Abstract
Background:
Alzheimer’s disease (AD) is a chronic neurodegenerative disease that accounts for more than 50% of all dementia cases worldwide. There is wide consensus on the risk factors of AD; however, a clear etiology remains unknown. Evidence suggests that the inflammatory-mediated disease model, such as that found with periodontal disease due to Porphyromonas gingivalis (P. gingivalis), plays a role in AD progression.
Objective:
This study aims to systematically review the literature on the association between P. gingivalis to AD, and to identify the homogeneity of the methods used across studies to measure P. gingivalis involvement in AD.
Methods:
We systematically searched studies on Cochrane library, Ovid Medline, PubMed, Web of Science, WHOLIS, Google Scholar databases, and reference lists of identified studies.
Results:
6 studies out of 636 identified records fulfilled all eligibility criteria. Results showed no clear pathophysiology of AD due to P. gingivalis and its various virulence factors. No consensus was found in the literature pertaining to the method of measurement of AD or P. gingivalis and its virulence factors.
Conclusion:
The included studies suggest that P. gingivalis bacteria play a role in the process of systemic inflammation which leads to cerebrospinal fluid inflammation and indirectly cause hastening of AD onset and progression. Our included studies revealed heterogeneity in the methodologies of measurement of AD and/or P. gingivalis and its virulence factors, which opens discussion about the benefits and weakness of possible standardization.
Keywords: Alzheimer’s disease, dementia, geriatrics, P. gingivalis, periodontitis, systematic review
INTRODUCTION
Alzheimer’s disease (AD) is an irreversible, progressive chronic neurodegenerative dementing disease [1–3]. AD is the most common cause of dementia, a broad term that describes memory loss and other cognitive ability impairments serious enough to interfere with a person’s daily life. Women seem to be more susceptible to AD than men [2]. AD is associated with neuronal degeneration and atrophy in the brain leading to death. In a normal non-AD diagnosed brain, amyloid-β (Aβ) proteins are found in-and-around the neurons. However, in AD, these proteins aggregate together and form clusters in the brain, called “amyloid plaques”. Another feature of AD is the abnormal deposits of the tau protein inside degenerating neurons. It is still unknown whether the plaques are the cause of AD or if they represent the brain’s response to neurodegeneration [4].
The complex-multifactorial causes of AD are still unknown [2, 4]. However, there is consensus in the literature that AD is an interaction between genetic and environmental elements, with age considered the main risk factor for AD [3, 5].
AD patients show signs of generalized inflammation and neuronal degeneration that are consistent with bacterial infections. These signs include complement, inflammasome, and microglial activation as well as altered cytokine profiles [6]. Recently, several studies have discovered periodontal pathogens in the brain and suggested their involvement in AD-associated inflammation [7–12].
Porphyromonas gingivalis (P. gingivalis) is a periodontopathic Gram-negative non-motile bacterium that is implicated in periodontal inflammation and periodontitis [13]. P. gingivalis is found in about 86% of subgingival plaque samples from patients with chronic periodontitis [13, 14]. It is also associated with several diseases and conditions, e.g., atherosclerosis, rheumatoid arthritis, non-alcoholic steatohepatitis, and squamous cell carcinoma due to the complex nature of its virulence factors [15]. The inflammatory nature of P. gingivalis and its virulence factors lead to stimulation of the inflammatory response which causes blood vessel damage [11, 16, 17]. One virulence factor highly associated with periodontal and inflammatory disease progression is gingipains. Gingipains are enzymes secreted by P. gingivalis that play a significant role in P. gingivalis survival, virulence, host invasion, and colonization [18]. Gingipains play a crucial role in degrading host immune response and tissue damage by the degradation of collagen, which in turn impede wound healing [19, 20]. Olsen and Singhrao explain that this happens through gingipains destroying complement through proteolytic degradation, and by inhibiting complement activation by binding to a complement inhibitor [21]. Moreover, P. gingivalis expression of gingipains also allows its persistence in the periodontal tissue and further complicates its elimination by the immune response [22]. Using tissue microarrays containing matched brain samples, Dominy et al. identified gingipains in the brains of AD patients. In the study, the levels of gingipains identified were found to be correlated to the extent of AD pathology [23].
Aside from the inflammation caused by P. gingivalis [24], blood vessel damage due to periodontal disease may act as a gateway for further pathogenic microorganisms to enter directly into the bloodstream, and subsequently to systematic circulation [20, 25]. Recently, P. gingivalis was shown to be present in the periodontium of AD patients [26, 27]. Another cross-sectional study reported finding P. gingivalis intracerebrally in four out of the ten AD-diagnosed brain samples, while none for all non-AD diagnosed brain samples [10].
An inflammatory-mediated model explaining the progression of AD has been described in the literature previously [28]. Innate immune inflammatory activity in the AD brain can result from the deposition of Aβ protein as well as from specific bacterial infections that tend to possess weak immunostimulatory responses [29]. Recently, numerous studies have discussed that periodontal inflammation may result in hastening of AD and/or dementia onset even though the mechanism is unclear [11, 30–35]. In a large cohort with follow-up of more than 10 years, subjects with periodontal disease were found to have a higher risk of developing AD and dementia [36]. Periodontal disease involvement in AD is further supported in a recent review by Kamer et al. that stated that an increased incidence of AD is evident in patients with periodontal disease [37].
The present study aims to systematically review and analyze the literature on the association between the periodontal pathogen, P. gingivalis, and the dementing condition of AD. The objectives of the study are to identify the risk of AD associated with P. gingivalis and its virulence factors and to explore the different tools and methods used across studies to measure P. gingivalis involvement in AD.
METHODS
This systematic literature review adhered to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines [38].
We applied the following inclusion-exclusion criteria: 1) Original research and studies using human populations and/or human specimens and published in peer-reviewed journals. Editorials, viewpoints, policy reviews, summaries, or discussion papers were excluded. 2) Studies with control groups. 3) Studies focusing on AD that has been diagnosed using clear definitions and/or criteria. 4) Studies including non-human sources or that focused only on specific populations such as HIV patients or psychiatric patients were excluded.
Two researchers independently conducted the literature search for studies published in English in Cochrane, OVID, PubMed, Web of Science, and WHOlis until 31 August 2020. The authors also used Google Scholar to search for relevant articles.
We used the following search terms to identify relevant studies: “Porphyromonas gingivalis” and “Alzheimer”. The authors AE and CI screened title-abstract and full texts of the references independently against the inclusion-exclusion criteria. In cases of disagreement, the issues were discussed among all authors.
