Abstract
Background
Acute cholangitis (AC) is a common emergency with a significant mortality risk. The Tokyo Guidelines (TG) provide recommendations for diagnosis, severity stratification, and management of AC. However, validation of the TG remains poor. This study aims to validate TG07, TG13, and TG18 criteria and identify predictors of in-hospital mortality in patients with AC.
Methods
This is a retrospective audit of patients with a discharge diagnosis of AC in the year 2016. Demographic, clinical, investigation, management and mortality data were documented. We performed a multinomial logistic regression analysis with stepwise variable selection to identify severity predictors for in-hospital mortality.
Results
Two hundred sixty-two patients with a median age of 75.9 years (IQR 64.8–82.8) years were included for analysis. TG13/TG18 diagnostic criteria were more sensitive than TG07 diagnostic criteria (85.1 vs. 75.2%; p < 0.006). The majority of the patients (n = 178; 67.9%) presented with abdominal pain, pyrexia (n = 156; 59.5%), and vomiting (n = 123; 46.9%). Blood cultures were positive in 95 (36.3%) patients, and 79 (83.2%) patients had monomicrobial growth. The 30-day, 90-day, and in-hospital mortality numbers were 3 (1.1%), 11 (4.2%), and 15 (5.7%), respectively. In multivariate analysis, type 2 diabetes mellitus (OR = 12.531; 95% CI 0.354–116.015; p = 0.026), systolic blood pressure <100 mm Hg (OR = 10.108; 95% CI 1.094–93.395; p = 0.041), Glasgow coma score <15 (OR = 38.16; 95% CI 1.804–807.191; p = 0.019), and malignancy (OR = 14.135; 95% CI 1.017–196.394; p = 0.049) predicted in-hospital mortality.
Conclusion
TG13/18 diagnostic criteria are more sensitive than TG07 diagnostic criteria. Type 2 diabetes mellitus, systolic blood pressure <100 mm Hg, Glasgow coma score <15, and malignant etiology predict in-hospital mortality in patients with AC. These predictors could be considered in acute stratification and treatment of patients with AC.
Keywords: Acute cholangitis, Biliary disease, Sepsis
Introduction
About 10–15% of the adult population will develop gallstones, and an estimated 10–15% of them will potentially develop common bile duct stones [1]. The incidence is higher in the elderly. The presence of common bile duct stones results in elevated intrabiliary pressures, and the resulting cholangiovenous reflux provides access for potentially infected bile to escape into the systemic circulation [2]. Thus, acute cholangitis (AC) is associated with gram-negative endotoxemia. AC is a critical care urgency and requires early diagnosis, prompt resuscitation, and early-to-urgent intervention to ensure good clinical outcomes. The management of patients with AC has evolved with refinement of critical-care protocols and an improved understanding of sepsis. The mortality outcomes of AC have improved over the past 5 decades [3], but even today severe AC is associated with a high mortality, primarily if not managed with urgency.
Diagnosis is an essential prerequisite to management. The Tokyo Guidelines (TG) guide the diagnosis and management of AC. Since the first publication in 2007 (TG07) [4], 2 updates have been made (TG13 and TG18) [5, 6]. Although it has been more than a decade since the first publication, validation of the TG is scarce [7, 8]. In a study including 120 AC patients, Sun et al. [7] showed that the TG07 is more reliable than Charcot triad for the diagnosis of AC but of limited prognostic value. In a study including 6,063 patients from Japan and Taiwan, Kiriyama et al. [7] showed that TG13 diagnostic and severity grading criteria not only improve diagnostic accuracy but also identify patients whose prognosis can improve with early biliary drainage [8]. The TG18 adopted the TG13 diagnostic and severity assessment criteria as there was no new evidence to add to the existing TG13 criteria. Besides, many units have adopted the new Sepsis-3 criteria, and this could influence the diagnosis and management of AC [9]. There is an unmet need to validate TG13/TG18 diagnostic and severity grading criteria not only to show that the criteria are useful in routine clinical practice but also to explore the new body of evidence. Thus, our study aims to validate TG13/TG18 diagnostic and severity assessment criteria and identify mortality predictors for AC.
Method
This is a retrospective cohort study of AC patients treated at a university-affiliated academic hospital from January 2016 to December 2016. All of the patients were identified electronically using the following ICD10 (International Statistical Classification of Disease) codes for primary or secondary diagnosis of AC: K83.0, K80.30, K80.31, K80.32, K 80.33, K80.34, K 80.35, K80.36, K80.37, and K74.3 [10]. We excluded all moribund patients or patients with a terminal illness, as the goals of care in both instances were palliation and comfort. We reviewed electronic medical records, endoscopy and imaging records, and discharge summaries for all AC patients to gather data. Patients in whom the diagnosis of AC could not be verified due to wrong coding or other significant primary concomitant illness were excluded from the analysis.
Locally, patients with AC can be admitted to the medical or surgical unit, depending on the judgment of the emergency department doctor. Patients with mild disease are admitted to the general ward. The on-duty team decides on admission to the critical care unit. Indications for high-dependency unit admission include support for single-organ failure, and indications for intensive care unit admission include support for multiorgan failure or patients requiring intubation or renal replacement therapy. All patients with AC had at least 1 set of blood cultures taken before the initiation of empirical broad-spectrum intravenous (i.v.) antibiotics. Based on local antibiograms, we administer a combination of amoxicillin with clavulanic acid along with a stat dose of gentamicin [11]. The specimens were processed for gram stain, bacterial cultures (aerobic and anaerobic), and tests for antibiotic susceptibility. Blood culture service attends to all patients with positive culture results and gives recommendations for antibiotic therapy according to culture and sensitivity results. All patients with positive blood cultures receive at least 7 days of i.v. antibiotics. The total duration of antibiotic therapy varies according to the clinical response. Patients with negative blood cultures receive empirical antibiotics based on a local antibiogram [12]. Ultrasound scan, computerized tomography (CT) scan, or magnetic resonance cholangiopancreatography scan of abdomen are done based on the judgment of the primary team. The medical gastroenterology unit provides emergency endoscopy service with capabilities for urgent biliary drainage by endoscopic retrograde cholangiopancreatography (ERCP). Similarly, emergency interventional radiology support is available for percutaneous biliary drainage. We recommend index admission cholecystectomy in patients with a biliary stone etiology and mild AC. Multidisciplinary care teams decide the management of patients with malignant disease.
