Abstract
Trigeminal nerve injuries are common and there is currently no consensus on both timing and type of intervention to achieve the best outcomes. A systematic review was performed to compare the outcomes of the many different types of therapeutic interventions for nerve injury. PubMed, EBSCO, and Cochrane Review databases were used to search for studies published from January 1, 2000 to December 31, 2019. Included studies detailed treatment of an injury to peripheral branches of the trigeminal nerve, either known transection or injury causing persistent alteration in sensation. The primary outcome was functional sensory recovery via the Medical Research Council scale. Twenty studies were included, detailing outcomes of 608 subjects undergoing intervention for 622 nerve injuries. Surgical interventions were able to achieve functional sensory recovery in approximately >80% or more of the subjects. There was heterogeneity among how procedures were performed, timing to intervention, and methods of measuring recovery. The data of this study supports the ability of surgical intervention to achieve functional sensory recovery in a significant number of subjects, and found evidence for better outcomes with intervention closer to the time of injury.
Keywords: trigeminal nerve, nerve injury, nerve repair, inferior alveolar nerve, lingual nerve, allograft, autograft, microsurgery
Introduction
Peripheral injuries and lesions of the trigeminal nerve are frequent and can result in permanent neurosensory disorder. The most common etiology of injuries are dental procedures, causing approximately 60% of all injuries, with the most common cause secondary to extraction and other oral surgery procedures. 1,2 The systematic review of Lin et al showed an incidence of altered sensation after mandibular implant surgery of 3% 2 with variable incidence described in literature included between 0 to 15% 3 and 0 to 40%. 4 Injuries of the inferior alveolar and lingual nerves are also well-described complications of both orthognathic surgery and mandibular resections due to benign or malignant pathologies. 2 The clinical presentation is variable from hypoesthesia to anesthesia, and includes bothersome symptoms such as paresthesia, dysesthesia and hyperalgesia, often defined with the more generic term of “altered sensation.” 5 Despite the frequency of these injuries there is no common and standardized therapeutic approach regarding treatment methods, biomaterials, or timing of repair. Treatment remains controversial, varying from a wait-and-see approach, to early surgical intervention, 6 delayed surgical treatment, or medical treatment. Surgical intervention creates additional risk for the patient, as well as further financial burden. The indications for intervention can vary widely among patients, as the need for surgery is often based on subject suffering balanced by what is acceptable to each patients’ quality of life. The primary aim of this systematic review is to evaluate in a population of patients with peripheral mandibular “sensory impairment” secondary to nerve injury, the different available surgical interventions and to contrast outcomes, specifically the ability to achieve a functional sensory recovery.
Materials and Methods
A systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA guidelines), 7 to review studies published from January 1, 2000 to December 31, 2019 [20 years] to evaluate all available treatment modalities and their ability to achieve a functional sensory recovery after oral and maxillofacial surgery procedures damaging the inferior alveolar or lingual nerves.
Search Methods
Studies were selected from a broad search of PubMed, EBSCO, and Cochrane Library with search keywords covering any variation of the terms peripheral trigeminal nerve, sensory disturbance/deficit/pain, all possible etiologies of injury, medical, laser, or surgical modalities. Search keywords used were: (“inferior alveolar nerve” or “mandibular nerve” or “trigeminal nerve” or “lingual nerve”) AND (“sensory disturbance” or “taste disorder” or “neurosensory deficit” “altered sensation” or “hyperalgesia” or “hypoesthesia” or “paresthesia” or “hypoesthesia” or “paraesthesia” or “injury” or “damage” or “lesion”) AND (“anesthesia” or “dentoalveolar surgery” or “sagittal split ramus osteotomy” or “orthognathic surgery” or “implant surgery” or “endodontic therapy”) AND (“repair” or “surgery” or “anastomosis” or “graft” or “sleeve” or “sliding” or “release” or “medical” or “antidepressant” or “antiepileptic” or “laser”) or (“trigeminal nerve repair”). Articles were selected independently at each stage of the review by 2 authors (A.W., R.P.), first based on inclusion criteria: 1) an injury to peripheral branches of the trigeminal nerve, either known transection or injury causing persistent alteration in sensation, and 2) studies with an objective measure of nerve recovery or patient satisfaction. Studies were excluded for the following: 1) cadaveric or non-human subjects, 2) studies treating suspected nerve injuries prophylactically, nerve injuries from trauma, or nerves that were planned to be transected and reconstructed at the same time for oncologic resection, 3) papers that reported pooled outcomes of multiple different interventions, making it impossible to differentiate which treatment/intervention correlated with which outcome data, 4) a full text copy was not available in English, and 5) abstracts, case reports, case series with <5 subjects, technical notes and letters to the editor. Disagreement between the 2 authors was decided by the senior author (S.S.). Included studies and results of manual search were then submitted to a third round for evaluation of the full text.
The primary outcome was functional sensory recovery (FSR) after intervention, as defined by the Medical Research Council scale (MRC), which is denoted by a score of S3 or higher 8,9 (Figure 1). This outcome was either provided in the study, or secondarily assessed by the authors based on available information in the results. Secondary outcomes were patient satisfaction, patient and surgeon subjective evaluation, neurosensory testing outcomes, and pain scores.
Figure 1.
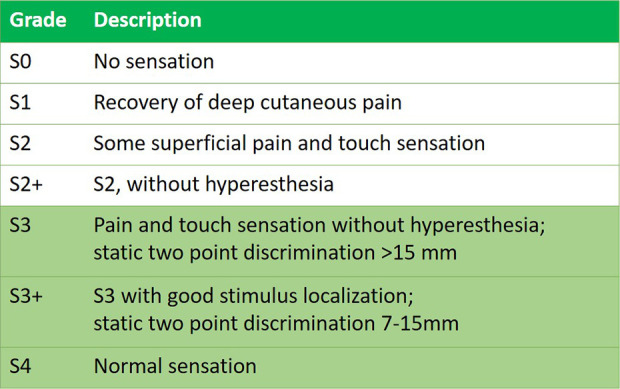
The British Medical Research Council (MRC) Score of Sensory Recovery. A postoperative score of S3 or higher indicates functional sensory recovery. This is the most commonly used scale for measuring nerve recovery.
