Abstract
Ascites is a common complication of several conditions, but it is rare in cases of Chlamydia trachomatis infection. We report a 36-year-old patient presenting with abdominal swelling for a week prior to hospitalization. An extensive workup excluded liver or heart disease and malignancy. A computed tomography scan demonstrated massive ascites and severe thickening of peritoneal reflections. Laboratory tests showed low serum-ascites albumin gradient, high total protein, and low adenosine. Diagnostic laparoscopy revealed inflammatory signs of both fallopian tubes. The histopathological results from peritoneal biopsy were consistent with lymphoid proliferation with reactive lymphoplasmacytic infiltrate. A gynecological investigation showed a positive DNA for C. trachomatis in the cervical swab. After treatment with doxycycline, there was a complete resolution of ascites.
Keywords: Pelvic inflammatory disease, Chlamydia trachomatis, Ascites
Introduction
Cirrhosis, heart failure, nephrotic syndrome, and peritoneal disease are the most common causes of ascites. The combination of serum-ascites albumin gradient (SAAG) and ascites total protein content has been used to help in the differential diagnosis of the etiology of ascites. SAAG >1.1 g/dL suggests liver or heart disease, whereas values <1.1 g/dL other causes of ascites. A high protein level of >2.5 mg/dL in ascitic fluid is usually found in heart failure or peritoneal disease [1].
Ascites associated with low SAAG values comprises a heterogeneous group of conditions that include pancreatic and renal disease, peritoneal malignancy, and infections such as tuberculosis or permeability changes (i.e., hypothyroidism and paraproteinemias) [2]. Chlamydia peritoneal infection is a rare cause of ascites with low SAAG in young people [3].
Chlamydia trachomatis is responsible for the most common sexually transmitted infectious disease in the world. There has been an increasing incidence and awareness over the last decades, likely due to the precise diagnosis with nucleic acid amplification testing. Although most of the patients remain asymptomatic, if left untreated, Chlamydia may cause chronic pelvic pain, infertility, and pelvic inflammatory disease (PID) [4]. The Centers for Disease Control and Prevention (CDC) current guidelines recommend routine screening in all sexually active women under 25 years to prevent complications [5].
However, due to the rarity of Chlamydia-induced ascites, there is no formal recommendation for performing routine testing in the workup diagnostic algorithms of ascites. Here, we report a case of a young woman presenting with ascites secondary to Chlamydia infection.
Case Report
A 36-year-old Caucasian female presented at the emergency department with a history of progressive abdominal distension and weight gain of 8 kg for the past 2 months, increasing from 60 to 68 kg. She also reported fatigue and reduced appetite for 1 week prior to hospitalization. A review of systems was negative for fever, night sweats, chills, jaundice, dark urine, vomiting, or diarrhea.
Her past medical history was remarkable for recurrent bacterial and yeast vulvovaginitis, but was asymptomatic over the last year. She denied known liver disease, alcohol abuse, or smoking. Her family history was uneventful.
Physical exam revealed normal vital signs with a blood pressure of 100/60 mm Hg and a heart rate of 90 beats per minute, and she was afebrile. She presented with a pale appearing, without jaundice. Moderate abdominal distension, with marked ascites, diffuse tenderness, and palpable liver edge 3 cm below the costal margin were the major findings. There were no peripheral stigmata of chronic liver disease or congestive heart failure.
Laboratory tests demonstrated iron deficiency anemia with a hemoglobin level of 9.5 mg/dL and a mean corpuscular volume of 75 fL. She had a normal white blood count of 6,050 mL/mm3 and a platelet count of 302 mL/mm3. C-reactive protein was 29 mg/dL and serum creatinine 0.74 mg/dL. She had normal liver enzymes: alanine transaminase 11 U/L, aspartate transaminase 22 U/L, alkaline phosphatase 51 U/L, and gamma-glutamyl transferase 9 U/L. Liver function tests showed a total bilirubin level of 0.2 mg/dL, serum albumin 3.6 g/dL, and an international normalized ratio 1.17. CA-125 was markedly elevated to 452 U/mL. An extensive workup diagnosis excluded hepatotropic virus infections, such as hepatitis A, B, and C, Epstein-Barr, and cytomegalovirus. Blood testing was seronegative for antinuclear, anti-smooth muscle, and antimitochondrial antibodies. Thyroid function was normal, and the B-hCG test was negative.