Demographic and clinical data, along with serum investigations, endoscopy records, operative records, morbidity, in-hospital mortality, 30-day mortality, and 90-day mortality, were documented. We retrospectively assigned TG07, TG13/18 diagnostic and severity grading criteria to patients. We assigned 3 diagnosis groups as follows: does not meet criteria, suspected diagnosis, and definite diagnosis, and 3 severity groups as follows: grade 1/mild, grade 2/moderate, and grade 3/severe. The upper limit of the normal reference range for serum investigations was taken from TG13 and TG07 guidelines for diagnosis and severity. We used the upper limit of standard reference ranges as per the quick sequential organ failure assessment (qSOFA) or the institution laboratory reference range for variables not included in TG13/TG18 guidelines. The international normalized ratio value had missing data for <10% patients, and imputation was done.
Mortality was defined as death from any cause during admission, within 30 days of admission, and within 90 days of admission as in-hospital mortality, 30 day mortality, and 90 day mortality, respectively. Ten patients had missing data, and they were excluded from the analysis.
We analyzed data according to all the 3 versions of the TG. Categorical data were presented as total numbers (n) and percentages (%), while continuous data were presented as either means (±SD) or medians (IQR), as required. The one-way ANOVA test for continuous variables and the χ2 for categorical variables (continuity corrected) were used as hypothesis test functions. Meanwhile, the Fisher exact t test for categorical variables and the Kruskal-Wallis rank-sum test for continuous variables were used for variables without a normal distribution. Post hoc tests were employed to compare significant variables. p < 0.05 was considered statistically significant. We performed a multinomial logistic regression analysis with stepwise variable selection to identify variables that were severity predictors based on TG13/18 severity grading, with in-hospital mortality as the outcome. p < 0.1 was used in the univariate analysis to indicate statistical significance and thereby inclusion into multivariate analysis. p < 0.05 was used to indicate statistical significance in the multivariate analysis. The statistical analysis was done with R Studio 1.2.5019 using the tableone package. IBM SPSS build 1.0.0.1347 (2018 version) was used for post hoc, univariate, and multivariate regression analyses.
Results
Two hundred seventy-two patients were treated for AC, and we excluded data of 10 patients due to missing items. The data of 262 patients were included in the analysis. One hundred twenty-nine (49.2%) patients were men, and the median age of the patients was 75.9 years (IQR 64.8–82.8). One hundred (38.2%) patients had type 2 diabetes mellitus (T2DM), 53 (20.2%) had ischemic heart disease, 12 (4.6%) had chronic obstructive pulmonary disease or asthma, 38 (14.5%) had a chronic renal impairment, and 136 (51.9%) had a history of biliary disease. The majority of the patients (n = 178, 67.9%) presented with abdominal pain, pyrexia (n = 156; 59.5%), and vomiting (n = 123; 46.9%). Upon physical examination, 136 (51.9%) patients had abdominal tenderness. Blood cultures were positive in 95 (36.3%) patients, and 79 (83.2%) patients had monomicrobial growth. Escherichia coli was the most common microbe (n = 66; 69.5%). An ultrasound scan was done in 71 (27.1%) patients, a CT scan was done in 104 (39.7%) patients (age range 35.3–94.4 years), and a magnetic resonance cholangiopancreatography scan was done in 116 (44.3%) patients. ERCP was done in 146 (55.7%) patients, mainly to achieve source control for biliary sepsis. The timing of ERCP was variable (range 1–6 days) and interventions included a combination of sphincterotomy, biliary stenting, and stone extraction. Index admission cholecystectomy was done in 14 (5.4%) patients. Table 1 shows the severity grading according to TG13/TG18 criteria, with 95 (36.3%), 94 (35.9%), and 73 (27.9%) patients graded as having mild, moderate, and severe AC, respectively. The 30-day, 90-day, and in-hospital mortality values were 3 (1.1%), 11 (4.2%), and 15 (5.7%), respectively.
Table 1.
Clinical characteristics compared by TG13 severity grading
| All (n = 262) | Grade 1: mild (n = 95) |
Grade 2: moderate (n = 94) |
Grade 3: Severe (n = 73) | p value | |
|---|---|---|---|---|---|
| Age, years | 75.9 (64.8–82.8) | 68.9 (61.4–78.3) | 78.1 (70.4–83.3) | 80.1 (66.3–83.8) | <0.001 |
| Male gender | 129 (49.2) | 43 (45.3) | 45 (47.9) | 41 (56.2) | 0.355 |
| Diabetes mellitus | 100 (38.2) | 32 (33.7) | 33 (35.1) | 35 (47.9) | 0.126 |
| Ischemic heart disease | 53 (20.2) | 16 (16.8) | 15 (16.0) | 22 (30.1) | 0.046 |
| COPD/asthma | 12 (4.6) | 4 (4.2) | 6 (6.4) | 2 (2.7) | 0.523 |
| Chronic renal impairment | 38 (14.5) | 12 (12.6) | 12 (12.8) | 14 (19.2) | 0.41 |
| History of biliary disease | 136 (51.9) | 46 (48.4) | 52 (55.3) | 38 (52.1) | 0.637 |
| Abdominal pain | 178 (67.9) | 67 (70.5) | 63 (67.0) | 48 (65.8) | 0.783 |
| Pyrexia | 156 (59.5) | 50 (52.6) | 60 (63.8) | 46 (63.0) | 0.227 |
