Abstract
Background:
Harmful alcohol use is a leading cause of morbidity and mortality in sub-Saharan Africa (sSA); however, the effects of non-pharmacological alcohol interventions in this region are unknown.
Design:
A systematic review and meta-analysis of the available literature through March 14, 2019 was undertaken. Two authors extracted and reconciled relevant data and assessed risk of bias. Meta-analyses were conducted. The review protocol is registered on PROSPERO (CRD42019094509).
Setting:
Studies conducted in sSA were eligible for inclusion.
Participants:
Individuals participating in interventions aimed at reducing alcohol use.
Interventions:
Randomized and non-randomized controlled trials testing non-pharmacological interventions (psychosocial and structural) on alcohol consumption in sSA.
Measurements:
Eligible outcomes included the Alcohol Use Disorders Identification Test (AUDIT) scores; alcohol abstinence; measures of drinking quantity and frequency; and biomarkers of alcohol consumption.
Findings:
Nineteen intervention trials (18 scientific manuscripts) testing psychosocial interventions (no structural intervention included), judged of moderate quality, were included in meta-analyses. A beneficial effect was identified for psychosocial interventions on alcohol abstinence at 3–6 months (OR=2.05, 95% CI=1.20–3.48, k=5, n=2,312, I2 = 79%) and 12–60 months (OR=1.91, 95% CI=1.40–2.61, k=6, n=2,737, I2 = 63%) follow-up. There were no statistically significant effects found for AUDIT score (2–3 month: MD= −1.13, 95% CI: −2.60–0.34, k=6, n=992, I2=85%; 6 month: MD= −0.83, 95% CI= −1.92–0.26, k=6, n=1081, I2=69%; 12 month: MD= −0.15, 95% CI = −1.66–1.36, k=4; n=677; I2 = 75%), drinks per drinking day (3 months: MD: −0.22, 95% CI = −2.51–2.07, k=2, n=359, I2=82%; 6–36 months: MD= −0.09, 95% CI= −0.49–0.30, k=3, n=1450, I2=60%), or percent drinking days (3 months: MD= −4.60, 95%= −21.14–11.94; k=2; n=361; I2 = 90%; 6–9 months: MD=1.96, 95% CI= −6.54–10.46; k=2; n=818; I2 = 88%).
Conclusion:
Psychosocial interventions show promise at increasing self-reported alcohol abstinence in sSA, but clinical, methodological, and statistical heterogeneity across meta-analytic outcomes suggests results should be interpreted with caution.
Introduction
Harmful alcohol use is the seventh leading risk factor for morbidity and mortality globally and has been causally linked to more than 230 diseases and injuries.1,2 Although the World Health Organization (WHO) Africa Region has relatively low alcohol per capita consumed (APC) (6.3 liters per person),2 APC is high among those who drink (18.4 liters) and is among the highest in the world in some sub-Saharan African (sSA) countries.2 Consequently, the region experiences a disproportionately high level of alcohol-related harms.3 Alcohol use is of special concern in sSA given the high prevalence of HIV and tuberculosis,4,5 for which alcohol is a risk factor for infection, a catalyst to disease progression, and interferes with treatment adherence and efficacy.6,7
With limited availability of pharmacologic alcohol treatments in low-income settings,8 feasible and effective non-pharmacological approaches are needed. Psychosocial interventions, or psychologically based approaches to alcohol reduction, are the most commonly studied non-pharmacological approaches to alcohol reduction (e.g., cognitive-behavioral therapy, brief interventions [BI], family therapy, 12-step programs).9 Systematic reviews and meta-analyses of psychosocial interventions have demonstrated efficacy for alcohol reduction in specific settings and subpopulations in resource-rich settings.9–12 To-date, one narrative review of BI for alcohol in sSA showed positive results for BIs in health care settings13 and a more recent scoping review assessed the amount and types of alcohol interventions in sSA.14 However, there have been no meta-analyses to quantitatively synthesize the effect of alcohol interventions on consumption.
Structural interventions are another important non-pharmacological approach to alcohol reduction, especially given the alcohol industry’s rapid expansion and limited regulation in Africa.15,16 Structural interventions aim to change the environments in which risk behavior occurs, such as limiting alcohol availability. Although no systematic reviews exist to-date to assess their effect on alcohol consumption, an increasing number of intervention trials have demonstrated success in structural approaches at reducing alcohol consumption and related problems in diverse populations and contexts.17
With an increasing number of studies focused on the evaluation of alcohol interventions in sSA,13,14 along with distinct patterns of drinking, comorbidities, and cultural and environmental contexts in this setting, a review focusing exclusively on these types of interventions in sSA is warranted. The consolidation of existing evidence can inform decisions on which interventions should be scaled up to reduce harmful drinking in these settings. Therefore, we conducted a systematic review and meta-analysis to assess the effect of non-pharmacological interventions on alcohol reduction in sSA settings.
Methods
Search strategy and selection criteria
In this systematic review and meta-analysis, Embase, Medline, PsycINFO, EBSCO, CINAHL, and Cochrane CENTRAL were searched on December 21, 2017 for published reports in English from the earliest available date per database. This search was rerun on March 14, 2019. The search protocol is provided in Table S1. Reports were also hand-searched and supplementary data sent by study authors was included.
Inclusion criteria
To be included, studies had to be a randomized or nonrandomized controlled trial, conducted in sSA, assessing a non-pharmacological intervention aimed at alcohol reduction, and measuring at least one alcohol consumption outcome at follow-up greater than one month post-intervention. Eligible comparator groups included interventions unrelated to alcohol, usual care for alcohol or other services, brief feedback on an alcohol screening tool, alcohol or other informational materials, wait-list, and nothing.
Eligible outcomes included alcohol biomarkers (i.e., urine/blood analysis, breathalyzer tests) and self-reported measures: total score for the Alcohol Use Disorders Identification Test (AUDIT),18 alcohol abstinence (i.e., no drinking vs. any drinking), measures of drinking quantity (e.g., average number of drinks in a specific time period, such as in the prior week or per drinking day) and frequency (e.g., percent drinking days in a specified time period), and drinking intensity (e.g., binge/heavy episodic drinking, such as 4–5 drinks in a 2 hour time period). However, only 3 of the 12 studies reporting binge drinking outcomes had similar definitions, for which the timeframes and standard drink definitions used were unclear. The variability in these outcomes warranted a narrative synthesis. Therefore, the review results were divided into two companion manuscripts to ensure adequate space for reporting. Specifically, the present meta-analysis and a systematic review for the non-pooled outcomes19 were submitted simultaneously for peer review.
Exclusion criteria
Reasons for exclusion included alcohol reduction not being a primary goal of the intervention; alcohol reduction only being addressed in the context of sexual behavior; no comparator condition; comparator was another evidence-based or ‘bona-fide’ alcohol intervention (i.e., non-inferiority trial) and studies without data to be included in the meta-analysis (i.e., not reported and not provided after requested from study authors).
Screening procedures
One author (KS) screened all titles and abstracts, which underwent a targeted review by a second author (AM). If there was disagreement, studies were included in the full-text review. Four authors (AM, JW, KS, SK) and two research assistants reviewed full-text reports and assessed their eligibility in pairs. Disagreements were resolved by discussion and consensus reached between the reviewers or by a third author.
Data extraction and quality assessment
Two reviewers (AM, KS) independently extracted all outcome data into standardized, piloted data collection forms. Characteristics of each study (e.g., design, intervention) were extracted by one reviewer and checked for accuracy by a second reviewer. Per GRADE handbook recommendations, the Population, Intervention, Comparator, Outcome (PICO) framework was used to inform the structure of the data extraction form.20 In this framework, every row of extracted data represents the components of a study essential to answering the review’s research questions. The data extraction form was stratified one step further by utilizing the intervention, outcome, population trio (IOPT) structure.21 In this structure, each row of extracted data represents a unique data point for analysis, reflecting one intervention and comparator combination (e.g., BI versus standard of care), one outcome (e.g., AUDIT score), in a specific population (e.g., male only, female only, both genders) at a given follow-up interval (e.g., 3 months). In most cases, multiple IOPTs were extracted from each study, which were then grouped together in the meta-analysis by outcome and follow-up interval. All outcome data were independently extracted by both reviewers, compared, and reconciled through discussion. Corresponding authors of studies were contacted to collect relevant data not reported in the paper. Of 15 data requests made, 13 authors responded (response rate: 86%).
Study quality was assessed using the Cochrane Collaboration Tool for Assessing Risk of Bias,20 and three additional bias categories from the GRADE handbook (see Table S2).22 Assessment of risk of bias occurred at the time of data extraction and was assessed at the IOPT level as well as the study level. Each reviewer (AM, KS) independently rated each of the items as low risk, high risk, or unclear. Discrepancies were resolved by discussion. If consensus could not be reached, a third author was asked to break the tie.
