Abstract
Adult homeostatic visual plasticity can be induced by short-term patching, heralded by a shift in ocular dominance in favor of the deprived eye after monocular occlusion. The potential to boost visual neuroplasticity with environmental enrichment such as exercise has also been explored; however, the results are inconsistent, with some studies finding no additive effect of exercise. Studies to date have only considered the effect of patching alone or in combination with exercise. Whether exercise alone affects typical outcome measures of experimental estimates of short-term visual neuroplasticity is unknown. We therefore measured binocular rivalry in 20 healthy young adults (20–34 years old) at baseline and after three 2-hour interventions: patching (of the dominant eye) only, patching with exercise, and exercise only. Consistent with previous work, the patching interventions produced a shift in ocular dominance toward the deprived (dominant) eye. Mild- to moderate-intensity exercise in the absence of patching had several effects on binocular rivalry metrics, including a reduction in the dominant eye percept. The proportion of mixed percept and the time to first switch (onset rivalry) did not change from baseline across all interventions. Thus, we demonstrate that exercise alone can impact binocular rivalry outcomes measures. We did not observe a synergistic effect between patching and exercise in our data.
Keywords: neuroplasticity, exercise, monocular deprivation, homeostatic plasticity, binocular rivalry
Introduction
In healthy adults, a compelling example of short-term visual neuroplasticity can be induced by temporary monocular deprivation, typically with 2 to 2.5 hours of patching (Binda, Kurzawski, Lunghi, Biagi, Tosetti, & Morrone, 2018; Finn, Baldwin, Reynaud, & Hess, 2019; Lunghi, Burr, & Morrone, 2011; Lunghi, Burr, & Morrone, 2013; Lunghi et al., 2019; Lunghi, Emir, Morrone, & Bridge, 2015; Lunghi & Sale, 2015; Min, Baldwin, & Hess, 2019; Sheynin, Chamoun, Baldwin, Rosa-Neto, Hess, & Vaucher, 2019; Sheynin, Proulx, & Hess, 2019; Steinwurzel, Animali, Cicchini, Morrone, & Binda, 2020; Yao, He, Wang, Lu, Qu, Zhou, & Hess, 2017; Zhou, Clavagnier, & Hess, 2013; Zhou, Reynaud, & Hess, 2017), although the effects can be observed with shorter durations of occlusion (Min, Baldwin, Reynaud, & Hess, 2018). After patch removal, the deprived eye temporarily becomes more dominant. Recently, the transient occlusion paradigm has garnered considerable interest, as it allows exploration of the homeostatic brain mechanisms that regulate input from the two eyes. There is also growing interest in boosting homeostatic plasticity with modifiable environmental factors, notably exercise (Baroncelli & Lunghi, 2020; Castaldi, Lunghi, & Morrone, 2020). Physical activity has been shown to enhance the ocular dominance shift induced by 2 hours of patching (Lunghi & Sale, 2015); however, not all studies have observed this effect of exercise (Finn et al., 2019; Zhou et al., 2017).
One possibility is that exercise boosts homeostatic plasticity by reducing inhibition. Although many neurotransmitters are involved in regulating visual cortical inhibition, magnetic resonance spectroscopy (MRS) has found a concomitant reduction in resting gamma-aminobutyric acid (GABA) levels in occipital cortex alongside the shift in ocular dominance after short-term monocular deprivation (Lunghi et al., 2015). In support of a reduction in inhibition driving the shift in ocular dominance plasticity, locomotion in animal models has been shown to alter the excitation–inhibition balance by decreasing GABA release (Baroncelli et al., 2012) or by inhibiting GABAergic interneuron activity (Kaneko & Stryker, 2014). On the other hand, MRS has detected increased levels of both GABA (inhibitory) and glutamate (excitatory) in human visual cortex following exercise (Maddock, Casazza, Buonocore, & Tanase, 2011; Maddock, Casazza, Fernandez, & Maddock, 2016), suggesting de novo synthesis of neurotransmitters during exercise that could impact on the excitation–inhibition balance in the opposite direction (i.e., increased net inhibition).
Because of the proposed impact of exercise on GABA, there has been recent study of the effect of exercise on other visual processes that are purported to be mediated (at least in part) by GABAergic inhibition. One such phenomenon is perceptual learning, which, when measured using a Vernier alignment task, is not enhanced when coupled with exercise (Campana, Fongoni, Astle, & McGraw, 2020). Furthermore, it has not been ascertained whether exercise by itself affects the typical outcome measures of studies investigating short-term visual neuroplasticity. Here, we address this knowledge gap by investigating the effects of temporary monocular deprivation and acute exercise on adult homeostatic visual neuroplasticity with three interventions: (1) patching only, (2) exercise and patching, and (3) exercise only. The first two interventions enabled replication of previous work (Finn et al., 2019; Lunghi & Sale, 2015; Zhou et al., 2017), and the third and novel intervention allowed investigation of the unexplored effect of exercise alone.
Short-term monocular deprivation effects have mainly been explored using one of two psychophysical tasks: binocular combination/fusion tasks, where stimuli are binocularly compatible and therefore fusible (Chadnova, Reynaud, Clavagnier, & Hess, 2017; Min et al., 2019; Min et al., 2018; Sheynin, Chamoun, et al., 2019; Yao et al., 2017; Zhou et al., 2013; Zhou et al., 2017), or binocular rivalry tasks, where stimuli are binocularly incompatible and so visual awareness alternates between the two eyes (Binda et al., 2018; Finn et al., 2019; Lunghi et al., 2011; Lunghi et al., 2013; Lunghi & Sale, 2015; Lunghi et al., 2015; Lunghi et al., 2019; Sheynin, Proulx, et al., 2019; Steinwurzel et al., 2020). Here, we chose to focus on binocular rivalry as a form of bistable perception that lends itself to the study of the balance between excitation and inhibition in visual cortex. Specifically, GABA has been both indirectly and directly linked to two distinct perceptual phenomena experienced during binocular rivalry: exclusive-dominance percepts (Pitchaimuthu, Wu, Carter, Nguyen, Ahn, Egan, & McKendrick, 2017; van Loon, Knapen, Scholte, St John-Saaltink, Donner, & Lamme, 2013) and mixed percepts (Mentch, Spiegel, Ricciardi, & Robertson, 2019). Similarly, inhibition is thought to be critically involved in modulating the initial period of dominance (suppression) before the first rivalry switch, or “onset rivalry” (Carter & Cavanagh, 2007; Stanley, Carter, & Forte, 2011; Stanley, Forte, Cavanagh, & Carter, 2011). If exercise alone reduces mutual inhibition between the percepts from the two eyes, then increased switch rate post-exercise may be expected. In many individuals, the percept from one eye is held more dominantly than its counterpart; that is, the percept for the non-dominant eye reaches some threshold for release more readily. This asymmetry raises the possibility that reducing inhibition through exercise may not affect the time spent in each percept symmetrically. In other words, exercise alone may influence measures of ocular dominance.
This study aimed to characterize binocular rivalry dynamics (exclusive-dominance percepts, mixed percepts, and onset rivalry) before and after exercise, with and without short-term monocular deprivation, in a single group of young healthy adults. We report the effects of occlusion with and without exercise to add to the evidence for and against additive effects of exercise on measures of short-term neuroplasticity. We additionally test the hypothesis that exercise alone changes the measured balance of percepts from the two eyes in a binocular rivalry task, in addition to altering other key aspects of binocular rivalry that rely on inhibition such as percept switch rate and the time to the onset of the first switch.
Methods
Participants
Experimental procedures were approved by the Human Research Ethics Committee of the University of Melbourne (#1851069) and complied with the tenets of the Declaration of Helsinki. Written informed consent was obtained from all participants prior to testing. Participants were recruited from advertisements placed around the University of Melbourne and from a database of previous laboratory participants who expressed interest in volunteering for future research. Twenty healthy adults (20–34 years old; mean ± SD, 25 ± 4 years) participated and met the following inclusion criteria: normal visual acuity (6/7.5 or better in each eye); normal ocular health as determined by a screening optometric examination; refractive error within ±5.00 diopter (D) sphere and –2.00 D cylinder; no history of amblyopia, eye surgery, or trauma; no systemic conditions (e.g., diabetes) or medications (e.g., antidepressants) that can affect visual or cognitive function.
