Abstract
Background:
Evidence-based recommendations on the optimal evaluation approach for dementia diagnostics are limited. This impedes a harmonized workup across clinics and nations.
Objective:
To evaluate the diagnostic performance of a multidisciplinary consensus conference compared to a single clinician approach.
Methods:
In this prospective study, we enrolled 457 patients with suspected cognitive decline, from two European memory clinics. A diagnostic evaluation was performed at baseline independently in two ways: 1) by a single clinician and 2) at a multidisciplinary consensus conference. A syndrome diagnosis and an etiological diagnosis was made. The confidence in the diagnosis was recorded using a visual analogue scale. An expert panel re-evaluation diagnosis served as reference for the baseline syndrome diagnosis and a 12-24-month follow-up diagnosis for the etiological diagnosis.
Results:
439 patients completed the study. We observed 12.5%discrepancy (k = 0.81) comparing the baseline syndrome diagnoses of the single clinician to the consensus conference, and 22.3%discrepancy (k = 0.68) for the baseline etiological diagnosis. The accuracy of the baseline etiological diagnosis was significantly higher at the consensus conference and was driven mainly by increased accuracy in the MCI group. Confidence in the etiological diagnosis at baseline was significantly higher at the consensus conference (p < 0.005), especially for the frontotemporal dementia diagnosis.
Conclusion:
The multidisciplinary consensus conference performed better on diagnostic accuracy of disease etiology and increased clinicians’ confidence. This highlights the importance of a multidisciplinary diagnostic evaluation approach for dementia diagnostics, especially when evaluating patients in the MCI stage.
Keywords: Alzheimer disease, clinical decision-making, dementia, differential diagnosis, frontotemporal dementia, Lewy body disease, vascular dementia
INTRODUCTION
The prevalence of dementia is increasing, largely due to a growing elderly population [1]. Higher prevalence and growing awareness of dementia in the population increases the number of patients entering into memory clinics. Thus, there is a need to increase diagnostic flow, but at the same time to maintain a sufficiently high diagnostic accuracy. Due to overlapping clinical presentations, conflicting biomarkers and mixed pathologies, dementia diagnostics can be challenging, especially in the early stages of neurodegenerative diseases [2–4]. Yet, treatment options and prognosis differ substantially between, e.g., Alzheimer’s disease (AD), Lewy body dementia (DLB), vascular dementia (VaD), and frontotemporal dementia (FTD), and an early and accurate diagnosis is therefore important to ensure optimal patient management. However, evidence-based recommendations on the optimal diagnostic evaluation approach for dementia diagnostics, are limited.
An integrated multidisciplinary approach is generally recommended [5]. However, the approach to diagnostic evaluation varies across memory clinics, and data on the performance of different diagnostic evaluation approaches, such as multidisciplinary consensus conference and single clinician decision-making, are limited [6]. One study found that based on clinical information of pathologically verified cases, a consensus panel diagnosis out-performed individual panel members’ diagnosis in terms of accuracy, when differentiating between AD or FTD [7]. Other studies have evaluated the agreement of a multidisciplinary consensus diagnosis compared to a monodisciplinary one but, given the lack of a reference diagnosis in these studies, the added value of the former could not be demonstrated [8, 9]. Data exist indicating increasing confidence in the diagnosis following a consensus panel approach [7], but generally data on diagnostic confidence is limited.
A consensus-based multidisciplinary approach to diagnostic decision-making is frequently used in other medical specialties, particularly in the diagnosis and management of cancer. Evidence suggests that this approach results in a higher likelihood of an accurate cancer staging and initiation of adjuvant therapy [10]. Based on the experience from cancer diagnostics, it may be hypothesized that a multidisciplinary consensus approach could be beneficial in terms of increasing the accuracy in dementia diagnostics also.
In this prospective study, we therefore assessed the accuracy of the diagnoses made at a multidisciplinary consensus conference and by a single clinician. In addition, the agreement between the diagnoses determined by the two diagnostic decision-making approaches and the confidence in the diagnoses were assessed.
METHODS
Subjects
Study participants were recruited as part of the PredictND project in two European memory clinics: the Alzheimer Center Amsterdam, Amsterdam UMC, location VU University Medical Center, Amsterdam, Netherlands (VUmc) [11, 12] and the Danish Dementia Research Centre, Copenhagen University Hospital, Rigshospitalet, Copenhagen, Denmark (RH) [13]. In these clinics, the routine diagnostic evaluation is based on a multidisciplinary consensus conference.
We included patients referred to the memory clinic who were suspected of having cognitive decline due to subjective cognitive decline (SCD), mild cognitive impairment (MCI), or dementia. Inclusion criteria were a baseline Mini-Mental State Examination (MMSE) ≥18, Clinical Dementia Rating (CDR) ≤1.0, and an available T1-weighted MRI at or above 1.5 Tesla within the last 6 months. Exclusion criteria were major psychiatric disorder, excessive alcohol intake or substance abuse within the last 2 years, and other brain disorders, which could explain the cognitive problems. In total, 457 patients were enrolled consecutively from March 2015 to June 2016. The study was approved to local Medical Ethical Committees in both centers and all patients gave written informed consent.
Clinical assessment
The standard screening program included medical history, relevant risk factors, neurological and physical examination, neuropsychological testing, blood screening, and an MRI brain scan. Cognitive testing was performed using a neuropsychological test battery which included MMSE [14], forward and backward performance on Digit span [15], the Trail Making Test A and B, The Category Fluency Test (letter and animal) [16], and clock drawing [17]. Verbal memory was tested by the Rey Auditory Verbal Learning Test (RAVLT) (VUmc) [18] and by the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) wordlist memory test (RH) [19]. The Geriatric Depression Scale (GDS) was used to assess symptoms of depression [20] and the Neuropsychiatric Inventory (NPI) to evaluate behavioral and psychological symptoms [21]. A global CDR score was estimated for all patients [22]. Activities of daily living were assessed by nursing staff in the majority of cases, using the Disability Assessment for Dementia (DAD) (VUmc) and the Instrumental Activities of Daily Living - Functional Activities Questionnaire (IADL-FAQ) (RH) [23, 24].