Data analysis
Meta-analysis was done to synthesize the effect estimate for alcohol reduction interventions. All analyses were conducted in RevMan version 5. Results of trials with comparable outcomes were pooled using the random effects model and 95% confidence intervals (CIs). For continuous outcomes, mean differences (MD) were calculated between the intervention and comparison groups with 95% CIs. For dichotomous outcomes, odds ratios (ORs) with 95% CIs are presented. Outcomes were compared at different follow-up points, categorizing 2–3 months as short-term, 6–9 months as medium-term, and 12 months or longer as long-term follow-up. To make the maximum number of comparisons between studies, categories were sometimes merged for meta-analysis (i.e., short-medium [3–6 months], medium-long [6–36 months]). In cases where multiple data points were available from one study within one follow-up interval, we used the longest follow-up period.
In cases where data (i.e., mean/standard deviations; n per outcome) were not available nor provided by the corresponding authors, effect estimates and standard errors (SE) were extracted, if available, or calculated using alternative statistics. Analysis was then conducted under the generic inverse variance outcome in RevMan. In some cases, data transformations were made to synthesize data (e.g., transforming a categorical drinking frequency variable that could not be synthesized with continuous frequency outcomes into no drinking vs. any drinking; transforming number of drinking days in prior 30 days into percent drinking days).
Most cluster randomized controlled trials (CRCTs) accounted for clustering in their analysis. However, too few reported comparable effect and variance measures for our outcomes to use the generic inverse variance method. Therefore, we calculated the design effect by extracting the average cluster size and the intra-cluster coefficient (ICC). We then divided the original sample size by the design effect to reduce the size of the trial to its “effective sample size.”20 The ICC was obtained from the published report or from the study authors.
Heterogeneity between comparable trials was tested using a standard chi-squared test and I2 statistics, using a p-value of 0.10 or less to determine heterogeneity.23,24 I2 values are interpreted as low (0%−40%), moderate (30%−60%), substantial (50%−90%), and considerable (75%−100%) heterogeneity.25 We did not conduct quantitative investigations of heterogeneity or subgroup analyses due to the small number of studies per outcome/follow-up interval with comparable results, as these analyses are not recommended with less than 10 studies.20 We qualitatively explore select factors that might affect heterogeneity through the visual assessment of funnel plots when possible. The symmetry of funnel plots were similarly only visually assessed for publication bias due to an insufficient number of studies (<10) to quantitatively test for symmetry.20 If sufficient information had been available, planned formal subgroup analyses (e.g., gender, intervention dose) were outlined in the study protocol, which was registered on January 5, 2019 with PROSPERO (CRD42019094509) after the initial search and review of studies commenced.
Role of the funding source
This study had no direct funding. Sponsors of the study authors had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The authors had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
Results
The database search identified a total of 1,282 unique citations after the exclusion of duplicates. Six additional studies were identified through hand-searching and correspondence with study authors. Of these citations, 77 reports underwent full-text screening, 53 were excluded for reasons outlined in Figure 1 (a full list of ineligible studies reviewed as full-text is available in Table S3). Of the 24 reports judged eligible for meta-analysis, 5 were excluded due to missing data on eligible outcomes. In total, 18 studies26–43 reported in 19 scientific manuscripts44 met criteria for inclusion and contributed data for meta-analysis for the following outcomes: AUDIT score (k=11),26,30–34,38,39,41–43 drinks per drinking day (DDD) (k=3),29,35,39 percent drinking days (PDD) (k=3),28,29,39 and alcohol abstinence (k=7).26–29,35–37 No eligible studies included alcohol biomarkers.
Figure 1. Studies included in systematic review and meta-analysis.
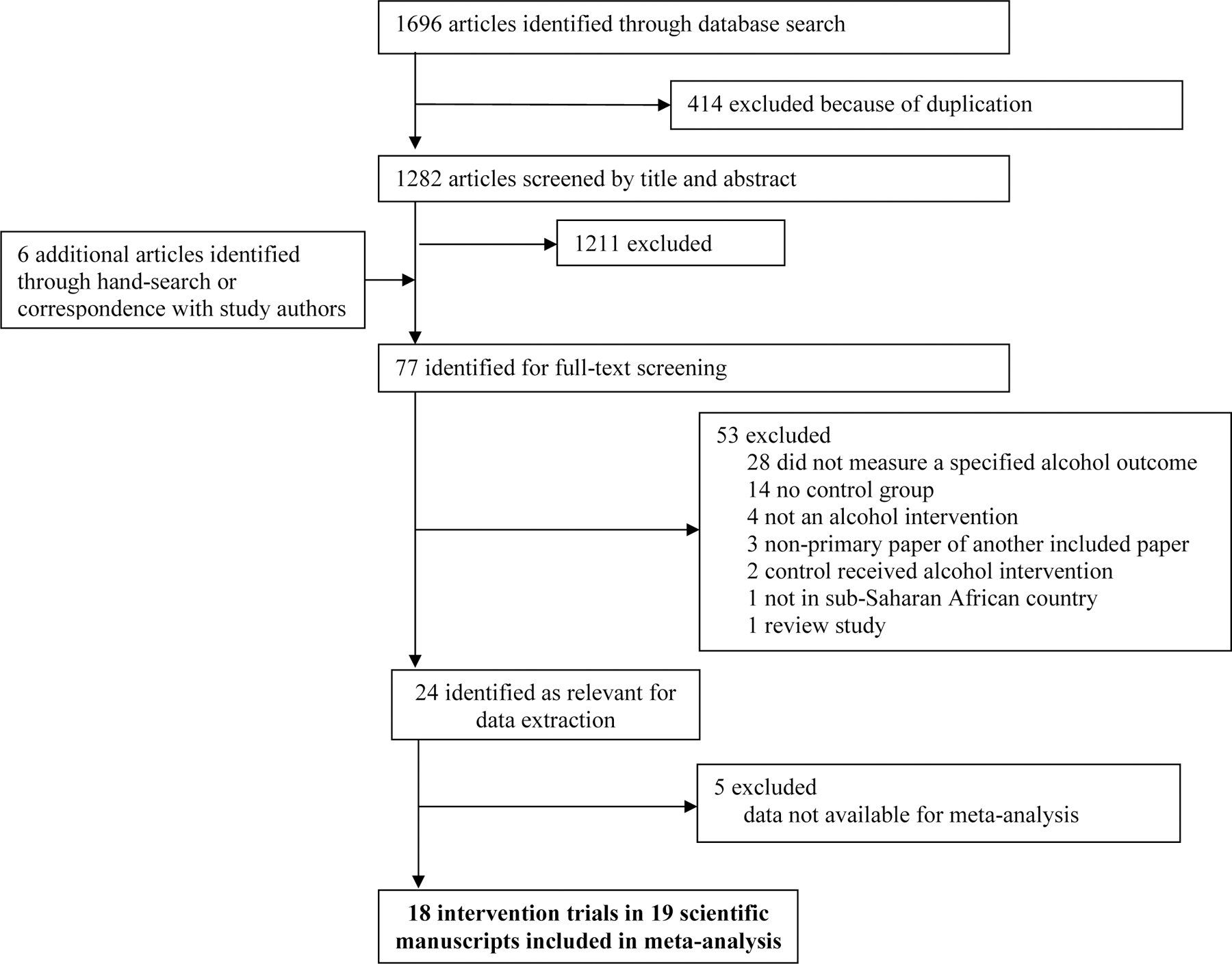
Adapted from the 2009 PRISMA Flow Diagram. Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred Reporting Items for Systematic Reviews and MetaAnalyses: The PRISMA Statement. PLoS Med 2009;6(7): e1000097. doi:10.1371/journal.pmed1000097
Summary of study characteristics
The 18 studies included spanned seven sSA countries: South Africa (k=9),30–36,40 Kenya (k=5),26–29,38 Namibia (k=1),37 Rwanda (k=1), Uganda (k=2),39 Tanzania (k=1),26 and Zambia (k=1)26 (including one multi-country study).26 Study designs were primarily RCTs (k=11).27–29,31–33,37,39–41,43 Eight studies tested interventions focused solely or primarily on alcohol reduction (k=5)30–32,38,39 or abstinence (k=3),27–29 as opposed to dual or multi-outcome focused interventions (k=10).26,33–37,40–43 The majority of studies included special subpopulations: people living with HIV (k=7),26,28,29,39–41,43 adolescents (n=3, including one with adolescents living with HIV),36,37,41 female sex workers (k=1),27 pregnant women (k=2),35,42 and TB patients (k=1).30
Nineteen interventions were tested across the 18 studies (one 3-armed study tested an intervention and an enhanced version of that intervention).38 All studies evaluated psychosocial interventions; one structural-level intervention was identified but was excluded because of a lack of appropriate data for meta-analysis. Seven interventions27,30–32,38,42 were based on or expanded upon WHO’s alcohol BI manual,18 five explicitly stated using motivational interviewing or motivational therapy (inclusive of two of the WHO BI studies),27,33,38,39,42 three were grounded in cognitive behavioral therapy,28,29,41 and four were informed by a behavior change model.30–32,37 The most common intervention setting was a health facility (k=8),26–30,39,41,42 followed by community venues (k=5),31–34,40 schools (k=3),36–38 and participants’ homes (k=2).35,43 Three interventions were delivered in a single-session,31,32,39 two did not reported the number of sessions,26,34 while all others were multi-session interventions. Comparator groups were: feedback on AUDIT results and/or general information or an educational leaflet on alcohol (k=6),30–33,38,42 standard-of-care for a range of services (k=7),26,29,35,39–41,43 nutrition/lifestyle intervention (k=4),27,28,36,40 and delayed intervention (k=2).34,37 Study design and intervention details are provided in Table 1.