Quality Assessment
The papers included after the final evaluation round were then appraised for quality according to the Cochrane reviewers’ handbook Section 6.7.1 to assess their risk of bias. 10 If the papers met 7 out of 7 criteria, the bias was low, 6 out of 7 moderate, and <6 out of 7 bias was high. All articles classified into 5 levels of evidence utilizing guidelines from the Journal of the American Medical Association.
Statistical Analysis
Data was extracted by 2 independent authors, and pooled after the accuracy of data collection was verified. Basic statistical analysis was performed utilizing MedCalc Statistical Software version 19.2 (MedCalc Software Ltd, Ostend, Belgium), via t-test, ANOVA, and Fisher exact test. P values <0.05 were considered statistically significant.
Results
Search Strategy
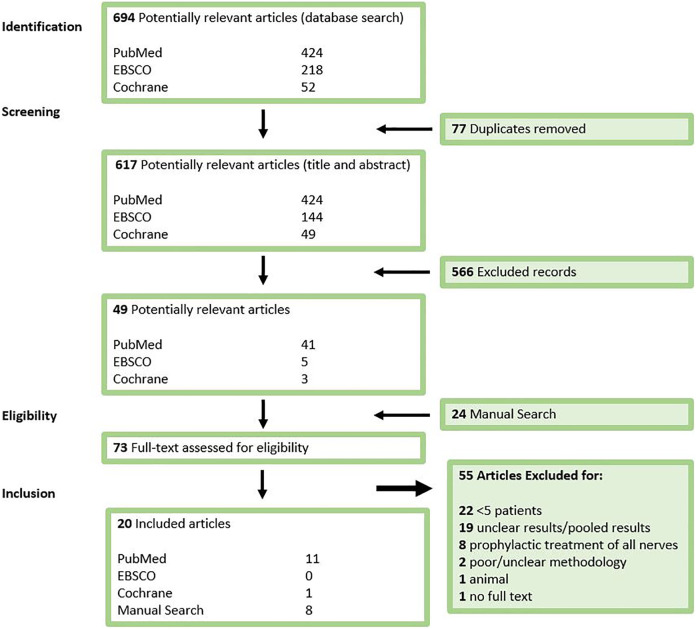
The search for articles, utilizing PubMed, EBSCO, and Cochrane was completed on 1 January 2020. A flowchart demonstrating included studies, according to PRISMA guidelines, at each stage of the systematic review is shown in Figure 2. PubMed was searched first and found 424 articles. EBSCO found 144 new articles (74 duplicates with PubMed), and Cochrane found 49 new articles (3 duplicates). This resulted in 617 possible studies after round 1.
Figure 2.
Flow chart of study selection. Systematic review was conducted in adherence to PRISMA guidelines.
Eligibility of the Studies
As part of the eligibility assessment, 566 articles were excluded after reviewing the title and abstract, leaving 49 articles. Manual search resulted in 24 additional studies, found through a search with Google Scholar, a review of the references of included papers, and gray literature, resulting in 73 possible studies in total after round 2. These 73 articles were then read in full, and at the end of this more thorough analysis a total of 20 papers were included for the final systematic review (Figure 2). The level of agreement between the 2 authors for the eligibility assessment was measured at kappa=0.96, an almost perfect level of agreement.
The risk of bias was considered high in 12 studies, moderate in 6, and low in 3. The source of bias in the majority of the studies was from poor description of patient selection process, follow-up and drop outs, and reporting of the clinical and demographic information. There was 1 level 1 evidence study, which was a randomized controlled trial. There were 9 level 3 evidence studies, all cohort studies, and 10 level 4 evidence studies, which were all case series, and most were retrospective. Of note, none of the studies were funded, but some did have one more surgeon participating in the study that was a paid consultant for Axogen. 11 –13
Data Extraction
From the 20 studies included it the systematic review, 12 were included from the main search, and 8 from the manual search. A summary of included studies is detailed in Table 1. The studies were all published between 2000 and 2018, and all but 1 was a single center study. From these 20 studies, 608 totals subjects were included, and in studies that specified the sex of patients, the majority were females (n = 414). A total of 622 nerves are evaluated, with 330 inferior alveolar nerves (IAN), 292 lingual nerves (LN). The papers were organized by type of intervention. Only 1 used low-level laser therapy. 13 Within the surgical intervention studies, 6 detailed direct repairs without grafts or conduits, 14 –19 using only surgical decompression, debridement, external/internal neurolysis, primary repair, or a combination of these procedures. There were 6 total studies about autografts in the preliminary search, 3 using nerve autografts, 20 –22 and 3 using veins as grafts/conduits. 23 –25 However, all 3 nerve autografts studies were in the setting of immediate repairs during oncologic surgery and then excluded. Three studies evaluated allografts, all using Avance® processed nerve allografts made by Axogen (AxoGen, Inc, Alachua, FL, USA). 11,12,26 Two of the 3 studies also used a conduit. Five studies used conduits in the setting of a primary repair, 9,27 –30 and they utilized NeuraGen® (Integra LifeSciences Co, Plainsboro, NJ, USA) type I collagen, Axoguard® (Axogen Inc) made of submucosa of porcine small intestine, Gortex, and polyglycolic acid filled tubes with collagen (PGA-c). Lastly, there were 3 studies that compared a variety of the already listed surgical methods. 12,31,32
Table 1.
Summary of Included Studies.