The abdominal computed tomography was remarkable for massive ascites, severe thickening, and enhancement of peritoneal reflections, stranding, and thickening of the omentum (Fig. 1). The liver parenchymal attenuation was preserved, while the main portal and hepatic veins were patent. An upper endoscopy ruled out esophageal varices.
Fig. 1.
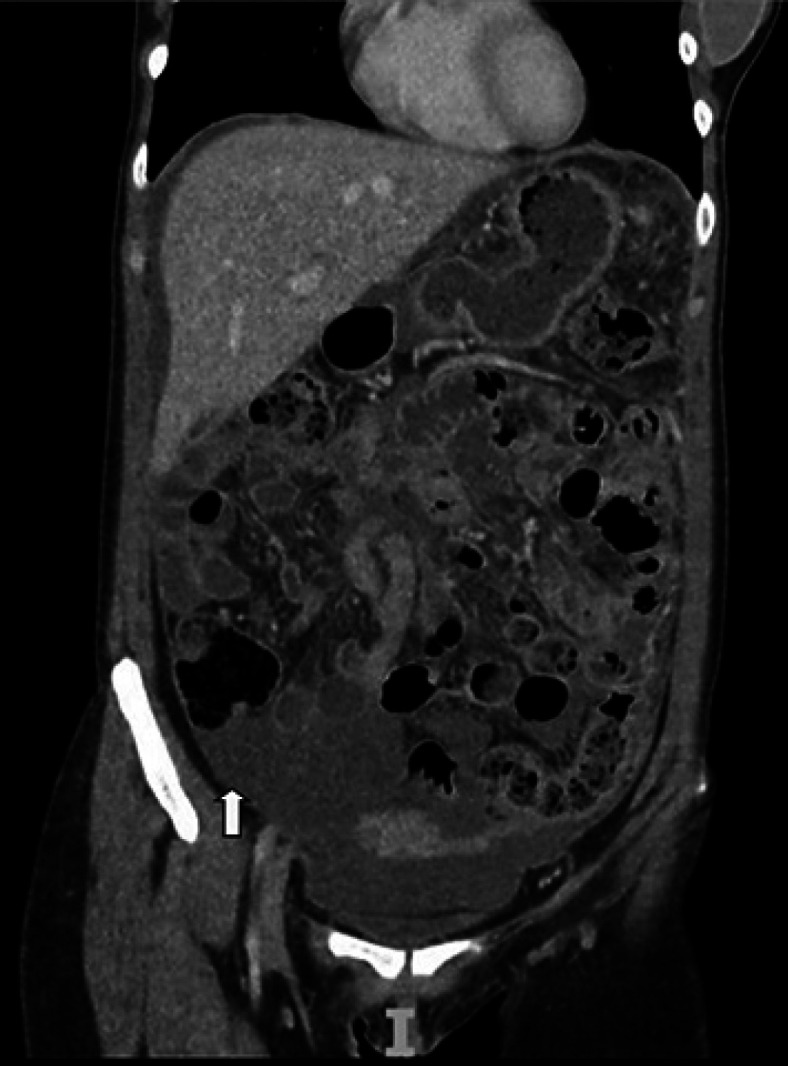
Coronal contrast-enhanced CT scan of the abdomen showed severe ascites, peritoneal thickening, and enhancement of peritoneal reflections and the omentum (yellow arrow).
A diagnostic paracentesis was performed, which showed a citrine-yellow fluid with an SAAG of 0.8 and a total protein of 2.8 g/dL, suggesting peritoneal disease. The total cytological count was 6,200 leukocytes/mL, 43% of neutrophils, 28% of lymphocytes, and 23% of monocytes. Adenosine deaminase of the fluid, polymerase chain reaction for tuberculosis, and oncotic cytology were all negative.
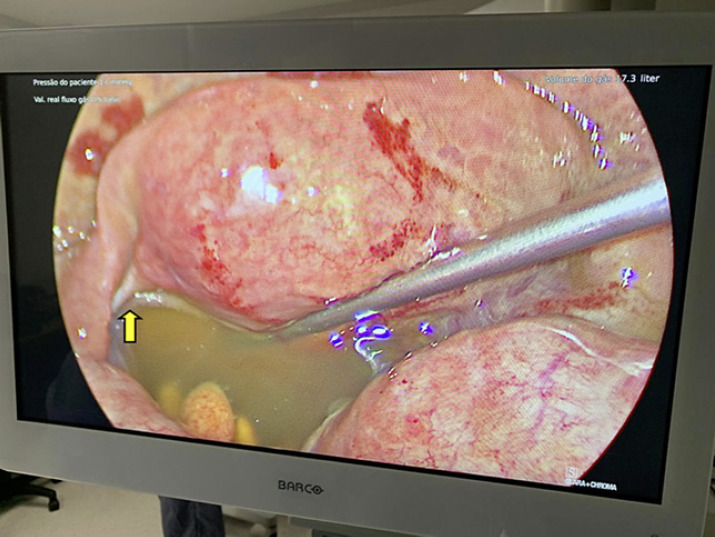
The patient was treated with intravenous antibiotics for spontaneous bacterial peritonitis without clinical improvement. She subsequently underwent a diagnostic laparoscopy that revealed macroscopically clear fluid and inflammatory signs of the tubes, suggesting PID. There were no neoplastic features, and the etiology of the ascites remained undetermined (Fig. 2).