Participants completed the Physical Activity Readiness Questionnaire for Everyone (PAR-Q+) (Warburton, Jamnik, Bredin, & Gledhill, 2011) to screen health history, current symptoms, and risk factors that could make physical activity unsafe or inappropriate. All but two participants were immediately cleared for physical activity. The remaining two participants (one reported a history of high blood pressure and the other reported a history of asthma) were deemed safe to participate in the study (i.e., “ready to become more physically active”) after completing the follow-up questions of the PAR-Q+.
Experimental design and procedure
Each participant attended three test sessions in total, completing three interventions in random order (Figure 1): (1) patching only, (2) patching and exercise, and (3) exercise only. Test sessions were scheduled at least 24 hours apart. At the beginning of each test session, baseline binocular rivalry was averaged from three consecutive runs (i.e., duration of ∼9 minutes). After each 2-hour intervention, a single binocular rivalry run was tested at the following time points: 0, 3, 6, 9, 12, 15, 30, 45, 60, and 90 minutes. Because previous studies have shown a significant effect on ocular dominance after patching at least over the first 15 minutes, which appears to plateau thereafter (e.g., Lunghi, Burr, & Morrone, 2013), we binned the data in ∼9-minute intervals to capture when we reasonably expected to observe an immediate effect of intervention compared to baseline. Three consecutive binocular rivalry runs from 0 to 9 minutes were averaged (at time points 0, 3, and 6 minutes) and runs from 9 to 18 minutes were averaged (at time points 9, 12, and 15 minutes). For 4.7% of the post-intervention data (17 runs of 20 participants × 3 conditions × 6 single time points = 360 runs), binocular rivalry dynamics were unable to be determined (see missing cells in Supplementary Material SB) due to a technical error where the button presses were not captured, despite the participant completing the task. In these instances, the final binned data available for analysis consisted of two binocular rivalry runs, rather than three repetitions.
Figure 1.
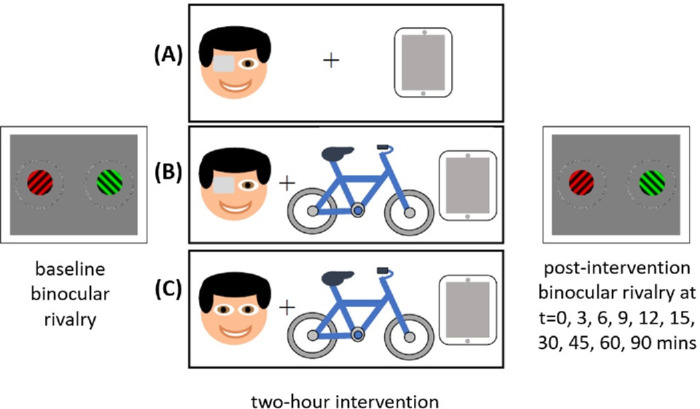
Schematic of the test sessions. At each test session, participants completed baseline binocular rivalry tests then a 2-hour intervention: (A) patching only, (B) patching and exercise, and (C) exercise only. Where patching occurred, the dominant eye was deprived for 120 minutes. Where cycling occurred, participants alternated between 10 minutes cycling and 10 minutes rest during the 120-minute period. Binocular rivalry was measured again at 0, 3, 6, 9, 12, 15, 30, 45, 60, and 90 minutes after each intervention. All participants completed the three interventions at separate test visits.
Binocular rivalry
Participants stabilized their head position with a chinrest and were refractively corrected for the 60-cm working distance. Stimuli were presented on a gamma-corrected Zowie XL2430-B liquid-crystal display monitor (100-Hz frame rate and 1920 × 1080-pixel screen resolution; BenQ, Taipei, Taiwan) with software written using PsychoPy 3.0 (Peirce et al., 2019). Prior to formal data collection, participants completed practice trials to familiarize themselves with the task.
At the beginning of each run, a pair of fusion rings (12° diameter, 0.3° width) constructed of random black and white dots with zero disparity were presented dichoptically using a mirror stereoscope (ScreenScope; Stereo Aids, Albany, Western Australia, Australia). Participants adjusted the stereoscope to see a central fixation cross (composed of a left vertical and right horizontal line, both 0.5° length) within a single fusion ring, signifying appropriate fusion. When fusion had been confirmed, the fixation cross disappeared at the onset of the binocular rivalry stimuli, but the fusion rings remained present throughout testing.
The binocular rivalry stimuli were equiluminant red and green circularly windowed grating patterns (see schematic in Figure 1; 2° diameter, 2-cycles/degree spatial frequency, 45° and 135° orientation) on a uniform gray background, each presented to one eye only. We chose to use red and green patterns rather than grayscale patterns because of the stronger effects of monocular deprivation with chromatic stimuli compared with achromatic stimuli (Lunghi et al., 2013). Additionally, we presented the gratings in a fixed construct (i.e., red always to the same eye, green always to the same eye) so that any individual differences in sensitivity to either red or green stimuli would be consistent among all runs and across all sessions. This enabled assessment of the effect of intervention without confounding changes in stimuli presented to the eyes under different conditions.
Binocular rivalry stimuli were presented for 150 seconds. Participants were instructed not to press any button at the beginning of the run and to start pressing the button only at the first perceptual switch. Time to first switch (indicating onset rivalry) was measured as the time between stimulus onset and first perceptual switch. During the subsequent sustained rivalry period, participants were asked to immediately press one of three keys as soon as a new percept appeared (red, green, or mixed percept). Participants chose the mixed-percept button if neither red nor green percept clearly dominated (i.e., some combination of red and green percept, either piecemeal or superimposed). Button presses were not continuous but indicated the time of each perceptual switch (change between any two percepts). Percept duration was determined from the time of button press (when a new percept appeared) to the time of the next button press (next perceptual switch). The sum of all percept durations for a given percept—deprived (dominant) eye percept, non-deprived (non-dominant) eye percept, and mixed percept—was divided by the total sustained rivalry time to give the percentage of total rivalry time spent in each percept. To determine total sustained rivalry time, we removed the time to first switch and the time between the last reported perceptual switch and the end of the trial (at 150 seconds).
To determine whether there was a shift in ocular dominance between baseline and post-intervention, an ocular dominance index was calculated, as per previous work (Lunghi et al., 2019) using Equation 1:
| (1) |
where Tdom is the total time spent seeing the deprived (dominant) eye percept and Tnondom is the total time spent seeing the non-deprived (non-dominant) eye percept, with values ranging from 0 (i.e., complete dominance of the non-deprived or non-dominant eye) to 1 (i.e., complete dominance of the deprived or dominant eye). We also calculated switch rate as the total number of perceptual switches divided by the total sustained rivalry time.
Temporary monocular deprivation
The dominant eye was covered with a translucent patch that completely degraded form perception. Sighting eye dominance was determined by asking the participant to keep both eyes open while pointing to a distant target and then alternately closing each eye to report which eye dominated the target view. For participants with a refractive correction, a double layer of Transpore surgical tape (31% light attenuation; 3M, Maplewood, MN) was fixed to a Halberg trial lens clip and placed over the participant's existing glasses to avoid taping directly onto a person's own lenses. Where no refractive correction was required, a soft eye patch with elastic fastening was customized with a hole covered by a double layer of LeukoFix tape (28% light attenuation; BSN Medical, Mulgrave, Victoria, Australia). While patched, participants could read, work at the computer, or watch a commercial video streaming service of their choice on a tablet device.
Mild to moderate exercise
For the exercise interventions, participants completed alternating 10-minute periods of mild to moderate physical activity using a stationary cycling machine, followed by rest. While cycling, participants simultaneously watched a commercial video streaming service to maintain visual input. Participants were required to maintain 50% to 70% of their maximum heart rate during the periods of exercise, where maximum heart rate was calculated as 220 minus age (i.e., aimed for 112–140 beats/minute for the age range tested). Heart rate was continuously monitored throughout the test session by an Alta HR heart rate and activity tracker (Fitbit, San Francisco, CA) attached to the wrist. During rest periods, participants sat comfortably and continued watching the same visual content as during the cycling periods.
