Abstract
Introduction
Targeted axillary dissection (TAD) has been proposed as an alternative method for the staging of patients with node-positive breast cancer who undergo neoadjuvant chemotherapy. However, not much is known yet about the false-negative rate (FNR) of the method and the subsequent risk of underestimation of residual axillary disease.
Methods
This study reviews published articles with calculations of false negativity of TAD and potential factors that may influence it.
Results
The FNR of TAD is usually reported as being <10%, but this calculation is usually based on small study populations. Lower FNR is a common finding along with lower N status, while not enough data are available yet for greater axillary involvement. When a marked node is revealed to be a sentinel lymph node (SLN) at surgery after neoadjuvant chemotherapy (NAC), this seems to be another factor that contributes to reliable TAD. With regard to the methods used to mark the positive node before chemotherapy and retrieval at surgery, there is no clear advantage of one over the other. The availability of relevant resources, the costs, and local legislation must all be taken into account for the selection of the optimal strategy.
Conclusion
Although still in its early days, the FNR of TAD can be low, at least in patients with relatively little axillary involvement and when the marked node is the SLN. All reported methods of lymph node marking seem reliable.
Keywords: Breast cancer, Targeted axillary dissection, Neoadjuvant chemotherapy, Sentinel lymph node
Introduction
Targeted axillary dissection (TAD) has been proposed as an alternative method for the staging of patients with node-positive breast cancer who undergo neoadjuvant chemotherapy (NAC). The positive node is marked before the commencement of NAC and is identified and removed at surgery. At surgery, sentinel-node biopsy (SNB) is also performed. The excision of the marked node in addition to any sentinel lymph nodes (SLNs) constitutes TAD.
The omission of axillary node clearance (ANC) for patients undergoing NAC for node-positive breast cancer and the reliance on SNB for the staging of the axilla bears the risk of underestimation of residual axillary cancer. The pattern of the response of the axillary tumor to NAC and the manner in which lymphatic channels alter due to tumor invasion and the treatment are not always predictable, so that what is identified as SLN after NAC may not represent the most probable sites of persisting malignancy. Several clinical trials have attempted to estimate when SNB post-NAC is reliable and reflects the actual axillary status. Of them, the SENTINA and Alliance Z1071 studies [1, 2] showed that removing <3 SLNs post-NAC might leave us with an unacceptable rate of underestimation of the axillary residual burden; the false-negative rate (FNR) of SNB in these cases (i.e., the cases where the SLN is free of cancer but other axillary nodes are positive) may be >10% if only 1 or 2 SLNs are removed.
The waters were further stirred by the Z1071 study where patients underwent SNB and axillary lymph node dissection (ALND) after NAC with a clip placed at the pre-NAC biopsied (and positive) node in some of the participants [3]. The conclusion was that, when the clipped node was found within the SNB specimen, the FNR of SNB was 7.2%; when the clipped node was different to the SLN, the FNR climbed to 26.9%. This confirms that nodes other than the classic SLN biopsied post-NAC hold information about the status of the axilla, and revisiting the node which was initially proven metastatic seemed a good idea.
Caudle et al. [4]were the pioneers of TAD; they described a cohort of 12 patients, 9 of whom had completed NAC and ANC. Four of these 9 had residual disease in the axilla and the marked node was invariably positive in all 4. Since then, there have been several other researchers applying TAD with variable outcomes.
In the fight to minimize the FNR of axillary staging for patients with node-positive breast cancer undergoing NAC, TAD appears to be a promising approach. It is, however, surprising how little we still know about the actual performance of the method, its sensitivity in particular, and at present there is only a handful of relevant publications available reporting on the FNR of TAD.
In this systematic review, we present what is currently known about the FNR of TAD and we examine the factors that potentially influence it.
