Abstract
Sustaining life requires efficient uptake of nutrients and conversion to usable forms. Almost everything about this process is dynamic. Nutrient availability fluctuates and changing environmental conditions impose new demands that can tip the metabolic equilibrium from biosynthesis and macromolecule storage to energy expenditure. At the same time, the organism itself changes, particularly during the rapid growth and differentiation in early development and also later in life as the adult ages. Here we review what has been learned from Drosophila melanogaster as an experimental model about the connections between external signals, signaling pathways, tissues and organs that allow animals to balance energy storage with expenditure in the face of change, both intrinsic and extrinsic.
Keywords: Drosophila, metabolism, energy storage, energy expenditure, nutrition, growth, development
Introduction
The growth, development and maturation of any animal requires that environmental nutrients are used to generate the energy and biomolecules required to drive processes ranging from cell proliferation and differentiation to tissue remodeling and even cell death. As a result, animal development must be capable of adapting to changes in nutrient availability and other environmental conditions. In the face of oscillating nutrient availability, multicellular organisms evolved specialized storage organs that confer the ability to tolerate wide variation in resource availability. Complex intra- and inter-organ signaling pathways coordinate the organismal responses to extrinsic cues, such as nutrient variability and environmental conditions, as well as internal metabolic needs. During the past two decades, Drosophila melanogaster has resurged in popularity as a model system to study the complex metabolic and physiologic networks at play during development and disease (Drummond-Barbosa and Tennessen, 2020; Galikova and Klepsatel, 2018; Musselman and Kuhnlein, 2018; Padmanabha and Baker, 2014; Sieber and Spradling, 2017). Here, we explore how development imposes stage-specific nutritional requirements and energetic demands upon animal physiology and highlight key systemic signals that coordinate flux through intermediary metabolism across multiple organ systems.
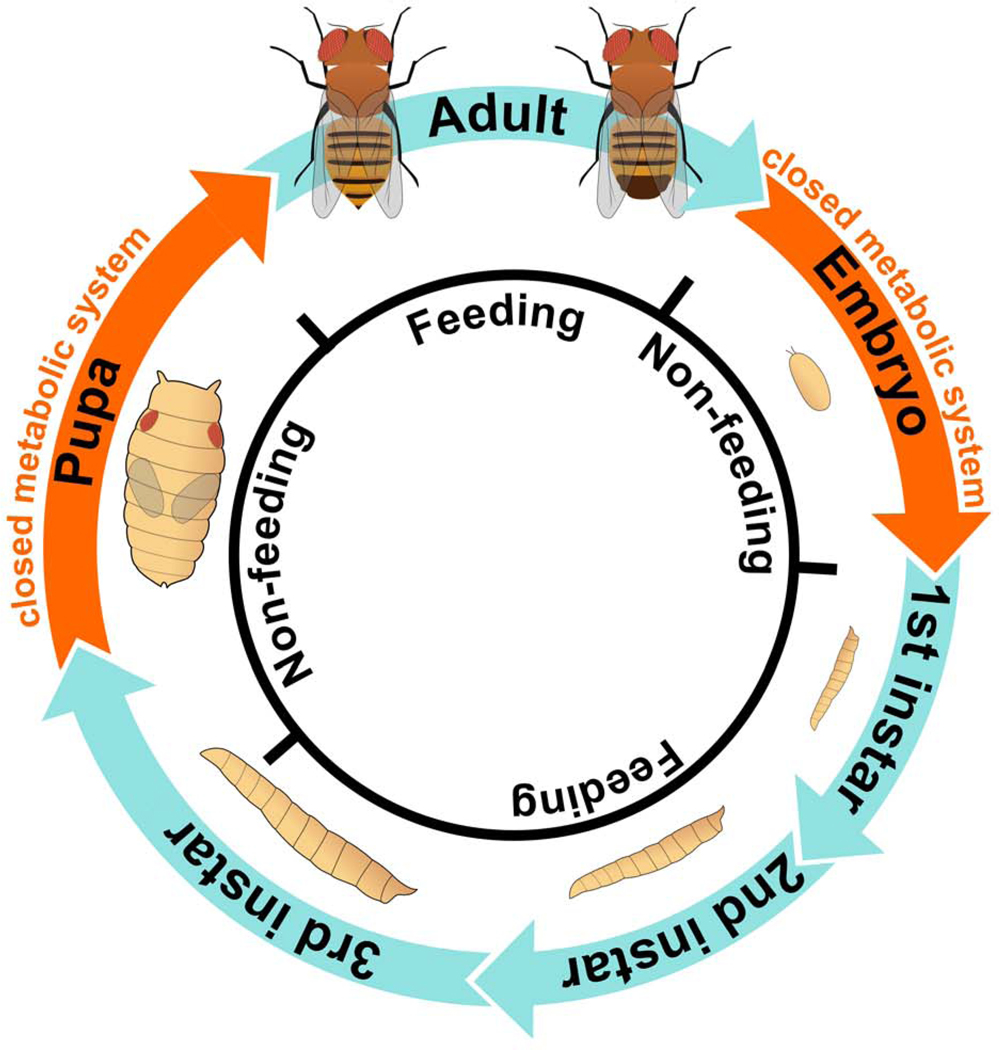
The four stages of the Drosophila life cycle – embryo, larva, pupa, and adult – have distinctly different energetic demands. Embryonic development, which lasts roughly 24 hours under optimal conditions, consists of rapid nuclear divisions followed by cellularization and dynamic cellular rearrangements. These biological processes are energetically expensive, and because the embryo is a closed system, they depend entirely upon inherited maternal resources. The larval stages that follow hatching are divided into 3 sub-stages, called instars (L1-L3), lasting a total of 4 days at 25 degrees. The larval stage is devoted to few major goals: attaining a minimal body mass that will ensure survival and reproduction during the adult stage, storing excess energy for use when morphogenesis begins and during the early days of adulthood, and serving as an incubator for the growing adult imaginal tissues (tissues that will later undergo metamorphosis to create adult structures). Metamorphosis occurs during the pupal stage, and during these 5 days, pupa solely rely on internal resources the meet the energetic requirements of extensive cell and tissue rearrangements. After these 10 days of development, flies eclose as mobile adults, however, metabolism in adults is also not static, but must adapt to specific life-stage demands and well as the sex-specific demands of reproduction. For example, young adult flies are especially dependent on a high-quality diet since developing fat-storage capacity requires another 5 days. These brief descriptions of the Drosophila life cycle illustrate how each stage of fly development has very specific metabolic demands, and progression throughout the different stages requires balanced metabolic shifts to suit changing needs (Figure 1). The sudden nature of these metabolic shifts means that a Drosophila researcher can study the mechanisms that redirect metabolic flux with a precision that is difficult or impossible to achieve in a mammalian system. Moreover, since both the embryo and pupa are closed metabolic systems while the larva and adult consume environmental nutrients, the fly life cycle provides a genetic system for studying very different metabolic questions, ranging from how organisms partition limited metabolite pools (embryos, pupa) to how growth and physiology adapt to sudden bouts of starvation (larva, adult).
Figure 1. Energetics during developmental transitions in the life cycle of Drosophila melanogaster.
Illustrations are not drawn to scale.
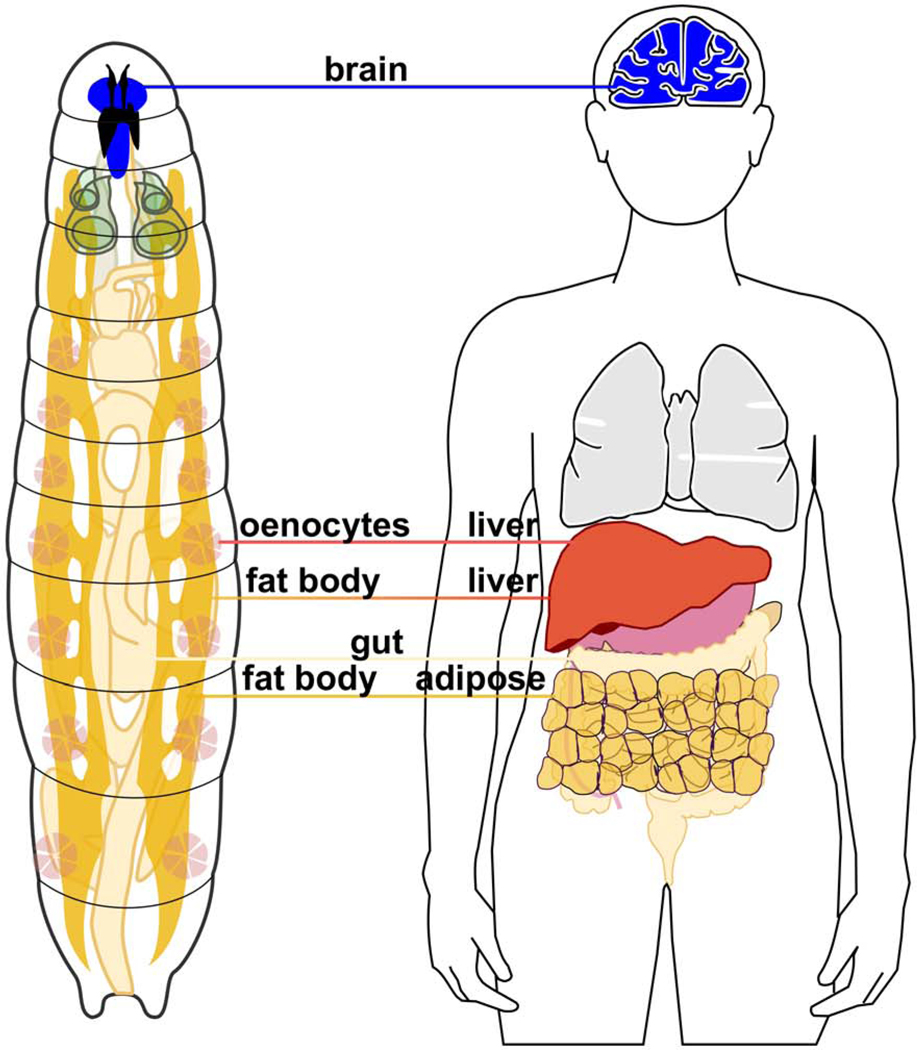
Beyond the ease with which metabolic transitions can be studied in Drosophila, the fly is also an appealing model for studying metabolism because the flypartitions metabolic processes in organs with direct functional homology to humans (Figure 2). This is true of both larvae and adults, which despite having very different body plans, rely on similar tissues to systemically regulate metabolism. The brain regulates complex feeding and locomotor behaviors. The heart is a contractile dorsal vessel that circulates nutrients and hormones via the hemolymph through an open circulatory system. The long contiguous gut is segmented into specialized compartments which function similarly to the multi-organ mammalian gastrointestinal tract. Lipid synthesis is regulated, in part, by hepatocyte-like cells known as oenocytes (Gutierrez et al., 2007), while fat and glycogen are primarily stored in the fat body, a specialized tissue that is functionally equivalent to mammalian white adipose tissue and the liver (Wigglesworth, 1949). Overall, the manner by which flies and mammals compartmentalize nutrient absorption, energy storage, and metabolic flux are strikingly similar and reveal how the overall strategies for regulating metabolism at a whole animal level arose early in animal evolution.