Table 1.
Summary of study characteristics of alcohol interventions in sub-Saharan Africa included in meta-analysis
| Author, year / peer-reviewed | Country | Data years | Study design | Population group | Total N* | Age* (SD) | %female* | Intervention focus | Counseling/theoretical approach | Dose | Format and setting | Comparator /time & attention matched? | Alcohol outcome(s) for meta-analysis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bachanas, 2016 /peer-reviewed | Kenya, Tanzania, Zambia | 2009–2011 | CRCT | PLHIV attending clinical care | 3538 | 36 (SD NR) | 58.10% | HIV prevention, with alcohol components | NR | NR | Individual and group format, delivered by health workers and lay workers, in a health facility | HIV standard-of-care / No | AUDIT score Abstinence |
| Chaudhury, 2016 / peer-reviewed | Rwanda | NR | RCT | HIV-infected caregivers with at least one school aged child; women and men; Alcohol analyses only run for those who drank at baseline | 62 | 41.03 (8.76) | 68.29% | Family strengthening with content on HIV, violence reduction, and alcohol reduction | Psychosocial family approaches related to resilience building, family communication, parenting skills, HIV psycho-education; engagement of formal and informal supports | 90 minutes (single session or carried out over several, plus additional follow-ups to check progress) | Individual and group (family) format delivered by a trained counselor in participants’ homes | HIV standard-of-care for social work support services / No | AUDIT score |
| L’Engle, 2014 /peer-reviewed | Kenya | 2011–2012 | RCT | Female Sex Workers, moderate risk drinkers (AUDIT = 7–19) | 818 | 27.5 (6.6) | 100.00% | Alcohol abstinence, some focus on alcohol and sexual risk | WHO Brief Intervention, Motivational Interviewing | 120 minutes (six 20-minute sessions over 6 months) | Individual format delivered by nurse counselors in a health facility setting | Nutrition control intervention / Yes | Abstinence |
| Marais, 2011 / peer-reviewed | South Africa | 2007–2008 | CRCT | Pregnant women reporting any drinking | 194 | 25 (SD NR) | 100.00% | Alcohol abstinence, pregnancy-focus | WHO Brief Intervention, Motivational Interviewing | 120 minutes (4 sessions: first session 1 hour, follow-ups 20 minutes) | Individual format delivered by trained research staff in a health facility setting | AUDIT screening and alcohol booklet (2 sessions) / No | AUDIT score |
| Papas, 2011/peer-reviewed | Kenya | 2009 | RCT | PLHIV reporting binge or hazardous drinking (AUDIT-C = 3 or 6 or more drinks per occasion at least monthly) | 75 | 37.07 (8.40) | NR (females and males included) | Alcohol abstinence | Cognitive Behavioral Therapy | 540 minutes (6 weekly 90-minute sessions) | Group format delivered by a trained counselor in a health facility setting | Routine medical care / No | Drinks per drinking day Percent drinking days Abstinence |
| Papas, 2017/not peer-reviewed | Kenya | 2012–2016 | RCT | PLHIV reporting binge or hazardous drinking (AUDIT-C = 3 or 6 or more drinks per occasion at least monthly) | 614 | NR | NR (females and males included) | Alcohol abstinence | Cognitive-Behavioral Therapy | 540 minutes (6 weekly 90-minute sessions) | Group format delivered by a trained counselor in a health facility setting | Group healthy lifestyles education control intervention / Yes | Percent drinking days |
| Peltzer, 2013/peer-reviewed | South Africa | 2011–2012 | CRCT | Tuberculosis outpatients misusing alcohol (AUDIT = 8 or more for men; AUDIT = 7 or more for women) | 1196 | 36.7 (10.9) | 25.70% | Alcohol reduction | WHO Brief Intervention + content informed by Information-Motivation-Behavioral Skills Model | 30–40 minutes(Two 15–20-minute sessions within 1 month) | Individual format delivered by a lay counselor in a health facility | Health education leaflet on responsible drinking / No | AUDIT score |
| Pengpid, 2013a /peer-reviewed | South Africa | 2011–2012 | RCT | University students screened as at-risk drinkers (AUDIT > 8) | 152 | 21.9 (3.5) | 12.70% | Alcohol reduction | WHO Brief Intervention + content informed by Information-Motivation-Behavioral Skills Model | 20 minutes (single session) | Individual format delivered by a nurse research assistant in a public venue | Feedback on AUDIT + health education leaflet on responsible drinking / No | AUDIT score |
| Pengpid, 2013b /peer-reviewed | South Africa | 2011–2012 | RCT | Outpatients screened as hazardous or harmful drinkers (AUDIT = 8–19 for men and 7–19 for women | 392 | 35.6 (11.45) | 27.60% | Alcohol reduction | WHO Brief Intervention + content informed by Information-Motivation-Behavioral Skills Model | 20 minutes (single session) | Individual format delivered by a nurse research assistant in a public venue | Health education leaflet on responsible drinking / No | AUDIT score |
| Rendall-Mkosi, 2013/peer-reviewed | South Africa | 2007–2008 | RCT | Women at high-risk of alcohol-affected pregnancy (not using contraceptives and engaging in risk drinking) | 165 | 29.8 (SD NR) | 100.00% | Fetal alcohol syndrome prevention | Motivational interviewing | 5 sessions (duration NR) | Individual format delivered by a lay worker in a venue of participants’ choice | Information pamphlet on fetal alcohol syndrome prevention and a handbook onwoman’s health / No | AUDIT score |
| Rotheram-Borus, 2015/peer-reviewed & Rotheram-Borus, unpublished manuscript/not peer reviewed | South Africa | 2009–2014 | CRCT | Pregnant women | 1238 | 26.53(5.63) | 100.00% | Maternal health package (HIV, TB, alcohol, mental health, breastfeeding, malnutrition healthy behaviorchange) | NR | 240 minutes(Eight 30-minute sessions, 4 prenatal and 4 postnatal) averaging about 30 minutes each | Individual format delivered by a Community Health Worker in participant’s homes | Standard-of-care services offered at health clinic within 5 km radius / No | Drinks per drinking day Abstinence (unpublished data) |
| Rotheram-Borus, 2016/peer-reviewed | South Africa | NR | CRCT | Unemployed young men (age 18–25) | 142 | 21.9 (1.9) | 0.00% (men only) | HIV prevention, alcohol and drug reduction | Psychosocial approaches (e.g., goal setting, problem solving, praise, social rewards). Subset of men received vocational training. | NR | Individual and group format delivered by trained soccer coaches in the context of a community soccer program | Delayed intervention / Yes | AUDIT score |
| Senyonyi, 2016 /peer-reviewed | Uganda | 2011 | RCT | HIV-infected adolescents | 171 | 15.24 (1.96) | 54.80% | Sexual risk and substance use reduction | Cognitive Behavioral Therapy | 640 minutes (Eight 80-minute sessions over 8 weeks) | Group format delivered by a trained counselor in a health facility | Standard group counseling / Yes | AUDIT score |
| Smith, 2008/peer-reviewed | South Africa | 2003 | NRCT | Adolescent students | 2176 | 14 (0.86) | 51.50% | Leisure, life skills, substance use, and sexuality education intervention | NR | 15 hours (Twelve 50-minute lessons in grade 8, followed by 6 booster lessons in grade 9) | Group format delivered by educators in a school setting | Standard-of-care (Life Orientation curriculum from the South AfricanDepartment of Education) / No | Abstinence |
| Stanton, 1998 /peer-reviewed | Namibia | 1996–1997 | RCT | Adolescents (aged 15–18) | 515 | 17 (medi an, SD NR) | 54.00% | HIV, sexual health, alcohol reduction, violence reduction | Social Cognitive Theory | 28 hours (14 sessions, 2 hours each) | Group format delivered by volunteer teachers and an out-of-school youth in an after-school program | Delayed intervention control / Yes | Abstinence |
| Takahashi, 2018 /peer-reviewed | Kenya | 2015 | NRCT | Hazardous or harmful drinkers (AUDIT = 8–19) | 161 | 43.6 (SD NR) | 18.25% | Alcohol reduction | Two intervention arms: 1) WHO Brief Intervention 2) WHO Brief Intervention + Motivational Therapy |
1) Brief Intervention Only: 15–60
minutes (three 5–20-minute sessions) 2) Brief Intervention + Motivational Therapy: 4 additional session, duration NR |
1) Brief Intervention Only: Individual
format delivered by a Community Health Worker in a local
school 2) Brief Intervention + Motivational Therapy: Individual + group format delivered by a Community Health Worker in a local school |
General information about alcohol / No | AUDIT score |
| Wandera, 2017 /peer-reviewed | Uganda | 2013–2014 | RCT | PLHIV identified as hazardous drinkers (AUDIT-C > 2) | 337 | 39 (medi an, IQR: 32–46) | 34.40% | Alcohol reduction | Motivational interviewing | 20–30 minutes (single session) | Individual format delivered by a trained counseling in a health facility | Standardized positive prevention counseling / Yes | AUDIT score Drinks per drinking day Percent drinking days |
| Zule, 2014/peer-reviewed | South Africa | 2008–2011 | RCT | WLHIV (age 18–33) who drink | 84 | 23.35 (3.95) | 100.00% | HIV-focused intervention, focused on: alcohol and drug use, sexual risk, violence, gender inequality | NR | 4 hours (Four 1-hour sessions delivered over 2 contact points) | Group format delivered by a peer educator in a community setting | Two control groups combined for analysis: Nutrition intervention and HIV Counseling and Testing group | Abstinence |
indicates as reported at baseline; RCT = randomized controlled trial; CRCT = cluster randomized controlled trial; NRCT = non-randomized quasi-experimental controlled trial; PLHIV=people living with HIV; women living with HIV=WLHIV; TB = Tuberculosis; AUDIT = Alcohol Use Disorders Identification Test; DDD = Mean drinks per drinking day; PDD = percentage of days drank; IQR = interquartile range; NR = Not reported
Meta-analysis results
The meta-analyses that focused on AUDIT scores found no statistically significant differences between intervention and comparator at 2–3 months, 6-months, or 12-months post-intervention (Figure 2). No statistically significant differences were found for DDD at 3 or 6–36 months or PDD at 3 or 6–9 months (Figures 3 & 4). The meta-analysis of trials on alcohol abstinence showed a beneficial effect of psychosocial interventions versus comparator at 3–6 months post-intervention. The effect on alcohol abstinence was also statistically significant for trials assessing long-term follow-up (12–60 months) (Figure 5).