Methods
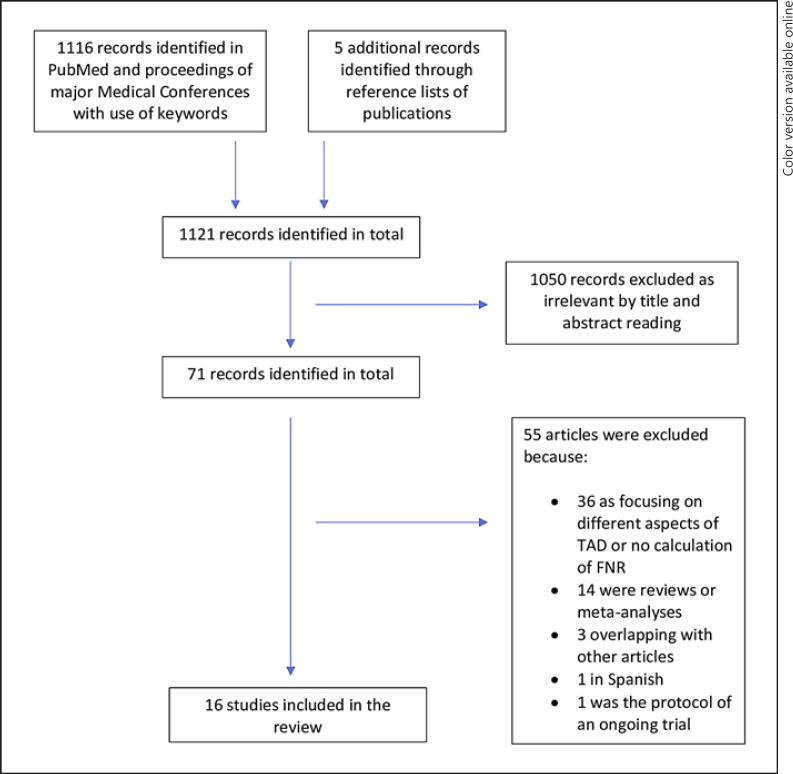
We searched PubMed and the proceedings of major relevant medical conferences for articles on TAD in breast cancer after NAC, with publication dates from 2010 to 2020. We used the following keywords: targeted axillary dissection, sentinel lymph node localization in breast cancer, sentinel lymph node localization in breast cancer after neoadjuvant, sentinel lymph node biopsy in breast cancer after neoadjuvant, sentinel lymph node marked breast cancer. The reference lists of the published articles were also screened. All articles were reviewed for consistency, duplicates, reviews, and meta-analyses, and exclusions were made as appropriate. More than one article by the same authors was accepted if the study period was different. Focus was given to the articles reporting FNRs in the study population. The selection process is presented in detail in Figure 1.
Fig. 1.
Selection process of eligible publications.
After the eligible articles were identified and carefully reviewed, we formulated 4 key questions that could potentially be answered by the available data:
What is the reported FNR of TAD?
Does higher N status at presentation increase the FNR?
Does the coincidence of the marked node and the SLN affect the FNR?
Is FNR affected by the technique of TAD?
Results
We identified 1,116 articles with the use of the selected keywords and a further 5 articles from the reference lists of previously identified ones. Of these, 1,050 duplicate and irrelevant articles were excluded after reading the titles and abstracts. The remaining 71 articles underwent a careful evaluation and a further 55 were excluded, 36 due to their focus on different aspects of TAD or lack of estimation of FNR, 14 due to being reviews or meta-analyses, 3 that overlapped with other articles, 1 that was written in Spanish, and 1 because it was the protocol of an ongoing trial. The remaining 16 articles comprised the body of the study and were used as the source of data for this review. A flow diagram shows the selection process of the included studies (Fig. 1).
Of the 16 articles, 13 were prospective and 2 were retrospective studies, and there was 1 case report with the number of participants undergoing TAD (not necessarily the total study population) between 1 and 191. Patients' ages ranged from 23 to 84 years. In some cases, TAD was performed as part of routine practice, and if no residual disease was found in the nodes, patients did not proceed with ANC; in others, TAD was applied within feasibility studies and completion of the ANC followed for all cases. Surgical methods, indications for TAD, the degree of axillary involvement at presentation, and the final success rate varied considerably. There was only 1 prospective, multicenter study [5].
