Abstract
One of the most prevalent symptoms after major surgery is pain. When postoperative pain treatment is unsatisfactory, it can lead to poor surgical recovery, decreased quality of life, and increased health care costs. Current analgesics, single or in combination, have limited efficacy due to low potency, limited duration of action, toxicities, and risk of addiction. The lack of nonaddictive strong analgesics along with the over prescription of opioids has led to an opioid epidemic in the United States. Therefore, there is an urgent need for the development of newer analgesics. Microribonucleic acids (miRNAs) are small noncoding RNA molecules that modulate protein synthesis in neurons and supporting cells (glia, leukocytes, and Schwann cells). The literature indicates that miRNA regulation is important in nociception. Here, we summarize the current evidence on the role of miRNAs on mechanisms involved in incisional, inflammatory, neuropathic, and cancer pain. We also discuss the role of modulating miRNA functions as potential therapeutic targets for analgesic use and opioid tolerance. Finally, we propose how the delivery of analog miRNAs (mimic-miRNAs or antago-miRNAs) could be introduced into clinical practice to provide analgesia in the perioperative period.
Despite the substantial use of prescription opioids and other less potent analgesics during and after major surgery, pain is still undertreated. Three unwanted consequences related to poorly managed pain and administration of high dosages of opioids include the risks of developing postoperative persistent pain, acute opioid-related adverse events, and addiction to persistent opioid use.1,2 Clinical studies indicate that 10%–50% of patients undergoing major procedures can develop postoperative persistent pain and may use opioids for 6–12 months after surgery.1,3 Both postoperative persistent pain and opioid use are risk factors for opioid addiction. Therefore, finding alternatives to opioids for treating perioperative pain could result in a decreased risk of long-term opioid addiction.
With the goal of reducing opioid consumption perioperatively, the recent consensus statements and guidelines from experts in the field of perioperative medicine emphasize the use of multimodal analgesic regimens.4,5 These strategies include a combination of regional anesthesia techniques with systemic nonopioid analgesics, such as oral gabapentinoids, intravenous lidocaine and ketamine, and nonsteroidal anti-inflammatory drugs.4,6 However, these systemic analgesics also have their own adverse events, such as sedation, bleeding, anastomotic gastrointestinal leaks, cardiovascular toxicities, and renal injury, that make them unsuitable for many patients.7,8 Furthermore, the actual duration of action of these analgesics is still limited to a few hours or days after major surgery, which ultimately results in the use of opioids. This notion highlights the importance of developing novel analgesic modalities that can effectively treat postoperative pain and decrease or eliminate the overprescription of opioids or high dosages of nonopioid analgesics.
In 1993, Ambros’s laboratory reported for the first time gene silencing by ribonucleic acid interference (RNAi) in Caenorhabditis elegans.9 RNAi has evolved as an ancient defense system against viruses and transposons and a mechanism to regulate endogenous gene expression. As a result of this interference, messenger RNA (mRNA) is prevented from translating into a protein.10 Notably, it is estimated that >60% of the mammalian genes are microribonucleic acid (miRNA) targets.11 There are 2 major mechanisms of short-stranded RNAi that have been studied extensively: small interference RNA (siRNA) and miRNA. Of these 2, mounting evidence indicates that miRNA-based treatments can be used to restore or repress miRNA expression and thus protein synthesis in different human conditions.12–14 In perioperative medicine, miRNAs regulate inflammation and immune responses, ischemia–reperfusion mechanisms, and the action of anesthetics and analgesics.15–17 Furthermore, miRNAs can serve as a means of cell-to-cell communication. As an example, Simeoli et al18 indicated that, after nerve injury, dorsal root ganglia (DRG) sensory neurons release extracellular vesicles containing miR-21, which then promotes M1 differentiation in macrophages.19 After lipopolysaccharide (LPS) stimulation, human-derived monocytic cells can release exosomes containing miR-532–3p. Remarkably, the intraplantar injection of these exosomes reduced thermal hyperalgesia.20 There is evidence to support the idea that the delivery of miRNAs could be used to modulate the signaling pathways and cellular elements involved in nociception.
In this narrative review, we will summarize the importance of miRNAs in the context of postsurgical pain and how “analog miRNAs” could be introduced to provide adequate and sustained pain relief after major surgery.
The Search for the “Ideal” Analgesic for Major Surgery
The current armamentarium of drugs and techniques available to provide analgesia after major surgery can be grouped into opioids and nonopioids and systemic or nonsystemic (local or regional analgesia). These currently available analgesics have limited efficacy and can cause significant adverse events. In the search for the “ideal” analgesic, we consider 4 criteria that must be fulfilled: (1) have ease of administration, (2) have long-lasting effects, (3) be well-tolerated (low toxicity), and (4) be nontolerant or nonaddictive. In that search, novel analgesic therapeutics (Table 1) are being investigated, including long-lasting local anesthetics (ie, liposomal bupivacaine), fatty acid amide hydrolase inhibitors, nitric oxide synthase inhibitors, cannabinoids, kappa and delta opioid agonists, biased opioid agonists that can preferentially activate G protein-coupled protein (GPCR) pathways, opioid vaccines, selective reversible inhibitor of microsomal prostaglandin synthase enzyme, and interleukin-6 antagonists.21–23 While some of these therapies have shown promising results in humans, others are still in experimental stages.21–23
Table 1.
Newer analgesics entering clinical trials
| Therapeutic agent | Mechanism of action | Comment |
|---|---|---|
Local anesthetics
|
Sodium channel blocker Sodium channel blocker + COX inhibition Sodium channel blocker |
These newer local anesthetics formulations are clinically used or in clinical trials to test safety and efficacy. |
Fatty acid amide hydrolase inhibitors
|
Inhibitor of the fatty acid amide hydrolase | Used in humans only in clinical studies for osteoarthritis pain, epilepsy and cannabis dependence. |
Nitric oxide synthase inhibitors
|
Inhibition of the production of nitric oxide
|
Only used in clinical trials. |
| MK8825, ubrogepant, olcegepant, telcagepant and rimegepant | CGRP receptor antagonist | Only used in clinical trials. |
| Tonabersat (SB-220453) | Gap junction modulator, anticonvulsant | Only used in clinical trials. |
| Tocilizumab | Interleukin 6 antagonist | Approved for rheumatoid arthritis, Castleman’s disease, giant cell arteritis, polyarticular and systemic juvenile idiopathic arthritis and chimeric antigen receptor T cell therapy-induced cytokine release syndrome. |
| Brivoligide | EGR1 - Transcription factor decoy | Only used in clinical trials. |
Abbreviations: COX, cyclooxygenase; EGR1, early growth factor gene; GPCR, G protein-coupled receptor.
One of these newer analgesic modalities is focused on RNAi technologies.10 In fact, a recent initiative by the US National Institutes of Health (https://grants.nih.gov/grants/guide/pa-files/PAR-18-742.html) is focused on understanding the role of epigenomics and noncoding RNAs on chronic pain, opioid use disorders, and opioid-induced hyperalgesia.
Acute and Persistent Postoperative Pain: Mechanisms
Surgical pain is evoked by tissue damage, has the goal of protecting the injured area, and plays an important role in initiating the healing process. Surgical pain after major procedures shares components of somatic, neuropathic, and visceral pain depending on patients’ predisposing factors and the location and extent of the tissue trauma.24 Somatic pain arises from trauma (ie, surgical incision) to tissues, such as abdominal wall structures (ie, muscle and parietal peritoneum), bones, or intra-articular structures (ie, capsule, ligaments, or tendons). Neuropathic pain is from nerve damage (ie, distention, ischemia, or transection) occurring during surgery, and visceral pain results from damage (ie, distention or ischemia) to organs such as the small or large intestine. As an example, postamputation pain has an element of acute somatic pain that can transition to chronic neuropathic pain (ie, postthoracotomy pain and postamputation phantom pain).25,26 On the other hand, pelvic exenterations share components of visceral, neuropathic, and somatic pain.25,26 The mixed component of different types of pain makes its treatment difficult, mainly after extensive surgical procedures.
