Abstract
Purpose
The purpose of this study was to investigate the choroidal luminal and interstitial stromal alterations using choroidal vascularity index (CVI) among different cohorts of dry age-related macular degeneration (dAMD) compared to healthy subjects.
Methods
Four distinct cohorts were collected: three different cohorts of patients with dAMD (i.e. drusen, reticular pseudodrusen [RPD], and geographic atrophy [GA]) and an age-matched cohort of healthy subjects (controls). CVI (the ratio between the luminal choroidal area [LCA] and the total choroidal area [TCA]) was calculated in the subfoveal 1000 µm area.
Results
One hundred twenty eyes (from 120 patients) were included (30 eyes in each cohort). The mean age was 76.6 ± 7.1 years. No statistical differences were disclosed in terms of age, axial length, and central macular thickness among study groups. TCA showed a different distribution among the four cohorts (P = 0.003), mainly due to the LCA changes (P = 0.001). Interestingly, CVI showed a different distribution among the four cohorts (P < 0.001). RPD showed a lower CVI in comparison to controls (P = 0.040), whereas GA showed a lower CVI in comparison to drusen, RPD, and controls (P = 0.001, P = 0.046, and P < 0.001, respectively).
Conclusions
Different cohorts of dAMD are characterized by different impairments of the choroidal vascular and stromal components, reflecting different degrees of AMD severity.
Translational Relevance
CVI provides insights for better understanding the pathogenesis of AMD.
Keywords: age-related macular degeneration (AMD), choroidal vascularity index, CVI, geographic atrophy (GA), reticular pseudodrusen (RPD)
Introduction
Age-related macular degeneration (AMD) is characterized by progressive retinal damage with genetic, environmental, and constitutional factors.1 AMD is classified into two different forms, neovascular AMD (nAMD) and non-neovascular or dry AMD (dAMD), based on the presence of macular neovascularization (MNV). In detail, early stages of dAMD are usually asymptomatic and they are characterized by the presence of drusen and/or reticular pseudodrusen (RPD).2,3 On the other hand, geographic atrophy (GA) is characterized by the loss of photoreceptors, retinal pigment epithelium (RPE), and choriocapillaris, leading to a progressive visual impairment.4 To date, the pathogenesis of the AMD is not yet completely understood. Previous histological studies have hypothesized that the primary damage could be at the level of the choroid.5,6 Furthermore, changes in the choroidal interstitial stroma were histologically reported in eyes affected by AMD,7 supporting the idea that AMD is a vascular disorder characterized by impairment of choroidal perfusion.8 However, histological samples are characterized by artifacts caused by the processing itself, complicating the vessel changes evaluation of the choroidal and retinal plexuse.9,10
In the last years, the introduction of optical coherence tomography angiography (OCT-A) in the clinical and research setting has allowed us to analyze the vascular changes in vivo in several retinal diseases.11–13 Particularly, different authors reported a reduction of the choriocapillaris perfusion density in patients affected by drusen, RPD, and GA, suggesting a crucial role of the whole choroidal impairment (and not only of the choriocapillaris) in the AMD pathogenesis.14–20 To date, thanks to the introduction of a new structural OCT tool, namely choroidal vascularity index (CVI), we are able to better investigate the choroidal changes, especially in the ratio between the stromal and luminal areas.21 The advantage of CVI is that it is a more sensitive biomarker in detecting choroidal changes as compared to choroidal thickness (ChT). Indeed, CVI, encompassing changes in both the choroidal vascular and stromal components, revealed more in-depth information. Furthermore, CVI is not associated with changes in ChT and with aging.22,23
Different studies reported CVI alterations specifically in patients affected by intermediate AMD (iAMD) or by GA.24–26 However, to date, no studies compared the CVI alterations among different cohorts of dAMD. The aim of this study is to investigate the luminal and interstitial stromal alterations of the choroid using CVI among different cohorts of dAMD compared to healthy subjects, in order to gather insights for better understanding the pathogenesis of AMD.
Methods
This was a retrospective cohort study, including consecutive patients affected by different stages of AMD. Clinical data of patients presenting between January 2020 and June 2020 at the Medical Retina & Imaging Unit of the Department of Ophthalmology of University Vita-Salute San Raffaele in Milan, Italy, were collected. The study was conducted in agreement with the Declaration of Helsinki for research involving human subjects and was approved by the Ethics Committee of San Raffaele Institute.
Inclusion criteria for all dAMD cohorts were: (1) age greater than 50 years, (2) diagnosis of AMD, and (3) clear ocular media to ensure proper image quality.
Exclusion criteria for all dAMD cohorts were: (1) presence of neovascular AMD, (2) any previous retinal treatments (e.g. intravitreal injections and photodynamic therapy), (3) history of any other chorioretinal disorder, (4) inadequate fixation to permit high-quality imaging, and (5) myopia greater than 6 diopters (D) of sphere or 3D of cylinder, and/or axial length >25.5 mm.
