Abstract
Background
Endoscopic resection of dysplastic lesions in early stages of cancer reduces mortality rates and is recommended by many national guidelines throughout the world. Snare polypectomy and endoscopic mucosal resection (EMR) are established techniques of polyp removal. The advantages of these methods are their relatively short procedure times and acceptable complication rates. The latter include delayed bleeding in 0.9% and a perforation risk of 0.4–1.3%, depending on the size and location of the resected lesion. EMR is a recent modification of endoscopic resection. A limited number of studies suggest that larger lesions can be removed en bloc with low complication rates and short procedure times. Novel techniques such as endoscopic submucosal dissection (ESD) are used to enhance en bloc resection rates for larger, flat, or sessile lesions. Endoscopic full-thickness resection (EFTR) is employed for non-lifting lesions or those not easily amenable to resection. Procedures such as ESD or EFTR are emerging standards for lesions inaccessible to EMR techniques.
Summary
Endoscopic treatment is now regarded as first-line therapy for benign lesions.
Key Message
Endoscopic resection of dysplastic lesions or early stages of cancer is recommended. A plethora of different techniques can be used dependent on the lesions.
Keywords: Endoscopic resection, Resection techniques, Early cancer, Complication rates
Introduction
Before the era of national screening programs, the majority of gastrointestinal cancers were diagnosed at an advanced stage, which necessitated major surgery and led to significant morbidity and mortality rates. In current times, cancer screening programs are not only established, but also widely accepted in most countries. The widespread use of modern high-definition endoscopes permits the detection of cancers in early stages of disease, with a low risk of lymph node metastases or distant spread. Consequently, the majority of the lesions can be managed via endoscopic approaches. Endoscopic detection devices have been paralleled by the development of new endoscopic treatment techniques in the last few decades.
Endoscopic mucosal resection (EMR) was pioneered in Japan by Soetikno et al. [1] for the management of early gastric cancer. The indications have been extended to include various gastrointestinal dysplastic and early cancer lesions. In expert hands, and based on the classifications detailed below in combination with (virtual) chromoendoscopy, the endoscopist is able to predict the aggressiveness of cancer and identify histologic subtypes, as well as investigate the lesion by endoscopy [2, 3].
The Paris classification of early cancer (Fig. 1) is helpful in stratifying the risk of submucosal invasion [4, 5, 6, 7, 8]. The pit pattern classification system proposed by Kudo, vascular patterns of the Sano classification, or more recently the Narrow-Band Imaging International Colorectal Endoscopic (NICE) criteria (Fig. 2), should be used to predict the grade of dysplasia [3, 9, 10].
Fig. 1.
Paris classification of early cancers.
Fig. 2.
NICE classifications.
Tubular adenomas typically have large or elongated pits (Kudo type III) and an organized brown capillary network surrounding the pits (Sano type 2/NICE type 2). Villous adenomas have more complex branching gyrus-like pits (Kudo type IV) [3, 10, 11, 12, 13]. Submucosal invasive cancer is suspected in patients with irregular mixed types, non-structural or absent pits (Kudo type V), irregular complex branching capillaries, or avascular areas (Sano type 3/NICE type 3).
A clear demarcation line is frequently seen between the regular background pattern of the non-invasive adenoma and the irregular area of the suspected invasive component. In 2014, the Japan Narrow Band Imaging Expert Team (JNET) classification was published and showed that the narrow-band imaging (NBI) diagnosis with magnification even had the potential to increase the confidence rate and enable endoscopists to differentiate malignant neoplasia from benign neoplasia [9, 14].
The JNET classification consists of 4 categories: JNET type 1, type 2A, type 2B, and type 3. Each type represents the histological features of the polyps, and they are categorized by focusing on 2 specific features: the vessel pattern and the surface pattern. JNET type 1 refers to hyperplastic polyps and sessile serrated polyps. Type 2A refers to LGIN and type 2B corresponds to HGIN or shallow submucosal invasive cancer (S-SMC). Type 3 refers to deep submucosal invasive cancer. The JNET classification is compatible with the NICE classification in that both classifications are divided into 3 major categories (type 1, 2, and 3) corresponding to the similar histopathology, but with the one special condition − that magnification has to be used. The sensitivity in differentiating neoplasia from non-neoplasia is reported to be 98.1–99.8%. The specificity in differentiating malignant neoplasia from benign neoplasia is 84.7–98.2% and the specificity in the differentiation of deep submucosal cancer from other neoplasia is 99.8–100.0%. This classification has the potential to enable endoscopists to identify almost all neoplasia, to appropriately determine whether to perform en bloc resection or not, and to avoid unnecessary surgery [9, 14, 15].
EMR is regarded as a curative measure for intramucosal and superficial submucosal cancers. In skilled hands, the technique yields very good outcomes and is associated with low complication rates. However, recurrent disease and the inadequate treatment of large lesions or early cancers are as yet unresolved issues. This has led to a further technical advancement in terms of one-piece excision even in cases of large neoplasms. The techniques range from knife-assisted snare resection (KAR) and endoscopic submucosal dissection (ESD) to full-thickness resection. In the following paragraphs we will review all of these resection techniques, analyze evidence of their success, and discuss future perspectives.
Endoscopic Mucosal Resection
EMR is an endoscopic technique developed for the removal of neoplasms confined to the superficial layers (mucosa and submucosa) of the gastrointestinal tract. It was introduced in 1955 for rigid sigmoidoscopy, and then in 1973 for flexible colonoscopy [16, 17]. EMR has gained wide acceptance as a therapeutic option for various gastrointestinal lesions. The indications have been extended to include the entire gastrointestinal tract. The technique can be learned easily and permits resection of large polyps which would otherwise require radical surgery.
Before commencing resection, the endoscopist should meticulously inspect the lesion, give due consideration to the abovementioned classifications, and distinguish resectable lesions from those potentially affected by submucosal invasion. This is very important because it may result in a different endoscopic approach or referral for surgical treatment. Before starting any EMR procedure, the endoscopist must perform a close visual inspection and delineate margins, especially those of flat lesions, because manipulation of the lesion may obscure landmarks. It may be helpful to highlight the margins of the target lesion with superficial cautery marks using the tip of a snare, or with argon plasma coagulation.
EMR procedures can be broadly divided into suck and cut (suction) or lift and cut (non-suction) techniques. The latter involve the injection of fluid into the submucosal space to lift the lesion away from the muscle (Fig. 3). Non-lifting signs of a lesion after submucosal injection are a predictor of deep invasion and indicate that the lesion is not amenable to endoscopic resection (Fig. 4) [18, 19]. However, sometimes the non-lifting sign is a result of submucosal fibrosis related to previous manipulation (previous biopsy or attempted/incomplete EMR) of the lesion [20]. In these cases, the full wall resection technique described below can be used.
Fig. 3.
EMR using the lift and cut technique with injection of fluid into the submucosal space to lift the lesion away from the muscle.
Fig. 4.
EMR with a central non-lifting sign as a predictor of deep invasion.
The suction technique, commonly used in Barrett's dysplasia, is based on the use of a multiband mucosectomy device without submucosal injection [21]. A pseudopolyp is formed by band ligation, which is then resected with a snare (Fig. 5). However, a submucosal injection is performed when cap-assisted EMR is used; the lesion is sucked into the cap and resected with a pre-looped snare. Currently, the lift and cut technique is most frequently used. Early work in Japan proved the feasibility and efficacy of the technique [21]. In the colorectum a modified endoscopic submucosal resection with band ligation has been used to remove small rectal neuroendocrine tumors. A multi-band ligation device is mounted on the scope. Submucosal saline solution is injected beneath the tumor to reduce the risk of perforation. The lesion is then aspirated into the ligation device and an elastic band is deployed. The snared lesion is then resected below the band. Carcinoid tumors <1 cm in the rectum have a low risk of metastatic disease and local treatment is considered curative. As the carcinoid extends to the submucosa, resection techniques must aim for complete resection. The use of a ligation device in the esophagus has shown a maximum thickness of submucosa resected up to 1,200 µm (median 800, range 500–1,200) [22]. The efficacy of endoscopic submucosal resection with band ligation in resecting small rectal carcinoids has been compared to that of standard EMR and ESD. Complete resection with histopathologically negative margins has been reported in 94.3–100% versus 75.7–80% of EMR cases [23, 24]. This technique has shown equivalent complete resection rates to those of ESD (100 vs. 92%, respectively), while offering the advantages of a shorter procedural duration and shorter (if any) hospital admission [25].
Fig. 5.
The suction technique with a multiband mucosectomy device without the need for submucosal injection creating a pseudopolyp by band ligation.
