Abstract
Objective
Fecal calprotectin (FC) is a promising marker for assessment of inflammatory bowel disease (IBD) activity. However, the utility of FC for predicting mucosal healing (MH) of IBD patients has yet to be clearly demonstrated. The objective of our study was to perform a meta-analysis evaluating the diagnostic accuracy of FC in predicting MH of IBD patients.
Methods
We systematically searched the databases for studies from inception to April 2020 that evaluated MH in IBD. The methodological quality of each study was assessed according to the Quality Assessment of Diagnostic Accuracy Studies checklist. The extracted data were pooled using a summary receiver operating characteristic curve model. Random-effects model was used to summarize the diagnostic odds ratio, sensitivity, specificity, positive likelihood ratio, and negative likelihood ratio.
Results
Sixteen studies comprising 1,682 ulcerative colitis (UC) patients and 4 studies comprising 221 Crohn's disease (CD) patients were included. The best performance of FC for predicting MH in UC was at cut-off range of 60–75 μg/g with area under the curve (AUC) of 0.88 and pooled sensitivity and specificity of 0.87 and 0.79, respectively. The pooled sensitivity and specificity values of cutoff range 180–250 μg/g for predicting MH in CD were 0.67 and 0.76, respectively. The AUC of 0.79 also revealed improved discrimination for identifying MH in CD with FC concentration.
Conclusion
Our meta-analysis has found that FC is a simple, reliable noninvasive marker for predicting MH in IBD patients. FC cutoff range 60–75 μg/g appears to have the best overall accuracy in UC patients.
Keywords: Inflammatory bowel disease, Ulcerative colitis, Fecal calprotectin, Fecal immunochemical test, Diagnostic accuracy, Meta-analysis
Introduction
Inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Crohn's disease (CD), have been empirically confirmed by clinical, pathological, endoscopic, and radiological features. The incidence and prevalence of IBD is increasing in worldwide [1]. The clinical course of IBD is characterized by repetitive cycles of clinical remissions and relapses. The patient's quality of life would be significantly affected during the relapse phrase. It is important to evaluate if the previous treatment is effective through clinical disease activity indices such as Mayo score, Crohn's Disease Activity Index (CDAI), and Harvey Bradshaw Index (HBI). However, the evaluation of symptom scoring for IBD patients is frequently incongruent with actual endoscopic findings. For example, Peyrin-Biroulet et al. [2] found that only 53% of patients with clinical remission by CDAI had achieved mucosal healing (MH). Recently, treatment of IBD is aimed at achieving MH, because the reduced risk of relapse has been shown in IBD patients with MH [2]. MH is defined as Mayo endoscopic subscore (MES) 0/0–1 or UC Endoscopic Index of Severity (UCEIS) score 0/0–1 or histologic remission for UC, Simple Endoscopic Score for CD (SES-CD) of 0 for CD. At the same time, the targeting of MH, rather than clinical remission, is related to improved long-term clinical outcomes for IBD, promoting the reduced risk of surgery, steroid dependence, and hospitalization, while advancing deep and sustained remission [3]. Therefore, evaluating the intestinal mucosa of IBD patients in clinical remission appears to be an important approach to tailoring therapy and predicting prognosis.
Nowadays, endoscopy remains the gold standard for assessment of intestinal inflammation of IBD [4]. However, endoscopy is not an ideal method for patients to experience repeated inspection because it is invasive, inconvenient, expensive, and related to a small but significant risk of procedural complications. Serum markers such as C-reactive protein, erythrocyte sedimentation rate, and leukocytes are used to reflect systemic inflammation of host responses. The disadvantage of these markers is not being specific for intestinal inflammation [5]. Hence, there is an urgent need for a reliable, convenient, rapid, inexpensive, reproducible, standardized noninvasive marker for MH in IBD. Fecal markers are highly accurate for detecting the mucosal inflammation and disease prognosis [6, 7]. Besides, they are also useful for detecting the lesions in the small intestine, which could not be easily observed with endoscopy [6, 7].
Fecal markers for mucosal inflammation are a potential noninvasive alternative that could be used to evaluate MH. The common fecal marker is fecal calprotectin (FC). FC is a cytosolic granulocyte protein associated with neutrophil migration to the intestinal tract. It is sensitive for identifying mucosal inflammation, prognosticating treatment response, and predicting disease relapse. However, the specificity of FC for MH has not been validated. Although the utility of FC in IBD has been evaluated in some studies, its exact role has yet to be quantified [8, 9, 10, 11, 12]. In this meta-analysis, we aim to evaluate the overall diagnostic accuracy of FC for predicting MH in IBD patients.
Methods
Literature Search
We comprehensively and systematically searched the databases including Medline (OvidSP), Web of Science, PubMed, Cochrane Library, Embase, and conference proceedings for eligible studies from inception to April 2020 that evaluated MH in IBD by FC. Language restrictions were not used. The search strategy used the following terms: (1) FC: “Fecal calprotectin”, “Faecal calprotectin”, “FC”, “FCP”; (2) IBD: “inflammatory bowel disease”, “IBD”, “ulcerative colitis”, “UC”, ”Crohn's disease”, “CD”, “colitis”, “enteritis”, “intestinal inflammation”. References and reviews of related literature were searched manually.
Study Selection
Articles were first screened by 2 independent reviewers (Cong Dai and Bing-Jie Xiang) based on the title and abstract. The full text of a potentially eligible study was then assessed independently. Disagreements were resolved by discussion. A study was included if it met the inclusion criteria as follows: (1) the study evaluated FC for predicting MH in IBD patients; (2) an endoscopic scoring system was used as reference standard to assess MH; (3) the study provided sufficient details to calculate true-positive (TP), false-positive (FP), false-negative (FN) and true-negative (TN) results.
Data Extraction and Quality Assessment
The 2 investigators (Cong Dai and Bing-Jie Xiang) who performed the database searches also independently extracted the relevant data. The retrieved data included the authors, the publication year, country, age, the reference criteria, patient characteristics, and the FC features (method, cutoff). The TP, FP, FN, and TN values were calculated for each included study.
The methodological quality of the included studies was independently assessed by 2 authors (Cong Dai and Bing-Jie Xiang) using the QUADAS-2 (Quality Assessment of Diagnostic Accuracy Studies) tool [13]. The QUADAS-2 tool comprises 4 key domains: patient selection, index test, reference standard, and flow and timing. Each domain is assessed in terms of the risk of bias, and the first 3 domains are additionally assessed in terms of concerns regarding applicability [14]. In any case of disagreement, a consensus was reached by consultation with the senior reviewer (Ming-Jun Sun).