The present study consists of three dAMD distinct cohorts: patients with drusen (drusen group), patients with RPD (RPD group), and patients with foveal involving GA (GA group). GA was defined as the absence of RPE in any demarcated unifocal/multifocal area larger than 175 µm.27 Patients with a mixed phenotype (i.e. drusen and RPD) were excluded from the study in order to obtain differential information in the dAMD cohorts investigated. A group of healthy age-matched subjects was enrolled as controls. If both eyes presented inclusion criteria, only one eye was included. The eye with the higher quality of structural OCT image was selected as the study eye. If both eyes had the same quality of OCT image, the included eye was selected by flipping a coin.
All patients underwent a complete ophthalmologic examination with a multimodal imaging approach as part of the standard clinical assessment of our unit. In detail, we recorded best-corrected visual acuity (BCVA) using Snellen charts, infrared reflectance (IR), short-wave fundus autofluorescence (FAF), structural spectral-domain OCT, and axial length measurement. All OCT examinations were performed using the enhanced depth imaging (EDI) OCT in order to achieve a good visualization of the choroid, and using the automatic real-time tracking (ART; minimum of 25 frames). Dye angiographies (fluorescein angiography [FA] and indocyanine green angiography [ICGA]) and/or OCT-A were also performed in case of clinical and multimodal imaging suspicion of MNV presence. IR, FAF, structural OCT, FA, and ICGA were performed using the Spectralis HRA + OCT (Heidelberg Engineering, Heidelberg, Germany). OCT-A examinations were performed using PLEX Elite 9000 (Carl Zeiss Meditec, Inc., Dublin, CA, USA). Axial length was measured using a noncontact biometry instrument (IOLMaster; Carl Zeiss Meditec AG, Jena, Germany).
Imaging Analysis
Subfoveal ChT, mean ChT, and central macular thickness (CMT) were recorded. Two trained examiners (authors M.B. and E.B.) measured the distance from the Bruch's membrane to the sclerochoroidal interface in the subfoveal location (subfoveal ChT) and the mean value was used for statistical analyses. Furthermore, the ChT was also measured 500 µm nasally and temporally to the fovea, and the mean value among the three measurements (subfoveal, 500 µm nasally, and 500 µm temporally to the fovea) was used in order to calculate the mean ChT. The CMT was defined as the vertical distance from the Bruch's membrane and vitreoretinal interface. CMT was recorded with the Spectralis Software (Heidelberg Eye Explorer, version 1.9.14.0, Heidelberg, Germany) in the central 1-mm-diameter circle.
The horizontal structural EDI OCT passing through the fovea was exported by the Spectralis Software and imported into the software FIJI (National Institute of Health, Bethesda, MD, USA) in order to calculate the CVI using a previously reported method.28,29 Briefly, a region of interest (ROI) of 1000 µm wide, centered on the fovea was selected using the polygon tool. The choroidal RPE junction and the sclerochoroidal junction were selected as the upper and lower boundary of the ROI. The brightness of the image was adjusted using the average value obtained from the lumen of three major choroidal vessels of the ROI, in order to consider the lumen of the choroidal vessels as real lumen area. Images were binarized using Niblack's autolocal threshold. Total choroidal area (TCA) was calculated as the total area of the ROI. Luminal choroidal area (LCA) was calculated as the dark pixels of the ROI after binarization. Stromal choroidal area (SCA) was calculated as the white pixels of the ROI after binarization. CVI was obtained as the ratio between LCA and TCA.
Statistical Analysis
Statistical analyses were performed using SPSS Statistics version 20 (IBM, Armonk, NY, USA). All values of descriptive analyses were expressed as counts and percentages for categorical variables, and as means ± standard deviations for quantitative variables. Frequencies were compared between the groups using the chi-square test. In all patients, BCVA was converted to logarithm of the minimum angle of resolution (logMAR) for statistical analysis. The agreement between individual measurements of ChT from both readers was performed using the intraclass correlation coefficient (ICC; 95% confidence interval [CI]).
Comparisons of BCVA, axial length, CMT, subfoveal ChT, mean ChT, TCA, LCA, SCA, and CVI among the four cohorts were performed using the analysis of variance (ANOVA) with Tukey post hoc analysis. In all analyses, P values < 0.05 were considered as statistically significant.
Results
Patient Demographics and Main Clinical Features
A total of 120 eyes of 120 Caucasian patients (30 eyes for each group) were included in the study. Seventy-one patients were women and 49 patients were men (17 women and 13 men in the drusen group, 18 women and 12 men in both the RPD and GA groups, and 17 women and 13 men in the controls group; P = 0.987).
The mean age of all patients was 76.6 ± 7.1 years (median = 77, range = 58–91 years), and the 4 cohorts of patients (i.e. drusen, RPD, GA, and controls) did not show statistical differences in terms of age population (P = 0.073; Table 1). Furthermore, the mean axial length was 23.62 ± 0.51 mm, and the mean CMT was 233 ± 42 µm. Of note, both axial length and CMT did not show statistical differences among study cohorts (P = 0.692 and P = 0.264, respectively; see Table 1). On the other hand, BCVA showed significant differences among study cohorts (P < 0.001). In detail, patients with GA showed a lower BCVA in comparison to drusen, RPD, and controls (P < 0.001 in all comparisons; see Table 1). All the demographics and main clinical features of the whole population and of each cohort are reported in Table 1.
Table 1.
