Abstract
Phthalates are used in many consumer products, leading to daily human exposure. Although many studies focus on single phthalates, humans are exposed to mixtures of phthalates. Our laboratory created a phthalate mixture consisting of six different phthalates and found that it negatively affected female reproduction and accelerated some biomarkers of reproductive aging. However, it was unknown if prenatal exposure to the mixture accelerates the natural decline in reproductive capacity and ovarian aging in mice. Therefore, we tested the hypothesis that prenatal exposure to a phthalate mixture accelerates the age-related decline in reproductive capacity and biomarkers of ovarian aging in the F1 generation of mice. Pregnant CD-1 dams were orally dosed with control or phthalate mixture (20 µg/kg/day–200 mg/kg/day) daily from gestational day 10—birth. The F1 female pups were aged to 11–13 months, and then estrous cyclicity and breeding trials were conducted at 11 and 13 months. Ovaries were collected from the F1 females at 13 months to examine biomarkers of ovarian aging. Prenatal exposure to the phthalate mixture decreased the time the F1 females spent in proestrus and the ability of the F1 females to give birth at 11 and 13 months of age compared to control. In contrast, prenatal exposure to the mixture did not affect biomarkers of direct aging of the ovary in the F1 generation. Collectively, our data show that prenatal phthalate mixture exposure accelerates the natural age-related decline in reproductive capacity but may not affect some biomarkers of ovarian aging in the F1 generation.
Keywords: ovary, multigenerational, phthalates, mixture, reproductive aging, fertility
Introduction
Phthalates are a group of chemicals abundantly used in the world today that have been shown to cause harmful effects to the endocrine system [1–3]. Phthalates are used to produce many consumer products, including nail polishes, perfumes, medical bags and intravenous (IV) tubing, children’s toys, and building materials [4, 5]. Additionally, phthalates have been found in household dust [6, 7]. With this constant exposure, humans are exposed daily via ingestion, inhalation, and dermal contact [8]. However, ingestion is the most common route of exposure [8].
Many toxicological studies have examined the effects of phthalates on reproduction, but many of these studies focus on single phthalate exposure [9–14] when in fact we are exposed to mixtures of different phthalates on a daily basis due to the widespread use of these chemicals. Therefore, our laboratory developed a mixture of six phthalates based on the phthalate metabolite levels in urine of pregnant women in central Illinois [15]. The phthalates in the mixture include diethyl phthalate (DEP), dibutyl phthalate (DBP), diisobutyl phthalate (DiBP), di(2-ethylhexyl) phthalate (DEHP), benzylbutyl phthalate (BzBP), and di-isononyl phthalate (DiNP). Our laboratory found that prenatal exposure to the phthalate mixture caused detrimental effects on female reproduction, including increased uterine weights, decreased anogenital distance, altered estrous cyclicity, and disrupted fertility ranging from postnatal day 0–9 months of age in a multiple and transgenerational manner in female mice [16, 17].
We recently determined that prenatal exposure to the mixture accelerated some biomarkers of reproductive aging in multiple generations of aging female mice [18]. We found that the mixture dysregulated hormone levels and increased cystic ovaries in the F1 generation, decreased the percentage of antral follicles and testosterone levels in the F2 generation, and it caused irregular cyclicity, decreased the percentage of ovarian follicles, and increased the occurrence of cysts in the F3 generation of mice [18]. Acyclicity, decreased sex steroid hormone and inhibin B levels, increased gonadotropin hormone levels, and a decreased ovarian follicle pool are key characteristics of reproductive aging in females [19–22]. In addition, rodents display cystic ovaries as they age [23]. Together, these biomarkers of reproductive aging can lead to decreased fertility.
In addition to the aforementioned characteristics of reproductive aging in females, the ovary also exhibits direct signs of aging. The direct signs of ovarian aging include a drastic decrease in the ovarian follicle pool and the quality of oocytes. Additionally, the aging ovary accumulates reactive oxygen species and has increased cell death and fibrosis [24–31]. Moreover, the health of the oocyte can deteriorate with aging due to altered cell cycle progression and DNA repair, increased apoptosis, and increased oxidative stress [24–28, 32]. Besides the oocyte, the ovarian stroma endures drastic effects with aging. The stroma has many different components, including immune cells, ovarian surface epithelium, tunica albuginea, rete ovarii, and the ovarian extracellular matrix [27, 29–31, 33]. Studies have shown that the stroma displays increased fibrosis with aging that could be due to decreases in the remodeling of the extracellular matrix in the ovary [27, 29–31].
Due to our previous findings that prenatal exposure to a phthalate mixture accelerated some biomarkers of reproductive aging [18], the goal of these studies was to examine if the phthalate mixture accelerates the natural age-related decline in fertility in aging female mice by monitoring their estrous cyclicity and performing breeding tests at 11 and 13 months of age. Additionally, because we previously determined that prenatal exposure to the phthalate mixture may accelerate some biomarkers of reproductive aging in the ovary (e.g. increased cysts and altered folliculogenesis) [18], we determined whether the phthalate mixture accelerates the aging of the ovary by decreasing the expression of antioxidant enzymes, increasing the expression of apoptotic factors and cell cycle regulators, altering the expression of DNA repair genes, and increasing the amount of fibrosis in the aging mouse ovary sooner than control animals. Thus, this study tested the hypothesis that prenatal exposure to an environmentally relevant phthalate mixture accelerates the natural age-related decline in reproductive capacity and the occurrence of biomarkers of direct aging of the ovary in the F1 generation of female mice.
Methods
Chemicals
DEP, DBP, DiBP, DEHP, BzBP, and DiNP were purchased from Sigma-Aldrich (St. Louis, MO). The phthalate mixture was made up of 35.22% DEP, 21.03% DEHP, 14.91% DBP, 15% DiNP, 8% DiBP, and 5.13% BzBP. The percentages of each phthalate in the mixture were calculated from levels of phthalate metabolites measured in urine from pregnant women in central Illinois (iKids study) [15–18]. The mixture was thoroughly mixed and diluted in tocopherol-stripped corn oil (vehicle control). The doses used for this study were 20 µg/kg/day, 200 µg/kg/day, and 200 mg/kg/day. Our laboratory has previously conducted multiple experiments using these doses of the phthalate mixture and found that they negatively affected female reproduction [16–18, 34]. Furthermore, our laboratory has determined that the individual phthalates in the mixture for the 20 and 200 µg/kg/day doses fall within the ranges of daily human exposure, infant exposure, and occupational exposure [18, 35, 36]. Specifically, in humans, the DEHP daily exposure range is 3–30 µg/kg/day, the DEP daily exposure range is 2.32–12 µg/kg/day, the BzBP daily exposure range is 0.26–0.88 µg/kg/day, the DBP daily exposure range is 0.84–5.22 µg/kg/day, and the DiBP daily exposure range is 0.12–1.4 µg/kg/day. In addition, the DiNP occupational exposure can reach 26 µg/kg/day, and it can reach 120 µg/kg/day in infants [17, 18]. The dose of DEHP used in the 20 µg mixture is about 4.2 µg (21.03% of the 20 µg mixture), which is in the range of daily human exposure. The dose of DEHP in the 200 µg mixture is about 42 µg (21.03% of the 200 µg mixture), which mimics daily human exposure. The dose of DEP used in the 20 µg mixture is about 7 µg (35.22% of the 20 µg mixture), which is in the range of daily human exposure. The dose of BzBP used in the 20 µg mixture is about 1 µg (5.13% of the 20 µg mixture), which is close to the reported range of daily human exposure. The dose of DBP used in the 20 µg mixture is about 3 µg (14.91% of the 20 µg mixture), which mimics daily human exposure. The dose of DiBP used in the 20 µg mixture is about 1.6 µg (8% of the 20 µg mixture), which is close to the reported range of daily human exposure. Finally, the dose of DiNP used in the 200 µg mixture is about 30 µg (15% of the 200 µg mixture), which mimics the occupational exposure level [17, 18]. In addition, the highest dose of 200 mg/kg/day was chosen so that we could compare our results to other studies examining single phthalate exposure on female reproduction [9–12, 37–40].