Data Synthesis and Statistical Analysis
The standard methods were used in this meta-analysis as recommended in the Cochrane handbook for systematic reviews of diagnostic test accuracy. For each study, sensitivity, specificity, the positive likelihood ratio (PLR), the negative likelihood ratio (NLR), and the diagnostic odds ratio (DOR) were calculated. The DOR combines both the PLRs and NLRs and is a global measure of test performance. A higher DOR value represents better discriminatory performance. For the data analysis, we estimated both summary receiver operating characteristic (SROC) curves and average operating points with each commonly applied threshold value, as they may complement each other in providing clinically useful summaries and powerful ways of detecting effects. An SROC curve with 95% confidence region and 95% prediction region was performed to examine the interaction between sensitivity and specificity. DOR and the area under the SROC curve (AUC) were calculated to evaluate the diagnostic performance of FC for MH in IBD patients. A DOR of 1 indicates that the test cannot discriminate between patients with MH and non-MH UC patients. A higher value indicates better test performance. AUC equals 1 for a perfect test and 0.5 for a completely uninformative test. When AUC was 0.5–0.7, it revealed there was a certain diagnostic efficacy. AUC >0.8 signified that diagnostic efficacy was outstanding, and AUC >0.9 signified that diagnostic efficacy was significantly outstanding. Pooled sensitivity, specificity, and their corresponding 95% CIs were calculated using a random effects model at each threshold. The heterogeneity was detected by a chi-square test or Q-statistic and Higgins I-squared statistic (I2). A p value of <0.1 was considered statistically significant heterogeneity for the chi-square or Q-statistics. The percentage of I2 represented the degree of heterogeneity. I2 percentages of 25, 50, and 75% indicated a low, moderate, and high degree of heterogeneity, respectively. We resolved the heterogeneity by sensitivity analysis. Publication bias was assessed using Deeks' test. p < 0.05 was considered to indicate statistically significant publication bias. We performed statistical analysis on Stata (version 14) and Review Manager (version 5.3).
Results
Study Characteristics
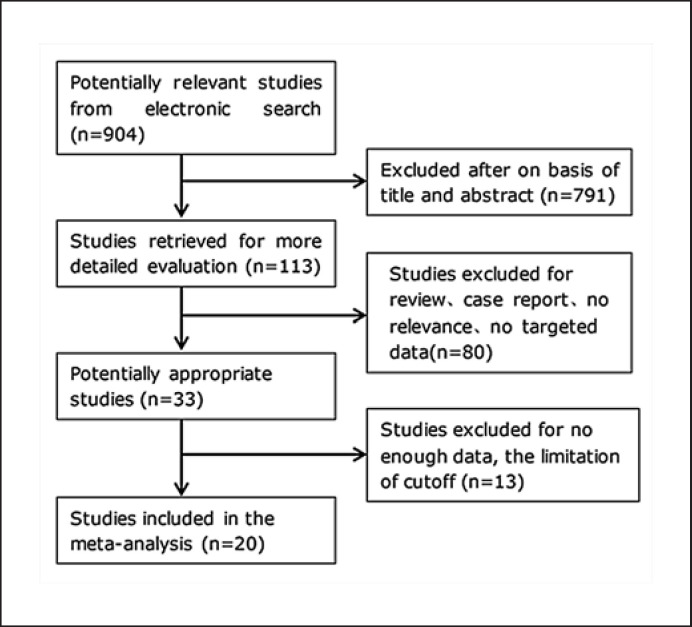
As Figure 1 shows, 904 articles are available after the initial search. After reading the titles and abstracts and reviewing the full texts, 16 publications including 1,682 UC patients and 4 publications including 221 CD patients were included in the final analysis. The clinical characteristics of the included studies are listed in Table 1. All studies enrolled patients with diagnosed UC or CD. Most studies except 3 studies [15, 16, 17] used a prospective study design. Seven of the studies were conducted in Asia (4 studies in Japan [8, 9, 11, 18], 3 studies in Korea [17, 19, 20]), 6 of the studies were in Europe (1 in Belgium [21], 3 in Norway [22, 23, 24], 2 in Denmark [15, 25]), 1 of the studies was in Canada [10], 2 of the studies were in the USA [16, 26], 1 of the studies was in Australia [27], and 3 of the studies were multicenter [28, 29, 30]. Reference standards of most included studies were based on endoscopy, including MES, UCEIS in MH evaluation of UC, and SES-CD in MH evaluation of CD. Reference standard of 2 studies [25, 26] was based on histologic inflammatory activity. Taking limitation of study number, cut-off of endoscopic score, and cut-off of FC into consideration, we evaluated 7 FC level cut-off ranges in UC as per availability of reports in the included studies: 25–50, 60–75, 96–125, 150–180, 192–201, 170–200, 250–259 μg/g. We evaluated 1 FC level cut-off range (180–250 μg/g) in CD as per availability of reports in the included studies. When MH was defined as MES 0, 4 studies [15, 24, 28, 30] (MH was based on histologic activity evaluation in 1 study[25]) about FC with cut-off range of 25–50 μg/g, 5 studies [16, 20, 22, 28, 30] about FC with cut-off range of 60–75 μg/g, 4 studies [20, 22, 24, 28] about FC with cut-off range of 96–125 μg/g, 5 studies [11, 17, 19, 28, 30] about FC with cutoff range of 150–180 μg/g, and 6 studies [8, 15, 19, 26, 28, 29] about FC with cut-off range of 192–201 μg/g for predicting MH in UC patients were included in the final meta-analysis (Table 1). When MH was defined as MES 0–1 or UCEIS 0–1, 4 studies [17, 26, 28, 29] (MH was based on histologic activity evaluation in 1 study [26]) about FC with cut-off range of 170–200 μg/g and 6 studies [8, 21, 22, 23, 26, 28] about FC with cut-off range of 250–259 μg/g for predicting MH in UC patients were included in the final meta-analysis (Table 1). When MH was defined as SES-CD 0, 4 studies [9, 10, 18, 27] about FC with cut-off range of 180–250 μg/g for predicting MH in CD patients were included in the final meta-analysis (Table 1).
Fig. 1.
Flow diagram of articles retrieved and inclusion progress through the stage of meta-analysis.
Table 1.