| Author | Year | Title | Journal | Level of Evidence | Study Dates | N Sex | Age (years) | |
|---|---|---|---|---|---|---|---|---|
| Laser Therapy | ||||||||
| 1 | Miloro | 2018 | Does low-level laser therapy affect recovery of lingual and inferior alveolar nerve injuries? | J Oral Maxillofac Surg | I | N/A | 28 9 M: 19 F | N/A |
| Surgical Intervention without Grafts or Conduits | ||||||||
| 2 | Robinson | 2000 | Prospective, quantitative study on the clinical outcome of lingual nerve repair | Br J Oral Maxillofac Surg | III | 1990-1998 | 53 15 M: 38 F | 30 (16-54) |
| 3 | Lam | 2003 | Patient satisfaction after trigeminal nerve repair | OOOE | III | 1997-2000 | 46 11 M: 35 F | 28 ± 12 |
| 4 | Greenwood | 2005 | Observations on the exploration and external neurolysis of injured inferior alveolar nerves | Int J Oral Maxillofac Surg | IV | N/A | 12 3 M: 9 F | 33.8 ± 10.4 |
| 5 | Strauss | 2006 | Outcome assessment of inferior alveolar nerve microsurgery: a retrospective review | J Oral Maxillofac Surg | IV | 1998-2003 | 28 11 M: 17 F | 38.5 (17-67) |
| 6 | Pogrel | 2007 | Damage to the inferior alveolar nerve as the result of root canal therapy | J Am Dent Assoc | III | 1991-2005 | 11 | N/A |
| 7 | Leung | 2016 | Longitudinal treatment outcomes of microsurgical treatment of neurosensory deficit after lower third molar surgery: a prospective case series | PLoS One | III | 2009-2012 | 12 2 M: 10 F | 28.3 ± 5.0 |
| Autograft Only Studies | ||||||||
| Vein Autograft | ||||||||
| 8 | Pogrel | 2001 | The use of autogenous vein grafts for inferior alveolar and lingual nerve reconstruction | J Oral Maxillofac Surg | IV | N/A | 15 | N/A |
| 9 | Jones | 2010 | The use of vein grafts in the repair of the IAN following surgery | Aust Dent | IV | N/A | 5 3 M: 2 F | N/A |
| 10 | Fujita | 2014 | Outcome following lingual nerve repair with vein graft cuff: a preliminary report | J Oral Maxillofac Surg | IV | 2002-2012 | 10 2 M: 8 F | 39.2 (17-55) |
| Allograft Only Studies | ||||||||
| 11 | Zuniga | 2015 | Sensory outcomes after reconstruction of lingual and inferior alveolar nerve discontinuities using processed nerve allograft—a case series | J Oral Maxillofac Surg | IV | 2007-2013 | 21 11 M: 10 F | 33.3 ± 17.0 |
| 12 | Yampolsky | 2017 | Efficacy of acellular nerve allografts in trigeminal nerve reconstruction | J Oral Maxillofac Surg | IV | 2008-2014 | 16 3 M: 13 F | 32.6 (16-62) |
| Conduit Only Studies | ||||||||
| 13 | Pitta | 2001 | Use of Gore-Tex tubing as a conduit for inferior alveolar and lingual nerve repair: experience with 6 cases | J Oral Maxillofac Surg | IV | N/A | 6 1 M: 5 F | 34 (21-49) |
| 14 | Farole | 2008 | A bioabsorbable collagen nerve cuff (NeuraGen) for repair of lingual and inferior alveolar nerve injuries: a case series. | J Oral Maxillofac Surg | IV | N/A | 9 1 M: 8 F | N/A |
| 15 | Erakat | 2013 | Interval between injury and lingual nerve repair as a prognostic factor for success using type I collagen conduit | J Oral Maxillofac Surg | III | 2000-2010 | 31 9 M: 32 F | 28.3 ± 8.3 |
| 16 | Seo | 2016 | Prognosis after surgical treatment of trigeminal neuropathy with a PGA-c tube: report of 10 cases | Pain Med | IV | 2005-2014 | 10 2 M: 8 F | 47.9 ± 12.6 |
| 17 | Wilson | 2017 | Lingual nerve microsurgery outcomes using 2 different conduits: a retrospective cohort study | J Oral Maxillofac Surg | III | 2007-2014 | 66 17 M: 49 F | 26.8 (13-50) |
| Multiple Surgical Technique Studies | ||||||||
| 18 | Bagheri | 2012 | Microsurgical repair of the inferior alveolar nerve: success rate and factors that adversely affect outcome | J Oral Maxillofac Surg | III | 1986-2005 | 167 41 M: 126 F | 38.7 (15-75) |
| 19 | Biglioli | 2015 | Surgical treatment of painful lesions of the inferior alveolar nerve | J Craniomaxillofac Surg | III | 2007-2013 | 19 | N/A |
| 20 | Miloro | 2015 | Lingual nerve repair: to graft or not to graft? | J Oral Maxillofac Surg | III | 2000-2012 | 43 18 M: 25 F | 28.3 (15-52) |
M: male; F: female; RCT: randomized controlled trial; N/A indicates information was not available in the study.
The most common way to describe nerve repair outcomes was with the Medical Research Council (MRC) scale, and it was used in 9 studies. Other methods of neurosensory testing were very heterogenous, as even studies using the same technique did not use similar methods. Evaluation methods included monofilament/Semmes-Weinstein/Von Frey Fibers, subjective patient satisfaction and reduction in pain, Pogrel neurosensory testing, 33 a subjective surgeon evaluation based on various neurosensory testing, and the 1 laser study based its success on having a greater than 1-unit improvement based on neurosensory testing and pain. Thus, most of the data was unusable when trying to pool outcome data to make a comparison.
Outcomes of the nerve injury interventions by study are detailed in Table 2. The injuries were caused by a wide range of etiologies. Tooth extractions (vast majority being third molars), caused 410 of the nerve injuries (65.9%). There was also a wide range in timing of intervention among the studies.
Table 2.
Summary of Outcome Data from Included Studies.