There were 4 articles in which the surgical technique was a variation of the previously described TAD. Kim et al. [6] described a method of axillary staging where node-positive breast cancer patients underwent dual localization of axillary nodes before and after NAC; a clip was placed in the positive node pre-NAC and any still-suspicious-looking nodes after NAC were tattooed with activated charcoal under ultrasound guidance. The clipped node was tattooed too. The same authors, in another publication [7], utilized post-NAC localization of the most suspicious axillary lymph node with activated charcoal; no pre-NAC marking was done in this case. Furthermore, some of the patients in both the above studies underwent a surgical axillary procedure, targeted axillary sampling (TAS), or extensive TAS which is slightly more extensive than TAD but less radical than ANC. This involved the removal of additional nodes in the vicinity of the SLN and the marked node. Donker et al. [8] described the MARI (Marking the Axilla with radioactive Iodine Seeds) technique, where only the marked node was removed at surgery and no SNB was performed; this means that, strictly speaking, no TAD was performed. Similarly, Lim et al. [9] did not perform SLB after chemotherapy but removed the clipped nodes only. We included their study, however, as it is the only one supplying information about how the FNR changes when >1 node is marked. Despite the obvious deviations, the above studies were included in the review.
In another article [10], the FNR reported represents the marked node biopsy performance only and not the TAD. However, as the FNR of the marked node biopsy was 0%, the FNR of the TAD would have the same value.
All but 2 of the selected studies presented data on FNR of TAD, with the deviations mentioned above. Two studies [3, 11] did not have data on FNR of TAD but are still included in this review, as they contain information about how the FNR of the SNB changes according to whether the marked node is an SLN or not (the third key question).
To calculate the FNR of the procedure, patients must undergo routine ANC after TAD, irrespective of the result of TAD. Because this was not the case for all the patients in the included studies, the respective study authors based the calculation of the FNR of TAD on a subgroup of the total study population. This means that the study population on which this FNR of TAD was calculated is often smaller than the total study population.
Finally, to answer all 4 key questions, only some studies were used for each one as the information relative to the question was not available in all of them.
Question 1: What Is the Reported FNR of TAD?
The FNR of TAD varies from 0 to 20% in the reported series. This may reflect differences in the techniques used, the characteristics of the study populations, or the surgeon's experience. Table 1 summarizes the data from the available studies with reports on the FNR of TAD.
Table 1.
Reported FNRs of TAD
| First author [ref.], year | Populationa, n | FNR | Comments |
|---|---|---|---|
| Reinisch [5], 2019 | 45 | FNR of TAD = 4.4% FNR of marked node only = 8% |
The only multicenter prospective study (SENTA) with 598 patients, 45 of whom were used for the FNR of TAD calculation |
|
| |||
| Kim [6], 2019 |
15 | FNR of TASb = 80% FNR of marked node biopsy or tattooed node biopsy = 67% for either |
28 cytologically proven N+ breast cancer patients underwent dual localization of axillary nodes before and after NAC with clip and activated charcoal At restaging US, 5 patients had suspicious nodes and 23 had no suspicious nodes Clipped node biopsy failed in 1 patient and tattooed node biopsy in none 20 patients underwent TAS and 8 underwent ALND |
|
| |||
| Kim [7], 2018 | 18 | FNR of TASb = 0 FNR of tattooed node biopsy only = 33% |
45 patients treated with NAC with <2 suspicious nodes at post-NAC restaging US All patients had clinically node-positive disease at initial staging and underwent post-NAC localization of the most suspicious axillary lymph node with activated charcoal Patients received TAS, extended TAS, or ANC. In 1 patient, the surgeon could not find the tattooed node |
|
| |||
| Donker [8], 2015 |
95 | 7% of the MARI node biopsy only |