Characteristics of included studies
| Study | Year | Country | Age, years | Type | Criteria | FC, µg/g | Method | TP | FP | FN | TN |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Carlsen et al. [30] | 2018 | Multicenter | 44 (29–52) | Prospective | MES 0 | 25 | ELISA | 10 | 6 | 15 | 64 |
| Goll et al. [24] | 2019 | Norway | NA | Prospective | MES 0 | 28 | ELISA | 16 | 11 | 26 | 51 |
| Theede et al. [25] | 2016 | Denmark | 39.3 (13.92) | Prospective | HS 0 | 45 | ELISA | 28 | 0 | 33 | 8 |
| Sandborn et al. [28] | 2016 | Multicenter | 39.9±15.1 | Prospective | MES 0 | 50 | ELISA | 29 | 77 | 4 | 84 |
| Patel et al. [16] | 2017 | USA | 26 (2–56) | Retrospective | PRO2 0 + MES 0 | 60 | ELISA | 12 | 2 | 2 | 52 |
| Kristensen et al. [22] | 2015 | Norway | 35.5 (18–72) | Prospective | MES 0 | 61 | ELISA | 37 | 3 | 7 | 15 |
| Kim et al. [20] | 2020 | Korea | 50 (18–81) | Prospective | MES 0 | 70 | ELISA | 58 | 18 | 7 | 44 |
| Carlsen et al. [30] | 2018 | Multicenter | 44 (29–52) | Prospective | MES 0 + Geboes 0/1 | 74 | ELISA | 11 | 26 | 1 | 51 |
| Sandborn et al. [28] | 2016 | Multicenter | 39.9±15.1 | Prospective | MES 0 | 75 | ELISA | 28 | 53 | 5 | 108 |
| Kristensen et al. [22] | 2015 | Norway | 35.5 (18–72) | Prospective | MES 0 | 96 | ELISA | 40 | 3 | 4 | 15 |
| Kim et al. [20] | 2020 | Korea | 50 (18–81) | Prospective | MES 0 | 100 | ELISA | 58 | 21 | 7 | 41 |
| Sandborn et al. [28] | 2016 | Multicenter | 39.9±15.1 | Prospective | MES 0 | 100 | ELISA | 27 | 53 | 6 | 108 |
| Goll et al. [24] | 2019 | Norway | NA | Prospective | MES 0 | 100 | ELISA | 36 | 25 | 6 | 37 |
| Lee et al. [19] | 2019 | Korea | 48.4±13.2 (23–80) | Prospective | MES 0 | 150 | QPOCT | 6 | 0 | 1 | 22 |
| Carlsen et al. [30] | 2018 | Multicenter | 44 (29–52) | Prospective | MES 0 + Geboes 0/1 | 150 | ELISA | 11 | 36 | 1 | 41 |
| Sandborn et al. [28] | 2016 | Multicenter | 39.9±15.1 | Prospective | MES 0 | 150 | ELISA | 26 | 41 | 7 | 120 |
| Ryu et al. [17] | 2019 | Korea | 47.2 (16–78) | Retrospective | MES 0 | 170 | ELISA | 40 | 31 | 11 | 92 |
| Hiraoka et al. [11] | 2018 | Japan | 32 (22–43) | Prospective | MES 0 | 180 | ELISA | 84 | 24 | 38 | 75 |
| Theede et al. [15] | 2015 | Denmark | 19–79 | Retrospective | MES 0 | 192 | ELISA | 24 | 10 | 8 | 78 |
| Yamaguchi et al. [29] | 2016 | Multicenter | 45 (36–54) | Prospective | MES 0 | 194 | ELISA | 37 | 22 | 15 | 31 |
| Takashima et al. [8] | 2015 | Japan | 35.5 (14–77) | Prospective | MES 0 | 200 | ELISA | 34 | 17 | 10 | 44 |
| Sandborn et al. [28] | 2016 | Multicenter | 39.9±15.1 | Prospective | MES 0 | 200 | ELISA | 23 | 29 | 10 | 132 |
| Mak et al. [26] | 2018 | USA | 36.0±18.7 | Prospective | MES 0 | 200 | ELISA | 4 | 11 | 1 | 45 |
| Lee et al. [19] | 2019 | Korea | 48.4±13.2 (23–80) | Prospective | MES 0 | 201 | ELISA | 5 | 0 | 2 | 22 |
| Ryu et al. [17] | 2019 | Korea | 47.2 (16–78) | Retrospective | UCEIS 0/1 | 170 | ELISA | 44 | 27 | 15 | 98 |
| Sandborn et al. [28] | 2016 | Multicenter | 39.9±15.1 | Prospective | MES 0–1 | 200 | ELISA | 26 | 69 | 7 | 92 |
| Yamaguchi et al. [29] | 2016 | Multicenter | 45 (36–54) | Prospective | MES 0–1 | 200 | ELISA | 63 | 1 | 31 | 10 |
| Mak et al. [26] | 2018 | USA | 36.0±18.7 | Prospective | HS | 200 | ELISA | 5 | 13 | 2 | 41 |
| Takashima et al. [8] | 2015 | Japan | 35.5 (14–77) | Prospective | MES 0–1 | 250 | ELISA | 45 | 14 | 19 | 27 |
| Kristensen et al. [23] | 2017 | Norway | 31 (18–60) | Prospective | MES 0–1 | 250 | ELISA | 16 | 0 | 3 | 1 |
| Sandborn et al. [28] | 2016 | Multicenter | 39.9±15.1 | Prospective | MES 0–1 | 250 | ELISA | 25 | 68 | 8 | 93 |
| D'Haens et al. [21] | 2012 | Belgium | 38 (30–46) | Prospective | MES 0/1 | 250 | ELISA | 14 | 3 | 4 | 18 |
| Mak et al. [26] | 2018 | USA | 36.0±18.7 | Prospective | MES 0–1 | 250 | ELISA | 3 | 13 | 2 | 43 |
| Kristensen et al. [22] | 2015 | Norway | 35.5 (18–72) | Prospective | MES 0–1 | 259 | ELISA | 25 | 9 | 5 | 23 |
| Arai et al. [18] | 2017 | Japan | 31.8 (15–69) | Prospective | SES-CD 0 | 180 | ELISA | 4 | 1 | 6 | 13 |
| Inokuchi et al. [9] | 2016 | Japan | 32 (22–43) | Prospective | SES-CD 0 | 180 | ELISA | 20 | 14 | 3 | 34 |
| Ma et al. [10] | 2017 | Canada | 47.9 (22–75) | Prospective | SES-CD 0 | 215 | ELISA | 36 | 12 | 7 | 31 |
| Zubin and Peter [27] | 2015 | Australia | 13.5 (12.2–13.88) | Prospective | SES-CD 0 | 250 | ELISA | 11 | 4 | 15 | 10 |
QPOCT, quantitative point-of-care test; ELISA, enzyme-linked immunosorbent assay; PRO2, patient-reported outcome measures; HS, histologic inflammatory activity score.