We assessed the quality of the included studies by using the National Heart, Lung, and Blood Institute (NHLBI) Study Quality Assessment Tools [39]. Use of randomization, the calculation of sample sizes, and the size of the unit of allocation impacted the quality assessment score given to the individual studies. A comparative analysis was done using analytical categories developed based on the methodology used to measure both AD and its progression, and the presence of P. gingivalis and its virulence factors. These categories were: 1) Mini-Mental State Examination (MMSE) average score of samples, 2) P. gingivalis antibody immunoglobulin (P. gingivalis Ab IgG) measures, 3) tumor necrosis factor alpha (TNF-α) measures, 4) P. gingivalis, virulence factor lipopolysaccharide (LPS), or gingipains measures or presence and 5) further analysis. As for 5), ‘further analysis’ is for the purpose of this review defined as variables the included-studies’ authors have reported to have a possible influence on the relation between AD and P. gingivalis. Some authors suggest that these variables may have also affected the obtained results or acted as confounders. We extracted the data using a data extraction sheet pre-designed and pre-tested for the objectives of our study. Information extracted included study aim, sample size, sampling strategy, statistical measures, and results.
RESULTS
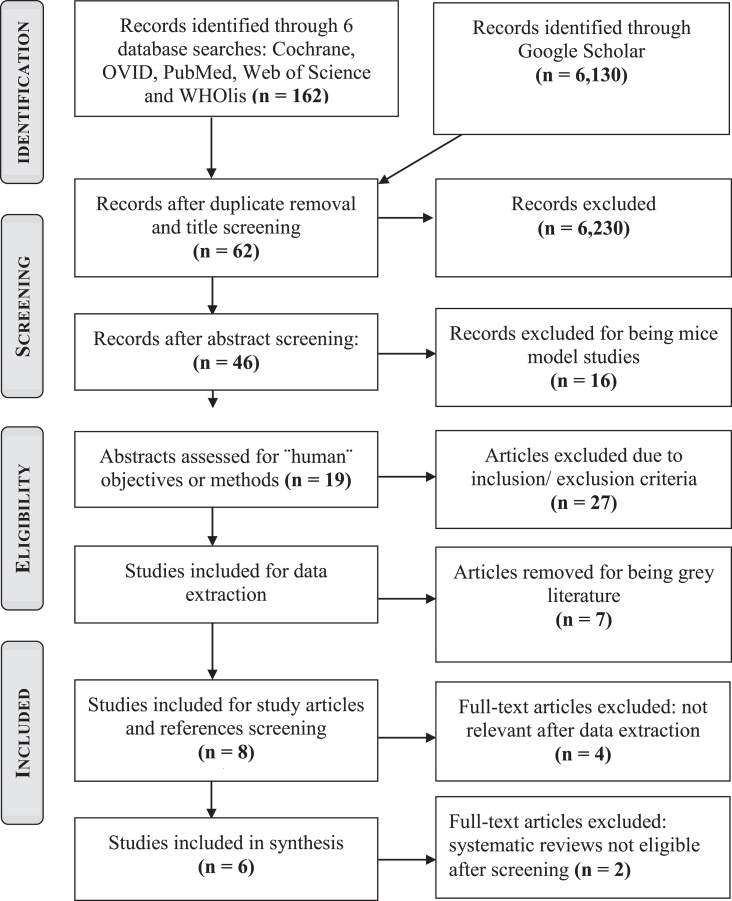
In total, the search yielded 6292 records from which, 46 articles were kept after duplicate removal and abstract screening. Applying full inclusion and exclusion criteria on these articles, 6 met the eligibility criteria. Detailed study selection process and reasons for exclusion are described in Fig. 1 (PRISMA Flowchart).
Fig. 1.
PRISMA flow chart showing the detailed study selection process.
Characteristics of selected studies
This systematic review comprised of four case-control and two cohort studies. A descriptive analysis of the included articles is presented in Table 1.
Table 1.
Characteristics of Included Studies
| Study | Country | Case Samples | Controls | Baseline Mean-Age | Gender | Study type | Measure used for P. gingivalis | Measure used for AD | Follow-up | Quality Assessment |
| (Noble et al., 2014) [42] | USA (Manhattan) | 110 incident AD cases | 109 without incident CI at last follow-up | Cases: 79 Controls: 72 | 67.8% Female | Case-cohort study | Serum IgG AB levels by checkboard immunoblotting | Series of neurological tests CARE- Diagnostic Interview | 5 years | 11/14 (79%) |
| (Ide et al., 2016) [40] | UK (South Hampton) | 22 mild/moderate AD Patients with PD | 37 mild AD Patients w/out PD | Cases: 74.9 Controls: 79.4 | 49.2% Female | Observational cohort study for 6 months | Venous blood sample for CRP, pro-cytokine TNFα, IL10, and AB to PG. Number of teeth and full periodontal chart for presence or absence of periodontitis | NINCDS-ARDRA1 ADAS-cog2 sMMSE | 6 months | 10/14 (71%) |
| (Poole et al., 2013) [10] | UK (New Castle) | 10 AD brain samples | 10 Non-AD brain samples | Cases: 80.2 Controls: 74.8 | N/A | Random case-control | Immunolabelling and immunoblotting with anti-PG | Confirmed AD brain samples | N/A3 | 7/11 (64%) |
| (Sparks Stein et al., 2012) [43] | USA (University of Kentucky) | 35 developed AD. (46 developed MCI, 81 in total) | 77 cognitively intact | Cases: 74.1 (for AD) Controls: 70 | Cases: 74.3% Female Control: 58.4% Female | case-control study nested within a cohort study. | Venous blood evaluation for levels of IgG AB to the oral bacteria | NINCDS-ARDRA1 McKann et al. Criteria: MMSE | Cases: 9.6–9.8 years Controls: 12.5 years | 8/12 (67%) |
| (Laugisch et al., 2018) [41] | Germany | 20 AD patients | 20 DEM-noAD5 patients | Cases: 58.3 Controls: 61.1 | Cases: 55% Female Control: 40% Female | pilot observational study | CSF by lumbar puncture Blood serum Full clinical periodontal examination Subgingival biofilm sample and gingival crevicular fluid | AD by:NIAA (2011) guideline4, MMSE CSF for biomarkers MRI CERAD | N/A1 | 8/12 (67%) |
| (Kamer et al., 2009) [44] | USA (New York) | 18 AD patients | 16 cognitively normal | Cases: 40–65 (n = 2), 66–79 (n = 6), > 80 (n = 10) Controls: 40–65 (n = 7), 66–79 (n = 6),>80 | Cases: 78% Female Control: 94% Female | Longitudinal case study | Fasting plasma for IgG AB and cytokine levels APOE genotyping using frozen whole blood. | NINCDS-ARDRA1 DSM-IV MMSE | N/A3 | 8/12 (67%) |
IgG, immunoglobulin; AD, Alzheimer’s disease; TD, Treponema denticola; TF, Treponema forsythia; PG, Porphyromonas gingivalis; AB, antibody; sMMSE, standardized Mini-Mental Examination; LPS, lipopolysaccharides; MRI, magnetic resonance imaging; CSF, cerebrospinal fluid; APOE, Apolipoprotein E; CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; DSM-IV, Diagnostic and Statistical Manual for Mental Diseases- 4th Edition. 1NINCDS-ADRDA National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association. One of the most commonly used criteria in the diagnosis of Alzheimer’s disease [70]. 2ADAS-COG, Alzheimer’s Disease Assessment Scale-cognitive subscale. 3Not Applicable or available. 4National Institutes of Health and the Alzheimer’s Association Guidelines published in 2011. It includes new revised diagnostic clinical criteria for Alzheimer’s disease. The new guidelines include a deeper understanding of the early stages of the disease [71]. 5In the study by Laugisch et al. [41], the controls used were patients diagnosed with dementing diseases other than Alzheimer’s disease.