Animals
Cycling adult female CD-1 mice and adult male CD-1 mice were purchased from Charles River Laboratories (Wilmington, Massachusetts) and housed in the animal facility at the College of Veterinary Medicine at the University of Illinois at Urbana-Champaign (Champaign, IL). Animals were housed individually at 25°C in polysulfone cages with a light/dark cycle of 12:12 hours. Facility temperature was maintained at 21.1 ± 2.2°C, and humidity was maintained at 50 ± 20%. Mice were given the Teklad Rodent Diet 8604 and reverse-osmosis filtered high-purity water ad libitum. The mice were acclimated to the facility for at least 1 week prior to beginning the experiment. All animal handling and animal procedures, including euthanasia and tissue collections, were approved by the University of Illinois Institutional Animal Care and Use Committee.
Dosing and Experimental Design
At 8 weeks of age, female mice (F0) were mated with nontreated males to create the F1 generation of mice. Females were monitored for the presence of a copulatory vaginal sperm plug. Once a vaginal sperm plug was observed, females were separated from males and individually housed. Females were weighed biweekly to confirm pregnancy. Once the pregnant females (F0) reached gestational day 10, oral dosing began at the same time every day with either the vehicle control (tocopherol-stripped corn oil) or one of the doses of the mixture (20 and 200 µg/kg/day and 200 mg/kg/day). Dosing continued every day until the birth of pups. The dosing regimen was chosen to mimic daily human exposure, and the dosing window was chosen because this is a critical time period for ovarian development in the mouse. Pups born from the pregnant F0 generation were considered the F1 generation, and these pups were exposed directly to vehicle or phthalate mixture as the developing pup within the F0 pregnant dam.
Each dam is considered the experimental unit. Thus, in this study, two F1 female mice from each dam (n = 9–10 dams per treatment group) were used for experiments. One F1 mouse per dam was designated for tissue collection at 13 months of age. This mouse was used to monitor fertility outcomes at 3, 6, and 9 months of age before tissue collections [16]. When mice reached 13 months of age, they were euthanized by CO2 asphyxiation followed by cervical dislocation during late metestrus or diestrus. Whole ovaries were collected from each mouse. One ovary was fixed in Dietrich’s fixative for the picrosirius red (PSR) staining, and the other ovary was immediately snap frozen and stored at −80°C for RNA extraction. Another F1 mouse from each dam was used for breeding trials and fertility indices at 11 and 13 months of age. These females were group housed with mice in the same treatment group until they reached 11 months of age. At 11 months of age, the mice were housed individually for at least 1 week to acclimate before monitoring estrous cyclicity.
Estrous Cyclicity
Mice were vaginally lavaged with 1× phosphate-buffered saline daily for 14 days before each breeding trial occurring at 11 and 13 months of age (n = 8–10 females per treatment group). Stages of estrus were determined by previously defined criteria [41]. Metestrus and diestrus were combined because they are similar in cytology and hormone profile. The percentage of days spent in each cycle was calculated by the number of days in each cycle divided by 14 and multiplying that value by 100.
Breeding Trials and Fertility Indices
At 11 and 13 months of age following the monitoring of the estrous cycle, F1 female mice were mated with untreated males to examine different fertility outcomes (n = 8–10 females per treatment group). The methods for breeding trials and fertility indices have been previously described by our laboratory [11, 13, 14, 16, 17]. Females were weighed on the day of mating and again on the day a copulatory sperm plug was found. Females were monitored twice a day for the presence of a sperm plug to indicate successful mating. If a sperm plug was found, the weight of the female was recorded, and the mouse was put into a new cage individually. Mice were given 14 days to become pregnant because they should have cycled three or four times in that period. The time to plug was recorded and analyzed. All female mice were weighed twice a week to monitor weight gain regardless of a sperm plug. If a vaginal sperm plug was not observed, females were still housed individually in a new cage and their weight was recorded after 14 days. Females were considered pregnant if: (i) a sperm plug was observed and they gained at least 4 g, (ii) a sperm plug was not observed, but they gained at least 4 g, or (iii) a sperm plug was not observed, but they gave birth to pups. Females were checked twice a day for the birth of pups. Length of gestation was calculated by recording the day of the observed sperm plug and the day of birth.
In addition, different F1 fertility indices were calculated, including mating index, pregnancy rate, fertility index, gestational index, and the index of the number of F1 females who gave birth. Specifically, mating index was calculated by taking the number of females with sperm plugs divided by the number of total females bred × 100. Pregnancy rate was determined by taking the number of pregnant females divided by the number of total females bred × 100. Fertility index was calculated by taking the number of pregnant females divided by the number of females with a sperm plug × 100. Gestational index was calculated by taking the number of females who gave birth to pups divided by the number of pregnant females × 100. Last, the index for females who gave birth was calculated by dividing the number of females who gave birth to pups by the number of total females bred × 100. If a female gave birth to pups (F2), the total number of pups, number of males and females, average weight of live pups, and number of dead pups were recorded. The percentage of females was calculated by taking the number of live female pups divided by the total number of pups × 100. Pup mortality was calculated by dividing the number of pups who died by the total number of pups in the litter × 100. If only dead pups or body parts were found, this was considered 100% mortality. Additionally, if the number of total pups or the number of females or males could not be determined due to cannibalism, data were not used for certain parameters. Last, the average weight of each litter was measured by weighing all live pups on postnatal day 0 and dividing the total weight by the number of live pups.
PSR Staining of Ovaries and Imaging
Ovaries from F1 females were collected at 13 months of age (n = 7–10 females per treatment group). After being fixed in Dietrich’s solution, ovaries were embedded in paraffin and sectioned at 5 µm. After sectioning, slides were stained with PSR. All slides were stained at the same time to minimize variability. We used the protocol for PSR staining and imaging adapted from Dr. Francesca Duncan’s laboratory at Northwestern University as previously described [29–31]. Slides were scanned using a 20× objective with the Hamamatsu NanoZoomer 2.0 HT (Model #C9600-12) using NDP.scan 3.2.15. To quantify the area of ovarian tissue that stained positively for PSR staining, ImageJ software was used to set a threshold based on the staining in the mouse that appeared to have the most PSR staining, and this was kept constant for all sections [29–31].
