Abstract
Vascular endothelial growth factor (VEGF) is a critical regulator of endothelial cell biology and vascular function. Chronic VEGF treatment has been shown to inhibit tumor necrosis factor–induced apoptosis in endothelial cells. However, the mechanism for this cell survival is unclear. Interestingly, VEGF also enhances the expression of X-linked inhibitor of apoptosis (XIAP), a well-established antiapoptotic factor. XIAP has been shown to suppress apoptosis by blocking caspase activity in cancer cells, but it remains under studied in the endothelium. Therefore, we hypothesized that VEGF affects important endothelial functions, such as apoptosis and cell migration, by regulating XIAP expression and downstream caspase activity. To test this hypothesis, caspase activity, apoptosis, and cell migration were assessed following XIAP overexpression or depletion in bovine aortic endothelial cells. Much like VEGF treatment, ectopic expression of XIAP blocked tumor necrosis factor–induced apoptosis. Surprisingly, the mechanism was caspase-independent. In addition, XIAP-associated cell survival was the result of enhanced nitric oxide (NO) production, and XIAP was partially localized in caveolae. In these lipid rafts, XIAP interacted with a regulator of NO production, caveolin-1, via a binding motif (FtFgtwiY, where the bold letters represent aromatic amino acids) in the baculoviral IAP repeat-3 domain. Endothelial NO synthase binding to caveolin-1 was competitively inhibited by XIAP, suggesting that XIAP acts as a modulator of NO production by releasing endothelial NO synthase from caveolin-1. Further studies showed that endothelial cell migration was also controlled by XIAP-dependent NO. Taken together, these results suggest that XIAP plays a novel role in endothelial cells, interacting with caveolin-1 and acting as a regulator of vascular antiatherogenic function.
Keywords: VEGF, XIAP, caveolin-1, nitric oxide, apoptosis
Extensive study of apoptosis has revealed a dichotomy of functions in the vascular system. In the endothelium, apoptosis contributes significantly to vascular pathology, as well as normal physiological function. In atherosclerotic plaques, apoptosis weakens the endothelial cell layer, promoting plaque rupture and atherothrombosis.1 In contrast, apoptosis also acts as an important physiological mediator, eliminating unbalanced, harmful cells and maintaining vascular homeostasis.2 Thus, the definition of apoptosis as an either physiological or pathological phenomenon requires careful dissection of distinct vascular apoptotic processes.
Vascular endothelial growth factor (VEGF) is an endothelial cytokine that acts through the activation of VEGF receptor tyrosine kinases, eg, flkA/KDR and flt-1.3 It is an important regulator of angiogenesis4 and cell survival, or antiapoptosis,5–7 and has been shown to act as a survival factor for newly formed blood vessels. Additional studies have found that VEGF also upregulates antiapoptotic proteins such as the inhibitors of apoptosis (IAPs) in human umbilical vein endothelial cells (HUVECs).8
Cellular IAP homologs have been identified in many organisms, from yeasts to higher-order animals.9 An important family member of IAPs, X-linked inhibitor of apoptosis (XIAP), has been linked with biologically significant cellular activities including antiapoptosis.10–11 Unlike other IAPs, XIAP is not homogeneously distributed in different tissues and cells12 but is strongly expressed in epidermal keratinocytes and the esophageal epithelium.12–13 Subcellularly, XIAP localizes predominantly in the cytoplasm but has been identified in the membrane of endometrial gland cells and in a granular supranuclear position in acinar exocrine cells.12
Previous work has shown that XIAP induces cell survival by inhibiting caspase-3, -7, and -9 through both molecular binding and ubiquitination.10,14–17 The XIAP protein is composed of 3 baculoviral IAP repeats (BIRs) and a carboxy-terminal RING finger domain. Caspase-3, -7, and -9 bind to XIAP via BIRs in the amino-terminal domains of XIAP. In addition, RING domains possessing E3 ubiquitin ligase activity initiate ubiquitination of caspases and synergistically promote cell survival.
Because of its potent antiapoptotic nature, XIAP is a target molecule in the development of therapeutic agents to treat malignant cancers.15,18–23 However, XIAP also modulates cell signaling molecules such as protein kinase B,24 nuclear factor-κB (NF-κB), transforming growth factor-β–activated kinase (TAK),25–28 and c-Jun N-terminal kinase (JNK),27 suggesting it is not limited to a cell survival role. In fact, XIAP domains for antiapoptotic activity are independent of the domains associated with other cell signaling pathways.27 Each functional domain possesses a distinct role, indicating that XIAP may act uniquely in a variety of cellular functions.
Caveolae, or lipid rafts, affect diverse vascular functions by controlling endothelial cell signaling in a local environment.29 Caveolin-1 is a principal protein in caveolae and confers vascular regulation by binding to various signaling molecules.30 The N-terminal domain of caveolin-1 contains an oligomerization domain (residues 61 to 101) and scaffolding domain (residues 81 to 101).30–31 The oligomerization domain is responsible for oligomerization of caveolin-1, whereas the scaffolding domain interacts with signaling molecules containing the caveolin-1 binding motifs φXXXXφ XXφ or φXφ XXXXφ, where φ represents an aromatic amino acid.32 Caveolin-1 has been shown to interact with heterotrimeric G proteins, Ras, Src, and endothelial nitric oxide synthase (eNOS).30,33–34
In this study, we present a unique endothelial apoptotic signaling pathway for the antiapoptotic molecule, XIAP. Our work shows that XIAP modulates endothelial apoptosis, interacting with caveolae/lipid rafts and caveolin-1 and enhancing nitric oxide (NO) production via eNOS. These novel findings clearly identify XIAP as a critical regulator of antiatherogenic function in the vasculature.
Materials and Methods
Cell Culture
Bovine aortic endothelial cells (BAECs) obtained from descending thoracic aortas were cultured at 37°C and 5% CO2 in DMEM (1 g/L glucose; Life Technologies Inc) containing 20% FBS (Wel GENE Inc) with antibiotics.35–36 Cells from passages 3 to 10 were used in these studies.