No perforations occurred in all the above studies. The results of a recent meta-analysis confirm that treatment of rectal carcinoid tumors with ESD or endoscopic submucosal resection with band ligation is superior to EMR, and that the efficacy is equivalent to ESD treatment [26].
In general, experienced endoscopists go through a relatively short learning curve for EMR [27, 28]. The outcome of endoscopic resection of colonic polyps was best summarized in a large meta-analysis of 50 studies [29], which revealed an initial success rate of 92%. Only 8% of the patients underwent a non-curative endoscopic resection. Perforation and delayed bleeding rates were 1.5 and 6.5%, respectively. However, recurrent disease was noted in 13.8% of patients, and covert cancer was found in 8% of the resection specimens.
The main drawback of EMR is the piecemeal nature of resection for polyps larger than 20 mm in size. This makes it difficult for the pathologist to evaluate the completeness of resection (Fig. 6). Multipiece resection is associated with a recurrence risk of 14–20% [30]. A prospective, multicenter, observational study from Australia targeting tumors larger than 20 mm in size comprised 479 patients who underwent EMR; the overall success rate was 89.2% and surgery was avoided in 83.7% [31]. The overall recurrence rate was 20.4%, and was even higher in patients with lesions larger than 40 mm in size (41 vs. 13%) or when >6 pieces were needed for complete resection (34.2 vs. 12.8%). At the same time, data from a large prospective multicenter study indicate that early recurrent or residual adenomas are typically unifocal and can be managed endoscopically in >90% of cases [32]. Furthermore, if the initial EMR was successful, and the EMR specimens did not reveal submucosal invasion requiring surgery, then with strict colonoscopy surveillance at 4 and 16 months for further endoscopic treatment as required, >98% of patients were adenoma-free and had avoided surgery at 16 months following EMR [32].
Fig. 6.
EMR with piecemeal resection of a large polyp.
Another recent study showed that post-EMR thermal ablation of the EMR margins with the tip of a snare using a soft coagulation current can significantly reduce recurrence rates [33]. Three hundred and ninety patients with lateral spreading tumors, 20 mm or larger in size, were randomized to receive thermal treatment of the EMR margin with snare tip soft coagulation or no treatment. At the first follow-up investigation, recurrence rates were 5.2% in the treatment group versus 21% in controls. This effect was even more pronounced in patients with polyps larger than 40 mm in size: recurrence rates were 36.4% in patients who underwent coagulation versus 3.3% in controls.
A study from the UK comprising 242 patients correlated the outcome of EMR with the size, morphology, site, and access score (SMSA) [34]. The SMSA system is simple, easy to use, and yields substantial information about the complexity of polyps, the risk of recurrence, and complications (Table 1). Complete endoscopic clearance was achieved in 90% of cases at the first attempt, and in 96% after subsequent resection attempts [35]. This was the first study to correlate the outcome of EMR with the SMSA grade. The SMSA grading system was designed to provide a structured framework for the management of large non-pedunculated colorectal polyps [35]. SMSA grade IV polyps required more attempts to achieve endoscopic clearance and were also associated with a higher risk of complications. A study comprising 2,675 lesions revealed failed single-session EMR for nearly 5% of lateral spreading lesions (LSL); the failure was predicted by the patients' SMSA score. Endoscopic recurrence at first surveillance was less likely for SMSA 2 (OR 0.19) and SMSA 3 (OR 0.33) lesions rather than SMSA 4 lesions [36]. Intraprocedural and clinically significant post-endoscopic bleeding was also significantly less common for SMSA 2 and SMSA 3 LSLs compared with SMSA 4 LSLs [30]. Risk factors for bleeding included lesion size, location of polyps in the right colon, and comorbidities [37, 38, 39].
Table 1.
SMSA scores with corresponding difficulty levels [36]
| Parameter | Score |
|---|---|
| Size | |
| <1 cm | 1 |
| 1–1.9 cm | 3 |
| 2–2.9 cm | 5 |
| 3–3.9 cm | 7 |
| >4 cm | 9 |
| Morphology | |
| Peduncultaed | 1 |
| Sessil | 2 |
| Flat | 3 |
| Site | |
| Left | 1 |
| Right | 2 |
| Access | |
| Easy | 1 |
| Difficult | 3 |
| Polyp level | |
| I | 4–5 |
| II | 6–8 |
| III | 9–12 |
| IV | >12 |
A further drawback of EMR is that it does not permit effective grading or cure of early cancer because of the multipiece nature of resection. The incidence of covert cancer has been reported to range between 8 and 10% in most large series [39]. The poor quality of specimens after piecemeal resection makes it difficult to ascertain the exact depth of dysplasia and the curativeness of resection [40].
However, we still believe that EMR is an effective and safe treatment modality for the large majority of polyps. The risk of recurrence can be minimized by experts who perform wide-field resection at high-volume centers. The advantages of EMR include relatively short procedure times (about 35 min for large lesions) and acceptable complication rates, with delayed bleeding in 0.9% and perforation rates of 0.4–1.3% [41, 42, 43].
Cold Snare Resection
Cold snare resection is the new standard for the treatment of small (< 5 mm) polyps and may also be used for the removal of non-cancerous polyps measuring up to 9 mm in size (Fig. 7). The procedure is associated with short procedure times and post-interventional complications, while ensuring the same efficacy as conventional hot snare resection.
Fig. 7.
Cold snare resection for the treatment of smaller (<5 mm) polyps.
Cold snare resection is performed with dedicated devices and has recently been employed for larger lesions as well. Additional electrocautery is not used. Several studies have been published on this subject. In a recent meta-analysis comprising 8 randomized controlled studies and 3,195 interventions [44], cold snare resection was as effective as conventional techniques, associated with significantly shorter procedure times and lower rates of delayed bleeding. Potential disadvantages were highlighted in a histopathology study comprising 184 polyps [45]: cold snare resection was associated with high rates of specimen damage, high rates of positive margins, and a smaller resection depth. Incomplete resection was mainly observed in patients with serrated/hyperplastic lesions [45]. Cold snare resection is considered unsuitable for malignant lesions [46].
Cold snare polypectomy has also been used in slightly larger lesions. As shown in a recent pooled analysis of 8 studies which included 522 colorectal polyps ≥10 mm (mean polyp size 17.5 mm), the complete resection rate with CsP was 99.3%, with an overall adverse event rate of 1.1%. Intra- and postprocedural bleeding rates were 0.7 and 0.5%, respectively [47]. These data indicate that CsP indeed exhibits an excellent success and adverse event rate even for polyps larger ≥10 mm.
A similar series of 41 lesions with a median size of 15 mm revealed no evidence of complications or recurrence after a follow-up period of 6 months [48]. The current European guidelines recommend cold snare polypectomy as the preferred technique for the removal of diminutive polyps (size ≤5 mm). The guidelines also recommend the technique for sessile polyps of 6–9 mm, although we lack formal evidence of its efficacy compared to conventional hot snare polypectomy in this setting [41]. It may be concluded that cold snare resection has become a standard treatment for diminutive polyps (up to 5 mm in size), and also small sessile non-cancerous polyps (up to 9 mm in size). Larger studies will be needed to determine the exact role of cold snare resection in the removal of larger polyps.
Underwater EMR
Conventional EMR employs gas insufflation for distension of the bowel. Better visualization of the field of surgery is offset by thinning of the bowel and a higher risk of perforation. Submucosal injection has been used to increase bowel thickness for safe snaring of the lesion. However, submucosal injection may hinder resection and prove difficult in patients with scarred polyps [49].
One potential solution to the problem is to immerse the polyp “underwater.” The technique is performed without a submucosal injection. Immersion of the polyps in water causes them to “float” away from the muscularis propria without over-distension of the gastrointestinal tract wall, thus reducing the risk of deep thermal injury and perforation [50]. The initial study was performed on 62 polyps with a mean size of 34 mm [51]. Delayed bleeding occurred in 3 patients, a recurrence was noted in 1 patient, and no perforation was encountered. Drawbacks included the limited number of cases, but more importantly the short period of follow-up and the uncontrolled nature of the study.
Although the technique is developed on the premise of reducing complication rates, one case of underwater perforation has been reported [52]. Underwater EMR appears to be an interesting alternative to conventional EMR, but published data on the subject are limited. A carefully designed comparison of underwater EMR and conventional EMR will be needed before the former may be suggested as a mainstream technique. A number of promising new techniques such as full wall resection are available for the treatment of scarred polyps not amenable to submucosal injection.
