Abstract
Objective
To examine the joint associations of daily time spent in different intensities of physical activity, sedentary behaviour and sleep with all-cause mortality.
Methods
Federated pooled analysis of six prospective cohorts with device-measured time spent in different intensities of physical activity, sedentary behaviour and sleep following a standardised compositional Cox regression analysis.
Participants
130 239 people from general population samples of adults (average age 54 years) from the UK, USA and Sweden.
Main outcome
All-cause mortality (follow-up 4.3–14.5 years).
Results
Studies using wrist and hip accelerometer provided statistically different results (I2=92.2%, Q-test p<0.001). There was no association between duration of sleep and all-cause mortality, HR=0.96 (95% CI 0.67 to 1.12). The proportion of time spent in moderate to vigorous physical activity was significantly associated with lower risk of all-cause mortality (HR=0.63 (95% CI 0.55 to 0.71) wrist; HR=0.93 (95% CI 0.87 to 0.98) hip). A significant association for the ratio of time spent in light physical activity and sedentary time was only found in hip accelerometer-based studies (HR=0.5, 95% CI 0.42 to 0.62). In studies based on hip accelerometer, the association between moderate to vigorous physical activity and mortality was modified by the balance of time spent in light physical activity and sedentary time.
Conclusion
This federated analysis shows a joint dose–response association between the daily balance of time spent in physical activity of different intensities and sedentary behaviour with all-cause mortality, while sleep duration does not appear to be significant. The strongest association is with time spent in moderate to vigorous physical activity, but it is modified by the balance of time spent in light physical activity relative to sedentary behaviour.
Keywords: physical activity, sleep, health
Introduction
Physical inactivity is associated with several chronic diseases,1 3.9–5.3 million annual premature deaths globally2 3 and a $67.5 billion per year cost to healthcare systems worldwide.4 Few people actually meet the recommended levels of physical activity.5 During the day, individuals engage mainly in sleep, sedentary behaviour (SB)6 and light physical activity (LIPA) such as walking.7 The health benefits of daily moderate to vigorous physical activity (MVPA) are well established but increasing evidence suggests that sleep, SB and LIPA also have important consequences for health.7–9
Canada has recently issued the first integrated 24-hour public health guidelines for adults and older adults10 following the introduction of 24-hour movement guidelines for children in Canada, Australia and UK several years ago.11 These 24-hour guidelines combine recommendations on sleep, SB and physical activity but are mainly based on evidence derived from studying the impact of each of the behaviours independently.
There is a lack of evidence about the joint association between time spent in sleep, SB and physical activity with health outcomes.12 Consequently, the WHO 2020 guidelines focus on physical activity and SB only.13 These guidelines highlight that some of the key remaining questions are how the combination of time spent in each of these basic elements of daily routine impacts our health, whether this reduces or enhances the benefits of recommended daily MVPA and whether an optimum daily combination of time exists.12 14 In order to answer this question, it is important to account for the finite nature of a day, given that time is limited to 24 hours in a day. Time spent in one movement behaviour necessarily influences the time that remains to be spent in the others. Therefore, time spent in sleep, SB, LIPA and MVPA are codependent. Compositional data analysis has been advocated as a methodological approach that accounts for this fact.15–18
Compositional data analysis is a well-established branch of statistics that deals with multivariate data that forms part of a finite whole.19 Exposure or recommendations expressed as time spent in movement behaviours and sleep per day or per week are compositional in nature as they form parts of a total, for example, the 24-hour day, the waking day, or a week. Consequently, compositional methods are starting to be used in physical activity research and epidemiology. The advantage of applying compositional methods, beyond properly dealing with the relative nature of the data, is that they allow the adjustment of models for time spent in all movement behaviours and enable the quantification of the joint associations between daily movement behaviours and health. This could provide evidence to underpin robust integrated movement behaviour guidelines. Janssen et al have systematically reviewed compositional analysis studies examining associations between sleep, SB and physical activity with health outcomes in adults as part of the evidence review for the Canadian 24-hour guidelines.20 Of the eight studies included in the review only one was prospective, demonstrating an urgent research need for further prospective compositional analysis evidence.21
Therefore, this study aimed to assess the prospective association of different combinations of time spent daily in physical activity, SB and sleep with mortality using compositional analysis applied to data from large-scale cohorts with device-measured time spent in movement behaviours. More precisely, this study aimed to estimate the joint effects of movement behaviours (sleep, SB and physical activity) on risk of all-cause mortality. In causal compositional analysis terms this refers to estimating the relative causal effect of each movement behaviour conditional on the other movement behaviours.18
Methods
Design
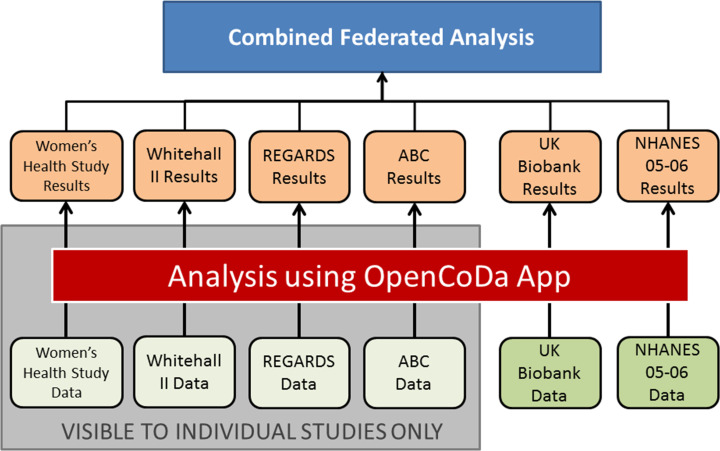
This study adopted a federated pooled analysis approach to maximise the use of available data (figure 1).22 23 Under such an approach, an analytical methodology was created and automated, then distributed to individual studies to perform the analysis without the need to share data access across teams, avoiding any associated ethical and legal issues.
Figure 1.
Structure of federated analysis employed. UK Biobank and National Health and Nutrition Examination Survey (NHANES) are open-source data sets.
Data sources and data set selection
To identify suitable data sets, we conducted a systematic search, following Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines,24 of four databases (PubMed, PsycINFO, Embase and Web of Science) from database inception until 28 February 2018 (a detailed search description is provided in the online supplemental file). We included prospective cohort studies, reported in the English language, that had individual-level exposure and outcome data, provided data on time spent in SB, LIPA and MVPA measured using a body-worn sensor over 7 consecutive days, and all-cause mortality. We identified whether data from each of the studies were available in accessible, open-source databases. When data for a study were not publicly available we contacted an author or principal investigator on at least two occasions between March and October 2018 and invited them to participate.
bjsports-2020-102345supp001.pdf (800.8KB, pdf)
Data analyses
We conducted the analysis according to Strengthening the Reporting of Observational Studies in Epidemiology guidelines.25 Authors with access to the individual-level data for each study analysed their data according to a standardised protocol implemented in R (R V.3.4.1, R Foundation for Statistical Computing, Vienna, Austria, 2017) and Shiny (Shiny V.1.0.5, RStudio, Boston, USA, 2017) that was made available through the OpenCoDa website (https://opencoda.net).