Five out of the six studies [40–43] enrolled AD patients as case samples. From those, Ide et al. [40] recruited AD patients with periodontitis as case samples. In the remaining study by Poole et al. [10], postmortem AD-diagnosed brain samples were used.
For controls, three studies [42–44] enlisted patients with the criteria of not having cognitive impairment. Poole et al. [10], however, similar to the case samples, used brain samples diagnosed to be AD-free. In the remaining two studies, Ide et al. [40] recruited controls with the criteria of AD patients without periodontitis while Laugisch et al. [41] recruited non-AD dementia patients as controls.
Risk of bias within studies
All articles were found to be of an average grade or above, and thus, none have been excluded. Quality Assessment scores of included studies are presented in Table 1.
Possible predictors of AD
Our primary analysis was built on the following: 1) MMSE average score of samples, 2) P. gingivalis antibody immunoglobulin (P. gingivalis Ab IgG) measures, 3) TNF-α, and 4) P. gingivalis or virulence factor LPS or gingipains measures, presence, or absence. Table 2 presents an overview of the reported results.
Table 2.
Main outcome measures reported by studies
| MMSE Average | PG AB IgG | TNF-α | PG or vir. factors | |||||
| (at baseline) | ||||||||
| Study | Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls |
| (Noble et al., 2014) [42] | – | – | Found in 25 | Found in 25 | – | – | – | – |
| (n = 110) | (n = 109) | |||||||
| (Ide et al., 2016) [40] | 19.5 | 21.0 | Mean average: | Mean average: | 0.29 pg/ml | 0.16 pg/ml | – | – |
| 0.38 Units | 0.37 Units | difference | difference | |||||
| (Poole et al., 2013) [10] | – | – | – | – | – | – | Found in 4 | Found in 0 |
| (n = 10) | (n = 10) | |||||||
| (Sparks Stein et al., 2012) [43] | 28.8 | 23.8 | Mean average: | Mean average: | – | – | – | – |
| 0.38 Units | 0.22 Units | |||||||
| (Laugisch et al., 2018) [41] | 22.1 | 23.8 | Found in 2 | Found in 3 | – | – | Not detected in | |
| (n = 20) | (n = 20) | serum or CSF | ||||||
| (Kamer et al., 2009) [44] | 19.5 | 29.3 | Found in 5 | Found in 6 | 13.0 pg/ml | 8.2 pm/ml | – | – |
| (n = 18)1 | (n = 16)2 | |||||||
AD, Alzheimer’s disease; PD, periodontal disease; CI, cognitive impairment; MMSE, Mini-Mental State Examination; PG AB IgG, Porphyromonas gingivalis antibody immunoglobulin; TNF-α, tumor necrosis factor alpha; CSF, cerebrospinal fluid. 1In the study by Kamer et al. [44], antibody immunoglobulin titers found were reported cumulatively. No separate results for AB IgG were presented.
Mini-Mental State Examination average score
In the recruited AD cases, the highest MMSE score of 28.8 was found in the study by Sparks Stein et al. [43], while two studies reported the same lowest score: 19.5 [40, 44]. In controls, the highest MMSE scores of 29.3 and 29.4 were reported by Kamer et al. [44] and Sparks Stein et al. [43], respectively. However, none of the studies claimed a statistically significant association between MMSE and P. gingivalis presence or prevalence. Only one study, by Poole et al. [10], did not report MMSE scores for participants.
P. gingivalis antibody immunoglobulin (P. gingivalis Ab IgG)
All studies, except one by Poole et al. [10], included measuring of P. gingivalis Ab IgG. However, three studies [41, 42, 44] only reported the number of participants that tested positive for this antibody. The study by Noble et al. [42] found that high levels of P. gingivalis Ab IgG were observed in only 23% of the participants while the other 77% had other periodontal bacteria antibodies other than those of P. gingivalis as the antibodies with highest levels measured. The association between P. gingivalis Ab IgG and AD were statistically insignificant in all five studies.
Although all studies, except Poole et al. [10], reported P. gingivalis AB IgG measurement, only the study by Noble et al. [42] reported the positive thresholds used in their investigations.
Tumor necrosis factor-alpha
Only the two articles by Ide et al. [40] and Kamer et al. [44] investigated TNF-α measures. Ide et al. [40] reported higher TNF-α rates among cases when compared to controls. The study by Kamer et al. [44] found similar results (TNF-α level reported for cases was 13.0 pg/ml, while in controls it was 8.2 pg/ml).
P. gingivalis or virulence factor lipopolysaccharides or gingipains
Out of the six studies, only Poole et al. [10] and Laugisch et al. [41] performed testing for presence of P. gingivalis or P. gingivalis virulence factors such as LPS and gingipains. Poole et al. [10] reported that four out of the ten AD-diagnosed samples tested positive for LPS, while all controls tested negative. On the other hand, gingipains were not detected in all control and AD-samples. The other study, conducted by Laugisch et al. [41], investigated the presence of P. gingivalis in the serum or cerebrospinal fluid of the participants. The authors reported that no P. gingivalis was detected in the serum or cerebrospinal fluid of both the cases and the controls.