Gene Expression Analysis
Frozen whole F1 ovaries collected at 13 months were used for quantitative real-time polymerase chain reaction (qPCR) analysis (n = 4–5 ovaries per treatment group). Total RNA (>100 ng) was extracted from the whole ovaries using the RNeasy Mini Kit (Qiagen, Austin, TX, USA) according to the manufacturer’s protocol, including DNase digestion. Total RNA (100 ng) was reverse transcribed to complementary DNA (cDNA) using the iScript RT Kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA) according to the manufacturer’s protocol. Each cDNA sample was diluted 1:8 using nuclease-free water prior to qPCR analysis. Analysis of qPCR was performed using the CFX96 C1000 Real-Time PCR Detection System and CFX Manager Software (Bio-Rad Laboratories, Inc., Hercules, CA, USA) according to the manufacturer’s protocol. All qPCR reactions were conducted in duplicate using 2 μL of cDNA, forward and reverse primers (5 pmol) for select genes, nuclease-free water, and SsoFastEvaGreen Supermix for a final reaction volume of 10 μL. The selected genes included the housekeeping gene beta-acin (Actb), the antioxidant enzymes superoxide dismutase 1 (Sod1), glutathione peroxidase (Gpx), catalase (Cat), and glutathione reductase (Gsr), the anti-apoptotic factor B cell leukemia/lymphoma 2 (Bcl2), the pro-apoptotic factor Bcl2-associated X protein (Bax), the cell cycle regulators cyclin-dependent kinase inhibitor 1A (Cdkn1a), cyclin-dependent kinase inhibitor 2A (Cdkn2a), cyclin-dependent kinase inhibitor 1B (Cdkn1b), and the DNA repair genes tumor suppressor gene p53 (p53) and breast cancer 2 (Brca2) (Table 1).
Table 1:
Sequences of primer sets used for gene expression analysis
| Primer sequence | |||
|---|---|---|---|
| Gene symbol | Gene name | Forward | Reverse |
| Bax | BCL2-associated X protein | TGAAGACAGGGGCCTTTTTG | AATTCGCCGGAGACACTCG |
| Bcl2 | B cell leukemia/lymphoma 2 | ATGCCTTTGTGGAACTATATGGC | GGTATGCACCCAGAGTGATGC |
| Cat | Catalase | GCAGATACCTGTGAACTGTC | GTAGAATGTCCGCACCTGAG |
| Gpx | Glutathione peroxidase | TTCGGACACCAGGAGAATGG | TAAAGAGCGGGTGAGCCTTC |
| Gsr | Glutathione reductase | CAGTTGGCATGTCATCAAGCA | CGAATGTTGCATAGCCGTGG |
| p53 | Tumor suppressor gene p53 | CTCTCCCCCGCAAAAGAAAAA | CGGAACATCTCGAAGCGTTTA |
| Sod1 | Superoxide dismutase 1 | TTCCGTCCGTCGGCTTCTCGT | CGCACACCGCTTTCATCGCC |
| Brca2 | Breast cancer 2 | GGGAGTCCCTTCACTTCAGC | GGCACTCGTCTGACAGGTAG |
| Cdkn1a | Cyclin-dependent kinase inhibitor 1A | TTAGGCAGCTCCAGTGGCAACC | ACCCCCACCACCACACACCATA |
| Cdkn2a | Cyclin-dependent kinase inhibitor 2A | GCTCTGGCTTTCGTGAACAT | CGAATCTGCACCGTAGTTGA |
| Cdkn1b | Cyclin-dependent kinase inhibitor 1B | GTTTCAGACGGTTCCCCGAA | TTCTTAATTCGGAGCTGTTTACGTC |
The CFX96 C1000 Real-Time PCR Detection machine quantifies the amount of PCR product generated by measuring SsoFastEvaGreen dye (Bio-Rad Laboratories, Inc., Hercules, CA) that fluoresces when bound to double-stranded DNA. The qPCR program consisted of an enzyme activation step (95°C for 1 min), an amplification and quantification program (40 cycles of 95°C for 10 s, 60°C for 10 s, single fluorescence reading), at 72°C for 5 min, a melt curve (65°C–95°C heating 0.5°C/s with continuous fluorescence readings), and a final step at 72°C for 5 min per the manufacturer’s protocol. All gene expression data were normalized to the housekeeping gene. Relative fold changes were calculated as the ratio of each treatment group to the control group level and analyzed using a mathematical model for relative quantification of real-time PCR data developed by Pfaffl [42].
Statistical Analysis
If data were normally distributed and met the assumption of homogeneity of variance, a one-way analysis of variance was performed and the post hoc test of Dunnett (two-sided) was used. However, if equal variances were not assumed, the post hoc test of Games–Howell was used. If data did not meet normality or were presented as percentages, independent sample Kruskal–Wallis H tests were used to compare each treatment group, followed by the Mann–Whitney U test. Nominal data were analyzed using a one-sided Fisher’s exact test. Statistical significance was assigned P ≤ 0.05, but when P-values were greater than 0.05, but less than 0.1, data were considered to exhibit a trend toward significance and were denoted by a caret. In the few instances where the treatment group n was less than 3 (i.e. 1 or 2), that treatment group was not included in statistical analysis. The software SPSS (SPSS Inc., Chicago, IL) was used for data analysis.
Results
Effect of Prenatal Exposure to the Phthalate Mixture on Estrous Cyclicity of F1 Female Mice at 11 and 13 Months
The estrous cycle was monitored for 14 days prior to fertility testing at 11 and 13 months of age in F1 female mice. At 11 months, the phthalate mixture (200 µg/kg/day) caused a borderline decrease in the time spent in proestrus, but it did not affect the time spent in estrus or metestrus/diestrus compared to control (Fig. 1A; n = 8–10 females per treatment group, ^ 0.05 < P < 0.1). At 13 months of age, the phthalate mixture (20 µg/kg/day and 200 mg/kg/day) significantly decreased the amount of time mice spent in proestrus, but it did not affect the time spent in estrus or metestrus/diestrus compared to control (Fig. 1B; n = 8–10 females per treatment group, *P ≤ 0.05).
Figure 1:
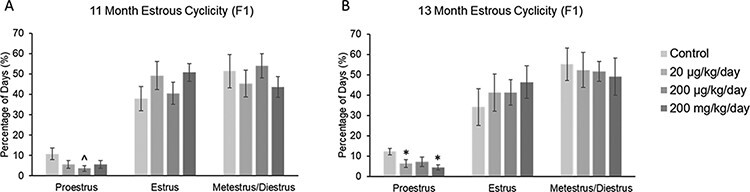
Effect of prenatal exposure to the phthalate mixture on estrous cyclicity at 11 and 13 months of age in the F1 generation of female mice. Percentage of days in proestrus, estrus, and metestrus/diestrus were calculated and compared to controls in each treatment group at 11 months (panel A: control = 10 females/treatment group, 20 µg/kg/day = 9 females/treatment group, 200 µg/kg/day = 8–9 females/treatment group, 200 mg/kg/day = 9 females/treatment group) and 13 months (panel B: control = 10 females/treatment group, 20 µg/kg/day = 9 females/treatment group, 200 µg/kg/day = 9 females/treatment group, 200 mg/kg/day = 8 females/treatment group). Graphs represent mean percentages ± SEM. Asterisks (*) indicate significant differences compared to the control (P < 0.05) and carets (^) indicate borderline significance compared to the control (0.05 < P < 0.1)
Effect of Prenatal Exposure to the Phthalate Mixture on F1 Fertility at 11 and 13 Months
At 11 months of age, prenatal exposure to the phthalate mixture did not affect mating index, pregnancy rate, fertility index, or gestational index in the F1 females compared to control (Fig. 2A–D; n = 6–10 females per treatment group). Furthermore, the phthalate mixture did not affect time to pregnancy or gestation length in the F1 females compared to control (Supplemental Fig. 1a and b). However, the phthalate mixture (200 µg/kg/day and 200 mg/kg/day) caused a borderline decrease in the percentage of F1 females who gave birth compared to control (Fig. 2E; n = 8–10 females per treatment group, ^ 0.05 < P < 0.1).