Endoscopic Submucosal Dissection
The disadvantage of EMR is the lesion size of approximately 20 mm. Large lesions cannot be resected en bloc and must be removed in fragments, with all of the abovementioned disadvantages. Incomplete endoscopic resection of adenomas is believed to be responsible for 20–25% of interval cancers [53]. Recurrence rates underline the need for endoscopic controls and compliance with follow-up endoscopy [54, 55].
ESD, developed in Japan in 2003 for the treatment of early gastric cancer, offers the option of en bloc resection for larger lesions. It involves submucosal injection and the use of an ESD knife to perform a mucosal incision, followed by submucosal dissection beneath the lesion to yield an en bloc R0 specimen with no size restriction (Fig. 8). This enhances the reliability of histological diagnosis and reduces recurrence rates.
Fig. 8.
ESD of an early gastric cancer.
However, ESD is technically more demanding than EMR and involves longer procedure times. A recent meta-analysis yielded a median procedure time of about 70 min [56, 57]. The level of complexity mainly depends on endoscopic access to the lesion, access to the submucosal compartment, and last but not least, the experience of the endoscopist [58].
For the abovementioned reasons, this method is not regarded as a standard in Europe. However, Japanese guidelines recommend ESD as the standard technique for large neoplastic lesions not amenable to en bloc resection, notwithstanding the fact that higher complication rates have been reported for ESD compared to EMR [59, 60, 61, 62]. A recent cost-effectiveness analysis of wide-field EMR versus ESD concluded that selective ESD is the most cost-effective option for high-risk colorectal lesions [63]. ESD is superior to laparoscopic surgery in many respects, although a formal head-to-head comparison is still lacking [64, 65, 66, 67].
In a frequently cited review, the authors examined 6,077 cases of ESD in 27 studies, compared outcomes with a meta-analysis of 11,873 EMR cases, and found significant differences between ESD and EMR in terms of recurrence rates (1.2 vs. 10.4%) and perforation (4.8 vs. 0.9%) [30]. The authors concluded that ESD was highly effective in ensuring en bloc resection with very low recurrence rates, but was achieved at the expense of a high rate of procedure-related complications. A further Japanese study reported a local recurrence rate of <2% after colorectal ESD [68]. Delayed bleeding was noted in 1% of cases, but the perforation rate (5.5%) was higher than that for EMR [69].
In summary, adverse events are more common in ESD than EMR. Perforation rates of approximately 4.8% (range 2–14) have been reported for ESD [70, 71]. However, the majority of perforations are minor and can be closed with standard clips during or at the end of the procedure. The rate of emergency surgery is relatively low (about 0.5%). Delayed bleeding is observed after 1.5–2.8% of interventions. Strictures are encountered occasionally after circumferential resection in the rectum [72].
In a large systematic review and meta-analysis, the authors reviewed 97 studies comprising 18,764 colorectal lesions removed by the standard ESD technique [73], reported an R0 resection rate of 82.9% (95% CI 80.4–85.1), and an en bloc resection rate of 91% (95% CI 89.2–92.5). Delayed bleeding was seen in 2.7% (95% CI 2.2–3.2), and perforation in 5.2% of procedures (95% CI 4.4–6.1%). The recurrence rate at 12 months was 2% (95% CI 1.3–3.0). It should be noted that all of the abovementioned data were derived from expert execution of the respective procedures.
In one study, the outcome of ESD differed significantly between Japan and Western countries (Europe and the USA; Table 2). The more frequent use of ESD in Japan was related to better training, skills, and careful lesion selection. Experience in gastric ESD is recommended prior to colorectal ESD. In a Japanese study, the authors analyzed success and complication rates in 180 colorectal ESDs performed by endoscopists with little or no experience in gastric ESD [74]. They demonstrated an en bloc and R0 resection rate of 88 and 75%, respectively, which improved with experience. Perforation rates also improved from 10.0 to 3.3%. This suggested that beginners should first work on rectal lesions and approach more challenging procedures after gaining experience.
Table 2.
Different ESD results between Asian and Western countries [107]
| Outcome rate | Non-Asian countries, % | Asian countries, % |
|---|---|---|
| En bloc resection | 81.2 | 93.0 |
| R0 resection | 71.3 | 85.6 |
| Need for surgery post-ESD | 3.1 | 0.8 |
| Adverse event | ||
| Delayed bleeding | 4.2 | 2.4 |
| Perforation | 8.6 | 4.5 |
Experience with regard to ESD is limited in the Western world. This is mainly due to the paucity of training opportunities, the high level of skills needed to perform colorectal ESD, the significantly longer procedure times for the method, and probably higher complication rates compared to piecemeal EMR [75]. However, under the supervision of Japanese ESD experts, an endoscopist will be able to achieve competence in ESD as well [76, 77, 78].
Data concerning ESD in the Western hemisphere are scarce and comprise small case series. Only 2 publications have addressed large patient populations (n >150) [79, 80]. Both en bloc and R0 resection rates were lower in these publications than in studies based on Asian populations.
An argument in favor of ESD is that en bloc excision serves as an effective cure for early cancer and is associated with a favorable prognosis (SM-1, well differentiated, L0, V0). Current ESGE guidelines recommend the use of ESD in cases of strong suspicion of submucosal invasion and in complex lesions for which en bloc resection with EMR would be unfeasible [81]. The ESGE guidelines state that LST-NG (lateral spreading tumor − non-granular type) lesions, those with a surface pattern suggestive of superficial invasion, and scarred polyps would qualify for ESD.
In the absence of appropriate training facilities and trainers for ESD in the Western world, a hybrid technique consisting of ESD and pre-cut EMR (EMR-PO) has been proposed as an alternative to ESD. Technically, these techniques may be grouped under KAR. A mucosal incision performed around the lesion is followed by snare resection. In a study from the UK comprising 170 patients who underwent KAR for large polyps (mean size 46 mm), the authors reported cure rates of 87% at the first resection attempt, and an en bloc resection rate of 41% [82]. Complication rates were similar to those for EMR, the perforation rate was 1%, and the delayed bleeding rate <5% [83].
Lee et al. [84] compared pre-cutting before EMR (EMR-P) with conventional EMR and ESD: 523 non-pedunculated colorectal tumors measuring >20 mm were removed with EMR, EMR-P, or ESD. En bloc resection rates were 42.9% (EMR), 65.2% (EMR-P), and 92.7% (ESD), and complete resection rates were 32.9% (EMR), 59.4% (EMR-P), and 87.6% (ESD) [84]. Although the investigation was not a randomized trial, it suggests that pre-cut EMR is technically superior to conventional EMR, yet still inferior to ESD. KAR or EMR-P appears to be a meaningful step in the endoscopist's transition from EMR to ESD. KAR serves as an intermediate step between EMR and ESD for Western endoscopists.
In summary, ESD is an effective technique in expert hands. Lesions highly suspicious of cancer and those larger than 20 mm in size are the best candidates for ESD.
Endoscopic Full-Thickness Resection
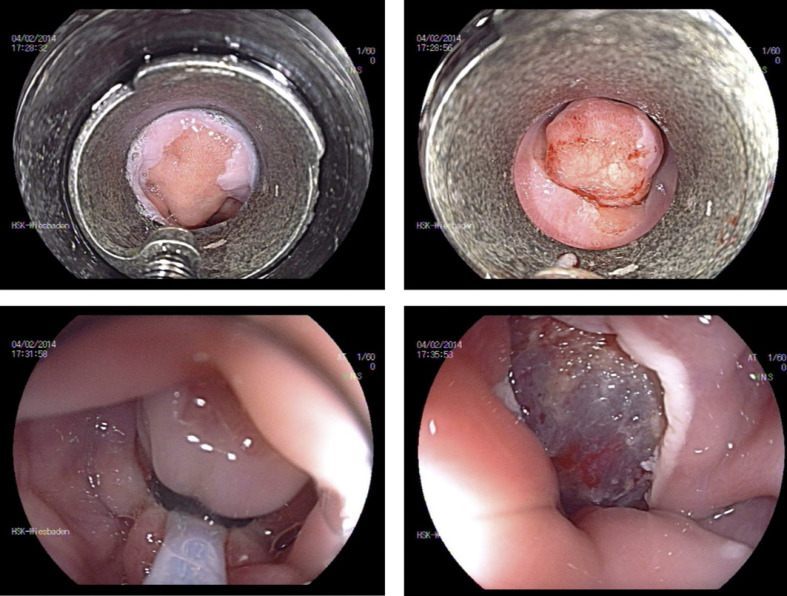
A commercially available full-thickness resection device was introduced recently in Europe (FTRD, Ovesco, Germany) [85, 86, 87, 88, 89]. The single-step full-thickness resection device combines a modified over-the-scope clip with a transparent cap and a preloaded snare. Technically, the colonoscope is retracted after marking the lesion, the FTRD is mounted and advanced to the target. The lesion is then pulled into the resection cap. After deployment of the clip, the snare is closed and electrocautery used to remove the full colonic wall above the clip (Fig. 9). The clip is provided with additional side pins for better anchorage at the margins of the defect.