Figure 2. Results of meta-analyses with Alcohol Use Disorders Identification Test (AUDIT) score by follow-up period.
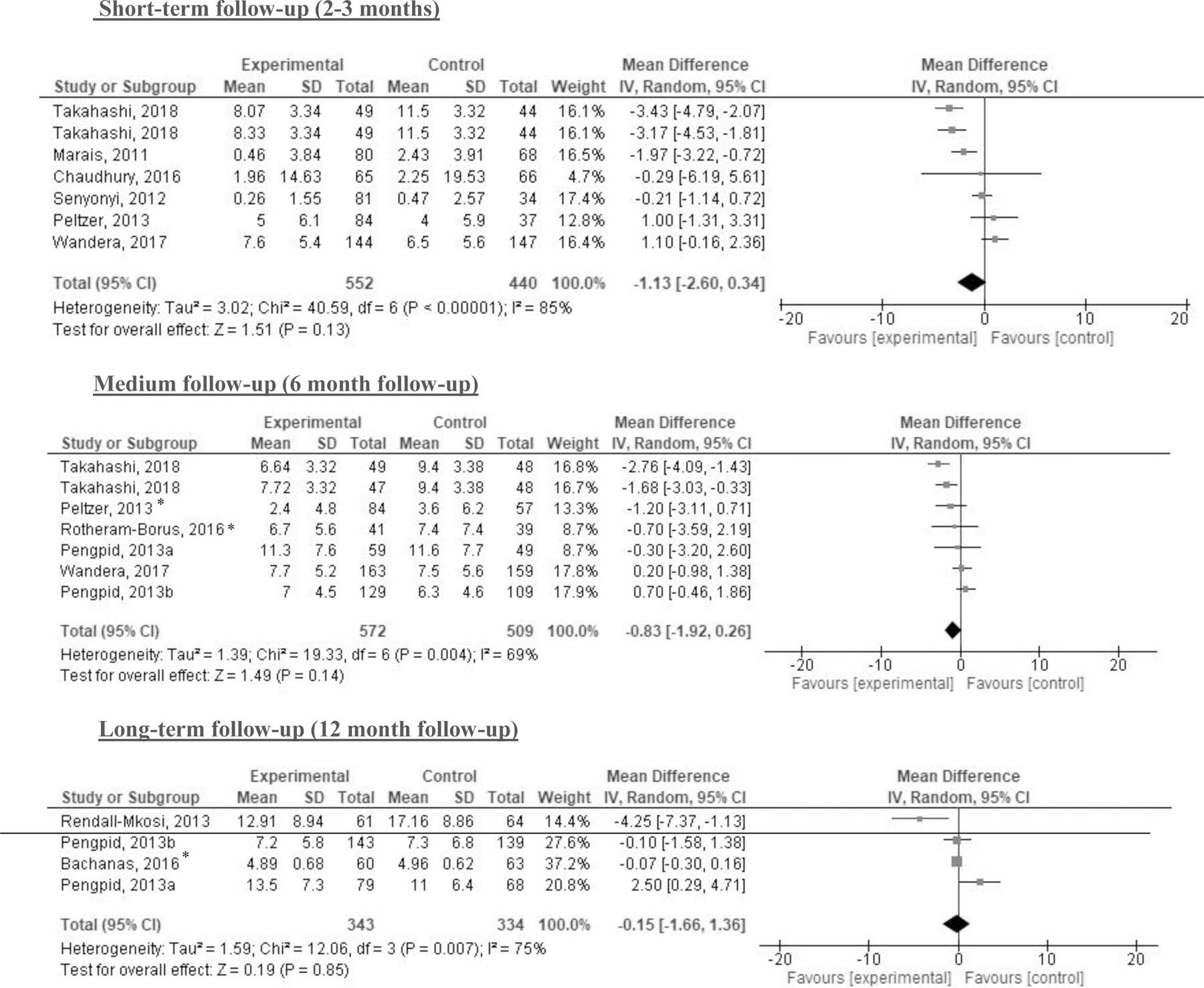
Note: *indicates cluster randomized controlled trials (CRCT) for which the sample size was adjusted by design effect
Figure 3. Results of meta-analyses with drinks per drinking day (DDD) by follow-up period.
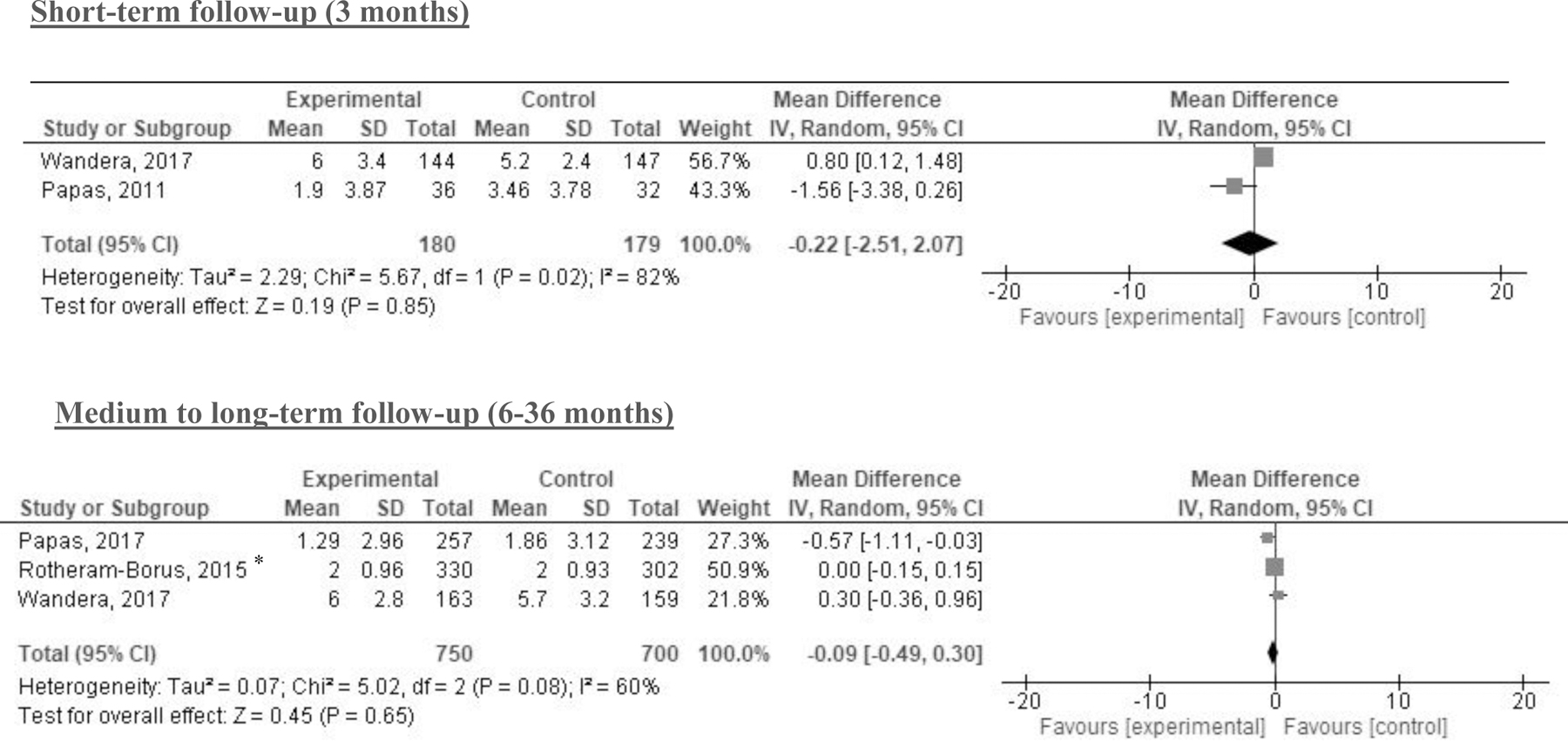
Note: *indicates cluster randomized controlled trials (CRCT) for which the sample size was adjusted by design effect
Figure 4.