Peripheral and central sensitization occur after activation and changes in the expression of nociceptors and secondary mediators located in sensory terminal, DRG cells, spinal neurons, and glial cells. At the level of the tissue injury (ie, skin and muscle), there is a significant increase in levels of inflammatory cytokines (ie, interleukin [IL]-1β and leukemia inhibitor factor [LIF]), complement mediators (C5a), growth factors (ie, nerve growth factor [NGF]), caspase 1, and the cyclooxygenase (COX) enzyme and a decrease in pH caused by accumulation of lactate.27 Inflammatory mediators such as IL-1β are released by invading leucocytes (ie, neutrophils and mastocytes), which are also critical cells in the resolution process of inflammation.28 At the DRG level, changes in activity and expression of nociceptive molecules and its receptors also occur and result in peripheral sensitization. The dorsal horn of the spinal cord is also a site of change in the activity and expression of mediators. Studies in animals demonstrate that a non-N-methyl-D-asparate (NMDA)/α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor, in particular, the GluR 1 subunit of the AMPA receptor, is critical in incisional pain. However, other molecules, such as proinflammatory cytokines, Toll-like receptor (TLR) 4, inducible nitric oxide synthase (iNOS), secondary messengers (PI3K, mitogen-activated protein kinase and mitogen activated protein protein [MEK], p38 kinase [p38], nuclear factor κ-light-chain-enhancer of activated B cells [NFκB]), and ion channels (acid sensing ion channel 3 [ASIC3], a2b subunit voltage-gated channels, and P2XR), also play a significant role in the development and maintenance of central sensitization.29 Epigenetic changes have also been implicated in surgical pain. DNA methylation, histone acetylation, and alterations in noncoding RNA expression occur after incision.30–32 For instance, in 24-hour incision, the miR206 levels are maximally reduced in rats, and it correlates with peaked expression of phospholipase A2 activating protein (PLAA).31
There are several considerations to make when studying the mechanisms of incisional pain. First, while most of the molecular changes occur acutely, they also persist days after surgery. For instance, the spinal levels of the enzyme COX2 acutely peak 4–6 hours after plantar incision, but they can remain elevated up to 3 days.33 Interestingly, this coincides as well with the duration of spinal cord glial cell activation after skin or muscle injury.34 Notably, in rats, some of the central changes in neurotransmission can be observed up to 3 weeks postoperatively in areas of nociceptive of processing in the brain.35 These time-related changes highlight the importance of developing strategies that can modulate nociceptive molecules beyond the few hours that a surgical input can last during surgery. Second, animal studies indicate that activation of different nociceptors may be responsible for evoked versus spontaneous pain or different modalities of hyperalgesia (mechanical versus thermal). As an example, mechanical hyperalgesia is effectively reduced by gabapentinoids, while inhibition of P2X purinoceptors has been shown to ameliorate thermal hyperalgesia.36,37 Furthermore, the ongoing spontaneous activity observed in sensory neurons after incision is responsible for nonevoked pain (pain at rest). This suggests that analgesics targeting nociceptors (ie, transient receptor potential cation channel subfamily V member 1 [TRPV1]) responsible for the increased spontaneous firing in small sensory neurons can effectively blockade pain at rest.27 Finally, the multiple changes occurring at expressional levels of different pronociceptive molecules explain why effective postoperative pain management strategies rely on the concept of multimodal analgesia.29
Mechanisms of RNAi: MiRNAs
In 1993, a seminal study from Ambros’s laboratory led to the discovery of miRNAs.38 MiRNAs are endogenous, single-strand, noncoding RNA molecules that act as posttranscriptional inhibitors or regulators of mRNA.15 An miRNA cluster is a polycistronic gene in which several miRNAs are encoded in a single primary transcript. MiRNAs are encoded by a complex network of genes and can regulate hundreds of genes.11
MiRNA transcription takes place at the nuclear level by RNA polymerase II. In the nucleus, the primary or pri-miRNA is processed by the Drosha/DGCR8 complex to give origin to the precursor miRNA (pre-miRNA) hairpins. After the cleavage, ~65- to 70-nucleotide-long pre-miRNAs are actively transported from the nucleus to cytoplasm by RAs related nuclear protein-guanosine triphosphate (Ran-GTP) and the export receptor exportin-5.39 Once the pre-miRNA is located in the cytoplasm, the double stranded RNA (dsRNA) is ready for loading onto RNA-induced silencing complex (RISC), which includes dsRNA-binding protein (dsRBP), Dicer, and Argonaute (Ago). The core components of RISC are the Ago protein family members. The Ago-2 possesses an active catalytic domain for cleavage activity in humans among the 8 members of this family. Translational repression and/or target mRNA destabilization occurs in multiprotein complexes that include GW182 proteins, deadenylases, and poly(A) binding proteins. Alteration in the Drosha, Dicer, and Ago protein family can modify the function of miRNAs.40 Then, a single-strand miRNA is formed by the activity of the RNA polymerase III.40
Depending on the number, type, and position of mismatches in the miRNA/mRNA pairing, it will trigger degradation or translation arrest.40 There are 2 ways to alter the functions of miRNAs: miRNA inhibition (antago-miRs) and miRNA replacement (miRNA mimics).11,41 Synthetic miRNAs, also called miRNA mimics, mimic the function of target endogenous mRNAs. In the case of antagonist miRNA or anti-microRNA (anti-miR), single-stranded synthetic miRNAs have antisense base pairs complementary with the target mRNA to inhibit the action of endogenous miRNA.42 These 2 approaches result in the inhibition/degradation of mRNA.11,43,44
The identification of miRNA targets is vital to develop safe and successful analgesic strategies. Validation studies to elucidate the target genes modulated by miRNA can be done in vitro or in silico. For validation in silico, there are many bioinformatic algorithms, including miRanda (http://microrna.sanger.ac.uk), TargetScan (http://www.targetscan.org), and PicTar (http://pictar.bio.nyu.edu), that can be queried to predict miRNA target sites. However, it is worth mentioning that in the search for the miRNA-binding sites, these sites should be carefully used due to possible discrepancies between results. These algorithms have been constructed to recognize elements in the 3′-untranslated region (UTR) of the miRNA and the complementary 3′-UTRs of orthologous genes. Computational analysis can also be used to predict off-target sites and the levels of inhibition of each target.11,45
Functional determination of the predicted miRNA/mRNA interaction can be performed in in vitro experiments using a reporter system.45 This assay has basis on the principle that the binding of the studied miRNA to its target mRNA will reduce the synthesis of the reporter protein, which is quantified and compared to a control. Once in silico and in vitro validation studies have been completed, signaling and cell function studies are needed to determine if the predicted miRNA/mRNA interaction translates into biological changes.45
MiRNAs in Surgical Pain.
Single miRNAs or miRNA clusters participate in mechanisms related to nociception and the response to analgesic drugs in animals and humans with different pain syndromes (Figure 1).12–14 Significant abnormalities in the expression of miRNAs at the peripheral (nerve endings and dorsal root ganglia) and central (spinal cord) levels have been described in surgical pain models, as well as in experimental paradigms of opioid-induced hyperalgesia and tolerance.31,46–53
Figure 1.
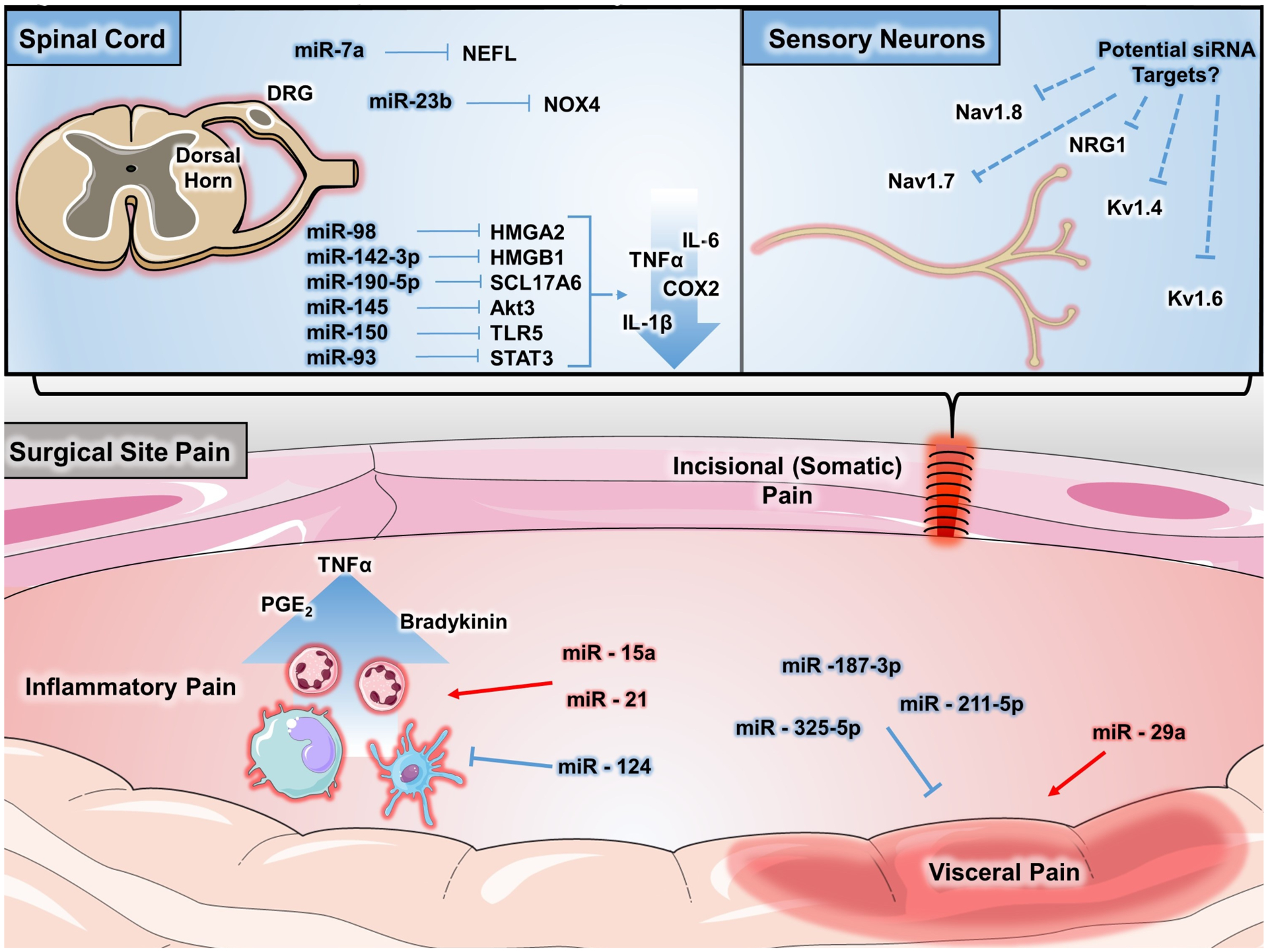
MiRNAs regulate several mechanisms of postoperative pain. Postoperative pain results from an accumulation of multiple sources, including central and peripheral nervous systems and inflammatory contributions. At several of these sites, miRNAs have demonstrated important roles in regulating mechanisms that can both promote (highlighted in red) and limit (highlighted in blue) pain. In the setting of somatic pain, such as pain occurring at the incisional site, miRNAs play regulatory roles in the central nervous system, such as miR-150 targeting of TLR5 and miR-93 targeting of STAT3, that result in decreased proinflammatory cytokine expression. In a similar fashion, miRNAs also regulate proinflammatory signaling from immune cells that contribute to inflammatory pain. These miRNAs include miR-15a and miR-21, which promote inflammation, and miR-124, which has an inhibitory role. Visceral pain has also been shown to be regulated by miRNAs in both positive and negative manners. At the level of sensory nerves, genetic studies and experimental siRNA-based therapies have implicated sodium and potassium ion channels as potential targets for analgesic treatments. Altogether, there is a growing accumulation of evidence implicating miRNA-mediated regulation of pain. COX indicates cyclooxygenase; DRG, dorsal root ganglia; IL, interleukin; miR, microRNA; miRNA, microribonucleic acid; NAV, voltage-gated sodium channel; NF-kB, nuclear factor kappa-light-chain-enhancer of activated B cells; NPY, neuropeptide Y; PLAA, phospholipase A2 activating protein; siRNA, small interference RNA; STAT3, signal transducer and activator of transcription 3; TLR, Toll-like receptor; TNF, tumor necrosis factor; TRPV, transient receptor potential cation channel subfamily V member.
Several miRNAs, mainly those regulating inflammation, appear to play a key role in incisional pain (Table 2).47,54–62 Low levels of miR-203 were found in the hind paw tissue of animals with incisional pain.31 The expressional change in that miRNA occurred acutely (2 hours) after surgery, lasted for 48 hours, and was inversely correlated with PLAA expression. High levels of PLAA were reversed after intraplanar injection of miR-203.31 A study by Li et al,63 demonstrated that the expression of miR-146 and the miR-183 cluster in the DRG and spinal cord was significantly reduced in a surgical model of osteoarthritis. Similarly, miR-16, miR-124–3p, and miR-141 were downregulated in the spinal cord of rats with inflammatory pain and correlated with mRNA upregulation of inflammatory mediators including member RAS oncogene family (RAB23), IL-6, IL-6R, IL-1B, and tumor necrosis factor (TNF)-α.60,64,65 Notably, the intrathecal administration of miR-16, miR-124–3p, and miR-141 mimics ameliorated inflammatory pain after complete Freund’s adjuvant (CFA) injection.60,64
Table 2.