Fig. 2.
Inflammatory signs of the fallopian tubes and ascites in laparoscopy (yellow arrow).
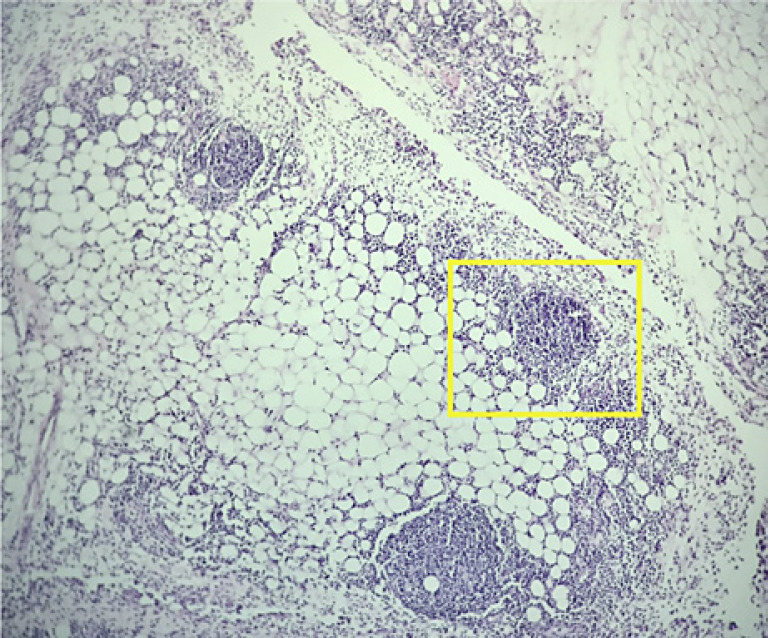
Histopathological results demonstrated fibrous tissue on multiple peritoneal and pelvic nodules, and the omental biopsy showed lymphoid proliferation (Fig. 3). The immunohistochemical staining demonstrated a reactive lymphoplasmacytic infiltrate.
Fig. 3.
Reactive lymphocytic aggregates and lymphoid follicles in the subperitoneal fat and connective tissue specimen (yellow box). Hematoxylin and eosin staining. ×4.
A gynecological investigation with a cervical swab showed a positive DNA for C. trachomatis. The combination of the findings in diagnostic laparoscopy, peritoneal biopsy, and ascitic fluid analysis led to the diagnosis of PID. The patient underwent treatment with oral doxycycline for 2 weeks, with complete resolution of all clinical manifestations. No recurrence of ascites was observed in the 3-month follow-up.
Discussion
We report the case of a young woman with recent-onset ascites without clear etiology. The ascitic fluid's workup showed a low SAAG and a high total protein, suggesting peritoneal disease. After an extensive investigation and exclusion of common causes of ascites, the detection of C. trachomatis DNA in the cervical swab combined with laparoscopy findings confirmed the diagnosis of PID. Treatment with doxycycline resulted in complete resolution of ascites.
PID is an inflammatory disorder of the upper genital tract in women due to infection. Many organisms might cause this condition, but Neisseria gonorrheae and C. trachomatis are the main pathogens. PID commonly presents as endometritis, salpingitis, tubo-ovarian abscess, and pelvic peritonitis. Ascites is considered a rare manifestation of the disease. The diagnosis can be challenging in clinical practice given the wide range of symptoms [6].
Since the first description of ascites caused by C. trachomatis in 1978, few cases were reported, but all occurred in young sexually active women, otherwise healthy. The largest series, by Muller-Schoop et al. [7], described 11 patients with abdominal pain and signs of peritonitis. Nine had strong serological evidence of acute infection by C. trachomatis, and 6 of them had ascites with negative results of ascitic fluid cultures [7].