Estimates of habitual physical activity
The short version of the International Physical Activity Questionnaire (IPAQ) was provided to all participants upon completion of the test sessions to assess frequency, intensity, and duration of physical activity (specifically, walking, moderate-intensity activities, and vigorous-intensity activities) in the last 7 days (Craig et al., 2003). Participants completed the IPAQ according to their habitual activities for a typical week. The IPAQ results were analyzed according to international guidelines (https://sites.google.com/site/theipaq/scoring-protocol) to give a continuous total score in metabolic equivalent of task (MET)-minutes/week. One MET-minute is equivalent to the amount of energy expended during a minute while performing an activity compared to being at rest; hence, light-intensity physical activity is associated with fewer MET-minutes than vigorous-intensity activities, and a higher total MET-minute/week score indicates greater physical activity. The IPAQ scoring also enables categorization of individuals into low, moderate, or high participants in physical activity.
Control experiment: effect of repeated binocular rivalry testing in the absence of any intervention
We additionally conducted a control experiment to determine whether there are effects of simply repeating the binocular rivalry task itself after a period of time. Nine participants (mean ± SD, 35 ± 8 years old; range, 28–51) completed the same experimental protocol described above (i.e., baseline binocular rivalry tests; 2 hours of rest, computer work, or screen time; then binocular rivalry tests at regular intervals), but without patching and exercise.
Statistical analysis
Statistical analysis was performed using SPSS Statistics 26.0 (IBM, Armonk, NY). Repeated-measures analysis of variance (RM-ANOVA) was conducted to compare baseline and 0 to 9 minutes and 9 to 18 minutes post-intervention. Post hoc Bonferroni tests for multiple comparisons were used to determine which time points were different from each other. Where Mauchly's test of sphericity determined non-homogeneity of variances, we adjusted the degrees of freedom and significance value with a Huynh–Feldt correction. Where the data were not normally distributed, Kruskal–Wallis and Mann–Whitney rank-sum tests were used to compare outcome baseline and post-intervention measures. Pearson's correlational analysis was conducted to explore the relationship between binocular rivalry dynamics and estimates of habitual physical activity. In addition, in order to demonstrate the immediate effect of each intervention, we normalized the binocular rivalry outcome measures obtained at 0 to 9 minutes to each individual's baseline and compared these normalized values among the three interventions. A p < 0.05 was considered statistically significant.
Results
Baseline binocular rivalry
Baseline measures of ocular dominance index across the three sessions (see scatterplots in Supplementary Material SA) showed good test–retest reliability based on absolute agreement-intraclass correlation coefficient (ICC) two-way mixed-effects model: ICC(3, 3) = 0.79 (Koo & Li, 2016). For all analyses described herein, we compared baseline binocular rivalry with the post-intervention binocular rivalry measures for each intervention (i.e., obtained at each session) separately.
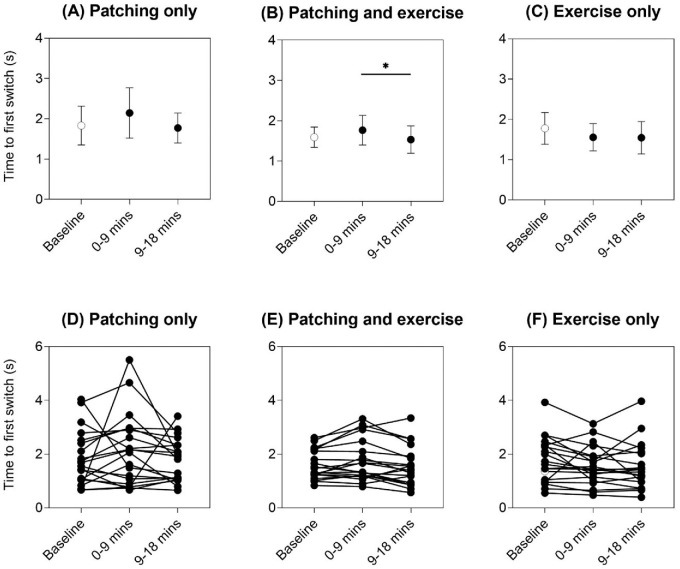
Onset rivalry: Time to first switch
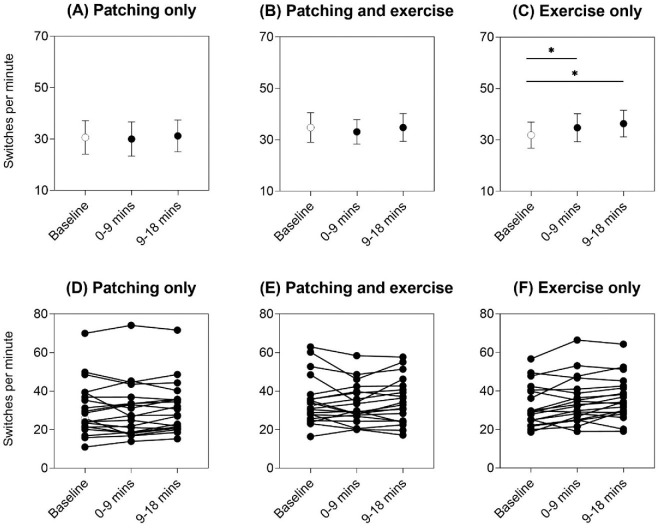
Figure 2 shows the group (top panels) and individual (bottom panels) time to first switch results as a measure of onset rivalry. There was no change in time to first switch from baseline after patching only, with RM-ANOVA main effect of intervention F(2, 38) = 1.15 and p = 0.33 (Figure 2A), or after exercise only, with RM-ANOVA main effect of intervention F(2, 38) = 2.40 and p = 0.10 (Figure 2C). Although patching and exercise did show an effect of intervention, with RM-ANOVA main effect of intervention F(2, 38) = 4.49, p = 0.018, and partial eta squared = 0.19 (Figure 2B), this was driven by the reduction in time to first switch at 9 to 18 minutes compared with 0 to 9 minutes (p = 0.031, post hoc Bonferroni comparison) (Figure 2B). Hence, relative to baseline (unfilled symbols in Figure 2), onset rivalry was not different after all three patching and exercise interventions.
Figure 2.
Time to first switch for the three interventions: (A) patching only, (B) patching and exercise, and (C) exercise only. Mean ± 95% confidence intervals of the mean are shown for each time point (baseline = unfilled symbol, 0–9 minutes and 9–18 minutes immediately post-intervention = filled symbols). Asterisks and horizontal lines indicate post hoc Bonferroni multiple comparisons between time points that were significant at p < 0.05. Individual data are shown in panels (D) to (F).
Sustained rivalry: Relative dominance of exclusive percepts
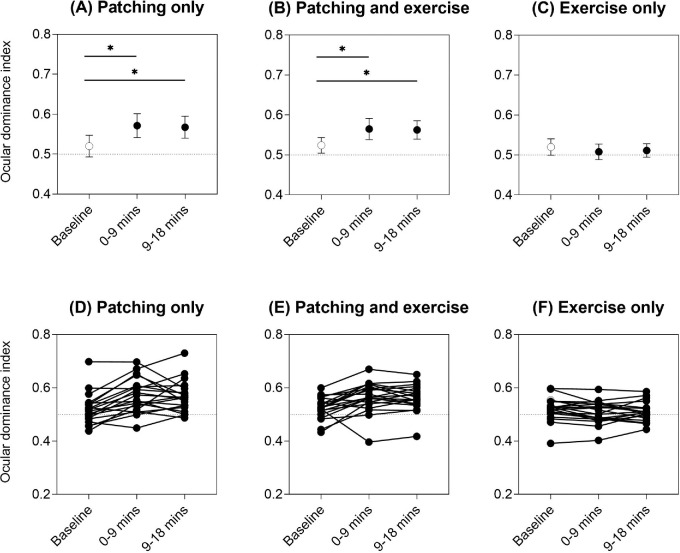
Figure 3 depicts the group and individual ocular dominance indices to indicate how exclusive percept dominance changed relative to baseline (unfilled symbols). After patching only, there was a shift in ocular dominance toward the deprived (dominant) eye as expected, with RM-ANOVA main effect of intervention F(2, 38) = 11.4, p < 0.001, and partial eta squared = 0.37 (baseline vs. 0–9 minutes, p < 0.001; baseline vs. 9–18 minutes, p = 0.007, post hoc Bonferroni multiple comparisons) (Figure 3A). The same pattern was seen for patching with exercise, with RM-ANOVA main effect of intervention Huynh–Feldt ɛ = 0.76, F(1.53, 29.0) = 10.7, p = 0.001, and partial eta squared = 0.36 (baseline vs. 0–9 minutes, p = 0.008; baseline vs. 9–18 minutes, p = 0.006, post hoc Bonferroni multiple comparisons) (Figure 3B). However, there was no change in ocular dominance index from baseline after exercise only, with RM-ANOVA main effect of intervention Huynh–Feldt ɛ = 0.81, F(1.62, 30.8) = 1.74, and p = 0.20 (Figure 3C).