Further results
In our analyses, we found that some authors considered several other characteristics (education, race, non-communicable diseases, smoking, and oral and medical history) that may also play a role in the relation between AD and P. gingivalis. Table 3 presents an overview of these variables and the results obtained from the included studies.
Table 3.
Additional Analysis and Data Extracted from Studies
| (Noble et al., 2014) [42] | (Ide et al., 2016) [40] | (Poole et al., 2013) [10] | (Sparks Stein et al., 2012) [43] | (Laugisch et al., 2018) [41] | (Kamer et al., 2009) [44] | |
| Education | Cases: 7.8 years | – | – | Cases: 16 years | – | Cases: 14.4 years |
| Controls: 11.9 years | Controls: 15.9 years | Controls: 15.6 years | ||||
| Race | 47% Hispanic | – | – | – | All Caucasian | Cases: 83% white |
| 26.5% Black | Controls: 81% white | |||||
| (no difference in cases against controls) | ||||||
| APOE Status | Cases: 24.1% Positive | – | (Siddiqui et al.) [68]1 | Cases: 37.1 % Positive | – | Measured |
| Controls: 26.4% Positive | Controls: 15.6% Positive | – | ||||
| Smoking | Cases: 7.6% | Only non-smokers recruited | – | Cases: 38.9% | All non-smokers | |
| Controls: 11.5% | Controls: 47.8% | |||||
| Diabetes | Cases: 21.8% Positive | – | – | Cases: 8.3% | All non-diabetic | No medical confounders2 |
| Controls: 15.6% Positive | Controls: 11.6% | |||||
| Hypertension | Cases: 57.3% Positive | – | – | – | – | |
| Controls: 56.9% Positive | ||||||
| Stroke History | Cases: 15.5% Positive | – | – | – | – | |
| Controls: 2.8% Positive | ||||||
| Oral Health Measures | Oral health status measured | Oral health status measured | – | – | Oral health status measured | |
| Participant Matching | By ethnicity and from the same community | From same community | By age | By age and gender | From same mental health unit |
APOE, Apolipoprotein. 1Although not mentioned in the study by Poole et al. [10], APOE genotypes of samples have been detected in a follow-up study by Siddiqui et al. [68] in 2019. The study states that 40% (4/10 samples) of the AD-diagnosed samples where positive of APOE 4 while 10% (1/10) of the control non-AD diagnosed samples was APOE positive [68]. 2The study states that all participants went through extensive diagnosis to rule out confounding medical, neurological, and psychiatric conditions.
Education
The educational level of participants was reported as years of schooling completed in three of the included studies [42–44]. Out of the three studies, only the study by Noble et al. [42] reported that AD cases had a lower average of education years completed. They also reported that this was a statistically significant association between education and AD.
Race and ethnicity
Three studies presented the ethnicity of their participants [41, 42, 44]. Laugisch et al. [41] only recruited samples of Caucasian origin while Kamer et al. [44] reported that 81% of the control subjects and 83% of the AD case subjects were of white ethnicity. Noble et al. [42] reported that 47.7% of the control subjects and 48.2% of the cases were Hispanic, while 27.5% of the control subjects and 25.5% of the cases were of non-Hispanic black ethnicity. Kamer et al. [44] and Noble et al. [42] reported no difference between populations affected by AD across the recruited ethnicities.
Apolipoprotein E Status (APOE)
APOE genotyping was performed in three of the six studies included [42–44]. Sparks Stein et al. [43] reported a significant difference due to APOE status between controls and AD-diagnosed cases while Noble et al. [42] reported no difference. Kamer et al. [44] did not report their findings regarding APOE.
Smoking status
Four studies reported the smoking status of participants [40–43]. The studies by Ide et al. [40] and Laugisch et al. [41] only recruited non-smokers and non-smokers for the past five years, respectively. The two other studies by Noble et al. [42] and Sparks Stein et al. [43] reported the smoking status of the participants as non-significant.
Diabetes, hypertension, and stroke history
Four out of the six studies investigated various general health conditions in their participants [41–44]. Noble et al. [42] included diabetes, hypertension, and stroke history of their participants. However, Sparks Stein et al. [43] only included and analyzed the diabetic condition. Laugisch et al. [41] reported that for their study, they only recruited non-diabetic participants. Kamer et al. [44] did not report any findings related to these conditions. In the four studies that mentioned general health measures, only Noble et al. [42] reported a statistically significant association between stroke history and AD progression and diagnosis.
Oral health measures
Three out of the six studies include a dental/oral health examination. Ide et al. [40] and Laugisch et al. [41] reported the mean scores of the number of teeth, bleeding on probing, measurement of pocket depth, and full-mouth plaque score. Ide et al. [40] and Laugisch et al. [41] reported significant association between oral health conditions and AD, while Sparks Stein et al. [43] found no difference.
DISCUSSION
Discussion of key findings
Several studies have identified an association between PD and AD; however, the nature of this relation remains unclear [23, 45]. Recent published articles suggest that P. gingivalis, which was recently found in AD-diagnosed brain autopsy specimens, is the link between those two inflammatory conditions [10, 41, 46]. Our study synthesized the evidence on the relationship between P. gingivalis and AD and contrary to hypothesis of direct causation between P. gingivalis and AD, we found that the presence of P. gingivalis virulence factors or antibodies has no proven direct association with AD. We also found that antibody levels detected in the included studies did not differ significantly between cases and controls. Moreover, this systematic review found no homogeneity in the methodology used across studies (besides the use of MMSE scale for AD progression). The results also highlight that there are divergent approaches with regards to P. gingivalis assessment in humans. Some of the included studies analyzed P. gingivalis AB IgG, yet they employed different threshold values.
Our review also found several inflammatory measures used across the studies that may indicate association between P. gingivalis and AD and in line with the hypothesis of AD being a direct cause of systemic inflammation [6, 28, 29, 47–50]. This is consistent with recent published literature that suggests that periodontal bacteria and their virulence factors, such as P. gingivalis and gingipains, caused neuronal impairment and inflammatory responses common to the pathological processes found in AD [23, 51, 52].