Results of meta-analyses with percentage of drinking days (PDD) by follow-up period
Figure 5. Results of meta-analyses with alcohol abstinence by follow-up period.
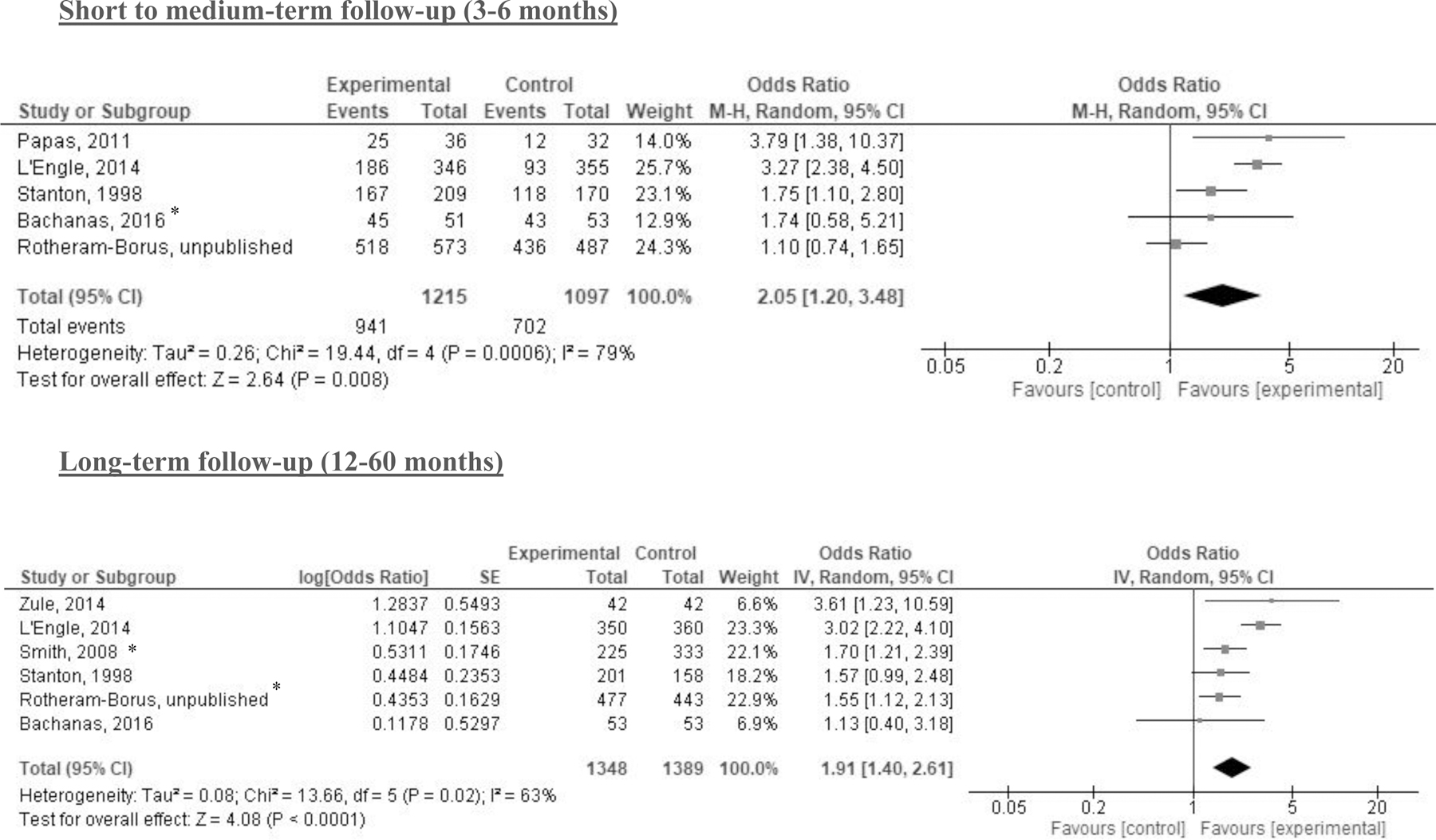
Note: *indicates cluster randomized controlled trials (CRCT) for which the sample size was adjusted by design effect; note the direction of the intervention effect differs from the previous continuous outcomes to reflect the desired outcome of greater abstinence.
A moderate to considerable level of heterogeneity was identified across all analyses (I² between 60% and 90%). Qualitative comparison identified one factor that appeared to drive differences in effects on abstinence. Exploratory funnel plots in Figure 6 demonstrates larger effect sizes for studies that included drinking (any drinking or specified risk-level) at baseline in their inclusion criteria compared to studies that did not.
Figure 6.
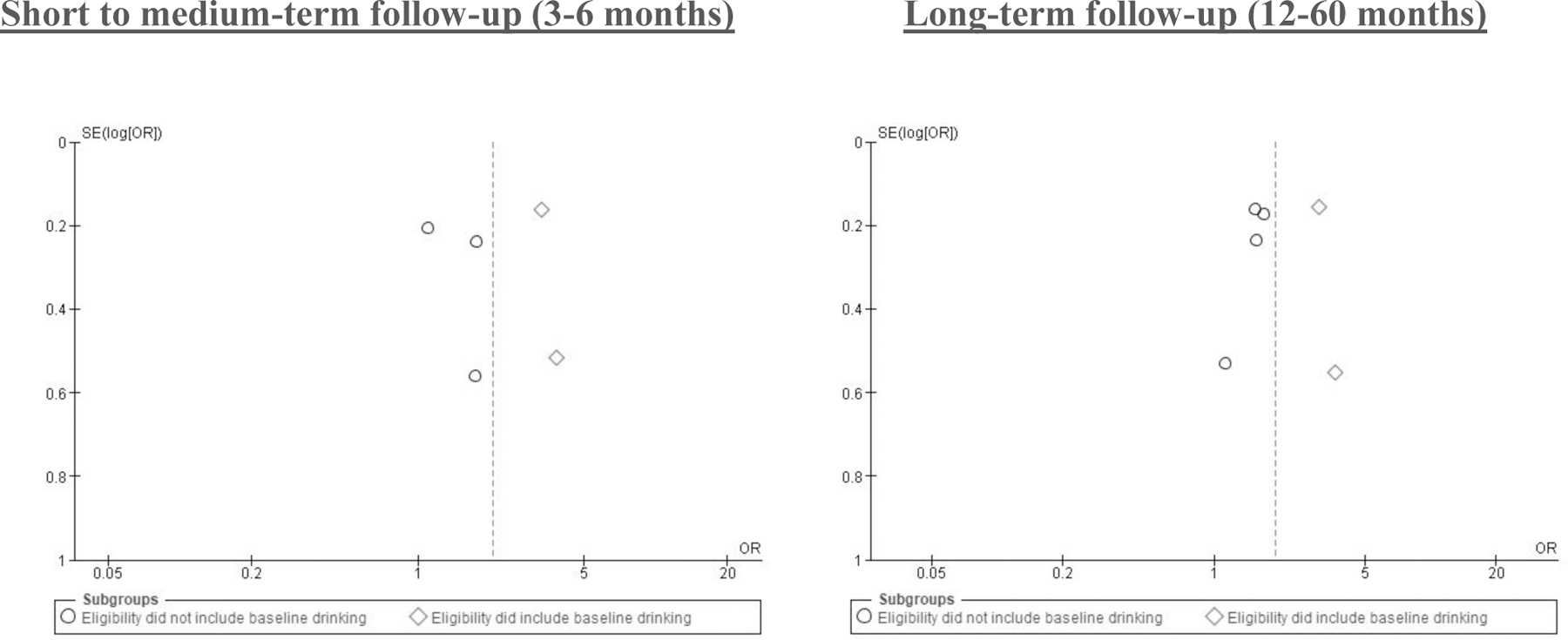
Funnel plot of comparison: Alcohol abstinence outcome by subgroups, eligibility did include baseline drinking vs. eligibility did not include baseline drinking
Publication bias results
Figure S1 presents funnel plots for AUDIT scores and abstinence outcomes to assess publication bias. Too few studies (k=2–3) were reported to make comparisons for PDD and DDD.20 The plots were overall symmetrical; therefore, no publication bias was detected. Studies tended to cluster at the top of the plot, indicating more publication of studies with larger sample sizes. Clustering at one end can indicate small study bias. However, this concern is mitigated; small studies with positive effects were not more likely to be published; rather, larger studies were more likely to be published regardless of effect.