Figure 3.
Ocular dominance index for the three interventions: (A) patching only, (B) patching and exercise, and (C) exercise only. Mean ± 95% confidence intervals of the mean are shown for each time point (baseline = unfilled symbol, 0–9 minutes and 9–18 minutes immediately post-intervention = filled symbols). Asterisks and horizontal lines indicate post hoc Bonferroni multiple comparisons between time points that were significant at p < 0.05. Individual data are shown in panels (D) to (F). Horizontal dotted reference lines at 0.05 on the y-axis indicate equal dominance of the two exclusive percepts. The ocular dominance index was calculated as per Equation 1. An ocular dominance index closer to 1 indicates a relative shift in exclusive-percept dominance toward the deprived (dominant) eye percept.
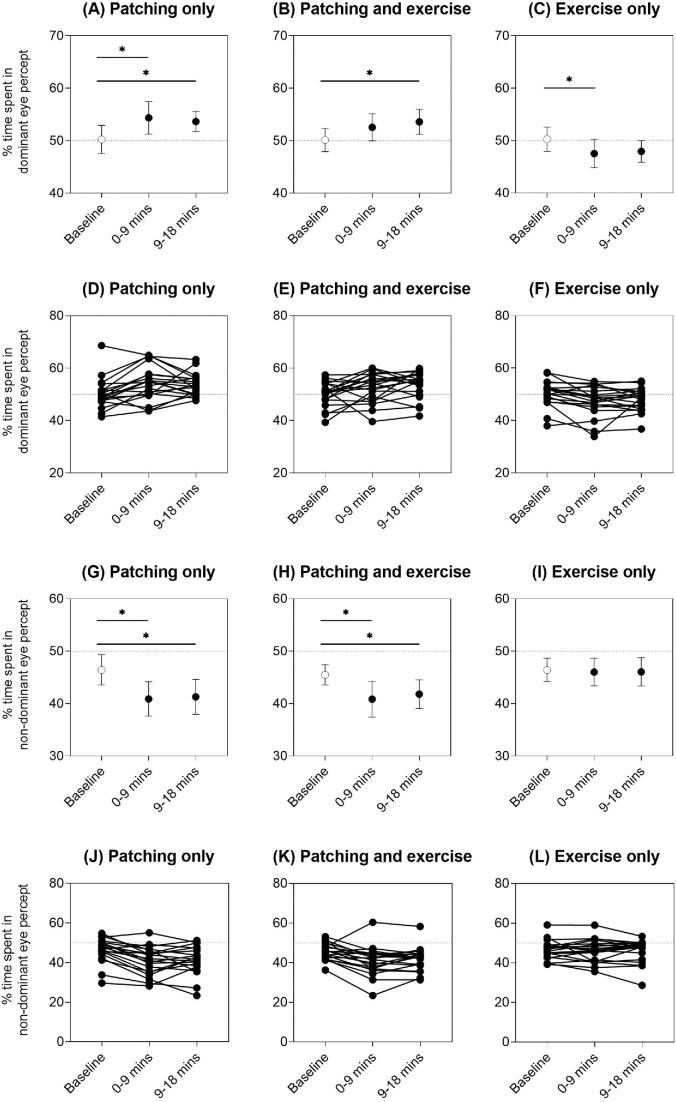
As the ocular dominance index depends on both the deprived (dominant) and the non-deprived (non-dominant) eye percepts, we explored these relative contributions further. There was an increase in the percentage of total rivalry time spent in the deprived (dominant) eye percept after patch removal with patching only, with RM-ANOVA main effect of intervention F(2, 38) = 6.73, p = 0.003, and partial eta squared = 0.26 (baseline vs. 0–9 minutes, p = 0.005; baseline vs. 9–18 minutes, p = 0.033, post hoc Bonferroni multiple comparisons) (Figure 4A), and when patching was combined with exercise, with RM-ANOVA main effect of intervention F(2, 38) = 4.54, p = 0.017, and partial eta squared = 0.19 (baseline vs. 0–9 minutes, p = 0.24; baseline vs. 9–18 minutes, p = 0.027, post hoc Bonferroni multiple comparisons) (Figure 4B). There was also a concomitant decrease in the time spent in the non-deprived (non-dominant) eye percept for the patching only, with RM-ANOVA main effect of intervention F(2, 38) = 12.9, p < 0.001, and partial eta squared = 0.40 (baseline vs. 0–9 minutes, p < 0.001; baseline vs. 9–18 minutes, p = 0.007, post hoc Bonferroni multiple comparisons) (Figure 4G), and patching with exercise interventions, with RM-ANOVA main effect of intervention F(2, 38) = 10.2, p < 0.001, and partial eta squared = 0.35 (baseline vs. 0–9 minutes, p = 0.006; baseline vs. 9–18 minutes, p = 0.010, post hoc Bonferroni multiple comparisons) (Figure 4H).
Figure 4.
Percentage of time spent in the deprived (dominant) eye percept for the three interventions: (A) patching only, (B) patching and exercise, and (C) exercise only. Percentage of time spent in the non-deprived (non-dominant) eye percept for the three interventions: (G) patching only, (H) patching and exercise, and (I) exercise only. Mean ± 95% confidence intervals of the mean are shown for each time point (baseline = unfilled symbol, 0–9 minutes and 9–18 minutes immediately post-intervention = filled symbols). Asterisks and horizontal lines indicate post hoc Bonferroni multiple comparisons between time points that were significant at p < 0.05. Individual data are shown in panels (D) to (F) and (J) to (L). Horizontal dotted reference lines at 50% on the y-axis indicate equal dominance of the two exclusive percepts.
Although the effect was subtle, exercise alone reduced the percentage of time spent in the dominant eye percept, which was opposite to the increase in dominant percept for the patching interventions, with RM-ANOVA main effect of intervention F(2, 38) = 5.41, p = 0.009, and partial eta squared = 0.22 (baseline vs. 0–9 minutes, p = 0.014; baseline vs. 9–18 minutes, p = 0.051, post hoc Bonferroni multiple comparisons) (Figure 4C), without changing the predominance of non-dominant eye percept, with RM-ANOVA main effect of intervention F(2, 38) = 0.15 and p = 0.86 (Figure 4I). The initial ocular dominance of the sighting dominant eye and the degree of exercise-induced plasticity (i.e., difference between dominant eye percept predominance between baseline and 0–9 minutes after exercise) were not correlated (Pearson's r = 0.14, R2 = 0.02, p = 0.57), indicating that the effect of exercise was independent of the baseline strength of ocular dominance.
Sustained rivalry: Mixed percept
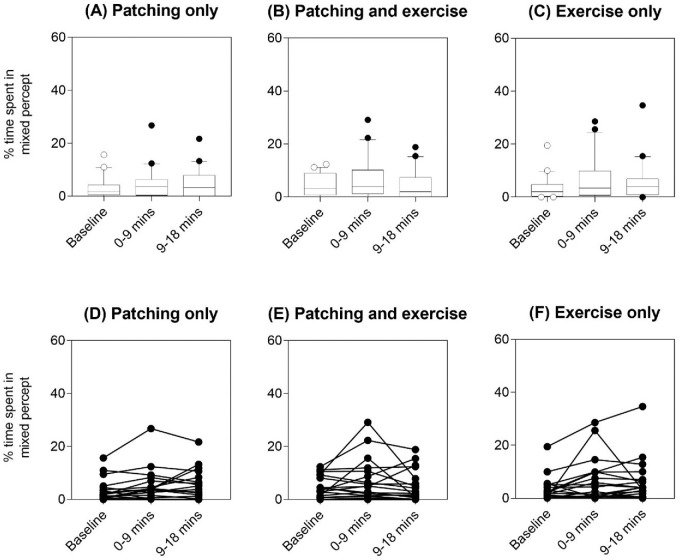
There was no difference in the percentage of time spent in mixed percept among baseline, 0 to 9 minutes, or 9 to 18 minutes after each of the three interventions (all p > 0.05, Kruskal–Wallis tests) (Figure 5).