Only MARI node biopsy was performed All patients were treated with ANC The marked node was identified in 97 patients 2 patients did not undergo subsequent ALND, leaving 95 patients for further analysis. |
|
| |||
| Lim [9], 2020 |
14 | FNR of TAD with 1 marked node = 7.1% FNR of TAD with 2 marked nodes = 0% | No SNB was done 9, 3, and 2 patients had 1, 2, and 3 positive nodes clipped, respectively |
|
| |||
| Hartmann [10], 2018 |
17 | 0% of marked node biopsy only | 30 patients were marked, 25 of whom were N+ All patients underwent completion ALND, and, if yiN0, SNB Wire localization of the marked nodes was possible in 24/30 patients, and the clip was inside the marked node specimen in 17/24 cases |
|
| |||
| Khallaf [12], 2020 |
19 | 8.3% | 20 patients had locally advanced disease with N0 axilla after NAC and received ANC at surgery The marked node was identified in 19 patients |
|
| |||
| García-Moreno [13], 2019 | 1 | 0 | Case report |
|
| |||
| Caudle [14], 2016 |
marked node evaluation: 191 TAD evaluation: 118 |
1.4% of TAD (4.2% of marked node biopsy only) |
Of 208 patients with marked nodes, 191 underwent ANC and 118 underwent SNB and ANC The clipped node was not identified in the surgical specimen in 5 patients, who were excluded from analysis |
|
| |||
| Flores-Funes [15], 2019 | 22 | 0% of TAD (0% of marked node only) |
23 patients had a marked node and all underwent ANC Marked node excision was successful in 22 cases |
|
| |||
| Siso [16], 2018 |
35 | 4.1% | The ILINA trial involved 35 patients who had a clipped node excised along with SLNs, followed by ANC |
|
| |||
| Park [17], 2018 |
10 | 0 | Patients with cytology-proven node metastases underwent charcoal tattooing before NAC Detection rate of tattooed nodes was 100% |
|
| |||
| Sutton [18], 2020 |
marked node evaluation: 24 TAD evaluation: 29 |
7% of TAD (17% of marked node biopsy only) |
29 patients with node-positive disease received NAC and TAD The clipped node was found in 25 patients Some, but not all, patients underwent ANC |
|
| |||
| Taback [19], 2018 |
19 | 0 of TAD (0 of marked node biopsy only) |
19 patients with N+ disease underwent fiducial reflector insertion in the positive node Some, but not all, patients underwent ANC The marked node was identified in all 19 patients in the reflector group |
N0, node-negative; N+, node-positive; TAD, targeted axillary dissection; SNB, sentinel-node biopsy; ALND, axillary lymph node dissection; ANC, axillary node dissection; NAC, neoadjuvant chemotherapy; US, ultrasound; MARI, marking the axilla with radioactive iodine seeds; TAS, targeted axillary sampling involving sentinel and marked nodes as well other nodes in the vicinity.
Reflects the number of patients used to calculate the FNR, or the best possible approximation.
Radioactive nodes and/or nodes containing blue dye, tattooed nodes, and clinically suspicious nodes at inspection or palpation during surgery were removed. In some patients, extended TAS was performed, i.e., the excision of several nodes around the sampled nodes.
With one exception, the FNR of TAD was <10%. In 1 publication, Kim et al. [6], with a study population of 15 patients who underwent dual localization of the suspicious nodes before and after NAC, reported a TAS sensitivity of 80% which is the lowest in the literature. It is worth noting though, that the sensitivity was highly dependent on the degree of the axillary involvement pre-NAC: for N1, N2, and N3, the sensitivity of TAS was 100, 67, and 0%, respectively. Similarly, Khallaf et al. [12], who reported the second-highest FNR for TAD, i.e., 8.3%, included patients with locally advanced disease pre-NAC in their study. The major disturbance of the lymphatic flow in the breast and the axilla due to the cancer invasion and the formation of scar tissue formation as a response to chemotherapy could be held responsible for the smaller success of TAD in these cases. For the MARI node biopsy, the FNR was reported as 7% even though SNB was not done [8]. Finally, the study by Lim et al. [9]highlighted that when 2 abnormal nodes were clipped and removed rather than only 1, the FNR dropped from 7.1 to 0%. However, their result was based on a population of 14 patients, with 9, 3, and 2 patients having 1, 2, and 3 malignant nodes clipped, respectively.