Figure 2:
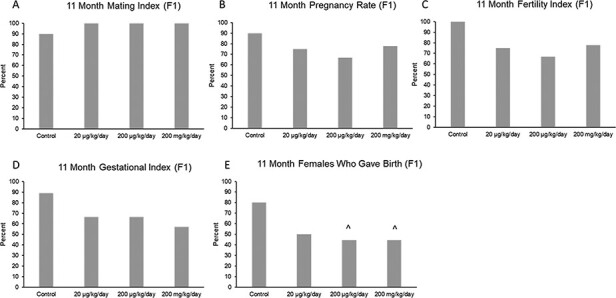
Effects of prenatal exposure to the phthalate mixture on fertility indices at 11 months of age in the F1 generation of female mice. Breeding was monitored by examining mating index (panel A: control = 10 females/treatment group, 20 µg/kg/day = 8 females/treatment group, 200 µg/kg/day = 9 females/treatment group, 200 mg/kg/day = 9 females/treatment group), pregnancy rate (panel B: control = 10 females/treatment group, 20 µg/kg/day = 8 females/treatment group, 200 µg/kg/day = 9 females/treatment group, 200 mg/kg/day = 9 females/treatment group), fertility index (panel C: control = 10 females/treatment group, 20 µg/kg/day = 8 females/treatment group, 200 µg/kg/day = 9 females/treatment group, 200 mg/kg/day = 9 females/treatment group), gestational index (panel D: control = 9 females/treatment group, 20 µg/kg/day = 6 females/treatment group, 200 µg/kg/day = 6 females/treatment group, 200 mg/kg/day = 7 females/treatment group), and females who gave birth (panel E: control = 10 females/treatment group, 20 µg/kg/day = 8 females/treatment group, 200 µg/kg/day = 9 females/treatment group, 200 mg/kg/day = 9 females/treatment group). Graphs represent mean percentages in the F1 generation at 11 months. Carets (^) indicate borderline significance compared to the control (0.05 < P < 0.1)
When examining the pups born to the F1 dams at 11 months, the phthalate mixture did not affect the litter size or the average live pup weight (Supplemental Fig. 1c and d), but the mixture (20 µg/kg/day and 200 mg/kg/day) decreased the F2% female pups and it (20 µg/kg/day) decreased the percentage of F2 pup mortality compared to control (Fig. 3A and B; n = 3–8 females per treatment group, except n = 2 in the 200 μg/kg/day group for percentage of female pups, *P ≤ 0.05, ^ 0.05 < P < 0.1).
Figure 3:
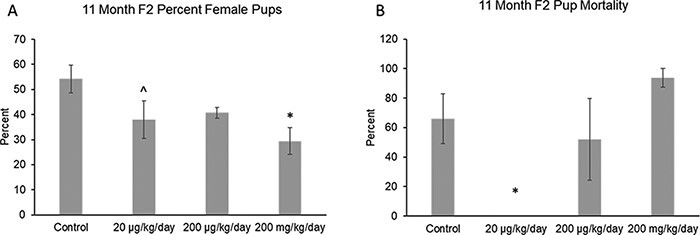
Effects of prenatal exposure to the phthalate mixture on F2 pup outcomes after breeding the F1 generation of female mice at 11 months of age. The percentage of female pups (panel A: control = 5 females/treatment group, 20 µg/kg/day = 4 females/treatment group, 200 µg/kg/day = 2 females/treatment group, 200 mg/kg/day = 3 females/treatment group) and pup mortality (panel B: control = 8 females/treatment group, 20 µg/kg/day = 4 females/treatment group, 200 µg/kg/day = 4 females/treatment group, 200 mg/kg/day = 4 females/treatment group) was analyzed. Graphs represent mean percentages ± SEM in the F1 generation at 11 months. Asterisks (*) indicate significant differences compared to the control (P < 0.05) and carets (^) indicate borderline significance compared to the control (0.05 < P < 0.1)
At 13 months of age, the phthalate mixture did not affect gestation length (Supplemental Fig. 2a), mating index, pregnancy rate, or fertility index in the F1 females compared to control (Fig. 4B–D; n = 8–10 females per treatment group). However, the phthalate mixture (200 µg/kg/day) decreased time to pregnancy, it (200 µg/kg/day) decreased gestational index, and it (200 µg/kg/day) decreased the percentage of F1 females who gave birth compared to control (Fig. 4A,E and F; n = 5–10 females per treatment group, *P ≤ 0.05, ^ 0.05 < P < 0.1).
Figure 4:
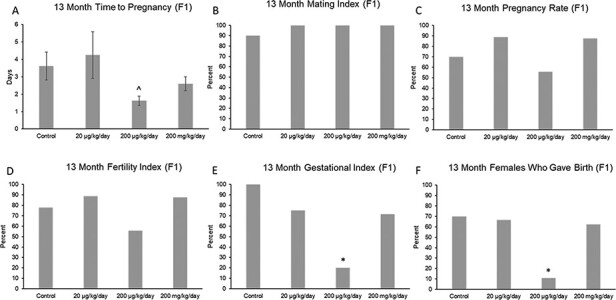
Effects of prenatal exposure to the phthalate mixture on time to mating and fertility indices at 13 months of age in the F1 generation of female mice. Breeding was monitored by examining time to pregnancy (panel A: control = 8 females/treatment group, 20 µg/kg/day = 8 females/treatment group, 200 µg/kg/day = 8 females/treatment group, 200 mg/kg/day = 5 females/treatment group), mating index (panel B: control = 10 females/treatment group, 20 µg/kg/day = 9 females/treatment group, 200 µg/kg/day = 9 females/treatment group, 200 mg/kg/day = 8 females/treatment group), pregnancy rate (panel C: control = 10 females/treatment group, 20 µg/kg/day = 9 females/treatment group, 200 µg/kg/day = 9 females/treatment group, 200 mg/kg/day = 8 females/treatment group), fertility index (panel D: control = 10 females/treatment group, 20 µg/kg/day = 9 females/treatment group, 200 µg/kg/day = 9 females/treatment group, 200 mg/kg/day = 8 females/treatment group), gestational index (panel E: control = 7 females/treatment group, 20 µg/kg/day = 8 females/treatment group, 200 µg/kg/day = 5 females/treatment group, 200 mg/kg/day = 7 females/treatment group), and females who gave birth (panel F: control = 10 females/treatment group, 20 µg/kg/day = 9 females/treatment group, 200 µg/kg/day = 9 females/treatment group, 200 mg/kg/day = 8 females/treatment group). Graphs represent mean percentages (± SEM for time to pregnancy) in the F1 generation at 13 months. Asterisks (*) indicate significant differences compared to the control (P < 0.05) and carets (^) indicate borderline significance compared to the control (0.05 < P < 0.1)
At 13 months, the phthalate mixture did not affect F2 litter size or average F2 live pup weight (Supplemental Fig. 2b and c), but it (20 µg/kg/day) decreased the F2% female pups compared to control (Fig. 5A; n = 3–5 females in each treatment group, except n = 1 in the 200 μg/kg/day and 200 mg/kg/day treatment groups, *P ≤ 0.05). Lastly, the phthalate mixture did not affect F2 pup mortality at 13 months of age compared to control (Fig. 5B; n = 5–6 females in each treatment group, except n =1 in the 200 μg/kg/day treatment group).