Methodological Quality Assessment
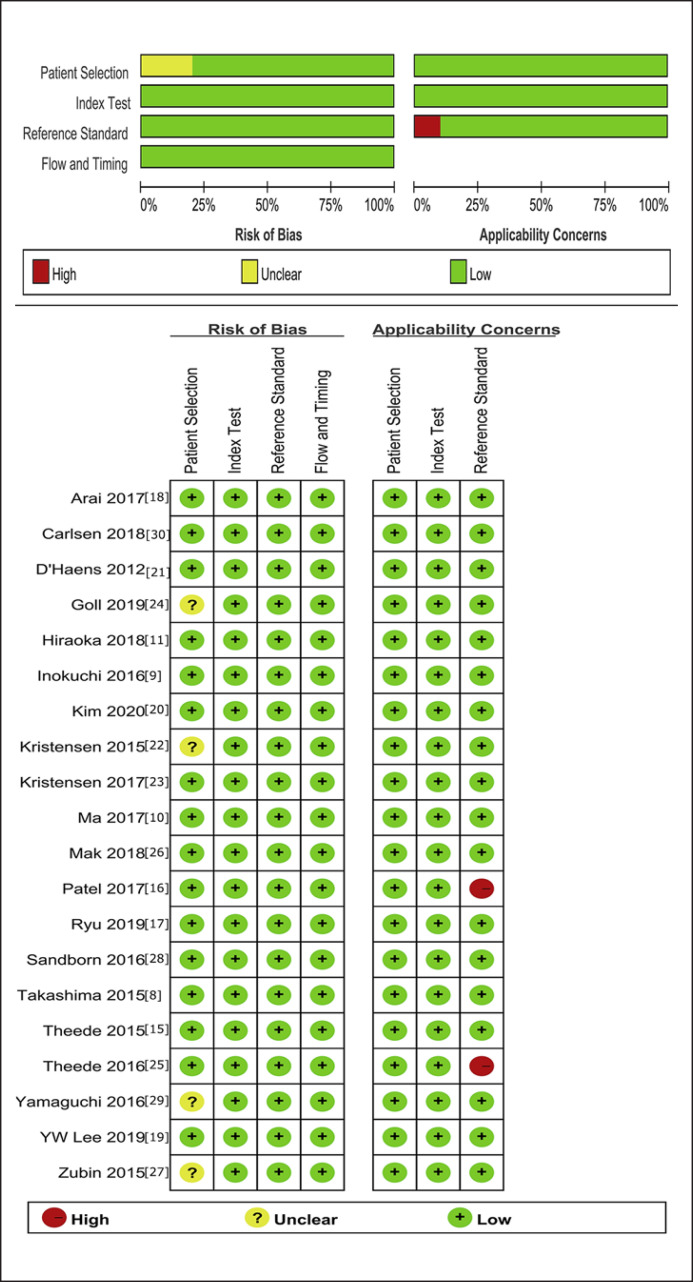
The 20 studies underwent quality assessment using the aforementioned QUADAS-2 tool. A summary of the results is presented in Figure 2. Most studies were deemed to have a representative spectrum of patients. The criteria for both MH were present in Table 1. Specifically, 2 studies [25, 26] defined MH in UC as histologic remission, and 1 study [16] defined MH in UC as MES 0 and patient-reported outcome measures (PRO2) 0. One study [30] defined MH in UC as MES 0 and Geboes 0/1. There was no evidence of commercial funding in any of the studies; however, not all of the manuscripts explicitly verified this with a funding statement.
Fig. 2.
Methodological quality graph and summary of the included studies based on the Cochrane handbook. +, low risk; −, high risk;?, unclear.
Diagnostic Accuracy Meta-Analyses
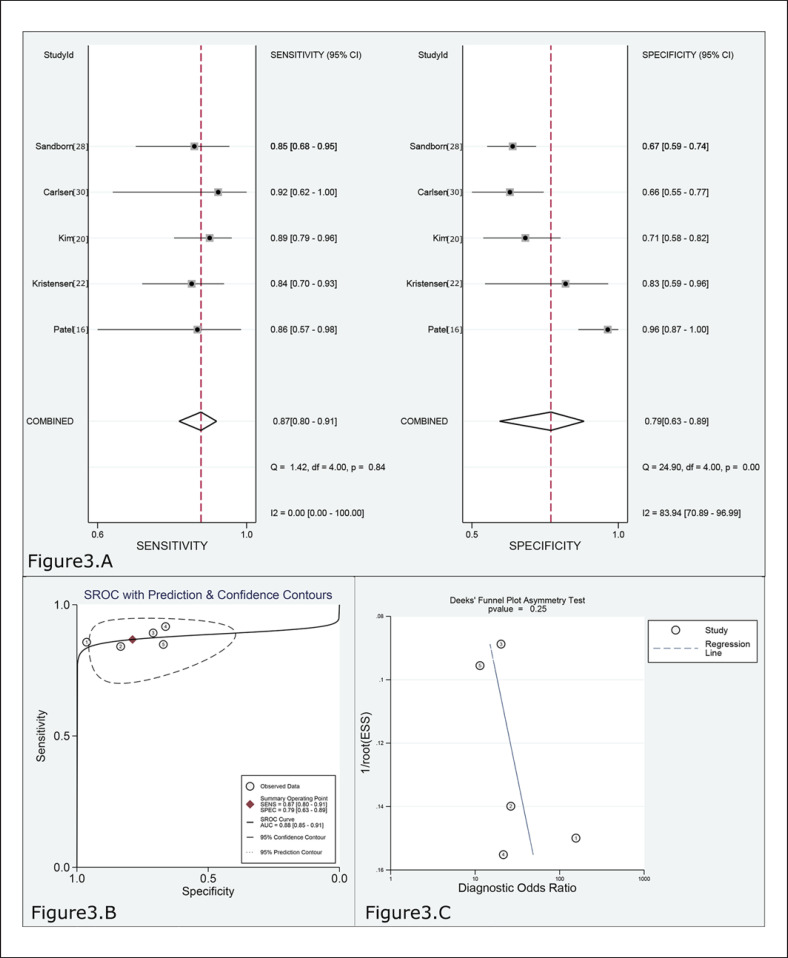
The pooled sensitivity and specificity values of 60–75 μg/g for predicting MES 0 in UC were 0.87 (95% CI 0.80–0.91, 5 studies, n = 540) and 0.79 (95% CI 0.63–0.89), respectively (Fig. 3A, Table 2). The FC level had a high rule-in value (PLR 4.1; 95% CI 2.2–7.5) and a low rule-out value (NLR 0.17; 95% CI 0.11–0.25) for predicting MH in UC. The results of the ROC curve analysis (AUC 0.88, 95% CI 0.85–0.91; Fig. 3B, Table 2) and DOR (24, 95% CI 11–56) also revealed outstanding discrimination for predicting MH in UC with FC concentration. I2 was 59% (p = 0.043); thus, the sensitivity analysis was performed after excluding 1 study and obtained the following results (online suppl. Fig. 2; for all online suppl. material, see www.karger.com/doi/10.1159/000514196). The pooled sensitivity and specificity were 0.88 (95% CI 0.80–0.93) and 0.78 (95% CI 0.59–0.90), respectively. PLR and NLR were 4.0 (95% CI 2.0–8.1) and 0.16 (95% CI 0.09–0.26), respectively. AUC and DOR were 0.89 (95% CI 0.86–0.91) and 25 (95% CI 9–71), respectively.
Fig. 3.
A Forest plot of the sensitivity and specificity for predicting MH in UC by FC. B SROC curve for predicting MH in UC by FC. C Deeks' funnel plot for evaluating publication bias (cut-off range = 60–75 μg/g).
Table 2.
Summary of analysis results
| Criteria | FC | Sensitivity | Specificity | DOR | PLR | NLR | AUC | I2, % |
|---|---|---|---|---|---|---|---|---|
| MES 0, HS 0 | 25–50 | 0.54 | 0.81 | 5 | 2.9 | 0.56 | 0.76 | 96 |
| MES 0 | 60–75 | 0.87 | 0.79 | 24 | 4.1 | 0.17 | 0.88 | 59 |
| MES 0 | 60–75* | 0.88 | 0.78 | 25 | 4.0 | 0.16 | 0.89 | 0 |
| MES 0 | 96–125 | 0.87 | 0.66 | 14 | 2.6 | 0.19 | 0.86 | 0 |
| MES 0 | 150–180 | 0.75 | 0.75 | 9 | 3.0 | 0.33 | 0.81 | 60 |
| MES 0 | 150–180* | 0.80 | 0.78 | 15 | 3.7 | 0.25 | 0.82 | 0 |
| MES 0 | 192–201 | 0.74 | 0.82 | 12 | 4.0 | 0.32 | 0.75 | 70 |
| MES 0 | 192–201* | 0.76 | 0.82 | 13 | 4.0 | 0.31 | 0.79 | 70 |
| MES/UCEIS 0–1/HS | 170–200 | 0.73 | 0.74 | 8 | 2.8 | 0.37 | 0.78 | 68 |
| MES 0–1 | 250–259 | 0.76 | 0.70 | 8 | 2.6 | 0.34 | 0.79 | 0 |
| SES-CD 0 | 180–250 | 0.67 | 0.76 | 7 | 2.9 | 0.43 | 0.79 | 79 |
Sensitivity analysis. MES, Mayo Endoscopic Subscore; HS, histologic inflammatory activity score.