It is also worth noting that the study populations were small. With the exception of Caudle et al. [14] (118 patients) and Donker et al. [8](95 patients), the studies based their calculations on groups not larger than 45 patients. Furthermore, many of the publications described projects not solely dedicated to TAD, but with other end points too. Finally, there was only 1 prospective, multicenter study. These are indications that reliable large-scale data on the oncological safety of TAD are still missing.
Question 2: Does Higher N Status at Presentation Increase the FNR?
Having a large number of axillary nodes affected by the same breast cancer, especially when these nodes are sizable or even matted together, does not necessarily mean that the response to NAC will be uniform for all of them. A higher N status at presentation may result in significant local anatomy changes, as lymphatics and blood vessels may get obstructed and the altered pharmacokinetics of the chemotherapeutic agents might preclude efficient treatment in the region. Even when there is a response, scar tissue may be left behind which means that the regional flow of blood and lymph are not always restored. Thus, what is identified as SLN post-NAC may not represent the biologically “first” node to receive the malignant cells from the breast. In addition, the majority of the authors mark just 1 of the affected nodes, even in N2 cases, presuming that this will be representative of the axillary status post-NAC (an assumption not really based on hard evidence).
It is rational to hypothesize that the higher the N status at presentation, the more probable it is for the TAD to miss a residual nodal metastasis. At present, there is no clear contraindication for TAD with regard to the degree of the involvement of the axilla, and authors use the method in cases with variance in axillary tumor burden. Few restrict TAD to small axillary involvement [19] only, and even fewer (if any) mark >1 suspicious node pre-NAC. Kim et al. [6, 7]in 2 publications, did take note that the FNR of TAD tended to increase along with higher clinical N status (Table 2).
Table 2.
FNR of TAD per N status at presentation
| First author [ref.], year | N status | Patients, n | FNR |
|---|---|---|---|
| Kim [6], 2019 | N1 | 10 | 0a |
| N2 | 3 | 33%a | |
| N3 | 2 | 100%a | |
| Kim [7], 2018 | 1 node | 2 | 0a, b |
| (0 when only marked and SLN were removed) | |||
|
|
|||
| 2–3 nodes | 8 | 0a, b | |
| (25% when only marked and SLN were removed) | |||
|
|
|||
| ≥4 nodes | 8 | 0a, b | |
| (25% when only marked and SLN were removed) | |||
Targeted axillary sampling: radioactive nodes and/or nodes containing blue dye, tattooed nodes, and clinically suspicious nodes at inspection or palpation during surgery were removed. In some patients, extended targeted axillary sampling was performed, i.e., excision of several nodes around the sampled nodes.
Median number of resected nodes was 3 in sampling and 7 in extended sampling.
It is worth mentioning that some researchers do not specifically report the FNR according to N status (N1, N2, etc.), but rather grant an overall FNR of 0% for all N statuses [10, 15, 17, 19]. This indirectly suggests that, for patients with axillary disease >N1, the FNR of TAD is still 0%. One can, however, not draw actual conclusions from this, but it is safe to say that TAD has been found reliable in a few selected cases with a higher axillary burden. Besides, the number of >N1 patients in published studies is usually very low.
With regard to the FNR in different N statuses, our conclusion would be that, for N1 patients at presentation, the sensitivity of TAD is acceptably high; however, there are not enough data available for >N1 patients yet to allow implementation in clinical routine.
Question 3: Does the Coincidence of the Marked Node and the SLN Affect the FNR?
There is a group of relevant studies where a clip was placed in the infiltrated node before NAC, and traditional SNB plus ANC were performed at surgery. TAD was not actually performed and therefore the FNR of TAD was not calculated. The value of these reports, however, lies in the observation that the FNR of post-NAC SNB depends heavily on whether the clipped node falls within the SNB specimen or not. A clipped node other than the post-NAC SLN means that the node initially deemed malignant will not be assessed histologically and any residual cancer deposit will be missed unless TAD is performed. In addition, due to the lymphatic flow alternations, the post-NAC SNB will be misguided to nodes that may have a different response to NAC from that of the marked node or were never actually positive.
A number of studies do confirm that when the marked node is not one of the SLNs, the FNR of post-NAC SNB is virtually tripled [3, 11, 14] (Table 3).