Figure 2. Anatomic comparisons between the Drosophila larva and the adult human.
Organs/tissues with similar roles in regulation of organismal metabolism are highlighted and connected by color-coded lines. Illustrations are not drawn to scale.
The parallels between fly and mammalian developmental metabolism also extend beyond the function of individual tissues. Metabolism must be coordinated across all cells within the animal, and regardless of developmental stage, the fly relies on a complex network of interorgan signals to coordinate growth and homeostasis with energy production and biosynthesis. Many of the peripheral tissues, including the muscle, fat body, oenocytes, brain, and imaginal tissues all release and respond to these signals. Moreover, specialized endocrine cells monitor and respond to signals from the peripheral tissues by secreting hormones into the open circulatory system, which in turn coordinately regulates physiology at a systemic level. While a complete description of these signals is beyond the scope of this manuscript, and we would guide our readers to more comprehensive overviews (Ahmad et al., 2020; Droujinine and Perrimon, 2016; Malita and Rewitz, 2020; Owusu-Ansah and Perrimon, 2015; Texada et al., 2020), here we highlight a few major cells and organs responsible for these systemic signals. First, several cell types synthesize and secrete Drosophila insulin-like peptides (dIlps) that control tissue growth and metabolism throughout the animal by activating a highly conserved insulin/insulin-like growth factor signaling (IIS) (Garofalo, 2002; Texada et al., 2020). The fly genome encodes eight dILP peptides, seven of which activate the Insulin Receptor (InR) (Nassel et al., 2013). The primary source of dILPs within the animal are the the Insulin Producing Cells (IPCs), which are located within the nodes of the brain as clusters of seven median neurosecretory cells (mNSC) (Nassel et al., 2013; Nassel and Vanden Broeck, 2016). However, some somatic tissues, such as the fat body and muscle (O’Brien et al., 2011; Okamoto et al., 2009; Slaidina et al., 2009; Suzawa et al., 2019), also produce and secrete dILPs for the purpose of regulating cell proliferation and developmental growth, thus demonstrating the complexity of endocrine signaling within the animal.
The second major source of systemic hormones is the ring gland, which in larvae, consists of the corpora cardiaca, corpora allata, and prothoracic gland arranged in a ring-like structure surround the aorta (King et al., 1966). The entire structure is located between the two larval brain lobes and connected to the brain by specific neurons, thus these tissues can respond to signals from both the circulatory and nervous system by secreting hormones that are readily distributed to the peripheral tissues. These hormones serve well-studied roles in controlling both metabolism and developmental progression and serve as functional homologs of mammalian hormones (Malita and Rewitz, 2020; Texada et al., 2020). For example, the corpora cardiaca secretes a glucagon-like hormone known as Adipokinetic hormone that serves an essential role in the starvation response (Galikova et al., 2015; Lee and Park, 2004). For the purpose of our review, however, we will largely focus on the prothoracic gland (PG). This endocrine tissue coordinately regulates gene expression across tissue throughout the organism by producing and secreting the steroid hormone ecdysone (Pan et al., 2020; Yamanaka et al., 2013). When this steroid is enzymatically converted to the active form, 20-hydroxyecdysone (20E), it regulates gene expression in peripheral tissues by controlling activity to a nuclear receptor heterodimeric complex composed of the Ecdysone Receptor (EcR) and the RXR homolog Ultraspirace (Usp) (King-Jones and Thummel, 2005). While 20E is well known as a key regulator of developmental transitions, recent finding demonstrate that this hormone is also a key regulator of metabolism.
The many similarities between developmental metabolism in Drosophila and mammals establishes fly development as a premier system to understand how nutrients and the systemic regulation of metabolism influence animal growth and development. Here we review recent studies of Drosophila development, highlighting important metabolic needs, nutrient-sensing mechanisms and how these are integrated to regulate growth, development, and other systemic functions such as lifespan and reproduction. Since fly metabolism is uniquely adapted to support specific developmental objectives that are often specific to a life-stage, below we divide our review to separately examine the metabolism of embryogenesis, larval growth, metamorphosis, and adulthood.
Embryonic Development – Regulating metabolic flux in a closed system
Embryos rely on maternally supplied nutrients for gastrulation and development. As a result, egg production and embryogenesis are exquisitely sensitive to the quality of maternal diet as well as genetic mutations that disrupt maternal deposition of proteins, carbohydrates, lipids, and other macromolecules that are required for energy production and biosynthesis. Since the mechanisms that control metabolite loading into Drosophila eggs are complex and have been the subject of recent reviews (Drummond-Barbosa, 2019; Sieber and Spradling, 2017), including a companion paper to this manuscript, we will instead focus on the energetic and biosynthetic demands of embryogenesis and the mechanisms that induce developmentally-regulated changes in metabolic flux.
During the course of development, maternally deposited molecules are consumed by intermediary metabolism for both energy production and biosynthesis. For example, the large pools of glycogen and triglyceride present within the early embryo are broken down in a regulated manner (An et al., 2014; Tennessen et al., 2014). Maternally deposited small molecules such as amino acids undergo predictable changes during the course of embryogenesis, reflecting how the metabolism of this closed system adapts to meet the biosynthetic and energetic demands of this developmental stage (An et al., 2014; Crone-Gloor, 1959; Tennessen et al., 2014). Finally, the embryo stores metabolic waste products in a manner that protects developing tissues, as evident by the accumulation of uric acid crystals within the developing Malpighian tubules (Skaer, 1993).
The predictable nature of Drosophila embryonic metabolism establishes this system as an ideal model to quantitatively study how energy production and biosynthesis drive developmental events. Since the embryo is a closed system, classic metabolic approaches such as calorimetry and respirometry can be used to precisely measure energetics in the context of this developmental stage. These techniques, together with quantitative measurements of macromolecular pools over time, have been used to build mathematical models that describe the thermodynamics of embryogenesis (Song and Shvartsman, 2020). The power of this approach was recently demonstrated by determining that Drosophila embryogenesis utilizes ~10 mJ of energy, most of which is generated by the oxidation of triglycerides and glycogen (Song et al., 2019). The production of this much energy raises an interesting question, as the major biosynthetic reactions that occur during embryogenesis require only ~10% of this amount (Song et al., 2019). What cellular processes use the remaining 90% of ATP generated remains an open and interesting question. One intriguing possibility is that this excess pool of ATP controls non-metabolic processes such as the regulation of gene expression by chromatin modification. Alternatively, such a seemingly high level of ATP production might ensure developmental success under a wide range of environmental conditions. Whatever the answer, the fly embryo provides a powerful system for understanding how energy production is budgeted in animal development.
The combination of quantitative metabolic measurements and mathematical modeling of embryonic metabolism provides an exciting opportunity to identify those metabolite pools that restrict the rate of developmental progression. For example, the rapid nuclear divisions that occur prior to the maternal-to-zygotic transition use large quantities of dNTPs in a very short time. The maternally-loaded dNTP pool, however, is only sufficient for 10–11 synchronous divisions, or 30–50% of the total nucleotides required to synthesize the genomic DNA in 6,000 nuclei (Song et al., 2017). As a result, the embyro must synthesize dNTPs “on the go,” which slows the nuclear divisions. The resulting delay is key for normal development, as embryos with excess dNTPs experience shortened interphase during nuclear cycles 12 and 13 (Djabrayan et al., 2019). The altered timing of these nuclear cycles results in decreased zygotic transcription and is lethal for the embryo (Djabrayan et al., 2019), demonstrating how metabolite pools can directly regulate key developmental events.
While the embryo provides an ideal tool for precisely studying developmental metabolism, this closed system also represents an opportunity to study metabolic plasticity. Variations in both maternal diet and temperature can induce dramatic changes in metabolism and embryonic development must adapt to these kinds of environmental stress. Moreover, embryogenesis, similar to other stages in the fly life cycle, can tolerate mutations that severely disrupt central carbon metabolism, including those that eliminate mitochondrial pyruvate transport (Bricker et al., 2012), fatty acid beta-oxidation (Strub et al., 2008), and the oxidative branch of the pentose phosphate pathway (Hughes and Lucchesi, 1977). In fact, embryonic development can proceed even in the absence of glycogen synthase and glycogen phosphorylase (Yamada et al., 2019). The fact that the embryo can adapt to loss of a major energy source highlights the remarkable ability of this system to adapt to metabolic stress.
Embryonic development also provides an ideal system for using a combination of gene expression analysis and metabolomics to probe the metabolic changes that occur as development progresses (An et al., 2014; Tennessen et al., 2014). The use of such systems biology approaches revealed that the embryo exhibits dramatic overall metabolic shifts during the course of development. Most notable among these changes is an up-regulation of genes involved in glycolysis that occurs midway through embryogenesis (Tennessen et al., 2014). At this time, the Drosophila Estrogen-Related Receptor (ERR) coordinately induces expression of genes that encode enzymes involved in glycolysis, the pentose phosphate pathway, as well as Lactate Dehydrogenase (Ldh) (Tennessen et al., 2011). The resulting metabolic program exhibits the hallmark features of a metabolic program that is ideally suited for biosynthesis and is commonly known as aerobic glycolysis or the Warburg effect. While the purpose of this transcriptional program in the embryo remains to be determined, the coordinated upregulation of these enzymes establishes a metabolic state that is essential in the next stage of the life cycle: larval development.
Larval development – rewiring metabolism for growth and energy storage
Larvae must store enough nutrients to sustain pupation and metamorphosis, whilst also increasing their body mass over 200-fold (Bakker, 1959). In order to support this dramatic growth, larval metabolism converts dietary nutrients into lipids, nucleotides, and amino acids required to support cell growth and proliferation. Moreover, a significant amount of the nutrients that enter the system are diverted to the growing pools of glycogen and triglycerides, which are used in the subsequent developmental stages (for excellent reviews on carbohydrate and lipid metabolism in Drosophila, see Heier and Kuhnlein, 2018; Mattila and Hietakangas, 2017). In order to support this level of growth, larval metabolism must be biased toward biosynthesis.
