Abstract
Immunotherapy in the critically ill is an appealing notion because of the apparent abnormal immune and inflammatory responses seen in so many patients. The administration of a medication that could alter immune responses and decrease mortality in patients with sepsis could represent a ‘magic bullet’. Various approaches have been tried over the last 20 yr: steroids; anti-endotoxin or anti-cytokine antibodies; cytokine receptor antagonists; and other agents with immune-modulating side-effects. However, in some respects, research along these lines has been unsuccessful or disappointing at best. The current state of knowledge is summarized with particular reference to sepsis and the acute respiratory distress syndrome.
Keywords: cytokines; immune system; intensive care; respiratory distress system, adult; systemic inflammatory response system
Drugs that alter immune function have been used therapeutically for at least 50 yr. The most commonly thought of immune system modifiers or immunomodulators are the glucocorticoids (GCs), which are synthetic forms of the physiologically active hormone cortisol. These are used frequently in the intensive care unit (ICU) in conditions where anti-inflammatory action or immunosuppression is required. For example, some patients have immune disorders that require treatment with GCs and happen to be admitted to ICU because of a requirement for organ support (e.g. renal failure resulting from immune vasculitis). Occasionally, corticosteroids are used to help prevent reactions to certain drugs and blood products. In other patients, GCs and other more powerful agents (e.g. methotrexate and ciclosporin) are used specifically for their immunosuppressive actions in patients undergoing transplantation. New immunotherapy drugs targeted specifically at critically ill patients on the ICU include immunostimulants to reverse the immunoparalysis seen in some critically ill patients, and immunosuppressive treatments for conditions such as sepsis and acute respiratory distress syndrome (ARDS). Many clinical trials of such agents have been undertaken and unfortunately few have shown positive results, despite some of the drugs being used successfully in more chronic inflammatory conditions.
Other drugs are commonly used which have immune-modulating actions or side-effects but are not thought of as immunotherapy and include non-steroidal anti-inflammatory agents (NSAIDs), histamine 2 (H2) receptor antagonists, anaesthetic or sedative agents, antibiotics, statins, and drugs given for analgesia including opioids.
Targets for immune modulation
The body possesses two main defences against infection—the innate immune response and the acquired immune response. Mechanical barriers, the skin, mucous secretion, ciliary action, and gastric acid constitute the innate or non-specific response. If these barriers are crossed, then micro-organisms are destroyed by soluble factors, such as lysozyme and complement, and by phagocytosis.
Cytokines
Activation of the innate immune response results in an inflammatory response regulated by soluble molecules called cytokines. This group of molecules includes chemokines, interleukins (ILs), growth factors, and interferons (IFNs). They are low-molecular-weight secreted proteins which regulate both the amplitude and the duration of the immune response. They have a transient action which is tightly regulated. Cytokines are highly active at very low concentrations, combining with small numbers of high affinity cell surface receptors and producing changes in the patterns of RNA and protein synthesis. They have multiple effects on growth and differentiation in a variety of cell types with considerable overlap and redundancy between them.
Chemokines are a family of molecules characterized by four conserved cysteine residues, which stimulate chemotaxis of immune cells to sites of infection. IFNs (α, β, γ) are a family of broad-spectrum antiviral agents which also modulate the activity of other cells, particularly IL-8 and platelet-activating factor (PAF), antibody production by B lymphocytes, and activation of cytotoxic macrophages. Growth factors regulate the differentiation, proliferation, activity, and function of specific cell types. The best known are colony stimulating factors which cause colony formation by haematogenic progenitor cells (e.g. granulocyte-macrophage colony stimulating factor or GM-CSF).
In addition to the low-molecular-weight protein mediators, there are also lipid mediators of inflammation which include PAF and arachidonic acid metabolites. PAF is released from a variety of cells and primes macrophages to other inflammatory mediators and also causes alterations of microvascular permeability. Arachidonic acid metabolites also have profound inflammatory and vascular actions, and may regulate and be regulated by other cytokines.
Tumour necrosis factor (TNF) α has a central role in initiating the network of other cytokines and factors that make up the immune response to infection. The wide variety of effects is attributable to the ubiquity of the TNF receptor, the ability to activate multiple signal transduction pathways, and the ability to induce or suppress an array of genes including those for growth factors, cytokines, transcription factors, receptors, and acute phase proteins.
The biological activities of cytokines are regulated by specific cellular receptors often comprising multiple subunits providing phased stages of activation. Soluble cytokine receptors have been identified which compete with membrane-bound receptors, thus regulating cytokine signals.74 In addition, some cytokines are also regulated by receptor antagonists. The receptor antagonist for IL-1 (IL-1ra) competes with cell receptors for IL-1, but when bound does not induce signalling. IL-1ra binds to cell receptors much more avidly than to soluble receptors, such that soluble receptors will have little effect on the inhibitory action of the receptor antagonist. IL-1ra is independently regulated by other cytokines as part of the inflammatory process.21
Progress of the inflammatory response
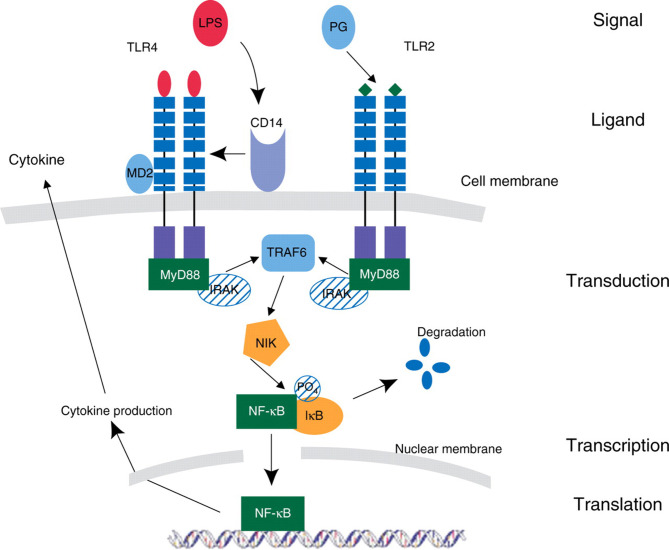
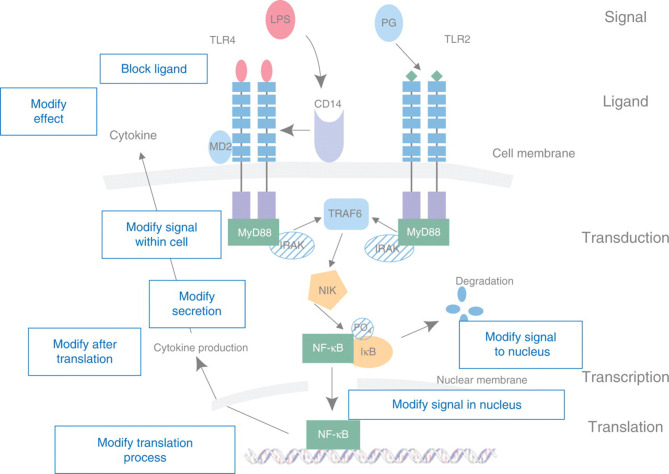
Severe infection leads to the appearance in the bloodstream of endotoxin or lipopolysaccharide (LPS) or similar bacterial products from Gram-positive organisms. This triggers the innate immune response leading to production of the inflammatory mediators, TNF, and IL-1. Secondary mediators, including other cytokines, prostaglandins, and PAF, are then released, with further activation of complement and the acute phase response, expression of adhesion molecules,16 T helper (Th) cell selection, and antibody production (Fig. 1 ). Figure 2 which is superimposed onto Figure 1 shows the various parts of the pathway that can be targeted as a potential for treatment and immune modulation.
Fig 1.