Each participating study examined the association between the daily composition of time spent in sleep, SB, LIPA and MVPA as exposure variables with all-cause mortality using a novel compositional Cox regression analysis.26 This method is an extension of standard Cox regression that enables computation of the association between the daily time composition, as an entire multicomponent exposure variable, with mortality (or any other time-to-event outcome variable) taking into account the codependence and interactions between behaviours making up the composition. The method is detailed below and R codes are available at www.opencoda.net.
In this study, the composition of the day was defined as the proportions of time spent in D=4 movement behaviours: MVPA, LIPA, SB and sleep (Sleep). How each cohort study measured the time spent in each behaviour is detailed in online supplemental table A1.
To be treated and interpreted correctly, information contained in parts of a composition needs to be expressed relative to the other parts as log ratios.19 Therefore, participant's times spent in sleep, SB, LIPA and MVPA were transformed into three isometric log ratio (ilr) coordinates27 given by equations 1–3:
| (1) |
| (2) |
| (3) |
These fully represent the composition and guarantee desirable formal properties, such as orthonormality and consistence if working with a subcomposition regardless of the scale of measurement of the data or the total time period considered. This is important, for example, when using the waking day as a subcomposition, due to variation in the length of the waking day.27 The ilr coordinates devised represent nested contrasts of relative importance between subsets of behaviours. The first coordinate represents time spent in sleep relative to the (geometric) mean of all the other behaviours. The second coordinate is the balance between time allocated to MVPA and time allocated to (the geometric mean of) LIPA and SB. The third coordinate accounts for the balance of time between LIPA and SB. Note that it is possible to consider other ilr coordinate systems in the Cox regression model to investigate the significance of other time balances of interest.26 However, the estimated responses and global parameters from the model would be the same. Our choice of the balance between sleep time and the other behaviours as the first coordinate facilitated the pooling of studies whether they measured sleep or not (detailed below). This set constrains the influence of sleep time to a single coordinate (in view of the presence of studies that did not measure Sleep time).
These coordinates were then used as explanatory variables to fit a Cox regression model of the form:
| (4) |
where , depending on time , is an unspecified baseline hazard function, the vectors and refer respectively to the ilr coordinates and any other covariates (with them all jointly forming the vector of explanatory variables ), and the vectors and are the corresponding regression coefficients. These coefficients were fitted in the usual manner by maximising the partial likelihood function.26 The term is the ordinary hazard function, which relates to probability of survival to time , , through equation 5:
| (5) |
The protocol provided a list of covariates to be included in the model based on the causal assumptions shown in online supplemental figure A1. These assumptions were informed by the compositional causal inference framework developed by Arnold et al 18 and the recent review in physical activity research.28 Each cohort selected the relevant data (detailed in online supplemental table A2) to represent these covariates: demographic information (age, sex and ethnicity), socioeconomic status, health status and/or pre-exiting conditions, health-related behaviours (smoking, diet and alcohol consumption). In order to address the lag in time between the measurement of the composition and covariates in the UK Biobank cohort we implemented the same methodology as Strain et al 29 to quantify the study covariates.
For each cohort, the estimated associations between the above time balances, as represented by the ilr coordinates, and mortality were expressed as HRs with their corresponding 95% CIs.26 In order to minimise the risk of reverse causation bias, we excluded deaths in the first 2 years of follow-up in each of the studies. The proportional hazards assumption was verified at the individual study level by examination of the Kaplan-Meier curves and a Grambsch-Therneau test.30
Because compositional data are expressed as log ratios, zero time recorded in a part of the composition creates a mathematical issue. Therefore, time budgets need to be preprocessed to deal with zeroes. For each study, any zeroes present in the compositional variables were assumed to represent unobserved small values, for example, resulting from rounding off, falling below detection thresholds or limited observation time, and were imputed using the log ratio EM algorithm implemented for this purpose in the function lrEM of the R package zCompositions.31 Less than 2% of individuals had zero-valued MVPA. Detection limits were set by study based on the measurement and epoch used, for example, 1 min for accelerometer measurements of physical activity based on 1 min epochs.
Pooling
Each individual study provided the estimated model coefficients and the variance-covariance matrix of these coefficients, which were used to obtain the pooled coefficients and SEs using the random effects model implemented in the R package mvmeta, whether a study measured sleep or not.32 As indicated, the ilr coordinates were chosen to restrict sleep to the first coordinate . To pool results, the coefficient for was set to zero in studies which did not measure sleep, and the variance was set to an arbitrarily high value. This ensured that the studies that did not measure sleep were not taken into account in the estimation of coefficients for . Their contribution to estimated coefficient for was thus made negligible in the pooled results.
The mortality HRs were estimated from the pooled model to ascertain the association between the daily four-behaviour time composition and all-cause mortality. The HR between any two compositions (say 1 and 2) expressed in ilr coordinates and respectively was calculated as
| (6) |
To select a reference composition, we fitted a normal distribution to the observed second and third ilr coordinates, then computed a 75% confidence region based on a contour of constant Mahalanobis distance (a multivariate distance accounting for the covariance structure).33 We then selected a point on this outer contour and applied inverse ilr transformation to obtain a reasonable composition of low MVPA and LIPA and sedentary time but that still lay within a region where our model was well supported by the data (online supplemental figure A2).
Heterogeneity and sensitivity analysis
We investigated heterogeneity between studies using the I2 statistics and Cochran’s Q-test. In order to ascertain the robustness of the results and to investigate potential sources of heterogeneity we repeated the pooled analysis with the following conditions: (A) we excluded one study in turn (leave-one-out procedure), (B) we excluded all studies using wrist-worn accelerometers, and (C) we excluded all studies using hip-worn accelerometers. We examined the difference in model coefficients for each ilr coordinate in each of these conditions.
Results
The systematic search identified 12 studies as eligible.34–46 Of these, six did not respond to our request to participate36 42–46 by the deadline of September 2019. Data from two studies were available publicly35 37 and four studies agreed to participate.34 39–41 These six studies were included in the analysis (table 1).
Table 1.