Fig. 9.
A–F EFTR.
Typical indications for endoscopic full-thickness resection (EFTR) include lesions with severe fibrosis due to scar formation in recurrent adenoma after previous resection. EFTR may also be used in patients with adenomas in specific anatomical locations associated with a significant risk of perforation, such as those close to a diverticulum or at the appendiceal orifice.
Despite the paucity of data concerning the efficacy of the method, FTRD has been successful and is being used increasingly often in Europe and other Western countries [90, 91, 92, 93]. In the largest prospective study published so far, the R0 resection rate was 77.7% for 127 patients with non-lifting adenomas. The overall complication rate was 9.9%, including perforation, fistula, bleeding, and appendicitis. The rate of emergency surgery was 2.2% [94]. Procedure times were longer than those for EMR, and were mainly related to the location of the lesion. Access to proximal locations may be difficult or even impossible. The size of the lesions is limited by the size of the cap. FTRD has been approved for the upper gastrointestinal tract and may also be useful for the resection of submucosal tumors, such as carcinoids, GISTs, or fibrotic polyps in the small bowel.
The principal feature of the procedure is single-step resection and simultaneous closure of the defect. EFTR is still in its infancy but the procedure is promising. Lesion size is a limitation of the technique [93]. As such, a large prospective multicenter study has shown that the R0 resection rate is significantly higher in lesions ≤2 vs. >2 cm (81.2 vs. 58.1%, respectively) [94]. Besides, flexibility and visibility are limited when the scope is advanced with the mounted cap.
Complications of Resection
Bleeding and perforation are the principal complications of all resection techniques. Usually intraprocedural bleeding occurs after resection, is typically controlled by diathermy or hemoclips, and rarely interferes with the outcome of the procedure. However, delayed bleeding is a significant complication after both ESD and EMR [95]. Several attempts have been made to reduce the risk of delayed bleeding. An American study of 524 polyps measuring 20 mm or more revealed that the risk of delayed bleeding after EMR can be reduced from 10% to nearly 2% by full clipping of the EMR defect [96]. However, this was a non-randomized, retrospective comparison with a historic cohort.
A recent randomized controlled trial performed by Zhang et al. [97] demonstrated significantly lower delayed bleeding rates of 1.1% in the clip closure group compared with 6.9% in the non-closure group. This study has been questioned due to the inclusion of small polyps (1–4 cm) and its inadequate power. A large randomized clinical trial on the subject was recently concluded in the USA [98].
One of the disadvantages of prophylactic clipping is the high cost of the procedure. Furthermore, while clipping is safe in experienced hands, closure of the entire defect may be technically challenging, increase the risk of perforation, prolong procedure time, and thus raise the cost of the procedure. Clipping should not be performed routinely, but may be a useful adjunct in a selected group of high-risk patients with several comorbidities who are likely to need anticoagulation or antiplatelet drugs after the procedure.
Future Perspectives
One of the challenges of ESD is the practical difficulty of retracting tissue to facilitate cutting. Ongoing efforts are being made to develop tissue retractors that permit faster and safer submucosal dissection.
Significant attention has been focused recently on enhancing the safety and efficacy of ESD [99, 100]. In a recent randomized clinical trial, conventional ESD was compared with traction ESD using a clip and thread [101]. A surgical thread is tied to the end of a clip, and the combination is used to grasp and lift the specimen. The dissection plane is seen clearly. Traction ESD was associated with an ESD time of 40 min compared to 70 min for conventional ESD. A commercially available device named the Endolifter was tested in the past, but did not gain acceptance because of its cumbersome handling and limited flexibility [102].
The devices currently in use include the tissue retractor system (TRS) developed by Boston Scientific (Marlborough, MA, USA), Dilumen C2 manufactured by Lumendi (High Wycombe, UK), Flex Robotic System by Medrobotics (Raynham, MA, USA), and the S-O clip. The TRS consists of an expandable and dynamically controlled intraluminal chamber mounted on a flexible overtube, and 2 articulating instrument guides (Fig. 3, 4) [103].
DiLumen, another commercially available device, permits quick and easy submucosal dissection [104]. ESD performed in animal models with the aid of a double balloon endoluminal device demonstrated a reduction of dissection time from 57 to 27 min.
These developments are promising in view of the fact that triangulation and the opening of dissection planes are challenging. The potential advantages of these approaches are undisputed, but we predict that the most effective, feasible, and least expensive technique will eventually triumph. The Master and Slave transluminal endoscopic robot (MASTER) is a new development in endoscopy. A pair of robotic “arms” are fixed to the end of the endoscope with an L-shaped hook and grasper. After initial testing on animal models, the MASTER was used in 5 live humans, yielded good results, and caused no complications [105, 106]. Further human trials are awaited.
Conclusion
Endoscopic treatment is now regarded as first-line therapy for benign lesions. En bloc resection is given preference over piecemeal resection. In view of the increasing number of failed resections and the presence of complex polyps, proficiency in performing ESD is a desirable goal for endoscopists. Promising data have been reported from Asia, but less so in the Western hemisphere. The range of lesions amenable to endoscopic treatment has been extended by new techniques, such as KAR or EFTR. Early submucosal invasive tumors are now candidates for ESD or full-thickness resection, although the notion was inconceivable a few years ago.
The steep learning curve for the technique remains its greatest challenge. Case selection is a crucial step. The best approach for effective treatment of a lesion should be determined with caution. The numerous challenges serve as an opportunity to reorganize training facilities and refine endoscopic skills. Promising new developments in the future are likely to make existing procedures even more feasible and efficient.
Conflict of Interest Statement
A.H., R.A., and. T.R have no conflicts of interest. M.F.N. has served as an advisor for Pentax, Janssen, PPM, Takeda, Roche, Boehringer, and Abbvie.
Funding Sources
There was no funding for this work.
Author Contributions
A.H. wrote the manuscript and was responsible for the literature research. R.A., T.R., and M.F.N. gave advice, especially in the molecular imaging section.