Demographics and Main Clinical Features of the Study Population
| AMD Patients | Healthy Subjects | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n = 90) | RPD (n = 30) | GA (n = 30) | Total (n = 30) | |||||||||
| Mean ± SD | P Valuea | Drusen (n = 30) Mean ± SD | Mean ± SD | P Valueb | Mean ± SD | P Valueb | P Valuec | Mean ± SD | P Valueb | P Valuec | P Valued | |
| Age, y | 76.6 ± 7.1 | 0.073 | 76.2 ± 8.9 | 78.2 ± 6.5 | 0.674 | 78.1 ± 5.2 | 0.719 | 1.000 | 74.0 ± 6.9 | 0.628 | 0.098 | 0.115 |
| BCVA, logMAR | 0.18 ± 0.25 | <0.001 | 0.07 ± 0.08 | 0.13 ± 0.08 | 0.562 | 0.48 ± 0.33 | <0.001 | <0.001 | 0.05 ± 0.06 | 0.938 | 0.241 | <0.001 |
| Axial length, mm | 23.62 ± 0.51 | 0.692 | 23.54 ± 0.46 | 23.59 ± 0.53 | 0.987 | 23.66 ± 0.52 | 0.840 | 0.959 | 23.69 ± 0.55 | 0.690 | 0.868 | 0.993 |
| CMT, µm | 233 ± 42 | 0.264 | 244 ± 27 | 234 ± 43 | 0.814 | 223 ± 57 | 0.214 | 0.707 | 230 ± 33 | 0.541 | 0.968 | 0.927 |
| Subfoveal ChT, µm | 191 ± 79 | <0.001 | 171 ± 48 | 192 ± 88 | 0.637 | 148 ± 77 | 0.577 | 0.071 | 253 ± 60 | <0.001 | 0.006 | <0.001 |
| Mean ChT, µm | 184 ± 78 | <0.001 | 161 ± 46 | 186 ± 88 | 0.516 | 143 ± 77 | 0.728 | 0.080 | 244 ± 55 | <0.001 | 0.007 | <0.001 |
n, number; SD, standard deviation; AMD, age-related macular degeneration; RPD, reticular pseudodrusen; GA, geographic atrophy; BCVA, best-corrected visual acuity; CMT, central macular thickness; ChT, choroidal thickness.
Statistical Analysis: Analysis of variance (ANOVA) for independent samples with Tukey post hoc analysis.
ANOVA for independent samples.
Comparison with drusen group.
Comparison with RPD group.
Comparison with GA group.
Choroidal Analysis
Comparing ChT among the four cohorts, subfoveal ChT and mean ChT showed significantly different values in both analyses (P < 0.001 in both analyses; see Table 1). Healthy patients (Fig. 1) showed a thicker mean subfoveal ChT in comparison to drusen (Fig. 2), RPD (Fig. 3), and GA cohorts (Fig. 4; P < 0.001, P = 0.006, and P < 0.001, respectively), and the same trend was disclosed in mean ChT (P < 0.001, P = 0.007, and P < 0.001, respectively). In detail, subfoveal ChT was 253 ± 60 µm, 171 ± 48 µm, 192 ± 88 µm, and 148 ± 77 µm in healthy, drusen, RPD, and GA cohort, respectively, and 244 ± 55 µm, 161 ± 46 µm, 186 ± 88 µm, and 143 ± 77 µm considering the mean ChT (see Table 1). Interobserver variability between readers was excellent for all measurements (ICC = 0.953, 95% CI = 0.923–0.983).
Figure 1.
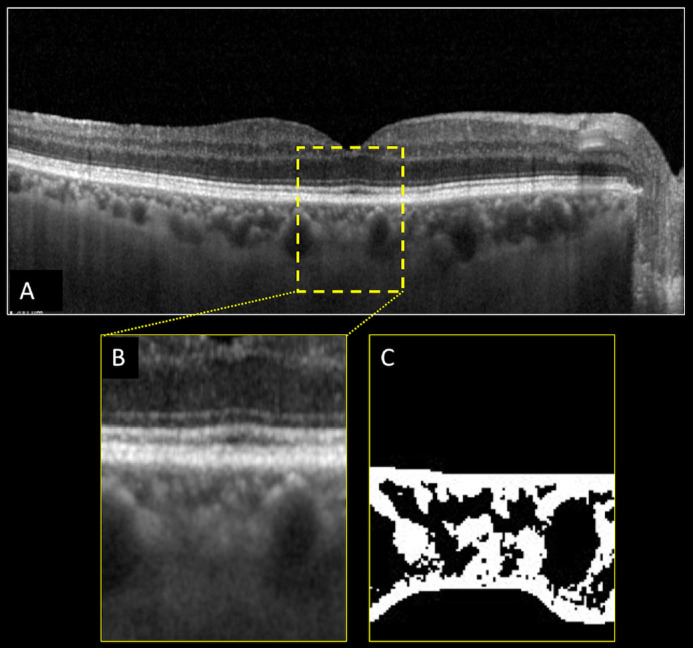
Structural optical coherence tomography (OCT) and choroidal vascularity index (CVI) calculation of the right eye of a healthy subject (control). Horizontal b-scan structural OCT passing through the fovea (A). A region of interest (ROI) of 1000 µm centered to the fovea was selected (B) and binarized (C) using Niblack's auto-local threshold in order to calculate the CVI. Dark pixels represent the luminal area, whereas white pixels the stromal area. CVI was calculated as the ratio between the luminal area and the total choroidal area.