| Vomiting | 123 (46.9) | 45 (47.4) | 42 (44.7) | 36 (49.3) | 0.833 |
| Jaundice | 67 (25.6) | 25 (26.3) | 21 (22.3) | 21 (28.8) | 0.627 |
| Abdominal tenderness | 136 (51.9) | 51 (53.7) | 45 (47.9) | 40 (54.8) | 0.614 |
| Temperature, °C | 37.94±1.10 | 37.68±0.98 | 38.10±1.10 | 38.07±1.18 | 0.014 |
| Heart rate, bpm | 91.77±19.09 | 85.64±17.06 | 91.56±15.27 | 100.01±22.84 | <0.001 |
| Systolic blood pressure, mm Hg | 123.22±25.97 | 127.84±22.09 | 125.56±22.46 | 114.19±32.23 | 0.004 |
| Respiratory rate, rpm | 17.41±1.21 | 17.49±0.89 | 17.26±0.85 | 17.50±1.81 | 0.191 |
| White blood cell count, n × 109/L | 12.79±5.87 | 10.92±4.77 | 14.27±4.91 | 13.32±7.48 | <0.001 |
| Platelet count, n × 109/L | 227.22±106.14 | 227.45±83.73 | 251.17±102.04 | 196.07±128.44 | 0.001 |
| AST, U/L | 253.11±287.26 | 275.45±277.24 | 251.01±345.95 | 227.07±206.78 | 0.255 |
| ALT, U/L | 195.84±171.72 | 219.22±165.04 | 196.98±201.92 | 163.96±130.08 | 0.09 |
| Bilirubin, μmol/L | 73.31±64.63 | 57.32±44.62 | 75.74±63.99 | 91±81.18 | 0.004 |
| ALP, U/L | 294.13±253.42 | 263.77±225.11 | 290.20±244.34 | 338.68±293.74 | 0.262 |
| GGT, U/L | 387.82±314.83 | 431.61±339.45 | 361.48±332.82 | 365.34±249.20 | 0.296 |
| Creatinine, μmol/L | 120.57±103.54 | 90.04±28.69 | 95.65±30.68 | 192.40±171.18 | <0.001 |
| Albumin, g/L | 31.79±6.24 | 34.65±4.79 | 30.71±6.15 | 29.44±6.67 | <0.001 |
| Total positive blood cultures, n | 95 | 28 | 31 | 36 | 0.001 |
| Monomicrobial | 79 (83.2) | 27 (96.4) | 28 (90.3) | 24 (66.7) | |
| Polymicrobial | 16 (16.8) | 1 (3.6) | 3 (9.7) | 12 (33.3) | |
| E. coli | 66 (69.5) | 17 (60.7) | 22 (71.0) | 27 (75.0) | 0.016 |
| Cholelithiasis | 186 (71.0) | 79 (83.2) | 64 (68.1) | 43 (58.9) | 0.002 |
| Malignancy | 45 (17.2) | 9 (9.5) | 17 (18.1) | 19 (26.0) | 0.018 |
| Ultrasound | 71 (27.1) | 26 (27.4) | 25 (26.6) | 20 (27.4) | 0.991 |
| CT scan | 104 (39.7) | 34 (35.8) | 42 (44.7) | 28 (38.4) | 0.441 |
| MRCP scan | 116 (44.3) | 48 (50.5) | 44 (46.8) | 24 (32.9) | 0.061 |
| ERCP | 146 (55.7) | 63 (66.3) | 50 (53.2) | 33 (45.2) | 0.020 |
| Index admission cholecystectomy | 14 (5.4)a | 10 (10.6)a | 3 (3.2) | 1 (1.4) | 0.039 |
| 30-day mortality | 3 (1.1) | 0 (0.0) | 0 (0.0) | 3 (4.1) | 0.020 |
| 90-day mortality | 11 (4.2) | 2 (2.1) | 3 (3.2) | 6 (8.2) | 0.122 |
| In-hospital mortality | 15 (5.7) | 1 (1.1) | 5 (5.3) | 9 (12.3) | 0.008 |
Values are presented as medians (IQR), numbers (%), or means (±SD), or numbers. COPD, chronic obstructive pulmonary disease; AST, aspartate aminotransferase; ALT, alanine transaminase; ALP, alkaline phosphatase; GGT, γ-glutamyl transferase, MRCP, magnetic resonance cholangiopancreatography. a There was a case of postcholecystectomy pancreatitis that was removed from the count.
Comparison across TG07 Diagnostic Criteria
One hundred ninety-seven (75.2%) patients fulfilled TG07 diagnostic criteria for AC. A history of biliary disease (p < 0.001), abdominal pain (p < 0.001), pyrexia (p < 0.001), and jaundice (p < 0.001) predicted patients with a suspected or definite diagnosis of AC. The absence of a prior history of arrhythmias was predictive (p = 0.01) of a suspected or definite diagnosis of AC, though only 3 (4.6%) patients had a history of arrhythmias and no definite conclusions should be drawn. There was a higher proportion of patients (n = 11; 26.8%) with polymicrobial results in the definite diagnosis cohort (p = 0.036) (Table 2).
Table 2.
TG07 diagnostic criteria
| Did not meet the criteria (n = 65) | Suspected diagnosis (n = 100) |
Definite diagnosis (n = 97) |
p value | |
|---|---|---|---|---|
| Age, years | 77.2 (64.8–82.5) | 76.9 (66.1–83.2) | 75.4 (62.2–82.4) | 0.525 |
| Male gender | 30 (46.2) | 52 (52.0) | 47 (48.5) | 0.75 |
| Diabetes mellitus | 25 (38.5) | 38 (38.0) | 37 (38.1) | 0.998 |
| Ischemic heart disease | 16 (24.6) | 22 (22.0) | 15 (15.5) | 0.311 |
| COPD/asthma | 2 (3.1) | 5 (5.0) | 5 (5.2) | 0.799 |
| Chronic renal impairment | 11 (16.9) | 11 (11.0) | 16 (16.5) | 0.448 |
| History of biliary disease | 7 (10.8) | 70 (70.0) | 59 (60.8) | <0.001 |
| Abdominal pain | 31 (47.7) | 71 (71.0) | 76 (78.4) | <0.001 |
| Pyrexia | 14 (21.5) | 62 (62.0) | 80 (82.5) | <0.001 |
| Vomiting | 36 (55.4) | 39 (39.0) | 48 (49.5) | 0.098 |
| Jaundice | 5 (7.7) | 25 (25.0) | 37 (38.1) | <0.001 |
| Abdominal tenderness | 30 (46.2) | 50 (50.0) | 56 (57.7) | 0.313 |
| Arrythmia | 3 (4.6) | 0 (0.0) | 0 (0.0) | 0.01 |
| Ionotrope use | 5 (7.7) | 6 (6.0) | 8 (8.2) | 0.821 |
| Total positive blood cultures | 26 | 28 | 41 | 0.036 |
| Monomicrobial | 23 (88.5) | 26 (92.9) | 30 (73.2) | |
| Polymicrobial | 3 (11.5) | 2 (7.1) | 11 (26.8) | |
| Cholelithiasis | 46 (70.8) | 69 (69.0) | 71 (73.2) | 0.809 |
| Malignancy | 10 (15.4) | 13 (13.0) | 22 (22.7) | 0.179 |
| Ultrasound | 22 (33.8) | 22 (22.0) | 27 (27.8) | 0.242 |
| CT scan | 27 (41.5) | 40 (40.0) | 37 (38.1) | 0.908 |
| MRCP scan | 30 (46.2) | 43 (43.0) | 43 (44.3) | 0.924 |
| ERCP | 30 (46.2) | 59 (59.0) | 57 (58.8) | 0.201 |
Values are presented as medians (IQR), numbers (%), or numbers. The total number of patients is 262. COPD, chronic obstructive pulmonary disease; MRCP, Magnetic resonance cholangiopancreatography.