HUVECs (Cambrex) were cultured at 37°C and 5% CO2 in EGM-2 Bullet Kit medium (Cambrex) containing 10% FBS (Wel GENE Inc). Cells from passage 4 to 7 were used for these studies.
An expanded Materials and Methods section is available in the online data supplement at http://circres.ahajournals.org.
Results
Chronic VEGF Treatment Blocks Tumor Necrosis Factor-α–, Resveratrol-, and Etoposide-Induced Endothelial Cell Apoptosis in a Caspase-Independent Manner
It is well accepted that TNFα induces endothelial cell apoptosis.37 To investigate whether chronic VEGF treatment has an effect on physiological endothelial cell apoptosis, BAECs were incubated with VEGF for 48 hours and subsequently treated with tumor necrosis factor (TNF)α. As shown in Figure 1A and 1B, significantly fewer apoptotic cells were found in the VEGF-treated group compared with untreated control. However, there was no difference in caspase-3 activity between groups, indicating a caspase-3-independent pathway for VEGF-induced cell survival (Figure 1C and 1D).
Figure 1.

Chronic VEGF treatment blocks TNFα-, resveratrol-, and etoposide-induced endothelial cell apoptosis in a caspase-independent manner. Confluent BAECs were pretreated with 100 ng/mL VEGF for 48 hours and then treated with various apoptotic agents as indicated. A, Apoptotic cells were monitored via optic microscope (top) or fluorescence microscope (bottom). The top images show representative apoptotic (small and rounded) cells. In the bottom images, the nuclei of BAECs stained with Hoechst 33258 were observed. B, BAECs were treated with either 50 ng/mL TNFα or 100 ng/mL VEGF or both. Apoptotic cells were quantified by counting cells in the same visual field. Results are shown line graphs (means±SE, n=3). *P<0.05, ** P<0.03. C, Following the indicated treatment, cells were lysed as described in Material and Methods. Proteins in cell lysates were resolved by SDS-PAGE, transferred to poly-(vinylidene difluoride) (PVDF) membrane, and immunoblotted with caspase-3 antibodies. D, Caspase-3 activity was measured with substrate Ac-DEVD-pNA using the Caspase-3 Colorimetric Activity Assay Kit. Caspase-3 activity is plotted as line graphs (means±SE). E, Apoptotic cells were monitored and quantified as described. Results are shown as bar graphs (means±SE, n=3). *P<0.05, ** P<0.03. F, Caspase-3 activity was measured as described in D.
Pharmaceutical drugs including resveratrol (Res) and etoposide (Eto) also induce endothelial cell apoptosis. In apoptosis studies, Res and Eto treatment induced 1.5-to 2.5-fold more apoptotic cells than TNFα, making such treatment ideal for subsequent cell survival assessments (Figure 1E). Despite this dramatic increase, VEGF pretreatment still significantly reduced apoptotic cell number, and this reduction did not correlate with caspase-3 activity (Figure 1F). In conjunction with TNFα studies, these findings further supported a VEGF-induced antiapoptotic pathway that does not involve caspase. As such, the goal of further studies was to reveal this unique mechanism of VEGF-dependent cell survival.
VEGF Promotes Expression of XIAP in Endothelial Cells
Previous studies have shown that VEGF upregulates the expression of the antiapoptotic protein XIAP, suggesting a role for XIAP in VEGF-dependent cell survival.8 To investigate this mechanism of cell survival, XIAP expression was evaluated in response to growth factor treatment. As shown in Figure 2A, XIAP levels increased in a time-dependent manner following treatment of BAECs with a physiological concentration of VEGF. In contrast, 2 other growth factors (basic fibroblast growth factor and insulin-like growth factor), did not induce XIAP expression in either BAECs or HUVECs. This finding was consistent with previous reports and suggested that VEGF-specific induction of XIAP likely contributes to endothelial cell survival.
Figure 2.
