Abstract
This study uses data from the Surveillance Epidemiology and End Results database to perform an age- and sex-specific time trend analysis of pancreatic cancer incidence rates in US adults.
Pancreatic cancer is the fourth leading cause of cancer death with a 5-year survival rate of approximately 10%.1 Noncomparative data from 2013 showed increasing incidence of pancreatic cancer in older White women and men and in younger non-Hispanic White women.2 However, there are limited data on recent trends in pancreatic cancer incidence.
The aim of this study was to perform an age- and sex-specific time trend analysis of pancreatic cancer incidence rates.
Methods
Pancreatic cancer incidence rates per 100 000 population were obtained (age-adjusted to the 2000 US population and adjusted for reporting delay) for 2000 to 2018 from the Surveillance, Epidemiology, and End Results (SEER) database. The SEER program collects information from cancer registries covering 37% of US population. The Cedars-Sinai institutional review board exempted the study because the data were deidentified and publicly available.
Time trends were quantified using Monte Carlo permutation analysis to fit the simplest joinpoint model using the incidence rate data.3 Annual percentage change and average annual percentage change (AAPC) were calculated. A 2-sided t test was performed to evaluate if annual percentage change was significant. Pairwise comparison between trends was performed to assess identicalness and equality.4
Age- and sex-specific analyses also were conducted. Younger adults were defined as those younger than 55 years and older adults were defined as those aged 55 years or older.5 A post hoc analysis also was performed for individuals aged 15 to 34 years and for those aged 35 to 54 years.
Version 8.3.9 of the SEER*Stat program (National Cancer Institute) and version 4.9 of the Joinpoint Regression program (National Cancer Institute) were used. A 2-sided P < .05 was considered statistically significant for the overall group. Multiple testing correction was used and P < .025 was considered statistically significant for the analyses of the younger and older age groups and P < .0125 was considered statistically significant for the age groups of 15 to 34 years and 35 to 54 years.
Results
From 2000 to 2018, a total of 283 817 cases of pancreatic cancer (50% women) were reported. Overall, the AAPCs of the total pancreatic cancer cases significantly increased in women (0.78% [95% CI, 0.68%-0.88%]; P < .001) and in men (0.90% [95% CI, 0.82%-0.99%]; P < .001) without a statistically significant between-group difference (0.12% [95% CI, 0%-0.25%]; P = .06).
The stratified analysis of different age groups showed significant variations in trends. Among individuals aged 55 years or older (251 360 cases; 51% women), the AAPCs increased among women (0.62% [95% CI, 0.51%-0.74%]; P < .001) and men (0.92% [95% CI, 0.82%-1.01%]; P < .001) with the nonequal trends (P < .001) suggesting a greater increase among men. The incidence rates were much lower among individuals younger than 55 years (32 369 cases [11.4% of cases]; 43% women) and the trends were reversed for sex-based incidence rates. A significantly greater relative increase in incidence (AAPC) occurred among women younger than 55 years (1.93% [95% CI, 1.57%-2.28%]; P < .001) compared with men younger than 55 years (0.77% [95% CI, 0.50%-1.05%]; P < .001) with nonequal trends (P = .002) (Table).
Table. Sex-Specific Trends for Pancreatic Cancer in the US Among Different Age Groups.
| Cancer cases (N = 283 817)a | Average annual percentage change (95% CI)b | Between-group difference, % (95% CI)c | P valued | |||
|---|---|---|---|---|---|---|
| Between-group difference | Coincidencee | Equalityf | ||||
| All ages | ||||||
| Men | 141 520 (49.8) | 0.90 (0.82-0.99) | 0.12 (0 to 0.25) | .06 | <.001 | .02 |
| Women | 142 297 (50.1) | 0.78 (0.68-0.88) | ||||
| Aged ≥55 y | ||||||
| Men | 123 156 (43.3) | 0.92 (0.82-1.01) | 0.29 (0.16 to 0.43) | <.001 | <.001 | <.001 |
| Women | 128 204 (45.1) | 0.62 (0.51-0.74) | ||||
| Aged <55 y | ||||||
| Men | 18 334 (6.5) | 0.77 (0.50-1.05) | −1.16 (−1.57 to −0.74) | <.001 | <.001 | .002 |
| Women | 14 035 (4.9) | 1.93 (1.57-2.28) | ||||
| Aged 35-54 y | ||||||
| Men | 17 692 (6.2) | 0.65 (0.38-0.91) | −0.91 (−1.29 to −0.53) | <.001 | <.001 | .004 |
| Women | 13 139 (4.6) | 1.56 (1.24-1.87) | ||||
| Aged 15-34 y | ||||||
| Men | 642 (0.2) | 4.20 (2.54-5.90) | −3.48 (−5.56 to −1.39) | .001 | <.001 | .01 |
| Women | 896 (0.3) | 7.68 (6.21-9.18) | ||||
Data are expressed as No. (%). The percentages in each row were calculated using the total number of cases as the denominator.