Figure 5:
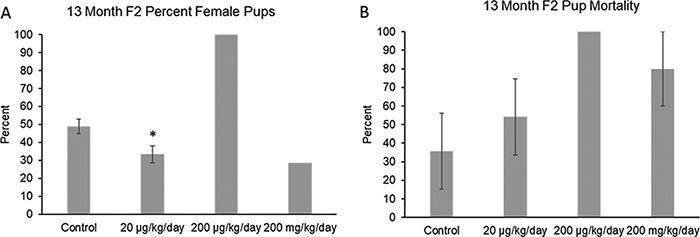
Effects of prenatal exposure to the phthalate mixture on F2 pup outcomes after breeding the F1 generation of female mice at 13 months of age. The percentage of female pups (panel A: control = 4 females/treatment group, 20 µg/kg/day = 3 females/treatment group, 200 µg/kg/day = 1 female/treatment group, 200 mg/kg/day = 1 female/treatment group) and pup mortality (panel B: control = 6 females/treatment group, 20 µg/kg/day = 6 females/treatment group, 200 µg/kg/day = 1 female/treatment group, 200 mg/kg/day = 5 females/treatment group) was analyzed. Graphs represent mean percentages ± SEM in the F1 generation at 13 months. Asterisks (*) indicate significant differences compared to the control (P < 0.05)
Effect of Prenatal Exposure to the Phthalate Mixture on Fibrosis of F1 Ovaries at 13 Months
Ovarian sections from 13-month-old mice were stained with PSR to examine the amount of fibrous collagen in the ovary (Fig. 6B). PSR staining (dark red staining) was observed in all ovaries. The phthalate mixture did not affect the percentage of PSR staining, or fibrosis, found in the F1 ovaries at 13 months of age compared to control (Fig. 6A; n = 7–10 females per treatment group).
Figure 6:
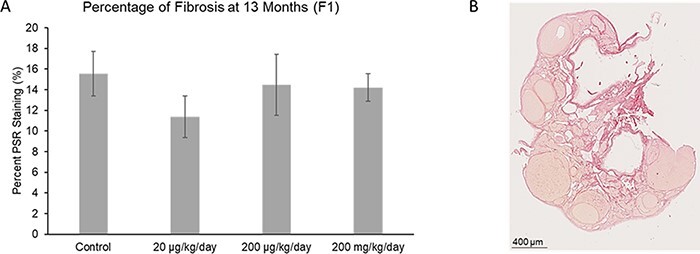
Effect of prenatal exposure to the phthalate mixture on the percentage of fibrosis in the ovary at 13 months of age in the F1 generation of female mice. Ovaries were sectioned and stained with PSR to evaluate the amount of fibrosis. The percentage of fibrosis was analyzed for each treatment group (panel A: control = 10 females/treatment group, 20 µg/kg/day = 6 females/treatment group, 200 µg/kg/day = 9 females/treatment group, 200 mg/kg/day = 9 females/treatment group). Graphs represent mean percentages ± SEM in the F1 generation of female mice. The image (panel B) shows an ovarian section, with dark red staining indicating fibrosis from a 13 month old mouse in the F1 generation
Effect of Prenatal Exposure to the Phthalate Mixture on Gene Expression in F1 Ovaries at 13 Months
The phthalate mixture did not affect the ovarian expression of the antioxidant enzymes Gpx, Cat, or Gsr, but it (200 mg/kg/day) caused a borderline increase in the expression of Sod1 compared to control in the F1 generation (Fig. 7A–D; n = 4–5 females per treatment group, ^ 0.05 < P < 0.1). Additionally, the phthalate mixture did not affect ovarian expression of the anti-apoptotic factor Bcl2, but it (200 mg/kg/day) caused a borderline increase in the expression of pro-apoptotic factor Bax compared to control in the F1 generation (Fig. 8A and B; n = 4–5 females per treatment group, ^ 0.05 < P < 0.1). The phthalate mixture (200 mg/kg/day) caused borderline increases in the ovarian expression of the DNA repair genes p53 and Brca2 compared to control in the F1 generation (Fig. 9A and B; n = 4–5 females per treatment group, ^ 0.05 < P < 0.1). Lastly, the phthalate mixture did not affect the ovarian expression of Cdkn1a, but it (20 µg/kg/day and 200 mg/kg/day) caused a borderline increase in the ovarian expression of Cdkn2a and Cdkn1b, and it (200 mg/kg/day) significantly increased the expression of Cdkn1b compared to control in the F1 generation (Fig. 10A–C; n = 4–5 females per treatment group, *P ≤ 0.05, ^ 0.05 < P < 0.1)
Figure 7:
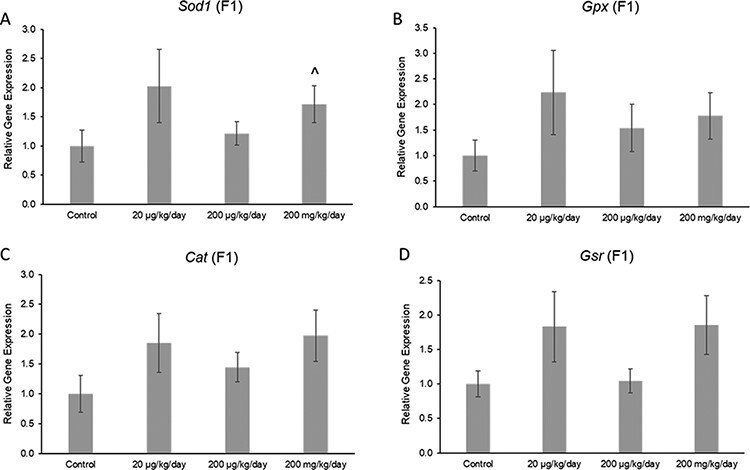
Effects of prenatal exposure to the phthalate mixture on the antioxidant enzymes Sod1 (panel A: control = 5 females/treatment group, 20 µg/kg/day = 5 females/treatment group, 200 µg/kg/day = 4 females/treatment group, 200 mg/kg/day = 5 females/treatment group), Gpx (panel B: control = 5 females/treatment group, 20 µg/kg/day = 5 females/treatment group, 200 µg/kg/day = 4 females/treatment group, 200 mg/kg/day = 5 females/treatment group), Cat (panel C: control = 5 females/treatment group, 20 µg/kg/day = 5 females/treatment group, 200 µg/kg/day = 4 females/treatment group, 200 mg/kg/day = 5 females/treatment group), and Gsr (panel D: control = 5 females/treatment group, 20 µg/kg/day = 5 females/treatment group, 200 µg/kg/day = 4 females/treatment group, 200 mg/kg/day = 5 females/treatment group) in ovaries from mice at 13 months of age in the F1 generation. All gene expression is relative to the housekeeping gene, Actb, and the relative fold change is normalized to 1 for control. Graphs represent means ± SEM. Carets (^) indicate borderline significance compared to the control (0.05 < P < 0.1)
Figure 8:
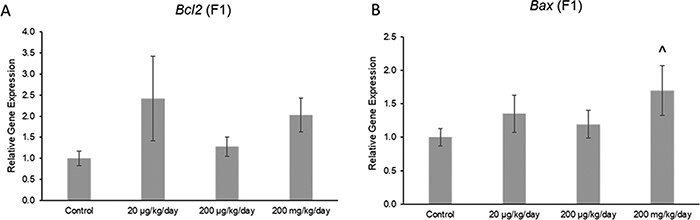
Effects of prenatal exposure to the phthalate mixture on the anti-apoptotic factor Bcl2 (panel A: control = 5 females/treatment group, 20 µg/kg/day = 5 females/treatment group, 200 µg/kg/day = 4 females/treatment group, 200 mg/kg/day = 5 females/treatment group) and the pro-apoptotic factor Bax (panel B: control = 5 females/treatment group, 20 µg/kg/day = 4 females/treatment group, 200 µg/kg/day = 4 females/treatment group, 200 mg/kg/day = 5 females/treatment group) in ovaries from mice at 13 months of age in the F1 generation. All gene expression is relative to the housekeeping gene, Actb, and the relative fold change is normalized to 1 for control. Graphs represent means ± SEM. Carets (^) indicate borderline significance compared to the control (0.05 < P < 0.1)
Figure 9:
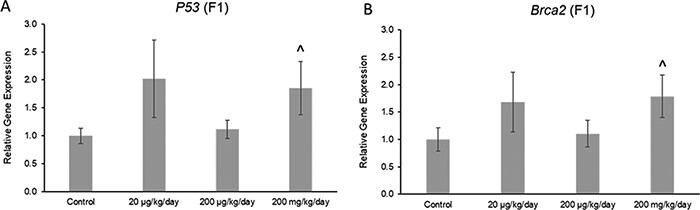
