered at a clinically approved dose to patients with ATTR cardiomyopathy. In a small study of ATTR patients treated with diflunisal, median TTR levels increased from 24 to 33 mg/dL, but this study was highly selective as to which patients received diflunisal: one-half the treated patients had variant TTR and only 12 patients of 33 treated had baseline and follow-up data (12). A detailed analysis of the effect of AG10 on TTR stabilization was performed on 49 subjects enrolled in a clinical trial (5). Thirty-two received AG10 (at a dose of either 400 mg twice daily or 800 mg twice daily) and 17 received placebo. The mean age was 74.6 years, slightly younger than our cohort, and 11 of 32 AG10-treated patients had variant TTR, predominantly the V122Ile sequence variant. TTR stabilization, measured by fluorescent probe exclusion, was >90% in both AG10-dose groups and in both variant and wild-type TTR. Despite the high TTR stability in both dose groups, patients receiving the higher dose of AG10 had a 50% increase in TTR levels compared to a 36% increase among those receiving the lower dose. This suggests that in vitro stability assays may not fully reflect the true stabilizing effects of a drug in vivo, most likely reflecting the nonphysiologic milieu of all current tests for assessing TTR stabilization. If this is the case, then it might be argued that the change in TTR levels after treatment, although an indirect marker of stabilization, may be a better reflection of true stabilizing effect than the assays.
Variant TTR vs wild-type TTR
An interesting feature of the AG10 study was the finding that patients with variant TTR had twice the percentage increase in TTR levels after treatment (67 ± 42%) than did those with wild-type ATTR cardiomyopathy (33 ± 20%) (5). Judge et al (5) suggest that this might be in part explained by the lower baseline serum TTR levels among variant ATTR patients than among wild-type TTR. Although this may be the case, we noted a similar effect in the small number of patients (n = 5) in our cohort who had variant TTR. Among these patients, the mean increase was 70.9% vs 31.1% in the wild-type TTR patients. Although the number of variant TTR patients is very small, the percentage increase in TTR is similar to that seen in the AG10 trial and is unlikely to just be accounted for by the slightly lower baseline TTR level in our variant patients. It might be hypothesized that a more rapid breakdown of TTR in variant patients results in greater TTR production by the liver. Once TTR is stabilized, this is reflected by a higher serum TTR level than might be expected with wild-type TTR and hence a higher percentage increase. Alternatively, both AG10 and tafamidis may have a greater stabilizing effect of variant TTR than on wild-type that is not captured by the current stabilization assays but is reflected in the response of TTR levels.
Failure to increase TTR levels
Reference to the Figure shows that only 3 patients had decreased TTR levels after tafamidis therapy, all with wild-type TTR. In 1 of these, the decrease could be explained by a severe interim illness and gastrointestinal bleed. A second patient had questionable drug compliance but was included because it was uncertain whether she had been compliant with the full dose, and there was no obvious explanation for the decrease in the third patient.
Effect on biomarkers
Patients receiving tafamidis in the phase 3, randomized, placebo-controlled ATTR-ACT trial showed a progressive increase in NT-proBNP over 30 months, although considerably less than in the placebo-treated group. We did not anticipate any significant change in NT-proBNP in our study given the short study period, relatively small number of subjects, and the large range of baseline NT-proBNP. Nevertheless, although there was no statistical change, there was a trend upward over the short study period of a few months, possibly representing disease progression. In contrast, high-sensitivity troponin T is generally a reproducible measure, with minor increases probably representing true cellular damage. Although the troponin changes were small from baseline to post-tafamidis, they did increase and suggest that, despite TTR stabilization, myocyte dysfunction may progress. This observation may have significance when considering the reason for tafamidis’ effectiveness in slowing (but not stopping) heart failure progression in the ATTR-ACT study. In vitro studies have now clearly shown that preamyloid oligomers, and possibly monomers derived from TTR breakdown, are toxic to neuronal tissue and probably to myocytes (13,14). If, as has been suggested, tafamidis meglumine/tafamidis stabilizes TTR nearly completely, then one would not anticipate further myocyte damage from toxicity and might even see a decrease in high-sensitivity troponin or NT-proBNP, as occurs when pathologic light chains are abolished by chemotherapy in amyloid light chain amyloidosis. On the other hand, if progression of the disease is caused by ongoing damage caused by neurohormonal activation or myocardial fibrosis in the setting of extensive prior amyloid damage, progressive elevation of troponin and NT-proBNP would be anticipated, despite slowing/abolition of further myocardial amyloid infiltration. Further investigation is needed to determine the mechanisms of increasing cardiac biomarkers and progressive heart dysfunction in this disease, particularly when infiltration is advanced.