miRNAs and miRNAs targets participating in incision and inflammatory pain
| Neuropathic pain | Incisional pain | Inflammatory pain | Visceral pain | Cancer bone pain | |||||
|---|---|---|---|---|---|---|---|---|---|
| Target | Target | Target | Target | Target | |||||
| Igfr1 | Traf6 | Rab23 | Rasgrp1 | Cav2.3 | |||||
| Nefl | Nfkb | Pomc | Klf5 | Synpo | |||||
| Fgf2/Fgfr1 | Tlr2 | Rankl | Sc5d | Creb | |||||
| Bace1 | Ngf | Il6r | Sert | MYMK | |||||
| Socs1 | Nav1.3 | Mrgpre | Htr7 | ||||||
| Nox4 | Trpv1 | Ptgs2 | Nfg | ||||||
| Mapk6 | Nav1.3 | Tnf | Il6 | ||||||
| Zeb1 | Trpv1 | Hmgb1 | Ptges3 | ||||||
| Scn9a | Plaa | p38 | Vgat | ||||||
| Tfg11 | Camkiiγ | Kcc-2 | |||||||
| Dusp5 | P2x7r | Zo1 | |||||||
| Nlrp3 | TLR4 | Ocln | |||||||
| Stat3 | Ugt11a1 | Ccl8 | |||||||
| Hmga2 | Gabra1 | ||||||||
| Sirt | Trpv1 | ||||||||
| Ezh2 | Arhgap12 | ||||||||
| Mecp2 | Erk | ||||||||
| GluA1 | Ccl2 | ||||||||
| Sn2b | Nk1r | ||||||||
| Piezo2 | TARGET? | ||||||||
| Twist1 | TARGET? | ||||||||
| Zeb1 | TARGET? | ||||||||
| Tnfaip1 | TARGET? | ||||||||
| Hmgb1 | |||||||||
| Hmgb1 | |||||||||
| Sn2b | |||||||||
| Trpm8 | |||||||||
| Rreb1 | |||||||||
| Akt1 | |||||||||
| Traf6 | |||||||||
| Irak1 | |||||||||
| Tlr5 | |||||||||
| Sirt | |||||||||
| Sgk3 | |||||||||
| Soc1 | |||||||||
| Ephb1 | |||||||||
| Bbnf | |||||||||
| Cacna2d1 | |||||||||
| Mtor | |||||||||
| Trek-1 | |||||||||
| Patch1 | |||||||||
| Bdnf | |||||||||
| Tlr5 | |||||||||
| Socs | |||||||||
| Hmgb1 | |||||||||
| Cxcr4 | |||||||||
| Trpa1 | |||||||||
| Kcnma1 | |||||||||
| Tpte | |||||||||
| Nr2b | |||||||||
Ifgr1: insulin like growth factor 1 receptor. Nefl: neurofilament, light polypeptide. Fgf2: fibroblast growth factor 2. Fgfr1: fibroblast growth factor receptor 1. Bace1: beta-secretase 1. Socs1: suppressor of cytokine signaling 1. Nox4: NADPH oxidase 4. Mapk6: Mitogen-activated protein 6. Zeb1: Zinc finger E-box binding homeobox 1. Scn9a: Sodium voltage-gated channel alpha subunit 9. Tgfb1: Transforming growth factor beta 1. Dusp5: Dual specificity phosphatase 5. Nlrp3: NLR family, pyrin domain containing 3. Stat3: Signal transducer and activator of transcription 3. Hmga2: High mobility group AT-hook 2. Sirt: Sirtuin 1. Ezh2: Enhancer of zeste 2 polycomb repressive complex 2 subunit. Mecp2: Methyl-CpG binding protein 2. Glua1: Glutamate ionotropic receptor AMPA type subunit 1. Piezo2: piezo type mechanosensitive ion channel component 2. Twist1: Twist family bHLH transcrpitor factor 1. TNFAIP1: Tumor necrosis factor, alpha-induced protein 1. Hmgb1: high mobility group box 1. Rrbe1: Ras responsive element binding protein 1. Akt1: Thymoma viral proto-oncogene 1. Traf: Tumor necrosis factor receptor associated factor 6. Irak1: Interleukin 1 receptor associated kinase 1. Tlr5: Toll-like receptor 5. Sgk3: serum/glucocorticoid regulated kinase family member 3. Ephb1: Eph receptor 1. Cacna2d1: calcium voltage-gated channel auxiliary subunit alpha2delta 1. Mtor: Mechanistic target of rapamycin kinase. TREK-1 (Kcnk2): Potassium channel, subfamily K, member 2. Cxcr4: chemokine (C-X-C motif) receptor 4. Ocln: Occludin. Nk1r: Tachykinin receptor 1. Rasgrp1: RAS guanyl releasing protein 1. Klf5: Kruppel like factor 5. Sc5d: sterol-C5-desaturase. Sert: Serotonin transporter. Htr7: 5-hydroxytryptamine (serotonin) receptor 7 Nfg: Nerve growth factor. Il6: Interleukin 6. Ptges3: Prostaglandin E synthase 3. Vgat: Vesicular GABA Transporter. Kcc-2: K+/Cl-Cotransporter. Zo1: Tight junction protein 1. Ccl8: C-C motif chemokine ligand 8. Grabra1: gamma-aminobutyric acid (GABA) A receptor, subunit alpha 1. Trpv1: Transient receptor potential cation channel subfamily V member. Erk: Extracellular regulated MAP kinase. CCL2: C-C motif chemokine ligand 2. Cav2.3: calcium channel, voltage-dependent, R type, alpha 1E. Creb: cAMP responsive element binding protein. Arhgap12: Rho GTPase activating protein 12. MYMK: myomaker, myoblast fusion factor. Synpo: Synaptopodyn. Traf6: Tumor necrosis factor receptor associated factor 6. Nfkb: Nuclear factor kappa-light-chain-enhancer of activated B cells. Tlr2: Toll-like receptor. Rankl: Receptor activator of nuclear factor kappa-B Ligand. Nav1.3: Sodium voltage-gated channel. Trpv1: Transient receptor potential cation channel subfamily V member 1. Plaa: Phospholipase A-2-activating protein. Rab23: Member RAS oncogene family. Pomc: Proopiomelanocortin. Il6r: Interleukin 6 receptor. Mrgpre: MAS-related GPR, member E. Ptgs2: Prostaglandin-endoperoxide synthase 2. Tnf: Tumor necrosis factor. Hmgb1: high mobility group box 1. P38: p38 kinase. Camkiiγ: Calcium/calmodulin-dependent protein kinase II gamma. P2x7r: purinergic receptor P2X, ligand-gated ion channel, 7. Ugt1a1: UDP glucuronosyltransferase family 1 member A1.
Analgesics commonly used to provide analgesia in the context of surgery can alter the expression of miRNAs. The daily intraperitoneal administration of gabapentin reduced miR-15a in DRG and partially reversed mechanical hyperalgesia in rodents with arthritic pain after CFA injection.66 In a similar animal model, the local coadministration of miR-124 plus ketoprofen-loaded particles was more effective than ketoprofen alone in reducing inflammation assessed by paw and ankle thickness.56 Western blot analysis indicated that animals treated with miR-124 plus ketoprofen-loaded particles had lower levels of the receptor activator of NFκB protein in the synovial tissue than those treated with either miR-124 or ketoprofen-loaded particles alone.56
Complex regional pain syndrome (CRPS) has been reported as a postoperative complication after major orthopedic surgery. The current evidence indicates that a deregulated inflammatory response linked to miRNA expressional changes is implicated in CRPS.54,55 TaqMan low-density array in the blood of CRPS patients demonstrated that 18 miRNAs were significantly altered. Among them, miR-532–3p was associated with CRPS type 2, pain intensity, IL1Ra, and vascular endothelial growth factor (VEGF). Pain also correlated with miR-296–5p, miR-361–3p, and miR-30d.47 McDonald et al20 conducted a study demonstrating the presence of exosomes containing miRNAs (miR-21–3p, miR-126–3p, and miR-212) in the blood of patients with CRPS. These miRNAs also increased after RAXW 264.7 cells were stimulated with LPS.
Opioid Tolerance and miRNAs.
The efficacy of opioids to treat surgical pain may vary from patient to patient, and its use can induce tolerance or opioid-induced persistent sensitization. Tapocik et al67 and other investigators have shown that multiple miRNAs are involved in opioid tolerance and sensitization.68–73 In the spinal cord or DRG of rodents, an increase in the expression of miR93–5p and a decrease in the levels of miR-365, miR-219–5p, and miR-338 or upregulation of miR-223–3p have been linked to induction of morphine tolerance in the presence or absence of pain.70,74,75 Wang et al70 measured the expression of miR-365 in the spinal cord of rats treated with 10 μg of morphine or saline intrathecally for 7 consecutive days. Downregulation of miR-365 was observed in morphine-tolerant animals compared to saline-treated rats. Notably, the intrathecal delivery of miR-365 using a lentivirus as a vector, partially reversed morphine tolerance. Similarly, overexpression of miR-219–5p in the spinal cord also reversed morphine tolerance by reducing the expression of calcium/calmodulin-dependent protein kinase II gamma (CaMKIIγ) and the NR1 subunit of the NDMA receptor.69 Changes in the expression of miRNAs also occurred in the DRG of mice acutely tolerant to morphine.76 Mice injected with a single dose of 100 mg of morphine subcutaneously showed downregulation of miR-375 in their DRG. Such change in miRNA expression inversely correlated with JAK2 levels and increased expression of brain-derived neurotrophic factor (BDNF). Interestingly, the intrathecal injection of miR-375 agomir reversed the tolerance.76
Neuropathic Pain.
Surgical pain with a neuropathic component is a common complication after mastectomy, thoracotomy, or hernia repairs. Laboratory studies indicate that miRNAs participate in mechanisms of peripheral and central sensitization after nerve injury (ie, spinal nerve ligation, chronic constriction injury, diabetic neuropathy, and chemotherapy-induced neuropathy).14,19,77–106 Furthermore, nerve damage triggers changes in the expression of different miRNAs that are observed at spinal and supraspinal levels. Liu et al107 demonstrated significant expressional changes in miR-3573–5p and miR-3074 in the spinal cord in animals with avulsion of the brachial plexus model while the expression of miR-30c-1–3p, miR-702–3p, miR-184, miR-25–5p, miR-873–5p, miR-93–3p, miR-455–3p, and miR-32–3p was altered in the thalamus and the anterior cingulate.107
Animal studies have also indicated that, after chronic injury of peripheral nerves, there is upregulation of spinal cord miRNAs (ie, miR-15b, miR-155, miR-196, and miR-30c-5p), while others are downregulated (ie, miR-96, miR-217, miR-34c, or miR-206–3p; Table 2).77–82,108–112 It is worth mentioning that the level of expression of the same miRNA can vary in different models of neuropathic pain. As an example, Chen et al113 demonstrated downregulation of miR-96 in the DRG of animals with sciatic nerve constriction injury, while it was upregulated in animals with spinal nerve ligation injury. This is evidence to illustrate the complexity of the epigenetic changes governing nociception after nerve injury. However, as shown in the next sections, 2 miRNAs that are significantly modulated during incisional and neuropathic pain are miR-146 and miR-183.