When clinically indicated, additional tests were performed including 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography (2-[18F]FDG-PET), cerebrospinal fluid (CSF) biomarkers (amyloid-beta1–42, total-tau, and phospho-tau), amyloid-PET, dopamine transporter single-photon emission computed tomography (DAT-SPECT), EEG and supplementary neuropsychological testing. An overview of the frequency of additional biomarker testing is provided in Table 1.
Table 1A.
Baseline characteristics
| Characteristic | SCD (n = 134) | MCI (n = 102) | Dementia (n = 212) | Significant differences between groups |
| Female, n (%) | 74 (55.2%) | 31 (30.4%) | 105 (49.5%) | SCD, dementia > MCI |
| Age, y | 62.4 (8.7) | 68.3 (8.0) | 70.5 (9.2) | SCD<MCI, dementia |
| Duration of symptoms, y | 4.1 (4.3) | 3.1 (3.5) | 2.7 (2.2) | none |
| MMSE | 28.7 (1.4) | 27.3 (2.4) | 24.9 (2.9) | SCD>MCI>dementia |
| Etiology, n (AD/VaD/DLB/MD/FTD/OT) | – | 49/13/8/3/3/26 | 111/19/17/19/21/25 | |
| Progression, n (MCI/Dementia) | 9/3 | –/23 | – |
Baseline characteristics of groups based on the syndrome diagnosis (as diagnosed by consensus conference). Differences between groups were assessed using one-way ANOVA with Tukey post-hoc testing (age), χ2 test (gender) and Kruskal-Wallis with Dunn post-hoc testing (duration of symptom and MMSE). Data are presented as mean (SD), unless otherwise specified. AD, Alzheimer’s disease; VaD, vascular dementia; DLB, dementia with Lewy bodies; MD, mixed dementia; FTD, frontotemporal dementia; OT, other dementia.
Table 1B.
Baseline biomarker status
| CSF analysis | FDG-PET | Amyloid-PET | DAT-SPECT | EEG | Biomarker status1 | Additional neuropsychology | |
| SCD (n = 134) | 21 (15.7%) | 47 (35.1%) | 1 (0.7%) | 1 (0.7%) | 68 (50.7%) | 116 (86.6%) | 133 (99.2%) |
| MCI (n = 102) | 25 (24.5%) | 46 (45.1%) | 1 (0.9%) | 1 (0.9%) | 46 (45.1%) | 92 (90.2%) | 98 (96.1%) |
| Dementia (n = 212) | 68 (32.1%) | 106 (50%) | 6 (2.8%) | 4 (1.9%) | 84 (39.6%) | 194 (91.5%) | 197 (92.9%) |
| Total (n = 448) | 114 (25.4%) | 199 (44.4%) | 8 (1.8%) | 6 (1.3%) | 198 (44.2%) | 402 (87.9%) | 428 (95.5%) |
Biomarkers available at baseline evaluation. Table stratified by syndrome diagnosis as diagnosed by the consensus conference. CSF analysis including beta-amyloid1–42, total-tau and phospho-tau. All included patients had an available MRI at baseline as part of inclusion criteria. 1 Biomarker status indicating at least one additional biomarker (CSF, FDG-PET, amyloid-PET, DAT-SPECT, EEG), besides MRI, available at baseline.
Diagnostic criteria
Patients were classified as SCD, MCI, or dementia. SCD was defined as cognitive complaints from the patient and/or caregiver, not fulfilling the criteria for MCI or dementia. The diagnostic criteria used for diagnosing dementia and MCI were based on the National Institute of Aging –Alzheimer’s Association (NIA-AA) criteria [4, 25]. The etiological diagnoses were based on the following criteria: the NIA-AA criteria for AD [4, 25], Neary and Snowden et al. or McKhann et al. criteria for FTD [26, 27] with Rascovsky et al. criteria for behavioral variant FTD (bvFTD) and Gorno-Tempini et al. criteria for semantic dementia (SD) and progressive non-fluent aphasia (PNFA) [28, 29], McKeith et al. criteria for DLB [30], NINDS-AIREN criteria for VaD [31], and the Dubois et al. criteria for mixed dementia with AD and evident vascular lesions [32]. All other causes of cognitive deficits (e.g., Parkinson’s disease with dementia or normal pressure hydrocephalus) were classified as “other dementia”. For statistical analysis, bvFTD, SD, and PNFA were grouped and labeled FTD.
Diagnostic procedure
For each patient, a baseline diagnosis (both syndrome and etiological diagnosis) was made as a consensus conference decision as well as by a single clinician with access to identical clinical and paraclinical information.
Consensus conference evaluation
The consensus conference included 3–5 physicians experienced in dementia diagnostics (most specialized in neurology), neuropsychologists, and specialized nurses. During the consensus meeting the following items were scored per included patient: 1) a syndrome diagnosis (SCD, MCI, or dementia), 2) in case of MCI or dementia: an etiological diagnosis, and 3) confidence in the etiological baseline diagnosis on a continuous scale based on a visual-analogue-scale (VAS) ranging from 0–100.
Single clinician evaluation
The single clinician had either not attended the consensus conference or performed the single clinician evaluation at least one month after the consensus conference. The single clinician rated the same items as were rated at the consensus conference. The single clinician evaluations were performed by four individual medical doctors; one specialized in neurology and one in geriatrics, both with more than five years of experience in dementia diagnostics, and two non-specialized doctors with approximately two years of experience in dementia diagnostics.