List characteristics of studies included in the federated analysis
| Study | Country; sample; deaths | Year of baseline assessment; mean follow-up | Device; method of sleep time assessment | Method of death ascertainment | Covariates |
| ABC | Sweden; n=841 (370 men, 471 women); 78 deaths | 2001–2002; 14.5 years |
ActiGraph 7164 (lower back); NA |
National death register | Age, sex, education level, alcohol consumption status, smoking status, self-assessed health |
| NHANES | USA; n=2927 (1453 men; 1474 women); 283 deaths | 2003–2006; 6.7 years |
ActiGraph 7164 (hip); NA |
National Death Index | Age, sex, ratio of family income to poverty, alcohol consumption, smoking status, energy intake, self-assessed health, physical limitations on movement, existing diagnosed medical condition (coronary heart disease, stroke, cancer, diabetes), hypertension |
| REGARDS | USA; n=7076 (3257 men; 3819 women); 477 deaths | 2003–2007; 5.7 years |
Actical (hip); NA |
Review of death certificates, medical records and administrative databases | Age, sex, BMI, education, race, region of residence, season accelerometer was worn, current smoking, alcohol use, diabetes, hypertension, dyslipidaemia, estimated glomerular filtration rate, atrial fibrillation, history of coronary heart disease, history of stroke, self-assessed health |
| UK Biobank | UK; n=98 819 (43 229 men; 55 590 women); 2411 deaths | 2013–2015; 5.4 years |
Axivity AX3 (dominant wrist); self-report |
National Health Service central registers | Age, sex, BMI, education, race, Townsend deprivation index, alcohol consumption, smoking status, self-assessed health, fruit and vegetable consumption, oily fish consumption, salt intake, red meat consumption, use of blood pressure or cholesterol medicine, physical limitations on movement, existing medical condition (cardiovascular disease, stroke, cancer), existing diagnosis of diabetes (self-reported diagnosis or use of insulin) |
| Whitehall II | UK; n=3900 (2891 men; 1009 women); 140 deaths | 2012–2013; 5.3 years |
GENEActiv Original (non-dominant wrist); accelerometer |
National Health Service central registers | Age, sex, occupational position, alcohol consumption, smoking status, fruit and vegetable consumption, health state (multimorbidity index made of history of diabetes, coronary heart disease, stroke, depression, cancers, arthritis, chronic obstructive pulmonary disease, dementia and Parkinson’s disease) |
| Women’s Health Study | USA; n=16 676 (all women); 503 deaths | 2011–2015; 4.3 years |
ActiGraph GT3X+ (hip); NA |
Review of medical records, death certificates or the National Death Index | Age, sex, income, smoking status, alcohol consumption, saturated fat intake, fibre intake, fruit and vegetable consumption, hormone therapy, family history of myocardial infarction, family history of cancer, general health, history of cardiovascular disease, history of cancer, results of cancer screen |
BMI, body mass index; NA, not applicable; NHANES, National Health and Nutrition Examination Survey.
These studies included 130 239 individuals who were followed on average from 4.3 to 14.5 years depending on the cohort, during which 3892 (2.98%) died. Two of the studies included data on sleep time assessed using wrist-worn devices40 and were entered in the analysis of the association of sleep time relative to other behaviours with all-cause mortality. Demographics and average (compositional centre) pattern of time in each behaviour for each study are presented in table 2.
Table 2.
Demographics and average daily time composition (computed as compositional geometric means) per study
| Study | % women | Average age (years) | MVPA (min/day) |
LIPA (min/day) |
SB (min/day) |
Sleep (min/day) |
| ABC | 44.0 | 52.8 | 23.9 | 379.8 | 556.3 | NA |
| NHANES 2003–2006 | 49.3 | 63.6 | 8.2 | 355.5 | 596.3 | NA |
| REGARDS | 54.0 | 63.4 | 5.2 | 193.2 | 761.7 | NA |
| UK Biobank | 56.2 | 62.3 | 61.8 | 122.6 | 818.3 | 437.3 |
| Whitehall II | 25.9 | 69.4 | 46.9 | 93.5 | 835.8 | 463.7 |
| Women’s Health Study | 100.0 | 72.0 | 7.0 | 303.5 | 649.5 | NA |
LIPA, light physical activity; MVPA, moderate to vigorous physical activity; NA, not applicable; NHANES, National Health and Nutrition Examination Survey; SB, sedentary behaviour.
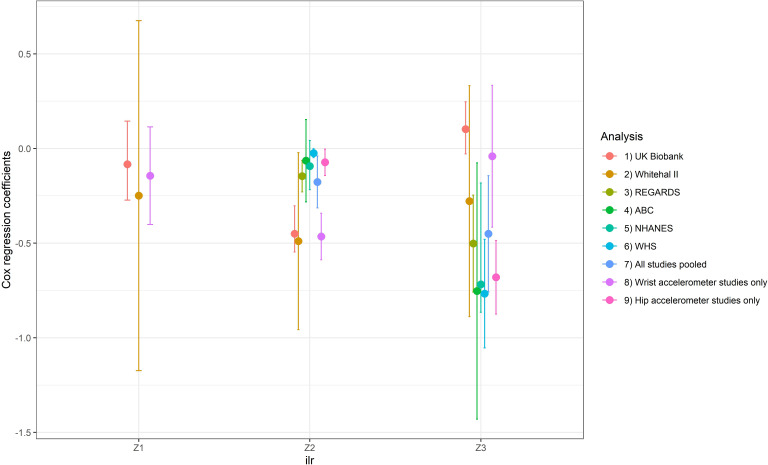
Meta-analysis pooling results from all studies showed significant heterogeneity (I2=92.2%, Q-test p<0.001). The sensitivity analysis revealed that the most important source of heterogeneity arose from accelerometer placement. Figure 2 shows that the model coefficients were significantly different when results were pooled according to accelerometer body placement. This strongly suggests that pooling results from studies using wrist accelerometers together with those using hip-mounted accelerometers might not be appropriate. Consequently, we present below results stratified according to accelerometer body placement (table 3). These stratified analyses showed low to moderate heterogeneity which was not significant according to Q-test (I2=16.3%, p=0.31 for wrist accelerometer studies and I2=30.3%, p=0.20 for hip accelerometer studies). Hip-mounted studies only measured waking day behaviour, therefore results for the first ilr coordinate (which includes sleep) are not presented.
Figure 2.
Results of the sensitivity analysis showing Cox regression model coefficients with 95% CI bars for each of the isometric log ratio (ilr) coordinates ( , , ) in models including all studies, leave-one-out models and in models pooling studies using wrist accelerometers only and hip accelerometers only. For hip accelerometers, only was not calculated. NHANES, National Health and Nutrition Examination Survey; WHS, Women’s Health Study.
Table 3.
Estimated coefficients for Cox regression model on isometric log ratio (ilr) coordinates of daily time composition pooled for studies using wrist accelerometers and studies using hip accelerometers, HRs, p values and 95% CIs
| Log ratio | Coefficient | Unit HR* | P value† | 95% CI lower bound | 95% CI upper bound |
| Wrist accelerometer studies | |||||
| −0.144 | 0.962 | 0.275 | −0.402 | 0.114 | |
| −0.465 | 0.628 | <0.001 | −0.588 | −0.342 | |
| −0.041 | 0.960 | 0.829 | −0.415 | 0.333 | |
| Hip-mounted accelerometer studies | |||||
| −0.073 | 0.930 | 0.040 | −0.143 | −0.003 | |
| −0.681 | 0.506 | <0.001 | −0.875 | −0.486 |
*This figure, calculated as exp(β coefficient), represents the HR in respect of an increase of 1 unit in the corresponding ilr coordinate. A value greater than 1 indicates that higher values of the behaviour balance are associated with increased mortality risk, and a value lower than 1 indicates that higher values of the behaviour balance are associated with decreased mortality risk.
†P values and 95% CIs are based on Wald test statistics.