MiRNAs also modulate genes implicated in the production of inflammatory cytokines after the damage of peripheral nerves (Table 2). The intrathecal administration of lentivirus-miR-mimics for miR-98, miR-142–3p, miR-190a-5p, miR-145, and miR-150, partially attenuated mechanical and thermal hyperalgesia in animals with neuropathic pain due to chronic constriction injury and diabetic neuropathy. A common mechanism for this finding was the downregulation of IL-6, TNF-α, COX 2, and IL-1β by directly targeting the mRNAs of high mobility group adenine-thymine hook2 (HMGA2), high mobility group box1 (HMGB1), protein kinase B (AKT3), voltage-glutamate transporter 1 (also known as SCL17A6), signal transducer and activator of transcription 3 (STAT3), and Toll-like receptor 5 (TLR5).48–53 In similar studies, Hori et al114 reported that miR-21, miR-431, and miR-511–3p induced hyperalgesia by increasing the production of IL-6 in the spinal dorsal horn, whereas the intrathecal administration (3 injections) of miR-30c-5p inhibitor and miR-32-p knockdown reversed hyperalgesia and allodynia in rats with spinal nerve ligation by suppressing the production of inflammatory cytokines.115
Human studies also demonstrate the aberrant expression of miRNAs in subjects with painful neuropathies and fibromyalgia syndrome.104 For instance, increased levels of miR-30c-5p in blood and cerebrospinal fluid (CSF) were a predictor of neuropathic pain in patients with diabetes mellitus.116 In a mixed cohort of patients with painful neuropathies, the expression of miR-21 and miR-132–3p was increased in the sural nerve and leucocytes, but they showed reduced levels of miR-146 and miR-155 in the affected skin.99,117 Heyn et al118 studied the expression of miR-124a and miR-155 in CD4+ T cells of 11 patients with neuropathic pain and 9 healthy subjects. The expression of both miRNAs was significantly higher in patients with pain than controls and downregulation of the expression of sirtuin 1 (SIRT). Notably, SIRT is a key controller of T regulatory cells differentiation, which has been shown to reduce pain sensitization.118
Visceral and Cancer Pain.
Visceral pain is common in certain conditions that might require surgery, such as endometriosis, or in patients with irritable and inflammatory bowel syndromes, chronic prostatitis, and bladder pain syndromes. Extensive research has been conducted to i dentify how miRNA modulates visceral nociception (Table 2).55,119–125 Increased expression of miR-146a-5p and downregulation of miR-211–5p and miR-325–5p were detected in the spinal cord and DRG of rats with chronic peritoneal and colonic inflammation, respectively.126–128 Notably, 4 consecutive intrathecal injections of miR-325-agomir and the systemic treatment with miR-211–5p reversed hypersensitivity to colonic distention and mechanical and thermal hyperalgesia.126,127 Li et al129 investigated the role of miR-187–3p in ischemia-reperfusion (IR) pain using an aortic cross-clamping model in rats. In IR animals, downregulation of miR-187–3p in the spinal cord was inversely correlated with the expression of purinergic receptor P2X, ligand-gated ion channel, 7 (P2X7R). Intrathecal treatment with miR-187–3p agomir reversed mechanical and thermal hyperalgesia and decreased inflammation at the level of the spinal cord.129
In men with chronic prostatitis/chronic pelvic pain syndrome, the expression of miR21–5p in their prostate fluid is higher than in healthy men.130 Similarly, in patients with irritable bowel syndrome, the colonic expression of miR-29a is increased and negatively correlated with the expression of 5-hydroxytryptamine (serotonin) receptor 7 (Htr7) mRNA. When the expression of miR-29a was in knockdown mice, the expression of HTR7 was increased and animals showed less visceral pain.131 The expression of miR-199a-5p is increased in patients with bladder pain syndrome. Monastyrskaya et al123 showed that miR-199a-5p downregulated the expression of LIN7C, ARH-GAP12, PALS1, RND1, and PVRL1, which in turn was associated with an increase in bladder epithelial permeability. Ciszek et al132 measured blood miRNA in women with and without chronic abdominal/pelvic pain. Blood levels of miR-484–5p, miR-1294, and miR-520f were significantly downregulated in those with pain, while blood levels of miR-520D-3p were increased compared to healthy controls. The authors linked the observed changes to deregulation in proinflammatory cytokines such as IL-8.
The most commonly used experimental model to study cancer pain consists of inoculating malignant cells into the long bones (ie, tibia) of rodents. Using this animal paradigm, Elramah et al133 showed a significant downregulation of miR-124 and upregulation of synaptopodin in the spinal dorsal horn ipsilateral to the tumors. Then, these authors elegantly treated animals with 2 μg of miR-124 mimic intrathecally using i-Fect reagent as a carrier for 3 consecutive days and found that the mimic miRNA significantly ameliorated pain in comparison to a C. elegans–specific miRNA.133 Cyclic adenosine monophosphate-responsive element-binding protein (CREB) is an important transcription factor that is activated after nociceptive inputs reach the spine dorsal horn. One of the mechanisms by which CREB participates in nociception is by promoting miR-132 upregulation as it was shown in the spinal cord of animals inoculated with NCTNC 2472 fibrosarcoma cells.134 MiR-34c-5p and its target calcium channel, voltage-dependent (Cav2.3), were observed in the DRG neurons of mice inoculated with the same fibrosarcoma cells.135
Transient musculoskeletal (MSK) pain is a common (approximately 30%) complication in chronic myeloid leukemia patients taking tyrosine kinase inhibitors (TKIs) such as imatinib. A recent study by Asano et al136 demonstrated the presence of circulating exosomes loaded with miRNA-140–3p in patients with MSK pain taking TKIs. The authors suggested that elevated miR-140–3p can reduce the expression of Myomarker (a critical gene for muscle regeneration), and this may be responsible for MSK symptoms.136
Strategies to Utilize miRNAs as Novel Postoperative Analgesic Therapies in Humans.
The interest in RNA-focused therapies has grown since the Fire and Mello discovery of RNAi.137 Since then, monogenic and polygenic targeting therapies have been proposed to cure different human pathologic conditions. To date, several biotechnology companies have been created to develop miRNA-based therapies in different medical fields, including cancer, cardiology, and inflammatory, infectious, and congenital diseases. As a result, multiple studies are ongoing to evaluate the efficacy and toxicokinetics of systemic miRNA delivery in humans.138 In 2016, Neudecker et al15 reviewed the role of miRNAs in the context of perioperative medicine. However, no study was tested in humans to determine the safety or efficacy of miRNAs in the context of surgery and pain treatment.
Several important questions should be considered for “analgesic-miRNA” or “analgo-miRNA” in the context of surgery (Figure 2). First, which miRNA should be targeted or administered? To answer this question, we should consider mimics of downregulated miRNA (synthetic double-standard miRNAs) or antagonists (chemically modified anti-miR oligo-nucleotides or sponges) of overexpressed miRNA to return miRNA to its physiological state along with the trajectory and characteristics of surgical pain. In rodents, miR-203, miR-146, and miR183 are all decreased postoperatively. If similar changes were occurring in humans, then the administration of analgo-miRs to restore the levels at the site of injury or centrally should be considered.31,63 In results from animal studies using miRNAs in models of neuropathic pain, one could consider examining these miRNAs in clinical studies targeting postoperative pain after procedures with high rates of postsurgical neuropathic pain (ie, mastectomies or thoracotomies). Monogenic targeting molecules might have a limited efficacy in the treatment of surgical pain because during peripheral and central sensitization, there is activation of redundant cellular pathways. Therefore, polygenic therapy regulation based on miRNAs (multitargeting therapy) is an attractive strategy to ameliorate surgical pain. Furthermore, miRNAs can be used to target “nondrugable genes” that also play a role in surgical pain. However, it is worth mentioning that miRNAs can activate genes or even bring pseudoactivity by themselves; therefore, careful selection of “analog miRNAs” is key to avoid unwanted adverse effects.
Figure 2.
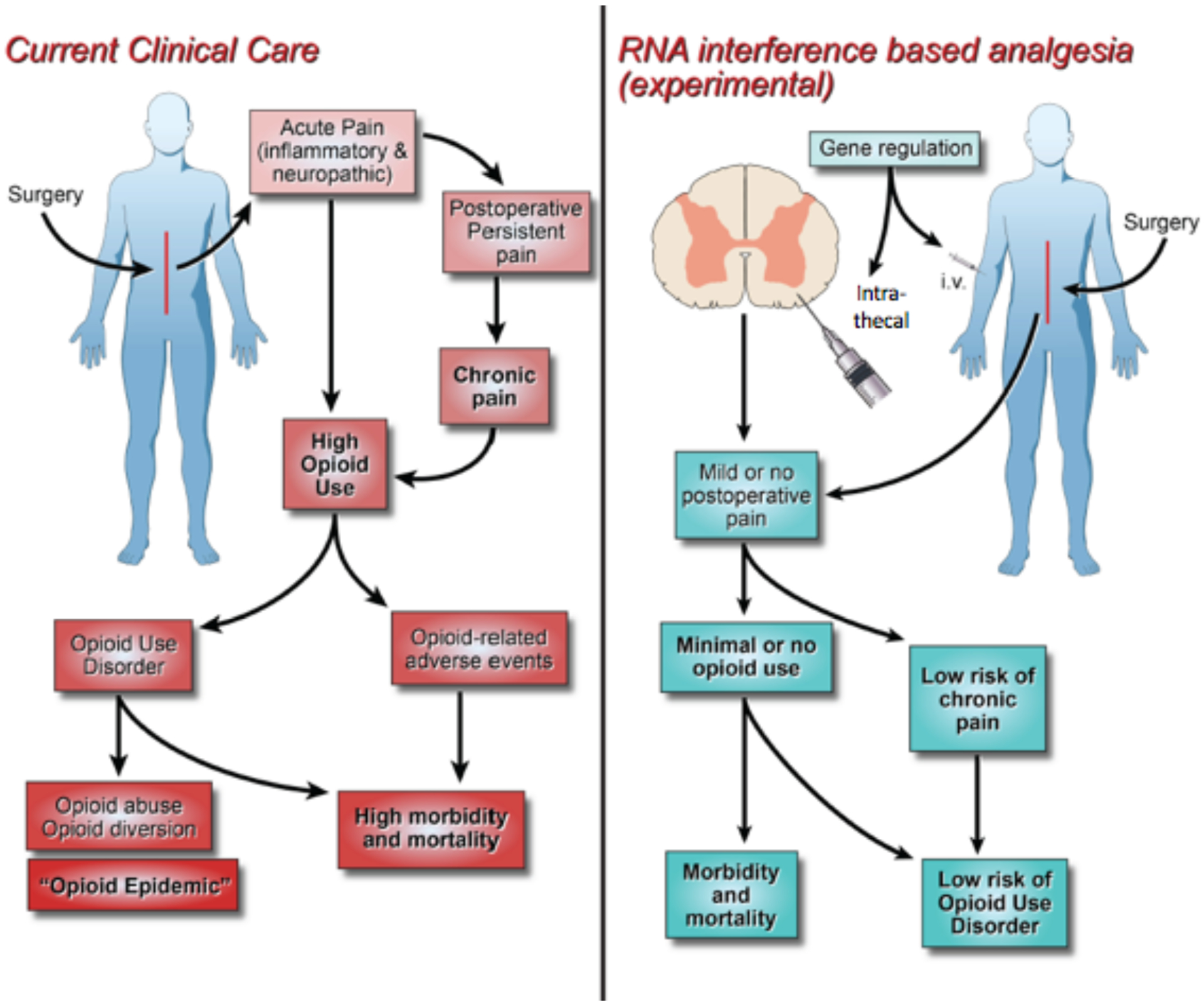
Analgo-miRNAs for surgical pain management. On the left, the current status of pain management (multimodal analgesia) for major surgery is displayed. On the right, a novel proposed concept of RNAi-based analgesia is depicted, which is currently being tested in the laboratory. miRNAs are single-stranded, noncoding RNA molecules that act as posttranscriptional inhibitors or regulators of the mRNA. miRNA therapies are in clinical trials for the treatment of some diseases. Here, we propose the administration of miRNAs perioperatively to provide pain relief. i.t. indicates intrathecal; i.v., intravenously; miRNA, microribonucleic acid; RNA, ribonucleic acid; RNAi, ribonucleic acid interference.