Reference diagnosis
An expert panel consisting of three senior level physicians experienced in dementia diagnostics (SH, KF, HR) re-evaluated all cases with discrepancy between the consensus conference and single clinician baseline syndrome diagnosis to define a syndrome reference diagnosis. The expert panel was blinded to the original baseline syndrome diagnosis.
All patients had a follow-up visit after a minimum of 12 months from baseline, and the diagnosis at follow-up was used as the reference etiological diagnosis. Moreover, patients with MCI or SCD had an additional follow-up visit at 18–24 months. Each follow-up visit included MMSE, CDR, and a face-to-face interview with the patient. If an in-person follow-up visit could not be performed, the evaluation was made based on a telephone interview (n = 40). A follow-up etiological diagnosis was determined by or in consultation with a senior level physician experienced in dementia diagnostics based on all available information. Blinding for the consensus conference diagnosis was not possible at the follow-up assessment.
Statistical analysis
Differences in baseline characteristics between groups were assessed using one-way analysis of variance (ANOVA), Kruskal–Wallis test, and Chi-square test, where appropriate.
First, for both baseline syndrome and etiological diagnoses, we assessed the agreement between the diagnoses made by the single clinician and the consensus conference by performing a Fleiss’ kappa test on paired categorical data with non-unique raters. In addition, individual kappa values were reported indicating the level of agreement for each separate diagnosis against all other diagnoses combined (e.g., MCI versus non-MCI). Kappa values≤0.20 indicates a “none to slight” agreement, 0.21 –0.40 indicates “fair”, 0.41 –0.60 indicates “moderate”, 0.61 –0.80 indicates “substantial”, and≥0.81 indicates a “almost perfect” strength of agreement [33].
Second, a McNemar test was used to test for equality of paired proportions in 2x2 contingency tables to study the diagnostic accuracy, again for both baseline syndrome and etiological diagnoses. Finally, we performed a paired sample t-test to assess the difference in diagnostic confidence on the VAS-scale (0–100). The level of significance was set at p < 0.05. SPSS software v. 25 (IBM, New York, NY) was used for statistical analyses.
RESULTS
In total, 448 patients were evaluated at baseline. Nine patients were lost to follow-up with 439 completing the study. At baseline, 134 patients (29.9%) were diagnosed with SCD, 102 (22.7%) with MCI, and 212 (47.3%) with dementia as diagnosed by the consensus conference. The most frequent etiology was AD (51.2%). Participants were generally mildly impaired with regards to cognition with a mean MMSE of 28.7 for SCD, 27.3 for MCI, and 24.9 for dementia.
All patients had an MRI available at baseline. At least one additional biomarker (CSF, PET-FDG, amyloid-PET, SPECT-DAT, EEG) was available in 87.9%of cases. Baseline characteristics and biomarker status according to the syndrome diagnosis at baseline are presented in Table 1.
In some instances, supplementary diagnostic tests were performed from baseline to follow-up. A CSF biomarker status was available at follow-up in 63.4%of patients.
The analyses were performed on merged data from the two memory clinics. Apart from a borderline significant difference in the syndrome diagnosis level of agreement, no significant differences in the results were seen between centers (data not shown).
Syndrome diagnosis agreement
In 87.5%(392/448) of the cases, there was an agreement between the single clinician and the consensus conference syndrome diagnosis at baseline, whereas in 12.5%(56/448) there was a disagreement. Measured by kappa values we found that the overall agreement was k = 0.81 (95%CI, 0.74–0.87). Based on individual kappa values, we observed a higher level of agreement for dementia (k = 0.84, 95%CI 0.75–0.93) and SCD (k = 0.89, 95%CI 0.80–0.98), and conversely lower agreement for MCI (k = 0.67, 95%CI 0.58–0.76). Table 2 presents a confusion matrix for the syndrome diagnoses.
Table 2.
Baseline syndrome diagnosis agreement
| Single clinician | |||||
| SCD | MCI | Dementia | Total | ||
| Consensus conference | SCD | 122 | 11 | 1 | 134 |
| MCI | 9 | 81 | 12 | 102 | |
| Dementia | 0 | 23 | 189 | 212 | |
| Total | 131 | 115 | 202 | 448 | |
Confusion matrix presenting baseline syndrome diagnosis agreement. SCD, subjective cognitive decline; MCI, mild cognitive impairment.
Syndrome diagnosis accuracy
In the 56 cases with discrepancy between the consensus conference and single clinician syndrome diagnosis, an expert panel of physicians experienced in dementia diagnostics re-evaluated the cases to define a reference diagnosis. Figure 1 displays the proportion of correct and incorrect syndrome diagnoses as compared to the re-evaluated reference diagnosis made by the expert panel. The difference in proportion did not reach statistical significance (p = 0.28).
Fig. 1.