Is in vitro stabilization likely to be the predominant marker of clinical outcome?
The concept that greater stabilization of TTR by therapeutic molecules with different binding sites may translate into better outcomes may be oversimplistic. In a recent publication, Nelson et al (15) performed a blinded potency comparison the ability of tafamidis, AG10, and tolcapone (a repurposed anti-Parkinsonian drug which stabilizes TTR) at levels equivalent to those obtained in the human in clinically used doses. They concluded that, although AG10 was 4 times more potent than tafamidis at a fixed plasma concentration, the oral administration in humans of 80 mg tafamidis as a single-dose daily (the U.S. Food and Drug Administration–approved dose) was equivalent to the clinical trial dose of 800 mg twice daily of AG10, as both doses reduce the rate of TTR wild-type tetramer dissociation by <96%. This raises the question as to whether any significant difference in clinical efficacy is likely to be found between AG10 and tafamidis despite the greater potency of AG10 and the different mechanism of TTR stabilization.
Study limitations
This study was observational and included a relatively small number of patients. Nevertheless, it is larger than other published studies with TTR stabilizers that reported TTR levels (5,12). Our data on TTR levels in a subgroup of patients within a year of the immediately pretafamidis TTR level show the stability of this level in patients not receiving a stabilizer and confirm the argument that increased levels post-tafamidis are a drug effect and not chance variation. Similarly, the subgroup of patients with follow-up levels after the initial post-tafamidis TTR level show that there was no further increase after a few months, consistent with the short half-life of TTR and the rapid onset of stabilization with tafamidis.
We have postulated that elevation of TTR levels is a marker for TTR stabilization of TTR and that it reflects the mechanism responsible for a positive outcome of tafamidis in the ATTR-ACT trial. Although it might be argued that tafamidis could have a nonstabilizing positive effect on outcome (and thus that changes in levels are merely a bystander phenomenon), we believe this to be very unlikely, as tafamidis was specifically designed to stabilize the TTR molecule and has no known or likely off-target effects that could affect the outcome of a heart failure trial.
Conclusions
In summary, in a non-trial, consecutive, unselected population of patients with ATTR cardiomyopathy predominantly caused by wild-type amyloid deposition, tafamidis had a consistent effect on increasing TTR levels by a mean of 34.5%, or 31.1% when 5 variant TTR patients were removed from analysis. This is virtually identical to the 33% increase seen in as study of AG10, even though those 32 patients were slightly younger and, by virtue of being in a clinical trial, were more highly selected (5). Whether the difference between the effect on TTR levels of tafamidis at clinically approved doses and AG10 at the higher dose is real and, if so, whether the slightly more potent effect of AG10 is of clinical significance cannot be determined by the current findings. However, our results suggest that there may not be much difference in clinical efficacy if it is predictable by change in TTR levels. This is emphasized by the finding in the ATTR-ACT trial that both 20 mg and 80 mg of tafamidis meglumine had identical benefits over placebo during the 30 months of the study despite prior data showing lesser stabilization with the lower dose.
We believe that the jury is still out as to whether a greater stabilization of TTR than that achieved by tafamidis will improve clinical outcomes. Forthcoming data from a randomized trial of AG10 will likely give further insight into the clinical benefit of a potentially more potent stabilizer, although the argument is unlikely ever to be resolved without the unlikely study of a direct comparison of stabilizers (8). In the interim, we believe that measurement of TTR levels before and after treatment is a simple and reasonable surrogate for TTR stabilization, and is useful to assure patients that, at least on the biochemical level, tafamidis is effective in its desired action.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Therapy for ATTR by the TTR stabilizer tafamidis has been shown in a pivotal clinical trial to slow disease progression. TTR breakdown is decreased by tafamidis resulting in an increase in serum levels of TTR. Because there is no current marker to determine the efficacy of tafamidis, measurement of TTR levels offers reassurance to the patient and clinician that the drug is having the desired biochemical effect of stabilization.
TRANSLATIONAL OUTLOOK: Future studies should evaluate various TTR stabilizers to determine whether there is a relationship between the degree of biochemical stabilization, the resultant increase in TTR levels, and the clinical outcome.