The diagnostic performances of other values including 25–50 μg/g, 96–125 μg/g, 150–180 μg/g, 192–201 μg/g, 170–200 μg/g, and 250–259 μg/g in predicting MH of UC are presented in Table 2 and online supplementary Figures 1, 3–9.
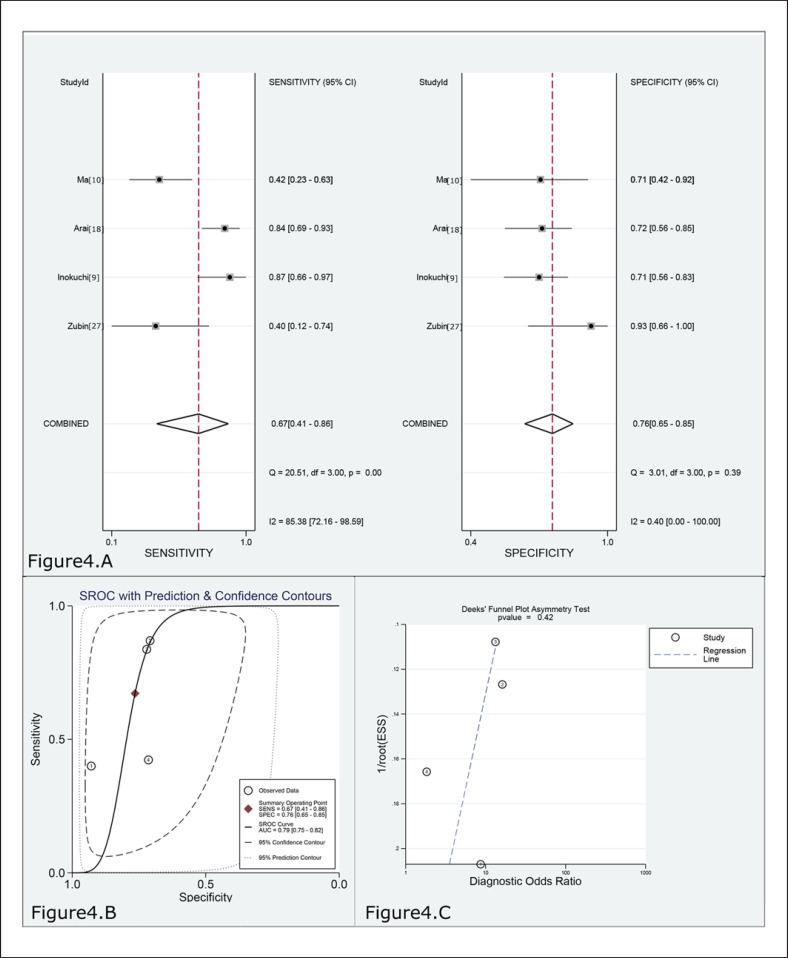
The pooled sensitivity and specificity values of 180–250 μg/g for predicting SES-CD 0 in CD were 0.67 (95% CI 0.41–0.86, 4 studies, n = 221) and 0.76 (95% CI 0.65–0.85), respectively (Fig. 4A, Table 2). The FC level had a moderate rule-in value (PLR 2.9; 95% CI 1.8–4.4) and a moderate rule-out value (NLR 0.43; 95% CI 0.22–0.86) for predicting MH in CD. The results of the ROC curve analysis (AUC 0.79, 95% CI 0.75–0.82; Fig. 4B, Table 2) and DOR (7, 95% CI 2–18) revealed diagnostic discrimination for predicting MH in CD with FC concentration. I2 was 79% (p < 0.01), but the sensitivity analysis could not be performed due to quantitative limitation of included studies.
Fig. 4.
A Forest plot of the sensitivity and specificity for predicting MH in CD by FC. B SROC curve for predicting MH in CD by FC. C Deeks' funnel plot for evaluating publication bias (cut-off range = 180–250 μg/g).
Publication Bias
Funnel plots for the analyses of publication bias were performed to compare the yield of FC levels for assessing MH in UC or CD. The Deeks' tests revealed no evidence of publication bias in all analyses (p = 0.19–0.77; online suppl. Figs. 3–10).
Discussion
To our knowledge, the present study was the first meta-analysis of assessment of MH in IBD patients using an FC assay. In this study, through a robust systematic review and an appropriately performed meta-analysis, FC has the best performance for predicting MH in UC at cutoff range of 60–75 μg/g, achieving a high sensitivity (0.87, 95% CI 0.80–0.91) and high specificity (0.81, 95% CI 0.76–0.85). The estimated DOR for the FC in predicting MH of UC was 24 in our meta-analysis. This means that for the FC, the odds for positivity among subjects with MH of UC patients is 24 times higher than the odds for positivity among subjects without MH of UC patients. Compared with DOR, likelihood ratios including PLR and NLR are considered more clinically useful. In our meta-analysis, the pooled PLR and NLR were 4.10 and 0.17, respectively, suggesting that UC patients with MH are 4-fold more likely to have lower FC levels. In contrast, if the FC level of the UC patients is above the cut-off value, the probability of non-MH is 17%.
FC has a moderate sensitivity (0.67, 95% CI 0.41–0.86) and a high specificity (0.76, 95% CI 0.65–0.85) for predicting MH in CD. The estimated DOR for the FC in predicting MH of CD was 7 in our meta-analysis. This means that for the FC, the odds for positivity among subjects with MH of CD patients is 7 times higher than the odds for positivity among subjects without MH of CD patients. Compared with DOR, likelihood ratios including PLR and NLR are considered more clinically useful. In our meta-analysis, the pooled PLR and NLR were 2.9 and 0.43, respectively, suggesting that CD patients with MH are approximately 3-fold more likely to have lower FC levels. In contrast, if the FC level of the CD patients is above the cutoff value, the probability of non-MH is 43%.