Comparison across TG13/TG18 Diagnostic Criteria
Two hundred twenty-three (85.1%) patients fulfilled TG13/TG18 diagnostic criteria for AC. TG13/TG18 diagnostic criteria were more sensitive than TG07 diagnostic criteria (85.1 vs. 75.2%; p < 0.006). One hundred ninety-five (74.4%) patients received a definite diagnosis of AC. T2DM (p = 0.027) and pyrexia (p < 0.001) predicted patients with a suspected or definite diagnosis of AC. Those with a definite diagnosis of AC were more likely to be treated with an ERCP (p = 0.003). Those who do not meet the diagnostic criteria for AC were more likely to have an ultrasound scan (p = 0.011) (Table 3).
Table 3.
TG13 and TG18 diagnostic criteria
| n = 262 | Did not meet the criteria (n = 39) |
Suspected diagnosis (n = 28) |
Definite diagnosis (n = 195) |
p value |
|---|---|---|---|---|
| Age, years | 75.4 (59.7–82.5) | 81.71 (66.3–83.1) | 75.5 (64.8–82.6) | 0.627 |
| Male gender | 16 (41.0) | 13 (46.4) | 100 (51.3) | 0.48 |
| Diabetes mellitus | 12 (30.8) | 17 (60.7) | 71 (36.4) | 0.027 |
| Ischemic heart disease | 8 (20.5) | 6 (21.4) | 39 (20.0) | 0.984 |
| COPD/asthma | 2 (5.1) | 1 (3.6) | 9 (4.6) | 0.955 |
| Chronic renal impairment | 3 (7.7) | 3 (10.7) | 32 (16.4) | 0.308 |
| History of biliary disease | 16 (41.0) | 17 (60.7) | 103 (52.8) | 0.248 |
| Abdominal pain | 29 (74.4) | 14 (50.0) | 135 (69.2) | 0.081 |
| Pyrexia | 13 (33.3) | 20 (71.4) | 123 (63.1) | 0.001 |
| Vomiting | 20 (51.3) | 15 (53.6) | 88 (45.1) | 0.593 |
| Jaundice | 7 (17.9) | 4 (14.3) | 56 (28.7) | 0.13 |
| Abdominal tenderness | 25 (64.1) | 8 (28.6) | 103 (52.8) | 0.014 |
| Arrythmia | 0 (0.0) | 0 (0.0) | 3 (1.5) | 0.594 |
| Ionotrope use | 1 (2.6) | 3 (10.7) | 15 (7.7) | 0.401 |
| Total positive blood cultures, n | 12 | 11 | 72 | 0.397 |
| Monomicrobial | 12 (100.0) | 10 (90.9) | 57 (79.2) | |
| Polymicrobial | 0 (0.0) | 1 (9.1) | 15 (20.8) | |
| Cholelithiasis | 28 (71.8) | 13 (46.4) | 145 (74.4) | 0.010 |
| Malignancy | 5 (12.8) | 1 (3.6) | 39 (20.0) | 0.072 |
| Ultrasound | 18 (46.2) | 5 (17.9) | 48 (24.6) | 0.011 |
| CT scan | 18 (46.2) | 11 (39.3) | 75 (38.5) | 0.668 |
| MRCP scan | 15 (38.5) | 11 (39.3) | 90 (46.2) | 0.578 |
| ERCP | 17 (43.6) | 9 (32.1) | 120 (61.5) | 0.003 |
Values are presented as medians (IQR), numbers (%), or numbers. COPD, chronic obstructive pulmonary disease; MRCP, magnetic resonance cholangiopancreatography.
Comparison across TG13/TG18 Severity Gradings
The median age of thd patients with severe AC was higher (mild AC, 68.9 years; moderate AC, 78.1 years; and severe AC, 80.1 years; p < 0.001). Ischemic heart disease was more common (p = 0.046) in patients with severe AC. Pyrexia (p = 0.014), tachycardia (p < 0.001), hypotension (p = 0.004), leucocytosis (p < 0.001), thrombocytopenia (p = 0.001), elevated bilirubin (p = 0.004), elevated creatinine (p < 0.001), hypoalbuminemia (p < 0.001), polymicrobial growth (p = 0.001), E. coli bacteremia (p = 0.016), and concomitant malignancy (p = 0.018) were more common in patients with severe AC. Cholelithiasis (p = 0.002), ERCP (p = 0.02), and index admission cholecystectomy (p = 0.039) were more common in patients with mild AC. Thirty-day mortality (p = 0.02) and in-hospital mortality (p = 0.008) were higher in patients with severe AC. Table 4 shows the variables that predicted in-hospital mortality. In the multivariate analysis, T2DM (OR = 12.531; 95% CI 0.354–116.015; p = 0.026), systolic blood pressure <100 mm Hg (OR = 10.108; 95% CI 1.094–93.395; p = 0.041), Glasgow coma score (GCS) <15 (OR = 38.16; 95% CI 1.804–807.191; p = 0.019), and malignancy (OR = 14.135; 95% CI 1.017–196.394; p = 0.049) predicted in-hospital mortality (Table 1).
Table 4.