In Chlamydia peritoneal infection, the ascitic fluid analysis shows a pattern of low SAAG, high protein levels, and lymphocytic predominance, mimicking other more common peritoneal diseases [8, 9]. Although high adenosine deaminase levels are often found in peritoneal tuberculosis, increased values have also been reported in Chlamydia infection [10].
Chlamydia ascites is a diagnosis not to be missed because of the long-term sequelae and disability associated with the infection. Although antibody titers may be measured in the ascitic fluid, the diagnosis more commonly relies on nucleic acid amplification testing on cervical mucus, urine, or serum, yielding higher accuracy rates for the former [6].
This report has described a rare manifestation of infection by C. trachomatis in a sexually active young woman. Clinicians should be aware of this condition when no clear cause for ascites is identified in the initial workup, particularly if the fluid analysis exhibits low SAAG and lymphocytic predominance.
Statement of Ethics
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. Ethical approval was not required for this study in accordance with local and national guidelines.
Conflict of Interest Statement
The authors of this manuscript do not have any conflicts of interest to declare.
Funding Sources
This manuscript did not receive any funding.
Author Contributions
All authors equally contributed to this study's conception, study and design, literature review and analysis, drafting, critical revision and editing, and final approval of the final version.
Data Availability Statement
The data that support the findings of this study cannot be shared due to the General Data Protection Regulation published by Brazilian laws and authorities. Queries regarding the data in this article should be addressed to B.L.L. (luisa.barros@hc.fm.usp.br).
References
- 1.Oey RC, van Buuren HR, de Man RA. The diagnostic work-up in patients with ascites: current guidelines and future prospects. Neth J Med. 2016 Oct;74((8)):330–5. [PubMed] [Google Scholar]
- 2.Farias AQ, Silvestre OM, Garcia-Tsao G, da Costa Seguro LF, de Campos Mazo DF, Bacal F, et al. Serum B-type natriuretic peptide in the initial workup of patients with new onset ascites: a diagnostic accuracy study. Hepatology. 2014 Mar;59((3)):1043–51. doi: 10.1002/hep.26643. [DOI] [PubMed] [Google Scholar]
- 3.Halford B, Piazza MB, Liu D, Obineme C. Chlamydia ascites: a call for sexually transmitted infection testing. BMJ Case Rep. 2018 Dec;11((1)):e226437. doi: 10.1136/bcr-2018-226437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gannon-Loew KE, Holland-Hall C. A review of current guidelines and research on the management of sexually transmitted infections in adolescents and young adults. Ther Adv Infect Dis. 2020 Oct 21;7:2049936120960664. doi: 10.1177/2049936120960664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Workowski KA. Centers for disease control and prevention sexually transmitted diseases treatment guidelines. Clin Infect Dis. 2015 Dec 15;61 Suppl 8:S759–62. doi: 10.1093/cid/civ771. [DOI] [PubMed] [Google Scholar]
- 6.Curry A, Williams T, Penny ML. Pelvic inflammatory disease: diagnosis, management, and prevention. Am Fam Physician. 2019 Sep 15;100((6)):357–64. [PubMed] [Google Scholar]
- 7.Müller-Schoop JW, Wang SP, Munzinger J, Schläpfer HU, Knoblauch M, Tammann RW. Chlamydia trachomatis as possible cause of peritonitis and perihepatitis in young women. Br Med J. 1978 Apr;1((6119)):1022–4. doi: 10.1136/bmj.1.6119.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brun R, Hutmacher J, Fink D, Imesch P. Erroneously suspected ovarian cancer in a 38-year-old woman with pelvic inflammatory disease and chlamydia. Case Rep Obstet Gynecol. 2017;2017:2514613. doi: 10.1155/2017/2514613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tondo-Steele K, Rath K, Niemeier L. Granulomatous peritonitis secondary to chlamydia trachomatis: a case report. Gynecol Oncol Rep. 2020 May;32:100558. doi: 10.1016/j.gore.2020.100558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang HM, Oh TH, Kang GH, Joen TJ, Seo DD, Shin WC, et al. [A case of chlamydia trachomatis peritonitis mimicking tuberculous peritonitis] Korean J Gastroenterol. 2011 Dec;58((2)):111–6. doi: 10.4166/kjg.2011.58.2.111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study cannot be shared due to the General Data Protection Regulation published by Brazilian laws and authorities. Queries regarding the data in this article should be addressed to B.L.L. (luisa.barros@hc.fm.usp.br).