Effects of prenatal exposure to the phthalate mixture on DNA repair genes p53 (panel A: control = 5 females/treatment group, 20 µg/kg/day = 5 females/treatment group, 200 µg/kg/day = 4 females/treatment group, 200 mg/kg/day = 5 females/treatment group) and Brca2 (panel B: control = 5 females/treatment group, 20 µg/kg/day = 5 females/treatment group, 200 µg/kg/day = 4 females/treatment group, 200 mg/kg/day = 5 females/treatment group) in ovaries from mice at 13 months of age in the F1 generation. All gene expression is relative to the housekeeping gene, Actb, and the relative fold change is normalized to 1 for control. Graphs represent means ± SEM. Carets (^) indicate borderline significance compared to the control (0.05 < P < 0.1)
Figure 10:
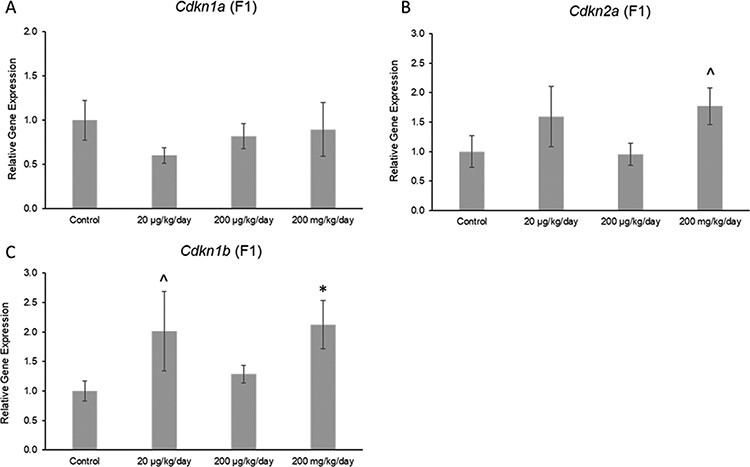
Effects of prenatal exposure to the phthalate mixture on the cell-cycle regulators Cdkn1a (panel A: control = 5 females/treatment group, 20 µg/kg/day = 4 females/treatment group, 200 µg/kg/day = 4 females/treatment group, 200 mg/kg/day = 5 females/treatment group), Cdkn2a (panel B: control = 5 females/treatment group, 20 µg/kg/day = 5 females/treatment group, 200 µg/kg/day = 4 females/treatment group, 200 mg/kg/day = 5 females/treatment group), and Cdkn1b (panel C: control = 5 females/treatment group, 20 µg/kg/day = 5 females/treatment group, 200 µg/kg/day = 4 females/treatment group, 200 mg/kg/day = 5 females/treatment group) in ovaries from mice at 13 months of age in the F1 generation. All gene expression is relative to the housekeeping gene, Actb, and the relative fold change is normalized to 1 for control. Graphs represent means ± SEM. Carets (^) indicate borderline significance compared to the control (0.05 < P < 0.1)
Discussion
This study tested the hypothesis that prenatal exposure to an environmentally relevant phthalate mixture accelerates the aging of the ovary and the age-related decline in reproductive capacity in the F1 generation of mice. Previously, we have shown that prenatal exposure to the phthalate mixture accelerated some biomarkers of reproductive aging, including increasing the time spent in metestrus/diestrus, altering folliculogenesis, and dysregulating hormones in the hypothalamic–pituitary–gonadal (HPG) axis expected with normal reproductive aging, and these effects occurred in a multiple and transgenerational manner in female mice [18]. The current study expands on our recent findings to further determine if the mixture accelerates the direct aging of the ovary by decreasing the expression of antioxidant enzymes, increasing the expression of apoptotic factors and cell cycle regulators, altering the expression of DNA repair genes, and increasing the amount of fibrosis in the aging mouse ovary. Additionally, this study examined the age-related decline in the reproductive capacity of aging female mice in the F1 generation. Our results show that prenatal exposure to the phthalate mixture decreased the time spent in proestrus and accelerated the age-related decline in some fertility indices, but it did not significantly affect the amount of fibrosis or factors involved in the direct aging ovary in the F1 generation.
At 11 and 13 months of age, we found that prenatal exposure to the phthalate mixture decreased the time spent in proestrus compared to control. When rodents are in the stage of proestrus, estradiol levels are usually increasing compared to other stages of the cycle. However, our previous study found that, at 13 months of age, the phthalate mixture did not significantly affect the levels of estradiol in the F1 generation of female mice [43]. In that previous study, we collected sera in the stage of diestrus. Thus, it is possible that we missed changing the levels of estradiol during proestrus because we collected samples in diestrus. In the future, it may be interesting to examine how prenatal exposure to the phthalate mixture affects hormone levels during the different stages of the estrous cycle.
Furthermore, the decrease in proestrus differs from previous studies showing that exposure to single phthalates do not affect estrous cyclicity. Specifically, adult exposure to DiNP and DEHP did not alter the time spent in proestrus at 12 and 15 months postdosing in female mice [14]. Furthermore, adult exposure to DiNP and DEHP did not drastically affect hormone levels that can influence estrous cyclicity at 12 and 15 months [18, 43]. The observed effects of phthalate exposure on estrous cycles could be due to difference in exposure windows (adult exposure vs. prenatal exposure) or differences in the effect of single phthalates versus a phthalate mixture.
At 11 and 13 months of age, prenatal exposure to the mixture did not affect gestation length, mating index, pregnancy rate, or fertility index in the F1 generation compared to control. Furthermore, the mixture did not affect F2 litter size or the F2 average live pup weight at 11 and 13 months. Similarly, when examining the effects of prenatal exposure to the phthalate mixture on fertility at 3, 6, and 9 months age in previous studies, the mixture did not cause changes in fertility indices compared to control in the F1 generation [16]. Moreover, another study similarly found that adult exposure to DEHP and DiNP did not affect most fertility indices 9 months after exposure compared to control [13]. Collectively, these data suggest that exposure to phthalates may not affect a female’s ability to mate with a male and become pregnant as they age.