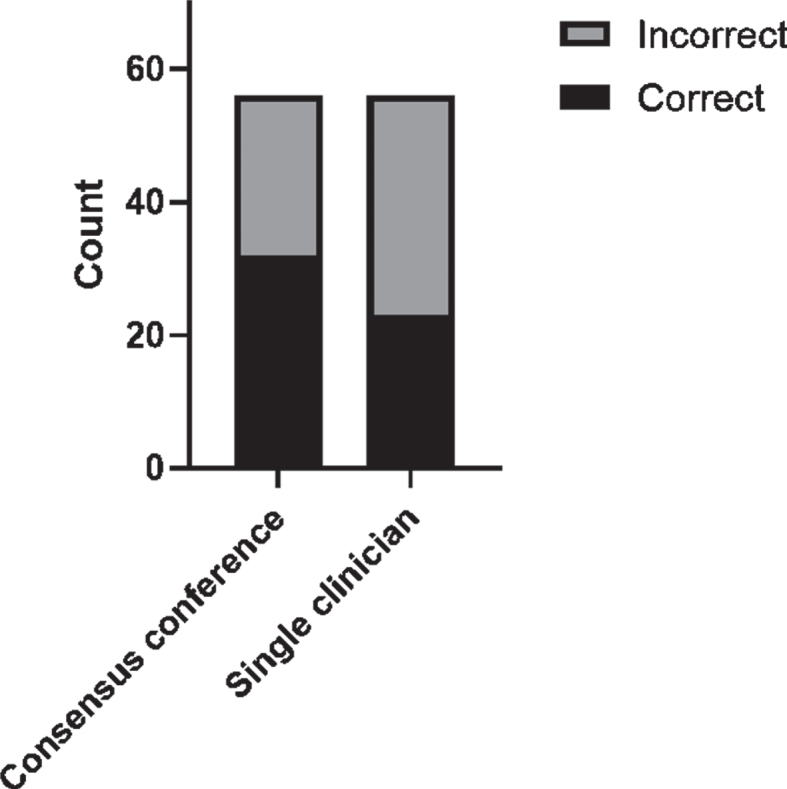
Diagnostic accuracy of baseline syndrome diagnosis. Only cases with discrepancy at baseline regarding syndrome diagnosis were re-evaluated by an expert panel to determine a reference diagnosis. Stacked bar chart displaying total number correct and incorrect diagnoses.
Etiological diagnosis agreement
In 305 of the MCI and dementia cases an etiological diagnosis was available for both the consensus conference and the single clinician at baseline. Cases diagnosed with SCD by either the consensus conference or the single clinician, were not assigned an etiological diagnosis and therefore not included in the analysis of etiological diagnosis agreement. In 77.7%(237/305) of the cases there were an agreement between the consensus conference and single clinician etiological diagnosis, whereas in 22.3%(68/305) there was a disagreement. Measured by kappa values we found that the overall level of agreement was k = 0.68 (95%CI, 0.62–0.74). The level of agreement for the etiological diagnosis was higher for patients with dementia (k = 0.73, 95%CI, 0.66–0.81) compared to MCI (k = 0.64, 95%CI 0.52–0.76).
Table 3 presents a confusion matrix for the etiological diagnoses. The consensus conference diagnosed a higher proportion of patients with AD and “Other dementia” compared to the single clinician where a higher number of patients were diagnosed with mixed dementia and FTD.
Table 3.
Baseline etiological diagnosis
| Single clinician | ||||||||
| AD | VaD | DLB | Mixed | FTD | Other | Total | ||
| Consensus conference | AD | 134 (40/94) | 2 (1/1) | 3 (0/3) | 11 (4/7) | 5 (1/4) | 4 (2/2) | 159 (48/111) |
| VaD | 0 | 25 (7/18) | 0 | 3 (2/1) | 0 | 3 (3/0) | 31 (12/19) | |
| DLB | 1 (0/1) | 1 (0/1) | 19 (6/13) | 1 (1/0) | 1 (0/1) | 1 (0/1) | 24 (7/17) | |
| Mixed | 2 (0/2) | 2 (0/2) | 0 | 17 (3/14) | 0 | 1 (0/1) | 22 (3/19) | |
| FTD | 5 (2/3) | 0 | 0 | 1 (1/0) | 16 (0/16) | 2 (0/2) | 24 (3/21) | |
| Other | 4 (3/1) | 5 (4/1) | 3 (0/3) | 0 | 7 (1/6) | 26 (12/14) | 45 (20/25) | |
| Total | 146 (45/101) | 35 (12/23) | 25 (6/19) | 33 (11/22) | 29 (2/27) | 37 (17/20) | 305 (93/212) | |
Confusion matrix presenting baseline etiological diagnosis agreement. Data presented as total (MCI/dementia). AD, Alzheimer’s disease; VaD, vascular dementia; DLB, dementia with Lewy bodies; Mixed, mixed dementia with AD and VaD; FTD, frontotemporal dementia; Other, Other dementia.
Table 4 presents an overview of the level of agreement stratified by diagnoses. The etiologies with highest level of agreement were AD and DLB. Conversely FTD and mixed dementia had the lowest level of agreement. Overall, the agreement of the etiologic diagnosis was lower for the MCI group compared to the dementia group. For the etiologies VaD, mixed dementia, and FTD, we observed the greatest change in level of agreement comparing MCI and dementia groups, although the total number of cases in these groups were small.
Table 4.
Level of agreement etiological diagnosis
| MCI + Dementia (n = 305) | Dementia (n = 189) | MCI (n = 81) | |
| AD | 0.76 (0.65–0.87) | 0.78 (0.635–0.920) | 0.73 (0.51–0.95) |
| VaD | 0.73 (0.62–0.84) | 0.91 (0.76–1) | 0.62 (0.40–0.84) |
| DLB | 0.76 (0.64–0.87) | 0.74 (0.60–0.88) | 0.90 (0.69–1) |
| Mixed | 0.58 (0.47–0.69) | 0.71 (0.57–0.85) | 0.51 (0.30–0.73) |
| FTD | 0.57 (0.45–0.68) | 0.64 (0.50–0.78) | –0.032 (–0.25–0.19) |
| Other | 0.58 (0.47–0.69) | 0.57 (0.43–0.71) | 0.58 (0.36–0.80) |
| Total | 0.68 (0.62–0.74) | 0.73 (0.66–0.81) | 0.64 (0.52–0.76) |
Table presenting the level of agreement including individual kappa values. First column includes all patients with MCI and dementia. Second column includes only patients with dementia as diagnosed by both consensus conference and single clinician. Third column includes only patients with MCI as diagnosed by both consensus conference and single clinician. Data presented as kappa value (95%CI). MCI, mild cognitive impairment; AD, Alzheimer’s disease; VaD, vascular dementia; DLB, dementia with Lewy bodies; Mixed, mixed dementia with AD and VaD; FTD, Frontotemporal dementia; Other, other dementia.