| Author Year | n | Mean Gap Length (mm) | Intervention | Etiology of Injury | Timing to Intervention (months) | Follow-up (months) | Outcome MRC “Recovery” | Other Outcomes | |
|---|---|---|---|---|---|---|---|---|---|
| Laser Therapy | |||||||||
| 1 | Miloro 2018 | 21 IAN 7 LN |
N/A | LLT | (22) Extraction (4) Needle (1) Implant (1) Other |
3-12; >12 | 3 | N/A |
≥ 1 unit improvement
LLT 7/15 (46.7%) Placebo 5/13 (38.5%) |
| Surgical Intervention without Grafts or Conduits | |||||||||
| 2 | Robinson 2000 | 53 LN | 9.5 (4-14) | Direct Repair | (53) 3rd molar ext | Mean 15 (4-47) | Median 13 | N/A |
Subjective Patient
“Value of operation” 33/53 (62.3%) scored a 7+ /10 |
| 3 | Lam 2003 | 10 IAN 36 LN | 14.3 ± 7.3 | Direct Repair | (46) 3rd molar ext | 6.8 ± 11.5 | Minimum 12 | N/A |
Subjective Patient
17/25 (68%) “good” or “better” |
| 4 | Greenwood 2005 | 12 IAN | 0 mm | External Neurolysis | (7) 3rd molar ext (4) Cyst (1) Orthognathic |
Mean 14.1 | 12 | N/A |
Subjective Patient
2/12 (16.7%) complete recovery 2/12 (16.7%) some improvement 1/12 (8.3%) resolved dysesthesia |
| 5 | Strauss 2006 | 28 IAN | N/A | (12) Primary Repair (11) External/internal neurolysis (5) External neurolysis |
(12) 3rd molar ext (5) RCT (4) Implant (2) Mandibular fx (1) Cyst (1) Apicoectomy (1) Orthognathic (1) Laceration (1) I&D |
Mean 6.6 (1-28) | 9.5 | N/A |
Subjective Surgeon
26/28 (92.9%) significant neurosensory improvement Subjective Patient 25/28 (89.3%) significant neurosensory improvement |
| 6 | Pogrel 2007 |
11 IAN | 0 mm | Debridement/ Decompression |
(11) RCT | 48 hours to 3 mo | N/A | N/A |
Subjective Patient
Early: 5/5 (100%) recovery Late: 4/6 (66.7%) partial recovery |
| 7 | Leung 2016 |
2 IAN 10 LN |
10.2 ± 5.5 (6-22) |
(11) Primary Repair (1) Decompression |
(12) 3rd molar ext | Mean 6.3 (1-14) |
12 | N/A |
Subjective Patient
11/12 (91.7%) improvement in numbness Pain VAS LN pre 7.2 to post 0.2 (change −7.0) IAN pre 6.5 to post 0 (change −6.5) |
| Autograft Studies | |||||||||
| Vein Autograft | |||||||||
| 8 | Pogrel 2001 |
6 IAN 10 LN |
2-14 | (16) Neuroma excision LN: Long saphenous vein conduit IAN: facial vein graft conduit |
(14) 3rd molar ext (1) Orthognathic (1) Endo |
4-10 | 22 (14-36) |
N/A | Pogrel Scale “Good return of sensation” LN Gap <5 mm= 1/3 (33.3%) Gap >5mm= 0/7 IAN Gap <5mm= 2/3 (66.7%) Gap >5mm= 1/3 (33.3%) |
| 9 | Jones 2010 |
5 IAN | N/A | Debridement/Decompression + Posterior Facial Vein Nerve Graft | (3) 3rd molar ext (1) Pathology (1) Orthognathic |
18 | 18 | N/A |
Subjective Surgeon
4/5 (80.0%) functional recovery |
| 10 | Fujita 2014 |
10 LN | 0 | (5) Direct repair (5) Direct repair +Inside out vein graft (external jugular) |
(10) 3rd molar ext | 4.4 ± 1.4 | 12 | 10/10 (100%) |
Pogrel Scale “Good return of sensation” 5/10 (50.0%) good improvement |
| Allograft Studies | |||||||||
| 11 | Zuniga 2015 |
8 IAN 15 LN |
32.4 ± 25.5 (8-70) |
(20) Allograft AVANCE + Axoguard Nerve Sheath (3) AVANCE Allograft only |
(13) 3rd molar ext (6) Malignant pathology (2) Orthognathic (2) Implant |
Mean 5 (0-17) |
12 | IAN Allograft= 7/8 (87.5%) LN Allograft= 13/15 (86.7%) |
— |
| 12 | Yampolsky 2017 |
8 IAN 8 LN |
<20 | Allograft AVANCE + Axoguard or Neuragen |
(9) 3rd molar ext (2) Implant (2) RCT (2) Extraction (1) Apicoectomy |
9 ± 8 | 12 (3-26) |
IAN= 7/8 (87.5%) LN= 8/8 (100%) |
— |
| Conduit Only Studies | |||||||||
| 13 | Pitta 2001 |
3 IAN 3 LN |
25 (10-50) |
Entubulization with Gortex Tube (Direct Repair) |
(4) 3rd molar ext (1) RCT (1) Apicoectomy |
21 (6-48) |
21 (12-36) |
2/6 (33.3%) |
Subjective Patient
Decrease in Pain 2/6 (33.3%) |
| 14 | Farole 2008 |
3 IAN 6 LN |
N/A | Nuragen Nerve Sheath | (9) 3rd molar ext | 3-7 | 12-30 | N/A | Pogrel Scale “Good return of sensation” 4/9 (44.4%) good improvement |
| 15 | Erakat 2013 |
42 LN | 0 | (22) Nuragen conduit: (12) neurolysis (10) primary repair (20) No conduit (6) neurolysis (14) direct repair |
(40) 3rd molar ext (1) Operculectomy (1) Injection |
5.18 ± 1.4 (conduit) 7.8 ± 5.6 (no conduit) |
9.7 (3.5-18) |
Conduit= 22/22 (100%) No conduit= 17/20 (85.0%) |
— |
| 16 | Seo 2016 |
8 IAN 2 LN |
N/A | (4) Bridging with PGA-c tube (6) Encircling with PGA-c tube |
(5) 3rd molar ext (3) implant (1) malignant pathology (1) infection |
45.7 ± 35.5 | 3-51 | Bridging= 3/4 (75.0%) Encircling = 3/6 (50.0%) |
— |
| 17 | Wilson 2017 |
43 LN | 0 | (28) Direct Repair +NURAGEN (15) Direct Repair + Axoguard |
(43) 3rd molar ext | 5.6 ± 2.6 | 9.3 (3-18) |
43/43 (100%) | — |
| Multiple Surgical Technique Studies | |||||||||
| 18 | Bagheri 2012 |
186 IAN | >20 | (97) Debride/Decompression (71) Autogenous Graft (GA>Sural) (18) Direct Repair |
(70) 3rd molar ext (31) Orthognathic (21) Mandibular fx (15) Implant (14) RCT (9) Pathology (26) Other |
10.7 (0-72) |
12 | Primary= 16/18 (88.9%) Decompression=74/97 (76.3%) Autograft= 62/71 (87.3%) |
— |
| 19 | Biglioli 2015 |
19 IAN | N/A | (9) Decompression or debridement (6) Autograft sural nerve (3) Vein graft conduit (1) Direct repair |
(4) 3rd molar ext (7) Implant (5) Endo (1) Benign tumor (1) Malignant path (1) Orthognathic |
Variable Immediate-12+ |
12 | N/A |
Subjective Patient
“Pain Free” Autograft 6/6 (100%) Decompression 5/9 (55.6%) Vein graft 2/3 (66.7%) |
| 20 | Miloro 2015 |
47 LN | 14.3 (10-30) |
(24) Autograft Sural Nerve (19) Primary Repair (4) AVANCE Allograft |
(34) 3rd molar ext (5) Pathology (3) Orthognathic (1) Implant |
Mean 3.2 (0.75-16.4) |
24 | Primary= 16/19 (84.2%) Autograft= 21/24 (87.5%) Allograft= 4/4 (100%) |
— |
Ext: extraction; RCT: root canal therapy; IAN: inferior alveolar nerve; LN: lingual nerve; LLT: low-level laser therapy; mo: months; fx: fracture; GA: greater auricular nerve; PGA-c: polyglycolic acid-collagen; VAS: visual analog scale; N/A: information was not available in the text.