The extent to which larval metabolism is rewired to promote growth remains understudied, and only recently computational models have begun to explore the metabolic basis of this growth phase (Schonborn et al., 2019). Nonetheless a few themes have emerged. First, larval metabolism exhibits high levels of glycolytic gene expression when compared with either embryos or pupae (Tennessen et al., 2011; White et al., 1999). Moreover, Ldh activity is elevated more than 10-fold in larvae when compared with all other points in the fly life cycle (Rechsteiner, 1970). The elevated level of Ldh activity observed during this time not only produces lactate but also generates exceptionally high levels of the oncometabolite L-2-hydroxyglutarate – a pro-growth molecule that potentially links larval metabolism with gene expression (Li et al., 2017). These observations suggest that larvae are well suited for both using carbohydrates to generate energy and biomass. Consistent with this hypothesis, decreased glycolytic metabolism disrupts muscle growth and differentiation (Bawa et al., 2020; Tixier et al., 2013). Similarly, the enzymes glycogen synthase (GlyS) and glycogen phosphorylase (GlyP), which encode the enzymes respectively required for glycogen production and breakdown, are essential for larval growth (Yamada et al., 2019). The other surprising trend to emerge from studies of larval metabolism is the observation that development can proceed in the absence of a complete tricarboxylic acid (TCA) cycle, as evident by the observation that Isocitrate Dehydrogenase 3b and Malate Dehydrogenase 2 mutants survive larval development and die at the onset of metamorphosis (Duncan et al., 2017; Wang et al., 2010). However, reduced oxidative phosphorylation, either due to hypoxia or mutations that decrease electron transport chain activity (Meiklejohn et al., 2013; Zhou et al., 2008), severely curtails growth. The discrepancy between phenotypes stemming from disruption of the TCA cycle and the electron transport chain reinforces the notion that larval metabolism is unique when compared with other life stages.
The second important trend to emerge from studies of larval development is the surprising level of metabolic plasticity present within the system. Larvae continue to develop despite nutrient deprivation (Tennessen and Thummel, 2011; Texada et al., 2020), large temperature shifts (Powsner, 1935), and even short-term exposure to anoxia (Callier et al., 2015). Larvae adapt to such extreme environmental stress by tapping into the stored pools of macromolecules that are synthesized during the larval stage. For example, larval starvation results in the breakdown of glycogen from the fat body and muscles (Matsuda et al., 2015). A major function of the glucose derived from these glycogen stores is the production of the disaccharide trehalose, which is consumed by larval tissues to maintain homeostasis in the absence of dietary nutrients. The requirement for mobilizing stored carbohydrates under starvation conditions is evident by the phenotypes associated with loss-of-function mutations in the enzymes required for the storage and utilization of glycogen as well as the production of trehalose. Although mutations in the gene trehalose-6-phosphate synthase (Tps1) render larvae unable to synthesize trehalose, the primary circulating sugar in larvae, Tps1 mutants successfully complete larval development (Matsuda et al., 2015). However, upon starvation Tps1 mutant larvae die rapidly, due to an inability to distribute carbohydrates to nutrient-deprived tissues (Matsuda et al., 2015).
While stored carbohydrates represent the first line of defense against starvation, when exposed to nutrient deprivation larvae will also utilize triglycerides, the other major pool of macromolecules (for an excellent review on this topic, see Heier and Kuhnlein, 2018). Since the major site of triglyceride storage is the fat body, starved animals exhibit a depletion of lipid droplets within this organ. By activating expression of genes required to break down both triglycerides and oxidize fatty acids, energy present within larval fat can sustain larvae for extended periods of starvation.
An illustrative example of how metabolic plasticity allows larvae to adapt to changing circumstances comes from detailed analysis of variations in dietary nutrition on metabolism in mutant animals. Specifically, larvae in which the RNA-binding protein Split ends (Spen) is depleted from the fat body are developmentally delayed and accumulate fat due to defects in liberating energy from storage forms (Hazegh et al., 2017). These animals survive by shifting to a generally catabolic mode of metabolism, including breakdown of protein (Hazegh et al., 2017). Accordingly, supplementing the diet with extra sources of protein partially reversed the increased adiposity and accelerated development (Gillette et al., 2020). While future work is required to understand at the molecular level how Spen regulates energy storage, these findings demonstrate how altered metabolic “set points” can have distinct consequences depending on environmental conditions. It is thus likely that in wild-type animals the same regulatory pathways help link metabolism to extrinsic factors like dietary content.
Since environmental factors can induce dramatic fluctuations in both the larval energy pools as well as growth rate, larvae utilize a series of developmental checkpoints that ensure that the animal has sufficient body mass and energy storage for adult survival and reproduction. The “minimum viable weight” refers to the mass at which the larvae are capable of surviving metamorphosis (Bakker, 1959). A period of growth follows this first checkpoint, and environmental factors such as poor nutrient can prolong the continuing growth phase. The next checkpoint is called “critical weight”, the size at which larvae commit to metamorphosis and further development proceeds independent of nutritional cues (Beadle et al., 1938; Church and Robertson, 1966; Nijhout, 2003; Robertson, 1963).
In Drosophila, the steroid hormone ecdysone is a master developmental regulator that controls progression from one life stage to the next (Pan et al., 2020; Texada et al., 2020). The key site of ecdysone biosynthesis is the prothoracic gland, a major endocrine organ located adjacent to the brain that consists of the corpora allata and corpora cardiaca. Ecdysone is generated from modified cholesterol in a process that shares conserved elements with synthesis of vertebrate steroid hormones. Once released into the hemolymph, ecdysone is converted into the active form 20-hydroxyecdysone (20E) which can then bind to Ecdysone Receptors in peripheral tissues (Gilbert et al., 2002). During larval development, pulses of 20E control molting and developmental progression between each of the three larval instars as well as the complex gene regulatory cascade that terminates larval growth and initiates the onset of metamorphosis (Riddiford et al., 2000; Riddiford and Truman, 2015).
These hormone pulses are regulated by a combination of nutritional status and hormone signaling (for more comprehensive reviews, see Malita and Rewitz, 2020; Pan et al., 2020; Texada et al., 2020). The most well described regulator of ecdysone release is a secreted peptide known as prothoracicotropic hormone (PTTH). In Drosophila PTTH-producing neurons directly innervate the prothoracic gland. While PTTH is thought to be the primary regulator of ecdysone, animals with ablated PTTH neurons managed to fully develop, albeit more slowly and becoming larger adults (McBrayer et al., 2007). Hence while PTTH is necessary for proper developmental timing, additional factors including nutritional signaling regulate ecdysone pulses. Upon reaching critical weight, larvae have a finite amount of time before pupation begins, regardless of ongoing nutritional status. Both fed and starved larvae undergo metamorphosis, with fed conditions yielding larger adults and starved yielding smaller, but still fertile, adults.
The ability of flies to complete development in the absence of nutrients highlights the manner by which larvae use the available energy to support the growth of specific organs at the expense of others. This phenomenon is often referred to as “organ sparing” and provides a unique opportunity to understand how growth factor signaling becomes uncoupled from the nutrient-dependent checkpoints (Lanet and Maurange, 2014). The classic example of an organ that is subject to the “organ sparing” phenomenon is the central nervous system (CNS), where specialized growth promoting mechanisms are used to ensure proper development regardless of nutrient status. One of the key molecular differences between the CNS and other larval issues is the use of anaplastic lymphoma kinase (Alk) signaling to regulate growth. This pathway suppresses the requirement for the amino-acid-sensitive Slimfast/Rheb/TOR complex 1 to promote growth and activates PI3-kinase signaling in place of IIS (Cheng et al., 2011). Thus, constitutive production of an Alk-activating ligand by cells comprising the niche that surrounds neuroblasts ensures proliferation within the brain even under starvation conditions that result in the cell cycle arrest of stem cells in other organs (Cheng et al., 2011). A similar mechanism exists in the visual system, which develops later than other parts of the nervous system and takes place in two stages, with each stage exhibiting different sensitivities to nutrient deprivation. In the first stage, the proliferation of neural stem cells is sensitive to diet in an insulin/PI3K/TOR-dependent manner, and a paucity of nutrients limits the number of symmetric cell divisions and thus the number of precursor cells (Lanet et al., 2013). Later, asymmetric neurogenic divisions are driven by ecdysone and occur independently of nutrients, ensuring a diversity of neuronal types in the adult visual system regardless of energy availability during development (Lanet et al., 2013).
Other than the CNS, the male genitalia also grow and develop in a manner that is nutrient insensitive. This trait is essential because changes in genitalia size would render the animal incapable of mating and is thus under evolutionary pressure to grow to a specific size, regardless of nutrient status. Intriguingly, the mechanism that ensures growth regardless of dietary stress differs between the CNS and genitalia, but still centers on IIS signaling. Unlike the CNS, where organ sparing relies on regulation of PI3-kinase signaling, the organ sparing within the genitalia results from decreased FoxO expression. FoxO is a transcription factor that promotes expression of genes involved in energy storage and cell cycle arrest (Tang et al., 2011), and FoxO activity is regulated by nutrients and the IIS pathway (Puig et al., 2003). FoxO levels are low in the genital disc, uncoupling adult genital size from nutrient availability during development (Tang et al., 2011). Overall, these studies illustrate how distinct signaling mechanisms in different tissues and organs promote the development of functional adults despite suboptimal nutrition in the larval stages.
Intrinsic and extrinsic growth-determining mechanisms in imaginal tissues.
The tissues that give rise to the adult organs such as the eye, wing, and legs all exist within the larvae as diploid imaginal tissues (in contrast to many larval-specific tissues, which are polypoid), and grow within the larval body prior to metamorphosis. For example, the wing imaginal disc begins as a cluster of 30–50 cells at the end of embryogenesis, and ends 1,000x larger (30,000–50,000 cells) upon pupation (Martin et al., 2009; Worley et al., 2013). The wing imaginal disc is preprogramed to reach a terminal size as evident by the fact that transplanting immature wing imaginal discs into the abdomen of adult females yielded properly sized discs (Bryant and Levinson, 1985). Curiously, starved larvae end up as smaller adults with wings of proportional size, thus demonstrating that nutrient-regulated growth factors coordinate wing development with diet-induced changes in body mass.