The common fecal markers in IBD include FC, fecal lactoferrin (FL) and fecal immunochemical test (FIT). FIT measures the amount of blood from the lesion in intestinal tract, whereas the fundamental of FC and FL estimating inflammation activity in intestinal tract is associated with the amount of inflammatory cells [31]. Calprotectin, a calcium and zinc-binding protein of 36.5 kDa, represents 60% of cytosolic proteins in the granulocytes and it appears on the surface of granulocytes [32, 33]. The concentration of calprotectin in stools is therefore correlated with the amount of neutrophil infiltrating in the inflamed mucosa of IBD [33, 34]. Lactoferrin is a 76-kDa transferrin with high affinity to iron ions. It is excreted by the majority of mucosal membranes and isolated from various secretions, such as saliva, breast milk, tears, nasal secretions, stool, and serum [35, 36, 37]. Polymorphonuclear neutrophils migrate to the mucosa during the inflammatory process of gastrointestinal tract, which promotes the increase of lactoferrin concentration in feces [38]. In distinction to FC, the use of FL testing has been mainly limited to research, probably because stability of lactoferrin is shorter than calprotectin at room temperature. FIT is a technique based on measurement of gut hemoglobin, not specific for intestinal inflammation. Thus, the elevation of FIT levels will results from any cause of increased red blood cells in bowel. Previous studies have shown that FIT is helpful for identifying MH in UC [11, 12]. However, the present study showed the performance of FC at cut-off range of 60–75 μg/g for predicting MH was better than that of FIT in previous meta-analysis [39], in which FIT had a pooled sensitivity, specificity, and DOR of 0.77, 0.81, and 18.8 for predicting MH in UC. Given a comparable accuracy to FIT and FL, FC has potential to become a promising tool for predicting MH in IBD patients.
FC has some disadvantages in predicting MH for IBD. First, dietary supplements such as vitamin D, fatty acids, zinc, environmental influences (intestinal bacterial flora), and genetic influences can affect FC levels. Second, FC is not generally obtainable in most countries due to higher expense and non-reimbursement compared with FIT. FIT assessment costs USD 26 in China, whereas FC approximately costs USD 200 [31]. Third, FC is analyzed by ELISA and the measurement requires 5–10 g of fecal material [40] and specific skills. Besides, the process takes several hours to perform. The stool processing steps also take a lot of time. Nowadays, 2 types of point-of-care tests have been developed: semi-quantitative tests that provide a range of FC levels and a rapid quantitative point-of-care test (QPOCT), which is as fast as the semi-quantitative tests but provides an exact number as the ELISA test [41, 42]. A good correlation between QPOCT and ELISA test was observed in previous studies, which also explored the ability of QPOCT to predict endoscopic activity in IBD patients [19, 43, 44]. Recently, Vinding et al. [45] have validated a home-based rapid test in the laboratory, but not in the hand of patients. Elkjaer et al. [46] have developed of a rapid home test by CALPRO, Inc., Oslo, Norway, and evaluated its ability in their laboratory. CalproSmart, a further development of the rapid home test, is performed by the patients themselves and can get the result within minutes. The fast and reliable tool allows for evaluation of gut inflammation by the patient themselves and the results thereby constitute a valuable addition to clinical evidence. But its reliability can be affected by educational level, which signifies that thorough instruction, guidance, and follow-up are needed if the tool is implemented in clinical practice. But these FC tests are only carried out in the laboratory, and future trials are warranted to verify these value in clinical.
This meta-analysis successfully determined the optimum cut-off range of FC for identifying MH in UC and confirmed the diagnostic accuracy of FC for identifying MH in CD. However, this meta-analysis has several limitations. First, most of the included studies were carried out in tertiary centers. Thus, the study was limited to IBD population that were enrolled in the primary cares. The usage of FC test was limited due to this spectrum bias in primary cares with a lower activity probability. More multicenter large-sample randomized controlled trials (RCTs) and strict patient access systems are required to precisely investigate the predictive performance of FC in IBD patients. Second, although the endoscopic evaluation was used as reference standard, there is still no consensus between endoscopic scoring systems for UC. For example, one study defined MH using UCEIS [17], whereas other studies defined MH using MES [28, 29]. Moreover, the definition of MH was based on histologic evaluation in 2 included studies [25, 26]. And definition of MH involved clinical activity or histologic activity in 2 studies [16, 30]. Third, the standardized measurement methods of FC have not been established. Various assay kits such as Calprest [24] and Calprotectin [22] ELISA were used to examine FC in IBD patients in these studies. Fourth, further information about clinical characteristics of patients is unavailable, which hindered us from investigating sources of heterogeneity by disease severity (mild, moderate, or severe disease) and disease location (distal or proximal involvement). For example, CD could not be classified into distal colonic, terminal ileal, or small intestinal group in this study due to insufficient data. It is hoped that more widespread multicenter large samples and implementation of the Standards for the Reporting of Diagnostic Accuracy studies will enable readers to directly extract desired information for assessment.
In conclusion, our study revealed that FC is a simple, reliable noninvasive marker for predicting MH in IBD patients, especially in UC patients, and the best performance of FC cutoff range for predicting MH in UC was 60–75 μg/g. Further appropriately designed studies (especially RCTs) are required to verify such benefits and to find the best strategy of FC to predict MH in IBD patients.
Statement of Ethics
The paper is exempt from ethical committee approval because this study is a meta-analysis.
Conflict of Interest Statement
The authors declare that they have no competing interests.
Funding Sources
This research was supported by Liaoning Science and Technology Foundation (No. 20170541052).
Author Contributions
C.D. and B.-J.X. carried out the literature search, selection, validity assessment, data abstraction, and data analysis. C.D., B.-J.X., and M.J. wrote the paper and incorporated the comments from other authors and peer reviewers. C.D. and M.-J.S. had the original idea for the paper, formulated the protocol, and contributed to data abstraction and analysis. All authors reviewed and approved the final draft of the paper.
Supplementary Material
Supplementary data
Acknowledgement
All authors were involved in the conception and design of the study, acquisition of data, data analysis, and the drafting, revision, and approval of the final version of the manuscript.