Risk of bias assessment results
In general, studies evaluated with the Cochrane risk of bias tool were of moderate quality (see Figure 7). Randomization procedures were properly described in 75% of studies, and half of the studies reported details on allocation concealment. No studies blinded both participants and study personnel, and less than 25% blinded outcome assessment – potential sources of performance and detection bias. Other weaknesses included a lack of published study protocols resulting in high risk for selective reporting bias, and flawed measurement of exposure (i.e., a lack of information on intervention dose and fidelity). See Figure S2 and Table S4 for the full risk of bias assessment per study and outcome.
Figure 7.
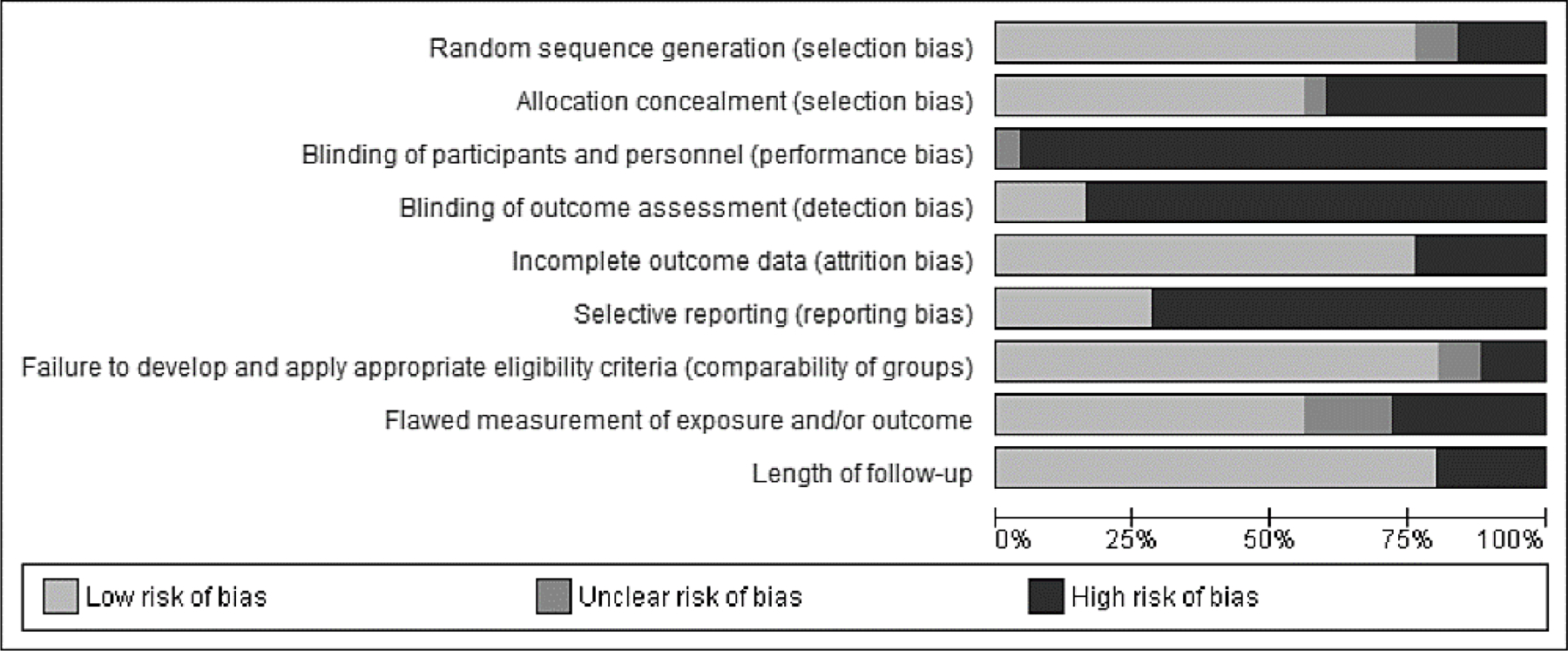
Risk of bias graph: review authors’ judgements about each risk of bias items presented as percentages across all included studies
Discussion
Despite high rates of alcohol-related morbidity and mortality in sSA,2 this is the only meta-analysis to compare the effect of psychosocial interventions versus a comparator on alcohol consumption among individuals in sSA to-date. Our results are specific to psychosocial interventions; only one structural intervention met our inclusion criteria,45 but did not have data available for meta-analysis. Two main findings emerged from this systematic review and meta-analysis. First, psychosocial interventions appear to have a benefit on alcohol abstinence at both short to medium and long-term follow up. Second, psychosocial interventions showed no significant effect on AUDIT score, DDD, and PDD.
Our review reinforces the need for research aimed to develop and test alcohol interventions in sSA. In line with Francis et al.’s scoping review,14 this review demonstrates that the number of studies with this aim is disproportionately low compared to the burden of alcohol use problems in the region and are heavily concentrated in South Africa and Kenya. Still, the beneficial effect identified for alcohol abstinence outcomes shows promise for the use of alcohol psychosocial interventions in sSA with this aim.
Potential sources of heterogeneity and varied effects
Drinking at baseline and alcohol-only vs. multi-component interventions
The heterogeneity identified across outcomes was moderate to high. While the total number of studies per outcome restricted quantitative comparisons, qualitative exploratory analysis identified larger effect sizes among studies that included any type of drinking at baseline as part of the study’s inclusion criteria compared to those that did not for alcohol abstinence (Figure 6). These differences may be attributed to regression to the mean, or more room for change for those already drinking at baseline. However, the same two studies in the short-medium term follow-up assessment that included those drinking at baseline were also the only two studies to focus solely on alcohol reduction. Therefore, we cannot tease apart the effects of these subcomponents within the short-medium analyses. It is possible that alcohol-focused interventions had a stronger impact than those with multiple outcomes, as we are unable to determine the intervention dose specific to alcohol in these studies. Given multiple alcohol-involved “syndemics” in African settings (i.e., two or more epidemics interacting synergistically to contribute to excess burden of disease in a population), about 45% of the interventions tested included alcohol reduction as a subcomponent of a multi-component intervention aimed at more than one health behavior.
Population
It is possible that the observed abstinence effects are influenced by over-reporting of self-reported alcohol abstinence due to social desirability, especially for certain populations (e.g., pregnant women).47 No clear patterns emerged in intervention effect by population, but the wide variability of populations included limited even qualitative comparison. However, studies measuring alcohol abstinence exclusively included sub-populations for which alcohol abstinence was an appropriate goal (i.e., HIV populations, female sex workers, pregnant women, adolescents). It is possible that these studies achieved greater effects than other alcohol outcomes given unique motivations to not drink among these subpopulations.
Measurement bias
The effect sizes for abstinence may be exaggerated by the binary nature of the measure.46 The null findings for AUDIT scores could also be an effect of measurement bias. The AUDIT is designed to identify high-risk drinking patterns, with half of the questions assessing occurrence of alcohol-related problems or negative consequences of alcohol use in the past year. Of the 8 studies reporting AUDIT change at less than 12 months follow-up, 2 did not explicitly state changing the timeframe of the questions to match their shorter follow-up period. These studies were included with the assumption that this change was made, but it is possible that the scale’s timeframe was not modified for all studies, reducing the likelihood that change would be observed in less than 12 months. Further, two of the AUDIT questions assess current or past lifetime harmful drinking. Thus, studies with less than 12 months follow-up may not show significant change in AUDIT scores even when the questions are modified to assess change in a 2 to 6 month timeframe. No studies included alcohol biomarkers, such as blood alcohol concentration (BAC) or Phosphatidylethanol (PEth), which has been shown to be more reliable than self-report in African cohort studies.47 Taken together, these findings highlight self-reported and inconsistent alcohol outcome measurement as a weakness of the alcohol-focused intervention literature in sSA.
Comparators
Comparators can drive effect size magnitude. The wide variability of comparators within each outcome assessment limited the ability to make any meaningful conclusions about their influence on effect size across outcomes. However, a number of BI studies and one CBT study found no significant differences between intervention participants compared to minimal intervention, but reductions in drinking were observed in both treatment arms. 31–33,41,42 This nuance is not apparent in our meta-analysis results but may be a driver of the AUDIT meta-analyses’ null effects.