Figure 2.
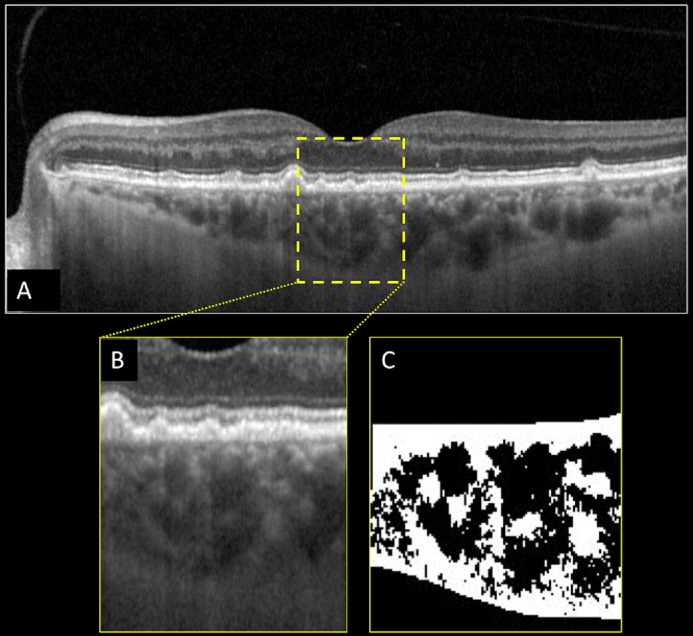
Structural optical coherence tomography (OCT) and choroidal vascularity index (CVI) calculation of the left eye of a patient affected by drusen secondary to age-related macular degeneration. Horizontal b-scan structural OCT passing through the fovea showing the presence of drusen (A). A region of interest of 1000 µm centered to the fovea was selected (B) and binarized (C) using Niblack's auto-local threshold in order to calculate the CVI. Dark pixels represent the luminal area, whereas white pixels the stromal area. CVI was calculated as the ratio between the luminal area and the total choroidal area.
Figure 3.
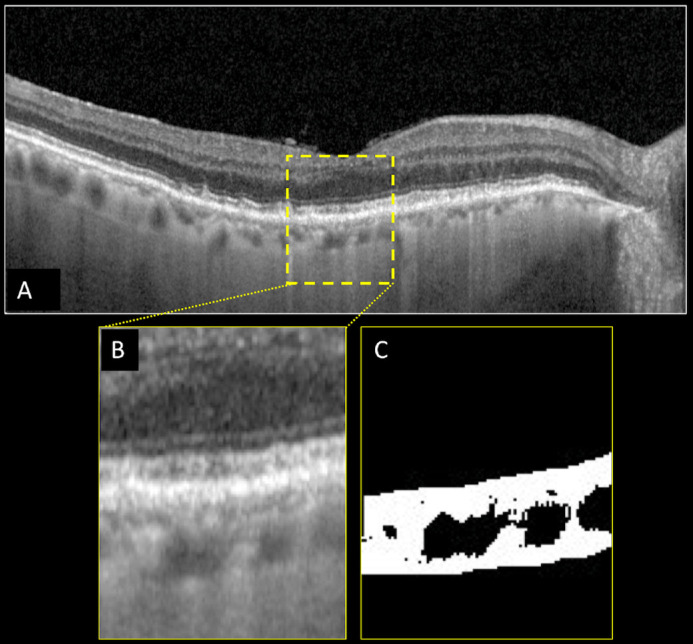
Structural optical coherence tomography (OCT) and choroidal vascularity index (CVI) calculation of the right eye of a patient affected by reticular pseudodrusen (RPD) secondary to age-related macular degeneration. Horizontal b-scan structural OCT passing through the fovea showing the presence of RPD (A). A region of interest of 1000 µm centered to the fovea was selected (B) and binarized (C) using Niblack's auto-local threshold in order to calculate the CVI. Dark pixels represent the luminal area, whereas white pixels the stromal area. CVI was calculated as the ratio between the luminal area and the total choroidal area.
Figure 4.
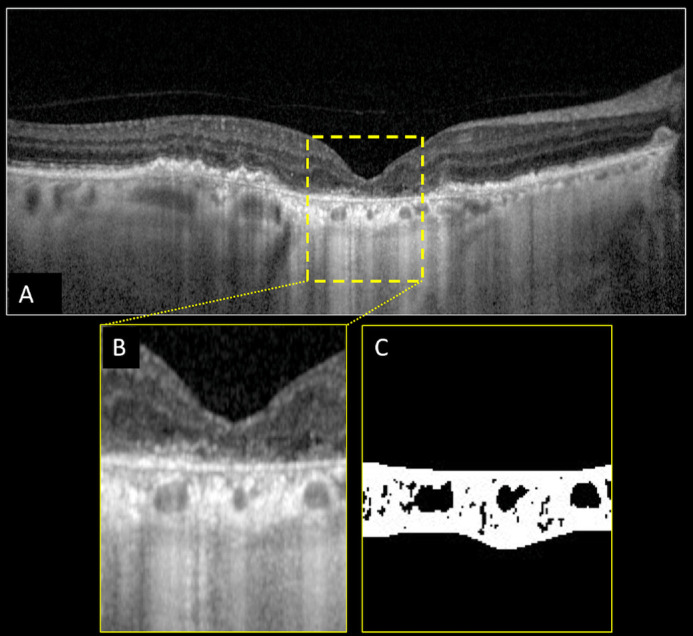
Structural optical coherence tomography (OCT) and choroidal vascularity index (CVI) calculation of the right eye of a patient affected by geographic atrophy (GA) secondary to age-related macular degeneration. Horizontal b-scan structural OCT passing through the fovea showing the presence of retinal pigment epithelium atrophy with increased signal transmission through the choroid and collapse or thinning of the outer retinal layer (i.e. GA) (A). A region of interest of 1000 µm centered to the fovea was selected (B) and binarized (C) using Niblack's auto-local threshold in order to calculate the CVI. Dark pixels represent the luminal area, whereas white pixels the stromal area. CVI was calculated as the ratio between the luminal area and the total choroidal area.