Risk of AD development due to P. gingivalis exposure
When establishing the risk of AD development due to P. gingivalis and its virulence factors, Ide et al. [40], Laugisch et al. [41], Noble et al. [42], and Kamer et al. [44] reported that there is an observed association between PD and AD; however, no proven direct associations between levels of P. gingivalis or P. gingivalis AB IgG in serum and AD were found. This may be deemed contrary to the results found in the study by Sparks Stein et al. [43], where the authors reported significantly elevated levels of P. gingivalis AB IgG in AD patients at baseline. Papapanou et al. [26] found P. gingivalis in 100% of periodontitis patients and 80% of the healthy controls. Thus, P. gingivalis AB IgG presence or prevalence may also be indicative of many other factors. One way to avoid such a drawback is to match participants according to oral condition or baseline P. gingivalis and P. gingivalis AB IgG levels. Several studies included in our analysis controlled for potential confounders, e.g., Ide et al. [40] differentiated between cases and controls using periodontitis status, while Kamer et al. [44] claimed that participants were matched according to several medical and dental diagnoses. Ide et al. [40] reported no significance between levels of P. gingivalis or P. gingivalis AB IgG in serum and AD. Kamer et al. [44] reported the cumulative level of antibody immunoglobulin to six different periodontal pathogens. In their study, Poole et al. [10] suggested that AD-affected brains are at a higher risk of secondary P. gingivalis infection. As memory typically deteriorates during AD, it may be harder for AD patients to maintain oral hygiene measures, which would also explain poor oral health conditions as AD advances [2, 3, 32].
Our findings suggest that no sufficient evidence in the literature is available that supports the hypothesis of direct connection between AD and P. gingivalis bacteria. These findings support previous statements by Leira et al. (2017) and Ranjan et al. (2018) that suggest that the current literature lacks evidence on the causal relation between P. gingivalis and AD [8, 11]. Regardless of these statements, we still found several inflammatory measures across the studies that may indicate association and in line with the hypothesis of AD being a direct cause of systematic inflammation [6, 28, 29, 47–50].
Inflammatory process hypothesis
Consistent with our results, other molecular and biological studies also emphasized the importance of inflammatory processes and infectious agents in developing localized inflammation in the brain [4, 24, 28, 29, 47, 49] as possible mechanisms that give rise to the landmark feature of AD in the brain, the amyloid plaques [4]. This hypothesis is specifically viable after the classification of these plaques as an antimicrobial peptide and an immune response [53]. Other studies have also identified P. gingivalis virulence factor, gingipains, in AD-diagnosed brain samples, where its intracranial levels have been found to be correlated with tau and ubiquitin pathology [23]. However, this was not supported by the findings of one of our included studies, by Poole et al. [10], which has not detected the presence of gingipains in all tested AD-diagnosed brain samples.
Oral health status
Recent studies have also investigated the association between periodontal disease such as periodontitis, and P. gingivalis, to AD and several inflammatory diseases [16, 20, 51, 54–57]. One of those studies conducted on the Nun Study, a continuing longitudinal study that examines the onset of AD [58], found that periodontal disease almost doubles the risk of AD onset [59]. By exclusively examining the risk of AD associated with the periodontal pathogen, P. gingivalis, and its virulence factors in humans, this study provides a recent and comprehensive analysis of available evidence.
Oral health measures were not fully explained in all studies included in this review. Several studies reported higher incidences of AD in participants with deteriorating oral health [7, 30, 34, 60–62]. Some authors suggest that inflammatory processes due to oral health infections cause immune system activation and release of cytokines in the bloodstream. The theory adds that some of these changes affect the permeability of the blood-brain barrier. This, the studies propose, leads to the passage of P. gingivalis virulence factors into the central nervous system. These factors then lead to plaque formation as a defensive mechanism in the brain [4, 20, 54]. Some longitudinal studies have shown a significant association between AD and poor oral health [7, 32, 52, 54, 63]. Whether the relationship is causal remains a question. Including the oral health status and parameters when investigating oral pathogens rather than merely focusing on specific bacteria, such as P. gingivalis, may provide the sought-after results regarding AD pathophysiology and diagnosis.
Sample characteristics
Our analysis found that age and gender are of high relevance when researching AD. This is due to AD being a disease that commonly appears in the elderly population [2, 3, 64]. Studies also show that AD is more prevalent in females than males [1–3, 64, 65]. We found that race is also seen as a risk factor for AD. According to a survey by Mayeda et al. in 2016, AD was found most prevalent in the Black race, followed by Hispanics, and then Caucasians [66].
Apolipoprotein ɛ4
Evidence in literature shows that one risk factor linked to increased incidence and earlier onset of AD is presence of apolipoprotein APOE ɛ4 [67]. Three of our included studies have mentioned performing APOE genotyping to their samples and only one, by Sparks Stein et al. [43] reported a significant difference due to APOE status between controls and AD-diagnosed cases. Although not reported in their study and mentioned in a subsequent study by Siddiqui et al. [68], APOE genotyping of the samples recruited in the study by Poole et al. [10] showed that 40% of AD-diagnosed samples and 10% of non-AD diagnosed samples were profiled as positive of APOE ɛ4 gene [68].
Limitations
Several limitations might have influenced the results obtained in our study. We included studies published only in the English language and therefore may have missed relevant articles published in other languages. All included studies were conducted either in the USA, the UK, or Germany demonstrating lacking evidence from other regions. Most of the included studies applied hospital-based and community-based sample selection methods; this may also lead to bias. Due to the high degree of subjectivity and different tools used to measure and diagnose AD in the included studies, we were not able to perform a formal meta-analysis. Furthermore, AD diagnosis requires demonstrated amyloid deposits and neurofibrillary tangles, either post-mortem or by a positron emission tomography examination, along with the presence of the clinical signs, which were not mentioned in the diagnosis criteria used for the AD cases in the studies included in this review [69]. Standardized diagnostic measures for AD would allow for better comparability across studies. Lastly, human error and subjected bias cannot be omitted.