Intervention
At this stage, the picture remains unclear on which intervention approaches show the most promise. Alcohol interventions for groups with special health concerns or other reasons not to drink (i.e., adolescents, female sex workers) that showed promise were conducted across a wide set of settings, using a range of psychosocial approaches, including CBT, MI, and other broad psychosocial group and individual-focused approaches. More research is needed to provide pointed policy and practice recommendations on which interventions work in different settings. Future research will also be needed to inform the cost-effectiveness and feasibility of scaling up these approaches in resource-limited settings. A cost-benefit analysis associated with the Kenya CBT study28 included in this review reported CBT can be effectively and economically task-shifted to paraprofessionals in Kenya.48 Additional costing studies, along with hybrid implementation studies that simultaneously assess implementation and effectiveness, can inform the feasible scale up of alcohol interventions in settings with resource-constraints.
Individual-level focus
This review demonstrates alcohol interventions in sSA to-date are overwhelmingly focused on individual, rather than structural-level, change. Despite a large number of BI studies based on MI and the WHO SBI guidelines, evidence for change in AUDIT scores using this approach remains limited in sSA, contrary to a body of literature supporting moderate effects using this approach in well-resourced settings.10 Beyond the measurement and methodological limitations already noted, a possible explanation for the underwhelming effects of these interventions may be their lack of focus on the social and physical environment.49 Alcohol outlet density,50 aggressive alcohol marketing,51 and lax alcohol regulation and policy enforcement52 are prevalent contributors to alcohol consumption in African settings. More rigorous research that tests interventions altering the social and physical environment, or other structural approaches, are needed.
Limitations
Limitations of this systematic review include challenges in the ability to synthesize all eligible studies due to the disparate measurement of alcohol outcomes at varying time points, resulting in low statistical power for some outcomes assessed. Low statistical power may have contributed to the null findings for DDD and PDD outcomes in particular. While variability of outcome measures is a known issue in the alcohol intervention field,53 our broad inclusion criteria likely also contributed to the broad set of outcomes identified. The especially variable measurement of heavy episodic/binge drinking limited our ability to present these findings alongside the outcomes in this review, which are included in a forthcoming narrative synthesis.19 In addition, we identified significant heterogeneity across studies with limitations in our ability to conduct comprehensive, quantitative assessments of differences by study design, intervention, and population, as discussed above. Our qualitative comparisons are exploratory in nature, and should be reviewed with caution as they include less than ten studies per outcome/timepoint.20 As the alcohol intervention literature in sSA continues to grow, this should be a focus of future reviews.
Our inclusion criteria allowed for both non-randomized and randomized controlled trials. Though only two non-randomized trials were included, they bring inherent risk of selection bias. Moreover, the risk of bias assessment identified risk in randomization and allocation concealment in a number of randomized studies. These and other risks of bias identified should be considered in the interpretation of our findings. Moreover, several interventions were “pilot” studies which may be less robust in design and intervention content – a reflection of the developmental stage of the alcohol intervention literature specific to sSA.29,34,40 The studies were judged as moderate quality, demonstrating a need for added rigor in the assessment of future alcohol interventions through randomized controlled trials.
Conclusion
This review highlights the need for more research testing alcohol interventions in sSA. Null findings were identified for interventions assessing change in AUDIT, DDD, and PDD across a range of sSA contexts. However, the review showed some promise for psychosocial interventions to promote alcohol abstinence. Given the wide scope of this review, significant heterogeneity was identified across studies. As the pool of research grows in this area, more direct investigations of differences across population, setting, design, and intervention type would provide more pointed guidance on the context-specific application of research to alcohol policy and programming in sSA.
With detrimental health and societal effects of harmful alcohol use affecting sSA and limited access to pharmacological alcohol interventions, research to develop acceptable and feasible non-pharmacological interventions for sSA should be prioritized. The literature on alcohol-focused interventions in sSA would benefit from more rigorous designs, consistency across alcohol outcomes, the inclusion of alcohol biomarker outcomes, and the systematic assessment of structural approaches to alcohol reduction in addition to the current literature focused on individual-level psychosocial interventions.
Supplementary Material
Acknowledgements
Janene Batten, Yale School of Nursing Librarian, helped to develop the search protocol and ran the search. Dr. Elizabeth Reed, Jessica Sibal, and Alice Wong at San Diego State University assisted us with the review of full-text articles. Tina Anh Huynh at the University of Texas at San Antonio provided editorial assistance. We thank these individuals for their contributions.
Funding:
KMS and APM were supported by a T32 Predoctoral Fellowship Award from NIDA (T32DA23356, PI: Strathdee). KMS was supported by a T32 Postdoctoral Fellowship Award from NIMH (5T32MH020031-18, PI: Kershaw). JAW was supported by a Career Development Award from NIAAA [7K01AA024068-05].
Footnotes
Declaration of competing interests: The authors have no conflicts of interests to disclose.
References
- 1.Griswold MG, Fullman N, Hawley C, Arian N, Zimsen SR, Tymeson HD, et al. Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the global burden of disease study 2016. The Lancet 2018; 392(10152): 1015–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. Global status report on alcohol and health. Geneva, Switzerland: WHO, 2018. [Google Scholar]
- 3.Shield K, Manthey J, Rylett M, Probst C, Wettlaufer A, Parry CD, et al. National, regional, and global burdens of disease from 2000 to 2016 attributable to alcohol use: a comparative risk assessment study. Lancet Public Health 2020; 5(1): e51–e61. [DOI] [PubMed] [Google Scholar]
- 4.WHO. Global Tuberculosis Report 2018. Geneva, Switzerland: World Health Organization; 2019. [Google Scholar]
- 5.UNAIDS. UNAIDS Data 2018. New York, New York, 2018. [Google Scholar]
- 6.Williams EC, Hahn JA, Saitz R, Bryant K, Lira MC, Samet JH. Alcohol use and human immunodeficiency virus (HIV) infection: current knowledge, implications, and future directions. Alcohol Clin Exp Res 2016; 40(10): 2056–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imtiaz S, Shield KD, Roerecke M, Samokhvalov AV, Lonnroth K, Rehm J. Alcohol consumption as a risk factor for tuberculosis: meta-analyses and burden of disease. Eur Respir J 2017; 50(1) pii: 1700216. doi: 10.1183/13993003.00216-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benegal V, Chand PK, Obot IS. Packages of care for alcohol use sisorders in low- and middle-income countries. PLOS Medicine 2009; 6(10): e1000170. doi: 10.1371/journal.pmed.1000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klimas J, Tobin H, Field CA, O’Gorman CS, Glynn LG, Keenan E, et al. Psychosocial interventions to reduce alcohol consumption in concurrent problem alcohol and illicit drug users. Cochrane Database Syst Rev 2014; (12): CD009269. doi: 10.1002/14651858.CD009269.pub4. [DOI] [PubMed] [Google Scholar]
- 10.Kaner EF, Beyer FR, Muirhead C, Campbell F, Pienaar ED, Bertholet N, et al. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database Syst Rev 2018; 2: CD004148. doi: 10.1002/14651858.CD004148.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beyer FR, Campbell F, Bertholet N, Daeppen JB, Saunders JB, Pienaar ED, et al. The Cochrane 2018 review on brief interventions in primary care for hazardous and harmful alcohol consumption: a distillation for clinicians and policy makers. Alcohol Alcohol 2019; 54(4): 417–27. [DOI] [PubMed] [Google Scholar]
- 12.Carney T, Myers BJ, Louw J, Okwundu CI. Brief school-based interventions and behavioural outcomes for substance-using adolescents. Cochrane Database Syst Rev 2016; (1): Cd008969. doi: 10.1002/14651858.CD008969.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peltzer K Brief intervention of alcohol problems in sub-Saharan Africa: a review. J Psychol Afr 2009; 19(3): 415–22. [Google Scholar]
- 14.Francis JM, Cook S, Morojele NK, Swahn MH. Rarity and limited geographical coverage of individual level alcohol interventions in sub Saharan Africa: findings from a scoping review. J Subst Use 2019: 1–9. doi: 10.1080/14659891.2019.1664662 [DOI] [Google Scholar]
- 15.Ferreira-Borges C, Parry CDH, Babor TF. Harmful use of alcohol: a shadow over sub-Saharan Africa in need of workable solutions. Int J Environ Res Public Health. 2017; 14(4): 346. doi: 10.3390/ijerph14040346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Babor TF, Robaina K, Jernigan D. The influence of industry actions on the availability of alcoholic beverages in the African region. Addiction 2015; 110(4): 561–71. [DOI] [PubMed] [Google Scholar]
- 17.Charlebois ED, Plenty AH, Lin J, Ayala A, Hecht J. Impact of a structural intervention to address alcohol use among gay bar patrons in San Francisco: the PACE study. AIDS Behav 2017; 21(Suppl 2): 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Babor TF, Higgins-Biddle JC. Brief interventions for hazardous and harmful drinking: a manual for use in primary care. Geneva: World Health Organization; 2001. [Google Scholar]
- 19.Sileo KM, Miller AP, Huynh TA, Kiene SM. A systematic review of interventions for reducing heavy episodic drinking and related outcomes in sub-Saharan African settings. Under peer review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins JPT, Green S. The Cochrane Handbook for Systematic Reviews of Interventions Version 510 [updated March 2011] The Cochrane Collaboration Available from: www.handbook.cochrane.org; 2011.