Analyzing the binarized images (see Figs. 123–4), we calculated the TCA, SCA, LCA, and the CVI. In particular, TCA showed a different distribution among the four cohorts (P = 0.003), mainly due to the changes of LCA among the four cohorts (P = 0.001). Indeed, SCA did not show a statistically different distribution among the four cohorts (P = 0.125; Table 2). Comparing each other, in all the four cohorts (using the Tukey post hoc analysis), we disclosed a significant reduction of the TCA and LCA mainly between the GA cohort and controls (P < 0.001 in both analyses).
Table 2.
Choroidal Analysis of the AMD Cohorts and Controls
| AMD Patients | Healthy Subjects | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n = 90) | RPD (n = 30) | GA (n = 30) | Total (n = 30) | |||||||||
| Mean ± SD | P Valuea | Drusen (n = 30) Mean ± SD | Mean ± SD | P Valueb | Mean ± SD | P Valueb | P Valuec | Mean ± SD | P Valueb | P Valuec | P Valued | |
| LCA, mm2 (mean ± SD) | 0.0497 ± 0.0303 | 0.001 | 0.0507 ± 0.0310 | 0.0503 ± 0.0323 | 1.000 | 0.0330 ± 0.0270 | 0.079 | 0.090 | 0.0648 ± 0.0225 | 0.225 | 0.204 | <0.001 |
| SCA, mm2 (mean ± SD) | 0.0745 ± 0.0170 | 0.125 | 0.0708 ± 0.0154 | 0.0757 ± 0.0158 | 0.679 | 0.0714 ± 0.0193 | 0.999 | 0.758 | 0.0801 ± 0.0165 | 0.152 | 0.747 | 0.198 |
| TCA, mm2 (mean ± SD) | 0.1242 ± 0.0431 | 0.003 | 0.1216 ± 0.0449 | 0.1260 ± 0.0452 | 0.975 | 0.1044 ± 0.0389 | 0.374 | 0.181 | 0.1449 ± 0.0348 | 0.131 | 0.291 | <0.001 |
| CVI, % (mean ± SD) | 37.03 ± 12.59 | <0.001 | 39.68 ± 7.87 | 36.09 ± 14.15 | 0.613 | 28.35 ± 14.68 | 0.001 | 0.046 | 43.99 ± 6.09 | 0.458 | 0.040 | <0.001 |
n, number; SD, standard deviation; AMD, age-related macular degeneration; RPD, reticular pseudodrusen; GA, geographic atrophy; LCA, luminal choroidal area; SCA, stromal choroidal area; TCA, total choroidal area; CVI, choroidal vascularity index
Statistical Analysis: Analysis of variance (ANOVA) for independent samples with Tukey post hoc analysis.
ANOVA for independent samples.
Comparison with drusen group.
Comparison with RPD group.
Comparison with GA group.
Interestingly, analyzing the ratio between the choroidal LCA and the TCA, the CVI showed a different distribution among the four cohorts (P < 0.001). Using the Tukey post hoc analysis, we disclosed that the RPD cohort was characterized by a lower CVI in comparison to controls (P = 0.040), and the GA cohort was characterized by a lower CVI in comparison to drusen, RPD, and controls (P = 0.001, P = 0.046, and P < 0.001, respectively). In other words, the LCA was impaired to a greater extent with the worsening of dAMD from drusen (see Fig. 2), to RPD (see Fig. 3), and to GA (see Fig. 4). All choroidal analyses were reported in Table 2.
Discussion
In this study, using the CVI, we have disclosed that the vascular choroidal area is significantly reduced in patients with AMD compared to healthy subjects. Furthermore, the CVI is variably impaired among the different cohorts of dAMD investigated (i.e. drusen, RPD, and GA), suggesting its potential role in characterizing different cohorts of the disease.