A diagram showing the intracellular signalling pathways and how an external inflammatory stimulus can induce cytokine synthesis. Bacterial products (lipopolysaccharide, LPS, and peptidoglycan, PG) bind to specific cell surface receptors (toll-like receptors, TLRs) and induce a downstream signalling pathway within the cell. The first induced complex is an adaptor molecule such as myeloid differentiation factor (MyD) 88, and a serine/threonine innate immunity kinase such as IL-1 receptor accessory protein kinase (IRAK). The activated TLR complex in turn activates the NFκB-inducing kinase (NIK) complex in a step mediated by TNF-receptor-associated factor (TRAF6). The NIK complex then causes the release of the inhibitory subunit (IB) of NFκB. NF-κB then moves to the nucleus, where it can activate target genes, resulting in enhanced production of pro- and anti-inflammatory cytokines. (Reproduced with the kind permission of the Royal College of Surgeons of Edinburgh.)13
Fig 2.
Superimposed on Figure 1 are the various sites at which immunotherapy can be altered by modification of the signalling pathway.
Acquired immunity
Acquired, or specific, immune responses occur upon second exposure to an antigen, inactivating pathogens with the assistance of antibodies that can activate the complement system, stimulate phagocytic cells, and specifically target micro-organisms. The acquired immune response is further classified into humoral and cell-mediated immunity. The humoral component involves interaction of B lymphocytes with antigen and their proliferation and differentiation into antibody-secreting plasma cells. Antibody is the effector of the humoral response via binding to the antigen, neutralizing, and facilitating its removal. This process also activates the complement system.
A key feature of the adaptive immune system compared with the innate system is the increased diversity.
The major histocompatibility complex (MHC) is a tightly linked cluster of genes located on chromosome 6 and associated with intercellular recognition and discrimination between self and non-self. The MHC encodes antigens (in man, known as human leucocyte antigens or HLA), which play an important role in antigen recognition by T-cells, and determines the response of an individual to infectious antigens and hence their susceptibility to disease.
MHC and T-cells
Foreign protein antigens must be degraded into small peptides then moved to the cell surface and complexed with class I or class II HLA molecules and held on the cell surface in a peptide-binding groove in order to be recognized by a T-cell. This is called antigen processing and antigen display. Regulation of the MHC plays a fundamental role in the immune system, since alterations in the expression of class I or II molecules on the surface of cells can greatly affect subsequent immune responses by T-cells. T-cells recognize infection by binding intracellular or phagocytosed peptide antigen bound in the groove of HLA. The T-cell receptor specifically binds antigen and the HLA molecule. The result of this is gene transcription and T-cell proliferation.
T-lymphocytes consist of two subsets: Th cells which recognize class II MHC molecules and which produce IFNγ and other macrophage-activating factors, and cytotoxic T (Tc) cells which recognize class I MHC molecules and also specific antigens. Th cells can be further classified according to the pattern of cytokines they produce.
Th cells are initially capable of production of many cytokines and are prompted into a more restricted and focused pattern of cytokine production depending on signals received at the outset of infection. Th1 cells secrete a characteristic set of cytokines (IL-2, IFNγ, and TNFβ) which push the system towards cellular cytotoxicity. Th2 cells are associated with humoral or antibody-mediated immunity and typically release IL-4, IL-5, IL-6, IL-9, IL-10, and IL-13. Polarization of Th cells towards either a Th1 or a Th2 response can significantly influence subsequent immune responses. It has also been recently reported that there is an IL-23-dependent Th cell population which produces IL-17 but not IFNγ or IL-4, the signature cytokines of Th1 and Th2 cells, respectively. This suggests a distinct third arm of T-cell effector repertoire: Th1, Th2, and Th17. Th17 cells are thought to have evolved to cope with a range of extracellular bacterial pathogens, and may be involved in autoimmune diseases.
Immunomodulation
Immunomodulatory drugs can either be immunosuppressants or be immunostimulants and can act upon different targets at different levels. They can act through inhibiting or increasing cytokine release, affecting cytokine receptor expression, or the response of cells to cytokines. They may affect phagocytic or cytotoxic actions of cells or regulate transcription or translation of protein mediators. Some drugs are used because they are immunomodulatory (e.g. GCs); some are given for another reason but happen to have effects upon the immune system which can be exploited (e.g. statins). Some are given to treat specific conditions but can have unwanted effects on the immune system (e.g. proton pump inhibitors).85 Other treatments affecting immune function in the critically ill include blood transfusion, nutritional interventions, antibiotics, analgesia, and sedation. Immunomodulation can therefore occur intentionally, incidentally, or inadvertently. Recent and ongoing trials of such agents are shown in Table 1 .
Table 1.
Recent and ongoing trials of immunotherapy. TLR4, toll-like receptor 4; TNF, tumour necrosis factor; IL-1ra, interleukin 1 receptor antagonist; rh, recombinant human; GM-CSF, granulocyte and monocyte colony-stimulating factor
| Drug | Target | Comments |
|---|---|---|
| Lipid emulsion | TLR4 | No benefit |
| E5564 | TLR4 | Ongoing/no results |
| TAK-242 | TLR4 | No results |
| TNF MoAb | TNF | No benefit |
| Etanercept | TNF | No benefit |
| IL-1ra | IL-1 | No benefit |
| Heparin | Coagulation | Ongoing/no report |
| rh-antithrombin | Coagulation | Ongoing/no report |
| rh-activated protein C | Coagulation | Approved but new study requested by EMA |
| rh-tissue factor pathway inhibitor | Coagulation | Ongoing/no report |
| Insulin | Hyperglycaemia | Conflicting results |
| Corticosteroids | Adrenal suppression | CORTICUS—no benefit |
| GM-CSF | Immunoparalysis | Ongoing/no report |
| Statins | Unknown | Ongoing/no report |
Intentional immunomodulation
Steroids
GCs are lipophilic and readily cross the cell membrane into the cell cytoplasm. Binding of GC to its receptor, GCR, results in translocation of the GC–GCR complex to the cell nucleus, where it has a variety of actions. Binding with GC response elements (GRE) on the promoter region of specific GC responsive genes results in activation of transcription and expression of several anti-inflammatory proteins.8 The GC–GCR complex can also down-regulate inflammatory mediators such as cytokines, adhesion molecules, and enzymes, through effects on transcription factors such as nuclear factor kappa B (NFκB) and activator protein 1 (AP)-1. Steroids have been shown to alter the production of TNF and IL-6 by a specific effect at the mRNA level.63
Anti-cytokine therapies
Both soluble receptors and monoclonal antibodies directed against receptors can be used to block the interaction of a cytokine with its receptor. This then prevents transduction of the appropriate biological signal in the target cell. The use of a recombinant soluble receptor might thus prevent the deleterious effect of excessive cytokine production. In addition to soluble receptors, monoclonal antibodies which block cellular cytokine receptors can be used as anti-cytokine therapy. However, it has been shown that in some cases, cytokine complexed to such binding proteins may be still available for receptor binding and thus act as agonists.40 Another approach is to blunt the final common pathways of damage (i.e. using either agents which decrease free radical production or antioxidants which inactivate free radicals as they are produced).
Incidental or inadvertent immunomodulation
Opioids
The immunomodulatory activity of opioids is by both a direct and an indirect action. A direct effect occurs through opioid receptors on cells of the immune system, whereas an indirect effect is via the central nervous system and the hypothalamic–pituitary axis. Morphine, for example, can increase plasma concentrations of corticotrophin-releasing hormone, adrenocorticotropin, and GCs.14 68 The effects of the opioids and the cell types on which they have this effect are shown in Table 2 .
Table 2.