In the current study, we found that the mixture decreased the F2% female pups born to the F1 dams in this study at 11 and 13 months. These data are consistent with studies that have shown that maternal age may contribute to an altered sex ratio [44, 45]. In addition, our laboratory previously has shown that phthalate exposure may be accelerating biomarkers of reproductive aging by decreasing the ovarian follicle pool, dysregulating the HPG axis, causing acyclicity, and increasing the occurrence of cystic ovaries in aging female mice [12, 18]. Thus, it is possible that phthalate exposure plus maternal age may be the cause of the observed altered sex ratio. Similarly, Chiang et al. found that adult exposure to DiNP and DEHP decreased the number of female pups born to the treated dams 9 and 12 months after treatment [13, 14]. It is interesting that different exposure windows and exposure to a single phthalate versus phthalate mixture are eliciting similar effects on the number of female pups born to each dam.
Our data show that phthalate mixture-exposed females had no issues mating with males or becoming pregnant, but we found that the phthalate mixture decreased the ability of F1 females to produce pups at 11 and 13 months, and it decreased the ability of F1 females to carry out their pregnancy at 13 months of age (decreased gestational index). It is possible that the mixture could be targeting the uterus in these aging female mice. In a collaborative study, Li et al. found that prenatal exposure to the phthalate mixture negatively affected the uterine morphology of 13-month-old mice in multiple generations [43]. The Li et al. study also found that the mixture increased many types of uterine abnormalities, higher incidence of myometrium intrusion in the endometrium, presence of myofibroblasts in the endometrium, and intrusion of endometrial tissues in the myometrium, and together these may be associated with adenomyosis or fibroids [43]. Adenomyosis is described as the abnormal growth of endometrial glands and stroma in the myometrium and has been associated with infertility in women [46, 47]. Furthermore, fibroids or uterine leiomyomas are benign tumors that arise from the myometrium and are linked to early pregnancy loss and infertility [46–48]. Therefore, the phthalate mixture may be affecting the proper function of the uterus, leading to decreased ability of these females to carry out their pregnancy and/or deliver pups.
We found that prenatal exposure to the phthalate mixture did not affect the amount of PSR staining, a marker of fibrosis, in the ovaries of 13-month-old F1 mice. Interestingly, control-treated mice also did not display large amounts of fibrosis. It is likely that fibrosis was not increased at 13 months of age because previous studies have found that increased fibrosis occurs around 22 months old in CD-1 mice and 17 months old in CB6F1 mice [29, 30]. Additionally, a recent study determined that even at 18 months of age, female C57BL/6 mice did not exhibit fibrotic ovaries [27]. Because we did not find drastic changes in the number of follicles in phthalate-treated mice in the F1 generation [18] or increased fibrosis, the phthalate mixture may not be accelerating the direct aging of the ovary at 13 months of age.
Prenatal exposure to the phthalate mixture caused minimal changes in ovarian gene expression related to antioxidant enzymes, apoptotic factors, DNA repair genes, and cell cycle regulators in the F1 generation. Because the phthalate mixture did not decrease the ovarian follicle pool [18] and fibrosis was not increased in these aging F1 female mice, the lack of effects on ovarian gene expression of factors directly related to ovarian aging is not surprising. Although the phthalate mixture did not significantly affect gene expression in ovaries from aging female mice, future studies should measure protein levels because some biomarkers are regulated posttranslationally.
In conclusion, this study found that prenatal exposure to an environmentally relevant phthalate mixture accelerates the age-related decline in reproductive capacity in the F1 generation of female mice. Specifically, the mixture decreased the ability of females to carry out their pregnancies and produce pups at 11 and 13 months of age. Furthermore, prenatal exposure to the phthalate mixture decreased the time spent in proestrus in F1 females. However, prenatal exposure to the mixture did not significantly affect key biomarkers of ovarian aging (fibrosis, antioxidants, apoptotic factors, DNA repair genes, or cell cycle regulators) in the F1 ovary. Additionally, because the phthalate mixture accelerated biomarkers of reproductive aging in the F2 and F3 generations of female mice [18], future studies should examine if prenatal exposure to the phthalate mixture directly accelerates the aging of the ovary in those generations. A previous study from our laboratory found that prenatal exposure to the single phthalate, DEHP, negatively affected the gene expression of key pathways important for proper ovarian function in a transgenerational manner and that gene expression may have been influenced by changes in DNA methyltransferases and 10 or 11 translocation enzymes [49]. Therefore, future studies should examine epigenetic mechanisms that contribute to the effects of phthalate mixtures on female reproduction in a multiple and transgenerational manner in female mice. Finally, while our results are important, there are some limitations in our study that make it difficult to compare with human fertility. Thus, future studies could examine fertility outcomes in treated mice after performing in vitro fertilization with embryo transfer.
Supplementary Material
Acknowledgements
The authors thank the members of the Flaws laboratory group for their assistance. This work was supported by R01 ES032163 (JF) and a Billie A. Field Fellowship (EB).
Contributor Information
Emily Brehm, Department of Comparative Biosciences, University of Illinois at Urbana-Champaign, 2001 S. Lincoln Ave. Urbana, IL 61802, USA.
Jodi A Flaws, Department of Comparative Biosciences, University of Illinois at Urbana-Champaign, 2001 S. Lincoln Ave. Urbana, IL 61802, USA.
Data availability
Raw data are available upon reasonable requests.
Supplementary data
Supplementary data is available at EnvEpig online.
Funding
No funds were received from governmental or private agencies to conduct this study.
None declared
Conflict of interest statement
Ethics statement
All animal work was approved by the IACUC at the University of Illinois at Urbana-Champaign (protocol number 20034).