Dry AMD is a multifactorial and complex disease, in which genetic, environmental, and constitutional factors are involved.1 Even though still not completely understood, an impairment of the choroid is one of the driving factors in AMD development. Functional impairment of the choroidal and retinal circulation in patients with AMD was previously well described.30,31 Berenberg et al.30 reported an association between decreased choroidal blood flow and increased drusen extension. Furthermore, Rabiolo et al.31 reported an impairment of retinal arterial dilation in patients affected by drusen and RPD. Starting from a histological point of view, Biesemeier et al.5 and Li et al.6 have hypothesized that the primary damage in patients with AMD could happen at the level of the choroid. In detail, Biesemeier et al.,5 analyzing the CC-RPE-retina complexes, reported that the CC breakdown precedes the RPE degeneration in patients with AMD, defining AMD as a vascular disease. The impairment of the choroidal layers was also confirmed in vivo by several groups in different dAMD cohorts. In detail, Borrelli et al.14 reported an increased CC flow impairment beneath and immediately surrounding drusen comparing 30 iAMD eyes with controls. Nesper et al.15 reported that eyes with RPD have a larger area of CC flow impairment compared with eyes affected by drusen and no RPD. Furthermore, greater CC impairment around the GA area and in the area that subsequently developed GA expansion was reported,16–19 suggesting the crucial role of the CC flow impairment in patients affected by GA.
Recently, CVI has been proposed as a novel OCT-based parameter to quantify choroidal structural changes in different disorders, including AMD.21,24–26 CVI was found to be reduced in patients affected by iAMD and by GA. In detail, Velaga et al.25 recently reported that multiple choroidal parameters, including CVI, seem to be altered in iAMD eyes in comparison to controls. Furthermore, comparing 34 GA eyes with 32 controls, Giannaccare et al.26 reported that CVI is reduced in patients affected by GA and that this parameter worsened during the follow-up period. However, no studies have compared, face to face, different cohorts of dAMD (i.e. drusen, RPD, and GA), in order to disclose the possible different status of vascular and stromal area of the choroid in different cohorts of dAMD. With this aim, we have compared different choroidal parameters in three cohorts of patients with AMD (i.e. drusen, RPD, and GA cohort) and controls. Based on our analyses, we disclosed a significant CVI reduction in RPD and GA cohorts in comparison to controls (P = 0.040 and P < 0.001, respectively). Moreover, comparing different AMD cohorts, there was a statistically significant lower CVI in patients with GA in comparison to drusen (P = 0.001), and to RPD (P = 0.046; see Table 2). One can argue that the worsening of the CVI was due to the different ChT of the three dAMD cohorts. However, Zhou et al.22 and Breher et al.23 demonstrated that CVI showed no significant variation associated with changes in mean ChT. The results of our analysis could help to better understand the pathogenesis of dAMD, and also could be relevant in the clinical setting. Indeed, this finding in turn means that the LCA was impaired to a greater extent with the worsening of dAMD. Recently, by means of en face structural OCT, it was demonstrated that different cohorts of dAMD (i.e. drusen, RPD, and GA) showed a specific distribution of vascular arrangement of the choroid.32 Based on reported findings, we have previously suggested that the different distribution of choroidal patterns could be associated with a different ratio between stromal and vascular components, leading to an increase in the fibrous matrix of the choroid as a possible explanation.32 The results of the current series are in agreement with this theory. Indeed, we have demonstrated here that patients with dAMD showed not only a decrease of the LCA but also a reduction of the CVI, supporting a progressive choroidal vascular degeneration with a stromal replacement in the pathogenesis and progression of dAMD. From this point of view, our data confirmed previously reported histological data. Indeed, McLeod et al.7 reported changes of the choroidal interstitial stroma in eyes affected by AMD in comparison to healthy patients. They reported that the percentage of the vascular area was significantly reduced in the edge and under GA lesions in comparison to healthy eyes due to a stromal replacement.7
Finally, CVI could have a clinically key prognostic role in patients affected by dAMD, as we have reported that different values of CVI are associated with different cohorts of dAMD, corresponding to different degrees of AMD progression risk. Patients affected by RPD are characterized by higher functional impairment and progression risk to GA in comparison to typical drusen.33,34 Indeed, RPD are characterized by a greater extent of reduced retinal sensitivity using microperimetry than drusen eyes.33 Furthermore, several studies have highlighted the role of RPD to accelerate the progression to GA or neovascular AMD.34 Therefore, by combining data from previous studies with our current findings, we suggest that CVI could be considered as a biomarker of dAMD progression.
One can argue that the same information could be obtained using the ChT analysis. However, the advantage of CVI is that it is a more sensitive biomarker in detecting choroidal changes as compared to ChT. Indeed, ChT is influenced by the age of patients, and this could be a greater confounding factor in the analysis of results. Another limitation of the ChT analysis is that it does not give structural information. Conversely, CVI, encompassing changes in both the choroidal vascular and stromal components, revealed more in-depth information.
Our study has several limitations that must be disclosed, mainly due to the relatively small sample size, the retrospective nature of the study, and the absence of longitudinal information. On the other hand, we minimized potential limitations by excluding patients with mixed phenotypes (i.e. drusen plus RPD) in order to obtain differential information in the dAMD cohorts investigated. Another limitation of our study was the possible influence of backscattering and shadowing effects due to the RPE alterations in patients with drusen and GA. However, in order to minimize this possible influence, we have used a previously validated compensated method of binarization28,29 based on two different strategies: first, the brightness of the image was adjusted using the average value obtained from the lumen of three major choroidal vessels of the ROI; and second, we have used an autolocal threshold.