Trends were quantified using version 4.9 of the Joinpoint Regression program (National Cancer Institute). Up to 4 joinpoints (5 line segments) were allowed. No joinpoints were identified. The annual percentage change over the whole period (2000-2018) is identical to the average among all subgroups.
A negative value was associated with a greater average annual percentage change in women.
Multiple testing correction was applied as follows: P < .05 was considered statistically significant for the all ages group, P < .025 was considered statistically significant for the age groups of 55 years or older and younger than 55 years, and P < .0125 was considered statistically significant for the age groups of 35 to 54 years and 15 to 34 years.
Tests whether sex-specific trends were identical. A significant P value indicates that the trends were not identical (ie, they had different incidence rates and coincidence was rejected).
Tests whether sex-specific trends were equal. A significant P value indicates that the trends were not equal (ie, they had different incidence rates and equality was rejected).
To evaluate this trend reversal further, the group of individuals younger than 55 years was divided into 2 equal age subgroups: 35 to 54 years (30 831 cases; 10.9%) and 15 to 34 years (1538 cases; 0.54%). Women aged 35 to 54 years had a greater relative increase in incidence rates (13 139 cases; AAPC, 1.56% [95% CI, 1.24%-1.87%]; P < .001) than men (17 692 cases; AAPC, 0.65% [95% CI, 0.38%-0.91%]; P < .001) with nonequal trends (P = .004). Women aged 15 to 34 years had a greater relative increase (896 cases; AAPC, 7.68% [95% CI, 6.21%-9.18%]; P < .001) than men (642 cases; AAPC, 4.20% [95% CI, 2.54%-5.90%]; P < .001) with nonequal trends (P = .01) (Table and Figure).
Figure. Sex-Specific Trends and Age-Adjusted Incidence Rates per 100 000 Population for Pancreatic Cancer in the US Among Different Age Groups.
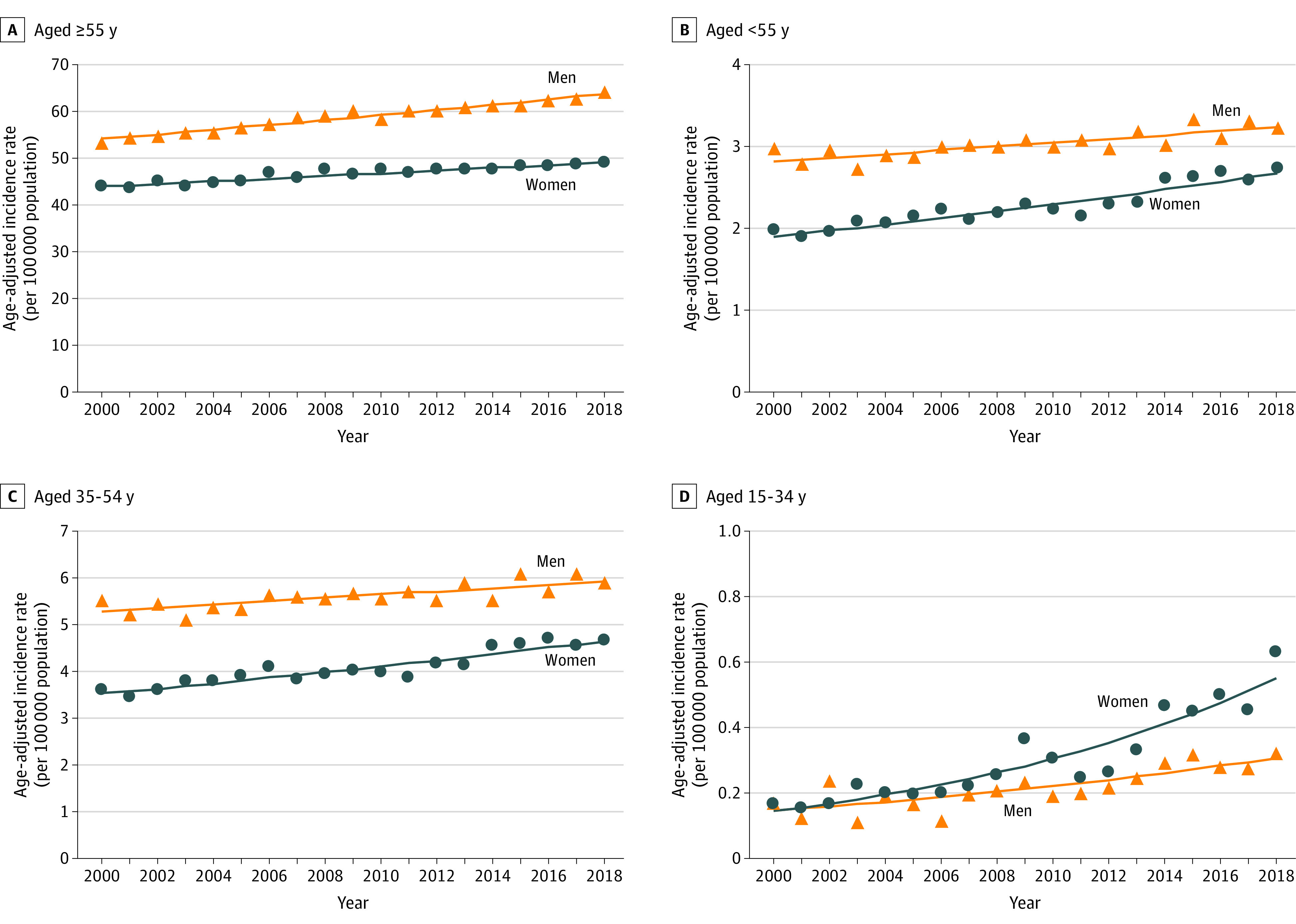
A, The average annual percentage change in men increased at a greater rate compared with women (0.92% vs 0.62%, respectively; P < .001 indicating nonequal trends) among people older than 55 years. B, The average annual percentage change in women relatively increased at a greater rate compared with men (1.93% vs 0.77%, respectively; P = .002 indicating nonequal trends) among people younger than 55 years. C, The average annual percentage change in women relatively increased at a higher rate compared with men (1.56% vs 0.65%, respectively; P = .004 indicating nonequal trends) among people aged 35 to 54 years. D, The average annual percentage change in women relatively increased at a greater rate compared with men (7.68% vs 4.20%, respectively; P = .01 indicating nonequal trends) among people aged 15 to 34 years.