Table 3.
FNR of post-NAC SNB when the marked node is or is not
Question 4: Is FNR Affected by the Technique of TAD?
As TAD is a new surgical method, there is no agreement yet on the optimal marking technique of the pre-NAC positive node. Furthermore, what makes a method largely successful depends on the local conditions each time, namely, the availability of materials and infrastructure as well as the costs and legislation regarding the use of radioactive materials or implantable medical devices. There is also significant variation in the methods used to retrieve the marked node. Finally, the post-NAC SNB may be done in different ways and the use of a dual tracer technique has been proposed by many as the optimal approach.
The use of a clip or tattoo to mark the pre-NAC malignant node is the most common technique and yields an FNR of <10%. Only Kim et al. [6] who marked the axilla with a clip before NAC and tattooed the suspicious-looking nodes after NAC reported a sensitivity of 80% for TAS (Table 4). Retrieving a clipped or a tattooed node requires meticulous visual inspection, the use of guide wires, or an intraoperative ultrasound scan. In one case, an iodine seed was placed at the clipped node so that it could be localized with a γ-probe. The use of radioactive or magnetic seeds and fiducial reflectors seems equally effective for marking the positive nodes. These techniques require special instrumentation to retrieve the marked node and, in some cases, their use is hindered by the higher cost of the respective devices or the restrictions in long-term implantation of radioactive materials.
Table 4.
Marking methods of the involved node pre-NAC and the FNR of TAD at surgery
| First author [ref.], year | Marking method | Marked node retrieval method | FNR for TAD, % |
|---|---|---|---|
| Khallaf [12], 2020 | tattoo | visual inspection | 8.3 |
| Park [17], 2018 | tattoo | visual inspection | 0 |
| Caudle [4], 2015 | clip | iodine seed and γ-probe | 1.4 |
| Flores-Funes [15], 2019 | clip | guide-wire | 0 |
| Hartmann [10], 2018 | clip | guide-wire | 0 |
| Siso [16], 2018 | clip | intraoperative US-guided | 4.1 |
| Sutton [18], 2020 | clip | 7 | |
| Taback [19], 2018 | fiducial reflector | electromagnetic probe | 0 |
| García-Moreno [13], 2019 | magnetic seed | magnetic probe | 0 (a case report) |
| Donker [8], 2015 | iodine seed | γ-probe | 7 |
| Kim [7], 2018 | tattoo post-NAC | visual inspection | 0 |
| Kim [6], 2019 | clip pre-NAC and tattoo post-NAC | visual inspection | 20 |
| Reinisch [5], 2019 | clip | 4.4 | |
| Lim [9], 2020 | clip | SMART1 | 7.1 when 1 clip was placed |
| 0 when 2 clips were placed |
SMART, skin mark clipped axillary-node removal technique; US, ultrasound.
The position of the clip was marked on the skin preoperatively with ultrasonography. Intraoperatively a needle was inserted through the skin marked site to localize the clip.
The technical details of the procedure as well as the identification rates of the marked node have not been analyzed here. It is prudent to mention that surgical methods vary heavily from hospital to hospital, and that, ultimately, a team will apply what suits each individual center best. Removing >2 SLNs [1, 2] or introducing regional radiotherapy [20] can also be valid treatment options in these cases.
Conclusion
TAD appears to be a promising tool in the management of node-positive breast cancer patients who undergo NAC. However, one size does not fit all. Although still in its early days, it seems that there are some identifiable cases where it can be used with safety. Overall, the FNR of TAD is reported as being <10%. N1 status at presentation (vs. N2 or N3, for which there are not yet enough data available), and a marked node being a SLN appear to be factors that contribute to a low FNR of TAD. There is also some very limited evidence that marking >1 positive node may improve the FNR, but this is still far from proven. With regard to the methods used to mark the positive node before NAC and then retrieve it at surgery, there is no clear advantage of one over the other. The availability of relevant resources, the costs, and local legislation must also be taken into account.