The control of adult organ size relative to body size is complex and has been subject to several recent reviews (Boulan and Leopold, 2021; Texada et al., 2020). Central to this regulation, however, is integration of 20E signaling with IIS and dTOR signaling pathways. IIS and dTOR pathways inactivate Thor (eukaryotic translation initiation factor 4E binding protein; 4E-BP), which inhibits translation. Thus, regulation of Thor represents a direct mechanism by which endocrine signaling controls biosynthesis. The importance of Thor serves an essential role in regulation of body size is further highlighted by the fact that Thor expression is high in early L3, inhibiting growth, but decreases toward late L3. This trend suggests that ecdysone promotes disc growth in mid to late L3 larvae through downstream components of the IIS/TOR signaling cascade (Herboso et al., 2015).
In conjunction with ecdysone, Drosophila also produces Juvenile Hormone (JH), which serves as a “status-quo” signal (Nijhout and Williams, 1974; Riddiford, 1994). JH is expressed continuously throughout larval development, and the presence of JH drives larva-to-larva molts. In the late L3 stage, JH titer drops, allowing the metamorphic molt (Riddiford et al., 2003). JH has emerged as an intriguing regulator of growth and metabolism in the fly. While this hormone is extensively studied in other insect orders (e.g., Lepidoptera), its function in Drosophila is only beginning to be understood (Riddiford et al., 2010). In fact, the Drosophila JH receptors were only recently identified (Jindra et al., 2015) – a discovery that will inevitably lead to significant advances this area or research. One clue to how this hormone might function in terms of development and metabolism comes from studies of the Drosophila insulin-like peptides (dILPs), which reveal that the JH-secreting corpora allata cells express the Insulin-like Receptor (InR) and are postulated to be responsive to dILP levels. Indeed, depleting InR causes a decrease in JH (Tu et al., 2005), suggesting that release of this hormone is sensitive to nutritional status of the animal.
Insulin, the fat body, and humoral control of growth
One of the key regulators of larval metabolism and growth are the Drosophila insulin-like peptides (dILP), which perform the growth-promoting functions of mammalian insulin-like growth factor (IGF) as well as the metabolic functions of insulins (Garofalo, 2002; Texada et al., 2020). As mentioned in the introduction, dILPs transduce signals through InR, which activates a conserved kinase cascade. IPCs produce four of eight dILPS (dILP1, 2, 3, and 5), the most abundant of which is dILP2 (Buch et al., 2008; Ikeya et al., 2002; Rulifson et al., 2002). Ablating or disrupting the function of the IPCs leads to systemic larval growth defects, supporting their role as regulators of tissue growth (Ikeya et al., 2002; Rulifson et al., 2002). Curiously, larval IPCs cannot sense circulating sugars, therefore dILP secretion is regulated by a number of external signaling factors that are produced by the peripheral tissues.
One of the key tissues that regulates dILP secretion from the IPCs is the fat body, which monitors hemolymph metabolite levels and secretes factors that remotely act on the IPCs, an ability that is illustrated by a classic genetic study of the gene slimfast (slif), which encodes an amino acid transporter. When slif levels are reduced in the fat body, amino acids are unable to enter the tissue, and larval growth is delayed in a manner that mimics a starvation state (Colombani et al., 2003). This unexpected result suggested that decreased amino acid levels within the fat body serve as a key regulator of larval development and raised the question as to how the fat body controlled systemic growth. One clue towards the underlying mechanism came from studies of dTor signaling within the fat body, which responds to changes in amino acid levels. The growth defects associated with loss of slif activity are partially rescued by overexpression of dS6k, a downstream target of the Drosophila dTOR signaling cascades, suggesting that disruption of amino acid import into the fat body leads to decreased dTor signaling. Consistent with this possibility, fat body-specific disruption of dTOR signaling, but not PI3K signaling, induced a systemic growth defect. These elegant observations presented a model in which decreased amino acid levels within the fat body lead to cell autonomous suppression of dTor signaling and systemic inhibition of growth (Colombani et al., 2003).
Studies of slif demonstrated that metabolites could indirectly influence growth and raised the question as to how the fat body influences larval development. A clue to this question came from a series of elegant ex vivo experiments, which demonstrated that larval fat bodies from fed, but not starved, animals could induce dILP secretion when cocultured with larval brains, suggesting that the fat body secretes a pro-growth factor in the fed state (Britton and Edgar, 1998; Davis and Shearn, 1977; Geminard et al., 2009). Moreover, the signal derived from the fat bodies of fed larvae is dependent on dTor signaling (Geminard et al., 2009), a result that was consistent with the earlier slif studies. Overall, these studies raised the question of what is the nutrient-dependent fat body signal that controls dILP secretion in larvae? The answer to this question was, in part, Unpaired 2 (Upd2), a Drosophila cytokine that has functional similarities to human Leptin (Rajan et al., 2017; Rajan and Perrimon, 2012). Fat body-secreted Upd2 circulates within the hemolymph and activates GABAergic neurons that locally inhibit IPC secretory function. Upd2 activates the GABAergic neuron Janus kinase (JAK)/Signal Transducer and Activator of Transcription (STAT) signaling, which represses the inhibitory effect of these neurons on IPCs, finally allowing dILP secretion. Upd2 mRNA expression increases on high-fat and high-sugar diets, suggesting it senses these two macronutrients, and based on their abundance, relays “fed” signals to the brain. Recent work in conditions of surplus energy stores identified changes in the structure of dILP-producing neurons due to altered activity of actin-based synapse reorganization complexes, ultimately affecting dILP secretion (Brent and Rajan, 2020). Upd2 secreted from the fat body was proposed to represent the key signal of energy surplus (Rajan et al., 2017; Rajan and Perrimon, 2012), though future work will be required to ask if IPCs directly respond to Upd2 of fat body origin. Another leptin analog, Upd1, is produced in the brain and controls satiety (Beshel et al., 2017). Upd1 presumably operates in neuronal circuits distinct from those involving Upd2.
Since the initial discovery of Upd2, a number of other secreted factors have been identified that control dILP secretion, including Growth-blocking peptide 1 and 2, and CCHa2 (Koyama and Mirth, 2016; Sano et al., 2015). One particularly interesting factor is stunted – a secreted peptide produced by the fat body with homology to the ε subunit of the mitochondrial F1F0-ATP synthase (Delanoue et al., 2016). Like the factors described above, secretion of stunted from the fat body regulates dILP secretion from the IPCs. However, the fact that stunted encodes a central component of energy production raises the question of whether other metabolic enzymes and associated proteins might serve similar and unappreciated roles.
Beyond the secreted cytokines that regulate dILP secretion from the IPCs, a number of other conserved molecules are secreted from the fat body and other peripheral tissues to control systemic growth. For example, in mammals, IGF-1 interacts with six IGF-binding molecules (IGFBPs) to form stable multimeric complexes that regulate availability and activity of IGF-1 (Duan and Xu 2005). Two putative IGFBPs have also been identified in the fly - Drosophila acid labial subunit (dALS) and Imaginal morphogenesis protein-late 2 (Imp-L2) (Colombani et al., 2003; Garbe et al., 1993). Upon amino acid restriction dALS expression decreases in the fat body. An in vitro study showed in an immunoprecipitation experiment that dALS and Imp-L2 associate. While dALS does not bind dILP2 alone, Imp-L2 appears to serve as a bridge allowing pulldown of the trimeric complex (Arquier et al., 2008). dALS binds to dILP2 in this trimeric complex and stabilizes dILP2, which can be beneficial to sustain growth in poor nutritional conditions. Conversely, formation of this complex is antagonistic to dILP growth-promoting function and overexpression of dALS can stunt growth (Arquier et al., 2008). These two molecules serve as examples of how metabolites present within individual peripheral tissues can influence secretion of molecules that fine tune growth factor stability and availability with the circulatory system.
Proper nutrient sensing is necessary to activate corresponding nutrient mobilization pathways and to regulate feeding behaviors. The fat body continues to serve as a key tissue in this regard. A primary intracellular carbohydrate sensor in both mammalian and Drosophila cells is the Mondo/Mlx complex (Havula and Hietakangas, 2012). This complex forms a transcription factor that regulates the expression of genes involved in carbohydrate metabolism that contain a Carbohydrate Response Element (ChoRE). Mlx loss-of-function mutants experience elevated circulating glucose and late larval lethality. Restoring Mlx specifically in the fat body was able to normalize circulating glucose levels in larvae, as well as rescue survival (Havula et al., 2013). This finding further demonstrates how the fat body ensures proper larval development by monitoring metabolite levels within the growing animal.
Beyond the peptide hormones and interacting molecules that regulate insulin secretion, 20E also serves to regulate IIS at a systemic level. The relationship between IIS and 20E is perhaps best illustrated by interplay in the fat body, where these two signaling pathways serve opposite roles in regulating systemic growth. While decreased insulin signaling within this tissue results in decreased growth, reduced 20E signaling within the fat body results in increased growth and final body size, suggesting that the fat body normally functions as a major relay for the growth-inhibitory effects of ecdysone signaling. In this regard, 20E signaling functions to antagonize IIS signaling, resulting in relocalization of dFOXO to the nucleus (Colombani et al., 2005).
The diversity of nutrient sensors and integrated humoral control of growth via the fat body provides insights into the systematic and coordinated growth of larvae. Unsurprisingly, most of these nutrient sensing mechanisms rely on IIS and dTOR signaling to transduce or inhibit pro-growth factors. Consistent with this trend, these factors continue to regulate growth even in wandering L3 larvae, when the animal has migrated away from the food to find a site to pupariate. During this time, production of the IGF-like peptide, dILP6 is induced by 20E and continues to promote growth of the imaginal discs (Okamoto et al., 2009; Slaidina et al., 2009). Interestingly, dILP6 can also promote limited tissue growth during times of low nutrient availability during early developmental stages as well. The end of larval development in holometabolous insects like Drosophila marks the end of growth and dictates the final body size of the adult. This final burst of growth is insensitive to nutrients.
Metamorphosis – building an adult body using larval metabolites
One of the most dramatic metabolic transitions that occurs in all of animal development is the metamorphosis of holometabolous insects, where larval-specific tissues are destroyed and the adult form arises from within the pupal case. The ease with which animals can be synchronized at the larval-to-prepupal and prepupal-to-pupal transitions allowed for precise dissection of how the 20E initiates a series of transcriptional cascades that control this dramatic developmental event. The same precision that allows for studies of gene expression also provides an opportunity to understand how metabolism changes during the onset of metamorphosis, when the animal transitions from a feeding and growth state to closed system.