References
- 1.Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018 Dec;390((10114)):2769–78. doi: 10.1016/S0140-6736(17)32448-0. [DOI] [PubMed] [Google Scholar]
- 2.Peyrin-Biroulet L, Ferrante M, Magro F, Campbell S, Franchimont D, Fidder H, et al. Scientific Committee of the European Crohn's and Colitis Organization Results from the 2nd Scientific Workshop of the ECCO. I: impact of mucosal healing on the course of inflammatory bowel disease. J Crohn's Colitis. 2011 Oct;5((5)):477–83. doi: 10.1016/j.crohns.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Reinink AR, Lee TC, Higgins PD. Endoscopic Mucosal Healing Predicts Favorable Clinical Outcomes in Inflammatory Bowel Disease: A Meta-analysis. Inflamm Bowel Dis. 2016 Aug;22((8)):1859–69. doi: 10.1097/MIB.0000000000000816. [DOI] [PubMed] [Google Scholar]
- 4.Oliva S, Di Nardo G, Hassan C, Spada C, Aloi M, Ferrari F, et al. Second-generation colon capsule endoscopy vs. colonoscopy in pediatric ulcerative colitis: a pilot study. Endoscopy. 2014 Jun;46((6)):485–92. doi: 10.1055/s-0034-1365413. [DOI] [PubMed] [Google Scholar]
- 5.Krzystek-Korpacka M, Neubauer K, Berdowska I, Boehm D, Zielinski B, Petryszyn P, et al. Enhanced formation of advanced oxidation protein products in IBD. Inflamm Bowel Dis. 2008 Jun;14((6)):794–802. doi: 10.1002/ibd.20383. [DOI] [PubMed] [Google Scholar]
- 6.Judd TA, Day AS, Lemberg DA, Turner D, Leach ST. Update of fecal markers of inflammation in inflammatory bowel disease. J Gastroenterol Hepatol. 2011 Oct;26((10)):1493–9. doi: 10.1111/j.1440-1746.2011.06846.x. [DOI] [PubMed] [Google Scholar]
- 7.Wright EK, Kamm MA, De Cruz P, Hamilton AL, Ritchie KJ, Keenan JI, et al. Comparison of Fecal Inflammatory Markers in Crohn's Disease. Inflamm Bowel Dis. 2016 May;22((5)):1086–94. doi: 10.1097/MIB.0000000000000671. [DOI] [PubMed] [Google Scholar]
- 8.Takashima S, Kato J, Hiraoka S, Nakarai A, Takei D, Inokuchi T, et al. Evaluation of Mucosal Healing in Ulcerative Colitis by Fecal Calprotectin Vs. Fecal Immunochemical Test. Am J Gastroenterol. 2015 Jun;110((6)):873–80. doi: 10.1038/ajg.2015.66. [DOI] [PubMed] [Google Scholar]
- 9.Inokuchi T, Kato J, Hiraoka S, Takashima S, Nakarai A, Takei D, et al. Fecal Immunochemical Test Versus Fecal Calprotectin for Prediction of Mucosal Healing in Crohn's Disease. Inflamm Bowel Dis. 2016 May;22((5)):1078–85. doi: 10.1097/MIB.0000000000000728. [DOI] [PubMed] [Google Scholar]
- 10.Ma C, Lumb R, Walker EV, Foshaug RR, Dang TT, Verma S, et al. Noninvasive Fecal Immunochemical Testing and Fecal Calprotectin Predict Mucosal Healing in Inflammatory Bowel Disease: A Prospective Cohort Study. Inflamm Bowel Dis. 2017 Sep;23((9)):1643–9. doi: 10.1097/MIB.0000000000001173. [DOI] [PubMed] [Google Scholar]
- 11.Hiraoka S, Inokuchi T, Nakarai A, Takashima S, Takei D, Sugihara Y, et al. Fecal Immunochemical Test and Fecal Calprotectin Results Show Different Profiles in Disease Monitoring for Ulcerative Colitis. Gut Liver. 2018 Mar;12((2)):142–8. doi: 10.5009/gnl17013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakarai A, Hiraoka S, Takahashi S, Inaba T, Higashi R, Mizuno M, et al. Simultaneous Measurements of Faecal Calprotectin and the Faecal Immunochemical Test in Quiescent Ulcerative Colitis Patients Can Stratify Risk of Relapse. J Crohn's Colitis. 2018 Jan;12((1)):71–6. doi: 10.1093/ecco-jcc/jjx118. [DOI] [PubMed] [Google Scholar]
- 13.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2 Group QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011 Oct;155((8)):529–36. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 14.Lundh A, Gøtzsche PC. Recommendations by Cochrane Review Groups for assessment of the risk of bias in studies. BMC Med Res Methodol. 2008 Apr;8((1)):22. doi: 10.1186/1471-2288-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Theede K, Holck S, Ibsen P, Ladelund S, Nordgaard-Lassen I, Nielsen AM. Level of Fecal Calprotectin Correlates With Endoscopic and Histologic Inflammation and Identifies Patients With Mucosal Healing in Ulcerative Colitis. Clin Gastroenterol Hepatol. 2015 Nov;13((11)):1929–36.e1. doi: 10.1016/j.cgh.2015.05.038. [DOI] [PubMed] [Google Scholar]
- 16.Patel A, Panchal H, Dubinsky MC. Fecal Calprotectin Levels Predict Histological Healing in Ulcerative Colitis. Inflamm Bowel Dis. 2017 Sep;23((9)):1600–4. doi: 10.1097/MIB.0000000000001157. [DOI] [PubMed] [Google Scholar]
- 17.Ryu DG, Kim HW, Park SB, Kang DH, Choi CW, Kim SJ, et al. Clinical implications of fecal calprotectin and fecal immunochemical test on mucosal status in patients with ulcerative colitis. Medicine (Baltimore) 2019 Sep;98((36)):e17080. doi: 10.1097/MD.0000000000017080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arai T, Takeuchi K, Miyamura M, Ishikawa R, Yamada A, Katsumata M, et al. Level of Fecal Calprotectin Correlates With Severity of Small Bowel Crohn's Disease, Measured by Balloon-assisted Enteroscopy and Computed Tomography Enterography. Clin Gastroenterol Hepatol. 2017 Jan;15((1)):56–62. doi: 10.1016/j.cgh.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 19.Lee YW, Lee KM, Lee JM, Chung YY, Kim DB, Kim YJ, et al. The usefulness of fecal calprotectin in assessing inflammatory bowel disease activity. Korean J Intern Med (Korean Assoc Intern Med) 2019 Jan;34((1)):72–80. doi: 10.3904/kjim.2016.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim ES, Lee HS, Kim SK, Kim EY, Jang BI, Kim KO, et al. Crohn's and Colitis Association in Daegu-Gyeongbuk (CCAiD) Fecal calprotectin is more accurate than fecal immunochemical test for predicting mucosal healing in quiescent ulcerative colitis: a prospective multicenter study. Scand J Gastroenterol. 2020 Feb;55((2)):163–8. doi: 10.1080/00365521.2020.1714716. [DOI] [PubMed] [Google Scholar]
- 21.D'Haens G, Ferrante M, Vermeire S, Baert F, Noman M, Moortgat L, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012 Dec;18((12)):2218–24. doi: 10.1002/ibd.22917. [DOI] [PubMed] [Google Scholar]
- 22.Kristensen V, Klepp P, Cvancarova M, Røseth A, Skar V, Moum B. Prediction of endoscopic disease activity in ulcerative colitis by two different assays for fecal calprotectin. J Crohn's Colitis. 2015 Feb;9((2)):164–9. doi: 10.1093/ecco-jcc/jju015. [DOI] [PubMed] [Google Scholar]