The immune effects of opioids
| Cell | Action |
|---|---|
| Neutrophils | Decreased phagocytosis |
| Decreased chemotaxis | |
| Monocytes/macrophages | Decreased phagocytosis |
| Changes in cytokine release | |
| Increased apoptosis | |
| Decreased adherence | |
| Lymphocytes | Decreased T-cell proliferation |
| Decreased natural killer cell activity | |
| Decreased antibody synthesis |
The direct action of opioids is thought to be predominantly at the μ3 receptor which is expressed predominantly on macrophages and other immune cells. Opioids such as fentanyl, alfentanil, and remifentanil are poor ligands for the μ3 receptor whereas morphine and its 6-glucuronide metabolite bind strongly. Binding to the μ3 receptor appears to induce immunosuppression by transcription factor stabilization of NFκB and AP-1 through an increase in cyclic adenosine monophosphate (cAMP) levels. This is associated with a concurrent up-regulation of the inducible cAMP early repressor/cAMP response element modulator and down-regulation of p-cAMP-response element-binding protein.77 78 It appears to be particularly important in T-cells and has been studied because of the immunosuppression seen in morphine addicts, but it has also been suggested to be of importance in the immunosuppression seen in the critically ill.81
Sedatives
In vitro studies have shown that at clinically relevant plasma concentrations, propofol suppresses neutrophil chemotaxis, phagocytosis, and respiratory burst activity and thus decreases the ability of the cells to kill and clear bacteria. Benzodiazepines also suppress chemotaxis, adhesion of immune cells, phagocytosis, respiratory burst activity, and natural killer (NK) cell activity. It is thought that this action is via the peripheral benzodiazepine receptors which are found on neutrophils and monocytes.27 In patients undergoing craniotomy, propofol anaesthesia prevented the decrease in Th1/Th2 cell ratio seen with isoflurane anaesthesia36 whereas in those undergoing prostate surgery, spinal anaesthesia increased the Th1/Th2 cell ratio compared with general anaesthesia.42 However, in patients with severe brain injury on ICU requiring long-term sedation, there was no difference between methohexital and propofol in terms of neutrophil function and various other aspects of immune status.34
Antibiotics
Many antibiotics are known to be immunomodulators acting on many immune cell types. Most evidence exists for the macrolides and the fluoroquinolones. These agents alter cytokine release in a stimulated cell culture model26 29 83 and a recent work has examined the effect of three different fluoroquinolones on cytokine release in an acute lung injury model in mice. Bronchoalveolar lavage concentrations of TNF, IL-1, and macrophage inflammatory protein-2 in the ciprofloxacin-treated group were significantly lower than those of controls after an endotoxin challenge. This was coupled with an improvement in 96 h survival.33
Non-steroidal anti-inflammatory agents
NSAIDs are routinely used in the ICU. There is some in vitro evidence that NSAIDs differentially exert immunomodulatory effects on activated macrophages and lymphocytes. In patients undergoing major urological surgery, administration of diclofenac was associated with lower IL-6 and higher IL-10 concentrations, and lower leucocyte count, C-reactive protein concentration, and temperature.46
Statins
Data from experimental animal and human models of sepsis suggest that the statins may modulate inflammatory and immune responses associated with sepsis. Although there have been no randomized controlled trials to test the efficacy of statins to prevent or treat sepsis, observational data support the notion of a beneficial effect. A recent review highlighted the effects of statins on a variety of immune functions using in vitro cell culture models.72 In 2310 patients undergoing non-cardiac vascular surgery, mortality was significantly lower in those patients receiving statins39 and patients receiving therapy with statins may have a lower incidence of severe sepsis.4
Immunotherapy in sepsis
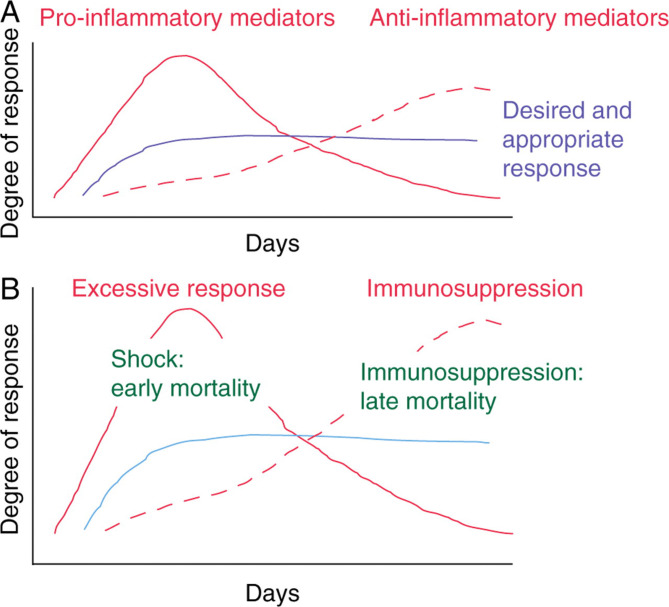
Despite considerable progress in general healthcare over the past decades, sepsis continues to be a major life-threatening condition. Although overall survival rates improved between 1979 and 2000, the total sepsis-related mortality increased from 22 to 44 per 100 000 population in the USA. It has been suggested that the immune response which occurs during sepsis is the result of the interplay of two contrasting phenomena relating to inflammatory status.13 The early phase, systemic inflammatory response syndrome, is characterized by excessive production of pro-inflammatory mediators (Fig. 3 ). This early response is then progressively suppressed by the development of the compensatory anti-inflammatory response syndrome. An intermediate phase, mixed anti-inflammatory response syndrome, has also been described. Table 3 shows the definitions of sepsis and septic shock.12
Fig 3.
The two phases of sepsis—an early pro-inflammatory phase follows onto a later anti-inflammatory phase. An ideal response occurs when these two phases are balanced (a). When there is an exaggerated early response, early mortality associated with shock may be the result; when there is an excessive later response, then immunoparalysis or immunosuppression may occur with later onset infections and multi-organ failure (b).
Table 3.
Definitions of sepsis and shock (consensus meeting of the American Thoracic Society and the American Society of Critical Care Medicine)12
| Infection is an inflammatory response to the presence of micro-organisms or the invasion of normally sterile host tissue by those organisms |
| Bacteraemia is the presence of viable bacteria in the blood |
| Septicaemia is a clinical term whose use is now discouraged |
| Sepsis is the systemic response to infection, manifested by two or more of the following conditions as a result of infection |
| Temperature >38°C or <36°C |
| Heart rate >90 beats min−1 |
| Ventilatory frequency >20 bpm or a requirement for artificial ventilation |
| White blood cell count >12 000 mm−3 or <4000 mm−3 |
| Severe sepsis is sepsis associated with organ dysfunction, hypoperfusion, or hypotension |
| Septic shock is sepsis-induced hypotension (systolic arterial pressure <90 mm Hg) or a requirement for vasoconstrictors, despite adequate fluid resuscitation |
| Multiple organ dysfunction syndrome is the presence of altered organ function such that homeostasis cannot be maintained without intervention |
Outcome from sepsis is not determined only by the infection but also by the intensity of the inflammatory response. This response is essential for the resolution of infection but may occur in an uncontrolled manner, causing damage. There is pronounced synergy and interaction of the components of the immune system such that either immunostimulation or immunosuppression may occur. Coordination is, therefore, vital for an optimum response and survival.
Glucocorticoids in sepsis
The history of GC use in sepsis and septic shock has waxed and waned over the years. Initially, high doses for short duration were recommended and then lower doses for longer durations with tapering of the dose. As new evidence has become available, the indications for the use of GCs in patients with sepsis have been refined.
In septic shock, GCs are thought to have effects on the immune response and on vascular responsiveness to catecholamines. The effect on the immune response is by a reduction in pro-inflammatory cytokine production and a reduction in the inflammatory response without causing immunosuppression. This is the result of effects at the transcriptional levels, causing a decrease in pro-inflammatory cytokine production and an increase in anti-inflammatory mediators, as described above. GCs also enhance vascular endothelial responsiveness to catecholamines by as yet unknown mechanisms, but suggestions include alteration in the prostaglandin or nitric oxide pathway, or an effect on the adrenoceptor itself.5 6
Early clinical studies used high doses of methylprednisolone, betamethasone, or dexamethasone for <24 h in patients with sepsis and septic shock. These studies were hampered by a lack of a definition of sepsis and septic shock and poor trial methodology. A study in 1976 showed a large mortality benefit from GC therapy, which was not replicated in other studies.67 In 1992, the Infectious Disease Society of North America recommended that GCs should not be used in septic shock. Two independent meta-analyses from 1995 included nine43 and 1017 adequate studies of GCs in sepsis and septic shock. In the meta-analysis by Lefering,43 the GC dose was equivalent to around 42 times that now routinely used (currently 200 mg day−1 of hydrocortisone), but nine of the 10 studies included only gave 24 h of therapy. Overall, there was no mortality benefit. There was also no difference in adverse events (gastrointestinal bleeding, superinfection, and hyperglycaemia) in the treatment groups. The other meta-analysis from the same year also found no statistically significant difference in mortality.17 However, the conclusion was that there was a trend to increased mortality, when GCs were used in patients with overwhelming infection and septic shock. During the late 1990s, there was a resurgence of interest in GC use in septic shock but with lower doses of hydrocortisone being used. However, a subsequent meta-analysis in 2004 reported a link between higher doses of GCs and increased mortality.52 This meta-analysis was conducted in two halves: published reports between 1988 and 2003 and those pre-1988. The MEDLINE search was performed using the terms glucocorticoids and survival or vasopressor requirements. The eight earlier trials showed a significant negative effect of steroids on survival whereas the five later trials showed a beneficial effect both on survival (although only modest, with confidence intervals of 1.01–1.50 and P=0.036) and vasopressor requirements.