Figure 5.
Percentage of total sustained rivalry time spent in mixed percept for the three interventions: (A) patching only, (B) patching and exercise, and (C) exercise only. Boxplots show the median and 25th and 75th percentiles, whiskers are the 10th and 90th percentiles, and symbols indicate the outliers for each time point (baseline = unfilled symbol, 0–9 minutes and 9–18 minutes immediately post-intervention = filled symbols). Individual data are shown in panels (D) to (F).
Sustained rivalry: Switch rate
For patching only, switch rate did not differ among baseline, to 9 minutes, or 9 to 18 minutes, with RM-ANOVA main effect of intervention F(2, 38) = 0.96 and p = 0.39 (Figure 6A). Neither was there a difference in switch rate among baseline, 0 to 9 minutes, or 9 to 18 minutes for the patching and exercise intervention, with RM-ANOVA main effect of intervention F(2, 38) = 1.69 and p = 0.20 (Figure 6B). Exercise only was associated with an increase in switch rate relative to baseline, with RM-ANOVA main effect of intervention Huynh–Feldt ɛ = 0.78, F(1.56, 29.7) = 8.73, p = 0.002, and partial eta squared = 0.32 (baseline vs. 0–9 minutes, p = 0.042; baseline vs. 9–18 minutes, p = 0.011, post hoc Bonferroni multiple comparisons) (Figure 6C).
Figure 6.
Switch rate for the three interventions: (A) patching only, (B) patching and exercise, and (C) exercise only. Mean ± 95% confidence intervals of the mean are shown for each time point (baseline = unfilled symbol, 0–9 minutes and 9–18 minutes immediately post-intervention = filled symbols). Asterisks and horizontal lines indicate post hoc Bonferroni multiple comparisons between time points that were significant at p < 0.05. Individual data are shown in panels (D) to (F).
Relationship to habitual physical activity
We considered whether the level of habitual physical activity in our participants could be related to the strength of the effects of exercise alone. Fourteen of 20 participants returned the IPAQ short form, representing a 70% response rate. Estimates of physical activity in terms of MET-minutes per week ranged from 462 to 10476 (median, 2961; interquartile range, 2600), which gave a range of low to high participation in habitual physical activity in our cohort. The level of habitual physical activity in a typical week was not correlated with the immediate effect of exercise only (i.e., 0–9-minute time point) on the percentage of time spent in the dominant eye percept (Pearson's r = –0.24, R2 = 0.06, p = 0.40; individual data from Figure 4F) nor with switch rate (Pearson's r = 0.10, R2 = 0.01, p = 0.73; individual data from Figure 6F).
Normalized effects of intervention
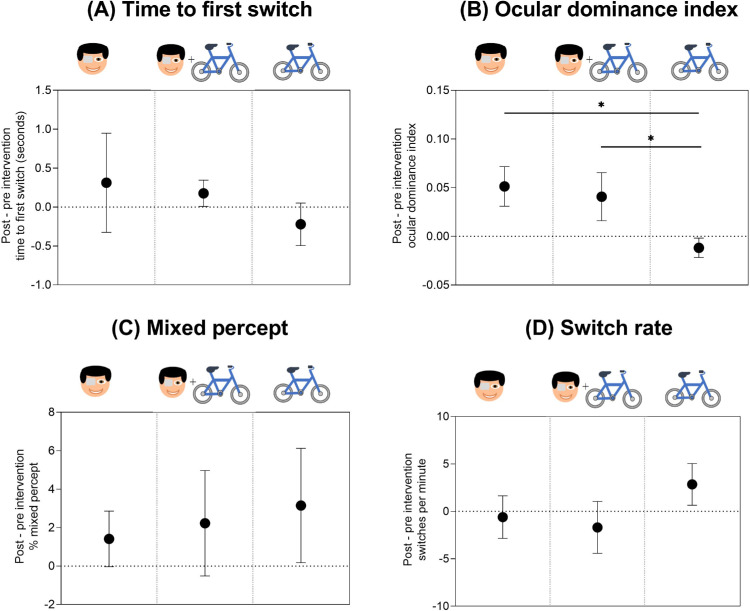
To directly compare the effect of each intervention (patching only, patching plus exercise, and exercise only), we normalized the time to first switch, ocular dominance index, percentage of time spent in mixed percept, and switch rate at 0 to 9 minutes to baseline for each individual (Figure 7). Comparing the normalized effects of the three interventions revealed no statistical difference between patching only and patching plus exercise for any of our binocular rivalry outcome measures (p > 0.05, post hoc Bonferroni multiple comparisons). However, the shift in ocular dominance index after exercise only was reduced (i.e., less shift in exclusive-percept dominance toward the dominant eye) relative to that observed with the two patching conditions, with RM-ANOVA main effect of intervention F(2, 38) = 16.00, p < 0.001, and partial eta squared = 0.46 (patching vs. exercise, p < 0001; patching plus exercise vs. exercise, p = 0.001, post hoc Bonferroni multiple comparisons) (Figure 7B). In addition, there was a trend for the exercise-only intervention to produce an increase in switch rate relative to the two patching interventions, with RM-ANOVA main effect of intervention F(2, 38) = 4.08, p = 0.025, and partial eta squared = 0.18 (patching vs. exercise, p = 0.05; patching plus exercise vs. exercise, p = 0.07, post hoc Bonferroni multiple comparisons) (Figure 7D).
Figure 7.
Normalized effects of the three interventions on (A) time to first switch, (B) ocular dominance index, (C) percentage of total sustained rivalry time spent in mixed percept, and (D) switch rate. A positive change on the y-axis indicates (A) delay in time to first switch, (B) greater shift in exclusive-percept dominance toward the deprived (dominant) eye percept, (C) greater proportion of mixed percept, and (D) faster switch rate after each intervention, relative to baseline. The three interventions are denoted by the symbols from left to right of each panel: patching only, patching and exercise, and exercise only. Mean ± 95% confidence intervals of the mean are plotted.
Control experiment: Effect of repeated binocular rivalry testing in the absence of any intervention
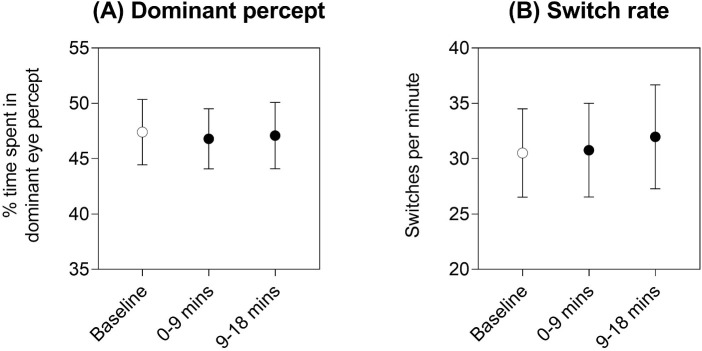
There was no effect of time on the percentage of time spent seeing the dominant eye percept, with RM-ANOVA main effect of time F(2, 16) = 0.12, p = 0.89 (Figure 8A) or on switch rate, with RM-ANOVA main effect of time F(2, 16) = 1.91, p = 0.18 (Figure 8B), indicating that our measures of binocular rivalry were not substantially impacted by repeated testing a few hours after baseline.
Figure 8.
Results of the control condition where neither patching nor exercise was conducted in the 2-hour interval between baseline and subsequent binocular rivalry measures: (A) percentage of time spent in the dominant eye percept, and (B) switch rate. Mean ± 95% confidence intervals of the mean are shown for each time point (baseline = unfilled symbol, 0–9 minutes and 9–18 minutes immediately post-intervention = filled symbols).
Supplementary results
In addition to the data presented above, which address our planned hypotheses regarding the immediate effect of each intervention, we present group data at each individual time point (measured at baseline and at 0, 3, 6, 9, 12, 15, 30, 45, 60, and 90 minutes after each intervention) in Supplementary Material SA. No formal statistics are presented for these later time points; however, raw data are available in Supplementary Material SB.