References
- 1.Soetikno RM, Gotoda T, Nakanishi Y, Soehendra N. Endoscopic mucosal resection. Gastrointest Endosc. 2003 Apr;57((4)):567–79. doi: 10.1067/mge.2003.130. [DOI] [PubMed] [Google Scholar]
- 2.Sano Y, Ikematsu H, Fu KI, Emura F, Katagiri A, Horimatsu T, et al. Meshed capillary vessels by use of narrow-band imaging for differential diagnosis of small colorectal polyps. Gastrointest Endosc. 2009 Feb;69((2)):278–83. doi: 10.1016/j.gie.2008.04.066. [DOI] [PubMed] [Google Scholar]
- 3.Hayashi N, Tanaka S, Hewett DG, Kaltenbach TR, Sano Y, Ponchon T, et al. Endoscopic prediction of deep submucosal invasive carcinoma: validation of the narrow-band imaging international colorectal endoscopic (NICE) classification. Gastrointest Endosc. 2013 Oct;78((4)):625–32. doi: 10.1016/j.gie.2013.04.185. [DOI] [PubMed] [Google Scholar]
- 4.Holt BA, Bourke MJ. Wide field endoscopic resection for advanced colonic mucosal neoplasia: current status and future directions. Clin Gastroenterol Hepatol. 2012 Sep;10((9)):969–79. doi: 10.1016/j.cgh.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 5.Lambert R, Lightdale CJ, Participants in the Paris Workshop The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003 Dec;58((6 Suppl)):S3–43. doi: 10.1016/s0016-5107(03)02159-x. [DOI] [PubMed] [Google Scholar]
- 6.Axon A, Diebold MD, Fujino M, et al. Endoscopic Classification Review Group Update on the paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy. 2005 Jun;37((6)):570–8. doi: 10.1055/s-2005-861352. [DOI] [PubMed] [Google Scholar]
- 7.Hurlstone DP, Sanders DS, Cross SS, Adam I, Shorthouse AJ, Brown S, et al. Colonoscopic resection of lateral spreading tumours: a prospective analysis of endoscopic mucosal resection. Gut. 2004 Sep;53((9)):1334–9. doi: 10.1136/gut.2003.036913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burgess NG, Hourigan LF, Zanati SA, Brown GJ, Singh R, Raftopoulos SC, et al. Gross morphology and lesion location stratify the risk of invasive disease in advanced mucosal neoplasia of the colon: results from a large multicenter cohort. Gastrointest Endosc. 2014;79((5)):AB556. [Google Scholar]
- 9.Sano Y, Tanaka S, Kudo SE, Saito S, Matsuda T, Wada Y, et al. Narrow-band imaging (NBI) magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Dig Endosc. 2016 Jul;28((5)):526–33. doi: 10.1111/den.12644. [DOI] [PubMed] [Google Scholar]
- 10.Kudo S, Kashida H, Nakajima T, Tamura S, Nakajo K. Endoscopic diagnosis and treatment of early colorectal cancer. World J Surg. 1997 Sep;21((7)):694–701. doi: 10.1007/s002689900293. [DOI] [PubMed] [Google Scholar]
- 11.Matsuda T, Saito Y, Nakajima T, Sakamoto T, Ikematsu H, Sano Y, et al. Macroscopic estimation of submucosal invasion in the colon. Tech Gastrointest Endosc. 2011;13((1)):24–32. [Google Scholar]
- 12.Matsuda T, Fujii T, Saito Y, Nakajima T, Uraoka T, Kobayashi N, et al. Efficacy of the invasive/non-invasive pattern by magnifying chromoendoscopy to estimate the depth of invasion of early colorectal neoplasms. Am J Gastroenterol. 2008 Nov;103((11)):2700–6. doi: 10.1111/j.1572-0241.2008.02190.x. [DOI] [PubMed] [Google Scholar]
- 13.Wada Y, Kashida H, Kudo SE, Misawa M, Ikehara N, Hamatani S. Diagnostic accuracy of pit pattern and vascular pattern analyses in colorectal lesions. Dig Endosc. 2010 Jul;22((3)):192–9. doi: 10.1111/j.1443-1661.2010.00983.x. [DOI] [PubMed] [Google Scholar]
- 14.Iwatate M, Sano Y, Tanaka S, Kudo SE, et al. Japan NBI Expert Team (JNET) Validation study for development of the Japan NBI Expert Team classification of colorectallesions. Dig Endosc. 2018;30:642–51. doi: 10.1111/den.13065. [DOI] [PubMed] [Google Scholar]
- 15.Hirata D, Kashida H, Iwatate M, Tochio T, Teramoto A, Sano Y, et al. Effective use of the Japan Narrow Band Imaging Expert Team classification based on diagnostic performance and confidence level. World J Clin Cases. 2019 Sep;7((18)):2658–65. doi: 10.12998/wjcc.v7.i18.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenberg N. Submucosal saline wheal as safety factor in fulguration or rectal and sigmoidal polypi. AMA Arch Surg. 1955 Jan;70((1)):120–2. doi: 10.1001/archsurg.1955.01270070122021. [DOI] [PubMed] [Google Scholar]
- 17.Deyhle P, Jenny S, Fumagalli I. [Endoscopic polypectomy in the proximal colon. A diagnostic, therapeutic (and preventive?) intervention] Dtsch Med Wochenschr. 1973 Feb;98((5)):219–20. doi: 10.1055/s-0028-1106782. [DOI] [PubMed] [Google Scholar]
- 18.Kato H, Haga S, Endo S, Hashimoto M, Katsube T, Oi I, et al. Lifting of lesions during endoscopic mucosal resection (EMR) of early colorectal cancer: implications for the assessment of resectability. Endoscopy. 2001 Jul;33((7)):568–73. doi: 10.1055/s-2001-15308. [DOI] [PubMed] [Google Scholar]
- 19.Ishiguro A, Uno Y, Ishiguro Y, Munakata A, Morita T. Correlation of lifting versus non-lifting and microscopic depth of invasion in early colorectal cancer. Gastrointest Endosc. 1999 Sep;50((3)):329–33. doi: 10.1053/ge.1999.v50.98591. [DOI] [PubMed] [Google Scholar]
- 20.Friedland S, Shelton A, Kothari S, Kochar R, Chen A, Banerjee S. Endoscopic management of nonlifting colon polyps. Diagn Ther Endosc. 2013;2013:412936. doi: 10.1155/2013/412936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peters FP, Kara MA, Curvers WL, Rosmolen WD, Fockens P, Krishnadath KK, et al. Multiband mucosectomy for endoscopic resection of Barrett's esophagus: feasibility study with matched historical controls. Eur J Gastroenterol Hepatol. 2007 Apr;19((4)):311–5. doi: 10.1097/MEG.0b013e328080ca90. [DOI] [PubMed] [Google Scholar]
- 22.Pouw RE, van Vilsteren FG, Peters FP, Alvarez Herrero L, Ten Kate FJ, Visser M, et al. Randomized trial on endoscopic resection-cap versus multiband mucosectomy for piecemeal endoscopic resection of early Barrett's neoplasia. Gastrointest Endosc. 2011 Jul;74((1)):35–43. doi: 10.1016/j.gie.2011.03.1243. [DOI] [PubMed] [Google Scholar]
- 23.Im YC, Jung SW, Cha HJ, Yang SS, Kim GY, Yi YA, et al. The effectiveness of endoscopic submucosal resection with a ligation device for small rectal carcinoid tumors: focused on previously biopsied tumors. Surg Laparosc Endosc Percutan Tech. 2014 Jun;24((3)):264–9. doi: 10.1097/SLE.0b013e3182901176. [DOI] [PubMed] [Google Scholar]
- 24.Ono A, Fujii T, Saito Y, Matsuda T, Lee DT, Gotoda T, et al. Endoscopic submucosal resection of rectal carcinoid tumors with a ligation device. Gastrointest Endosc. 2003 Apr;57((4)):583–7. doi: 10.1067/mge.2003.142. [DOI] [PubMed] [Google Scholar]
- 25.Niimi K, Goto O, Fujishiro M, Kodashima S, Ono S, Mochizuki S, et al. Endoscopic mucosal resection with a ligation device or endoscopic submucosal dissection for rectal carcinoid tumors: an analysis of 24 consecutive cases. Dig Endosc. 2012 Nov;24((6)):443–7. doi: 10.1111/j.1443-1661.2012.01303.x. [DOI] [PubMed] [Google Scholar]
- 26.He L, Deng T, Luo H. Efficacy and safety of endoscopic resection therapies for rectal carcinoid tumors: a meta-analysis. Yonsei Med J. 2015 Jan;56((1)):72–81. doi: 10.3349/ymj.2015.56.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iishi H, Tatsuta M, Kitamura S, Narahara H, Iseki K, Ishiguro S. Endoscopic resection of large sessile colorectal polyps using a submucosal saline injection technique. Hepatogastroenterology. 1997 May-Jun;44((15)):698–702. [PubMed] [Google Scholar]
- 28.Kudo S, Tamegai Y, Yamano H, Imai Y, Kogure E, Kashida H. Endoscopic mucosal resection of the colon: the Japanese technique. Gastrointest Endosc Clin N Am. 2001 Jul;11((3)):519–35. [PubMed] [Google Scholar]