Etiological diagnosis accuracy
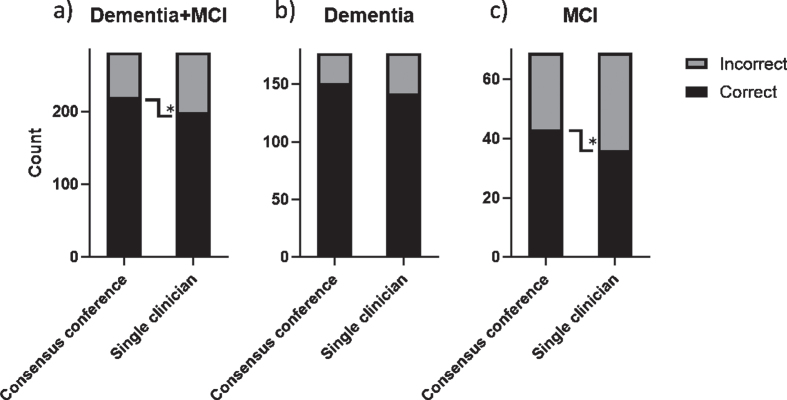
In 281 of the MCI and dementia cases an etiological diagnosis was available for both the consensus conference and the single clinician at baseline and at follow-up. Figure 2 displays the proportion of correct and incorrect etiological diagnoses for all patients, as compared to the follow-up reference diagnosis, stratified by the baseline syndrome diagnosis of dementia and MCI. When comparing the etiological diagnoses for all patients the proportion of correct diagnoses was significantly higher for the consensus conference (p = 0.003) (Fig. 2a). We observed that the proportion of correct etiological diagnoses was lower for patients with MCI compared to dementia for both consensus conference and single clinician (p = 0.001 and p < 0.001 respectively). Furthermore, the accuracy of the etiological diagnoses made by the consensus conference and a single clinician differed significantly for MCI patients (p = 0.039), but not for dementia patients (p = 0.12) (Fig. 2b, c).
Fig. 2.
Diagnostic accuracy of baseline etiological diagnosis. Stacked bar chart displaying total number correct and incorrect diagnoses. *p < 0.05.
Rater experience
As a subanalysis, we assessed if the level of agreement was influenced by the level of experience of the single clinicians. The syndrome diagnosis level of agreement was lower for single clinician raters with less experience (k = 0.70, 95%CI 0.60–0.80) compared to more experienced raters (k = 0.89; 95%CI 0.80–0.98). The etiological diagnosis agreement was not influenced by the single clinician raters experience (k = 0.67 and k = 0.69).
Confidence in the diagnosis
Finally, we found that a mean VAS value for confidence in the etiological diagnosis at consensus conference and single clinician was 77.7 (±14.9) and 73.5 (±17.5), respectively. With a mean difference of 4.2 (±15.8) confidence in the diagnosis was significantly higher for the consensus conference (p < 0.005). Stratified by etiology, we observed that the confidence in the diagnosis was lowest for the FTD diagnosis compared to other etiologies, when rated by the single clinician. The confidence in the diagnosis for the consensus conference was independent of the assigned etiology (see Table 5).
Table 5.
Confidence in diagnosis
| AD | VaD | DLB | Mixed | FTD | Other | Total | |
| Consensus conference VAS | 78 (76–80) | 78 (73–83) | 70 (64–76) | 75 (70–81) | 75 (69–81) | 67 (62–72) | 78 (76–79) |
| Single clinician VAS | 76 (74–79) | 74 (69–80) | 64 (55–74) | 71 (66–75) | 59 (50–67) | 56 (50–61) | 74 (72–75) |
VAS confidence in diagnosis stratified by assigned etiology. Data presented as mean (95%CI). AD, Alzheimer’s disease; VaD, vascular dementia; DLB, dementia with Lewy bodies; Mixed, mixed dementia with AD and VaD; FTD, frontotemporal dementia; Other, other dementia.
DISCUSSION
In this prospective study, we compared a single clinician versus a multidisciplinary consensus conference approach for dementia diagnostics. We found that the two diagnostic approaches showed substantial agreement for syndrome diagnoses, whereas the agreement was lower for the etiological diagnosis especially in the MCI group. Furthermore, we found that the diagnostic accuracy of the etiological diagnosis and confidence in the diagnosis were significantly higher for the consensus conference.
In line with previous studies [8], we found a substantial overall agreement (k = 0.81) for the baseline syndrome diagnosis suggesting comparability between the two evaluation approaches for the syndrome diagnosis. A syndrome diagnosis discordancy of 12.5%may, however, be clinically problematic since the syndrome diagnosis has significant implications on planned follow-up, level of care and also potentially for treatment of the patient. Confirming the results of previous studies, we observed a lower level of agreement (k = 0.67) between single clinician and consensus conference diagnosis with regards to MCI versus non-MCI (SCD/dementia) indicating that interrater agreement of borderline cognitively impaired patients are generally lower [34]. The syndrome diagnosis is solely based on information from the patient and/or caregiver and cognitive testing. Subtle details in the history can sometimes shift the diagnosis from MCI to dementia or vice versa and some degree of uncertainty is to be expected and may be influenced by clinical experience, as our findings indicate. When exploring the diagnostic accuracy for the syndrome diagnosis, a significantly added value of the consensus conference could not be demonstrated indicating that the two evaluation approaches performed similarly well. The etiology of MCI and dementia may have even greater implications for the individual patient in terms of prognosis, care and treatment. Thus, focusing on an early etiologic diagnosis is increasingly important.