Mean MRC functional recovery was not reported for any of the non-surgical intervention studies. A summary of outcomes via the MRC scale for the surgical interventions is available in Tables 2 and 3. There was only a statistically significant difference among the treatment interventions of autograft v allograft, and autograft v conduit, when evaluating using the MRC scale. The results of the alternative outcomes measured showed very mixed results, where the same subjects evaluated with neurosensory testing methods described by Pogrel 33 and Zuniga et al 34 showed outcomes to be less favorable than when reported with the MRC scale. Outcomes reported based on etiology are shown in Table 4. The vast majority of injured nerves were secondary to third molar extraction, and the best outcomes were seen with intervention after dental extractions and malignant pathology, and the worst after infection and implants.
Table 3.
Comparison of Outcome Results Among Treatment Modalities.
| FSR via MRC | |
|---|---|
| Low-Level Laser Therapy | N/A |
| Primary Surgery (no graft/conduit) | 123/154 (80%) |
| Autograft-Vein | 10/10 (100%) |
| Allograft | 39/43 (91%) |
| Autograft Sural/GA | 70/95 (74%) |
| Conduit Only | 73/81 (90%) |
| p-value | |
| Primary Surgery v Autograft-vein | 0.118 |
| Primary v Autograft sural/GA | 0.276 |
| Primary v Allograft | 0.095 |
| Primary v Conduit | 0.051 |
| Autograft sural/GA v Allograft | 0.025 |
| Allograft v Conduit | 0.858 |
| Autograft sural/GA v Conduit | 0.001 |
P-value calculated via Fisher exact test.
GA-Greater auricular nerve.
Table 4.
“Improvement” Outcomes by Etiology.
| 3rd Molar | Endodontics | Benign | Malignant | Implant | Orthognathic | Infection | |
|---|---|---|---|---|---|---|---|
| Direct Repair | 33/5314 | 10/1118 | 1/416 | 0/116 | |||
| 4/716 | |||||||
| 11/1219 | |||||||
| Vein Autograft | 2/325 | 1/125 | 1/125 | ||||
| 10/1024 | |||||||
| Allograft | 7/1311 | 6/611 | 0/211 | 1/211 | |||
| Conduits | 2/428 | 0/228 | 0/129 | 1/329 | 0/129 | ||
| 8/930 | |||||||
| 5/529 | |||||||
| 43/439 | |||||||
| Total (n) | 146 | 13 | 5 | 7 | 5 | 4 | 1 |
| % Improved | 87.4% | 76.9% | 40.0% | 85.7% | 20.0% | 50.0% | 0.0% |
Improvement defined as MRC S3 or greater or a reported subjective patient improvement from baseline.
Timing of Repair
For all nerve injuries, time is “precious.” 35 and immediate repair at the moment of the damage guarantees the best functional recovery. 12 Obviously, immediate action is not always possible, as in many studies the patients were referred to the surgeon by another provider. 35 The surgeons’ skill, patient compliance, availability of the operating room and availability of adjunctive grafts and conduits can influence the decision of timing. 36 Most nerve injuries were not witnessed and first noted at postoperative follow-up. When time has passed and symptoms persist, repair time is a controversial topic, with studies showing mixed results. A systematic review by Kushnerev et al found many authors recommend surgery when neurosensory deficits showed no improvement 90 days post-diagnosis. 37 Alternatively, Nizam et al proposed a therapeutic diagnostic algorithm where follow-up is performed for up to 3 months. At this point, if there is no evidence of recovery of nerve function, then surgery is indicated within 6 months. They report having obtained statistically significant better results if the repair is carried out before 6 months, particularly for IAN injuries. 36 Randomized controlled trials are needed to standardize the surgical timing as there still is no universally accepted consensus on timing. The timing to intervention was reported in a variety of ways among the papers, thus it is not possible to calculate the average time to intervention among all of the papers. However, 4 studies found no significant difference between early and later repairs 14,15,17,28 , and 3 reported early repairs to be superior to late. 18,27,31
Lasers
For this modality, only 1 study met inclusion criteria, and it was also the only RCT of this systematic review. The one included study failed to show any significant improvement in subjects receiving low laser therapy, and this modality also requires approximately 20 sessions to complete therapy, thus it is not likely that this will not emerge as a primary treatment modality for nerve injury.
Decompression/Debridement/Neurolysis/Primary Repair
The foundation of a successful surgical nerve repair, regardless of method, relies upon proper debridement of foreign bodies and scar tissues surrounding the nerve, proper resection of injured neural tissue, and performing a tension free repair. Thus, there are multiple surgical interventions one can perform without use of a graft or conduit. Direct repair or neurorrhaphy is used when the 2 ends of the nerve can be approximated via epineural sutures without tension, commonly for defects less than 1 cm. Direct suturing is favorable, as the length of regeneration is minimized and there is only 1 anastomosis to complete (versus 2 for grafts). In this review, these methods were the most commonly employed out of all treatment methods. Many studies show favorable results, which could be due to these injuries being less complex than those requiring grafts at baseline. Overall, most subjects experienced some improvement after surgery. It was very difficult to compare these studies as most authors relied on patient subjective scores, were treating a heterogeneous group in regard to cause of injury and severity, and there was not consistent timing of the intervention.