- 29.Hassan C, Repici A, Sharma P, Correale L, Zullo A, Bretthauer M, et al. Efficacy and safety of endoscopic resection of large colorectal polyps: a systematic review and meta-analysis. Gut. 2016 May;65((5)):806–20. doi: 10.1136/gutjnl-2014-308481. [DOI] [PubMed] [Google Scholar]
- 30.De Ceglie A, Hassan C, Mangiavillano B, Matsuda T, Saito Y, Ridola L, et al. Endoscopic mucosal resection and endoscopic submucosal dissection for colorectal lesions: A systematic review. Crit Rev Oncol Hematol. 2016 Aug;104:138–55. doi: 10.1016/j.critrevonc.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 31.Moss A, Bourke MJ, Williams SJ, Hourigan LF, Brown G, Tam W, et al. Endoscopic mucosal resection outcomes and prediction of submucosal cancer from advanced colonic mucosal neoplasia. Gastroenterology. 2011 Jun;140((7)):1909–18. doi: 10.1053/j.gastro.2011.02.062. [DOI] [PubMed] [Google Scholar]
- 32.Moss A, Williams SJ, Hourigan LF, Brown G, Tam W, Singh R, et al. Long-term adenoma recurrence following wide-field endoscopic mucosal resection (WF-EMR) for advanced colonic mucosal neoplasia is infrequent: results and risk factors in 1000 cases from the Australian Colonic EMR (ACE) study. Gut. 2015 Jan;64((1)):57–65. doi: 10.1136/gutjnl-2013-305516. [DOI] [PubMed] [Google Scholar]
- 33.Klein A, Tate DJ, Jayasekeran V, Hourigan L, Singh R, Brown G, et al. Thermal ablation of mucosal defect margins reduces adenoma recurrence after colonic endoscopic mucosal resection. Gastroenterology. 2019 Feb;156((3)):604–613.e3. doi: 10.1053/j.gastro.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Longcroft-Wheaton G, Duku M, Mead R, Basford P, Bhandari P. Risk stratification system for evaluation of complex polyps can predict outcomes of endoscopic mucosal resection. Dis Colon Rectum. 2013 Aug;56((8)):960–6. doi: 10.1097/DCR.0b013e31829193e0. [DOI] [PubMed] [Google Scholar]
- 35.Rutter MD, Chattree A, Barbour JA, Thomas-Gibson S, Bhandari P, Saunders BP, et al. British Society of Gastroenterology/Association of Coloproctologists of Great Britain and Ireland guidelines for the management of large non-pedunculated colorectal polyps. Gut. 2015 Dec;64((12)):1847–73. doi: 10.1136/gutjnl-2015-309576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sidhu M, Tate DJ, Desomer L, Brown G, Hourigan LF, Lee EY, et al. The size, morphology, site, and access score predicts critical outcomes of endoscopic mucosal resection in the colon. Endoscopy. 2018 Jul;50((7)):684–92. doi: 10.1055/s-0043-124081. [DOI] [PubMed] [Google Scholar]
- 37.Bahin FF, Rasouli KN, Byth K, Hourigan LF, Singh R, Brown GJ, et al. Prediction of Clinically Significant Bleeding Following Wide-Field Endoscopic Resection of Large Sessile and Laterally Spreading Colorectal Lesions: A Clinical Risk Score. Am J Gastroenterol. 2016 Aug;111((8)):1115–22. doi: 10.1038/ajg.2016.235. [DOI] [PubMed] [Google Scholar]
- 38.Belderbos TD, Leenders M, Moons LM, Siersema PD. Local recurrence after endoscopic mucosal resection of nonpedunculated colorectal lesions: systematic review and meta-analysis. Endoscopy. 2014 May;46((5)):388–402. doi: 10.1055/s-0034-1364970. [DOI] [PubMed] [Google Scholar]
- 39.Briedigkeit A, Sultanie O, Sido B, Dumoulin FL. Endoscopic mucosal resection of colorectal adenomas [{GT}] 20 mm: risk factors for recurrence. World J Gastrointest Endosc. 2016 Mar;8((5)):276–81. doi: 10.4253/wjge.v8.i5.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burgess NG, Hourigan LF, Zanati SA, Brown GJ, Singh R, Williams SJ, et al. Risk stratification for covert invasive cancer among patients referred for colonic endoscopic mucosal resection: a large multi-center cohort. Gastroenterology. 2017 Sep;153((3)):732–742.e1. doi: 10.1053/j.gastro.2017.05.047. [DOI] [PubMed] [Google Scholar]
- 41.Schmiegel W, Buchberger B, Follmann M, Graeven U, Heinemann V, Langer T, et al. S3-Leitlinie − Kolorektales Karzinom. Z Gastroenterol. 2017 Dec;55((12)):1344–498. doi: 10.1055/s-0043-121106. [DOI] [PubMed] [Google Scholar]
- 42.Moss A, Williams SJ, Hourigan LF, Brown G, Tam W, Singh R, et al. Long-term adenoma recurrence following wide-field endoscopic mucosal resection (WF-EMR) for advanced colonic mucosal neoplasia is infrequent: results and risk factors in 1000 cases from the Australian Colonic EMR (ACE) study. Gut. 2015 Jan;64((1)):57–65. doi: 10.1136/gutjnl-2013-305516. [DOI] [PubMed] [Google Scholar]
- 43.Holmes I, Friedland S. Endoscopic Mucosal Resection versus Endoscopic Submucosal Dissection for Large Polyps: A Western Colonoscopist's View. Clin Endosc. 2016 Sep;49((5)):454–6. doi: 10.5946/ce.2016.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shinozaki S, Kobayashi Y, Hayashi Y, Sakamoto H, Lefor AK, Yamamoto H. Efficacy and safety of cold versus hot snare polypectomy for resecting small colorectal polyps: systematic review and meta-analysis. Dig Endosc. 2018 Sep;30((5)):592–9. doi: 10.1111/den.13173. [DOI] [PubMed] [Google Scholar]
- 45.Ito A, Suga T, Ota H, Tateiwa N, Matsumoto A, Tanaka E. Resection depth and layer of cold snare polypectomy versus endoscopic mucosal resection. J Gastroenterol. 2018 Nov;53((11)):1171–8. doi: 10.1007/s00535-018-1446-2. [DOI] [PubMed] [Google Scholar]
- 46.Ferlitsch M, Moss A, Hassan C, Bhandari P, Dumonceau JM, Paspatis G, et al. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2017 Mar;49((3)):270–97. doi: 10.1055/s-0043-102569. [DOI] [PubMed] [Google Scholar]
- 47.Thoguluva Chandrasekar V, Spadaccini M, Aziz M, Maselli R, Hassan S, Fuccio L, et al. Cold snare endoscopic resection of nonpedunculated colorectal polyps larger than 10 mm: a systematic review and pooled-analysis. Gastrointest Endosc. 2019 May;89((5)):929–936.e3. doi: 10.1016/j.gie.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 48.Tate DJ, Awadie H, Bahin FF, Desomer L, Lee R, Heitman SJ, et al. Wide-field piecemeal cold snare polypectomy of large sessile serrated polyps without a submucosal injection is safe. Endoscopy. 2018 Mar;50((3)):248–52. doi: 10.1055/s-0043-121219. [DOI] [PubMed] [Google Scholar]
- 49.Tajika M, Niwa Y, Bhatia V, Kondo S, Tanaka T, Mizuno N, et al. Comparison of endoscopic submucosal dissection and endoscopic mucosal resection for large colorectal tumors. Eur J Gastroenterol Hepatol. 2011 Nov;23((11)):1042–9. doi: 10.1097/MEG.0b013e32834aa47b. [DOI] [PubMed] [Google Scholar]
- 50.Cao Y, Liao C, Tan A, Gao Y, Mo Z, Gao F. Meta-analysis of endoscopic submucosal dissection versus endoscopic mucosal resection for tumors of the gastrointestinal tract. Endoscopy. 2009 Sep;41((9)):751–7. doi: 10.1055/s-0029-1215053. [DOI] [PubMed] [Google Scholar]
- 51.Binmoeller KF, Weilert F, Shah J, Bhat Y, Kane S. “Underwater” EMR without submucosal injection for large sessile colorectal polyps (with video) Gastrointest Endosc. 2012 May;75((5)):1086–91. doi: 10.1016/j.gie.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 52.Ponugoti PL, Rex DK. Perforation during underwater EMR. Gastrointest Endosc. 2016 Sep;84((3)):543–4. doi: 10.1016/j.gie.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 53.Robertson DJ, Lieberman DA, Winawer SJ, Ahnen DJ, Baron JA, Schatzkin A, et al. Colorectal cancers soon after colonoscopy: a pooled multicohort analysis. Gut. 2014 Jun;63((6)):949–56. doi: 10.1136/gutjnl-2012-303796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Belderbos TD, Leenders M, Moons LM, Siersema PD. Local recurrence after endoscopic mucosal resection of nonpedunculated colorectal lesions: systematic review and meta-analysis. Endoscopy. 2014 May;46((5)):388–402. doi: 10.1055/s-0034-1364970. [DOI] [PubMed] [Google Scholar]