More recently, smaller doses of corticosteroids, for longer periods of time with a tapering regimen, have become popular. The study by Annane and colleagues6 changed practice in managing patients with septic shock, despite concerns such as the use of etomidate (known to suppress endogenous cortisol production), changes to inclusion criteria during the trial, and the fact that the only statistically significant results were from a post hoc subgroup analysis. The CORTICUS study published in 2008 has still not answered the question definitively as to whether steroids reduce mortality, as it was stopped early and had a lower than expected control group mortality and is therefore underpowered.69
At present, there is evidence that hydrocortisone therapy in septic shock reduces time to reversal of shock, but this has not shown a benefit in survival. The 2008 Surviving Sepsis Campaign guidelines still suggest considering hydrocortisone therapy (<300 mg day−1, tapered once vasopressors have been stopped) in the patient with septic shock, if hypotension which is poorly responsive to fluids and vasopressors is present.18 The evidence, however, is graded as weak and of low quality.
Anti-cytokine therapies
Most of these agents have been based on our improving knowledge of the biochemical signalling pathways which control the inflammatory and immune response to infection. These range from specifically targeted agents such as monoclonal antibodies directed against TNF, receptor blockers, and soluble receptors (such as IL-1ra, and sTNF-R), and colony stimulating factors, to more generally acting compounds such as lipid emulsions, statins, and insulin. To date, all trials using such agents have either not shown a beneficial effect or are still ongoing.
TNF has been targeted as a therapeutic modality in sepsis largely because it has been found to be elevated in almost all patients with sepsis. One of the first agents to be investigated was a monoclonal antibody to TNFα. This proved to be most efficacious in preventing the mortality of endotoxaemia in a primate model.73 However, subsequent large studies in humans did not show a mortality benefit.1 3 15 64 An initial North American study called NORASEPT enrolled 971 patients and showed no difference in mortality with the use of the monoclonal antibody to TNFα but did show a trend to an improvement in mortality in those patients who were shocked; this was most evident at 3 days.3 An ongoing ‘rest of the world’ study (INTERSEPT) was changed to enrol only those patients with septic shock. In total, 564 patients were enrolled and again there was no mortality difference; although this time, there was a trend towards more rapid reversal of shock.15 The follow-up North American study (NORASEPT II) enrolled 1879 patients with septic shock and again showed no mortality benefit (40.3% of trial compound patients died at 28 days vs 42.8% of those given placebo, P=0.27).1
The therapeutic use of IL-1 receptor antagonist (IL-1ra) has also been studied, first, in an endotoxaemia model in rabbits, where a highly significant mortality benefit was seen,56 and then in two large clinical trials, where no significant effect was seen.25 57 The first reported study in 1994 looked at 893 patients with sepsis syndrome and showed no mortality benefit of recombinant human (rh) IL-1ra.25 However, a retrospective analysis suggested that treatment with rhIL-1ra resulted in a dose-related increase in survival time in those patients with sepsis who had organ dysfunction (i.e. those with severe sepsis), a predicted risk of mortality of 24% or greater, or both. A second study was conducted, which enrolled 696 patients with severe sepsis, but again mortality was similar in both groups (33.1% in the rhIL-1ra group vs 36.4% in the placebo group, P=0.36).57 The use of soluble TNF receptors has also been evaluated in sepsis, again with no clinical benefit.24
Specific chemical antagonists (e.g. against PAF) have also been evaluated in patients with sepsis, again without effect.19 58 75 The PAF receptor antagonist BN 52021 (Ginkgolide B) was evaluated in 262 patients with sepsis syndrome who received standard supportive care and antimicrobial therapy, in addition to the PAF receptor antagonist or placebo. The 28 day mortality rate was 51% for the placebo group and 42% for the PAF receptor antagonist group (P=0.17). However, the efficacy of PAF receptor antagonist was significantly greater in patients with Gram-negative sepsis (test for interaction, P=0.03). In a separate analysis of patients with and without Gram-negative sepsis, the 28 day mortality rate was 57% for the patients receiving placebo (30 deaths of 53 patients) and 33% for patients receiving PAF receptor antagonist (22 deaths of 67 patients; P=0.01).19 Like many studies performed both before and after this one, a post hoc analysis produced significant results. This prompted a study using a different agent, the PAF receptor antagonist BB-882, in 152 patients with sepsis.75 The 28 day mortality was 45% in the placebo group and 53% in the treatment group, P=0.32. But also most notably, there were no differences in circulating TNFα, TNF receptor, or IL-6 concentrations between the groups.
Activated protein C
The inflammatory and pro-coagulant host responses to infection are intricately linked. Infectious agents, endotoxin, and cytokines such as TNFα and IL-1β activate coagulation by stimulating the release of tissue factor from monocytes and endothelial cells. Up-regulation of tissue factor leads to the formation of thrombin and a fibrin clot. Although cytokines are capable of activating coagulation and inhibiting fibrinolysis, thrombin is capable of stimulating several inflammatory pathways. The end result may be widespread injury to the vascular endothelium, multiorgan dysfunction, and ultimately death. Protein C is an endogenous protein, a vitamin K-dependent serine protease, which promotes fibrinolysis, while inhibiting thrombosis and inflammatory responses.
Activated protein C (APC) can intervene at multiple points during the systemic response to infection. It exerts an anti-thrombotic effect limiting the generation of thrombin. As a result of decreased thrombin levels, the thrombin-mediated inflammatory, pro-coagulant, and anti-fibrinolytic response is attenuated. In vitro data indicate that APC exerts an anti-inflammatory effect by inhibiting the production of TNFα, IL-1β, and IL-6 by monocytes and limiting monocyte and neutrophil adhesion to the endothelium. Reduced levels of protein C are found in the majority of patients with sepsis and are associated with an increased risk of death.
Administration of APC was shown in the PROWESS trial to reduce mortality in patients with severe sepsis,11 but the trial was criticized because of a lack of effect in all patient groups and excess side-effects. A similar repeat trial is underway.23 Although it is accepted that APC has immunomodulatory effects and the PROWESS trial found lower circulating IL-6 concentrations in those patients treated with APC, other evidence is largely a result of in vitro studies. In vitro studies of LPS-stimulated human neutrophils showed that APC decreased chemotaxis and IL-6 production whereas other neutrophil functions were unaffected.28 APC decreased NFκB binding at target sites in cultured endothelial cells and directly suppressed expression of p50 and p52 NFκB subunits and blocked downstream targets, including vascular cell adhesion molecule-1 and E-selectin.38
Trials of other agents that act on the coagulation system (such as tissue factor pathway inhibitor and antithrombin) did not show a survival benefit.2 80
Failure of immunomodulatory therapies in sepsis
Blockade of any single or combined inflammatory mediator may not be successful for a number of reasons. First, the immuno-inflammatory process is a normal response to infection and is essential not only for the resolution of infection but also for the initiation of other adaptive stress responses required for host survival (e.g. acute phase and heat shock responses).60 Secondly, the profound redundancy of action of many cytokines means that there are many overlapping pathways for cellular activation and further mediator release. In addition, the synergism of actions and effects of many cytokines suggests that imbalance in the process of the immune response may be adversely affected by inhibition of a single agent. Exogenously administered anti-cytokine therapy may have hitherto unrecognized effects due to interaction with naturally occurring immunomodulators or their receptors.40 Finally, the timing of any potential anti-cytokine therapy is clearly crucial. Strategies designed to predict the activation of specific components of the inflammatory response may thus be useful. It is also possible that specific cellular targeting of such therapy may be more beneficial than global inhibition. Preliminary animal studies suggest that the therapeutic use of anti-inflammatory cytokines such as IL-10 and IL-13 may be beneficial in sepsis, although as yet there have been no confirmatory clinical studies.53
The possible reasons for the lack of efficacy shown in the various trials have been enumerated and there has been much discussion about the continuing need for more studies.54 Trials of drugs which have been used successfully to treat chronic inflammation and autoimmune diseases (for example, rheumatoid arthritis and inflammatory bowel disease) are of benefit and the agents are now in common use (examples include the anti-TNF drugs etanercept and infliximab and the IL-6 receptor antagonist tocilizumab).30 In chronic inflammatory conditions, combined blockade of cytokines (e.g. TNF and IL-1) has been used with success and this may be another approach to the treatment of severe sepsis.