Funding Support and Author Disclosures
All authors were supported, in part, by the Antonio Elmaleh and Anne Williams Cardiac Amyloidosis Fund, the Appleby Cardiac Amyloidosis Fund, the Sean McDonough Celebrity Golf Classic Fund for Cardiac Amyloidosis and the Demarest Lloyd Jr. Foundation. Dr Falk has received consulting fees from Ionis Pharmaceuticals, Alnylam Pharmaceuticals, and Caelum Biosciences; and has received research funding from GlaxoSmithKline, Eidos, Akcea, and Pfizer. Dr Dorbala has received research grants from Pfizer/GE Healthcare/Attralus; and has received consulting fees from Pfizer/GE Healthcare/Ionis. Dr Cuddy has received an investigator-initiated research grant from Pfizer; and has received research funding from Ionis and Alnylam.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Adamski-Werner S.L., Palaninathan S.K., Sacchettini J.C., Kelly J.W. Diflunisal analogues stabilize the native state of transthyretin. Potent inhibition of amyloidogenesis. J Med Chem. 2004;47:355–374. doi: 10.1021/jm030347n. [DOI] [PubMed] [Google Scholar]
- 2.Berk J.L., Suhr O.B., Obici L. Repurposing diflunisal for familial amyloid polyneuropathy: a randomized clinical trial. JAMA. 2013;310:2658–2667. doi: 10.1001/jama.2013.283815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maurer M.S., Grogan D.R., Judge D.P. Tafamidis in transthyretin amyloid cardiomyopathy: effects on transthyretin stabilization and clinical outcomes. Circ Heart Fail. 2015;8:519–526. doi: 10.1161/CIRCHEARTFAILURE.113.000890. [DOI] [PubMed] [Google Scholar]
- 4.Maurer M.S., Schwartz J.H., Gundapaneni B. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379:1007–1016. doi: 10.1056/NEJMoa1805689. [DOI] [PubMed] [Google Scholar]
- 5.Judge D.P., Heitner S.B., Falk R.H. Transthyretin stabilization by AG10 in symptomatic transthyretin amyloid cardiomyopathy. J Am Coll Cardiol. 2019;74:285–295. doi: 10.1016/j.jacc.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Bulawa C.E., Connelly S., Devit M. Tafamidis, a potent and selective transthyretin kinetic stabilizer that inhibits the amyloid cascade. Proc Natl Acad Sci U S A. 2012;109:9629–9634. doi: 10.1073/pnas.1121005109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falk R.H. Tafamidis for transthyretin amyloid cardiomyopathy: the solution or just the beginning of the end? Eur Heart J. 2019;40:1009–1012. doi: 10.1093/eurheartj/ehy697. [DOI] [PubMed] [Google Scholar]
- 8.Eidos Therapeutics. Efficacy and Safety of AG10 in Subjects With Transthyretin Amyloid Cardiomyopathy. 2019. Accessed April 14, 2021. https://ClinicalTrials.gov/show/NCT03860935
- 9.Miller M., Pal A., Albusairi W. Enthalpy-driven stabilization of transthyretin by AG10 mimics a naturally occurring genetic variant that protects from transthyretin amyloidosis. J Med Chem. 2018;61:7862–7876. doi: 10.1021/acs.jmedchem.8b00817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou S., Ge S., Zhang W. Conventional molecular dynamics and metadynamics simulation studies of the binding and unbinding mechanism of TTR stabilizers AG10 and tafamidis. ACS Chem Neurosci. 2020;11:3025–3035. doi: 10.1021/acschemneuro.0c00338. [DOI] [PubMed] [Google Scholar]
- 11.Lockwood P.A., Le V.H., O'Gorman M.T. The bioequivalence of tafamidis 61-mg free acid capsules and tafamidis meglumine 4 × 20-mg capsules in healthy volunteers. Clin Pharmacol Drug Dev. 2020;9:849–854. doi: 10.1002/cpdd.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lohrmann G., Pipilas A., Mussinelli R. Stabilization of cardiac function with diflunisal in transthyretin (ATTR) cardiac amyloidosis. J Card Fail. 2020;26:753–759. doi: 10.1016/j.cardfail.2019.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dasari A.K.R., Arreola J., Michael B., Griffin R.G., Kelly J.W., Lim K.H. Disruption of the CD loop by enzymatic cleavage promotes the formation of toxic transthyretin oligomers through a common transthyretin misfolding pathway. Biochemistry. 2020;59:2319–2327. doi: 10.1021/acs.biochem.0c00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dasari A.K.R., Hughes R.M., Wi S. Transthyretin aggregation pathway toward the formation of distinct cytotoxic oligomers. Sci Rep. 2019;9:33. doi: 10.1038/s41598-018-37230-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson L.T., Paxman R.J., Xu J., Webb B., Powers E.T., Kelly J.W. Blinded potency comparison of transthyretin kinetic stabilisers by subunit exchange in human plasma. Amyloid. 2021;28:24–29. doi: 10.1080/13506129.2020.1808783. [DOI] [PMC free article] [PubMed] [Google Scholar]