CONCLUSION
This study analyzed available literature on the association between P. gingivalis bacteria and AD and its progression. Our findings show insufficient data to evaluate the association between P. gingivalis and AD and its development. However, all included studies suggest the probability of P. gingivalis bacteria, especially through its virulence factors, in playing a role in the process of systematic inflammation. Moreover, our results show a lack of homogeneity in AD and P. gingivalis diagnosis and measuring across the included studies.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
REFERENCES
- [1]. Nichols E, Szoeke CEI, Vollset SE, Abbasi N, Abd-Allah F, Abdela J, Aichour MTE, Akinyemi RO, Alahdab F, Asgedom SW, Awasthi A, Barker-Collo SL, Baune BT, Béjot Y, Belachew AB, Bennett DA, Biadgo B, Bijani A, Bin Sayeed MS, Brayne C, Carpenter DO, Carvalho F, Catalá-López F, Cerin E, Choi J-YJ, Dang AK, Degefa MG, Djalalinia S, Dubey M, Duken EE, Edvardsson D, Endres M, Eskandarieh S, Faro A, Farzadfar F, Fereshtehnejad S-M, Fernandes E, Filip I, Fischer F, Gebre AK, Geremew D, Ghasemi-Kasman M, Gnedovskaya E V., Gupta R, Hachinski V, Hagos TB, Hamidi S, Hankey GJ, Haro JM, Hay SI, Irvani SSN, Jha RP, Jonas JB, Kalani R, Karch A, Kasaeian A, Khader YS, Khalil IA, Khan EA, Khanna T, Khoja TAM, Khubchandani J, Kisa A, Kissimova-Skarbek K, Kivimäki M, Koyanagi A, Krohn KJ, Logroscino G, Lorkowski S, Majdan M, Malekzadeh R, März W, Massano J, Mengistu G, Meretoja A, Mohammadi M, Mohammadi-Khanaposhtani M, Mokdad AH, Mondello S, Moradi G, Nagel G, Naghavi M, Naik G, Nguyen LH, Nguyen TH, Nirayo YL, Nixon MR, Ofori-Asenso R, Ogbo FA, Olagunju AT, Owolabi MO, Panda-Jonas S, Passos VM de A, Pereira DM, Pinilla-Monsalve GD, Piradov MA, Pond CD, Poustchi H, Qorbani M, Radfar A, Reiner RC, Robinson SR, Roshandel G, Rostami A, Russ TC, Sachdev PS, Safari H, Safiri S, Sahathevan R, Salimi Y, Satpathy M, Sawhney M, Saylan M, Sepanlou SG, Shafieesabet A, Shaikh MA, Sahraian MA, Shigematsu M, Shiri R, Shiue I, Silva JP, Smith M, Sobhani S, Stein DJ, Tabarés-Seisdedos R, Tovani-Palone MR, Tran BX, Tran TT, Tsegay AT, Ullah I, Venketasubramanian N, Vlassov V, Wang Y-P, Weiss J, Westerman R, Wijeratne T, Wyper GMA, Yano Y, Yimer EM, Yonemoto N, Yousefifard M, Zaidi Z, Zare Z, Vos T, Feigin VL, Murray CJL (2019) Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990– 2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18, 88–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].(2019) 2019 Alzheimer’s disease facts and figures. Alzheimers Dement 15, 321–387. [Google Scholar]
- [3]. Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E (2011) Alzheimer’s disease. Lancet 377, 1019–1031. [DOI] [PubMed] [Google Scholar]
- [4]. Cummings JL, Vinters H V., Cole GM, Khachaturian ZS (1998) Alzheimer’s disease: Etiologies, pathophysiology, cognitive reserve, and treatment opportunities.S2-S. Neurology 51, 17. [DOI] [PubMed] [Google Scholar]
- [5]. Galimberti D, Scarpini E (2012) Progress in Alzheimer’s disease. J Neurol 259, 201–211. [DOI] [PubMed] [Google Scholar]
- [6]. Wyss-Coray T, Rogers J (2012) Inflammation in Alzheimer disease–a brief review of the science and clinical literature.a006346-a. Cold Spring Harb Perspect Med 2, 006346–basic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Chen C-K, Wu Y-T, Chang Y-C (2017) Association between chronic periodontitis and the risk of Alzheimer’s disease: A retrospective, population-based, matched-cohort study. Alzheimers Res Ther 9, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Leira Y, Domínguez C, Seoane J, Seoane-Romero J, Pías-Peleteiro JM, Takkouche B, Blanco J, Aldrey JM (2017) Is periodontal disease associated with Alzheimer’s disease? A systematic review with meta-analysis. Neuroepidemiology 48, 21–31. [DOI] [PubMed] [Google Scholar]
- [9]. Wu Z, Ni J, Liu Y, Teeling JL, Takayama F, Collcutt A, Ibbett P, Nakanishi H (2017) Cathepsin B plays a critical role in inducing Alzheimer’s disease-like phenotypes following chronic systemic exposure to lipopolysaccharide from Porphyromonas gingivalis in mice. Brain Behav Immun 65, 350–361. [DOI] [PubMed] [Google Scholar]
- [10]. Poole S, Singhrao SK, Kesavalu L, Curtis MA, Crean S (2013) Determining the presence of periodontopathic virulence factors in short-term postmortem Alzheimer’s disease brain tissue. J Alzheimers Dis 36, 665–677. [DOI] [PubMed] [Google Scholar]
- [11]. Ranjan R, Abhinay A, Mishra M (2018) Can oral microbial infections be a risk factor for neurodegeneration? A review of the literature. Neurol India 66, 344. [DOI] [PubMed] [Google Scholar]
- [12]. Tonsekar PP, Jiang SS, Yue G (2017) Periodontal disease, tooth loss and dementia: Is there a link? A systematic review. Gerodontology 34, 151–163. [DOI] [PubMed] [Google Scholar]
- [13]. How KY, Song KP, Chan KG (2016) Porphyromonas gingivalis: An overview of periodontopathic pathogen below the gum line. Front Microbiol 7, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Datta HK, Ng WF, Walker JA, Tuck SP, Varanasi SS (2008) The cell biology of bone metabolism. J Clin Pathol 61, 577–587. [DOI] [PubMed] [Google Scholar]
- [15]. Olsen I, Yilmaz Ö (2016) Modulation of inflammasome activity by Porphyromonas gingivalis in periodontitis and associated systemic diseases. J Oral Microbiol 8, 30385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Singhrao SK, Olsen I (2019) Assessing the role of Porphyromonas gingivalis in periodontitis to determine a causative relationship with Alzheimer’s disease. J Oral Microbiol 11, 1563405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Rokad F, Moseley R, Hardy RS, Chukkapalli S, Crean S, Kesavalu L, Singhrao SK (2017) Cerebral oxidative stress and microvasculature defects in TNF-α expressing transgenic and Porphyromonas gingivalis-infected ApoE-/- mice. J Alzheimers Dis 60, 359–369. [DOI] [PubMed] [Google Scholar]