In conclusion, we have demonstrated that different cohorts of dAMD are characterized by different impairments of the ratio between vascular and stromal components of the choroid. In detail, CVI decreased comparing drusen, RPD, and GA cohorts, reflecting different degrees of AMD severity, and providing insights for better understanding the pathogenesis of AMD. Further longitudinal studies should be performed in order to investigate the potential role of this novel OCT parameter in the prognosis of patients with dAMD.
Acknowledgments
Disclosure: R. Sacconi, Novartis, Basel, Switzerland (C), and Zeiss, Dublin, CA, USA (C). G. Vella, None; M. Battista, None; E. Borrelli, Novartis, Basel, Switzerland (C), and Zeiss, Dublin, CA, USA (C). S. Balasubramanian, None; L. Querques, None. F. Bandello, Alcon, Fort Worth, TX, USA (C), Alimera Sciences, Alpharetta, GA, USA (C), Allergan Inc., Irvine, CA, USA (C), Farmila-Thea, Clermont-Ferrand, France (C), Bayer Shering-Pharma, Berlin, Germany (C), Bausch & Lomb, Rochester, NY, USA (C), Genentech, San Francisco, CA, USA (C), Hoffmann-La-Roche, Basel, Switzerland (C), NovagaliPharma, Évry, France (C), Novartis, Basel, Switzerland (C), Sanofi-Aventis, Paris, France (C), Thrombogenics, Heverlee, Belgium (C), and Zeiss, Dublin, CA, USA (C). G. Querques, Alimera Sciences, Alpharetta, GA, USA (C), Allergan Inc., Irvine, CA, USA (C), Amgen, Thousand Oaks, CA, USA (C), Heidelberg, Germany (C), KBH, Chengdu, China (C), LEH Pharma, London, UK (C), Lumithera, Poulsbo, WA, USA (C), Novartis, Basel, Switzerland (C), Bayer Shering-Pharma, Berlin, Germany (C), Sandoz, Berlin, Germany (C), Sifi, Catania, Italy (C), Soof-Fidia, Albano, Italy (C), and Zeiss, Dublin, CA, USA (C)
References
- 1. Chew EY, Clemons TE, Agrón E, et al.. Ten-year follow-up of age-related macular degeneration in the age-related eye disease study: AREDS report no. 36. JAMA Ophthalmol . 2014; 132: 272–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ferris FL III, Wilkinson CP, Bird A, et al.. Clinical classification of age-related macular degeneration. Ophthalmology . 2013; 120: 844–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rabiolo A, Sacconi R, Cicinelli MV, Querques L, Bandello F, Querques G.. Spotlight on reticular pseudodrusen. Clin Ophthalmol . 2017; 11: 1707–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sacconi R, Corbelli E, Querques L, Bandello F, Querques G.. A Review of Current and Future Management of Geographic Atrophy. Ophthalmol Ther . 2017; 6(1): 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Biesemeier A, Taubitz T, Julien S, Yoeruek E, Schraermeyer U.. Choriocapillaris breakdown precedes retinal degeneration in age-related macular degeneration. Neurobiol Aging . 2014; 35: 2562–2573. [DOI] [PubMed] [Google Scholar]
- 6. Li M, Huisingh C, Messinger J, et al.. Histology of geographic atrophy secondary to age-related macular degeneration a multilayer approach. Retina . 2018; 38: 1937–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McLeod DS, Grebe R, Bhutto I, et al.. Relationship between RPE and choriocapillaris in age-related macular degeneration. Invest Ophthalmol Vis Sci . 2009; 50: 4982–4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Friedman E. The pathogenesis of age-related macular degeneration. Am J Ophthalmol . 2008; 146: 348–349. [DOI] [PubMed] [Google Scholar]
- 9. Anger EM, Unterhuber A, Hermann B, et al.. Ultrahigh resolution optical coherence tomography of the monkey fovea. Identification of retinal sublayers by correlation with semithin histology sections. Exp Eye Res . 2004; 78: 1117–1125. [DOI] [PubMed] [Google Scholar]
- 10. Branchini LA, Adhi M, Regatieri CV, et al.. Analysis of choroidal morphologic features and vasculature in healthy eyes using spectral-domain optical coherence tomography. Ophthalmology . 2013; 120: 1901–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sacconi R, Battista M, Borrelli E, et al.. OCT-A characterisation of recurrent type 3 macular neovascularisation. Br J Ophthalmol . 2021; 105(2): 222–226. [DOI] [PubMed] [Google Scholar]
- 12. Sacconi R, Corbelli E, Carnevali A, et al.. Optical coherence tomography angiography in pseudophakic cystoid macular oedema compared to diabetic macular oedema: qualitative and quantitative evaluation of retinal vasculature. Br J Ophthalmol . 2018; 102: 1684–1690. [DOI] [PubMed] [Google Scholar]
- 13. Sacconi R, Sarraf D, Garrity S, et al.. Nascent Type 3 Neovascularization in Age-Related Macular Degeneration. Ophthalmol Retina . 2018; 2: 1097–1106. [DOI] [PubMed] [Google Scholar]
- 14. Borrelli E, Shi Y, Uji A, et al.. Topographic Analysis of the Choriocapillaris in Intermediate Age-related Macular Degeneration. Am J Ophthalmol . 2018; 196: 34–43. [DOI] [PubMed] [Google Scholar]