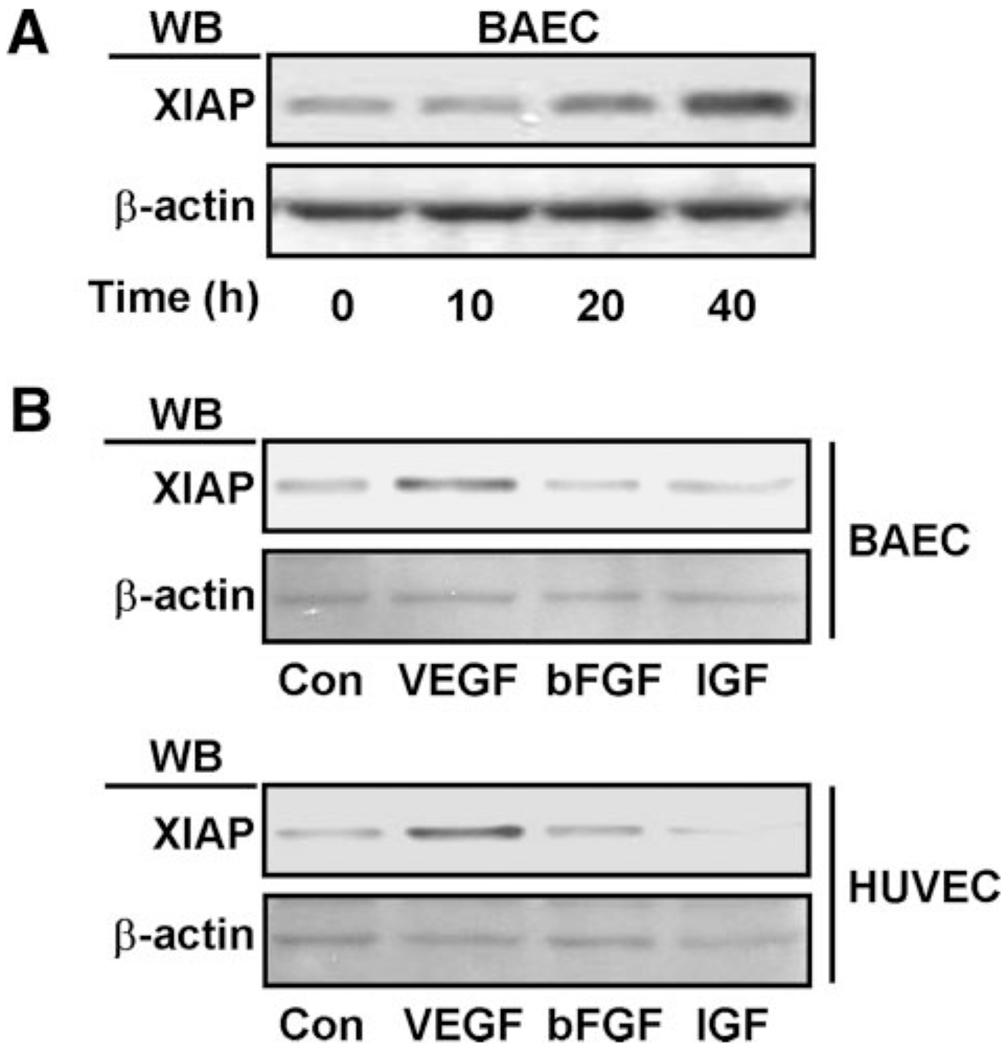
VEGF specifically upregulates the expression of an antiapoptotic protein, XIAP. A, BAECs were treated with 100 ng/mL VEGF as indicated. B, BAECs and HUVECs were treated with 100 ng/mL VEGF, basic fibroblast growth factor (bFGF), and insulin-like growth factor (IGF). After cell lysis, proteins in cell lysates were resolved by SDS-PAGE, transferred to a PVDF membrane, and immunoblotted with XIAP antibodies.
Ectopic Expression of XIAP Inhibits Res- and Eto-Induced Endothelial Cell Apoptosis in a Caspase-3–Independent Manner
To define the role of XIAP in the endothelium, apoptosis was assessed in response to ectopic XIAP expression. As illustrated in Figure 3A, treatment with the pharmaceutical drugs Res and Eto promoted endothelial cell apoptosis. As expected, overexpression of XIAP inhibited this increase in cell death, reducing the number of apoptotic cells by 20% to 25% (Figure 3B).
Figure 3.

XIAP plays an important role in VEGF-induced antiapoptosis. BAECs were transfected with vector alone, vector containing the xiap gene (wt-XIAP), scrambled siRNA, or xiap siRNAs. Then, confluent cells were treated with apoptosis-inducing agents (TNFα, etoposide, or resveratrol), as indicated. A, Apoptotic cells were detected as described in Figure 1A. B, Apoptotic cells were counted as described in Figure 1B and are plotted as bar graphs (means±SE, n=3 to 5). C, After cells were lysed, proteins in cell lysates were resolved by SDS-PAGE, transferred to PVDF membrane, and immunoblotted with caspase-3 antibodies. D, Caspase-3 activity was measured with substrate Ac-DEVD-pNA using the Caspase-3 Colorimetric Activity Assay Kit. The caspase-3 activity for various cell lysates are plotted as bar graphs (means±SE). *P<0.05. E, *P<0.05. E, Apoptotic cells are plotted as line graphs (mean±SE). *P<0.05.
In various cancer cell lines, it has been shown that XIAP inhibits apoptosis by blocking caspase activity.14–22 Both Res and Eto increased caspase-3 activity in endothelial cells. However, unlike apoptosis, this caspase-3 activation was not affected by ectopic XIAP expression (Figure 3C). Moreover, the caspase-3 inhibitor Ac-DEVD-CHO blunted Res- and Eto-induced caspase-3 activity but had no effect on XIAP-dependent endothelial cell survival (Figure 3B and 3D). Taken together, these results indicated that caspase-3 is not involved in drug-stimulated endothelial cell apoptosis. Thus, XIAP expression likely reverses Res and Eto-induced apoptosis via a unique caspase-independent mechanism in the vasculature.
XIAP Knockdown Increases Apoptosis Induced by TNFα and Eto
The abovementioned studies, as shown in Figure 3, identified XIAP as an important antiapoptotic factor by an ectopic expression of XIAP. To investigate whether XIAP play this critical role endogenously, XIAP-depletion studies were performed in BAECs. XIAP-specific small interfering (si)RNA was used to transiently knockdown endogenous protein expression. Compared with control siRNA, XIAP siRNA considerably reduced XIAP levels (Figure 3E, bottom). In addition, functional studies showed that siRNA depletion of XIAP increased TNFα- and Eto-induced apoptosis (Figure 3E, top). These data further implicated XIAP in endothelial cell survival, but the mechanism behind this role remained unclear.
XIAP Increases Cell Survival in Endothelial Cells by Enhancing NO Production
Previous work by us has shown that NO, an important vasodilator, can significantly block Res-stimulated endothelial apoptosis.38 To confirm this finding, we pretreated BAECs with the NO-producing agent S-nitroso-N-acetyl penicillamine (SNAP) and, consecutively, with proapoptotic agents Res and Eto. Consistent with our previous report, Resand Eto-induced apoptosis was inhibited by SNAP in a dose-dependent manner, indicating that NO has an inhibitory effect on endothelial cell apoptosis (Figure 4A).
Figure 4.