Further research is certainly needed to shed light on areas with still unresolved issues, namely, how breast cancer subtypes may affect the FNR due to their different chemotherapy response patterns, whether severe axillary involvement constitutes a contraindication for TAD (and where the actual limit is), and what is the optimal number of nodes that should be clipped, if positive.
Conflict of Interest Statement
The authors report no conflict of interests.
Funding Sources
This work received no funding.
Author Contributions
G.K.: data collection, data analysis, and writing; A.C.: data analysis and review; M.K.: writing and review.
References
- 1.Kuehn T, Bauerfeind I, Fehm T, Fleige B, Hausschild M, Helms G, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013 Jun;14((7)):609–18. doi: 10.1016/S1470-2045(13)70166-9. [DOI] [PubMed] [Google Scholar]
- 2.Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, et al. Alliance for Clinical Trials in Oncology Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013 Oct;310((14)):1455–61. doi: 10.1001/jama.2013.278932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boughey JC, Ballman KV, Le-Petross HT, McCall LM, Mittendorf EA, Ahrendt GM, et al. Identification and Resection of Clipped Node Decreases the False-negative Rate of Sentinel Lymph Node Surgery in Patients Presenting With Node-positive Breast Cancer (T0-T4, N1-N2) Who Receive Neoadjuvant Chemotherapy: Results From ACOSOG Z1071 (Alliance) Ann Surg. 2016 Apr;263((4)):802–7. doi: 10.1097/SLA.0000000000001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caudle AS, Yang WT, Mittendorf EA, Black DM, Hwang R, Hobbs B, et al. Selective surgical localization of axillary lymph nodes containing metastases in patients with breast cancer: a prospective feasibility trial. JAMA Surg. 2015 Feb;150((2)):137–43. doi: 10.1001/jamasurg.2014.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reinisch M, Heil J, Rüland A, Seiberling C, Harrach H, Schindowski D, et al. Prospective, multicenter registry trial to evaluate the clinical feasibility of targeted axillary dissection (TAD) in patients (pts) with breast cancer (BC) and core biopsy proven axillary involvement (cN1) Ann Oncol. 2019;30(suppl 5):v55–98. [Google Scholar]
- 6.Kim WH, Kim HJ, Kim SH, Jung JH, Park HY, Lee J, et al. Ultrasound-guided dual-localization for axillary nodes before and after neoadjuvant chemotherapy with clip and activated charcoal in breast cancer patients: a feasibility study. BMC Cancer. 2019 Aug;19((1)):859. doi: 10.1186/s12885-019-6095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim WH, Kim HJ, Jung JH, Park HY, Lee J, Kim WW, et al. Ultrasound-guided restaging and localization of axillary lymph nodes after neoadjuvant chemotherapy for guidance of axillary surgery in breast cancer patients: experience with activated charcoal. Ann Surg Oncol. 2018 Feb;25((2)):494–500. doi: 10.1245/s10434-017-6250-3. [DOI] [PubMed] [Google Scholar]
- 8.Donker M, Straver ME, Wesseling J, Loo CE, Schot M, Drukker CA, et al. Marking axillary lymph nodes with radioactive iodine seeds for axillary staging after neoadjuvant systemic treatment in breast cancer patients: the MARI procedure. Ann Surg. 2015 Feb;261((2)):378–82. doi: 10.1097/SLA.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 9.Lim GH, Gudi M, Teo SY, Ng RP, Yan Z, Lee YS, et al. Would removal of all ultrasound abnormal metastatic lymph nodes without sentinel lymph node biopsy be accurate in patients with breast cancer with neoadjuvant chemotherapy? Oncologist. 2020 Jun;25((11)) doi: 10.1634/theoncologist.2020-0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartmann S, Reimer T, Gerber B, Stubert J, Stengel B, Stachs A. Wire localization of clip-marked axillary lymph nodes in breast cancer patients treated with primary systemic therapy. Eur J Surg Oncol. 2018 Sep;44((9)):1307–11. doi: 10.1016/j.ejso.2018.05.035. [DOI] [PubMed] [Google Scholar]