The metabolic changes that mark the end of larval development begin during the wandering L3 stage, when the genes that encode glycolytic enzymes are coordinately down-regulated (White et al., 1999). Consistent with this decreased emphasis on carbohydrate metabolism, L-2-hydroxyglutarate, a non-canonical product of Ldh activity, also exhibits a dramatic decline in concentration during this period (Li et al., 2017). Since these changes occur before the histolysis of larval tissue, this transition must be pre-programmed and can’t simply be attributed to the death of larval tissues. In nearly all larval tissues, the significance of this metabolic change as well as the mechanisms that trigger these changes in carbohydrate metabolism, however, remain poorly understood.
As the animal exits larval development and initiates metamorphosis, progression through both the puparium and pupal stages is associated with dramatic changes in metabolite abundance (An et al., 2017; Nishimura, 2020). These observations indicate that changes in metabolic flux significantly contribute to death and remodeling of larval tissues and the growth of adult structures. Consistent with this possibility, several studies have demonstrated that ecdysteroid pulses during both pupariation and pupation induce metabolic changes that are required for normal development. In this regard, the disaccharide trehalose serves a unique role in pupariation (Nishimura, 2020). Not only do trehalose levels exhibit a significant decrease during the 24 hours following pupariation, but ecdysteroid pulse that precedes the prepupal-to-pupal transition also transiently activates expression of the genes that encode the trehalose transporter (Tret1) and the enzyme trehalase (Treh), which cleaves the disaccharide trehalose into two glucose molecules (Nishimura, 2020). This induction of trehalose catabolism is essential for development: loss-of-function mutations in Treh or the gene Tps1, which encodes the enzyme required for trehalose synthesis, leads decrease ecdysteroid signaling during the prepupal to pupal transition and defects in pupal development. In contrast, glycogen metabolism seems to play a minor role during this time. Unlike trehalose, levels of glycogen remain relatively stable during the 24 hours after puparium formation and mutations in either GlyS or GlyP had no influence on the timing of pupation and metamorphosis. Thus trehalose, but not glycogen, serves an essential role in promoting developmental progression at beginning of metamorphosis.
Individual tissues also exhibit unique metabolic needs during both the prepupal and pupal stages. For example, the prepupal ecdysone pulse of activates cell death within the larval salivary glands. While many of the molecular mechanisms that control death in the fly are well studied (Banerjee et al., 2012; Tracy and Baehrecke, 2013), two metabolic enzymes have surprisingly appeared as being essential regulators of cell death in the salivary gland. Mutations in the genes encoding the TCA cycle enzymes Idh3b and Mdh2 inhibit salivary gland cell death (Duncan et al., 2017; Wang et al., 2010), indicating that the destruction of this tissue is tightly associated with changes in mitochondrial metabolism. Similarly, changes in neuroblast growth and differentiation are regulated by the pre-pupal to pupal ecdysone pulse, which is associated with a metabolic rewiring of these cells (for review, see Sood et al., 2020). In a metabolic shift that is reminiscent to what is seen in the salivary gland, neuroblasts during this time activate oxidative phosphorylation in order to exit the cell cycle and terminally differentiate (Homem et al., 2014), once again highlighting a unique requirement for oxidative metabolism in tissues at the onset of metamorphosis.
As highlighted above, recent studies of the early events in metamorphosis have uncovered essential links between metabolic flux and developmental progression. However, the metabolism of this developmental stage remains largely unexplored. In this regard, a nearly century-old question remains as to how metabolism and development are coordinated during the middle and late phase of metamorphosis, when oxidative metabolism appears to be suppressed. Some of the earliest mechanistic studies of metamorphosis revealed that pupal oxidative metabolism exhibits a U-shaped curve (Wolsky, 1938), indicating a down-regulation of mitochondrial metabolism and energy production. These early observations have been confirmed using modern techniques and further expanded upon to demonstrate that the pupa exhibits decreased mitochondrial enzyme activity and also relies on lipid stores for energy generation (Merkey et al., 2011). While the basis of this phenomenon requires further investigation, citrate synthase activity follows a similar U-shaped curve during the course of pupal development, suggesting that oxygen consumption at any given time during metamorphosis reflects the amount of active aerobic tissue present within that animal (Merkey et al., 2011).
Metabolic adaptations in young adults – buffering the new organism against environmental stress
While the metabolic changes that occur at the transition from late metamorphosis to early adulthood remain understudied and poorly understood, a number of studies demonstrate that the lipids stored during the larval stage serve a key role in early adult physiology and survival. Despite the fact that the larval fat body disassociates at the onset of metamorphosis, individual fat body cells persist into the early adult stage, where these larval lipid stores serve several essential function (Aguila et al., 2007). First, larval fat stores serve as an essential energy source in young adults, which are immobile for ~8 hours after eclosion and must rely on internal metabolite stores (Aguila et al., 2007; H.C., 1963). Moreover, since the food source that supported larval growth may have disappeared during pupal development, larval fat supports adult metabolism during the search for new nutrient sources (Aguila et al., 2007). Finally, adults use lipids from the larval fat cells to produce very long chain fatty acids (VLCFAs) and other hydrocarbons to waterproof the cuticle and prevent desiccation (Storelli et al., 2019).
Considering the important of larval fat in young adults, the newly eclosed must closely regulate the release of lipids from these remaining juvenile cells. In this regard, the oenocyte has emerged a key regulator of larval lipid processing, utilization, and storage in the young adult. For example, HNF4 acts within the young adult oenocytes to regulate expression of the enzymes involved in VLCFA synthesis (Storelli et al., 2019). The other major regulator to emerge is the Drosophila homolog of the platelet-derived growth factor (PDGF)/Vascular endothelial growth factor (VEGF) family members (Pvfs). Within the young adult, Pvf produced by the muscle inhibit lipid synthesis in the oenocytes by activating Pi3K/Akt1/TOR signaling (Ghosh et al., 2020). This mechanism acts as a governor on lipid accumulation in young adults, allowing growth of adult adipose tissue to gradually reach the appropriate size.
Balancing reproduction and longevity in adults
As sexually reproductive adults, Drosophila must balance reproductive fitness with longevity. Nutrition and metabolism play a central role in both phenomena and have been intensely studied for well over a decade. In this regard, studies in the fly have uncovered conserved mechanisms that control the interplay between nutrition and lifespan as well as the link between growth factor signaling, metabolite trafficking, and egg production. Both of these topics are beyond the scope of our manuscript and we would point the interested reader to recent comprehensive reviews on these topics (Drummond-Barbosa, 2019; Mirth et al., 2019; Piper and Partridge, 2018). Instead, we will briefly cover key topics in these fields and review a few emerging areas that warrant special mention.
In mated females it is well documented that increased egg laying is deleterious to lifespan (Chippindale et al., 1993). This phenomenon, prioritizing fecundity over longevity, is common across metazoans (Partridge et al., 2005). Many hypothesize that the detriment of sexual reproduction comes from competing utilization of limited resources for the various processes in adulthood, including somatic maintenance, repair, and complex behaviors (Kirkwood et al., 1979; Williams, 1966). Another theory is that reproduction directly harms somatic systems (Barnes and Partridge 2003). Given the differences in male and female reproductive systems, one might predict sexual dimorphisms in pathways controlling energy balance, and indeed recent studies have identified sex-specific differences in metabolism and physiology. For example, the expression of the gene brummer, a key lipase that controls triglyceride homeostasis, is controlled in a sex-specific manner (Wat et al., 2020). Moreover, there are key sex-specific differences in dILP expression and secretion (Rideout et al., 2015), as evident by the fact that changes in dILP express induce sex-specific growth phenotypes (Liao et al., 2020; Millington et al., 2020).
Mated female longevity is greatest when the protein to carbohydrate (P:C) nutritional intake ratio is low (1:16), while maximal egg production is most robust at 1:4 (Lee et al., 2008). An excess of protein beyond the 1:4 ratio is deleterious to both biological imperatives. Allowed to consume ad libitum sources of sugar- or protein-containing media, mated females trend naturally toward to the 1:4 ratio suggesting that they compromise lifespan to ensure reproductive potential. Increased protein consumption increases reproductive rate (Lee, 2015).
The metabolic demands of egg production may drive, in part, this shift in dietary preference in mated females. For example, diets enriched in yeast drive increased egg yolk protein (vitellogenins) production in the fat body, which are then shuttled to the developing egg chamber (Bownes et al., 1983; Carlson and Harshman, 1999). While mechanistically unclear, altering the composition of the diet allows the fat body to further support egg production. The fat body, as mentioned previously, can regulate complex feeding behaviors through endocrine signaling involving Upd2 stimulation of dILP2 production in IPCs, which in turn increases feeding behaviors.
The ability to manipulate the amounts of specific nutrients in diet is key for the study of nutritional effects on development. Changes in the duration of developmental stages can be used to learn about the necessity and sufficiency of dietary components to fuel developmental transitions. Use of an improved defined diet – in which larval development is nearly as rapid as in rich, undefined medium – demonstrated that adding extra protein accelerated development whereas extra carbohydrates slowed development (Reis, 2016). Extra protein also extended adult lifespan (Reis, 2016), suggesting that the developmental acceleration did not come at any obvious cost later in life. Additional insights are likely to be gained from the use of defined diets that can, when nutritionally balanced, support efficient development and full longevity.
One way to extend lifespan, and in some instances healthspan, is to force caloric restriction (CR). These benefits ultimately come with costs, such as decreased fecundity. Perhaps one of the most well studied, and highly contested, lifespan-extending proteins is Sir2. Sir2 is a highly conserved NAD+-dependent histone deacetylase (Imai et al., 2000). Initial studies found that systemic or neuronal overexpression of Sir2 increased lifespan, up to 57% in one of the crosses tested (Rogina and Helfand, 2004). Moderate overexpression of Sir2 in the fat body alone was sufficient to promote life extension, seemingly independent of the effects of CR (Hoffmann et al., 2013). Another study found that Sir2 overexpression had no effect on lifespan (Burnett et al., 2011), while yet another showed that Sir2 increased lifespan in a dose-dependent manner (Whitaker et al., 2013). Moderate Sir2 overexpression in the fat body depletes lipid stores, and higher fat body overexpression arrests larval development, consistent with a role for Sir2 in setting the balance between energy catabolism and metabolism in fat body cells (Reis et al., 2010). Systemically depleting Sir2 negated the lifespan-extending effect of caloric restriction (Rogina and Helfand, 2004).