Autograft
Autografts can be divided into 2 modalities: 1) an autogenous nerve graft, and 2) an autogenous vein graft which is used similarly to a conduit in conjunction with a direct nerve repair. Like other surgical interventions, the decision to use an autograft is based on the gap length of the nerve injury, as a tension free repair is mandatory. There are many different nerves available for autograft but commonly the sural nerve is used as its diameter and fascicular pattern best matches the trigeminal nerve, 38 or the greater auricular nerve because of its ease of harvest and proximity to the reconstruction site. The greater auricular nerve however is smaller and more prone to bifurcate early. 39 Autografts can be advantageous as they are more cost effective than allografts, and in some cases 22 the donor nerve may already be accessible near the primary surgical site. 39 Multiple studies have also shown that sural nerve harvest is well tolerated by the majority of patients, as the nerve supplies a relatively insignificant dermatome. 20,38 However, it is still important to consider the tradeoff in sacrificing sensation from one part of the body, to repair altered sensation in another. No studies demonstrating autografting of nerve qualified for this systematic review, as the studies all were in the context of a planned resection of mandible for oncologic resection. 40
Vein autografts detailed in this review functioned more as a barrier membrane or conduit than a graft bridging a gap. The veins are often turned inside out, hoping to benefit from the growth factors found on the external surface. 25 Pogrel et al found this technique was more successful with shorter gaps, and Fujita et al found faster recovery with the vein autograft, when compared to primary repair alone. Two studies were able to harvest vein from the head and neck, avoiding a second surgical site. 24,25
Allografts
All studies used Avance® processed nerve allografts by Axogen, which are available in a variety of diameters and lengths, and in this review, 2 out of 3 authors also used the allograft in conjunction with a conduit. Outcomes were similar among studies that did and did not use a conduit. In theory, a direct repair is always thought to be superior to an allograft repair, however Miloro et al 12 suggests that poor outcomes in nerves repaired directly may result from attempting to avoid an allograft, either by not performing a complete resection of neuroma to allow for a primary repair without tension, or settling for a primary repair with some tension. Therefore, using an allograft can give the surgeon freedom to perform a complete resection of the nerve to reach healthy fascicles. Miloro’s study did find superior results with both auto- and allograft, when compared to direct nerve repair, which was attributed to this reasoning. Additionally, this review does prove that allografts are successful, with comparable rates to autografts, while avoiding donor site morbidity. Rate of FSR was similar among LN and IANs compared in this study (87.5% IAN and 92.6% for LN). Although results are promising and have demonstrated success, use of allografts may be cost prohibitive for some centers.
Conduits Without Grafting
All conduits detailed were used with graft-less surgical intervention, however conduits were also utilized after repair with both auto- and allografts. Conduits provide a more consistent technical alignment of the nerve repair, as proper alignment of the fascicles is key for repair, particularly for less experienced surgeons. 41 The limitation of this method is when used without grafting for gaps >6 mm. 41,42 Conduits are more of an adjunctive method enhancing primary or grafted repairs, and are not a reliable method alone for gap reconstruction. Axoguard® and NeuraGen® performed better than Gortex and PGA, achieving FSR in 100% of cases, versus 60% with PGA and 33.3% with Gore-Tex. 43 However, the Gortex study was the only included study with a significant gap. 28 There was high bias when pooling results of these studies as there were vast differences in how the procedures were performed among studies.
Discussion
Treatment of nerve injuries can be difficult as patient symptoms after injury are quite subjective, and often an invasive surgical exploration is needed just to diagnose the severity of injury, and determine appropriate treatment. Intervention is often selected based on surgeon experience and preference, as few maxillofacial surgeons primarily specialized in nerve repair. Thus, this study is important to familiarize surgeons with available treatments and outcome data to utilize evidence-based treatment decisions. One of the most important findings of this study is the need to standardize the way we evaluate and treat nerve injuries. The MRC scale was the most commonly used measure of nerve recovery. It is appropriate and easy to use, and addresses the primary goal of intervention, which is functional recovery, not a full return to baseline.
As there is no gold standard of treatment, likely because the heterogeneity of etiologies and subjectivity of the postoperative symptoms experienced and tolerated by patients, surgeons have many options when planning nerve repair. Based on the synthesis of this systematic review the best results appear to occur when the repair is initiated sooner, with the best time being as soon as the injury is discovered. Ideal treatment will be patient dependent, as it is unacceptable to some patients to lose sensation in another area of the body to recover sensation elsewhere, and for others allografts may be cost prohibitive. Primary repair that is truly tension free is ideal, but if there is any doubt then allograft/autograft should be used to span the gap. Conduits should not be used if there is a gap, and only as an adjunct to better approximate the free nerve ends, considering use of autograft over allograft if the tissues are readily accessible from the surgical access.
Cost is an important consideration in nerve repair. Avance® was the only allograft used in this systematic review, and it comes in various lengths and diameters. In 2020, the average price in the United State, when factoring in all available sizes, and using the pricing for those who routinely use and keep product in stock was $3,790.00 ± $1,630.51 (Range $1,887-6,254). However, purchasing a graft on consignment, or on demand for a single case is significantly more expensive, with prices ranging from $2,417-7,950. The average price of all available Axoguard® sizes is $1,534.70 ± $441.93 (range $1,030-2,283). Again, purchasing a conduit for an individual case is more expensive with prices ranging from $,1260-1,851. In addition, many surgeons utilize both Avance® and Axoguard® for each nerve repair. Although expensive, allografts can prevent potentially greater costs being incurred if there are complications at the donor site.
Limitations of this study include heterogeneity of surgeons, institutions, surgical methods, timing to intervention, different conduit materials, and most importantly, method of reporting outcomes. There was also a paucity of higher-level evidence studies to review. Data was pooled to show general trends, but needs to be evaluated with critical judgment because of the biases mentioned.
Conclusion
There are many studies reporting outcomes management of trigeminal nerve injuries, but most provide low-level evidence. Many different treatment modalities are currently available, however the decision to perform surgical repair should depend first on the ability to complete a tension free repair, and then evaluate the need for adjuvant graft materials. The data of this study supports the ability of surgical intervention to achieve functional sensory recovery in a significant number of subjects, and found evidence for better outcomes with intervention closer to the time of injury. We propose that future studies on this topic should be designed as prospective controlled trials, standardizing timing to intervention, surgical procedures, and use the MRC scale to measure postoperative outcome.
Footnotes
Ethical Approval: IRB approval not needed, study is systematic review, no human or animal subjects.