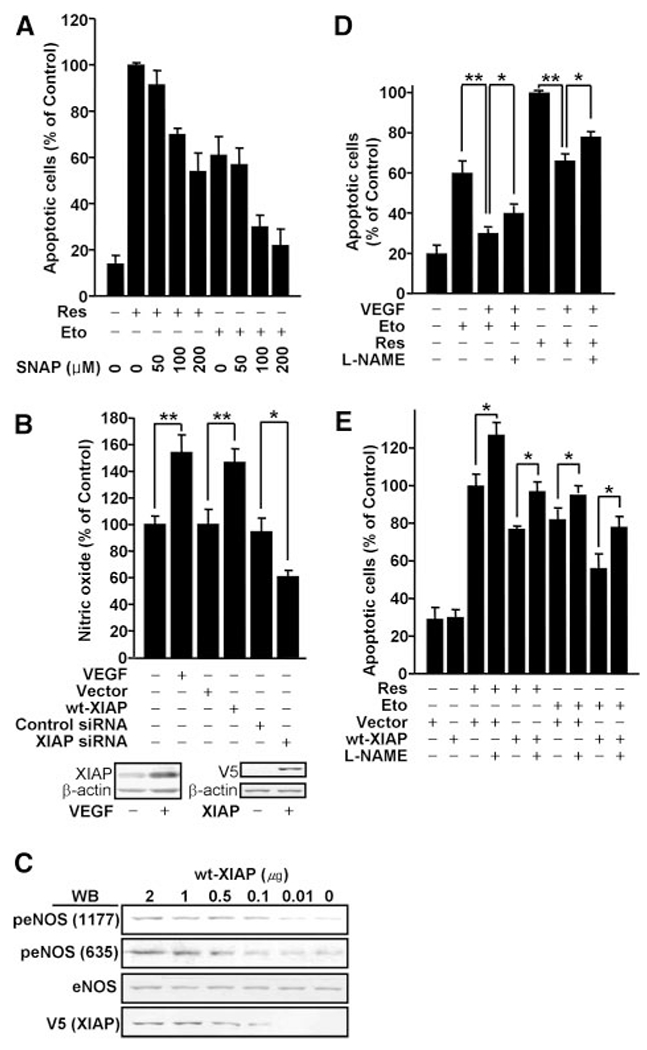
NO, promoted by VEGF or ectopic XIAP expression, is a key regulator for VEGF-induced cell survival. BAECs were transfected with vector alone, vector containing the xiap gene (wt-XIAP), scrambled siRNA, or xiap siRNAs. A, To examine an antiapoptotic effect of an NO donor (SNAP), BAECs were treated with Eto or Res and SNAP. Apoptotic cells were also counted and plotted as bar graphs (means±SE, n=3 to 5). B, Confluent cells were treated with a Ca2+ ionophore, A23187, and then NO was measured as described in Materials and Methods. NO levels are plotted as bar graphs (means±SE, n=3). *P<0.05, ** P<0.03. C, BAECs were transfected with vector alone or vector containing the xiap gene (wt-XIAP). eNOS phosphorylation was monitored by Western Blot. D and E, Following transfection and treatment with 1 mmol/L L-NAME, apoptotic cells were counted. The number of apoptotic cells are plotted as bar graphs. *P<0.05 (D and E), **P<0.03 (D).
To test whether NO participates in the unique mechanism for XIAP-induced cell survival, NO levels were evaluated in response to both ectopic XIAP expression and XIAP siRNA. Because of a limited sensitivity threshold, we were unable to detect differences between NO production in treated versus control cells (data not shown). To magnify the NO signal, cells were stimulated with A23187 (Ca2+ ionophore) before NO monitoring. As shown in Figure 4B, ectopic XIAP expression enhanced NO production, and XIAP-induced NO levels were comparable to those following VEGF treatment. In contrast, NO levels were reduced by XIAP knockdown via XIAP siRNA. Together, these data suggested that XIAP is able to regulate NO production in endothelial cells.
XIAP likely induces NO production via the enzyme endothelial NO synthase, which catalyzes the synthesis of NO. Increasing concentrations of XIAP caused concomitant phosphorylation of eNOS in a dose-dependent manner (Figure 4C). These results indicated that XIAP is an important vascular regulator, activating eNOS to promote NO production in endothelial cells.
To directly connect XIAP-dependent NO production to VEGF-dependent cell survival, we pretreated BAECs with the eNOS inhibitor NG-nitro-l-arginine methyl ester (L-NAME) before inducing apoptosis. As previously described, VEGF decreased the number of apoptotic cells present following Res and Eto treatment. In the presence of L-NAME, this effect was significantly reduced, indicating NO plays an important role in VEGF-dependent cell survival (Figure 4D). A similar effect was observed with ectopic XIAP expression. As shown in Figure 4E, XIAP reduced apoptosis, whereas L-NAME blunted this effect. Collectively, these results demonstrated a mechanism by which VEGF induces XIAP expression and, in turn, enhances NO production via eNOS to restrict endothelial cell apoptosis. Further studies focused on the mechanism of XIAP action of eNOS activity.
XIAP Is Expressed in Caveolae or Lipid Rafts
Interaction with caveolin-1, a principal protein of caveolae, is an important component of eNOS activity. Caveolin-1, the caveolae scaffolding protein, binds to and negatively regulates eNOS activity.29,39 Based on our finding that XIAP expression can induce eNOS activation, we hypothesized that ectopically expressed XIAP localizes in caveolae and interacts with caveolin-1 to activate eNOS. To test this hypothesis, lipid raft fractions were obtained and probed for XIAP with specific antibodies (Figure 5A). Fifteen to 20 percent of total cellular XIAP was found in the caveolar or lipid raft fraction. Immunostaining studies also supported this finding. Endogenous XIAP and exogenous V5-XIAP colocalized with caveolin-1, indicating a portion of cellular XIAP is located in lipid rafts (Figure 5B and 5C). Although this data clearly supported our hypothesis, further studies were necessary to definitively establish the molecular interaction between caveolin-1 and XIAP.
Figure 5.