A study published in 200222 investigated whether a relationship between the risk of death and treatment effect could explain the disparate results between the preclinical and the clinical sepsis trials of a range of anti-inflammatory agents published over the preceding decade. The preclinical studies showed that the treatment effects of the agents were highly dependent on risk of death (P=0.0001) and that animals were almost always studied at significantly higher control mortality rates than that observed in humans [median (inter-quartile range), 88% (79–96%) vs 39% (32–42%), P=0.0001]. Furthermore, analysis of the clinical trials showed that anti-inflammatory agents were also significantly more efficacious in septic patients with higher risk of death (P=0.002) and were harmful in those with low risk.
In fact, the paradigm of an excess pro-inflammatory response followed a few days later by an excessive anti-inflammatory response in sepsis is now being questioned. In fact, there is little evidence to support this progression. In experimental sepsis in animals, there is a mediator surge within 6 h of the insult which peaks at about 12 h. In lethal sepsis, there is simultaneous release of both pro- and anti-inflammatory mediators into the circulation.47 59 In addition, the pre-morbid state of the patient may be important; unlike animals, humans with sepsis may have been ill for days or weeks. In addition, in humans, the duration of treatment for sepsis has usually been limited to 24–96 h.
Nasraway54 detailed the potential reasons for the failure of the various trials and these are given in Table 4 .
Table 4.
The problems and challenges of immunotherapy in human sepsis54
| Runaway inflammation may not be responsible for the mortality of sepsis and therefore immunotherapy alone would not be expected to work. The wrong hypothesis |
| Study design may have been flawed. Right question, wrong study design |
| Given the complexity of the inflammatory cascade along with the inbuilt redundancy within the system means that either we need to know precisely which phase of the response the patient is currently in or we risk making matters worse. Or at best the patient is able to compensate because of the overlap between the various regulators of inflammation. Right question, wrong agent at wrong time |
| Enrolment criteria have largely been based on non-specific clinical signs and have resulted in a mixed population to study. Right question, wrong target group |
Future of immunotherapy for sepsis
The point has been made that treatment with the correct antibiotic in a timely manner along with attention to tissue perfusion will make more difference to outcome than any immunomodulator therapy.35 41 Study design is clearly very important and larger numbers with more strictly defined entry criteria resulting in a more homogeneous study group may be needed. It may be necessary to establish at which stage in the inflammatory process, the patient is actually in, using perhaps bedside tests for cytokines and other mediators. If we accept that we cannot influence the inflammatory process up-stream, then perhaps we should modify matters downstream.31 In an LPS–peptidoglycan rat model of the organ dysfunction that occurs during sepsis, treatment with an antioxidant targeted to mitochondria, MitoQ, resulted in lower levels of biochemical markers of acute liver and renal dysfunction.44 MitoQ also reduced pro-inflammatory cytokine responses and increased anti-inflammatory responses in an endothelial model of sepsis.44 These findings suggest that the use of mitochondria-targeted antioxidants such as MitoQ may be beneficial in sepsis.
Immunotherapy and ARDS
GCs are used in many pulmonary disorders in the critically ill. Of particular relevance are ARDS, pneumonia, and post-extubation stridor. GCs may also have a role in the management of acute respiratory failure due to asthma, chronic obstructive pulmonary disease, alveolar haemorrhage syndromes, acute lupus pneumonitis, bronchiolitis obliterans organizing pneumonia, radiation pneumonitis, and pulmonary drug toxicity.37 However, for some of these conditions, this is controversial and based on limited evidence.
ARDS is a syndrome of pulmonary inflammation which can develop in response to a variety of factors in the critically ill.9 79 ARDS has a mortality rate of 30–50%.7 66 Pathologically, ARDS is divided into an early exudative phase (where the alveolar spaces fill with an inflammatory exudate of neutrophils and macrophages) and late proliferative phases (characterized by progressive pulmonary fibrosis).
Corticosteroids are attractive as a therapy in ARDS, as they should, theoretically, reduce inflammation and prevent late fibrotic lung disease.50 In unresolving ARDS, Meduri and colleagues51 demonstrated that methylprednisolone therapy suppressed the activity of NFκB, reduced production of the pro-inflammatory cytokines TNFα, IL-1β, and IL-6, and increased GC receptor-mediated activity.
The use of high-dose corticosteroids for a short duration in early ARDS was studied in the 1980s, with no evidence of a beneficial effect on mortality.9 10 12 45 In a small randomized controlled trial published in 1998, Meduri and colleagues49 gave lower dose methylprednisolone for a longer duration in 24 patients with unresolving ARDS (>7 days). Methylprednisolone 2 mg kg−1 was used as a bolus followed by 2 mg kg−1 day−1 for 14 days then a slowly tapering dose until day 32. There was an improved Pao2:F i o2 ratio, lung injury score, and a reduction in ICU mortality from 63% to 13% in the methylprednisolone-treated patients. However, this trial was small and featured a cross-over design which meant that 20 of the 24 patients received corticosteroids.
The 2006 ARDSnet trial studied the use of methylprednisolone in late (>7 days) ARDS.70 A total of 180 patients were studied, and there was no difference in mortality at 60 days. The secondary outcomes of ventilator-free days, shock-free days, and ICU-free days were lower in the treatment group at 28 days. This was not maintained at 180 days, when there was an increased ICU readmission rate in the treatment group, perhaps related to the increased rate of neuromuscular weakness. The authors also studied the subgroup which commenced methylprednisolone therapy after 14 days of ARDS, and found an increased mortality (35% vs 8%) in post hoc analysis.
Meduri48 has also studied the use of methylprednisolone 1 mg kg−1 day−1 for up to 28 days in ARDS of <72 h duration. There were 91 patients included in the study, and there was a statistically significant difference in the number of patients with a reduction of one point in the lung injury score in the treatment group. More patients were ventilator-free at 7 days in the treatment group. Limitations of this study are the high proportion (66%) of patients who also had septic shock and the small sample size. GCs may have a role in the treatment of ARDS of <14 days duration, when it can increase ventilator-free days and may reduce mortality.61 This, however, is at the expense of increased infection rates, and regular surveillance by bronchoalveolar lavage should be considered if using GCs for ARDS.