Patient Consent: Not needed per IRB.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Ashleigh Weyh, MD, DMD, MPH  https://orcid.org/0000-0001-9745-1083
https://orcid.org/0000-0001-9745-1083
Resi Pucci, MD  https://orcid.org/0000-0001-6237-2334
https://orcid.org/0000-0001-6237-2334
References
- 1. Tay ABG, Zuniga JR. Clinical characteristics of trigeminal nerve injury referrals to a university centre. Int J Oral Maxillofac Surg. 2007;36(10):922–927. doi:10.1016/j.ijom.2007.03.012 [DOI] [PubMed] [Google Scholar]
- 2. Lin C, Wu S, Huang H, Lai Y. Systematic review and meta-analysis on incidence of altered sensation of mandibular implant surgery. PLoS ONE. 2016;11(4):e0154082. doi:10.1371/journal.pone.0154082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bagheri SC, Meyer RA. When to refer a patient with a nerve injury to a specialist. J Am Dent Assoc. 2014;145(8):859–861. doi:10.14219/jada.2014.45 [DOI] [PubMed] [Google Scholar]
- 4. Juodzbalys G, Kubilius M. Clinical and radiological classification of the jawbone anatomy in endosseous dental implant treatment. J Oral Maxillofac Res. 2013;4(2):e2. https://www.ncbi.nlm.nih.gov/pubmed/24422030. doi:10.5037/jomr.2013.4202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coulthard P, Kushnerev E, Yates JM, et al. Interventions for iatrogenic inferior alveolar and lingual nerve injury. Cochrane Database Syst Rev. 2014;(4):CD005293. doi:10.1002/14651858.CD005293.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jones RHB. Repair of the trigeminal nerve: A review. Aust Dent J. 2010;55(2):112–119. doi:10.1111/j.1834-7819.2010.01216.x [DOI] [PubMed] [Google Scholar]
- 7. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ. 2009;339: b2700. doi:10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang Y, Sunitha M, Chung KC. How to measure outcomes of peripheral nerve surgery. Hand Clin. 2013;29(3):349–361. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3746316/. doi:10.1016/j.hcl.2013.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wilson MT, Chuang S, Ziccardi VB. Lingual nerve microsurgery outcomes using 2 different conduits: A retrospective cohort study. J Oral Maxillofac Surg. 2017;75(3):609–615. doi:10.1016/j.joms.2016.09.022 [DOI] [PubMed] [Google Scholar]
- 10. Higgins JP, Altman DG. Assessing risk of bias in included studies. In: Cochrane handbook for systematic reviews of interventions. John Wiley & Sons, Ltd; 2008:187–241. https://onlinelibrary.wiley.com/doi/abs/10.1002/9780470712184.ch8 . Accessed April 10, 2020. [Google Scholar]
- 11. Zuniga JR. Sensory outcomes after reconstruction of lingual and inferior alveolar nerve discontinuities using processed nerve allograft—a case series. J Oral Maxillofac Surg. 2015;73(4):734–744. doi:10.1016/j.joms.2014.10.030 [DOI] [PubMed] [Google Scholar]
- 12. Miloro M, Ruckman P, Kolokythas A. Lingual nerve repair: To graft or not to graft? J Oral Maxillofac Surg. 2015;73(9):1844–1850. doi:10.1016/j.joms.2015.03.018 [DOI] [PubMed] [Google Scholar]
- 13. Miloro M, Criddle T. Does low-level laser therapy affect recovery of lingual and inferior alveolar nerve injuries? J Oral Maxillofac Surg. 2018;76(12):2669–2675. doi:10.1016/j.joms.2018.06.001 [DOI] [PubMed] [Google Scholar]
- 14. Robinson PP, Loescher AR, Smith KG. A prospective, quantitative study on the clinical outcome of lingual nerve repair. Br J Oral Maxillofac Surg. 2000;38(4):255–263. http://www.sciencedirect.com/science/article/pii/S0266435600904637. doi:10.1054/bjom.2000.0463 [DOI] [PubMed] [Google Scholar]
- 15. Lam NP, Donoff RB, Kaban LB, Dodson TB. Patient satisfaction after trigeminal nerve repair. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95(5):538–543. doi:10.1067/moe.2003.163 [DOI] [PubMed] [Google Scholar]
- 16. Greenwood M, Corbett IP. Observations on the exploration and external neurolysis of injured inferior alveolar nerves. Int J Oral Maxillofac Surg. 2005;34(3):252–256. https://www.ijoms.com/article/S0901-5027(04)00194-8/abstract. doi:10.1016/j.ijom.2004.06.002 [DOI] [PubMed] [Google Scholar]
- 17. Strauss ER, Ziccardi V, Janal MN. Outcome assessment of inferior alveolar nerve microsurgery: a retrospective review. J Oral Maxillofac Surg. 2004;62(12):47–48. https://www.joms.org/article/S0278-2391(04)00709-8/abstract. doi:10.1016/j.joms.2004.05.185 [DOI] [PubMed] [Google Scholar]
- 18. Pogrel MA. Damage to the inferior alveolar nerve as the result of root canal therapy. J Am Dent Assoc. 2007;138(1):65–69. https://www.meta.org/papers/damage-to-the-inferior-alveolar-nerve-as-the/17197403 [DOI] [PubMed] [Google Scholar]
- 19. Leung YY, Cheung LK. Longitudinal treatment outcomes of microsurgical treatment of neurosensory deficit after lower third molar surgery: A prospective case series. PLoS One. 2016;11(3):e0150149. doi:10.1371/journal.pone.0150149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chang Y, Rodriguez ED, Chu Y, Tsai C, Wei F. Inferior alveolar nerve reconstruction with interpositional sural nerve graft: A sensible addition to one-stage mandibular reconstruction. J Plast Reconstr Aesthet Surg. 2012;65(6):757–762. doi:10.1016/j.bjps.2011.12.028 [DOI] [PubMed] [Google Scholar]
- 21. Schultes G, Gaggl A, Kärcher H. Vascularized transplantation of the long thoracic nerve for sensory reinnervation of the lower lip. Br J Oral Maxillofac Surg. 2000;38(2):138–141. doi:10.1054/bjom.1999.0334 [DOI] [PubMed] [Google Scholar]