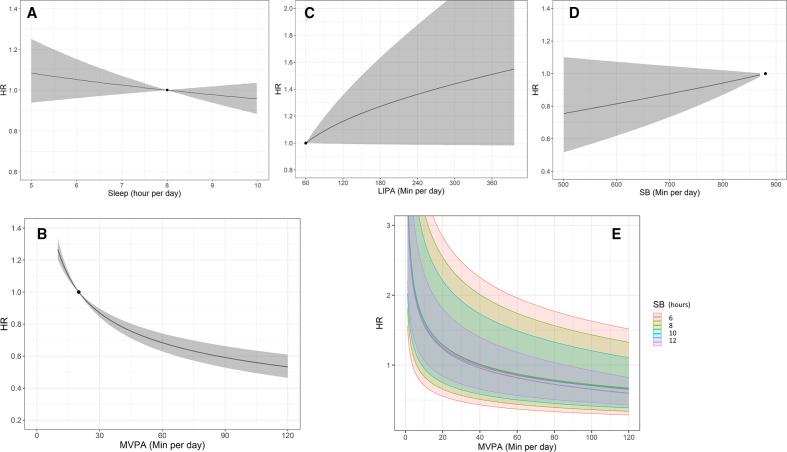
Pooled analysis using wrist accelerometers
The proportion of time spent sleeping relative to the other behaviours was not significantly associated with all-cause mortality (table 3, ). This is illustrated in figure 3A showing that the dose–response relationship between the risk of all-cause mortality and sleep time is nearly flat. For the waking day behaviours, there was a statistically significant association between the time spent in MVPA compared with other waking day behaviours ( ) and all-cause mortality. MVPA had a curvilinear dose–response relationship with lower risk of all-cause mortality at higher time spent in MVPA (figure 3B). The third ilr coordinate ( ), involving the ratio of time spent in LIPA and SB, was not statistically significant in this analysis (table 3), hinting that the balance of time between SB and LIPA is not associated with all-cause mortality. The dose–response curves for LIPA and sedentary time are shown in figure 3C, D. While these show trends towards higher risk with higher time spent in either of these behaviours, they are not statistically significant. The joint association between the waking day behaviours and all-cause mortality is presented in figure 3E. This curve shows that the association between MVPA and all-cause mortality was not significantly modified by the balance of time spent in LIPA or SB. In figure 3E, the similarity of the dose–response curves of MVPA across a range of values of sedentary time (and thus also across a range of different values of time spent in LIPA as it constitutes the remaining time of the waking day) shows that the relationship was not significantly modified by time spent in LIPA.
Figure 3.
Results from pooled studies using wrist accelerometers. Dose–response relationship (with 95% CI—ribbons) between time spent in (A) sleep (relative to all other behaviours), (B) moderate to vigorous physical activity (MVPA) (relative to all other behaviours), (C) light physical activity (LIPA) (relative to all other behaviours), (D) sedentary behaviour (SB) (relative to all other behaviours), (E) joint association between time in MVPA, SB (presented as different levels) and LIPA (implied as it makes up the remaining time in the waking day time in LIPA=16 hours−time in MVPA−time in SB). HRs were computed with respect to the following reference composition defined as described in the Methods section: MVPA=20 min/day, LIPA=60 min/day, SB=14 hours and 40 min/day, sleep=8 hours/day (marked as a solid black dot). Compositions with different values in time in the primary behaviour were reported such that the remaining behaviours were in the same ratio as for the reference composition.
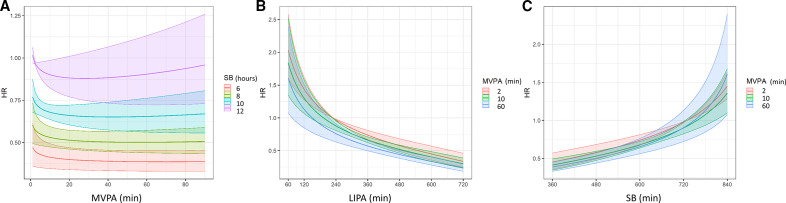
Pooled analysis using hip accelerometers
For pooled studies using hip accelerometers, the analysis also revealed a statistically significant association between the proportion of time spent in MVPA compared with other waking day behaviours ( ) and all-cause mortality. Additionally, there was a statistically significant association between the proportion of time spent in LIPA compared with sedentary time ( ) and all-cause mortality (table 3). MVPA had a curvilinear dose–response relationship with lower risk of all-cause mortality at higher time spent in MVPA relative to other waking day behaviours which tended to flatten after around 20 min/day (figure 4A). However, the benefits are lower at higher levels of sedentary time (and therefore lower levels of LIPA as it is the remaining time of the waking day) and fully attenuated for daily sedentary time exceeding 11–12 hours/day. Time spent in LIPA shows a curvilinear dose–response with lower risk of all-cause mortality at higher levels of LIPA (figure 4B) that is not significantly modified by the time spent in MVPA. In contrast, higher sedentary time is associated with higher mortality risk (figure 4C) and this is not significantly modified by the time spent in MVPA.
Figure 4.
Results from pooled studies using hip accelerometers. Dose–response relationship (with 95% CI—ribbons) for waking day behaviours between time spent in (A) moderate to vigorous physical activity (MVPA) for different levels of sedentary time (with light physical activity (LIPA) making up the remaining time in the waking day), (B) LIPA for different levels of MVPA (with sedentary time making up the remaining time in the waking day), and (C) sedentary behaviour (SB) for different levels of MVPA (with LIPA making up the remaining time in the waking day). HRs were computed with respect to the following reference composition defined as described in the Methods section: MVPA=2 min/day, LIPA=229 min/day, SB=729 min/day, sleep=8 hours/day.
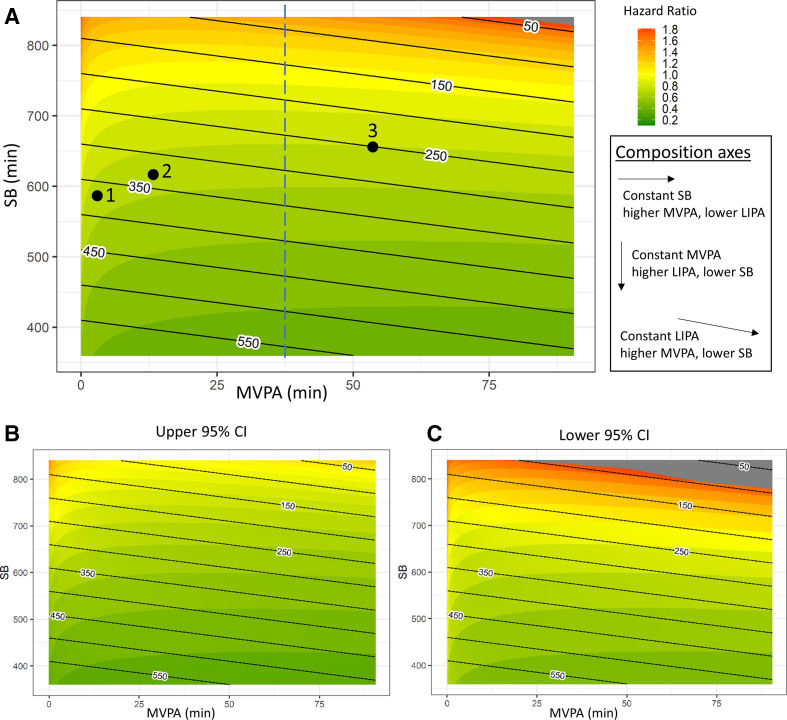
Heat maps (figure 5) show the joint associations between time spent in MVPA, LIPA and SB with all-cause mortality in more detail. HRs for waking day behaviour are shown against MVPA and SB. For each data point in the heat maps, the remaining time in the waking day is made up of LIPA, and LIPA isotime lines have been added to the graph for ease of interpretation. Heat maps of the upper and lower bounds of the 95% CIs are provided in figure 5B, C. MVPA does not appear to be the only behaviour that affects risk. For example, spending 30 min in MVPA is associated with a wide range of HR from 1.6 to 0.2, depending on how the remaining waking time is divided across SB and LIPA (figure 5A, blue dashed line). A range of different combinations of time spent in waking day behaviours are associated with similar lower risk of all-cause mortality. For example, point 1 (MVPA=3 min/day, LIPA=375 min/day, SB=582 min/day), point 2 (MVPA=13 min/day, LIPA=330 min/day, SB=617 min/day) and point 3 (MVPA=55 min/day, LIPA=250 min/day, SB=655 min/day) in figure 5A all correspond to HR=0.70 (95% CI 0.65 to 0.75). How the dose–response between MVPAs is modified by other waking day behaviours is shown in figure 5A. Generally, compositions with higher MVPA and LIPA and lower sedentary time are associated with lower risk of all-cause mortality. This suggests that displacing sedentary time with physical activity of any intensity is beneficial. Table 4 shows differences in compositions associated with a 10% lower risk of all-cause mortality compared with three reference compositions. At low levels of MVPA (eg, 2 min/day), displacing sedentary time with MVPA appears to be six times more time efficient compared with displacing sedentary time with LIPA. Above 30 min/day of moderate-to-vigorous physical activity, displacing sedentary time with LIPA was just as time efficient as displacing sedentary time with MVPA.