Endogenous XIAP proteins are partially localized in caveolae. The lipid raft (caveolae) fractions of BAECs were obtained by a discontinuous sucrose gradient ultracentrifugation. Fractions were immunoblotted with appropriate antibodies. A, BAECs were also immunostained with caveolin-1 and XIAP antibodies. Caveolin-1 partially colocalized with endogenous (B) or exogenous (C) XIAP.
XIAP Interacts with Caveolin-1
Interestingly, the XIAP molecule contains a caveolin-1 binding motif (FtFgtwiY, where the bold letters represent aromatic amino acids) (Figure 6A).32 To determine whether this binding motif plays an important role in molecular interaction between XIAP and caveolin-1, we constructed 2 XIAP mutants, ΔRING and BIR1-2, with and without the caveolin-1 binding domain, respectively. In vitro studies showed that wild-type (wt) XIAP was able to bind caveolin-1 (Kd=127±70 nmol/L), whereas the BIR1-2 mutant was not (Figure 6B and 6C). Immunoprecipitation experiments supported this observation. As illustrated in Figure 6D, both wt XIAP and ΔRING coimmunoprecipitated with caveolin-1, whereas the BIR1-2 deletion mutant did not. Together, these data indicated that the BIR3 domain of XIAP is necessary for binding between caveolin-1 and XIAP.
Figure 6.

XIAP interacts with caveolin-1. A, Schematic diagram of the XIAP deletion mutants used in this study. Residues 270 to 277 (FtFgtwiY) represent a theoretical caveolin-1 binding domain (φXφXXXXφ, where φ represents an aromatic amino acid). B, In vitro binding assay of caveolin-1 with the XIAP deletion mutants (XIAP or BIR1-2). C, Binding assay blots were quantified by densitometry and plotted as line graphs. The Kd value for wt XIAP is 127±70 nmol/L. D, V5-tagged xiap mutant genes were transfected into BAECs. Cell lysates were immunoprecipitated with V5 antibodies and immunoblotted with caveolin-1 antibodies.
XIAP Activates eNOS by Competitively Binding to Caveolin-1 in Caveolae or Lipid Rafts
The oligomerization domain (residues 61 to 101) of caveolin-1 is an essential binding domain for eNOS and other caveolin binding proteins, suggesting a potential competition between eNOS and XIAP for caveolin-1.32 In in vitro binding assays, eNOS binding to caveolin-1 was inhibited as the concentration of wt XIAP was increased, whereas increasing the BIR1-2 mutant had no effect (Figure 7A). In addition, immunoprecipitation studies revealed that both ectopic XIAP and ΔRING expression inhibited eNOS binding to caveolin-1, whereas BIR1-2 did not (Figure 7B). In conjunction with colocalization studies, these results demonstrated that XIAP and eNOS compete for caveolin-1 in caveolae or lipid rafts, suggesting a critical role of caveolin-1 in the XIAP-dependent physiological effects.
Figure 7.

XIAP activates eNOS by competitively binding to caveolin-1 in caveolae or lipid rafts. A, BAEC lysates were incubated with various concentrations of exogenous XIAP. Mixtures were pulled down with caveolin-1 antibodies and then immunoblotted with eNOS antibodies. Whereas wt XIAP inhibited the interaction between caveolin-1 and eNOS in a dose-dependent manner, BIR1-2 did not. B, Vector alone or xiap deletion mutant (ΔRING, BIR1-2) genes (1.5 μg each) were transfected into BAECs. Cell lysates were immunoprecipitated with polyclonal caveolin-1 antibodies or nonimmune IgG and immunoblotted with eNOS antibodies. C, BAECs were transfected with vector containing the xiap gene (wt-XIAP) and scrambled siRNA or caveolin-1 siRNAs. Top, Western blots. Bottom, Bar graphs for NO production. D, NO production was measured in BAECs transfected with xiap or mutant genes. *P<0.05. E, Apoptotic cells were detected as described in Figure 1. The number of apoptotic cells is plotted as bar graphs. *P<0.05.
This function for caveolin-1 was further explored using cavolin-1–specific siRNA. As shown in Figure 7C, caveolin-1 knockdown increased NO levels compared with control. However, this NO level was not different from that induced by exogenous XIAP expression or by a concomitant XIAP expression and caveolin-1 depletion. Additionally, only wt XIAP and ΔRING vectors were able to promote increased NO levels (Figure 7D). The BIR1-2 deletion mutant lacking the caveolin-1 binding domain did not. These findings suggested that the release of eNOS from caveolin-1, whether via competitive binding of XIAP or depletion of caveolin-1, promotes activation of eNOS. Furthermore, this mechanism also seemed to apply to XIAP-dependent cell survival. Caveolin-1 depletion blunted apoptosis in an eNOS-dependent manner, similar to wt XIAP expression or wt XIAP expression and caveolin-1 knockdown together (Figure 7E). Together, these studies showed that both XIAP and caveolin-1 act through NO to regulate endothelial cell functions.
XIAP Promotes the Endothelial Cell Migration via NO
It has been well established that VEGF is a proangiogenic factor and that NO induces endothelial cell migration, a proangiogenic property.40–42 Combined with the present finding that XIAP is a functional mediator for VEGF-induced cell survival, these studies imply that XIAP expression may affect endothelial migration. To test this hypothesis, we evaluated endothelial cell migration in response to XIAP expression. Overexpression of XIAP, generated by either chronic VEGF treatment or ectopic expression, significantly enhanced endothelial cell migration, whereas XIAP siRNA reduced migration (Figure 8A and 8B). As shown in Figure 8B, increased migration was inhibited by the L-NAME pretreatment, implicating NO in XIAP-promoted endothelial cell migration. Overall, this work showed that XIAP acts as an important regulator for vascular biology through VEGF- and NO-dependent pathways.
Figure 8.