The second question is when and where “analog miRNAs” should be delivered. miRNA “mimics” or “antago-miRNAs” have a short half-life and rapidly degrade by nucleases, which limits their duration of action. The concentration of nucleases in the CSF is lower than in blood, thus making the intrathecal administration more attractive for the use of “analog miRNAs.” In addition, nuclease-resistant delivery systems have been developed for patient treatment, which could further increase the duration of action of “analog miRNAs.”139 Preclinical studies have shown that both anti-miRs and miRNA mimetics can effectively be delivered intrathecally. However, the current evidence is not clear regarding the differences in efficacy between anti-miRs and miRNA mimetics, which can be explained by the fact that most animal studies have focused on interventions modulating single miRNAs. While the intrathecal delivery is clearly the most studied route of administration of miRNAs in animals, it should be taken into consideration that it might not be suitable for everyday use in many patients. Therefore, new studies should focus on other alternatives for miRNA delivery, such as local. In fact, the intraplanar injection of miR-203 reversed surgical-induced pain in animals with plantar incision.31
Time of miRNA delivery is also a clinically relevant consideration. There are no clinical studies demonstrating the efficacy of preoperative versus postoperative administration of “analog miRNAs” for surgical pain control. Also, the available preclinical evidence is not clear on whether there are differences in the duration of action between intrathecally delivered anti-miRs and miRNA mimetics. However, animal studies suggest that patients could require the administration of multiple injections of “analog miRNAs” hours or days before surgery to facilitate the silencing or modulation of preformed or highly expressed protein before surgery. Contrarily, silencing of molecules only expressed after surgical insult could be achieved with single injections of miRNAs. Finally, the coadministration miRNA antagonists or miRNA mimics with local anesthetics could be an attractive alternative.
The last question is how miRNAs should be delivered to provide effective analgesia. Several barriers exist to successfully deploy miRNA to target cells.41 There are 2 main methods of carrying miRNAs: viral and nonviral vectors.10,140 Nonviral vectors can also be grouped into inorganics, organic (peptides, chitosan, lipids/liposomes, and aptamers), and polymer-based nanomaterials. Among the nonviral carriers, cationic lipids are used due to their biocompatibility, biodegradability, enhanced cellular entry of miRNAs, easy production, moderate toxicity, and immunogenicity.141 Inorganic carriers include gold, grapheme, quantum dot, and silica nanoparticles.141 Except for quantum dots (highly toxic), low toxicity and high biocompatibility are features of these inorganic nanoparticles. Finally, polymers such as polyethyleneimine, dendrimers, and polylactic-coglycolic acid have been extensively investigated to deliver miRNAs. One of the main advantages of polymer is high stability in body fluids, in addition to low cytotoxicity.141 The type of vector or carrier is of particular importance, since the spread of the “analog miRNAs” in the CSF will depend on the density, specific gravity, and baricity of the delivered mimic or antago-miR and the vector solution, which ultimately will determine the dermatomal level of analgesia.
SiRNAs could be considered as an alternative to analog miRNAs. SiRNAs are short (21–23 base pairs), double-stranded RNAs with multiple biological functions, including posttranscriptional repression and heterochromatin formation. While miRNA and siRNA cause RNA-induced translational silencing, there are major differences among them. First, siRNAs are considered exogenous, while miRNAs are naturally occurring. Second, siRNAs bind perfectly to their mRNA targets, while miRNAs can attach to multiple mRNAs.142 Effector phases of posttranscriptional siRNA silencing occur primarily in the cytoplasm.40 Chemical synthesis of siRNA results in purer and more stable siRNAs than those generated by gene expression. Synthetic siRNAs are typically 19–21 base pairs in length with 2-nucleotide single-stranded overhangs at their 3′ ends. After incorporation into the cytoplasm, synthetic siRNAs are incorporated into the RNAi machinery to initiate protein inhibition. However, there are “off-target” effects of siRNAs. This appears to occur when there is an imperfect match between the siRNA and the target mRNAs. siRNA has been used to repress synthesis of a wide variety of proteins involved in nociceptive mechanisms. For instance, Li et al143 demonstrated that spinal downregulation of the Cavβ3 subunit of the voltage-activated calcium channel via siRNA reversed hypersensitivity to mechanical stimulation in animals with spinal nerve ligation. The available literature suggests that effective downregulation of nociceptors can be achieved after multiple injections, after which the duration of changes in nociceptor protein levels appears to be long lasting (days). There is no evidence of siRNA use in humans with the goal of providing analgesia in the context of surgery. Contrarily, siRNAs have been effectively administered in humans with malignancies.144
CONCLUSIONS AND FUTURE PERSPECTIVES
Peripheral and central sensitization are associated with epigenetic alterations occurring in the DRG and spinal cord and mediated by activation of leucocytes, myeloid cells, neurons, and glial cells. Among those epigenetic aberrations, research has demonstrated downregulation and upregulation of miRNAs. Animals studies convincingly demonstrate that mimic-miR/antago-miRs administered before a surgical insult can ameliorate nociceptive behaviors. This exciting research using RNAi technologies based on miRNA mimics or antago-miRNAs has shown promising results and could bring new hope in the care of thousands of patients undergoing major surgery. However, there is a need for the development of better animal models that can reproduce pain related to specific procedures, such as craniotomies or labor.145
New discoveries in delivery systems will allow clinicians to safely administer mimic-miR/antago-miRs. Single or multiple analog miRNAs could be used to suppress proteins involved in surgical pain. Therefore, it can be theorized that this novel analgesic modality could (1) be administered preoperatively, (2) provide long-lasting analgesia, (3) diminish or prevent the chronification of pain, and (4) avoid or spare the use of other analgesics such as opioids. In addition, when given intrathecally, analog miRNAs could avoid systemic adverse events. On the other hand, off-target effects and still unclear rational selection of best miRs/anti-miRs can be considered as disadvantages. Based on the premises presented above, we hope to see future clinical studies evaluating the safety and efficacy of analog miRNAs as newer analgesics emerge in the context of major surgery.
ACKNOWLEDGMENTS
We want to acknowledge the SMART SERVIER MEDICAL ART as we have adapted the components of Figure 1 from its Clip Art Library under the license (https://creativecommons.org/licenses/by/3.0/). Special thanks to Kelli Wallen, MPH, McGovern Medical School, for the editorial assistance.
Funding:
National Institute of Health (NIH) Grants R01 DK097075, POI-HL114457, RO1-HL109233, RO1-DK109574, RO1-HL119837 and RO1-HL133900 to H.K.E.
GLOSSARY
- Ago
Argonaute
- Akt1
thymoma viral proto-oncogene
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- anti-miR
anti-microRNA
- Arhgap12
Rho GTPase activating protein 12
- ASIC3
acid sensing ion channel 3
- Bace1
beta-secretase 1
- BDNF
brain-derived neurotrophic factor
- Cacna2d1
calcium voltage-gated channel auxiliary subunit alpha2delta 1
- Camkiiγ
calcium/calmodulin-dependent protein kinase II gamma
- cAMP
cyclic adenosine monophosphate
- Cav2.3
calcium channel, voltage-dependent
- CCL
C-C motif chemokine ligand
- CFA
complete Freund’s adjuvant
- COX
cyclooxygenase
- Creb
cyclic adenosine monophosphate-responsive element-binding protein
- CRPS
complex regional pain syndrome
- CSF
cerebrospinal fluid
- Cxcr4
chemokine (C-X-C motif) receptor 4
- DRG
dorsal root ganglia
- dsRBP
dsRNA-binding protein
- dsRNA
double stranded RNA
- Dusp5
dual specificity phosphatase 5
- Ephb1
Eph receptor
- Erk
extracellular-regulated MAP kinase
- Ezh2
enhancer of zeste 2 polycomb repressive complex 2 subunit
- Fgf2
fibroblast growth factor 2
- Fgfr1
fibroblast growth factor receptor 1
- GluA1
glutamate ionotropic receptor AMPA type subunit 1
- GPCR
G protein-coupled receptor
- GTP
guanosine triphosphate
- Hmga2
high mobility group adenine-thymine-hook 2
- HMGB1
high mobility group box 1
- Htr7
5-hydroxytryptamine (serotonin) receptor 7
- IL
interleukin
- Il6r
interleukin 6 receptor
- iNOS
inducible nitric oxide synthase
- IR
ischemia-reperfusion
- Irak1
interleukin 1 receptor–associated kinase 1
- i.v.
intravenous
- Kcc-2
K+/Cl-cotransporter
- Kcnma1
potassium calcium-activated channel subfamily M alpha 1
- Klf5
Kruppel like factor 5
- LIF
leukemia inhibitor factor
- LPS
lipopolysaccharide
- MAP
mitogen-activated protein
- Mapk6
mitogen-activated protein 6
- Mecp2
methyl-CpG binding protein 2
- MEK
mitogen activated protein kinase kinase
- miR
miRNA
- miRNA
microRNA
- MRGPRE
mastocyte-related G protein-coupled receptors, member E
- mRNA
messenger ribonucleic acid
- MSK
musculoskeletal
- Mtor
mechanistic target of rapamycin kinase
- MYMK
myomaker, myoblast fusion factor
- Nav
voltage-gated sodium channel
- Nefl
neurofilament, light polypeptide
- NFκB
nuclear factor kappa-light-chain-enhancer of activated B cells
- Nfg
nerve growth factor
- NGF
nerve growth factor
- Nk1r
tachykinin receptor 1
- Nlrp3
nod-like receptor family, pyrin domain containing 3
- Nox4
nicotinamide adenine dinucleotide phosphate oxidase 4
- NPY
neuropeptide Y
- NR1
subunit NR1 NMDA receptor
- OCLN
occludin
- P2X7R
purinergic receptor P2X, ligand-gated ion channel, 7
- P38
p38 kinase
- PI3K
phosphoinositide 3-kinase
- Piezo2
piezo type mechanosensitive ion channel component 2
- PLAA
phospholipase A2 activating protein
- POMC
proopiomelanocortin
- pre-miRNA
precursor miRNA
- PTGES2
prostaglandin-endoperoxide synthase 2
- Ptgs2
prostaglandin-endoperoxide synthase 2
- RAB23
member RAS oncogene family
- Ran-GTP
RAs related nuclear protein-guanosine triphosphate
- Rankl
receptor activator of nuclear factor kappa-B ligand
- RANL
receptor activator of nuclear factor kappa-B ligand
- Rasgrp1
RAS guanyl releasing protein 1
- RISC
RNA-induced silencing complex
- RNA
ribonucleic acid
- RNAi
ribonucleic acid interference
- SC5D
sterol-C5-desaturase
- Scn9a
sodium voltage-gated channel alpha subunit 9
- Sert
serotonin transporter
- Sgk3
serum/glucocorticoid regulated kinase family member 3
- SIRT
sirtuin 1
- siRNA
small interference RNA
- Socs1
suppressor of cytokine signaling 1
- Stat3
signal transducer and activator of transcription 3
- Synpo
synaptopodyn
- Tgfb1
transforming growth factor beta 1
- TKIs
tyrosine kinase inhibitors
- TLR
Toll-like receptor
- TNF
tumor necrosis factor
- Tnfaip1
tumor necrosis factor, alpha-induced protein 1
- Tpte
transmembrane phosphatase with tensin homology
- TRAF6
tumor necrosis factor receptor associated factor 6
- TREK-1 (Kcnk2)
potassium channel, subfamily K, member
- TRK
tropomyosin receptor kinase
- Trpa1
transient receptor potential cation channel, subfamily A, member 1
- TRPV1
transient receptor potential cation channel subfamily V member 1
- Twist1
twist family basic helix-loop-helix transcrpitor factor 1
- Ugt1a1
Uridine diphosphate glucuronosyltransferase family 1 member A1
- UTR
untranslated region
- VEGF
vascular endothelial growth factor
- Vgat
vesicular gamma aminobutyric acid transporter
- Zeb1
zinc finger E-box binding homeobox 1
- Zo1
tight junction protein 1
Footnotes
The authors declare no conflicts of interest.