- [18]. Li N, Collyer CA (2011) Gingipains from Porphyromonas gingivalis — complex domain structures confer diverse functions. Eur J Microbiol Immunol 1, 41–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Guo Y, Nguyen K-A, Potempa J (2010) Dichotomy of gingipains action as virulence factors: From cleaving substrates with the precision of a surgeon’s knife to a meat chopper-like brutal degradation of proteins. Periodontol 2000 54, 15–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Bostanci N, Belibasakis GN (2012) Porphyromonas gingivalis: An invasive and evasive opportunistic oral pathogen. FEMS Microbiol Lett 333, 1–9. [DOI] [PubMed] [Google Scholar]
- [21]. Olsen I, Singhrao SK (2020) Is there a link between genetic defects in the complement cascade and Porphyromonas gingivalis in Alzheimer’s disease?. J Oral Microbiol 12, 1676486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Hajishengallis G, Liang S, Payne MA, Hashim A, Jotwani R, Eskan MA, McIntosh ML, Alsam A, Kirkwood KL, Lambris JD, Darveau RP, Curtis MA (2011) Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe 10, 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Dominy SS, Lynch C, Ermini F, Benedyk M, Marczyk A, Konradi A, Nguyen M, Haditsch U, Raha D, Griffin C, Holsinger LJ, Arastu-Kapur S, Kaba S, Lee A, Ryder MI, Potempa B, Mydel P, Hellvard A, Adamowicz K, Hasturk H, Walker GD, Reynolds EC, Faull RLM, Curtis MA, Dragunow M, Potempa J (2019) Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors.eaau. Sci Adv 5, 3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Tornwall R, Chow AK (2012) The association between periodontal disease and the systemic inflammatory conditions of obesity, arthritis, Alzheimer’s and renal diseases. Can J Dent Hyg 46, 115–123. [Google Scholar]
- [25]. Saini R, Marawar P, Shete S, Saini S (2009) Periodontitis, a true infection. J Glob Infect Dis 1, 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Papapanou PN, Neiderud A, Papadimitriou A, Sandros J, Dahlén G (2000) “Checkerboard” assessments of periodontal microbiota and serum antibody responses: A case-control study. J Periodontol 71, 885–897. [DOI] [PubMed] [Google Scholar]
- [27]. Kumar PS (2017) From focal sepsis to periodontal medicine: A century of exploring the role of the oral microbiome in systemic disease. J Physiol 595, 465–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Sochocka M, Zwolińska K, Leszek J (2017) The infectious etiology of Alzheimer’s disease. Curr Neuropharmacol 15, 996–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Olsen I, Singhrao SK (2016) Inflammasome Involvement in Alzheimer’s disease. J Alzheimers Dis 54, 45–53. [DOI] [PubMed] [Google Scholar]
- [30]. Gaur S, Agnihotri R (2015) Alzheimer’s disease and chronic periodontitis: Is there an association?. Geriatr Gerontol Int 15, 391–404. [DOI] [PubMed] [Google Scholar]
- [31]. Sochocka M, Sobczyński M, Sender-Janeczek A, Zwolińska K, Błachowicz O, Tomczyk T, Ziętek M, Leszek J (2017) Association between periodontal health status and cognitive Abilities. The role of cytokine profile and systemic inflammation. Curr Alzheimer Res 14, 978–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Abbayya K, Chidambar Y, Naduwinmani S, Puthanakar N (2015) Association between periodontitis and Alzheimer’s disease. N Am J Med Sci 7, 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Wu Z, Nakanishi H (2014) Connection between periodontitis and Alzheimer’s disease: Possible roles of microglia and leptomeningeal cells. J Pharmacol Sci 126, 8–13. [DOI] [PubMed] [Google Scholar]
- [34]. Gurav AN (2014) Alzheimer’s disease and periodontitis - an elusive link. Rev Assoc Med Bras 60, 173–180. [DOI] [PubMed] [Google Scholar]
- [35]. Aarabi G, Thomalla G, Heydecke G, Seedorf U (2019) Chronic oral infection: An emerging risk factor of cerebral small vessel disease. Oral Dis 25, 710–719. [DOI] [PubMed] [Google Scholar]
- [36]. Choi S, Kim K, Chang J, Kim SM, Kim SJ, Cho H, Park SM (2019) Association of chronic periodontitis on Alzheimer’s disease or vascular dementia. J Am Geriatr Soc 67, 1234–1239. [DOI] [PubMed] [Google Scholar]
- [37]. Kamer AR, Craig RG, Niederman R, Fortea J, de Leon MJ (2020) Periodontal disease as a possible cause for Alzheimer’s disease. Periodontol 2000 83, 242–271. [DOI] [PubMed] [Google Scholar]
- [38]. Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 339, 332–336. [PMC free article] [PubMed] [Google Scholar]
- [39].National Heart, Lung, and Blood Institute (NHLBI). Study Quality Assessment Tools. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- [40]. Ide M, Harris M, Stevens A, Sussams R, Hopkins V, Culliford D, Fuller J, Ibbett P, Raybould R, Thomas R, Puenter U, Teeling J, Perry VH, Holmes C (2016) Periodontitis and cognitive decline in Alzheimer’s disease.e. PLoS One 11, 0151081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41]. Laugisch O, Johnen A, Maldonado A, Ehmke B, Bürgin W, Olsen I, Potempa J, Sculean A, Duning T, Eick S (2018) Periodontal pathogens and associated intrathecal antibodies in early stages of Alzheimer’s disease. J Alzheimers Dis 66, 105–114. [DOI] [PubMed] [Google Scholar]
- [42]. Noble JM, Scarmeas N, Celenti RS, Elkind MSV, Wright CB, Schupf N, Papapanou PN (2014) Serum IgG antibody levels to periodontal microbiota are associated with incident Alzheimer disease.e. PLoS One 9, 114959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43]. Sparks Stein P, Steffen MJ, Smith C, Jicha G, Ebersole JL, Abner E, Dawson D (2012) Serum antibodies to periodontal pathogens are a risk factor for Alzheimer’s disease. Alzheimers Dement 8, 196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44]. Kamer AR, Craig RG, Pirraglia E, Dasanayake AP, Norman RG, Boylan RJ, Nehorayoff A, Glodzik L, Brys M, de Leon MJ (2009) TNF-α and antibodies to periodontal bacteria discriminate between Alzheimer’s disease patients and normal subjects. J Neuroimmunol 216, 92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45]. Ryder MI (2020) Porphyromonas gingivalis and Alzheimer disease: Recent findings and potential therapies. J Periodontol 91, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46]. Beydoun MA, Beydoun HA, Hossain S, El-Hajj ZW, Weiss J, Zonderman AB (2020) Clinical and bacterial markers of periodontitis and their association with incident all-cause and Alzheimer’s disease dementia in a large national survey. J Alzheimers Dis 75, 157–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47]. Kamer AR, Craig RG, Dasanayake AP, Brys M, Glodzik-Sobanska L, de Leon MJ (2008) Inflammation and Alzheimer’s disease: Possible role of periodontal diseases. Alzheimers Dement 4, 242–250. [DOI] [PubMed] [Google Scholar]