XIAP plays an important role in the endothelial cell migration. Confluent endothelial cells were treated with 2 mmol/L thymidine twice for 18 and 17 hours and then scraped with a razor blade. The cells were subsequently incubated for an additional 16 hours in starvation media containing various agents (100 ng/mL VEGF, 1 mmol/L L-NAME) as indicated. Migrated cells were observed with microscopy. A, Images are representative of at least 3 different observations. The solid line indicates the boundary line immediately after scraping. B, Quantification was performed by counting the number of cells migrated in the same field. Bar graphs represent means±SE (n≥3). *P<0.05, ** P<0.03.
Discussion
Through exogenous XIAP expression and siRNA depletion studies, we have shown that XIAP regulates important vascular functions in endothelial cells. Our work provides evidence for the following mechanism: (1) VEGF upregulates XIAP in both BAECs and HUVECs; (2) XIAP competitively binds to caveolin-1, releasing and activating eNOS; and (3) eNOS activation results in NO production which promotes both cell survival and cell migration.
Previous reports have stated that XIAP cannot be detected in the endothelium when monitored by immunohistochemistry.12 Here, we were able to identify XIAP in both HUVECs and BAECs by Western blots. This is consistent with previous reports.8,26 These conflicting results are likely attributable to a low expression level in non–VEGF-treated cells.
In other studies, XIAP has been shown to block apoptosis in cancer cells by inhibiting caspase activity.14–22 Although these previous reports indicated that VEGF upregulates XIAP in endothelial cells, until now, the physiological effect of XIAP in endothelial cells was not well understood.8 Through a number of functional studies, we have shown that VEGF-induced XIAP blunts apoptosis and acts as a potent antiatherogenic molecule. In addition, our work has shown that XIAP blocks TNF-, and Res-, and Eto-induced apoptosis in a caspase-independent manner.
The pathway for TNF-induced apoptosis is well described and is initiated by TNF and TNF receptor binding.37 Subsequent recruitment of serial adaptor proteins, including TNF receptor-1–associated death domain protein (TRADD) and Fas-associated death domain protein (FADD), results in capase-8 activation. Caspase-8 then activates other caspases, including caspase-3, to induce apoptotic cell processes. Based on this established signaling pathway, a caspase-3–independent mechanism for VEGF/XIAP-induced cell survival is not easily understood. A model for the caveolin-1–dependent regulation of TNF-induced apoptosis is more consistent with our findings. Previously, an essential role of caveolin-1 in the regulation of TNF signaling has been documented.43 In addition, caveolin-1 was suggested to play an important role in the regulation of apoptosis via a phosphatidylinositol 3-kinase/Akt signaling pathway.44 Thus, our findings support this caveolin-associated mechanism and further suggest an important role of XIAP interacting with caveolin-1 in the regulation of endothelial apoptosis or TNF signaling.
Via 2 distinct fractionation assays, we found that ≈20% of endothelial XIAP was localized to detergent-insoluble membrane raft fractions. Our unpublished observations using β-methyl cyclodextrin indicate that caveolar or lipid raft fraction of XIAP can be dynamically changed by certain stimuli, such as shear stress, to affect target molecules, such as ERK (unpublished data, 2008). Together, these data imply that XIAP is compartmentalized in endothelial cells to define function according to location. Like XIAP, caveolin-1 is not ubiquitously expressed in all tissues and cells. These observations suggest that in caveolin-1–expressing cells, like endothelial cells, XIAP behaves differently based on its location, with caveolin-associated functions or caspase-dependent functions occurring independently. The finding that XIAP promotes eNOS activation and NO production further supports this concept.
We have established that XIAP blunts cell death in an NO-dependent manner. Although it is not clear how NO functions as an inhibitor for TNF-dependent apoptosis, several studies support such a protective role. For example, Hida et al reported that NO plays a role in mitochondria and acts as an antiapoptotic factor.45 Thus, it is possible that NO promotes cell survival by inhibiting signaling molecules downstream of caspase-3 in the TNF-induced pathway.
Beyond cell survival, association of XIAP with other important molecules suggests a broader role in vascular function. It has been established that XIAP is related to endothelial cell survival28 and activation of cell signaling molecules including NF-κB. Because NF-κB regulates inflammatory responses, cell proliferation, and cell survival, correspondingly, XIAP likely participates in several physiological functions in the vascular system.28 Via in vitro and the in vivo assays, we showed that XIAP not only colocalizes with caveolin-1 but also interacts with it. Caveolin-1 is involved in modulation of vascular tone, angiogenesis, and atherosclerosis. The molecular interaction between caveolin-1 and XIAP provides insight into a wider spectrum of vascular functions exerted by XIAP. In this study, we have shown that XIAP expression is associated with important vascular functions such as NO production, apoptosis, and migratory responses. These vascular functions are essential to maintain homeostasis of blood vessels and to prevent disease. For instance, NO modulates basal vascular tone, endothelial cell proliferation and survival, and angiogenesis.46 Reduction in NO bioavailability induces a number of major cardiovascular diseases such as arterial hypertension and dyslipidemia47 and inhibition of angiogenesis.46 However, overexpression of NO has also been related to infectious disease and immune disease mediation.48 These different physiological or pathophysiological roles for NO are dependent on NO concentration and cell type. Given that immune cells stimulated by infection produce 10-fold more NO than quiescent cells49 and XIAP induces less than a 2-fold increase in NO, XIAP is likely involved in maintaining vascular physiology rather than vascular disease. We were able to verify that XIAP does indeed participate in vascular functions other than cell survival. Scratch assay results showed that ectopically expressed XIAP modulated endothelial cell migration as well.
Endothelial cell migration is critical to vascular remodeling, inflammation, wound healing, and angiogenesis. The finding that XIAP participates not only in VEGF-induced cell survival but also in cell migration suggests that XIAP is an important vascular factor. Although these studies were limited to bovine aortic endothelial cells and investigation of other cell types is warranted, this work clearly identifies a novel role for XIAP in vessel physiology. In conclusion, XIAP can now be studied as a significant functional regulator in the cardiovascular system.
Supplementary Material
Sources of Funding
This study was supported by Korea Research Foundation grant 2003-070-C00031.