- 55.Briedigkeit A, Sultanie O, Sido B, Dumoulin FL. Endoscopic mucosal resection of colorectal adenomas [{GT}] 20 mm: risk factors for recurrence. World J Gastrointest Endosc. 2016 Mar;8((5)):276–81. doi: 10.4253/wjge.v8.i5.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang J, Zhang XH, Ge J, Yang CM, Liu JY, Zhao SL. Endoscopic submucosal dissection vs endoscopic mucosal resection for colorectal tumors: a meta-analysis. World J Gastroenterol. 2014 Jul;20((25)):8282–7. doi: 10.3748/wjg.v20.i25.8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arezzo A, Passera R, Marchese N, Galloro G, Manta R, Cirocchi R. Systematic review and meta-analysis of endoscopic submucosal dissection vs endoscopic mucosal resection for colorectal lesions. United European Gastroenterol J. 2016 Feb;4((1)):18–29. doi: 10.1177/2050640615585470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takeuchi Y, Iishi H, Tanaka S, Saito Y, Ikematsu H, Kudo SE, et al. Factors associated with technical difficulties and adverse events of colorectal endoscopic submucosal dissection: retrospective exploratory factor analysis of a multicenter prospective cohort. Int J Colorectal Dis. 2014 Oct;29((10)):1275–84. doi: 10.1007/s00384-014-1947-2. [DOI] [PubMed] [Google Scholar]
- 59.Tanaka S, Kashida H, Saito Y, Yahagi N, Yamano H, Saito S, et al. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc. 2015 May;27((4)):417–34. doi: 10.1111/den.12456. [DOI] [PubMed] [Google Scholar]
- 60.Conio M, Repici A, Demarquay JF, Blanchi S, Dumas R, Filiberti R. EMR of large sessile colorectal polyps. Gastrointest Endosc. 2004 Aug;60((2)):234–41. doi: 10.1016/s0016-5107(04)01567-6. [DOI] [PubMed] [Google Scholar]
- 61.Oka S, Tanaka S, Kaneko I, Mouri R, Hirata M, Kawamura T, et al. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 2006 Dec;64((6)):877–83. doi: 10.1016/j.gie.2006.03.932. [DOI] [PubMed] [Google Scholar]
- 62.Yamasaki M, Kume K, Yoshikawa I, Otsuki M. A novel method of endoscopic submucosal dissection with blunt abrasion by submucosal injection of sodium carboxymethylcellulose: an animal preliminary study. Gastrointest Endosc. 2006 Dec;64((6)):958–65. doi: 10.1016/j.gie.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 63.Bahin FF, Heitman SJ, Rasouli KN, Mahajan H, McLeod D, Lee EY, et al. Wide-field endoscopic mucosal resection versus endoscopic submucosal dissection for laterally spreading colorectal lesions: a cost-effectiveness analysis. Gut. 2018 Nov;67((11)):1965–73. doi: 10.1136/gutjnl-2017-313823. [DOI] [PubMed] [Google Scholar]
- 64.Kiriyama S, Saito Y, Yamamoto S, Soetikno R, Matsuda T, Nakajima T, et al. Comparison of endoscopic submucosal dissection with laparoscopic-assisted colorectal surgery for early-stage colorectal cancer: a retrospective analysis. Endoscopy. 2012 Nov;44((11)):1024–30. doi: 10.1055/s-0032-1310259. [DOI] [PubMed] [Google Scholar]
- 65.Ahlenstiel G, Hourigan LF, Brown G, Zanati S, Williams SJ, Singh R, et al. Australian Colonic Endoscopic Mucosal Resection (ACE) Study Group Actual endoscopic versus predicted surgical mortality for treatment of advanced mucosal neoplasia of the colon. Gastrointest Endosc. 2014 Oct;80((4)):668–76. doi: 10.1016/j.gie.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 66.Nakamura F, Saito Y, Sakamoto T, Otake Y, Nakajima T, Yamamoto S, et al. Potential perioperative advantage of colorectal endoscopic submucosal dissection versus laparoscopy-assisted colectomy. Surg Endosc. 2015 Mar;29((3)):596–606. doi: 10.1007/s00464-014-3705-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jayanna M, Burgess NG, Singh R, Hourigan LF, Brown GJ, Zanati SA, et al. Cost Analysis of Endoscopic Mucosal Resection vs Surgery for Large Laterally Spreading Colorectal Lesions. Clin Gastroenterol Hepatol. 2016;14:271–278. doi: 10.1016/j.cgh.2015.08.037. e1–2. [DOI] [PubMed] [Google Scholar]
- 68.Fujishiro M, Yahagi N, Kakushima N, Kodashima S, Muraki Y, Ono S, et al. Outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms in 200 consecutive cases. Clin Gastroenterol Hepatol. 2007 Jun;5((6)):678–83. doi: 10.1016/j.cgh.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 69.Repici A, Hassan C, De Paula Pessoa D, Pagano N, Arezzo A, Zullo A, et al. Efficacy and safety of endoscopic submucosal dissection for colorectal neoplasia: a systematic review. Endoscopy. 2012 Feb;44((2)):137–50. doi: 10.1055/s-0031-1291448. [DOI] [PubMed] [Google Scholar]
- 70.Kim YJ, Kim ES, Cho KB, Park KS, Jang BK, Chung WJ, et al. Comparison of clinical outcomes among different endoscopic resection methods for treating colorectal neoplasia. Dig Dis Sci. 2013 Jun;58((6)):1727–36. doi: 10.1007/s10620-013-2560-x. [DOI] [PubMed] [Google Scholar]
- 71.Bhatt A, Abe S, Kumaravel A, Vargo J, Saito Y. Indications and Techniques for Endoscopic Submucosal Dissection. Am J Gastroenterol. 2015 Jun;110((6)):784–91. doi: 10.1038/ajg.2014.425. [DOI] [PubMed] [Google Scholar]
- 72.Maple JT, Abu Dayyeh BK, Chauhan SS, Hwang JH, Komanduri S, Manfredi M, et al. ASGE Technology Committee Endoscopic submucosal dissection. Gastrointest Endosc. 2015;81((6)):1311–25. doi: 10.1016/j.gie.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 73.Fuccio L, Hassan C, Ponchon T, Mandolesi D, Farioli A, Cucchetti A, et al. Clinical outcomes after endoscopic submucosal dissection for colorectal neoplasia: a systematic review and meta-analysis. Gastrointest Endosc. 2017 Jul;86((1)):74–86.e17. doi: 10.1016/j.gie.2017.02.024. [DOI] [PubMed] [Google Scholar]
- 74.Shiga H, Ohba R, Matsuhashi T, Jin M, Kuroha M, Endo K, et al. Feasibility of colorectal endoscopic submucosal dissection (ESD) carried out by endoscopists with no or little experience in gastric ESD. Dig Endosc. 2017 Apr;29(Suppl 2):58–65. doi: 10.1111/den.12814. [DOI] [PubMed] [Google Scholar]
- 75.Watanabe K, Ogata S, Kawazoe S, Watanabe K, Koyama T, Kajiwara T, et al. Clinical outcomes of EMR for gastric tumors: historical pilot evaluation between endoscopic submucosal dissection and conventional mucosal resection. Gastrointest Endosc. 2006 May;63((6)):776–82. doi: 10.1016/j.gie.2005.08.049. [DOI] [PubMed] [Google Scholar]
- 76.Uraoka T, Parra-Blanco A, Yahagi N. Colorectal endoscopic submucosal dissection: is it suitable in western countries? J Gastroenterol Hepatol. 2013 Mar;28((3)):406–14. doi: 10.1111/jgh.12099. [DOI] [PubMed] [Google Scholar]
- 77.Yang DH, Jeong GH, Song Y, Park SH, Park SK, Kim JW, et al. The Feasibility of Performing Colorectal Endoscopic Submucosal Dissection Without Previous Experience in Performing Gastric Endoscopic Submucosal Dissection. Dig Dis Sci. 2015 Nov;60((11)):3431–41. doi: 10.1007/s10620-015-3755-0. [DOI] [PubMed] [Google Scholar]
- 78.Berr F, Wagner A, Kiesslich T, Friesenbichler P, Neureiter D. Untutored learning curve to establish endoscopic submucosal dissection on competence level. Digestion. 2014;89((3)):184–93. doi: 10.1159/000357805. [DOI] [PubMed] [Google Scholar]
- 79.Sauer M, Hildenbrand R, Oyama T, Sido B, Yahagi N, Dumoulin FL. Endoscopic submucosal dissection for flat or sessile colorectal neoplasia [{GT}] 20 mm: A European single-center series of 182 cases. Endosc Int Open. 2016 Aug;4((8)):E895–900. doi: 10.1055/s-0042-111204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Probst A, Ebigbo A, Märkl B, Schaller T, Anthuber M, Fleischmann C, et al. Endoscopic submucosal dissection for early rectal neoplasia: experience from a European center. Endoscopy. 2017 Mar;49((3)):222–32. doi: 10.1055/s-0042-118449. [DOI] [PubMed] [Google Scholar]
- 81.Pimentel-Nunes P, Dinis- Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: ESGE guideline. Endoscopy. 2015;47:829–54. doi: 10.1055/s-0034-1392882. [DOI] [PubMed] [Google Scholar]