Discussion
Our study confirms the well-established phenomenon of short-term visual neuroplasticity in adults induced by 2 hours of monocular contrast deprivation. As per previous work using binocular rivalry methods (Binda et al., 2018; Finn et al., 2019; Lunghi & Sale, 2015; Lunghi et al., 2011; Lunghi et al., 2013; Lunghi et al., 2015; Lunghi et al., 2019; Sheynin, Proulx, et al., 2019; Steinwurzel et al., 2020), we report a shift in ocular dominance in favor of the deprived (dominant) eye when participants were exposed to temporary monocular deprivation. The shift in ocular dominance was driven by an increase in time spent in the deprived (dominant) eye percept and a concomitant decrease in time spent in the non-deprived (non-dominant) eye percept. Previous studies of ocular dominance homeostatic plasticity show conflicting results when comparing a patching-only intervention and a patching plus exercise intervention. One binocular rivalry study showed that exercise enhanced the shift in ocular dominance toward the deprived eye (Lunghi & Sale, 2015), whereas another study (Finn et al., 2019) replicated the previous methodology but did not find a significant effect of physical activity. Our direct comparison of the three interventions (see Figure 7, normalized to baseline data) did not find evidence for an interaction between patching and exercise that increases the patching effect on ocular dominance, nor for any other binocular rivalry outcome measure. In contrast, exercise in the absence of patching reduced the contribution of the dominant eye percept (see Figure 4C) and produced less shift in ocular dominance index compared with patching (see Figure 7B). Thus, although the effect of temporary monocular deprivation was clearly robust, the inconsistent outcomes of patching plus exercise on short-term visual neuroplasticity may have arisen because exercise, in and of itself, changes perceptual rivalry.
We were interested in exploring three distinct perceptual phenomena experienced during a binocular rivalry task—exclusive dominance percepts, mixed percept, and onset rivalry—as these may have different mechanistic underpinnings. Binocular rivalry models involve interocular competition and mutual inhibition between two populations of neurons as the brain alternates between two exclusive-dominance percepts (Blake, 1989; Blake & Logothetis, 2002; Klink, Brascamp, Blake, & van Wezel, 2010; Said & Heeger, 2013; Tong, Meng, & Blake, 2006). Indeed, longer percept phases, or slower rivalry switch rates, correlate with higher resting-state GABA levels (i.e., increased inhibitory neurotransmitter) in occipital cortex, measured using MRS (Pitchaimuthu et al., 2017; van Loon et al., 2013). A second, distinct inhibitory pathway has been proposed to govern the prominence of mixed percept, given recent findings that direct pharmacological agonism of specific GABA-subtype receptors is associated with increased mixed percept relative to exclusive-dominance percepts (Mentch et al., 2019). Furthermore, exclusive percept durations are not strongly correlated with either the proportion or duration of mixed percept (Steinwurzel et al., 2020), implying that exclusive-dominance percepts and mixed percepts are differently modulated. In this study, we found that all interventions produced shifts in ocular dominance (patching increased the dominance of the deprived or dominant eye, whereas exercise alone decreased the dominance of the dominant eye) during the period of sustained rivalry; however, there was no significant change in mixed percept nor onset rivalry relative to baseline.
Our study was designed to provide further insight into the debate regarding whether exercise enhances neuroplasticity effects by studying whether there are effects on binocular rivalry ocular dominance from exercise alone. The exercise-only intervention produced unique effects on ocular dominance not seen in the two patching interventions—namely, a reduction in the proportion of time spent in the dominant eye percept. Assuming that exercise results in a general shift in the balance between inhibition and excitation, why might ocular dominance be altered by exercise alone? The metric used to assess dominance is the amount of time spent during the binocular rivalry runs in the percept of each eye. In most individuals, one eye is more dominant than the other. With mutual inhibition, the population of neurons that represent the dominant eye percept inhibits the non-dominant eye population of neurons, leading to suppression of the weaker percept until the dominant percept is released. Given this baseline dominant versus non-dominant eye asymmetry in mutual inhibition, reducing inhibition through exercise may potentially affect the two exclusive percepts asymmetrically (i.e., the dominant eye may reach the threshold for release more readily with exercise). Our data support this idea—that exercise alone may influence measures of ocular dominance.
We also demonstrated that exercise alone increased the binocular rivalry switch rate, which was not observed in the exercise and patching condition. There are several potential mechanisms for increased switch rate. If exercise reduces the level of GABA-ergic inhibition in the cortex (Baroncelli et al., 2012; Kaneko & Stryker, 2014), then the rate of release of mutual inhibition between the two eye percepts would be predicted to increase. However, it should be noted that there is also evidence for increased, rather than decreased, levels of GABA in visual cortex following acute cycling exercise in young adults (Maddock et al., 2016). Clearly, we do not have a direct measure of any change in neurochemical status in our participants; however, on balance, the strong trend for an increase in switch rate in our participants after exercise was more consistent with reduced rather than increased inhibition. A further possibility is that exercise might lead to increased arousal or attention. Visual attention is considered necessary for binocular rivalry (reviewed by Dieter, Brascamp, Tadin, & Blake, 2016), and attentional modulation is a key component of newer models of binocular rivalry (Li, Rankin, Rinzel, Carrasco, & Heeger, 2017). One measurable indicator of a change in arousal or attention is pupil size, with increased arousal or attention resulting in pupil dilation, mediated predominantly via noradrenaline released from the locus coeruleus (Joshi & Gold, 2020; Yoshitomi, Ito, & Inomata, 1985). Previous studies have shown that, under typical rivalry conditions, pupil diameter is increased at the time of a perceptual switch and cannot be explained by a motor response (Einhauser, Stout, Koch, & Carter, 2008). Further insight into the role of attentional modulation by exercise could be gained by future study of pupil dynamics during binocular rivalry after exercise intervention. However, there is no clear explanation of why the increase in switch rate did not occur in the presence of patching. Previous work suggests that monocular deprivation acts by upregulation of cortical gain control primarily in the dominant eye (Lunghi et al., 2011); perhaps this change in gain control with patching is strong enough to hold the dominant eye percept for a longer period of time such that any effect of increased switch rate with exercise is masked.
Unlike the present study where we explored a direct exercise intervention, there is recent evidence pointing to a possible link between body metabolism and short-term adult visual plasticity through the examination of baseline body mass index levels. Morbidly obese individuals show a less pronounced shift in ocular dominance and increased mixed percept following short-term monocular deprivation (Lunghi et al., 2019). The authors speculated that there may be stronger GABAergic inhibition in people with higher body mass index (Lunghi et al., 2019), given that the shift in ocular dominance with short-term monocular deprivation has been linked to decreased GABA levels in visual cortex (Lunghi et al., 2015). However, this interpretation is likely to be overly simplistic, as it does not fully explain why the binocular rivalry outcomes associated with obesity (Lunghi et al., 2019) are partly consistent with a relative increase in inhibition (less ocular dominance shift compared to normal-weighted individuals) and partly consistent with a reduction in inhibition (more mixed percept; see Mentch et al., 2019). In terms of linking to our study, we did not measure body mass index but participants knew in advance that they were volunteering for a study involving riding an exercise bike, likely resulting in some self-selection bias. Our results are considered representative of a general healthy population (with a range of low to high habitual participation in physical activity) and demonstrate in these individuals that direct exercise intervention can lead to a reduction in ocular dominance with no impact on mixed percept.
In this study, we chose to use a mild to moderate exercise regime rather than vigorous or maximal physical activity for three main reasons: (1) to replicate the level of exercise in previous studies of short-term monocular deprivation (Finn et al., 2019; Lunghi & Sale, 2015; Zhou et al., 2017); (2) because mild to moderate exercise intensities are achievable by most people; and (3) to avoid known effects on brain metabolic activity with vigorous exercise (e.g., lactate metabolism; see Dalsgaard, Quistorff, Danielsen, Selmer, Vogelsang, & Secher, 2004). Our heart rate measures were comparable to previous studies of mild to moderate exercise intervention; here, we achieved an average 126 beats/minute, other studies between 124 beats/minute (Zhou et al., 2017) and 129 beats/minute (Finn et al., 2019; Lunghi & Sale, 2015), suggesting that differences in the outcomes of studies do not arise from this factor. Other population differences may be important, such as exercise habits, although our preliminary analysis of this factor in our dataset did not reveal any strong relationships.