Figure 5.
Results from pooled studies using hip accelerometers. HRs for different compositions of the waking day are presented as a heat map in (A). Moderate to vigorous physical activity (MVPA) and sedentary time are shown on the x-axis and y-axis, respectively. The remaining time in the waking day is made up of light physical activity (LIPA), black lines represent LIPA isotime lines. The dashed blue line represents composition with 30 min of MVPA. Heat maps of the lower and upper bounds of the 95% CIs are shown in (B) and (C). HRs were computed with respect to the following composition: MVPA=2 min/day, LIPA=229 min/day, SB=729 min/day, sleep=8 hours/day. SB, sedentary behaviour.
Table 4.
Estimated time difference in waking day composition associated with a risk reduction of 10% in all-cause mortality (HR=0.90) with respect to the reference composition
| Reference composition | MVPA=2 min/day LIPA=358 min/day SB=10 hours/day |
MVPA=10 min/day LIPA=350 min/day SB=10 hours/day |
MVPA=30 min/day LIPA=330 min/day SB=10 hours/day |
| Composition difference | |||
| More MVPA and less SB (LIPA is fixed) | 8 min (95% CI 3 to 91) | 29 min (95% CI 13 to 95) | 52 min (95% CI 31 to 98) |
| More LIPA and less SB (MVPA is fixed) | 51 min (95% CI 39 to 79) | 50 min (95% CI 39 to 72) | 49 min (95% CI 37 to 70) |
Computations are based on hip accelerometer data.
LIPA, light physical activity; MVPA, moderate to vigorous physical activity; SB, sedentary behaviour.
Discussion
This large federated analysis, comprising 130 239 participants across six prospective cohort studies, provides important data about the joint associations between daily movement behaviours and health that could inform public health recommendations and interventions targeting movement behaviours.
We observed a curvilinear dose response between time spent in MVPA and risk of all-cause mortality, with lower risk associated with higher time spent in MVPA. The shape of the relationship suggests that there are diminishing returns in engaging in MVPA. Most of the benefits are seen below 30 min of daily MVPA. This supports the large existing body of research showing the beneficial effect of physical activity.2 47
However, there was a clear heterogeneity of results between studies using wrist- and hip-mounted accelerometers. Studies using a wrist accelerometer tended to result in stronger association for MVPA and suggest that it is only time spent in this behaviour relative to the others that is associated with all-cause mortality. On the contrary, studies using hip accelerometers showed a beneficial association between LIPA and all-cause mortality, which supports recent suggestions that LIPA could be beneficial for health7 8 48 and a detrimental association of time spent in SB with all-cause mortality which is consistent with a growing body of evidence about the detrimental effect of SB on health.9 These results are in good agreement with a recent similar study48 and add new evidence about joint associations. Notably, we show that the beneficial association of time spent in MVPA with all-cause mortality depends on the balance of time spent in LIPA and SB. At high sedentary time (over 11–12 hours) and therefore lower time spent in LIPA, the benefits of MVPA might be completely attenuated. This is in contradiction with reports based on self-reported data49 but in good agreement with more recent studies based on accelerometer data.50
Our results suggest that there might be different behavioural pathways to achieve health benefits through encouraging the displacement of sedentary time by either MVPA or LIPA. Interventions, guidelines or policies advocating increases in LIPA and decreases in SB could be useful in reducing the health burden of inactivity, considering how few people achieve the recommended guidelines for MVPA.51 This could open the door to interventions and recommendations tailored to individual circumstances and capacity. LIPA could be important for people who cannot engage in MVPA, although this is potentially between two and six times less efficient in terms of time investment (see table 4). On the other end of the spectrum, LIPA appears just as beneficial as MVPA for people who engage in over 30 min/day in MVPA.
This difference in results between wrist-worn and hip-worn accelerometers is not entirely surprising as differences in accelerometer placement have been hotly debated to find the right balance between accuracy and increasing sample size through better compliance to measurement protocols. It asks the question about which of these results are the most likely. In this study all the cohorts used threshold-based methods to classify time spent in the different behaviours. Recent validation studies show that threshold methods for both hip and wrist accelerometers provide valid estimates of sleep time.52 On the contrary, poor correlation between wrist and hip accelerometer count-based metrics has been widely reported.53 54 The validation studies tend to find that hip accelerometers provide more accurate estimates than wrist accelerometer based on count threshold classification methods.55 Particularly, threshold-based classification of wrist accelerometer data leads to overestimation of time spent in MVPA and a large misclassification between SB and LIPA.56 57 These misclassifications of sedentary time as LIPA and overestimation of time spent in MVPA are likely to significantly attenuate the association for LIPA and SB in the results of the wrist accelerometer studies. Despite larger sample sizes in the studies using wrist accelerometers, the results of the hip accelerometer studies are at face value more likely to reflect the relationships for waking day behaviours. This might not be the case if other methods of behaviour classification are used. Indeed, wrist accelerometers are more likely to assess sporadic movement in addition to physical activities, particularly with a resolution of the acceleration signal at 5 s. Although these movements generate energy expenditure, their benefit for health might not be similar to those from sustained activities. Further studies should investigate the importance of bout duration in wrist accelerometer data for inference in health research.
We did not find a clear indication that sleep time was associated with all-cause mortality. This is, however, based on only two studies and a narrow range of sleep time recorded in these studies. In contrast, several studies report a dose–response between sleep and mortality, although the shape of the dose–response is contested.8 A possible explanation is that the observed association in previous research could be due to the lack of adjustment for waking day behaviour which is accounted for in our analysis. It is also important to note that a variety of measurement techniques were employed to measure sleep time across the various studies. In addition, sleep time is not regarded as a good indicator of healthy sleep behaviour,58 so our results do not preclude other dimensions of sleep being important for health.