- 23.Kristensen V, Røseth A, Ahmad T, Skar V, Moum B. Fecal Calprotectin: A Reliable Predictor of Mucosal Healing after Treatment for Active Ulcerative Colitis. Gastroenterol Res Pract. 2017;2017:2098293. doi: 10.1155/2017/2098293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goll R, Heitmann R, Moe OK, Carlsen K, Florholmen J. Head to head comparison of two commercial fecal calprotectin kits as predictor of Mayo endoscopic sub-score and mucosal TNF expression in ulcerative colitis. PLoS One. 2019 Dec;14((12)):e0224895. doi: 10.1371/journal.pone.0224895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Theede K, Holck S, Ibsen P, Kallemose T, Nordgaard-Lassen I, Nielsen AM. Fecal Calprotectin Predicts Relapse and Histological Mucosal Healing in Ulcerative Colitis. Inflamm Bowel Dis. 2016 May;22((5)):1042–8. doi: 10.1097/MIB.0000000000000736. [DOI] [PubMed] [Google Scholar]
- 26.Mak WY, Buisson A, Andersen MJ, Jr, Lei D, Pekow J, Cohen RD, et al. Fecal Calprotectin in Assessing Endoscopic and Histological Remission in Patients with Ulcerative Colitis. Dig Dis Sci. 2018 May;63((5)):1294–301. doi: 10.1007/s10620-018-4980-0. [DOI] [PubMed] [Google Scholar]
- 27.Zubin G, Peter L. Predicting Endoscopic Crohn's Disease Activity Before and After Induction Therapy in Children: A Comprehensive Assessment of PCDAI, CRP, and Fecal Calprotectin. Inflamm Bowel Dis. 2015 Jun;21((6)):1386–91. doi: 10.1097/MIB.0000000000000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sandborn WJ, Panés J, Zhang H, Yu D, Niezychowski W, Su C. Correlation Between Concentrations of Fecal Calprotectin and Outcomes of Patients With Ulcerative Colitis in a Phase 2 Trial. Gastroenterology. 2016 Jan;150((1)):96–102. doi: 10.1053/j.gastro.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Yamaguchi S, Takeuchi Y, Arai K, Fukuda K, Kuroki Y, Asonuma K, et al. Fecal calprotectin is a clinically relevant biomarker of mucosal healing in patients with quiescent ulcerative colitis. J Gastroenterol Hepatol. 2016 Jan;31((1)):93–8. doi: 10.1111/jgh.13061. [DOI] [PubMed] [Google Scholar]
- 30.Carlsen K, Riis LB, Elsberg H, Maagaard L, Thorkilgaard T, Sørbye SW, et al. The sensitivity of fecal calprotectin in predicting deep remission in ulcerative colitis. Scand J Gastroenterol. 2018 Jun - Jul;53((7)):825–30. doi: 10.1080/00365521.2018.1482956. [DOI] [PubMed] [Google Scholar]
- 31.Kato J, Hiraoka S, Nakarai A, Takashima S, Inokuchi T, Ichinose M. Fecal immunochemical test as a biomarker for inflammatory bowel diseases: can it rival fecal calprotectin? Intest Res. 2016 Jan;14((1)):5–14. doi: 10.5217/ir.2016.14.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut. 2006 Mar;55((3)):426–31. doi: 10.1136/gut.2005.069476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manceau H, Chicha-Cattoir V, Puy H, Peoc'h K. Fecal calprotectin in inflammatory bowel diseases: update and perspectives. Clin Chem Lab Med. 2017 Mar;55((4)):474–83. doi: 10.1515/cclm-2016-0522. [DOI] [PubMed] [Google Scholar]
- 34.Schoepfer AM, Trummler M, Seeholzer P, Seibold-Schmid B, Seibold F. Discriminating IBD from IBS: comparison of the test performance of fecal markers, blood leukocytes, CRP, and IBD antibodies. Inflamm Bowel Dis. 2008 Jan;14((1)):32–9. doi: 10.1002/ibd.20275. [DOI] [PubMed] [Google Scholar]
- 35.Baveye S, Elass E, Mazurier J, Spik G, Legrand D. Lactoferrin: a multifunctional glycoprotein involved in the modulation of the inflammatory process. Clin Chem Lab Med. 1999 Mar;37((3)):281–6. doi: 10.1515/CCLM.1999.049. [DOI] [PubMed] [Google Scholar]
- 36.Mendoza JL, Abreu MT. Biological markers in inflammatory bowel disease: practical consideration for clinicians. Gastroenterol Clin Biol. 2009 Jun;33(Suppl 3):S158–73. doi: 10.1016/S0399-8320(09)73151-3. [DOI] [PubMed] [Google Scholar]
- 37.Borkowska A, Liberek A, Łuczak G, Jankowska A, Plata-Nazar K, Korzon M, et al. Fecal lactoferrin, a marker of intestinal inflammation in children with inflammatory bowel disease. Acta Biochim Pol. 2015;62((3)):541–5. doi: 10.18388/abp.2015_982. [DOI] [PubMed] [Google Scholar]
- 38.Guerrant RL, Araujo V, Soares E, Kotloff K, Lima AA, Cooper WH, et al. Measurement of fecal lactoferrin as a marker of fecal leukocytes. J Clin Microbiol. 1992 May;30((5)):1238–42. doi: 10.1128/jcm.30.5.1238-1242.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dai C, Jiang M, Sun MJ, Cao Q. Fecal immunochemical test for predicting mucosal healing in ulcerative colitis patients: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2018 May;33((5)):990–7. doi: 10.1111/jgh.14121. [DOI] [PubMed] [Google Scholar]
- 40.Costa F, Mumolo MG, Bellini M, Romano MR, Ceccarelli L, Arpe P, et al. Role of faecal calprotectin as non-invasive marker of intestinal inflammation. Dig Liver Dis. 2003 Sep;35((9)):642–7. doi: 10.1016/s1590-8658(03)00381-5. [DOI] [PubMed] [Google Scholar]
- 41.Dolci A, Panteghini M. Comparative study of a new quantitative rapid test with an established ELISA method for faecal calprotectin. Clinica chimica acta; international journal of clinical chemistry. 2012 Jan 18;413((1-2)):350–351. doi: 10.1016/j.cca.2011.09.030. [DOI] [PubMed] [Google Scholar]
- 42.Wassell J, Wallage M, Brewer E, J W Evaluation of the Quantum Blue® rapid test for faecal calprotectin. Ann Clin Biochem. 2012 Jan;49((Pt 1)):55–8. doi: 10.1258/acb.2011.011106. [DOI] [PubMed] [Google Scholar]
- 43.Kolho KL, Turner D, Veereman-Wauters G, Sladek M, de Ridder L, Shaoul R, et al. Rapid test for fecal calprotectin levels in children with Crohn disease. J Pediatr Gastroenterol Nutr. 2012 Oct;55((4)):436–9. doi: 10.1097/MPG.0b013e318253cff1. [DOI] [PubMed] [Google Scholar]
- 44.Sydora MJ, Sydora BC, Fedorak RN. Validation of a point-of-care desk top device to quantitate fecal calprotectin and distinguish inflammatory bowel disease from irritable bowel syndrome. J Crohn's Colitis. 2012 Mar;6((2)):207–14. doi: 10.1016/j.crohns.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 45.Vinding KK, Elsberg H, Thorkilgaard T, Belard E, Pedersen N, Elkjaer M, et al. Fecal Calprotectin Measured By Patients at Home Using Smartphones—A New Clinical Tool in Monitoring Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2016 Feb;22((2)):336–44. doi: 10.1097/MIB.0000000000000619. [DOI] [PubMed] [Google Scholar]
- 46.Elkjaer M, Burisch J, Voxen Hansen V, Deibjerg Kristensen B, Slott Jensen JK, Munkholm P. A new rapid home test for faecal calprotectin in ulcerative colitis. Aliment Pharmacol Ther. 2010 Jan;31((2)):323–30. doi: 10.1111/j.1365-2036.2009.04164.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data