Footnotes
Circulation Research is available at http://circres.ahajournals.org
Disclosures
None.
References
- 1.Napoli C Oxidation of LDL, atherogenesis, and apoptosis. Ann N Y Acad Sci. 2003;1010:698–709. [DOI] [PubMed] [Google Scholar]
- 2.Sakamaki K Regulation of endothelial cell death and its role in angiogenesis and vascular regression. Curr Neurovasc Res. 2004;1:305–315. [DOI] [PubMed] [Google Scholar]
- 3.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- 4.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. [DOI] [PubMed] [Google Scholar]
- 5.Benjamin LE, Golijanin D, Itin A, Pode D, Keshet E. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J Clin Invest. 1999;103:159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benjamin LE, Keshet E. Conditional switching of vascular endothelial growth factor (VEGF) expression in tumors: induction of endothelial cell shedding and regression of hemangioblastoma-like vessels by VEGF withdrawal. Proc Natl Acad Sci U S A. 1997;94:8761–8766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alon T, Hemo I, Itin A, Pe’er J, Stone J, Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995;1:1024–1028. [DOI] [PubMed] [Google Scholar]
- 8.Tran J, Rak J, Sheehan C, Saibil SD, LaCasse E, Korneluk RG, Kerbel RS. Marked induction of the IAP family antiapoptotic proteins survivin and XIAP by VEGF in vascular endothelial cells. Biochem Biophys Res Commun. 1999;264:781–788. [DOI] [PubMed] [Google Scholar]
- 9.Miller LK. An exegesis of IAPs: salvation and surprises from BIR motifs. Trends Cell Biol. 1999;9:323–328. [DOI] [PubMed] [Google Scholar]
- 10.Deveraux QL, Reed JC. IAP family proteins: suppressors of apoptosis. Genes Dev. 1999;13:239–252. [DOI] [PubMed] [Google Scholar]
- 11.Holcik M, Korneluk RG. XIAP, the guardian angel. Nat Rev Mol Cell Biol. 2001;2:550–556. [DOI] [PubMed] [Google Scholar]
- 12.Vischioni B, van der Valk P, Span SW, Kruyt FA, Rodriguez JA, Giaccone G. Expression and localization of inhibitor of apoptosis proteins in normal human tissues. Hum Pathol. 2006;37:78–86. [DOI] [PubMed] [Google Scholar]
- 13.Bowen AR, Hanks AN, Allen SM, Alexander A, Diedrich MJ, Grossman D. Apoptosis regulators and responses in human melanocytic and keratinocytic cells. J Invest Dermatol. 2003;120:48–55. [DOI] [PubMed] [Google Scholar]
- 14.Roy N, Deveraux QL, Takahashi R, Salvesen GS, Reed JC. The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. EMBO J. 1997;16:6914–6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deveraux QL, Takahashi R, Salvesen GS, Reed JC. X-linked IAP is a direct inhibitor of cell-death proteases. Nature. 1997;388:300–304. [DOI] [PubMed] [Google Scholar]
- 16.Deveraux QL, Roy N, Stennicke HR, Van Arsdale T, Zhou Q, Srinivasula SM, Alnemri ES, Salvesen GS, Reed JC. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J. 1998;17:2215–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi R, Deveraux Q, Tamm I, Welsh K, Assa-Munt N, Salvesen GS, Reed JC. A single BIR domain of XIAP sufficient for inhibiting caspases. J Biol Chem. 1998;273:7787–7790. [DOI] [PubMed] [Google Scholar]
- 18.Hu S, Yang X. Cellular inhibitor of apoptosis 1 and 2 are ubiquitin ligases for the apoptosis inducer Smac/DIABLO. J Biol Chem. 2003;278:10055–10060. [DOI] [PubMed] [Google Scholar]
- 19.Huang HK, Joazeiro CAP, Bonfoco E, Kamada S, Leverson JD, Hunter T. The inhibitor of apoptosis, cIAP2, functions as a ubiquitin-protein ligase and promotes in vitro monoubiquitination of caspases 3 and 7. J Biol Chem. 2000;275:26661–26664. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Yang Y, Ashwell JD. TNF-RII and c-IAP1 mediate ubiquitination and degradation of TRAF2. Nature. 2002;416:345–347. [DOI] [PubMed] [Google Scholar]
- 21.Yang Y, Fang S, Jensen JP, Weissman AM, Ashwell JD. Ubiquitin protein ligase activity of IAPs and their degradation in proteasomes in response to apoptotic stimuli. Science. 2000;288:874–877. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki Y, Nakabayashi Y, Takahashi R. Ubiquitin-protein ligase activity of X-linked inhibitor of apoptosis protein promotes proteasomal degradation of caspase-3 and enhances its anti-apoptotic effect in Fas-induced cell death. Proc Natl Acad Sci U S A. 2001;98:8662–8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schimmer AD, Dalili S, Batey RA, Riedl SJ. Targeting XIAP for the treatment of malignancy. Cell Death Differ. 2006;13:179–188. [DOI] [PubMed] [Google Scholar]
- 24.Dan HC, Sun M, Kaneko S, Feldman RI, Nicosia SV, Wang HG, Tsang BK, Cheng JQ. Akt phosphorylation and stabilization of X-linked inhibitor of apoptosis protein (XIAP). J Biol Chem. 2004;279:5405–5412. [DOI] [PubMed] [Google Scholar]
- 25.Stehlik C, de Martin R, Kumabashiri I, Schmid JA, Binder BR, Lipp J. Nuclear factor (NF)-B-regulated X-chromosome-linked IAP gene expression protects endothelial cells from tumor necrosis factor-induced apoptosis. J Exp Med. 1998;188:211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levkau B, Garton KJ, Ferri N, Kloke K, Nofer JR, Baba HA, Raines EW, Breithardt G. xIAP induces cell-cycle arrest and activates nuclear factor-B: new survival pathways disabled by caspase-mediated cleavage during apoptosis of human endothelial cells. Circ Res. 2001;88:282–290. [DOI] [PubMed] [Google Scholar]