- [48]. Liu Y, Wu Z, Nakanishi Y, Ni J, Hayashi Y, Takayama F, Zhou Y, Kadowaki T, Nakanishi H (2017) Infection of microglia with Porphyromonas gingivalis promotes cell migration and an inflammatory response through the gingipain-mediated activation of protease-activated receptor-2 in mice. Sci Rep 7, 11759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49]. McCaulley ME, Grush KA (2015) Alzheimer’s disease: Exploring the role of inflammation and implications for treatment. Int J Alzheimers Dis 2015, 515248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50]. Zhao N, Liu C-C, Qiao W, Bu G (2018) Apolipoprotein E, receptors, and modulation of Alzheimer’s disease. Biol Psychiatry 83, 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51]. Ding Y, Ren J, Yu H, Yu W, Zhou Y (2018) Porphyromonas gingivalis, a periodontitis causing bacterium, induces memory impairment and age-dependent neuroinflammation in mice. Immun Ageing 15, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52]. Ilievski V, Zuchowska PK, Green SJ, Toth PT, Ragozzino ME, Le K, Aljewari HW, O’Brien-Simpson NM, Reynolds EC, Watanabe K (2018) Chronic oral application of a periodontal pathogen results in brain inflammation, neurodegeneration and amyloid beta production in wild type mice.e. PLoS One 13, 0204941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53]. Gosztyla ML, Brothers HM, Robinson SR (2018) Alzheimer’s amyloid-β is an antimicrobial peptide: A review of the evidence. J Alzheimers Dis 62, 1495–1506. [DOI] [PubMed] [Google Scholar]
- [54]. Carter CJ, France J, Crean S, Singhrao SK (2017) The Porphyromonas gingivalis/host interactome shows enrichment in GWASdb genes related to Alzheimer’s disease, diabetes and cardiovascular diseases. Front Aging Neurosci 9, 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55]. Olsen I, Taubman MA, Singhrao SK (2016) Porphyromonas gingivalis suppresses adaptive immunity in periodontitis, atherosclerosis, and Alzheimer’s disease. J Oral Microbiol 8, 33029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56]. Singhrao SK, Harding A, Poole S, Kesavalu L, Crean S (2015) Porphyromonas gingivalis periodontal infection and its putative links with Alzheimer’s disease. Mediators Inflamm 2015, 137357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57]. Singhrao SK, Harding A, Simmons T, Robinson S, Kesavalu L, Crean S (2014) Oral inflammation, tooth loss, risk factors, and association with progression of Alzheimer’s disease. J Alzheimers Dis 42, 723–737. [DOI] [PubMed] [Google Scholar]
- [58]. Brayne C (2002) Aging with grace: What the Nun Study teaches us about leading longer, healthier and more meaningful lives. Int J Epidemiol 31, 879–879. [Google Scholar]
- [59]. Stein PS, Desrosiers M, Donegan SJ, Yepes JF, Kryscio RJ (2007) Tooth loss, dementia and neuropathology in the Nun Study. J Am Dent Assoc 138, 1314–1322. [DOI] [PubMed] [Google Scholar]
- [60]. Cerajewska TL, Davies M, West NX (2015) Periodontitis: A potential risk factor for Alzheimer’s disease. Br Dent J 218, 29–34. [DOI] [PubMed] [Google Scholar]
- [61]. Harding A, Gonder U, Robinson SJ, Crean S, Singhrao SK (2017) Exploring the association between Alzheimer’s disease, oral health, microbial endocrinology and nutrition. Front Aging Neurosci 9, 398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62]. Harding A, Robinson S, Crean S, Singhrao SK (2017) Can better management of periodontal disease delay the onset and progression of Alzheimer’s disease?. J Alzheimers Dis 58, 337–348. [DOI] [PubMed] [Google Scholar]
- [63]. Ishida N, Ishihara Y, Ishida K, Tada H, Funaki-Kato Y, Hagiwara M, Ferdous T, Abdullah M, Mitani A, Michikawa M, Matsushita K (2017) Periodontitis induced by bacterial infection exacerbates features of Alzheimer’s disease in transgenic mice. NPJ Aging Mech Dis 3, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64]. Patterson C (2018) World Alzheimer Report 2018. The state of the art of dementia research: New frontiers. Alzheimer’s Disease International, London.
- [65]. Campbell-Taylor I, James B, Leurgans S, Bennett D (2014) Contribution of Alzheimer disease to mortality in the United States. Neurology 83, 1302. [DOI] [PubMed] [Google Scholar]
- [66]. Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA (2016) Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement 12, 216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67]. Ward A, Crean S, Mercaldi CJ, Collins JM, Boyd D, Cook MN, Arrighi HM (2012) Prevalence of apolipoprotein E4 genotype and homozygotes (APOE e4/4) among patients diagnosed with Alzheimer’s disease: A systematic review and meta-analysis. Neuroepidemiology 38, 1–17. [DOI] [PubMed] [Google Scholar]
- [68]. Siddiqui H, Eribe ER, Singhrao SK, Olsen I (2019) High throughput sequencing detect gingivitis and periodontal oral bacteria in Alzheimer’s disease autopsy brains. Neuro Res 1, 3. [Google Scholar]
- [69]. Jack CR, Albert MS, Knopman DS, McKhann GM, Sperling RA, Carrillo MC, Thies B, Phelps CH (2011) Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70]. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group* under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34, 939–939. [DOI] [PubMed] [Google Scholar]
- [71]. Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R, Elliott C, Masliah E, Ryan L, Silverberg N (2018) NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14, 535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]