Lower level of agreement for the etiological dementia diagnoses has previously been found by a systematic review assessing the added value of a multidisciplinary team approach, however, only specifying AD or VaD diagnoses [8]. Similar results were observed in our study as we found a considerably lower level of agreement for the etiological diagnosis (k = 0.68) compared to the syndrome diagnosis (k = 0.81). The highest level of agreement was seen for the AD diagnosis, whereas the lowest level of agreement was observed for the FTD and mixed dementia diagnosis. The diagnosis of FTD can be challenging to determine as several core features overlap with other neurodegenerative diseases and there are no specific biomarkers for FTD [27, 28]. Especially bvFTD can be difficult to diagnose in the early stages of disease, and in this study the level of cognitive impairment was relatively mild. FTD is a heterogeneous disease where all subtypes were evaluated as one diagnostic group in this study, and we can therefore not conclude if the level of agreement depends on the FTD subtype. The relatively low level of agreement regarding mixed dementia could be explained by the lack of clear criteria for this condition [32].
We observed that accuracy of the etiological baseline diagnosis was significantly higher for the consensus conference compared to the single clinician. Interestingly, the accuracy was not significantly different when only considering patients with a baseline diagnosis of dementia, but the significance retained when only considering patients with MCI. Thus, the increased accuracy of consensus conference was driven mainly by a difference in patients with mild impairment. This indicates that the strength of the multidisciplinary team lies in the evaluation and discussion of difficult borderline cases of, e.g., MCI, where valuable insights from, e.g., neuropsychologist, may increase the diagnostic accuracy. An early and precise diagnosis is important for optimal patient and caregiver guidance on prognosis, care and management since these may differ depending on the disease etiology. Furthermore, as new treatment options, for especially AD, are expected to emerge in the future, an early accurate etiologic diagnosis becomes increasingly important [35].
Finally, we found that the confidence in the etiological diagnosis was significantly higher at the consensus conference compared to the single clinician, especially for patients with FTD. The confidence in the diagnosis is important in the general management of the patient and the decision to start treatment. Also, increased confidence in the diagnosis is associated with decreased tendency to request supplementary tests [36]. Thus, increased confidence in the diagnosis can possibly reduce the need for additional diagnostic testing that may be unnecessary or invasive. Further, we hypothesize that increased confidence in the diagnosis can prompt early initiation of treatment, appropriate patient care and caregiver guidance, improving patient related outcome.
The strengths of this study were the relatively large prospectively recruited cohort from two large tertiary memory clinics. Moreover, we considered the etiologic diagnosis of all the common types of dementia disorders. However, a concern for consideration is that we chose to assign a suspected etiology to all patients with MCI, although diagnostic criteria for this group probably is less well adopted besides for AD [4]. The included subjects were relatively mildly impaired with a large proportion of SCD patients, possibly reflecting site characteristics of a tertiary center in an urban area. This may affect the generalizability of the results to memory clinics with a population of more severely impaired patients.
The main limitation of our results is the lack of a pathologically verified reference diagnosis of dementia etiology. Up to 17%of patients fulfilling clinical criteria for AD do not have evidence of AD pathology on postmortem examination [37]. The concordance between the clinical and pathological diagnosis for other neurodegenerative diseases are even lower [38]. A great proportion of subjects had supplementary test performed at baseline, including CSF sampling in 25.4%of patients. In 63.4%of patients CSF biomarker status was available at follow-up, thus the reference diagnosis used to determine the accuracy of the baseline diagnosis was largely supported by CSF biomarkers. Obtaining a systematic biomarker profile on all patients at follow-up may have increased the accuracy of the reference etiological diagnosis. Additionally, including amyloid-beta42/40-ratio could have increased specificity further [39]. Though, to optimize the reference diagnosis, all follow-up evaluations were performed by a senior physician experienced in dementia diagnostics. One important point to be noted is that this physician performing the follow-up assessment, was not blinded for the consensus conference baseline diagnosis, thus possibly introducing bias in the diagnostic assessment. Compared to the follow-up reference diagnosis the consensus conference performed significantly better than single clinician when considering only MCI patients, but the difference was not significant for dementia patients. We argue that this indicates that the bias of the follow-up assessment was of minor importance. Finally, in some instances, the clinician performing the single clinician evaluation also attended the consensus conference. It is therefore possible that some raters evaluated the same patient on both occasions. However, the single clinician evaluation was performed at least one month after the consensus conference evaluation and given the large flow of patients at a major memory clinic we believe that this did not contribute to bias the results.
A multidisciplinary consensus conference approach to dementia diagnostic offers several possible advantages. It serves as a platform for education for less experienced clinicians and a consensus conference functions to streamline the diagnostic decision-making and patients care ensuring that every patient is offered the same standard of evaluation and care management. Specialized nursing staff is also an important component of the multidisciplinary team with valuable insights to, e.g., the psychosocial aspects of dementia and to the level of care needed for the individual patient.
The possible disadvantages are the increased cost and organizational complexity. However, a study has shown that compared to general practitioners an integrated approach to dementia diagnostics was not demonstrably more expensive, but more complex socioeconomic aspects still needs to be explored [40]. The increased diagnostic confidence at the multidisciplinary consensus conference demonstrated in our study, can possibly lower the need for additional testing and thereby lower overall cost. The optimal composition of the multidisciplinary team in terms of number of participants and diversity of expertise was not assessed in our study and may be a topic for future research. Finally, the impact of the multidisciplinary consensus approach on prognosis, management and disease burden may be explored in future studies.
In conclusion, we compared a multidisciplinary consensus conference diagnostic evaluation approach with a single clinician approach regarding dementia diagnostics and observed that the consensus conference increased the accuracy of the etiological diagnosis. Moreover, the consensus conference reported significantly higher confidence in the diagnosis. Our findings support the importance of a multidisciplinary consensus conference approach, especially when evaluating patients with mild symptoms in the early stage of the disease.