Univariate and multivariate analysis of patient variables against in-hospital mortality
| Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Age ≥75 years | 1.045 (0.368–2.971) | 0.934 | − | − |
| Male gender | 4.444 (1.224–16.139) | 0.023 | 4.543 (0.603–34.248) | 0.142 |
| Diabetes mellitus | 0.287 (0.095–0.865) | 0.027 | 12.531 (0.354–116.015) | 0.026 |
| Ischemic heart disease | 3.733 (0.480–29.049) | 0.208 | − | − |
| COPD/asthma | 1.513 (0.185–12.728) | 0.693 | − | − |
| Chronic renal impairment | 1.514 (0.407–5.639) | 0.536 | − | − |
| History of biliary disease | 1.921 (0.638–5.782) | 0.246 | − | − |
| Abdominal pain | 4.676 (1.545–14.152) | 0.006 | 0.297 (0.050–1.756) | 0.181 |
| Vomiting | 6.242 (1.380–28.241) | 0.017 | 0.098 (0.007–1.341) | 0.082 |
| Jaundice | 0.670 (0.221–2.037) | 0.480 | − | − |
| Abdominal tenderness | 1.062 (0.374–3.020) | 0.909 | − | − |
| Heart rate >90 bpm | 4.517 (1.244–16.403) | 0.022 | 3.551 (0.456–27.676) | 0.226 |
| Systolic blood pressure ≤100 mm Hg | 5.128 (1.752–15.014) | 0.003 | 10.108 (1.094–93.395) | 0.041 |
| Respiratory rate | 1.079 (0.743–1.566) | 0.690 | − | − |
| High fever ≥39°C | 0.894 (0.243–3.281) | 0.865 | − | − |
| White blood cell count >12,000/m3 | 1.029 (0.362–2.924) | 0.958 | − | − |
| White blood cell count <4,000/m3 | 3.457 (0.378–31.628) | 0.272 | − | − |
| Hyperbilirubinemia ≥85.5 μmol/L | 2.351 (0.821–6.734) | 0.111 | − | − |
| Hypoalbuminemia <28 g/L | 11.11 (3.393–36.393) | <0.001 | 0.728 (0.064–8.285) | 0.798 |
| Cardiovascular (inotropes used) | 3.609 (0.924–14.103) | 0.065 | 0.642 (0.047–8.829) | 0.741 |
| Neurologic (GCS <15) | 9.616 (2.559–36.136) | 0.001 | 38.160 (1.804–807.191) | 0.019 |
| Renal (creatinine ≥176.8) | 4.440 (1.405–14.028) | 0.011 | 0.848 (0.076–9.475) | 0.894 |
| Hepatic (INR >1.5) | 7.567 (2.445–23.415) | <0.001 | 2.361 (0.298–18.691) | 0.416 |
| Hematologic (platelets <100) | 2.221 (0.461–10.704) | 0.320 | − | − |
| AST >45 U/L | 0.287 (0.092–0.895) | 0.032 | 0.144 (0.007–2.880) | 0.205 |
| ALT >55 U/L | 0.227 (0.078–0.662) | 0.007 | 1.042 (0.066–16.446) | 0.977 |
| ALP >120 U/L | 1.448 (0.316–6.643) | 0.634 | − | − |
| GGT >90 U/L | 1.038 (0.224–4.802) | 0.962 | − | − |
| Monomicrobial | 0.352 (0.068–1.821) | 0.213 | − | − |
| Polymicrobial | 0.473 (0.083–2.685) | 0.398 | − | − |
| Cholelithiasis | 11.44 (3.127–41.832) | <0.001 | 0.349 (0.027–4.578) | 0.349 |
| Malignancy | 12.11 (3.907–37.558) | <0.001 | 14.135 (1.017–196.394) | 0.049 |
| qSOFA score >1 | 7.446 (1.317–42.092) | 0.023 | 0.146 (0.001–25.697) | 0.466 |
The total in-hospital mortality number was 15. INR, international normalized ratio; AST, aspartate aminotransferase; ALT, alanine transaminase; ALP, alkaline phosphatase; GGT, γ-glutamyl transferase; COPD, chronic obstructive pulmonary disease.
Discussion
TG13/18 diagnostic criteria are more sensitive than TG07 diagnostic criteria. T2DM, systolic blood pressure <100 mm Hg, GCS <15, and malignant disease predict in-hospital mortality in patients with AC.
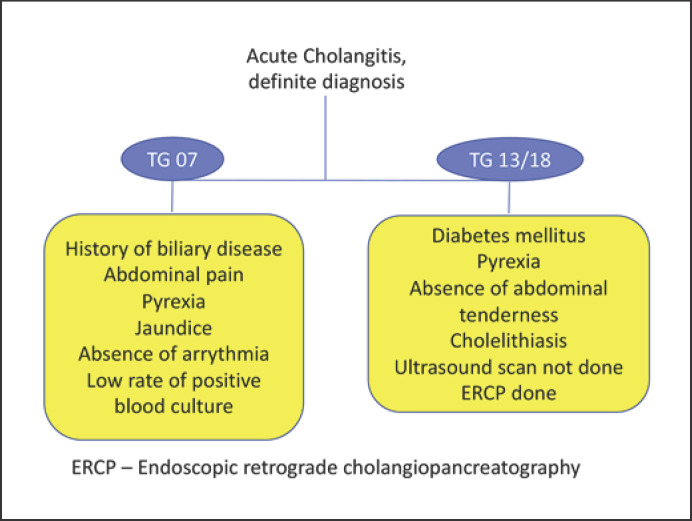
The TG07 clarified the diagnostic criteria, severity assessment, and treatment of AC. However, validation of the TG07 remains poor outside Japan [7, 8]. The Charcot triad is traditionally considered diagnostic for AC; however, it has a low sensitivity [13, 14]. In addition to the Charcot triad, TG07 diagnostic criteria include a history of biliary disease, and it has an increased diagnostic sensitivity for the diagnosis of AC [4, 15]. It is known that patients with complicated acute cholecystitis also present with similar symptoms and signs, and therefore TG07 diagnostic criteria lack specificity. Thus, the accuracy of diagnostic criteria could be enhanced by exclusion of variables that confound the presence of acute cholecystitis. Patients with acute cholecystitis may have abdominal tenderness more than patients with AC. In our study, abdominal tenderness and ultrasound scan negatively predicted the diagnosis of AC based on TG13/TG18 diagnostic criteria (Fig. 1). This could be due to liberal policy of early CT scans in patients presenting with abdominal sepsis. Our study showed that a history of biliary disease, abdominal pain, pyrexia, and jaundice are significant variables for the diagnosis of AC based on the TG07, but only pyrexia was significantly associated with a diagnosis of AC.