Initially, it was proposed that the life extending effects of Sir2 worked through the same mechanism as CR, however other reports refute this claim (Banerjee et al., 2012; Hoffmann et al., 2013; Rogina and Helfand, 2004). Emerging evidence suggests that the life-extending effects of Sir2 is not based in calorie restriction, but rather in the ability of Sir2 to maintain IIS signaling during aging (Palu and Thummel, 2016). Hepatocyte Nuclear Factor 4 (dHNF4) is a nuclear receptor that is deacetylated and stabilized by Sir2, and restoration of dHNF4 function in sir2 mutant animals restores IIS signaling response (Palu and Thummel, 2016). Overall, the current literature support incompletely understood effects of Sir2 manipulation on the coupling between nutritional status and systemic regulation of metabolism and aging.
Broader impacts.
Drosophila has been a powerhouse model organism for nearly a century. Many of the nutrient-sensing pathways and their mechanisms of action have been found to operate in other biological contexts. For example, embryonic and larval development utilize metabolic programming employed by numerous cancer types to fuel rapid biomass accumulation. Further characterization of the metabolic programming at this developmental stage may provide new insights into these cancer contexts.
Concluding statements.
Development is a complex process, and frequently individual processes are difficult to isolate. When questions concerning development are extended to external factors, such as nutritional environment, the interplay of cell autonomous responses to systematic humoral regulation grow increasingly complex. The high conservation of metabolic regulation though systems like IIS/dTOR, steroid hormones and receptors, and additional intracellular transcription factors makes Drosophila an attractive model to answer these fundamental, but difficult to parse out, questions. This review focuses on insights into how Drosophila integrate nutrient-sensing mechanisms with whole organismal regulation of growth, as well as aspects of the unique energy flux needs across the different stages of Drosophila development.
Acknowledgements
We would like to thank Luca McMurray and Michael McMurray for graphical design and Michael McMurray for editorial support. J.M.T. is supported by the National Institute of General Medical Sciences of the National Institutes of Health under a R35 Maximizing Investigators’ Research Award (MIRA; 1R35GM119557). T.R. is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK106177).
LITERATURE CITED
- Aguila JR, Suszko J, Gibbs AG, Hoshizaki DK, 2007. The role of larval fat cells in adult Drosophila melanogaster. J Exp Biol 210, 956–963. [DOI] [PubMed] [Google Scholar]
- Ahmad M, He L, Perrimon N, 2020. Regulation of insulin and adipokinetic hormone/glucagon production in flies. Wiley Interdiscip Rev Dev Biol 9, e360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An PN, Yamaguchi M, Bamba T, Fukusaki E, 2014. Metabolome analysis of Drosophila melanogaster during embryogenesis. PLoS One 9, e99519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An PNT, Yamaguchi M, Fukusaki E, 2017. Metabolic profiling of Drosophila melanogaster metamorphosis: a new insight into the central metabolic pathways. Metabolomics 13, 29. [Google Scholar]
- Arquier N, Geminard C, Bourouis M, Jarretou G, Honegger B, Paix A, Leopold P, 2008. Drosophila ALS regulates growth and metabolism through functional interaction with insulin-like peptides. Cell metabolism 7, 333–338. [DOI] [PubMed] [Google Scholar]
- Bakker K, 1959. Feeding period, growth, and pupation in larvae of Drosophila melanogaster. Ent Exp Appl 2, 171–186. [Google Scholar]
- Banerjee KK, Ayyub C, Ali SZ, Mandot V, Prasad NG, Kolthur-Seetharam U, 2012. dSir2 in the adult fat body, but not in muscles, regulates life span in a diet-dependent manner. Cell Rep 2, 1485–1491. [DOI] [PubMed] [Google Scholar]
- Bawa S, Brooks DS, Neville KE, Tipping M, Sagar MA, Kollhoff JA, Chawla G, Geisbrecht BV, Tennessen JM, Eliceiri KW, Geisbrecht ER, 2020. Drosophila TRIM32 cooperates with glycolytic enzymes to promote cell growth. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beadle GW, EL T, Clancy CW, 1938. Food level in relation to rate of development and eye pigmentation in Drosophila melanogaster. Biol. Bull 75, 447–462. [Google Scholar]
- Beshel J, Dubnau J, Zhong Y, 2017. A Leptin Analog Locally Produced in the Brain Acts via a Conserved Neural Circuit to Modulate Obesity-Linked Behaviors in Drosophila. Cell Metab 25, 208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulan L, Leopold P, 2021. What determines organ size during development and regeneration? Development 148. [DOI] [PubMed] [Google Scholar]
- Bownes M, Dempster M, Blair M, 1983. The regulation of yolk protein gene expression in Drosophila melanogaster. Ciba Found Symp 98, 63–79. [DOI] [PubMed] [Google Scholar]
- Brent AE, Rajan A, 2020. Insulin and Leptin/Upd2 Exert Opposing Influences on Synapse Number in Fat-Sensing Neurons. Cell Metab 32, 786–800 e787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricker DK, Taylor EB, Schell JC, Orsak T, Boutron A, Chen YC, Cox JE, Cardon CM, Van Vranken JG, Dephoure N, Redin C, Boudina S, Gygi SP, Brivet M, Thummel CS, Rutter J, 2012. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science 337, 96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton JS, Edgar BA, 1998. Environmental control of the cell cycle in Drosophila: nutrition activates mitotic and endoreplicative cells by distinct mechanisms. Development 125, 2149–2158. [DOI] [PubMed] [Google Scholar]
- Bryant PJ, Levinson P, 1985. Intrinsic growth control in the imaginal primordia of Drosophila, and the autonomous action of a lethal mutation causing overgrowth. Dev Biol 107, 355–363. [DOI] [PubMed] [Google Scholar]
- Buch S, Melcher C, Bauer M, Katzenberger J, Pankratz MJ, 2008. Opposing effects of dietary protein and sugar regulate a transcriptional target of Drosophila insulin-like peptide signaling. Cell Metab 7, 321–332. [DOI] [PubMed] [Google Scholar]
- Burnett C, Valentini S, Cabreiro F, Goss M, Somogyvari M, Piper MD, Hoddinott M, Sutphin GL, Leko V, McElwee JJ, Vazquez-Manrique RP, Orfila AM, Ackerman D, Au C, Vinti G, Riesen M, Howard K, Neri C, Bedalov A, Kaeberlein M, Soti C, Partridge L, Gems D, 2011. Absence of effects of Sir2 overexpression on lifespan in C. elegans and Drosophila. Nature 477, 482–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callier V, Hand SC, Campbell JB, Biddulph T, Harrison JF, 2015. Developmental changes in hypoxic exposure and responses to anoxia in Drosophila melanogaster. J Exp Biol 218, 2927–2934. [DOI] [PubMed] [Google Scholar]
- Carlson KA, Harshman LG, 1999. Extended longevity lines of Drosophila melanogaster: abundance of yolk protein gene mRNA in fat body and ovary. Exp Gerontol 34, 173–184. [DOI] [PubMed] [Google Scholar]
- Cheng LY, Bailey AP, Leevers SJ, Ragan TJ, Driscoll PC, Gould AP, 2011. Anaplastic lymphoma kinase spares organ growth during nutrient restriction in Drosophila. Cell 146, 435–447. [DOI] [PubMed] [Google Scholar]
- Chippindale AK, Leroi AM, Kim SB, Rose MR, 1993. Phenotypic plasticity and selection in Drosophila life-history evolution. I. Nutrition and the cost of reproduction, Methuselah Flies, pp. 122–144. [Google Scholar]
- Church RB, Robertson FW, 1966. A biochemical study of the growth of Drosophila melanogaster. J. Exp. Zool. 162, 337–351. [Google Scholar]
- Colombani J, Bianchini L, Layalle S, Pondeville E, Dauphin-Villemant C, Antoniewski C, Carre C, Noselli S, Leopold P, 2005. Antagonistic actions of ecdysone and insulins determine final size in Drosophila. Science 310, 667–670. [DOI] [PubMed] [Google Scholar]
- Colombani J, Raisin S, Pantalacci S, Radimerski T, Montagne J, Leopold P, 2003. A nutrient sensor mechanism controls Drosophila growth. Cell 114, 739–749. [DOI] [PubMed] [Google Scholar]
- Crone-Gloor U, 1959. Quantitative untersuchung der Freien Aminosäuren und Polypeptide während der Embryonalentwicklung von Drosophila melanogaster. Journal of Insect Physiology 3, 50–56. [Google Scholar]
- Davis KT, Shearn A, 1977. In vitro growth of imaginal disks from Drosophila melanogaster. Science 196, 438–440. [DOI] [PubMed] [Google Scholar]
- Delanoue R, Meschi E, Agrawal N, Mauri A, Tsatskis Y, McNeill H, Leopold P, 2016. Drosophila insulin release is triggered by adipose Stunted ligand to brain Methuselah receptor. Science 353, 1553–1556. [DOI] [PubMed] [Google Scholar]
- Djabrayan NJ, Smits CM, Krajnc M, Stern T, Yamada S, Lemon WC, Keller PJ, Rushlow CA, Shvartsman SY, 2019. Metabolic Regulation of Developmental Cell Cycles and Zygotic Transcription. Curr Biol 29, 1193–1198 e1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droujinine IA, Perrimon N, 2016. Interorgan Communication Pathways in Physiology: Focus on Drosophila. Annu Rev Genet 50, 539–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond-Barbosa D, 2019. Local and Physiological Control of Germline Stem Cell Lineages in Drosophila melanogaster. Genetics 213, 9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond-Barbosa D, Tennessen JM, 2020. Reclaiming Warburg: using developmental biology to gain insight into human metabolic diseases. Development 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan DM, Kiefel P, Duncan I, 2017. Mutants for Drosophila Isocitrate Dehydrogenase 3b Are Defective in Mitochondrial Function and Larval Cell Death. G3 (Bethesda) 7, 789–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galikova M, Diesner M, Klepsatel P, Hehlert P, Xu Y, Bickmeyer I, Predel R, Kuhnlein RP, 2015. Energy Homeostasis Control in Drosophila Adipokinetic Hormone Mutants. Genetics 201, 665–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galikova M, Klepsatel P, 2018. Obesity and Aging in the Drosophila Model. Int J Mol Sci 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbe JC, Yang E, Fristrom JW, 1993. IMP-L2: an essential secreted immunoglobulin family member implicated in neural and ectodermal development in Drosophila. Development 119, 1237–1250. [DOI] [PubMed] [Google Scholar]