Overall, our study suggests that future research should further investigate the relationship between the balance of time spent daily in these behaviours and health using different methods of accelerometer data processing to harmonise hip and wrist accelerometer data and/or use coordinated pooling of postural data.59 60 In particular, further prospective studies are required to provide evidence to underpin 24-hour movement behaviour recommendations.
Our analysis has several strengths. First, the use of accelerometers avoids the well-known pitfalls of self-report data. However, it should be noted that neither hip nor wrist accelerometers quantify actual sedentary time according to its established definition as time spent below 1.5 metabolic equivalents in a sitting or reclining posture6 as they do not measure posture. The large sample size allowed for a combined analysis of the dose–response associations among SB, physical activity and mortality that provided precise estimates with relatively narrow CIs. Mortality ascertainment varied across studies, but all used official national or regional registers which are likely to reflect a complete and accurate record. Lastly, and perhaps most importantly, the utilisation of a compositional data analysis approach ensures that the interplay between the different behaviours is properly accounted for.
The study has also some limitations. First, all of the studies were conducted in the USA and Western Europe and our search was limited to articles published in English. Thus, the results may not be generalisable beyond these populations. In addition, our search was systematic but not exhaustive of all possible databases, so it is possible that we have missed some data sources. However, we have checked the results of our search against the results of the exhaustive search conducted for the American physical activity guidelines.1 Second, residual confounding may exist. Although we aimed for broadly consistent covariates, differences exist between the studies, and difference in measurement of confounders might have distorted our results. We did not account for all potential confounders in our analysis, for example, genetic factors, environmental factors, body mass index and fitness were not considered (online supplemental material S1). Third, we attempted to minimise bias from reverse causation (ie, illness causing individuals to become sedentary) by restricting our analysis to free-living individuals, adjusting for the subject’s state of health at outset and by excluding death within the first 2 years in sensitivity analysis. However, we cannot fully rule out this bias.61 It is possible that ill individuals are more likely to die prematurely, as a consequence of engaging in less activity and more SB and sleep. This could result in overestimation of the association, but a recent study showed that this is unlikely to lead to entirely spurious associations.62
Our analysis did not examine specifically time spent in vigorous physical activity which was considered in a single category of movement behaviour together with moderate physical activity. Yet, recent research hints that the proportion of time spent in vigorous physical activity might be important for reducing mortality risk.29 63 Future research should consider daily time compositions with time spent in vigorous physical activity to understand the joint association between time spent in vigorous physical activity and other movement behaviours with mortality risk.
In conclusion, this federated analysis indicates that the daily balance of time spent in waking day behaviour physical activity of different intensity and SB shows a joint dose–response association with all-cause mortality. In general, combinations of time with lower SB and higher physical activity (of any intensity) are associated with lower all-cause mortality risk. The strongest association is with time spent in MVPA which should remain the focus of interventions and policy. However, our results also suggest that a number of different approaches could be taken towards health promotion in different populations. To reduce the risk of premature mortality, avoiding very long sedentary time and displacing SB with LIPA could also be a suitable strategy.
What are the findings?
In this study, sleep time was not associated with all-cause mortality.
Wrist and hip accelerometer data when processed using threshold classification methods lead to different results.
Several different combinations of time spent in physical activities, sedentary behaviours and sleep are associated with a similar lower mortality risk.
Replacing sedentary time with light physical activity provides health benefits but increasing moderate to vigorous physical activity requires less time for similar benefits.
How might it impact on clinical practice in the future?
The results provide evidence for the development of integrated 24-hour movement guidelines.
The results suggest that combined and flexible public health recommendations, policy and interventions tailored to an individual’s circumstances and capacities could be adopted. Future guidelines could be expressed in terms of a precise healthy balance of time spent in different waking day behaviours.
As sedentary behaviour is so prevalent and most people are constrained to remain sedentary during the day at work, for example, it would allow them to match their activity levels to their levels of sedentary behaviour.
Acknowledgments
We would like to thank first the participants of the cohort studies. We also would like to thank Neville Owen, Emmanuel Stamatakis and Ulf Ekelund for fruitful discussions that shaped up this work. We would like to extend our gratitude to Tessa Strain, Søren Brage and Katrien Wijndaele for their help and guidance in dealing with the UK Biobank data.
Footnotes
Twitter: @SebChastin, @epiAgeing
SC and DM contributed equally.
Contributors: SC conceived the study. DM, SC, PD and JPA contributed to the design of the study. DM and SC did the literature search. JPA provided guidance on the statistical elements of the study and compositional data analysis expertise. DM performed the federated analysis. DM, SC and PD wrote the first draft of the report. All authors analysed the data. All authors contributed to analysis and interpretation of the data, and critically reviewed the report.
Funding: This work was supported by the Scottish Government’s Rural and Environment Science and Analytical Services Division (to DM and JPA) and the Spanish Ministry of Science, Innovation and Universities (CODAMET RTI2018-095518-B-C21, 2019-2021) (to JPA). The ABC study was supported by Stockholm County Council, Swedish National Centre for Research in Sports, and project ALPHA, which received funding from the European Union in the framework of the Public Health Programme and Folksam Research Foundation, Sweden (to MH and PvR). The REGARDS study was supported by a cooperative agreement U01 NS041588 cofunded by the National Institute of Neurological Disorders and Stroke (NINDS) and the National Institute on Aging (NIA), National Institutes of Health (NIH), Department of Health and Human Service. Additional funding was provided by an investigator-initiated grant R01-NS061846 from the NINDS/NIH (to SH) and an unrestricted research grant from The Coca-Cola Company. The Whitehall II study was supported by grants from the UK Medical Research Council (K013351, R024227, S011676); the British Heart Foundation (PG/11/63/29011 and RG/13/2/30098); the British Health and Safety Executive; the British Department of Health; the National Heart, Lung, and Blood Institute (R01HL036310); the NIA, NIH (R01AG056477, R01AG034454); and the Economic and Social Research Council (ES/J023299/1). MSY and SS were supported by the French National Research Agency (ANR-19-CE36-0004-01). The Women’s Health Study was supported by the NIH grants: CA154647, CA047988, CA182913, HL043851, HL080467 and HL099355 (to EJS and IML).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available in a public, open-access repository. Data may be obtained from a third party and are not publicly available. Some of the data sets included in this study are open access and others are curated by the studies. This is detailed in the manuscript and supplemental material.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
All included cohort studies were approved by a local ethics committee and participants signed informed consent.