Discussion
This study found that pancreatic cancer incidence increased among both sexes between 2000 and 2018. However, a greater relative increase was observed among women younger than 55 years, especially among those aged 15 to 34 years. Even though the reason for this relative increasing trend among younger women is unclear, it may imply a sex-based disproportional exposure to known or unknown risk factors. The observed trend can offer clues to researchers to gain better insight into pathogenesis of pancreatic cancer.
A limitation of this study is the small number of patients with pancreatic cancer who were younger than 55 years. In addition, limited covariates and coding reliability have been noted in the SEER database.6 Future studies should validate these findings in other large population-based cohorts.
Section Editors: Jody W. Zylke, MD, Deputy Editor; Kristin Walter, MD, Associate Editor.
References
- 1.National Cancer Institute; Surveillance, Epidemiology, and End Results Program . Cancer stat facts: pancreatic cancer. Accessed September 28, 2021. https://seer.cancer.gov/statfacts/html/pancreas.html
- 2.Gordon-Dseagu VL, Devesa SS, Goggins M, Stolzenberg-Solomon R. Pancreatic cancer incidence trends: evidence from the Surveillance, Epidemiology and End Results (SEER) population-based data. Int J Epidemiol. 2018;47(2):427-439. doi: 10.1093/ije/dyx232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335-351. doi: [DOI] [PubMed] [Google Scholar]
- 4.Kim HJ, Fay MP, Yu B, Barrett MJ, Feuer EJ. Comparability of segmented line regression models. Biometrics. 2004;60(4):1005-1014. doi: 10.1111/j.0006-341X.2004.00256.x [DOI] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A. Colorectal cancer mortality rates in adults aged 20 to 54 years in the United States, 1970-2014. JAMA. 2017;318(6):572-574. doi: 10.1001/jama.2017.7630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park HS, Lloyd S, Decker RH, Wilson LD, Yu JB. Limitations and biases of the Surveillance, Epidemiology, and End Results database. Curr Probl Cancer. 2012;36(4):216-224. doi: 10.1016/j.currproblcancer.2012.03.011 [DOI] [PubMed] [Google Scholar]