Our findings also add to the growing evidence that onset rivalry and sustained rivalry are governed by different mechanisms (Attarha & Moore, 2015; Carter & Cavanagh, 2007; Kalisvaart & Goossens, 2013; Stanley, Forte, et al., 2011). Figure 2 shows no effect of any invention on the time to first switch, whereas the patching intervention clearly shifted dominance toward the patched eye during sustained rivalry (Figure 4). Although research continues to highlight the idiosyncratic nature of onset rivalry, the current results are consistent with previous studies emphasizing the role of eye dominance, luminance, and color in determining onset dominance compared with higher level factors (Dieter, Sy, & Blake, 2017; Stanley, Forte, & Carter, 2019; Stanley, Forte, et al., 2011). As the occlusion procedure involved a translucent patch that degraded form perception but largely preserved luminance and color, the occlusion procedure may have spared the neural signaling processes that are most relevant to the period of onset rivalry. In contrast, the process of mutual inhibition between orientation selective neurons corresponding to each eye will have been impacted by the occlusion period.
Compared to previous reports, we found a smaller magnitude of the effect of short-term monocular deprivation. Consequently, the previously reported enhancement of homeostatic visual plasticity (approximately 12% shift in ocular dominance) with additional exercise (Lunghi & Sale, 2015) could have been more difficult to detect here due to a smaller patching effect in the first instance. For example, the mean ocular dominance plasticity effect measured by Lunghi et al. (2019), evaluated by the difference between sensory eye dominance measured before and after 2 hours of patching, was approximately 0.13 (n = 20, 19–53 years of age). For our data, using the same calculation, we show a plasticity effect of patching of approximately 0.05 (n = 20, 20–34 years of age), or approximately half that reported previously. We speculate that the difference in effect size could partly be due to methodological differences such as the use of a stereoscope versus shutter goggles to induce dichoptic viewing (although we have no direct evidence to that effect), or the method by which the “dominant” eye was determined at the beginning of the first of three test sessions (here we used a clinical sighting eye dominance test). For logistical reasons, we did not choose the more dominant eye based on binocular rivalry performance at baseline in order to keep session durations to a minimum (the overall time commitment of participants was up to 12 hours over three sessions). Furthermore, we did not analyze any binocular rivalry data until after all sessions were completed to avoid potential bias during the data collection phase.
Albeit subtle, our post-exercise effects on ocular dominance (i.e., the rebalancing of dominance by reducing the dominant eye percept) are interesting from the perspective of treating neurodevelopmental disorders of vision, namely adult amblyopia. Because some degree of plasticity is retained in the adult amblyopic visual system, short-term monocular deprivation has been suggested as a means to restore visual function (visual acuity) and rebalance ocular dominance. Furthermore, the addition of exercise has also been explored as a possible adjunct to enhance the therapeutic effect. In adult anisometropic amblyopes, Lunghi et al. (2019) recently demonstrated that, when exercise was simultaneously performed while undergoing short-term inverse occlusion (i.e., patching of the amblyopic, or completely non-dominant eye), there was double the improvement in visual acuity of the amblyopic (deprived) eye. In addition, the sensory dominance of the amblyopic (deprived) eye increased after patching and became progressively more dominant with each training session (amblyopic eye occlusion plus exercise); that is, physical exercise further promoted the rebalancing of ocular dominance with patching, possibly in a dose-dependent manner. Here, we studied those with normal visual development, where the percentage of time spent in the dominant eye is substantially less than in the amblyopic situation. Presently, we have insufficient data to understand whether the response of the visual system to various interventions, such as those used in our study, differs in expression between those with normal visual development and those with amblyopia. Nevertheless, our data suggest that exercise alone in those with normal vision may shift the balance away from the previously dominant eye percept. Given that amblyopia therapy can be considered as aiming to reduce the dominance of a very dominant eye, our exercise-alone data provide some additional support to the possible utility of adding exercise to amblyopia therapy.
Conclusions
Here, we demonstrate for the first time, to the best of our knowledge, that exercise alone can impact ocular dominance in the absence of patching. By considering the separate and combined effects of patching and mild to moderate exercise, our findings support previous studies that binocular rivalry dynamics are influenced by short-term monocular deprivation but do not support the concept that exercise necessarily enhances that effect. In our cohort of young and healthy adults, neither short-term monocular deprivation nor patching influenced onset rivalry and mixed-percept predominance. Our results suggest distinct mechanistic differences among onset rivalry, mixed percept, and exclusive-dominance percept under conditions that induce short-term visual neuroplasticity, despite all three binocular rivalry features being linked to suppression/inhibition. We believe it is critical to consider all three distinct outcome measures of binocular rivalry in future work.
Supplementary Material
Acknowledgments
The authors thank Menaka Malavita and Yu Man Chan for their assistance in collecting part of the data.
Supported by a grant from the Australian Research Council Discovery Project (DP180102596 to AMM and OLC).
Commercial relationships: none.
Corresponding author: Allison M. McKendrick.
Email: allisonm@unimelb.edu.au.
Address: Department of Optometry and Vision Sciences, The University of Melbourne, Parkville, Victoria, Australia.
Appendix
Group binocular rivalry outcomes measures (time to first switch; ocular dominance index; percent time spent in the dominant eye, non-dominant eye, and mixed percept; switch rate) at all time points (baseline and 0, 3, 6, 9, 12, 15, 30, 45, 60, and 90 minutes post-intervention) are plotted for the three interventions (patching only, patching and exercise, exercise only) in Supplementary Material SA. Individual data are provided in spreadsheet form in Supplementary Material SB.
References
- Attarha, M., & Moore, C. M. (2015). Onset rivalry: Factors that succeed and fail to bias selection. Attention, Perception, & Psychophysics, 77(2), 520–535. [DOI] [PubMed] [Google Scholar]
- Baroncelli, L., Bonaccorsi, J., Milanese, M., Bonifacino, T., Giribaldi, F., Manno, I., Cenni, M. C., Berardi, N., Bonanno, G., Maffei, L., & Sale, A. (2012). Enriched experience and recovery from amblyopia in adult rats: Impact of motor, social and sensory components. Neuropharmacology, 62(7), 2388–2397. [DOI] [PubMed] [Google Scholar]
- Baroncelli, L., & Lunghi, C. (2020). Neuroplasticity of the visual cortex: In sickness and in health. Experimental Neurology, 335, 113515. [DOI] [PubMed] [Google Scholar]
- Binda, P., Kurzawski, J. W., Lunghi, C., Biagi, L., Tosetti, M., & Morrone, M. C. (2018). Response to short-term deprivation of the human adult visual cortex measured with 7T BOLD. Elife, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake, R. (1989). A neural theory of binocular rivalry. Psychological Review, 96(1), 145–167. [DOI] [PubMed] [Google Scholar]
- Blake, R., & Logothetis, N. (2002). Visual competition. Nature Reviews Neuroscience, 3(1), 13–21. [DOI] [PubMed] [Google Scholar]
- Campana, G., Fongoni, L., Astle, A., & McGraw, P. V. (2020). Does physical exercise and congruent visual stimulation enhance perceptual learning? Ophthalmic & Physiological Optics, 40(5), 680–691. [DOI] [PubMed] [Google Scholar]
- Carter, O., & Cavanagh, P. (2007). Onset rivalry: brief presentation isolates an early independent phase of perceptual competition. PLoS One, 2(4), e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaldi, E., Lunghi, C., & Morrone, M. C. (2020). Neuroplasticity in adult human visual cortex. Neuroscience & Biobehavioral Reviews, 112, 542–552. [DOI] [PubMed] [Google Scholar]