References
- 1. Piercy KL, Troiano RP, Ballard RM, et al. The physical activity guidelines for Americans. JAMA 2018;320:2020–8. 10.1001/jama.2018.14854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee I-M, Shiroma EJ, Lobelo F, et al. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet 2012;380:219–29. 10.1016/S0140-6736(12)61031-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Strain T, Brage S, Sharp SJ, et al. Use of the prevented fraction for the population to determine deaths averted by existing prevalence of physical activity: a descriptive study. Lancet Glob Health 2020;8:e920–30. 10.1016/S2214-109X(20)30211-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ding D, Lawson KD, Kolbe-Alexander TL, et al. The economic burden of physical inactivity: a global analysis of major non-communicable diseases. Lancet 2016;388:1311–24. 10.1016/S0140-6736(16)30383-X [DOI] [PubMed] [Google Scholar]
- 5. Guthold R, Stevens GA, Riley LM, et al. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1·9 million participants. Lancet Glob Health 2018;6:e1077–86. 10.1016/S2214-109X(18)30357-7 [DOI] [PubMed] [Google Scholar]
- 6. Tremblay MS, Aubert S, Barnes JD, et al. Sedentary Behavior Research Network (SBRN) - Terminology Consensus Project process and outcome. Int J Behav Nutr Phys Act 2017;14:75. 10.1186/s12966-017-0525-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chastin SFM, De Craemer M, De Cocker K, et al. How does Light-intensity physical activity associate with adult cardiometabolic health and mortality? systematic review with meta-analysis of experimental and observational studies. Br J Sports Med 2019;53:370–6. 10.1136/bjsports-2017-097563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of sleep medicine and sleep research Society. Sleep 2015;38:843–4. 10.5665/sleep.4716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Biswas A, Oh PI, Faulkner GE, et al. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: a systematic review and meta-analysis. Ann Intern Med 2015;162:123. 10.7326/M14-1651 [DOI] [PubMed] [Google Scholar]
- 10. Ross R, Chaput J-P, Giangregorio LM, et al. Canadian 24-hour movement guidelines for adults aged 18-64 years and adults aged 65 years or older: an integration of physical activity, sedentary behaviour, and sleep. Appl Physiol Nutr Metab 2020;45:S57–102. 10.1139/apnm-2020-0467 [DOI] [PubMed] [Google Scholar]
- 11. Tremblay MS, Carson V, Chaput J-P, et al. Canadian 24-hour movement guidelines for children and youth: an integration of physical activity, sedentary behaviour, and sleep. Appl Physiol Nutr Metab 2016;41:S311–27. 10.1139/apnm-2016-0151 [DOI] [PubMed] [Google Scholar]
- 12. DiPietro L, Al-Ansari SS, Biddle SJH, et al. Advancing the global physical activity agenda: recommendations for future research by the 2020 WHO physical activity and sedentary behavior guidelines development group. Int J Behav Nutr Phys Act 2020;17:143. 10.1186/s12966-020-01042-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bull FC, Al-Ansari SS, Biddle S, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med 2020;54:1451–62. 10.1136/bjsports-2020-102955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ekelund U, Dalene KE, Tarp J, et al. Physical activity and mortality: what is the dose response and how big is the effect? Br J Sports Med 2020;54:1125–6. 10.1136/bjsports-2019-101765 [DOI] [PubMed] [Google Scholar]
- 15. Katzmarzyk PT. Studies of sedentary behavior, activity, and mortality: duplication or replication? Med Sci Sports Exerc 2016;48:1302. 10.1249/MSS.0000000000000919 [DOI] [PubMed] [Google Scholar]
- 16. Chastin SFM, Palarea-Albaladejo J, Dontje ML, et al. Combined effects of time spent in physical activity, sedentary behaviors and sleep on obesity and Cardio-Metabolic health markers: a novel compositional data analysis approach. PLoS One 2015;10:e0139984. 10.1371/journal.pone.0139984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pedišić Ž . Measurement issues and poor adjustments for physical activity and sleep undermine sedentary behaviour research—The focus should shift to the balance between sleep, sedentary behaviour, standing and activity. Kinesiology 2014;46:135–46. [Google Scholar]
- 18. Arnold KF, Berrie L, Tennant PWG, et al. A causal inference perspective on the analysis of compositional data. Int J Epidemiol 2020;49:1307–13. 10.1093/ije/dyaa021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aitchinson J. The Statistical Analysis of Compositional Data. Caldwell, NJ, USA: Blackburn Press, 2003. [Google Scholar]
- 20. Janssen I, Clarke AE, Carson V, et al. A systematic review of compositional data analysis studies examining associations between sleep, sedentary behaviour, and physical activity with health outcomes in adults. Appl Physiol Nutr Metab 2020;45:S248–57. 10.1139/apnm-2020-0160 [DOI] [PubMed] [Google Scholar]
- 21. Stamatakis E, Bauman AE. The bold sedentary behavior recommendations in the new Canadian guidelines: are they evidence-based? Response to "Sedentary Behavior Research Network members support new Canadian 24-Hour Movement Guideline recommendations". J Sport Health Sci 2020;9:482–4. 10.1016/j.jshs.2020.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jones EM, Sheehan NA, Masca N, et al. DataSHIELD – shared individual-level analysis without sharing the data: a biostatistical perspective. Nor Epidemiol 2012;21:231–9. 10.5324/nje.v21i2.1499 [DOI] [Google Scholar]
- 23. Wolfson M, Wallace SE, Masca N, et al. DataSHIELD: resolving a conflict in contemporary bioscience--performing a pooled analysis of individual-level data without sharing the data. Int J Epidemiol 2010;39:1372–82. 10.1093/ije/dyq111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006–12. 10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 25. von Elm E, Altman DG, Egger M, et al. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806–8. 10.1136/bmj.39335.541782.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McGregor DE, Palarea-Albaladejo J, Dall PM, et al. Cox regression survival analysis with compositional covariates: application to modelling mortality risk from 24-h physical activity patterns. Stat Methods Med Res 2020;29:1447–65. 10.1177/0962280219864125 [DOI] [PubMed] [Google Scholar]
- 27. Egozcue JJ, Pawlowsky-Glahn V. Groups of parts and their balances in compositional data analysis. Math Geol 2005;37:795–828. 10.1007/s11004-005-7381-9 [DOI] [Google Scholar]
- 28. Kujala UM. Is physical activity a cause of longevity? it is not as straightforward as some would believe. A critical analysis. Br J Sports Med 2018;52:914–8. 10.1136/bjsports-2017-098639 [DOI] [PubMed] [Google Scholar]
- 29. Strain T, Wijndaele K, Dempsey PC, et al. Wearable-device-measured physical activity and future health risk. Nat Med 2020;26:1385–91. 10.1038/s41591-020-1012-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994;81:515–26. 10.1093/biomet/81.3.515 [DOI] [Google Scholar]
- 31. Palarea-Albaladejo J, Martín-Fernández JA. zCompositions — R package for multivariate imputation of left-censored data under a compositional approach. Chemometrics and Intelligent Laboratory Systems 2015;143:85–96. 10.1016/j.chemolab.2015.02.019 [DOI] [Google Scholar]
- 32. Gasparrini A, Armstrong B, Kenward MG. Multivariate meta-analysis for non-linear and other multi-parameter associations. Stat Med 2012;31:3821–39. 10.1002/sim.5471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mahalanobis P. On the generalised distance in statistics. Proc Natl Inst Sci India 1936;2:49–55. [Google Scholar]