- 15. Nesper PL, Soetikno BT, Fawzi AA.. Choriocapillaris Nonperfusion is Associated With Poor Visual Acuity in Eyes With Reticular Pseudodrusen. Am J Ophthalmol . 2017; 174: 42–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sacconi R, Corbelli E, Borrelli E, et al.. Choriocapillaris flow impairment could predict the enlargement of geographic atrophy lesion. Br J Ophthalmol . 2021; 105(1): 97–102. [DOI] [PubMed] [Google Scholar]
- 17. Sacconi R, Corbelli E, Carnevali A, Querques L, Bandello F, Querques G.. Optical coherence tomography angiography in geographic atrophy. Retina. 2018; 38(12): 2350–2355. [DOI] [PubMed] [Google Scholar]
- 18. Nassisi M, Shi Y, Fan W, et al.. Choriocapillaris impairment around the atrophic lesions in patients with geographic atrophy: A swept-source optical coherence tomography angiography study. Br J Ophthalmol . 2018; 103(7): 911–917. [DOI] [PubMed] [Google Scholar]
- 19. Thulliez M, Zhang Q, Shi Y, et al.. Correlations between Choriocapillaris Flow Deficits around Geographic Atrophy and Enlargement Rates Based on Swept-Source OCT Imaging. Ophthalmol Retina. 2019; 3(6): 478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Corbelli E, Sacconi R, Rabiolo A, et al.. Optical Coherence Tomography Angiography in the Evaluation of Geographic Atrophy Area Extension. Invest Ophthalmol Vis Sci . 2017; 58(12): 5201–5208. [DOI] [PubMed] [Google Scholar]
- 21. Iovino C, Pellegrini M, Bernabei F, et al.. Choroidal Vascularity Index: An In-Depth Analysis of This Novel Optical Coherence Tomography Parameter. J Clin Med . 2020; 9(2): 595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhou H, Dai Y, Shi Y, et al.. Age-Related Changes in Choroidal Thickness and the Volume of Vessels and Stroma Using Swept-Source OCT and Fully Automated Algorithms. Ophthalmol Retina . 2020; 4: 204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Breher K, Terry L, Bower T, Wahl S.. Choroidal Biomarkers: A Repeatability and Topographical Comparison of Choroidal Thickness and Choroidal Vascularity Index in Healthy Eyes. Transl Vis Sci Technol . 2020; 9(11): 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wei X, Ting DSW, Ng WY, et al.. Choroidal vascularity index - a novel optical coherence tomography based parameter in patients with exudative age-related macular degeneration. Retina . 2017; 37: 1120–1125. [DOI] [PubMed] [Google Scholar]
- 25. Velaga SB, Nittala MG, Vupparaboina KK, et al.. Choroidal vascularity index and choroidal thickness in eyes with reticular pseudodrusen. Retina . 2020; 40: 612–617. [DOI] [PubMed] [Google Scholar]
- 26. Giannaccare G, Pellegrini M, Sebastiani S, et al.. Choroidal vascularity index quantification in geographic atrophy using binarization of enhanced-depth imaging optical coherence tomographic scans. Retina . 2020; 40: 960–965. [DOI] [PubMed] [Google Scholar]
- 27. Bird AC, Bressler NM, Bressler SB, et al.. An international classification and grading system for age-related maculopathy and age-related macular degeneration. Surv Ophthalmol . 1995; 39(5): 367–374. [DOI] [PubMed] [Google Scholar]
- 28. Sonoda S, Sakamoto T, Yamashita T, et al.. Luminal and stromal areas of choroid determined by binarization method of optical coherence tomographic images. Am J Ophthalmol . 2015; 159: 1123–1131.e1. [DOI] [PubMed] [Google Scholar]
- 29. Sonoda S, Sakamoto T, Yamashita T, et al.. Choroidal structure in normal eyes and after photodynamic therapy determined by binarization of optical coherence tomographic images. Investig Ophthalmol Vis Sci . 2014; 55: 3893–3898. [DOI] [PubMed] [Google Scholar]
- 30. Berenberg TL, Metelitsina TI, Madow B, et al.. The association between drusen extent and foveolar choroidal blood flow in age-related macular degeneration. Retina . 2012; 32: 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rabiolo A, Benatti L, Tomasso L, et al.. Retinal arterial dilation is impaired in eyes with drusen and reticular pseudodrusen. Retina . 2019; 39: 2205–2211. [DOI] [PubMed] [Google Scholar]
- 32. Sacconi R, Cicinelli MV, Borrelli E, et al.. Haller's vessels patterns in non-neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol . 2020; 258: 2163–2171. [DOI] [PubMed] [Google Scholar]
- 33. Querques G, Massamba N, Srour M, Boulanger E, Georges A, Souied EH.. Impact of reticular pseudodrusen on macular function. Retina . 2014; 34,321–329. [DOI] [PubMed] [Google Scholar]
- 34. Zweifel SA, Imamura Y, Spaide TC, Fujiwara T, Spaide RF.. Prevalence and significance of subretinal drusenoid deposits (reticular pseudodrusen) in age-related macular degeneration. Ophthalmology . 2010; 117,1775–1781. [DOI] [PubMed] [Google Scholar]