- Chadnova, E., Reynaud, A., Clavagnier, S., & Hess, R. F. (2017). Short-term monocular occlusion produces changes in ocular dominance by a reciprocal modulation of interocular inhibition. Scientific Reports, 7, 41747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig, C. L., Marshall, A. L., Sjostrom, M., Bauman, A. E., Booth, M. L., Ainsworth, B. E., Pratt, M., Ekelund, U., Yngve, A., Sallis, J. F., & Oja, P. (2003). International physical activity questionnaire: 12-country reliability and validity. Medicine & Science in Sports & Exercise, 35(8), 1381–1395. [DOI] [PubMed] [Google Scholar]
- Dalsgaard, M. K., Quistorff, B., Danielsen, E. R., Selmer, C., Vogelsang, T., & Secher, N. H. (2004). A reduced cerebral metabolic ratio in exercise reflects metabolism and not accumulation of lactate within the human brain. Journal of Physiology, 554(pt 2), 571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieter, K. C., Brascamp, J., Tadin, D., & Blake, R. (2016). Does visual attention drive the dynamics of bistable perception? Attention, Perception, & Psychophysics, 78(7), 1861–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieter, K. C., Sy, J. L., & Blake, R. (2017). Persistent biases in binocular rivalry dynamics within the visual field. Vision (Basel), 1(3), 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einhauser, W., Stout, J., Koch, C., & Carter, O. (2008). Pupil dilation reflects perceptual selection and predicts subsequent stability in perceptual rivalry. Proceedings of the National Academy of Sciences, USA, 105(5), 1704–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn, A. E., Baldwin, A. S., Reynaud, A., & Hess, R. F. (2019). Visual plasticity and exercise revisited: No evidence for a “cycling lane”. Journal of Vision, 19(6):21, 1–10, 10.1167/19.6.21. [DOI] [PubMed] [Google Scholar]
- Joshi, S., & Gold, J. I. (2020). Pupil size as a window on neural substrates of cognition. Trends in Cognitive Sciences, 24(6), 466–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisvaart, J. P., & Goossens, J. (2013). Influence of retinal image shifts and extra-retinal eye movement signals on binocular rivalry alternations. PLoS One, 8(4), e61702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko, M., & Stryker, M. P. (2014). Sensory experience during locomotion promotes recovery of function in adult visual cortex. eLife, 3, e02798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klink, P. C., Brascamp, J. W., Blake, R., & van Wezel, R. J. (2010). Experience-driven plasticity in binocular vision. Current Biology, 20(16), 1464–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo, T. K., & Li, M. Y. (2016). A guideline of selecting and reporting intraclass correlation coefficients for reliability research. Journal of Chiropractic Medicine, 15(2), 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H. H., Rankin, J., Rinzel, J., Carrasco, M., & Heeger, D. J. (2017). Attention model of binocular rivalry. Proceedings of the National Academy of Sciences, USA, 114(30), E6192–E6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunghi, C., Burr, D. C., & Morrone, C. (2011). Brief periods of monocular deprivation disrupt ocular balance in human adult visual cortex. Current Biology, 21(14), R538–539. [DOI] [PubMed] [Google Scholar]
- Lunghi, C., Burr, D. C., & Morrone, M. C. (2013). Long-term effects of monocular deprivation revealed with binocular rivalry gratings modulated in luminance and in color. Journal of Vision, 13(6):1, 1–10, 10.1167/13.6.1. [DOI] [PubMed] [Google Scholar]
- Lunghi, C., Daniele, G., Binda, P., Dardano, A., Ceccarini, G., Santini, F., Del Prato, S., & Morrone, M. C. (2019). Altered visual plasticity in morbidly obese subjects. iScience, 22, 206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunghi, C., Emir, U. E., Morrone, M. C., & Bridge, H. (2015). Short-term monocular deprivation alters GABA in the adult human visual cortex. Current Biology, 25(11), 1496–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunghi, C., & Sale, A. (2015). A cycling lane for brain rewiring. Current Biology, 25(23), R1122–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock, R. J., Casazza, G. A., Buonocore, M. H., & Tanase, C. (2011). Vigorous exercise increases brain lactate and Glx (glutamate+glutamine): a dynamic 1H-MRS study. NeuroImage, 57(4), 1324–1330. [DOI] [PubMed] [Google Scholar]
- Maddock, R. J., Casazza, G. A., Fernandez, D. H., & Maddock, M. I. (2016). Acute modulation of cortical glutamate and GABA content by physical activity. Journal of Neuroscience, 36(8), 2449–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentch, J., Spiegel, A., Ricciardi, C., & Robertson, C. E. (2019). GABAergic inhibition gates perceptual awareness during binocular rivalry. Journal of Neuroscience, 39(42), 8398–8407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min, S. H., Baldwin, A. S., & Hess, R. F. (2019). Ocular dominance plasticity: A binocular combination task finds no cumulative effect with repeated patching. Vision Research, 161, 36–42. [DOI] [PubMed] [Google Scholar]
- Min, S. H., Baldwin, A. S., Reynaud, A., & Hess, R. F. (2018). The shift in ocular dominance from short-term monocular deprivation exhibits no dependence on duration of deprivation. Scientific Reports, 8(1), 17083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce, J., Gray, J. R., Simpson, S., MacAskill, M., Hochenberger, R., Sogo, H., Kastman, E., & Lindelov, J. K. (2019). PsychoPy2: Experiments in behavior made easy. Behavior Research Methods, 51(1), 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitchaimuthu, K., Wu, Q. Z., Carter, O., Nguyen, B. N., Ahn, S., Egan, G. F., & McKendrick, A. M. (2017). Occipital GABA levels in older adults and their relationship to visual perceptual suppression. Scientific Reports, 7(1), 14231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said, C. P., & Heeger, D. J. (2013). A model of binocular rivalry and cross-orientation suppression. PLoS Computational Biology, 9(3), e1002991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheynin, Y., Chamoun, M., Baldwin, A. S., Rosa-Neto, P., Hess, R. F., & Vaucher, E. (2019). Cholinergic potentiation alters perceptual eye dominance plasticity induced by a few hours of monocular patching in adults. Frontiers in Neuroscience, 13, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheynin, Y., Proulx, S., & Hess, R. F. (2019). Temporary monocular occlusion facilitates binocular fusion during rivalry. Journal of Vision, 19(5):23, 1–17, 10.1167/19.5.23. [DOI] [PubMed] [Google Scholar]
- Stanley, J., Carter, O., & Forte, J. (2011). Color and luminance influence, but can not explain, binocular rivalry onset bias. PLoS One, 6(5), e18978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley, J., Forte, J. D., & Carter, O. (2019). Rivalry onset in and around the fovea: The role of visual field location and eye dominance on perceptual dominance bias. Vision (Basel), 3(4), 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley, J., Forte, J. D., Cavanagh, P., & Carter, O. (2011). Onset rivalry: The initial dominance phase is independent of ongoing perceptual alternations. Frontiers in Human Neuroscience, 5, 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinwurzel, C., Animali, S., Cicchini, G. M., Morrone, M. C., & Binda, P. (2020). Using psychophysical performance to predict short-term ocular dominance plasticity in human adults. Journal of Vision, 20(7):6, 1–13, 10.1167/jov.20.7.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong, F., Meng, M., & Blake, R. (2006). Neural bases of binocular rivalry. Trends in Cognitive Sciences, 10(11), 502–511. [DOI] [PubMed] [Google Scholar]
- van Loon, A. M., Knapen, T., Scholte, H. S., St John-Saaltink, E., Donner, T. H., & Lamme, V. A. (2013). GABA shapes the dynamics of bistable perception. Current Biology, 23(9), 823–827. [DOI] [PubMed] [Google Scholar]
- Warburton, D. E. R., Jamnik, V. K., Bredin, S. S. D., & Gledhill, N. (2011). The Physical Activity Readiness Questionnaire for Everyone (PAR-Q+) and Electronic Physical Activity Readiness Medical Examination (ePARmed-X+). Health and Fitness Journal of Canada, 4(2), 3–23. [Google Scholar]
- Yao, Z., He, Z., Wang, Y., Lu, F., Qu, J., Zhou, J., & Hess, R. F. (2017). Absolute not relative interocular luminance modulates sensory eye dominance plasticity in adults. Neuroscience, 367, 127–133. [DOI] [PubMed] [Google Scholar]
- Yoshitomi, T., Ito, Y., & Inomata, H. (1985). Adrenergic excitatory and cholinergic inhibitory innervations in the human iris dilator. Experimental Eye Research, 40(3), 453–459. [DOI] [PubMed] [Google Scholar]
- Zhou, J., Clavagnier, S., & Hess, R. F. (2013). Short-term monocular deprivation strengthens the patched eye's contribution to binocular combination. Journal of Vision, 13(5):12, 1–10, 10.1167/13.5.12. [DOI] [PubMed] [Google Scholar]
- Zhou, J., Reynaud, A., & Hess, R. F. (2017). Aerobic exercise effects on ocular dominance plasticity with a phase combination task in human adults. Neural Plasticity, 2017, 4780876. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.