Immunostimulation
The rationale for immunostimulation in the critically ill is based on clinical observations which suggest that during critical illness, host defence may be impaired and the susceptibility to infection increased; so-called anergy or immunoparalysis.76 Several studies have shown that neutrophil function in such patients may be abnormal, including reduced migration, decreased superoxide anion production, and defective bacterial-killing.71 Abnormalities such as these have been shown to be potentially reversible under the influence of G-CSF or IFNγ.20 71
However, G-CSF is potentially a double-edged sword; although augmenting neutrophil function, enhancing host defence, and improving microbial clearance, G-CSF may also worsen inflammatory tissue injury.82 In several studies in patients either with complicated community-acquired pneumonia or with pneumonia and sepsis, G-CSF was not beneficial overall.55 65 84
Two small clinical trials have been conducted investigating the effects of prophylactic G-CSF in non-neutropenic critically ill patients. In patients with major brain trauma or cerebral haemorrhage, prophylactic G-CSF increased circulating neutrophils and decreased the incidence of bacteraemia but did not decrease mortality rates, length of stay, or the incidence of nosocomial infection.32 In another study, prophylactic G-CSF given over 7 days, although safe, also showed no benefit in decreasing nosocomial infections, mortality rates, or organ dysfunction.62
In a small study of septic patients where IFNγ was given to those with low monocyte HLA-DR expression, there was restoration of HLA-DR expression and in vitro LPS-induced TNFα secretion. Recovery of monocyte function resulted in resolution of sepsis in eight of the nine patients. These data suggest that IFNγ treatment in carefully selected septic patients may be a useful therapeutic strategy.20
Conclusions
Like all therapies, immunomodulation has both beneficial and undesirable effects. The level of immunomodulation appears important—high dose vs low dose, short duration vs long duration; and the timing may well be crucial. It may be necessary to evaluate the stage in the inflammatory process that the patient is at and then tailor the therapy to the individual patient. It may also be necessary to use more than one agent at any time in a similar fashion to that used in chronic inflammatory conditions. Further, well-designed studies using a more focused patient group are required. Enhanced knowledge of the molecular biology of inflammation along with improved bedside testing techniques to evaluate patient response may also result in improved treatment modalities for these difficult to treat critically ill patients.
Funding
The authors are in receipt of research funding from the Medical Research Council, the Intensive Care Society and the British Journal of Anaesthesia.
References
- 1.Abraham E, Anzueto A, Gutierrez G. Double-blind randomised controlled trial of monoclonal antibody to human tumour necrosis factor in treatment of septic shock. NORASEPT II Study Group. Lancet. 1998;351:929–933. [PubMed] [Google Scholar]
- 2.Abraham E, Reinhart K, Opal S. Efficacy and safety of tifacogin (recombinant tissue factor pathway inhibitor) in severe sepsis: a randomized controlled trial. J Am Med Assoc. 2003;290:238–247. doi: 10.1001/jama.290.2.238. [DOI] [PubMed] [Google Scholar]
- 3.Abraham E, Wunderink R, Silverman H. Efficacy and safety of monoclonal antibody to human tumor necrosis factor alpha in patients with sepsis syndrome. A randomized, controlled, double-blind, multicenter clinical trial. TNF-alpha MAb Sepsis Study Group. J Am Med Assoc. 1995;273:934–941. [PubMed] [Google Scholar]
- 4.Almog Y, Shefer A, Novack V. Prior statin therapy is associated with a decreased rate of severe sepsis. Circulation. 2004;110:880–885. doi: 10.1161/01.CIR.0000138932.17956.F1. [DOI] [PubMed] [Google Scholar]
- 5.Annane D. Glucocorticoids in the treatment of severe sepsis and septic shock. Curr Opin Crit Care. 2005;11:449–453. doi: 10.1097/01.ccx.0000176691.95562.43. [DOI] [PubMed] [Google Scholar]
- 6.Annane D, Sebille V, Bollaert PE. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. J Am Med Assoc. 2002;288:862–871. doi: 10.1001/jama.288.7.862. [DOI] [PubMed] [Google Scholar]
- 7.ARDSnet Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 8.Barnes PJ. How corticosteroids control inflammation: quintiles prize lecture 2005. Br J Pharmacol. 2006;148:245–254. doi: 10.1038/sj.bjp.0706736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernard GR, Artigas A, Brigham KL. The American–European Consensus Conference on ARDS: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 10.Bernard GR, Luce JM, Sprung CL. High dose corticosteroids in patients with the adult respiratory distress syndrome. N Engl J Med. 1987;317:1565–1570. doi: 10.1056/NEJM198712173172504. [DOI] [PubMed] [Google Scholar]
- 11.Bernard GR, Vincent JL, Laterre PF. Efficacy and safety of recombinant human activated protein C for sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 12.Bone RC, Balk RA, Cerra FB. ACCP/SCCM consensus conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 13.Boontham P, Chandran P, Rowlands B, Eremin O. Surgical sepsis: dysregulation of immune function and therapeutic implications. Surg J R Coll Surg Edinb Irel. 2003;1:187–206. doi: 10.1016/s1479-666x(03)80018-5. [DOI] [PubMed] [Google Scholar]
- 14.Cabot PJ. Immune-derived opioids and peripheral antinociception. Clin Exp Pharmacol Physiol. 2001;28:230–232. doi: 10.1046/j.1440-1681.2001.03425.x. [DOI] [PubMed] [Google Scholar]
- 15.Cohen J, Carlet J. INTERSEPT: an international, multicenter, placebo-controlled trial of monoclonal antibody to human tumor necrosis factor-alpha in patients with sepsis. International Sepsis Trial Study Group. Crit Care Med. 1996;24:1431–1440. doi: 10.1097/00003246-199609000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Cowley HC, Heaney D, Gearing A, Webster NR. Increased circulating adhesion molecule concentrations in patients with the systemic inflammatory response syndrome: a prospective cohort study. Crit Care Med. 1994;22:651–657. doi: 10.1097/00003246-199404000-00022. [DOI] [PubMed] [Google Scholar]
- 17.Cronin L, Cook DJ, Carlet J. Corticosteroid treatment for sepsis: a critical appraisal and meta-analysis of the literature. Crit Care Med. 1995;23:1430–1439. doi: 10.1097/00003246-199508000-00019. [DOI] [PubMed] [Google Scholar]
- 18.Dellinger RP, Levy MM, Carlet J. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock. Intensive Care Med. 2008;34:17–60. doi: 10.1007/s00134-007-0934-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhainaut JF, Tenaillon A, Le Tulzo Y. Platelet-activating factor receptor antagonist BN 52021 in the treatment of severe sepsis: a randomized, double-blind, placebo-controlled, multicenter clinical trial. BN 52021 Sepsis Study Group. Crit Care Med. 1994;22:1720–1728. [PubMed] [Google Scholar]
- 20.Docke WD, Randow F, Syrbe U. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat Med. 1997;3:678–681. doi: 10.1038/nm0697-678. [DOI] [PubMed] [Google Scholar]
- 21.Dripps DJ, Brandhuber BJ, Thompson RC, Eisenberg SP. Interleukin 1 (IL-1) receptor antagonist binds to the 80 kDa IL-1 receptor but does not initiate IL-1 signal transduction. J Biol Chem. 1991;266:10331–10336. [PubMed] [Google Scholar]
- 22.Eichacker PQ, Parent C, Kalil A. Risk and the efficacy of antiinflammatory agents: retrospective and confirmatory studies of sepsis. Am J Respir Crit Care Med. 2002;166:1197–1205. doi: 10.1164/rccm.200204-302OC. [DOI] [PubMed] [Google Scholar]