- 34. Dohrn I-M, Kwak L, Oja P, et al. Replacing sedentary time with physical activity: a 15-year follow-up of mortality in a national cohort. Clin Epidemiol 2018;10:179–86. 10.2147/CLEP.S151613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Matthews CE, Keadle SK, Troiano RP, et al. Accelerometer-measured dose-response for physical activity, sedentary time, and mortality in US adults. Am J Clin Nutr 2016;104:1424–32. 10.3945/ajcn.116.135129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ensrud KE, Blackwell TL, Cauley JA, et al. Objective measures of activity level and mortality in older men. J Am Geriatr Soc 2014;62:2079–87. 10.1111/jgs.13101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Doherty A, Jackson D, Hammerla N, et al. Large scale population assessment of physical activity using wrist worn Accelerometers: the UK Biobank study. PLoS One 2017;12:e0169649. 10.1371/journal.pone.0169649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. LaMonte MJ, Buchner DM, Rillamas-Sun E, et al. Accelerometer-Measured physical activity and mortality in women aged 63 to 99. J Am Geriatr Soc 2018;66:886–94. 10.1111/jgs.15201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Diaz KM, Howard VJ, Hutto B, et al. Patterns of sedentary behavior in US middle-age and older adults: the REGARDS study. Med Sci Sports Exerc 2016;48:430–8. 10.1249/MSS.0000000000000792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sabia S, van Hees VT, Shipley MJ, et al. Association between questionnaire- and accelerometer-assessed physical activity: the role of sociodemographic factors. Am J Epidemiol 2014;179:781–90. 10.1093/aje/kwt330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee I-M, Shiroma EJ, Evenson KR, et al. Accelerometer-Measured physical activity and sedentary behavior in relation to all-cause mortality: the women's health study. Circulation 2018;137:203–5. 10.1161/CIRCULATIONAHA.117.031300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jefferis BJ, Parsons TJ, Sartini C, et al. Objectively measured physical activity, sedentary behaviour and all-cause mortality in older men: does volume of activity matter more than pattern of accumulation? Br J Sports Med 2019;53:1013–20. 10.1136/bjsports-2017-098733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Aresu M, Bécares L, Brage S. Health survey for England: physical activity and fitness. NHS Inf Cent Heal Soc care 2009. 10.1016/j.ridd.2011.01.017 [DOI] [PubMed] [Google Scholar]
- 44. Koolhaas CM, Dhana K, van Rooij FJA, et al. Sedentary time assessed by actigraphy and mortality: the Rotterdam study. Prev Med 2017;95:59–65. 10.1016/j.ypmed.2016.11.021 [DOI] [PubMed] [Google Scholar]
- 45. Henson J, Yates T, Biddle SJH, et al. Associations of objectively measured sedentary behaviour and physical activity with markers of cardiometabolic health. Diabetologia 2013;56:1012–20. 10.1007/s00125-013-2845-9 [DOI] [PubMed] [Google Scholar]
- 46. Murabito JM, Pedley A, Massaro JM, et al. Moderate-to-vigorous physical activity with accelerometry is associated with visceral adipose tissue in adults. J Am Heart Assoc 2015;4:e001379. 10.1161/JAHA.114.001379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wen CP, Wai JPM, Tsai MK, et al. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet 2011;378:1244–53. 10.1016/S0140-6736(11)60749-6 [DOI] [PubMed] [Google Scholar]
- 48. Ekelund U, Tarp J, Steene-Johannessen J, et al. Dose-response associations between accelerometry measured physical activity and sedentary time and all cause mortality: systematic review and harmonised meta-analysis. BMJ 2019;366:l4570. 10.1136/bmj.l4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ekelund U, Steene-Johannessen J, Brown WJ, et al. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet 2016;388:1302–10. 10.1016/S0140-6736(16)30370-1 [DOI] [PubMed] [Google Scholar]
- 50. Ekelund U, Tarp J, Fagerland MW, et al. Joint associations of accelero-meter measured physical activity and sedentary time with all-cause mortality: a harmonised meta-analysis in more than 44 000 middle-aged and older individuals. Br J Sports Med 2020;54:1499–506. 10.1136/bjsports-2020-103270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hallal PC, Andersen LB, Bull FC, et al. Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet 2012;380:247–57. 10.1016/S0140-6736(12)60646-1 [DOI] [PubMed] [Google Scholar]
- 52. Full KM, Kerr J, Grandner MA, et al. Validation of a physical activity accelerometer device worn on the hip and wrist against polysomnography. Sleep Health 2018;4:209–16. 10.1016/j.sleh.2017.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Migueles JH, Cadenas-Sanchez C, Rowlands AV, et al. Comparability of accelerometer signal aggregation metrics across placements and dominant wrist cut points for the assessment of physical activity in adults. Sci Rep 2019;9:18235. 10.1038/s41598-019-54267-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kamada M, Shiroma EJ, Harris TB, et al. Comparison of physical activity assessed using hip- and wrist-worn accelerometers. Gait Posture 2016;44:23–8. 10.1016/j.gaitpost.2015.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rosenberger ME, Haskell WL, Albinali F, et al. Estimating activity and sedentary behavior from an accelerometer on the hip or wrist. Med Sci Sports Exerc 2013;45:964–75. 10.1249/MSS.0b013e31827f0d9c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Marcotte RT, Petrucci GJ, Cox MF, et al. Estimating sedentary time from a Hip- and Wrist-Worn Accelerometer. Med Sci Sports Exerc 2020;52:225–32. 10.1249/MSS.0000000000002099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Leinonen A-M, Ahola R, Kulmala J, et al. Measuring physical activity in free-living Conditions-Comparison of three Accelerometry-Based methods. Front Physiol 2016;7:681. 10.3389/fphys.2016.00681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Groeger JA, Zijlstra FRH, Dijk D-J. Sleep quantity, sleep difficulties and their perceived consequences in a representative sample of some 2000 British adults. J Sleep Res 2004;13:359–71. 10.1111/j.1365-2869.2004.00418.x [DOI] [PubMed] [Google Scholar]
- 59. Stamatakis E, Koster A, Hamer M, et al. Emerging Collaborative research platforms for the next generation of physical activity, sleep and exercise medicine guidelines: the prospective physical activity, sitting, and sleep Consortium (ProPASS). Br J Sports Med 2020;54:435–7. 10.1136/bjsports-2019-100786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dall PM, Skelton DA, Dontje ML, et al. Characteristics of a protocol to collect objective physical activity/sedentary behaviour data in a large study: seniors USP (understanding sedentary patterns). J Meas Phys Behav 2018;1:26–31. 10.1123/jmpb.2017-0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Allison DB, Heo M, Flanders DW, et al. Examination of "early mortality exclusion" as an approach to control for confounding by occult disease in epidemiologic studies of mortality risk factors. Am J Epidemiol 1997;146:672–80. 10.1093/oxfordjournals.aje.a009334 [DOI] [PubMed] [Google Scholar]
- 62. Matthews CE, Troiano RP, Salerno EA, et al. Exploration of confounding due to poor health in an Accelerometer–Mortality study. Med Sci Sport Exerc 2020;52:2546–53. 10.1249/MSS.0000000000002405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. O'Donovan G, Stamatakis E, Stensel DJ, et al. The importance of Vigorous-Intensity leisure-time physical activity in reducing cardiovascular disease mortality risk in the obese. Mayo Clin Proc 2018;93:1096–103. 10.1016/j.mayocp.2018.01.016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bjsports-2020-102345supp001.pdf (800.8KB, pdf)
Data Availability Statement
Data are available in a public, open-access repository. Data may be obtained from a third party and are not publicly available. Some of the data sets included in this study are open access and others are curated by the studies. This is detailed in the manuscript and supplemental material.