Fig. 1.
Factors associated with a definite diagnosis of AC.
In our study, patients with a definite diagnosis of AC were more likely to receive ERCP. However, it was interesting to note that, compared to severe AC, patients with a mild diagnosis receive ERCP more often. This could be due to a reluctance to do ERCP among sick patients with severe AC. ERCP may be delayed in patients with severe AC with the intent of restoring hemodynamics, normalizing the deranged serum biochemistry, correcting the coagulopathy, or seeking specialist cardiology or anesthesia opinions about risks-benefits [2]. However, with optimization of oxygenation and fluids, reduction and restriction of the sedative dose, and experienced personnel, early ERCP is safe and feasible, even among the elderly [16]. Even though timely and liberal ERCP policies could improve the clinical outcomes of patients with biliary sepsis, no significant differences were noted between the 3 groups stratified using TG07 diagnostic criteria (p = 0.201; Table 2). This was unlike TG13 diagnostic criteria, where there was an observable trend toward ERCP use in the definite-diagnosis group (p = 0.003; Table 2). It is our opinion that biliary decompression is a part of resuscitation in sick patients and should not be delayed. In patients in whom ERCP is not feasible or is deemed unsafe, percutaneous transhepatic cholangiography remains an option. However, percutaneous transhepatic cholangiography is associated with the risk of bleeding in patients with coagulopathy and technically challenging in patients with nondilated intrahepatic biliary ducts. In selected high-risk patients, percutaneous cholecystostomy can achieve biliary decompression. Percutaneous cholecystostomy can be done under local anesthesia and it has been shown to restore hemodynamics within 48 h in critically ill patients [17]. After acute illness management, patients warrant a definitive cholecystectomy to prevent recurrent biliary events or stent-related morbidity [18].
Our results show that T2DM predicts in-hospital mortality in patients with AC. This is not surprising as T2DM has been shown to predict mortality in sepsis. In a study of 286 diabetic patients, Gornik et al. [19] reported that elevated HbA1C independently predicts mortality in patients with sepsis (OR = 1.36). The cause-and-effect relationship of T2DM with mortality is multifactorial. T2DM impairs neutrophil function and affects innate and adaptive immune mechanisms [20]. T2DM is associated with a lower incidence of abdominal pain and thus may cause a delay in seeking medical attention, leading to treatment delays [21]. Hyperglycemia resulting from T2DM reduces the bactericidal activity of neutrophils. Further, poor control of T2DM is associated with end-organ dysfunction, and patients may have an increased risk of acute kidney injury. Elevated serum creatinine is associated with inferior outcomes in acute illnesses like acute pancreatitis, perforated peptic ulcer, and pyogenic liver abscess [22, 23, 24].
In this study, serum albumin, bilirubin, and creatinine did not predict in-hospital mortality, but mental obtundation (GCS <15) and systolic blood pressure <100 mm Hg independently predicted mortality. An altered mental state and septic shock are components of the Reynold pentad [25], indicating severe AC. Kiriyama et al. [8] reported that mental obtundation was an independent predictor of mortality (OR = 4.86; 95% CI 2.94–7.73). Mental obtundation is a part of many risk prediction models. In an analysis of 11 risk prediction models, Schneider et al. [26] reported that the Random Forest model was accurate in predicting mortality (area under the curve: 91.5%) and mental confusion had the highest odds for mortality prediction.
Mental obtundation and a low systolic blood pressure are included when computing the bedside quick sequential organ failure assessment (qSOFA) score [27]. The qSOFA criteria include at least 2 of the following: an altered mental state, a respiratory rate ≥22/min, and systolic blood pressure ≤100 mm Hg. In non-intensive care unit patients, a qSOFA score of 2 or more was associated with a 3- to 14-fold increase in in-hospital mortality [28]. However, our analysis shows that, in univariate analysis, a qSOFA score >1 predicted in-hospital mortality (OR = 7.446; 95% CI 1.317–42.092; p = 0.023) and in multivariate analysis a qSOFA score >1 did not predict in-hospital mortality (OR = 0.146; 95% CI 0.001–25.697; p = 0.466; Table 4). A low GCS also predicts mortality in acute illness [29, 30]. Goodacre et al. [29] reported that low GCS independently predict mortality (OR = 2.10; p < 0.001). The relevance of hypotension in sepsis is well known. In a study including 8,782 patients, Maheshwari et al. [31] reported an 11.4% increase in mortality for a unit drop in the mean arterial pressure. Malignancy-related AC is distinct from biliary lithiasis as long-term outcomes are determined by the stage and extent of the tumor. In a study including 574 patients, Shi et al. [32] reported an association of malignancy with mortality in patients with acute cholecystitis and cholangitis (OR = 9.6). In another report of 775 consecutive AC patients, Tan et al. [33] reported that malignant obstruction predicted the 30-day mortality (OR = 1.11). However, our 30-day mortality was significantly lower (1.1 vs. 12%). This could be due to the inclusion of a 25-year sample by Tan et al. [33] or a higher proportion of patients with a malignant etiology (17.2 vs. 42%).
Our study has several limitations. This was a retrospective study, and hence the cause-effect relation of mortality predictors cannot be established as such conclusions can rightfully be derived from a prospective study. We did not record compliance with the sepsis bundle, the timing of antibiotic administration, antibiotic regimens, or the duration of the antibiotic course. We also did not study the cause of in-hospital mortality. Mortality may be driven by patient co-morbidity and not directly attributed to AC. However, regardless, it is essential to determine the all-cause mortality as this information assists in patient counselling regarding risk. Only 55.7% of the patients in this study received ERCP, and indications were not specified. We did not collect data about other options for biliary drainage, e.g., percutaneous transhepatic cholangiography and percutaneous cholecystostomy. Some patients might have passed a stone with natural relief from obstruction. Lastly, we are unable to comment on the specificity of TG07 or TG13/18 criteria from our dataset. Despite its limitations, this study has merits. There are very few reports about AC, and data from this study add to the current body of evidence. Further, validation of the TG is necessary outside of Japan and Taiwan, and hence this study fulfills an unmet need. The multivariate analysis attempted to reduce the bias and thus enhance the reliability of the results. In conclusion, TG13/18 diagnostic and severity assessment criteria demonstrated improvements compared to TG07 criteria. T2DM, systolic blood pressure <100 mm Hg, GCS <15, and malignant etiology predict in-hospital mortality in patients with AC.