- 21.Malekinejad M, Barker R, Wu J, Loi J, Mirzazadeh A. Systematic review [internet]. Global Health Decisions: University of California San Franscisco: [accessed 2019 Oct]. Available from: http://globalhealthdecisions.org/methods/systematic-review/ [Google Scholar]
- 22.Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from www.guidelinedevelopment.org/handbook. [Google Scholar]
- 23.Huedo-Medina TB, Sanchez-Meca J, Marin-Martinez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods 2006; 11(2): 193–206. [DOI] [PubMed] [Google Scholar]
- 24.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21(11): 1539–58. [DOI] [PubMed] [Google Scholar]
- 25.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327(7414): 557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bachanas P, Kidder D, Medley A, Pals SL, Carpentar D, Howard A, et al. Delivering prevention interventions to people living with HIV in clinical care settings: results of a cluster randomized trial in Kenya, Namibia, and Tanzania. AIDS Behav 2016; 20(9): 2110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.L’Engle KL, Mwarogo P, Kingola N, Sinkele W, Weiner DH. A randomized controlled trial of a brief intervention to reduce alcohol use among female sex workers in Mombasa, Kenya. J Acquir Immune Defic Syndr 2014; 67(4): 446–53. [DOI] [PubMed] [Google Scholar]
- 28.Papas RK, Gakinya BN, Mwaniki MM, Lee H, Keter AK, Klein MP, et al. Successful treatment outcomes from a stage 2 randomized clinical trial of CBT to reduce alcohol use among HIV-infected outpatients in Western Kenya. Alcohol Clin Exp Res 2017; 41: 253A. [Google Scholar]
- 29.Papas RK, Sidle JE, Gakinya BN, Baliddawa JB, Martino S, Mwaniki MM. Treatment outcomes of a stage 1 cognitive-behavioral trial to reduce alcohol use among HIV-infected outpatients in western Kenya. Addiction 2011; 106(12): 2156–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peltzer K, Naidoo P, Louw J, Matseke G, Zuma K, McHunu G, et al. Screening and brief interventions for hazardous and harmful alcohol use among patients with active tuberculosis attending primary public care clinics in South Africa: results from a cluster randomized controlled trial. BMC Public Health 2013; 13: 699. doi: 10.1186/471-2458-13-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pengpid S, Peltzer K, Skaal L, Van der Heever H. Screening and brief interventions for hazardous and harmful alcohol use among hospital outpatients in South Africa: results from a randomized controlled trial. BMC Public Health 2013; 13: 644. doi: 10.1186/471-2458-13-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pengpid S, Peltzer K, Van der Heever H, Skaal L. Screening and brief interventions for hazardous and harmful alcohol use among university students in South Africa: results from a randomized controlled trial. Int J Environ Res Public Health 2013; 10(5): 2043–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rendall-Mkosi K, Morojele N, London L, Moodley S, Singh C, Girdler-Brown B. A randomized controlled trial of motivational interviewing to prevent risk for an alcohol-exposed pregnancy in the Western Cape, South Africa. Addiction 2013; 108(4): 725–32. [DOI] [PubMed] [Google Scholar]
- 34.Rotheram-Borus MJ, Tomlinson M, Durkin A, Baird K, DeCelles J, Swendeman D. Feasibility of using soccer and job training to prevent drug abuse and HIV. AIDS Behav 2016; 20(9): 1841–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rotheram-Borus MJ, Tomlinson M, Roux IL, Stein JA. Alcohol use, partner violence, and depression: a cluster randomized controlled trial among urban South African mothers over 3 years. Am J Prev Med 2015; 49(5): 715–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith EA, Palen LA, Caldwell LL, Flisher AJ, Graham JW, Mathews C, et al. Substance use and sexual risk prevention in Cape Town, South Africa: an evaluation of the healthwise program. Prev Sci 2008; 9(4): 311–21. [DOI] [PubMed] [Google Scholar]
- 37.Stanton BF, Li X, Kahihuata J, Fitzgerald AM, Neumbo S, Kanduuombe G, et al. Increased protected sex and abstinence among Namibian youth following a HIV risk-reduction intervention: a randomized, longitudinal study. AIDS 1998; 12(18): 2473–80. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi R, Wilunda C, Magutah K, Mwaura-Tenambergen W, Atwoli L, Perngparn U. Evaluation of alcohol screening and community-based brief interventions in rural western Kenya: a quasi-experimental study. Alcohol Alcohol 2018; 53(1): 121–8. [DOI] [PubMed] [Google Scholar]
- 39.Wandera B, Tumwesigye NM, Nankabirwa JI, Mafigiri DK, Parkes-Ratanshi RM, Kapiga S, et al. Efficacy of a single, brief alcohol reduction intervention among men and women living with HIV/AIDS and using alcohol in Kampala, Uganda: a randomized trial. J Int Assoc Provid AIDS Care 2017; 16(3): 276–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zule W, Myers B, Carney T, Novak SP, McCormick K, Wechsberg WM. Alcohol and drug use outcomes among vulnerable women living with HIV: results from the western cape women’s health coop. AIDS Care 2014; 26(12): 1494–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Senyonyi RM, Underwood LA, Suarez E, Musisi S, Grande TL. Cognitive behavioral therapy group intervention for HIV transmission risk behavior in perinatally infected adolescents. Health 2012; 4(12): 1334–1345. [Google Scholar]
- 42.Marais S, Jordaan E, Viljoen D, Olivier L, de Waal J, Poole C. The effect of brief interventions on the drinking behaviour of pregnant women in a high-risk rural South African community: a cluster randomised trial. Early Child Dev Care 2011; 181(4): 463–74. [Google Scholar]
- 43.Chaudhury S, Brown F, Kirk C, Mukunzi S, Nyirandagijimana B, Mukandanga J, et al. Exploring the potential of a family-based prevention intervention to reduce alcohol use and violence within HIV-affected families in Rwanda. AIDS care 2016; 28(Suppl 2): 118–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rotheram-Borus MJ et al. The association of maternal alcohol use and paraprofessional home visiting with children’s health: a randomized controlled trial. Unpublished data and report provided from study authors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cubbins LA, Kasprzyk D, Montano D, Jordan LP, Woelk G. Alcohol use and abuse among rural Zimbabwean adults: a test of a community-level intervention. Drug Alcohol Dep 2012; 124(3): 333–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanner-Smith EE, Risser MD. A meta-analysis of brief alcohol interventions for adolescents and young adults: variability in effects across alcohol measures. Am J Drug Alcohol Abuse 2016; 42(2): 140–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bajunirwe F, Haberer JE, Boum Y II, Hunt P, Mocello R, Martin JN, et al. Comparison of self-reported alcohol consumption to phosphatidylethanol measurement among HIV-infected patients initiating antiretroviral treatment in southwestern Uganda. PLoS One 2014; 9(12): e113152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Galárraga O, Gao B, Gakinya BN, Klein DA, Wamai RG, Sidle JE, et al. Task-shifting alcohol interventions for HIV+ persons in Kenya: a cost-benefit analysis. BMC health Serv Res 2017; 17(1): 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sileo K, Kintu M, Chanes-Mora P, Kiene SM. “Such behaviors are not in my home village, I got them here”: a qualitative study of the influence of contextual factors on alcohol and HIV risk behaviors in a fishing community on Lake Victoria, Uganda. AIDS Behav 2016; 20(3): 537–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leslie HH, Ahern J, Pettifor AE, Twine R, Kahn K, Gomez-Olive FX, et al. Collective efficacy, alcohol outlet density, and young men’s alcohol use in rural South Africa. Health Place 2015; 34: 190–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bryden A, Roberts B, McKee M, Petticrew M. A systematic review of the influence on alcohol use of community level availability and marketing of alcohol. Health Place 2012; 18(2): 349–57. [DOI] [PubMed] [Google Scholar]
- 52.Casswell S, Morojele N, Williams PP, Chaiyasong S, Gordon R, Gray-Phillip G, et al. The alcohol environment protocol: a new tool for alcohol policy. Drug Alcohol Rev 2018; 37 Suppl 2: S18–s26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shorter GW, Bray JW, Giles EL, O’Donnell AJ, Berman AH, Holloway A, et al. The variability of outcomes used in efficacy and effectiveness trials of alcohol brief interventions: a systematic review. J Stud Alcohol Drugs 2019; 80(3): 286–98. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