- 82.Bhattacharyya R, Chedgy FJ, Kandiah K, Longcroft-Wheaton G, Bhandari P. Knife-assisted snare resection (KAR) of large and refractory colonic polyps at a Western centre: Feasibility, safety and efficacy study to guide future practice. United European Gastroenterol J. 2016 Jun;4((3)):466–73. doi: 10.1177/2050640615615301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chedgy FJ, Bhattacharyya R, Kandiah K, Longcroft-Wheaton G, Bhandari P. Knife-assisted snare resection: a novel technique for resection of scarred polyps in the colon. Endoscopy. 2016 Mar;48((3)):277–80. doi: 10.1055/s-0035-1569647. [DOI] [PubMed] [Google Scholar]
- 84.Lee EJ, Lee JB, Lee SH, Youk EG. Endoscopic treatment of large colorectal tumors: comparison of endoscopic mucosal resection, endoscopic mucosal resection-precutting, and endoscopic submucosal dissection. Surg Endosc. 2012 Aug;26((8)):2220–30. doi: 10.1007/s00464-012-2164-0. [DOI] [PubMed] [Google Scholar]
- 85.Schmidt A, Damm M, Caca K. Endoscopic full-thickness resection using a novel over-the-scope device. Gastroenterology. 2014 Oct;147((4)):740–742.e2. doi: 10.1053/j.gastro.2014.07.045. [DOI] [PubMed] [Google Scholar]
- 86.Schmidt A, Meier B, Caca K. Endoscopic full-thickness resection: current status. World J Gastroenterol. 2015 Aug;21((31)):9273–85. doi: 10.3748/wjg.v21.i31.9273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vitali F, Naegel A, Siebler J, Neurath MF, Rath T. Endoscopic full-thickness resection with an over-the-scope clip device (FTRD) in the colorectum: results from a university tertiary referral center. Endosc Int Open. 2018 Jan;6((1)):E98–103. doi: 10.1055/s-0043-124079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aepli P, Criblez D, Baumeler S, Borovicka J, Frei R. Endoscopic full thickness resection (EFTR) of colorectal neoplasms with the Full Thickness Resection Device (FTRD): clinical experience from two tertiary referral centers in Switzerland. United European Gastroenterol J. 2018 Apr;6((3)):463–70. doi: 10.1177/2050640617728001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schurr MO, Baur FE, Krautwald M, Fehlker M, Wehrmann M, Gottwald T, et al. Endoscopic full-thickness resection and clip defect closure in the colon with the new FTRD system: experimental study. Surg Endosc. 2015 Aug;29((8)):2434–41. doi: 10.1007/s00464-014-3923-x. [DOI] [PubMed] [Google Scholar]
- 90.Fähndrich M, Sandmann M. Endoscopic full-thickness resection for gastrointestinal lesions using the over-the-scope clip system: a case series. Endoscopy. 2015 Jan;47((1)):76–9. doi: 10.1055/s-0034-1377975. [DOI] [PubMed] [Google Scholar]
- 91.Richter-Schrag HJ, Walker C, Thimme R, Fischer A. [Full thickness resection device (FTRD). Experience and outcome for benign neoplasms of the rectum and colon] Chirurg. 2016 Apr;87((4)):316–25. doi: 10.1007/s00104-015-0091-z. [DOI] [PubMed] [Google Scholar]
- 92.Backes Y, Kappelle WF, Berk L, Koch AD, Groen JN, de Vos Tot Nederveen Cappel WH, et al. T1 CRC Working Group Colorectal endoscopic full-thickness resection using a novel, flat-base over-the-scope clip: a prospective study. Endoscopy. 2017 Nov;49((11)):1092–7. doi: 10.1055/s-0043-114730. [DOI] [PubMed] [Google Scholar]
- 93.Al-Bawardy B, Rajan E, Wong Kee Song LM. Over-the-scope clip-assisted endoscopic full-thickness resection of epithelial and subepithelial GI lesions. Gastrointest Endosc. 2017 May;85((5)):1087–92. doi: 10.1016/j.gie.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 94.Schmidt A, Beyna T, Schumacher B, Meining A, Richter-Schrag HJ, Messmann H, et al. Colonoscopic full-thickness resection using an over-the-scope device: a prospective multicentre study in various indications. Gut. 2018 Jul;67((7)):1280–9. doi: 10.1136/gutjnl-2016-313677. [DOI] [PubMed] [Google Scholar]
- 95.Fujiya M, Tanaka K, Dokoshi T, Tominaga M, Ueno N, Inaba Y, et al. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc. 2015 Mar;81((3)):583–95. doi: 10.1016/j.gie.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 96.Liaquat H, Rohn E, Rex DK. Prophylactic clip closure reduced the risk of delayed postpolypectomy hemorrhage: experience in 277 clipped large sessile or flat colorectal lesions and 247 control lesions. Gastrointest Endosc. 2013 Mar;77((3)):401–7. doi: 10.1016/j.gie.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 97.Zhang QS, Han B, Xu JH, Gao P, Shen YC. Clip closure of defect after endoscopic resection in patients with larger colorectal tumors decreased the adverse events. Gastrointest Endosc. 2015 Nov;82((5)):904–9. doi: 10.1016/j.gie.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 98.Feagins LA, Smith AD, Kim D, Halai A, Duttala S, Chebaa B, et al. Efficacy of Prophylactic Hemoclips in Prevention of Delayed Post-Polypectomy Bleeding in Patients With Large Colonic Polyps. Gastroenterology. 2019 Oct;157((4)):967–976.e1. doi: 10.1053/j.gastro.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 99.Toyonaga T, Man-i M, Chinzei R, Takada N, Iwata Y, Morita Y, et al. Endoscopic treatment for early stage colorectal tumors: the comparison between EMR with small incision, simplified ESD, and ESD using the standard flush knife and the ball tipped flush knife. Acta Chir Iugosl. 2010;57((3)):41–6. doi: 10.2298/aci1003041t. [DOI] [PubMed] [Google Scholar]
- 100.Yamamoto K, Michida T, Nishida T, Hayashi S, Naito M, Ito T. Colorectal endoscopic submucosal dissection: recent technical advances for safe and successful procedures. World J Gastrointest Endosc. 2015 Oct;7((14)):1114–28. doi: 10.4253/wjge.v7.i14.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yamasaki Y, Takeuchi Y, Uedo N, Kanesaka T, Kato M, Hamada K, et al. Efficacy of traction-assisted colorectal endoscopic submucosal dissection using a clip-and-thread technique: A prospective randomized study. Dig Endosc. 2018 Jul;30((4)):467–76. doi: 10.1111/den.13036. [DOI] [PubMed] [Google Scholar]
- 102.Teoh AY, Chiu PW, Hon SF, Mak TW, Ng EK, Lau JY. Ex vivo comparative study using the Endolifter® as a traction device for enhancing submucosal visualization during endoscopic submucosal dissection. Surg Endosc. 2013 Apr;27((4)):1422–7. doi: 10.1007/s00464-012-2583-y. [DOI] [PubMed] [Google Scholar]
- 103.Kantsevoy SV, Bitner M, Piskun G. New endoscopic platform for endoluminal en bloc tissue resection in the gastrointestinal tract (with videos) Surg Endosc. 2016 Jul;30((7)):3145–51. doi: 10.1007/s00464-015-4544-8. [DOI] [PubMed] [Google Scholar]
- 104.Sharma S, Momose K, Hara H, East J, Sumiyama K, Nakajima K, et al. Facilitating endoscopic submucosal dissection: double balloon endolumenal platform significantly improves dissection time compared with conventional technique (with video) Surg Endosc. 2019 Jan;33((1)):315–21. doi: 10.1007/s00464-018-6336-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang Z, Phee SJ, Lomanto D, Goel R, Rebala P, Sun ZL, et al. Endoscopic submucosal dissection of gastric lesions by using a master and slave transluminal endoscopic robot: an animal survival study. Endoscopy. 2012 Jul;44((7)):690–4. doi: 10.1055/s-0032-1309404. [DOI] [PubMed] [Google Scholar]
- 106.Chiu PW, Phee SJ, Bhandari P, Sumiyama K, Ohya T, Wong J, et al. Enhancing proficiency in performing endoscopic submucosal dissection (ESD) by using a prototype robotic endoscope. Endosc Int Open. 2015 Oct;3((5)):E439–42. doi: 10.1055/s-0034-1393178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hossain E, Alkandari A, Bhandari P. Future of Endoscopy: brief review of current and future endoscopic resection techniques for colorectal lesions. Dig Endosc. 2020 May;32((4)):503–11. doi: 10.1111/den.13475. [DOI] [PubMed] [Google Scholar]