References
- 1. Gore AC, Chappell VA, Fenton SE. et al. EDC-2: the endocrine society’s second scientific statement on endocrine-disrupting chemicals. Endocr Rev 2015;36:E1–E150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rattan S, Zhou C, Chiang C. et al. Exposure to endocrine disruptors during adulthood: consequences for female fertility. J Endocrinol 2017;233:R109–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brehm E, Flaws JA. Transgenerational effects of endocrine-disrupting chemicals on male and female reproduction. Endocrinology 2019;160:1421–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Johns LE, Cooper GS, Galizia A. et al. Exposure assessment issues in epidemiology studies of phthalates. Environ Int 2015;85:27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang Y, Zhu H, Kannan K. A review of biomonitoring of phthalate exposures. Toxics 2019;7:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hwang H-M, Park E-K, Young TM. et al. Occurrence of endocrine-disrupting chemicals in indoor dust. Sci Total Environ 2008;404:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sears CG, Lanphear BP, Calafat AM. et al. Lowering urinary phthalate metabolite concentrations among children by reducing contaminated dust in housing units: a randomized controlled trial and observational study. Environ Sci Technol 2020;54:4327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wittassek M, Koch HM, Angerer J. et al. Assessing exposure to phthalates - the human biomonitoring approach. Mol Nutr Food Res 2011;55:7–31. [DOI] [PubMed] [Google Scholar]
- 9. Hannon PR, Peretz J, Flaws JA. Daily exposure to Di(2-ethylhexyl) phthalate alters estrous cyclicity and accelerates primordial follicle recruitment potentially via dysregulation of the phosphatidylinositol 3-kinase signaling pathway in adult mice. Biol Reprod 2014;90:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hannon PR, Niermann S, Flaws JA. Acute exposure to Di(2-Ethylhexyl) phthalate in adulthood causes adverse reproductive outcomes later in life and accelerates reproductive aging in female mice. Toxicol Sci 2016;150:97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rattan S, Brehm E, Gao L. et al. Di(2-Ethylhexyl) phthalate exposure during prenatal development causes adverse transgenerational effects on female fertility in mice. Toxicol Sci 2018;163:420–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brehm E, Rattan S, Gao L. et al. Prenatal exposure to Di(2-Ethylhexyl) phthalate causes long-term transgenerational effects on female reproduction in mice. Endocrinology 2018;159:795–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chiang C, Flaws JA. Subchronic exposure to Di(2-ethylhexyl) phthalate and diisononyl phthalate during adulthood has immediate and long-term reproductive consequences in female mice. Toxicol Sci 2019;168:620–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chiang C, Lewis LR, Borkowski G. et al. Late-life consequences of short-term exposure to di(2-ethylhexyl) phthalate and diisononyl phthalate during adulthood in female mice. Reprod Toxicol 2020;93:28–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yazdy MM, Coull BA, Gardiner JC. et al. A possible approach to improving the reproducibility of urinary concentrations of phthalate metabolites and phenols during pregnancy. J Expo Sci Environ Epidemiol 2018;28:448–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou C, Gao L, Flaws JA. Prenatal exposure to an environmentally relevant phthalate mixture disrupts reproduction in F1 female mice. Toxicol Appl Pharmacol 2017;318:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhou C, Gao L, Flaws JA. Exposure to an environmentally relevant phthalate mixture causes transgenerational effects on female reproduction in mice. Endocrinology 2017;158:1739–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brehm E, Zhou C, Gao L. et al. Prenatal exposure to an environmentally relevant phthalate mixture accelerates biomarkers of reproductive aging in a multiple and transgenerational manner in female mice. Reprod Toxicol 2020;98:260–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wise PM, Krajnak KM, Kashon ML. Menopause: the aging of multiple pacemakers. Science 1996;273:67–70. [DOI] [PubMed] [Google Scholar]
- 20. te Velde ER, Pearson PL. The variability of female reproductive ageing. Hum Reprod Update 2002;8:141–54. [DOI] [PubMed] [Google Scholar]
- 21. Finch CE. The menopause and aging, a comparative perspective. J Steroid Biochem Mol Biol 2014;142:132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hall JE. Endocrinology of the menopause. Endocrinol Metab Clin North Am 2015;44:485–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vidal J, Wood CE, Colman K. et al. Reproductive System and Mammary Gland, in Toxicologic Pathology . New York: Humana, CRC Press, 2013, 717–830. [Google Scholar]
- 24. Lim J, Luderer U. Oxidative damage increases and antioxidant gene expression decreases with aging in the mouse ovary. Biol Reprod 2011;84:775–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Velarde MC, Menon R. Positive and negative effects of cellular senescence during female reproductive aging and pregnancy. J Endocrinol 2016;230:R59–76. [DOI] [PubMed] [Google Scholar]
- 26. Huang Y, Hu C, Ye H. et al. Inflamm-aging: a new mechanism affecting premature ovarian insufficiency. J Immunol Res 2019;2019:8069898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lliberos C, Liew SH, Zareie P. et al. Evaluation of inflammation and follicle depletion during ovarian ageing in mice. Sci Rep 2021;11:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maidarti M, Anderson RA, Telfer EE. Crosstalk between PTEN/PI3K/Akt signalling and DNA damage in the oocyte: implications for primordial follicle activation, oocyte quality and ageing. Cells 2020;9:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Briley SM, Jasti S, McCracken JM. et al. Reproductive age-associated fibrosis in the stroma of the mammalian ovary. Reproduction 2016;152:245–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mara JN, Zhou LT, Larmore M. et al. Ovulation and ovarian wound healing are impaired with advanced reproductive age. Aging 2020;12:9686–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Amargant F, Manuel SL, Tu Q. et al. Ovarian stiffness increases with age in the mammalian ovary and depends on collagen and hyaluronan matrices. Aging Cell 2020;19:e13259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Laisk T, Tšuiko O, Jatsenko T. et al. Demographic and evolutionary trends in ovarian function and aging. Hum Reprod Update 2019;25:34–50. [DOI] [PubMed] [Google Scholar]
- 33. Kinnear HM, Tomaszewski CE, Chang FL. et al. The ovarian stroma as a new frontier. Reproduction 2020;160:R25–R39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhou C, Flaws JA. Effects of an environmentally relevant phthalate mixture on cultured mouse antral follicles. Toxicol Sci 2017;156:217–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kavlock R, Boekelheide K, Chapin R. et al. NTP Center for the Evaluation of Risks to Human Reproduction: phthalates expert panel report on the reproductive and developmental toxicity of di(2-ethylhexyl) phthalate. Reprod Toxicol 2002;16:529–653. [DOI] [PubMed] [Google Scholar]
- 36. Koch HM, Calafat AM. Human body burdens of chemicals used in plastic manufacture. Philos Trans R Soc Lond B Biol Sci 2009;364:2063–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pocar P, Fiandanese N, Secchi C. et al. Exposure to di(2-ethyl-hexyl) phthalate (DEHP) in utero and during lactation causes long-term pituitary-gonadal axis disruption in male and female mouse offspring. Endocrinology 2012;153:937–48. [DOI] [PubMed] [Google Scholar]
- 38. Neier K, Cheatham D, Bedrosian LD. et al. Longitudinal metabolic impacts of perinatal exposure to phthalates and phthalate mixtures in mice. Endocrinology 2019;160:1613–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu X, Craig ZR. Environmentally relevant exposure to dibutyl phthalate disrupts DNA damage repair gene expression in the mouse ovarydagger. Biol Reprod 2019;101:854–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rattan S, Brehm E, Gao L. et al. Prenatal exposure to di(2-ethylhexyl) phthalate disrupts ovarian function in a transgenerational manner in female mice. Biol Reprod 2018;98:130–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hartman CG. Some new observations on the vaginal smear of the rat. Yale J Biol Med 1944;17:99–112. [PMC free article] [PubMed] [Google Scholar]
- 42. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001;29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li K, Liszka M, Zhou C. et al. Prenatal exposure to a phthalate mixture leads to multigenerational and transgenerational effects on uterine morphology and function in mice. Reprod Toxicol 2020;93:178–90. [DOI] [PubMed] [Google Scholar]
- 44. Ruckstuhl KE, Colijn GP, Amiot V. et al. Mother’s occupation and sex ratio at birth. BMC Public Health 2010;10:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rapaport T, Villaseñor FA, Altman RM. et al. Sex ratio and maternal age in a natural fertility, subsistence population: Daughters, sons, daughters. Am J Phys Anthropol 2019;169:368–76. [DOI] [PubMed] [Google Scholar]
- 46. Chodankar R, Critchley HOD. Biomarkers in abnormal uterine bleedingdagger. Biol Reprod 2019;101:1155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hur C, Rehmer J, Flyckt R. et al. Uterine factor infertility: a clinical review. Clin Obstet Gynecol 2019;62:257–70. [DOI] [PubMed] [Google Scholar]
- 48. Pritts EA, Parker WH, Olive DL. Fibroids and infertility: an updated systematic review of the evidence. Fertil Steril 2009;91:1215–23. [DOI] [PubMed] [Google Scholar]
- 49. Rattan S, Beers HK, Kannan A. et al. Prenatal and ancestral exposure to di(2-ethylhexyl) phthalate alters gene expression and DNA methylation in mouse ovaries. Toxicol Appl Pharmacol 2019;379:114629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data are available upon reasonable requests.