DISCLOSURE STATEMENT
Authors’ Competings available online (https://www.j-alz.com/manuscript-disclosures/21-0278r1).
REFERENCES
- [1]. World Health Organization (2020) Dementia Fact sheet.
- [2]. Schoonenboom NS, Reesink FE, Verwey NA, Kester MI, Teunissen CE, van de Ven PM, Pijnenburg YA, Blankenstein MA, Rozemuller AJ, Scheltens P, van der Flier WM (2012) Cerebrospinal fluid markers for differential dementia diagnosis in a large memory clinic cohort. Neurology 78, 47–54. [DOI] [PubMed] [Google Scholar]
- [3]. Kapasi A, DeCarli C, Schneider JA (2017) Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol 134, 171–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH (2011) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Rabins PV, Blacker D, Rovner BW, Rummans T, Schneider LS, Tariot PN, Blass DM, McIntyre JS, Charles SC, Anzia DJ, Cook IA, Finnerty MT, Johnson BR, Nininger JE, Schneidman B, Summergrad P, Woods SM, Berger J, Cross CD, Brandt HA, Margolis PM, Shemo JP, Blinder BJ, Duncan DL, Barnovitz MA, Carino AJ, Freyberg ZZ, Gray SH, Tonnu T, Kunkle R, Albert AB, Craig TJ, Regier DA, Fochtmann LJ (2007) American Psychiatric Association practice guideline for the treatment of patients with Alzheimer’s disease and other dementias. Second edition. Am J Psychiatry 164, 5–56. [PubMed] [Google Scholar]
- [6]. Konijnenberg E, Fereshtehnejad SM, Kate MT, Eriksdotter M, Scheltens P, Johannsen P, Waldemar G, Visser PJ (2017) Early-onset dementia: Frequency, diagnostic procedures, and quality indicators in three European tertiary referral centers. Alzheimer Dis Assoc Disord 31, 146–151. [DOI] [PubMed] [Google Scholar]
- [7]. Gabel MJ, Foster NL, Heidebrink JL, Higdon R, Aizenstein HJ, Arnold SE, Barbas NR, Boeve BF, Burke JR, Clark CM, Dekosky ST, Farlow MR, Jagust WJ, Kawas CH, Koeppe RA, Leverenz JB, Lipton AM, Peskind ER, Turner RS, Womack KB, Zamrini EY (2010) Validation of consensus panel diagnosis in dementia. Arch Neurol 67, 1506–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Wolfs CA, Dirksen CD, Severens JL, Verhey FR (2006) The added value of a multidisciplinary approach in diagnosing dementia: A review. Int J Geriatr Psychiatry 21, 223–232. [DOI] [PubMed] [Google Scholar]
- [9]. Wolfs CA, Kessels A, Dirksen CD, Severens JL, Verhey FR (2008) Integrated multidisciplinary diagnostic approach for dementia care: Randomised controlled trial. Br J Psychiatry 192, 300–305. [DOI] [PubMed] [Google Scholar]
- [10]. Pillay B, Wootten AC, Crowe H, Corcoran N, Tran B, Bowden P, Crowe J, Costello AJ (2016) The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: A systematic review of the literature. Cancer Treat Rev 42, 56–72. [DOI] [PubMed] [Google Scholar]
- [11]. van der Flier WM, Pijnenburg YA, Prins N, Lemstra AW, Bouwman FH, Teunissen CE, van Berckel BN, Stam CJ, Barkhof F, Visser PJ, van Egmond E, Scheltens P (2014) Optimizing patient care and research: The Amsterdam Dementia Cohort. J Alzheimers Dis 41, 313–327. [DOI] [PubMed] [Google Scholar]
- [12]. van der Flier WM, Scheltens P (2018) Amsterdam Dementia Cohort: Performing research to optimize care. J Alzheimers Dis 62, 1091–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Bruun M, Frederiksen KS, Rhodius-Meester HFM, Baroni M, Gjerum L, Koikkalainen J, Urhemaa T, Tolonen A, van Gils M, Tong T, Guerrero R, Rueckert D, Dyremose N, Andersen BB, Simonsen AH, Lemstra A, Hallikainen M, Kurl S, Herukka SK, Remes AM, Waldemar G, Soininen H, Mecocci P, van der Flier WM, Lotjonen J, Hasselbalch SG (2019) Impact of a clinical decision support tool on dementia diagnostics in memory clinics: The PredictND Validation Study. Curr Alzheimer Res 16, 91–101. [DOI] [PubMed] [Google Scholar]
- [14]. Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12, 189–198. [DOI] [PubMed] [Google Scholar]
- [15]. Lindeboom J, Matto D (1994) [Digit series and Knox cubes as concentration tests for elderly subjects]. Tijdschr Gerontol Geriatr 25, 63–68. [PubMed] [Google Scholar]
- [16]. Van der Elst W, Van Boxtel MP, Van Breukelen GJ, Jolles J (2006) Normative data for the Animal, Profession and Letter M Naming verbal fluency tests for Dutch speaking participants and the effects of age, education, and sex. J Int Neuropsychol Soc 12, 80–89. [DOI] [PubMed] [Google Scholar]
- [17]. Mathuranath PS, Nestor PJ, Berrios GE, Rakowicz W, Hodges JR (2000) A brief cognitive test battery to differentiate Alzheimer’s disease and frontotemporal dementia. Neurology 55, 1613–1620. [DOI] [PubMed] [Google Scholar]
- [18]. Schmidt M (1996) Rey auditory verbal learning test: A handbook. Western Psychological Services, Los Angeles. [Google Scholar]
- [19]. Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C (1989) The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 39, 1159–1165. [DOI] [PubMed] [Google Scholar]
- [20]. Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO (1982) Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res 17, 37–49. [DOI] [PubMed] [Google Scholar]
- [21]. Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J (1994) The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology 44, 2308–2314. [DOI] [PubMed] [Google Scholar]