Reprints will not be available from the authors.
REFERENCES
- 1.Brescia AA, Harrington CA, Mazurek AA, et al. Factors associated with new persistent opioid usage after lung resection. Ann Thorac Surg. 2019;107:363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schug SA, Lavandʼhomme P, Barke A, Korwisi B, Rief W, Treede RD; IASP Taskforce for the Classification of Chronic Pain. The IASP classification of chronic pain for ICD-11: chronic postsurgical or posttraumatic pain. Pain. 2019;160:45–52. [DOI] [PubMed] [Google Scholar]
- 3.Brat GA, Agniel D, Beam A, et al. Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: retrospective cohort study. BMJ. 2018;360:j5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beverly A, Kaye AD, Ljungqvist O, Urman RD. Essential elements of multimodal analgesia in Enhanced Recovery After Surgery (ERAS) guidelines. Anesthesiol Clin. 2017;35:e115–e143. [DOI] [PubMed] [Google Scholar]
- 5.Buggy DJ, Borgeat A, Cata J, et al. Consensus statement from the BJA workshop on cancer and anaesthesia. Br J Anaesth. 2015;114:2–3. [DOI] [PubMed] [Google Scholar]
- 6.Eltzschig HK, Lieberman ES, Camann WR. Regional anesthesia and analgesia for labor and delivery. N Engl J Med. 2003;348:319–332. [DOI] [PubMed] [Google Scholar]
- 7.Cata JP, Guerra CE, Chang GJ, Gottumukkala V, Joshi GP. Non-steroidal anti-inflammatory drugs in the oncological surgical population: beneficial or harmful? A systematic review of the literature. Br J Anaesth. 2017;119:750–764. [DOI] [PubMed] [Google Scholar]
- 8.Memtsoudis S, Cozowicz C, Zubizarreta N, et al. Risk factors for postoperative delirium in patients undergoing lower extremity joint arthroplasty: a retrospective population-based cohort study. Reg Anesth Pain Med. 2019. [DOI] [PubMed] [Google Scholar]
- 9.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. [DOI] [PubMed] [Google Scholar]
- 10.Chen X, Mangala LS, Rodriguez-Aguayo C, Kong X, Lopez-Berestein G, Sood AK. RNA interference-based therapy and its delivery systems. Cancer Metastasis Rev. 2018;37:107–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pritchard CC, Cheng HH, Tewari M. MicroRNA profiling: approaches and considerations. Nat Rev Genet. 2012;13:358–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrari D, Bianchi N, Eltzschig HK, Gambari R. MicroRNAs modulate the purinergic signaling network. Trends Mol Med. 2016;22:905–918. [DOI] [PubMed] [Google Scholar]
- 13.Sakai A, Saitow F, Maruyama M, et al. MicroRNA cluster miR-17–92 regulates multiple functionally related voltage-gated potassium channels in chronic neuropathic pain. Nat Commun. 2017;8:16079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng C, Li L, Zhang MD, et al. miR-183 cluster scales mechanical pain sensitivity by regulating basal and neuropathic pain genes. Science. 2017;356:1168–1171. [DOI] [PubMed] [Google Scholar]
- 15.Neudecker V, Brodsky KS, Kreth S, Ginde AA, Eltzschig HK. Emerging roles for microRNAs in perioperative medicine. Anesthesiology. 2016;124:489–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neudecker V, Yuan X, Bowser JL, Eltzschig HK. MicroRNAs in mucosal inflammation. J Mol Med (Berl). 2017;95:935–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neudecker V, Haneklaus M, Jensen O, et al. Myeloid-derived miR-223 regulates intestinal inflammation via repression of the NLRP3 inflammasome. J Exp Med. 2017;214:1737–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simeoli R, Montague K, Jones HR, et al. Exosomal cargo including microRNA regulates sensory neuron to macrophage communication after nerve trauma. Nat Commun. 2017;8:1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park CK, Xu ZZ, Berta T, et al. Extracellular microRNAs activate nociceptor neurons to elicit pain via TLR7 and TRPA1. Neuron. 2014;82:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDonald MK, Tian Y, Qureshi RA, et al. Functional significance of macrophage-derived exosomes in inflammation and pain. Pain. 2014;155:1527–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viscusi ER, Webster L, Kuss M, et al. A randomized, phase 2 study investigating TRV130, a biased ligand of the μ-opioid receptor, for the intravenous treatment of acute pain. Pain. 2016;157:264–272. [DOI] [PubMed] [Google Scholar]
- 22.Schmid CL, Kennedy NM, Ross NC, et al. Bias factor and therapeutic window correlate to predict safer opioid analgesics. Cell. 2017;171:1165–1175.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohia-Nwoko O, Kosten TA, Haile CN. Animal models and the development of vaccines to treat substance use disorders, animal models for medications screening to treat addiction. Int Rev Neruobiol. 2016;126:263–291. [DOI] [PubMed] [Google Scholar]
- 24.Lovich-Sapola J, Smith CE, Brandt CP. Postoperative pain control. Surg Clin North Am. 2015;95:301–318. [DOI] [PubMed] [Google Scholar]
- 25.Collins KL, Russell HG, Schumacher PJ, et al. A review of current theories and treatments for phantom limb pain. J Clin Invest. 2018;128:2168–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young JM, Badgery-Parker T, Masya LM, et al. Quality of life and other patient-reported outcomes following exenteration for pelvic malignancy. Br J Surg. 2014;101:277–287. [DOI] [PubMed] [Google Scholar]
- 27.Xu J, Brennan TJ. The pathophysiology of acute pain: animal models. Curr Opin Anaesthesiol. 2011;24:508–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carreira EU, Carregaro V, Teixeira MM, et al. Neutrophils recruited by CXCR1/2 signalling mediate post-incisional pain. Eur J Pain. 2013;17:654–663. [DOI] [PubMed] [Google Scholar]
- 29.Pogatzki-Zahn EM, Segelcke D, Schug SA. Postoperative pain-from mechanisms to treatment. Pain Rep. 2017;2:e588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun Y, Sahbaie P, Liang DY, et al. Epigenetic regulation of spinal CXCR2 signaling in incisional hypersensitivity in mice. Anesthesiology. 2013;119:1198–1208. [DOI] [PubMed] [Google Scholar]
- 31.Sun Y, Li XQ, Sahbaie P, et al. miR-203 regulates nociceptive sensitization after incision by controlling phospholipase A2 activating protein expression. Anesthesiology. 2012;117:626–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun Y, Sahbaie P, Liang D, et al. DNA methylation modulates nociceptive sensitization after incision. PLoS One. 2015;10:e0142046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao J, Wang PK, Tiwari V, et al. Short-term pre- and postoperative stress prolongs incision-induced pain hypersensitivity without changing basal pain perception. Mol Pain. 2015;11:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ying YL, Wei XH, Xu XB, et al. Over-expression of P2X7 receptors in spinal glial cells contributes to the development of chronic postsurgical pain induced by skin/muscle incision and retraction (SMIR) in rats. Exp Neurol. 2014;261:836–843. [DOI] [PubMed] [Google Scholar]
- 35.Romero A, Rojas S, Cabañero D, et al. A 18F-fluorodeoxyglucose microPET imaging study to assess changes in brain glucose metabolism in a rat model of surgery-induced latent pain sensitization. Anesthesiology. 2011;115:1072–1083. [DOI] [PubMed] [Google Scholar]
- 36.Cheng JK, Pan HL, Eisenach JC. Antiallodynic effect of intrathecal gabapentin and its interaction with clonidine in a rat model of postoperative pain. Anesthesiology. 2000;92:1126–1131. [DOI] [PubMed] [Google Scholar]
- 37.Füredi R, Bölcskei K, Szolcsányi J, Petho G. Comparison of the peripheral mediator background of heat injury- and plantar incision-induced drop of the noxious heat threshold in the rat. Life Sci. 2010;86:244–250. [DOI] [PubMed] [Google Scholar]
- 38.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. [DOI] [PubMed] [Google Scholar]
- 39.Yi R, Qin Y, Macara IG, Cullen BR. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003;17:3011–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahmadzada T, Reid G, McKenzie DR. Fundamentals of siRNA and miRNA therapeutics and a review of targeted nanoparticle delivery systems in breast cancer. Biophys Rev. 2018;10:69–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.American Cancer Society. Overview: Breast Cancer. Survival rates for breast cancer. Available at: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts-and-figures-2019-2020.pdf. Accessed March 25, 2020.