- 27.Lewis J, Burstein E, Reffey SB, Bratton SB, Roberts AB, Duckett CS. Uncoupling of the signaling and caspase-inhibitory properties of X-linked inhibitor of apoptosis. J Biol Chem. 2004;279:9023–9029. [DOI] [PubMed] [Google Scholar]
- 28.Warbinek RH, Schmid JA, Stehlik C, Binder BR, Lipp J, Martin RD. Activation of NF-κB by XIAP, the X chromosome-Linked inhibitor of apoptosis, in endothelial cells involves TAK1. J Biol Chem. 2000;275:22064–22068. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez E, Kou R, Lin AJ, Golan DE, Michel T. Subcellular targeting and agonist-induced site-specific phosphorylation of endothelial nitric-oxide synthase. J Biol Chem. 2002;277:39554–39560. [DOI] [PubMed] [Google Scholar]
- 30.Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J Biol Chem. 1998;273:5419–5422. [DOI] [PubMed] [Google Scholar]
- 31.Park H, Go YM, Darji R, Choi JW, Lisanti MP, Maland MC, Jo H. Caveolin-1 regulates shear stress-dependent activation of extracellular signal-regulated kinase. Am J Physiol Heart Circ Physiol. 2000;278: H1285–H1293. [DOI] [PubMed] [Google Scholar]
- 32.Cohen AW, Hnasko R, Schubert W, Lisanti MP. Role of caveolae and caveolins in health and disease. Physiol Rev. 2004;84:1341–1379. [DOI] [PubMed] [Google Scholar]
- 33.Engelman JA, Zhang X, Galbiati F, Volonte D, Sotgia F, Pestell RG, Minetti C, Scherer PE, Okamoto T, Lisanti MP. Molecular genetics of the caveolin gene family: implications for human cancers, diabetes, Alzheimer disease, and muscular dystrophy. Am J Hum Genet. 1998;63:1578–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murata M, Peranen J, Schreiner R, Wieland F, Kurzchalia TV, Simons K. VIP21/caveolin is a cholesterol-binding protein. Proc Natl Acad Sci U S A. 1995;92:10339–10343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park H, Go YM, St John PL, Maland MC, Lisanti MP, Abrahamson DR, Jo H. Plasma membrane cholesterol is a key molecule in shear stress-dependent activation of extracellular signal-regulated kinase. J Biol Chem. 1998;273:32304–32311. [DOI] [PubMed] [Google Scholar]
- 36.Jo H, Sipos K, Go YM, Law R, Rong J, McDonald JM. Differential effect of shear stress on extracellular signal-regulated kinase and N-terminal Jun kinase in endothelial cells. Gi2- and Gbeta/gamma-dependent signaling pathways. J Biol Chem. 1997;272:1395–1401. [DOI] [PubMed] [Google Scholar]
- 37.Madge LA, Pober JS. TNF signaling in vascular endothelial cells. Exp Mol Pathol 2001;70:317–325. [DOI] [PubMed] [Google Scholar]
- 38.Kim JM, In KM, Choi SM, Pyee JH, Park H. Resveratrol-induced apoptosis is inhibited by nitric oxide in endothelial cells. J Nano Bio Tech. 2006;3:89–92. [Google Scholar]
- 39.Feron O, Saldana F, Michel JB, Michel T. The endothelial nitric-oxide synthase-caveolin regulatory cycle. J Biol Chem. 1998;273:3125–3128. [DOI] [PubMed] [Google Scholar]
- 40.Ferrara N, Houck K, Jakeman L, Leung DW. Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr Rev. 1992;13:18–32. [DOI] [PubMed] [Google Scholar]
- 41.Isner JM, Asahara T. Angiogenesis and vasculogenesis as therapeutic strategies for postnatal neovascularization. J Clin Invest. 1999;103:1231–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Michaud SE, Dussault S, Groleau J, Haddad P, Rivard A. Cigarette smoke exposure impairs VEGF-induced endothelial cell migration: role of NO and reactive oxygen species. J Mol Cell Cardiol. 2006;41:275–284. [DOI] [PubMed] [Google Scholar]
- 43.D’Alessio A, Al-Lamki RS, Bradley JR, Pober JS. Caveolae participate in tumor necrosis factor receptor 1 signaling and internalization in a human endothelial cell line. Am J Pathol. 2005;166:1273–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carver LA, Schnitzer JE. Caveolae: mining little caves for new cancer targets. Nat Rev Cancer. 2003;3:571–581. [DOI] [PubMed] [Google Scholar]
- 45.Hida A, Kawakami A, Miyashita T, Yamasaki S, Nakashima K, Tanaka F, Izumi Y, Tamai M, Huang M, Ida H, Nakamura H, Origuchi T, Ueki Y, Eguchi K. Nitric oxide acts on the mitochondria and protects human endothelial cells from apoptosis. J Lab Clin Med. 2004;144:148–155. [DOI] [PubMed] [Google Scholar]
- 46.Cooke JP. NO and angiogenesis. Atheroscler Suppl. 2003;4:53–60. [DOI] [PubMed] [Google Scholar]
- 47.Cook S Coronary artery disease, nitric oxide and oxidative stress: the “Yin-Yang” effect–a Chinese concept for a worldwide pandemic. Swiss Med Wkly. 2006;136:103–113. [DOI] [PubMed] [Google Scholar]
- 48.Bogdan C Nitric oxide and the immune response. Nat Immunol. 2001;2:907–916. [DOI] [PubMed] [Google Scholar]
- 49.Yadav M, Dubey ML, Gupta I, Malla N. Nitric oxide radicals in leucocytes and vaginal washes of Trichomonas vaginalis-infected symptomatic and asymptomatic women. Parasitology. 2006;132:1–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