- Garofalo RS, 2002. Genetic analysis of insulin signaling in Drosophila. Trends Endocrinol Metab 13, 156–162. [DOI] [PubMed] [Google Scholar]
- Geminard C, Rulifson EJ, Leopold P, 2009. Remote control of insulin secretion by fat cells in Drosophila. Cell Metab 10, 199–207. [DOI] [PubMed] [Google Scholar]
- Ghosh AC, Tattikota SG, Liu Y, Comjean A, Hu Y, Barrera V, Ho Sui SJ, Perrimon N, 2020. Drosophila PDGF/VEGF signaling from muscles to hepatocyte-like cells protects against obesity. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert LI, Rybczynski R, Warren JT, 2002. Control and biochemical nature of the ecdysteroidogenic pathway. Annu Rev Entomol 47, 883–916. [DOI] [PubMed] [Google Scholar]
- Gillette CM, Hazegh KE, Nemkov T, Stefanoni D, D’Alessandro A, Taliaferro JM, Reis T, 2020. Gene-Diet Interactions: Dietary Rescue of Metabolic Effects in spen-Depleted Drosophila melanogaster. Genetics 214, 961–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez E, Wiggins D, Fielding B, Gould AP, 2007. Specialized hepatocyte-like cells regulate Drosophila lipid metabolism. Nature 445, 275–280. [DOI] [PubMed] [Google Scholar]
- HC C, 1963. Tactic reactions of young adults of Drosophila melanogaster. The American Midland Naturalist 70, 329–338. [Google Scholar]
- Havula E, Hietakangas V, 2012. Glucose sensing by ChREBP/MondoA-Mlx transcription factors. Semin Cell Dev Biol 23, 640–647. [DOI] [PubMed] [Google Scholar]
- Havula E, Teesalu M, Hyotylainen T, Seppala H, Hasygar K, Auvinen P, Oresic M, Sandmann T, Hietakangas V, 2013. Mondo/ChREBP-Mlx-regulated transcriptional network is essential for dietary sugar tolerance in Drosophila. PLoS Genet 9, e1003438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazegh KE, Nemkov T, D’Alessandro A, Diller JD, Monks J, McManaman JL, Jones KL, Hansen KC, Reis T, 2017. An autonomous metabolic role for Spen. PLoS Genet 13, e1006859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heier C, Kuhnlein RP, 2018. Triacylglycerol Metabolism in Drosophila melanogaster. Genetics 210, 1163–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herboso L, Oliveira MM, Talamillo A, Perez C, Gonzalez M, Martin D, Sutherland JD, Shingleton AW, Mirth CK, Barrio R, 2015. Ecdysone promotes growth of imaginal discs through the regulation of Thor in D. melanogaster. Sci Rep 5, 12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann J, Romey R, Fink C, Yong L, Roeder T, 2013. Overexpression of Sir2 in the adult fat body is sufficient to extend lifespan of male and female Drosophila. Aging (Albany NY) 5, 315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homem CCF, Steinmann V, Burkard TR, Jais A, Esterbauer H, Knoblich JA, 2014. Ecdysone and mediator change energy metabolism to terminate proliferation in Drosophila neural stem cells. Cell 158, 874–888. [DOI] [PubMed] [Google Scholar]
- Hughes MB, Lucchesi JC, 1977. Genetic rescue of a lethal “null” activity allele of 6-phosphogluconate dehydrogenase in Drosophila melanogaster. Science 196, 1114–1115. [DOI] [PubMed] [Google Scholar]
- Ikeya T, Galic M, Belawat P, Nairz K, Hafen E, 2002. Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila. Current Biology 12, 1293–1300. [DOI] [PubMed] [Google Scholar]
- Imai S, Armstrong CM, Kaeberlein M, Guarente L, 2000. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403, 795–800. [DOI] [PubMed] [Google Scholar]
- Jindra M, Uhlirova M, Charles JP, Smykal V, Hill RJ, 2015. Genetic Evidence for Function of the bHLH-PAS Protein Gce/Met As a Juvenile Hormone Receptor. PLoS Genet 11, e1005394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King RC, Aggarwal SK, Bodenstein D, 1966. The comparative submicroscopic morphology of the ring gland of Drosophila melanogaster during the second and third larval instars. Z Zellforsch Mikrosk Anat 73, 272–285. [DOI] [PubMed] [Google Scholar]
- King-Jones K, Thummel CS, 2005. Nuclear receptors--a perspective from Drosophila. Nat Rev Genet 6, 311–323. [DOI] [PubMed] [Google Scholar]
- Kirkwood TBL, Holliday R, Smith JM, Holliday R, 1979. The evolution of ageing and longevity. Proceedings of the Royal Society of London. Series B. Biological Sciences 205, 531–546. [DOI] [PubMed] [Google Scholar]
- Koyama T, Mirth CK, 2016. Growth-Blocking Peptides As Nutrition-Sensitive Signals for Insulin Secretion and Body Size Regulation. PLoS Biol 14, e1002392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanet E, Gould AP, Maurange C, 2013. Protection of neuronal diversity at the expense of neuronal numbers during nutrient restriction in the Drosophila visual system. Cell Rep 3, 587–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanet E, Maurange C, 2014. Building a brain under nutritional restriction: insights on sparing and plasticity from Drosophila studies. Front Physiol 5, 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G, Park JH, 2004. Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics 167, 311–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KP, 2015. Dietary protein:carbohydrate balance is a critical modulator of lifespan and reproduction in Drosophila melanogaster: a test using a chemically defined diet. J Insect Physiol 75, 12–19. [DOI] [PubMed] [Google Scholar]
- Lee KP, Simpson SJ, Clissold FJ, Brooks R, Ballard JW, Taylor PW, Soran N, Raubenheimer D, 2008. Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proc Natl Acad Sci U S A 105, 2498–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Chawla G, Hurlburt AJ, Sterrett MC, Zaslaver O, Cox J, Karty JA, Rosebrock AP, Caudy AA, Tennessen JM, 2017. Drosophila larvae synthesize the putative oncometabolite L-2-hydroxyglutarate during normal developmental growth. Proc Natl Acad Sci U S A 114, 1353–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao S, Post S, Lehmann P, Veenstra JA, Tatar M, Nassel DR, 2020. Regulatory Roles of Drosophila Insulin-Like Peptide 1 (DILP1) in Metabolism Differ in Pupal and Adult Stages. Front Endocrinol (Lausanne) 11, 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malita A, Rewitz K, 2020. Interorgan communication in the control of metamorphosis. Curr Opin Insect Sci 43, 54–62. [DOI] [PubMed] [Google Scholar]
- Martin FA, Herrera SC, Morata G, 2009. Cell competition, growth and size control in the Drosophila wing imaginal disc. Development 136, 3747–3756. [DOI] [PubMed] [Google Scholar]
- Matsuda H, Yamada T, Yoshida M, Nishimura T, 2015. Flies without trehalose. J Biol Chem 290, 1244–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila J, Hietakangas V, 2017. Regulation of Carbohydrate Energy Metabolism in Drosophila melanogaster. Genetics 207, 1231–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBrayer Z, Ono H, Shimell M, Parvy JP, Beckstead RB, Warren JT, Thummel CS, Dauphin-Villemant C, Gilbert LI, O’Connor MB, 2007. Prothoracicotropic hormone regulates developmental timing and body size in Drosophila. Developmental cell 13, 857–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiklejohn CD, Holmbeck MA, Siddiq MA, Abt DN, Rand DM, Montooth KL, 2013. An Incompatibility between a mitochondrial tRNA and its nuclear-encoded tRNA synthetase compromises development and fitness in Drosophila. PLoS Genet 9, e1003238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkey AB, Wong CK, Hoshizaki DK, Gibbs AG, 2011. Energetics of metamorphosis in Drosophila melanogaster. J Insect Physiol 57, 1437–1445. [DOI] [PubMed] [Google Scholar]
- Millington JW, Brownrigg GP, Basner-Collins PJ, Sun Z, Rideout EJ, 2020. Genetic manipulation of insulin/insulin-like growth factor signaling pathway activity has sex-biased effects on Drosophila body size. bioRxiv, 2020.2009.2004.283382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirth CK, Nogueira Alves A, Piper MD, 2019. Turning food into eggs: insights from nutritional biology and developmental physiology of Drosophila. Curr Opin Insect Sci 31, 49–57. [DOI] [PubMed] [Google Scholar]
- Musselman LP, Kuhnlein RP, 2018. Drosophila as a model to study obesity and metabolic disease. J Exp Biol 221. [DOI] [PubMed] [Google Scholar]
- Nassel DR, Kubrak OI, Liu Y, Luo J, Lushchak OV, 2013. Factors that regulate insulin producing cells and their output in Drosophila. Front Physiol 4, 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassel DR, Vanden Broeck J, 2016. Insulin/IGF signaling in Drosophila and other insects: factors that regulate production, release and post-release action of the insulin-like peptides. Cell Mol Life Sci 73, 271–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijhout HF, 2003. The control of body size in insects. Developmental biology 261, 1–9. [DOI] [PubMed] [Google Scholar]
- Nijhout HF, Williams CM, 1974. Control of moulting and metamorphosis in the tobacco hornworm, Manduca sexta (L.): cessation of juvenile hormone secretion as a trigger for pupation. J Exp Biol 61, 493–501. [DOI] [PubMed] [Google Scholar]
- Nishimura T, 2020. Feedforward Regulation of Glucose Metabolism by Steroid Hormones Drives a Developmental Transition in Drosophila. Curr Biol 30, 3624–3632 e3625. [DOI] [PubMed] [Google Scholar]
- O’Brien LE, Soliman SS, Li X, Bilder D, 2011. Altered modes of stem cell division drive adaptive intestinal growth. Cell 147, 603–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto N, Yamanaka N, Yagi Y, Nishida Y, Kataoka H, O’Connor MB, Mizoguchi A, 2009. A fat body-derived IGF-like peptide regulates postfeeding growth in Drosophila. Dev Cell 17, 885–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owusu-Ansah E, Perrimon N, 2015. Stress signaling between organs in metazoa. Annu Rev Cell Dev Biol 31, 497–522. [DOI] [PubMed] [Google Scholar]
- Padmanabha D, Baker KD, 2014. Drosophila gains traction as a repurposed tool to investigate metabolism. Trends Endocrinol Metab 25, 518–527. [DOI] [PubMed] [Google Scholar]
- Palu RA, Thummel CS, 2016. Sir2 Acts through Hepatocyte Nuclear Factor 4 to maintain insulin Signaling and Metabolic Homeostasis in Drosophila. PLoS Genet 12, e1005978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X, Connacher RP, O’Connor MB, 2020. Control of the insect metamorphic transition by ecdysteroid production and secretion. Curr Opin Insect Sci 43, 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge L, Gems D, Withers DJ, 2005. Sex and death: what is the connection? Cell 120, 461–472. [DOI] [PubMed] [Google Scholar]