- [22]. Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL (1982) A new clinical scale for the staging of dementia. Br J Psychiatry 140, 566–572. [DOI] [PubMed] [Google Scholar]
- [23]. Gelinas I, Gauthier L, McIntyre M, Gauthier S (1999) Development of a functional measure for persons with Alzheimer’s disease: The disability assessment for dementia. Am J Occup Ther 53, 471–481. [DOI] [PubMed] [Google Scholar]
- [24]. Pfeffer RI, Kurosaki TT, Harrah CH Jr., Chance JM, Filos S (1982) Measurement of functional activities in older adults in the community. J Gerontol 37, 323–329. [DOI] [PubMed] [Google Scholar]
- [25]. McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr., Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH (2011) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF (1998) Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology 51, 1546–1554. [DOI] [PubMed] [Google Scholar]
- [27]. McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ (2001) Clinical and pathological diagnosis of frontotemporal dementia: Report of the Work Group on Frontotemporal Dementia and Pick’s Disease. Arch Neurol 58, 1803–1809. [DOI] [PubMed] [Google Scholar]
- [28]. Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, van Swieten JC, Seelaar H, Dopper EG, Onyike CU, Hillis AE, Josephs KA, Boeve BF, Kertesz A, Seeley WW, Rankin KP, Johnson JK, Gorno-Tempini ML, Rosen H, Prioleau-Latham CE, Lee A, Kipps CM, Lillo P, Piguet O, Rohrer JD, Rossor MN, Warren JD, Fox NC, Galasko D, Salmon DP, Black SE, Mesulam M, Weintraub S, Dickerson BC, Diehl-Schmid J, Pasquier F, Deramecourt V, Lebert F, Pijnenburg Y, Chow TW, Manes F, Grafman J, Cappa SF, Freedman M, Grossman M, Miller BL (2011) Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134, 2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Ogar JM, Rohrer JD, Black S, Boeve BF, Manes F, Dronkers NF, Vandenberghe R, Rascovsky K, Patterson K, Miller BL, Knopman DS, Hodges JR, Mesulam MM, Grossman M (2011) Classification of primary progressive aphasia and its variants. Neurology 76, 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, Aarsland D, Galvin J, Attems J, Ballard CG, Bayston A, Beach TG, Blanc F, Bohnen N, Bonanni L, Bras J, Brundin P, Burn D, Chen-Plotkin A, Duda JE, El-Agnaf O, Feldman H, Ferman TJ, Ffytche D, Fujishiro H, Galasko D, Goldman JG, Gomperts SN, Graff-Radford NR, Honig LS, Iranzo A, Kantarci K, Kaufer D, Kukull W, Lee VMY, Leverenz JB, Lewis S, Lippa C, Lunde A, Masellis M, Masliah E, McLean P, Mollenhauer B, Montine TJ, Moreno E, Mori E, Murray M, O’Brien JT, Orimo S, Postuma RB, Ramaswamy S, Ross OA, Salmon DP, Singleton A, Taylor A, Thomas A, Tiraboschi P, Toledo JB, Trojanowski JQ, Tsuang D, Walker Z, Yamada M, Kosaka K (2017) Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 89, 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, Amaducci L, Orgogozo JM, Brun A, Hofman A, et al. (1993) Vascular dementia: Diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology 43, 250–260. [DOI] [PubMed] [Google Scholar]
- [32]. Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, DeKosky ST, Gauthier S, Selkoe D, Bateman R, Cappa S, Crutch S, Engelborghs S, Frisoni GB, Fox NC, Galasko D, Habert MO, Jicha GA, Nordberg A, Pasquier F, Rabinovici G, Robert P, Rowe C, Salloway S, Sarazin M, Epelbaum S, de Souza LC, Vellas B, Visser PJ, Schneider L, Stern Y, Scheltens P, Cummings JL (2014) Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet Neurol 13, 614–629. [DOI] [PubMed] [Google Scholar]
- [33]. Altman DG (1999) Practical statistics for medical research. Chapman & Hall/CRC Press, New York. [Google Scholar]
- [34]. Tuokko HA, Gabriel G (2006) Neuropsychological detection of cognitive impairment: Inter-rater agreement and factors affecting clinical decision-making. J Int Neuropsychol Soc 12, 72–79. [DOI] [PubMed] [Google Scholar]
- [35]. Schneider L (2020) A resurrection of aducanumab for Alzheimer’s disease. Lancet Neurol 19, 111–112. [DOI] [PubMed] [Google Scholar]
- [36]. Meyer AN, Payne VL, Meeks DW, Rao R, Singh H (2013) Physicians’ diagnostic accuracy, confidence, and resource requests: A vignette study. JAMA Intern Med 173, 1952–1958. [DOI] [PubMed] [Google Scholar]
- [37]. Beach TG, Monsell SE, Phillips LE, Kukull W (2012) Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005-2010. J Neuropathol Exp Neurol 71, 266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]. Brunnstrom H, Englund E (2009) Clinicopathological concordance in dementia diagnostics. Am J Geriatr Psychiatry 17, 664–670. [DOI] [PubMed] [Google Scholar]
- [39]. Hansson O, Lehmann S, Otto M, Zetterberg H, Lewczuk P (2019) Advantages and disadvantages of the use of the CSF amyloid β (Aβ) 42/40 ratio in the diagnosis of Alzheimer’s Disease. Alzheimers Res Ther 11, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40]. Wolfs CA, Dirksen CD, Kessels A, Severens JL, Verhey FR (2009) Economic evaluation of an integrated diagnostic approach for psychogeriatric patients: Results of a randomized controlled trial. Arch Gen Psychiatry 66, 313–323. [DOI] [PubMed] [Google Scholar]