- 43.van Rooij E, Purcell AL, Levin AA. Developing microRNA therapeutics. Circ Res. 2012;110:496–507. [DOI] [PubMed] [Google Scholar]
- 44.Bader AG, Brown D, Winkler M. The promise of microRNA replacement therapy. Cancer Res. 2010;70:7027–7030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuhn DE, Martin MM, Feldman DS, Terry AV Jr, Nuovo GJ, Elton TS. Experimental validation of miRNA targets. Methods. 2008;44:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andersen HH, Duroux M, Gazerani P. MicroRNAs as modulators and biomarkers of inflammatory and neuropathic pain conditions. Neurobiol Dis. 2014;71:159–168. [DOI] [PubMed] [Google Scholar]
- 47.Orlova IA, Alexander GM, Qureshi RA, et al. MicroRNA modulation in complex regional pain syndrome. J Transl Med. 2011;9:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y, Su Z, An LJ, et al. miR-98 acts as an inhibitor in chronic constriction injury-induced neuropathic pain via downregulation of high-mobility group AT-hook 2. J Cell Biochem. 2019;120:10363–10369. [DOI] [PubMed] [Google Scholar]
- 49.Shi J, Jiang K, Li Z. MiR-145 ameliorates neuropathic pain via inhibiting inflammatory responses and mTOR signaling pathway by targeting Akt3 in a rat model. Neurosci Res. 2018;134:10–17. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y, Mou J, Cao L, Zhen S, Huang H, Bao H. MicroRNA-142–3p relieves neuropathic pain by targeting high mobility group box 1. Int J Mol Med. 2018;41:501–510. [DOI] [PubMed] [Google Scholar]
- 51.Yang D, Yang Q, Wei X, et al. The role of miR-190a-5p contributes to diabetic neuropathic pain via targeting SLC17A6. J Pain Res. 2017;10:2395–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ji LJ, Shi J, Lu JM, Huang QM. MiR-150 alleviates neuropathic pain via inhibiting toll-like receptor 5. J Cell Biochem. 2018;119:1017–1026. [DOI] [PubMed] [Google Scholar]
- 53.Yan XT, Ji LJ, Wang Z, et al. MicroRNA-93 alleviates neuropathic pain through targeting signal transducer and activator of transcription 3. Int Immunopharmacol. 2017;46:156–162. [DOI] [PubMed] [Google Scholar]
- 54.Ramanathan S, Douglas SR, Alexander GM, et al. Exosome microRNA signatures in patients with complex regional pain syndrome undergoing plasma exchange. J Transl Med. 2019;17:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liao XJ, Mao WM, Wang Q, Yang GG, Wu WJ, Shao SX. MicroRNA-24 inhibits serotonin reuptake transporter expression and aggravates irritable bowel syndrome. Biochem Biophys Res Commun. 2016;469:288–293. [DOI] [PubMed] [Google Scholar]
- 56.Yu C, Zhang X, Sun X, et al. Ketoprofen and MicroRNA-124 Co-loaded poly (lactic-co-glycolic acid) microspheres inhibit progression of adjuvant-induced arthritis in rats. Int J Pharm. 2018;552:148–153. [DOI] [PubMed] [Google Scholar]
- 57.Dong Z, Jiang H, Jian X, Zhang W. Change of miRNA expression profiles in patients with knee osteoarthritis before and after celecoxib treatment. J Clin Lab Anal. 2019;33:e22648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun X, Zhang H. miR-451 elevation relieves inflammatory pain by suppressing microglial activation-evoked inflammatory response via targeting TLR4. Cell Tissue Res. 2018;374:487–495. [DOI] [PubMed] [Google Scholar]
- 59.Zhou LL, Zhu YM, Qian FY, Yuan CC, Yuan DP, Zhou XP. MicroRNA-143-3p contributes to the regulation of pain responses in collagen-induced arthritis. Mol Med Rep. 2018;18:3219–3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu CC, Cheng JT, Li TY, Tan PH. Integrated analysis of microRNA and mRNA expression profiles in the rat spinal cord under inflammatory pain conditions. Eur J Neurosci. 2017;46:2713–2728. [DOI] [PubMed] [Google Scholar]
- 61.Dong Y, Li P, Ni Y, Zhao J, Liu Z. Decreased microRNA-125a-3p contributes to upregulation of p38 MAPK in rat trigeminal ganglions with orofacial inflammatory pain. PLoS One. 2014;9:e111594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pan Z, Zhu LJ, Li YQ, et al. Epigenetic modification of spinal miR-219 expression regulates chronic inflammation pain by targeting CaMKIIγ. J Neurosci. 2014;34:9476–9483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li X, Kroin JS, Kc R, et al. Altered spinal microRNA-146a and the microRNA-183 cluster contribute to osteoarthritic pain in knee joints. J Bone Miner Res. 2013;28:2512–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shen WS, Xu XQ, Zhai NN, Zhou ZS, Shao J, Yu YH. Potential mechanisms of microRNA-141–3p to alleviate chronic inflammatory pain by downregulation of downstream target gene HMGB1: in vitro and in vivo studies. Gene Ther. 2017;24:353–360. [DOI] [PubMed] [Google Scholar]
- 65.Chen W, Guo S, Wang S. MicroRNA-16 alleviates inflammatory pain by targeting ras-related protein 23 (RAB23) and inhibiting p38 MAPK activation. Med Sci Monit. 2016;22:3894–3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun D, Yang J, Wang D, et al. Gabapentin regulates expression of FGF2 and FGFR1 in dorsal root ganglia via microRNA-15a in the arthritis rat model. J Orthop Sci. 2017;22:1112–1119. [DOI] [PubMed] [Google Scholar]
- 67.Tapocik JD, Ceniccola K, Mayo CL, et al. MicroRNAs are involved in the development of morphine-induced analgesic tolerance and regulate functionally relevant changes in serpini1. Front Mol Neurosci. 2016;9:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang J, Wang J, Guo Q, Zou W. Emerging roles of microRNAs in morphine tolerance. J Pain Res. 2019;12:1139–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang J, Xu W, Shao J, et al. miR-219–5p targets CaMKIIγ to attenuate morphine tolerance in rats. Oncotarget. 2017;8:28203–28214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang J, Xu W, Zhong T, et al. miR-365 targets β-arrestin 2 to reverse morphine tolerance in rats. Sci Rep. 2016;6:38285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grace PM, Strand KA, Galer EL, Maier SF, Watkins LR. MicroRNA-124 and microRNA-146a both attenuate persistent neuropathic pain induced by morphine in male rats. Brain Res. 2018;1692:9–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xie XJ, Ma LG, Xi K, et al. Effects of microRNA-223 on morphine analgesic tolerance by targeting NLRP3 in a rat model of neuropathic pain. Mol Pain. 2017;13:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ni J, Gao Y, Gong S, Guo S, Hisamitsu T, Jiang X. Regulation of μ-opioid type 1 receptors by microRNA134 in dorsal root ganglion neurons following peripheral inflammation. Eur J Pain. 2013;17:313–323. [DOI] [PubMed] [Google Scholar]
- 74.Hu XM, Cao SB, Zhang HL, et al. Downregulation of miR-219 enhances brain-derived neurotrophic factor production in mouse dorsal root ganglia to mediate morphine analgesic tolerance by upregulating CaMKIIgamma. Mol Pain. 2016;12:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xiao WF, Li YS, Lou W, et al. MicroRNA-93–5p may participate in the formation of morphine tolerance in bone cancer pain mouse model by targeting Smad5. Oncotarget. 2016;7:52104–52114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li H, Tao R, Wang J, Xia L. Upregulation of miR-375 level ameliorates morphine analgesic tolerance in mouse dorsal root ganglia by inhibiting the JAK2/STAT3 pathway. J Pain Res. 2017;10:1279–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang X, Wang H, Zhang T, et al. Inhibition of microRNA-195 alleviates neuropathic pain by targeting patched1 and inhibiting SHH signaling pathway activation. Neurochem Res. 2019;44:1690–1702. [DOI] [PubMed] [Google Scholar]
- 78.Xu L, Wang Q, Jiang W, Yu S, Zhang S. MiR-34c ameliorates neuropathic pain by targeting NLRP3 in a mouse model of chronic constriction injury. Neuroscience. 2019;399:125–134. [DOI] [PubMed] [Google Scholar]
- 79.Wen J, He T, Qi F, Chen H. MiR-206–3p alleviates chronic constriction injury-induced neuropathic pain through targeting HDAC4. Exp Anim. 2019;68:213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jiang W, Wang Q, Yu X, Lu T, Zhang P. MicroRNA-217 relieved neuropathic pain through targeting toll-like receptor 5 expression. J Cell Biochem. 2019;120:3009–3017. [DOI] [PubMed] [Google Scholar]
- 81.Fang B, Wei L, Dong K, Niu X, Sui X, Zhang H. miR-202 modulates the progression of neuropathic pain through targeting RAP1A. J Cell Biochem. 2019;120:2973–2982. [DOI] [PubMed] [Google Scholar]
- 82.Zhong L, Fu K, Xiao W, Wang F, Shen LL. Overexpression of miR-98 attenuates neuropathic pain development via targeting STAT3 in CCI rat models. J Cell Biochem. 2018. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 83.Zhang Y, Liu HL, An LJ, et al. miR-124–3p attenuates neuropathic pain induced by chronic sciatic nerve injury in rats via targeting EZH2. J Cell Biochem. 2019;120:5747–5755. [DOI] [PubMed] [Google Scholar]
- 84.Shen F, Zheng H, Zhou L, Li W, Zhang Y, Xu X. LINC00657 expedites neuropathic pain development by modulating miR-136/ZEB1 axis in a rat model. J Cell Biochem. 2019;120:1000–1010. [DOI] [PubMed] [Google Scholar]
- 85.Ji LJ, Su J, Xu AL, Pang B, Huang QM. MiR-134–5p attenuates neuropathic pain progression through targeting Twist1. J Cell Biochem. 2018. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 86.Zhan LY, Lei SQ, Zhang BH, et al. Overexpression of miR-381 relieves neuropathic pain development via targeting HMGB1 and CXCR4. Biomed Pharmacother. 2018;107:818–823. [DOI] [PubMed] [Google Scholar]
- 87.Zhang Y, Su Z, Liu HL, et al. Effects of miR-26a-5p on neuropathic pain development by targeting MAPK6 in in CCI rat models. Biomed Pharmacother. 2018;107:644–649. [DOI] [PubMed] [Google Scholar]
- 88.Wang Z, Liu F, Wei M, et al. Chronic constriction injury-induced microRNA-146a-5p alleviates neuropathic pain through suppression of IRAK1/TRAF6 signaling pathway. J Neuroinflammation. 2018;15:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pan Z, Shan Q, Gu P, et al. miRNA-23a/CXCR4 regulates neuropathic pain via directly targeting TXNIP/NLRP3 inflammasome axis. J Neuroinflammation. 2018;15:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yan XT, Zhao Y, Cheng XL, et al. Inhibition of miR-200b/miR-429 contributes to neuropathic pain development through targeting zinc finger E box binding protein-1. J Cell Physiol. 2018;233:4815–4824. [DOI] [PubMed] [Google Scholar]
- 91.Zhao Y, Li S, Xia N, Shi Y, Zhao CM. Effects of XIST/miR-137 axis on neuropathic pain by targeting TNFAIP1 in a rat model. J Cell Physiol. 2018;233:4307–4316. [DOI] [PubMed] [Google Scholar]
- 92.Su S, Shao J, Zhao Q, et al. MiR-30b attenuates neuropathic pain by regulating voltage-gated sodium channel nav1.3 in rats. Front Mol Neurosci. 2017;10:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lu S, Ma S, Wang Y, Huang T, Zhu Z, Zhao G. Mus musculus-microRNA-449a ameliorates neuropathic pain by decreasing the level of KCNMA1 and TRPA1, and increasing the level of TPTE. Mol Med Rep. 2017;16:353–360. [DOI] [PubMed] [Google Scholar]
- 94.Ding M, Shen W, Hu Y. The role of miR-539 in the anterior cingulate cortex in chronic neuropathic pain. Pain Med. 2017;18:2433–2442. [DOI] [PubMed] [Google Scholar]