- Piper MDW, Partridge L, 2018. Drosophila as a model for ageing. Biochim Biophys Acta Mol Basis Dis 1864, 2707–2717. [DOI] [PubMed] [Google Scholar]
- Powsner L, 1935. The Effects of Temperature on the Durations of the Developmental Stages of Drosophila Melanogaster. Physiological Zoology 8, 474–520. [Google Scholar]
- Puig O, Marr MT, Ruhf ML, Tjian R, 2003. Control of cell number by Drosophila FOXO: downstream and feedback regulation of the insulin receptor pathway. Genes Dev 17, 2006–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan A, Housden BE, Wirtz-Peitz F, Holderbaum L, Perrimon N, 2017. A Mechanism Coupling Systemic Energy Sensing to Adipokine Secretion. Dev Cell 43, 83–98 e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan A, Perrimon N, 2012. Drosophila cytokine unpaired 2 regulates physiological homeostasis by remotely controlling insulin secretion. Cell 151, 123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechsteiner MC, 1970. Drosophila lactate dehydrogenase and alpha-glycerolphosphate dehydrogenase: distribution and change in activity during development. J Insect Physiol 16, 1179–1192. [DOI] [PubMed] [Google Scholar]
- Reis T, 2016. Effects of Synthetic Diets Enriched in Specific Nutrients on Drosophila Development, Body Fat, and Lifespan. PLoS One 11, e0146758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis T, Van Gilst MR, Hariharan IK, 2010. A buoyancy-based screen of Drosophila larvae for fat-storage mutants reveals a role for Sir2 in coupling fat storage to nutrient availability. PLoS Genet 6, e1001206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddiford LM, 1994. Cellular and Molecular Actions of Juvenile Hormone I. General Considerations and Premetamorphic Actions, Advances in Insect Physiology. Academic Press, pp. 213–274. [Google Scholar]
- Riddiford LM, Cherbas P, Truman JW, 2000. Ecdysone receptors and their biological actions. Vitam Horm 60, 1–73. [DOI] [PubMed] [Google Scholar]
- Riddiford LM, Hiruma K, Zhou X, Nelson CA, 2003. Insights into the molecular basis of the hormonal control of molting and metamorphosis from Manduca sexta and Drosophila melanogaster. Insect Biochem Mol Biol 33, 1327–1338. [DOI] [PubMed] [Google Scholar]
- Riddiford LM, Truman JW, 2015. Hormone Receptors and the Regulation of Insect Metamorphosis1. American Zoologist 33, 340–347. [Google Scholar]
- Riddiford LM, Truman JW, Mirth CK, Shen YC, 2010. A role for juvenile hormone in the prepupal development of Drosophila melanogaster. Development 137, 1117–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rideout EJ, Narsaiya MS, Grewal SS, 2015. The Sex Determination Gene transformer Regulates Male-Female Differences in Drosophila Body Size. PLoS Genet 11, e1005683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson F, 1963. The ecological genetics of growth in Drosophila. 6. The genetic correlation between the duration of the larval period and body size in relation to larval diet. Genet Res 4, 74–92. [Google Scholar]
- Rogina B, Helfand SL, 2004. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci U S A 101, 15998–16003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rulifson EJ, Kim SK, Nusse R, 2002. Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science 296, 1118–1120. [DOI] [PubMed] [Google Scholar]
- Sano H, Nakamura A, Texada MJ, Truman JW, Ishimoto H, Kamikouchi A, Nibu Y, Kume K, Ida T, Kojima M, 2015. The Nutrient-Responsive Hormone CCHamide-2 Controls Growth by Regulating Insulin-like Peptides in the Brain of Drosophila melanogaster. PLoS Genet 11, e1005209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonborn JW, Jehrke L, Mettler-Altmann T, Beller M, 2019. FlySilico: Flux balance modeling of Drosophila larval growth and resource allocation. Sci Rep 9, 17156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieber MH, Spradling AC, 2017. The role of metabolic states in development and disease. Curr Opin Genet Dev 45, 58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaer H, 1993. The alimentary canal, in: Martinez M.B.a.A. (Ed.), The Development of Drosophila melanogaster. Cold Spring Harbor Laboratory Press, Plainview, NY, pp. 941–1012. [Google Scholar]
- Slaidina M, Delanoue R, Gronke S, Partridge L, Leopold P, 2009. A Drosophila insulin-like peptide promotes growth during nonfeeding states. Dev Cell 17, 874–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Marmion RA, Park JO, Biswas D, Rabinowitz JD, Shvartsman SY, 2017. Dynamic Control of dNTP Synthesis in Early Embryos. Dev Cell 42, 301–308 e303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Park JO, Tanner L, Nagano Y, Rabinowitz JD, Shvartsman SY, 2019. Energy budget of Drosophila embryogenesis. Curr Biol 29, R566–R567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Shvartsman SY, 2020. Chemical Embryology Redux: Metabolic Control of Development. Trends Genet 36, 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood C, Doyle SE, Siegrist SE, 2020. Steroid hormones, dietary nutrients, and temporal progression of neurogenesis. Curr Opin Insect Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storelli G, Nam HJ, Simcox J, Villanueva CJ, Thummel CS, 2019. Drosophila HNF4 Directs a Switch in Lipid Metabolism that Supports the Transition to Adulthood. Dev Cell 48, 200–214 e206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strub BR, Parkes TL, Mukai ST, Bahadorani S, Coulthard AB, Hall N, Phillips JP, Hilliker AJ, 2008. Mutations of the withered (whd) gene in Drosophila melanogaster confer hypersensitivity to oxidative stress and are lesions of the carnitine palmitoyltransferase I (CPT I) gene. Genome 51, 409–420. [DOI] [PubMed] [Google Scholar]
- Suzawa M, Muhammad NM, Joseph BS, Bland ML, 2019. The Toll Signaling Pathway Targets the Insulin-like Peptide Dilp6 to Inhibit Growth in Drosophila. Cell Rep 28, 1439–1446 e1435. [DOI] [PubMed] [Google Scholar]
- Tang HY, Smith-Caldas MS, Driscoll MV, Salhadar S, Shingleton AW, 2011. FOXO regulates organ-specific phenotypic plasticity in Drosophila. PLoS Genet 7, e1002373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennessen JM, Baker KD, Lam G, Evans J, Thummel CS, 2011. The Drosophila estrogen-related receptor directs a metabolic switch that supports developmental growth. Cell Metab 13, 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennessen JM, Bertagnolli NM, Evans J, Sieber MH, Cox J, Thummel CS, 2014. Coordinated Metabolic Transitions During Drosophila Embryogenesis and the Onset of Aerobic Glycolysis. G3: Genes|Genomes|Genetics 4, 839–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennessen JM, Thummel CS, 2011. Coordinating growth and maturation - insights from Drosophila. Curr Biol 21, R750–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Texada MJ, Koyama T, Rewitz K, 2020. Regulation of Body Size and Growth Control. Genetics 216, 269–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tixier V, Bataille L, Etard C, Jagla T, Weger M, Daponte JP, Strahle U, Dickmeis T, Jagla K, 2013. Glycolysis supports embryonic muscle growth by promoting myoblast fusion. Proc Natl Acad Sci U S A 110, 18982–18987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy K, Baehrecke EH, 2013. The role of autophagy in Drosophila metamorphosis. Curr Top Dev Biol 103, 101–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu MP, Yin CM, Tatar M, 2005. Mutations in insulin signaling pathway alter juvenile hormone synthesis in Drosophila melanogaster. Gen Comp Endocrinol 142, 347–356. [DOI] [PubMed] [Google Scholar]
- Wang L, Lam G, Thummel CS, 2010. Med24 and Mdh2 are required for Drosophila larval salivary gland cell death. Dev Dyn 239, 954–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wat LW, Chao C, Bartlett R, Buchanan JL, Millington JW, Chih HJ, Chowdhury ZS, Biswas P, Huang V, Shin LJ, Wang LC, Gauthier ML, Barone MC, Montooth KL, Welte MA, Rideout EJ, 2020. A role for triglyceride lipase brummer in the regulation of sex differences in Drosophila fat storage and breakdown. PLoS Biol 18, e3000595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker R, Faulkner S, Miyokawa R, Burhenn L, Henriksen M, Wood JG, Helfand SL, 2013. Increased expression of Drosophila Sir2 extends life span in a dose-dependent manner. Aging (Albany NY) 5, 682–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White KP, Rifkin SA, Hurban P, Hogness DS, 1999. Microarray Analysis of Drosophila Development During Metamorphosis. Science 286, 2179–2184. [DOI] [PubMed] [Google Scholar]
- Wigglesworth VB, 1949. The utilization of reserve substances in Drosophila during flight. J Exp Biol 26, 150–163, illust. [DOI] [PubMed] [Google Scholar]
- Williams GC, 1966. Natural Selection, the Costs of Reproduction, and a Refinement of Lack’s Principle. The American Naturalist 100, 687–690. [Google Scholar]
- Wolsky A, 1938. The Effect of Carbon Monoxide on the Oxygen Consumption of Drosophila Melanogaster Pupae. Journal of Experimental Biology 15, 225–234. [Google Scholar]
- Worley MI, Setiawan L, Hariharan IK, 2013. TIE-DYE: a combinatorial marking system to visualize and genetically manipulate clones during development in Drosophila melanogaster. Development 140, 3275–3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Habara O, Yoshii Y, Matsushita R, Kubo H, Nojima Y, Nishimura T, 2019. The role of glycogen in development and adult fitness in Drosophila. Development 146. [DOI] [PubMed] [Google Scholar]
- Yamanaka N, Rewitz KF, O’Connor MB, 2013. Ecdysone control of developmental transitions: lessons from Drosophila research. Annu Rev Entomol 58, 497–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Xue J, Lai JC, Schork NJ, White KP, Haddad GG, 2008. Mechanisms underlying hypoxia tolerance in Drosophila melanogaster: hairy as a metabolic switch. PLoS Genet 4, e1000221. [DOI] [PMC free article] [PubMed] [Google Scholar]