- 22. Shimizu F, Ooatari M, Uehara M, Takahashi Y, Kawano K. Effect of concurrent mental nerve reconstruction at the same time as mandibular reconstruction using a fibula osteoseptocutaneous flap. J Plast Reconstr Aesthet Surg. 2015;68(9):1228–1234. doi:10.1016/j.bjps.2015.05.010 [DOI] [PubMed] [Google Scholar]
- 23. Pogrel MA, Maghen A. The use of autogenous vein grafts for inferior alveolar and lingual nerve reconstruction. J Oral Maxillofac Surg. 2001;59(9):985–993. doi:10.1053/joms.2001.25821 [DOI] [PubMed] [Google Scholar]
- 24. Fujita S, Tojyo I, Yamada M, Go Y, Matsumoto T, Kiga N. Outcome following lingual nerve repair with vein graft cuff: A preliminary report. J Oral Maxillofac Surg. 2014;72(7): 1433.e1–7. doi:10.1016/j.joms.2014.03.018 [DOI] [PubMed] [Google Scholar]
- 25. Jones RHB. The use of vein grafts in the repair of the inferior alveolar nerve following surgery. Aust Dent J. 2010;55(2):207–213. doi:10.1111/j.1834-7819.2010.01215.x [DOI] [PubMed] [Google Scholar]
- 26. Yampolsky A, Ziccardi V, Chuang S. Efficacy of acellular nerve allografts in trigeminal nerve reconstruction. J Oral Maxillofac Surg. 2017;75(10):2230–2234. doi:10.1016/j.joms.2017.02.015 [DOI] [PubMed] [Google Scholar]
- 27. Erakat MS, Chuang S, Shanti RM, Ziccardi VB. Interval between injury and lingual nerve repair as a prognostic factor for success using type I collagen conduit. J Oral Maxillofac Surg. 2013;71(5):833–838. doi:10.1016/j.joms.2011.11.026 [DOI] [PubMed] [Google Scholar]
- 28. Pitta MC, Wolford LM, Mehra P, Hopkin J. Use of Gore-Tex tubing as a conduit for inferior alveolar and lingual nerve repair: Experience with 6 cases. J Oral Maxillofac Surg. 2001;59(5):493–496; discussion 497. doi:10.1053/joms.2001.22671 [DOI] [PubMed] [Google Scholar]
- 29. Seo K, Terumitsu M, Inada Y, Nakamura T, Shigeno K, Tanaka Y. Prognosis after surgical treatment of trigeminal neuropathy with a PGA-c tube: Report of 10 cases. Pain Med. 2016;17(12):2360–2368. doi:10.1093/pm/pnw088 [DOI] [PubMed] [Google Scholar]
- 30. Farole A, Jamal BT. A bioabsorbable collagen nerve cuff (NeuraGen) for repair of lingual and inferior alveolar nerve injuries: A case series. J Oral Maxillofac Surg. 2008;66(10):2058–2062. http://www.sciencedirect.com/science/article/pii/S0278239108010471. doi:10.1016/j.joms.2008.06.017 [DOI] [PubMed] [Google Scholar]
- 31. Bagheri SC, Meyer RA, Cho SH, Thoppay J, Khan HA, Steed MB. Microsurgical repair of the inferior alveolar nerve: Success rate and factors that adversely affect outcome. J Oral Maxillofac Surg. 2012;70(8):1978–1990. doi:10.1016/j.joms.2011.08.030 [DOI] [PubMed] [Google Scholar]
- 32. Biglioli F, Allevi F, Lozza A. Surgical treatment of painful lesions of the inferior alveolar nerve. J Craniomaxillofac Surg. 2015;43(8):1541–1545. doi:10.1016/j.jcms.2015.07.036 [DOI] [PubMed] [Google Scholar]
- 33. Pogrel MA. The results of microneurosurgery of the inferior alveolar and lingual nerve. J Oral Maxillofac Surg. 2002;60(5):485–489. doi:10.1053/joms.2002.31841 [DOI] [PubMed] [Google Scholar]
- 34. Zuniga JR, Meyer RA, Gregg JM, Miloro M, Davis LF. The accuracy of clinical neurosensory testing for nerve injury diagnosis. J Oral Maxillofac Surg. 1998;56(1):2–8. doi:10.1016/s0278-2391(98)90904-1 [DOI] [PubMed] [Google Scholar]
- 35. Ricciardi L, Stifano V, Pucci R, et al. Comparison between VII-to-VII and XII-to-VII systematic review and pooled analysis of the functional outcomes. Neurosurg Rev. 2020;44(1):153–161. doi:10.1007/s10143-019-01231-z [DOI] [PubMed] [Google Scholar]
- 36. Nizam SA, Ziccardi VB. Trigeminal nerve injuries: Avoidance and management of iatrogenic injury. Oral Maxillofac Surg Clin North Am. 2015;27(3):411–424. doi:10.1016/j.coms.2015.04.006 [DOI] [PubMed] [Google Scholar]
- 37. Kushnerev E, Yates JM. Evidence-based outcomes following inferior alveolar and lingual nerve injury and repair: A systematic review. J Oral Rehabil. 2015;42(10):786–802. doi:10.1111/joor.12313 [DOI] [PubMed] [Google Scholar]
- 38. Miloro M, Stoner JA. Subjective outcomes following sural nerve harvest. J Oral Maxillofac Surg. 2005;63(8):1150–1154. doi:10.1016/j.joms.2005.04.031 [DOI] [PubMed] [Google Scholar]
- 39. Wessberg GA, Wolford LM, Epker BN. Experiences with microsurgical reconstruction of the inferior alveolar nerve. J Oral Maxillofac Surg. 1982;40(10):651–655. https://www.joms.org/article/0278-2391(82)90115-X/abstract. doi:10.1016/0278-2391(82)90115-X [DOI] [PubMed] [Google Scholar]
- 40. Kanaya F, Firrell J, Tsai TM, Breidenbach WC. Functional results of vascularized versus nonvascularized nerve grafting. Plast Reconstr Surg. 1992;89(5):924–930. doi:10.1097/00006534-199205000-00024 [DOI] [PubMed] [Google Scholar]
- 41. Isaacs J, Safa B, Evans PJ, Greenberg J. Technical assessment of connector-assisted nerve repair. J Hand Surg Am. 2016;41(7):760–766. doi:10.1016/j.jhsa.2016.04.015 [DOI] [PubMed] [Google Scholar]
- 42. Ducic I, Yoon J. Reconstructive options for inferior alveolar and lingual nerve injuries after dental and oral surgery: An evidence-based review. Ann Plast Surg. 2019;82(6):653–660. doi:10.1097/SAP.0000000000001783 [DOI] [PubMed] [Google Scholar]
- 43. Safa B, Buncke G. Autograft substitutes: Conduits and processed nerve allografts. Hand Clin. 2016;32(2):127–140. doi:10.1016/j.hcl.2015.12.012 [DOI] [PubMed] [Google Scholar]