Conflict of Interest Statement
The authors have no conflict of interests to declare.
Funding Sources
There are no funding sources to be declared.
Statement of Ethics
The subjects (or their parents or guardians) gave written informed consent. This study was approved by the local institutional review board (NHG Domain-Specific Review Board; DSRB 2017/00200).
Authors Contributions
Ramkumar Mohan was involved in data analysis, interpretation, drafting, and critical revision of this article. Stefanie Goh and Guan Wei Tan were involved in data collection, and interpretation. Yen Pin Tan, Sameer P. Junnarkar, Cheong Wei Terence Huey, and Jee Keem Low were involved in critical revisions of this article. Vishal G. Shelat was involved in conception of this study, critical revisions, and final approval of the version to be published.
References
- 1.Kama NA, Atli M, Doganay M, Kologlu M, Reis E, Dolapci M. Practical recommendations for the prediction and management of common bile duct stones in patients with gallstones. Surg Endosc. 2001 Sep;15((9)):942–5. doi: 10.1007/s00464-001-0005-7. [DOI] [PubMed] [Google Scholar]
- 2.Wang Z, Ahmed S, Shelat VG, Acute Cholangitis . In: Abdominal Sepsis: A Multidisciplinary Approach. Sartelli M, Bassetti M, Martin-Loeches I, editors. Springer International Publishing; 2018. pp. pp. 65–81. [Google Scholar]
- 3.Andrew DJ, Johnson SE. Acute suppurative cholangitis, a medical and surgical emergency. A review of ten years experience emphasizing early recognition. Am J Gastroenterol. 1970 Aug;54((2)):141–54. [PubMed] [Google Scholar]
- 4.Wada K, Takada T, Kawarada Y, Nimura Y, Miura F, Yoshida M, et al. Diagnostic criteria and severity assessment of acute cholangitis: tokyo Guidelines. J Hepatobiliary Pancreat Surg. 2007;14((1)):52–8. doi: 10.1007/s00534-006-1156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiriyama S, Takada T, Strasberg SM, Solomkin JS, Mayumi T, Pitt HA, et al. Tokyo Guidelines Revision Committee New diagnostic criteria and severity assessment of acute cholangitis in revised Tokyo Guidelines. J Hepatobiliary Pancreat Sci. 2012 Sep;19((5)):548–56. doi: 10.1007/s00534-012-0537-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiriyama S, Kozaka K, Takada T, Strasberg SM, Pitt HA, Gabata T, et al. Tokyo Guidelines 2018: diagnostic criteria and severity grading of acute cholangitis (with videos) J Hepatobiliary Pancreat Sci. 2018 Jan;25((1)):17–30. doi: 10.1002/jhbp.512. Available from: https://doi-org.libproxy1.nus.edu.sg/10.1002/jhbp.512. [DOI] [PubMed] [Google Scholar]
- 7.Sun G, Han L, Yang YS, Linghu EQ, Li W, Cai FC, et al. Verification of the Tokyo guidelines for acute cholangitis secondary to benign and malignant biliary obstruction: experience from a Chinese tertiary hospital. Hepatobiliary Pancreat Dis Int. 2013 Aug;12((4)):400–7. doi: 10.1016/s1499-3872(13)60062-4. [DOI] [PubMed] [Google Scholar]
- 8.Kiriyama S, Takada T, Hwang TL, Akazawa K, Miura F, Gomi H, et al. Clinical application and verification of the TG13 diagnostic and severity grading criteria for acute cholangitis: an international multicenter observational study. J Hepatobiliary Pancreat Sci. 2017 Jun;24((6)):329–37. doi: 10.1002/jhbp.458. [DOI] [PubMed] [Google Scholar]
- 9.Mak M., Low J. K., Junnarkar S. P., Huey T., Shelat V. G. A prospective validation of Sepsis-3 guidelines in acute hepatobiliary sepsis: qSOFA lacks sensitivity and SIRS criteria lacks specificity (Cohort Study). International journal of surgery (London, England) 2019;72:71–77. doi: 10.1016/j.ijsu.2019.10.022. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization . ICD-10: International statistical classification of diseases and related health problems: Tenth revision. 5th ed. World Health Organization; 2016. [Google Scholar]
- 11.Shelat VG, Chia CL, Yeo CS, Qiao W, Woon W, Junnarkar SP. Pyogenic Liver Abscess: Does Escherichia Coli Cause more Adverse Outcomes than Klebsiella Pneumoniae? World J Surg. 2015 Oct;39((10)):2535–42. doi: 10.1007/s00268-015-3126-1. [DOI] [PubMed] [Google Scholar]
- 12.Shelat VG, Wang Q, Chia CL, Wang Z, Low JK, Woon WW. Patients with culture negative pyogenic liver abscess have the same outcomes compared to those with Klebsiella pneumoniae pyogenic liver abscess. Hepatobiliary Pancreat Dis Int. 2016 Oct;15((5)):504–11. doi: 10.1016/s1499-3872(16)60127-3. [DOI] [PubMed] [Google Scholar]
- 13.Miura F, Okamoto K, Takada T, Strasberg SM, Asbun HJ, Pitt HA, et al. Tokyo Guidelines 2018: initial management of acute biliary infection and flowchart for acute cholangitis. J Hepatobiliary Pancreat Sci. 2018 Jan;25((1)):31–40. doi: 10.1002/jhbp.509. [DOI] [PubMed] [Google Scholar]
- 14.Tinusz B, Szapáry L, Paládi B, Tenk J, Rumbus Z, Pécsi D, et al. Short-Course Antibiotic Treatment Is Not Inferior to a Long-Course One in Acute Cholangitis: A Systematic Review. Dig Dis Sci. 2019 Feb;64((2)):307–15. doi: 10.1007/s10620-018-5327-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dinc T, Kayilioglu SI, Coskun F. Evaluation and Comparison of Charcot's Triad and Tokyo Guidelines for the Diagnosis of Acute Cholangitis. Indian J Surg. 2017 Oct;79((5)):427–30. doi: 10.1007/s12262-016-1512-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tohda G, Ohtani M, Dochin M. Efficacy and safety of emergency endoscopic retrograde cholangiopancreatography for acute cholangitis in the elderly. World J Gastroenterol. 2016 Oct;22((37)):8382–8. doi: 10.3748/wjg.v22.i37.8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeo CS, Tay VW, Low JK, Woon WW, Punamiya SJ, Shelat VG. Outcomes of percutaneous cholecystostomy and predictors of eventual cholecystectomy. J Hepatobiliary Pancreat Sci. 2016 Jan;23((1)):65–73. doi: 10.1002/jhbp.304. Available from: https://doi-org.libproxy1.nus.edu.sg/10.1002/jhbp.304. [DOI] [PubMed] [Google Scholar]