- 11.Cabıoğlu N, Karanlık H, Kangal D, Özkurt E, Öner G, Sezen F, et al. Improved False-Negative Rates with Intraoperative Identification of Clipped Nodes in Patients Undergoing Sentinel Lymph Node Biopsy After Neoadjuvant Chemotherapy. Ann Surg Oncol. 2018 Oct;25((10)):3030–6. doi: 10.1245/s10434-018-6575-6. [DOI] [PubMed] [Google Scholar]
- 12.Khallaf E, Wessam R, Abdoon M. Targeted axillary dissection of carbon-tattooed metastatic lymph nodes in combination with post-neo-adjuvant sentinel lymph node biopsy using 1% methylene blue in breast cancer patients. Breast J. 2020 May;26((5)):1061–3. doi: 10.1111/tbj.13736. [DOI] [PubMed] [Google Scholar]
- 13.García-Moreno JL, Benjumeda-Gonzalez AM, Amerigo-Góngora M, Landra-Dulanto PJ, Gonzalez-Corena Y, Gomez-Menchero J. Targeted axillary dissection in breast cancer by marking lymph node metastasis with a magnetic seed before starting neoadjuvant treatment. J Surg Case Rep. 2019 Dec;2019((11)):rjz344. doi: 10.1093/jscr/rjz344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caudle AS, Yang WT, Krishnamurthy S, Mittendorf EA, Black DM, Gilcrease MZ, et al. Improved Axillary Evaluation Following Neoadjuvant Therapy for Patients With Node-Positive Breast Cancer Using Selective Evaluation of Clipped Nodes: Implementation of Targeted Axillary Dissection. J Clin Oncol. 2016 Apr;34((10)):1072–8. doi: 10.1200/JCO.2015.64.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flores-Funes D, Aguilar-Jiménez J, Martínez-Gálvez M, Ibáñez-Ibáñez MJ, Carrasco-González L, Gil-Izquierdo JI, et al. Validation of the targeted axillary dissection technique in the axillary staging of breast cancer after neoadjuvant therapy: preliminary results. Surg Oncol. 2019 Sep;30:52–7. doi: 10.1016/j.suronc.2019.05.019. [DOI] [PubMed] [Google Scholar]
- 16.Siso C, de Torres J, Esgueva-Colmenarejo A, Espinosa-Bravo M, Rus N, Cordoba O, et al. Intraoperative Ultrasound-Guided Excision of Axillary Clip in Patients with Node-Positive Breast Cancer Treated with Neoadjuvant Therapy (ILINA Trial): A New Tool to Guide the Excision of the Clipped Node After Neoadjuvant Treatment. Ann Surg Oncol. 2018 Mar;25((3)):784–91. doi: 10.1245/s10434-017-6270-z. [DOI] [PubMed] [Google Scholar]
- 17.Park S, Koo JS, Kim GM, Sohn J, Kim SI, Cho YU, et al. Feasibility of Charcoal Tattooing of Cytology-Proven Metastatic Axillary Lymph Node at Diagnosis and Sentinel Lymph Node Biopsy after Neoadjuvant Chemotherapy in Breast Cancer Patients. Cancer Res Treat. 2018 Jul;50((3)):801–12. doi: 10.4143/crt.2017.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sutton TL, Johnson N, Garreau JR. Adequate sentinel node harvest is associated with low false negative rate in breast cancer managed with neoadjuvant chemotherapy and targeted axillary dissection. Am J Surg. 2020 May;219((5)):851–4. doi: 10.1016/j.amjsurg.2020.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Taback B, Jadeja P, Ha R. Enhanced Axillary Evaluation Using Reflector-Guided Sentinel Lymph Node Biopsy: A Prospective Feasibility Study and Comparison With Conventional Lymphatic Mapping Techniques. Clin Breast Cancer. 2018 Oct;18((5)):e869–74. doi: 10.1016/j.clbc.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Comparison of Axillary Lymph Node Dissection With Axillary Radiation for Patients With Node-Positive Breast Cancer Treated With Chemotherapy https://clinicaltrials.gov/ct2/show/record/NCT01901094, accessed 1 June 1st 2020. [Google Scholar]