- 23.Finfer S, Ranieri VM, Thompson BT. Design, conduct, analysis and reporting of a multi-national placebo controlled trial of activated protein C for persistent septic shock. Intensive Care Med. 2008;34:1935–1947. doi: 10.1007/s00134-008-1266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fisher CJ, Agosti JM, Opal SM. Treatment of septic shock with the tumor necrosis factor receptor: Fc fusion protein. The Soluble TNF Receptor Sepsis Study Group. N Engl J Med. 1996;334:1697–1702. doi: 10.1056/NEJM199606273342603. [DOI] [PubMed] [Google Scholar]
- 25.Fisher CJ, Dhainaut JF, Opal SM. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. Phase III rhIL-1ra Sepsis Syndrome Study Group. J Am Med Assoc. 1994;271:1836–1843. [PubMed] [Google Scholar]
- 26.Galley HF, Dhillon JK, Paterson RL, Webster NR. Effect of ciprofloxacin on the activation of the transcription factors NFκB, AP-1 and NF-IL-6, and interleukin-6 and interleukin-8 mRNA expression in a human endothelial cell line. Clin Sci. 2000;99:405–410. [PubMed] [Google Scholar]
- 27.Galley HF, Dubbels AM, Webster NR. The effect of midazolam and propofol on interleukin-8 from human polymorphonuclear leukocytes. Anesth Analg. 1998;86:1289–1293. doi: 10.1097/00000539-199806000-00030. [DOI] [PubMed] [Google Scholar]
- 28.Galley HF, El Sakka NE, Webster NR, Lowes DA, Cuthbertson BH. Activated protein C inhibits chemotaxis and interleukin-6 release by human neutrophils without affecting other neutrophil functions. Br J Anaesth. 2008;100:815–819. doi: 10.1093/bja/aen079. [DOI] [PubMed] [Google Scholar]
- 29.Galley HF, Nelson SJ, Dubbels AM, Webster NR. Effect of ciprofloxacin on the accumulation of interleukin-6, interleukin-8 and nitrite from an endothelial cell model of sepsis. Crit Care Med. 1997;25:1392–1395. doi: 10.1097/00003246-199708000-00029. [DOI] [PubMed] [Google Scholar]
- 30.Genovese MC, McKay JD, Nasonov EL. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum. 2008;58:2968–2980. doi: 10.1002/art.23940. [DOI] [PubMed] [Google Scholar]
- 31.Goode HF, Webster NR. Free radicals and antioxidants in sepsis. Crit Care Med. 1993;21:1770–1776. doi: 10.1097/00003246-199311000-00029. [DOI] [PubMed] [Google Scholar]
- 32.Heard SO, Fink MP, Gamelli RL. Effect of prophylactic administration of recombinant human granulocyte colony-stimulating factor (filgrastim) on the frequency of nosocomial infections in patients with acute traumatic brain injury or cerebral hemorrhage. Crit Care Med. 1998;26:748–754. doi: 10.1097/00003246-199804000-00027. [DOI] [PubMed] [Google Scholar]
- 33.Huang HC, Shieh CC, Yu WL. Comparing the protective effects of ciprofloxacin, moxifloxacin and levofloxacin in mice with lipopolysaccharide-induced acute lung injuries. Respirology. 2008;13:47–52. doi: 10.1111/j.1440-1843.2007.01192.x. [DOI] [PubMed] [Google Scholar]
- 34.Huettemann E, Jung A, Vogelsang H, Hout N, Sakka SG. Effects of propofol vs methohexital on neutrophil function and immune status in critically ill patients. J Anesth. 2006;20:86–91. doi: 10.1007/s00540-005-0377-2. [DOI] [PubMed] [Google Scholar]
- 35.Ibrahim EH, Sherman G, Ward S. The influence of inadequate antimicrobial treatment of blood stream infections on patient outcomes in the ICU setting. Chest. 2000;118:146–155. doi: 10.1378/chest.118.1.146. [DOI] [PubMed] [Google Scholar]
- 36.Inada T, Yamanouchi Y, Jomura S. Effect of propofol and isoflurane anaesthesia on the immune response to surgery. Anaesthesia. 2004;59:954–959. doi: 10.1111/j.1365-2044.2004.03837.x. [DOI] [PubMed] [Google Scholar]
- 37.Jantz MA, Sahn SA. Corticosteroids in acute respiratory failure. Am J Respir Crit Care Med. 1999;160:1079–1100. doi: 10.1164/ajrccm.160.4.9901075. [DOI] [PubMed] [Google Scholar]
- 38.Joyce DE, Gelbert L, Ciaccia A, DeHoff B, Grinnell BW. Gene expression profile of antithrombotic protein C defines new mechanisms modulating inflammation and apoptosis. J Biol Chem. 2001;276:11199–11203. doi: 10.1074/jbc.C100017200. [DOI] [PubMed] [Google Scholar]
- 39.Kertai MD, Boersma E, Klein J, van Urk H, Poldermans D. Optimising the prediction of perioperative mortality in vascular surgery by using a customized probability model. Arch Intern Med. 2005;165:898–904. doi: 10.1001/archinte.165.8.898. [DOI] [PubMed] [Google Scholar]
- 40.Klein B, Brailly H. Cytokine binding proteins: stimulating antagonists. Immunology Today. 1995;16:216–220. doi: 10.1016/0167-5699(95)80161-8. [DOI] [PubMed] [Google Scholar]
- 41.Kollef MH. Inadequate antimicrobial treatment: an important determinant of outcome for hospitalized patients. Clin Infect Dis. 2000;31:S131–S138. doi: 10.1086/314079. [DOI] [PubMed] [Google Scholar]
- 42.LeCras AE, Galley HF, Webster NR. Spinal but not general anesthesia increases the ratio of T helper 1 to T helper 2 cell subsets in patients undergoing transurethral resection of the prostate. Anesth Analg. 1998;87:1421–1425. doi: 10.1097/00000539-199812000-00041. [DOI] [PubMed] [Google Scholar]
- 43.Lefering R, Neugebauer EAM. Steroid controversy in sepsis and septic shock: a meta-analysis. Crit Care Med. 1995;23:1294–1303. doi: 10.1097/00003246-199507000-00021. [DOI] [PubMed] [Google Scholar]
- 44.Lowes DA, Thottakam BMVJ, Webster NR, Murphy MP, Galley HF. The mitochondria-targeted antioxidant MitoQ protects against organ damage in a lipopolysaccharide–peptidoglycan model of sepsis. Free Rad Biol Med. 2008;45:1559–1565. doi: 10.1016/j.freeradbiomed.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 45.Luce JM, Montgomery AB, Marks JD, Turner J, Metz CA, Murray JF. Ineffectiveness of high-dose methylprednisolone in preventing parenchymal lung injury and improving mortality in patients with septic shock. Am Rev Respir Dis. 1988;138:62–68. doi: 10.1164/ajrccm/138.1.62. [DOI] [PubMed] [Google Scholar]
- 46.Mahdy AM, Galley HF, Abdel-Wahed MA, El-Korny KF, Sheta SA, Webster NR. Differential modulation of interleukin-6 and interleukin-10 by diclofenac in patients undergoing major surgery. Br J Anaesth. 2002;88:797–802. doi: 10.1093/bja/88.6.797. [DOI] [PubMed] [Google Scholar]
- 47.Martin C, Boisson C, Haccoun M, Thomachot L, Mege JL. Patterns of cytokine evolution (tumor necrosis factor-alpha and interleukin-6) after septic shock, hemorrhagic shock, and severe trauma. Crit Care Med. 1997;25:1813–1819. doi: 10.1097/00003246-199711000-00018. [DOI] [PubMed] [Google Scholar]
- 48.Meduri GU, Golden E, Freire AX. Methylprednisolone infusion in early severe acute respiratory distress syndrome: results of a randomised controlled trial. Chest. 2007;131:954–963. doi: 10.1378/chest.06-2100. [DOI] [PubMed] [Google Scholar]
- 49.Meduri GU, Headley AS, Golden E. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome. N Engl J Med. 1998;280:159–165. doi: 10.1001/jama.280.2.159. [DOI] [PubMed] [Google Scholar]
- 50.Meduri GU, Tolley EA, Chinn A, Stentz F, Postlethwaite A. Procollagen types I and III aminoterminal propeptide levels during acute respiratory distress syndrome and in response to methylprednisolone treatment. Am J Respir Crit Care Med. 1998;158:1432–1441. doi: 10.1164/ajrccm.158.5.9801107. [DOI] [PubMed] [Google Scholar]
- 51.Meduri GU, Tolley EA, Chrousos GP, Stentz F. Prolonged methylprednisolone treatment suppresses systemic inflammation in patients with unresolving acute respiratory distress syndrome. Am J Respir Crit Care Med. 2002;165:983–991. doi: 10.1164/ajrccm.165.7.2106014. [DOI] [PubMed] [Google Scholar]
- 52.Minneci PC, Deans KJ, Banks SM. Meta-analysis: the effect of steroids on survival and shock during sepsis depends on the dose. Ann Intern Med. 2004;141:47–56. doi: 10.7326/0003-4819-141-1-200407060-00014. [DOI] [PubMed] [Google Scholar]