- 18.Kwan JR, Low KS, Lohan R, Shelat VG. Percutaneous transhepatic biliary drainage catheter fracture: A case report. Ann Hepatobiliary Pancreat Surg. 2018 Aug;22((3)):282–6. doi: 10.14701/ahbps.2018.22.3.282. Available from: https://doi-org.libproxy1.nus.edu.sg/10.14701/ahbps.2018.22.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gornik I, Gornik O, Gasparović V. HbA1c is outcome predictor in diabetic patients with sepsis. Diabetes Res Clin Pract. 2007 Jul;77((1)):120–5. doi: 10.1016/j.diabres.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 20.Hatanaka E, Monteagudo PT, Marrocos MS, Campa A. Neutrophils and monocytes as potentially important sources of proinflammatory cytokines in diabetes. Clin Exp Immunol. 2006 Dec;146((3)):443–7. doi: 10.1111/j.1365-2249.2006.03229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chia CL, Lee AY, Shelat VG, Ahmed S, Junnarkar SP, Woon WW, et al. Does diabetes mellitus affect presentation, stage and survival in operable pancreatic cancer? Hepatobiliary Surg Nutr. 2016 Feb;5((1)):38–42. doi: 10.3978/j.issn.2304-3881.2015.07.04. Available from: https://doi-org.libproxy1.nus.edu.sg/10.3978/j.issn.2304-3881.2015.07.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiat TT, Gunasekaran SK, Junnarkar SP, Low JK, Woon W, Shelat VG. Are traditional scoring systems for severity stratification of acute pancreatitis sufficient? Ann Hepatobiliary Pancreat Surg. 2018 May;22((2)):105–15. doi: 10.14701/ahbps.2018.22.2.105. Available from: https://doi-org.libproxy1.nus.edu.sg/10.14701/ahbps.2018.22.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anbalakan K, Chua D, Pandya GJ, Shelat VG. Five year experience in management of perforated peptic ulcer and validation of common mortality risk prediction models - are existing models sufficient? A retrospective cohort study. International journal of surgery (London, England) 2015;14:38–44. doi: 10.1016/j.ijsu.2014.12.022. https://doi-org.libproxy1.nus.edu.sg/10.1016/j.ijsu.2014.12.022 . [DOI] [PubMed] [Google Scholar]
- 24.Danson Y, Yuan TM, Shelat VG. Pyogenic Liver Abscess. In: Sartelli M, Bassetti M, Martin-Loeches I, editors. Abdominal Sepsis: A Multidisciplinary Approach. Springer International Publishing; 2018. pp. pp. 83–93. [Google Scholar]
- 25.Reynolds BM, Dargan EL. Acute obstructive cholangitis; a distinct clinical syndrome. Ann Surg. 1959 Aug;150((2)):299–303. doi: 10.1097/00000658-195908000-00013. Available from: https://doi-org.libproxy1.nus.edu.sg/10.1097/00000658-195908000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneider J, Hapfelmeier A, Thöres S, Obermeier A, Schulz C, Pförringer D, et al. Mortality Risk for Acute Cholangitis (MAC): a risk prediction model for in-hospital mortality in patients with acute cholangitis. BMC Gastroenterol. 2016 Feb;16((1)):15. doi: 10.1186/s12876-016-0428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016 Feb;315((8)):801–10. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016 Feb;315((8)):762–74. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goodacre S, Turner J, Nicholl J. Prediction of mortality among emergency medical admissions. Emergency medicine journal: EMJ. 2006;23((5)):372–375. doi: 10.1136/emj.2005.028522. https://doi-org.libproxy1.nus.edu.sg/10.1136/emj.2005.028522 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grmec S, Gasparovic V. Comparison of APACHE II, MEES and Glasgow Coma Scale in patients with nontraumatic coma for prediction of mortality. Acute Physiology and Chronic Health Evaluation. Mainz Emergency Evaluation System. Crit Care. 2001;5((1)):19–23. doi: 10.1186/cc973. Available from: https://doi-org.libproxy1.nus.edu.sg/10.1186/cc973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maheshwari K, Nathanson BH, Munson SH, Khangulov V, Stevens M, Badani H, et al. The relationship between ICU hypotension and in-hospital mortality and morbidity in septic patients. Intensive Care Med. 2018 Jun;44((6)):857–67. doi: 10.1007/s00134-018-5218-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi H, Wan J, Xu SP, Liao L. [A retrospective analysis of clinical characteristics and mortality risks in elderly patients with acute cholecystitis and cholangitis] Zhonghua Nei Ke Za Zhi. 2019 Jun;58((6)):415–8. doi: 10.3760/cma.j.issn.0578-1426.2019.06.003. Available from: https://doi-org.libproxy1.nus.edu.sg/10.3760/cma.j.issn.0578-1426.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 33.Tan M, Schaffalitzky de Muckadell OB, Laursen SB. Unchanged mortality in patients with acute cholangitis despite an increase in malignant etiologies - a 25-year epidemiological study. Scand J Gastroenterol. 2019 Mar;54((3)):335–41. doi: 10.1080/00365521.2019.1585568. Available from: https://doi-org.libproxy1.nus.edu.sg/10.1080/00365521.2019.1585568. [DOI] [PubMed] [Google Scholar]