- 95.Xie X, Ma L, Xi K, Zhang W, Fan D. MicroRNA-183 suppresses neuropathic pain and expression of AMPA receptors by targeting mTOR/VEGF signaling pathway. Cell Physiol Biochem. 2017;41:181–192. [DOI] [PubMed] [Google Scholar]
- 96.Sun W, Zhang L, Li R. Overexpression of miR-206 ameliorates chronic constriction injury-induced neuropathic pain in rats via the MEK/ERK pathway by targeting brain-derived neurotrophic factor. Neurosci Lett. 2017;646:68–74. [DOI] [PubMed] [Google Scholar]
- 97.Pang X, Tang Y, Zhang D. Role of miR-145 in chronic constriction injury in rats. Exp Ther Med. 2016;12:4121–4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shao J, Cao J, Wang J, et al. MicroRNA-30b regulates expression of the sodium channel Nav1.7 in nerve injury-induced neuropathic pain in the rat. Mol Pain. 2016;12:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Leinders M, Üçeyler N, Pritchard RA, Sommer C, Sorkin LS. Increased miR-132–3p expression is associated with chronic neuropathic pain. Exp Neurol. 2016;283:276–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xia L, Zhang Y, Dong T. Inhibition of microRNA-221 alleviates neuropathic pain through targeting suppressor of cytokine signaling 1. J Mol Neurosci. 2016;59:411–420. [DOI] [PubMed] [Google Scholar]
- 101.Wang C, Jiang Q, Wang M, Li D. MiR-19a targets suppressor of cytokine signaling 1 to modulate the progression of neuropathic pain. Int J Clin Exp Pathol. 2015;8:10901–10907. [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang J, Zhang H, Zi T. Overexpression of microRNA-141 relieves chronic constriction injury-induced neuropathic pain via targeting high-mobility group box 1. Int J Mol Med. 2015;36:1433–1439. [DOI] [PubMed] [Google Scholar]
- 103.Lu Y, Cao DL, Jiang BC, Yang T, Gao YJ. MicroRNA-146a-5p attenuates neuropathic pain via suppressing TRAF6 signaling in the spinal cord. Brain Behav Immun. 2015;49:119–129. [DOI] [PubMed] [Google Scholar]
- 104.Zhang R, Huang M, Cao Z, Qi J, Qiu Z, Chiang LY. MeCP2 plays an analgesic role in pain transmission through regulating CREB/miR-132 pathway. Mol Pain. 2015;11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bali KK, Hackenberg M, Lubin A, Kuner R, Devor M. Sources of individual variability: miRNAs that predispose to neuropathic pain identified using genome-wide sequencing. Mol Pain. 2014;10:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lin CR, Chen KH, Yang CH, Huang HW, Sheen-Chen SM. Intrathecal miR-183 delivery suppresses mechanical allodynia in mononeuropathic rats. Eur J Neurosci. 2014;39:1682–1689. [DOI] [PubMed] [Google Scholar]
- 107.Liu Y, Wang L, Lao J, Zhao X. Changes in microRNA expression in the brachial plexus avulsion model of neuropathic pain. Int J Mol Med. 2018;41:1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ito N, Sakai A, Miyake N, et al. miR-15b mediates oxaliplatin-induced chronic neuropathic pain through BACE1 down-regulation. Br J Pharmacol. 2017;174:386–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.El-Lithy GM, El-Bakly WM, Matboli M, Abd-Alkhalek HA, Masoud SI, Hamza M. Prophylactic L-arginine and ibuprofen delay the development of tactile allodynia and suppress spinal miR-155 in a rat model of diabetic neuropathy. Transl Res. 2016;177:85–97.e1. [DOI] [PubMed] [Google Scholar]
- 110.Liu S, Zhu B, Sun Y, Xie X. MiR-155 modulates the progression of neuropathic pain through targeting SGK3. Int J Clin Exp Pathol. 2015;8:14374–14382. [PMC free article] [PubMed] [Google Scholar]
- 111.Tan Y, Yang J, Xiang K, Tan Q, Guo Q. Suppression of microRNA-155 attenuates neuropathic pain by regulating SOCS1 signalling pathway. Neurochem Res. 2015;40:550–560. [DOI] [PubMed] [Google Scholar]
- 112.Yang FR, Chen J, Yi H, Peng LY, Hu XL, Guo QL. MicroRNA-7a ameliorates neuropathic pain in a rat model of spinal nerve ligation via the neurofilament light polypeptide-dependent signal transducer and activator of transcription signaling pathway. Mol Pain. 2019;15:1744806919842464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen HP, Zhou W, Kang LM, et al. Intrathecal miR-96 inhibits Nav1.3 expression and alleviates neuropathic pain in rat following chronic construction injury. Neurochem Res. 2014;39:76–83. [DOI] [PubMed] [Google Scholar]
- 114.Hori N, Narita M, Yamashita A, et al. Changes in the expression of IL-6-mediated microRNAs in the dorsal root ganglion under neuropathic pain in mice. Synapse. 2016;70:317–324. [DOI] [PubMed] [Google Scholar]
- 115.Yan T, Zhang F, Sun C, et al. miR-32–5p-mediated Dusp5 downregulation contributes to neuropathic pain. Biochem Biophys Res Commun. 2018;495:506–511. [DOI] [PubMed] [Google Scholar]
- 116.Zhou XL, Zhang CJ, Wang Y, et al. EphrinB-EphB signaling regulates spinal pain processing via PKCγ. Neuroscience. 2015;307:64–72. [DOI] [PubMed] [Google Scholar]
- 117.Leinders M, Üçeyler N, Thomann A, Sommer C. Aberrant microRNA expression in patients with painful peripheral neuropathies. J Neurol Sci. 2017;380:242–249. [DOI] [PubMed] [Google Scholar]
- 118.Heyn J, Luchting B, Hinske LC, Hübner M, Azad SC, Kreth S. miR-124a and miR-155 enhance differentiation of regulatory T cells in patients with neuropathic pain. J Neuroinflammation. 2016;13:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhou Q, Yang L, Larson S, et al. Decreased miR-199 augments visceral pain in patients with IBS through translational upregulation of TRPV1. Gut. 2016;65:797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fourie NH, Peace RM, Abey SK, et al. Elevated circulating miR-150 and miR-342–3p in patients with irritable bowel syndrome. Exp Mol Pathol. 2014;96:422–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hou Q, Huang Y, Zhu S, et al. MiR-144 increases intestinal permeability in IBS-D rats by targeting OCLN and ZO1. Cell Physiol Biochem. 2017;44:2256–2268. [DOI] [PubMed] [Google Scholar]
- 122.Wright KR, Mitchell B, Santanam N. Redox regulation of microRNAs in endometriosis-associated pain. Redox Biol. 2017;12:956–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Monastyrskaya K, Sánchez-Freire V, Hashemi Gheinani A, et al. miR-199a-5p regulates urothelial permeability and may play a role in bladder pain syndrome. Am J Pathol. 2013;182:431–448. [DOI] [PubMed] [Google Scholar]
- 124.Sengupta JN, Pochiraju S, Pochiraju S, et al. MicroRNA-mediated GABA Aα−1 receptor subunit down-regulation in adult spinal cord following neonatal cystitis-induced chronic visceral pain in rats. Pain. 2013;154:59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang J, Yu J, Kannampalli P, et al. MicroRNA-mediated downregulation of potassium-chloride-cotransporter and vesicular γ-aminobutyric acid transporter expression in spinal cord contributes to neonatal cystitis-induced visceral pain in rats. Pain. 2017;158:2461–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wu R, Zhang PA, Liu X, et al. Decreased miR-325–5p contributes to visceral hypersensitivity through posttranscriptional upregulation of CCL2 in rat dorsal root ganglia. Neurosci Bull. 2019;35:791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sun L, Zhou J, Sun C. MicroRNA-211–5p enhances analgesic effect of dexmedetomidine on inflammatory visceral pain in rats by suppressing ERK signaling. J Mol Neurosci. 2019;68:19–28. [DOI] [PubMed] [Google Scholar]
- 128.Lu Y, Cao DL, Zhao LX, Han Y, Zhang YL. MicroRNA-146a-5p attenuates visceral hypersensitivity through targeting chemokine CCL8 in the spinal cord in a mouse model of colitis. Brain Res Bull. 2018;139:235–242. [DOI] [PubMed] [Google Scholar]
- 129.Li X-Q, Yu Q, Zhang Z-L, Sun X-J, Ma H. MiR-187–3p mimic alleviates ischemia-reperfusion-induced pain hypersensitivity through inhibiting spinal P2X7R and subsequent mature IL-1β release in mice. Brain Behav Immun. 2019;79:91–101. [DOI] [PubMed] [Google Scholar]
- 130.Chen Y, Chen S, Zhang J, et al. Expression profile of microRNAs in expressed prostatic secretion of healthy men and patients with IIIA chronic prostatitis/chronic pelvic pain syndrome. Oncotarget. 2018;9:12186–12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhu H, Xiao X, Chai Y, Li D, Yan X, Tang H. MiRNA-29a modulates visceral hyperalgesia in irritable bowel syndrome by targeting HTR7. Biochem Biophys Res Commun. 2019;511:671–678. [DOI] [PubMed] [Google Scholar]
- 132.Ciszek BP, Khan AA, Dang H, et al. MicroRNA expression profiles differentiate chronic pain condition subtypes. Transl Res. 2015;166:706–720.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Elramah S, López-González MJ, Bastide M, et al. Spinal miRNA-124 regulates synaptopodin and nociception in an animal model of bone cancer pain. Sci Rep. 2017;7:10949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hou B, Cui X, Liu Y, et al. Positive feedback regulation between microRNA-132 and CREB in spinal cord contributes to bone cancer pain in mice. Eur J Pain. 2016;20:1299–1308. [DOI] [PubMed] [Google Scholar]
- 135.Gandla J, Lomada SK, Lu J, Kuner R, Bali KK. miR-34c-5p functions as pronociceptive microRNA in cancer pain by targeting Cav2.3 containing calcium channels. Pain. 2017;158:1765–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Asano M, Umezu T, Katagiri S, et al. Up-regulated exosomal miRNA-140–3p in CML patients with musculoskeletal pain associated with discontinuation of tyrosine kinase inhibitors. Int J Hematol. 2017;105:419–422. [DOI] [PubMed] [Google Scholar]
- 137.Bernards R. The nobel prize in physiology or medicine for 2006 for the discovery of RNA interference. Ned Tijdschr Geneeskd. 2006;150:2849–2853. [PubMed] [Google Scholar]
- 138.van der Ree MH, van der Meer AJ, van Nuenen AC, et al. Miravirsen dosing in chronic hepatitis C patients results in decreased microRNA-122 levels without affecting other microRNAs in plasma. Aliment Pharmacol Ther. 2016;43:102–113. [DOI] [PubMed] [Google Scholar]
- 139.Chakraborty C, Sharma AR, Sharma G, Doss CGP, Lee SS. Therapeutic miRNA and siRNA: moving from bench to clinic as next generation medicine. Mol Ther Nucleic Acids. 2017;8:132–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG. Non-viral vectors for gene-based therapy. Nat Rev Genet. 2014;15:541–555. [DOI] [PubMed] [Google Scholar]
- 141.Chaudhary V, Jangra S, Yadav NR. Nanotechnology based approaches for detection and delivery of microRNA in healthcare and crop protection. J Nanobiotechnology. 2018;16:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lam JKW, Chow MYT, Zhang Y, Leung SWS. siRNA versus miRNA as therapeutics for gene silencing. Mol Ther Nucleic Acids. 2015;4:e252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li L, Cao XH, Chen SR, et al. Up-regulation of Cavβ3 subunit in primary sensory neurons increases voltage-activated Ca2+ channel activity and nociceptive input in neuropathic pain. J Biol Chem. 2012;287:6002–6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Davis ME, Zuckerman JE, Choi CH, et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464:1067–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mogil JS, Davis KD, Derbyshire SW. The necessity of animal models in pain research. Pain. 2010;151:12–17. [DOI] [PubMed] [Google Scholar]