- 53.Muchamuel T, Menon S, Pisacane P, Howard MC, Cockayne DA. IL-13 protects mice from lipopolysaccharide-induced lethal endotoxemia: correlation with down-modulation of TNF-alpha, IFN-gamma, and IL-12 production. J Immunol. 1997;158:2898–2903. [PubMed] [Google Scholar]
- 54.Nasraway SA. The problems and challenges of immunotherapy in sepsis. Chest. 2003;123:451S–459S. doi: 10.1378/chest.123.5_suppl.451s. [DOI] [PubMed] [Google Scholar]
- 55.Nelson S, Heyder AM, Stone J. A randomized controlled trial of filgrastim for the treatment of hospitalized patients with multilobar pneumonia. J Infect Dis. 2000;182:970–973. doi: 10.1086/315775. [DOI] [PubMed] [Google Scholar]
- 56.Ohlsson K, Bjork P, Bergenfeldt M. Interleukin 1 receptor antagonist reduces mortality from endotoxin shock. Nature. 1990;348:550–552. doi: 10.1038/348550a0. [DOI] [PubMed] [Google Scholar]
- 57.Opal SM, Fisher CJ, Dhainaut JF. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. The Interleukin-1 Receptor Antagonist Sepsis Investigator Group. Crit Care Med. 1997;25:1115–1124. doi: 10.1097/00003246-199707000-00010. [DOI] [PubMed] [Google Scholar]
- 58.Opal S, Laterre PF, Abraham E. Recombinant human platelet-activating factor acetylhydrolase for treatment of severe sepsis: results of a phase III, multicenter, randomized, double-blind, placebo-controlled, clinical trial. Crit Care Med. 2004;32:332–341. doi: 10.1097/01.CCM.0000108867.87890.6D. [DOI] [PubMed] [Google Scholar]
- 59.Osuchowski MF, Welch K, Siddiqui J, Remick DG. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J Immunol. 2006;177:1967–1974. doi: 10.4049/jimmunol.177.3.1967. [DOI] [PubMed] [Google Scholar]
- 60.Perlmutter DH, Dinarello CA, Punsal PI, Cohen HR. Cachectin/tumour necrosis factor regulates hepatic acute phase gene expression. J Clin Invest. 1986;78:1349–1354. doi: 10.1172/JCI112721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peter JV, John P, Graham PL. Corticosteroids in the prevention and treatment of acute respiratory distress syndrome (ARDS) in adults: meta-analysis. Br Med J. 2008;336:1006–1009. doi: 10.1136/bmj.39537.939039.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pettila V, Takkunen O, Varpula T. Safety of granulocyte colony-stimulating factor (filgrastim) in intubated patients in the intensive care unit: interim analysis of a prospective, placebo-controlled, double-blind study. Crit Care Med. 2000;28:3620–3625. doi: 10.1097/00003246-200011000-00011. [DOI] [PubMed] [Google Scholar]
- 63.Quante T, Ng YC, Ramsay EE. Corticosteroids reduce IL-6 in ASM cells via up-regulation of MKP-1. Am J Respir Cell Mol Biol. 2008;39:208–217. doi: 10.1165/rcmb.2007-0014OC. [DOI] [PubMed] [Google Scholar]
- 64.Reinhart K, Menges T, Garlund B. Randomized, placebo-controlled trial of the anti-tumor necrosis factor antibody fragment afelimomab in hyperinflammatory response during severe sepsis: The RAMSES Study. Crit Care Med. 2001;29:765–769. doi: 10.1097/00003246-200104000-00015. [DOI] [PubMed] [Google Scholar]
- 65.Root RK, Dale DC. Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor: comparisons and potential for use in the treatment of infections in nonneutropenic patients. J Infect Dis. 1999;179:S342–S352. doi: 10.1086/513857. [DOI] [PubMed] [Google Scholar]
- 66.Rubenfeld GD, Caldwell E, Peabody E. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 67.Schumer W. Steroids in the treatment of clinical septic shock. Ann Surg. 1976;184:333–341. doi: 10.1097/00000658-197609000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sharp BM, Roy S, Bidlack JM. Evidence for opioid receptors on cells involved in host defense and the immune system. J Neuroimmunol. 1998;83:45–56. [PubMed] [Google Scholar]
- 69.Sprung CL, Annane D, Keh D. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358:111–124. doi: 10.1056/NEJMoa071366. [DOI] [PubMed] [Google Scholar]
- 70.Steinberg KP, Hudson LD, Goodman RB. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006;354:1671–1684. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 71.Stephan F, Yang K, Tankovic J. Impairment of polymorphonuclear neutrophil functions precedes nosocomial infections in critically ill patients. Crit Care Med. 2002;30:315–322. doi: 10.1097/00003246-200202000-00009. [DOI] [PubMed] [Google Scholar]
- 72.Terblanche M, Almog Y, Rosenson RS, Smith TS, Hackam DG. Statins: panacea for sepsis? Lancet Infect Dis. 2006;6:242–248. doi: 10.1016/S1473-3099(06)70439-X. [DOI] [PubMed] [Google Scholar]
- 73.Tracey K, Fong Y, Hesse DG. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330:662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- 74.Van Zee KJ, Kohno T, Fischer E, Rock CS, Moldawer LL, Lowry SF. Tumor necrosis factor soluble receptors circulate during experimental and clinical inflammation and can protect against excessive tumor necrosis factor alpha in vitro and in vivo. Proc Natl Acad Sci USA. 1992;89:4845–4849. doi: 10.1073/pnas.89.11.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vincent JL, Spapen H, Bakker J, Webster NR, Curtis L. Phase II multicenter clinical study of the platelet-activating factor receptor antagonist BB-882 in the treatment of sepsis. Crit Care Med. 2000;28:638–642. doi: 10.1097/00003246-200003000-00006. [DOI] [PubMed] [Google Scholar]
- 76.Volk HD, Reinke P, Docke WD. Clinical aspects: from systemic inflammation to immunoparalysis. Chem Immunol. 2000;74:162–177. doi: 10.1159/000058753. [DOI] [PubMed] [Google Scholar]
- 77.Wang J, Barke RA, Charboneau R, Loh HH, Roy S. Morphine negatively regulates interferon-gamma promoter activity in activated murine T cells through two distinct cyclic AMP-dependent pathways. J Biol Chem. 2003;278:37622–37631. doi: 10.1074/jbc.M301224200. [DOI] [PubMed] [Google Scholar]
- 78.Wang J, Barke RA, Roy S. Transcriptional and epigenetic regulation of interleukin-2 gene in activated T cells by morphine. J Biol Chem. 2007;282:7164–7171. doi: 10.1074/jbc.M604367200. [DOI] [PubMed] [Google Scholar]
- 79.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 80.Warren BL, Eid A, Singer P. Caring for the critically ill patient. High-dose antithrombin III in severe sepsis: a randomized controlled trial. J Am Med Assoc. 2001;286:1869–1878. doi: 10.1001/jama.286.15.1869. [DOI] [PubMed] [Google Scholar]
- 81.Weinert CR, Kethireddy S, Roy S. Opioids and infections in the intensive care unit should clinicians and patients be concerned? J Neuroimmune Pharmacol. 2008;3:218–229. doi: 10.1007/s11481-008-9124-4. [DOI] [PubMed] [Google Scholar]
- 82.Weiss M, Gross-Weege W, Schneider M. Enhancement of neutrophil function by in vivo filgrastim treatment for prophylaxis of sepsis in surgical intensive care patients. J Crit Care. 1995;10:21–26. doi: 10.1016/0883-9441(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 83.Williams AC, Galley HF, Watt AM, Webster NR. Differential effects of three antibiotics on T helper cell cytokine expression. J Antimicrob Chemother. 2005;56:502–506. doi: 10.1093/jac/dki251. [DOI] [PubMed] [Google Scholar]
- 84.Wunderink R, Leeper K, Schein R. Filgrastim in patients with pneumonia and severe sepsis or septic shock. Chest. 2001;119:523–529. doi: 10.1378/chest.119.2.523. [DOI] [PubMed] [Google Scholar]
- 85.Zedtwitz-Liebenstein K, Wenisch C, Patruta S, Parschalk B, Daxböck F, Graninger W. Omeprazole treatment diminishes intra- and extracellular neutrophil reactive oxygen production and bactericidal activity. Crit Care Med. 2002;30:1118–1122. doi: 10.1097/00003246-200205000-00026. [DOI] [PubMed] [Google Scholar]